Abstract
Background
There is a paucity of information about treatment and mortality trends following acute myocardial infarction (AMI) for cancer survivors (CS).
Methods
This was a population-based study to compare temporal trends of treatments and outcomes (mortality, non-fatal cardiovascular outcomes), among CS and non-cancer patients (NCP) with AMI in Ontario (Canada), using inverse probability treatment weight (IPTW) adjusted modeling.
Results
Of 270,089 patients with AMI (22,907 CS; 247,182 NCP; 1995-2013; median follow-up 10.1 and 11.0 years, respectively), use of invasive coronary strategy and pharmacotherapies increased, and mortality declined for CS and NCP (all p-for-trend<0.001). At 30 days following AMI, there was no difference between CS and NCP for use of coronary angiography [incidence risk ratio (IRR) 0.98, 95% confidence interval (CI) 0.96-1.01, p=0.23], percutaneous coronary intervention (IRR 0.98, 95%CI 0.94-1.02, p=0.29), and bypass (IRR 0.93, 95%CI 0.85-1.02, p=0.11). At 90 days following AMI, there was no difference for usage of β-blockers, clopidogrel, or nitrates, but CS were prescribed less ACEi/ARB and statins. CS had higher all-cause mortality at 30 days [adjusted hazard ratio (HR) 1.12, 95%CI 1.07-1.17, p<0.001], 1-year (1.16, 95%CI 1.12-1.20, p<0.001) and long-term (HR 1.21, 95%CI 1.17-1.25, p<0.001), and greater risk of heart failure (HF) (HR 1.08, 95%CI 1.03-1.14, p=0.001), but not myocardial re-infarction (HR 0.98, 95%CI 0.95-1.01, p=0.22) or stroke (HR 1.06, 95% CI 0.97-1.16, p=0.18).
Conclusions
Among CS and NCP with AMI in Ontario, similar improvements in mortality and use of treatments were observed between 1995 and 2013. However, compared with NCP, CS had a higher risk of mortality and HF.
Keywords: myocardial infarction, cardiovascular outcomes during cancer survivorship, mortality outcomes, cancer survivorship, temporal trend
Introduction
Cardiovascular disease and cancer are leading causes of mortality worldwide.1 Over the last two decades, deaths due to cancer have declined due to earlier detection and modern treatment regimens. Many patients diagnosed with cancer will not die from it; often, their cancer is either cured or becomes a chronic disease when cure cannot be achieved, leading to a growing population of cancer survivors.2 Likewise, there has been a global decline in deaths from acute myocardial infarction (AMI), associated with an increase in the use of guideline-indicated prevention strategies and treatments.3, 4
Data suggest that non-cancer related mortality such as cardiovascular disease has become increasingly important during cancer survivorship.5, 6 Many types of cancer has been associated with increased risk of coronary artery disease.7 Cancer and coronary artery disease have many shared risk factors, including tobacco and sedentary lifestyle;8–10 cancer is associated with hypercoagulability and atherosclerosis,11 and cardiovascular events such as heart failure (HF) and ischemia.12–14
Management of cardiovascular diseases among cancer survivors pose a unique challenge to clinicians.15, 16 In part, this is because there is a paucity of detailed, yet generalizable information regarding cardiovascular care and outcomes following AMI in cancer survivors compared with non-cancer patients. Certainly, preventative care and treatment of cardiovascular comorbidities is critically important to improve outcomes during survivorship in this vulnerable population. Multisource electronic health records provide an opportunity to perform high resolution investigations of common diseases. Accordingly, providing insights into longer-term outcomes for cancer survivors with AMI may highlight opportunities for enhanced delivery of cardiovascular care. Herein, we sought to investigate whether the previously reported increase in use of treatments for AMI (in non-cancer patients) and associated decline in mortality were evident among Ontario (Canada) cancer survivors with AMI.
Methods
Data sources
Patient characteristics, chemotherapies, medications, and outcome information were obtained from the following Ontario databases: Registered Persons Database (RPDB) for demographics and vital status; Ontario Cancer Registry (OCR) for cancer diagnosis; Canadian Institute of Health Information Discharge Abstract Database (CIHI-DAD) for admission and discharge data; Ontario Health Insurance Plan (OHIP) for cardiac risk factors and comorbidities; Ontario Drug Benefits (ODB) for medication data for patients ≥65 years; and CIHI-National Ambulatory Care Reporting System (CIHI-NACRS) for emergency department visits; New Drug Funding Program (NDFP), for adjuvant systemic therapy details. Detailed information are reported in Supplementary Table S1.
Cohort selection
Although there is no uniform definition for cancer survivors,17 we aimed to identify a subset of cancer patients in clinical remission,18, 19 to address whether a diagnosis of cancer in the past results in unjustifiable biases in cardiovascular care, and to delineate any actionable care gaps. Accordingly, our main objective was to evaluate cardiovascular care and outcomes following an AMI that occurred during cancer survivorship in those with no apparent recurrent or metastatic disease (to isolate the effect of a past history of cancer diagnosis from active malignancy).
Cancer survivors who were unlikely to be in cancer relapse were identified by the following criteria: (1) index AMI occurring at least 1 year after initial cancer diagnosis (cancer types in Supplementary Table S3); (2) survived beyond 1 year from date of cancer diagnosis without a second primary cancer diagnosis; (3) no receipt of chemotherapy or radiation therapy beyond 1 year after diagnosis (surrogate of relapse and/or progression).
For cohort derivation, all patients aged ≥18 with AMI during index hospital admission between January 1995 and December 2013 in Ontario, Canada were identified through CIHI-DAD20 and stratified as a cancer survivor (solid or hematologic) or non-cancer patient (Supplementary Figure S1). The International Classification of Diseases diagnostic codes were used to ascertain AMI cases (Supplementary Table S2; ICD-9 410, ICD-10 I20, ICD-10 I21, ICD-10 I22, and ICD-10 I25). Following AMI, patients’ treatments received and clinical outcomes observed were tracked by linkage of their unique code to the seven databases, and upon provision of data for analysis, patient information was de-identified.
The primary outcome was all-cause mortality at 30-days, 1-year, and at final follow-up December 2014. The secondary outcomes were: non-fatal cardiovascular hospital admission for HF (ascertained using a validated algorithm for identifying HF21), myocardial re-infarction or stroke that occurred during overall follow-up and within 1-year of AMI; rates of use of an invasive coronary strategy including coronary angiography, percutaneous coronary intervention (PCI), and coronary artery bypass graft (CABG) surgery that occurred within 30 days of AMI; cardiovascular pharmacotherapies prescribed within 90 days (only conducted for patients who were ≥65 years old, as no reliable medication data were available for patients <65 years old). Subsequently, the temporal relationship of the above outcomes with time (from 1995 to 2013) were examined and compared between cancer survivors and non-cancer patients.
Ethical Approval
Research Ethics Board (REB) approval was obtained from Sunnybrook Health Sciences Centre (REB number 033-2013, approved April 2013).
Statistical Analysis
Unadjusted and adjusted baseline characteristics were described as percentages for all baseline categorical variables.
Given the potential for confounding by indication, we employed propensity score derived inverse probability of treatment weights (IPTW) to directly compare outcomes in cancer survivors and non-cancer patients. First, propensity scores were calculated using logistic regression and comprised age, gender, rural residence, income, geographical location, hypertension, diabetes, dyslipidemia, anemia, gastrointestinal bleed, stroke, peripheral vascular disease, arrhythmias, Charlson comorbidity score, aggregated disease groups (ADG), baseline cardiovascular medications, and receipt of coronary angiography, PCI, or CABG surgery prior to index AMI. Subsequently, IPTWs were computed as the reciprocal of the propensity score.
Time-to-first non-fatal cardiovascular outcomes and mortality were compared between cancer survivors and non-cancer patients using IPTW-adjusted Cox proportional hazard modeling. Patients who did not develop the corresponding endpoint by the end of observation period (December 2014) were censored. Exploratory analyses were conducted to compare HF risk in breast cancer survivors, many of whom likely received anthracycline-based chemotherapy with or without trastuzumab (well-known to cause type 1 and type 2 cardiotoxicity, respectively13), and non-breast cancer survivors to non-cancer patients. The use of an invasive coronary strategy within 30 days and pharmacotherapies within 90 days following AMI, was estimated using IPTW-adjusted modified Poisson regression.22
For temporal trends of IPTW-adjusted rates of cardiac care and outcomes following AMI between 1995 and 2013, Poisson regression was used to estimate annual average percent change (AAPC) and p-for-trend for both cancer survivors and non-cancer patients. Ancillary analyses were conducted to delineate whether a significant interaction exists between cancer survivor status and time for each outcome, by using logistic regression to obtain p-for-interaction.
To ascertain the influence of the length of time from cancer diagnosis to index AMI, we conducted subgroup analysis which segregated cancer patients into those who had AMI 1-5 years within cancer diagnosis or more than 5 years after cancer diagnosis. Adjusted comparison of mortality and non-fatal cardiovascular outcomes were conducted as above.
For all analysis, a two-tailed P<0.05 was considered statistically significant. Statistical analysis was performed using SAS v9.4 (SAS, Inc., Cary, NC).
Results
Patient characteristics
We identified 270,089 AMI patients, of whom 22,907 (8.5%) were cancer survivors [the most prevalent cancers were prostate 25.7% (5886), colorectal 16.5% (3788) and breast 16.1% (3684)] and 247,182 (91.5%) non-cancer patients. There were more men than women in both cancer (58.4%) and non-cancer group (62.5%). Before weighted adjustment of baseline characteristics (Table 1), cancer survivors were older (87.8% >65 years old for cancer survivors vs. 56.2% >65 years for non-cancer patients). Cancer survivors also had more comorbidities, including diabetes mellitus (33.7% vs. 28.8%), hypertension (73.6% vs. 58.4%), renal disease (18.4% vs. 9.8%), HF (26.5% vs. 15.5%), and stroke (9.7% vs. 6.0%) prior to AMI. Compared to non-cancer patients, cancer survivors were more likely to have had prior coronary angiography, PCI, and CABG surgery.
Table 1. Unadjusted and adjusted baseline characteristics of cancer survivors (n=22,907) and non-cancer patients (n=247,182).
| Characteristic | Non-cancer patients, unadjusted % | Cancer patients, unadjusted % | Non-cancer patients, IPTW adjusted % | Cancer survivors, IPTW adjusted % | IPTW standardized difference, % |
|---|---|---|---|---|---|
| Sex (female) | 37.5 | 41.6 | 37.8 | 38.9 | 2.2 |
| Age, years | |||||
| < 45 | 6.5 | 0.6 | 5.8 | 5.8 | 0.19 |
| 45-64 | 37.3 | 11.6 | 35.2 | 34.2 | 2.1 |
| > 65 | 56.2 | 87.8 | 59.0 | 60.0 | 2.0 |
| Year of AMI | |||||
| 1995-1999 | 28.4 | 23.8 | 26.7 | 26.0 | 1.4 |
| 2000-2004 | 29.5 | 28.8 | 27.9 | 28.4 | 1.0 |
| 2005-2009 | 23.7 | 25.6 | 24.8 | 24.3 | 1.1 |
| 2010-2013 | 18.4 | 21.8 | 20.6 | 21.2 | 1.6 |
| Income quintiles | |||||
| Q1 | 22.5 | 22.1 | 22.5 | 22.5 | 0 |
| Q2 | 21.5 | 21.3 | 21.5 | 21.2 | 0.68 |
| Q3 | 20.0 | 19.8 | 20.0 | 20.1 | 0.21 |
| Q4 | 18.7 | 18.7 | 18.7 | 18.8 | 0.23 |
| Q5 | 17.3 | 18.2 | 17.4 | 17.5 | 0.27 |
| Rural population | 16.0 | 16.3 | 15.9 | 15.7 | 0.42 |
| Charlson Comorbidity Index (>2) | 10.5 | 17.0 | 10.5 | 11.6 | 3.3 |
| ADG (10+) | 22.3 | 35.6 | 22.4 | 23.1 | 1.8 |
| Previous comorbidities | |||||
| Diabetes mellitus | 28.8 | 33.7 | 29.2 | 29.9 | 1.7 |
| Hypertension | 58.4 | 73.6 | 59.7 | 60.2 | 0.89 |
| Dyslipidemia | 39.3 | 39.4 | 39.4 | 39.2 | 0.40 |
| Heart failure | 15.5 | 26.5 | 16.5 | 17.5 | 2.7 |
| Stroke | 6.0 | 9.7 | 6.3 | 6.8 | 2.1 |
| PVD | 4.5 | 7.6 | 4.7 | 5.1 | 1.5 |
| Atrial fibrillation | 7.3 | 14.2 | 7.9 | 8.4 | 1.6 |
| Bradyarrhythmia | 2.9 | 5.7 | 3.1 | 3.3 | 1.1 |
| Other arrhythmia | 5.9 | 10.1 | 6.2 | 6.4 | 0.81 |
| Anemia | 18.9 | 34.6 | 20.2 | 21.7 | 3.6 |
| Renal disease | 9.8 | 18.4 | 10.6 | 11.8 | 4.0 |
| GI bleed | 9.8 | 17.7 | 10.5 | 11.0 | 1.4 |
| COPD | 23.2 | 34.4 | 24.2 | 25.6 | 3.4 |
| Prior coronary procedures | |||||
| Coronary angiography | 12.0 | 15.4 | 12.3 | 12.6 | 1.2 |
| PCI | 3.8 | 4.8 | 3.9 | 4.0 | 0.34 |
| CABG surgery | 3.9 | 4.8 | 4.0 | 4.2 | 0.67 |
| Baseline cardiovascular medications | |||||
| Clopidogrel | 3.9 | 6.8 | 4.1 | 4.2 | 0.46 |
| ACEi/ARB | 32.9 | 52.3 | 36.8 | 38.0 | 2.3 |
| Spironolactone | 11.9 | 20.1 | 12.6 | 13.1 | 1.6 |
| β-blockers | 29.0 | 47.3 | 30.6 | 31.4 | 1.8 |
| CCB | 29.9 | 48.6 | 31.5 | 32.5 | 2.1 |
| Lipid lowering agents | 24.2 | 36.3 | 25.3 | 25.9 | 1.5 |
| Diuretics | 19.3 | 34.9 | 20.7 | 21.8 | 2.8 |
| Nitrates | 26.4 | 44.1 | 28.0 | 28.8 | 1.8 |
| Oral anticoagulants | 8.5 | 16.2 | 9.2 | 9.9 | 2.6 |
Abbreviations: ACEi, angiotensin converting enzyme inhibitor; ADG, aggregated disease groups; AMI, acute myocardial infarction; ARB, angiotensin II receptor blocker; CABG, coronary artery bypass graft surgery; CCB, calcium channel blocker; COPD, chronic obstructive pulmonary disease; GI, gastrointestinal; HF, heart failure; HR, hazard ratio; IPTW, inverse probability treatment weight; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease.
The median follow-up was 10.1 years [interquartile range (IQR) 5.7-14.7 years] and 11.0 years (IQR 6.2-15.7 years) for cancer survivors and non-cancer patients, respectively. The median time from cancer diagnosis to AMI was 8.1 years (IQR 4.1-14.5 years).
Invasive coronary strategy
In 1995 and 2013, the IPTW-adjusted use of coronary angiography (1995: 20.4% vs. 16.2%; 2013: 79.5% vs. 80.3%), PCI (1995: 5.1% vs. 4.3%; 2013: 58.4% vs. 54.4%), and CABG surgery (1995: 3.2% vs. 3.3%; 2013: 8.2% vs. 8.1%) was similar for cancer survivors and non-cancer patients. Likewise, IPTW-adjusted incidence rate ratios (IRR) of procedure rates did not differ between the two groups (Table 2).
Table 2. Comparison of coronary procedure rates and cardiovascular medications prescribed between cancer survivors and non-cancer patients, using adjusted Modified Poisson regression.
| Outcomes | Cancer survivors vs non-cancer patients† | |
|---|---|---|
| Adjusted RR (95% CI) | P-value | |
| Coronary procedure within 30 days of AMI | ||
| Coronary angiography | 0.98 (0.96-1.01) | 0.23 |
| PCI | 0.98 (0.94-1.02) | 0.29 |
| CABG surgery | 0.93 (0.85-1.02) | 0.11 |
| Medications prescribed within 90 days of AMI | ||
| Clopidogrel | 0.98 (0.95-1.00) | 0.05 |
| ACEi/ARB | 0.98 (0.97-0.99) | 0.0015 |
| Spironolactone | 0.98 (0.92-1.04) | 0.50 |
| β-blockers | 0.99 (0.98-1.00) | 0.21 |
| CCB | 1.00 (0.97-1.03) | 0.87 |
| Lipid lowering agents | 0.96 (0.95-0.98) | <0.0001 |
| Diuretics | 0.98 (0.96-1.01) | 0.17 |
| Nitrates | 1.00 (0.99-1.01) | 0.73 |
| Oral anticoagulants | 0.93 (0.89-0.97) | 0.0003 |
Abbreviations: ACEi, angiotensin converting enzyme inhibitor; ADG, aggregated disease groups; AMI, acute myocardial infarction; ARB, angiotensin II receptor blocker; CABG, coronary artery bypass graft surgery; CCB, calcium channel blocker; HF, heart failure; PCI, percutaneous coronary intervention; RR, relative risk.
Temporal trends in an invasive coronary strategy
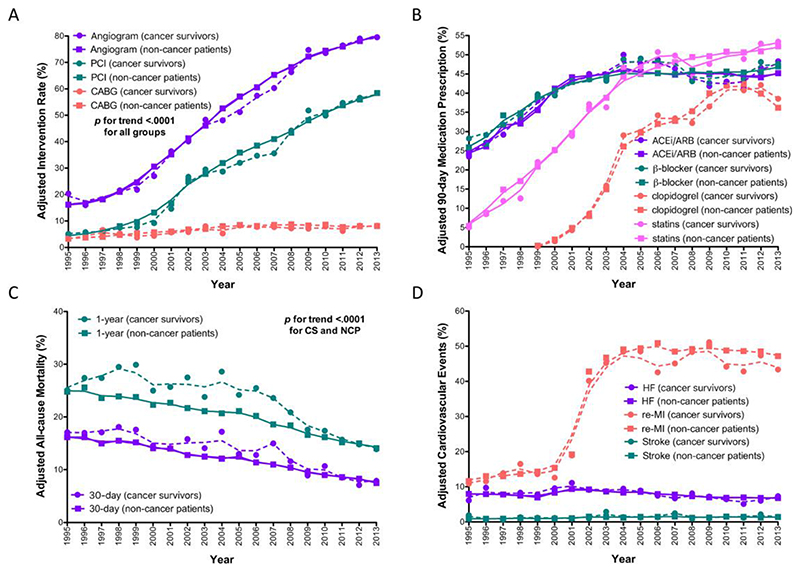
Between 1995 and 2013, the IPTW-adjusted use of an invasive coronary strategy within 30 days following AMI increased equally among cancer survivors and non-cancer patients (p-for-trend <0.0001; p-for-interaction 0.93, 0.23, 0.46 for coronary angiography, PCI, and CABG surgery, respectively) (Figure 1A). For cancer survivors and non-cancer patients alike, the greatest increase was for PCI (AAPC 13.4%, 95% CI 11.4-15.4% and 12.8%, 95%CI 10.9-14.7%, respectively), followed by coronary angiography (AAPC 9.1%, 95% CI 7.7-10.5% and 9.0%, 95% CI 7.7-10.4%, respectively), and CABG surgery (AAPC 3.7%, 95% CI 0.4-7.2% and 4.4%, 95% CI 1.1-7.8%, respectively).
Figure 1.
Inverse probability treatment weight adjusted temporal trends in a retrospectively identified population cohort of cancer survivors with acute myocardial infarction (AMI) between 1995 and 2013, compared with a control group of non-cancer patients with AMI. (A) Invasive coronary procedure received within 30 days of AMI, including angiography, percutaneous coronary intervention, or bypass surgery. (B) Evidence-based cardiac medications received within 90 days of AMI. (C) 30-day and 1-year all-cause mortality. (D) Non-fatal cardiovascular events of heart failure, myocardial re-infarction, and stroke.
Cardiovascular pharmacotherapies
At 90 days following index AMI, the use of pharmacotherapies in cancer survivors compared with non-cancer patients (age ≥65) was similar for β-blockers, calcium-channel blockers, clopidogrel, nitrates, and spironolactone (Table 2). Conversely, cancer survivors were prescribed marginally less angiotensin converting enzyme inhibitor (ACEi)/angiotensin II receptor blocker (ARB) (IRR 0.98, 95% CI 0.97-0.99, p=0.0015), statins (IRR 0.96, 95% CI 0.95-0.98, p<0.0001), and oral anticoagulants (IRR 0.93, 95% CI 0.89-0.97, p=0.0003).
Temporal trends in cardiovascular pharmacotherapies
Between 1995 and 2013, the IPTW-adjusted prescription of pharmacotherapies within 90 days of AMI increased similarly among cancer survivors and non-cancer patients (aged ≥65 years) for β-blockers and clopidogrel (all p-for-trend <0.001; p-for-interaction 0.82 and 0.15 for β-blockers and clopidogrel, respectively) (Figure 1B). For the temporal trend of ACEis/ARBs and statins usage, the p-for-interaction between cancer survivors and non-cancer patients was significant (p-for-interaction 0.0096 and 0.0048 for ACEi/ARB and statins, respectively).
For cancer survivors and non-cancer patients alike, the greatest increase was prescription of clopidogrel (AAPC 14.1%, 95% CI 11.2-17.0% and 14.2%, 95% CI 11.3-17.1%, respectively), followed by statin (AAPC 8.4%, 95% CI 6.8-10.0% and 8.0%, 95% CI 6.5-9.6%, respectively), ACEi/ARB (AAPC 2.5%, 95% CI 1.2-3.8% and 2.5%, 95% CI 1.2-3.8%, respectively), and β-blockers (AAPC 2.3%, 95% CI 1.0-3.6% and 2.3%, 95% CI 1.0-3.7%, respectively) for cancer survivors and non-cancer patients, respectively.
All-cause mortality
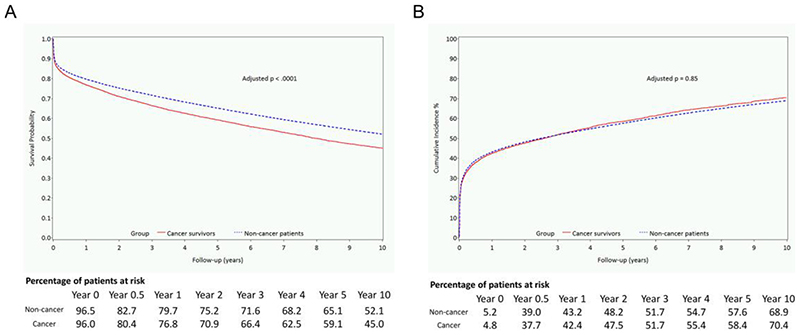
Cancer diagnosis prior to AMI was associated with higher 30-day mortality (HR 1.12, 95% CI 1.07-1.17, p<0.0001), 1-year mortality (HR 1.16, 95% CI 1.12-1.20, p<0.0001), and worse overall survival (OS) (HR 1.21, 95% CI 1.17-1.25, p<0.0001), whereby at 1-year the adjusted survival estimates were 76.8% for cancer survivors and 79.7% for non-cancer patients (Figure 2A). The IPTW-adjusted 30-day, 1-year, and long-term all-cause mortality rates for cancer survivors and non-cancer patients were 13.6%, 23.4%, and 54.8%, respectively, and 12.3%, 20.4%, and 49.0%, respectively.
Figure 2.
Survival curve of long-term all-cause mortality and non-fatal cardiovascular events during entire follow-up in a retrospectively identified population cohort of cancer survivors with acute myocardial infarction (AMI) between 1995 and 2013, compared with a control group of noncancer patients with AMI. (A) Inverse probability treatment weight adjusted all-cause mortality. (B) IPTW-adjusted composite outcome of heart failure, stroke, or myocardial re-infarction.
Cancer survivors, whose cancer diagnosis was made 5 years or more from the time of AMI, had significantly higher risk of 30-day mortality (HR 1.13, 95% CI 1.07-1.20, p<0.0001), while those with a cancer diagnosis within 1-5 years of AMI did not (HR 1.09, 95%CI 1.00-1.18, p=0.054) (Supplementary Table S4). For 1-year mortality and OS, an increased mortality risk was observed for cancer survivors irrespective of their time of cancer diagnosis to index AMI (Supplementary Table S4).
Temporal trends in mortality
At the start and end of the study period, the IPTW-adjusted 30-day mortality rates were similar between cancer survivors and non-cancer patients (17.1% vs. 16.2% in 1995 and 7.9% vs. 7.5% for in 2013, respectively) (Figure 1C). Similarly, IPTW-adjusted 1-year mortality rates in 1995 and 2013 were similar between cancer survivors and non-cancer patients (25.1% vs. 24.8% and 13.9% vs. 14.1%) (Figure 1C).
Between 1995 and 2013, 30-day and 1-year mortality declined for cancer survivors and non-cancer patients. Moreover, the 30-day mortality following AMI decreased similarly for cancer survivors and non-cancer patients (AAPC -4.2%, 95% CI -2.0% to -6.3% and -4.0%, 95% CI -1.7% to -6.3%, respectively; p-for-trend <0.001, p-for-interaction=0.58). The decline in 1-year all-cause mortality following AMI was also similar between cancer survivors and non-cancer patients (AAPC -3.3%, 95% CI -1.6% to -5.0% and -3.0%, 95% CI -1.2% to -4.8%, respectively; p-for-trend <0.001, p-for-interaction=0.26).
Non-fatal cardiovascular outcomes
Over the follow-up, cancer survivors had a higher risk of HF compared with non-cancer patients (HR 1.08, 95% CI 1.03-1.14, p=0.0011), but not myocardial re-infarction or stroke (Table 3). The composite of heart failure/myocardial re-infarction/stroke did not significantly differ between the two groups (HR 1.00, 95% CI 0.97-1.04, p=0.85; Figure 2B). Over follow-up period, breast cancer survivors had higher HF risk (HR 1.33, 95% CI 1.11-1.58, p=0.0016) compared to non-cancer patients than non-breast cancer survivors (HR 1.07, 95% CI 1.02-1.12, p=0.014).
Table 3. Adjusted Cox proportional hazards model evaluating the hazard of cancer diagnosis on outcomes following AMI.
| Outcomes (Time-to-first) | Cancer survivors vs non-cancer patients | |
|---|---|---|
| Adjusted HR (95% CI) | P-value | |
| 30-day mortality | 1.12 (1.07-1.17) | <0.0001 |
| 1-year mortality | 1.16 (1.12-1.20) | <0.0001 |
| All-cause mortality; full follow-up | 1.21 (1.17-1.25) | <0.0001 |
| Overall heart failure | 1.08 (1.03-1.14) | 0.0011 |
| Overall myocardial re-infarction | 0.98 (0.95-1.01) | 0.22 |
| Overall stroke | 1.06 (0.97-1.16) | 0.18 |
Abbreviations: AMI, acute myocardial infarction; HR, hazard ratio.
With regards to the trend of cardiovascular outcomes that occurred within 1-year of AMI, between 1995 and 2013, the adjusted rates for HF (cancer survivor AAPC -1.6%, 95% CI -4.5% to 1.3%, p-for-trend=0.27; non-cancer group AAPC 0.9%, 95% CI -3.8% to 2.1%, p-for-trend=0.54; p-for-interaction=0.095) and stroke (cancer survivor AAPC 1.9%, 95% CI -4.9% to 9.2%, p-for-trend=0.59; non-cancer group AAPC 2.5%, 95% CI -4.5 to 10.4%, p-for-trend=0.51; p-for-interaction=0.67) were similar between cancer survivors and non-cancer patients (Figure 1D). For myocardial re-infarction, there was an increase in rates from 1995 to 2002 for both groups that stabilized thereafter (cancer survivors AAPC 7.5%, 95% CI 6.0% to 9.1%, p-for-trend<0.0001; non-cancer patients APPC 7.8%, 95% CI 6.3% to 9.4%, p-for-trend< 0.0001; p-for-interaction=0.055).
Discussion
This population-based study of 270,089 AMI patients, investigated trends in cardiac care and outcomes between 1995 and 2013, amongst a subset of cancer patients in clinical remission (defined as cancer survivors in this study) compared to patients with no history of cancer. Over the 18-year study period, we observed an increase in the use of coronary procedures and cardiovascular medications, and similar rates of decline in 30-day and 1-year mortality in cancer survivors and non-cancer patients. While we did not identify major differences in the delivery of cardiovascular care between patients with and without cancer, compared with non-cancer patients, cancer survivors with AMI had a significantly higher risk of mortality and heart failure, but not myocardial re-infarction and stroke. This study reveals that cancer survivors with AMI may have worse outcomes compared to non-cancer patients, and emphasizes the need for clinical attention to this expanding population.
The rate of decline in mortality observed in this study is consistent with those reported in the US and other European countries.23, 24 However, our study suggests that these improvements in outcomes follow the timeline of increases in the utilization of evidence-based therapies, and align with the results of studies among patients without cancer for AMI.25 We found that the rates of an invasive coronary strategy after AMI did not differ between cancer survivors and non-cancer patients, suggesting that the latter had similar access to cardiac procedures. This finding is of note when there have been inconsistent findings with respect to the impact of coronary procedures on outcomes in patients with cancer. Analysis of a multicenter registry suggested that patients with a cancer diagnosis up to, but not longer than 6 months before AMI who received PCI had significantly worse 1-year mortality compared with non-cancer patients.26 Another study showed that patients with cancer up to 1 year prior to receipt of PCI did not experience worse long-term cardiovascular outcomes compared with patients without cancer.27
We found that cancer survivors aged 65 years or older received similar rates of post-AMI cardiovascular pharmacotherapies except for marginally less ACEi/ARB, oral anticoagulants, and statins. The precise reason for less prescription of these medications is difficult to discern from our dataset, given lack of granular data. Cancer survivors in our cohort were older and more comorbid than the control group. We postulate that among cancer survivors, the higher prevalence of gastrointestinal bleed and anemia might have potentiated the bleeding risk associated with oral anticoagulation, while renal disease might have precluded the use of an ACEi/ARB.
In terms of outcomes, cancer survivors had significantly worse survival compared to those without malignancy. A diagnosis of cancer prior to AMI had a 12% and 21% higher risk for 30-day and long-term all-cause mortality, respectively. There are a number of possible reasons for this. The acute phase reactants and inflammation induced by cancer in the form of chemokines, cytokines, and platelet activation may accelerate atherosclerosis.28 Radiation therapy is a well-established risk factor for CAD, the consequence of which may become clinically significant many years later.29, 30 Certain cancer therapies such as anthracyclines, antimetabolites, hormonal therapies, and new targeted and biological agents can have adverse cardiac effects.31 We found that cancer survivors had a higher risk of HF, but not stroke or myocardial re-infarction. This latent risk for HF is may be due to the cardiotoxic effects of commonly used cancer regimens (including anthracyclines, kinase inhibitors, and trastuzumab32), though we cannot exclude the possibility that there was excess myocardial damage at index AMI in cancer patients despite similar rates of treatment. Anthracyclines have been associated with acute, early, and late cardiac events due to type 1 cardiotoxicity. Similarly, although trastuzumab is known to cause reversible type 2 cardiotoxicity, long-term cardiovascular complications remain pertinent in survivorship.33 Although HF was not a main endpoint in this study, in an exploratory analysis, we found that post-AMI HF risk was higher in breast cancer survivors than non-breast cancer survivors. These results may be consistent with the notion that pre-existing cardiac dysfunction may lead to worse outcomes post-AMI. Lastly, cancer survivors tended to be anemic at baseline, possibly due to chemotherapy-induced bone marrow toxicity, nutritional deficiencies, and increased hepcidin,34 which may have contributed to HF and ischemia risk. 35, 36
Cardiovascular prognosis among cancer survivors is complex, and traditionally categorized as phases of survivorship.37 Given that cardiovascular events occurring during initial period of time following cancer treatment may have different prognostic impact from those occurring late after cancer diagnosis,38, 39 we conducted subgroup analysis by cancer diagnosis 1-4 versus ≥5 years prior to AMI. We found no difference in mortality risk with regards to time from cancer diagnosis to AMI, highlighting the importance of diligent cardiovascular surveillance during sustained cancer survivorship.
This study has clinical implications. While the continuing decline in mortality following AMI is encouraging, cardiovascular disease likely continues to be a leading cause of death among cancer survivors.40 It is reassuring cancer diagnosis is not a deterrent to receiving invasive coronary strategy or pharmacotherapies following AMI. However, our results also highlight the importance of continued vigilance in cardiac risk surveillance during survivorship. It is crucial that cancer survivors continue to be evaluated for their risk of latent cardiovascular events and future studies are needed to evaluate the effectiveness of such monitoring.
Although our study results are noteworthy, they are not without limitation. We could not attain the full array of covariates that may impact upon outcomes, such as type of surgery, all chemotherapies and other treatments received, as well as granular details of radiation therapy. Hence, we could not stratify our outcomes according to type of treatment intervention received. The observational nature of this study may also result in unmeasured confounding that remained unaccounted for in IPTW. The cancer survivors in this study were a heterogeneous population with a variety of cancer types and stages. While this allowed for delineation of pre-existing malignancy as a risk factor for adverse outcomes overall, our study sample size lacked power to evaluate each cancer type individually. Additionally, changes in cancer stage during follow-up may not be fully accounted for due to inherent limitations of administrative databases. We aimed at excluding cancer relapse by excluding those with re-initiation of systemic therapies during follow-up. We recognize that our study might have included stage IV patients surviving at 1-year post diagnosis who did not receive additional therapy. There is a possibility of misclassification of cancer survivor as a non-cancer patient. Inability to capture events that occurred outside of Ontario might have resulted in under-reporting of outcomes. Our study precluded the opportunity to evaluate outcomes based on severity of myocardial damage, as we could not collect granular clinical data to accurately adjust for post-AMI prognosticators. Cause-specific mortality was also lacking in our dataset; however, it is recognized that ascertainment of cause of death is likely inaccurate for several reasons, and that all-cause mortality may be the most objective unbiased endpoint.41 Analyses of pharmacotherapies were limited to patients 65 or older, due to lack of medication information in those younger than 65 (recognized limitation of Ontario administrative database). Ontario provides universal health care; hence, results herein may not be generalizable to other parts of the world where access to healthcare may be more limited. Moreover, characteristics of the cancer survivor population may vary regionally and additional studies are required to confirm our results in other regions of the world. Our observational study sought to evaluate the association of cancer diagnosis with outcomes to provide insight into appropriate management strategies to enhance survivorship, rather than establishing causality per se.
In conclusion, our findings demonstrate that over time, the use of coronary procedures increased and mortality decreased comparably in patients with AMI, irrespective of previous cancer diagnosis. However, despite improvements in mortality trends, cancer survivors had excess number of short-term and long-term mortality events compared to non-cancer patients. The critically important message of our study is that optimal evidence-based surveillance guidelines should be implemented with the goal of preventing morbidity and mortality in the growing population of cancer survivors with cardiovascular disease. To achieve this, future studies are needed to identify long-term follow-up strategies to provide targeted cardiovascular education, surveillance, management, and timely intervention as appropriate to improve longterm outcomes.
Supplementary Material
Funding
This research was supported through provision of data by ICES and Cancer Care Ontario (CCO) and through funding support to ICES from an annual grant by the Ministry of Health and Long-Term Care (MOHLTC) and the Ontario Institute for Cancer Research (OICR). The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES, CCO, OICR or the Government of Ontario is intended or should be inferred.
Footnotes
Precis
There is a paucity of information about treatment and mortality trends following acute myocardial infarction for cancer survivors. Our study results highlight the susceptibility of cancer survivors with acute myocardial infarction to worse outcomes, and emphasizes the need for clinical attention to this emerging population of cancer survivors.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Author contribution:
Conceptualization: IYG, ATY, KKWC
Data curation: KKWC
Formal analysis: IYG, ATY, KKWC
Funding acquisition: KKWC
Investigation: IYG, ATY, KKWC
Methodology: all authors
Project administration, resources, software, supervision, validation, visualization: ATY, KKWC Writing - original draft: IYG, ATY, KKWC
Writing - review and editing: all authors
References
- 1.Organization WH. [accessed February 10, 2017];Global status report on noncommunicable diseases 2014. Available from URL: http://apps.who.int/iris/bitstream/10665/148114/1/9789241564854eng.pdf?ua=1.
- 2.Ryerson AB, Eheman CR, Altekruse SF, et al. Annual Report to the Nation on the Status of Cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122:1312–1337. doi: 10.1002/cncr.29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox KA, Steg PG, Eagle KA, et al. Decline in rates of death and heart failure in acute coronary syndromes, 1999-2006. JAMA. 2007;297:1892–1900. doi: 10.1001/jama.297.17.1892. [DOI] [PubMed] [Google Scholar]
- 4.Hall M, Dondo TB, Yan AT, et al. Association of Clinical Factors and Therapeutic Strategies With Improvements in Survival Following Non-ST-Elevation Myocardial Infarction, 2003-2013. JAMA. 2016;316:1073–1082. doi: 10.1001/jama.2016.10766. [DOI] [PubMed] [Google Scholar]
- 5.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 6.Colzani E, Liljegren A, Johansson AL, et al. Prognosis of patients with breast cancer: causes of death and effects of time since diagnosis, age, and tumor characteristics. J Clin Oncol. 2011;29:4014–4021. doi: 10.1200/JCO.2010.32.6462. [DOI] [PubMed] [Google Scholar]
- 7.Zoller B, Ji J, Sundquist J, Sundquist K. Risk of coronary heart disease in patients with cancer: a nationwide follow-up study from Sweden. Eur J Cancer. 2012;48:121–128. doi: 10.1016/j.ejca.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Dreyer L, Olsen JH. Cancer risk of patients discharged with acute myocardial infarct. Epidemiology. 1998;9:178–183. [PubMed] [Google Scholar]
- 9.Dreyer L, Olsen JH. Risk for non-smoking-related cancer in atherosclerotic patients. Cancer Epidemiol Biomarkers Prev. 1999;8:915–918. [PubMed] [Google Scholar]
- 10.Fernberg P, Odenbro A, Bellocco R, et al. Tobacco use, body mass index, and the risk of leukemia and multiple myeloma: a nationwide cohort study in Sweden. Cancer Res. 2007;67:5983–5986. doi: 10.1158/0008-5472.CAN-07-0274. [DOI] [PubMed] [Google Scholar]
- 11.Blann AD, Dunmore S. Arterial and venous thrombosis in cancer patients. Cardiol Res Pract. 2011;2011:394740. doi: 10.4061/2011/394740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeh ET. Cardiotoxicity induced by chemotherapy and antibody therapy. Annu Rev Med. 2006;57:485–498. doi: 10.1146/annurev.med.57.121304.131240. [DOI] [PubMed] [Google Scholar]
- 13.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53:2231–2247. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 14.Truong J, Yan AT, Cramarossa G, Chan KK. Chemotherapy-induced cardiotoxicity: detection, prevention, and management. Can J Cardiol. 2014;30:869–878. doi: 10.1016/j.cjca.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 15.Herrmann J, Lerman A, Sandhu NP, Villarraga HR, Mulvagh SL, Kohli M. Evaluation and management of patients with heart disease and cancer: cardio-oncology. Mayo Clin Proc. 2014;89:1287–1306. doi: 10.1016/j.mayocp.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zamorano JL, Lancellotti P, Rodriguez Munoz D, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 17.Feuerstein M. Defining cancer survivorship. J Cancer Surviv. 2007;1:5–7. doi: 10.1007/s11764-006-0002-x. [DOI] [PubMed] [Google Scholar]
- 18.McCabe MS, Bhatia S, Oeffinger KC, et al. American Society of Clinical Oncology statement: achieving high-quality cancer survivorship care. J Clin Oncol. 2013;31:631–640. doi: 10.1200/JCO.2012.46.6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Earle CC, Neville BA. Under use of necessary care among cancer survivors. Cancer. 2004;101:1712–1719. doi: 10.1002/cncr.20560. [DOI] [PubMed] [Google Scholar]
- 20.Tu JV, Austin PC, Walld R, Roos L, Agras J, McDonald KM. Development and validation of the Ontario acute myocardial infarction mortality prediction rules. J Am Coll Cardiol. 2001;37:992–997. doi: 10.1016/s0735-1097(01)01109-3. [DOI] [PubMed] [Google Scholar]
- 21.Schultz SE, Rothwell DM, Chen Z, Tu K. Identifying cases of congestive heart failure from administrative data: a validation study using primary care patient records. Chronic Dis Inj Can. 2013;33:160–166. [PubMed] [Google Scholar]
- 22.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 23.Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. N Engl J Med. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 24.Levi F, Lucchini F, Negri E, La Vecchia C. Trends in mortality from cardiovascular and cerebrovascular diseases in Europe and other areas of the world. Heart. 2002;88:119–124. doi: 10.1136/heart.88.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox KA, Anderson FA, Jr, Goodman SG, et al. Time course of events in acute coronary syndromes: implications for clinical practice from the GRACE registry. Nat Clin Pract Cardiovasc Med. 2008;5:580–589. doi: 10.1038/ncpcardio1302. [DOI] [PubMed] [Google Scholar]
- 26.Velders MA, Boden H, Hofma SH, et al. Outcome after ST elevation myocardial infarction in patients with cancer treated with primary percutaneous coronary intervention. Am J Cardiol. 2013;112:1867–1872. doi: 10.1016/j.amjcard.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 27.Hess CN, Roe MT, Clare RM, et al. Relationship Between Cancer and Cardiovascular Outcomes Following Percutaneous Coronary Intervention. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. 2015;126:582–588. doi: 10.1182/blood-2014-08-531582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schultz-Hector S, Trott KR. Radiation-induced cardiovascular diseases: is the epidemiologic evidence compatible with the radiobiologic data? Int J Radiat Oncol Biol Phys. 2007;67:10–18. doi: 10.1016/j.ijrobp.2006.08.071. [DOI] [PubMed] [Google Scholar]
- 30.Heidenreich PA, Kapoor JR. Radiation induced heart disease: systemic disorders in heart disease. Heart. 2009;95:252–258. doi: 10.1136/hrt.2008.149088. [DOI] [PubMed] [Google Scholar]
- 31.Yeh ET, Chang HM. Oncocardiology-Past, Present, and Future: A Review. JAMA Cardiol. 2016;1:1066–1072. doi: 10.1001/jamacardio.2016.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ewer MS, Ewer SM. Cardiotoxicity of anticancer treatments. Nat Rev Cardiol. 2015;12:620. doi: 10.1038/nrcardio.2015.133. [DOI] [PubMed] [Google Scholar]
- 33.Gong IY, Verma S, Yan AT, et al. Long-term cardiovascular outcomes and overall survival of early-stage breast cancer patients with early discontinuation of trastuzumab: a populationbased study. Breast Cancer Res Treat. 2016;157:535–544. doi: 10.1007/s10549-016-3823-y. [DOI] [PubMed] [Google Scholar]
- 34.Birgegard G, Aapro MS, Bokemeyer C, et al. Cancer-related anemia: pathogenesis, prevalence and treatment. Oncology. 2005;68(Suppl 1):3–11. doi: 10.1159/000083128. [DOI] [PubMed] [Google Scholar]
- 35.Felker GM, Adams KF, Jr, Gattis WA, O’Connor CM. Anemia as a risk factor and therapeutic target in heart failure. J Am Coll Cardiol. 2004;44:959–966. doi: 10.1016/j.jacc.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 36.Rousseau M, Yan RT, Tan M, et al. Relation between hemoglobin level and recurrent myocardial ischemia in acute coronary syndromes detected by continuous electrocardiographic monitoring. Am J Cardiol. 2010;106:1417–1422. doi: 10.1016/j.amjcard.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 37.Mullan F. Seasons of survival: reflections of a physician with cancer. N Engl J Med. 1985;313:270–273. doi: 10.1056/NEJM198507253130421. [DOI] [PubMed] [Google Scholar]
- 38.Lenihan DJ, Cardinale DM. Late cardiac effects of cancer treatment. J Clin Oncol. 2012;30:3657–3664. doi: 10.1200/JCO.2012.45.2938. [DOI] [PubMed] [Google Scholar]
- 39.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 40.Fidler MM, Reulen RC, Winter DL, et al. Long term cause specific mortality among 34 489 five year survivors of childhood cancer in Great Britain: population based cohort study. BMJ. 2016;354:i4351. doi: 10.1136/bmj.i4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith Sehdev AE, Hutchins GM. Problems with proper completion and accuracy of the cause-of-death statement. Arch Intern Med. 2001;161:277–284. doi: 10.1001/archinte.161.2.277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.