Abstract
Ion-sensitive field-effect transistors (ISFETs) in combination with unmodified complementary metal oxide semiconductors present a point-of-care platform for clinical diagnostics and prognostics. This work illustrates the sensitive and specific detection of two circulating mRNA markers for prostate cancer, the androgen receptor and the TMPRSS2-ERG fusion using a target-specific loop-mediated isothermal amplification method. TMPRSS2-ERG and androgen receptor RNA were detected down to 3x101 and 5x101 copies respectively in under 30 minutes. Administration of these assays onto the ISFET Lab-on-chip device was successful and the specificity of each marker was corroborated with mRNA extracted from prostate cancer cell lines.
Index Terms: Biosensors, ISFETs, RNA, Prostate cancer, RT-LAMP, Lab-on-Chip
I. Introduction
Prostate cancer (PCa) is the fourth most common cancer worldwide [1]. The most advanced and lethal stage of PCa is metastatic castration-resistant prostate cancer (mCRPC) where the tumour exhibits resistance to androgen deprivation therapy. Alternatively, PCa can be asymptomatic for years, which is why for early-stage disease active surveillance is often preferred over more invasive treatment [2], [3]. Despite the discovery of biomarkers relevant to PCa progression, determining which patients will exhibit aggressive or indolent phenotypes remains challenging.
Both TMPRSS2-ERG and the androgen receptor (AR) are established oncogenic drivers of PCa. The AR is the main target for therapeutic options in inoperable PCa since aberrant AR signalling contributes to proliferation, migration and survival of PCa tumour cells [4]–[6]. qPCR detection of over 10 copies of AR mRNA in circulating tumour cells per mL of blood in mCRPC patients was associated with a reduced overall survival while quantification of AR mRNA in the blood has prognostic signifance for mCRPC patients treated with abiraterone or enzalutamide [7], [8]. TMPRSS2-ERG is a prostate cancer specific gene fusion present in up to 50 % of all PCa patients. The fusion results in the increased androgen-regulated expression of the oncogene ERG, which propagates TMPRSS2-ERG mediated invasion of PCa cell lines [9]. Detection of TMPRSS2-ERG mRNA in circulating tumour cells has shown potential as a biomarker for docetaxel resistance in mCRPC patients [10]. Elucidation of these mRNA biomarkers circulating in the blood could present a novel and minimally invasive prognostic test for advanced PCa patients.
Ion-sensitive field-effect transistors (ISFETs) can be utilised to measure ion concentrations in solution due to the inherent pH sensing potential of the Si3N4 passivation layer. Equation 1 illustrates the relationship between the chemical voltage of the ISFET (Vchem) and pH, where γ is a constant chemical term, SN is the Nernstian sensitivity and α is the sensitivity deviation from the Nernstian sensitivity.
| (1) |
ISFETs contain several non-idealities as a biosensor including sensor drift and trapped charge [11], [12]. Despite this, the combination of ISFET biosensors and nucleic acid amplification tests (NAATs) has previously been successful [13]–[16]. In particular, loop-mediated isothermal amplification (LAMP) requires a constant temperature between 60-65 oC and therefore does not limit the NAAT to a laboratory setting. Other NAATs, including the polymerase chain reaction, require thermal cycling which is challenging to implement at point-of-care. In combination, the LAMP NAAT and ISFET biosensors present a relevant technological platform for reliable detection of nucleic acid biomarkers directly within the clinic. This work utilises a handheld ISFET biosensor that outputs data directly to a mobile phone which has been previously described [17]. This ISFET sensor in combination with unmodified complementary metal oxide semiconductor technology has a pH sensitivity of 11.91 mV / pH and a pH resolution of 0.019 pH units [17].
II. Development Of Rt-Lamp Assays
Synthetic RNA detection
Reverse transcriptase LAMP (RT-LAMP) primer development was initially conducted with primer explorer v5 (https://primerexplorer.jp/e/). Front inner primer (FIP) and back inner primer (BIP) lengths were adapted to ensure time to positives (TTPs) were optimised. Table 1 indicates the primer sequences used for AR and TMPRSS2-ERG RT-LAMP reactions. Previously, the BCR-ABL1 fusion gene has been detected using RT-LAMP and Q-LAMP for leukaemia diagnosis [18], [19]. However, there remains a limited number of RT-LAMP assays capable of detecting fusion gene mRNA. The developed TMPRSS2-ERG assay targets the TMPRSS2 exon 1 and ERG exon 4 fusion, which is present in approximately 80% of TMPRSS2-ERG cases [20]. Since this is the most common TMPRSS2-ERG fusion subtype it was chosen as a target for the developed RT-LAMP assay. A previous isothermal technique has been utilised to detect this TMPRSS2-ERG fusion mRNA with a detection limit of 105 copies per reaction in urine [21]. The AR RT-LAMP primers span over exons 5 and 6. This prevents amplification of the AR-567es variant, which can be present in prostate cancer cells and omits exons 5 to 7 [22].
Table 1. AR and TMPRSS2-ERG RT-pHLAMP primers.
| Primer Sequences 5’ -- > 3’ | ||
|---|---|---|
| TMPRSS2-ERG | AR | |
| B3 | GGTGACCCTGGCTGGGGG | AATTCCTGGGGGGTGATT |
| F3 | GCAGGAGGCGGAGGCG | ATGGGGCTCATGGTGTT |
| FIP | ACAACGACTGGTCCTC GAGGGGCGGGGAGC |
AACCAGATCAGGGGC TGGCGATCCTTCACC |
| BIP | GAGTGTGCCTACGGAAC TCCTGCTGAGGGACGCG |
TTCAATGAGTACCGC ATGCACAAGTGCCATCC AAACTCTTGAGAGAGGTG |
| LF | ATAAGGCTTC CTGCCGCGCT |
GCATCCTGGAG TTGACATTGGT |
| LB | GGCTAAGACA GAGATGACCGCG |
AGCCAGTGTG TCCGAATGAGG |
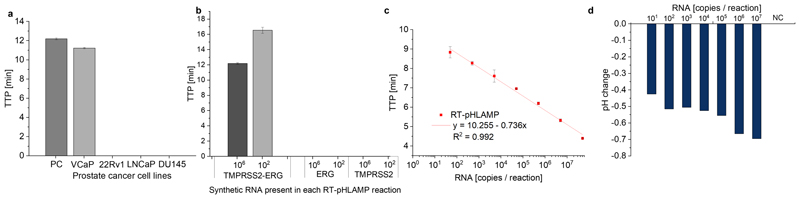
Adjustment of RT-LAMP assays to provide a pH signal is crucial for ISFET detection of positive amplification signals. This is achievable through the removal of the buffering system of tris(hydroxymethyl)aminomethane in a standard RT-LAMP reaction (RT-pHLAMP). During double stranded DNA synthesis, one proton is released per nucleotide added to the DNA strand [23]. Large formation of dsDNA in RT-LAMP reactions results in a relevant pH change for the ISFET biosensor. Sensitivity of the developed AR RT-pHLAMP assay is shown in Figure 1 c. Both assays show sensitive limits of detection with synthetic RNA, 30 copies per reaction is achievable for the TMPRSS2-ERG reaction, while quantitative detection of AR mRNA was observed down to 50 copies per reaction within 10 minutes. The TMPRSS2-ERG RT-pHLAMP reaction exhibited limited quantitative potential (R2 = 0.75). In this instance, the lack of quantitation does not significantly diminish the value of the developed assay. Since TMPRSS2-ERG is a specific gene fusion to prostate cancer, detection of the presence of the marker alone confers a potential prognostic benefit. Both assays registered pH change outputs between -0.4 and -0.7 pH units. Figure 1 d suggests that the pH change of the AR reaction attenuates at lower concentrations of synthetic RNA in the AR reaction. Despite this, pH change is sufficient for compatibility with the ISFET biosensor. Both TMPRSS2 and ERG mRNA are present in healthy cells. Therefore, the RT-pHLAMP assays must detect only the fusion mRNA to avoid false positives results. Figure 1 b indicates that the RT-pHLAMP reaction does not detect synthetic RNA regions of TMPRSS2 and ERG where the fusion presides even at high concentrations of synthetic target (3x106 copies). As such, the TMPRSS2-ERG assay was taken forward for testing with extracted mRNA from prostate cancer cell lines.
Fig. 1.
(a) TMPRSS2-ERG RT-pHLAMP reactions conducted with 1 ng per reaction of mRNA extracted from prostate cancer cell lines. b The specificity of the TMPRSS2-ERG RT-pHLAMP reaction with synthetic fragments of TMPRSS2-ERG, TMPRSS2 and ERG mRNA tested at concentrations of 3 x 106 and 3 x 102 copies per reaction. (c) The standard curve for the AR RT-pHLAMP assay from 5 x 107 to 5 x 101 synthetic copies per reaction. (d) The pH change readout of the AR assay across serial dilutions varying from 5x107 copies to 5x101 copies per reaction. The starting pH of the AR RT-pHLAMP benchtop reaction was between 8.0-8.6 pH units for all recorded reactions.
Extracted RNA detection
Confirmation of detection of endogenous expression of mRNA in PCa cell lines is required to corroborate the validity of these RT-pHLAMP assays. AR mRNA is expressed in VCaP, LNCaP and 22Rv1 cell lines [24]. However, the DU145 cell line is androgen independent therefore, little to no AR mRNA is present [25], [26]. Using 1 ng per reaction of mRNA extracted from prostate cancer cell lines resulted in rapid detection of AR mRNA in androgen-sensitive cell lines. Time to positives in VCaPs, LNCaPs and 22Rv1s were achieved in 6.61 ± 0.02, 6.33 ± 0.08 and 7.99 ± 0.10 min respectively. However, in DU145s no amplification signal was observed after 35 min.
The TMPRSS2-ERG fusion is present in the VCaP cell line but not in 22Rv1, DU145s or LNCaPs [27]. Figure 1 a illustrates that detection of TMPRSS2-ERG mRNA is only observed in the VCaP cell line. LNCaP cells express both TMPRSS2 and ERG mRNA, confirming the RT-pHLAMP assay is specific to the fusion mRNA alone. Since both assays exhibited sufficient sensitivity and specificity for reliable detection, they were subsequently tested on the ISFET Lab-on-Chip device.
III. Isfet Lab-On-Chip Detection
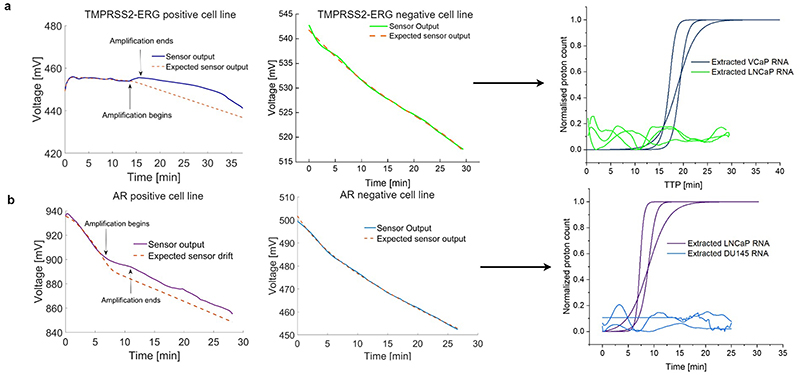
Synthetic detection of RT-pHLAMP reactions at 106 and 101 copies for both AR and TMPRSS2-ERG was carried out. During an amplification event (i.e. a release of protons) the ISFET sensor output increases in voltage relative to the inherent drift. The sigmoidal amplification curves generated in Figure 2 for positive TMPRSS2-ERG and AR samples were produced by subtracting the sensor output by the approximated drift rate followed by sigmoidal fitting [14], [28]. Conversion from voltage to proton count was conducted using equation 1. Negative samples were not sigmoidally fitted on account of little to no discrepancies between sensor output and expected sensor output. For AR detection at 5x106 and 5x101 RNA copies per reaction occurred within 5.42 ± 1.04 min and 9.31 ± 0.31 min respectively. At each copy number value Lab-on-Chip TTP values did not deviate meaningfully from the benchtop RT-pHLAMP reactions. This indicates that both the speed and the sensitivity of the ISFET biosensor reactions are commensurate with experiments which required a specialised laboratory.
Fig. 2.
(a) The sensor outputs of positive and negative RT-pHLAMP reactions for TMPRSS2-ERG detection and the sigmoidal curves generated from the sensor outputs of the Lab-on-Chip device. (b) The sensor outputs of positive and negative RT-pHLAMP reactions for AR detection and the sigmoidal curves generated from the sensor outputs of the Lab-on-Chip device.
Similarly, detection of synthetic TMPRSS2-ERG RNA was observed at 3x106 and 30 copies per reaction with the ISFET biosensor in 18.97 ± 1.18 min and 23.85 ± 2.30 min respectively. The TMPRSS2-ERG assay’s TTP values vary more greatly between the benchtop and ISFET biosensor reactions than in the AR assays. It is likely that the reaction chamber in the ISFET biosensor slightly reduces the efficiency of the RT-pHLAMP reactions, increasing the TTP of slower reactions.
Triplicate ISFET biosensor reactions were additionally completed with mRNA extracted from prostate cancer cell lines (Figure 2). AR RT-pHLAMP Lab-on-Chip reactions containing 1 ng of DU145 (AR negative) and LNCaP (AR positive) mRNA were conducted (Figure 2 b). Rapid detection of AR mRNA was observed in LNCaP extracted mRNA within 6.61 ± 0.34 min. Presence of DU145 mRNA however, resulted in no evidence of amplification. Correspondingly, no relevant change in the ISFET biosensor drift was ascertained in the TMPRSS2-ERG reaction containing 1 ng of RNA extracted from the LNCaP cell line. Presence of VCaP RNA however, resulted in amplification in 16.54 ± 1.41 min (Figure 2 a). pH changes for positive reactions were between -0.4 and -0.7 pH units and 0 to -0.2 pH units for negative reactions.
IV. Discussion
To our knowledge, this work has culminated in the first RT-LAMP assay capable of detection of AR and significantly improves upon previous sensitivity when detecting TMPRSS2-ERG RNA. Both of the developed RT-pHLAMP assays are sensitive and specific to their respective targets with amplification occuring within 30 minutes. Implementation of these assays onto an ISFET based Lab-on-Chip device rendered comparable sensitivity and TTPs to the benchtop reactions, demonstrating its potential to be utilised at a point-of-care setting, allowing for rapid detection of circulating mRNA biomarkers for prostate cancer prognosis. Detection of circulating AR and TMPRSS2-ERG mRNA could result in adjustment of treatment options to improve patient outcomes. Further optimisation of the developed RT-pHLAMP assays for direct plasma testing could reduce the time taken from biofluid extraction to test result and reduce the necessity of specialised personnel. Further expansion of RT-pHLAMP assays to target other biomarkers for PCa, could increase the validity of a multipanel test for point-of-care PCa prognostics.
Acknowledgment
The authors thank members of Georgiou and Bevan laboratories for insightful discussions. Funding for this research includes Cancer Research UK Convergence PhD Studentship (J.B and T.PM C24523/A27435) and Prostate Cancer UK grants (MA-COE18-001 / RIA17-ST2-017).
References
- [1].Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- [2].Drost FJH, Roobol MJ. The need for active surveillance for low risk prostate cancer. Expert Review of Anti cancer Therapy. 2017;17(6):487–89. doi: 10.1080/14737140.2017.1319767. [DOI] [PubMed] [Google Scholar]
- [3].Bul M, Zhu X, Valdagni R, Pickles T, Kakehi Y, Rannikko A, Bjartell A, Van Der Schoot DK, Cornel EB, Conti GN, Boevé ER, et al. Active surveillance for low-risk prostate cancer worldwide: The PRIAS study. European Urology. 2013;63(4):597–603. doi: 10.1016/j.eururo.2012.11.005. [DOI] [PubMed] [Google Scholar]
- [4].Castoria G, D’Amato L, Ciociola A, Giovannelli P, Giraldi T, Sepe L, Paolella G, Barone MV, Migliaccio A, Auricchio F. Androgen-induced cell migration: Role of androgen receptor/filamin A association. PLoS ONE. 2011;6(2) doi: 10.1371/journal.pone.0017218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Haapala K, Kuukasjärvi T, Hyytinen E, Rantala I, Helin HJ, Koivisto PA. Androgen receptor amplification is associated with increased cell proliferation in prostate cancer. Human Pathology. 2007;38(3):474–478. doi: 10.1016/j.humpath.2006.09.008. [DOI] [PubMed] [Google Scholar]
- [6].Shanmugam I, Cheng G, Terranova PF, Thrasher JB, Thomas CP, Li B. Serum/glucocorticoid-induced protein kinase-1 facilitates androgen receptordependent cell survival. Cell Death and Differentiation. 2007;14(12):2085–2094. doi: 10.1038/sj.cdd.4402227. [DOI] [PubMed] [Google Scholar]
- [7].Cattrini C, Rubagotti A, Zinoli L, Cerbone L, Zanardi E, Capaia M, Barboro P, Boccardo F. Role of circulating tumor cells (CTC), androgen receptor full length (AR-FL) and androgen receptor splice variant 7 (AR-V7) in a prospective cohort of castration-resistant metastatic prostate cancer patients. Cancers. 2019;11(9):1–10. doi: 10.3390/cancers11091365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Silberstein J, Luber B, Wang H, Lu C, Chen Y, Zhu Y, Taylor MN, Carducci MA, Eisenberger MA, Luo J, Antonarakis ES. Clinical significance of AR mRNA quantification from circulating tumor cells (CTCs) in men with metastatic castration-resistant prostate cancer (mCRPC) treated with abiraterone (Abi) or enzalutamide (Enza) Journal of Clinical Oncology. 2017;35(6_suppl):132. doi: 10.1200/JCO.2017.35.6_suppl.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tomlins SA, Laxman B, Varambally S, Cao X, Yu J, Helgeson BE, Cao Q, Prensner JR, Rubin MA, Shah RB, Mehra R, et al. Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia. 2008;10(2):177–188. doi: 10.1593/neo.07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Reig M Marín-Aguilera, Carrera G, Jiménez N, Paré L, García-Recio S, Gaba L, Pereira MV, Fernández P, Prat A, Mellado B. TMPRSS2-ERG in Blood and Docetaxel Resistance in Metastatic Castration-resistant Prostate Cancer. European Urology. 2016;70(5):709–713. doi: 10.1016/j.eururo.2016.02.034. [DOI] [PubMed] [Google Scholar]
- [11].Moser N, Keeble L, Rodriguez-Manzano J, Georgiou P. ISFET arrays for lab-on-chip technology: A review; 2019 26th IEEE International Conference on Electronics, Circuits and Systems, ICECS 2019; 2019. pp. 57–60. [Google Scholar]
- [12].Tripathi P, Gulli C, Broomfield J, Alexandrou G, Kalofonou M, Bevan C, Moser N, Georgiou P. Classification of nucleic acid amplification on ISFET arrays using spectrogram-based neural networks. Computers in Biology and Medicine. 2023 April;161:107027. doi: 10.1016/j.compbiomed.2023.107027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Toumazou C, Shepherd LM, Reed SC, Chen GI, Patel A, Garner DM, Wang CJA, Ou CP, Amin-Desai K, Athanasiou P, Bai H, Brizido IM, et al. Simultaneous DNA amplification and detection using a pH-sensing semiconductor system. Nature Methods. 2013;10(7):641–646. doi: 10.1038/nmeth.2520. [DOI] [PubMed] [Google Scholar]
- [14].Rodriguez-Manzano J, Malpartida-Cardenas K, Moser N, Pennisi I, Cavuto M, Miglietta L, Moniri A, Penn R, Satta G, Randell P, Davies F, et al. Handheld point-of-care system for rapid detection of SARS-CoV-2 extracted RNA in under 20 min. ACS Central Science. 2021;7(2):307–317. doi: 10.1021/acscentsci.0c01288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Alexandrou G, Moser N, Rodriguez-Manzano J, Georgiou P, Shaw J, Coombes C, Toumazou C, Kalofonou M. Detection of Breast Cancer ESR1 p.E380Q Mutation on an ISFET Lab-on-Chip Platform; IEEE-ISCAS; 2020. pp. 1–5. [Online]. Available: https://ieeexplore.ieee.org/document/9180622. [DOI] [PubMed] [Google Scholar]
- [16].Broomfield J, Kalofonou M, Pataillot-Meakin T, Powell SM, Fernandes RC, Moser N, Bevan CL, Georgiou P. Detection of YAP1 and AR-V7 mRNA for Prostate Cancer Prognosis Using an ISFET Lab-On-Chip Platform. ACS sensors. 2022;11:2022.08.04.502773. doi: 10.1021/acssensors.2c01463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Moser N, Rodriguez-Manzano J, Lande TS, Georgiou P. A scalable ISFET sensing and memory array with sensor auto-calibration for on-chip real-time DNA detection. IEEE Transactions on Biomedical Circuits and Systems. 2018;12(2):390–401. doi: 10.1109/TBCAS.2017.2789161. [DOI] [PubMed] [Google Scholar]
- [18].Stella S, Gottardi EM, Favout V, Gonzalez EB, Errichiello S, Vitale SR, Fava C, Luciano L, Stagno F, Grimaldi F, Pironi L, et al. The Q-LAMP method represents a valid and rapid alternative for the detection of the BCR-ABL1 rearrangement in Philadelphia-positive Leukemias. International Journal of Molecular Sciences. 2019;20(24):1–12. doi: 10.3390/ijms20246106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Marin AM, Zanette DL, Nardin JM, Munhoz EC, Blanes L, Soligo Sanchuki HB, Boçon de Araújo Munhoz F, de Oliveira Coelho B, Aoki MN. Fluorescent and colorimetric RT-LAMP as a rapid and specific qualitative method for chronic myeloid leukemia diagnosis. Analytical Biochemistry. 2021 December;641 doi: 10.1016/j.ab.2021.114541. 2022. [DOI] [PubMed] [Google Scholar]
- [20].Shao L, Tekedereli I, Wang J, Yuca E, Tsang S, Sood A, Lopez-Berestein G, Ozpolat B, Ittmann M. Highly specific targeting of the TMPRSS2/ERG fusion gene using liposomal nanovectors. Clinical Cancer Research. 2012;18(24):6648–6657. doi: 10.1158/1078-0432.CCR-12-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Koo KM, Wee EJ, Trau M. Colorimetric TMPRSS2-ERG gene fusion detection in prostate cancer urinary samples via recombinase polymerase amplification. Theranostics. 2016;6(9):1415–1424. doi: 10.7150/thno.15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Haile S, Sadar MD. Androgen receptor and its splice variants in prostate cancer. Cellular and Molecular Life Sciences. 2011;68(24):3971–3981. doi: 10.1007/s00018-011-0766-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sawaya MR, Prasad R, Wilson SH, Kraut J, Pelletier H. Crystal structures of human DNA polymerase ß complexed with gapped and nicked DNA: Evidence for an induced fit mechanism. Biochemistry. 1997;36(37):11 205–11 215. doi: 10.1021/bi9703812. [DOI] [PubMed] [Google Scholar]
- [24].Ma Y, Luk A, Young FP, Lynch D, Chua W, Balakrishnar B, de Souza P, Becker TM. Droplet digital PCR based androgen receptor variant 7 (AR-V7) detection from prostate cancer patient blood biopsies. International Journal of Molecular Sciences. 2016;17(8) doi: 10.3390/ijms17081264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Alimirah F, Chen J, Basrawala Z, Xin H, Choubey D. DU-145 and PC-3 human prostate cancer cell lines express androgen receptor: Implications for the androgen receptor functions and regulation. FEBS Letters. 2006;580(9):2294–2300. doi: 10.1016/j.febslet.2006.03.041. [DOI] [PubMed] [Google Scholar]
- [26].Song C, Kim Y, Min GE, Ahn H. Dihydrotestosterone enhances castration-resistant prostate cancer cell proliferation through STAT5 activation via glucocorticoid receptor pathway. Prostate. 2014;74(12):1240–1248. doi: 10.1002/pros.22841. [DOI] [PubMed] [Google Scholar]
- [27].Yin L, Rao P, Elson P, Wang J, Ittmann M, Heston WD. Role of TMPRSS2-ERG gene fusion in negative regulation of PSMA expression. PLoS ONE. 2011;6(6):1–7. doi: 10.1371/journal.pone.0021319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rodriguez-Manzano J, Moser N, Malpartida-Cardenas K, Moniri A, Fisarova L, Pennisi I, Boonyasiri A, Jauneikaite E, Abdolrasouli A, Otter JA, Bolt F, et al. Rapid Detection of Mobilized Colistin Resistance using a Nucleic Acid Based Lab-on-a-Chip Diagnostic System. Scientific Reports. 2020;10(1):1–9. doi: 10.1038/s41598-020-64612-1. [DOI] [PMC free article] [PubMed] [Google Scholar]