Summary
Profound changes are occurring in the epidemiology of schistosomiasis, a neglected tropical disease caused by a chronic infection with parasitic helminths of the genus Schistosoma. Schistosomiasis affects 240 million people worldwide, mostly in sub-Saharan Africa. The advent and proliferation of mass drug administration (MDA) programs using the drug praziquantel (PZQ) is resulting in substantial increases in the number of people, mainly school-aged children, being effectively treated, approaching the point where the majority of people in endemic areas will receive one or more treatments during their lifetimes. PZQ treatment not only cures infection but also frees the host from the powerful immunomodulatory action of the parasites and simultaneously enhances exposure to key parasite antigens, accelerating the development of protective acquired immunity, which takes many years to develop naturally. At a population level these changes constitute a substantial alteration to schistosome ecology in that the parasites are more likely to be exposed not only to PZQ directly but also to hosts with altered immune phenotypes. Here, we consider the consequences of this for schistosome biology and immuno-epidemiology and for public health. We anticipate that there could be significant impacts on chronic pathology, natural immunity, vaccine development strategies, immune disorders and drug efficacy. This makes for a complex picture that only will become apparent over timescales of decades. We recommend careful monitoring and evaluation to accompany the roll-out of MDA programs in order to ensure that the considerable health benefits to populations are achieved and sustained.
Introduction
One of the greatest advances in improving human health in the tropics, particularly child health and development, has been the development of anthelminthic drugs and their subsequent distribution in mass drug administration (MDA) programs. MDA refers to the drug treatment of targeted populations regardless of individual infection status. MDA was first used in the 1930s in attempts to control malaria with the drug Plasmoquine, with mixed results1. MDA campaigns for schistosomiasis were carried out in Egypt in the 1950s using tartar emetic2, but because tarter emetic had limited efficacy and needed many daily injections, this was a false dawn for schistosome MDA. When praziquantel (PZQ) became available in the 1980s, MDA against schistosomiasis was slowly adopted as the major control strategy. Praziquantel is safe and efficacious, with cure rates as high as 100% as determined by parasitological methods of egg counts using urine filtration3 or Kato Katz 4. The drug is given as oral tablets at a dose of 40mg/kg body weight in Africa5 and 50mg/kg in adults and 60mg/kg in children in South America6.
Current global initiatives following the guidelines for preventative chemotherapy set out by the World Health Organization (WHO), have implemented regular school-based de-worming strategies in order to reduce development of severe morbidity, promote school-child health and improve the developmental potential of children. Many affected countries have now formulated helminth and trachoma control master plans targeting multiple diseases and specific vulnerable groups within the population. These programs are primarily focusing on onchocerciasis, lymphatic filariasis (LF), trachoma, schistosomiasis and soil transmitted helminths (STH) and are aimed at reducing infection and morbidity. LF and onchocerciasis programs are both aiming for elimination, but schistosomiasis and STH programs lag well behind. One exception is Zanzibar, where success in reducing the prevalence of schistosome infection has suggested the possibility of elimination7.
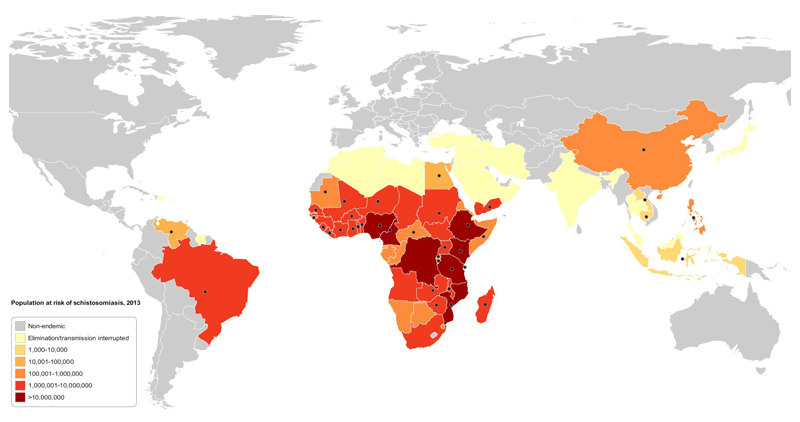
In 2014, 56 million people in 27 countries (20 of them in Africa) received PZQ treatment for schistosomiasis and the aim is to increase this number steadily over the years 2016-20. In Sub-Saharan Africa alone, it is estimated that some 100 million school-aged children require treatment for schistosomiasis8, and Merck Soreno have pledged to donate 250 million tablets of PZQ annually to meet the need for treatment. With additional PZQ also available from World Vision, the World Bank and the UK Department for International Development, sufficient amounts should be available to deliver about 130 million treatments annually. Whether countries will have the funds and capacity to deliver these drugs to the rural populations will be the factor which determines whether schistosomiasis will be effectively controlled in the near future. Indeed, better implementation has been highlighted as a potential reason for the greater effectiveness of MDA programs in China compared to the Philippines9. However, it is clear that we are fast approaching the point where, for the first time in history, the majority of schistosome-infected people will receive treatment at some point during their lives (see Figure 1). In any event, it will be important to investigate the consequences of this massive MDA not only for measuring the success of the control programs and infection/transmission reduction, but also for understanding any changes in the epidemiology of schistosomiasis resulting from unprecedented levels of drug pressure.
Figure 1.
Heat map showing the estimated number of school age children requiring annual treatment for schistosomiasis (preventative chemotherapy) in countries where the disease is endemic. Black dots indicate countries where there has been a MDA program using Praziquantel within the past 5 years (2010-2015). Data obtained from the World Health Organisation Preventative Chemotherapy databank (http://www.who.int/neglected_diseases/preventive_chemotherapy/sch/en/).
We have been involved in implementing as well as monitoring and evaluating schistosome control programs in several countries in Africa. Concurrently, we have been undertaking basic scientific research to inform the development and delivery of helminth interventions, including schistosomiasis control strategies. Our research has led us to contemplate what the human health and parasite population landscape will look like in both the short and longer terms following widespread MDA to control schistosome infection and morbidity.
Malaria MDA programs offer valuable lessons on how to anticipate both failure and success in population-based parasite control programs (see review10). However, interpretation of the impact of malaria MDA programs is complicated by the fact that many malaria campaigns employed both MDA and integrated vector control approaches. For schistosomiasis, most people recognise that improvements in water supplies, sanitation and hygiene (WASH), will be needed if disease is to be eliminated and historically some countries, notably Egypt, have included WASH, as well as mollusciding to reduce numbers of intermediate host snails, though with mixed outcomes2. Current schistosomiasis control programs, however, predominantly rely on PZQ treatment which allows the effects of MDA to be assessed in the absence of the confounding effects of other interventions. To date, there here have been just two studies investigating the potential long term effects of MDA on the epidemiology of schistosomiasis, one study using infection data11, and the other using both infection data and immunology data12. Here, we extend this work by discussing the potential effects of schistosomiasis MDA on other aspects of both the human and parasite populations.
Studies in human populations have shown that protective immunity against schistosome infection does develop naturally, albeit over several years13. This slow development of protective acquired immunity has been partly attributed to the undoubted ability of the worms to modulate host immune responses14, thereby allowing the parasites to establish chronic infections. PZQ alters the schistosome calcium transport channels in the schistosome adult worm tegument increasing cellular ion permeability thus inducing spastic muscular paralysis15. This causes morphological changes in the schistosome tegument allowing greater exposure of parasite antigens and thereby driving stronger schistosome-specific antibody and cellular responses16. This damage to the tegument allows the host’s immune system to attack the worms leading to their death15. In addition, the clearance of the parasites also removes the cause of immunological down-modulation, so increasing the proportion and activation phenotype of antigen presenting cells, CD4+ effector and CD4+ memory T cell17, 18. These changes occur in both schistosome-specific and non-schistosome-specific cells and mechanistic studies in experimental models have shown that the relationship is causal (see review19)
The consequences of these two PZQ-related effects on the intrinsic immune response of the infected host are that the antigen threshold required to mount protective immune responses is achieved20 and the cellular hypo-responsiveness induced by the parasites is reversed21. Furthermore, PZQ treatment can reverse early pathology in human infections22. This benefits the host in that both re-infection rates and subsequent immunopathology are reduced. Since resistance to schistosomiasis is only partial, people do still become re-infected but repeated treatment has been shown to have greater impact on reducing re-infection rates23.
Although there is some heterogeneity in the effector responses induced by treatment24, data from field immuno-epidemiological studies suggest that the immune responses induced by treatment mirror protective immunity that develops naturally, indicating that PZQ treatment accelerates a process that takes years to occur in untreated individuals25.
The frequency of PZQ MDA has been prescribed for primary school children by the WHO and ranges from once every 1-2 years to once over the 7 primary school years for each child depending on levels of transmission26. In countries such as Zimbabwe, ministries of health have embarked on an annual treatment for every schistosome-exposed school child regardless of the level of transmission, so every child will receive up to 5 treatments, but children who miss one year may well be treated in the following year, thus ensuring good coverage. However, as has occurred in other countries, MDA programs run for a specified pre-defined period, for example Zimbabwe’s MDA programme is running for 5 years from 2012 to 2016. Other MDA programs may not be continued, for various reasons including financial and political willpower9, 27. In either case, residual foci of transmission may lead to people in affected areas carrying infections without the benefit of treatment28. Importantly, it is still not known how long immune responses will persist or decay once the source of antigenic stimulation is removed.
Our prediction is that, in addition to substantially reducing levels of infection, the MDA programs will initially boost schistosome-specific protective responses in the school-aged population and this will contribute to a reduction in re-infection rates12. This prediction is supported by work on Kenyan car washers23 and Zimbabwean children29 who showed significant reductions in re-infection rates (accompanied by significant changes in their immune phenotype) during the 91 and 75 weeks following treatment respectively
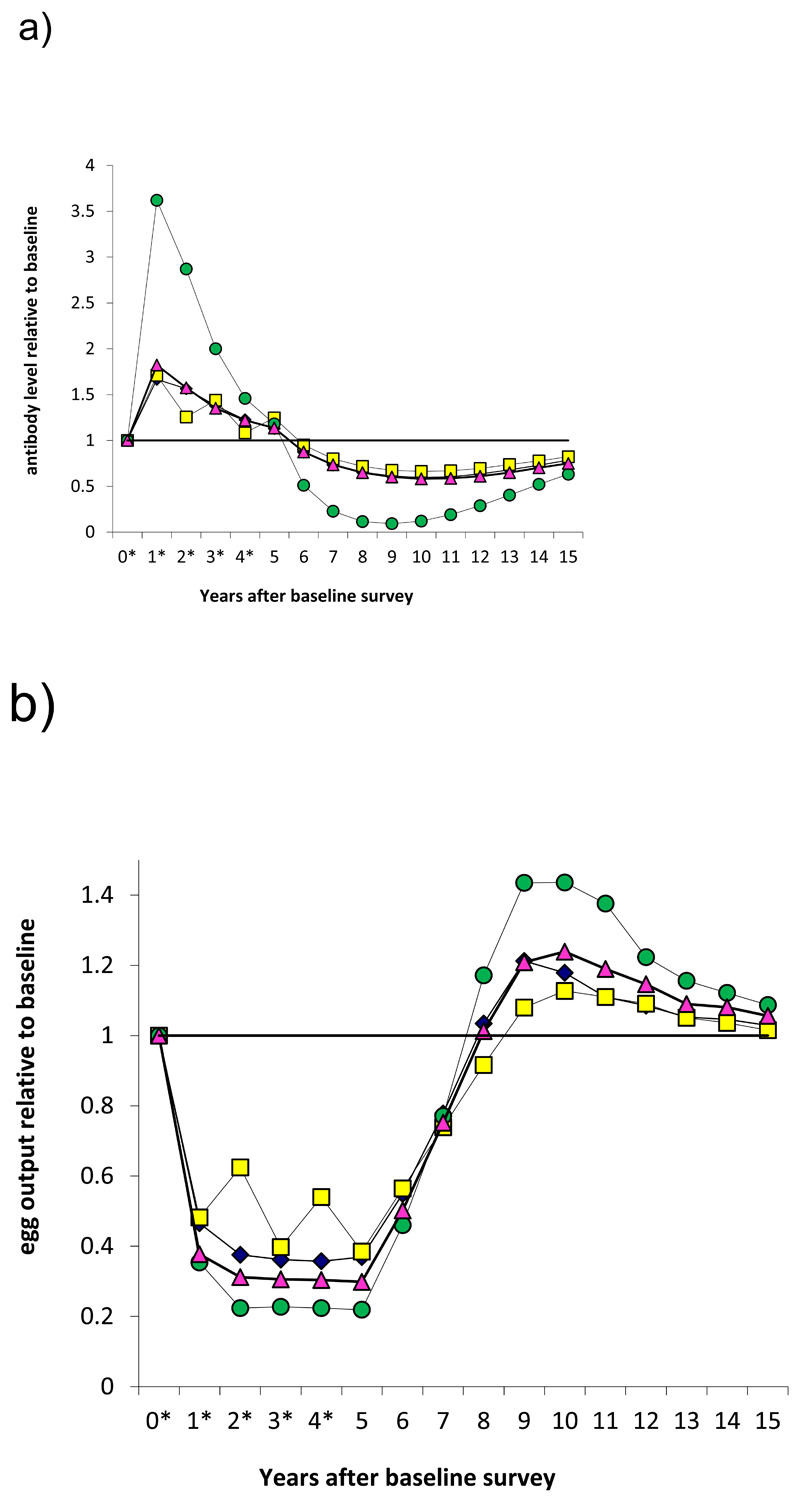
However, it remains unclear for how long this level of acquired resistance will be maintained in the face of reduced natural exposure to infection to boost protective immunity as well as in the absence of large amounts of antigen released through the killing of worms by PZQ16. The long-term effects of PZQ treatment on host immune phenotype remain unexplored as there have not been any studies investigating the effects of these changes several years after cessation of MDA. Nonetheless, in the case of schistosome-specific responses and susceptibility to re-infection, we have demonstrated that cessation of MDA after 5 years in areas where there has not been a sustained reduction in overall transmission rate (as might occur in the absence of additional complementary interventions) can impact on the development of schistosome-specific responses12. There has already been a precedent in malaria, where following cessation of MDA in Tanzanian children, among whom those who had received malaria chemoprophylaxis had higher rates of pathology (severe malaria) and anaemia compared to non-chemoprophylaxis groups, a difference attributed to the lack of development of naturally acquired protection in the treated group30. Decay in protective immunity could lead to a rebound in schistosome infection to levels higher than those before treatment once drug protection ceases (see Figure 3). Indeed a rebound in schistosome infection and morbidity after MDA cessation has already been reported from Pemba and Mali 27,31, and this is consistent with predictions from quantitative analyses12.
Figure 3.
Model-predicted dynamics of protective antibody and egg output during and after MDA for schistosomiasis. Treatment applied at yearly intervals for 5 years (asterisks) to a large population of school-aged children (6-15 years old) with 75% coverage. (a) Antibody levels. (b) Egg output. Figures compare results when MDA reduces transmission by 0, 50 or 100%. This model assumes a relatively short-lived memory response and a worm life span of 6.5 years. Full details are given in ref 12.
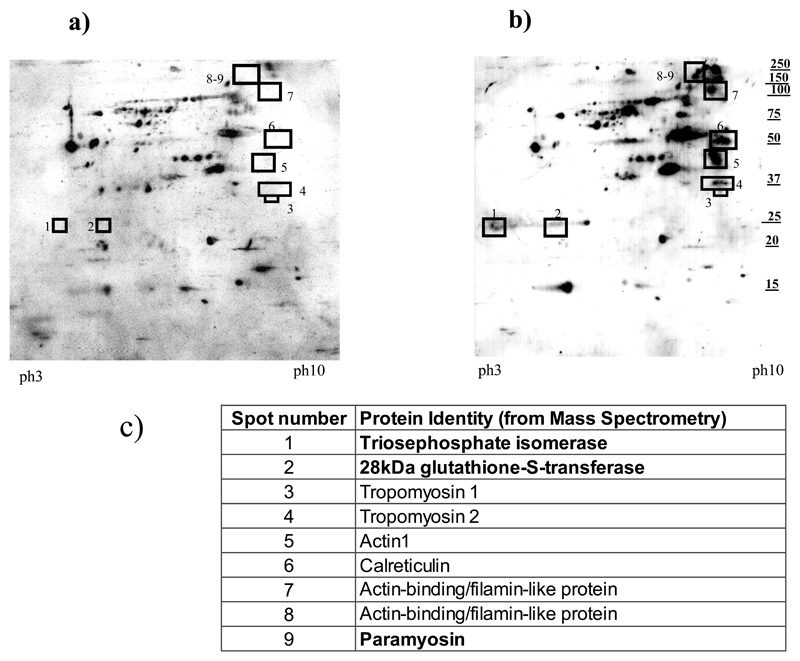
There is also a need to predict the effect of superimposing an anti-schistosome vaccine, when one becomes available, on populations already naturally exposed to the parasite and treated with PZQ, and thus having some partial protection against infection/re-infection. Interestingly, we have shown16 that PZQ treatment enhances immune responses against every WHO schistosome vaccine candidate antigen, including the 28-kDa glutathione S transferase (28kDa GST) formulated as Bilhvax© (see Figure 2) which has undergone Phase III clinical trials. Consequently, we have highlighted that the choice of study design and controls in the post-MDA schistosome vaccine trials would need very careful consideration in order to detect the effects of vaccination above and beyond those of treatment32.
Figure 2. Effects of PZQ on human schistosome specific antibody responses (adopted from ref 16).
2-dimensional Western blot analyses of serological reactivity of the treated cohort comparing pre- and post-treatment responses. The intensity of the IgG1 reactivity against adult schistosome worm antigens in serum pooled from 112 people (male and female) aged 5-42 was compare before and 12 weeks after treatment with the standard dose of Praziquantel (PZQ) of 40mg/kg body weight. (Full details are given in ref16)
a) Spots (antigens) reacting with sera collected at time 0 (before PZQ treatment). Boxes represent the position where additional spots absent in this Figure are present in Figure 2b.
b) Spots reacting with sera collected 12 weeks post-treatment. In addition to enhanced recognition of antigens recognised before treatment, additional antigens highlighted in the numbered boxes were identified.
c) Protein identities of the antigens identified by sera after treatment. The proteins highlighted in bold are on the current World Health Organisation list of schistosome vaccine candidates (see http://www.who.int/immunization/diseases/en/)
In experimental models, regulatory responses induced as part of the immunomodulatory mechanisms employed by helminths to evade host immune attack, reduce the severity or incidence of immune disorders such as colitis and allergic airway inflammation (see review19). Several human studies including our own, have shown that PZQ treatment increases immune reactivity against allergens and self- or autoantigens. In theory, this gives rise to the potential for PZQ MDA to exacerbate the clinical manifestations of these immune disorders19, generating concerns that MDA programs could lead to increased levels of these immune disorders in treated populations. However, in practice, these concerns have not been realised. Broadly, the immunological changes in allergic and/or autoimmune reactivity are quantitative uplifts that do not translate to clinical disease. Indeed there are several studies documenting that sensitization to one or more allergens may be highly prevalent without manifesting as a clinically relevant allergy33, 34. Similarly, autoreactivity alone does not indicate clinical autoimmune disease. This suggests the existence of complex features of the immune system preventing the translation of immunological changes to clinical pathology in helminth-infected individuals, some of which we have studied in affected human populations: for example, the uncoupling of allergic reactivity and clinical disease through higher ratios of total to allergen-specific serum IgE and the epitope specificity of the IgE35. It is also worth factoring in other homeostatic features of the immune system that influence the impact of helminth infections on host health such as the gut microbiome36. For example, we have recently demonstrated that in children aged 6 months to 10 years, the gut microbiome abundance and diversity are refractory to PZQ treatment of S. haematobium infection37. Hence, there is a need to continue monitoring the long-term effects of PZQ treatment on these responses and clinical disease status in the relevant MDA target populations with the understanding that immunological changes may take months or years to manifest themselves given the complex dynamics of each component in the immune response.
Also worthy of consideration is the converse side of these immunological changes; i.e. the different type of environment for schistosomes that a treated host constitutes and the potential for this new environment to drive selection for different schistosome genotypes/phenotypes. Experimental studies show that schistosome worms show plasticity in their development depending on the host immune phenotype; for example, the development of male worms is dependent on signals from the host immune system38. The effects of MDA on the developmental plasticity of schistosomes remain to be investigated, particularly to determine if schistosome transmission and life cycle propagation are altered under MDA pressure as a result of changes in the host immunological environment. Although parasite transmission should be reduced, in theory, there may be an overall increase in fecundity or virulence; an experimental study with Plasmodium chabaudi found that parasites developing in the face of immunity were more pathogenic when transferred to naïve hosts39.
In a recent meta-analysis of 55 reports of trials of PZQ efficacy, egg reduction rates ranged from 72% to 100% and cure rates ranged from 48% to 100%40. Although this heterogeneity arises from several factors, not least pre-treatment infection levels, the effects of differences in host factors cannot be excluded and there is a need to determine whether certain human phenotypes or genotypes will harbour a reservoir of schistosome parasites despite PZQ treatment. Such human refugia might need supplementary targeting, and immunological and/or biochemical profiling could help predict individuals in whom treatment failures will occur and these can be targeted by selective population chemotherapy. This may require infection diagnostics more sensitive than parasitological methods, such as antigen-based diagnostics, to ensure identification of people harbouring a reservoir of parasites41.
An important host variable is likely to be in pharmacokinetics, which may affect the amount of therapeutic PZQ in the host. One explanation proposed for lower re-infection rates in children treated with 60mg compared to 40mg/kg body weight was potential differences in immune responses resulting from treatment with different PZQ doses5. PZQ is metabolised by the cytochrome P450 (CYP) enzyme system in the liver42. Differential expression of these enzymes makes its availability susceptible to variability arising from individual pharmacokinetic heterogeneity, interactions with drugs/substances that induce/inhibit specific isoenzymes of the CYP system taken concomitantly with PZQ, and liver function (e.g. rifampicin used to treat Mycobacterium tuberculosis infection can reduce the amount of PZQ released in the host to below therapeutic levels43). To date, no extensive studies have been conducted to ascertain the relative contribution of these variables in the metabolism and efficacy of PZQ, and the resultant break-through of parasites despite treatment. Similarly, the level of polymorphism in the isoenzymes of the CYP system44 has not been documented in the MDA target populations, nor has there been quantification of the scale of people taking PZQ concurrently with drugs that affect the bioavailability of PZQ.
One of the main concerns for any parasite control program heavily reliant on drug intervention is possible selection for drug-resistant parasites. The effects of PZQ MDA on schistosome genetic diversity remain inconclusive45. So far, there have been no validated reports of drug resistance to PZQ amongst schistosome worms in human populations, and both field and quantitative studies refute the rapid development of PZQ resistance46. Nonetheless, heritable variation in PZQ sensitivity has been shown in schistosome worms experimentally, thus indicating that schistosome worms have the capacity to develop some level of resistance to PZQ47, 48, 49. Phylogenetic analyses and indices of schistosome parasite population differentiation have been able to distinguish parasites acquired through re-infection from those which have survived following PZQ treatment50. This, combined with host genotype characterisation will be useful for indicating putatively resistant parasites and hosts non-responsive to PZQ treatment. The advent of schistosome resistance to Oxamniquine in the 1970s that halted the S. mansoni MDA program in Brazil using this drug51 indicates a need for complementary interventions for long-term schistosome control programs.
Consequences of MDA on schistosome population biology include the potential for hybridisation between anthroponotic and zoonotic schistosomes, for example S. haematobium worms can hybridise with S. intercalatum or S. mansoni52 as well as with S. curassoni and S. bovis53. The ability of the ‘human’ schistosomes to hybridise with the animal schistosomes not only provides the potential for extending the host range, but may also affect pathogenicity52. Field studies have already shown that in areas co-endemic for S. mansoni and S. haematobium, the efficacy of PZQ can differ between the species54, and that competition for mates and ‘over-spill’ can result in hybridisation55. Taking the different efficacy of PZQ in the two main human schistosome species that are co-endemic in some African regions (S. mansoni and S. haematobium), hybridisation and its effects on pathology/morbidity require monitoring and further investigation.
In order to track the effects of MDA on overall host health and parasite population biology and, more crucially, to make predictions that can inform public health interventions and policy, it is essential that host and parasite schistosome epidemiology are closely monitored and related to molecular and genetic characteristics of each. This means following the effects of PZQ MDA on the classical schistosome epidemiological patterns13 and determining whether MDA programs (1) increase (or decrease) heterogeneity of host immune responses, (2) increase or decrease aggregation of infection, and (3) alter the age distribution of infection. It is also important to determine the effects of MDA frequency on these patterns in the populations exposed to different schistosome transmission levels56.
With the ultimate goal being improvement of both the short- and long-term health status of at-risk populations, the effects and strategies of schistosome interventions must continually be monitored and evaluated in the light of accumulating scientific evidence. Schistosomes have been shown to survive for many years in the host and host responses to schistosome infection develop over similar time scales. As a consequence, population-level shifts in schistosome immune-epidemiology take place slowly, over time-scales of decades13. Changes in schistosome genetics may occur over even longer time scales. Current evidence indicates that there are significant health benefits to receiving PZQ MDA, with very little downside to MDA, but it is important that we learn how to sustain these benefits for future generations.
Acknowledgments
We thank T. Mduluza, N. Midzi, and the staff of the National Institutes of Health Research Zimbabwe and the Department of Biochemistry at the University of Zimbabwe for their contributions to the work discussed here. The authors thank the Medical Research Council (UK), the Wellcome Trust, The University of Edinburgh ISSF Fund, the World Health Organisation and the Thrasher Research Fund for support.
Footnotes
Contributors
FM conceived of the idea. FM, RM, AF and MEJW contributed equally to the content of the paper, helped in the preparation of draft manuscript and reviewed the final version submitted to the journal.
Conflicts of Interest
We declare that we have no conflicts of interest.
References
- 1.Barber MA, Rice JB, Broen JY. Malaria studies on the Firestone rubber plantation in Liberia, West Africa. Am J Hyg. 1932;16:601–33. [Google Scholar]
- 2.Barakat RM. Epidemiology of Schistosomiasis in Egypt: Travel through time: review. J Ad Res. 2013;4(5):425–32. doi: 10.1016/j.jare.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mott KE. A reusable polyamide filter for diagnosis of S. haematobium infection by urine filtration. Bull SocPathol Exot. 1983;76:101–4. [PubMed] [Google Scholar]
- 4.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick smear technique in schistosomiasis mansoni. Rev Instit Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 5.Olliaro PL, Vaillant MT, Belizario VJ, et al. A multicentre randomized controlled trial of the efficacy and safety of single-dose praziquantel at 40 mg/kg vs. 60 mg/kg for treating intestinal schistosomiasis in the Philippines, Mauritania, Tanzania and Brazil. PLoS Negl Trop Dis. 2011;5(6):e1165. doi: 10.1371/journal.pntd.0001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piero O. Praziquantel: Getting the dosage right 2008. [accessed 7 September 2016]. http://www.who.int/tdr/news/2008/praziquantel-dosing/en/
- 7.Knopp S, Person B, Ame SM, et al. Elimination of schistosomiasis transmission in Zanzibar: baseline findings before the onset of a randomized intervention trial. PLoS Negl Trop Dis. 2013;7(10):e2474. doi: 10.1371/journal.pntd.0002474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. Schistosomiasis progress report 2001–2011 and Strategic Plan 2012–2020. World Health Organisation; Geneva, Switzerland: 2012. [Google Scholar]
- 9.Wei Wang, Liang Y. Mass drug administration (MDA) for schistosomiasis. J Infec Dis. 2014;211(5):848–849. doi: 10.1093/infdis/jiu506. [DOI] [PubMed] [Google Scholar]
- 10.von Seidlein L, Greenwood BM. Mass administrations of antimalarial drugs. Trends Parasitol. 2003;19(10):452–60. doi: 10.1016/j.pt.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Gurarie D, Yoon N, Li E, et al. Modelling control of Schistosoma haematobium infection: predictions of the long-term impact of mass drug administration in Africa. Parasit Vectors. 2015;8:529. doi: 10.1186/s13071-015-1144-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell KM, Mutapi F, Mduluza T, Midzi N, Savill NJ, Woolhouse ME. Predicted impact of mass drug administration on the development of protective immunity against Schistosoma haematobium. PLoS Negl Trop Dis. 2014;8(7):e3059. doi: 10.1371/journal.pntd.0003059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woolhouse ME, Taylor P, Matanhire D, Chandiwana SK. Acquired immunity and epidemiology of Schistosoma haematobium. Nature. 1991;351(6329):757–9. doi: 10.1038/351757a0. [DOI] [PubMed] [Google Scholar]
- 14.Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3(9):733–44. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 15.Harnett W. The antihelminthic action of praziquantel. Parasitol Today. 1988;4(5):144–6. doi: 10.1016/0169-4758(88)90192-5. [DOI] [PubMed] [Google Scholar]
- 16.Mutapi F, Burchmore R, Foucher A, et al. Praziquantel treatment of people exposed to Schistosoma haematobium enhances serological recognition of defined parasite antigens. J Infect Dise. 2005;192(6):1108–18. doi: 10.1086/432553. [DOI] [PubMed] [Google Scholar]
- 17.Nausch N, Appleby LJ, Sparks AM, Midzi N, Mduluza T, Mutapi F. Group 2 innate lymphoid cell proportions are diminished in young helminth infected children and restored by curative anti-helminthic treatment. PLoS Negl Trop Dis. 2015;9(3):e0003627. doi: 10.1371/journal.pntd.0003627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watanabe K, Mwinzi PN, Black CL, et al. T regulatory cell levels decrease in people infected with Schistosoma mansoni on effective treatment. Am J Trop Med Hyg. 2007;77(4):676–82. [PMC free article] [PubMed] [Google Scholar]
- 19.McSorley HJ, Maizels RM. Helminth infections and host immune regulation. Clin Microbiol Rev. 2012;25(4):585–608. doi: 10.1128/CMR.05040-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell KM, Mutapi F, Savill NJ, Woolhouse ME. Protective immunity to Schistosoma haematobium infection is primarily an anti-fecundity response stimulated by the death of adult worms. Proc Natl Acad Sci U S A. 2012;109(33):13347–52. doi: 10.1073/pnas.1121051109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmiedel Y, Mombo-Ngoma G, Labuda LA, et al. CD4+CD25hiFOXP3+ Regulatory T cells and cytokine responses in human schistosomiasis before and after treatment with praziquantel. PLoS Negl Trop Dis. 2015;9(8):e0003995. doi: 10.1371/journal.pntd.0003995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Subramanian AK, Mungai P, Ouma JH, et al. Long-term suppression of adult bladder morbidity and severe hydronephrosis following selective population chemotherapy for Schistosoma haematobium. Am J Trop Med Hyg. 1999;61(3):476–81. doi: 10.4269/ajtmh.1999.61.476. [DOI] [PubMed] [Google Scholar]
- 23.Black CL, Mwinzi PN, Muok EM, et al. Influence of exposure history on the immunology and development of resistance to human Schistosomiasis mansoni. PLoS Negl Trop Dis. 2010;4(3):e637. doi: 10.1371/journal.pntd.0000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mutapi F. Heterogeneities in anti-schistosome humoral responses following chemotherapy. Trends Parasitol. 2001;17(11):518–24. doi: 10.1016/s1471-4922(01)02118-3. [DOI] [PubMed] [Google Scholar]
- 25.Mutapi F, Burchmore R, Mduluza T, Midzi N, Turner CM, Maizels RM. Age-related and infection intensity-related shifts in antibody recognition of defined protein antigens in a schistosome-exposed population. J Infect Dis. 2008;198(2):167–75. doi: 10.1086/589511. [DOI] [PubMed] [Google Scholar]
- 26.WHO. Prevention and control of schistosomiasis and soil-transmisted helminthiasis. World Health Organisation; Geneva: 2002. [Google Scholar]
- 27.Guidi A, Andolina C, Makame Ame S, Albonico M, Cioli D, Juma Haji H. Praziquantel efficacy and long-term appraisal of schistosomiasis control in Pemba Island. Trop Med Int Health. 2010;15(5):614–8. doi: 10.1111/j.1365-3156.2010.02488.x. [DOI] [PubMed] [Google Scholar]
- 28.Elmorshedy H, Bergquist R, El-Ela NE, Eassa SM, Elsakka EE, Barakat R. Can human schistosomiasis mansoni control be sustained in high-risk transmission foci in Egypt? Parasit Vectors. 2015;8:372. doi: 10.1186/s13071-015-0983-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bourke CD, Nausch N, Rujeni N, et al. Integrated analysis of innate, Th1, Th2, Th17, and regulatory cytokines identifies changes in immune polarisation following treatment of human schistosomiasis. J Infect Dis. 2013;208(1):159–69. doi: 10.1093/infdis/jis524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menendez C, Kahigwa E, Hirt R, et al. Randomised placebo-controlled trial of iron supplementation and malaria chemoprophylaxis for prevention of severe anaemia and malaria in Tanzanian infants. Lancet. 1997;350(9081):844–50. doi: 10.1016/S0140-6736(97)04229-3. [DOI] [PubMed] [Google Scholar]
- 31.Clements AC, Bosque-Oliva E, Sacko M, et al. A comparative study of the spatial distribution of schistosomiasis in Mali in 1984-1989 and 2004-2006. PLoS Negl Trop Dis. 2009;3(5):e431. doi: 10.1371/journal.pntd.0000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bourke CD, Nausch N, Rujeni N, et al. Cytokine responses to the anti-schistosome vaccine candidate antigen glutathione-S-transferase vary with host age and are boosted by praziquantel treatment. PLoS Negl Trop Dis. 2014;8(5):e2846. doi: 10.1371/journal.pntd.0002846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flohr C, Tuyen LN, Quinnell RJ, et al. Reduced helminth burden increases allergen skin sensitization but not clinical allergy: a randomized, double-blind, placebo-controlled trial in Vietnam. Clin Exp Allergy. 2009 doi: 10.1111/j.1365-2222.2009.03346.x. [DOI] [PubMed] [Google Scholar]
- 34.Wiria AE, Hamid F, Wammes LJ, et al. The effect of three-monthly albendazole treatment on malarial parasitemia and allergy: a household-based cluster-randomized, double-blind, placebo-controlled trial. PLoS One. 2013;8(3):e57899. doi: 10.1371/journal.pone.0057899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rujeni N, Nausch N, Midzi N, et al. Soluble CD23 levels are inversely associated with atopy and parasite-specific IgE levels but not with polyclonal IgE levels in people exposed to helminth infection. Int Arch Allergy Immunol. 2013;161(4):333–41. doi: 10.1159/000346545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reynolds LA, Finlay BB, Maizels RM. Cohabitation in the intestine: interactions among helminth parasites, bacterial microbiota, and host immunity. J Immunol. 2015;195(9):4059–66. doi: 10.4049/jimmunol.1501432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kay GL, Millard A, Sergeant MJ, et al. Differences in the faecal microbiome in Schistosoma haematobium infected children vs. uninfected children. PLoS Negl Trop Dis. 2015;9(6):e0003861. doi: 10.1371/journal.pntd.0003861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davies SJ, Grogan JL, Blank RB, Lim KC, Locksley RM, McKerrow JH. Modulation of blood fluke development in the liver by hepatic CD4+ lymphocytes. Science. 2001;294(5545):1358–61. doi: 10.1126/science.1064462. [DOI] [PubMed] [Google Scholar]
- 39.Mackinnon MJ, Read AF. Immunity promotes virulence evolution in a malaria model. PLoS Biol. 2004;2(9):E230. doi: 10.1371/journal.pbio.0020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zwang J, Olliaro PL. Clinical efficacy and tolerability of praziquantel for intestinal and urinary schistosomiasis-a meta-analysis of comparative and non-comparative clinical trials. PLoS Negl Trop Dis. 2014;8(11):e3286. doi: 10.1371/journal.pntd.0003286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mwinzi PN, Kittur N, Ochola E, et al. Additional evaluation of the point-of-contact circulating cathodic antigen assay for Schistosoma mansoni infection. Frontiers Pub Health. 2015;3:48. doi: 10.3389/fpubh.2015.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li XQ, Bjorkman A, Andersson TB, Gustafsson LL, Masimirembwa CM. Identification of human cytochrome P(450)s that metabolise anti-parasitic drugs and predictions of in vivo drug hepatic clearance from in vitro data. Eur J Clin Phamacol. 2003;59(5-6):429–42. doi: 10.1007/s00228-003-0636-9. [DOI] [PubMed] [Google Scholar]
- 43.Ridtitid W, Wongnawa M, Mahatthanatrakul W, Punyo J, Sunbhanich M. Rifampin markedly decreases plasma concentrations of praziquantel in healthy volunteers. Clin Pharmacol Ther. 2002;72(5):505–13. doi: 10.1067/mcp.2002.129319. [DOI] [PubMed] [Google Scholar]
- 44.Preissner SC, Hoffmann MF, Preissner R, Dunkel M, Gewiess A, Preissner S. Polymorphic cytochrome P450 enzymes (CYPs) and their role in personalized therapy. PLoS One. 2013;8(12):e82562. doi: 10.1371/journal.pone.0082562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huyse T, Van den Broeck F, Jombart T, et al. Regular treatments of praziquantel do not impact on the genetic make-up of Schistosoma mansoni in Northern Senegal. Infect Genet Evol. 2013;18:100–5. doi: 10.1016/j.meegid.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 46.King CH, Muchiri EM, Ouma JH. Evidence against rapid emergence of praziquantel resistance in Schistosoma haematobium Kenya. Emerg Infect Dis. 2000;6(6):585–94. doi: 10.3201/eid0606.000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mwangi IN, Sanchez MC, Mkoji GM, et al. Praziquantel sensitivity of Kenyan Schistosoma mansoni isolates and the generation of a laboratory strain with reduced susceptibility to the drug. Int J Parasitol Drugs Drug Res. 2014;4(3):296–300. doi: 10.1016/j.ijpddr.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coeli R, Baba EH, Araujo N, Coelho PM, Oliveira G. Praziquantel treatment decreases Schistosoma mansoni genetic diversity in experimental infections. PLoS Negl Trop Dis. 2013;7(12):e2596. doi: 10.1371/journal.pntd.0002596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Couto FF, Coelho PM, Araujo N, et al. Schistosoma mansoni: a method for inducing resistance to praziquantel using infected Biomphalaria glabrata snails. Mem Inst Oswaldo Cruz. 2011;106(2):153–7. doi: 10.1590/s0074-02762011000200006. [DOI] [PubMed] [Google Scholar]
- 50.Norton AJ, Gower CM, Lamberton PH, et al. Genetic consequences of mass human chemotherapy for Schistosoma mansoni: population structure pre- and post-praziquantel treatment in Tanzania. Am J Trop Med Hyg. 2010;83(4):951–7. doi: 10.4269/ajtmh.2010.10-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Utzinger J, Xiao S, Keiser J, Chen M, Zheng J, Tanner M. Current progress in the development and use of artemether for chemoprophylaxis of major human schistosome parasites. Curr Med Chem. 2001;8(15):1841–60. doi: 10.2174/0929867013371581. [DOI] [PubMed] [Google Scholar]
- 52.King KC, Stelkens RB, Webster JP, Smith DF, Brockhurst MA. Hybridization in parasites: consequences for adaptive evolution, pathogenesis, and public health in a changing world. PLoS Pathog. 2015;11(9):e1005098. doi: 10.1371/journal.ppat.1005098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Webster BL, Diaw OT, Seye MM, Webster JP, Rollinson D. Introgressive hybridization of Schistosoma haematobium group species in Senegal: species barrier break down between ruminant and human schistosomes. PLoS Negl Trop Dis. 2013;7(4):e2110. doi: 10.1371/journal.pntd.0002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.King CH, Olbrych SK, Soon M, Singer ME, Carter J, Colley DG. Utility of repeated praziquantel dosing in the treatment of schistosomiasis in high-risk communities in Africa: a systematic review. PLoS Negl Trop Dis. 2011;5(9):e1321. doi: 10.1371/journal.pntd.0001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cunin P, Tchuem Tchuente LA, Poste B, Djibrilla K, Martin PM. Interactions between Schistosoma haematobium and Schistosoma mansoni in humans in north Cameroon. Trop Med Int Health. 2003;8(12):1110–7. doi: 10.1046/j.1360-2276.2003.01139.x. [DOI] [PubMed] [Google Scholar]
- 56.Woolhouse ME, Chandiwana SK. The epidemiology of schistosome infections of snails: taking the theory into the field. Parasitol Today. 1990;6(3):65–70. doi: 10.1016/0169-4758(90)90211-l. [DOI] [PubMed] [Google Scholar]