Abstract
Hypoxia is increasingly recognized as an important physiological driving force. A specific transcriptional program, induced by a decrease in oxygen (O2) availability, for example, inspiratory hypoxia at high altitude, allows cells to adapt to lower O2 and limited energy metabolism. This transcriptional program is partly controlled by and partly independent of hypoxia-inducible factors. Remarkably, this same transcriptional program is stimulated in the brain by extensive motor-cognitive exercise, leading to a relative decrease in O2 supply, compared to the acutely augmented O2 requirement. We have coined the term “functional hypoxia” for this important demand-responsive, relative reduction in O2 availability. Functional hypoxia seems to be critical for enduring adaptation to higher physiological challenge that includes substantial “brain hardware upgrade,” underlying advanced performance. Hypoxia-induced erythropoietin expression in the brain likely plays a decisive role in these processes, which can be imitated by recombinant human erythropoietin treatment. This article review presents hints of how inspiratory O2 manipulations can potentially contribute to enhanced brain function. It thereby provides the ground for exploiting moderate inspiratory plus functional hypoxia to treat individuals with brain disease. Finally, it sketches a planned multistep pilot study in healthy volunteers and first patients, about to start, aiming at improved performance upon motor-cognitive training under inspiratory hypoxia.
Keywords: brain EPO circle, erythropoietin, functional hypoxia, HIF, human pilot study, hyperoxia, inspiratory oxygen manipulations, motor-cognitive performance, PBMC, translation
1. Inspiratory or Functional Hypoxia and the Brain Erythropoietin Circle
Normal neuronal activity depends on adequate tissue oxygenation. Oxygen (O2) homeostasis is determined via a balanced O2 supply and its consumption by mitochondria. Mitochondrial oxidative phosphorylation utilizes molecular O2 as the final electron acceptor to generate adenosine triphosphate (ATP). Hypoxia arises when the cellular O2 demand, required to generate sufficient levels of ATP to enable all physiological requirements, exceeds the available supply. Despite its inherent challenge to homeostasis, encompassing numerous mechanisms, hypoxia is frequently encountered and associated with physiological conditions including fetal development or adaptation towards moderate to high altitude. Known cellular environments requiring or experiencing hypoxia include stem cell niches, seminal tubuli, the renal papilla, inflammatory tissue, or the inner mass of solid tumors. Previously interpreted as principally pathological, for instance, upon cardiac arrest, hypoxia is thus increasingly recognized also as an important physiological driving force.1–15
In 2019, P. J. Ratcliffe, W. G. Kaelin, and G. L. Semenza jointly received the Nobel Prize in Physiology and Medicine for their pivotal discoveries of how mammalian cells sense and adapt to altering O2 availability. A specific transcriptional program, induced by hypoxia, allows cells to acclimate to lower O2 levels and/or to limited metabolic support.1–11,16,17 The transcription is partly controlled by hypoxia-inducible factors (HIFs) binding to hypoxia-responsive elements to modulate gene expression of potent growth factors such as erythropoietin (EPO)10,18–28 and is partly HIFindependent.10,28 Amazingly, this same transcriptional program is induced by extensive motor-cognitive exercise during a complex running wheel task in mice and seems to be fundamental for a lasting adaptation of the brain in general and the hippocampus in particular to increased physiological challenge. We have coined the term “functional hypoxia” for this important prerequisite of physicocognitive, demand-responsive “brain hardware upgrade.”29
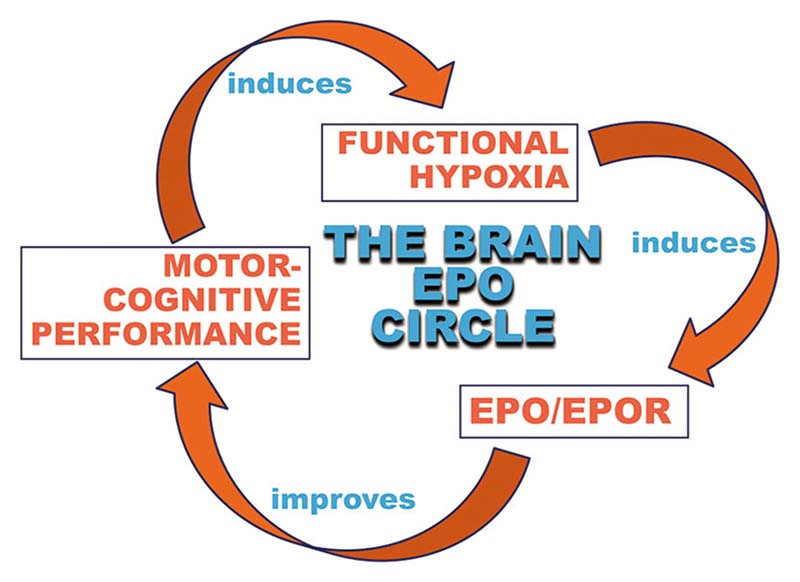
Extensive physical activity and cognitive challenges are known to lead to widespread brain activation, brain volume increases, and improved global brain function ranging from cognition to mood.30–32 We hypothesize that activity-induced, “functional hypoxia” of neurons and brain-expressed EPO play essential roles in all these circumstances in the sense of “brain doping.” Similar effects of a “brain hardware upgrade” are seen upon inspiratory hypoxia.18,29,33–35 In other words, strong motor-cognitive activity leads to neuronal activation and functional hypoxia, inducing HIF stabilization, followed by EPO transcription (among other transcripts) in pyramidal neurons, which in turn grow more dendritic spines and simultaneously stimulate their neighboring cells, ready to become neurons, to differentiate within the hippocampus. This brain hardware upgrade includes an EPO induced ~20% increase in pyramidal neurons and oligodendrocytes in cornu ammonis hippocampi, all occurring in the absence of elevated DNA synthesis.18,36 In parallel to mediating this novel form of swift adult neurogenesis, EPO reduces microglia numbers and dampens their activity and metabolism as prerequisites for undisturbed neuronal differentiation and maturation.34,37 This “brain EPO circle” (Figure 1) contributes to improved brain function including boosted cognition on demand. Application of recombinant human (rh)EPO imitates the effects of brain-expressed EPO, perfectly explaining the strong neuroprotective and procognitive action of this treatment.29 Mechanistically, hypoxia-induced EPO (as much as rhEPO) may also have an impact, for example, on regulating oxidative metabolism and mitochondrial function.38
Figure 1.
The brain EPO circle. Motor-cognitive activity induces functional hypoxia in the brain. A hypoxia-induced transcriptional program in brain cells includes upregulation of the expression of erythropoietin (EPO) and its receptor (EPOR), which in turn lead to improvement in motor-cognitive performance. This circle can essentially be entered anywhere, starting with inspiratory (instead of functional) hypoxia or with recombinant human (rh)EPO treatment (i.e., exogenous EPO)—both ultimately leading to improved motor-cognitive performance as well.
Still unfamiliar with the long-recognized brain-expressed EPO system,39,40 some critics keep wondering why the “blood hormone” EPO should play such a remarkable protective and procognitive role in the brain. In this context, we propose that evolution has extended an existing and very precise O2 sensing system that originally enhanced EPO synthesis for improved motor-cognitive performance, neuroprotection, and neuroregeneration, to increase erythropoiesis in conditions of reduced oxygenation such as blood loss or exposure to altitude. In other words, the original properties of EPO found in species without hematopoiesis have obviously been conserved. Of note, we recently provided evidence that the O2 sensing system (prolyl hydroxylase domain protein, PHD2/HIF-2/EPO axis) is very precise and accurate, allowing detection of subtle O2 alterations occurring already at low to moderate altitude, that is, between 200 and 2000 m above sea level.41 In fact, we observed that every 300 m of altitude increment led to a modest but distinct increase in hemoglobin levels in healthy young men. The question arises as to why humans are equipped with such a precise sensing mechanism to control hematopoiesis. We hypothesize that from an evolutionary point of view, the O2 sensing mechanism did not originally evolve to increase red blood cell production by elevating EPO synthesis. For example, mosquitos that do not produce red blood cells are furnished with HIF pathway components including PHD-1.42 In addition, the fact that EPO-like genes are found in invertebrates suggests that an EPO-like protein evolved about 550 million years ago43,44 and indicates that this O2-dependent EPO pathway has been kept and continued to be modified by evolution. Along these lines, rhEPO induced protection and enhanced regeneration of neuronal cells isolated from grasshoppers.44
2. Exploiting O2 Manipulations for Improving Brain Function: Effects of Hypoxia Versus Hyperoxia
We are just starting to understand the effects of O2 manipulations on brain performance and brain disease, and how they can potentially be exploited for novel, nonpharmacological treatment approaches. There is a huge amount of literature on purposeful O2 manipulations in both sports and the health system. Most papers document the positive or beneficial effects of targeted exposures,45,46 while only a few report on potential negative aspects.47 Humans have been exposed to various kinds of inspiratory hypoxia or hyperoxia, either in a chronic fashion or in a swiftly alternating way, as applicable via face masks. To make it even more complicated, different conditions for applying air pressure changes were introduced and have to be considered. Whereas exposure to high altitude results in hypobaric hypoxia, the so-called hypoxia chambers allow the application of normobaric hypoxia, and various approaches were taken by researchers, among others in the armed forces, to even apply hyperbaric hyperoxia. In this context, multiple variables, such as the duration, the total number of exposures, the kind of training, and the cessation of exposure, descending from high altitude versus simply stepping out of a chamber, have to be taken into account.48–54
From all this work, we learn that the effects and consequences of these variable exposures are obviously diverse, including measured target functions (cognition, motor performance, others), evaluated gene expression levels, for example, from blood cells, or assessed physiological parameters. The data obtained from rodent models or even other species is certainly highly informative, but not always completely translatable to humans, apart from the fact that truly systematic investigations are rare. Drawbacks in published human studies include the highly inconsistent experimental conditions, the small numbers of individuals tested, and the sometimes insufficient exposure time to altered O2 levels or too low degrees of O2 changes, to just name a few.54–57
A whole array of different methods of modulating O2 availability has been described to influence brain functions in health and disease. Of note, potential health benefits may not be immediate consequences of hypoxia or hyperoxia per se, but may be a result of adaptations evoked by these stimuli.58–60 Although several studies demonstrated favorable health effects associated with living at moderate to high altitudes, that is, hypobaric hypoxia, they include other environmental and socioeconomic factors that are difficult to control.61–64 Clearly defined prophylactic or therapeutic effects have been attributed to the use of normobaric hypoxia. In contrast to rather few reports on the application of hypoxia in patients suffering from multiple sclerosis, Parkinson's or Huntington's disease,55,56,65,66 there are several studies suggesting beneficial impacts of hypoxia on cognitive performance, dementia, and Alzheimer's disease.48,67–71 Moreover, hypoxic exposure has been suggested as a potentially effective treatment for human diseases associated with mitochondrial dysfunction.72
Neuroprotection by calibrated hypoxia programs may be fostered by increasing cerebral perfusion and oxygenation, the reduction of cardio- and cerebrovascular risk factors, for example, systemic hypertension, dyslipidemia, and glucose intolerance, the upregulation of neuroprotectants, for example, EPO, vascular endothelial growth factor (VEGF), nitric oxide, and/or antioxidants, but also by suppressing neuronal apoptosis.73–75
Hyperoxia, the rise in inspiratory O2, is never physiological, but represents an artificial, rather pharmacological intervention. It increases brain oxygenation as O2 easily diffuses across the blood–brain barrier and may possess therapeutic potential after, for example, brain injuries.76,77 In fact, the exclusive use of hyperoxia looks back on a long tradition in the clinical therapy of illnesses characterized by hypoxemia.78 For example, normobaric hyperoxia represents a principle measure in the acute management of circulatory shock, especially in trauma patients,79 but is also the application of choice in hypoxemic respiratory failure to be treated in the intensive care unit.80 It has been suggested as a simple and widely accessible therapeutic strategy, associated with improved clinical outcomes in stroke patients.81 Beneficial effects of normobaric hyperoxia for brain protection are considered to primarily result from improvements in brain metabolism and the resistance to cell damage.82,83 Moderate normobaric hyperoxia (10×2h at 37% O2) in healthy volunteers apparently improves cognitive performance and increases blood levels of antioxidant enzymes and neurotrophic factors.84 Advances in normobaric hyperoxia application, particularly derived from animal studies evaluating effects in experimental stroke and brain trauma, have recently been reviewed.85
While normobaric hyperoxia is commonly used in the settings mentioned above, the use of hyperbaric O2 therapy seems to be more effective in other circumstances, for example, acute severe traumatic brain injury,86 or carbon monoxide poisoning.87 Noteworthy in this context, in severe burns, beneficial effects of hyperbaric O2 therapy may include the attenuation of central sensitization.88 Further examples are recent work on hyperbaric O2 therapy that reported alleviation of vascular dysfunction and amyloid burden in an Alzheimer’s disease mouse model and also in elderly patients.89
Similarly, intermittent hypoxic–hyperoxic training in geriatric subjects67 and patients with mild cognitive impairment48 showed beneficial effects on cognition. Intermittent hypoxia or hypoxia–hyperoxia conditioning programs commonly apply multiple exposures to hypoxia (3–8 min, 10%–16% O2), interspersed by brief (2–5 min) exposures to normoxic (21% O2) or hyperoxic (30%–40% O2) conditions; with a total exposure duration of 30–40 min per session; applied at 1- or 2-day intervals over 2–8 weeks.48,67,90–93 Findings from preclinical and clinical experiments provide valuable hints on the neuroprotective potential of such intermittent programs.73,94,95
The combined use of hypoxia and hyperoxia might evoke complementary and therapeutically more favorable effects than either one alone, consistent with sort of a hyperoxic–hypoxic paradox.58,96 This is supported by recent intermediate interventions,48,67 with benefits likely resulting from overlaps of gene transcription programs, for example, HIF (see also below) or an emerging regulator of cellular resistance to oxidants, nuclear factor erythroid 2-related factor 2 (Nrf2), inducing more robust adaptive processes than hypoxia or hyperoxia alone.58,94
Also, upon chronic moderate normobaric hyperoxia (50% O2 for 3 weeks), increased stabilization of the α-subunits of HIF-1 and HIF-2, as well as elevated expression of VEGF and EPO has been reported in the mouse brain,97 pointing to some overlap in downstream mechanisms of hypoxia and hyperoxia, reflected or mediated, for example, by excess reactive oxygen species.96,98,99 Moreover, underlying molecular mechanisms include variable changes in the expression of caspase-3, matrix metalloproteinase-9, aquaporin-4, and Na+/H+ exchanger-1.85,100,101
On the other hand, contrasting effects of hypoxia versus hyperoxia were found, for example, in a mouse model of Friedreich's ataxia where breathing of 11% O2 attenuated the progression of ataxia, whereas breathing 55% O2 hastened it.102 Effects of hypoxia versus hyperoxia on gene expression and functional or structural neuroplasticity have not been studied yet systematically and back-to-back. Pilot data from our laboratories indicate that this will be essential to understand and exploit the underlying mechanisms. In preliminary work, using a transgenic reporter of transient hypoxia (CaMKIIα-CreERT2-ODD::R26R-tdTomato mice, expressing the HIF-1α oxygen-dependent degradation-domain, ODD, fused to CreERT2-recombinase103 for persistent activation of a fluorescent reporter after hypoxia), we surprisingly found a mild increase in red-labeled (tdTomato+) neurons also after exposure to inspiratory hyperoxia (unpublished observations). It is unclear, however, whether this was due to the stabilization of CreERT2-ODD also at high O2 concentration or to a rapidly sensed “relative hypoxia” after cessation of hyperoxia. Therefore, using, for example, sophisticated ultrastructural analyses,104–106 we are presently testing the hypothesis that the intracellular mechanisms of hypoxia and hyperoxia are in part similar, affect functional and structural neuroplasticity, and involve the activation of EPO/EPOR signaling in the brain. In fact, synaptic transmission is highly energy demanding relying on oxidative metabolism.107 Excitability of hippocampal CA1 pyramidal cell synapses, for instance, is dependent on O2 tension.108 Plasticity-associated proteins such as presynaptic calcium channels and active zone scaffolds as well as postsynaptic glutamate receptors are likely candidates to be altered upon hypoxia or hyperoxia. Indeed, structural changes in hippocampal CA1 synapses after hypoxia resemble activity-dependent plasticity, including calcium and NMDA receptor-dependent remodeling of postsynaptic spines and the formation of presynaptic filopodia.109,110 Putative modifiers of structural plasticity may include O2 sensors such as HIF-1/ HIF-297 and PHD2,111 as well as nitric oxide110 and growth factors like brain-derived neurotrophic factor (BDNF)112 and EPO.18,33,97
3. Moderate Inspiratory Hypoxia Plus Motor-Cognitive Training to Treat Patients with Brain Disease: Where we are and Where we are Going
In contrast to acute mountain sickness, usually observed after ≥6 h of exposure to hypobaric hypoxia,113 it has been known for many years from sports medicine and altitude research that short and moderate inspiratory O2 manipulations are well tolerated by human subjects and can lead to improved performance. However, truly systematic studies to understand the effect under strictly defined conditions are widely lacking. In particular, controlled hypoxia studies in patients are still rare and highly heterogeneous in terms of type (O2 concentration under normo-, hypo- or hyperbaric conditions), application (hypoxia chamber, generators with masks), and respective duration of inspiratory O2 manipulations, and usually include only a few subjects and often no proper controls. In addition, there is frequently a lack of monitoring and follow-up with convincing parameters/ biomarkers of success. Some studies show that EPO is elevated in blood under these conditions.114,115 This is also in agreement with our own studies in mice, which, moreover, show that a significant increase in EPO expression in the brain can be expected even with short exposure to hypoxia for a few hours.33,34
We are presently preparing for systematic studies to investigate the effect of inspiratory hypoxia on human motor-cognitive performance. In parallel, we aim to carefully examine human blood cells for their hypoxia response. In fact, HIF-dependent changes in metabolism profoundly affect the phenotype and function of immune cells.116 A new fluorescent antibody cell sorting device with thus far unique possibilities to characterize and isolate human blood cell subpopulations is now up and running in one of our laboratories (HE; BD FACSymphony™ S6 cell sorter with 7 lasers). Blood cell studies include the determination of relative numbers and cell types, but also single-cell or nuclei transcriptome analyses. These in turn will help select transcripts that might be useful for response prediction. To achieve this goal, translational approaches from rodents to humans are planned. Healthy wild-type mice will—analogously to humans (see below)—either be exposed for 3 weeks to daily 3.5 h of hypoxia (90 min down from 16% to 12%; then 2 h at 12%) in a rodent hypoxia chamber or serve as normoxia controls. Immediately after the last exposure, brain (cortex, hippocampus, cerebellum, brainstem) and peripheral blood mononuclear cells (PBMCs) will be prepared for single-cell/nuclei RNA-sequencing as described.18,33 This will allow the identification of PBMC transcripts in mice that correlate with the observed response of brain regions/cell types to hypoxia. We expect sufficient overlap of intraspecies PBMC and brain transcripts117 to approach interspecies analyses next. Identified PBMC markers that correspond to the brain hypoxia response in mice will be used to infer the human brain response from human hypoxia-induced PBMC gene expression. Trans-species analogously hypoxia-stimulated transcripts in the brain (mouse) and PBMC (mouse and human) will be identified in health, and later exploited for disease. Novel bioinformatic tools of small conditional RNA-sequencing data analyses will—based on these data—allow the generation of various prediction models of a beneficial (e.g., procognitive, regenerative, remyelinating, or anti-inflammatory) response also in humans to moderate hypoxia.118–122 This will also include effects on energy metabolism,116 which is part of the glial support of neuronal network functions.123,124 Astrocytes are a local source of lactate for glutamatergic synapses,125 and myelinating oligodendrocytes have a similar function when metabolically supporting spiking axons with lactate/pyruvate.126–128 Thus, the bulk of neuronal ATP production in the brain is mitochondrial oxidative phosphorylation, whereas the lactate-producing glial cells operate at least in part by aerobic glycolysis.126,129 The major function of HIF is the adjustment of the organism to hypoxia, which includes the upregulation of genes for glucose import and glycolysis. In cancer cells which—similar to glial cells —switch to aerobic glycolysis (Warburg effect), the hypoxia-induced enzyme pyruvate kinase M2 even serves as a feedforward transcriptional coactivator for HIF-1 expression/stabilization.130 Thus, with respect to the cellular energy balance under functional hypoxia, the critical question arises whether a possible dampening effect on mitochondrial respiration is outweighed by an upregulation of glycolytic ATP and lactate production. Interestingly, combined functional magnetic resonance imaging and 15O positron emission tomography (PET) imaging studies in humans showed, for the visual system, that the neurovascular responses to neuronal activity are not closely coupled to the actual decreases of tissue oxygenation and O2 requirements.131,132 Thus, it is plausible that functional hypoxia at the cellular level can increase glucose consumption and overall energy production in a HIF-dependent fashion well before a lack of O2 puts a break on overall ATP production. Here, the influence of hypoxia-induced EPO on mitochondrial function may play a crucial role.38
To test mild inspiratory hypoxia together with functional hypoxia, induced by physicocognitive exercise, as a synergistic approach in humans, hypoxia training chambers (HÖHENBALANCE GmbH, Going, Austria) with floor areas of approximately 16–20 m2 have been installed in our institutes, that is, thus far the MPI-NAT outpatient research clinic (HE) and the Department of Psychology, University of Copenhagen (KM). The chambers are equipped with computer desks, set up for cognitive training (Happyneuron, Humansmatter, Lyon, France), as well as bicycle ergometer and treadmill (h/p/cosmos sports & medical GmbH, Nussdorf-Traunstein, Germany). The physical training devices will have large screens in the front that can be used to apply entertaining movies, keeping test subjects stimulated for workouts, or even cognitively challenging online tests, thus allowing simultaneous motor-cognitive challenges. Comprehensive online data monitoring will be professionally performed and supervised to allow wide-ranging structured analyses (Datico Sport & Health GmbH. Burghausen, Germany).
4. Pilot Study on Motor-Cognitive Training Under Inspiratory Hypoxia about to Start: Multistage Procedure Planned
Due to the still exploratory nature of the planned project, the following pilot study is launched, which builds on two decades of own basic and clinical EPO (H. E., M. G., A. L.-S., K.-A. N., and K. M.) and high-altitude research (M. G. and M. B.) and with which first own experience with the hypoxia chamber can be acquired in a multistep procedure:
-
(1)
In the first step, the researchers themselves will— as healthy volunteers—test the altitude training chamber, and initially experience different O2 concentrations for different periods of time up to several hours daily. At the same time, the O2 saturation in the blood is closely monitored using pulse oximeters. After convenient adaptation to the reduced O2 concentration in the atmosphere, the first physical and cognitive training sessions under hypoxia are offered. For this purpose, the training devices (ergometer, treadmill) can be combined with neuropsychological tasks that are communicated via screens. Most importantly, however, probands can choose to sit at a computer desk for intensive cognitive training. This first step aims at free exploration and has no fixed schedule. Subjects are just encouraged for an optimal outcome to practice using all three alternative options as much as they can. The observations will then feed into step 2.
-
(2)
In the next step, 8–10 healthy volunteers will, after adequate habituation, be exposed to 3.5 h of hypoxia daily over the course of 3 weeks. The researchers assume that healthy adult subjects will show improvements in cognition and performance as active participants in the training chamber. The subjects enter the normobaric chamber, which is stably set to 16% O2. Then, the O2 is slowly reduced within 90 min and replaced by nitrogen until 12% O2 is reached. The duration of this habituation phase is anticipated to last from days 1 to 3 of the 3-week experiment, with some expected inter-individual differences in convenience and speed of adaptation. This has to be tested individually and optimal conditions will have to be compiled. Ultimately, probands should enter the chamber at 16%, experience a decrease to 12% within 90 min, and keep training under 12% O2, which continues for 2 h daily over 3 weeks. Its intensity, that is, the frequency of ergometer or treadmill use, can be decided by each participant during this exploration phase of the pilot study, that is, is left up to the individual and should be done according to motivation and well-being, but will be recorded very precisely. Later, a standardized plan will be derived from this information.
-
(3)
In case of success, that is, hints of the expected intraindividual improvement of cognition and motor performance of volunteers, and good tolerability, first patients suffering from multiple sclerosis, chronic schizophrenia, or autism will be allowed to undergo a similar procedure in the sense of compassionate use approaches after intensive informed consent. Patients are already waiting to participate (approximately 8–10 subjects per diagnosis will ultimately be recruited). Exclusion criteria include, for example, heart or lung disease and epilepsy.
-
(4)
In case of a positive outcome of this pilot study, that is, a measurable intraindividual improvement in function, selected parameters will be specifically and systematically changed in a series of subsequent steps. These include, for example, the exposure time: Can it be shorter? The training mode: How much training is most helpful? Follow-up examinations of cognition and motor function (as well as blood cells) over a few weeks to months after completion of the 3-week hypoxia training are intended, too.
Throughout the entire period of the pilot study, the researchers will be consulted and guided by an advisory board of experts (altitude researchers, sports physicians, cardiologists) providing advice and exchange. Interdisciplinary and international cooperations (e.g., with colleagues in Harvard, New York, Innsbruck, Zürich, Cambridge, Oxford, etc.) are already being initiated. The first publishable results are expected in about 1–2 years after the start. Based on this preliminary work, the ERC Consolidator Project ALTIBRAIN (awarded to K. M., with HE as the collaborator) will start soon. In parallel studies in healthy individuals, patients with affective disorders and mice, we will investigate whether intermittent moderate hypoxia (12%) combined with motor-cognitive training over 3 weeks is sufficient to enduringly increase cognitive performance and hippocampal volume and function, as well as the maturation of neural progenitor cells and dendritic spines. This will be measured (1) in humans with PET, using synaptic vesicle glycoprotein 2A UCB-J binding to reflect dendritic spine density, and (2) in mice (which also allows determining the induced expression of brain EPO) with immunohistochemistry, for example, Golgi staining and Light sheet microscopy. The findings can lead to a breakthrough in the understanding of mechanisms underlying enduring neuroplasticity and to novel treatment strategies targeting cognitive decline.
Moreover, our planned “high-altitude” training of autistic children and their parents in our hypoxia chamber will commence in due course (L. P. with H. E.). This is a worldwide unique pilot study in a frequent condition, challenging diagnostic competences as well as reliability of follow-up measures,133,134 and thus far lacking biology-based treatments.135 We hypothesize that intensive motor-cognitive training within a defined social setting (family condition) that consists of 3 h daily for 3 weeks in a hypoxia training chamber (12% O2; conditions comparable as described above for adults) will lastingly improve autistic symptoms and motor performance as well as improve cognitive dysfunction in children diagnosed with autism and/or intellectual disability. Among the principal mediators of the beneficial hypoxia effects, we see, in particular, the hypoxia-induced brain EPO system. We expect that this early intervention in a period of still the highest brain plasticity will enable enduring amelioration or substantial corrections of developmental trajectories at risk. This requires developmental alterations to be partly reversible, which was actually confirmed in models of syndromic ASD, that is, Syngap1 haploinsufficiency and Rett syndrome,136,137 as well as in early intervention studies.138 Parents (as concurrently challenged adults in the chamber) will show profits for their own cognition and general performance. Moreover, we expect advantageous effects also for the parent–child interaction and synchrony as another central feature of favorable outcome and prognosis. We anticipate that the beneficial outcome will overall exceed that of identical training conditions under normoxia.
Based on these studies, it will eventually be possible to initiate the planning of larger trials, which will then have realistic chances of receiving appropriate financial support from public funds. If successful, requests for follow-up studies will emerge quickly due to the considerable worldwide need for disease-modifying therapies for brain diseases. Since most likely, the pharmaceutical industry may not be too much interested in nonpharmacological approaches, new financing strategies and ideas will have to be pursued. Only dissemination and potential economic exploitation will allow broader accessibility of these nonpharmacological treatments to the healthcare system and general public. At the same time, effective replication studies and multicentric endeavors for scientific consolidation of treatment success demand standardized and highly comparable methodological approaches. It may be worthwhile even considering franchising as a means of expanding and protecting successful protocols.
The various expected positive impacts of inspiratory and functional hypoxia on brain function but also other physiological parameters might deliver a broad variety of potential health benefits. Setting the scene for applications via structured and scientifically assessed trials using modern methodologies of digital data processing and sophisticated laboratory testing can improve the convergence of scientific results also into economically attractive treatment protocols as prerequisite of translatability into the real world. Differentiated knowledge regarding hypoxia effects on human performance may lead to more effective treatment procedures. Even after acute events like, for example, stroke, with the necessity of rapid invasive pharmacology, subsequent hypoxia training may prove efficient for recovery, as well as cost-saving with lower side effects. Respective therapeutic offers might later be aligned with health, vacation, or rehabilitation centers, for instance, in the mountains or in favorable seawater environments. In this sense, also prevention could gain increasing significance.
Highlights.
This review focuses on the brain and sketches hypoxia as a physiological driving force, inducing specific transcriptional programs.
Moderate inspiratory hypoxia may improve brain function and performance.
Our concept of “functional hypoxia” is introduced as a demand-responsive mediator of “brain hardware upgrade” on extensive motor-cognitive exercise.
Hypoxia-induced erythropoietin (EPO) expression in the brain plays a decisive role in these processes, constituting what we coined the “brain EPO circle.”
The brain EPO circle underlies the adaptation to challenge, that is, enhanced brain function including cognition and accomplishment on demand: “Brain doping.”
Recombinant human EPO imitates the effects of brain-expressed EPO, explaining the strong neuroprotective and procognitive impact of this treatment.
A pilot study in healthy volunteers and first patients is outlined, exploiting moderate inspiratory plus functional hypoxia in humans for improving cognition.
Acknowledgments
This work has been supported by the Max Planck Society, the Max Planck Förderstiftung, the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), via DFG-Center for Nanoscale Microscopy and Molecular Physiology of the Brain (CNMPB), and DFG-TRR 274/1 2020—408885537. M. G. acknowledges the Swiss National Science Foundation for the support of this project. In 2022, H. E. received a European Research Council (ERC) Advanced Grant under the European Union's Horizon Europe research and innovation programme (BREPOCI; grant agreement no. 101054369) and K. M. an ERC Consolidator Grant (ALTIBRAIN). Moreover, H. E. holds at present a grant from Roche Pharma AG, Grenzach-Wyhlen, enabling the continuation and extension of the GRAS (Göttingen Research Association for Schizophrenia) Data Collection in direction of Multiple Sclerosis (GRAMS, Göttingen Research Association for MS). The GRAMS project has also received support through a donation from Merck Serono GmbH.
Funding information
This work has been supported by the Max Planck Society, the Max Planck Förderstiftung, the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), via DFG-Center for Nanoscale Microscopy & Molecular Physiology of the Brain (CNMPB) and DFG-TRR 274/1 2020 – 408885537. MG acknowledges the Swiss National Science Foundation for support of this project. In 2022, HE received an European Research Council (ERC) Advanced Grant under the European Union’s Horizon Europe research and innovation programme (BREPOCI; grant agreement No 101054369) and KM an ERC Consolidator Grant (ALTIBRAIN). Moreover, HE holds at present a grant from Roche Pharma AG, Grenzach-Wyhlen, enabling the continuation and extension of the GRAS (Göttingen Research Association for Schizophrenia) Data Collection in direction of Multiple Sclerosis (GRAMS, Göttingen Research Association for MS). The GRAMS project has also received support by a donation of Merck Serono GmbH
Footnotes
Author Contributions
Hannelore Ehrenreich: Conceptualization (lead); funding acquisition (equal); investigation (equal); methodology (equal); project administration (equal). All authors were involved in the conceptualization of this Brief Review and Perspective article, comprehensive literature search, and manuscript writing.
Conflict of Interest Statement
The authors declare no conflict of interest.
Ethics Statement
Ethical approval has been obtained or ethical proposal been submitted where applicable.
Data Availability Statement
Not applicable.
References
- 1.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30(4):393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Ratcliffe PJ. Oxygen sensing and hypoxia signalling pathways in animals: the implications of physiology for cancer. J Physiol. 2013;591(8):2027–2042. doi: 10.1113/jphysiol.2013.251470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3(10):721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 4.Kullmann JA, Trivedi N, Howell D, et al. Oxygen tension and the VHL-Hif1α pathway determine onset of neuronal polarization and cerebellar germinal zone exit. Neuron. 2020;106(4):607–623.e5. doi: 10.1016/j.neuron.2020.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ivashkiv LB. The hypoxia–lactate axis tempers inflammation. Nat Rev Immunol. 2020;20(2):85–86. doi: 10.1038/s41577-0190259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang X, Trinh T, Aljoufi A, Broxmeyer HE. Hypoxia signaling pathway in stem cell regulation: good and evil. Curr Stem Cell Rep. 2018;4(2):149–157. doi: 10.1007/s40778-018-0127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morikawa T, Takubo K. Hypoxia regulates the hematopoietic stem cell niche. Pflügers Archiv Eur J Physiol. 2016;468(1):13–22. doi: 10.1007/s00424-015-1743-z. [DOI] [PubMed] [Google Scholar]
- 8.Taylor CT, Colgan SP. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat Rev Immunol. 2017;17(12):774–785. doi: 10.1038/nri.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baik AH, Jain IH. Turning the oxygen dial: balancing the highs and lows. Trends Cell Biol. 2020;30(7):516–536. doi: 10.1016/j.tcb.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee P, Chandel NS, Simon MC. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat Rev Mol Cell Biol. 2020;21(5):268–283. doi: 10.1038/s41580-0200227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuen TJ, Silbereis JC, Griveau A, et al. Oligodendrocyte-encoded HIF function couples postnatal myelination and white matter angiogenesis. Cell. 2014;158(2):383–396. doi: 10.1016/j.cell.2014.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gassmann M, Fandrey J, Bichet S, et al. Oxygen supply and oxygen-dependent gene expression in differentiating embryonic stem cells. Proc Natl Acad Sci USA. 1996;93(7):2867–2872. doi: 10.1073/pnas.93.7.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bichet S, Wenger RH, Camenisch G, et al. Oxygen tension modulates β-globin switching in embryoid bodies. FASEB J. 1999;13(2):285–295. doi: 10.1096/fasebj.13.2.285. [DOI] [PubMed] [Google Scholar]
- 14.Gassmann NN, van Elteren HA, Goos TG, et al. Pregnancy at high altitude in the Andes leads to increased total vessel density in healthy newborns. J Appl Physiol. 2016;121(3):709–715. doi: 10.1152/japplphysiol.00561.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tissot van Patot MC, Gassmann M. Hypoxia: adapting to high altitude by mutating EPAS-1, the gene encoding HIF-2α. High Alt Med Biol. 2011;12(2):157–167. doi: 10.1089/ham.2010.1099. [DOI] [PubMed] [Google Scholar]
- 16.Fandrey J, Gorr T, Gassmann M. Regulating cellular oxygen sensing by hydroxylation. Cardiovasc Res. 2006;71(4):642–651. doi: 10.1016/j.cardiores.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Gassmann M, Muckenthaler MU. Adaptation of iron requirement to hypoxic conditions at high altitude. J Appl Physiol. 2015;119(12):1432–1440. doi: 10.1152/japplphysiol.00248.2015. [DOI] [PubMed] [Google Scholar]
- 18.Wakhloo D, Scharkowski F, Curto Y, et al. Functional hypoxia drives neuroplasticity and neurogenesis via brain erythropoietin. Nat Commun. 2020;11(1):1313. doi: 10.1038/s41467-020-15041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sirén A-L, Fratelli M, Brines M, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci USA. 2001;98(7):4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaelin WG., Jr Proline hydroxylation and gene expression. Annu Rev Biochem. 2005;74(1):115–128. doi: 10.1146/annurev.biochem.74.082803.133142. [DOI] [PubMed] [Google Scholar]
- 21.Kenneth NS, Rocha S. Regulation of gene expression by hypoxia. Biochem J. 2008;414(1):19–29. doi: 10.1042/BJ20081055. [DOI] [PubMed] [Google Scholar]
- 22.Marti HH. Erythropoietin and the hypoxic brain. J Exp Biol. 2004;207(18):3233–3242. doi: 10.1242/jeb.01049. [DOI] [PubMed] [Google Scholar]
- 23.Adamcio B, Sperling S, Hagemeyer N, Walkinshaw G, Ehrenreich H. Hypoxia inducible factor stabilization leads to lasting improvement of hippocampal memory in healthy mice. Behav Brain Res. 2010;208(1):80–84. doi: 10.1016/j.bbr.2009. [DOI] [PubMed] [Google Scholar]
- 24.Brines M, Cerami A. Emerging biological roles for erythropoietin in the nervous system. Nat Rev Neurosci. 2005;6(6):484–494. doi: 10.1038/nrn1687. [DOI] [PubMed] [Google Scholar]
- 25.Suresh S, Rajvanshi PK, Noguchi CT. The many facets of erythropoietin physiologic and metabolic response. Front Physiol. 2020;10:1534. doi: 10.3389/fphys.2019.01534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuler B, Vogel J, Grenacher B, Jacobs RA, Arras M, Gassmann M. Acute and chronic elevation of erythropoietin in the brain improves exercise performance in mice without inducing erythropoiesis. FASEB J. 2012;26(9):3884–3890. doi: 10.1096/fj.11-191197. [DOI] [PubMed] [Google Scholar]
- 27.Jelkmann W. Erythropoietin: structure, control of production, and function. Physiol Rev. 1992;72(2):449–489. doi: 10.1152/physrev.1992.72.2.449. [DOI] [PubMed] [Google Scholar]
- 28.Jain IH, Calvo SE, Markhard AL, et al. Genetic screen for cell fitness in high or low oxygen highlights mitochondrial and lipid metabolism. Cell. 2020;181(3):716–727.e11. doi: 10.1016/j.cell.2020.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehrenreich H, Garcia-Agudo LF, Steixner-Kumar AA, Wilke JBH, Butt UJ. Introducing the brain erythropoietin circle to explain adaptive brain hardware upgrade and improved performance. Mol Psychiatry. 2022;27(5):2372–2379. doi: 10.1038/s41380-02201551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn Sci. 2007;11(8):342–348. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Erickson KI, Hillman CH, Kramer AF. Physical activity, brain, and cognition. Curr Opin Behav Sci. 2015;4:27–32. doi: 10.1016/j.cobeha.2015.01.005. [DOI] [Google Scholar]
- 32.Pajonk F-G, Wobrock T, Gruber O, et al. Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry. 2010;67(2):133–143. doi: 10.1001/archgenpsychiatry.2009.193. [DOI] [PubMed] [Google Scholar]
- 33.Butt UJ, Steixner-Kumar AA, Depp C, et al. Hippocampal neurons respond to brain activity with functional hypoxia. Mol Psychiatry. 2021;26(6):1790–1807. doi: 10.1038/s41380-02000988-w11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandez Garcia-Agudo L, Steixner-Kumar AA, Curto Y, et al. Brain erythropoietin fine-tunes a counterbalance between neurodifferentiation and microglia in the adult hippocampus. Cell Rep. 2021;36(8):109548. doi: 10.1016/j.celrep.2021.109548. [DOI] [PubMed] [Google Scholar]
- 35.Gassmann M, Heinicke K, Soliz J, Ogunshola OO. Non-erythroid functions of erythropoietin. Adv Exp Med Biol. 2003;543:323–330. doi: 10.1007/978-1-4419-8997-0_22. [DOI] [PubMed] [Google Scholar]
- 36.Hassouna I, Ott C, Wüstefeld L, et al. Revisiting adult neurogenesis and the role of erythropoietin for neuronal and oligodendroglial differentiation in the hippocampus. Mol Psychiatry. 2016;21(12):1752–1767. doi: 10.1038/mp.2015.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khalid K, Frei J, Aboouf MA, et al. Erythropoietin stimulates GABAergic maturation in the mouse hippocampus. eNeuro. 2021;8(1):ENEURO.0006-21.2021. doi: 10.1523/ENEURO.000621.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, Di L, Noguchi CT. Erythropoietin, a novel versatile player regulating energy metabolism beyond the erythroid system. Int J Biol Sci. 2014;10(8):921–939. doi: 10.7150/ijbs.9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marti HH, Wenger RH, Rivas LA, et al. Erythropoietin gene expression in human, monkey and murine brain. Eur J Neurosci. 1996;8(4):666–676. doi: 10.1111/j.1460-9568.1996.tb01252.x. [DOI] [PubMed] [Google Scholar]
- 40.Digicaylioglu M, Bichet S, Marti HH, et al. Localization of specific erythropoietin binding sites in defined areas of the mouse brain. Proc Natl Acad Sci USA. 1995;92(9):3717–3720. doi: 10.1073/pnas.92.9.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Staub K, Haeusler M, Bender N, et al. Hemoglobin concentration of young men at residential altitudes between 200 and 2000 m mirrors Switzerland’s topography. Blood. 2020;135(13):1066–1069. doi: 10.1182/blood.2019004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valzania L, Coon KL, Vogel KJ, Brown MR, Strand MR. Hypoxia-induced transcription factor signaling is essential for larval growth of the mosquito Aedes aegypti. Proc Natl Acad Sci USA. 2018;115(3):457–465. doi: 10.1073/pnas.1719063115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wickramasinghe SN, Shiels S, Wickramasinghe PS. Immunoreactive erythropoietin in teleosts, amphibians, reptiles, and birds: evidence that the teleost kidney is both an erythropoietic and erythropoietin-producing organ. Ann NY Acad Sci. 1994;718(1):366–370. doi: 10.1111/j.1749-6632.1994.tb55742.x. [DOI] [PubMed] [Google Scholar]
- 44.Ostrowski D, Ehrenreich H, Heinrich R. Erythropoietin promotes survival and regeneration of insect neurons in vivo and in vitro. Neuroscience. 2011;188:95–108. doi: 10.1016/j.neuroscience.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 45.Drigas A, Mitsea E, Skianis C. Intermittent oxygen fasting & digital technologies: from antistress & hormones regulation to wellbeing, bliss & higher mental states. Technium BioChemMed. 2022;3(2):55–72. doi: 10.47577/biochemmed.v3i2.6628. [DOI] [Google Scholar]
- 46.Behrendt T, Bielitzki R, Behrens M, Herold F, Schega L. Effects of intermittent hypoxia–hyperoxia on performance-and health-related outcomes in humans: a systematic review. Sports Med Int Open. 2022;8(1):70. doi: 10.1186/s40798-022-00450-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.March-Diaz R, Lara-Ureña N, Romero-Molina C, et al. Hypoxia compromises the mitochondrial metabolism of Alzheimer’s disease microglia via HIF1. Nat Aging. 2021;1(4):385–399. doi: 10.1038/s43587-021-00054-2. [DOI] [PubMed] [Google Scholar]
- 48.Serebrovska ZO, Serebrovska TV, Kholin VA, et al. Intermittent hypoxia-hyperoxia training improves cognitive function and decreases circulating biomarkers of Alzheimer’s disease in patients with mild cognitive impairment: a pilot study. Int J Mol Sci. 2019;20(21):5405. doi: 10.3390/ijms20215405. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Treml B, Gatterer H, Burtscher J, Kleinsasser A, Burtscher M. A focused review on the maximal exercise responses in hypo-and normobaric hypoxia: divergent oxygen uptake and ventilation responses. Int J Environ Res Public Health. 2020;17(14):5239. doi: 10.3390/ijerph17145239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Viscor G, Torrella JR, Corral L, et al. Physiological and biological responses to short-term intermittent hypobaric hypoxia exposure: from sports and mountain medicine to new biomedical applications. Front Physiol. 2018;9:814. doi: 10.3389/fphys.2018.00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brugniaux JV, Coombs GB, Barak OF, Dujic Z, Sekhon MS, Ainslie PN. Highs and lows of hyperoxia: physiological, performance, and clinical aspects. Am J Physiol Regul Integr Comp Physiol. 2018;315(1):R1–R27. doi: 10.1152/ajpregu.00165.2017. [DOI] [PubMed] [Google Scholar]
- 52.Coppel J, Hennis P, Gilbert-Kawai E, Grocott MP. The physiological effects of hypobaric hypoxia versus normobaric hypoxia: a systematic review of crossover trials. Extrem Physiol Med. 2015;4:1–20. doi: 10.1186/s13728-014-0021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan AQ, Barth S, Trumbower RD. Acute intermittent hypoxia as a potential adjuvant to improve walking following spinal cord injury: evidence, challenges, and future directions. Curr Phys Med Rehabil Rep. 2020;8(3):188–198. doi: 10.1007/s40141-020-00270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Albertus-Cámara I, Ferrer-López V, Martínez-González-Moro I. The effect of normobaric hypoxia in middle-and/or long-distance runners: systematic review. Biology. 2022;11(5):689. doi: 10.3390/biology11050689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mähler A, Balogh A, Csizmadia I, et al. Metabolic, mental and immunological effects of normoxic and hypoxic training in multiple sclerosis patients: a pilot study. Front Immunol. 2018;9:2819. doi: 10.3389/fimmu.2018.02819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zrzavy T, Pfitzner A, Flachenecker P, Rommer P, Zettl UK. Effects of normobaric hypoxic endurance training on fatigue in patients with multiple sclerosis: a randomized prospective pilot study. JNeurol. 2021;268(12):4809–4815. doi: 10.1007/s00415021-10596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boos CJ, O'Hara JP, Mellor A, et al. A four-way comparison of cardiac function with normobaric normoxia, normobaric hypoxia, hypobaric hypoxia and genuine high altitude. PLoS One. 2016;11(4):e0152868. doi: 10.1371/journal.pone.0152868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burtscher J, Mallet RT, Pialoux V, Millet GP, Burtscher M. Adaptive responses to hypoxia and/or hyperoxia in humans. Antioxid Redox Signal. 2022;37(13–15):887–912. doi: 10.1089/ars.2021.0280. [DOI] [PubMed] [Google Scholar]
- 59.Serebrovskaya TV. Intermittent hypoxia research in the former Soviet Union and the Commonwealth of Independent States: history and review of the concept and selected applications. High Alt Med Biol. 2002;3(2):205–221. doi: 10.1089/15270290260131939. [DOI] [PubMed] [Google Scholar]
- 60.Samaja M, Milano G. Adaptation to hypoxia: a chimera? Int J Mol Sci. 2020;21(4):1527. doi: 10.3390/ijms21041527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Faeh D, Gutzwiller F, Bopp M. Lower mortality from coronary heart disease and stroke at higher altitudes in Switzerland. Circulation. 2009;120(6):495–501. doi: 10.1161/CIRCULATIONAHA.108.819250. [DOI] [PubMed] [Google Scholar]
- 62.Burtscher J, Millet GP, Burtscher M. Does living at moderate altitudes in Austria affect mortality rates of various causes? An ecological study. BMJ Open. 2021;11(6):e048520. doi: 10.1136/bmjopen-2020-048520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ezzati M, Horwitz MEM, Thomas DSK, et al. Altitude, life expectancy and mortality from ischaemic heart disease, stroke, COPD and cancers: national population-based analysis of US counties. J Epidemiol Community Health. 2012;66(7):e17. doi: 10.1136/jech.2010.112938. [DOI] [PubMed] [Google Scholar]
- 64.Thiersch M, Swenson ER, Haider T, Gassmann M. Reduced cancer mortality at high altitude: the role of glucose, lipids, iron and physical activity. Exp Cell Res. 2017;356(2):209–216. doi: 10.1016/j.yexcr.2017.03.048. [DOI] [PubMed] [Google Scholar]
- 65.Janssen Daalen JM, Meinders MJ, Giardina F, et al. Multiple N-of-1 trials to investigate hypoxia therapy in Parkinson’s disease: study rationale and protocol. BMC Neurol. 2022;22(1):262. doi: 10.1186/s12883-022-02770-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burtscher J, Maglione V, Di Pardo A, Millet GP, Schwarzer C, Zangrandi L. A rationale for hypoxic and chemical conditioning in Huntington’s disease. Int J Mol Sci. 2021;22(2):582. doi: 10.3390/ijms22020582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bayer U, Likar R, Pinter G, et al. Intermittent hypoxic–hyperoxic training on cognitive performance in geriatric patients. Alzheimer’s Dement. 2017;3(1):114–122. doi: 10.1016/j.trci.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schega L, Peter B, Törpel A, Mutschler H, Isermann B, Hamacher D. Effects of intermittent hypoxia on cognitive performance and quality of life in elderly adults: a pilot study. Gerontology. 2013;59(4):316–323. doi: 10.1159/000350927. [DOI] [PubMed] [Google Scholar]
- 69.Schega L, Peter B, Brigadski T, et al. Effect of intermittent normobaric hypoxia on aerobic capacity and cognitive function in older people. J Sci Med Sport. 2016;19(11):941–945. doi: 10.1016/j.jsams.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 70.Wang H, Shi X, Schenck H, et al. Intermittent hypoxia training for treating mild cognitive impairment: a pilot study. Am J Alzheimers Dis Other Demen. 2020;35:1533317519896725. doi: 10.1177/1533317519896725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Manukhina EB, Downey HF, Shi X, Mallet RT. Intermittent hypoxia training protects cerebrovascular function in Alzheimer’s disease. Exp Biol Med. 2016;241(12):1351–1363. doi: 10.1177/1535370216649060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jain IH, Zazzeron L, Goli R, et al. Hypoxia as a therapy for mitochondrial disease. Science. 2016;352(6281):54–61. doi: 10.1126/science.aad9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burtscher J, Mallet RT, Burtscher M, Millet GP. Hypoxia and brain aging: neurodegeneration or neuroprotection? Ageing Res Rev. 2021;68:101343. doi: 10.1016/j.arr.2021.101343. [DOI] [PubMed] [Google Scholar]
- 74.Jung ME, Simpkins JW, Wilson AM, Downey HF, Mallet RT. Intermittent hypoxia conditioning prevents behavioral deficit and brain oxidative stress in ethanol-withdrawn rats. J Appl Physiol. 2008;105(2):510–517. doi: 10.1152/japplphysiol.90317.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lyamina NP, Lyamina SV, Senchiknin VN, Mallet RT, Downey HF, Manukhina EB. Normobaric hypoxia conditioning reduces blood pressure and normalizes nitric oxide synthesis in patients with arterial hypertension. J Hypertens. 2011;29(11):2265–2272. doi: 10.1097/HJH.0b013e32834b5846. [DOI] [PubMed] [Google Scholar]
- 76.Ejaz S, Emmrich JV, Sitnikov SL, et al. Normobaric hyperoxia markedly reduces brain damage and sensorimotor deficits following brief focal ischaemia. Brain. 2016;139(3):751–764. doi: 10.1093/brain/awv391. [DOI] [PubMed] [Google Scholar]
- 77.Menzel M, Doppenberg EMR, Zauner A, Soukup J, Reinert MM, Bullock R. Increased inspired oxygen concentration as a factor in improved brain tissue oxygenation and tissue lactate levels after severe human head injury. J Neurosurg. 1999;91(1):1–10. doi: 10.3171/jns.1999.91.1.0001. [DOI] [PubMed] [Google Scholar]
- 78.Allardet-Servent J, Sicard G, Metz V, Chiche L. Benefits and risks of oxygen therapy during acute medical illness: just a matter of dose. Rev Méd Intern. 2019;40(10):670–676. doi: 10.1016/j.revmed.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 79.Hafner S, Beloncle F, Koch A, Radermacher P, Asfar P. Hyperoxia in intensive care, emergency, and peri-operative medicine: Dr. Jekyll or Mr. Hyde? A 2015 update. Ann Intensive Care. 2015;5(1):42. doi: 10.1186/s13613-015-0084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schjørring OL, Klitgaard TL, Perner A, et al. Lower or higher oxygenation targets for acute hypoxemic respiratory failure. N Engl J Med. 2021;384(14):1301–1311. doi: 10.1056/NEJMoa2032510. [DOI] [PubMed] [Google Scholar]
- 81.Qi Z, Liu W, Luo Y, Ji X, Liu KJ. Normobaric hyperoxia-based neuroprotective therapies in ischemic stroke. Med Gas Res. 2013;3(1):2. doi: 10.1186/2045-9912-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chazalviel L, David HN, Haelewyn B, et al. The underestimated effect of normobaric hyperoxia on cerebral blood flow and its relationship to neuroprotection. Brain. 2016;139(11):e62. doi: 10.1093/brain/aww178. [DOI] [PubMed] [Google Scholar]
- 83.Tessema B, Sack U, Serebrovska Z, König B, Egorov E. Effects of hyperoxia on aging biomarkers: a systematic review. Front Aging. 2021;2:783144. doi: 10.3389/fragi.2021.783144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kujawski S, Słomko J, Morten KJ, et al. Autonomic and cognitive function response to normobaric hyperoxia exposure in healthy subjects. Medicina. 2020;56(4):172. doi: 10.3390/medicina56040172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen Z, Ding Y, Ji X, Meng R. Advances in normobaric hyperoxia brain protection in experimental stroke. Front Neurol. 2020;11:50. doi: 10.3389/fneur.2020.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Daly S, Thorpe M, Rockswold S, et al. Hyperbaric oxygen therapy in the treatment of acute severe traumatic brain injury: a systematic review. J Neurotrauma. 2018;35(4):623–629. doi: 10.1089/neu.2017.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Casillas S, Galindo A, Camarillo-Reyes LA, Varon J, Surani SR. Effectiveness of hyperbaric oxygenation versus normobaric oxygenation therapy in carbon monoxide poisoning: a systematic review. Cureus. 2019;11(10):e5916. doi: 10.7759/cureus.5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rasmussen VM, Borgen AE, Jansen EC, Rotbøll Nielsen PH, Werner MU. Hyperbaric oxygen therapy attenuates central sensitization induced by a thermal injury in humans. Acta Anaesthesiol Scand. 2015;59(6):749–762. doi: 10.1111/aas.12492. [DOI] [PubMed] [Google Scholar]
- 89.Shapira R, Gdalyahu A, Gottfried I, et al. Hyperbaric oxygen therapy alleviates vascular dysfunction and amyloid burden in an Alzheimer's disease mouse model and in elderly patients. Aging. 2021;13(17):20935–20961. doi: 10.18632/aging.203485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Serebrovskaya TV, Manukhina EB, Smith ML, Downey HF, Mallet RT. Intermittent hypoxia: cause of or therapy for systemic hypertension? Exp Biol Med. 2008;233(6):627–650. doi: 10.3181/0710-MR-267. [DOI] [PubMed] [Google Scholar]
- 91.Navarrete-Opazo A, Alcayaga J, Sepúlveda O, Rojas E, Astudillo C. Repetitive intermittent hypoxia and locomotor training enhances walking function in incomplete spinal cord injury subjects: a randomized, triple-blind, placebo-controlled clinical trial. J Neurotrauma. 2017;34(9):1803–1812. doi: 10.1089/neu.2016.4478. [DOI] [PubMed] [Google Scholar]
- 92.Glazachev O, Kopylov P, Susta D, Dudnik E, Zagaynaya E. Adaptations following an intermittent hypoxia–hyperoxia training in coronary artery disease patients: a controlled study. Clin Cardiol. 2017;40(6):370–376. doi: 10.1002/clc.22670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dudnik E, Zagaynaya E, Glazachev OS, Susta D. Intermittent hypoxia–hyperoxia conditioning improves cardiorespiratory fitness in older comorbid cardiac outpatients without hematological changes: a randomized controlled trial. High Alt Med Biol. 2018;19(4):339–343. doi: 10.1089/ham.2018.0014. [DOI] [PubMed] [Google Scholar]
- 94.Mallet RT, Burtscher J, Manukhina EB, et al. In: The Neuroscience of Dementia. ch. 47. Martin Colin R, Preedy Victor R, editors. Academic Press; 2020. Diagnosis and management in dementia; pp. 745–760. [Google Scholar]
- 95.Tessema B, Sack U, König B, Serebrovska Z, Egorov E. Effects of intermittent hypoxia in training regimes and in obstructive sleep apnea on aging biomarkers and age-related diseases: a systematic review. Front Aging Neurosci. 2022;14:878278. doi: 10.3389/fnagi.2022.878278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hadanny A, Efrati S. The hyperoxic–hypoxic paradox. Biomolecules. 2020;10(6):958. doi: 10.3390/biom10060958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Benderro GF, Sun X, Kuang Y, LaManna JC. Decreased VEGF expression and microvascular density, but increased HIF-1 and 2α accumulation and EPO expression in chronic moderate hyperoxia in the mouse brain. Brain Res. 2012;1471:46–55. doi: 10.1016/j.brainres.2012.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol. 2006;91(5):807–819. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- 99.Semenza GL. Life with oxygen. Science. 2007;318(5847):62–64. doi: 10.1126/science.1147949. [DOI] [PubMed] [Google Scholar]
- 100.Yang D, Ma L, Wang P, et al. Normobaric oxygen inhibits AQP4 and NHE1 expression in experimental focal ischemic stroke. Int J Mol Med. 2019;43(3):1193–1202. doi: 10.3892/ijmm.2018.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.You P, Lin M, Li K, Ye X, Zheng J. Normobaric oxygen therapy inhibits HIF-1α and VEGF expression in perihematoma and reduces neurological function defects. Neuroreport. 2016;27(5):329–336. doi: 10.1097/WNR.0000000000000542. [DOI] [PubMed] [Google Scholar]
- 102.Ast T, Meisel JD, Patra S, et al. Hypoxia rescues frataxin loss by restoring iron sulfur cluster biogenesis. Cell. 2019;177(6):1507–1521.e16. doi: 10.1016/j.cell.2019.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Butt UJ, Hassouna I, Fernandez Garcia-Agudo L, et al. CaMKIIα expressing neurons to report activity-related endogenous hypoxia upon motor-cognitive challenge. Int J Mol Sci. 2021;22(6):3164. doi: 10.3390/ijms22063164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lichter K, Paul MM, Pauli M, et al. Ultrastructural analysis of wild-type and RIM1α knockout active zones in a large cortical synapse. Cell Rep. 2022;40(12):111382. doi: 10.1016/j.celrep.2022.111382. [DOI] [PubMed] [Google Scholar]
- 105.Mrestani A, Pauli M, Kollmannsberger P, et al. Active zone compaction correlates with presynaptic homeostatic potentiation. Cell Rep. 2021;37(1):109770. doi: 10.1016/j.celrep.2021.109770. [DOI] [PubMed] [Google Scholar]
- 106.Pauli M, Paul MM, Proppert S, et al. Targeted volumetric single-molecule localization microscopy of defined presynaptic structures in brain sections. Commun Biol. 2021;4(1):407. doi: 10.1038/s42003-021-01939-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Harris JJ, Jolivet R, Attwell D. Synaptic energy use and supply. Neuron. 2012;75(5):762–777. doi: 10.1016/j.neuron.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 108.Garcia AJ, III, Putnam RW, Dean JB. Hyperbaric hyperoxia and normobaric reoxygenation increase excitability and activate oxygen-induced potentiation in CA1 hippocampal neurons. J Appl Physiol. 2010;109(3):804–819. doi: 10.1152/japplphysiol.91429.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399(6731):66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- 110.Nikonenko I, Jourdain P, Muller D. Presynaptic remodeling contributes to activity-dependent synaptogenesis. J Neurosci. 2003;23(24):8498–8505. doi: 10.1523/JNEUROSCI.23-24-08498.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Segura I, Lange C, Knevels E, et al. The oxygen sensor PHD2 controls dendritic spines and synapses via modification of filamin A. Cell Rep. 2016;14(11):2653–2667. doi: 10.1016/j.celrep.2016.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tsai Y-W, Yang Y-R, Sun SH, Liang K-C, Wang R-Y. Post ischemia intermittent hypoxia induces hippocampal neurogenesis and synaptic alterations and alleviates long-term memory impairment. J Cereb Blood Flow Metab. 2013;33(5):764–773. doi: 10.1038/jcbfm.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bärtsch P, Swenson ER. Acute high-altitude illnesses. N Engl J Med. 2013;368(24):2294–2302. doi: 10.1056/NEJMcp1214870. [DOI] [PubMed] [Google Scholar]
- 114.Knaupp W, Khilnani S, Sherwood J, Scharf S, Steinberg H. Erythropoietin response to acute normobaric hypoxia in humans. J Appl Physiol. 1992;73(3):837–840. doi: 10.1152/jappl.1992.73.3.837. [DOI] [PubMed] [Google Scholar]
- 115.Gore CJ, Rodríguez FA, Truijens MJ, Townsend NE, Stray-Gundersen J, Levine BD. Increased serum erythropoietin but not red cell production after 4 wk of intermittent hypobaric hypoxia (4,000–5,500 m) J Appl Physiol. 2006;101(5):1386–1393. doi: 10.1152/japplphysiol.00342.2006. [DOI] [PubMed] [Google Scholar]
- 116.Taylor CT, Scholz CC. The effect of HIF on metabolism and immunity. Nat Rev Nephrol. 2022;18(9):573–587. doi: 10.1038/s41581-022-00587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rollins B, Martin MV, Morgan L, Vawter MP. Analysis of whole genome biomarker expression in blood and brain. Am J Med Genet B. 2010;153B(4):919–936. doi: 10.1002/ajmg.b.31062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nature Biotechnol. 2018;36(5):411–420. doi: 10.1038/nbt.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Trapnell C, Cacchiarelli D, Grimsby J, et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat Biotechnol. 2014;32(4):381–386. doi: 10.1038/nbt.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Efremova M, Vento-Tormo M, Teichmann SA, Vento-Tormo R. CellPhoneDB: inferring cell–cell communication from combined expression of multi-subunit ligand–receptor complexes. Nat Protoc. 2020;15(4):1484–1506. doi: 10.1038/s41596-0200292-x. [DOI] [PubMed] [Google Scholar]
- 121.Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ashburner M, Ball CA, Blake JA, et al. Gene ontology: tool for the unification of biology. Nat Genet. 2000;25(1):25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Magistretti PJ, Allaman I. Lactate in the brain: from metabolic end-product to signalling molecule. Nat Rev Neurosci. 2018;19(4):235–249. doi: 10.1038/nrn.2018.19. [DOI] [PubMed] [Google Scholar]
- 124.Nave K-A. Myelination and the trophic support of long axons. Nat Rev Neurosci. 2010;11(4):275–283. doi: 10.1038/nrn2797. [DOI] [PubMed] [Google Scholar]
- 125.Pellerin L, Magistretti PJ. Sweet sixteen for ANLS. J Cereb Blood Flow Metab. 2012;32(7):1152–1166. doi: 10.1038/jcbfm.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fünfschilling U, Supplie LM, Mahad D, et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485(7399):517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Saab AS, Tzvetavona ID, Trevisiol A, et al. Oligodendroglial NMDA receptors regulate glucose import and axonal energy metabolism. Neuron. 2016;91(1):119–132. doi: 10.1016/j.neuron.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Trevisiol A, Saab AS, Winkler U, et al. Monitoring ATP dynamics in electrically active white matter tracts. eLife. 2017;6:e24241. doi: 10.7554/eLife.24241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Supplie LM, Düking T, Campbell G, et al. Respiration-deficient astrocytes survive as glycolytic cells in vivo. J Neurosci. 2017;37(16):4231–4242. doi: 10.1523/JNEUROSCI.0756-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Luo W, Hu H, Chang R, et al. Pyruvate kinase M2 is a PHD3stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145(5):732–744. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mintun MA, Lundstrom BN, Snyder AZ, Vlassenko AG, Shulman GL, Raichle ME. Blood flow and oxygen delivery to human brain during functional activity: theoretical modeling and experimental data. Proc Natl Acad Sci USA. 2001;98(12):6859–6864. doi: 10.1073/pnas.111164398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Buxton RB. The thermodynamics of thinking: connections between neural activity, energy metabolism and blood flow. Philos Trans R Soc Ser B. 2021;376(1815):20190624. doi: 10.1098/rstb.2019.0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nees F, Banaschewski T, Bokde ALW, et al. Global and regional structural differences and prediction of autistic traits during adolescence. Brain Sci. 2022;12(9):1187. doi: 10.3390/brainsci12091187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schulte-Rüther M, Kulvicius T, Stroth S, et al. Using machine learning to improve diagnostic assessment of ASD in the light of specific differential and co-occurring diagnoses. J Child Psychol Psychiatry. 2022;64(1):16–26. doi: 10.1111/jcpp.13650. [DOI] [PubMed] [Google Scholar]
- 135.Konicar L, Radev S, Prillinger K, et al. Volitional modification of brain activity in adolescents with autism spectrum disorder: a Bayesian analysis of slow cortical potential neurofeedback. Neuroimage. 2021;29:102557. doi: 10.1016/j.nicl.2021.102557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sztainberg Y, Chen H, Swann JW, et al. Reversal of phenotypes in MECP2 duplication mice using genetic rescue or antisense oligonucleotides. Nature. 2015;528(7580):123–126. doi: 10.1038/nature16159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Creson TK, Rojas C, Hwaun E, et al. Re-expression of SynGAP protein in adulthood improves translatable measures of brain function and behavior. eLife. 2019;8:e46752. doi: 10.7554/eLife.46752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dawson G, Jones EJ, Merkle K, et al. Early behavioral intervention is associated with normalized brain activity in young children with autism. J Am Acad Child Adolesc Psychiatry. 2012;51(11):1150–1159. doi: 10.1016/j.jaac.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.