Abstract
Lymphopoiesis is the process in which B, T, and innate lymphoid cells (ILC) develop from hematopoietic progenitors that exhibit early lymphoid priming. The branching points where lymphoid-primed human progenitors are further specified to B/T/ILC differentiation trajectories remain unclear. Here, we discuss the emerging role of IRF8 as a key factor to bridge human lymphoid and dendritic cell (DC) differentiation and the current evidence for the existence of circulating and tissue-resident CD123+CD127+ lymphoid progenitors. We propose a model whereby DC/B/T/ILC lineage programs in circulating CD123+CD127+ lymphoid progenitors are expressed in balance. Upon tissue seeding, the tissue microenvironment tilts this molecular balance towards a specific lineage, thereby determining in vivo lineage fates. Finally, we discuss the translational implication of these lymphoid precursors.
New perspectives on human lymphopoiesis in the era of single cell genomics
Over the past five years, single cell genomics has been increasingly applied to dissect human lymphopoiesis across anatomical sites. These valuable resources collectively provide an unprecedented high-resolution perspective on the composition of various hematopoietic tissues at the single cell level. In this Opinion article, we discuss how these timely resources guided us to revise the branching points of human lymphoid differentiation and highlight an underappreciated role for interferon regulatory factor 8 (IRF8) in connecting and coordinating human DC and lymphoid differentiation. Based on these novel findings, we put forward a hematopoietic model in which circulating IRF8-expressing CD123+CD127+ lymphoid progenitors initiate DC and/or lymphoid differentiation via tissue-seeding. Lastly, we discuss how a better understanding of CD123+CD127+ lymphoid progenitors could guide the development of stem cell mobilizers that may improve the kinetics of lymphoid reconstitution in clinical transplantation.
Revisiting the branching points of human lymphoid development
Hematopoietic stem cells (HSC) reside at the apex of the hematopoietic hierarchy and sustain the lifelong production of all blood cell types via downstream progenitors that are initially primed towards multiple lineages and then commit to produce specific blood cell types (uni-lineage priming (See Glossary)) [1, 2]. During lineage specification, expression of certain lineage-specific programs is reinforced, and expression of downstream differentiation modules is triggered. This drives progressive lineage restriction of hematopoietic progenitors towards lineage commitment and the corresponding phenotypic manifestation [3]. To date, EPCR (endothelial protein C receptor)+ HSCs (CD34+CD38-CD45RA-CD90-/+CD49f+EPCR+) represent the most homogeneous human HSC population with the highest self-renewal capacity (Box 1) and are present in cord blood (CB) and adult bone marrow (BM) [4]. In a primary xenotransplantation model using NSG mice, human EPCR+ HSCs generated balanced myeloid and lymphoid differentiation outputs whereas the immediate downstream CD34+CD38-CD45RA-CD90+CD49f-/+EPCR- (hereafter CD90+EPCR-) and multipotent progenitor (MPP: CD34+CD38-CD45RA-CD90-EPCR-) progeny fractions were lymphoid-biased [4]. This conforms with earlier studies showing that lymphoid lineage restriction is already present within the HSC/MPP compartment that is transcriptionally and epigenetically heterogeneous at the single cell level [5–7]. One group reported that the earliest loss of erythroid potential happens within a CD49f+CLEC9Alo human CB HSC subset (presumably part of CD90+EPCR- progenitors) [6], upstream of lymphoid-primed multipotent progenitors (LMPP: CD34+CD38-CD45RA+CD90-CD10-) [8] and multilymphoid progenitors (MLP: CD34+CD38-CD45RA+CD90-CD10+) [9], both populations retaining myeloid differentiation potential. Hence, the differentiation of EPCR+ HSCs towards lymphopoiesis is associated with a developmental transition from multipotency to gradual lymphoid lineage enforcement and restriction.
Glossary.
- CITE-seq
cellular indexing of transcriptomes and epitopes by sequencing is a method in which oligonucleotide-labeled antibodies are used to integrate cell surface protein and transcriptome measures into a single-cell readout.
- CellPhoneDB
a public repository of ligands, receptors and their interactions that enables prediction of biologically relevant interacting ligand-receptor partners from single-cell data.
- Co-culture assay
a method to measure cell potential in which HSPCs are co-cultured with stromal cells and induced to differentiate with a lineage-specific cytokine cocktail.
- Dormancy
a deep quiescent cellular state where HSCs are non-cycling (G0) and divide extremely infrequently. Dormant HSCs have been shown to have the highest self-renewal capacity and significantly contribute to hematopoiesis only in times where high levels of blood regeneration are needed (transplantation, chemotherapy, etc...).
- ILC
innate lymphoid cells comprise of five subsets, including NK cells, helper ILCs (ILC1s, ILC2s and ILC3s) and lymphoid tissue-inducer cells.
- ITGB7
integrin beta-7 can pair with an alpha4 chain or an alphaE chain to form heterodimeric integral membrane proteins that play a role in lymphocyte adhesion.
- Lineage commitment
a stage of differentiation where progenitors are fully committed to produce a specific cell lineage and lose the ability to adopt differentiation trajectories of other cell lineages when placed in alternative lineage-promoting environments.
- Lineage priming
a process whereby HSPC subsets express low amounts of lineage associated genes. This is considered the first step that licenses progenitors to adopt a specific differentiation trajectory.
- Lineage specification
a stage of differentiation where progenitors have reinforced a specific cell lineage program but retain the ability to adopt differentiation trajectories of other cell lineages when placed in alternative lineage-promoting environments.
- Methylcellulose colony-forming unit assay
a method to measure the ability of HSPCs to proliferate and differentiate into colonies in a semi-solid media in response to a lineage-specific cytokine cocktail.
- NBSGW mice
NOD,B6.SCID Il2rγ-/- KitW41/W41 mice harbor a mutant KitW41 allele and support engraftment of human HSPCs in the absence of irradiation.
- NicheNet
a computational method to infer the effects of sender-cell ligands on receiver-cell gene expression based on prior knowledge on signaling and gene regulatory network.
- NSG mice
non-obese diabetic, severe combined immunodeficiency, gamma chain null mice that support engraftment of human HSPCs in the presence of irradiation.
- Partition-based graph abstraction
a trajectory-inference algorithm that generates a topology-preserving map of single cells and allows prediction of developmental trajectories and tracking of gene expression changes along the trajectories.
- Primary hematopoietic sites
human hematopoiesis takes place mainly in liver from 5-6 post conception weeks until the middle of the second trimester and subsequently in bone marrow from around 20 post conception weeks and throughout postnatal life.
- Pseudotime analysis
a computational method to map high dimensional asynchronous single-cell data on a one-dimensional pseudotemporal progression scale in order to allow modeling of the differentiation of single-cells.
- SCID repopulating cell
cell that has the ability to initiate multilineage engraftment which is indicated by the presence of human myeloid and lymphoid cells in bone marrow of NSG mice after 12 to 20 weeks of transplantation.
- Self-renewal
a distinct feature of bona fide HSCs measured by the capacity to produce all blood cell types upon serial transplantation. For human HSCs, this is assessed using immuno-compromised mice, most often NSG.
- Severe combined immunodeficiency
a type of primary immune deficiency disease caused by mutations in different genes involved in the development and function of immune cells.
- SIRPA
signal regulatory protein alpha is also known as CD172a that acts as a immunoglobulin-like cell surface receptor for CD47.
- STEMNET
a trajectory-inference algorithm that computes the probabilities of progenitors to differentiate towards distinct developmental endpoints that are projected on the edge of a circle.
- UMAP
an algorithm that reduces the high dimensionality of single-cell data for visualization while preserving the global structure.
- V(D)J recombination
a somatic event mediated by RAG recombinase to rearrange variable (V), diversity (D), and joining (J) genes that encode antigen receptor molecules to allow the formation of a diverse repertoire of TCRs in T cells and BCRs in B cells.
Box 1. Identification of human HSC subsets with varying self-renewal capacity.
The past 10 years have seen great advances in our understanding of the very first steps of hematopoiesis that occur within the most primitive CD34+ hematopoietic compartment in humans. These include identification and prospective purification of HSC subsets which differ in their self-renewal and differentiation capacities [75], as well as the delineation of the cellular intermediates that lead to the specification of the erythroid and myeloid lineages. How these multipotent progenitors specify to distinct lymphoid lineages is less clear. The HSC subset with the highest self-renewal capacity has historically been identified within the CD34+CD38-CD45RA-CD90+ compartment [76]; this has been further enriched using the CD49f+ marker that improves the SCID repopulating cell (SRC) frequency from 1 in 20 cells to 1 in 10.5 cells [77]. Recently, one research group reported that EPCR+ HSCs possess the highest SRC frequency (1 in 3.1 cells) [4]. Accordingly, others identified EPCR as a marker that enriches for increased engraftment potential within human FL HSCs [78]. Furthermore, EPCR+ HSCs express the highest amount of GPRC5C - a dormancy marker established in human adult BM HSCs [79], compared to CD90+EPCR- progenitors and MPP [4].
Development of B/T/ILC lineages occurs in multiple anatomical sites. Immature B cells arise in primary hematopoietic sites and migrate to peripheral lymphoid tissues as mature naïve B cells. In humans, putative B lineage-specified progenitors (CD34+CD19-CD22+) that phenotypically emerge before B lineage-committed pre-pro-B progenitors (CD34+CD19+CD10-) were recently identified and are present in fetal tissues (liver and BM), CB, and adult BM [10]. However, functional characterization of these CD34+CD19-CD22+ cells will be required to validate their hierarchical position in the B-lineage differentiation trajectory. Of note, despite the lack of myeloid/T/natural killer (NK) potential, pre-pro-B progenitors that are enriched in fetal BM were found to continue to express low amounts of stem cell, myeloid, and T cell (CD244, CD7 and CD3D)-associated genes, which is intriguing because pre-pro-B progenitors are fully committed to the B-lineage differentiation trajectory [11]. Hence, B cells, at least in the fetal ontogenetic stage, might originate from multipotent progenitors that are also transcriptionally primed towards the myeloid and T lineages. Accordingly, recent studies in mice [12] and humans [13] indicate that T cell differentiation is initiated in hematopoietic progenitors at primary sites before the cells colonize the thymus via blood circulation. In humans, two subsets of thymus seeding progenitors (TSP1: CD34+CD44+CD10+CD7-CD123-CD127- and TSP2: CD34+CD44+CD10-CD7+CD123+CD127+) were identified via integrated analyses of single-cell RNA-sequencing (scRNA-seq) datasets from human postnatal CD34+ thymocytes, CD34+ BM cells, and immature peripheral blood mononuclear cells (PBMC) [13, 14]. In contrast to TSP1s that are Notch-naïve, TSP2s express Notch target genes such as CD7 and CD3E [13]. Hence, TSP2s are likely to have received Notch stimulation, which is crucial for inducing T cell lineage specification, prior to their seeding in the thymus. Co-culture assays revealed NK potential within human postnatal T lineage-specified progenitors as these cells could generate CD56+ NK cells in vitro in the presence of interleukin (IL)-15 [15]. However, scRNA-seq analyses of human postnatal CD34+ thymocytes did not identify any intrathymic NK lineage differentiation trajectories [13, 16]. This indicates that postnatal TSPs also possess NK-lineage priming but are directed to the T-lineage fate in vivo in the thymus.
In contrast to T cells, ILCs develop in both primary hematopoietic sites and peripheral tissues. In the latter, differentiation of human ILCs is sustained by the seeding of circulating ILC lineage-committed progenitors (CD34-CD7+CD117+CD127+CD45RA+) that lack T and B lineage potential [17]. The origin of these progenitors was partially clarified by the identification of human fetal ILC lineage-specified progenitors [18]. Using scRNA-seq, two distinct human lymphoid progenitor clusters (LP1 and LP2) that were CD34+CD127+CD123+ were identified in liver, thymus, spleen, intestine, skin, and lung [18]. Based on computational analyses (Partition-based graph abstraction and STEMNET), LP1s were predicted to correspond to multipotent lymphoid progenitors (downstream of hematopoietic stem and progenitor cells (HSPC)), giving rise to LP2s which in turn were predicted to constitute ILC lineage-specified progenitors upstream of ILC lineage-committed progenitors [18]. Indeed, CD34+CD127+CD123+ cells isolated from the human fetal liver (FL) were functionally validated to possess T, B, ILC, and myeloid potential through co-culture assays that support in vitro generation of CD4+CD8+ T cells, CD19+ B cells, CD56+ NK cells, IFNγ/IL-13-producing ILCs or CD33+ myeloid cells [18]. These cells were immunophenotypically similar to the CD127- and CD123-expressing TSP2s that have been identified in human postnatal thymus, which is intriguing because fetal ILCs and postnatal T cells therefore appear to be derived from a common CD123+CD127+ hematopoietic progenitors [13, 14]åCollectively, these studies suggest that T/B/ILC lineage programs have been initiated in early lymphoid-primed progenitors at the primary hematopoietic sites and that additional signals are likely to be acquired during specification, leading to the segregation of these distinct lymphoid lineages.
Unlike B cells and T cells, ILCs lack rearranged, antigen specific receptors. Hence, V(D)J recombination of B and T cell receptors is a hallmark of adaptive immune cell development. Based on the analysis of adaptive immune receptor sequencing datasets (scVDJ-seq) from prenatal human tissues, expression of non-reproductive T cell receptor beta locus (TRB) recombination was observed in the most immature double negative (DN) CD4-CD8- stages of T cell development as expected, but also in NKs, ILC2s, ILC3s, pre-pro B and B1 cells which was surprising because genomic recombination of T cell receptors is generally perceived to occur during T-lineage specification [19]. Furthermore, like DN thymocytes, ILCs but not B cells also expressed non-reproductive recombination of TRG and TRD [19]. Future studies will be required to clarify the observation that TRB recombination appears to occur first during the fetal T-lineage differentiation trajectory, whereas it has been shown that TRD recombination can precede the other TCR loci during postnatal T cell development [20, 21]. In each case, these recombination events suggest that fetal B cells, T cells, and ILCs share a common developmental stage and that B cells might deviate first from T/ILC development.
Mounting evidence for a common cellular origin of B/T/ILC lineages raises two questions. First, how does a multipotent progenitor express these distinct and seemingly antagonistic lymphoid programs in balance? Second, what triggers enforcement of a specific lineage program and leads to lineage segregation? Here, we propose a hematopoietic model (Figure 1, Key figure) where the expression of the transcription factor IRF8, which is essential for the development of monocytes and DCs [22–25], is also indispensable for the development of CD123+CD127+ lymphoid progenitors [14] and marks the priming of B/T/ILC lineage programs in them. Based on scRNA-seq analyses of hematopoietic progenitors isolated from multiple human tissues, these IRF8- expressing lymphoid progenitors have been identified as the starting points for ILC- [18] and T-lineage differentiation trajectories [13, 14, 26]. We propose that enforcement of a specific lineage program in these lymphoid progenitors occurs upon seeding in the tissues and is driven by tissue-specific microenvironmental cues that lead to lineage segregation and induce in vivo lineage fate. Below, we discuss evidence that supports the existence of such human B/T/ILC lymphoid progenitors in primary hematopoietic sites, peripheral blood (PB), and different tissues.
Figure 1. Circulating CD123+CD127+ lymphoid progenitors sustain human ILC and T cell development.
In this model of hematopoiesis, CD123+CD127+ lymphoid progenitors in the peripheral blood express IRF8 and are DC- and lymphoid-primed. Additional signals from the tissue microenvironment are likely to enforce specific lineage programs in these lymphoid progenitors and lead to lineage segregation that establish their in vivo cell fates. Recent studies indicate that circulating CD123+CD127+ lymphoid progenitors give rise to fetal innate lymphoid cell lineage-specified progenitors [18], as well as to fetal [26] and postnatal [13, 14] thymus seeding progenitors upon tissue seeding. These tissue-resident progenies of circulating CD123+CD127+ lymphoid progenitors have increased expression of IRF8 and CD123. Some elements in this figure were created using BioRender.com.
Emerging role of IRF8 as a key factor connecting lymphoid and DC lineages in humans
IRF8 is known as the key lineage determining transcription factor in hematopoiesis that promotes the development of DCs and monocytes but inhibits neutrophilic differentiation [22–25]. As such, monocytopenia, DC deficiency and neutrophilia are the most prominent dysregulations found in patients harboring bi-allelic IRF8 mutations [27, 28]. In humans, IRF8 has been documented to regulate the development of DCs and monocytes via two distinct differentiation trajectories in the CD34+ progenitor compartment, downstream of HSC/MPP [29]. In the lymphoid-primed CD123+IRF8hi pathway, LMPPs from adult BM gave rise to plasmacytoid DC (pDC) and classical DCs (cDC1 and cDC2) via CD33-CD123-/lo granulocyte-macrophage progenitors (GMP: CD34+CD38hiCD10-CD45RA+) and subsequently, CD123+IRF8hi GMPs [29]. In contrast, monocytes and DC3s, that were previously thought to be a subpopulation of cDC2, were generated from CD33+ GMPs along a common myeloid IRF8loSIRPA+ pathway [29]. Hence, DC-lineage potential (pDC and cDC) is imprinted by the expression of IRF8 during lymphoid priming and differentiation of HSCs/MPPs to LMPPs [29–31]. For illustration purposes, we discuss a previous report that has comprehensively characterized the transcriptome of human adult HSPCs in BM, spleen, and PB at steady state and post G-CSF mobilization using scRNA-seq [32]. In BM, expression of IRF8 was mainly observed in lymphoid and myeloid progenitors as well as in monocyte/DC progenitors (Figure 2A,B) [32]. However, in steady state PB that harbored a distinct HSPC composition compared to BM [32], the expression of IRF8 was limited to circulating lymphoid and monocyte/DC progenitors (Figure 2A,B) [32]. Iin addition to the extramedullary tissues where human ILC [18] and T cells [13, 14, 16, 26, 33] develop, IRF8-expressing lymphoid progenitors were also found in human adult spleen [32] (Figure 2C,D). Also, in the same study, HSPC mobilization by granulocyte colony-stimulating factor (G-CSF) negatively affected the presence of IRF8-expressing lymphoid progenitors in PB which was important as it suggested that G-CSF stimulation could alter the cellular composition of HSPCs collected for clinical transplantation (Figure 2A,B) [32].
Figure 2. scRNA-sequencing studies clarify the expression of IRF8 in human HSPCs.
For illustrative purposes, previous published data were compiled and presented as: (A) A Uniform Manifold Approximation and Projection (UMAP) of adult CD19-CD34+ HSPCs isolated from bone marrow (BM), steady state peripheral blood (PB) and G-CSF mobilized PB [32]. (B) Expression of IRF8 in (A). (C) UMAP of adult CD19-CD34+ HSPCs isolated from spleen [32]. (D) Expression of IRF8 in (C). (E) UMAP of CD34+ HSPCs isolated from fetal liver, fetal BM, and cord blood [37]. (F) Expression of IRF8 in (E). HSC/MPP, hematopoietic stem cell/multipotent progenitor; MEMBP, megakaryocyte/erythroid/mast cell/basophil progenitor; MyP, myeloid progenitor; EryP, erythroid progenitor; MDP, monocyte/dendritic cell progenitor; LyP, lymphoid progenitor; ND, not defined cluster; MEP, megakaryocyte/erythroid progenitor; EoBasoMC, eosinophil/basophil/mast cell progenitor; CLP, common lymphoid progenitor; DC, dendritic cell. (A-D) were generated from a publicly accessible ShinyCell web app [85]http://bioinf.stemcells.cam.ac.uk:3838/laurenti/mobPB_HSPCs/ and http://bioinf.stemcells.cam.ac.uk:3838/laurenti/ExtramedHSPCs/. (E,F) were generated by Tom Putteman based on the CITE-seq dataset from [37].
Besides its well-studied role in DC and monocyte development, IRF8 also contributes to early steps of lymphoid differentiation from HSCs. In mice, IRF8 and its interacting protein partner PU.1 upregulate the expression of Ebf1 to promote B cell lineage specification and the development of pre-pro-B progenitors [34–36]. Accordingly, IRF8 was reported to be expressed in human fetal B lineage-associated progenitors, in addition to DC and myeloid progenitors (Figure 2E,F) [37]. Furthermore, common progenitors (CD34+CD38+CD45RA+CD10+CD7−CD19−CD117+CD127+) for human B cells and pDCs were recently found in CB [38]. Based on pseudotime analysis of lymphoid progenitors from human FL and early T cell progenitors (ETP), IRF8-expressing fetal TSPs were identified and predicted to differentiate toward the expected T cell lineage or the alternative B cell lineage. However, human postnatal TSP2s also expressed IRF8 and were predicted by the STEMNET algorithm to possess the highest probability of giving rise to T cells followed by DC and B cells [13], suggesting that IRF8 is involved in the development of both DC and lymphoid lineages. Subsequently, IRF8 was reported to be indispensable for the development of TSP2s as the phenotypic emergence of CD34+CD123loCD127+ precursors, that displayed T- and DC-lineage potential in vitro in co-cultures, was severely impaired in an organoid model system following hematopoietic induction in IRF8 knockout human induced pluripotent stem cells (iPSC) [14]. Furthermore, limiting dilution co-culture assays revealed that iPSC-derived human TSP2s were DC-biased as 1 in 68.1 cells gave rise to DCs only whereas 1 in 140.6 cells gave rise to both DCs and T cells, and 1 in 1033.3 cells gave rise to T cells only [14]. TSP2s, DC progenitors, and mature DCs in the human postnatal thymus also contained comparable frequencies of Dδ2–Dδ3 recombination within the TRD locus [14]. Consistent with the role of TSP2s in supporting human T cell lineage specification [14], patients with IRF8 deficiency were found to present with a subtle decrease in peripheral T cells relative to healthy control subjects [27, 39]. Lastly, others found that the expression of IRF8, rather than TCF7 (the latter being essential for the development of murine ILC progenitors [40]), gradually increased during human fetal ILC lineage specification (from HSPCs to LP1s: multipotent lymphoid progenitors to LP2s: ILC lineage-specified progenitors) [18]. Altogether, these studies indicate that in addition to unlocking DC potential, IRF8 likely plays an active role in the development of lymphoid progenitors at primary hematopoietic sites which is supported by the detection of IRF8-expressing TSP2s in the BM and PB [13]. The pleiotropic role of IRF8 in hematopoiesis might be attributed to IRF8 function, which in terms of DNA binding and transcriptional activity (Box 2), depends on its heterodimeric association with different interacting protein partners [41]. This suggests that IRF8 function is restricted and modulated by the expression of its interacting protein partners, and it is likely that during HSC differentiation, IRF8 contributes to either DC or lymphoid lineage differentiation via formation of distinct heterodimers.
Box 2. DNA binding and transcriptional activity of IRF8.
Human IRF8 is a 426 amino acid protein with a N-terminal DNA-binding domain (DBD) and a C-terminal IRF association domain (IAD) [41]. The DBD allows IRF8 to recognize the interferon-stimulated response element (ISRE: 5’-(A/G)NGAAANNGAAA-3’) [80]. However, IRF8 has low intrinsic DNA binding affinity and is thus unable to bind to ISRE motif on its own. Hence, the DNA binding and transcriptional activity of IRF8 is dependent on its heterodimeric association with other interacting-protein partners via the IAD. For example, IRF8 can form heterodimers with other IRF family members such as IRF1, IRF2 and IRF4 to bind to the ISRE motif [81]. In addition, formation of heterodimers with non-IRF partners also allows IRF8 to recognize and to bind to distinct composite DNA sequences. IRF8-PU.1 heterodimers bind to the Ets-IRF composite element (EICE: 5’-GGAANNGAAA-3’), IRF-ETS composite sequence (IECS: 5’-GAAANA[N]GGA-3’) or ETS-IRF response element (EIRE: 5’-GGAAANNGAAA-3’) [82]. IRF8-BATF heterodimers bind to two AP-1-IRF composite elements (AICE: 5’-TTTCNNNNTGA(G/C)T(C/A)A-3’ and 5’-GAAATGA(G/C)T(C/A)A-3’) [83]. A new binding composite element has been identified in IRF4 where it interacts with Ikaros to bind to zinc finger-IRF composite elements (ZICE: 5’-GGGAAANNGAAA-3’) [84]. It remains to be investigated if IRF8 might also interact with Ikaros.
Circulating and tissue-resident CD123+CD127+ human lymphoid progenitors
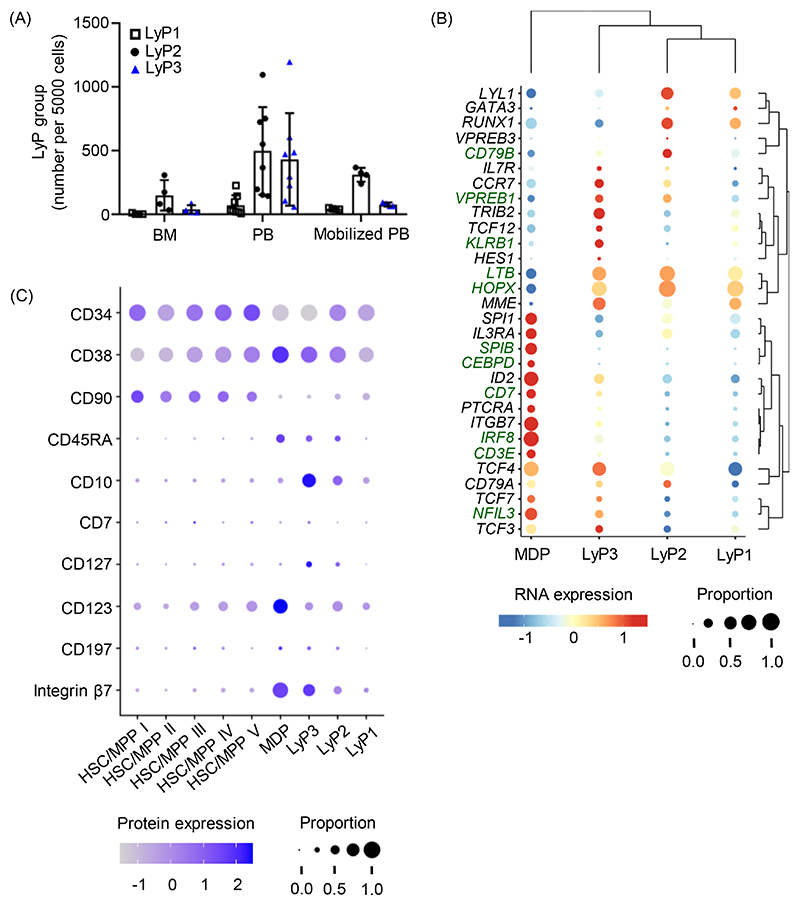
From several single cell profiling studies, there is accumulating evidence of CD123+CD127+ lymphoid progenitors circulating and seeding tissues in humans. For instance, in adults, scRNA-seq of human CD19-CD34+ HSPCs (Figure 2A) identified three clusters of lymphoid progenitors (LyP1-3) in BM, steady state PB, and G-CSF mobilized PB (Figure 3A) [32]. To determine which lymphoid clusters corresponded to IRF8-expressing lymphoid progenitors (Figure 2B) and for illustrative purposes, our laboratory groups took a closer look at IRF8 expression and B/T/ILC lineage-related genes from this scRNA-seq dataset [32]. In contrast to IRF8-expressing monocyte/DC progenitors (Figure 2B), none of the lymphoid clusters seemed to express SPIB and CEBPD, which were associated with the myeloid lineage (Figure 3B). Compared to other lymphoid clusters, LyP3 cells seemed to express IRF8, Notch target genes (CD7, CD3E) and genes related to B (CD79B, VPREB1), T (LTB, HOPX), and ILC (KLRB1, NFIL3) lineages (Figure 3B). Of note, LyP3 cells are relatively rare in BM, but they are more abundant than their LyP1/LyP2 counterparts in steady state PB (Figure 3A) [32]. The CITE-seq analysis in the same study also showed that LyP3 cells expressed lymphoid-associated cell surface markers, including CD45RA, CD127, and Integrin β7 (Figure 3C) [32]. Of note, the cells captured in the LyP3 cluster are likely IRF8-expressing circulating adult lymphoid progenitors that are depleted in G-CSF mobilized PB (Figure 2B and 3A) [32].
Figure 3. Single-cell profiling of human HSPCs supports the existence of circulating adult CD123-/loCD127+ lymphoid progenitors.
For illustrative purposes, previously published data on single-cell profiling of human HSPCs [32] were compiled and presented as: (A) Relative number of the three clusters of lymphoid progenitors (LyP1-3) in each adult tissue. (B) Bubble plot illustrates the relative expression of genes that are associated with myeloid, DC and lymphoid lineages. (C) Bubble plot illustrates the relative expression of cell surface markers that are associated with hematopoietic stem cells/multipotent progenitors (HSC/MPP) and LyP. BM, bone marrow; PB, peripheral blood; MDP, monocyte/dendritic cell progenitor. (A) was generated based on the normalized cell counts from the Supplemental File 5 of [32]. (B) was generated from ShinyCell web app http://bioinf.stemcells.cam.ac.uk:3838/laurenti/mobPB_HSPCs/. (C) was generated by Ahmed Waraky based on the CITE-seq dataset from [32].
IRF8-expressing lymphoid progenitors also exist during fetal and neonatal life [37, 42]. In a CITE-seq dataset that consists of CD34+ HSPCs from FL, fetal BM and CB (Figure 2E,F) [37], the cellular cluster annotated by researchers as LyP I in ref [37] might represent the fetal counterpart of the adult IRF8-expressing lymphoid progenitor as identified in the adult dataset described above [32] (matching gene and cell surface marker expression profiles, Figure 4A-B). However, in accordance with literature [43, 44], IRF8-expressing lymphoid progenitors appear to switch cell surface expression from CD7 (Figure 4B: fetal LyP I) to CD10 (Figure 3C: adult LyP3) during ontogenetic development, although this remains to be further tested experimentally.
Figure 4. Single-cell profiling of human HSPCs supports the existence of fetal CD123-/loCD127+ lymphoid progenitors.
For illustrative purposes, previously published data of single-cell profiling of human HSPCs [37] were compiled and presented (A) Bubble plot illustrates the relative expression of genes that are associated with myeloid, DC, and lymphoid lineages in selected clusters of fetal HSPCs. (B) Bubble plot illustrates the relative expression of cell surface markers that are associated with hematopoietic stem cells/multipotent progenitors (HSC/MPP) and lymphoid progenitors (LyP). CLP, common lymphoid progenitor; DC, dendritic cell. (A,B) were generated by Tom Putteman based on the CITE-seq dataset from [37].
In addition to CD127, our laboratory groups have observed subtle expression of CD123 (encoded by IL3RA) which is a surrogate marker for IRF8 expression [14, 29] in both fetal [37] and adult [32] IRF8-expressing lymphoid progenitors (Figure 3C and 4B). Hence, CD123-/loCD127+ lymphoid progenitors that have initiated IRF8 expression are present in primary hematopoietic sites and in circulation [32, 37]. Historically, CD123 and CD127 have been used separately as markers to identify respectively human myeloid [8] and lymphoid [45] progenitors within HSPCs. Thus, it may appear counterintuitive that IRF8-expressing CD127+ lymphoid progenitors also express CD123. However, CD123+ GMPs are heterogeneous for other markers of lymphoid priming such as CD62L (L-selectin) [8, 46], and lymphoid potential within human CB GMPs can be enriched by low expression of CD38 [8]. In addition, CD123-/lo GMPs from human adult BM are comprised of a predominant CD33+ population and a minor CD33- population that give rise to pDCs and cDCs via the lymphoid-primed IRF8hi pathway [29]. A recent study also demonstrated that, in the presence of Notch stimulation, IL-3 which signals through CD123 can synergize with soluble tumor necrosis factor to promote in vitro generation of human T cell precursors from CB HSPCs [47]. Thus, CD123 expression does not necessarily restrict precursor cells from harboring lymphoid potential, and rather reflects the expression of IRF8 by CD127+ lymphoid progenitors. Caution is therefore needed in the use of CD123 as lineage-associated cell surface marker.
IRF8-expressing CD123-/loCD127+ lymphoid progenitors are phenotypically similar to CD127+ early lymphoid progenitors (CD127+ ELP: CD34+CD45RA+ITGB7+CD127+) that are present in human fetal and adult BM [43]. Of note, CD127+ ELPs were reported to lack T cell potential because CD127+ ELPs isolated from a xenotransplantation model (NSG mice engrafted with human CB HSPCs) could not generate CD4+CD8+ T cells in a co-culture assay [43]. However, in contrast to these findings and based on scRNA-seq studies of human HSPCs from multiple human tissues, we propose that circulating CD123-/loCD127+ lymphoid progenitors give rise to fetal ILC lineage-specified progenitors [18], as well as to fetal [26] and postnatal [13, 14] TSPs that have T cell potential upon tissue seeding. For human fetal ILC development, both multipotent lymphoid progenitors (LP1 cluster) and their downstream ILC lineage-specified progenitors (LP2 cluster) were identified as CD34+CD127+CD123+ [18]. In comparison to the LP1s, LP2s have increased expression of IRF8, IL3RA and ILC lineage associated genes such as RUNX3, NFIL3, CD7 and IL7R [18]. For human fetal T cell development, intrathymic TSPs were identified to be transcriptionally similar to CD127+ FL lymphoid progenitors but with increased expression of IRF8 and IL3RA [26]. For human postnatal T cell development, integrated analyses of scRNA-seq datasets from CD34+ thymocytes and immature PBMCs identified transcriptionally similar circulating counterparts of thymic TSP2s that are both Notch-primed and express IRF8 [13], likely corresponding to the circulating CD123-/loCD127+ lymphoid progenitors. However, human postnatal IRF8-expressing CD123+CD127+ thymic TSP2s had similar expression of Notch target genes (CD7 and CD3E) compared to ETPs which require intrathymic Notch signaling for their development (Figure 5A,B) [13]. Hence, upon seeding the thymus, circulating CD123-/loCD127+ lymphoid progenitors might receive further Notch stimulation to give rise to thymic TSP2s. Consistent with literature showing that Notch signaling upregulates IRF8 expression [14] and promotes DC development [48, 49], human postnatal thymic TSP2s could generate CD7+CD5+ ETPs, HLA-DR+CD1c+ cDCs and HLA-DR+CD123+ pDCs in co-culture assays [14]. Also, endothelial cells in human FL express the Notch ligand DLL4 [50]. Increased expression of IRF8 during fetal ILC lineage specification is likely to be driven by intrahepatic Notch signaling, which is relevant because the Notch target TCF7 [51], which is a key factor for murine ILC development [40], is poorly expressed during this stage of human ILC development. However, this remains to be tested. Based on scRNA-seq and CITE-seq analyses, human adult spleen was also shown to harbor IRF8-expressing CD123+CD127+ lymphoid progenitors (LyP3 cells, Figure 5C-E) [32]. Although ILCs [52] and DCs [53] are present in human adult spleen, the contribution of these CD123+CD127+ lymphoid progenitors to intrasplenic ILC or DC development remains to be experimentally addressed.
Figure 5. Single-cell profiling of human postnatal immature thymocytes and adult splenic HSPCs suggests the existence of tissue-seeding CD123+CD127+ lymphoid progenitors.
For illustrative purposes, previously published data of single-cell profiling of human postnatal immature thymocytes [13] and adult splenic HSPCs [32] were compiled and presented as: (A) the schematic illustrates the developmental relationship between thymus seeding progenitor (TSP) subset 1, TSP2, early T cell progenitor (ETP) and IRF8hi-expressing granulocyte/macrophage progenitors (GMP). (B) Bubble plot illustrates the relative expression of genes that are associated with myeloid, DC, and lymphoid lineages in (A). (C) The relative number of the three clusters of lymphoid progenitors (LyP1-3) in adult spleen are shown. (D) Bubble plot illustrates the relative expression of genes associated with myeloid, DC, and lymphoid lineages in selected clusters from adult splenic HSPCs. (E) Bubble plot illustrates the relative expression of cell surface markers associated with hematopoietic stem cells/multipotent progenitors (HSC/MPP) and LyP. MDP, monocyte/dendritic cell progenitor. (B) was generated by Tom Putteman based on the scRNA-seq dataset from [13]. (C) was generated based on the normalized cell counts from the Supplemental File 5 of [32]. (D) was generated by Hugo P. Bastos based on the scRNA-seq dataset from [32]. (E) was generated by Ahmed Waraky based on the CITE-seq dataset from [32].
In contrast to the human DC-lineage differentiation trajectory where IRF8 expression gradually increases [29, 31], IRF8 expression is tightly regulated during the distinct stages of human lymphoid differentiation [14, 18]. For human fetal ILC development, one scRNA-seq study showed that IRF8 expression was upregulated during ILC lineage specification but subsequently silenced in ILC lineage-committed progenitors [18]. With regards to human postnatal T cell development, gene perturbation experiments and co-culture assays with genetically modified cells demonstrated that IRF8 expression is silenced by the T-lineage molecular driver GATA3 to allow completion of T cell lineage commitment [14]. Finally, scRNA-seq of human fetal HSPCs also revealed expression of IRF8 in B lineage-associated progenitors (Figure 2E,F) [37]. Altogether, from the studies discussed above, we propose a model (Figure 1) in which IRF8 expression in human circulating CD123+CD127+ lymphoid progenitors initially supports balanced maintenance of the DC/B/T/ILC lineage programs and in which tissue-specific microenvironmental cues, for example by Notch signaling [14] or lineage-specific transcription factors such as GATA3 [14], subsequently further modulate IRF8 expression in tissue-resident CD123+CD127+ lymphoid progenitors to tilt the molecular balance towards one particular cell fate.
Implication of CD123+CD127+ human lymphoid progenitors
HSC transplantation is a curative treatment for blood cancers and certain immune disorders such as severe combined immunodeficiency. The procedure depends on the ability to collect sufficient HSPCs from HLA-matching donors. Of note, G-CSF mobilized PB has largely replaced BM as the primary HSPC source in adults because pharmacological mobilization of HSPCs from BM to PB allows circulating HSPCs to be collected by leukapheresis which is much less invasive than BM aspiration. HSPCs derived from G-CSF mobilized PB also have faster engraftment kinetics for neutrophils and platelets compared to BM [54]. However, the impact of the different sources of HSPCs on the kinetics of lymphoid reconstitution is less clear. Given the pivotal role of G-CSF in granulopoiesis [55], G-CSF stimulation may alter the composition and/or lineage programming of HSPCs and lead to mobilization of specific HSPC populations from the BM. Indeed, scRNA-seq analyses of human adult BM from healthy donors before and after G-CSF treatment revealed that G-CSF stimulation expanded the GMP cluster and led to a proportional decrease in the lymphoid cluster [56]. This suggests that patients transplanted with G-CSF mobilized PB HSPCs may receive fewer lymphoid progenitors than those receiving BM HSPCs. Using non-conditioned NBSGW mice as a transplantation model, human HSPCs from adult G-CSF-mobilized PB were demonstrated to give rise to more neutrophils at the expense of B cells compared to the output of transplanted adult BM HSPCs [57]. Accordingly, human extramedullary HSC/MPPs (from steady-state PB, G-CSF mobilized PB or spleen) shared a transcriptional identity with features of activation and lineage priming, compared to BM HSC/MPPs [32]. G-CSF mobilized PB HSC/MPPs specifically expressed higher amounts of genes that were linked to neutrophil function and IFN signaling [32]. In addition, CD123+CD127+ lymphoid progenitors were relatively less abundant in G-CSF mobilized PB compared with steady state PB (Figure 3A) [32]. Given the putative role of these lymphoid progenitors in ILC [18] and T cell [13, 14, 26] development, we propose that using a HSPCs source that maximizes the presence of CD123+CD127+ lymphoid progenitors might help to improve the kinetics of lymphoid reconstitution.
Of note, Plerixafor is a CXCR4 inhibitor used clinically in combination with G-CSF to improve HSPC mobilization in patients whose HSPC mobilization post-G-CSF treatment is likely to be poor or which may pose a high risk for certain patients [58]. This agent induces mobilization of a distinct subset of HSPCs compared to G-CSF [59–62]. One report showed that human G-CSF/Plerixafor-mobilized PB HSPCs harbored more CD127+ progenitors and generated more ILCs in vitro than G-CSF mobilized PB HSPCs. This is of clinical interest because it implies that G-CSF/Plerixafor-mobilized PB HSPCs could improve the kinetics of ILC reconstitution upon transplantation [59]. In addition, plerixafor has been reported to preferentially mobilize CD34+CD45RA+CD123+ progenitors that express IRF8 and lack myeloid and erythroid potential in a methylcellulose colony-forming unit assay [60]. Recently, scRNA-seq was used to characterize human adult peripheral HSPCs from healthy donors that were mobilized with G-CSF or CXCR4 inhibitors (plerixafor or motixafortide) [61]. Cellular clusters annotated as common lymphoid progenitors and monocyte/DC progenitors were significantly more abundant in HSPCs mobilized with CXCR4 inhibitors than in HSPCs mobilized with G-CSF [61]. Overall, these studies suggested that CXCR4 inhibitors, compared to G-CSF, could more efficiently mobilize lymphoid progenitors with features of CD123+CD127+ lymphoid progenitors than G-CSF. However, future work is needed to experimentally address whether these HSPC mobilization protocols, impact the kinetics of lymphoid reconstitution for therapeutic benefit.
Concluding remarks
Various progenitor subsets with different cell surface phenotypes and distinct lymphoid or combined lymphoid and myeloid potential have been described in various hematopoietic tissues. The lack of consistency in phenotypic markers and experimental models that were used to test their differentiation capacity has made it difficult to understand the relationship between all these precursor subsets. Recent advances including single cell genomics now render it more feasible to compare cells across tissues. Here, we propose that IRF8-expressing CD123+CD127+ lymphoid precursors act as circulating precursors that seed various hematopoietic tissues across human development where they ultimately differentiate into lineage-restricted cells under the influence of the local microenvironment. Understanding the functional role of this cellular population might help to reconcile some of the current knowledge in the field about human lymphoid differentiation across anatomical sites [43, 44, 46, 63–66] (see Outstanding Questions). Furthermore, analysis of the tissue-dependent differentiation of circulating CD123+CD127+ lymphoid progenitors may help to clarify the functional heterogeneity of tissue-resident intermediate precursor subsets that have been identified in the past [13, 16, 26, 33, 67, 68]. In addition, the expression of IRF8 in these precursors [13, 14, 18, 26] and their capacity to differentiate into DCs and particularly pDCs [13, 14] might resolve or further fuel the ongoing debate regarding the origin and function of pDCs as myeloid or lymphoid cells [69–71].
Outstanding questions.
How is the expression of IRF8 initiated during the development of CD123+CD127+ lymphoid progenitors at primary hematopoietic sites? This might allow us to refine the staging where the HSC/MPP compartment exhibits lymphoid lineage restriction.
What is the impact of disease-causing IRF8 mutations on the development of CD123+CD127+ lymphoid progenitors and their subsequent differentiation to the distinct lymphoid lineages? This might explain the widespread immune dysregulation found in patients with IRF8 deficiency.
How different is the protein interactome of IRF8 between circulating and tissue-seeding CD123+CD127+ lymphoid progenitors? This will reveal if and how the formation of distinct IRF8 heterodimers coordinates human DC and lymphoid differentiation.
Can IRF8 drive human fetal ILC lineage specification in which TCF7 is poorly expressed? This could be another unique molecular mechanism that drives lymphoid differentiation in humans compared to mice.
What is the role of IRF8 in human B cell development? This will further clarify the role of IRF8 during human lymphoid lineage priming, specification and commitment.
Can we uniformly define CD123+CD127+ lymphoid progenitors across tissues and track their in vivo lineage cell fate? This will allow us to validate the role of CD123+CD127+ precursors as key initiators of lymphoid differentiation across tissues.
How different are fetal and adult circulating CD123+CD127+ lymphoid progenitors in their contribution to the development of innate and adaptive immune system? This will provide important insights into the ontogeny of human lymphoid cells.
Do splenic CD123+CD127+ lymphoid progenitors actively contribute to lymphoid or DC production in the spleen? This may provide novel insights into the role of the spleen as a hematopoietic organ.
What regulates the physiological mobilization of CD123+CD127+ lymphoid progenitors from bone marrow to peripheral blood? This will guide the development of pharmacological agents that mobilize HSPCs with high abundance of CD123+CD127+ lymphoid progenitors.
Does the presence of CD123+CD127+ lymphoid progenitors in stem cell grafts accelerate lymphoid reconstitution post-transplantation? This will provide a rationale to revise the current HSPC mobilization protocols for therapeutic benefit.
To further substantiate our hypothesis on the role of CD123+CD127+ precursors as key initiators of lymphoid differentiation in various hematopoietic tissues, it will be important to obtain more extensive multi-omic insights on these progenitors at single-cell resolution and to further optimize the current computational tools to improve the integration of various datasets from different tissues across development. While in vivo data will be important to validate our model, xenograft studies in mice may not accurately recapitulate what happens in humans due to species-specific differences. Indeed, IRF8 expression in early T cell development is a distinct human feature [14, 16] and the spectrum of IRF8 dependence in monocytes and DC development is also distinct between mice and humans [29, 72]. Of note, other human lymphoid progenitors that do not express IRF8 have been found in the blood circulation via scRNA-seq analyses (Figure 3B) [13, 32] and our hematopoietic model does not account for the possible contribution of these circulating progenitors to the various hematopoietic tissues. Nevertheless, it will be inspiring to further clarify the role of IRF8 in human lymphopoiesis and to improve our understanding on the development and differentiation of circulating and tissue-resident human lymphoid progenitors. It will be particularly intriguing to delineate the precise tissue-specific molecular mechanisms that dictate their downstream lineage cell fate using bioinformatics tools such as CellPhoneDB [73] and Nichenet [74], in addition to spatial transcriptomics. In conclusion, our perspective on human lymphopoiesis lays the framework for future studies on circulating and tissue-resident IRF8-expressing CD123+CD127+ lymphoid progenitors. Bridging this gap in our knowledge in human hematopoiesis will certainly translate into a better understanding of a variety of diseases and improvements of stem cell transplantation and emerging cell therapies.
Significance.
The hematopoietic model proposed here highlights an underappreciated role for IRF8 in unlocking human DC and B/T/ILC potential in CD123+CD127+ lymphoid progenitors. Understanding the development and differentiation of CD123+CD127+ lymphoid progenitors across tissues has important basic and translational implications.
Highlights.
Several single cell profiling studies of human HSPCs indicate the existence of circulating CD123+CD127+ lymphoid progenitors that express IRF8 and that are DC- and lymphoid (B/T/ILC)-primed transcriptionally.
IRF8-expressing CD123+CD127+ lymphoid progenitors are also found in tissues that support ILC, DC and T cell development, suggesting that downstream lineage specification and commitment are driven by the specific tissue microenvironment.
Expression of IRF8 unlocks DC lineage potential in these human lymphoid progenitors but is differentially regulated in downstream steps of DC and B/T/ILC differentiation. Expression of CD123, a surrogate cell surface marker for IRF8, does not preclude hematopoietic progenitors from harboring lymphoid potential.
The abundance of CD123+CD127+ lymphoid progenitors in peripheral blood is affected by stem cell mobilizers. This may impact lymphoid reconstitution following transplantation with mobilized peripheral blood.
Acknowledgments
We apologize to colleagues whose work could not be cited due to space limitations. We thank Ahmed Waraky, Hugo P. Bastos and Tom Putteman for their assistance in making the figures. Work in the Taghon laboratory on this topic is funded by the Ghent University (Bijzonder Onderzoeksfonds), the Chan-Zuckerberg Initiative, the Fund for Scientific Research Flanders (FWO) and the Foundation Against Cancer (STK). E.L. is supported by a Sir Henry Dale Fellowship from Wellcome/Royal Society (107630/Z/15/Z). Generation of the data reanalysed here in E.L.’s laboratory is/was supported by Wellcome (107630/Z/15/Z), BBSRC (BB/P002293/1) and by core support grants by Wellcome and MRC to the Wellcome-MRC Cambridge Stem Cell Institute (203151/Z/16/Z). This research was funded in whole, or in part, by the Wellcome Trust. For the purpose of open access, the author has applied for a Creative Commons Attribution (CC BY) licence to any Author Accepted Manuscript version arising from this submission.
Footnotes
Declaration of interests
No interests are declared.
References
- 1.Jacobsen SEW, Nerlov C. Haematopoiesis in the era of advanced single-cell technologies. Nat Cell Biol. 2019;21:2–8. doi: 10.1038/s41556-018-0227-8. [DOI] [PubMed] [Google Scholar]
- 2.Laurenti E, Gottgens B. From haematopoietic stem cells to complex differentiation landscapes. Nature. 2018;553:418–426. doi: 10.1038/nature25022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Velten L, et al. Human haematopoietic stem cell lineage commitment is a continuous process. Nat Cell Biol. 2017;19:271–281. doi: 10.1038/ncb3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anjos-Afonso F, et al. Single cell analyses identify a highly regenerative and homogenous human CD34+ hematopoietic stem cell population. Nat Commun. 2022;13:2048. doi: 10.1038/s41467-022-29675-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranzoni AM, et al. Integrative Single-Cell RNA-Seq and ATAC-Seq Analysis of Human Developmental Hematopoiesis. Cell Stem Cell. 2021;28:472–487.:e477. doi: 10.1016/j.stem.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belluschi S, et al. Myelo-lymphoid lineage restriction occurs in the human haematopoietic stem cell compartment before lymphoid-primed multipotent progenitors. Nat Commun. 2018;9:4100. doi: 10.1038/s41467-018-06442-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buenrostro JD, et al. Integrated Single-Cell Analysis Maps the Continuous Regulatory Landscape of Human Hematopoietic Differentiation. Cell. 2018;173:1535–1548.:e1516. doi: 10.1016/j.cell.2018.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karamitros D, et al. Single-cell analysis reveals the continuum of human lympho-myeloid progenitor cells. Nat Immunol. 2018;19:85–97. doi: 10.1038/s41590-017-0001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doulatov S, et al. Revised map of the human progenitor hierarchy shows the origin of macrophages and dendritic cells in early lymphoid development. Nat Immunol. 2010;11:585–593. doi: 10.1038/ni.1889. [DOI] [PubMed] [Google Scholar]
- 10.Bueno C, et al. CD34+CD19-CD22+ B-cell progenitors may underlie phenotypic escape in patients treated with CD19-directed therapies. Blood. 2022;140:38–44. doi: 10.1182/blood.2021014840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Byrne S, et al. Discovery of a CD10-negative B-progenitor in human fetal life identifies unique ontogeny-related developmental programs. Blood. 2019;134:1059–1071. doi: 10.1182/blood.2019001289. [DOI] [PubMed] [Google Scholar]
- 12.Chen ELY, et al. RBPJ-dependent Notch signaling initiates the T cell program in a subset of thymus-seeding progenitors. Nat Immunol. 2019;20:1456–1468. doi: 10.1038/s41590-019-0518-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavaert M, et al. Integrated scRNA-Seq Identifies Human Postnatal Thymus Seeding Progenitors and Regulatory Dynamics of Differentiating Immature Thymocytes. Immunity. 2020;52:1088–1104.:e1086. doi: 10.1016/j.immuni.2020.03.019. [DOI] [PubMed] [Google Scholar]
- 14.Liang KL, et al. Intrathymic dendritic cell-biased precursors promote human T cell lineage specification through IRF8-driven transmembrane TNF. Nat Immunol. 2023;24:474–486. doi: 10.1038/s41590-022-01417-6. [DOI] [PubMed] [Google Scholar]
- 15.Van de Walle I, et al. GATA3 induces human T-cell commitment by restraining Notch activity and repressing NK-cell fate. Nat Commun. 2016;7:11171. doi: 10.1038/ncomms11171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le J, et al. Single-Cell RNA-Seq Mapping of Human Thymopoiesis Reveals Lineage Specification Trajectories and a Commitment Spectrum in T Cell Development. Immunity. 2020;52:1105–1118.:e1109. doi: 10.1016/j.immuni.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim AI, et al. Systemic Human ILC Precursors Provide a Substrate for Tissue ILC Differentiation. Cell. 2017;168:1086–1100.:e1010. doi: 10.1016/j.cell.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 18.Liu C, et al. Delineating spatiotemporal and hierarchical development of human fetal innate lymphoid cells. Cell Res. 2021;31:1106–1122. doi: 10.1038/s41422-021-00529-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suo C, et al. Dandelion uses the single-cell adaptive immune receptor repertoire to explore lymphocyte developmental origins. Nat Biotechnol. 2023 doi: 10.1038/s41587-023-01734-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dik WA, et al. New insights on human T cell development by quantitative T cell receptor gene rearrangement studies and gene expression profiling. J Exp Med. 2005;201:1715–1723. doi: 10.1084/jem.20042524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Themeli M, et al. iPSC-Based Modeling of RAG2 Severe Combined Immunodeficiency Reveals Multiple T Cell Developmental Arrests. Stem Cell Reports. 2020;14:300–311. doi: 10.1016/j.stemcr.2019.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Becker AM, et al. IRF-8 extinguishes neutrophil production and promotes dendritic cell lineage commitment in both myeloid and lymphoid mouse progenitors. Blood. 2012;119:2003–2012. doi: 10.1182/blood-2011-06-364976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurotaki D, et al. IRF8 inhibits C/EBPalpha activity to restrain mononuclear phagocyte progenitors from differentiating into neutrophils. Nat Commun. 2014;5:4978. doi: 10.1038/ncomms5978. [DOI] [PubMed] [Google Scholar]
- 24.Giladi A, et al. Single-cell characterization of haematopoietic progenitors and their trajectories in homeostasis and perturbed haematopoiesis. Nat Cell Biol. 2018;20:836–846. doi: 10.1038/s41556-018-0121-4. [DOI] [PubMed] [Google Scholar]
- 25.Murakami K, et al. A RUNX-CBFbeta-driven enhancer directs the Irf8 dose-dependent lineage choice between DCs and monocytes. Nat Immunol. 2021;22:301–311. doi: 10.1038/s41590-021-00871-y. [DOI] [PubMed] [Google Scholar]
- 26.Zeng Y, et al. Single-Cell RNA Sequencing Resolves Spatiotemporal Development of Pre-thymic Lymphoid Progenitors and Thymus Organogenesis in Human Embryos. Immunity. 2019;51:930–948.:e936. doi: 10.1016/j.immuni.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Bigley V, et al. Biallelic interferon regulatory factor 8 mutation: A complex immunodeficiency syndrome with dendritic cell deficiency, monocytopenia, and immune dysregulation. J Allergy Clin Immunol. 2018;141:2234–2248. doi: 10.1016/j.jaci.2017.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hambleton S, et al. IRF8 mutations and human dendritic-cell immunodeficiency. N Engl J Med. 2011;365:127–138. doi: 10.1056/NEJMoa1100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cytlak U, et al. Differential IRF8 Transcription Factor Requirement Defines Two Pathways of Dendritic Cell Development in Humans. Immunity. 2020;53:353–370.:e358. doi: 10.1016/j.immuni.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Helft J, et al. Dendritic Cell Lineage Potential in Human Early Hematopoietic Progenitors. Cell Rep. 2017;20:529–537. doi: 10.1016/j.celrep.2017.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J, et al. Lineage specification of human dendritic cells is marked by IRF8 expression in hematopoietic stem cells and multipotent progenitors. Nat Immunol. 2017;18:877–888. doi: 10.1038/ni.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mende N, et al. Unique molecular and functional features of extramedullary hematopoietic stem and progenitor cell reservoirs in humans. Blood. 2022;139:3387–3401. doi: 10.1182/blood.2021013450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cordes M, et al. Single-cell immune profiling reveals thymus-seeding populations, T cell commitment, and multilineage development in the human thymus. Sci Immunol. 2022;7:eade0182. doi: 10.1126/sciimmunol.ade0182. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, et al. IRF8 regulates B-cell lineage specification, commitment, and differentiation. Blood. 2008;112:4028–4038. doi: 10.1182/blood-2008-01-129049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiu Z, et al. Generation and characterization of the Emicro-Irf8 mouse model. Cancer Genet. 2020;245:6–16. doi: 10.1016/j.cancergen.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, et al. A reporter mouse reveals lineage-specific and heterogeneous expression of IRF8 during lymphoid and myeloid cell differentiation. J Immunol. 2014;193:1766–1777. doi: 10.4049/jimmunol.1301939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jardine L, et al. Blood and immune development in human fetal bone marrow and Down syndrome. Nature. 2021;598:327–331. doi: 10.1038/s41586-021-03929-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagaharu K, et al. A bifurcation concept for B-lymphoid/plasmacytoid dendritic cells with largely fluctuating transcriptome dynamics. Cell Rep. 2022;40:111260. doi: 10.1016/j.celrep.2022.111260. [DOI] [PubMed] [Google Scholar]
- 39.Dang D, et al. Identification of a novel IRF8 homozygous mutation causing neutrophilia, monocytopenia and fatal infection in a female neonate. Infect Genet Evol. 2021;96:105121. doi: 10.1016/j.meegid.2021.105121. [DOI] [PubMed] [Google Scholar]
- 40.Harly C, et al. The transcription factor TCF-1 enforces commitment to the innate lymphoid cell lineage. Nat Immunol. 2019;20:1150–1160. doi: 10.1038/s41590-019-0445-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moorman HR, et al. IRF8: Mechanism of Action and Health Implications. Cells. 2022;11 doi: 10.3390/cells11172630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sommarin MNE, et al. Single-cell multiomics of human fetal hematopoiesis defines a developmental specific population and a fetal signature. Blood Adv. 2023 doi: 10.1182/bloodadvances.2023009808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alhaj Hussen K, et al. Molecular and Functional Characterization of Lymphoid Progenitor Subsets Reveals a Bipartite Architecture of Human Lymphopoiesis. Immunity. 2017;47:680–696.:e688. doi: 10.1016/j.immuni.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 44.Keita S, et al. Distinct subsets of multi-lymphoid progenitors support ontogeny-related changes in human lymphopoiesis. Cell Rep. 2023;42:112618. doi: 10.1016/j.celrep.2023.112618. [DOI] [PubMed] [Google Scholar]
- 45.Pang WW, et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci U S A. 2011;108:20012–20017. doi: 10.1073/pnas.1116110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kohn LA, et al. Lymphoid priming in human bone marrow begins before expression of CD10 with upregulation of L-selectin. Nat Immunol. 2012;13:963–971. doi: 10.1038/ni.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edgar JM, et al. Multi-objective optimization reveals time- and dose-dependent inflammatory cytokine-mediated regulation of human stem cell derived T-cell development. NPJ Regen Med. 2022;7:11. doi: 10.1038/s41536-022-00210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Balan S, et al. Large-Scale Human Dendritic Cell Differentiation Revealing Notch-Dependent Lineage Bifurcation and Heterogeneity. Cell Rep. 2018;24:1902–1915.:e1906. doi: 10.1016/j.celrep.2018.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kirkling ME, et al. Notch Signaling Facilitates In Vitro Generation of Cross-Presenting Classical Dendritic Cells. Cell Rep. 2018;23:3658–3672.:e3656. doi: 10.1016/j.celrep.2018.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wesley BT, et al. Single-cell atlas of human liver development reveals pathways directing hepatic cell fates. Nat Cell Biol. 2022;24:1487–1498. doi: 10.1038/s41556-022-00989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harly C, et al. A Shared Regulatory Element Controls the Initiation of Tcf7 Expression During Early T Cell and Innate Lymphoid Cell Developments. Front Immunol. 2020;11:470. doi: 10.3389/fimmu.2020.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Audia S, et al. Altered distribution and function of splenic innate lymphoid cells in adult chronic immune thrombocytopenia. J Autoimmun. 2018;93:139–144. doi: 10.1016/j.jaut.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 53.Petvises S, et al. Delineation of a novel dendritic-like subset in human spleen. Cell Mol Immunol. 2016;13:443–450. doi: 10.1038/cmi.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stem Cell Trialists’ Collaborative, G. Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic malignancies: an individual patient data meta-analysis of nine randomized trials. J Clin Oncol. 2005;23:5074–5087. doi: 10.1200/JCO.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mehta HM, Corey SJ. G-CSF, the guardian of granulopoiesis. Semin Immunol. 2021;54:101515. doi: 10.1016/j.smim.2021.101515. [DOI] [PubMed] [Google Scholar]
- 56.You G, et al. Decoding lymphomyeloid divergence and immune hyporesponsiveness in G-CSF-primed human bone marrow by single-cell RNA-seq. Cell Discov. 2022;8:59. doi: 10.1038/s41421-022-00417-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hess NJ, et al. Different Human Immune Lineage Compositions Are Generated in Non-Conditioned NBSGW Mice Depending on HSPC Source. Front Immunol. 2020;11:573406. doi: 10.3389/fimmu.2020.573406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crees ZD, et al. Innovations in hematopoietic stem-cell mobilization: a review of the novel CXCR4 inhibitor motixafortide. Ther Adv Hematol. 2023;14:20406207231174304. doi: 10.1177/20406207231174304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moretta F, et al. The generation of human innate lymphoid cells is influenced by the source of hematopoietic stem cells and by the use of G-CSF. Eur J Immunol. 2016;46:1271–1278. doi: 10.1002/eji.201546079. [DOI] [PubMed] [Google Scholar]
- 60.Schroeder MA, et al. Mobilization of allogeneic peripheral blood stem cell donors with intravenous plerixafor mobilizes a unique graft. Blood. 2017;129:2680–2692. doi: 10.1182/blood-2016-09-739722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crees ZD, et al. Motixafortide and G-CSF to mobilize hematopoietic stem cells for autologous transplantation in multiple myeloma: a randomized phase 3 trial. Nat Med. 2023;29:869–879. doi: 10.1038/s41591-023-02273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lidonnici MR, et al. Plerixafor and G-CSF combination mobilizes hematopoietic stem and progenitors cells with a distinct transcriptional profile and a reduced in vivo homing capacity compared to plerixafor alone. Haematologica. 2017;102:e120–e124. doi: 10.3324/haematol.2016.154740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hao QL, et al. Identification of a novel, human multilymphoid progenitor in cord blood. Blood. 2001;97:3683–3690. doi: 10.1182/blood.v97.12.3683. [DOI] [PubMed] [Google Scholar]
- 64.Haddad R, et al. Molecular characterization of early human T/NK and B-lymphoid progenitor cells in umbilical cord blood. Blood. 2004;104:3918–3926. doi: 10.1182/blood-2004-05-1845. [DOI] [PubMed] [Google Scholar]
- 65.Hoebeke I, et al. T-, B-and NK-lymphoid, but not myeloid cells arise from human CD34(+)CD38(-)CD7(+) common lymphoid progenitors expressing lymphoid-specific genes. Leukemia. 2007;21:311–319. doi: 10.1038/sj.leu.2404488. [DOI] [PubMed] [Google Scholar]
- 66.Six EM, et al. A human postnatal lymphoid progenitor capable of circulating and seeding the thymus. J Exp Med. 2007;204:3085–3093. doi: 10.1084/jem.20071003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hao QL, et al. Human intrathymic lineage commitment is marked by differential CD7 expression: identification of CD7-lympho-myeloid thymic progenitors. Blood. 2008;111:1318–1326. doi: 10.1182/blood-2007-08-106294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haddad R, et al. Dynamics of thymus-colonizing cells during human development. Immunity. 2006;24:217–230. doi: 10.1016/j.immuni.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 69.Ziegler-Heitbrock L, et al. Reclassifying plasmacytoid dendritic cells as innate lymphocytes. Nat Rev Immunol. 2023;23:1–2. doi: 10.1038/s41577-022-00806-0. [DOI] [PubMed] [Google Scholar]
- 70.Reizis B, et al. Reclassification of plasmacytoid dendritic cells as innate lymphocytes is premature. Nat Rev Immunol. 2023;23:336–337. doi: 10.1038/s41577-023-00864-y. [DOI] [PubMed] [Google Scholar]
- 71.Ziegler-Heitbrock L, et al. Reply to ‘Reclassification of plasmacytoid dendritic cells as innate lymphocytes is premature’. Nat Rev Immunol. 2023;23:338–339. doi: 10.1038/s41577-023-00866-w. [DOI] [PubMed] [Google Scholar]
- 72.Collin M. Reading the Ts and DCs of thymopoiesis. Nat Immunol. 2023;24:385–386. doi: 10.1038/s41590-023-01439-8. [DOI] [PubMed] [Google Scholar]
- 73.Efremova M, et al. CellPhoneDB: inferring cell-cell communication from combined expression of multi-subunit ligand-receptor complexes. Nat Protoc. 2020;15:1484–1506. doi: 10.1038/s41596-020-0292-x. [DOI] [PubMed] [Google Scholar]
- 74.Browaeys R, et al. NicheNet: modeling intercellular communication by linking ligands to target genes. Nat Methods. 2020;17:159–162. doi: 10.1038/s41592-019-0667-5. [DOI] [PubMed] [Google Scholar]
- 75.Anjos-Afonso F, Bonnet D. Human CD34+ hematopoietic stem cell hierarchy: how far are we with its delineation at the most primitive level? Blood. 2023 doi: 10.1182/blood.2022018071. [DOI] [PubMed] [Google Scholar]
- 76.Majeti R, et al. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell. 2007;1:635–645. doi: 10.1016/j.stem.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Notta F, et al. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333:218–221. doi: 10.1126/science.1201219. [DOI] [PubMed] [Google Scholar]
- 78.Vanuytsel K, et al. Multi-modal profiling of human fetal liver hematopoietic stem cells reveals the molecular signature of engraftment. Nat Commun. 2022;13:1103. doi: 10.1038/s41467-022-28616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang YW, et al. Hyaluronic acid-GPRC5C signalling promotes dormancy in haematopoietic stem cells. Nat Cell Biol. 2022;24:1038–1048. doi: 10.1038/s41556-022-00931-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Driggers PH, et al. An interferon gamma-regulated protein that binds the interferon-inducible enhancer element of major histocompatibility complex class I genes. Proc Natl Acad Sci U S A. 1990;87:3743–3747. doi: 10.1073/pnas.87.10.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Langlais D, et al. The macrophage IRF8/IRF1 regulome is required for protection against infections and is associated with chronic inflammation. J Exp Med. 2016;213:585–603. doi: 10.1084/jem.20151764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Eisenbeis CF, et al. Pip, a novel IRF family member, is a lymphoid-specific, PU.1-dependent transcriptional activator. Genes Dev. 1995;9:1377–1387. doi: 10.1101/gad.9.11.1377. [DOI] [PubMed] [Google Scholar]
- 83.Glasmacher E, et al. A genomic regulatory element that directs assembly and function of immune-specific AP-1-IRF complexes. Science. 2012;338:975–980. doi: 10.1126/science.1228309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ochiai K, et al. Zinc finger-IRF composite elements bound by Ikaros/IRF4 complexes function as gene repression in plasma cell. Blood Adv. 2018;2:883–894. doi: 10.1182/bloodadvances.2017010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ouyang JF, et al. ShinyCell: simple and sharable visualization of single-cell gene expression data. Bioinformatics. 2021;37:3374–3376. doi: 10.1093/bioinformatics/btab209. [DOI] [PubMed] [Google Scholar]