Abstract
Group 3 innate lymphoid cells (ILC3s) are critical for maintaining gut epithelial integrity and tissue repair. Recent research identifies mechanisms by which circadian machinery and feeding behavior regulate enteric ILC3s to maintain gut homeostasis.
The enteric immune system is crucial for gut epithelium protection against pathogen invasion and ingestion-associated chemical and physical abrasions, as well as for general integrity and maintenance of the intestinal epithelium. Within the gut ecosystem, type 3 innate lymphoid cells (ILC3s) are highly enriched at mucosal sites where they act as major regulators of mucosal defense. In this issue of Nature Immunology and in a recent Letter in Nature, Seillet et al.1 and Godinho-Silva et al.2 demonstrate how integration of local food-induced neuronal signaling and light-entrained systemic circadian circuits regulates intrinsic ILC3 rhythmic cycles important for gut protection.
ILC3s express the transcription factor RORγt and secrete cytokines such as interleukin-22 (IL-22), IL-17, granulocyte–macrophage colony-stimulating factor (GM-CSF) and tumor necrosis factor (TNF-α), mirroring the functional characteristics of type 17 helper T cells (TH17 cells)3. ILC3-derived cytokines directly interact with intestinal epithelial cells, and also modulate other immune cell functions to induce tissue reorganization4. While ILC3s produce cytokines crucial for barrier protection, uncontrolled activation can disrupt intestinal homeostasis and lead to severe gut pathologies such as inflammatory bowel disease, Crohn’s disease and bowel cancer. Therefore, it is critical that this defense mechanism is sensitive to environmental cues that constantly challenge the immunological balance within the intestine, such as the diurnal cycle, food intake and commensal microbes.
Almost all mammalian cells are equipped with circadian clock genes that allow physiological adaption by anticipating environmental cues using the brain’s master circadian clock5, and clock genes in intestinal epithelial cells regulate barrier integrity6. However, it was not clear whether such a clock exists in ILC3s. To investigate this, Seillet et al.1 assessed cytokine production in ILCs isolated from various murine organs at different stages of the light–dark cycle. During the dark (active) phase, Seillet et al. found increased numbers of IL-22-producing ILC3s in the gut, while IL-17-expressing ILC3s remained unaffected. Furthermore, IL-22 production in intestinal ILC3s exhibited a diurnal pattern and peaked between t4 (4 h after light onset) and t16 (4 h after light offset). Transcriptomic analysis of purified intestinal ILC3s at various timepoints showed a rhythmic pattern in the expression of major clock genes (Per1–3, Dpb, Cry1 and Nr1d1) and genes involved in inflammatory and metabolic pathways. Consistently, Godinho-Silva et al.2 also reported circadian patterns in the expression of clock genes in ILC3s. Collectively, data from both studies clearly indicate that ILC3s possess circadian machinery.
Both groups examined the role of the master clock gene, Arntl, in ILC3 function using bone marrow chimera studies. Seillet et al.1 generated mixed chimeric mice by transplanting a 1:1 ratio of lymphoidspecific Arntl-deficient (ArntlΔIL7R) and wild-type bone marrow into lethally irradiated wild-type recipients. Godinho-Silva et al.2 transferred wild-type and hematopoietic-cell-specific Arntl-deficient (ArntlΔVav1) bone marrow into irradiated alymphoid hosts. The advantage of these models is that reconstituted Arntl-sufficient and Arntl-deficient ILC subsets are examined in the same host. Notably, both studies observed reductions in the number of ILC3s and ILC3-derived IL-22 in the Arntl-deficient compartment compared to wild type, indicating that ILC3 functionality requires intrinsic clock signals.
To precisely define the functional effects of ILC3-intrinsic circadian patterns, Godinho-Silva et al.2 generated conditional knockout mice (ArntlΔRorgt), in which Arntl is deleted in RORγt-expressing cells. ArntlΔRorgt mice are more prone to bacterial infection and display profound gut inflammation, impaired epithelial integrity, altered lipid metabolism and body fat accumulation. ILC3s express surface receptors for chemokines and integrins (also known as postcode receptors), which are essential for migration to the base of intestinal villi7. Notably, Godinho-Silva et al. observed that ArntlΔRorgt ILC3s had a marked reduction in the expression of gut postcode receptors and disrupted migration to the gut, suggesting that intrinsic circadian signals are essential for ILC3 physiology, mucosal defense and gut homeostasis. In contrast, Seillet et al.1 observed that genetic ablation of Arntl reduces the amplitude of IL-22 production in ILC3s in the small intestine, but the oscillatory expression pattern is not completely abolished. These data indicate that ILC3 rhythmicity is partially controlled by the intrinsic circadian machinery and may be impacted by other extrinsic factors.
Seillet et al.1 showed that one week of time-restricted feeding during the dark phase induced only a twofold increase in the constitutive production of IL-22 in enteric ILC3s, compared to restricted feeding during the light phase only. Even with antibiotic treatment, dark-phase-fed mice exhibited a marked increase in enteric IL-22-producing ILC3s, indicating mechanistic independence from the gut microbiome. To explore the underlying mechanism through which feeding regulates ILC3 function, Seillet et al. used single-cell RNA sequencing to characterize the subtypes and molecular signatures of enteric ILCs. Transcriptomic analysis revealed several novel subsets of ILC3s, one of which expresses the intestinal neuroendocrine receptor VIPR2. These cells also highly express IL-22. VIPR2 is a receptor for the neuropeptide VIP, which is released in high amounts by enteric neurons to coordinate intestinal motility with pancreatic enzyme release. Furthermore, VIP has been reported to drive oscillatory production of IL-5 by intestinal type 2 innate lymphoid cells (ILC2s) following food consumption8, suggesting that VIP–VIPR2 signaling may play a crucial role in coordinating innate lymphoid responsiveness with food intake.
To determine the role of VIP in ILC3 activity, Seillet et al.1 exposed purified enteric ILC3s from fed mice to VIP or a VIPR2 agonist and observed increased secretion of IL-22, whereas a VIPR2 antagonist blocked this response. In vivo, global deletion of VIPR2 in Vipr2–/– mice significantly reduced constitutive IL-22 production by ILC3s in both fed and fasted conditions. In addition, VIP did not induce expression of IL-22 in ILC3s isolated from Vipr2–/– mice. These data demonstrate that the VIP–VIPR2 axis drives ILC3s to produce IL-22 in vivo. To link the VIP–VIPR2 axis to enteric defense, Seillet et al. treated wild-type and Vipr2–/– mice with low-dose dextran sodium sulfate for 5 days. Vipr2–/– mice were more susceptible to colitis and ulcerations, and exhibited impaired epithelial integrity, marked weight loss and a reduced number of IL-22-expressing ILC3s compared to wild-type mice. Overall, this study demonstrates that ILC3 function is regulated by intrinsic circadian rhythms, as well as extrinsic feeding habits, such that VIP–VIPR2 signaling directly regulates IL-22 production in ILC3s in response to food to protect against gut inflammation.
Whereas Seillet et al. reported a cyclical pattern of food intake as a major regulator of ILC3 function, Godinho-Silva et al. found that feeding regimen alterations minorly impacted circadian ILC3 oscillations. Specifically, light–dark cycle reversal completely upended the ILC3 circadian rhythm2. These rhythmic changes were maintained afterward despite constant darkness, confirming light as a major extrinsic cue that entrains ILC3 circadian oscillations. Furthermore, Godinho-Silva et al.2 demonstrated that the brain circadian clock impacted enteric ILC3 activity. Surgical lesion of, or genetic ablation of Arntl in, the suprachiasmatic nuclei in mice caused severe circadian arrhythmicity in ILC3s and subsequent intestinal homeostatic disruption. Thus, Godinho-Silva et al. show that ILC3s integrate extrinsic light-entrained and brain-tuned signals with the cell-intrinsic circadian machinery to maintain enteric defense and metabolic homeostasis.
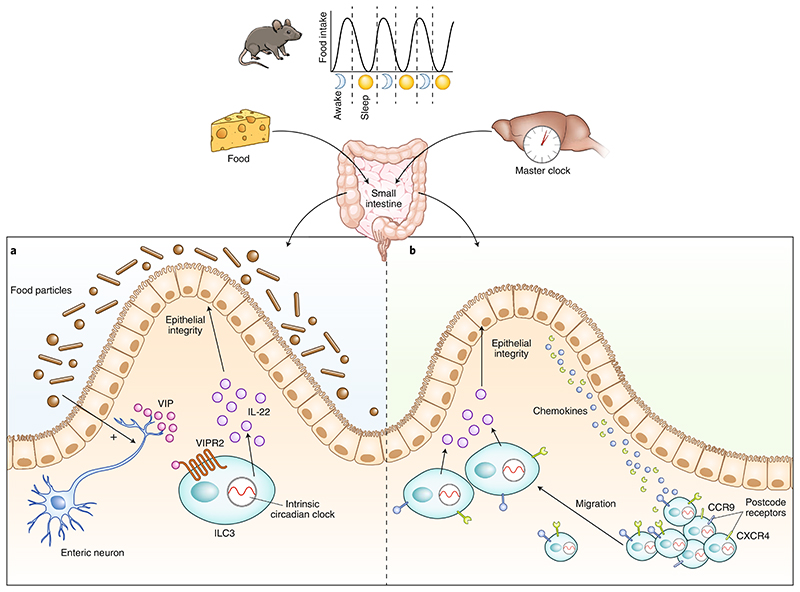
Although it was reported that ILC3s interact with glia-derived neurotrophic factors to protect against microbial infection9, the findings of Seillet et al. and Godinho-Silva et al. expand our insights into the crosstalk between neurons and ILC3s. While Seillet et al.1 identified that local food-induced neuronal VIP signaling regulates IL-22 production in ILC3s for mucosal barrier protection, Godinho-Silva et al.2 discovered that central circadian-clock-tuned signals inform the ILC3 intrinsic circadian machinery to regulate the migration of ILC3s to the gut (Fig. 1).
Fig. 1. Neuroimmune regulation of ILC3s in the gut.
ILC3s require a cell-intrinsic circadian clock for their activity. Local and systemic neuronal signaling interacts with the circadian machinery of ILC3s to shape gut homeostasis. a, Seillet et al.1 show that gut ILC3s are located in proximity to enteric neurons at the base of the lamina propria. Food enhances VIP release from enteric neurons. VIP interacts with VIPR2 receptors on the surface of ILC3s to induce the secretion of IL-22, which maintains intestinal epithelial integrity and gut homeostasis. b, Godinho-Silva et al.2 demonstrate that light-entrained signals tuned by the master circadian clock in the brain integrate with the ILC3s’ intrinsic circadian machinery, which in turn promotes the expression of postcode receptors on ILC3s (such as CCR9 or CXCR4) that facilitate chemokine-mediated migration of ILC3s to the base of the intestinal villi to mediate mucosal defense and host homeostasis. ILC3s, type 3 innate lymphoid cells; VIP, vasoactive intestinal polypeptide; VIPR2, vasoactive intestinal polypeptide receptor 2.
Notably, a study under review by Talbot et al.10 reports that food-induced VIP–VIPR2-mediated activation of chemokine receptor CCR6+ ILC3s inhibits IL-22 production. In turn, reduced IL-22 levels inhibit the production of the antimicrobial peptide RegIIIγ by intestinal epithelial cells (IECs), which affects epithelial barrier function. Interestingly, Talbot et al.10 show that such VIP–VIPR2-mediated inhibition of IL-22 production enhances the expression of lipid transporters by IECs to expedite intestinal lipid absorption. Currently, it is not possible to reconcile the differences of these findings with those of Seillet et al. due to distinctions in experimental methods, such as ILC3 gating strategies and the circadian timepoints used. Therefore, it will be of great interest to explore the underlying mechanisms that determine the opposite roles of VIP–VIPR2 signaling in ILC3s following food consumption.
In addition, the complete circuitry connecting the local and systemic neuronal pathways with the intrinsic signaling cascades in ILC3s remains to be determined. It is still unclear how the suprachiasmatic nuclei communicates with peripheral ILC3s. VIPR2 is also highly expressed in the suprachiasmatic nuclei, where it conveys extrinsic cues to regulate central circadian oscillations11. It would be interesting to address whether central VIP signaling has a role in orchestrating the intrinsic clock of ILC3s. Although Seillet et al. and Godinho-Silva et al. have clearly demonstrated fluctuations in IL-22 activity in enteric ILC3s in response to feeding or diurnal changes, it remains to be elucidated whether similar regulatory mechanisms are present in ILCs in other systems. Visceral white adipose tissue is highly enriched in ILCs, which contribute to inflammation in obesity12. Future studies could therefore explore whether the ILC-derived cytokines in adipose tissue fluctuate with food intake, and thus coordinate adipose function with feeding behavior via immune regulation.
There is increasing interest in the impact of circadian rhythms on intestinal physiology. Disrupted circadian rhythms have been reported to cause profound gastrointestinal tract inflammation and result in several digestive pathologies and metabolic disorders; however, the underlying mechanisms are still unclear. The data of Seillet et al.1 and Godinho-Silva et al.2 have shed new light on the molecular mechanisms through which circadian disruptions induce inflammatory bowel diseases, metabolic syndrome and bowel cancer, and these studies undoubtedly open new possibilities for targeting this neuroimmune axis for novel therapeutic strategies.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Seillet C, et al. Nat Immunol. 2019 doi: 10.1038/s41590-019-0567-y. [DOI] [Google Scholar]
- 2.Godinho-Silva C, et al. Nature. 2019;574:254–258. doi: 10.1038/s41586-019-1579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spits H, et al. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 4.von Burg N, Turchinovich G, Finke D. Front Immunol. 2015;6:416. doi: 10.3389/fimmu.2015.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes ME, et al. PLoS Genet. 2012;8:e1002835. doi: 10.1371/journal.pgen.1002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukherji A, Kobiita A, Ye T, Chambon P. Cell. 2013;153:812–827. doi: 10.1016/j.cell.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 7.Mackley EC, et al. Nat Commun. 2015;6:5862. doi: 10.1038/ncomms6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nussbaum JC, et al. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ibiza S, et al. Nature. 2016;535:440–443. doi: 10.1038/nature18644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talbot J, et al. Preprint at bioRxiv. 2019 doi: 10.1101/721464. [DOI] [Google Scholar]
- 11.Maywood ES, et al. Proc Natl Acad Sci USA. 2013;110:9547–9552. doi: 10.1073/pnas.1220894110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Sullivan TE, et al. Immunity. 2016;45:428–441. doi: 10.1016/j.immuni.2016.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]