Abstract
Objective
The question of whether depression is associated with worse survival in people with cancer remains unanswered because of methodological criticism of the published research on the topic. We aimed to study the association in a large methodologically robust study.
Methods
We analysed data on 20,582 patients with breast, colorectal, gynaecological, lung and prostate cancers who had attended cancer outpatient clinics in Scotland, UK. Patients had completed two-stage screening for major depression as part of their cancer care. These data on depression status were linked to demographic, cancer and subsequent mortality data from national databases. We estimated the association of major depression with survival for each cancer using Cox regression. We adjusted for potential confounders and interactions between potentially time-varying confounders and the interval between cancer diagnosis and depression screening, and used multiple imputation for missing depression and confounder data. We pooled the cancer-specific results using fixed-effects meta-analysis.
Results
Major depression was associated with worse survival for all cancers, with similar adjusted hazard ratios: breast cancer (HR 1.42, 95% CI 1.15-1.75), colorectal cancer (HR 1.47, 95% CI 1.11-1.94), gynaecological cancer (HR 1.36, 95% CI 1.08-1.71), lung cancer (HR 1.39, 95% CI 1.24-1.56), prostate cancer (HR 1.76, 95% CI 1.08-2.85). The pooled hazard ratio was 1.41 (95% CI 1.29-1.54, p<0.001, I2=0%). These findings were not materially different when we only considered the deaths (90%) that were attributed to cancer.
Conclusions
Major depression is associated with worse survival in patients with common cancers. The mechanisms of this association and the clinical implications require further study.
Keywords: Depression, Cancer, Neoplasms, Survival, Mortality, Cohort
Introduction
The question of whether depression is associated with worse survival in people with cancer remains unanswered. Whilst many relevant studies have been published (1–23), their methods have been have been criticised (24–26). The methodological limitations described include: (a) the use of small unrepresentative samples of patients; (b) poorly defined cancer diagnoses, including often a reliance on self-report; (c) frequently an inadequate determination of depression status, often using questionnaire scores rather than a diagnostic interview; (d) incomplete follow-up of participants to determine their survival; (e) a lack of data on cause of death; (f) inadequate statistical methods; and (g) a failure to adequately control for the factors that may confound an association between major depression and survival, including cancer severity and demographic factors such as social deprivation (24–26).
We had the opportunity to address the question in a methodologically robust study by analysing prospectively collected data from a large cohort of patients with common cancers (breast, colorectal, gynaecological, lung and prostate cancers) who had completed systematic screening for major depression as part of their cancer care and for whom we also had data on subsequent deaths. Our aim was therefore to investigate the association between major depression and subsequent survival in patients with common cancers.
Methods
Study design and patients
We analysed data from patients who had attended the outpatient clinics of the Edinburgh, Glasgow and Dundee National Health Service (NHS) cancer centres in Scotland, UK, and participated in screening for major depression as part of their cancer care. Each of these publically funded cancer centres provides a full range of diagnostic and treatment services through teaching hospitals and outreach clinics. Together the three centres serve a geographically defined area of approximately four million people and provide specialist care for the vast majority of patients who have been diagnosed with cancer in this region.
We included a patient’s data in this analysis if: (a) they had attended an outpatient oncology consultation in a central or outreach cancer clinic between May 12, 2008 and August 24 2011; (b) they had participated in the routine major depression screening programme that operated in these clinics; (c) they had given consent for their relevant clinical data to be used for research; (d) we could obtain their matched demographic and clinical data from the Scottish National Cancer Registry; and (e) they had a primary breast, colorectal, gynaecological, lung or prostate cancer. We chose these cancers because they are the most common, they often form the basis for multidisciplinary cancer care (therefore the association between major depression and survival in each group is clinically useful) and the number of patients with each cancer was sufficient to estimate this association with acceptable accuracy.
Measures
Major depression
Screening for major depression was carried out as part of usual clinical care; 80% of patients attending the relevant cancer clinics completed depression screening (the main reason that patients did not complete screening was that their oncology appointment had begun before they could do so). The screening used a conventional two-stage procedure to ensure efficiency of the diagnostic process; this procedure is described in detail in previous publications (27, 28). In brief, the first stage of screening used the Hospital Anxiety and Depression Scale (HADS) self-rated questionnaire to identify those patients who required a diagnostic interview (those with a HADS total score ≥15) (29, 30). In the second stage, patients with a high score on the HADS were assessed using the depression section of the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (SCID) to determine whether they met criteria for major depression (31, 32). Interviews were carried out over the telephone to the patient’s home usually within several days of clinic attendance; telephone SCID interviews have good agreement with face-to-face interviews (33). Telephone interviews were audio-recorded with patients’ permission.
In order to ensure the validity and reliability of the diagnosis of major depression, the following procedures were used: (a) Interviewers (psychology graduates and nurses) received four weeks’ training from consultation-liaison psychiatrists with expertise in the use of the SCID. (b) Interviewers had to complete at least 20 satisfactory interviews which resulted in accurate diagnoses (that is, 100% inter-rater reliability between interviewer and psychiatrist) before conducting interviews independently. (c) The psychiatrists provided ongoing individual and group supervision of interviewers informed by regular review of at least 10% of interviews.
The diagnosis of major depression was made using the standard inclusive approach (all relevant symptoms counted towards the diagnosis of depression without attempting to establish whether they should be attributed to depression or to cancer); this is the most reliable method and has been found to not significantly overestimate depression in the medically ill (34). To minimise the misdiagnosis of adjustment disorder as a depressive disorder, major depression was only diagnosed if the patient described relevant symptoms of at least four weeks’ duration. If they reported symptoms between two weeks (the usual minimum duration required for a diagnosis of major depression) and four weeks, the patient was re-interviewed two weeks later. We classified patients as ‘depressed’ if they met criteria for major depression at the diagnostic interview.
Demographic and cancer data
We obtained data on patients’ demographic and cancer characteristics from the NHS Scotland Cancer Registry. The Registry systematically collects information from hospitals throughout Scotland for all recorded cases of cancer. The data included sex, date of cancer diagnosis (the date on which the cancer was first diagnosed whether by histopathological, radiological or other clinical methods), age at cancer diagnosis, social deprivation score (calculated using the Scottish Index of Multiple Deprivation, which provides a relative measure of deprivation, based on area of residence at the time of cancer diagnosis; see appendix, Supplemental Digital Content, for details), primary cancer (see appendix for details) and initial cancer treatment objective (curative or palliative). We used cancer treatment objective as a proxy for cancer severity because it could be applied across all the cancers studied, whereas staging systems differ between and within the five common cancers.
Mortality data
We obtained data on the date and recorded cause of death of each patient from the National Records of Scotland (NRS) database.
Data linkage
To ensure data security and confidentiality the dataset of patients’ depression status was sent to the Information Services Division of NHS Scotland for linkage using unique patient identification numbers (Community Health Index numbers) and dates of birth. All identifying data were then removed in a one-way linkage to produce the anonymised dataset that was used for analysis. The study was approved by the South East Scotland Research Ethics Committee, the NHS Scotland Caldicott Guardian Forum and the NHS Scotland Privacy Advisory Committee.
Statistical analyses
For each patient, we calculated the time to their death from the date they attended the cancer clinic and took part in depression screening. If a patient had attended the cancer clinic and participated in depression screening more than once during the study period, we used the data relating to the earliest of their clinic attendances. In the primary analysis, we considered deaths from all causes, censoring patients who had left Scotland (at their date of emigration) and patients who were not known to have died or to have emigrated at the latest date on which data were available (April 30, 2012). We also censored one patient whose mortality status was unknown on April 30, 2012 at their last known appointment date. In a secondary analysis, we considered only deaths attributed to cancer, additionally censoring non-cancer deaths at the date of death.
We used Cox proportional hazards models to estimate the effect of major depression on survival from the time of depression screening for each of the primary cancers (breast, colorectal, gynaecological, lung, and prostate). We assigned patients who had multiple primary cancers according to the cancer diagnosis that most closely preceded the clinic appointment (11 patients who were given two different cancer diagnoses on the same day were included in the analyses of both cancers).
The models adjusted for the following potential confounders: sex, age at cancer diagnosis, social deprivation score, initial cancer treatment objective and the interval between cancer diagnosis and depression screening. Full details of the statistical models are given in the online appendix. In brief, we modelled the confounding effects of the continuous variables using fractional polynomials, using the method described by Benner in order to allow for non-linear effects (35).
A further refinement was made because age at cancer diagnosis, social deprivation score and initial cancer treatment objective were measured at the time of patients’ cancer diagnoses, and it is likely that the magnitude of their confounding effects on survival may change according to the time interval between cancer diagnosis and depression screening. To allow for this possibility the models also included interactions between the fractional polynomial terms for this time interval and initial cancer treatment objective, and between the fractional polynomial terms for this time interval and the fractional polynomial terms for age at cancer diagnosis and social deprivation score respectively.
We used multiple imputation to deal with missing data on initial cancer treatment objective (2,606 patients) and on depression status (1,081 patients who had a high HADS score at stage one of depression screening but did not undergo a diagnostic interview, mainly because they declined or could not be contacted). The propensity for data to be missing was associated with both the HADS score and with subsequent survival. To attempt to reduce potential bias in our estimates we imputed these data using the substantive model compatible fully conditional specification (SMCFCS) method in order to properly account for interactions and non-linear effects (36). For each cancer we performed 20 imputations, fitted the Cox regression models on each imputed dataset and combined the coefficients using Rubin’s rules (see appendix for further details) (37).
We pooled the combined log hazard ratios for each cancer using the inverse variance method in a fixed-effects meta-analysis (as noted above, eleven patients were included in the analysis of two separate cancers but this number is small relative to the cohort size and the impact of this on the pooled result is negligible). We also conducted a sensitivity meta-analysis omitting lung cancer. We did this because patients with lung cancer had: a much worse prognosis than those with the other cancers, as expected (38); a substantially shorter average time interval between cancer diagnosis and depression screening; and the most missing (and therefore imputed) depression data.
All analyses were carried out in R version 3.5.1 using the packages “mfp”, “smcfcs” and “mitools” (35, 36, 39, 40).
Results
We included data from 20,582 patients in the analysis (see Table 1 for a description of their characteristics). 6,099 patients died (from all causes) during the period of follow-up. Most of the deaths (more than 90%) were recorded as being due to cancer (see online appendix for details of the primary causes of death).
Table 1. Demographics, depression status and survival of patients included in the analysis.
| Breast cancer | Colorectal cancer | Gynaecological cancer | Lung cancer | Prostate cancer | |
|---|---|---|---|---|---|
| Total | 8679a | 2807a | 3052a | 4476a | 1579a |
| Male | 0 (0%) | 1616 (58%) | 0 (0%) | 2363 (53%) | 1579 (100%) |
| Age at cancer diagnosis [median years, IQR] | 58 [49, 66] | 65 [58, 72] | 61 [51, 69] | 68 [61, 74] | 67 [62, 72] |
| Missing | 1 (0%) | 1 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Missing | 1498 (17%) | 237 (8%) | 407 (13%) | 199 (4%) | 266 (17%) |
| Time interval between cancer diagnosis & depression screening [median years, IQR] | 2.0 [0.4, 5.2] | 1.0 [0.3, 2.5] | 1.0 [0.4, 3.0] | 0.3 [0.1, 0.9] | 2.0 [0.8, 4.5] |
| Pre-imputation | |||||
| Major depression | 9% | 6% | 11% | 13% | 5% |
| Time from depression screening to death or censoring [median years, IQR] | 2.3 [1.6, 3.0] | 1.8 [1.2, 2.7] | 1.9 [1.2, 2.7] | 0.8 [0.4, 1.5] | 2.2 [1.7, 3.1] |
| Died during study period | 1036 (12%) | 876 (31%) | 865 (28%) | 3029 (68%) | 299 (19%) |
| Died of cancer during study period | 912 (11%) | 813 (29%) | 816 (27%) | 2799 (63%) | 257 (16%) |
Data are n (%) unless stated otherwise.
11 patients are included in this table twice because they were diagnosed with more than one primary cancer on the same day: 3 had breast & gynaecological cancers, 3 had colorectal & gynaecological cancers, 2 had breast & lung cancers, 1 had breast & colorectal cancers, 1 had colorectal & lung cancers, 1 had colorectal & prostate cancers.
Scottish Index of Multiple Deprivation quintile score: 1=most deprived, 5=least deprived.
mean after 20 imputations.
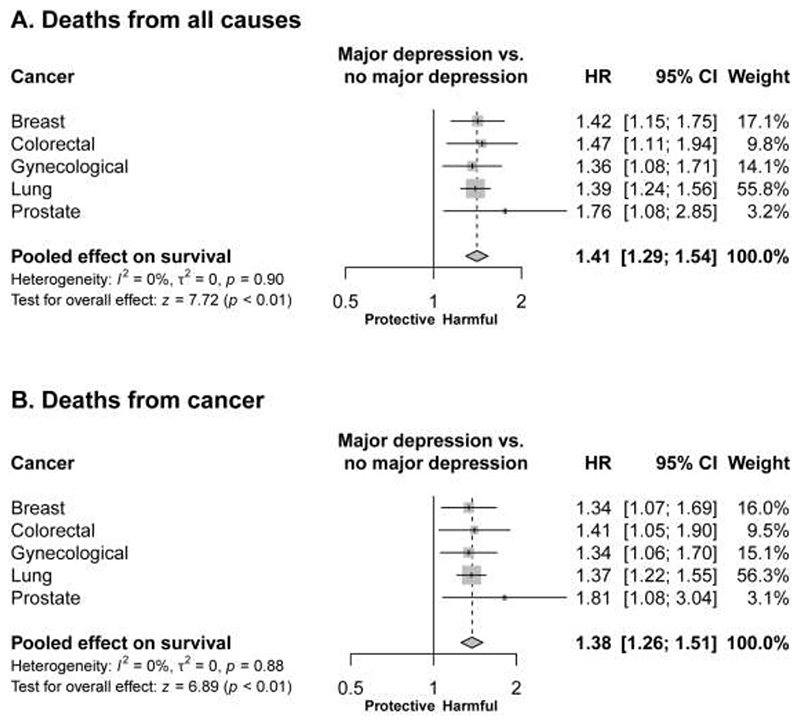
In our primary analysis (Figure 1, panel A) where we considered all-cause mortality, major depression was associated with worse survival in all five cancers (p ranged from 4.0 x 10-8 for patients with lung cancer to 2.2 x 10-2 for patients with prostate cancer). The hazard ratios comparing the survival of patients with a diagnosis of major depression with that of patients who did not have major depression were similar for all five cancers: breast cancer (HR 1.42, 95% CI 1.15, 1.75), colorectal cancer (HR 1.47, 95% CI 1.11, 1.94), gynaecological cancer (HR 1.36, 95% CI 1.08, 1.71), lung cancer (HR 1.39, 95% CI 1.24, 1.56) and prostate cancer (HR 1.76, 95% CI 1.08, 2.85)(see Table 2 for all parameter estimates from these models). There was no evidence of heterogeneity in these estimates (I2=0%). The estimated hazard ratio pooled for all cancers was 1.41 (95% CI 1.29, 1.54, p<0.001) and was similar when we omitted lung cancer (HR 1.43, 95% CI 1.26, 1.63).
Figure 1. Association of major depression with survival: estimated hazard ratios (95% confidence intervals).
Table 2. The association between depression and mortality risk: Parameter estimates from the primary survival analysis models stratified by type of cancer.
| Parameter | Hazard Ratio | 95% CI | p-value |
|---|---|---|---|
| Breast cancer | |||
| Major depression diagnosis | 1.42 | 1.15, 1.75 | 0.001 |
| Years between cancer diagnosis and depression screeninga | 0.96 | 0.46, 1.99 | 0.905 |
| Age at cancer diagnosisb | 0.28 | 0.19, 0.41 | <0.001 |
| Age at cancer diagnosisb squared | 1.13 | 1.10, 1.17 | <0.001 |
| SIMDb | 1.09 | 1.05, 1.14 | <0.001 |
| Therapy intent (palliative v curative) | 7.80 | 6.57, 9.26 | <0.001 |
| Years between cancer diagnosis and depression screeninga × Age at cancer diagnosisb | 1.17 | 0.91, 1.52 | 0.218 |
| Years between cancer diagnosis and depression screeninga × Age at cancer diagnosisb squared | 0.98 | 0.96, 1.01 | 0.157 |
| Years between cancer diagnosis and depression screeninga × SIMDb | 1.02 | 0.99, 1.05 | 0.123 |
| Years between cancer diagnosis and depression screeninga × therapy intent (palliative v curative) | 0.74 | 0.65, 0.84 | <0.001 |
| Colorectal cancer | |||
| Major depression diagnosis | 1.47 | 1.11, 1.94 | 0.007 |
| Years between cancer diagnosis and depression screeningc | 1.06 | 0.88, 1.27 | 0.553 |
| Age at cancer diagnosisb | 1.14 | 1.06, 1.24 | <0.001 |
| Sex (male v female) | 1.13 | 0.98, 1.29 | 0.087 |
| SIMDb | 1.02 | 0.97, 1.08 | 0.378 |
| Therapy intent (palliative v curative) | 7.39 | 6.10, 8.94 | <0.001 |
| Years between cancer diagnosis and depression screeningc × Age at cancer diagnosisb | 1.00 | 0.98, 1.03 | 0.793 |
| Years between cancer diagnosis and depression screeningc × SIMDb | 1.01 | 0.99, 1.03 | 0.444 |
| Years between cancer diagnosis and depression screeningc × therapy intent (palliative v curative) | 0.79 | 0.71, 0.87 | <0.001 |
| Gynaecological cancer | |||
| Major depression diagnosis | 1.36 | 1.08, 1.71 | 0.010 |
| Years between cancer diagnosis and depression screeningd | 1.00 | 0.95, 1.06 | 0.896 |
| Years between cancer diagnosis and depression screeninge | 1.00 | 0.98, 1.02 | 0.997 |
| Age at cancer diagnosisb | 1.18 | 1.11, 1.26 | <0.001 |
| SIMDb | 1.04 | 0.99, 1.09 | 0.148 |
| Therapy intent (palliative v curative) | 5.03 | 4.14, 6.11 | <0.001 |
| Years between cancer diagnosis and depression screeningd × Age at cancer diagnosisb | 1.00 | 0.99, 1.01 | 0.794 |
| Years between cancer diagnosis and depression screeninge × Age at cancer diagnosisb | 1.00 | 1.00, 1.00 | 0.761 |
| Years between cancer diagnosis and depression screeningd × SIMDb | 1.00 | 0.99, 1.01 | 0.487 |
| Years between cancer diagnosis and depression screeninge × SIMDb | 1.00 | 1.00, 1.00 | 0.407 |
| Years between cancer diagnosis and depression screeningd × therapy intent (palliative v curative) | 0.96 | 0.88, 1.03 | 0.252 |
| Years between cancer diagnosis and depression screeninge × therapy intent (palliative v curative) | 1.01 | 0.98, 1.05 | 0.425 |
| Lung cancer | |||
| Major depression diagnosis | 1.39 | 1.24, 1.56 | <0.001 |
| Years between cancer diagnosis and depression screeninga | 0.81 | 0.62, 1.06 | 0.133 |
| Age at cancer diagnosisb | 1.09 | 1.04, 1.15 | <0.001 |
| Sex (male v female) | 1.13 | 1.05, 1.21 | 0.001 |
| SIMDb | 1.02 | 1.00, 1.05 | 0.075 |
| Therapy intent (palliative v curative) | 2.16 | 1.93, 2.41 | <0.001 |
| Years between cancer diagnosis and depression screeninga × Age at cancer diagnosisb | 1.03 | 1.00, 1.08 | 0.078 |
| Years between cancer diagnosis and depression screeninga × SIMDb | 1.00 | 0.98, 1.02 | 0.819 |
| Years between cancer diagnosis and depression screeninga × therapy intent (palliative v curative) | 0.69 | 0.63, 0.75 | <0.001 |
| Prostate cancer | |||
| Major depression diagnosis | 1.76 | 1.08, 2.85 | 0.022 |
| Years between cancer diagnosis and depression screeningc | 0.76 | 0.47, 1.22 | 0.254 |
| Age at cancer diagnosisb cubed | 0.93 | 0.89, 0.97 | 0.001 |
| Age at cancer diagnosisb cubed × loge(age at cancer diagnosisb) | 1.03 | 1.01, 1.05 | <0.001 |
| SIMDb | 1.17 | 1.06, 1.30 | 0.003 |
| Therapy intent (palliative v curative) | 3.11 | 2.01, 4.82 | <0.001 |
| Years between cancer diagnosis and depression screeningc × age at cancer diagnosisb cubed | 1.01 | 1.00, 1.02 | 0.080 |
| Years between cancer diagnosis and depression screeningc × age at cancer diagnosisb cubed × loge(age at cancer diagnosisb) | 1.01 | 1.00, 1.02 | 0.080 |
| Years between cancer diagnosis and depression screeningc × SIMDb | 0.98 | 0.96, 1.00 | 0.027 |
| Years between cancer diagnosis and depression screeningc × therapy intent (palliative v curative) | 0.98 | 0.90, 1.06 | 0.551 |
The hazard ratios of primary importance (shown in bold) relate to the comparison between those patients with and without major depression. The hazard ratios for other variables and interactions between pairs of such variables are for (sometimes) transformed covariates that make up the fractional polynomial model used for covariate adjustment. The transformations are
loge (X+0.1);
X/10;
X+0.1;
(X+0.1)^2 and
([X+0.1]^2)×loge[X+0.1].
The results of a secondary analysis (Figure 1, panel B) which considered only deaths attributed to cancer (censoring follow-up at the time of death for deaths from other causes) were not materially different. For this analysis the estimated hazard ratio pooled for all cancers was 1.38 (95% CI 1.26, 1.51, p<0.001).
Discussion
We addressed the question of whether comorbid major depression is associated with worse subsequent survival in a methodologically robust study of a large cohort of patients with breast, colorectal, gynaecological, lung or prostate cancer. We found that the survival of patients with major depression was worse than that of patients who did not have major depression. This association of major depression with worse survival remained even when a number of potential confounders were adjusted for. Notably, the observed association was of a similar magnitude for all of the cancers studied and our findings were not materially different when we omitted lung cancer from the meta-analysis or when we only considered the deaths that were attributed to cancer.
Our estimated hazard ratio of 1.41 might be considered small to medium in magnitude (41). It is interpretable as a 41% increase in the mortality rate throughout follow-up for patients with major depression compared with the mortality rate for those without major depression. The prevalence of major depression in our cohort varied by cancer from five to 13 percent, which is similar to that reported by a systematic review of interview-based studies of cancer outpatients (42). This means that this increase in mortality rate affects a modest but significant number of patients.
The previous literature on the association of depression with worse subsequent survival in people with cancer has been subject to methodological criticism. Whilst two published meta-analyses of the literature (22, 23), concluded that depression does predict worse survival, these conclusions have been disputed (24, 26, 43). More recent studies have failed to resolve the dispute as some found depression to be associated with worse survival (1–6, 8, 10–16, 19–21) and others did not (7, 9, 17, 18). A recent overview concluded that the inherent methodological limitations prevented any clear conclusions from being drawn (25).
We had the opportunity to address this question in a robust study that was able to address the methodological critiques of the published literature listed in the introduction. The strengths of our study were: (a) it used data on a large representative sample of patients with common cancers attending cancer centres that served geographically defined areas; (b) the diagnosis of major depression was made by diagnostic interview; (c) cancer diagnosis and severity assessment was made by oncologists; (d) there was almost complete follow-up using individually linked national registry data, including data on cause of death; and (e) the analysis addressed missing data and controlled for most potential confounders, including not only age and sex but also social deprivation (determined by the patient’s address) and initial cancer severity (determined by recorded treatment objective).
Our study also had limitations however. These were: (a) uncertain generalisability to other populations (such as patients attending different healthcare settings and patients diagnosed with cancer many years ago who no longer attend clinics); (b) incomplete patient participation in the screening programme that determined depression status, although participation was high at 80 percent; (c) missing data on depression status and on initial cancer treatment objective which we addressed with multiple imputation; (d) the assessment for major depression occurring at varying intervals after initial cancer diagnosis, which we allowed for in our analysis; (e) lack of information on the time-course of depression either prior to or subsequent to the depression diagnostic assessment; (f) availability of data on deaths for a mean of only two years after depression assessment; (g) potentially inadequate adjustment for all confounders. In particular we had to rely on initial treatment objective as a measure of cancer severity. We were also unable to control for other medical comorbidities that may have affected survival, although almost all patient deaths were attributed to cancer and the findings were similar when we considered only deaths from cancer. In summary, despite its limitations, this study was able to address most of the limitations of previous research on the topic and consequently provides strong support for the hypothesised association between major depression and worse subsequent survival of patients with common cancers.
Why might there be an association between major depression and subsequent survival? One clinically important possible explanation is that depression has a negative causal influence on cancer prognosis, for example by reducing patients’ adherence to anticancer treatment or by directly influencing the progression of their cancer (21, 44, 45). Whilst this explanation is a tantalising one, with potentially important implications for practice, it must be regarded with some caution. In particular, we should note that there is currently little good evidence that treating comorbid major depression in patients with cancer improves their survival (46).
Another possible explanation is that people who are dying from cancer are more likely to be depressed as a result and the association reflects reverse causation; that is dying from cancer causes depression. However, this is an unlikely explanation of the findings of this, and other studies, in which there was a substantial interval between the diagnosis of major depression and subsequent death.
Yet another possible explanation is that there are common factors that lead both to the development of major depression and to worse survival. Such factors might include biological processes such as inflammation, immunological activity and the effect of stress on physiological systems, as well as behavioural factors known to be risk factors for both depression and cancer such as low physical inactivity and high alcohol intake (47).
In conclusion, this study of a large prospectively assessed cohort of cancer patients adds weight to the accumulating but disputed evidence that major depression is associated with the worse subsequent survival of patients with common cancers. The mechanisms underlying this association remain unknown and clearly require further investigation. Importantly, we do not currently have good evidence that treating comorbid depression in patients with cancer lengthens their life. However, we do have evidence that treating depression in patients with cancer improves the quality of their lives (48). Whilst more research into the effect of treating depression on survival is needed, we already have sufficient evidence to justify the identification and active treatment of depression in people suffering from cancer.
Supplementary Material
Acknowledgments
This work was funded by the UK National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care Oxford at Oxford NHS Foundation Trust (the views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health) and by the charity Cancer Research UK (grant no. C5547/A7375).
Abbreviations
- NHS
National Health Service
- HADS
Hospital Anxiety and Depression Scale
- SCID
Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition
- NRS
National Records of Scotland
- SMCFCS
substantive model compatible fully conditional specification
- HR
hazard ratio
- CI
confidence interval
Footnotes
Conflicts of Interest and Sources of Funding:
The authors declare that they have no conflicts of interest.
References
- 1.Antoni MH, Jacobs JM, Bouchard LC, Lechner SC, Jutagir DR, Gudenkauf LM, Blomberg BB, Gluck S, Carver CS. Post-surgical depressive symptoms and long-term survival in non-metastatic breast cancer patients at 11-year follow-up. Gen Hosp Psychiatry. 2017;44:16–21. doi: 10.1016/j.genhosppsych.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cash E, Duck CR, Brinkman C, Rebholz W, Albert C, Worthen M, Jusufbegovic M, Wilson L, Bumpous JM. Depressive symptoms and actigraphy-measured circadian disruption predict head and neck cancer survival. Psychooncology. 2018;27:2500–7. doi: 10.1002/pon.4862. [DOI] [PubMed] [Google Scholar]
- 3.Chan CM, Wan Ahmad WA, Yusof MM, Ho GF, Krupat E. Effects of depression and anxiety on mortality in a mixed cancer group: a longitudinal approach using standardised diagnostic interviews. Psychooncology. 2015;24:718–25. doi: 10.1002/pon.3714. [DOI] [PubMed] [Google Scholar]
- 4.Chen ML, Chen MC, Yu CT. Depressive symptoms during the first chemotherapy cycle predict mortality in patients with advanced non-small cell lung cancer. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2011;19:1705–11. doi: 10.1007/s00520-010-1005-8. [DOI] [PubMed] [Google Scholar]
- 5.Jansen F, Verdonck-de Leeuw IM, Cuijpers P, Leemans CR, Waterboer T, Pawlita M, Penfold C, Thomas SJ, Waylen A, Ness AR. Depressive symptoms in relation to overall survival in people with head and neck cancer: a longitudinal cohort study. Psychooncology. 2018 doi: 10.1002/pon.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim SA, Roh JL, Lee SA, Lee SW, Kim SB, Choi SH, Nam SY, Kim SY. Pretreatment depression as a prognostic indicator of survival and nutritional status in patients with head and neck cancer. Cancer. 2016;122:131–40. doi: 10.1002/cncr.29693. [DOI] [PubMed] [Google Scholar]
- 7.Liang X, Margolis KL, Hendryx M, Reeves K, Wassertheil-Smoller S, Weitlauf J, Danhauer SC, Chlebowski RT, Caan B, Qi L, Lane D, et al. Effect of depression before breast cancer diagnosis on mortality among postmenopausal women. Cancer. 2017;123:3107–15. doi: 10.1002/cncr.30688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin PH, Liu JM, Hsu RJ, Chuang HC, Chang SW, Pang ST, Chang YH, Chuang CK, Lin SK. Depression Negatively Impacts Survival of Patients with Metastatic Prostate Cancer. International journal of environmental research and public health. 2018;15 doi: 10.3390/ijerph15102148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Peh C, Chua SM, Mehandran R. Emotional distress in newly diagnosed cancer outpatients: do depression and anxiety predict mortality and psychosocial outcomes after 1 year? Journal of depression and anxiety. 2017:S11:005 [Google Scholar]
- 10.Mols F, Husson O, Roukema J-A, van de Poll-Franse LV. Depressive symptoms are a risk factor for all-cause mortality: results from a prospective population-based study among 3,080 cancer survivors from the PROFILES registry. Journal of Cancer Survivorship [journal article] 2013;7:484–92. doi: 10.1007/s11764-013-0286-6. [DOI] [PubMed] [Google Scholar]
- 11.Onitilo AA, Nietert PJ, Egede LE. Effect of depression on all-cause mortality in adults with cancer and differential effects by cancer site. Gen Hosp Psychiatry. 2006;28:396–402. doi: 10.1016/j.genhosppsych.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Prasad SM, Eggener SE, Lipsitz SR, Irwin MR, Ganz PA, Hu JC. Effect of depression on diagnosis, treatment, and mortality of men with clinically localized prostate cancer. J Clin Oncol. 2014;32:2471–8. doi: 10.1200/JCO.2013.51.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reyes CC, Anderson KO, Gonzalez CE, Ochs HC, Wattana M, Acharya G, Todd KH. Depression and survival outcomes after emergency department cancer pain visits. BMJ supportive palliative care. 2018 doi: 10.1136/bmjspcare-2018-001533. [DOI] [PubMed] [Google Scholar]
- 14.Rieke K, Schmid KK, Lydiatt W, Houfek J, Boilesen E, Watanabe-Galloway S. Depression and survival in head and neck cancer patients. Oral Oncology. 2017;65:76–82. doi: 10.1016/j.oraloncology.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Y, Vedsted P, Fenger-Grøn M, Wu CS, Bech BH, Olsen J, Benros ME, Vestergaard M. Cancer Mortality in People Treated with Antidepressants before Cancer Diagnosis: A Population Based Cohort Study. PLOS ONE. 2015;10:e0138134. doi: 10.1371/journal.pone.0138134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suppli NP, Johansen C, Kessing LV, Toender A, Kroman N, Ewertz M, Dalton SO. Survival After Early-Stage Breast Cancer of Women Previously Treated for Depression: A Nationwide Danish Cohort Study. J Clin Oncol. 2017;35:334–42. doi: 10.1200/JCO.2016.68.8358. [DOI] [PubMed] [Google Scholar]
- 17.Vodermaier A, Linden W, Rnic K, Young SN, Ng A, Ditsch N, Olson R. Prospective associations of depression with survival: a population-based cohort study in patients with newly diagnosed breast cancer. Breast Cancer Research and Treatment. 2014;143:373–84. doi: 10.1007/s10549-013-2795-4. [journal article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vodermaier A, Lucas S, Linden W, Olson R. Anxiety After Diagnosis Predicts Lung Cancer-Specific and Overall Survival in Patients With Stage III Non-Small Cell Lung Cancer: A Population-Based Cohort Study. J Pain Symptom Manage. 2017;53:1057–65. doi: 10.1016/j.jpainsymman.2016.12.338. [DOI] [PubMed] [Google Scholar]
- 19.Yang L, Korhonen K, Moustgaard H, Silventoinen K, Martikainen P. Pre-existing depression predicts survival in cardiovascular disease and cancer. Journal of Epidemiology and Community Health. 2018;72:617–22. doi: 10.1136/jech-2017-210206. [DOI] [PubMed] [Google Scholar]
- 20.Yu H, Wang Y, Ge X, Wu X, Mao X. Depression and survival in Chinese patients with gastric cancer: a prospective study. Asian Pacific journal of cancer prevention : APJCP. 2012;13:391–4. doi: 10.7314/apjcp.2012.13.1.391. [DOI] [PubMed] [Google Scholar]
- 21.Zimmaro LA, Sephton SE, Siwik CJ, Phillips KM, Rebholz WN, Kraemer HC, Giese-Davis J, Wilson L, Bumpous JM, Cash ED. Depressive symptoms predict head and neck cancer survival: Examining plausible behavioral and biological pathways. Cancer. 2018;124:1053–60. doi: 10.1002/cncr.31109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115:5349–61. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- 23.Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med. 2010;40:1797–810. doi: 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garssen B. Letter to the Editor: Depression linked to cancer mortality not convincingly demonstrated. Psychological Medicine. 2011;41:1338–42. doi: 10.1017/S0033291711000250. [DOI] [PubMed] [Google Scholar]
- 25.Miloyan B, Fried E. A reassessment of the relationship between depression and all-cause mortality in 3,604,005 participants from 293 studies. World Psychiatry. 2017;16:219–20. doi: 10.1002/wps.20439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Machado MO, Veronese N, Sanches M, Stubbs B, Koyanagi A, Thompson T, Tzoulaki I, Solmi M, Vancampfort D, Schuch FB, Maes M, et al. The association of depression and all-cause and cause-specific mortality: an umbrella review of systematic reviews and meta-analyses. BMC Medicine. 2018;16:112. doi: 10.1186/s12916-018-1101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker J, Wanat M, Fielding J, Martin P, Petit A, Burke K, Sharpe M. Screening Medical Patients for Depression: Lessons From a National Program in Cancer Clinics. Psychosomatics. 2017;58:274–80. doi: 10.1016/j.psym.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Walker J, Hansen CH, Martin P, Symeonides S, Ramessur R, Murray G, Sharpe M. Prevalence, associations, and adequacy of treatment of major depression in patients with cancer: a cross-sectional analysis of routinely collected clinical data. Lancet Psychiat. 2014;1:343–50. doi: 10.1016/S2215-0366(14)70313-X. [DOI] [PubMed] [Google Scholar]
- 29.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 30.Walker J, Postma K, McHugh GS, Rush R, Coyle B, Strong V, Sharpe M. Performance of the Hospital Anxiety and Depression Scale as a screening tool for major depressive disorder in cancer patients. J Psychosom Res. 2007;63:83–91. doi: 10.1016/j.jpsychores.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 31.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. DSMIV: American Psychiatric Association; 1994. [Google Scholar]
- 32.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders: Biometrics Research. New York State Psychiatric Institute; USA: 1999. [Google Scholar]
- 33.Simon GE, Revicki D, Von Korff M. Telephone assessment of depression severity. Journal of Psychiatric Research. 1993;27:247–52. doi: 10.1016/0022-3956(93)90035-z. [DOI] [PubMed] [Google Scholar]
- 34.Simon GE, Von Korff M. Medical co-morbidity and validity of DSM-IV depression criteria. Psychological Medicine. 2006;36:27–36. doi: 10.1017/S0033291705006136. [DOI] [PubMed] [Google Scholar]
- 35.Ambler G, Benner A. mfp: Multivariable Fractional Polynomials. Available from: https://CRAN.R-project.org/package=mfp2015.
- 36.Bartlett J. smcfcs: Multiple Imputation of Covariates by Substantive Model Compatible Fully Conditional Specification. https://CRAN.R-project.org/package=smcfcs2016 . [DOI] [PMC free article] [PubMed]
- 37.Rubin D. Multiple Imputation for Nonresponse in Surveys. New York: Wiley; 1987. [Google Scholar]
- 38.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 39.Team RDC. R: A Language and Environment for Statistical Computing. Vienna, Austria: The R Foundation for Statistical Computing; 2017. [Google Scholar]
- 40.Lumley T. mitools: Tools for multiple imputation of missing data. Available from: https://CRAN.R-project.org/package=mitools2014.
- 41.Azuero A. A note on the magnitude of hazard ratios. Cancer. 2016;122:1298–9. doi: 10.1002/cncr.29924. [DOI] [PubMed] [Google Scholar]
- 42.Walker J, Holm Hansen C, Martin P, Sawhney A, Thekkumpurath P, Beale C, Symeonides S, Wall L, Murray G, Sharpe M. Prevalence of depression in adults with cancer: a systematic review. Ann Oncol. 2013;24:895–900. doi: 10.1093/annonc/mds575. [DOI] [PubMed] [Google Scholar]
- 43.Schneider S, Moyer A. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2010;116:3304. doi: 10.1002/cncr.25318. author reply-5. [DOI] [PubMed] [Google Scholar]
- 44.Spiegel D, Giese-Davis J. Depression and cancer: mechanisms and disease progression. Biol Psychiatry. 2003;54:269–82. doi: 10.1016/s0006-3223(03)00566-3. [DOI] [PubMed] [Google Scholar]
- 45.Saint Onge JM, Krueger PM, Rogers RG. The Relationship Between Major Depression and Nonsuicide Mortality for U.S. Adults: The Importance of Health Behaviors. (The Journals of Gerontology: Series B).2014;69:622–32. doi: 10.1093/geronb/gbu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mulick A, Walker J, Puntis S, Burke K, Symeonides S, Gourley C, Wanat M, Frost C, Sharpe M. Does depression treatment improve the survival of depressed patients with cancer? A long-term follow-up of participants in the SMaRT Oncology-2 and 3 trials. Lancet Psychiat. 2018;5:321–6. doi: 10.1016/S2215-0366(18)30061-0. [DOI] [PubMed] [Google Scholar]
- 47.Bortolato B, Hyphantis TN, Valpione S, Perini G, Maes M, Morris G, Kubera M, Köhler CA, Fernandes BS, Stubbs B, Pavlidis N, et al. Depression in cancer: The many biobehavioral pathways driving tumor progression. Cancer Treatment Reviews. 2017;52:58–70. doi: 10.1016/j.ctrv.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Sharpe M, Walker J, Holm Hansen C, Martin P, Symeonides S, Gourley C, Wall L, Weller D, Murray G Team SMO- Integrated collaborative care for comorbid major depression in patients with cancer (SMaRT Oncology-2): a multicentre randomised controlled effectiveness trial. Lancet. 2014;384:1099–108. doi: 10.1016/S0140-6736(14)61231-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.