Abstract
The human nasopharynx contains a stable microbial ecosystem of commensal and potentially pathogenic bacteria, which can elicit protective primary and secondary immune responses. Experimental intranasal infection of human adults with the commensal Neisseria lactamica produced safe, sustained pharyngeal colonization. This has potential utility as a vehicle for sustained release of antigen to the human mucosa, but commensals in general are thought to be immunologically tolerated. Here, we show that engineered Neisseria lactamica, chromosomally transformed to express a heterologous vaccine antigen safely induces systemic, antigen-specific immune responses during carriage in humans. When N. lactamica expressing the meningococcal antigen Neisseria Adhesin A (NadA) was inoculated intranasally into human volunteers, all colonized participants carried the bacteria asymptomatically for at least 28 days, with the majority (86%) still carrying the bacteria at 90 days. Compared to an otherwise isogenic, but phenotypically wild-type strain, colonization with NadA-expressing N. lactamica generated NadA-specific IgG- and IgA-secreting plasma cells within 14 days of colonization and NadA-specific IgG memory B cells within 28 days of colonization. NadA-specific IgG memory B cells were detected in peripheral blood of colonized participants for at least 90 days. Over the same period, there was seroconversion against NadA and generation of serum bactericidal antibody activity against a NadA-expressing meningococcus. The controlled infection was safe, and there was no transmission to adult bedroom-sharers during the 90 day period. Genetically modified N. lactamica could therefore be used to generate beneficial immune responses to heterologous antigens during sustained pharyngeal carriage.
Introduction
Natural protective immunity to invasive disease caused by nasopharyngeal pathobionts including Streptococcus pneumoniae, Haemophilus influenzae and Neisseria meningitidis (Nmen) is a consequence of repetitive, transient, asymptomatic carriage commencing in infancy. In each case, carriage is associated with seroconversion against cognate antigens (1–3). This natural mechanism could be harnessed using safe, genetically modified, live bacterial vectors as mucosal vaccines. The human commensal bacterium Neisseria lactamica (Nlac) is a harmless colonizer of infants and young children. The frequency of colonization wanes during early childhood with niche replacement by the closely related pathobiont Nmen (4). Controlled human intranasal infection of wild-type (WT) Nlac results in safe colonization of human volunteers, which is sustained for at least 6 months, and is accompanied by both humoral and mucosal immune responses (5) and reduced natural acquisition of Nmen (6). During experimental human colonization, the genome of Nlac remains stable (7). This, together with the inherent adjuvant properties of its outer membrane components (8) suggests that this bacterium could be adapted as a microbial factory or delivery platform, producing molecules of biological or therapeutic relevance, such as vaccine antigens, in situ following colonization (9–11). However, commensal bacteria have long been considered to be immunologically tolerated in symbiotic mutualism with the host (12), which could defeat this objective.
Here we report the pre-clinical quality and safety evaluation, and deployment of recombinant strains of Nlac in a first-in-man, controlled human infection model experiment (CHIME) to determine whether an engineered commensal can elicit beneficial immune responses to heterologous antigen. Co-primary objectives of the CHIME were (i) establishing safety of genetically modified Nlac (GM-Nlac), and (ii) measuring NadA-specific immunity in healthy volunteers following nasal inoculation with NadA-expressing Nlac, compared to healthy volunteers inoculated with a control genetically modified strain.
Results
Functional NadA is constitutively expressed on the surface of Nlac strain 4NB1
The expression of nadA in the meningococcus is phase variable (13) and regulated by the NadR repressor protein, which becomes de-repressed by salivary concentrations of the metabolite 4-hydroxyphenylacetic acid (4HPA) (14). Longitudinal analysis of serial Nmen isolates recovered from the human nasopharynx reveals that NadA expression decreases over time, likely a result of seroconversion against NadA and antibody-mediated selective pressure against NadA expression (15). To prevent the gene from becoming phase “OFF” in strain 4NB1 during the CHIME, transcription of the nadA gene in the construct is driven by a hybrid, non-phase variable porA/porB promoter. The transcription activity of this hybrid promoter is enhanced by 200 nucleotides of “upstream activation sequence” (UAS) of the WT porA promoter, which is optimal for increasing gene expression (fig. S1).
Flow cytometry using anti-NadA monoclonal antibody (mAb) 6e3 (16) demonstrates that Nlac strain 4NB1 expresses NadA on its surface, in contrast to both WT Y92-1009 and the genetically modified but phenotypically WT control strain 4YB2 (Fig. 1A). Visualization of 4NB1 membrane proteins separated by SDS-PAGE reveals that NadA is expressed as a multimer, most likely a trimer given its apparent molecular weight of approximately 120 kDa (Fig. 1B).
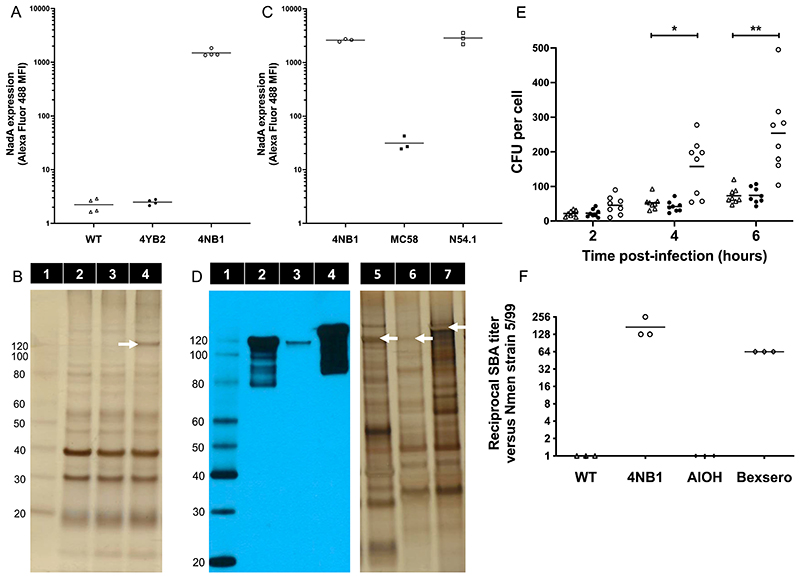
Figure 1. N. lactamica-expressed NadA functions as an adhesin and is immunogenic in mice.
(A) The median fluorescence intensity (MFI) of cultures of WT Nlac (Δ), 4YB2 (●) and 4NB1 (◦), labeled with murine anti-NadA monoclonal antibody 6e3 and goat-derived, anti-mouse IgG conjugated to Alexa Fluor 488 are shown. Bars represent mean, n = 4 replicates per group. (B) Membrane preparations derived from WT Nlac (Lane 2), 4YB2 (Lane 3) and 4NB1 (Lane 4), were separated by SDS-PAGE and visualized by silver staining (total protein = 2 μg per lane). The band representing NadA multimer is indicated with a white arrow. Molecular weight (in kDa) of protein standards (Lane 1) are shown. (C) Graph showing MFI of cultures of GM Nlac strain 4NB1 (◦) and Nmen strains MC58 (■) and N54.1 (□), labeled with rabbit anti-NadA polyclonal antibody [PE-JRL-3] and goat-derived, anti-rabbit IgG conjugated to Alexa Fluor 488. Bars represent mean, n = 3 replicates per group. (D) Membrane preparations derived from GM-Nlac strain 4NB1 (Lanes 2 & 5) and Nmen strains MC58 (Lanes 3 & 6) and N54.1 (Lanes 4 & 7) (total protein = 5 μg per lane), were separated by SDS-PAGE and either visualized by silver staining (Lanes 5 through 7), or transferred to PVDF membrane and probed in a western blot using rabbit anti-NadA polyclonal antibody [PE-JRL-3] (Lanes 1 through 4). Bands representing NadA multimers on silver stained gel are indicated with white arrows. Molecular weight (in kDa) of protein standards (Lane 1) are shown. (E) Association of WT Nlac (Δ), 4YB2 (●) and 4NB1 (◦) with monolayers of HEP-2 epithelial cells over time in hours (h), presented as colony forming units (CFU) per cell. Bars indicate mean. *p ≤ 0.05, **p ≤ 0.01 by two-way ANOVA with Dunnett’s multiple comparisons test versus WT at each time point (n = 8). (F) Reciprocal endpoint serum bactericidal antibody titer measured in triplicate assays of pooled mouse sera versus the NadA-overexpressing Nmen strain 5/99. Mice (n = 10 per group) were immunized intraperitoneally with either (i) dOMV derived from either WT Nlac (Δ) or 4NB1 (◦), supplemented with aluminium hydroxide (AlOH) as adjuvant, or (ii) AlOH alone (◆). Serum NA9136, derived from whole blood extracted from a human male 28 days after vaccination with Bexsero (♢), was included as a positive control with each assay replicate. Bars represent mean.
Similarly, flow cytometry using an affinity-purified, anti-NadA polyclonal antibody mixture (pAb), [PE-JRL-3] (Fig. 1B), demonstrated that the abundance of NadA expression on the surface of strain 4NB1 is similar to that of Nmen strain N54.1, a serogroup Y WT carriage isolate shown to strongly express NadA (allele 2/3) (17). Both strain 4NB1 and N54.1 express substantially more NadA than the widely-studied Nmen strain MC58 (allele 1), itself a serogroup B WT carriage isolate (Fig. 1C). These data, confirmed by western blot (Fig. 1D), suggest the heterologous expression of the nadA gene by Nlac results in presentation of trimeric NadA on the bacterial surface, at an abundance equivalent to that of naturally circulating, NadA-expressing meningococci. Consistent with the role of NadA as an adhesin (18), and supportive of its functional expression in 4NB1, this strain exhibits increased association with confluent monolayers of HEp-2 epithelial cells as compared to WT Nlac (Fig. 1E).
Immunization of mice with deoxycholate-extracted outer membrane vesicles (dOMV) derived from strain 4NB1 (4NB1-dOMV) generated potent serum bactericidal antibody (SBA) activity against Nmen strain 5/99, a NadA-overexpressing reference strain (19). In contrast, immunizing mice with WT Y92-1009 dOMV generates no SBA (Fig. 1F). This is consistent with previous observations in humans that antibody responses directed against the native outer membrane proteins of Nlac are not bactericidal (20), and suggests that the SBA activity is instead linked to the presence of antibody targeting NadA. This is supported by the SBA activity of control serum NA9136, which was extracted from whole blood taken from a human donor 28 days after immunization with Bexsero (Fig. 1F).
The pathogenic potential of recombinant Nlac is equivalent to WT Nlac
To gain approval for Deliberate Release of GM-Nlac in a CHIME, it was necessary to demonstrate no increase in the pathogenic potential of these strains over WT. Nmen expresses polysaccharide capsule (21), which affords resistance to killing by human serum and constitutes the key determinant of the organism’s ability to cause invasive disease. It is plausible that Nlac might become more pathogenic if it could deposit polysaccharide capsule on its surface. The most likely potential source of ectopic capsule synthesis genes during human colonization is Nmen, which shares the nasopharyngeal niche with Nlac. The uptake and assimilation of an antibiotic resistance marker (gene aphA3, coding kanamycin resistance or KanR) from three different genomic sources into the chromosome of WT Nlac strain Y92-1009, GM-Nlac strain 4NB1 and Nmen strain MC58 was therefore measured (Fig. 2A). Genomic DNA (gDNA) derived from Y92-1009 ΔnlaIII efficiently transformed both WT Y92-1009 and strain 4NB1, consistent with donor and recipient strains having homologous patterns of DNA methylation, which precludes degradation of the gDNA by restriction endonuclease activities and increases transformation efficiency (TE). The same gDNA also transformed Nmen strain MC58, although less efficiently. Conversely, whereas gDNA derived from mutants MC58ΔsiaD (in which the KanR gene inactivates a capsule biosynthesis gene) and MC58ΔnadA (in which the KanR gene inactivates the endogenous nadA gene) was able to transform MC58; neither gDNA was able to generate a single Nlac transformant (N = 5 experiments). The TE of 4NB1 with Nmen gDNA, under conditions ideal for transformation, is therefore < 1 transformant per 2 x 109 CFU, and is the same as the TE of the parental strain. We conclude that the likelihood of GM-Nlac assimilating capsule synthesis genes from Nmen remains as low as the WT parental strain and that the risk of this occurring is negligible.
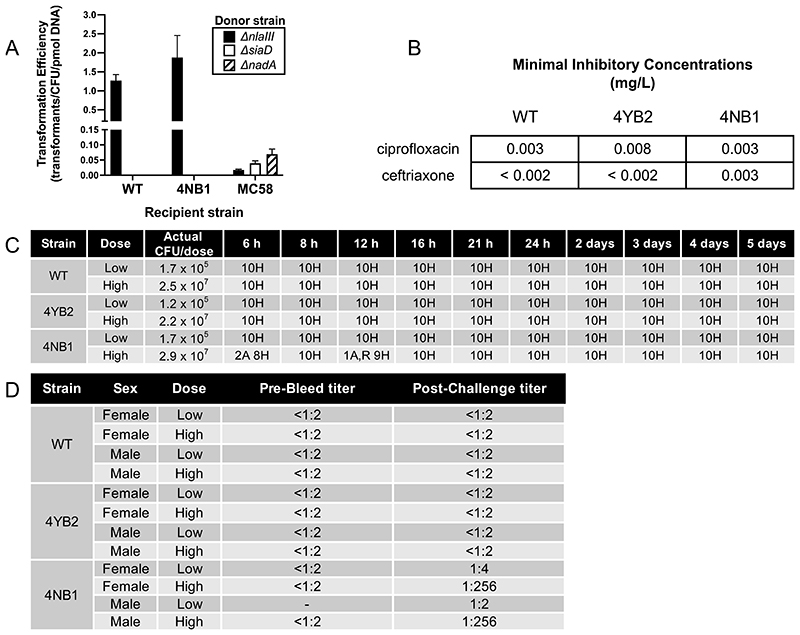
Figure 2. Genetically modified N. lactamica is no more likely to become transformed than its wild-type parental strain, remains susceptible to clinically relevant antibiotics, and is both non-pathogenic and immunogenic in a murine model of systemic infection.
(A) Transformation efficiency (TE) of WT Nlac Y92-1009, the NadA-expressing GM-Nlac strain 4NB1 and WT Nmen strain MC58 with 0.0001 pmol of gDNA from one of three Neisseria strains: (i) Nlac Y92-1009Δnlalll:aphA3 (Δnlalll, black bars), (ii) Nmen MC58ΔsiaD:aphA3 (ΔsiaD, white bars) and (iii) Nmen MC58ΔnadA:aphA3 (ΔnadA, hatched bars). Bars represent mean ± SD (n ≥ 5 replicates). (B) Minimal inhibitory concentrations (MICs, mg L-1) of antibiotics used clinically to treat meningococcal disease versus GM-Nlac strain 4NB1, as determined by e-test. Tests carried out according to European Committee on Antimicrobial Susceptibility Testing (EUCAST) methodology. Note that a strain is considered susceptible to ciprofloxacin if its measured MIC is less than 0.03 mg L-1 and susceptible to ceftriaxone if its measured MIC is less than 0.125 mg L-1. (C) Qualitative health scores for groups of mice inoculated intraperitoneally with high or low doses of one of WT Nlac Y92-1009, or the GM-Nlac strains 4YB2 and 4NB1. Bacteria were injected with 10 mg human holo-transferrin on Day 0. A second injection of 10 mg human holo-transferrin was administered at 24 hours post initial injection. Mice were regularly monitored over 5 days. The number of mice assessed as exhibiting normal or healthy (H) behavior, and the number of mice exhibiting classic signs of mild discomfort such as having an arched (A) or ruffled (R) appearance were recorded. After the first day no mice exhibited any signs of discomfort. (D) Reciprocal endpoint serum bactericidal antibody titers versus Nmen strain 5/99 in sera from individual mice challenged as described above. Serum samples wherein there was insufficient volume to perform the SBA measurement are denoted as -.
Both GM-Nlac strains retained susceptibility to standard antibiotics for the treatment of meningococcal disease (Fig. 2B). Antimicrobial susceptibility tests demonstrated that the minimal inhibitory concentrations (MICs) of ciprofloxacin and ceftriaxone for GM-Nlac were well below the European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints for Nmen and those previously established in 286 Nlac isolates (22). Neither 4NB1 or 4YB2 proved any more pathogenic than WT Y92-1009 in a mouse model of systemic infection at two different inoculum doses (Fig. 2C). However, systemic infection with the larger dose of strain 4NB1 resulted in the generation of SBA responses against Nmen strain 5/99 (Fig. 2D).
GM-Nlac safely colonizes the human upper respiratory tract in a CHIME
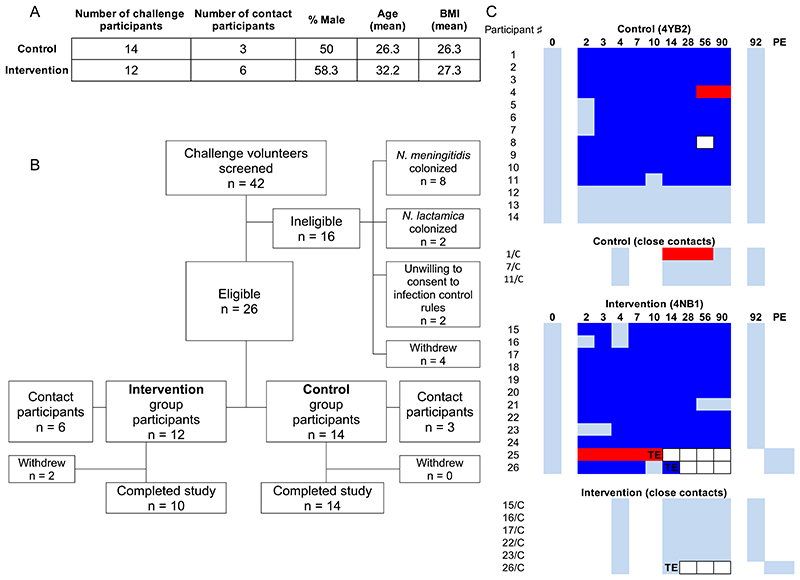
Volunteers aged 18-45 years provided informed consent and were recruited for preliminary screening of Neisseria species carriage. Amongst 42 volunteers screened, 26 were free from Nlac and Nmen carriage and eligible for participation (Fig. 3A). Participants were assigned, initially in 1-person but subsequently in up-to-5-person blocks, to either the intervention (strain 4NB1) or control (strain 4YB2) study arm (Fig. 3B). Participants were admitted to a hospital clinical research facility (CRF) for the first 4.5 days after inoculation. Volunteers were deemed colonized by GM-Nlac following recovery of one or more colonies of GM-Nlac from either nasal wash fluid or throat swabs at any point within 14 days of inoculation. Eligible volunteers were inoculated in each study block until at least 11 volunteers had been successfully colonized. The colonization status of Nlac and Nmen for each participant during the study period of 90 days is shown in Fig. 3C. Note that neither the Nmen strain acquired by Participant 25 prior to challenge, nor the Nmen strain acquired after Day 28 by Participant 4 were shown to express NadA. The latter point is important because it meant that samples from this participant could be included in analyses of NadA-specific immunity.
Figure 3. Controlled human infection model experiment using genetically modified N. lactamica.
(A) Demographic data of participants challenged with the control, WT-equivalent strain of GM-Nlac (4YB2, Control) or the NadA-expressing strain of GM-Nlac (4NB1, Intervention). The size of the inocula in each arm (mean ± SD) was (3.26 ± 0.84 x 105) CFU of strain 4YB2 (Control) and (3.74 ± 1.22 x 105) CFU of strain 4NB1 (Intervention) (p = 0.2595, unpaired, two-tailed t test). (B) Study flow diagram showing allocation to groups and study completion. (C) Pattern of Neisseria species carriage for all volunteers and their close contacts throughout the course of the study, showing Control and Intervention groups. Participant numbers are assigned arbitrarily. Participants and their close contacts share the same number where appropriate, with contacts denoted by an additional “/C”. Days post-inoculation at time of sampling are shown in bold. Color blocks represent: nonattendance (white, bordered), no GM-Nlac or Nmen cultured (light blue), 1+ colonies of PCR-verified GM-Nlac cultured (dark blue), Nmen cultured in the absence of Nlac/GM-Nlac (red). TE, triggered eradication following withdrawal from study; PE, post-eradication visit to certify clearance of GM-Nlac ( = TE + 2 days).
In the event of withdrawal from the study (n = 2) or at day 90 post-inoculation (n = 24), a single dose of oral ciprofloxacin (500 mg) was administered, which eliminated carriage of both strains of GM-Nlac within 2 days (Fig. 3C). During both the admission period (fig. S2) and the follow-up period (fig. S3), there were only a small number of solicited or unsolicited symptoms reported, and similarly few clinically observed (fig. S4) or laboratory test-identified (fig. S5) adverse events recorded. Upon clinical review, none of these events were deemed to be related or likely-related to carriage of either GM-Nlac strain, and the number of adverse events in the intervention study arm was similar to the control study arm. There were no serious adverse events, no antibiotic eradication or treatment given due to adverse events, and no study withdrawal due to adverse events. At no point throughout the study was GM-Nlac detected in exhaled breath samples or on surgical facemasks worn prior to each visit to the CRF. Additionally, there was no detectable transmission of GM-Nlac to bedroom-sharing contacts of volunteers, as established by culture of oropharyngeal swab taken from these individuals at Day 4, Day 14, Day 28, Day 56 or Day 90 (N = 9, Fig. 3C).
Colonization with NadA-expressing GM-Nlac expanded circulating Nlac-specific and NadA-specific antibody secreting cells
Two enzyme-linked immunospot (ELISpot) assays were employed to detect either IgG-secreting cells or IgA-secreting cells, collectively termed antibody-secreting cells (ASC), in the peripheral blood of participants with specificity to one of the following: (i) dOMV derived from strain 4YB2 (4YB2-dOMV), (ii) 4NB1-dOMV, (iii) the soluble domain of NadA (sNadA). We compared the maximum number of ASC spot-forming units (SFU) per 200,000 peripheral blood mononuclear cells (PBMC) developed against each antigen post-inoculation, assuming one SFU equated to one ASC. In view of the rapidity and transience of circulation of plasmablasts following infection or vaccination (23), peak responses were measured in samples taken at either day 7 or day 14 post-inoculation. Analysis of the number of IgG-secreting cells (Fig. 4, A to F) was conducted separately to the analysis of IgA-secreting cells (Fig. 4, G to L).
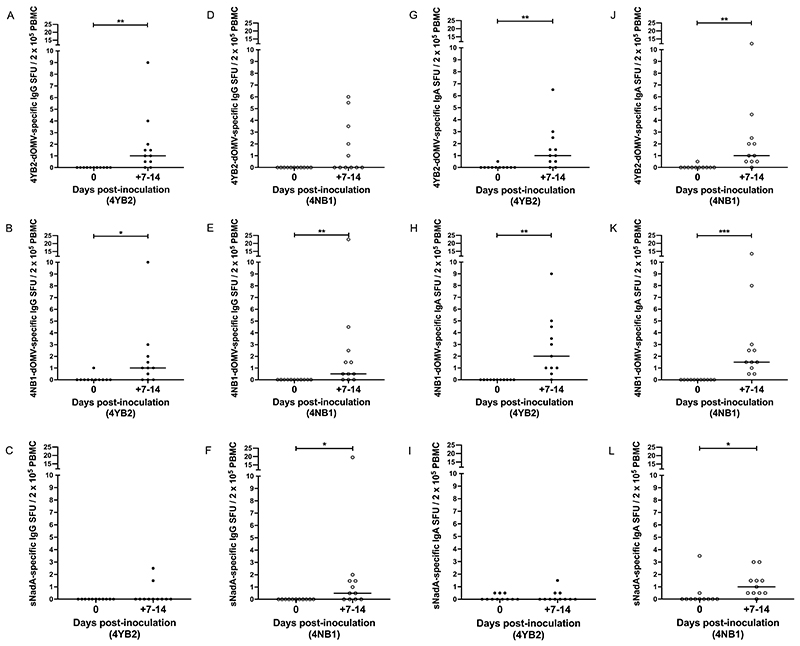
Figure 4. Colonization with NadA-expressing genetically modified N. lactamica was associated with an increase in the circulation of IgG-secreting plasma cells and IgA-secreting plasma cells specific for NadA.
PBMC isolated from whole blood at baseline and on Days 7 & 14 post-challenge with either control, WT-equivalent GM-Nlac (4YB2, ●) or NadA-expressing GM-Nlac (4NB1, ○) were assessed by ELISpot for the presence of IgG-secreting plasma cells (A to F) or IgA-secreting plasma cells (G to L) specific for 4YB2-dOMV (A, D, G and J), 4NB1-dOMV (B, E, H and K) or sNadA (C, F, I and L). Antibody secreting cells were visualized as spot forming units (SFU) and adjusted for non-specific SFU by deducting the appropriate average number of SFU from keyhole limpet hemocyanin (KLH)-coated membranes. Data are presented as the number of antigen-specific IgG or IgA SFU per 2 x 105 PBMC. For each participant, the largest number of antigen-specific IgG or IgA SFU per 2 x 105 PBMC (universally measured at either Day 7 or Day 14) is shown (+7-14). Bars indicate median. *p ≤ 0.05, ** p ≤ 0.01, ***p ≤ 0.001 by Wilcoxon matched-pairs signed rank test (n = 11).
In 4YB2-colonized participants, there was consensus between the pattern of changes in both the IgG-secreting and IgA-secreting cell datasets. Specifically, there was a significant increase in the number of cells with specificity to either 4YB2-dOMV (P = 0.0039, Fig. 4A and P = 0.0039, 4G) or 4NB1-dOMV (P = 0.0234, Fig. 4B and P = 0.002, 4H) at peak in comparison to baseline, but no change in the number of cells with specificity to sNadA was observed (Fig. 4C and 4I).
In 4NB1-colonized participants however, the two datasets were discrepant. Although there was no difference between the baseline and peak measurements of 4YB2-dOMV-specific, IgG-secreting cells (Fig. 4D), there was a significant increase between the baseline and peak measurements of 4YB2-dOMV-specific, IgA-secreting cells (P = 0.002, Fig. 4J). In contrast, the peak number of 4NB1-dOMV-specific cells was significantly increased in both datasets from these participants compared to baseline (P = 0.0078, Fig. 4E and P = 0.001, 4K). Similarly, the peak number of sNadA-specific cells was significantly increased over baseline in these participants (P = 0.0156, Fig. 4F and P = 0.0449, 4L).
Colonization with NadA-expressing GM-Nlac elicits seroconversion against NadA and stimulates anti-meningococcal serum bactericidal antibody activity
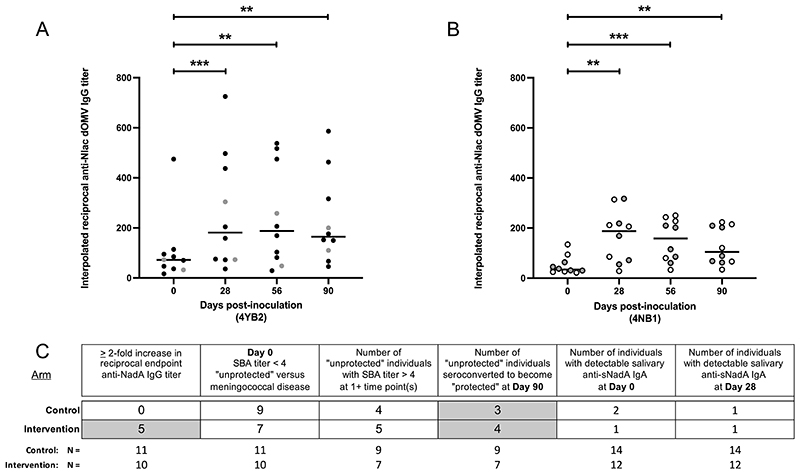
Colonization with either strain of GM-Nlac led to a sustained increase in the interpolated reciprocal titer of anti-WT Nlac dOMV-specific IgG (hereafter, anti-Nlac IgG) present in the sera of volunteers at Days 28, 56 and 90 compared to baseline (Fig. 5). As expected, in 4YB2-colonized participants (Fig. 5A), there was an increase in the titer of anti-Nlac IgG from baseline (Median = 72.3; Range = 16.7 – 474.6) to Day 90 (Median = 164.6; Range = 46.0 – 586.3). This includes a median maximum fold change in anti-Nlac IgG of 2.9 (range: 1.5 – 16.9). Compared to baseline, the increases in the titers of anti-Nlac IgG at all time points were significant (P = 0.0004, Day 28; P = 0.003, Day 56 and P = 0.003, Day 90). Similarly in 4NB1-colonized participants (Fig. 5B), there was a sustained increase in the titer of anti-Nlac IgG from baseline (Median = 34.3; Range = 21.7 – 134.5) to Day 90 (Median = 104.5; Range = 34.2 – 223.6), including a median maximum fold change of 2.8 (range: 1.4 – 12.8). Compared to baseline, the increases in the titers of anti-Nlac IgG at all time points were significant (P = 0.0055, Day 28; P = 0.0004, Day 56 and P = 0.0016, Day 90). Anti-Nlac IgG titers calculated for sera taken from 4YB2-colonized participants were not different to those taken from 4NB1-colonized participants at the same time points. These data indicate that both sets of participants were capable of generating immunological responses and that there was no generalized defect in either: (i) the ability of the participants in one or other arm to respond immunologically to infection with GM-Nlac, or (ii) one or other of the GM-Nlac strains to elicit an immunological response from the participants. These data confirmed that GM-Nlac-colonized participants were generally responsive to the presence of the colonizing bacteria and generate IgG against epitopes found on the surface of WT Nlac.
Figure 5. Colonization with genetically modified N. lactamica was associated with increased serum concentrations of IgG directed against N. lactamica surface epitopes, including NadA when present and stimulates vaccine-like serum bactericidal antibody responses versus meningococcal strain 5/99 in a subset of participants.
(A and B) Sera from volunteers colonized with (A) 4YB2 (Control, ●) or (B) 4NB1 (Intervention, ○) were assayed for IgG with specificity to epitopes present on WT Nlac dOMV (anti-Nlac dOMV IgG). Titers of anti-Nlac dOMV IgG in serum samples were interpolated with reference to serum NA9136. Bars denote median. **p ≤ 0.01, ***p ≤ 0.001 by Friedman’s twoway Analysis of Variance by Ranks with Dunn’s multiple comparisons test versus Day 0. Only complete data sets at all time points were analyzed (4YB2, 4NB1: n = 10). Participants in whose serum there was a detectable reciprocal endpoint titer of anti-sNadA IgG (a reciprocal titer ≥ 2) at one or more time points are depicted in gray. (C) Summary of antibody responses measured in serum and saliva of participants, showing the numbers of participants from both arms of the study with: (i) a ≥ 2-fold increase in the reciprocal endpoint anti-sNadA IgG titer, (ii) a baseline SBA titer < 4 (unprotected against meningococcal disease); (iii) baseline SBA titers < 4 who were shown to have SBA titer(s) ≥ 4 at one or more subsequent timepoints; (iv) baseline SBA titers < 4 who were shown to become protected against meningococcal disease and remained so until Day 90; (v) a detectable titer of salivary anti-sNadA IgA at baseline and (vi) a detectable titer of salivary anti-sNadA IgA at Day 90. Participants were considered to have a 2-fold increase in anti-NadA IgG titer if we measured a 2-fold increase in anti-sNadA IgG in at least one post-baseline serum sample. Sample sizes (N) of the Control and Intervention groups for each antibody response are shown beneath each column.
The titer of anti-sNadA IgG was also measured in each serum sample, using an endpoint enzyme-linked immunosorbent assay (ELISA) (endpoint = optical density at 490nm (OD490nm) ≥ 1.4, equivalent to an interpolated reciprocal titer of anti-sNadA IgG = 17.245 in reference serum NA9136, fig. S6). Prior to inoculation, the majority of participants had undetectable concentrations of anti-sNadA IgG in their sera (reciprocal titer: < 2) (fig. S7), consistent with the absence of colonization by NadA-expressing Nmen at enrolment. Analysis of the maximum fold changes in anti-sNadA IgG shows that, although there is no increase in anti-sNadA IgG measurable in the sera of 4YB2-colonized participants (fig. S7), we measured at least a 2-fold rise in the reciprocal endpoint titer of anti-sNadA IgG at one or more time points in 5 of the 10 4NB1-colonized participants (Fig. 5C).
Additionally, each serum sample was assayed for serum bactericidal antibody activity versus the NadA-overexpressing Nmen strain 5/99 using human complement (fig. S8). The SBA titers of participants at baseline varied in both arms of the study. In the Control arm, 9 of the 11 participants had a Day 0 interpolated SBA titer less than 4 (non-protective against 5/99-induced meningococcal disease) (24); whereas 7 of the 10 participants in the Intervention arm were similarly not protected at Day 0 (Fig. 5C). Across the 90 day course of the study, colonization with GM-Nlac resulted in participants having a protective SBA titer of greater than 4. This occurred in 4/9 participants (44.4 %) in the Control arm and 5/7 in the Intervention arm (71.4 %) (Fig. 5C). The numbers of initially unprotected participants at Day 0 who became and remained protected at Day 90 were 3 and 4 in the Control and Intervention arms, respectively (Fig. 5C). Importantly, one of the 3 participants in the Control arm was the same individual that had lost carriage of GM-Nlac after Day 28 and acquired NadA-negative Nmen carriage at some point before their visit at Day 56 (fig. S8).
Despite the expansion of sNadA-specific IgA-secreting plasma cells in 4NB1-colonized participants (Fig. 4), the presence of anti-sNadA IgA was only measured in the saliva of three individuals at Day 0, and in two individuals at Day 28 (Fig. 5C). Although total IgA titers were measured in the majority of saliva samples, measurement of sNadA-specific IgA was confounded by non-specific signals in the antigen-specific IgA ELISA (fig. S9).
Colonization with NadA-expressing GM-Nlac increases the circulating sNadA-specific memory B cell pool
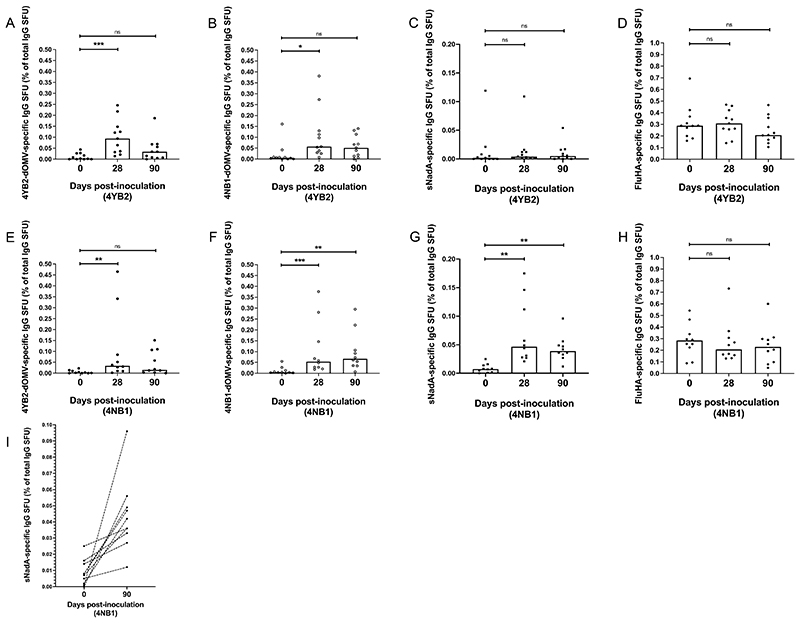
IgG B cell memory to GM-Nlac and specifically to NadA was assessed by ELISpot assay (25), assuming that one SFU in this assay equated to one memory B cell (fig. S10). In 4YB2-colonized participants, there was a significant but temporary increase in the percentage of the total number of IgG-secreting memory B cells (hereafter, percentage of memory B cells) with specificity to either 4YB2-dOMV (P = 0.0009) or 4NB1-dOMV (P = 0.0284) at Day 28, as compared to baseline. These increases were no longer evident at 90 days post-inoculation (Fig. 6A and B). Importantly, there were no differences between the percentage of memory B cells with specificity to sNadA measured at any point in these participants (Fig. 6C), and no change in IgG memory B cells with specificity to influenza hemagglutinin (FluHA, the positive control antigen) (Fig. 6D).
Figure 6. Colonization with genetically modified N. lactamica expressing NadA results in increased numbers of circulating, NadA-specific IgG memory B cells.
(A to H) PBMC isolated from whole blood at baseline and on Days 28 & 90 post-challenge with either control, WT-equivalent GM-Nlac (4YB2, A to D) or NadA-expressing GM-Nlac (4NB1, E to H) were polyclonally stimulated and assessed by ELISpot for the presence of IgG-secreting cells specific for: 4YB2-dOMV (●, A and E), 4NB1-dOMV (○, B and F), sNadA (■, C, G and I) and FluHA (control antigen) (◆, D and H). IgG-secreting cells were visualized as spot forming units (SFU) and averages were adjusted for non-specific SFU by deducting the appropriate average number of SFU from KLH-coated membranes. Antigen-specific IgG-secreting SFU are shown as a percentage of the total number of IgG-secreting SFU. Columns represents median values. ns: p> 0.05, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, by Friedman’s twoway Analysis of Variance by Ranks with Dunn’s multiple comparisons test versus Day 0. (I) Percentages of NadA-specific SFU as proportions of the total number of IgG-secreting SFU at baseline and Day 90. Points derived from the same participant are linked by dashed lines.
The same significant but transient increase in the percentage of memory B cells specific for 4YB2-dOMV was evident in 4NB1-colonized participants (P = 0.0073) (Fig. 6E), but interestingly there was also a significant increase in the percentage of IgG memory B cells specific for 4NB1-dOMV at Day 90 (P = 0.0035) (Fig. 6F). In contrast to participants in the Control arm, there was a increase in the percentage of IgG memory B cells with specificity to sNadA measured at both Day 28 (P = 0.0016) and Day 90 (P = 0.0016), compared to baseline (Fig. 6G). Again, the proportion of IgG memory B cells with specificity to influenza hemagglutinin remained unchanged (Fig. 6H), indicating stability of the assay over time and that changes to the composition of the circulating memory B cell pool in response to Nlac-dOMV or sNadA were specific to the experimental intervention. Importantly, in every participant that was colonized with strain 4NB1, there was a relative increase in the percentage of memory B cells with specificity to sNadA (Fig. 6I).
Discussion
In this study, we demonstrated the utility of GM-Nlac as a platform for the presentation of antigen to the upper respiratory tract of humans and shown its ability to elicit antigen-specific immunity during a 90-day period of asymptomatic colonization. GM-Nlac can be deployed safely, survive in its biological niche, and be effectively eradicated as necessary without transmitting to other adults living in close proximity to the study participants. Future translation of this could generate vaccines that exploit the natural biological process of colonization, or delivery systems for therapeutics or experimental medicine investigation.
We found that a single, intranasal dose of the recombinant human commensal expressing a heterologous vaccine antigen (NadA), expands antigen-specific IgG memory B cells in all recipients by 90 days after inoculation. In contrast, expansion of circulating memory B cell responses to non-NadA surface antigens was relatively transient. This could plausibly represent the difference between an immunological priming event and recall of a memory response, consistent with evidence that natural carriage of Nlac during childhood does not result in immunological priming against Nlac surface antigens (26) and the observation that NadA-specific IgG memory B cells were detected in PBMC harvested at baseline in a majority of participants. Although there is some evidence that antigen-specific plasma cells are found in peripheral blood 6 to 7 days after a booster immunization, in contrast to the appearance of such cells after day 10 in response to primary immunization (27), the dynamics are likely influenced by the route of administration and effective dose of the antigen; both of which differ from parenteral vaccination in our nasally-inoculated CHIME.
Memory B cells do not have a primary antibody-secreting function, but promptly differentiate into antibody-secreting plasma cells upon effective re-stimulation by cognate antigen (28). Commensurate with this, presentation of NadA to the upper respiratory tract mucosa by strain 4NB1 elicits increases in the numbers of both IgG-secreting and IgA-secreting, sNadA-specific plasma cells, which are largely absent when a participant is colonized by the control strain, 4YB2. Interestingly, individuals with large sNadA-specific plasma cell responses also tended to have larger 4YB2- and 4NB1-dOMV-specific plasma cell responses, suggesting that Nmen-specific memory B cells (and, by extension, plasma cells) are cross-reactive with the surface antigens of Nlac. Regarding the small number of 4YB2-colonized individuals in whom sNadA-specific plasma cells were detected, we speculate these events represent either displacement of long-lived plasma cells specific for sNadA from their niche in the bone marrow (29) or capture of plasma cells with cross reactive specificity to a closely related antigen, such as plasma cells targeting epitopes in the YadA domain of other type V autotransporters (30). The expansion of IgA-secreting plasma cells broadly reflects the patterns observed for IgG-secreting plasma cells (Figure 4), with the exception that the peak number of IgA-secreting plasma cells recognizing 4YB2-dOMV antigens in 4NB1-colonized participants was increased over baseline, whereas no change was observed in the numbers of IgG-secreting, 4YB2-dOMV-specific plasma cells. This difference could be attributed to compartmentalization of the mucosal immune response (31) or to slightly different dynamics in plasma cell formation and circulation between the IgG and IgA memory B cell pools in certain individuals.
In five of the 4NB1-colonised participants, we observed at least a two-fold increase in their reciprocal endpoint anti-sNadA IgG titer after inoculation. In the absence of an adjuvant, NadA protein was shown by other investigators to be poorly immunogenic (32). Here, we show that prolonged nasopharyngeal colonization of human volunteers with NadA-expressing GM-Nlac leads to relatively modest increases in anti-sNadA IgG titers in a subset of participants, suggesting the recombinant organism can act as both antigen presentation platform and adjuvant (20). These antibody titers, though modest, are less than a single log10 lower than titers we observed in serum after parenteral vaccination of an individual with the NadA-containing meningococcal vaccine Bexsero. Some normal human adults with no history of meningococcal disease have similar anti-NadA IgG titers to children convalescing from meningococcal infection with NadA-expressing strains (33), providing further evidence that repeated, asymptomatic colonization with upper respiratory tract commensals throughout a lifetime can lead to seroconversion and, potentially, protection from disease.
Regarding efficacy of meningococcal vaccines, an SBA reciprocal titer greater than or equal to 4 is accepted internationally as a correlate of protection (24). Parenterally-delivered vaccines such as the glycoconjugate vaccines and the non-capsular (protein-based) vaccines such as Bexsero elicit high SBA titers (34). In contrast, natural nasopharyngeal colonisation by meningococci elicits modest and variable SBA titers (35). In this study, we measured SBA activity of all colonized study participants against Nmen reference strain 5/99. Though the study was not powered to test the effect of NadA expression by GM-Nlac on SBA titers a priori, our data suggest that colonization with strain 4NB1 could potentially protect against meningococcal disease caused by NadA-expressing Nmen. In individuals in either study arm with a ‘protective’ baseline SBA titer (≥ 4), colonization for 90 days resulted in a no more than 2.9-fold increase in SBA titer, which is consistent with previous studies of intranasal re-challenge of immunized individuals with cognate antigen (36). However, individuals with non-protective baseline SBA titers became and then remained protected against meningococcal disease at Day 90 more frequently when colonized with strain 4NB1. Although these individuals did not all have detectable anti-sNadA IgG titers, it should be noted that not all anti-NadA antibody clones are bactericidal (16, 37) and that SBA responses to Bexsero are conferred by synergistic action of multiple antibody clones targeting multiple bacterial antigens (38). Therefore, the ability of a given participant to mount an SBA response is likely conditioned by their history of prior exposure to various clones of meningococci.
Some limitations of our work should be discussed. Firstly, our analyses of plasma cell numbers made no attempt to discriminate between putative priming and recall responses on the basis of the dynamics of plasma cell formation, instead comparing the dynamics-agnostic peak value of plasma cells in each participant. The observed responses could therefore represent either priming or recall, but discrimination will require further studies with strains expressing different, perhaps synthetic, antigens to which the majority of humans are immunologically naïve.
However, our data suggests at least a booster-like potential for this technology, especially as a means to provoke anamnestic responses against established vaccine antigens with which recipients have already been immunized. Secondly, the presence of NadA-specific IgG memory B cells in the circulation beyond 90 days (the duration of the study) has not been established. Such cells may exist in a senescent state for years until reactivated by exposure to cognate antigen (39), although the context of the immunization (such as colonization of a mucosal surface with a bacterial commensal versus intramuscular injection) could play a role in the dynamics of memory B cell availability and reactivation (40, 41). In this study, the eradication of carriage of each GM-Nlac strain with antibiotics at Day 90 was considered necessary, given the experimental nature of the challenge agent and the potential for the genetically modified commensal to be shed into the environment and to spread between humans. Thirdly, although the lack of shedding of GM-Nlac or onward transmission to bedroom-sharing adults is reassuring in terms of reducing the potential to inadvertently infect vulnerable, immunocompromised members of the community, the snapshots of these processes taken from a small number of participants and their close contacts may not reflect the transmissibility of the GM organism from all participants across the length of the study period. Finally, IgA-secreting plasma cells specific for NadA were detected in the blood of 4NB1-colonised participants and from this we infer the likelihood of salivary IgA being generated in these individuals. We previously demonstrated production of salivary IgA against WT Nlac dOMV following a CHIME with WT Nlac (5) but in the current study the volumes of saliva recovered were small. Although we were able to detect sNadA-specific IgA in 3 participants colonized with GM-Nlac, in other participants, the criteria for confirmation of anti-sNadA IgA were not met. Although this may be an artifact due to the methodological requirement for dilution (anti-sNadA IgA might be present in saliva, but at a reciprocal titer less than 3), this result is also biologically plausible due to sequestration of antigen-specific IgA onto the surface of colonizing bacteria, as has been suggested in relation to antibody against capsular polysaccharide in a Streptococcus pneumoniae CHIME (42).
The findings of this study showcase one application for the use of a genetically modified commensal capable of colonizing the upper respiratory tract, but the ability to deploy a safe GMO expressing heterologous antigen has wider potential utility in translational medicine. Examples include (i) analysis of the role of individual microbial gene products in the processes of colonization, carriage, and systemic and mucosal immunological responses, (ii) the impact of vaccination on the carriage of bacteria expressing cognate antigen, (iii) as an experimental platform to manipulate the composition of the upper respiratory tract microbiome and (iv) as a means of potentially inducing immunological tolerance to specific antigens or allergens. In conclusion, we have shown that intranasal inoculation of a live, genetically modified commensal, N. lactamica, can safely elicit beneficial immune responses to a pathogen-derived heterologous antigen during sustained pharyngeal carriage.
Materials and Methods
Study Design
The clinical protocol for the CHIME has been published elsewhere, including the study rationale, objectives, inclusion and exclusion criteria, and stopping rules (43). This was a prospective controlled human infection model experiment (NCT03630250) with co-primary outcomes of establishing the safety of GM-Nlac for human inoculation and NadA-specific immunogenicity in colonized participants. Standard deviations (SD) associated with serological responses to WT Nlac derived from our first Nlac CHIME were utilized to inform the sample size calculation (5). This gave SDs on a log-10 scale of 0.26 for serum IgG. Using the SD of 0.26, it was calculated that a 4-fold rise in Nlac-specific IgG titer would be confirmed with 10 carriers of (GM-)Nlac with 90% power using analysis of variance. The study was reviewed by the South Central Oxford A Research Ethics Committee (reference: 18/SC/0133). Informed consent for participation in the study was obtained from healthy volunteers aged 18-45 years. Key exclusion criteria included (i) colonization by Neisseria spp. in throat swabs at the screening (one week prior to inoculation) and challenge day visits, (ii) active cigarette or illicit drug smoking, (iii) previous vaccination with a NadA containing vaccine such as Bexsero. The study was single-blinded and block-randomized. Consent by participants to undergo enhanced hygiene training and to limit social activities for the duration of the study were obtained a priori, as was informed consent of a single, nominated bedroom-sharing close contact of inoculated participants (where applicable). Immunological data generation and analysis was done by a blinded laboratory study team. Staff responsible for blinding longitudinal samples ensured samples from the same participant were analyzed on the same plate to minimize variation. No outliers were excluded. Deliberate Release of these genetically modified organisms in a CHIME was approved by the UK government Department for Environment, Food and Rural Affairs (reference: 17/R50/01). Animal experiments were approved by the Public Health England, Porton Down Ethical Review Committee and authorized under an appropriate UK Home Office project license under the Animals (Scientific Procedures) Act 1986.
Bacterial isolates and routine culture methods
All stocks of Nlac, Nmen, and their mutant derivatives (table S1) were maintained as frozen glycerol stocks at -80 °C. Upon recovery from cryostorage, Nlac was cultured on tryptone soya agar (TSA) (Oxoid) supplemented with 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal) (40 μg ml-1) (Sigma-Aldrich) where appropriate. Nmen was cultured on Columbia agar supplemented with horse blood (5 % v/v) (CBA) (Southern Group Laboratories), supplemented with kanamycin (50 μg ml-1) (Sigma-Aldrich) where appropriate. Routine liquid culture of Nlac was performed in tryptone soya broth (Oxoid) supplemented with 0.2% yeast extract (Sigma-Aldrich) (TSB) at 37 °C, 5 % CO2.
Nmen serogroup B strain MC58 and its mutant derivatives (MC58ΔnadA& MC58ΔsiaD) were cultured in Mueller-Hinton broth (MHB) (Oxoid), supplemented with kanamycin (50 μg ml-1) and 5 mM 4-hydroxyphenylacetic acid (4HPA) (Sigma-Aldrich) as appropriate. Nmen serogroup Y strain N54.1 was cultured in MHB supplemented with 4HPA. Nmen NadA-overexpressing strain 5/99 was cultured in modified Frantz medium (44).
For determination of clinically relevant antibiotic MICs, Nlac was cultured as a lawn on Mueller-Hinton agar (MHA) supplemented with horse blood (5 % v/v) (Oxoid) in the presence of the appropriate e-test strips: ciprofloxacin and ceftriaxone (Biomerieux). Note that as no interpretative criteria exist for determining antibiotic susceptibility of Nlac (WT or GM), the lowest value for each antibiotic from two reference criteria were used. The first was the European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints for Nmen and the effective treatment of meningococcal disease and the second was the antibiotic susceptibility profile of 286 isolates of WT Nlac (22).
Detection and identification of Neisseria species in clinical samples
Neisseria species were isolated from clinical samples using commercially made gonococcal selective (GC) agar plates, supplemented with 10 % (v/v) lysed horse blood, VCAT selective supplement (1 % v/v), Vitox supplement (2 % v/v), glucose (0.4 % w/v), Amphotericin B (10 μg ml-1) and X-gal (40 μg ml-1) (GC+Xgal) (Southern Group Laboratories). Pre-clinical tests demonstrated that only Nlac grew as blue-colored colonies. For each participant at each time point, up to 10 colonies of blue, putative GM-Nlac were subcultured onto fresh GC+X-gal plates for the preparation of stocks and lysates for diagnostic PCR. White-colored, putative Nmen colonies were tested for oxidase activity using oxidase detection strips (Oxoid). All oxidase positive, Gram-negative diplococci were subcultured onto fresh Columbia agar plates supplemented with chocolated horse blood (5 % v/v) (CHOC) (Southern Group Laboratories), incubated overnight at 37 °C, 5 % CO2 and then identified using API NH kits (Biomerieux), performed according to the manufacturer’s instructions. Bacterial stocks were created of all carriage Nmen isolates identified during the study.
Culture methods for preparation of human challenge inocula
To prepare the inocula, seed cultures of the appropriate GM-Nlac strain were grown overnight at 37 °C, 5 % CO2 on TSA plates supplemented with X-gal. Blue-colored GM-Nlac colonies were subcultured in TSB at 37 °C, 5 % CO2 to an optical density at 600nm (OD600nm) of 1.5 arbitrary units. Bacteria were washed and then resuspended in Frantz medium:glycerol (70 % : 30 % v/v). Aliquots were stored in numbered “Master Stock” cryovials at -80 °C. Master stock viability and purity was determined by culture on TSA, CBA and CHOC plates overnight at 37 °C, 5 % CO2. Subsequently, contaminant-free “Master Stock” vials were diluted into ice-cold Frantz medium:glycerol (70% : 30% v/v) to a final concentration of 5 x 106 CFU ml-1. One-milliliter aliquots were then stored in numbered “Inoculum” cryovials at -80 °C. The average viability of the “Inoculum” vials was calculated as 4.8 x 106 CFU ml-1 for strain 4YB2 (n = 7) and 4.8 x 106 CFU ml-1 (n = 8) for strain 4NB1.
Preparation of inoculum for intranasal administration
A 1 ml “Inoculum” vial containing the appropriate GM-Nlac strain was thawed at room temperature, vortexed, and 500 μl was diluted into 4.5 ml sterile phosphate buffered saline (PBS) pH 7.4 (Severn Biotech). Intranasal inoculation of participants occurred within 30 min of defrosting the vial and was performed with sterile Pasteur pipettes (1 per nostril). The purity and viability of the inoculation dose was determined by culture on CHOC agar.
Preparation of gDNA
Bacterial gDNA was harvested from mid log phase cultures of each bacterial strain using a GeneJET Genomic DNA Purification kit (Thermo Fisher Scientific) according to Protocol D (Gram-negative bacteria) in the manufacturer’s instructions. The concentration of gDNA was measured using a Nanodrop spectrophotometer (Shimadzu).
Assessment of transformation efficiency in Neisseria species
Bacteria were pelleted from mid-log phase liquid cultures of Neisseria species, washed in PBS and resuspended in TSB supplemented with 10 mM MgSO4 at an OD600nm = 0.3. One milliliter aliquots of each bacterial suspension were incubated at 37 °C, 5 % CO2 for 3 hours with 1 x 104 pmoles of gDNA harvested from one of the following Nlac or Nmen mutant strains: (i) Nlac strain Y92-1009Δnlalll, (ii) Nmen strain MC58 ΔnadA, (iii) Nmen strain MC58ΔsiaD. Aliquots of each suspension were spread on CBA plates supplemented with kanamycin (50 μg ml-1). The total viability (in CFU ml-1) of each suspension was determined through serial dilution and plating on CBA. The number of kanamycin resistant colonies (in CFU ml-1) was divided by the total number of bacteria (in CFU ml-1) and normalized to 1 pmol gDNA to give transformation efficiency.
Diagnostic Polymerase Chain Reaction (PCR)
Bacterial isolates from clinical samples were identified as GM-Nlac using multiplex PCR. Briefly, lysates from putative GM-Nlac colonies were used as template DNA to amplify a GM signature sequence (Band3d, 284 bp) or the 16S rRNA gene (Band1, 469 bp). Genomic DNA from GM-Nlac strain 4NB1 (50 ng ml-1) was used as a Band3d positive control, gDNA from WT Nmen strain H44/76 (50 ng ml-1) was used as a Band1 positive control, and RNAse-free H2O was used as a negative control. Each PCR reaction volume (20 μl) comprised one of: control gDNA, test lysate or RNAse-free H2O (1 μl), plus Platinum II Taq Hot-Start DNA polymerase Master mix (2x) (Thermo Fisher Scientific) (10 μl), Band1FOR (10 μM) (0.8 μl), Band1REV (10 μM) (0.8 μl), Band3dFOR (5 μM) (0.4 μl), Band3dREV (5 μM) (0.4 μl) and RNAse-free H2O (6.6 μl). Primer sequences are listed in table S2. PCR amplification used the following, 2-step thermal cycling parameters: [1 x 2 minutes @ 95 °C] followed by [35 x (5 seconds @ 98 °C, 15 seconds @ 60 °C and 15 seconds @ 68 °C)]. Amplicons were analyzed by 2% agarose gel electrophoresis and visualized under ultraviolet transillumination using a Gel Doc XR+ (Bio Rad). GM-Nlac was positively identified if two amplicons of 284 bp and 469 bp were present. A single amplicon of 469 bp was determined to be something other than GM-Nlac. PCR yielding zero amplicons was considered to have failed, and was repeated until definitively identified as being, or not being, GM-Nlac.
Flow cytometry
Surface expression of NadA on Neisseria spp. was assessed by flow cytometry of fixed cells. Briefly, bacteria (2 x 107 CFU) were labeled with either: (i) a 1:100 dilution of anti-NadA mAb 6e3 (16) or (ii) a 1:50 dilution of anti-NadA pAb [JRL-PE-3] in wash buffer (PBS + 5 % fetal calf serum (FCS)) for 30 minutes at 4 °C. Cells were then labeled with: (i) a 1:100 dilution of goat-derived, anti-mouse IgG-Alexa Fluor488 pAb (Jackson Immunoresearch Laboratories) or (ii) a 1:100 dilution of goat-derived, anti-rabbit IgG-Alexa Fluor488 pAb (Jackson Immunoresearch Laboratories) in wash buffer for 30 minutes at 4 °C, and then fixed in formalin for 15 minutes. Bacteria were gated based upon forward and side scatter profiles and a total of 10,000 events were acquired on a BD FACSAria II (BD Biosciences). Relative NadA expression was measured as the median fluorescence intensity (MFI) of Alexa Fluor488.
Anti-NadA immunoblotting
Expression of the NadA protein was verified by western blot. GM-Nlac strain 4NB1 and WT Nmen strains MC58 and N54.1 were grown to OD600nm = 0.4 in the presence of 4HPA, centrifuged and then washed once in PBS before final resuspension into 500 μl bacterial lysis buffer (50 mM Tris HCl (pH 8.0) + 10 % (v/v) glycerol + 0.1 % Triton X-100) (BLB) supplemented with 100 μg ml-1 lysozyme (Sigma-Aldrich) and protease inhibitor cocktail (Sigma-Aldrich). Samples were incubated for 30 min at 30 °C. Bacteria were then transferred to ice before lysis through sonication (3 x 15 sec bursts). Sonicated lysates were supplemented with 1 mM phenylmethylsulfonyl fluoride (PMSF), 2 mM MgSO4 and recombinant DNAse (Thermo Fisher Scientific), and then incubated at 30 °C for 30 min. Crude membrane preparations were collected by centrifugation of each sample at 17,000 g for 1 h at 4 °C. The pellet was solubilized in 1x Reducing LDS Sample Buffer (Thermo Fisher Scientific) at 4 μl mg-1 wet weight, then heated to 95 °C for 10 min. Following a final centrifugation to remove insoluble material, the protein concentration of each sample was determined using the RC DC Protein Assay (BioRad) according to the manufacturer’s instructions and in comparison to bovine serum albumin (BSA) Standards in LDS sample buffer. Five micrograms (5 μg) of protein were loaded per lane onto a NuPAGE 4-12 % Bis Tris gel (Thermo Fisher Scientific) and separated by electrophoresis. The proteins were transferred to methanol-activated polyvinylidene difluoride (PVDF) membrane (BioRad), which was then blocked using a solution of 5 % fat-free milk powder in PBS (5 % MPBS). The membrane was probed overnight at 4 °C with a 1:250 dilution of anti-NadA pAb [JRL-PE-3] in 5 % MPBS. The secondary antibody was a 1:20,000 dilution of goat-derived, anti-rabbit IgG conjugated to horseradish peroxidase (HRP) (Jackson ImmunoResearch Laboratories) in 5 % MPBS. The blot was developed onto photographic film using ECL reagents (Pierce).
Adherence assay
HEP-2 cells (2 x 105 cells well-1) were seeded into Corning Costar 24-well plates and cultured for 2 days in Dulbecco’s Modified Eagle’s medium (DMEM) (Gibco, Invitrogen) supplemented with 10 % FCS at 37 °C, 5 % CO2. HEP-2 cells were then washed twice in sterile PBS, then infected at a multiplicity of infection (MOI) = 100 with the relevant bacterial strain. Plates were incubated at 37 °C, 5 % CO2 and at 2, 4 and 6 hours post infection, supernatants were aspirated and wells washed five times with PBS. Two percent (w/v) saponin (Sigma-Aldrich) in PBS (250 μl well-1) was added and plates were incubated for 15 minutes at 37 °C, 5 % CO2. Wells were supplemented with PBS to a final volume of 1 ml and cells were mechanically agitated through pipetting. The number of viable bacteria was determined by plating serially-diluted saponized cell lysate onto CBA plates. The viability of each lysate was normalized to the number of HEP-2 cells per well, which was estimated from the cell count of duplicate wells.
Isolation of Outer Membrane Vesicles
OMV were deoxycholate-extracted from bacterial cell pellets grown in modified Caitlin (MC7) medium (44). Briefly, Nlac were cultured overnight at 37 °C, 5 % CO2 to OD600nm > 2.0 and pelleted by centrifugation at 4500 g for 1 hour. Cell pellets were resuspended using a glass homogenizer into 0.1 M Tris-HCl (pH 8.6) with 10 mM EDTA and 0.5 % (w/v) deoxycholic acid sodium salt (Sigma-Aldrich). These suspensions were centrifuged at 20,000 g for 30 minutes at 4 °C, and the supernatant was retained. The homogenization procedure was performed twice. dOMV were harvested from the combined supernatants by ultracentrifugation at 100,000 g for 2 h at 4 °C. Pelleted dOMV were gently resuspended into 50 mM Tris-HCl (pH 8.6) with 2 mM EDTA, 20 % (w/v) sucrose and 1.2 % deoxycholic acid sodium salt. dOMV were again pelleted by ultracentrifugation at 100,000 g for 2 hours at 4 °C, before resuspension into 50 mM Tris-HCl with 3 % sucrose, which formed the final formulation. The protein content of the dOMV formulation was measured by the DC Protein assay (BioRad), with reference to a BSA standard curve.
Murine Immunizations with dOMV
Groups of ten 6-8-week-old, female BALB/c mice (Envigo) were immunized by subcutaneous injection with three doses of 10 μg Nlac dOMV containing 0.33% Alhydrogel (45) at day 0, 21 and 28. Sera were collected on day 35.
Murine intraperitoneal challenge model
Bacteria were pelleted from mid-log phase cultures of WT Nlac strain Y92-1009 and the GM-Nlac strains 4YB2 and 4NB1 and resuspended at different concentrations into 50 μl PBS supplemented with 10 mg of human holo-Transferrin (Sigma-Aldrich). Six groups of NIH/OLA mice (6-8 weeks), comprised of 5 male and 5 female mice per group (Envigo), were challenged with “high” or “low” intraperitoneal doses of the Nlac or GM-Nlac strains. Viability of each dose was determined by colony count on CBA plate cultures. Twenty-four hours after challenge, the mice were given an additional intraperitoneal dose of 10 mg human holo-Transferrin. The health of the mice was assessed based on their outward appearance and behavior and was recorded every 4 hours in the first 2 days and then every 6 to 8 hours up to 5 days post-challenge. Behaviorally normal mice were considered healthy (recorded as H), whereas those experiencing mild discomfort presented as arched (recorded as A) or ruffled (recorded as R).
Serum bactericidal assays
All SBA assays were performed against Nmen strain 5/99, resuspended in bactericidal buffer (BB; Hanks buffered saline solution (Invitrogen) supplemented 1% (v/v) with BSA) at 6 x 104 CFU ml-1. Two-fold dilutions of heat-inactivated sera were prepared in microtiter plates (20 μl), to which Nmen (10 μl) was added followed by IgG-depleted pooled human plasma as an exogenous human complement source (10 μl) (25% final concentration). Plates were incubated at 37°C for 1 hour with shaking at 900 rpm. Each sample and control well was then plated onto CBA using the tilt method and incubated overnight at 37 °C, 5% CO2. The following day colonies were counted and percent survival was determined in comparison to colony numbers in the t = zero control. Dilutions of sera were deemed bactericidal if bacterial survival rates were ≤ 50 %. SBA reciprocal titers were determined as the reciprocal of the most diluted serum sample that was bactericidal.
Recombinant expression of the soluble domain of NadA
Recombinant expression of sNadA was carried out commercially by BioServUK, broadly consistent with the methodology described by Cecchini and colleagues (46). Briefly, the coding sequence for nadA allele 1, minus the sequence coding for the C-terminal YadA domain (nadAΔ285-364), was cloned from pJL0017 into pRSETα to include a C-terminal 6His-tag. Plasmid sequences are listed in table S3. Protein secreted into growth medium by the transformed E. coli strain BL21 (DE3) was purified using HiFliQ column chromatography and buffer exchanged into PBS (pH 7.5). Analysis of purified sNadA by SDS-PAGE showed a single band by silver staining under reducing and denaturing conditions, and three bands, consistent with homotrimer formation, under denaturing but non-reducing conditions. Purified sNadA was cross-reactive with anti-NadA mAb 6e3 (16) in western blotting analysis. Purified sNadA was stored at -80 °C and diluted in PBS to make working stocks (1 mg ml-1), which were stored at -20 °C.
ELISA for measurement of human anti-Nlac IgG
Nunc Maxisorb 96-well plates were coated overnight at 5 °C with WT Nlac Y92-1009 dOMV (10 μg ml-1) in carbonate coating buffer (pH 9.6). Coated plates were washed five times with PBS containing 1 % Tween 20 (PBST) (Skatron SkanWasher 400) and blocked with PBST containing 5 % (v/v) FCS for 90 min at room temperature. Plates were washed five times with PBST and then loaded in duplicate with serially diluted test samples and reference serum (NA9136). Dilutions were prepared in Sterilin Serowell (low binding) plates with a starting dilution of 1:25 followed by three-fold serial dilution across the plate. Loaded plates were incubated with shaking at room temperature for 90 minutes. Plates were then washed five times with PBST prior to incubation at room temperature for 90 minutes with goat anti-human IgG Fcγ-fragment-specific alkaline phosphatase conjugate (Jackson Immunoresearch Laboratories) diluted 1:1750 in PBST + 5 % (v/v) FCS. This was followed by a wash step as above, and addition of p-nitrophenyl phosphate (AP yellow) substrate (BioFX Laboratories) for 55 minutes at room temperature. The reaction was stopped with 3M NaOH. Optical density (OD) was read using a VersaMax plate reader at 405nm with a reference wavelength of 690nm. SoftMax PRO Enterprise software (Molecular Devices) was used to fit an un-weighted five parameter logistic (5PL) log curve to titrations of the reference serum for each plate. Test sample titers were calculated by interpolation from the reference serum dose response curve (interpolated titer multiplied by dilution factor). A mean of the acceptable values from all dilutions was then taken as the final value for each test serum.
Antigen-specific immunoglobulin detection by ELISA
A similar method was performed to detect both: (i) anti-sNadA IgG in participant serum and (ii) anti-sNadA IgA in participant saliva samples. For both assays, Corning Costar EIA/RIA 96 well plates were coated at 4 °C overnight with one of: sNadA (10 μg ml-1) in carbonate coating buffer (pH 9.6), bovine serum albumin (BSA) (10 μg ml-1) in carbonate coating buffer (pH 9.6), or carbonate coating buffer (pH 9.6) alone (uncoated wells). Coated plates were washed three times with PBS and blocked with 5 % FCS-PBS at 37 °C for 2 h. Plates were then washed four times with PBS. Appropriately-coated wells were then loaded with: (i) twofold serial dilutions of participant sera, or serum NA9136 diluted 1:16 in 5 % FCS-PBS as a positive control (50 μl); or (ii) a threefold dilution of participant saliva, or a 1:4 dilution of saliva NP12803 (found to contain anti-BSA IgA) in ChonBlock Sample Diluent/Blocking buffer (SD/B buffer) (Chondrex Inc.) (50 μl). Loaded plates were incubated at 37 °C, 5 % CO2 for 1 hour, then washed five times with PBS supplemented with 0.05 % Tween 20 (PBS-T). Dilutions of primary antibody were then added to each well: (i) a 1:1000 dilution of biotinylated, rat-derived anti-human IgG mAb (M1310G05) (BioLegend) in 5 % FCS-PBS (50 μl); or (ii) a 1:1000 dilution of mouse-derived anti-human IgA mAb, [3B7] (Abcam) in SD/B buffer (50 μl). Plates were incubated at 37 °C, 5 % CO2 for 1 hour then washed five times with PBS-T. Dilutions of secondary antibody, conjugated to horseradish peroxidase (HRP) were then added to each well: (i) a 1:1000 dilution of streptavidin-HRP (Biolegend) in 5 % FCS-PBS (50 μl); or (ii) a 1:1000 dilution of rat-derived, anti-mouse IgG mAb [SB77e] conjugated to HRP (abcam) in Chonblock detection antibody dilution buffer (Chondrex Inc.) (50 μl). Plates were incubated at 37 °C, 5 % CO2 for 1 hour then washed five times with PBS-T and a single wash of PBS.
Chromogenic substrate, o-phenylenediamine dihydrochloride (OPD) (Thermo Fisher Scientific), was prepared by dissolving 1 OPD tablet into 9 ml of dH2O supplemented with 1 ml stable peroxide substrate buffer (Thermo Fisher Scientific). OPD was added to each well (100 μl) and incubated at 32 °C, 5 % CO2 for exactly 20 minutes, at which point the reaction was stopped by addition of 2N H2SO4 (50 μl). Color change was quantified on a Versamax plate reader (Molecular Devices) measuring at OD490nm. The reciprocal endpoint titer of each test serum was considered to be the reciprocal titer of the least diluted serum sample tested that generated an OD490nm in excess of 1.4 arbitrary units in this assay. The threshold for a positive signal in a sNadA-coated well was an OD490nm reading equal to or in excess of 0.07, equivalent to the mean + 3SD of the OD490nm of anti-sNadA IgA-negative saliva samples in sNadA-coated wells (0.04518 ± 0.008153 (N = 45)). The threshold for a positive signal in uncoated wells was an OD490nm reading equal to or in excess of 0.068, equivalent to the mean + 3SD of the OD490nm of anti-sNadA IgA-negative saliva samples in uncoated wells 0.04511 ± 0.007796 (N = 45). For a saliva sample to be considered “anti-sNadA IgA positive” in this ELISA (to be considered as containing anti-sNadA IgA at a reciprocal titer equal to or in excess of 3), we required a positive signal in the sNadA-coated well and a negative signal in the uncoated well. Positive signals in uncoated wells were considered to result from non-specific interactions of the sample with another component of the ELISA.
ELISA for measurement of total human sIgA
Corning Costar EIA/RIA 96 well plates were coated at 4 °C with murine anti-human IgA mAb [3B7] (20 μg ml-1) in carbonate coating buffer (pH 9.6) (50 μl). Saliva samples were diluted x30, x300 and x3000-fold in SD/B buffer. Solutions of human IgA (isolated from colostrum) (Sigma-Aldrich) were prepared at 1 mg ml-1, 0.1 mg ml-1 and 0.01 mg ml-1. Following plate washing (3 x PBS) and well blocking with 5 % FCS-PBS for 2 hours at 37 °C, 50 μl aliquots of each standard and sample dilution were loaded into duplicate wells and incubated for 1 hour at 37 °C. Plates were washed five times with PBS-T. Murine anti-human IgA mAb [1H9], conjugated to HRP, was diluted 1:1000 times in Chonblock detection antibody dilution buffer and added to each well (50 μl). Plates were incubated at 37 °C, 5 % CO2 for 1 hour, then washed five times with PBS-T and a single wash with PBS. The plate was incubated with OPD substrate (100 μl well-1) at 32 °C for exactly 20 minutes. Signal development was stopped with 2 N H2SO4 (50 μl well-1), and the color-change quantified on a Versamax plate reader (Molecular Devices) measuring at OD490nm. The average of duplicate OD490nm readings was used to interpolate the concentration of IgA present in each saliva sample with reference to the IgA standards. Where more than one interpolation was possible, the IgA concentration was calculated as the average value of the interpolations.
Isolation of peripheral blood mononuclear cells
Whole blood was collected from CHIME participants at day 0, day 28 and day 90 post infection. PBMC were isolated from blood by density gradient centrifugation and used immediately in the plasma cell ELISpot assay. The remainders were stored in LN2 for later use in the memory B cell ELISpot assay.
Polyclonal stimulation of peripheral blood mononuclear cells
PBMC were cultured and polyclonally stimulated as outlined previously (47). Briefly, PBMC isolated from the whole blood of participants were cultured separately in 96-well tissue culture plates (Thermo Fisher Scientific) at 2 x 105 cells per well in 200 μl AIM/V + albumax medium (Gibco, Invitrogen) supplemented with 10 % FCS and 50 μM β-mercaptoethanol (hereafter, AIM/V+). Cultures were supplemented with 3 μg ml-1 human phosphorothioate-modified oligodeoxynucleotide containing CpG motifs (ODN2006: 5’-TCG TCG TTT TGT CGT TTT GTC GTT-3’) (InvivoGen), 10 ng ml-1 interleukin (IL)-2 (R&D Systems), and 10 ng ml-1 IL-10 (Pharmingen, BD), and incubated for 5 days at 37 °C, 5 % CO2.
ELISpot Assay
Ninety-six-well ELISpot plates (Multiscreen HTS plate, Merck Millipore) were activated with 70 % (v/v) ethanol, washed three times with PBS and coated with 100 μl PBS containing one of the following antigens: keyhole limpet hemocyanin (KLH) (Sigma-Aldrich) (10 μg ml-1), tetanus toxoid (TT) (2.5 level of flocculation (LOF) units /ml), influenza hemagglutinin (FluHA) (influenza antigen reagent 09/174, H1N1, NIBSC) (0.5 μg ml-1), dOMV derived from GM-Nlac strain 4YB2 (4YB2-dOMV) (10 μg ml-1), dOMV derived from GM-Nlac strain 4NB1 (4NB1-dOMV) (10 μg ml-1), sNadA (10 μg ml-1) or rat-anti-human IgG mAb (clone M1310G05, IgG2a, k, BioLegend) (anti-IgG mAb) (10 μg ml-1). All plates were stored at 4 °C overnight. Prior to use, plates were washed four times with PBS and blocked by incubation at 37 °C for 2 hours with AIM-V medium supplemented with 10 % FCS. Blocked plates were washed four times with PBS before adding PBMC to each well and incubating for 18 hours at 37 °C, 5 % CO2. For the plasma cell ELISpot, PBMC were freshly isolated and plated in duplicate at 2 x 105 cells well-1 in AIM-V medium + 10 % FCS. For the memory B cell assay, polyclonally-stimulated PBMC were harvested and washed in AIM/V+ media by centrifugation at 300 g. Cells were resuspended in AIM/V+ medium at a concentration of 5 x 106 cells ml-1, serially diluted, and seeded into ELISpot plate wells (200 μl volume) in duplicate or triplicate at concentrations of 5 x 106 cells ml-1, 2 x 106 cells ml-1, 1 x 106 cells ml-1 and 2.5 x 105 cells ml-1.
Following incubation, plates were washed four times with PBS-T and cells were lysed by washing three times with dH2O, followed by a 5 minute incubation at room temperature in dH2O. Plates were washed as before and incubated for 1 hour at 37 °C, 5 % CO2, with alkaline phosphatase-conjugated anti-human IgG pAb (Sigma-Aldrich), prepared in PBS supplemented with 0.01 % Tween 20 and 1 % goat serum (Binding Buffer). Unbound pAb was removed by washing with PBS-T, rinsing the backs of the PVDF membranes gently with tap water and a final three washes with PBS. Plates were then incubated at room temperature with 50 μl well-1 of 5-bromo-4-chloro-3-indolyl phosphate (BCIP) substrate solution (prepared from SigmaFAST BCIP tablets (Sigma-Aldrich) in dH2O) for 13 minutes, before washing with tap water. ELISpot plates were dried and imaged using an AID ELISpot reader. IgG spot-forming units (SFU) were counted using the AID ELISpot software package, version 3.5. For the memory B cell assay, antigen-specific IgG SFU frequencies were derived for NadA, 4NB1-dOMV, and 4YB2-dOMV-coated wells from the lowest input cell concentration where a mean of 1-20 IgG SFU were counted following subtraction of KLH background. These antigen-specific SFU were expressed as a percentage of total IgG SFUs, as described previously (48).
Statistical Analysis
All statistical analyses were performed using GraphPad Prism software (Version 8.0) (Graphpad Software Inc.). Normality tests were performed using Shapiro-Wilk, and nonnormally distributed data (P < 0.05) were analyzed using a nonparametric two-tailed Wilcoxon matched pairs signed rank test when comparing two groups or a Friedman test with Dunn’s multiple comparison posttest when assessing more than two groups. Statistical analysis of normally distributed data was carried out using a standard t test, or two-way ANOVA with Dunett’s multiple comparisons test. Parametrically and non-parametrically-distributed continuous variables are summarized with mean ± standard deviation (SD) or median and range, respectively. All p values were two-tailed and p values ≤ 0.05 were considered statistically significant.
Supplementary Material
One Sentence Summary:
An engineered commensal bacteria with a surface-expressed vaccine target provoked antigen-specific antibodies during pharyngeal carriage in humans.
Acknowledgements
We thank all of our study participants and the staff at the NIHR Clinical Research Facility at Southampton General Hospital for delivery of the clinical component of this study, notably Nursing Lead Sarah Horswill, Human Challenge Coordinator Sara Hughes and the CRF laboratory support staff. We thank Caroline Barker and the members of the Southampton Public and Patient Involvement group for their assistance in shaping the national press release that preceded our application to DEFRA. We thank Martin Cannell and Ivy Wellman from DEFRA and Richard Lockey from University of Southampton for guiding us through the Deliberate Release application process. We also thank Mariagrazia Pizza and colleagues at GlaxoSmithKline for antibody reagents, and Chris Bayliss for kindly providing meningococcal strain N54.1. We are grateful to Professor Chistoph Tang and Professor Joel Ernst for their valuable insights.
Funding
This work was funded by the following Medical Research Council (MRC) grants: MR/N013204/1 (to R.C.R.) and MR/N026993/1 (to R.C.R.), in addition to the MRC Confidence in Concept Award (to J.R.L.). Additional funding was from the Wessex Institute for Vaccines and Infectious Disease (to R.C.R.and J.R.L.). R.C.R. is an NIHR Senior Investigator (NF-SI-0617-10010). A.P.D. is supported by the Wellcome Trust Research Training Fellowship (203581/Z/16/Z). The work is also supported by the National Institute for Health Research Southampton Biomedical Research Centre (IS-BRC-1215-20004).
Footnotes
Author contributions: J.R.L., D.G., A.P.D., M.C.M., S.N.F., A.R.G. and R.C.R. conceptualized and designed the study. D.G., H.D.G., S.N.F. and R.C.R. wrote the clinical protocol. J.R.L., Z.C.P., G.B., and K.B. derived and characterized the recombinant strains of GM Nlac. H.E.H. performed pre-clinical immunizations and the pre-clinical infection model. J.R.L., C.N.W. and E.F.R. manufactured the challenge inocula. D.G., J.R.L., C.N.W., E.F.R., M.M.I., A.R.H.efefsf D.W.C. and A.K.P. conducted or supported the clinical study. E.F.R. and A.R.H. performed the plasma cell ELISpot assays and analyzed the plasma cell ELISpot data. H.E.H., L.A. and J.R.L. performed the serological ELISAs and analyzed the serological ELISA data. A.P.D. and J.R.L. performed the memory B cell ELISpots and analyzed the memory B cell ELISpot data. J.M.G., M.A. and J.R.L. performed the salivary ELISAs and analyzed the salivary ELISA data. H.E.H. and L.A. performed the SBA assays. J.R.L, A.P.D., A.R.H., D.G. and R.C.R. wrote the paper.
Competing interests: J.R.L. and R.C.R. are listed as inventors on a patent for genetic manipulation of Nlac (WO2017103593A1: Meningococcal infection and modified Neisseria lactamica.). Personal fees for advisory board participation or grants for contract commercial clinical trials were paid to S.N.F.’s institution (with no personal payment of any kind) from AstraZeneca/Medimmune, Sanofi, Pfizer, Sequrius, Sandoz, Merck, GSK, J&J, Merck and Valneva, outside the submitted work. All other authors declare no competing interests.
Data Availability
All data associated with this study are present in the paper or the Supplementary Materials. Materials are available upon request from the corresponding author.
References
- 1.Goldblatt D, Hussain M, Andrews N, Ashton L, Virta C, Melegaro A, Pebody R, George R, Soininen A, Edmunds J, Gay N, et al. Antibody responses to nasopharyngeal carriage of Streptococcus pneumoniae in adults: a longitudinal household study. J Infect Dis. 2005;192:387–393. doi: 10.1086/431524. [DOI] [PubMed] [Google Scholar]
- 2.Hall DB, Lum MK, Knutson LR, Heyward WL, Ward JI. Pharyngeal carriage and acquisition of anticapsular antibody to Haemophilus influenzae type b in a high-risk population in southwestern Alaska. Am J Epidemiol. 1987;126:1190–1197. doi: 10.1093/oxfordjournals.aje.a114758. [DOI] [PubMed] [Google Scholar]
- 3.Jones GR, Christodoulides M, Brooks JL, Miller AR, Cartwright KA, Heckels JE. Dynamics of carriage of Neisseria meningitidis in a group of military recruits: subtype stability and specificity of the immune response following colonization. J Infect Dis. 1998;178:451–459. doi: 10.1086/515622. [DOI] [PubMed] [Google Scholar]
- 4.Gold R, Goldschneider I, Lepow ML, Draper TF, Randolph M. Carriage of Neisseria meningitidis and Neisseria lactamica in infants and children. J Infect Dis. 1978;137:112–121. doi: 10.1093/infdis/137.2.112. [DOI] [PubMed] [Google Scholar]
- 5.Evans CM, Pratt CB, Matheson M, Vaughan TE, Findlow J, Borrow R, Gorringe AR, Read RC. Nasopharyngeal colonization by Neisseria lactamica and induction of protective immunity against Neisseria meningitidis. Clin Infect Dis. 2011;52:70–77. doi: 10.1093/cid/ciq065. [DOI] [PubMed] [Google Scholar]
- 6.Deasy AM, Guccione E, Dale AP, Andrews N, Evans CM, Bennett JS, Bratcher HB, Maiden MC, Gorringe AR, Read RC. Nasal Inoculation of the Commensal Neisseria lactamica Inhibits Carriage of Neisseria meningitidis by Young Adults: A Controlled Human Infection Study. Clin Infect Dis. 2015;60:1512–1520. doi: 10.1093/cid/civ098. [DOI] [PubMed] [Google Scholar]
- 7.Pandey A, Cleary DW, Laver JR, Gorringe A, Deasy AM, Dale AP, Morris PD, Didelot X, Maiden MCJ, Read RC. Microevolution of Neisseria lactamica during nasopharyngeal colonisation induced by controlled human infection. Nat Commun. 2018;9:4753. doi: 10.1038/s41467-018-07235-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sardinas G, Reddin K, Pajon R, Gorringe A. Outer membrane vesicles of Neisseria lactamica as a potential mucosal adjuvant. Vaccine. 2006;24:206–214. doi: 10.1016/j.vaccine.2005.07.064. [DOI] [PubMed] [Google Scholar]
- 9.Chi H, Wang X, Shao Y, Qin Y, Deng Z, Wang L, Chen S. Engineering and modification of microbial chassis for systems and synthetic biology. Synth Syst Biotechnol. 2019;4:25–33. doi: 10.1016/j.synbio.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michon C, Langella P, Eijsink VG, Mathiesen G, Chatel JM. Display of recombinant proteins at the surface of lactic acid bacteria: strategies and applications. Microb Cell Fact. 2016;15:70. doi: 10.1186/s12934-016-0468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Z, Chen X, Sheng H, Shen X, Sun X, Yan Y, Wang J, Yuan Q. Engineering probiotics as living diagnostics and therapeutics for improving human health. Microb Cell Fact. 2020;19:56. doi: 10.1186/s12934-020-01318-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 13.van der Ende A, Hopman CT, Dankert J. Multiple mechanisms of phase variation of PorA in Neisseria meningitidis. Infect Immun. 2000;68:6685–6690. doi: 10.1128/iai.68.12.6685-6690.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fagnocchi L, Pigozzi E, Scarlato V, Delany I. In the NadR regulon, adhesins and diverse meningococcal functions are regulated in response to signals in human saliva. J Bacteriol. 2012;194:460–474. doi: 10.1128/JB.06161-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alamro M, Bidmos FA, Chan H, Oldfield NJ, Newton E, Bai X, Aidley J, Care R, Mattick C, Turner DP, Neal KR, et al. Phase variation mediates reductions in expression of surface proteins during persistent meningococcal carriage. Infect Immun. 2014;82:2472–2484. doi: 10.1128/IAI.01521-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertoldi I, Faleri A, Galli B, Lo Surdo P, Liguori A, Norais N, Santini L, Masignani V, Pizza M, Giuliani MM. Exploiting chimeric human antibodies to characterize a protective epitope of Neisseria adhesin A, one of the Bexsero vaccine components. FASEB J. 2016;30:93–101. doi: 10.1096/fj.15-273813. [DOI] [PubMed] [Google Scholar]
- 17.Bidmos FA, Neal KR, Oldfield NJ, Turner DP, Ala ’Aldeen DA, Bayliss CD. Persistence, replacement, and rapid clonal expansion of meningococcal carriage isolates in a 2008 university student cohort. J Clin Microbiol. 2011;49:506–512. doi: 10.1128/JCM.01322-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capecchi B, Adu-Bobie J, Di Marcello F, Ciucchi L, Masignani V, Taddei A, Rappuoli R, Pizza M, Arico B. Neisseria meningitidis NadA is a new invasin which promotes bacterial adhesion to and penetration into human epithelial cells. Mol Microbiol. 2005;55:687–698. doi: 10.1111/j.1365-2958.2004.04423.x. [DOI] [PubMed] [Google Scholar]
- 19.Masignani V, Pizza M, Moxon ER. The Development of a Vaccine Against Meningococcus B Using Reverse Vaccinology. Front Immunol. 2019;10:751. doi: 10.3389/fimmu.2019.00751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorringe AR, Taylor S, Brookes C, Matheson M, Finney M, Kerr M, Hudson M, Findlow J, Borrow R, Andrews N, Kafatos G, et al. Phase I safety and immunogenicity study of a candidate meningococcal disease vaccine based on Neisseria lactamica outer membrane vesicles. Clin Vaccine Immunol. 2009;16:1113–1120. doi: 10.1128/CVI.00118-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrison OB, Claus H, Jiang Y, Bennett JS, Bratcher HB, Jolley KA, Corton C, Care R, Poolman JT, Zollinger WD, Frasch CE, et al. Description and nomenclature of Neisseria meningitidis capsule locus. Emerg Infect Dis. 2013;19:566–573. doi: 10.3201/eid1904.111799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arreaza L, Salcedo C, Alcala B, Vazquez JA. What about antibiotic resistance in Neisseria lactamica? J Antimicrob Chemother. 2002;49:545–547. doi: 10.1093/jac/49.3.545. [DOI] [PubMed] [Google Scholar]
- 23.Fink K. Origin and Function of Circulating Plasmablasts during Acute Viral Infections. Front Immunol. 2012;3:78. doi: 10.3389/fimmu.2012.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borrow R, Carlone GM, Rosenstein N, Blake M, Feavers I, Martin D, Zollinger W, Robbins J, Aaberge I, Granoff DM, Miller E, et al. Neisseria meningitidis group B correlates of protection and assay standardization--international meeting report Emory University, Atlanta, Georgia, United States, 16-17 March 2005. Vaccine. 2006;24:5093–5107. doi: 10.1016/j.vaccine.2006.03.091. [DOI] [PubMed] [Google Scholar]
- 25.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods. 2004;286:111–122. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Vaughan AT, Gorringe A, Davenport V, Williams NA, Heyderman RS. Absence of mucosal immunity in the human upper respiratory tract to the commensal bacteria Neisseria lactamica but not pathogenic Neisseria meningitidis during the peak age of nasopharyngeal carriage. J Immunol. 2009;182:2231–2240. doi: 10.4049/jimmunol.0802531. [DOI] [PubMed] [Google Scholar]
- 27.Blanchard-Rohner G, Pulickal AS, Jol-van der Zijde CM, Snape MD, Pollard AJ. Appearance of peripheral blood plasma cells and memory B cells in a primary and secondary immune response in humans. Blood. 2009;114:4998–5002. doi: 10.1182/blood-2009-03-211052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weisel F, Shlomchik M. Memory B Cells of Mice and Humans. Annu Rev Immunol. 2017;35:255–284. doi: 10.1146/annurev-immunol-041015-055531. [DOI] [PubMed] [Google Scholar]
- 29.Odendahl M, Mei H, Hoyer BF, Jacobi AM, Hansen A, Muehlinghaus G, Berek C, Hiepe F, Manz R, Radbruch A, Dorner T. Generation of migratory antigen-specific plasma blasts and mobilization of resident plasma cells in a secondary immune response. Blood. 2005;105:1614–1621. doi: 10.1182/blood-2004-07-2507. [DOI] [PubMed] [Google Scholar]
- 30.Domina M, Lanza Cariccio V, Benfatto S, D’Aliberti D, Venza M, Borgogni E, Castellino F, Biondo C, D’Andrea D, Grassi L, Tramontano A, et al. Rapid profiling of the antigen regions recognized by serum antibodies using massively parallel sequencing of antigen-specific libraries. PLoS One. 2014;9:e114159. doi: 10.1371/journal.pone.0114159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimoda M, Nakamura T, Takahashi Y, Asanuma H, Tamura S, Kurata T, Mizuochi T, Azuma N, Kanno C, Takemori T. Isotype-specific selection of high affinity memory B cells in nasal-associated lymphoid tissue. J Exp Med. 2001;194:1597–1607. doi: 10.1084/jem.194.11.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bowe F, Lavelle EC, McNeela EA, Hale C, Clare S, Arico B, Giuliani MM, Rae A, Huett A, Rappuoli R, Dougan G, et al. Mucosal vaccination against serogroup B meningococci: induction of bactericidal antibodies and cellular immunity following intranasal immunization with NadA of Neisseria meningitidis and mutants of Escherichia coli heat-labile enterotoxin. Infect Immun. 2004;72:4052–4060. doi: 10.1128/IAI.72.7.4052-4060.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Litt DJ, Savino S, Beddek A, Comanducci M, Sandiford C, Stevens J, Levin M, Ison C, Pizza M, Rappuoli R, Kroll JS. Putative vaccine antigens from Neisseria meningitidis recognized by serum antibodies of young children convalescing after meningococcal disease. J Infect Dis. 2004;190:1488–1497. doi: 10.1086/424464. [DOI] [PubMed] [Google Scholar]
- 34.Read RC. Neisseria meningitidis and meningococcal disease: recent discoveries and innovations. Curr Opin Infect Dis. 2019;32:601–608. doi: 10.1097/QCO.0000000000000606. [DOI] [PubMed] [Google Scholar]
- 35.Trotter C, Findlow J, Balmer P, Holland A, Barchha R, Hamer N, Andrews N, Miller E, Borrow R. Seroprevalence of bactericidal and anti-outer membrane vesicle antibodies to Neisseria meningitidis group B in England. Clin Vaccine Immunol. 2007;14:863–868. doi: 10.1128/CVI.00102-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wing JB, Smart L, Borrow R, Findlow J, Findlow H, Heath AW, Read RC. Kinetics of immune responses to nasal challenge with meningococcal polysaccharide one year after serogroup-C glycoconjugate vaccination. Clin Infect Dis. 2011;52:1317–1323. doi: 10.1093/cid/cir198. [DOI] [PubMed] [Google Scholar]
- 37.Malito E, Biancucci M, Faleri A, Ferlenghi I, Scarselli M, Maruggi G, Lo Surdo P, Veggi D, Liguori A, Santini L, Bertoldi I, et al. Structure of the meningococcal vaccine antigen NadA and epitope mapping of a bactericidal antibody. Proc Natl Acad Sci U S A. 2014;111:17128–17133. doi: 10.1073/pnas.1419686111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giuliani M, Bartolini E, Galli B, Santini L, Lo Surdo P, Buricchi F, Bruttini M, Benucci B, Pacchiani N, Alleri L, Donnarumma D, et al. Human protective response induced by meningococcus B vaccine is mediated by the synergy of multiple bactericidal epitopes. Sci Rep. 2018;8:3700. doi: 10.1038/s41598-018-22057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 40.Hapfelmeier S, Lawson MA, Slack E, Kirundi JK, Stoel M, Heikenwalder M, Cahenzli J, Velykoredko Y, Balmer ML, Endt K, Geuking MB, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328:1705–1709. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 2011;331:1203–1207. doi: 10.1126/science.1201730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitsi E, Roche AM, Reine J, Zangari T, Owugha JT, Pennington SH, Gritzfeld JF, Wright AD, Collins AM, van Selm S, de Jonge MI, et al. Agglutination by anti-capsular polysaccharide antibody is associated with protection against experimental human pneumococcal carriage. Mucosal Immunol. 2017;10:385–394. doi: 10.1038/mi.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gbesemete D, Laver JR, de Graaf H, Ibrahim M, Vaughan A, Faust S, Gorringe A, Read RC. Protocol for a controlled human infection with genetically modified Neisseria lactamica expressing the meningococcal vaccine antigen NadA: a potent new technique for experimental medicine. BMJ Open. 2019;9:e026544. doi: 10.1136/bmjopen-2018-026544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mukhopadhyay TK, Halliwell D, O’Dwyer C, Shamlou PA, Levy MS, Allison N, Gorringe A, Reddin KM. Rapid characterization of outer-membrane proteins in Neisseria lactamica by SELDI-TOF-MS (surface-enhanced laser desorption ionization-time-of-flight MS) for use in a meningococcal vaccine. Biotechnol Appl Biochem. 2005;41:175–182. doi: 10.1042/BA20040098. [DOI] [PubMed] [Google Scholar]
- 45.Gorringe AR, Reddin KM, Funnell SG, Johansson L, Rytkonen A, Jonsson AB. Experimental disease models for the assessment of meningococcal vaccines. Vaccine. 2005;23:2214–2217. doi: 10.1016/j.vaccine.2005.01.053. [DOI] [PubMed] [Google Scholar]
- 46.Cecchini P, Tavano R, Polverino de Laureto P, Franzoso S, Mazzon C, Montanari P, Papini E. The soluble recombinant Neisseria meningitidis adhesin NadA(Delta351-405) stimulates human monocytes by binding to extracellular Hsp90. PLoS One. 2011;6:e25089. doi: 10.1371/journal.pone.0025089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buisman AM, de Rond CG, Ozturk K, Ten Hulscher HI, van Binnendijk RS. Long-term presence of memory B-cells specific for different vaccine components. Vaccine. 2009;28:179–186. doi: 10.1016/j.vaccine.2009.09.102. [DOI] [PubMed] [Google Scholar]
- 48.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pandey AK, Cleary DW, Laver JR, Maiden MCJ, Didelot X, Gorringe A, Read RC. Correction to: Neisseria lactamica Y92-1009 complete genome sequence. Stand Genomic Sci. 2018;13:4. doi: 10.1186/s40793-017-0288-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qiang BQ, Schildkraut I. Two unique restriction endonucleases from Neisseria lactamica. Nucleic Acids Res. 1986;14:1991–1999. doi: 10.1093/nar/14.5.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tettelin H, Saunders NJ, Heidelberg J, Jeffries AC, Nelson KE, Eisen JA, Ketchum KA, Hood DW, Peden JF, Dodson RJ, Nelson WC, et al. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science. 2000;287:1809–1815. doi: 10.1126/science.287.5459.1809. [DOI] [PubMed] [Google Scholar]
- 52.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this study are present in the paper or the Supplementary Materials. Materials are available upon request from the corresponding author.