Abstract
The health burden of acute coronary syndrome (ACS) and frailty is high, but the impact of frailty on ACS treatment and outcomes is uncertain. In this structured literature review, we investigated the relationship between frailty, ACS treatment and outcomes. Between 2000 and 2016, we identified only a small number of primary research studies investigating frailty and ACS care (n= 10). Frailty was independently associated with increased mortality following ACS (adjusted all-cause mortality hazard ratios for patients with frailty ranged from 1.54 – 5.39). Older people with frailty were significantly less likely to receive guideline-indicated ACS care, including percutaneous coronary intervention (PCI) (Rates ranged from 6.7% -43.7% vs. 30.4%-69.5%). Available data for PCI indicated a gap between treatment recommended by international guidelines and clinical practice. Further research is warranted to investigate methods for identifying frailty in the acute setting and opportunities for improving care among older people with frailty presenting with ACS.
Keywords: Frailty, acute coronary syndrome (ACS), quality of care, mortality
Introduction
Over half of all people admitted to hospital with acute coronary syndrome (ACS) are elderly and many have substantial multi-morbidity. 1 Moreover, about 10% of those over the age of 65 years and 25-50% of those over the age of 85 years are considered frail.2, 3
Definition of frailty
Frailty is a condition characterised by loss of biological reserves, which leads to failure of homeostatic mechanisms following stressor events .4 An acute myocardial infarction is an example of a stressor event from which an older person with frailty may be at greater risk of adverse outcomes, compared to a fit older person.
Frailty is best understood as a long-term condition but it is especially problematic because it often remains invisible to health and care services until revealed by an unforeseen event. In addition, the severity of frailty at an individual level is important because it is a more reliable predictor for adverse outcomes than chronological age.5, 6 Therefore, UK and international guidelines have recommended routine identification of frailty as part of clinical encounters,7, 8 but this has not yet become embedded as part of routine clinical care, including in the context of acute coronary syndrome.
Frailty models
The phenotype model9 and the cumulative deficit model10 are the two best-established international frailty models. Both have been extensively validated in large epidemiological studies, but are less practical for use in day-to-day clinical practice. The phenotype model identifies frailty on the basis of five physical characteristics: weight loss; exhaustion; low energy expenditure; slow gait speed; and reduced grip strength. People with no characteristics are identified as fit; those with one or two identified as pre-frail; those with three or more are identified as frail. The cumulative deficit model identifies frailty on the basis of a range of 'deficits', which can be clinical signs, symptoms, diseases and disabilities. A frailty index (FI) score is calculated as a proportion of the number of deficits present to the total possible in the model (e.g. if 9/36 deficits are present, the FI score = 0.25). The model is useful as it is very flexible - it has been established that a minimum of 30 deficits are required for a model to be valid.
Simple frailty tools and questionnaires
A range of simple frailty tools and questionnaires are available and validated for use in clinical practice.11 The 2016 UK National Institute for Health and Care Excellence (NICE) multimorbidity guideline12 recommends using one of: gait speed <0.8 m/s; timed up and go test score <12 seconds; self-reported health status score <6; PRISMA-7 questionnaire ≥ 3; self-reported physical activity scale in the elderly (PASE) score ≤56 for men or ≤59 for women to identify the presence of frailty. The NICE guideline cautions against using a performance-based tool in people who are acutely unwell because frailty and acute illness can be conflated using, for example, gait speed. However, the Clinical Frailty Scale10 and Reported Edmonton Frail Scale13 have been validated for use in secondary care, and are potentially useful for inpatient cardiology care. More recently, an electronic frailty index (eFI) has been developed and validated using routine electronic health record data, which is supported in NICE guidance, and may have future application to identify frailty in the context of ACS.11
Frailty and ACS
Although there are well defined pathways for the management of ACS (based upon class 1 guideline recommendations), these have predominantly been based on randomised controlled trial evidence that is not necessarily generalisable to older people with frailty. Existing evidence indicates adherence to guideline recommendations for the management of ACS is suboptimal in both older people and those with multi-morbidity, and resultant outcomes are poor.1, 14, 15 Yet, what is unknown is how frailty interplays with the provision of treatments and subsequent clinical outcomes among patients with ACS. Indeed, to date there is no international consensus as to how patients with frailty and ACS should be managed. Development of new models of ACS care for older people based on individual frailty should be informed by robust research evidence.
No previous reviews have explored the relationship between frailty, quality of treatment and outcomes in older people who experience ACS. We therefore undertook a structured literature review of observational studies and randomised controlled trials to investigate the relationship between frailty, ACS treatment and outcomes.
Methods
We followed the PRISMA guidelines to undertake a structured literature review. A Medline search strategy was developed and adapted for CINAHL, EMBASE, Web of Science and AMED. All databases were searched from 1st January, 2000 to the 26th September, 2016. The search was restricted to English language publications. The full search strategy is available in Appendix 1.
Eligibility Criteria
Eligible studies were those using a validated model to assess frailty in patients during their admission with ACS, defined as AMI (either ST-segment elevation, STEMI, or non-ST-segment elevation myocardial infarction, NSTEMI) and unstable angina. All abstracts were reviewed by two reviewers (OB, FS) and potentially eligible studies retrieved. These articles were reviewed in full against the stated eligibility criteria and reference lists were searched to identify additional articles.
Results
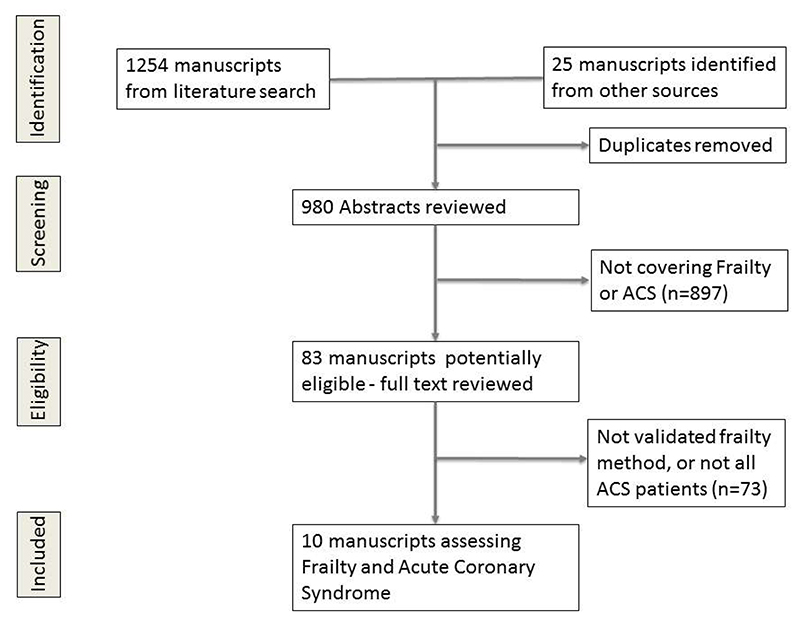
The search strategy identified 980 papers (Figure 1). Of these, 83 were retrieved for detailed evaluation and ten were considered eligible for inclusion based on the stated eligibility criteria.16–25 Three papers studied frailty in a percutaneous coronary intervention (PCI) population that contained both ACS and stable angina patients and were, therefore, not included in the main review.26–28 There was one sub-study of the TRIUMPH registry which assessed gait speed one month after an AMI this was not included as the frailty assessment occurred one month after the index event.29
Figure 1. PRISMA diagram results of literature search.
The ten included papers reported data from a total of 8,773 patients participating in nine individual cohort studies and one randomised controlled trial (RCT).16–25 Two papers reported data from one cohort investigating 30 day and one year mortality.16, 17
Definition of frailty
The studies defined frailty and pre-frailty using a range of validated tools. Two used the phenotype model, or its modified version.24, 25 Four used the Canadian Study of Health and Ageing Clinical Frailty Scale.16, 17, 19, 22 One applied the Edmonton Frail Scale;18 one used the Tilburg Frailty index;20 one used gait speed;21 one used the SHARE-FI index23 (which has been validated in the primary care setting) and one used the Green score, which is a validated frailty tool including measures of grip strength, gait speed and activities of daily living.24 (Supplementary table 1)
Patient population
The papers reported on a range of patient populations. Four papers reported on AMI.16, 17, 21, 22 Two studied whether patients with NSTEMI had the opportunity to receive all appropriate therapies.16, 17 One studied patients with non ST-elevation acute coronary syndrome (NSTEACS) who only received medical management.25 One considered patients with STEMI.21 Six studied patients following hospitalisation with ACS.18–20, 23–25 Table 1 reports the main study characteristics and outcome measures.
Table 1. Study Characteristics.
| Authors | Number patients | Type study | Location | Year Published | Type ACS | Frailty Measure | Age | Prevalence (Frailty) | Prevalence (pre-frailty) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Ekerstad et al. 16 | 307 | Observational Cohort (Three Centres) | Sweden | 2011 | NSTEMI | CFS (Rockwood) | >75 | 48.5% | not defined | All-cause mortality |
| Ekerstad et al. 17 | 307 | Observational Cohort (Three Centres) | Sweden | 2013 | NSTEMI | CFS (Rockwood) | >75 | 48.5% | not defined | All-cause mortality |
| Graham et al.18 | 183 | Prospective Cohort (Single Centre) | Canada | 2013 | ACS | Edmonton | >65 | 30.0% | 36.0% | All-cause mortality |
| Kang et al.19 | 352 | Prospective Cohort (Single Centre) | China | 2015 | ACS | CFS (Rockwood) | >65 | 43.2% | not defined | All-cause mortality |
| Lisiak et al.20 | 91 | Prospective Cohort (Single Centre) | Poland | 2016 | ACS | Tilburg Frailty Indicator | >65 | 82.4% | not defined | Quality of life |
| Matsuwaza et al.21 | 472 | Prospective Cohort (Single Centre) | Japan | 2013 | STEMI | Gait speed | Mean 63.1 (SD 11.8) | 33.5% | not defined | All-cause mortality and recurrent cardiac events |
| Myers et al.22 | 1521 | Observational Cohort (Eight Centres) | Israel | 2014 | post MI | CFS (Rockwood) | Mean 55 (SD 7) | 5.1% | 34.7% | All-cause mortality |
| Salinas et al.23 | 202 | Observational Cohort (Four Centers) | Spain | 2016 | ACS | SHARE-FI | >75 | 35.1% | 36.6% | All-cause mortality and MACE |
| Sanchis et al.24 | 342 | Prospective Cohort (Single Centre) | Spain | 2014 | ACS | Fried and Green | >65 | 47.0% | not defined | All-cause mortality |
| White et al.25 | 4996 | Sub study of TRILOGY | Multi Centre | 2015 | ACS | Fried | >65 | 4.7% | 23.0% | Cardiovascular death, MI or stroke |
Abbreviations; Ml (myocardia infarction); ACS (Acute Coronary Syndrome); NSTEMI (Non ST elevation myocardial infarction); STEMI (ST elevation myocardial infarction); CFS (Clinical Frailty Scale); MACE (major adverse cardiovascular events); SHARE-FI (Survey of Health, Ageing and Retirement in Europe – Frailty Index).
Five studies reported higher prevalence of frailty amongst female patients16–18, 22, 25, and three demonstrated higher prevalence in men.19, 21, 24 However, none of the differences were statistically significant. Frail participants were typically older (74.6 years) than non-frail participants (mean age 69.8 years). 16, 17, 22, 25
Prevalence of frailty
The median reported prevalence of frailty across the studies was 31.5% (range 4.7% to 82.4%).20, 25 The median reported prevalence of pre-frailty was 35.4% (range 23.0% to 36.6%).18, 25 The lowest prevalence was seen in the TRILOGY ACS randomised controlled trial, at 4.7% for frailty and 23.0% for pre-frailty, defined using the phenotype model.25
Mortality
Nine manuscripts described a statistically significantly higher mortality rate in participants with frailty compared to those defined as non-frail.16–19, 21–25 One manuscript did not report mortality.20 Mortality was measured at a variety of points from in hospital23 to 13 years (Table 2).22 Mortality was adjusted for age, sex and clinical variables. Several studies employed the coronary artery disease (CAD) specific index as a measure of comorbidity, which includes current smoker, hypertension, and history of cerebrovascular disease, diabetes, chronic obstructive pulmonary disease, peripheral vascular disease, malignancy, and chronic kidney disease. Three studies reported cardiovascular mortality in addition to all-cause mortality. All showed higher rates in older people with frailty compared with those who were not frail.21, 22, 25 One study reported that faster walking speed, used to identify fit older people, was independently associated with reduced mortality. For every 0.1m/s increment in gait speed significant reductions in all-cause mortality (HR 0.71, 95% CI 0.62 to 0.82) and cardiovascular mortality (HR 0.65, 95% CI 0.55 to 0.81) were observed.
Table 2.
Adjusted risk of mortality for older people with frailty following acute coronary syndromes and percutaneous coronary intervention. For all outcomes, the comparator is older people defined as fit.
| Study | Frailty prevalence (%) | Study Population | Follow-up | Adjusted risk of all-cause mortality† | Adjusted cardiovascular mortality |
|---|---|---|---|---|---|
| Salinas et al.23 | 35.1% | ACS | In-hospital | OR 12.1 (95% CI 1.4-103) | Not defined |
| Ekerstad et al.16 | 48.5% | NSTEMI | 30 days | OR 2.17 (95% CI 1.28-3.67) | Not defined |
| Kang et al.19 | 43.2% | ACS | 4 months | HR 5.39 (95% CI 1,48-19.69) | Not defined |
| White et al.25 | 4.7% | ACS | 17 months | HR 1.54 (95% CI 1.13-2.08) | HR 1.41 (95% CI 1.00-1.99) |
| Sanchis et al.24 | 47.0% | ACS | 25 months | HR 3.4 (95% CI 1.8-6.2) | Not defined |
| Ekerstad et al.17 | 48.5% | NSTEMI | 1 year | HR 4.3 (95% CI 2.4-7.8) | Not defined |
| Graham et al.18 | 30.0% | ACS | 1 year | HR 3.49 (95% CI 1.08-7.61) | Not defined |
| Matsuwaza et al.21 | 33% | STEMI | 5.5 years | For increasing 0.1m/s gait speed HR 0.71 (95% CI 0.62-0.82) | For increasing 0.1m/s gait speed HR 0.65 (95% CI 0.55-0.81) |
| Myers et al.22 | 5.1% | MI | 13 years | HR 2.02 (95% CI 1.46-2.79) | 2.38 (95% CI 1.49-3.82) |
Abbreviations: MI (myocardial infarction); ACS (Acute Coronary Syndrome); NSTEMI (Non ST elevation myocardial infarction); STEMI (ST elevation myocardial infarction); OR, odds ratio; HR, hazard ratio; CI, confidence interval.
Adjustment was performed for age, sex, gender and clinical variables. Myers et al. also adjusted for socioeconomic status
Invasive Coronary Procedures
Seven papers reported the use of invasive coronary procedures among participants with frailty, and those defined as non-frail. 16–19, 21, 23, 25
Coronary angiography
Six papers reported on coronary angiography. When the populations were subdivided, the rates of angiography among participants defined as frail and non-frail were 75.7% vs. 85.0% (P=0.027),19 15.4% vs. 46.2% (P<0.001),16, 17 66.2% vs. 93.1% (P<0.001),23 and 58.2% vs. 88.9% (P<0.001).18 The one randomised controlled trial found no statistically significant difference in the rate of angiography pre- or post-randomisation to either clopidogrel or prasugrel; rates of angiography were 53.2% for those with frailty; 45.9% for those with pre-frailty; 48.2% for those with no frailty.25
One study considered the characteristics of those frail patients who received angiography against those who did not. The only statistically significant variable was age (mean age 86 vs. 80 years, P<0.001), however, in general those who received angiography were younger, more likely to be male and less likely to have dementia, congestive heart failure or severe renal disease (GFR<30ml/min).16
Percutaneous coronary intervention
Three papers investigating use of PCI in older people following ACS reported rates of 6.7 vs. 30.4%,16 16.4 vs. 36.5%,18 and 43.7% vs. 69.5%23 in frail and non-frail patients respectively. One manuscript investigated the use of coronary artery stent implantation in those receiving PCI and reported a non-statistically significant decrease in use for those with frailty compared with non-frail patients (67.1% vs. 69.7%, P=0.83).21 One study suggested that frail patients were less likely to receive complete revascularisation than their non-frail counterparts (28.2% vs. 46.6%, P<0.001).23 In another study, of whom 82.4% were reported to be frail, receipt of PCI was associated with a better quality of life than those who were managed conservatively (P=0.043).20
Coronary artery bypass grafting
One paper offered details about coronary artery bypass grafting rates and found no significant difference in rates of coronary artery bypass grafting among participants with frailty (9.1% vs. 12.7%, P=0.364).18
Pharmacological management
Two articles investigated pharmacological management among frail older people with ACS. The first a sub study of the Trilogy-ACS trial which compared the use of prasugrel with clopidogrel, compared medication management by frailty status; this was only studied at point of randomisation (patients assigned to either clopidogrel or prasugrel) and not at hospital discharge. All patients in this study received medical management. Increasing frailty was associated with a decrease in use of statins (P=0.011) and angiotensin converting enzyme inhibitors (ACEi) or angiotensin II receptor blockers (ARB) (P<0.001). There was no statistically significant difference in use of β-blockers between frail and non-frail patients (P=0.141). People with frailty were less likely to receive proton pump inhibitors (P=0.009).25 Older people with frailty randomised to prasugrel experienced lower rates of the composite endpoint of cardiovascular death, MI or stroke, and lower all-cause mortality than those randomised to clopidogrel. However, older people with frailty were more likely to have bleeding events on prasugrel, compared with clopidogrel.25
The other study found that the prescription of medications at discharge from hospital did not vary across tertiles of gait speed.21 Those with slower gait speeds were less likely to be prescribed an ACEi/ARB (P=0.001) and statins (P=0.003), though there was no statistically significant difference in prescription of β-blockers (P=0.18). The study reported additional characteristics of those who were not prescribed ACEi/ARB and statins. Participants who did not receive these medications were older, had reduced renal function and lower levels of low-density lipoproteins (LDL).21 Neither study demonstrated a difference in the rate of prescription of aspirin for frail and non-frail patients.
Hospital stay
Four studies provided information on where patients received their treatment.16, 17, 19, 22 Three identified that older people with frailty were less likely to receive treatment in an intensive cardiac unit,16, 17, 22 whilst one reported that frail patients were more likely to be cared for in an intensive cardiac unit than non-frail (32.9% vs. 20.5% P=0.009).19
Three studies reported that frail patients had longer length of stay, compared to non-frail patients (13.4 vs. 7.5 bed days, P<0.00116, 17 and 12.7 vs. 7.0 bed days, P=0.03018). Whilst another study found no statistically significant difference in lengths of hospital stay between the frail and non-frail (6.8 vs. 10.0 bed days, P=0.666).23
Re-admission
Four papers provided information on unplanned re-admissions.16, 17, 19, 22 One study reported no statistically significant difference in readmission for people with frailty compared to fit older people at either one month (29.9% vs. 21.9%, P=0.138) or 12 months (61.7% vs. 67.7%, P=0.28).16 17 One demonstrated a statistically significant increase in readmission (12.0% vs. 32.2% P<0.001).19 Another study with 5.5 year follow-up data reported higher rates of re-admission among older people with frailty compared with those who were not frail (rate ratio of 3.31, 95% CI 2.57-4.27); an effect which persisted after adjustment for age, sex, clinical and socioeconomic variables (adjusted rate ratio 2.14, 95% CI 1.63-2.81).22 Two studies provided information on the reasons for readmission. A cardiovascular cause was identified in 14% and 50% of patients in these studies, respectively.17, 19
Quality of life
One study focused on the effects of the ‘frailty syndrome’ on quality of life (as assessed by the MacNEW Heart disease Health related Quality of Life questionnaire) it was completed prior to discharge from hospital, the authors demonstrated a negative correlation between frailty and quality of life in patients experiencing an ACS.20
Discussion
Our review has identified that frailty and pre-frailty are common in older people experiencing ACS, with a median prevalence estimate of 31.5% for frailty and 35.4% for pre-frailty across included studies. We have also summarised evidence indicating that the presence of frailty and pre-frailty identifies patients at increased risk of mortality following admission to hospital with ACS.
A range of validated frailty assessment tools were used in the studies. Although clinicians might wish to select tools to align with local service requirements, we recommend the Clinical Frailty Scale as a tool that is practical, validated in a secondary care setting, identified as the most commonly applied standardised frailty assessment in an ACS research context, and is predictive of adverse ACS outcomes. Alternative simple tools, such as gait speed, might be considered for ACS patients who are ambulant, or as part of an outpatient workup. The eFI is a frailty identification tool that has been developed using routine data.11 It has been widely implemented in the UK but is based on international standard coding systems, so has potential for future global implementation, depending on future validation.
Despite evidence for increased risk of mortality, rates of coronary angiography and PCI among older people with frailty are low. There was no evidence to indicate differences in use of aspirin or β-blockers for those with frailty, but evidence indicates lower rates of ACEi, ARB and statin prescription.
Previous research has demonstrated improved outcomes for those invasively managed following ACS.15 Our review has identified that people with frailty were less likely to receive an invasive coronary strategy.16, 18, 25 One RCT reported similar rates of PCI for those with frailty, pre-frailty and no frailty, but prevalence estimates for frailty in this study were low, indicating that the study population may not be representative. Furthermore, the tightly controlled RCT environment may have precluded deviation from the trial protocol for participants based on clinical judgment regarding suitability for PCI, limiting generalisability of findings to routine clinical practice.
People with frailty who received coronary angiography had lower revascularisation rates than less frail counterparts. 16, 18, 23 This may be, in part, a reflection of the more complex coronary artery disease identified in those with frailty, who were more likely to have left main stem disease, three vessel disease or proximal disease, which may not be amenable to PCI.27, 28 However, it is also possible that lower rates reflect an aversion to a perceived risk of invasive management in frailty, whereby those with potential to gain benefit may have been deemed not appropriate for coronary intervention.
The review has identified a possible difference in rates of management on intensive cardiac units depending on individual frailty. Three studies recorded lower rates of admission to intensive cardiac units16, 17, 22 whilst one demonstrated higher rates of admission to such units for people with frailty.19 Those which showed a negative association with frailty were conducted in 1992-9322 and 2009-10,16, 17 whilst the one with a positive association was conducted in 2014-15.19 It is possible that the differences observed may be due in part to the increasingly frail hospital demographic in modern healthcare systems, but also may indicate potential changes in recognition and understanding of frailty amongst clinicians over this time period.
Evidence from this review indicates that older people with frailty have a longer length of hospital stay, and that there is uncertainty regarding the association between frailty and rehospitalisation following ACS; rates vary between 14% and 50% for cardiovascular-related readmissions.
There was also a paucity of information on medication management for those with frailty and ACS. The two available studies identified lower rates of ACEi, ARB and statin prescribing following ACS. Prescribing decisions are especially complex for those with frailty, who are at increased risk of medication-related side effects. Careful clinical judgment is required to weigh up the compromise between risk of harm due to side effects and longer-term benefit based on likely duration of treatment.21 Some older people with advanced frailty may be entering the terminal phase of life, and a decision to withhold a medication that may not provide overall benefit and may increase polypharmacy burden and risk of side effects may be considered appropriate.
The 2016 NICE multimorbidity guideline includes a database of treatment effects summarising the benefits/harms of a range of medications in multimorbid patients. 30 In addition to standard numbers needed to treat (NNTs) the database includes additional information on treatment time horizon, which enables calculation of annualised numbers needed to treat (ANNTs), which is the number of people requiring treatment per year to receive benefit. ANNT estimates are especially helpful for making judicious treatment decisions for older people with advancing frailty, some of whom may be in the terminal stage of life. Treating 1000 people with statins for one year would result in four fewer cardiovascular deaths.30
Lower levels of provision of ACS care may be the result of a lack of randomised evidence to guide ACS management in the context of frailty. We identified only one RCT that used a validated tool to assess frailty. Within this study, prevalence of frailty was notably low at 4.7%, compared to our median prevalence estimate across studies of 38.1%.25 In this trial, rates of cardiovascular and all-cause mortality for people with frailty were lower than reported in observational studies. This may be because the trial participants did not include people with advanced frailty in the terminal phase of life, which would be consistent with the relatively low study prevalence of frailty. The study did show that frail patients benefited more with respect to outcomes if they received prasugrel, however, this group had higher rates of bleeding complications. The bleeding complications may in part be due to a lower use of proton pump inhibitors in the frail group.25
Only one study assessed quality of life outcomes showing that those with frailty have worse quality of life outcomes than those without frailty. This is supported by a study of a mixed population of ACS and stable angina patients reporting health-related quality of life in participants immediately after PCI using the Short-Form 36 item health questionnaire (SF36) and quality of life scale of the Seattle Angina Questionnaire (SAQ). Those with frailty had lower physical and mental health-related quality of life summary scores on the SF36 (P<0.001) and lower SAQ quality of life scores (P=0.013), compared with those defined as fit.26 Greater consideration of non-mortality driven outcomes, such as morbidity, healthcare utilisation and quality of life are desirable when assessing the efficacy of ACS interventions among this group.
To our knowledge, this is the first review reporting international data about the prevalence, management and outcomes of ACS in older people with well-defined frailty. The review was supported by a robust search strategy that has enabled a comprehensive review of the available literature. We only included data from studies that used a validated tool to identify frailty in participants and had full text available for assessment. An important limitation of this review is that ambiguity remains regarding whether the association between frailty and mortality represents the loss of biological reserves associated with the condition, or is the result of under treatment of this high-risk group. Furthermore, we excluded three studies that investigated outcomes of PCI in mixed ACS and stable angina populations but did not report results by subgroup. These studies reported no clear difference in mortality at 30 days between those with frailty undergoing PCI for ACS or stable angina, compared to those without.26 Three-year rates of MI or mortality for those with frailty were higher, but increased mortality rates in people with frailty at more distant time points are not necessarily unexpected, and cannot be reliably associated with PCI treatment.27 The investigation of outcomes of PCI in frail and non-frail older people with ACS is an important area for future investigation because, should similar outcomes be confirmed, this may indicate that the higher mortality following ACS for older people with frailty is potentially modifiable through appropriate treatment. We also excluded a study that performed frailty assessments one month after the index event; they demonstrated that slow gait speed (≤0.8m/s) was present in 53.6% of patients and that those with slow gait speed had worse outcomes (including mortality and readmission) at one year. However, they did note that readmission was the predominant driver of their composite endpoint and that only 41.9% of the readmissions in the slow gait speed group were for a cardiovascular cause.29
Future challenges
Presently, there is only preliminary evidence to guide decision making in the management of frail patients with ACS, and establishment of a more robust evidence base is required. The under-representation of older people with frailty in RCTs of ACS interventions risks excluding those at greatest risk of adverse outcomes following ACS and limits the generalisability of trial findings. The one RCT that was included in the review reported similar drop-out rates for patients with both frailty and pre-frailty, but these groups demonstrated significantly higher drop-out rates than the non-frail group. These estimates should be considered when designing future RCTs of ACS interventions involving people with frailty. Within the UK, the RINCAL study aims to look at revascularisation versus a conservative strategy in patients >80, although there it is unclear whether there will be a frailty assessment involved within the study.31 The ICON1 study is a prospective observational study that follows patients with NSTEMI >75 years, which utilises both the phenotype model and cumulative deficit frailty index to assess frailty.32 The Italian STORM study used the Gold standards framework (GSF) as a surrogate for frailty and similarly to our study found a reduction in the rates of PCI. Use of the GRACE risk score (as per existing guidelines33, 34) was determined to be accurate at predicting cardiovascular events, however did not predict death from other causes.35
Design of future RCTs of ACS interventions should include methods to select and stratify participants on the basis of individual frailty to help guide appropriate decision-making based on an individual balance of risk and benefit. Resultant evidence can then contribute to the development of clinical guidelines for ACS management that consider the complex challenges that are commonly encountered by clinicians caring for older people with frailty.
Conclusion
This structured review found that of the limited studies to date, nearly a third of older people presenting to hospital with ACS are to be frail or pre-frail. These people, at increased risk of mortality following ACS, are less likely to receive an invasive coronary strategy and pharmacological therapies. To inform new models of ACS care that consider individual frailty, research investigating the association between frailty, coronary interventions, pharmacological therapy and outcomes will be necessary. In addition, there is a need for a frailty assessment tool for cardiovascular patients that can be used in the acute setting to help guide appropriate care to achieve optimal patient-centred outcomes.
Supplementary Material
Acknowledgments
We wish to thank Deirdre Andre, University of Leeds, for her help in designing and performing the literature search.
Funding
There was no external funding for this review.
None
Footnotes
Competing interests: There are no competing interests
Ethics: n/a
Conflicts of interest
The authors report no conflicts of interest.
References
- 1.Gale CP, Cattle B, Woolston A, et al. Resolving inequalities in care? Reduced mortality in the elderly after acute coronary syndromes. The Myocardial Ischaemia National Audit Project 2003-2010. Eur Heart J. 2011:ehr381. doi: 10.1093/eurheartj/ehr381. [DOI] [PubMed] [Google Scholar]
- 2.Campbell AJ, Buchner DM. Unstable disability and the fluctuations of frailty. Age Ageing. 1997;26:315–8. doi: 10.1093/ageing/26.4.315. [DOI] [PubMed] [Google Scholar]
- 3.Song X, Mitnitski A, Rockwood K. Prevalence and 1 O-Year Outcomes of Frailty in Older Adults in Relation to Deficit Accumulation. J Am Geriatr Soc. 2010;58:681–7. doi: 10.1111/j.1532-5415.2010.02764.x. [DOI] [PubMed] [Google Scholar]
- 4.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–62. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson TN, Wu DS, Pointer L, Dunn CL, Cleveland JC, Moss M. Simple frailty score predicts postoperative complications across surgical specialties. The American Journal of Surgery. 2013;206:544–50. doi: 10.1016/j.amjsurg.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winograd CH, Gerety MB, Chung M, Goldstein MK, Dominguez F, Vallone R. Screening for frailty: criteria and predictors of outcomes. Journal of the American Geriatrics Society. 1991;39:778–84. doi: 10.1111/j.1532-5415.1991.tb02700.x. [DOI] [PubMed] [Google Scholar]
- 7.Turner G, Clegg A. Best practice guidelines for the management of frailty: a British Geriatrics Society, Age UK and Royal College of General Practitioners report. Age and ageing. 2014;43:744–7. doi: 10.1093/ageing/afu138. [DOI] [PubMed] [Google Scholar]
- 8.Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. Journal of the American Medical Directors Association. 2013;14:392–7. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults evidence for a phenotype. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2001;56:M146–M57. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 10.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. Canadian Medical Association Journal. 2005;173:489–95. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clegg A, Bates C, Young J, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age and ageing. 2016;45:353–60. doi: 10.1093/ageing/afw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE (NICE) Multimorbidity: clinical assessment and management (NG56) NICE; London: 2016. [Google Scholar]
- 13.Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age and ageing. 2006;35:526–9. doi: 10.1093/ageing/afl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simms AD, Batin PD, Kurian J, Durham N, Gale CP. Acute coronary syndromes: an old age problem. J Geriatr Cardiol. 2012;9:192. doi: 10.3724/SP.J.1263.2012.01312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alabas OA, Allan V, McLenachan JM, Feltbower R, Gale CP. Age dependent improvements in survival after hospitalisation with acute myocardial infarction: An analysis of the Myocardial Ischemia National Audit Project (MINAP) Age Ageing. 2014;43:779–85. doi: 10.1093/ageing/aft201. [DOI] [PubMed] [Google Scholar]
- 16.Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation. 2011;124:2397–404. doi: 10.1161/CIRCULATIONAHA.111.025452. [DOI] [PubMed] [Google Scholar]
- 17.Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with 1-year mortality for elderly patients with non-ST-segment elevation myocardial infarction. Eur JPrev Cardiol. 2013:2047487313490257. doi: 10.1177/2047487313490257. [DOI] [PubMed] [Google Scholar]
- 18.Graham MM, Galbraith PD, O’Neill D, Rolfson DB, Dando C, Norris CM. Frailty and outcome in elderly patients with acute coronary syndrome. Can J Cardiol. 2013;29:1610–5. doi: 10.1016/j.cjca.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Kang L, Zhang SY, Zhu WL, et al. Is frailty associated with short-term outcomes for elderly patients with acute coronary syndrome? J Geriatr Cardiol. 2015;12:662–7. doi: 10.11909/j.issn.1671-5411.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lisiak M, Uchmanowicz I, Wontor R. Frailty and quality of life in elderly patients with acute coronary syndrome. Clin IntervAging. 2016;11:553. doi: 10.2147/CIA.S99842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuzawa Y, Konishi M, Akiyama E, et al. Association between gait speed as a measure of frailty and risk of cardiovascular events after myocardial infarction. J Am Coll Cardiol. 2013;61:1964–72. doi: 10.1016/j.jacc.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 22.Myers V, Drory Y, Gerber Y. Clinical relevance of frailty trajectory post myocardial infarction. Eur JPrev Cardiol. 2014;21:758–66. doi: 10.1177/2047487312462828. [DOI] [PubMed] [Google Scholar]
- 23.Salinas GLA, Fernández MS, Izco MP, et al. Frailty is a short-term prognostic marker in acute coronary syndrome of elderly patients. Eur Heart J Acute Cardiovasc Care. 2016:2048872616644909. doi: 10.1177/2048872616644909. [DOI] [PubMed] [Google Scholar]
- 24.Sanchis J, Bonanad C, Ruiz V, et al. Frailty and other geriatric conditions for risk stratification of older patients with acute coronary syndrome. Am Heart J. 2014;168:784–91. doi: 10.1016/j.ahj.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 25.White HD, Westerhout CM, Alexander KP, et al. Frailty is associated with worse outcomes in non-ST-segment elevation acute coronary syndromes: Insights from the TaRgeted platelet Inhibition to cLarify the Optimal strateGy to medicallY manage Acute Coronary Syndromes (TRILOGY ACS) trial. Eur Heart J Acute Cardiovasc Care. 2015 doi: 10.1177/2048872615581502. [DOI] [PubMed] [Google Scholar]
- 26.Gharacholou SM, Roger VL, Lennon RJ, et al. Comparison of frail patients versus nonfrail patients >65 years of age undergoing percutaneous coronary intervention. Am J Cardiol. 2012;109:1569–75. doi: 10.1016/j.amjcard.2012.01.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh M, Rihal CS, Lennon RJ, Spertus JA, Nair KS, Roger VL. Influence of frailty and health status on outcomes in patients with coronary disease undergoing percutaneous revascularization. Circ Cardiovasc Qual Outcomes. 2011;4:496–502. doi: 10.1161/CIRCOUTCOMES.111.961375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murali-Krishnan R, Iqbal J, Rowe R, et al. Impact of frailty on outcomes after percutaneous coronary intervention: a prospective cohort study. Open Heart. 2015;2:e000294. doi: 10.1136/openhrt-2015-000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dodson JA, Arnold SV, Gosch KL, et al. Slow Gait Speed and Risk of Mortality or Hospital Readmission After Myocardial Infarction in the Translational Research Investigating Underlying Disparities in Recovery from Acute Myocardial Infarction: Patients’ Health Status Registry. J Am Geriatr Soc. 2016 doi: 10.1111/jgs.14016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Institute For Health And Care Excellence. Multimorbidity: clinical assessment and management; Tools and resources. NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE, (NICE); London: 2016. Database detailing beneifts/harms of medications in multimorbid patients. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.