Abstract
Objective
To study clinical, surgical characteristics and the relationship between endometriosis lesion types and conception rate after surgery in infertile women with endometriosis.
Methods
A prospective, multicenter, cohort of 204 women (age 20–35 years) with endometriosis was followed up post-surgery between November 2017 and February 2020 at three tertiary-care hospitals.
Results
Based on the severity of endometriosis lesion type, deep infiltrating endometriosis (DIE) (81/204, 39.7%) was the most common lesion; followed by ovarian endometriosis (OMA) (64/204, 31.4%), and superficial peritoneal endometriosis (SUP) (59/204, 28.9%). Endometriosis patients had a single lesion type (94/204, 46.1%), two lesion types (77/204, 37.7%), or three lesion types (33/204, 16.2%) with significant differences between regions (P < 0.001). Around 40% (37/95) of obese women had SUP (P = 0.003) whereas 78% (14/18) of underweight women had DIE (P < 0.001). Significant differences in mean Endometriosis Fertility Index scores between endometriosis lesion types and patients with one, two, and three types of lesions were observed (P < 0.001). The majority (22/32, 68.8%) of the women conceived naturally after the surgery. Half (16/32; 50%) of the women with a single lesion type conceived after the surgery; of which most (13/16, 81.2%) had SUP, followed by OMA (2/16, 12.5%), and DIE (1/16, 6.3%).
Conclusion
Women with SUP and only one type of endometriotic lesion were more likely to conceive post-surgery.
Keywords: anatomical distribution, deep infiltrating endometriosis, endometrioma, infertility, management, superficial peritoneal endometriosis
1. Introduction
Endometriosis is an estrogen-dependent, benign, chronic inflammatory condition defined as the presence of endometrium-like tissue at extrauterine sites.1 It is estimated to affect approximately 247 million women of reproductive age worldwide and 42 million women in India.2 Its etiology remains unclear and many theories have been proposed, but retrograde menstruation remains the most accepted theory.3 Epigenetics,4 genetics,5 hormones, autoimmunity,6 and inflammation7 are reported to be of significance in the etiology of endometriosis. Endometriosis has wide-ranging clinical presentations from asymptomatic to symptoms such as chronic pelvic pain, dysmenorrhea, dyspareunia, dyschezia, and dysuria. Between 30% and 50% of women with endometriosis experience infertility.3 Several mechanisms have been proposed to explain fertility impairment; they include distortion of pelvic anatomy,1 altered folliculogenesis leading to ovulatory dysfunction and poor oocyte quality, alteration of hormonal and cell-mediated functions, and impaired implantation.3
Laparoscopy is the reference standard for the diagnosis of endometriosis with histologic confirmation.3 Different classification systems have been developed such as disease staging based on laparoscopic visualization of lesions,8 with three distinct endometriosis phenotypes: superficial peritoneal endometriosis (SUP), cystic ovarian endometriosis or endometrioma (OMA), and deep infiltrating endometriosis (DIE); and the endometriosis fertility index (EFI), which can predict clinical outcome among surgically reported endometriosis in women with infertility.3 However, performing laparoscopy in endometriosis-associated infertility is still debated, probably because of the invasive nature of the surgery followed by the complications and settings of the surgical procedures. In contrast, treatment of early-stage endometriosis through laparoscopy exhibits promising results in improving fertility.3 Although evaluation of infertility has been standardized and simplified, affected couples, especially women from low- and middle-income countries, continue to bear an overwhelming burden on social, economic and personal well-being. The high cost of assisted reproductive technologies, lack of infrastructure and laboratory facilities, and limited personnel in public sectors, as well as poor health-seeking culture, social stigma, and mental and physical violence, contribute to the burden.9 In addition, endometriosis patients experience emotional difficulties and anger because of disease recurrence, with the uncertainty of the future impacting negatively on their sexual relationship, social life, education, and employment.10 This has resulted in a lack of information on endometriosis-associated infertility as well as the association between endometriosis phenotypes and infertility. Therefore, there is limited information on endometriosis lesion phenotypes and infertility in low- and middle-income countries.11,12 The severity of endometriosis is categorized according to the location, the extent, and the depth of penetration of the lesions.13 Despite the stage of endometriosis, women have reported similar ages at menarche and similar menstrual patterns along with showing no differences in lifestyle factors.14 It is still unclear whether these demographic, anthropometric, clinical, and reproductive characteristics are associated with location, multiple lesions, or extent of penetration of the lesions. The present study aimed to investigate demographic, anthropometric, clinical and surgical characteristics among different endometriosis phenotypes in women undergoing laparoscopic surgery. We also investigated the relationship between endometriosis lesion types and conception rate after surgical management in infertile women with endometriosis.
2. Materials and Methods
2.1. Study population
A prospective, multicenter cohort study was conducted between November 2017 and February 2020 at three tertiary-care hospitals located in the eastern, northern, and southern regions of India. The study was approved by the Institutional Ethics Committee of Indian Council of Medical Research (ICMR)–National Institute for Research in Reproductive and Child Health (NIRRCH), Mumbai, India, and by the Institutional Ethics Committees of participating study sites (Spectrum Clinic and Endoscopy Research Institute, Kolkata; Sree Avittam Thirunal Hospital, Thiruvananthapuram and King George’s Medical University, Lucknow). All the study participants provided written informed consent before data collection. Initially, 250 women undergoing laparoscopy were screened by experienced laparoscopic surgeons, and 204 women with a confirmed diagnosis of endometriosis on laparoscopy in the age group of 20–35 years and wanting conception were enrolled as per the inclusion criteria. Histopathologic confirmation of endometriosis was possible in 71% (144/204) of the women and in the remaining 29% (60/204) of women, endometriosis was confirmed by laparoscopy. All women were married and the majority of them (72%, 144/201) were diagnosed with primary infertility, while 28% (57/201) had secondary infertility. We excluded women undergoing any hormonal treatment in the previous 3 months.
2.2. Data collection
The details of the data collection and quality controls are reported elsewhere.2 Data were collected as per the WERF EPHect Endometriosis Patient Questionnaire15 and the Standard Surgical Form (SSF).16 Body mass index (BMI; calculated as weight in kilograms divided by the square of height in meters) was classified as per the ICMR-National Institute of Nutrition (NIN), India, criteria.17 The women included in the present study were regularly followed up post-surgery every 3 months to check pregnancy status. Post-conception data were collected using an interview-based follow-up questionnaire. The questionnaire included details on gestational age, ultrasonography findings, blood pressure, sugar levels, urine examination, pregnancy complications, delivery, and post-delivery. The collaborators and the trained research staff interviewed the patients every 3 months after the surgery, either by contacting them via telephone or whenever the patients visited the hospitals for their follow-up in person, to the collaborating centers.
2.3. Patient stratification
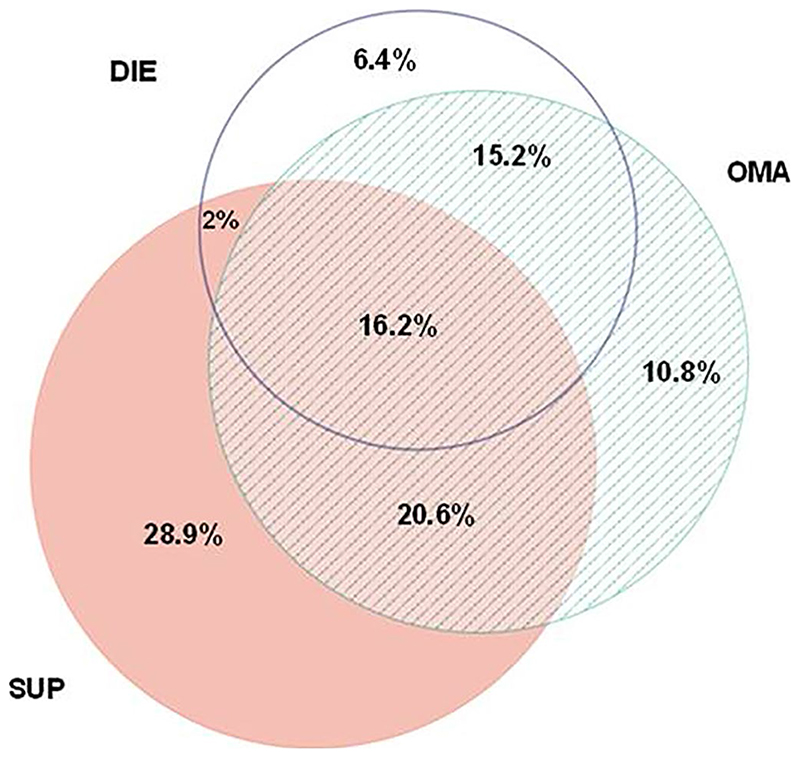
Women with endometriosis were classified as per the location of the lesion as left, right, and central pelvic regions (Table S1). The details on endometriotic lesions were captured in sections VIII, IX, and X of WERF EPHect SSF during the surgery or immediately after the surgery. After the analysis of the SSF data, endometriosis was classified as minimal, mild, moderate, and severe disease (revised American Society for Reproductive Medicine Stages: I [77/204; 38%], II [22/204; 11%], III [21/204; 10%], and IV [84/204; 41%]).8 The women were also categorized by lesion type (SUP, OMA, DIE), and number of lesion types (One type, Two types, Three types) across the three regions of India. Lesion types were classified as follows: DIE is any patient with DIE and any other lesion type; OMA is any patient having OMA with or without SUP, but no evidence of DIE; and SUP is any patient with SUP, but no DIE or OMA lesion types (Figure 1). The demographic, anthropometric, and clinical characteristics, and outcomes were evaluated among lesion classifications (SUP, OMA, DIE) (Table 1).
Figure 1.
Distribution of endometriosis cases as per endometriosis lesion classification (n = 204). DIE is any patient with DIE and any other lesion type; OMA is any patient with OMA ± SUP, but no evidence of DIE; and SUP is any patient with SUP, but no other lesion type. Abbreviations: DIE, deep infiltrating endometriosis; OMA, ovarian endometriosis; SUP, superficial peritoneal endometriosis
Table 1. Demographic and clinical characteristics as per endometriosis lesion classificationa.
| Age, years | 28.9 ± 3.7 | 28.8 ± 3.6 | 29.1 ± 3.7 | 28.9 ± 3.9 | 0.907b | |
| Study sites | Eastern | 127 (62.3) | 52 (40.9) | 42 (33.1) | 33 (26) | <0.001c |
| Northern | 29 (14.2) | 2 (6.9) | 3 (10.3) | 24 (82.8) | ||
| Southern | 48 (23.5) | 5 (10.4) | 19 (39.6) | 24 (50) | ||
| BMI | Underweight (≤18.5) | 18 (8.8) | 1 (5.6) | 3 (16.7) | 14 (77.8) | 0.002c |
| Normal (>18.5-<23) | 53 (26) | 9 (17) | 20 (37.7) | 24 (45.3) | ||
| Overweight (>23–<25) | 38 (18.6) | 12 (31.6) | 13 (34.2) | 13 (34.2) | ||
| Obese (≥25) | 95 (46.6) | 37 (38.9) | 28 (29.5) | 30 (31.6) | ||
| Education level (n = 191) | Up to Secondary | 24 (12.6) | 4 (16.7) | 3 (12.5) | 17 (70.8) | 0.024c |
| Higher Secondary | 38 (19.9) | 12 (31.6) | 11 (28.9) | 15 (39.5) | ||
| Graduate and above | 129 (67.5) | 39 (30.2) | 45 (34.9) | 45 (34.9) | ||
| Employment (n = 177) | Government/ Private job | 12 (6.8) | 3 (25) | 3 (25) | 6 (50) | 0.139c |
| Self employed | 14 (7.9) | 3 (21.4) | 1 (7.1) | 10 (71.4) | ||
| Unemployed | 151 (85.3) | 46 (30.5) | 48 (31.8) | 57 (37.7) | ||
| Annual family income (in ₹) (n = 135) | ≤100000 | 41 (30.4) | 5 (12.2) | 7 (17.1) | 29 (70.7) | <0.001c |
| >100000-500000 | 74 (54.8) | 16 (21.6) | 26 (35.1) | 32 (43.2) | ||
| ≥500000 | 20 (14.8) | 10 (50) | 7 (35) | 3 (15) | ||
| Comorbidities | Yes | 69 (33.8) | 22 (31.9) | 25 (36.2) | 22 (31.9) | 0.258c |
| No | 135 (66.2) | 37 (27.4) | 39 (28.9) | 59 (43.7) | ||
| Menstrual characteristicsd | ||||||
| Age at menarche, years (n = 201) | ≤12 | 78 (38.8) | 22 (28.2) | 27 (34.6) | 29 (37.2) | 0.798c |
| >12 | 123 (61.2) | 37 (30.1) | 37 (30.1) | 49 (39.8) | ||
| Regularity of menses (n = 197) | Regular | 163 (82.7) | 44 (27) | 56 (34.4) | 63 (38.7) | 0.230c |
| Irregular | 34 (17.3) | 13 (38.2) | 7 (20.6) | 14 (41.2) | ||
| Menstrual bleeding duration, days | n = 193 | n = 55 | n = 61 | n = 77 | 0.911b | |
| 4.68 ± 2.3 | 4.7 ± 2.0 | 4.6 ± 1.2 | 4.7 ± 3.1 | |||
| Menstrual cycle length, days (n = 203) | 24–31 | 162 (79.8) | 40 (24.7) | 58 (35.8) | 64 (39.5) | 0.004c |
| >31 | 41 (20.2) | 19 (46.3) | 5 (12.2) | 17 (41.5) | ||
| Menstrual blood loss, mL (n = 204) | At Heaviest | 41.9 ± 13.6 | 39 ± 13.8 | 41.7 ± 11.2 | 44.3 ± 14.6 | 0.077b |
| On average | 30.9 ± 13.9 | 29.9 ± 14.2 | 30.6 ± 12.4 | 31.8 ± 14.8 | 0.708b | |
| Pain symptomse | ||||||
| Dysmenorrhea (n = 115) | Yes | 94 (81.7) | 20 (21.3) | 29 (30.9) | 45 (47.9) | 0.916c |
| No | 21 (18.3) | 5 (23.8) | 7 (33.3) | 9 (42.9) | ||
| Dyspareunia (n = 115) | Yes | 50 (43.5) | 6 (12) | 16 (32) | 28 (56) | 0.067c |
| No | 65 (56.5) | 19 (29.2) | 20 (30.8) | 26 (40) | ||
| Chronic pelvic pain (n = 114) | 17 (14.9) | 3 (12) | 5 (14.7) | 9 (16.4) | 0.878c | |
| Infertility (n = 201)f | Primary | 144 (71.6) | 41 (28.5) | 48 (33.3) | 55 (38.2) | 0.764c |
| Secondary | 57 (28.4) | 18 (31.6) | 16 (28.1) | 23 (40.4) | ||
| Gravidity (n = 201) | Nulligravida | 144 (71.6) | 41 (28.5) | 48 (33.3) | 55 (38.2) | 0.869c |
| Primigravida | 36 (17.9) | 10 (27.8) | 11 (30.6) | 15 (41.7) | ||
| Multigravida | 21 (10.4) | 8 (38.1) | 5 (23.8) | 8 (38.1) | ||
| Parity (n = 201)g | Nullipara | 179 (89.9) | 53 (29.6) | 59 (33) | 67 (37.4) | 0.430c |
| Primipara | 20 (10.1) | 6 (30) | 4 (20) | 10 (50) | ||
| Recurrent endometriosis | 25 (12.3) | 4(6.8) | 10 (15.6) | 11 (13.6) | 0.293c | |
| EFI | 6.5 ± 2 | 7.9 ± 1.5 | 6.5 ± 1.8 | 5.4 ± 1.9 | <0.001b | |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by the square of height in meters); DIE, deep infiltrating endometriosis; EFI, Endometriosis Fertility Index; OMA, ovarian endometriosis; SUP, superficial peritoneal endometriosis.
Data are presented as mean ± standard deviation or as number (percentage).
One-way analysis of variance.
Pearson’s χ2 test.
The menstrual characteristics included age at menarche, regularity of menses, the volume of menstrual flow defined as the amount of bleeding experienced every 4 h during menstruation when it is “at its heaviest” and “on average” further categorized as: (1) Spotting, (2) Light, (3) Moderate, and (4) Heavy using a previously validated menstrual pictogram 25 and menstrual cycle length.
The existence of pelvic pain symptoms was defined as the presence of dysmenorrhea and/or dyspareunia from moderate to severe level for at least 6 months and chronic pelvic pain was defined as pelvic pain in general for 6 months or longer.
The existence and duration of infertility (primary or secondary) were defined as a ≥12-month history of unprotected intercourse without pregnancy.
Sample size for multipara (n = 2) was small and were not comparable.
As more than half of the women in the present study were observed to have more than one lesion type (Figure 1), we also evaluated the demographic, anthropometric, and clinical characteristics, and outcomes among patients who presented with one lesion type (SUP or OMA, or DIE only), two lesion types (SUP + OMA or SUP + DIE, or OMA + DIE) or three lesion types (SUP + OMA + DIE) (Table 2).
Table 2. Demographic and clinical characteristics of women having one, two and three types of endometriosis lesionsa.
| Age, years | 28.9 ± 3.7 | 28.8 ± 3.8 | 28.8 ± 3.6 | 29.6 ± 4 | 0.553b | |
| Study sites | Eastern | 127 (62.3) | 82 (64.6) | 37 (29.1) | 8 (6.3) | <0.001c |
| Northern | 29 (14.2) | 3 (10.3) | 13 (44.8) | 13 (44.8) | ||
| Southern | 48 (23.5) | 9 (18.8) | 27 (56.3) | 12 (25.0) | ||
| BMI | Underweight (≤18.5) | 18 (8.8) | 4 (22.2) | 8 (44.4) | 6 (33.3) | 0.193c |
| Normal (>18.5-<23) | 53 (26) | 21 (39.6) | 23 (43.4) | 9 (17) | ||
| Overweight (>23–<25) | 38 (18.6) | 19 (50) | 14 (36.8) | 5 (13.2) | ||
| Obese (≥25) | 95 (46.6) | 50 (52.6) | 32 (33.7) | 13 (13.7) | ||
| Education level (n = 191) | Up to Secondary | 24 (12.6) | 7 (29.2) | 11 (45.8) | 6 (25) | 0.473c |
| Higher Secondary | 38 (19.9) | 17 (44.7) | 15 (39.5) | 6 (15.8) | ||
| Graduate and above | 129 (67.5) | 63 (48.8) | 47 (36.4) | 19 (14.7) | ||
| Employment (n = 177) | Government/ Private job | 12 (6.8) | 4 (33.3) | 5 (41.7) | 3 (25) | 0.253c |
| Self employed | 14 (7.9) | 3 (21.4) | 7 (50) | 4 (28.6) | ||
| Unemployed | 151 (85.3) | 74 (49) | 55 (36.4) | 22 (14.6) | ||
| Annual family income (in ≥) (n = 135) | ≤100000 | 41 (30.4) | 9 (22) | 21 (51.2) | 11 (26.8) | 0.008c |
| >100000-500000 | 74 (54.8) | 26 (35.1) | 32 (43.2) | 16 (21.6) | ||
| ≥500000 | 20 (14.8) | 14 (70) | 5 (25) | 1 (5) | ||
| Comorbidities | Yes | 69 (33.8) | 35 (50.7) | 24 (34.8) | 10 (14.5) | 0.633c |
| No | 135 (66.2) | 59 (43.7) | 53 (39.3) | 23 (17) | ||
| Menstrual characteristicsd | ||||||
| Age at menarche, years (n = 201) | ≤12 | 78 (38.8) | 35 (44.9) | 29 (37.2) | 14 (17.9) | 0.820c |
| >12 | 123 (61.2) | 58 (47.2) | 47 (38.2) | 18 (14.6) | ||
| Regularity of menses (n = 197) | Regular | 163 (82.7) | 67 (41.1) | 67 (41.1) | 29 (17.8) | 0.018c |
| Irregular | 34 (17.3) | 23 (67.6) | 8 (23.5) | 3 (8.8) | ||
| Menstrual bleeding duration, days | n = 193 | n = 86 | n = 74 | n = 33 | 0.426b | |
| 4.68 ± 2.3 | 4.7 ± 1.9 | 4.8 ± 2.9 | 4.2 ± 1.5 | |||
| Menstrual cycle length, days (n = 203) | 24-31 | 162 (79.8) | 65 (40.1) | 67 (41.4) | 30 (18.5) | 0.005c |
| >31 | 41 (20.2) | 28 (68.3) | 10 (24.4) | 3 (7.3) | ||
| Menstrual blood loss, ml (n = 204) | At heaviest | 41.9 ± 13.6 | 39.3 ± 13.1 | 43.2 ± 14.4 | 46.6 ± 11.8 | 0.019b |
| On average | 30.9 ± 13.9 | 30.1 ± 13.4 | 30.5 ± 13.8 | 33.8 ± 15.4 | 0.406b | |
| Pain symptomse | ||||||
| Dysmenorrhea (n = 115) | Yes | 94 (81.7) | 29 (30.9) | 41 (43.6) | 24 (25.5) | 0.296c |
| No | 21 (18.3) | 5 (23.8) | 13 (61.9) | 3 (14.3) | ||
| Dyspareunia (n = 115) | Yes | 50 (43.5) | 12 (24) | 24 (48) | 14 (28) | 0.424c |
| No | 65 (56.5) | 22 (33.8) | 30 (46.2) | 13 (20) | ||
| Chronic pelvic pain (n = 114) | 17 (14.9) | 4 (11.8) | 7 (13.5) | 6 (21.4) | 0.630c | |
| Infertility (n = 201)f | Primary | 144 (71.6) | 64 (44.4) | 57 (39.6) | 23 (16) | 0.712c |
| Secondary | 57 (28.4) | 28 (49.1) | 19 (33.3) | 10 (17.5) | ||
| Gravidity (n = 201) | Nulligravida | 144 (71.6) | 64 (44.4) | 57 (39.6) | 23 (16) | 0.325c |
| Primigravida | 36 (17.9) | 16 (44.4) | 11 (30.6) | 9 (25) | ||
| Multigravida | 21 (10.4) | 12 (57.1) | 8 (38.1) | 1 (4.8) | ||
| Parityg (n = 201) | Nullipara | 179 (89.9) | 82 (45.8) | 71 (39.7) | 26 (14.5) | 0.023c |
| Primipara | 20 (10.1) | 10 (50) | 3 (15) | 7 (35) | ||
| Recurrent endometriosis | 25 (12.3) | 7 (7.4) | 12 (15.6) | 6 (18.2) | 0.143c | |
| EFI | 6.5 ± 2 | 7.5 ± 1.5 | 5.7 ± 2.0 | 5.5 ± 1.9 | <0.001b | |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by the square of height in meters); DIE, deep infiltrating endometriosis; EFI, Endometriosis Fertility Index; OMA, ovarian endometriosis; SUP, superficial peritoneal endometriosis.
Data are presented as mean ± standard deviation or as number (percentage).
One-way analysis of variance.
Pearson’s χ2 test.
The menstrual characteristics included age at menarche, regularity of menses, the volume of menstrual flow defined as the amount of bleeding experienced every 4 h during menstruation when it is “at its heaviest” and “on average” further categorized as: (1) Spotting, (2) Light, (3) Moderate, and (4) Heavy using a previously validated menstrual pictogram 25 and menstrual cycle length.
The existence of pelvic pain symptoms was defined as the presence of dysmenorrhea and/or dyspareunia from moderate to severe level for at least 6 months and chronic pelvic pain was defined as pelvic pain in general for 6 months or longer.
The existence and duration of infertility (primary or secondary) were defined as a ≥12-month history of unprotected intercourse without pregnancy.
Sample size for multipara (n = 2) was small and were not comparable.
2.4. Data analysis
All statistical analyses were performed using SPSS software version 19 (SPSS South Asia Pvt. Ltd.) and MS EXCEL. The categorical data such as study sites, BMI, education, employment, annual family income, comorbidities, age at menarche, regularity of menses, menstrual cycle length, pain symptoms, infertility, gravidity, parity, recurrent endometriosis, surgical procedures, endometriosis classification, number and location of endometriotic lesions and adhesions were recorded as proportions. Continuous variables such as age, BMI, menstrual bleeding duration, menstrual blood loss, and EFI were expressed as mean ± standard deviation or as median (interquartile range [IQR]). Pearson χ2 and Fisher exact tests were used to compare proportions of demographics and clinical characteristics among the lesion classifications, lesion types, and preliminary analysis of post-conception follow-up data. One-way analysis of variance was used to compare continuous parametric variables and the Kruskal–Wallis test for continuous non-parametric variables. McNemar χ2 test was used to evaluate the laterality of endometriosis by assuming that the chances of left endometriosis are equal to non-left endometriosis. A P value less than 0.05 was considered statistically significant. Post hoc analyses were performed for comparisons that were statistically significant.
3. Results
3.1. Endometriosis lesion types and classification
Superficial peritoneal endometriosis (59/204, 28.9%) was the most common isolated lesion type, followed by OMA (22/204, 10.8%) and DIE (13/204, 6.4%). There was an overlap of endometriosis lesion type seen in 54% (110/204) of women. SUP + OMA were observed in 20.6% of patients (42/204) and SUP + OMA + DIE lesions in 16.2% of patients (33/204) (Figure 1).
Grouping patients by the most severe lesion type, DIE was reported in 39.7% (81/204) of the patients, followed by OMA (without DIE) and SUP (without DIE or OMA) in 31.4% (64/204) and 28.9% (59/204), respectively (Table 1). There was a significant difference in the distribution of endometriosis lesion types at three geographically different study sites (P < 0.001). Women recruited in eastern India had the highest proportion of SUP (52/127, 40.9%). OMA was most commonly seen in the southern Indian population (19/48, 39.6%), followed by the north Indian population (42/127, 33.1%). The highest proportion of DIE lesions was observed in women recruited in the northern region of India (24/29, 82.8%) (Table 2). Full results of the post hoc analyses for lesion classification are shown in Table S2.
Nearly half of the women were observed to have a single lesion type (94/204; 46.1%), followed by two lesion types (77/204; 37.7%) and three lesion types (33/204; 16.2%) with significant differences between regions (P < 0.001) (Table 2). Women from eastern India were commonly diagnosed with one lesion type (82/127, 64.6%, P < 0.001), whereas southern Indian women had two lesion types (27/48, 56.3%, P = 0.003) compared with eastern Indian women (37/127, 29.1%); and three lesion types were common (13/29, 44.8%, P < 0.001) in northern Indian women compared with eastern India (8/127, 6.3%). Full results of the post hoc analyses for lesion type are shown in Table S3.
3.2. BMI, socio-economic status, and endometriosis
The mean age of women with endometriosis was 28.9 ± 3.7 years with a mean BMI of 24.8 ± 5.1. Significant differences were observed in the distributions of endometriosis lesion types for BMI, education, and economic status (Tables 1 and 2). Obese women appeared to be more commonly diagnosed with SUP (37/95, 38.9%, P = 0.0037), whereas the highest proportion of DIE (14/18, 77.8%, P < 0.001) was diagnosed among underweight women (Table S2). Women up to the secondary level of education were mostly diagnosed with DIE (17/24, 70.8%, P = 0.001). Half of the women with high annual family income appeared to have the highest proportion of SUP (P = 0.002), whereas women with a low annual family income had DIE (29/41, 70.7%, P < 0.001) (Table S2). The majority of women with high annual family income were diagnosed with one lesion type (14/20, 70%, P = 0.001) (Table S3).
3.3. Menstrual characteristics, pain symptoms, and fertility history
The majority (163/197, 82.7%) of the women had regular menstrual cycles. Irregular menstrual cycles were significantly higher in women with one lesion type (23/34, 67.6%) (P = 0.005). There were significant differences among the endometriosis lesion types for menstrual cycle length (P = 0.004; Table 1). Significantly more women with one lesion type (28/41, 68.3%) had longer menstrual cycles (>31 days) compared with shorter menstrual cycles (<24 to 31 days; 65/162, 40.1%, P = 0.001) (Table 1). Women with OMA lesions more frequently reported shorter cycle lengths (<24 to 31 days; 58/162, 35.8%, P = 0.004), whereas women with SUP lesions reported long cycle lengths (>31 days; 19/41, 46.3%, P = 0.007) (Table S2). A higher amount of menstrual blood loss was observed among women with three lesion types compared with women with one lesion type (P = 0.025) (Table S3). Dysmenorrhea was the most common pain symptom (94/115, 81.7%) followed by dyspareunia (50/115, 43.5%) and chronic pelvic pain (17/114, 14.9%). Around 72% (144/201) of women in our cohort had primary infertility and nearly 90% (179/201) of women with endometriosis were nulliparous (P = 0.023) (Table 2).
3.4. Comorbidities and endometriosis
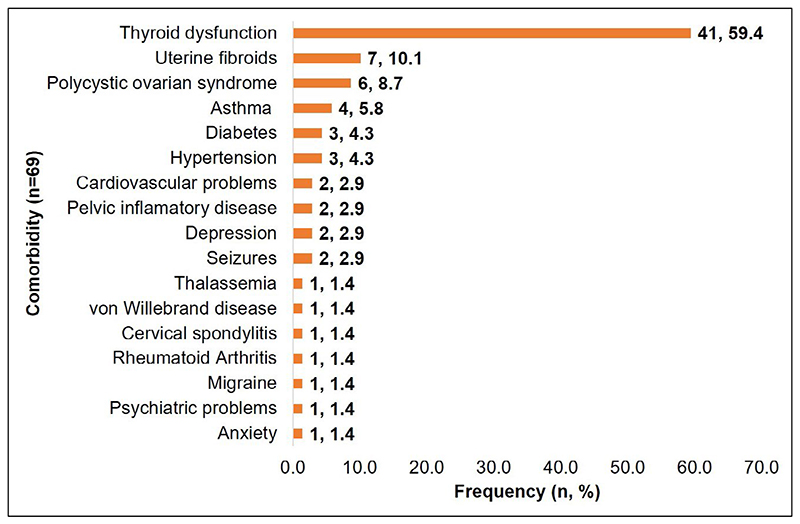
Sixty-nine women reported comorbidities and thyroid dysfunction (59%) was the most common comorbid condition with the highest incidence in eastern India (31/41; 75.6%) (Figure 2). There were 10 cases of adenomyosis associated with DIE, where five cases were associated with isolated DIE, three with SUP + OMA + DIE, and two with OMA + DIE. Uterine anomalies such as septate uterus (n = 2), unicornuate uterus (n = 1), bicornuate uterus (n = 2), and diverticulum (n = 1) were also reported in 3.4% (7/204) of endometriosis cases. The majority of uterine anomalies were associated with SUP (5/7; 71.4%). There was no association between the lesion classifications and comorbid conditions (P = 0.258).
Figure 2. Types of comorbidities reported among the endometriosis patients.
3.5. Surgical management of endometriosis
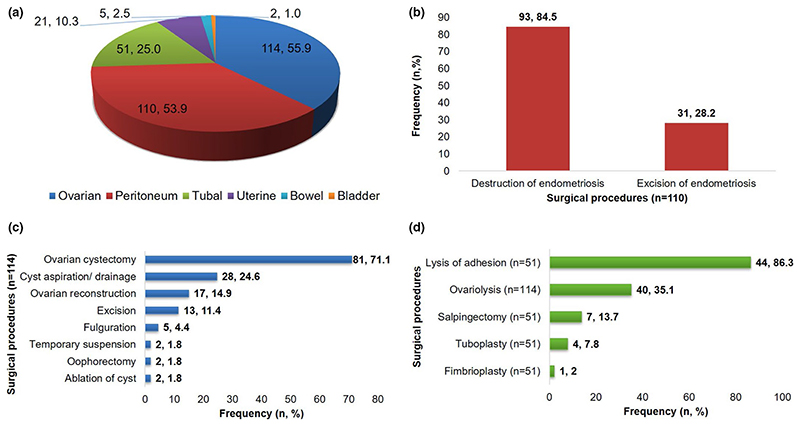
The surgical procedures performed for the treatment of endometriosis are indicated in Figure 3. The median operative time for surgical procedures was 60 min (IQR 39–120 min; n = 123). The median operative times for surgery among OMA (90 min, IQR 60–120 min) and DIE (90 min, IQR 48.8–120 min) were higher than for SUP (30 min, IQR 30–55 min; P < 0.001).
Figure 3. Surgical procedures.
(a) Types of surgeries performed on endometriosis patients. (b) Peritoneal surgeries performed to treat peritoneal endometriosis. Destruction of endometriosis performed through monopolar or bipolar electrosurgery and excision of endometriosis performed using scissors or harmonic scalpel. (c) Ovarian surgeries performed to treat ovarian endometriosis. (d) Surgeries performed to treat blocked fallopian tubes and adhesions
3.6. Laterality of endometriosis lesions and adhesions
Left laterality was higher for endometriosis lesions and adhesions in 203 women with endometriosis (Left 175/203, 86% versus Non-left 28/203, 14%; P = 0.001) (Table 3). The median number of lesions per patient was 3 (IQR 2–5) and the median number of adhesions per patient was 3 (IQR 2–6). Endometriosis lesions were reported more in the left region (368/963, 38.2%), followed by the right (318/963, 33%) and central (277/963, 29%) regions. Similarly, adhesions were observed more in the left region (118/335, 35.2%), followed by the right (112/335, 33.4%) and central (105/335, 31.3%) regions. Significantly more left endometrioma were observed than right endometrioma (Left 79/102, 77.5% vs. Non-left 23/102, 22.5%; P = 0.001). Seventy-five patients (37%) reported a total of 79 Allen-Masters peritoneal defects, which were frequently associated with OMA (34/75; 45.3%) and DIE (36/75; 48%). Recurrence of endometriosis was more commonly reported in women with previous surgery for OMA (8/13; 61.5%) (Table 1).
Table 3. Anatomical distribution of endometriotic lesions and adhesions in women with endometriosisa.
| Left pelvic sidewall | 57 (28.2) | 99 (10.3) | 16 (4.8) |
| Left uterosacral ligament | 125 (61.9) | 123 (12.8) | 30 (9) |
| Left ovary, serosa | 102 (50.5) | 105 (10.9) | 43 (12.8) |
| Left tube, serosa | 50 (24.8) | 36 (3.7) | 26 (7.8) |
| Other | 7 (3.5) | 5 (0.5) | 3 (0.9) |
| Right pelvic sidewall | 51 (25.2) | 84 (8.7) | 14 (4.2) |
| Right uterosacral ligament | 102 (50.5) | 98 (10.2) | 29 (8.7) |
| Right ovary, serosa | 91 (45) | 100 (10.4) | 36 (10.7) |
| Right tube, serosa | 45 (22.3) | 29 (3) | 28 (8.4) |
| Other | 10 (5) | 7 (0.7) | 5 (1.5) |
| Uterovesical pouch/anterior cul-de-sac | 30 (14.9) | 54 (5.6) | 8 (2.4) |
| Pouch of Douglas/posterior cul-de-sac | 117 (57.9) | 105 (10.9) | 60 (17.9) |
| Uterus, serosa | 72 (35.6) | 96 (10) | 18 (5.4) |
| Bladder, deep infiltrating | 2 (1) | 3 (0.3) | None |
| Bladder, serosa | 9 (4.5) | 10 (1) | 2 (0.6) |
| Colon, deep infiltrating | 5 (2.5) | 1 (0.1) | 3 (0.9) |
| Colon, serosa | 17 (8.4) | 5 (0.5) | 13 (3.9) |
| Vagina | 2 (1) | 1 (0.1) | 1 (0.3) |
| Rectovaginal septum | 2 (1) | 2 (0.2) | None |
Data are presented as number (percentage).
Overall number of women who reported with endometriotic lesions and adhesions was calculated irrespective of the overlaps.
Adhesions were present in 77/203 women.
3.7. Endometriosis fertility index and outcome
The average EFI and median least function scores were 6.5 ± 2 and 5 (IQR 3–7), respectively. There was a significant difference in mean EFI score between endometriosis lesion classifications (P < 0.001) (Table 1). Post-surgery, 19.3% (32/166) of patients conceived within 5 months (IQR 2-11.5 months) of surgery.
Significant differences for mean EFI were observed between patients with one, two, and three lesion types (P < 0.001) (Table 1). Fifty per cent (16/32) of women with endometriosis who conceived after surgery had a single lesion type. Among these women, 81.2% (13/16) had SUP with mean EFI 7.6 ± 2.10, followed by OMA (12.5%, 2/16; EFI 5 ± 2.2) and DIE (6.3%, 1/16; EFI 7).
The majority of women with endometriosis (22/32; 68.8%) conceived naturally following the surgical treatment. There were no significant differences for any of the clinical and surgical parameters among pregnant and non-pregnant groups (Table S4).
4. Discussion
This multicenter study demonstrates that women with SUP lesions or with only one type of endometriotic lesion have better chances of conception compared with women with OMA or DIE or women having two or three types of lesions. In the present cohort, 52% of the women had an EFI score between 6 and 8, suggesting that these women have a higher chance of conception. Moreover, the infertile women with SUP lesions reported a higher EFI score than those with OMA and DIE lesions, which was validated among the women who conceived post-surgery, suggesting that women with SUP lesions had higher chances of conceiving than women with OMA and DIE lesions. We also observed that natural conceptions were higher among the women who conceived within 12 months post-surgery, which was in accordance with a previous study on external validation of EFI,18 indicating that surgery might enhance the chances of natural conception.
The heterogeneous distribution of endometriosis lesion types was observed in infertile women with endometriosis. Based on the severe form of the endometriosis lesion types, DIE (39.7%) was the most common lesion type followed by OMA (31.4%) and SUP (28.9%) in our study. The frequency of lesion types irrespective of other lesions in the same patient were reported as SUP (67.7%), OMA (62.8%), and DIE (39.8%). In China and Russia, SUP was the most common endometriosis lesion reported (84% and 77%); followed by OMA (22% and 23%); and DIE (1% and 2%) whereas SUP was the most common lesion type (46%) followed by OMA (41%) and DIE (16%) in French women.19 Higher incidence of single endometriosis lesion type (46.1%) was observed in our cohort. Isolated DIE lesion was reported in only 6% of women and the majority had DIE lesions that had coexisted with OMA (31%) and/or SUP (18%) lesions, similar to the Italian population.20
We observed strong evidence for differences in both the lesion types and lesion classifications in the eastern, northern, and southern Indian populations. We also observed differences in the distributions of endometriosis lesion types associated with annual family income. These differences could be due to the socio-economic status, ascertainment of patients in different clinics or ethnicity, which could be a barrier for accessing healthcare services for endometriosis, similar to a study reported in the UK.21 Additionally, clinical settings, laparoscopic expertise, disease awareness, and socio-economic status also influenced the diagnosis of endometriosis.
Surgical treatment is the basis of endometriosis management that aims at removing endometriotic tissue, providing adequate tissue for histologic assessment, and preserving the maximum amount of normal ovarian tissue where fertility is desired.3 Laparoscopic cystectomy is considered as the reference standard for endometriosis-associated with pelvic pain and infertility22 and is most commonly performed for ovarian endometriosis. Vercellini et al.23 reported cystectomy of endometriomas leading to high pregnancy rates for 30%–67% of endometriosis patients, with an average of 50%. Left-sided unilateral oophorectomy was performed in two cases, possibly because of the high prevalence of left-side endometriosis. However, the effects of laterality on unilateral oophorectomy have not yet been investigated. Moreover, it was observed that the spontaneous reproductive potential was similar among women who underwent unilateral oophorectomy and those who had two ovaries.24 In our study, hysteroscopy was performed in 89% of women along with laparoscopy. The improved fertility performance and restoration by this combined hysteroscopy-laparoscopy surgical treatment for endometriosis-related infertility is one of the better options currently available, despite some limitations.25,26 A previous study reported higher pregnancy rates (81.3%) and live birth rates (94.2%) after surgery and also indicated that surgical expertise and patient’s age play an important role in fertility performance rather than the endometriosis stage.25 Ovarian endometriosis was found to be diagnosed preoperatively on ultrasonography in the majority of OMA cases, but other endometriotic lesions were undetected, which agreed with previous observations.27 Recurrence of OMA was reported among women with a previous diagnosis of OMA, which corroborated with a recent hospital-based cohort study.28
We reported the association of BMI with endometriosis in Indian women. Obese women had a higher frequency of SUP lesions and underweight women had a higher frequency of DIE lesions. A previous case–control study reported a higher incidence of DIE in women with the lowest BMI (<18.5).29 It is still unclear why lower BMI is associated with DIE lesions and why SUP lesions are seen more commonly in obese women. Rahmioglu et al.,30 demonstrated a shared genetic basis between endometriosis and fat distribution, highlighting the novel loci in/near KIFAP3 and CAB39L and showing significant evidence of trait association sharing. Further investigations on larger populations are required to understand the biologic basis of the association between BMI and endometriosis lesion types.
There were significant differences in the regularity of menses, menstrual blood loss, and menstrual cycle length among the lesion types, suggesting that menstrual characteristics might be influenced by location or number of lesions rather than the severity of the disease. We found higher rates of dysmenorrhea (81.7% vs. 69%) and dyspareunia (56.5% vs. 45.2%) compared with a recent single-center cohort study of Warzecha et al.31 Thyroid disorders were the most commonly observed comorbidity in our study. Peyneau et al.32 demonstrated an association of thyroid disorders with severe forms of endometriosis; however, we found no such association. It is well known that thyroid disorders are prevalent among women of reproductive age, and infertility is common with thyroid dysfunction, which possibly could be the reason for higher incidences of thyroid disease in our cohort.33 Moreover, an epidemiologic study in eight Indian cities found that the incidence of hypothyroidism was higher in the inland cities including Kolkata compared with coastal areas,34 which corroborated our results.
Our observations of a higher percentage of endometrioma, endometriotic implants, and adhesions on left side confirmed the earlier observations of the left lateral predisposition of endometriosis.35 We also observed pelvic DIE lesions more commonly in the posterior compartment compared with the anterior compartment.
The key strengths of our study are the systematic, prospective data collection using globally standardized and validated research instruments, histologically confirmed diagnosis of endometriosis and inclusion of tertiary-care hospitals having surgeons and clinicians highly skilled in endometriosis diagnosis and management, and the inclusion of patients from multiple centers to reduce the selection bias. However, limitations include small sample size within specific age groups leading to possible bias for the management of endometriosis-related infertility in older women. We did not include women on hormonal treatment, but this was intentional because we aimed to investigate surgical management of endometriosis. However, combined surgical treatment, hormonal treatment, and in vitro fertilization are recommended to improve reproductive outcomes in some cases of infertility,3 which might also be the reason for low pregnancy rates in our study. Lastly, follow up was carried out for only a short time and majorly via telephonic communication, because the majority of patients opted for gynecology consultations in their hometowns.
The study raised several important issues on clinical characteristics and reproductive outcomes being influenced by severity of endometriosis or location and number of endometriotic lesions. It is still unclear why all lesion types are present in some woman and why there exist no well-accepted guidelines for endometriosis classification with more than one lesion type, suggesting an urgent need to undertake future studies. The significant differences observed in the distribution of endometriosis lesion types across three geographic regions in India suggest the influence of ethnicity and environmental factors in the etiopathogenesis of endometriosis. The moderate to high EFI score reported in our study shows that laparoscopy surgery by a skilled surgeon and stricter management by experienced team might increase the reproductive outcomes. Our study showed that women diagnosed with SUP lesions or with only one type of lesion are more likely to conceive along with higher rates of natural conception. Therefore, we recommend that infertile women with endometriosis, younger than 35 years of age with SUP or with only one lesion type should be encouraged towards natural conception or assisted reproductive technology within 6 months after surgery. Our data on factors influencing poor reproductive outcomes suggest that more emphasis should be placed on infertility counseling both before and after surgery.
Supplementary Material
Acknowledgments
The authors thank Dr. Smita D. Mahale, Dr. Amalan Kanti Ray, Mr. Vaibhav Shinde, Ms. Trupti Bendigeri, Dr. Periyasamy Kuppusamy, Ms. Merlin Pious, and Ms. Sanaa Shaikh. Data collection was facilitated by and conducted in compliance with the World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonization Project (WERF EPHect). Rahul K Gajbhiye is an awardee of the DBT Wellcome India alliance clinical and public health intermediate fellowship (Grant no. IA/CPHI/18/1/503933). The study was partially funded by Department of Science and Technology, Government of India (EEQ/2016/000206), and Indian Council of Medical Research-National Institute for Research in Reproductive and Child Health, Ministry of Health and Family Welfare, Government of India (ICMR-NIRRH/MS/RA/803/09-2019).
Footnotes
Author Contributions
RG conceptualized, designed and implemented the study, obtained funding and ethics approvals, coordinated the study and monitored data collection, quality controls, data analysis, writing of the manuscript, and revision of the manuscript. AB was involved in co-ordination with study sites, data acquisition, data entry, analysis, interpretation, and drafting and revision of manuscript. PDM, SB, NC, and VD were involved in implementation of the study at their respective study sites, recruitment of study participants, data collection and manuscript preparation. SAK was involved in co-ordination and data acquisition. GS was involved in co-ordination and manuscript preparation. SCV, AS, and AM coordinated the recruitment of study participants, data entry and manuscript preparation. AP and AB conducted the statistical analysis. GWM and SM were involved in data analysis, interpretation, and drafting and revision of the manuscript. All authors approved the final version of the manuscript. All authors had full access to all of the data in the study (including statistical reports and tables) and take responsibility for the integrity of the data and accuracy of the data analysis.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available because of privacy or ethical restrictions.
References
- 1.Giudice LC. Clinical practice endometriosis. N Engl J Med. 2010;362:2389–2398. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gajbhiye RK, Montgomery G, Pai MV, et al. Protocol for a case–control study investigating the clinical phenotypes and genetic regulation of endometriosis in Indian women: the ECGRI study. BMJ Open. 2021;11:e050844. doi: 10.1136/bmjopen-2021-050844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P. Endometriosis. Nat Rev Dis Primers. 2018;4:9. doi: 10.1038/s41572-018-0008-5. [DOI] [PubMed] [Google Scholar]
- 4.Mortlock S, Restuadi R, Levien R, et al. Genetic regulation of methylation in human endometrium and blood and gene targets for reproductive diseases. Clin Epigenetics. 2019;11:49. doi: 10.1186/s13148-019-0648-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gajbhiye R, Fung JN, Montgomery GW. Complex genetics of female fertility. NPJ Genom Med. 2018;3:29. doi: 10.1038/s41525-018-0068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gajbhiye R, Bendigeri T, Ghuge A, et al. Panel of autoimmune markers for noninvasive diagnosis of minimal–mild endometriosis: a Multicenter Study. Reprod Sci. 2017;24:413–420. doi: 10.1177/1933719116657190. [DOI] [PubMed] [Google Scholar]
- 7.Gajbhiye R, McKinnon B, Mortlock S, Mueller M, Montgomery G. Genetic variation at chromosome 2q13 and its potential influence on endometriosis susceptibility through effects on the IL-1 family. Reprod Sci. 2018;25:1307–1317. doi: 10.1177/1933719118768688. [DOI] [PubMed] [Google Scholar]
- 8.Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817–821. doi: 10.1016/s0015-0282(97)81391-x. [DOI] [PubMed] [Google Scholar]
- 9.Sharma S, Mittal S, Aggarwal P. Management of infertility in low resource countries. BJOG. 2009;116:77–83. doi: 10.1111/j.1471-0528.2009.02311.x. [DOI] [PubMed] [Google Scholar]
- 10.Nnoaham KE, Hummelshoj L, Webster P, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96:366–373.:e8. doi: 10.1016/j.fertnstert.2011.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moini A, Malekzadeh F, Amirchaghmaghi E, et al. Risk factors associated with endometriosis among infertile Iranian women. Arch Med Sci. 2013;9:506–514. doi: 10.5114/aoms.2013.35420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaghar MI. Evaluation of lifestyle and endometriosis in infertile women referring to the selected hospital of Tehran university medical sciences. J Family Med Prim Care. 2019;8:3574–3577. doi: 10.4103/jfmpc.jfmpc_496_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubuisson J-B, Chapron C. Classification of endometriosis: the need for modification. Hum Reprod. 1994;9:2214–2216. doi: 10.1093/oxfordjournals.humrep.a138425. [DOI] [PubMed] [Google Scholar]
- 14.Sinaii N, Plumb K, Cotton L, et al. Differences in characteristics among 1,000 women with endometriosis based on extent of disease. Fertil Steril. 2008;89:538–545. doi: 10.1016/j.fertnstert.2007.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vitonis AF, Vincent K, Rahmioglu N, et al. World endometriosis research foundation endometriosis phenome and biobanking harmonization project: II. Clinical and covariate phenotype data collection in endometriosis research. Fertil Steril. 2014;102:1223–1232. doi: 10.1016/j.fertnstert.2014.07.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker CM, Laufer MR, Stratton P, et al. World endometriosis research foundation endometriosis phenome and biobanking harmonisation project: I. surgical phenotype data collection in endometriosis research. Fertil Steril. 2014;102:1213–1222. doi: 10.1016/j.fertnstert.2014.07.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dietary Guidelines for Indians: A Manual. 2nd edn. Indian Council of Medical Research-National Institute of Nutrition; 2011. [Accessed December 9, 2021]. [ICMR-NIN website]. https://www.nin.res.in/downloads/DietaryGuidelinesforNINwebsite.pdf. [Google Scholar]
- 18.Tomassetti C, Geysenbergh B, Meuleman C, Timmerman D, Fieuws S, D’Hooghe T. External validation of the endometriosis fertility index (EFI) staging system for predicting non-ART pregnancy after endometriosis surgery. Hum Reprod. 2013;28:1280–1288. doi: 10.1093/humrep/det017. [DOI] [PubMed] [Google Scholar]
- 19.Chapron C, Santulli P, Cabri P. The pain and daily consequences of living with endometriosis: a qualitative online survey of women in China, France and Russia. J Endometr Pelvic Pain Disord. 2015;7:89–94. [Google Scholar]
- 20.Somigliana E. Association rate between deep peritoneal endometriosis and other forms of the disease: pathogenetic implications. Hum Reprod. 2004;19:168–171. doi: 10.1093/humrep/deg513. [DOI] [PubMed] [Google Scholar]
- 21.Smith GD, Chaturvedi N, Harding S, Nazroo J, Williams R. Ethnic inequalities in health: a review of UK epidemiological evidence. Crit Public Health. 2000;10:375–408. [Google Scholar]
- 22.Ünlü C, Yildirim G. Ovarian cystectomy in endometriomas: combined approach. J Turk Ger Gynecol Assoc. 2014;15:177–189. doi: 10.5152/jtgga.2014.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vercellini P, Somigliana E, Viganò P, et al. Surgery for endometriosis-associated infertility: a pragmatic approach. Hum Reprod. 2009;24:254–269. doi: 10.1093/humrep/den379. [DOI] [PubMed] [Google Scholar]
- 24.Gasparri ML, Ruscito I, Braicu EI, et al. Biological impact of unilateral oophorectomy: does the number of ovaries really matter? Geburtshilfe Frauenheilkd. 2021;81:331–338. doi: 10.1055/a-1239-3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ekine AA, Fülöp I, Tekse I, et al. The surgical benefit of Hysterolaparoscopy in endometriosis-related infertility: a single Centre retrospective study with a minimum 2-year follow-up. J Clin Med. 2020;9:507. doi: 10.3390/jcm9020507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varlas V, Rhazi Y, Cloţea E, Borş RG, Mirică RM, Bacalbaşa N. Hysterolaparoscopy: a gold standard for diagnosing and treating infertility and benign uterine pathology. J Clin Med. 2021;10:3749. doi: 10.3390/jcm10163749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore J, Copley S, Morris J, Lindsell D, Golding S, Kennedy S. A systematic review of the accuracy of ultrasound in the diagnosis of endometriosis. Ultrasound Obstet Gynecol. 2002;20:630–634. doi: 10.1046/j.1469-0705.2002.00862.x. [DOI] [PubMed] [Google Scholar]
- 28.Nirgianakis K, Ma L, McKinnon B, Mueller MD. Recurrence patterns after surgery in patients with different endometriosis subtypes: a long-term hospital-based cohort study. J Clin Med. 2020;9:496. doi: 10.3390/jcm9020496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lafay Pillet M-C, Schneider A, Borghese B, et al. Deep infiltrating endometriosis is associated with markedly lower body mass index: a 476 case-control study. Hum Reprod. 2012;27:265–272. doi: 10.1093/humrep/der346. [DOI] [PubMed] [Google Scholar]
- 30.Rahmioglu N, Macgregor S, Drong AW, et al. Genome-wide enrichment analysis between endometriosis and obesity-related traits reveals novel susceptibility loci. Hum Mol Genet. 2015;24:1185–1199. doi: 10.1093/hmg/ddu516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warzecha D, Szymusik I, Wielgos M, Pietrzak B. The impact of endometriosis on the quality of life and the incidence of depression—a cohort study. Int J Environ Res Public Health. 2020;17:3641. doi: 10.3390/ijerph17103641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peyneau M, Kavian N, Chouzenoux S, et al. Role of thyroid dysimmunity and thyroid hormones in endometriosis. Proc Natl Acad Sci U S A. 2019;116:11894–11899. doi: 10.1073/pnas.1820469116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dosiou C. Thyroid and fertility: recent advances. Thyroid. 2020;30:479–486. doi: 10.1089/thy.2019.0382. [DOI] [PubMed] [Google Scholar]
- 34.Unnikrishnan AG, Kalra S, Sahay RK, et al. Prevalence of hypothyroidism in adults: an epidemiological study in eight cities of India. Indian J Endocrinol Metab. 2013;17:647–652. doi: 10.4103/2230-8210.113755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Audebert A, Petousis S, Margioula-Siarkou C, Ravanos K, Prapas N, Prapas Y. Anatomic distribution of endometriosis: a reappraisal based on series of 1101 patients. Eur J Obstet Gynecol. 2018;230:36–40. doi: 10.1016/j.ejogrb.2018.09.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available because of privacy or ethical restrictions.