Abstract
Objective
To investigate the incidence and predictors of viraemia among individuals on antiretroviral therapy (ART) in Harare, Zimbabwe.
Methods
Children (0–19 years) and adults (>19 years) starting ART between 2013 and 2015 were followed for a median of 2.8 and 2.7 years, respectively. The incidence rates of virological failure (VF), low-level viraemia (LLV), and viral blips were assessed and the predictors of viraemia were determined using logistic and parametric survival regression analyses.
Results
A total of 630 individuals initiated ART, and 19.7% of children and 5.6% of adults did not achieve viral suppression by 12 months. Younger age and CD4 count ≤200 cells/mm3 at baseline were associated with not being virally suppressed at 12 months in adults. Among those who achieved viral suppression during the follow-up period, the incidence of VF was higher in children (4.0/100 person-years vs. 0.4/100 person-years in adults; p < 0.001), as was the incidence of LLV (1.9/100 person-years vs. 0.3/100 person-years in adults; p = 0.03). The incidence rate of blips was 10.9 per 100 person-years in children and 4.0 per 100 person-years in adults.
Conclusions
Children are less likely to reach viral suppression and are at higher risk of viraemia while on ART than adults. The significance of LLV and blips needs further study.
Keywords: Antiretroviral therapy, HIV, Sub-Saharan Africa, Viral blip, Viral load, Viraemia
Introduction
Access to antiretroviral therapy (ART) has substantially increased survival and improved quality of life for HIV-infected individuals worldwide (Antiretroviral Therapy Cohort C, 2017; De La Mata et al., 2016). Sustained viral suppression achieved on ART reduces the risk of immunodeficiency, clinical progression, and HIV transmission (Cohen et al., 2016). According to World Health Organization (WHO) guidelines, an HIV viral load (VL) of ≥1000 copies/ml should prompt enhanced counselling and a repeat VL test within 6 weeks, and two sequential VL measurements of ≥1000 copies is considered virological failure (VF) and should lead to regimen change (World Health Organization, 2016).
A proportion of individuals on ART experience low-level viraemia (LLV) and/or transient viraemia; for example, viral blips have been reported in up to 40% of HIV-positive individuals on ART (Grennan et al., 2012; Havlir et al., 2001; Sorstedt et al., 2016). Viral blips have been shown to be associated with higher HIV pre-treatment VL and lower CD4 count at the time of ART initiation (Farmer et al., 2016; Havlir et al., 2001; Sorstedt et al., 2016). There is some evidence that they may impair CD4 cell recovery and maintain ongoing low-grade immune activation (Taiwo et al., 2013; Zoufaly et al., 2014).
Data on detectable viraemia after ART initiation are scarce and particularly the incidence and significance of transient and/or LLV in low-income settings, where – unlike high-income settings - regular VL monitoring is not the norm (Haas et al., 2015). The few studies that have been conducted in low-income countries have focused mainly on adults (Kanapathipillai et al., 2014). However, children are potentially at a higher risk of developing viraemia due to weight-based dosing, which may lead to variable drug levels, poor tolerability of drugs, and suboptimal adherence (Easterbrook et al., 2002; Young et al., 2015). Moreover, psychosocial factors including dependence on a caretaker and delayed disclosure of HIV status to the child may put children at higher risk of remaining viraemic after ART initiation (Lall et al., 2015). Adolescence is a specific high-risk period for poor adherence in many chronic conditions (Taddeo et al., 2008).
In this study, the incidence of and risk factors for detectable viraemia including VF, LLV, and blips were investigated in a cohort of HIV-infected children and adults initiated on ART in Harare, Zimbabwe.
Methods
A retrospective cohort study was conducted using data collected from patients who attended Newlands Clinic, Harare, Zimbabwe, a not-for-profit HIV clinic that provides care for children and adults. ART is provided free of charge according to national guidelines. Routine 6-monthly VL monitoring is performed (during the study period, this was done using the Roche COBAS AmpliPrep/COBAS TaqMan48 version 2.0 test; Roche Molecular Systems, Pleasanton, CA, USA). Adherence and psycho-social counselling is provided at each clinic visit (at least every 3 months). Adherence is assessed by pill count for each antiretroviral drug at every clinic visit. In the case of suspected VF (a VL ≥1000 copies/ml), the patient receives intensified adherence counselling for 6 weeks and repeated VL testing. Those with a VL ≥ 1000 copies/ml in two consecutive VL measurements are considered to have VF and are switched to second-line ART (protease inhibitor (PI)-based regimen and change of at least one nucleoside reverse transcriptase inhibitor (NRTI)). Those on second-line ART who have a VL ≥1000 copies/ml despite counselling undergo HIV drug resistance testing and are considered for third-line ART.
Individuals who were ART-naïve and initiated ART between August 2013 and August 2015 and who had at least two VL tests after ART initiation were included in the study. The following data were extracted: age, sex, date of ART initiation, ART regimen, adherence, height, weight, clinical history (WHO HIV disease stage, history of tuberculosis, opportunistic infections, chronic comorbidities), and laboratory parameters (VL, CD4 count, haemoglobin) at the time of ART initiation and during the follow-up period until September 28, 2017.
Data analysis
All patient data are stored in a secure electronic database. The data were anonymized prior to analysis. Statistical analyses were performed in Stata 14 (StataCorp LLC, College Station, TX, USA). The outcomes were the incidence of VF, LLV, and viral blips. Viral blip was defined as a VL measurement ≥50 copies/ml preceded and followed by a VL below the limit of detection (<50 copies/ml) (Havlir et al., 2001; Kanapathipillai et al., 2014; Martinez et al., 2005). LLV was defined as a VL ≥50 to <1000 copies/ml in at least two consecutive VL tests. The WHO definition for VF was used (VL ≥1000 copies/ml in two consecutive VL measurements) (World Health Organization, 2016).
The proportion of participants who did not achieve viral suppression by 12 months of ART (the 12-month cut-off was chosen to allow for a VL test to confirm suppression at month 12) was estimated and the factors associated with virological nonsuppression were studied using logistic regression.
The incidence rates of viral blips, LLV, and VF among those who had achieved viral suppression during the follow-up period and had at least two VL tests after initial VL suppression were estimated. Nelson–Aalen cumulative hazard curves were plotted to evaluate the incidence of VF, LLV, and viral blips after VL suppression by age at ART initiation (children aged 0–19 years and adults aged >19 years).
The factors associated with the occurrence of viral blips were investigated using survival analysis. For the model, participants were included in the analysis at time 0 (time of first suppressed VL test after ART initiation) and followed until a viral blip occurred, or for those patients who remained suppressed until the last VL test available, by the end of the follow-up. Participants who reported treatment interruption in ART for more than 2 weeks were excluded from this analysis. Since the estimated cumulative hazard of blips increased exponentially with time, we fitted the parametric survival regression with Weibull distribution stratified by age group. A value of P (the shape parameter) > 1 confirmed that the hazard of failure (viral blip) increased with time.
Age, sex, body mass index (BMI), stunting (in children only), pre-treatment VL, CD4 count, anaemia, WHO clinical stage at ART initiation, history of tuberculosis before ART initiation, chronic comorbidities (adults only), ART regimen, and average adherence were investigated as predictors of detectable viraemia. Stunting was defined as a height-for-age Z-score of <–2 (children only). Height-for-age Z-scores and BMI-for-age Z-scores in children were calculated using WHO reference standards. Anaemia was defined according to WHO criteria (haemoglobin <11 g/dl for children <5 years; haemoglobin <11.5 g/dl for children 5–11.99 years; haemoglobin <12 g/dl for children 12–14.99 years; haemoglobin <12 g/dl for females aged ≥15 years; haemoglobin <13 g/dl for males aged ≥15 years). Adherence was calculated as a percentage of the number of tablets dispensed at the last visit minus the number of tablets returned at the current visit divided by number of tablets that should have been consumed between visits. The average adherence over the study period was calculated for each participant. Age, sex, and CD4 count were adjusted for a priori. All statistical tests were two-tailed and p-values of <0.05 were considered statistically significant.
Ethical approval for the study was obtained from the Newlands Clinic Research Committee, the Medical Research Council of Zimbabwe, and Regional Committee for Medical and Health Research Ethics (Norway).
Results
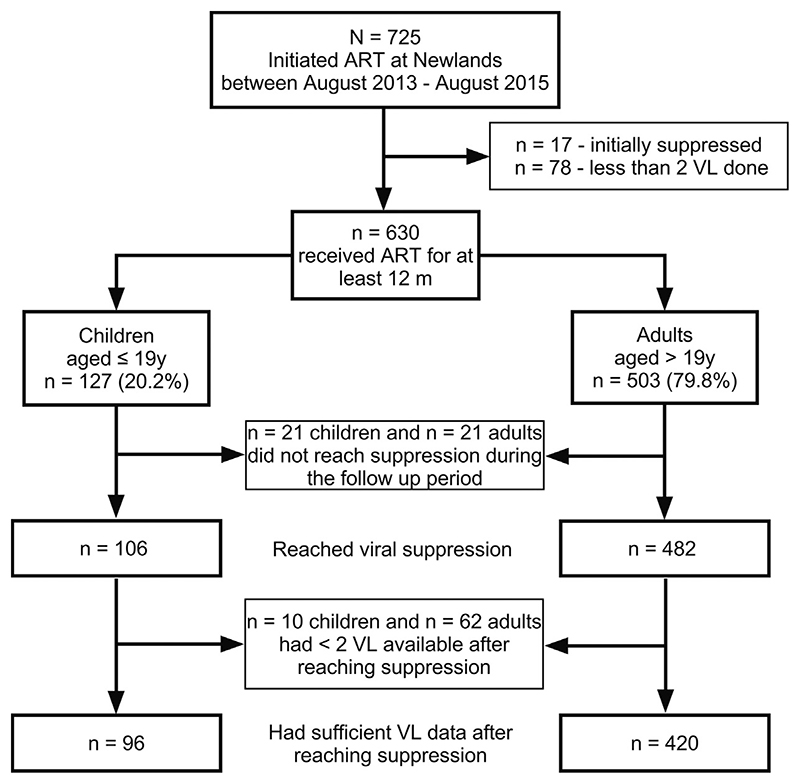
Of the 725 participants who initiated ART during the study period, 17 were excluded as they were suppressed at baseline (and thus may have received ART previously) and 78 were excluded for having <2 VL measurements available following ART initiation (Figure 1). The baseline demographic, clinical, and laboratory characteristics of the remaining 630 participants (127 children and 503 adults) are shown in Table 1. The median follow-up time was 2.8 (interquartile range (IQR) 2.3–3.2) years for children and 2.7 (IQR 1.8–2.8) years for adults. The sex distribution was equal in the paediatric group (49.6% female vs. 50.4% male), while there were more adult female than male participants (62.2% female vs. 37.8% male), consistent with studies in Sub-Saharan Africa, which have shown higher HIV treatment coverage among women (UNAIDS, 2013). Twenty-one children, all aged below 3 years at ART initiation, were commenced on PI-based regimens, as per the national guidelines, which recommend PIs as part of the first-line regimen in children below 3 years. Ten adults initiated treatment with a PI-based regimen based on clinician judgement, with reasons including severe anaemia, Kaposi sarcoma, WHO stage 4, third trimester of pregnancy, and breastfeeding.
Figure 1. Flow chart of participant recruitment.
Table 1. Characteristics of study participants. Results are presented as the number (percentage), or as the median (interquartile range), unless indicated otherwise.
| Characteristics | Children (n= 127) | Adults (n = 503) |
|---|---|---|
| Demographic | ||
| Male | 64 (50.4%) | 190 (37.8%) |
| Age at ART initiation, years | 10 (3–15) | 37 (31–44) |
| Stunted at ART initiation (height-for-age Z-score <–2) | 24 (18.9%) | |
| Underweight at ART initiation (BMI Z-score <–2 for children or BMI <18.5 kg/m2 for adults) | 13 (10.2%) | 40 (8.0%) |
| Clinical | ||
| History of tuberculosis | 10 (7.9%) | 59 (11.7%) |
| Prevalence of chronic comorbiditiesa | 2 (1.6%) | 90 (17.9%) |
| Opportunistic infectionsb | 13 (10.2%) | 75 (14.9%) |
| WHO clinical stage 3 or 4 at ART initiationc | 31 (24.8%) | 144 (28.9%) |
| ART regimen at treatment initiation | ||
| 2NRTI + NNRTI | 106 (83.5%) | 493 (98.0%) |
| PI-based | 21 (16.5%) | 10 (2.0%) |
| Average adherence to ART by pill-count | 98.4% | 99.6% |
| Years on ART | 2.8 (2.3–3.2) | 2.8 (1.8–2.8) |
| Laboratory | ||
| CD4 count at ART initiation, cells/mm3 | 341 (137–733) | 220 (104–334) |
| Viral load at ART initiation, log10 copies/mld | 4.8 (4.4–5.3) | 4.8 (4.3–5.2) |
| Anaemia at ART initiation | 82 (64.6%) | 248 (49.4%) |
ART, antiretroviral therapy; BMI, body mass index; WHO, World Health Organization; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Chronic comorbidities included hypertension, diabetes, or renal failure diagnosed before inclusion in the study.
After ART initiation, had at least one episode of one of the following: oral candidiasis, necrotizing gingivitis, herpes zoster, histoplasmosis, cryptococcal meningitis, molluscum contagiosum, genital warts, tonsillitis.
Data missing for two children and five adults.
Data missing for 42 children and 159 adults.
Of the 630 participants, significantly more children than adults did not achieve viral suppression by 12 months post ART initiation (19.7% children vs. 5.6% adults; p < 0.001). Younger age and CD4 count ≤200cells/mm3 at baseline were associated with not achieving viral suppression in adults (Table 2). Over the followup period, 106 (83.5%) children and 482 (95.8%) adults reached VL suppression, with the median time to VL suppression being 0.5 (IQR 0.4–1.3) years for children and 0.5 (IQR 0.2–0.8) years for adults.
Table 2. Logistic regression analysis of the risk factors for not achieving viral suppression by 12 months of ART.
| Characteristic | Children (≤19 years of age) (n = 127) | Adults (>19 years of age) (n = 503) | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted analysis | Adjusted analysisa | Unadjusted analysis | Adjusted analysisa | |||||
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Female | 1.69 (0.69–4.11) | 0.25 | 1.71 (0.69–4.19) | 0.24 | 0.59 (0.27–1.26) | 0.17 | 0.72 (0.33–1.60) | 0.43 |
| Age at ART initiation (per 1 year older) | 1.05 (0.98–1.13) | 0.18 | 1.06 (0.96–1.18) | 0.23 | 0.97 (0.93–1.01) | 0.15 | 0.95 (0.91–1.00) | 0.04 |
| Stunted at ART initiation (height-for-age Z-score <–2), yes vs. no | 0.53 (0.14–1.93) | 0.33 | - | - | - | - | - | - |
| BMI Z-score (children) or BMI kg/m2 (adults) at ART initiation | 0.78 (0.57–1.07) | 0.12 | 0.79 (0.56–1.13) | 0.21 | 0.91 (0.84–0.98) | 0.01 | 0.94 (0.87–1.03) | 0.22 |
| History of TB, yes vs. no | - | - | - | - | 1.70 (0.62–4.64) | 0.30 | 0.92 (0.31–2.74) | 0.89 |
| Chronic comorbiditiesb, yes vs. no | - | - | - | - | 0.53 (0.16–1.81) | 0.32 | 0.86 (0.24–3.16) | 0.83 |
| WHO clinical stage 3 or 4 at ART initiation, yes vs. no | 1.23 (0.46–3.30) | 0.68 | 1.44 (0.51–4.06) | 0.49 | 2.24 (1.04–4.84) | 0.04 | 1.18 (0.50–2.83) | 0.70 |
| ART regimen at treatment initiation (Ref. 2NRTI + NNRTI)c | 0.64 (0.17–2.36) | 0.50 | 1.06 (0.17–6.72) | 0.95 | - | - | - | - |
| Average adherence to ART by pill-count, % | 0.88 (0.72–1.07) | 0.21 | 0.85 (0.69–1.05) | 0.14 | 0.97 (0.89–1.06) | 0.54 | 1.00 (0.91–1.09) | 0.92 |
| CD4 count at ART initiation, cells/mm3 | ||||||||
| ≤200 cells/mm3 | 2.55 (1.02–6.36) | 0.04 | 2.21 (0.78–6.29) | 0.14 | 5.66 (2.11–15.1) | 0.001 | 5.92 (2.18–16.1) | <0.001 |
| >200 cells/mm3 | Ref. | Ref. | Ref. | Ref. | ||||
| Viral load at ART initiation, log10 copies/ml | 1.15 (0.63–2.11) | 0.65 | 1.48 (0.73–3.00) | 0.27 | 1.78 (1.00–3.18) | 0.05 | 1.26 (0.68–2.33) | 0.46 |
| Anaemia at ART initiation, yes vs. no | 1.53 (0.58–4.00) | 0.38 | 1.46 (0.55–3.88) | 0.45 | 2.26 (1.00–5.10) | 0.05 | 1.73 (0.72–4.19) | 0.22 |
ART, antiretroviral therapy; OR, odds ratio; CI, confidence interval; BMI, body mass index; TB, tuberculosis; WHO, World Health Organization; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Adjusted for CD4 count at ART initiation, age, and sex.
Chronic comorbidities included hypertension, diabetes, or renal failure diagnosed before inclusion in the study.
PI-based regimen as the exposure.
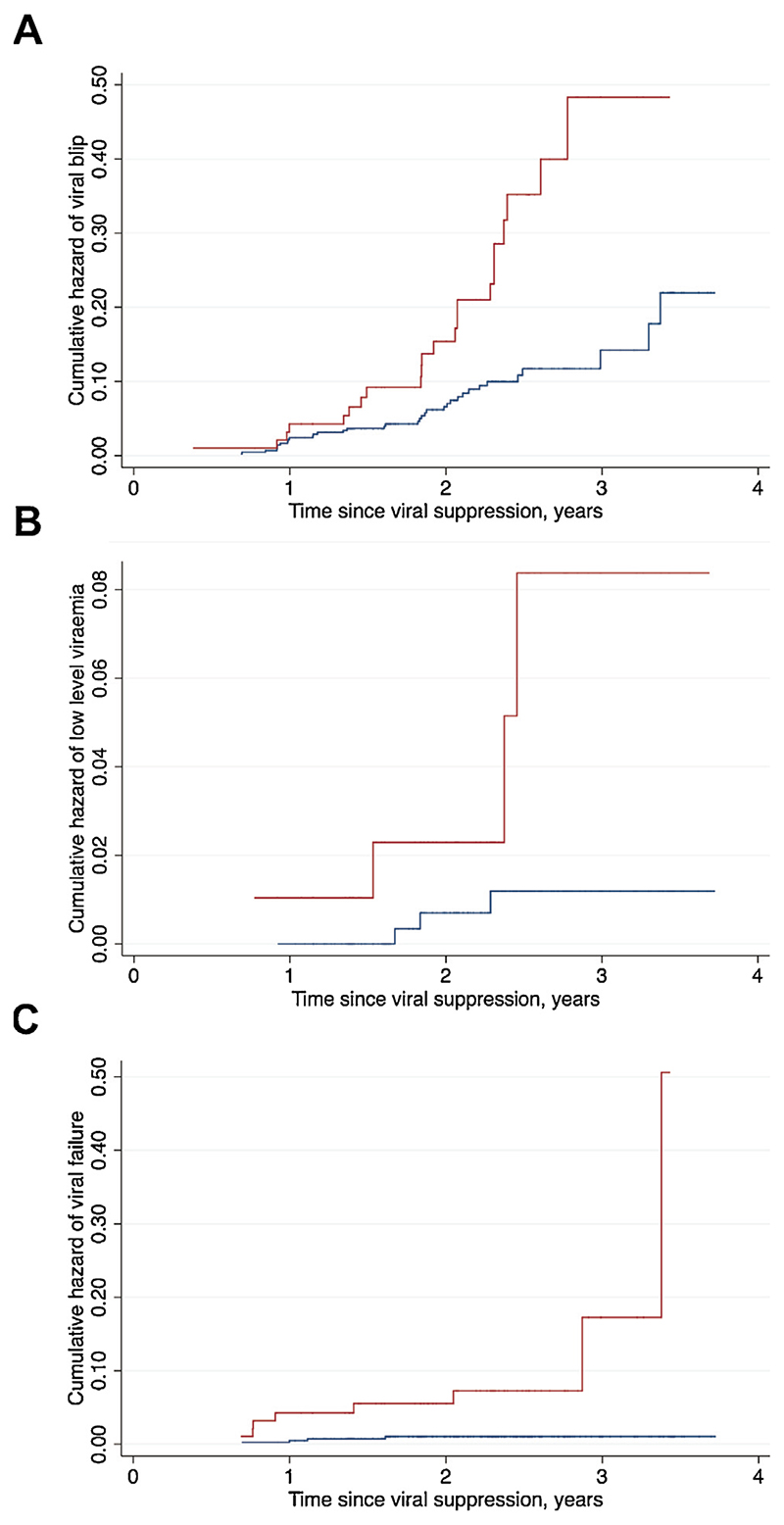
Of the 588 participants who achieved viral suppression after ART initiation, 516 had sufficient VL data to study the incidence of detectable viraemia. Over the follow-up period, 57 (11%) participants experienced a viral blip. Blips occurred more commonly in children than in adults (22.9% vs. 8.3%; p < 0.001), with the incidence rate being 10.9 (95% confidence interval (CI) 7.2–16.6) per 100 person-years in children and 4.0 (95% CI 2.8–5.5) per 100 person-years in adults (Figure 2A). Fifty percent of blips in children and 71.4% of blips in adults were of low magnitude (50–199 copies/ ml). The median time from viral suppression to a blip was 1.9 (IQR 1.4–2.3) years in children and 1.8 (IQR 1.0–2.1) years in adults.
Figure 2.
Nelson–Aalen cumulative hazard curves for the development of (A) viral blips, (B) low-level viraemia (LLV), and (C) virological failure (VF) after viral load suppression. Curves for children are presented in red and curves for adults in blue.
Seven (1.4%) participants (four children and three adults) experienced LLV and 12 (2.3%) participants (eight children and four adults) developed VF after initial suppression during follow-up. The incidence of LLV was 1.9 (95% CI 0.7-5.1) per 100 person-years in children and 0.3 (95% CI 0.1-1.0) per 100 person-years in adults (Figure 2B). VF was more common in children compared to adults (8.3% vs. 0.9%; p < 0.001). The incidence of VF was 4.0 (95% CI 2.0–7.9) per 100 person-years in children and 0.4 (95% CI 0.2–1.2) per 100 person-years in adults (Figure 2C). Notably, 2.2% of children <10 years and 13.7% of those ≥10 years developed VF (p = 0.05). All four adults with VF had re-suppression (three following a switch from a non-nucleoside reverse transcriptase inhibitor (NNRTI)-based to a PI-based regimen, one remaining on the same NNRTI- based regimen), while only three children had re-suppression (two following a switch from an NNRTI-based to a PI-based regimen, one remaining on the same NNRTI-based regimen) during the follow-up period.
Four out of 22 children (18.2%) experienced both viral blips and VF during the follow-up. Blips were followed by VF in two participants, while blips occurred after VF and re-suppression in the other two cases. In those participants with blips followed by VF, the magnitude of blips was low, while in those who had blips after VF, blips were of medium (200–499 copies/ml) and high (>500 copies/ml) magnitude. Among two children with blips after VF, one had re-suppression on the same ART regimen with adherence counselling, while another had re-suppression after a switch to a PI-based regimen. Two children with blips followed by VF remained unsuppressed by the end of follow-up.
Due to low rates of LLV in the study participants after initial VL suppression, risk factors for only viral blips were investigated in the survival analysis. The survival analysis included 507 participants (94 in the paediatric group and 413 in the adult group). No baseline characteristics were found to be associated with an increased risk of viral blips in children or adults (Table 3).
Table 3. Risk factors for the development of viral blips by age.
| Characteristic | Children (≤19 years) | Adults (>19 years) | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted analysis | Adjusted analysis’a | Unadjusted analysis | Adjusted analysis’a | |||||
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Female | 1.41 (0.60–3.32) | 0.43 | 1.08 (0.42–2.76) | 0.87 | 0.79 (0.39–1.58) | 0.50 | 0.76 (0.37–1.56) | 0.45 |
| Age at ART initiation, years | 0.99 (0.91–1.07) | 0.74 | 1.06 (0.92–1.22) | 0.43 | 1.00 (0.96–1.04) | 0.87 | 1.00 (0.96–1.04) | 0.91 |
| Stunted at ART initiation (height-for-age Z-score <–2) | 0.46 (0.13–1.67) | 0.24 | 0.39 (0.08–2.01) | 0.26 | – | – | – | – |
| BMI Z-score (children) or BMI kg/m2 (adults) at ART initiation | 1.12 (0.84–1.49) | 0.44 | 0.87 (0.55–1.37) | 0.54 | 0.97 (0.92–1.03) | 0.31 | 0.97 (0.91–1.03) | 0.33 |
| History of TB | 2.17 (0.60–7.94) | 0.24 | 2.99 (0.87–10.3) | 0.08 | 0.87 (0.27–2.81) | 0.82 | 0.85 (0.24–3.00) | 0.80 |
| Chronic comorbiditiesb | – | – | – | – | 1.17 (0.50–2.76) | 0.71 | 1.19 (0.50–2.86) | 0.69 |
| WHO clinical stage 3 or 4 at ART initiation | 0.62 (0.19–1.98) | 0.42 | 0.75 (0.21–2.74) | 0.66 | 0.78 (0.33–1.83) | 0.57 | 0.78 (0.31–1.94) | 0.60 |
| ART regimen at treatment initiation (Ref. 2NRTI + NNRTI)c | 1.92 (0.72–5.11) | 0.19 | 1.65 (0.44–6.26) | 0.46 | 0.94 (0.13–6.97) | 0.95 | 0.99 (0.13–7.42) | 0.99 |
| Average adherence to ART by pill-count, % | 0.91 (0.67–1.24) | 0.56 | 0.90 (0.66–1.22) | 0.50 | 1.16 (0.86–1.56) | 0.34 | 1.17 (0.86–1.58) | 0.31 |
| Months to viral load suppression | 1.06 (0.99–1.14) | 0.09 | 1.06 (0.98–1.16) | 0.14 | 1.03 (0.96–1.12) | 0.38 | 1.03 (0.96–1.11) | 0.35 |
| CD4 count at ART initiation, log™ cells/mm3 | 2.21 (0.84–5.82) | 0.11 | 3.83 (0.53–27.5) | 0.18 | 1.19 (0.57–2.50) | 0.64 | 1.29 (0.61–2.73) | 0.50 |
| Viral load at ART initiation, log™ copies/ml | 1.01 (0.43–2.34) | 0.99 | 1.06 (0.41–2.73) | 0.91 | 1.58 (0.95–2.62) | 0.08 | 1.56 (0.98–2.50) | 0.06 |
| Anaemia at ART initiation | 2.33 (0.78–6.97) | 0.13 | 1.99 (0.65–6.10) | 0.23 | 0.91 (0.46–1.81) | 0.79 | 0.97 (0.44–2.13) | 0.94 |
HR, hazard ratio; CI, confidence interval; ART, antiretroviral therapy; BMI, body mass index; TB, tuberculosis; WHO, World Health Organization; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Adjusted for CD4 count at ART initiation, age, and sex.
Chronic comorbidities included hypertension, diabetes, or renal failure diagnosed before inclusion in the study.
Pl-based regimen as the exposure.
Discussion
Among individuals starting ART, a significantly higher proportion of children compared to adults did not achieve viral suppression by 12 months. Likely reasons include poor tolerability to antiretroviral drugs, inadequate ART dosing, or suboptimal adherence (Boerma et al., 2016). This is in line with the findings of Jobanputra et al., who showed that being a child or adolescent is associated with detectable viraemia (Jobanputra et al., 2015). Some individuals may require longer periods to achieve suppression, as shown in the present study, where over the follow-up period, 83.5% of children and 95.8% of adults did achieve viral suppression. One possibility is that perinatally HIV-infected young children tend to have sustained high VL in the first years of life with slow reduction of peak VL with increasing age (McIntosh et al., 1996). This potentially could contribute to incomplete initial VL suppression and a higher risk of viraemia. Yet the present study results do not support this, as the exclusion of children aged ≤3 years did not change the rates of viral non-suppression in the paediatric study group. Lower CD4 count at baseline was associated with not being suppressed at 12 months in adults, a finding consistent with other studies (Anude et al., 2013; Collaboration of Observational et al., 2008; Mujugira et al., 2016; Samuel et al., 2014).
This study found a significantly higher incidence of viral blips among children compared to adults. The reported incidence of blips varies in different populations (Farmer et al., 2016; Grennan et al., 2012; Havlir et al., 2001; Kanapathipillai et al., 2014; Sorstedt et al., 2016), largely due to different definitions and variability of the assays for VL. We used a common definition of viral blip, as a single VL measurement ≥50 copies/ml preceded and followed by VL <50 copies/ml (Fung et al., 2012; Ryscavage et al., 2014). Compared to studies that have used a similar definition, it was found that a lower proportion of adults in the present study experienced blips: 8.3% during 2.1 years of follow-up compared to 18.6–40% followed up from 1.08 to 1.58 years (Havlir et al., 2001; Martinez et al., 2005; Sklar et al., 2002). Several studies have shown that patients on PI regimens are more prone to developing viral blips (Grennan et al., 2012; Sorstedt et al., 2016). Havlir et al. (2001) reported a high incidence of blips (40%) in a cohort of patients who received an unboosted PI-based treatment regimen in a high-income country. In addition, the median number of VL measurements was 17 per participant, compared to an average of six measurements in the present study in which only 2.1% of adults were receiving a ritonavir-boosted PI-based regimen.
It is likely that more frequent VL testing will increase the probability of detecting blips, which may explain the discrepancies between our findings and those of studies conducted in high-income settings. However, in a study comparing the incidence of blips in high-, middle-, and low-income countries, 21% of the individuals in the first category experienced blips, while only 11% of participants did so in the middle/low income group, despite a similar ratio of blips to the number of VL measurements across the settings (Kanapathipillai et al., 2014). HIV plasma RNA measurements are subject to pre-analytic and laboratory errors, variations in cut-off point, and variations in lower limit of detection among the different VL assays, which may have an impact on the incidence of blips (Grennan et al., 2012; Young et al., 2015). Even though good agreement between the different assays is observed at high VL levels, there is substantial variability at low VL levels (Ruelle et al., 2012; Swenson et al., 2014a).
There appear to be limited data on the incidence of viral blips in children in the low-income setting (Jobanputra et al., 2015; Szubert et al., 2017). In a retrospective analysis of VL tests among children who initiated ART in Uganda and Zimbabwe, 46% of children experienced viral blips (Szubert et al., 2017). This is considerably higher than the results of the present study, but may be explained by the use of WHO 2006 criteria for ART initiation, which relied on clinical and/or immunological assessment and not just the diagnosis of HIV infection. The majority of studies in low-income countries have investigated the incidence of any detectable viraemia in one or two VL measurements. For example, a cross-sectional study among adolescents with HIV conducted in an urban setting in Cameroon utilized two consecutive VL measurements to detect sustained VL suppression (VL < 50 copies/ml in two VL tests) (Fokam et al., 2017). In that study, 18.6% of participants had a VL measurement above 50 copies/ml in a single test. Another study conducted in Ethiopia found detectable viraemia (defined as an HIV-1 RNA of 41–1000 copies/ml) in a single VL measurement in 13% of HIV-positive children who had received first-line ART for a median of 24 months (Mulu et al., 2014). In a study by Jobanputra et al., 38% of children aged <10 years and 34% of children aged 10–19 years had single detectable VL (>100 copies/ml) followed by VL re-suppression (Jobanputra et al., 2015). Although the results from these studies are not directly comparable to the present study findings, they show that detectable viraemia is common in paediatric patients. A multicentre cohort study of data on children with HIV in the UK and Ireland showed that 22% of participants with sustained viral suppression experienced transient viraemia (defined as a single VL >50 copies/ml between two VL tests below 50 copies/ml), with an incidence of 12 per 100 person-years, similar to the findings in our study (Lee et al., 2007).
The incidence of LLV and VF after reaching VL suppression was also significantly higher in children than in adults. Furthermore, in the present study, adolescents (those aged ≥10 years) were more likely to experience VF compared to younger children, a finding that has also been reported by other studies conducted in Africa (Makadzange et al., 2015). Adolescence is a period of particularly high risk for poor adherence (Mukui et al., 2016; Nachega et al., 2009). Taken together, these emphasize the need for investigating and addressing factors that impede viral suppression among adolescents.
The rate of VF in children who reached VL suppression is lower than those reported by other studies (Makadzange et al., 2015; Salou et al., 2016). A possible reason for the higher VF rates reported in other studies is the cross-sectional design of studies and lack of information regarding initial VL suppression prior to inclusion in the study (Fokam et al., 2017; Salou et al., 2016). Due to regular VL monitoring and the longitudinal design of our study, it was possible to distinguish between those who developed VF and those who never reached suppression. Another explanation for low VF in our cohort is the high standard of care provided at Newlands Clinic, with routine VL testing, continuous adherence monitoring support, and a rapid response with intensified adherence counselling and/or ART switch among those who have a non-suppressed VL (Haas et al., 2015). Routine VL testing, which is not available as standard of care in many low-income settings, can detect VF earlier than targeted VL monitoring, i.e., VL testing prompted by clinical or immunological deterioration.
A number of studies have shown that episodes of LLV and viral blips are associated with an increased risk of subsequent VF in adults (Antiretroviral Therapy Cohort C et al., 2015; Grennan et al., 2012; Laprise et al., 2013; Leierer et al., 2016). While some studies have suggested that episodes of transient viraemia may result in the selection of drug-resistant HIV strains, leading to an increased risk of VF (Clutter et al., 2016; Gonzalez-Serna et al., 2014; Swenson et al., 2014b), others have found no link between blips and HIV progression (Havlir et al., 2001; Kanapathipillai et al., 2014; Nettles et al., 2005). Young et al. found a gradual increase in the risk of VF with increasing blip magnitude (Young et al., 2015). In another study, only blips of 500–999 copies/ml magnitude were associated with the increased risk of viral rebound (Grennan et al., 2012). In contrast, other studies have shown no evidence of an association between blips and VF in adults (Kanapathipillai et al., 2014; Martinez et al., 2005; Sklar et al., 2002). However, these studies have been conducted largely in adults. In the present study, not only did children have a higher risk of VF, but they also had a higher incidence of viral blips as well as LLV, and the significance of these in predicting VF needs further study. Furthermore, careful virological monitoring is warranted in children.
The strengths of this study are the use of a standardized definition for viral blips, regular VL monitoring, availability of detailed clinical data including adherence data, and good follow-up rates. The limitations of the study include the relatively small sample size and low number of blips in each age group, and there may therefore be inadequate power to detect an association between the covariates and the incidence of viral blips. Furthermore, the follow-up period was not long enough to investigate whether blips or LLV increase the risk of subsequent VF. Adherence may have been over-estimated, as the pill count is not the optimal measure of adherence.
In conclusion, this study demonstrated that detectable viraemia is common among children, although its role with regard to long-term outcomes necessitates further study in low-income settings, especially where HIV prevalence is high and the availability of VL monitoring remains limited.
Acknowledgements
We are grateful to the staff and patients of Newlands Clinic for their assistance.
Funding
This study was supported by grant from HelseNord (HNF 1387-17). RAF is funded through a Wellcome Trust Senior Fellowship in Clinical Science (206316/Z/17/Z). The funding sources were not involved in the analysis, interpretation of the data, writing of the report, or in the decision to submit the article for publication.
Footnotes
Ethical approval
Ethical approval was given by Newlands Clinic Research Committee, the Medical Research Council of Zimbabwe (MRCZ/E/188), and the Regional Committee for Medical and Health Research Ethics, Norway (REK 2015/1650).
Conflict of interest
None declared.
References
- UNAIDS. Global report 2013. UNAIDS; Geneva: 2013. [Google Scholar]
- Antiretroviral Therapy Cohort C. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 2017;4(8):e349–56. doi: 10.1016/S2352-3018(17)30066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antiretroviral Therapy Cohort C. Vandenhende MA, Ingle S, May M, Chene G, Zangerle R, et al. Impact of low-level viremia on clinical and virological outcomes in treated HIV-1-infected patients. AIDS. 2015;29(3):373–83. doi: 10.1097/QAD.0000000000000544. [DOI] [PubMed] [Google Scholar]
- Anude CJ, Eze E, Onyegbutulem HC, Charurat M, Etiebet MA, Ajayi S, et al. Immuno-virologic outcomes and immuno-virologic discordance among adults alive and on anti-retroviral therapy at 12 months in Nigeria. BMC Infect Dis. 2013;13:113. doi: 10.1186/1471-2334-13-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerma RS, Boender TS, Bussink AP, Calis JC, Bertagnolio S, Rinke de Wit TF, et al. Suboptimal viral suppression rates among HIV-infected children in low- and middle-income countries: a meta-analysis. Clin Infect Dis. 2016;63(12):1645–54. doi: 10.1093/cid/ciw645. [DOI] [PubMed] [Google Scholar]
- Clutter DS, Jordan MR, Bertagnolio S, Shafer RW. HIV-1 drug resistance and resistance testing. Infect Genet Evol. 2016;46:292–307. doi: 10.1016/j.meegid.2016.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375(9):830–9. doi: 10.1056/NEJMoa1600693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaboration of Observational HIVERESG. Sabin CA, Smith CJ, d'Arminio Monforte A, Battegay M, Gabiano C, et al. Response to combination antiretroviral therapy: variation by age. AIDS. 2008;22(12):1463–73. doi: 10.1097/QAD.0b013e3282f88d02. [DOI] [PubMed] [Google Scholar]
- De La Mata NL, Kumarasamy N, Khol V, Ng OT, Van Nguyen K, Merati TP, et al. Improved survival in HIV treatment programmes in Asia. Antivir Ther. 2016;21(6):517–27. doi: 10.3851/IMP3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterbrook PJ, Ives N, Waters A, Mullen J, O’Shea S, Peters B, et al. The natural history and clinical significance of intermittent viraemia in patients with initial viral suppression to 400 copies/ml. AIDS. 2002;16(11):1521–7. doi: 10.1097/00002030-200207260-00009. [DOI] [PubMed] [Google Scholar]
- Farmer A, Wang X, Ganesan A, Deiss RG, Agan BK, O’Bryan TA, et al. Factors associated with HIV viral load “blips” and the relationship between self-reported adherence and efavirenz blood levels on blip occurrence: a case-control study. AIDS Res Ther. 2016;13:16. doi: 10.1186/s12981-016-0100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fokam J, Billong SC, Jogue F, Moyo Tetang Ndiang S, Nga Motaze AC, Paul KN, et al. Immuno-virological response and associated factors amongst HIV-1 vertically infected adolescents in Yaounde-Cameroon. PLoS One. 2017;12(11):e0187566. doi: 10.1371/journal.pone.0187566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung IC, Gambhir M, van Sighem A, de Wolf F, Garnett GP. The clinical interpretation of viral blips in HIV patients receiving antiviral treatment: are we ready to infer poor adherence? J Acquir Immune Defic Syndr. 2012;60(1):5–11. doi: 10.1097/QAI.0b013e3182487a20. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Serna A, Min JE, Woods C, Chan D, Lima VD, Montaner JS, et al. Performance of HIV-1 drug resistance testing at low-level viremia and its ability to predict future virologic outcomes and viral evolution in treatment-naive individuals. Clin Infect Dis. 2014;58(8):1165–73. doi: 10.1093/cid/ciu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grennan JT, Loutfy MR, Su D, Harrigan PR, Cooper C, Klein M, et al. Magnitude of virologic blips is associated with a higher risk for virologic rebound in HIV-infected individuals: a recurrent events analysis. J Infect Dis. 2012;205(8):1230–8. doi: 10.1093/infdis/jis104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas AD, Keiser O, Balestre E, Brown S, Bissagnene E, Chimbetete C, et al. Monitoring and switching of first-line antiretroviral therapy in adult treatment cohorts in sub-Saharan Africa: collaborative analysis. Lancet HIV. 2015;2(7):e271-8. doi: 10.1016/S2352-3018(15)00087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havlir DV, Bassett R, Levitan D, Gilbert P, Tebas P, Collier AC, et al. Prevalence and predictive value of intermittent viremia with combination hiv therapy. JAMA. 2001;286(2):171–9. doi: 10.1001/jama.286.2.171. [DOI] [PubMed] [Google Scholar]
- Jobanputra K, Parker LA, Azih C, Okello V, Maphalala G, Kershberger B, et al. Factors associated with virological failure and suppression after enhanced adherence counselling, in children, adolescents and adults on antiretroviral therapy for HIV in Swaziland. PLoS One. 2015;10(2):e0116144. doi: 10.1371/journal.pone.0116144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanapathipillai R, McManus H, Kamarulzaman A, Lim PL, Templeton DJ, Law M, et al. The significance of HIV ‘blips’ in resource-limited settings: is it the same? analysis of the treat Asia HIV Observational Database (TAHOD) and the Australian HIV Observational Database (AHOD) PLoS One. 2014;9(2):e86122. doi: 10.1371/journal.pone.0086122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lall P, Lim SH, Khairuddin N, Kamarulzaman A. Review: an urgent need for research on factors impacting adherence to and retention in care among HIV-positive youth and adolescents from key populations. J Int AIDS Soc. 2015;18(2 Suppl. 1):19393. doi: 10.7448/IAS.18.2.19393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprise C, de Pokomandy A, Baril JG, Dufresne S, Trottier H. Virologic failure following persistent low-level viremia in a cohort of HIV-positive patients: results from 12 years of observation. Clin Infect Dis. 2013;57(10):1489–96. doi: 10.1093/cid/cit529. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Shingadia D, Pillay D, Walker AS, Riordan A, Menson E, et al. Transient viral load increases in HIV-infected children in the U.K. and Ireland: what do they mean? Antivir Ther. 2007;12(6):949–56. [PubMed] [Google Scholar]
- Leierer G, Grabmeier-Pfistershammer K, Steuer A, Sarcletti M, Geit M, Haas B, et al. A single quantifiable viral load is predictive of virological failure in human immunodeficiency virus (HIV)-infected patients on combination antiretroviral therapy: the Austrian HIV cohort study. Open Forum Infect Dis. 2016;3(2):ofw089. doi: 10.1093/ofid/ofw089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makadzange AT, Higgins-Biddle M, Chimukangara B, Birri R, Gordon M, Mahlanza T, et al. Clinical, virologic, immunologic outcomes and emerging HIV. Drug resistance patterns in children and adolescents in public ART care in Zimbabwe. PLoS One. 2015;10(12):e0144057. doi: 10.1371/journal.pone.0144057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez V, Marcelin AG, Morini JP, Deleuze J, Krivine A, Gorin I, et al. HIV-1 intermittent viraemia in patients treated by non-nucleoside reverse transcriptase inhibitor-based regimen. AIDS. 2005;19(10):1065–9. doi: 10.1097/01.aids.0000174453.55627.de. [DOI] [PubMed] [Google Scholar]
- McIntosh K, Shevitz A, Zaknun D, Kornegay J, Chatis P, Karthas N, et al. Age- and time-related changes in extracellular viral load in children vertically infected by human immunodeficiency virus. Pediatr Infect Dis J. 1996;15(12):1087–91. doi: 10.1097/00006454-199612000-00006. [DOI] [PubMed] [Google Scholar]
- Mujugira A, Celum C, Tappero JW, Ronald A, Mugo N, Baeten JM. Younger age predicts failure to achieve viral suppression and virologic rebound among HIV-1-infected persons in serodiscordant partnerships. AIDS Res Hum Retroviruses. 2016;32(2):148–54. doi: 10.1089/aid.2015.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukui IN, Ng’ang’a L, Williamson J, Wamicwe JN, Vakil S, Katana A, et al. Rates and predictors of non-adherence to antiretroviral therapy among HIV-positive individuals in Kenya: results from the second Kenya AIDS indicator survey, 2012. PLoS One. 2016;11(12):e0167465. doi: 10.1371/journal.pone.0167465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulu A, Liebert UG, Maier M. Virological efficacy and immunological recovery among Ethiopian HIV-1 infected adults and children. BMC Infect Dis. 2014;14:28. doi: 10.1186/1471-2334-14-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachega JB, Hislop M, Nguyen H, Dowdy DW, Chaisson RE, Regensberg L, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa. J Acquir Immune Defic Syndr. 2009;51(1):65–71. doi: 10.1097/QAI.0b013e318199072e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettles RE, Kieffer TL, Kwon P, Monie D, Han Y, Parsons T, et al. Intermittent HIV-1 viremia (Blips) and drug resistance in patients receiving HAART. JAMA. 2005;293(7):817–29. doi: 10.1001/jama.293.7.817. [DOI] [PubMed] [Google Scholar]
- Ruelle J, Debaisieux L, Vancutsem E, De Bel A, Delforge ML, Pierard D, et al. HIV-1 low-level viraemia assessed with 3 commercial real-time PCR assays show high variability. BMC Infect Dis. 2012;12:100. doi: 10.1186/1471-2334-12-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryscavage P, Kelly S, Li JZ, Harrigan PR, Taiwo B. Significance and clinical management of persistent low-level viremia and very-low-level viremia in HIV-1-infected patients. Antimicrob Agents Chemother. 2014;58(7):3585–98. doi: 10.1128/AAC.00076-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salou M, Dagnra AY, Butel C, Vidal N, Serrano L, Takassi E, et al. High rates of virological failure and drug resistance in perinatally HIV-1-infected children and adolescents receiving lifelong antiretroviral therapy in routine clinics in Togo. J Int AIDS Soc. 2016;19(1):20683. doi: 10.7448/IAS.19.1.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel M, Jose S, Winston A, Nelson M, Johnson M, Chadwick D, et al. The effects of age on associations between markers of HIV progression and markers of metabolic function including albumin, haemoglobin and lipid concentrations. HIV Med. 2014;15(5):311–6. doi: 10.1111/hiv.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar PA, Ward DJ, Baker RK, Wood KC, Gafoor Z, Alzola CF, et al. Prevalence and clinical correlates of HIV viremia (‘blips’) in patients with previous suppression below the limits of quantification. AIDS. 2002;16(15):2035–41. doi: 10.1097/00002030-200210180-00008. [DOI] [PubMed] [Google Scholar]
- Sorstedt E, Nilsson S, Blaxhult A, Gisslen M, Flamholc L, Sonnerborg A, et al. Viral blips during suppressive antiretroviral treatment are associated with high baseline HIV-1 RNA levels. BMC Infect Dis. 2016;16:305. doi: 10.1186/s12879-016-1628-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson LC, Cobb B, Geretti AM, Harrigan PR, Poljak M, Seguin-Devaux C, et al. Comparative performances of HIV-1 RNA load assays at low viral load levels: results of an international collaboration. J Clin Microbiol. 2014a;52(2):517–23. doi: 10.1128/JCM.02461-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson LC, Min JE, Woods CK, Cai E, Li JZ, Montaner JS, et al. HIV drug resistance detected during low-level viraemia is associated with subsequent virologic failure. AIDS. 2014b;28(8):1125–34. doi: 10.1097/QAD.0000000000000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szubert AJ, Prendergast AJ, Spyer MJ, Musiime V, Musoke P, Bwakura-Dangarembizi M, et al. Virological response and resistance among HIV-infected children receiving long-term antiretroviral therapy without virological monitoring in Uganda and Zimbabwe: observational analyses within the randomised ARROW trial. PLoS Med. 2017;14(11):e1002432. doi: 10.1371/journal.pmed.1002432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddeo D, Egedy M, Frappier JY. Adherence to treatment in adolescents. Paediatr Child Health. 2008;13(1):19–24. doi: 10.1093/pch/13.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiwo B, Hunt PW, Gandhi RT, Ellingson A, McKenna M, Jacobson JM, et al. CD8+ T-cell activation in HIV-1-infected patients experiencing transient low-level viremia during antiretroviral therapy. J Acquir Immune Defic Syndr. 2013;63(1):101–4. doi: 10.1097/QAI.0b013e3182895af4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Second edition. World Health Organization; Geneva, Switzerland: 2016. [PubMed] [Google Scholar]
- Young J, Rickenbach M, Calmy A, Bernasconi E, Staehelin C, Schmid P, et al. Transient detectable viremia and the risk of viral rebound in patients from the Swiss HIV Cohort Study. BMC Infect Dis. 2015;15:382. doi: 10.1186/s12879-015-1120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoufaly A, Kiepe JG, Hertling S, Hufner A, Degen O, Feldt T, et al. Immune activation despite suppressive highly active antiretroviral therapy is associated with higher risk of viral blips in HIV-1-infected individuals. HIV Med. 2014;15(8):449–57. doi: 10.1111/hiv.12134. [DOI] [PubMed] [Google Scholar]