Abstract
Identification of genes dysregulated during the hepatitis B virus (HBV)-host cell interaction adds to the understanding of underlying molecular mechanisms and aids in discovering effective therapies to improve prognosis in hepatitis B virus (HBV)-infected individuals. Through bioinformatics analyses of transcriptomics data, this study aimed to identify potential genes involved in the cross-talk of human hepatocytes expressing the HBV viral protein HBx with endothelial cells. Transient transfection of HBV viral gene X (HBx) was performed in THLE2 cells using pcDNA3 constructs. Through mRNA Sequencing (RNA Seq) analysis, differentially expressed genes (DEGs) were identified. THLE2 cells transfected with HBx (THLE2x) were further treated with conditioned medium from cultured human umbilical vein derived endothelial cells (HUVEC-CM). Gene Ontology (GO) enrichment analysis revealed that interferon and cytokine signaling pathways were primarily enriched for the downregulated DEGs in THLE2x cells treated with HUVEC-CM. One significant module was selected following protein-protein interaction (PPI) network generation, and thirteen hub genes were identified from the module. The prognostic values of the hub genes were evaluated using Kaplan–Meier (KM) plotter, and three genes (IRF7, IFIT1, and IFITM1) correlated with poor disease specific survival (DSS) in HCC patients with chronic hepatitis. A comparison of the DEGs identified in HUVEC-stimulated THLE2x cells with four publicly available HBV-related HCC microarray datasets revealed that PLAC8 was consistently downregulated in all four HCC datasets as well as in HUVEC-CM treated THLE2x cells. KM plots revealed that PLAC8 correlated with worse relapse free survival and progression free survival in HCC patients with hepatitis B virus infection. This study provided molecular insights which may help develop a deeper understanding of HBV–host stromal cell interaction and open avenues for future research.
Keywords: Endothelial cells, HBV X gene (HBx), Transcriptome, THLE2, HUVEC, Conditioned medium, Hepatitis B
1. Introduction
The hepatitis B virus (HBV) is a major human pathogen affecting >240 million patients worldwide and leading to cycles of liver inflammation and significant deaths from liver failure and hepatocellular carcinoma (HCC) [1]. Upon entering hepatocytes, the partially double-stranded viral DNA genome is converted into covalently closed circular DNA (cccDNA), which serves as the template for transcription of viral genes. cccDNA is very stable and is considered a major cause of viral persistence. Integration of HBV DNA can result in chromosomal instability and insertional mutagenesis [2,3]. Viral proteins interfere strongly with cell signaling and transcription in host hepatocytes, affecting cell cycle and growth. Moreover, HBV patients often develop chronic liver inflammation resulting from immune responses against infected hepatocytes. This persistent inflammation may lead to hepatocellular damage and fibrosis, resulting in liver cirrhosis, a major risk factor for HCC [4]. The HBV genome comprises four open reading frames, including the preS/S, P, C, and X genes. The HBV X (HBx) gene guides the HBx protein synthesis that interacts with many host proteins and plays important roles in virus replication, apoptosis induction, and the triggering of inflammatory responses. Although HBx has been demonstrated to exert effects on the regulation of cell proliferation, the detailed regulatory mechanisms of the HBx protein are yet to be established.
Liver sinusoidal endothelial cells (LSECs) form the wall of the hepatic sinusoids and comprise the highest proportion of non-parenchymal cells in the liver. LSECs are fenestrated and lack a basement membrane in their native state. However, they do not allow the passage of large molecules while physically separating the sinusoidal blood from the hepatocytes. Hepatotropic viruses pass through the protective filter constructed by LSECs to the liver parenchyma by passive diffusion or active transport. In addition to forming an anatomical barrier, LSECs also exert scavenger functions clearing macromolecules from the sinusoidal blood. C-type lectin receptors present on LSECs have a role in facilitating viral clearance and reducing liver inflammation. The absence of LSEC lectin led to the accumulation of intrahepatic effector cytotoxic T lymphocytes in a mouse model of HBV infection. However, experiments with fluorescence-tagged viral particles and coated gold particles revealed that LSECs can efficiently uptake specific hepatitis B viruses from the bloodstream and release them into adjacent hepatocytes via transcytosis [5]. It has been argued that the virus in LSECs may serve as a reservoir for endogenous reinfection, as indicated by a study where non-cytopathic clearance of lymphocytic choriomeningitis virus (LCMV) infection was observed only in hepatocytes but not in non-parenchymal liver cells [6]. Processing of viral proteins for major histocompatibility class I presentation in LSEC may further help the virus escape the immune system. LSECs cross-present exogenous antigens to naïve CD4+ cells [7] and CD8+ T cells [8] in a unique manner that shifts the hepatic immune balance towards tolerance rather than immunity. On the contrary, viral infections may also disrupt the tolerance induction by LSECs [9]. S Huang and coworkers showed that LSEC maturation following nucleotide-binding oligomerization domain (NOD1) activation promotes HBV-specific T cell responses and controls HBV replication in immunocompetent models. Diaminopimelic acid (DAP) treatment has been shown to stimulate the Ag-presenting ability of LSECs. It triggered the activation of CD4+ and CD8+ T cells in the liver, suggesting that NOD-like receptor (NLR) agonists may be used as immunomodulatory agents to control viral infection. Importantly, the DAP-induced anti-HBV effect was impaired in the LSEC-depleted mice [10].
The most crucial function of LSECs in chronic liver disease and tumor development is angiogenesis, which is particularly relevant to hyper-vascular HCC tumors. Chronic HBV infection is a significant risk factor for cirrhosis and hepatocellular carcinoma. The viral gene encoding the regulatory X protein (HBx) of the HBV genome has gained much attention as it is required for viral replication and is strongly implicated in hepatocarcinogenesis [11,12]. Moreover, the X gene is the most frequently integrated portion of HBV DNA found in hepatocyte chromosomes during HCC development [13]. Previous studies have demonstrated that viral HBx protein induces the synthesis of pro-angiogenic molecules such as nitric oxide (NO) [14], vascular endothelial growth factor (VEGF) [15,16], and Ang-2 [17]. HBx stimulated angiogenesis-related factors primarily through the stabilization and transcriptional activation of the hypoxia inducible factor 1α (HIF-1α) [18,19]. Furthermore, in the context of HBV-associated HCC, endothelial cells also play a role in developing a particularly malicious form of intrahepatic metastasis, the portal vein tumor thrombus (PVT). HBV infection potentially predisposes HCC patients to develop PVTT, possibly through creating an immune-subversive microenvironment [20].
The clinical complexity of HBV infection due to heterogeneity in disease stages and individual patients poses a challenge to comprehensively understanding the HBV-induced molecular signaling. However, several investigations have been carried out in cell-based HBV replication systems. A plasmid-based replicative HBV genome can be introduced in various types of cells by transient transfection followed by analyzing the cellular response as a consequence [21]. The protein profiling of rat primary hepatocytes (RPHs) and HCC-derived HepG2 cells transfected with the replicative HBV genome indicated that fumarate hydratase (FH) was down-regulated in both RPHs and HepG2 cells. At the same time, tryptophanyl-tRNA synthetase (TrpRS) was only upregulated in HepG2 transfected with the replicative HBV genome. [22]
Using a transwell co-culture system of induced pluripotent cell (iPSC)-derived hepatocytes and LSECs, paracrine regulation for HBV receptor during HBV infection was studied. Chen et al. showed that LSECs secreted EGF that enhanced HBV infection at a low dose, whereas EGF at a high dose suppressed HBV infection. EGFR is co-receptor of NTCP and EGF and is known to be internalized by clathrin-mediated endocytosis (CME) and clathrin-independent endocytosis (CIE) path ways depending on the dose of EGF. At a high dose, the EGFR is endocytosed via clathrin-independent endocytosis (CIE), which is degraded in the lysosome [23].
In this study, we did a transient transfection of an immortalized normal liver epithelial cell line (THLE-2) with the HBx viral gene. In-direct co-culture of the hepatocyte derived THLE-2 expressing HBx gene with primary human endothelial cells derived from the umbilical cords was then performed. To analyze how endothelial cells communicate with HBx-expressing hepatocytes, we analysed the RNA Seq profile of the HBx-transfected human hepatocytes treated with HUVEC conditioned medium. This was followed by evaluating differentially expressed genes and signaling pathways in the HBx expressing hepatocytes stimulated by endothelial cell conditioned culture medium. Our findings provide insights into hepatitis B viral gene–host cell interaction that may add to understanding the mechanisms underlying the development and progression of HBV-associated HCC.
2. Material and methods
2.1. Cell lines and cell culture
Human Umbilical Vein Endothelial Cells were purchased from Lonza and were propagated in EGM-2 Bullet kit. Briefly, EBM-2 basal medium was supplemented with 2% fetal bovine serum (FBS-10 ml), human epidermal growth factor (hEGF-0.5 ml), hydrocortisone (0.2 ml), vascular endothelial growth factor (VEGF-0.5 ml), antibiotic (gentamycin, amphotericin B-0.5 ml), human fibroblast growth factor-beta (hFGFβ-2 ml), insulin-like growth factor-1 (R3-IGF-1-0.5 ml), ascorbic acid (0.5 ml), and heparin (0.5 ml), according to the manufacturer's protocols. Human normal hepatocyte cell line THLE-2 was purchased from ATCC and cultured in LHC-8 medium (Thermo Fisher Scientific) supplemented with 10% FBS (HiMedia), 70 ng/ml of phosphoethanolamine (TCI), 5 ng/ml of epidermal growth factor (Thermo Fisher Scientific), and insulin-transferrin-selenium solution diluted to 1 (Gibco). HepG2 cells were obtained from the Institute of Liver and Biliary Sciences, New Delhi, India. HepG2 cells were routinely cultured in Dulbecco Modified Eagle Medium (DMEM) which was supplemented with 10% FBS. All cell cultures were maintained at 37 °C in a humidified chamber (humidity-95%) with 5% CO2 in a CO2 incubator (Thermo Fisher Scientific) with 100 IU/ml penicillin and 100 μg/ml streptomycin at the cell culture laboratory at Amity Institute of Molecular Medicine and Stem Cell Research. HUVEC conditioned medium (HUVEC-CM) was collected by incubating HUVECs in EGM2 for 24 h. Unconditioned EGM2 incubated at 37 °C for 24 h was used as a negative control.
2.2. Transient transfection of HBx in THLE-2 or HepG2cells
pcDNA3, pcDNA3-HBx and pEGFP-C3 constructs were kindly gifted by Dr. Syed Kazim Naqvi, Centre for Interdisciplinary Research in Basic Sciences, Jamia Millia Islamia, New Delhi, India [24], Supplementary Fig. S1a-c). According to manufacturer's protocol, Lipofectamine 3000 (Invitrogen) was used to transiently transfect pcDNA3 or pEGFP constructs in THLE2 or HepG2cells. The HBx expression by cytomegalovirus promoter-enhancer, used by pcDNA3 is reported to be within the physiological limits of HBV-infected cells [25,26].
2.3. Flow cytometry
THLE2 cells harvested 24 h following co-transfection with pcDNA3-HBx and pEGF-C3 were trypsinised, collected, and analysed for GFP positive cells at 488 nm using FACS Aria-III flow cytometer (BD Bioscience, US).
2.4. Western blot analysis
Cells were washed with ice-cold PBS and harvested in cell lysis RIPA (Radioimmunoprecipitation assay) buffer [50 mM Tris-HCl - pH 8, 150 mM NaCl, 0.25% Sodium deoxycholate, 1 mM EDTA, 1% NP-40] supplemented with protease inhibitor cocktail (Roche, Mannheim, Germany). Cells in RIPA were incubated on ice with intermittent vortexing for 50 min. The homogenate was then centrifuged for 30 min at 12,000g at 4 °C. The supernatant was removed and collected as whole-cell protein. Total protein content was determined by the Bradford protein estimation procedure with bovine serum albumin (HiMedia) as the standard. 50 μg of protein samples with 1 Laemmli buffer were heated at 95 °C for 5 min. Protein samples were resolved in 10% SDS (sodium dodecyl sulphate)-polyacrylamide gel electrophoresis under reducing conditions, and electro-transferred to polyvinyl difluoride (PVDF) membrane (BioRad). After blocking with 5% non-fat milk in TBS (tris buffer saline) containing 0.05% Tween-20 (TBS-Tween) for 2 h, the membrane was incubated with the specific primary antibodies overnight. After washing with TBS-Tween, the membrane was incubated for 2 h at room temperature with horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit IgG antibodies (ImmunoJackson). Chemiluminescent signals were detected using Pierce ECL western blotting substrate according to the manufacturer's protocol. Bands were quantitatively analysed by using ImageJ software. GAPDH (Cell Signaling Technology) was used as loading control.
2.5. RNA library construction and sequencing
Total RNA was isolated using Trizol according to manufacturer's instructions. mRNA quality control (QC) was done using Qubit, gel electrophoresis, and Bioanalyser. mRNA library kit (Qiagen) was used for library preparation. Briefly, RNA was fragmented, converted to DNA, and adapters/barcodes were ligated. Libraries that passed QC through Bioanalyser and Qubit were sequenced on Illumina Novaseq/HiSeq 4500 platform. Read Length was 150 2. The RNA-Seq analysis was done with a pipeline consisting of: QC of raw reads > trimming > aligning > counting > normalization > DEG analysis across conditions.
2.6. Identification of DEGs
Genes with |log2 fold change (FC)| >1 and adj P-value <0.05 (<0.01 for GEO datasets including human tissue samples) were identified using the limma package of R software and categorized as differentially expressed genes (DEGs) between different comparative groups in a pairwise manner. Ggplot2 package of R was applied to make volcano plots to visualize the identified DEGs in all datasets. The online tool Venny2.1.0 (https://bioinfogp.cnb.csic.es/tools/venny/) was used to generate common DEGs by overlapping different datasets.
2.7. Functional enrichment analysis
To classify the DEGs based on their function and understand their biological characteristics, GO (Gene Ontology) function was executed using FunRich [27] and ShinyGO software that offer a broad set of functional annotations.
2.8. PPI network construction and module analysis
The Search Tool for the Retrieval of Interacting Genes (STRING;http://string.embl.de/) was employed to build a PPI network of genes based on known and predicted PPIs followed by analyzing the functional associations among proteins [28]. After providing a gene list on the STRING online tool, PPI networks with a high confidence score (≥0.7) were selected. The PPI network was then visualized using Cytoscape software (version 3.5.1), and the Molecular Complex Detection (MCODE) plugin [29] was applied to generate the significant modules. The advanced options were set as follows: degree cutoff=2, K-Core=2, and Node Score Cutoff=0.2. The connectivity degree of each protein node was determined by computing the total number of nodes that interact directly with one specific node using the CytoHubba plugin [30]. Finally, the top genes with the maximum connectivity degrees were selected as hub genes.
2.9. Real-time quantitative polymerase chain reaction (RT-qPCR)
For RT-qPCR analysis, cDNA was synthesized from the total RNA templates by using RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific). cDNA templates diluted to 0.1⨯ were used for RT-qPCR, which was performed using the SYBR Green PCR Master Mix (Thermo Fisher Scientific) and the ABI Prism 7500 (Applied Bio-systems). The PCR conditions were as follows: 95 °C for 3 min, then 30 cycles of 95 °C for 30 s followed by 52.9 °C for 30 s, and 72 °C for 30 s. The final extension was performed at 72 °C for 10 min. Beta-actin gene (ACTB) was taken as the internal control. Relative quantitation was done by comparing the threshold cycle (Ct) values of each sample gene to the Ct values of ACTB. ∆Ct corresponds to the difference between the Ct of the gene of interest and the Ct of ACTB. Data is presented in terms of the fold change of mRNA expression, which was calculated from the 2-ΔΔCt method. The gene specific primers were designed using Primer3 Plus [31] (Supplementary Table. S6).
2.10. Survival analysis of Hub genes
The Kaplan–Meier plotter (http://kmplot.com/analysis/) can calculate the outcome of submitted genes on overall as well as relapse-free survival with information from GEO, TCGA, and EGA databases [32]. This tool allows the comparison of survival rates between patient cohorts with low and high expression of a specific gene (as compared to the median value of expression in the cohort), and it estimates the log rank value and hazard ratio (HR) with 95% confidence intervals. In liver cancer patients with hepatitis infection, the Kaplan-Meier plotter mRNA liver cancer database [33] was applied to predict prognostic values for the hub genes identified. Patients were divided based on auto-select best cutoff (all potential cutoff values as assessed from lower to upper quartiles, and the most effective threshold was used as a cutoff). Hepatitis virus as a risk factor was incorporated in the analysis, whereas the alcohol consumption factor was disregarded. The survival rates of individual hub genes were examined through the Kaplan–Meier plotter in HCC patients.
2.11. Statistics
All data were presented as mean ±S.D. (standard deviation) from at least three separate experiments. Student's t-test was applied to evaluate the differences between treated and control groups. Data from multiple groups were analysed by one-way ANOVA; multiple groups with different conditions were analysed by two-way ANOVA. For all the tests, significant values were P < 0.05.
3. Results
3.1. Intracellular expression of HBx in THLE2 cells from pcDNA3 constructs
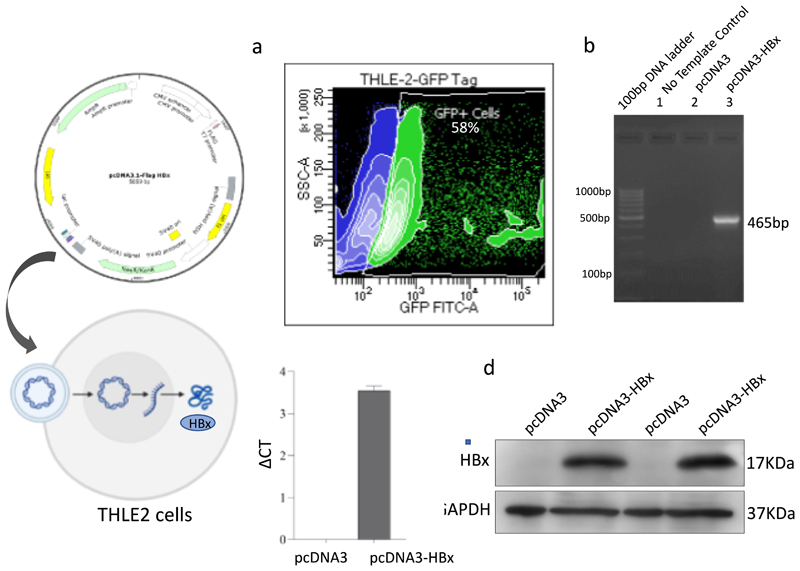
Transient transfection of pcDNA3 and pcDNA3-HBx in THLE2 cells was performed as previously described. THLE2 cells transfected with pcDNA3-HBx gene are referred to as THLE2x cells from here onwards. Transfection efficiency in THLE2x cells was anlysed by co-transfecting with pEGFP-C3. Approximately 60% of the cells were found positive for GFP signal using flow cytometry (Fig. 1a). Amplicons of 465 bp were successfully PCR amplified using HBx-specific primers and cDNA from THLE2x transfectants. No amplification was observed from cDNA derived from THLE2 cells transfected with empty-pcDNA3 (Fig. 1b). Real time mRNA expression of HBx gene in THLE2x cells was confirmed by RT-qPCR (Fig. 1c). HBx-specific bands in whole cell lysates of THLE2x were observed by western blotting (Fig. 1d). Empty pcDNA3 transfected cells served as control for all experiments. The pcDNA3-HBx construct was confirmed by sequencing by Eurofins Genomics (Bangalore, India) using M13 reverse primers (Supplementary Fig. S2). These results confirmed the successful intracellular expression of HBx from pcDNA3 constructs in THLE2 cells.
Fig. 1. Intracellular expression of HBx gene in THLE2 cells from pcDNA3 constructs.
a. Transfection efficiency was measured to be around 60% by analyzing the percent of GFP positive THLE2 cells co-transfected with pcDNA3-HBx and pEGFP-C3 b. PCR product from THLE2 cells transfected with empty-pcDNA3 and pcDNA3-HBx analysed by gel electrophoresis on 2% agarose. HBx gene (465 bp long) corresponding to 0.5-kb band of marker was successfully amplified from cells transfected with pcDNA3-HBx. c. Graph representing the real time expression of HBx gene in cells transfected with pcDNA3-HBx. d. Western blot representing HBx-specific bands in protein samples from THLE2 cells transfected with pcDNA3-HBx.
3.2. Overview of the transcriptome fragment libraries
A total of six transcriptome fragment libraries were constructed from THLE2-pcDNA3, THLE2x, and HUVEC-conditioned THLE2x cells, with two repeats assigned to each group. These were sequenced by Illumina Hiseq3000 (150 bp paired end read length) sequencing platform. >140 million paired end reads were generated from all the samples, ranging from 19 to 30 million reads from the individual sample libraries. >90% high-quality reads were satisfactorily mapped in each library. Mapping rates ranged from 91.70% to 95.10% for the six libraries, and the unique mapping rates ranged from 82.7% to 87.10%, respectively (Supplementary Table. S1). The raw sequence data can be downloaded from SRA database using PRJNA960847 BioProject accession number.
3.3. Transcriptome difference in THLE2-pcDNA3 cells and THLE2x cells
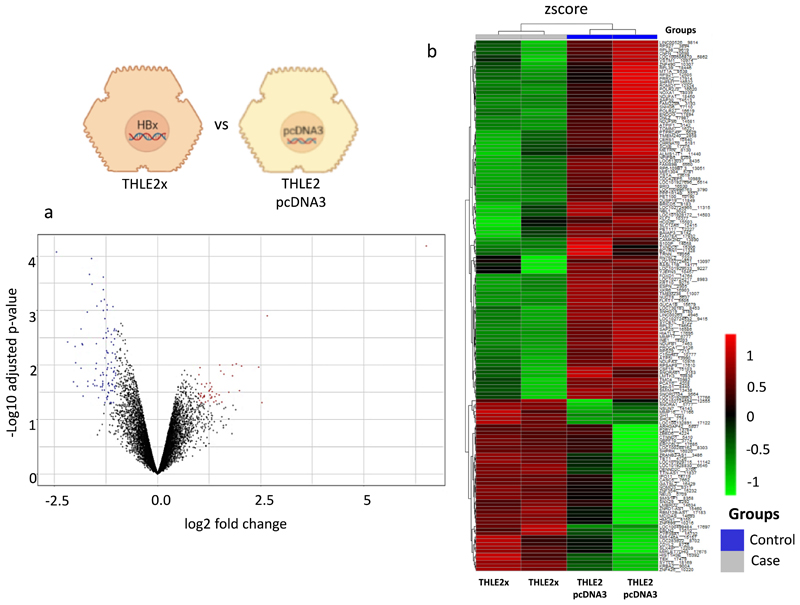
Differential gene expression between different groups was analysed pairwise using the limma package of R and represented in Supplementary Tables. S2 and S3a-c. With adjusted p value <0.05 as the threshold and |log2fold change (FC)| >1, 148 DEGs were identified in the THLE2x group compared to THLE2-pcDNA3. These included 48 up-regulated genes and 100 downregulated genes (Supplementary Table. S3 a) that are represented in the volcano plot and heat map in Fig. 2a-b. CTNND1 and ZBED6 genes were found to be up-regulated in the THLE2x group. These genes have a potential role in malignant transformation of the hepatocytes. On the other hand, MT1A and RPS27 genes were downregulated in the THLE2x group. TMC4 was also found to be downregulated in the THLE2x group, but its role in liver disease is controversial [34]. The potential implications of these genes in liver disease has been elaborated in the discussion section.
Fig. 2. Identification of genes differentially regulated in THLE2x cells compared to control THLE2-pcDNA3 cells.
a. Volcano plot of the total expression of genes in a THLE2x vs THLE2-pcDNA3 group. The x-axis represents the log2 (fold change) values, and the y-axis represents the -xlog10 (adjusted p values) values for gene expression. Each dot represents a gene identified from the RNA-seq data. Red dots indicate 48 up-regulated DEGs, blue dots indicate 100 downregulated DEGs, and black dots indicate nondifferential expressed genes. b. Heatmap of 148 DEGs in the THLE2x cells compared to THLE2-pcDNA3 cells. The output thresholds were |log2FC| > 1 and p-value< 0.05.
3.4. RNA-seq analysis reveals differentially expressed genes in HBx transfected hepatocytes stimulated with HUVEC-CM
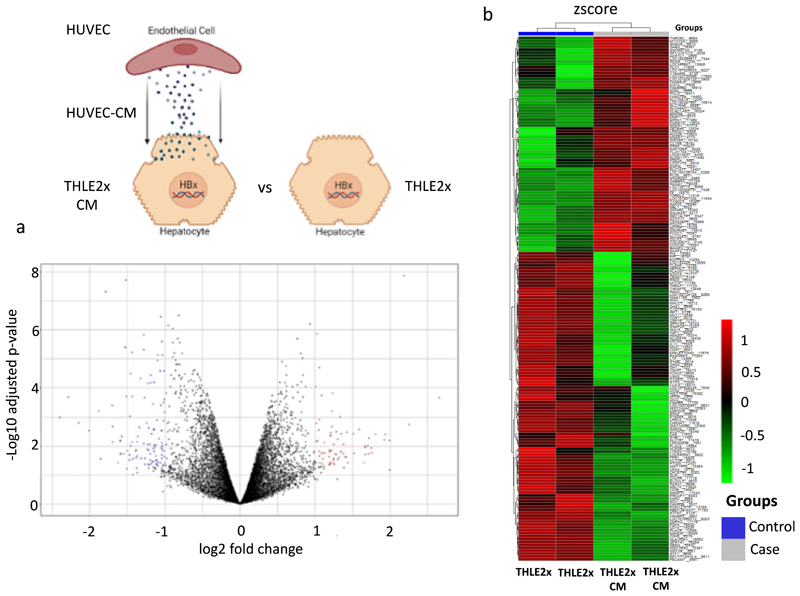
To investigate the genes and pathways stimulated by the cross-talk between endothelial cells and hepatocytes expressing HBx viral gene of HBV, THLE2x cells were incubated with HUVEC conditioned culture media for 24 h. HUVEC culture stimulated THLE2x cells, referred to as THLE2x-CM, were then analysed for differential gene expression in THLE2×-CM, compared to the THLEx group, were enriched in the compared to non-treated THLE2x cells. A total of 180 DEGs were identified, including 74 up-regulated genes and 106 downregulated genes represented in volcano plot and heat map in Fig. 3a-b (Supplementary Table. S3b).
Fig. 3. Identification of genes differentially regulated in THLE2x cells treated with HUVEC-CM (THLE2x-CM) compared to THLE2x cells.
a. Volcano plot of the total expression of genes in THLE2x-CM group vs THLE2-x group. The x-axis represents the log2 (fold change) values, and the y-axis represents the -log10 (adjusted p values) values for gene expression. Red dots indicate 74 up-regulated DEGs, blue dots indicate 106 downregulated DEGs, and black dots indicate nondifferential expressed genes. b. Heatmap of 180 DEGs in the THLE2x-CM cells compared to THLE2x cells. The output thresholds were |log2FC| > 1 and p-value< 0.05.
3.5. Functional enrichment of genes in THLE2x regulated by the treatment of HUVEC-CM
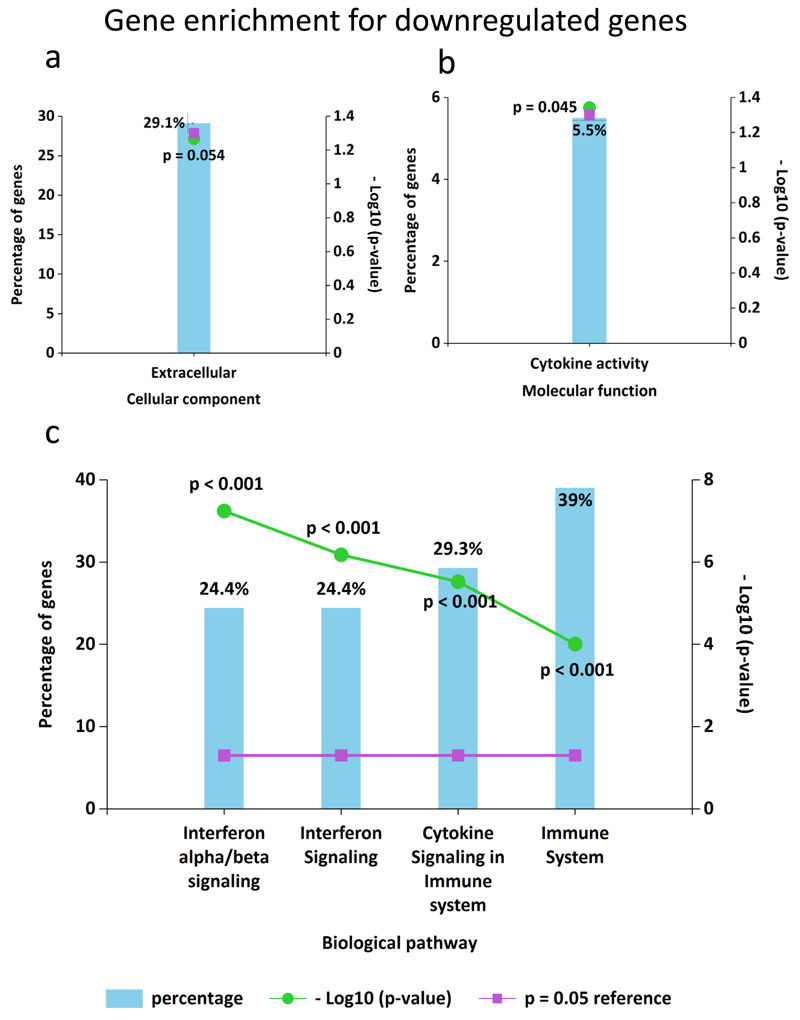
To further elucidate the functional roles of the 180 DEGs, GO pathway enrichment analyses were performed by FunRich software and ShinyGO tool to search for significantly overrepresented categories. Up-regulated genes in the THLE2x-CM group compared to THLE2x were not found to be significantly enriched for GO terms. GO terms for downregulated genes belonged to three categories: cellular component, molecular function, and biological pathways (Fig. 4, Supplementary Fig. S2a-d, Supplementary Table. S4a-d). The downregulated DEGs were principally enriched in the extracellular space and their molecular function enrichment term was mainly correlated with the cytokine activity (Fig. 4a-b). In addition, it was found that the downregulated DEGs biological pathways that regulate interferon signaling and cytokine signaling (Fig. 4c).
Fig. 4.
GO (Gene Ontology) enrichment analysis for DEGs in THLE2x-CM cells compared THLE2x using FunRich Downregulated DEGs in THLE2x cells when treated with HUVEC-CM were enriched in the GO categories “cellular component” (a), “molecular function” (b), and “biological pathways”(c). p values <0.05 were statistically significant.
3.6. PPI analysis revealed a significant cluster of genes downregulated in THLE2 ⨯ cells by HUVEC-CM treatment
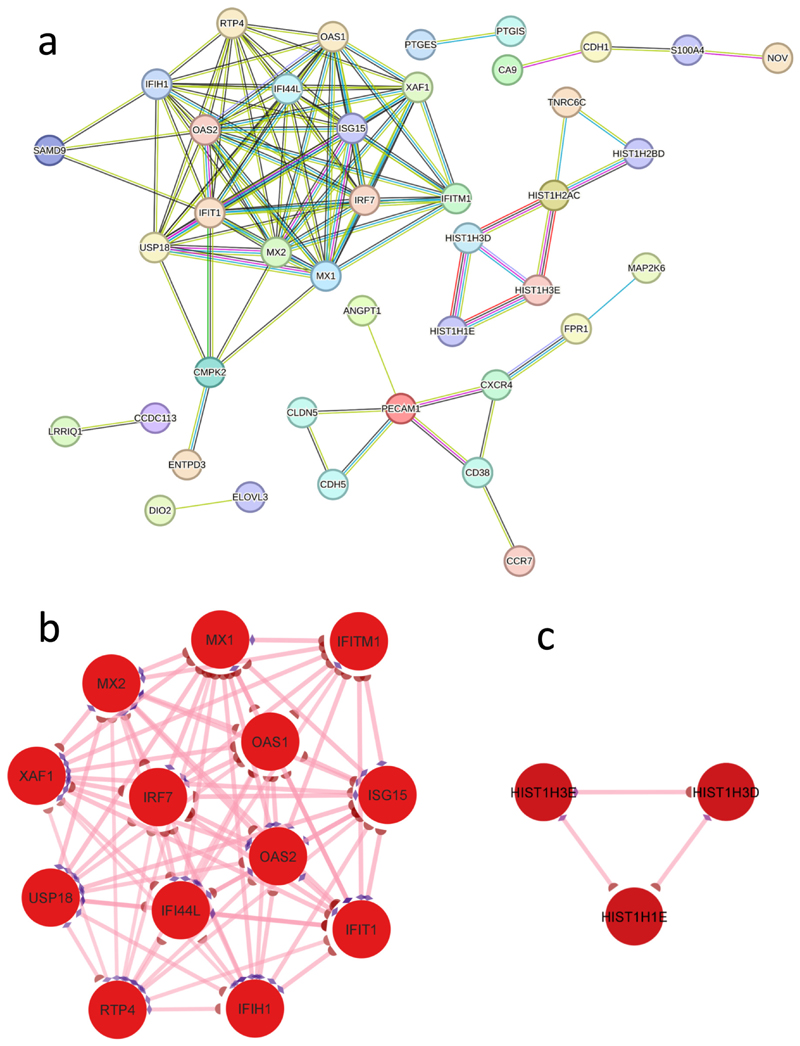
PPI analysis of the DEGs in the HUVEC-CM stimulated THLE-2x cells revealed 148 nodes and 106 interactions in total (Fig. 5a). These proteins were chosen on the basis of their collective high confidence score (≥ 0.7) in STRING analysis. One significant module (module-1) with a score ≥ 5 was selected through MCODE where ISG15, IFITM1, IFI44L,IFIH1, MX1, USP18, IRF7, OAS2, MX2, XAF1, RTP4, IFIT1, and OAS1 were identified as hub nodes (Fig. 5b, Supplementary Table. S5). An additional module (module-2) with a score ≥3 was also determined by MCODE, represented in Fig. 5c. All the identified nodes were downregulated DEGs in the network. The hub genes from module-1 were chosen for further analysis due to their higher connectivity degree.
Fig. 5. Protein-protein interaction (PPI) network and identification of protein clusters with significant modules.
a. PPI network was generated by STRING with up-regulated and downregulated DEGs identified in THLE2x cells treated with HUVEC-CM compared to THLE2x cells. b. One significant module, Cluster 1 (MCODE score = 12) was identified using the molecular complex detection (MCODE) method. c. Protein cluster with MCODE score = 3 was identified as Cluster 2. Red nodes in the protein clusters indicate downregulated genes.
3.7. RT-qPCR validation of the identified hub genes identified in THLE2⨯ cells downregulated by HUVEC-CM treatment
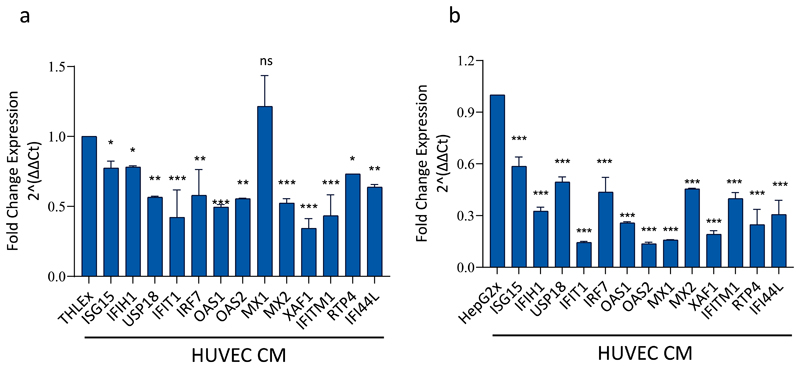
To verify the RNA-seq expression results of the 13 hub genes identified by PPI analysis, we further determined the expression levels of these genes by RT-qPCR analysis. RT-qPCR results confirmed that HUVEC conditioned culture medium treatment significantly downregulated the expression of ISG15, IFITM1, IFI44L, IFIH1, USP18, IRF7, OAS2, MX2, XAF1, RTP4, IFIT1, and OAS1 genes in THLE-2 cells expressing the HBx gene of the HBV. However, MX1 gene expression in our RT-qPCR results was found to be non-significant (Fig. 6a). The expression levels of these 13 genes were also investigated in HepG2cells transfected with pcDNA3-HBx expression vector (referred to as HepG2x cells) that were treated with HUVEC-CM compared to non-treated HepG2x cells. All 13 genes were found to be consistently and significantly downregulated in HepG2x cells treated with HUVEC-CM compared to control HepG2x cells (Fig. 6 b). The downregulation of all genes by HUVEC-CM in HepG2x cells was substantially higher than THLE2x cells. The RT-qPCR data validate the gene expression results obtained by RNA-Seq analysis of THLE2x-CM cells.
Fig. 6.
Relative fold change expression of 13 hub genes by RT-qPCR in THLE2x and HepG2x cells after treatment with HUVEC-CM. Relative fold change in mRNA expression of ISG15, IFITM1, IFI44L, IFIH1, MX1, USP18, IRF7, OAS2, MX2, XAF1, RTP4, IFIT1, and OAS1 analysed in THLE2x cells (a), and HepG2x cells (b) treated with HUVEC-CM compared to non-treated control cells. The expression levels of the genes were normalised to the expression of ACTB. *p < 0.05; **p < 0.01; *** < 0.001; ns, non-significant.
3.8. Disease specific survival analysis of the hub genes revealed that low expression of IRF7, IFITM1, and IFIT1 is implicated in hepatitis-related HCC
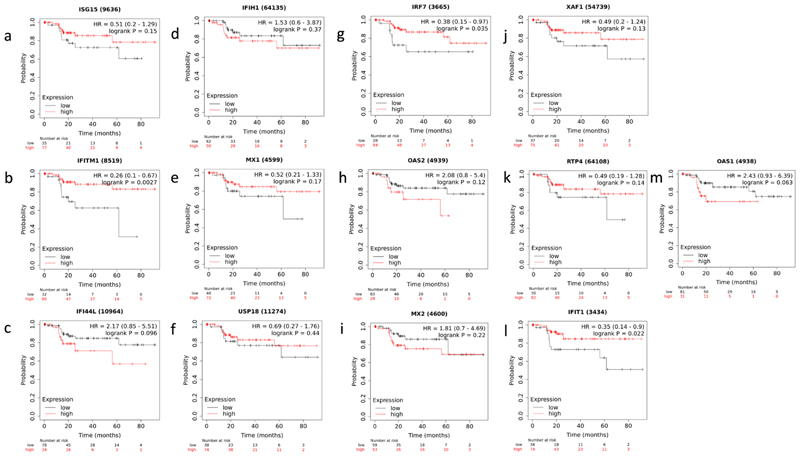
Using the Kaplan-Meier plotter, the prognostic significance of the 13 hub genes was examined in liver cancer patients. The analysis included the hepatitis virus infection as a risk factor while leaving out the influence of alcohol consumption. A comparison of survival status between groups with high expression and those with low expression revealed that low expression of 3 out of the 13 genes, i.e., IRF7, IFITM1, IFIT1 showed a significant decrease in disease specific survival (DSS) in HCC patients with chronic hepatitis infection (Fig. 7 a-m, Supplementary Table S7). Kaplan-Meier plots revealed that low expression of IRF7, IFITM1, and IFIT1 is related to worse outcomes in DSS of hepatitis associated HCC patients.
Fig. 7. Kaplan–Meier survival curves of the 13 hub genes.
a-m. Graph representing disease specific survival (DSS) in HCC patients grouped according to low and high expression of ISG15, IFITM1, IFI44L, IFIH1, MX1, USP18, IRF7, OAS2, MX2, XAF1, RTP4, IFIT1, and OAS1 genes. p values <0.05 were considered as statistically significant.
3.9. PLAC8 gene was found significantly downregulated in four HBV related HCC datasets, and HUVEC-CM treated THLE2x cells
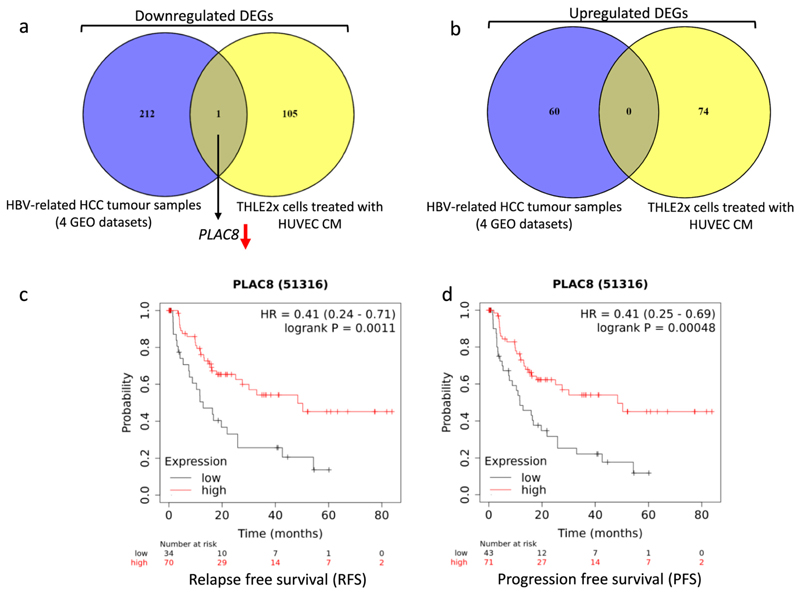
Four datasets (GSE47197, GSE121248, GSE55092, and GSE62232) were analysed to identify genes that were differentially expressed between HBV-associated HCC and non-HCC liver tissues. Altogether from these datasets, a total of 191 non-tumoral samples and 180 HBV-infected HCC samples were analysed (Supplementary Table. S8). Following the cutoff criteria as mentioned before, 638 DEGs (135 up-regulated, 503 down-regulated) in GSE47197, 1020 DEGs (373 up-regulated and 647 down-regulated) in GSE121248, 1950 DEGs (814 up-regulated and 1136 down-regulated) in GSE55092, and 2076 DEGs (1267 up-regulated and 809 down-regulated) in GSE62232 were found between non-tumor tissues and HBV-associated HCC tissues as represented in volcano plots (Supplementary Fig. S4a-d). Further analysis of these DEGs using Venn diagrams revealed that 273 DEGs, including 60 up-regulated and 213 down-regulated genes, were consistently found in all four HBV-associated datasets (Supplementary Fig. S4 e-f). All 273 DEGs are listed in Supplementary Table. S9. Among the genes common to all four HBV-associated HCC datasets, only PLAC8 was also found differentially downregulated in THLE2x cells when treated by HUVEC-CM (Fig. 8 a-b). Kaplan-Meier plots revealed that low expression of PLAC8 did not correlate with a significant decrease in disease specific survival (DSS) in HCC patients with chronic hepatitis infection (figure not shown). However, low expression of PLAC8 is related to worse outcomes in relapse free survival (RFS) and progression free survival (PFS) in hepatitis associated HCC patients (Fig. 8 c-d).
Fig. 8. PLAC8 was differentially downregulated in THLE2x-CM group and in HBV-related HCC datasets.
a-b. Venn diagram showing that PLAC8 gene is downregulated in HBV associated HCC datasets and in THLE2x cells treated by HUVEC CM c-d. Graph representing relapse free survival (RFS), (c), and progression free survival (PFS), (d) in HCC patients grouped according to low and high expression of PLAC8 gene. p values <0.05 were considered as statistically significant.
4. Discussion
HBV and HCV infection are major risk factors for developing HCC. Although the relationship between HCC and persistent HBV infection is well documented, a comprehensive understanding of the underlying mechanisms leading to HCC is difficult to establish as HBV-associated carcinogenesis can be seen as a multifactorial process that includes both direct and indirect mechanisms that might act synergistically [35].
The clearance of HBV is noncytopathic, i.e., the infection does not trigger innate immunity at a detectable level due to initial replication not involving hepatocytes [36] but requires T-lymphocytes to activate cell death. In fact, adaptive immune responses make or break the fate of infected hepatocytes by mediating viral clearance as well as progression of the disease to cirrhosis and HCC [37]. Specifically, robust CD8+ T cell response is required to kill infected cells and secrete antiviral lymphokines such as interferon gamma, a member of a class of cytokines named for their capability to interfere with viral replication [38]. CD4+ T lymphocytes activate and maintain CD8+ T cell pools as well as induce the production of neutralizing antibodies to target residual infection. Chronic infection occurs due to viral persistence as a result of failure of this adaptive immune response, the functional restoration of which may lead to rare but spontaneous resolution of the infection. Meanwhile, chronic infection triggers necroinflammatory liver disease that can progress to hepatocellular carcinoma (HCC) [39].
During sustained antigen stimulation, HBV-specific CD8+ T cells fail to differentiate into cytolytic effector T cells that secrete cytotoxic signaling molecules such as TNF and IFNg, a phenomenon known as T-cell exhaustion [37]. Such an exhausted T-lymphocyte milieu is more common in HBV-associated HCC as compared to nonviral etiologies of HCC, [40] and the tumor microenvironment is dominated by regulatory T cells (Tregs).
There is emerging evidence that several proinflammatory cytokines, such as interferon-γ, IL-1β, and tumor necrosis factor, stabilize HIF-1α, even in the absence of hypoxia [41,42]and the same cytokines can induce HBx expression. Ideally, any viral nucleic acids, such as HBV's relaxed circular DNA, covalently closed circular DNA (cccDNA), and RNA intermediates should be detected by pattern recognition receptors (PRRs) to induce interferon (IFN) responses. However, as a “stealth” virus, little or no IFN production is detected during HBV infection, regardless of the viral load. IFN and IFN-stimulated gene (ISG) expression are in fact downregulated in HBV-infected individuals due to the virus having developed strategies to counteract innate immunity by interfering with either PRRs or their downstream signals, and due to progressive T-cell exhaustion in chronic infection. [43] Therefore, it makes sense that many of the differentially regulated genes in our RNASeq data of our in vitro model of HBV-associated HCC are ISGs.
IFN-stimulated 15 kDa protein (ISG15) is a ubiquitin-like molecule that acts as a direct anti-HBV effector of Type I IFN-inducible response s [44] and has been reported to be a biomarker for HBV HCC [45–47] wherein it promotes the proliferation and migration of HCC through stabilizing Survivin protein by sequestering XIAP and preventing it from interacting with Survivin [46].
IFITM1 (interferon-induced transmembrane protein 1) is downregulated during HCV [48] and HBV [49] infection. IFN signaling mediated by IFITM1 is known to also downregulate proliferation in both, hepatocellular carcinoma cells as well as non-malignant hepatocytes [50–52]. Additionally, IFITM1 is the downstream target of the tumor suppressing chromatin remodeling gene, ARID2, which is commonly mutated in HCV-associated HCC, as well as hepatitis B virus (HBV)-associated HCC, alcohol-associated HCC and HCC with no known etiology. [52,53] However, while hypermethylation of the IFITM1 promoter has been reported in HCC [54], the molecular mechanisms by which IFITM1 influences the development and progression of HCC in general and HBV-associated HCC specifically are largely under-researched. A series of in vitro experiments by Wu et al. suggest that IFITM1 influences EMT and stem cell-like properties in hepatoma cells by modulating important genes regulating HCC development and progression, such as STAT3, membrane metalloproteases, p53, and caspases [50].
The interferon-induced protein 44-like gene in HBV-HCC acts as a tumor suppressor by downregulating cancer stemness, drug resistance, and metastasis [55] However, it is up-regulated in monocytic myeloid-derived suppressor cells (MDSCs) of patients with a high viral load [56]. It is hypothesized that IFI44L methylation may play an important role in phase transition and pathogenesis of virus-host interaction, and it has been established that IFI44L methylation in DNA of WBCs can be a biomarker for predicting risk of HCC development [57,58].
The gene IFIH1 (FN induced with helicase C domain 1) encodes MDA5, that senses intracellular RNA intermediates generated during the HBV life cycle [43]. Chronic HBV-infected (CHB) patients are unable to properly respond to hepatitis B virus (HBV) due to significant downregulation of MDA5 activity [59].
MX1 (myxovirus resistance protein 1), also known as interferon-induced GTP-binding protein 1, is one of the IFN-stimulated genes (ISG). In humans, it is a cytoplasmic protein that is active against many viruses that have a cytoplasmic replication phase, such as the HBV virions [44,60]. As an antiviral ISG, it is conventionally used as a marker for the induction of cellular countermeasures to combat viral infection [61,62]. MX2 is similar to MX1 in function but the molecular basis of its antiviral activity is underexplored, with some evidence suggesting it impairs the conversion of relaxed circular DNA of HBV to cccDNA. [63]
Ubiquitin-specific peptidase 18 (USP18) is yet another ISG closely associated with hepatitis B virus (HBV) [64] and is up-regulated during the tumorigenesis and progression of HBV-associated HCC [65–68]. It promotes HBV replication by inhibiting type I IFN signaling, which is independent of its activity as a cysteine protease that deconjugates IFN stimulated gene 15 (ISG15) from host or pathogen proteins.
IRF7 (Interferon regulatory factor 7) is a virus-inducible transcription factor which modulates type I IFN secretion activated by the pathogen recognition receptor (PRR) TLR9 in plasmacytoid dendritic cells (pDCs) [69]. Meanwhile, in hepatocytes, in hepatocytes, IRF7 switches on the retinoic acid-inducible gene-I (RIG-I)/mitochondrial antiviral protein (MAVS) pathway [70] that also involves another of our hits, IFIH1/MDA5. HBV has coevolved a few measures to combat the antiviral activity switched on by IRF7. The Hepatitis B X antigen (HBx) acts as a deubiquitinating enzyme to inhibit ubiquitination of IRF7, and therefore suppressing its antiviral activity [71]. Additionally, HBV surface antigen (HBsAg) reduces the stability IRF7 mRNA to decrease transcription of the inflammasome component AIM2 in Kupffer cells. [72]
The genes OAS1 and OAS2 encodes the enzyme and viral sensor, 2′-5′-oligoadenylate synthetase 1 and 2, respectively, that activate RNase L, responsible for viral RNA degradation and therefore the inhibition of viral replication. OAS2 is a tumor suppressor not just in virus-mediated cancers, but also those not involving any viral infection [73]. In HBV-associated HCC, OAS1 and 2 act as effectors of type I IFN-mediated antiviral signaling [74]. They are also up-regulated in HCV-associated HCC [75].
The tumor suppressor gene X-linked inhibitor of apoptosis (XIAP)-associated factor 1 (XAF1) is known to induce apoptosis, inhibit angiogenesis, as well as tumor growth in hepatocellular carcinoma [76]. Its downregulation or promoter hypermethylation can be used as a biomarker to differentiate HCC from noncancerous tissue [77] or to predict recurrence of the tumor [78].
RTP4 (Receptor Transporter Protein 4) is one of the ISGs suppressed during HCV [79] and HBV infections [80]. However, its role in HBV-associated HCC remains to be explored.
IFIT1 encodes the cytoplasmic interferon induced protein with tetratricopeptide repeats 1. Sequestration by IFIT1 impairs the translation of 2′O-unmethylated capped RNA generated by HBV, thereby impairing its ability to use host translational machinery. [81] Not much is known about its role in the progression of chronic HBV infection to HBV associated HCC, but our study suggests it might play a role during tumorigenesis and disease progression.
PLAC8, also known as Onzin, is significantly decreased in HCC, and higher expression is correlated with poor prognosis [82]. Its downregulation is known to promote cell viability, proliferation and tumor formation, both in vitro and in vivo [83]. The molecular mechanism behind its tumor-suppressing effects involves the inhibition of PI3K/Akt/GSK3β and Wnt/β-catenin signaling. Interestingly, PLAC8 is known to be regulated by miR-1228-3p, which is transferred from Cancer associated fibroblasts (CAFs) into HCC cells by CAF-derived extracellular vesicles [84]. Since our study found it to be up-regulated in HUVEC CM-treated HCC, it is possible that a similar mechanism is responsible for the modulation of PLAC8. Interestingly, PLAC8 inhibits interferon gamma production by T cells [85], but on the other hand up-regulates PD-L1 in the presence of IFN-γ [86] in triple negative breast cancer.
Based on these studies, we suggest that IFITM1 and PLAC8 may be promising markers and predictors for clinical drug selection, immuno therapy response and tumor prognosis. Their underlying mechanisms should be determined in the future.
To the best of our knowledge, this is the first transcriptome analysis of an in vitro model of the interaction between endothelial cells and HBV-infected hepatocytes which may later undergo oncogenic transformation. Many known HBV-associated signatures were identified in our RNASeq data comparing THLE2 transfected with HBx as compared to empty vector-transfected THLE2. These include CTNND1 that regulates EMT via modulation of Wnt signaling [87–89], the transcription factor ZBED6 [90], the cell proliferation and apoptosis-regulating gene MT1A [91],the chloride channel TMC4 [92]and the ribosomal protein RPS27 [93]. Therefore, this model may be used to simulate HBV infection for the purpose of developing and testing therapeutic strategies.We acknowledge that the cell signaling pathways involved in the interaction between LSECs and hepatocytes during HBV infection and associated tumorigenesis may be very complicated and not easily replicated by a more limited protein milieu represented in our in vitro attempt to recapitulate the disease (HUVEC CM-treated THLE2x). In that case, concentrated solutions of the complete secretome or partial secretomal size fractions should be studied for their therapeutic potential.
5. Conclusion
Our findings provide molecular insights into cross-talk between endothelial cells and hepatocytes with HBV X protein expression. The study may help in the deeper understanding of the hepatitis B virus–host interaction and open avenues for future research.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ygeno.2023.110642.
Supplementary Material
Acknowledgement
The authors would like to thank Dr. Syed Kazim Naqvi, Centre for Interdisciplinary Research in Basic Sciences, Jamia Millia Islamia, New Delhi, India for kindly gifting pcDNA3, pcDNA3-HBx and pEGFP-C3 plasmids. Flow cytometry experiments were helped by Manoj Gupta at the, Department of Science and Technology (DST) and FIST sponsored (SR/FST/LS-II/2017/115) flow cytometry facility at AIMMSCR, Amity University, Noida. Lab members, Basundhara Das and Jaydeb Pal helped with their constructive inputs and discussions
Funding
This work was supported by fellowships and grant supports from the University Grants Commission (UGC Ref no-581/CSIR-UGC-NET June 2017) to S.M. Ghufran, Council of Scientific and Industrial Research [CSIR-09/915(0020)/2021-EMR-1] to Prachi Sharma, Department of Biotechnology (DBT-102/IFD/SAN/3003/2017–2018 to S. Biswas) and Science and Engineering Research Board (SERB) (CRG/2018/003918 to S. Biswas and SERB-YSS/2015/000092 to S. Ghose) Indian Council of Medical Research (ICMR-2019-1306/SCR/ADHOC-BMS to S. Biswas), India. The authors wish to acknowledge the DBT/Wellcome Trust India Alliance Fellowship [AUUP/2021–22/590] awarded to S. Sengupta for financial support.
Footnotes
Ethics approval and consent to participate
No human samples were utilized in the study and publicly available datasets were used for analyses, therefore the ethics approval for the datasets was not applicable.
Consent for publication
Not applicable.
Authors' contributions
Shaikh Maryam Ghufran: Conceptualization, Investigation, Methodology. Software, Writing – original draft. Prachi Sharma: Methodology, Data curation. Bornika Roy: Methodology, Data Curation. Shivani Jaiswal: Software, Formal analysis. Mehreen Aftab: Software, Formal Analysis. Shinjinee Sengupta: Data Curation, Formal Analysis, Validation. Sampa Ghose: Data curation, Funding acquisition. Subhrajit Biswas: Investigation, Funding acquisition, Project administration, Resources, Supervision, Writing-review & editing.
Declaration of Competing Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability
The datasets analysed during the current study are publicly available in the Gene Expression Omnibus (GEO) repository, https://www.ncbi.nlm.nih.gov/gds/, RNA sequencing data can be accessed from SRA database using PRJNA960847 BioProject accession number. Information from the datasets analysed during this study are included in this article and its supplementary information files. The raw data supporting the conclusions of this article will be made available on request to the authors.
References
- [1].Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Lancet. 10003. Vol. 386. Elsevier; 2015. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013; pp. 1546–1555. [DOI] [PubMed] [Google Scholar]
- [2].Di Bisceglie AM. Hepatitis B and hepatocellular carcinoma. Hepatology. 2009 May;49(5 Suppl):S56–S60. doi: 10.1002/hep.22962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007 Jun;132(7):2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- [4].Chisari FV. Viruses, Immunity, and Cancer: Lessons from Hepatitis B - PMC. [cited 2023 Jan 25];2000 doi: 10.1016/s0002-9440(10)64980-2. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Breiner KM, Schaller H, Knolle PA. Hepatology. 4. Vol. 34. Wiley Online Library; 2001. Endothelial cell–mediated uptake of a hepatitis B virus: a new concept of liver targeting of hepatotropic microorganisms; pp. 803–808. [DOI] [PubMed] [Google Scholar]
- [6].Guidotti LG, Borrow P, Brown A, McClary H, Koch R, Chisari FV. J Exp Med. 10. Vol. 189. The Rockefeller University Press; 1999. Noncytopathic clearance of lymphocytic choriomeningitis virus from the hepatocyte; pp. 1555–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Knolle PA, Schmitt E, Jin S, Germann T, Duchmann R, Hegenbarth S, Gerken G, Lohse AW. Gastroenterology. 6. Vol. 116. Elsevier; 1999. Induction of cytokine production in naive CD4+ T cells by antigen-presenting murine liver sinusoidal endothelial cells but failure to induce differentiation toward Th1 cells; pp. 1428–1440. [DOI] [PubMed] [Google Scholar]
- [8].Limmer A, Ohl J, Kurts C, Ljunggren HG, Reiss Y, Groettrup M, Momburg F, Arnold B, Knolle PA. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat Med. 2000 Dec;6(12):1348–1354. doi: 10.1038/82161. [DOI] [PubMed] [Google Scholar]
- [9].Kern M, Popov A, Scholz K, Schumak B, Djandji D, Limmer A, Eggle D, Sacher T, Zawatzky R, Holtappels R, Reddehase MJ, et al. Gastroenterol. 1. Vol. 138. Elsevier; 2010. Jan 1, Virally infected mouse liver endothelial cells trigger CD8+ T-cell immunity; pp. 336–346. [DOI] [PubMed] [Google Scholar]
- [10].Huang S, Zou S, Chen M, Gao X, Chen L, Yang X, Yu Q, Zhao X, Du Y, Yang X. Local stimulation of liver sinusoidal endothelial cells with a NOD1 agonist activates T cells and suppresses hepatitis B virus replication in mice. J Immunol Am Assoc Immnol. 2018;200(9):3170–3179. doi: 10.4049/jimmunol.1700921. [DOI] [PubMed] [Google Scholar]
- [11].Murakami S. Hepatitis B virus X protein: a multifunctional viral regulator. J Gastroenterol. 2001 Oct;36(10):651–660. doi: 10.1007/s005350170027. [DOI] [PubMed] [Google Scholar]
- [12].Zhang X, Zhang H, Ye L. Effects of hepatitis B virus X protein on the development of liver cancer. J Lab Clin Med. 2006 Feb 1;147(2):58–66. doi: 10.1016/j.lab.2005.10.003. [DOI] [PubMed] [Google Scholar]
- [13].Koike K, Moriya K, Iino S, Yotsuyanagi H, Endo Y, Miyamura T, Kurokawa K. High-level expression of hepatitis B virus HBx gene and hepatocarcinogenesis in transgenic mice. Hepatology. 1994;19(4):810–819. [PubMed] [Google Scholar]
- [14].Majano PL, García-Monzón C, López-Cabrera M, Lara-Pezzi E, Fernández-Ruiz E, García-Iglesias C, Borque MJ, Moreno-Otero R. Inducible nitric oxide synthase expression in chronic viral hepatitis. Evidence for a virus-induced gene upregulation. The journal of clinical investigation. Am Soc Clin Investig. 1998;101(7):1343–1352. doi: 10.1172/JCI774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lee SW, Lee YM, Bae SK, Murakami S, Yun Y, Kim KW. Biochem Biophys Res Commun. 2. Vol. 268. Elsevier; 2000. Human hepatitis B virus X protein is a possible mediator of hypoxia-induced angiogenesis in hepatocarcinogenesis; pp. 456–461. [DOI] [PubMed] [Google Scholar]
- [16].Yun C, Lee JH, Wang JH, Seong JK, Oh SH, Yu DY, Cho H. Biochem Biophys Res Commun. 5. Vol. 296. Elsevier; 2002. Expression of hepatitis B virus X (HBx) gene is up-regulated by adriamycin at the post-transcriptional level; pp. 1157–1163. [DOI] [PubMed] [Google Scholar]
- [17].Sanz-Cameno P, Martín-Vílchez S, Lara-Pezzi E, Borque MJ, Salmerón J, de Rueda PM, Solís JA, López-Cabrera M, Moreno-Otero R. Am J Pathol. 4. Vol. 169. Elsevier; 2006. Hepatitis B virus promotes angiopoietin-2 expression in liver tissue: role of HBV x protein; pp. 1215–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Moon EJ, Jeong CH, Jeong JW, Rok Kim K, Yu DY, Murakami S, Woo Kim C, Kim KW. The FASEB journal. 2. Vol. 18. Wiley online, Library; 2004. Hepatitis B virus X protein induces angiogenesis by stabilizing hypoxia-inducible factor-1α; pp. 1–16. [DOI] [PubMed] [Google Scholar]
- [19].Yoo YG, Oh SH, Park ES, Cho H, Lee N, Park H, Kim DK, Yu DY, Seong JK, Lee MO. Hepatitis B virus X protein enhances transcriptional activity of hypoxia-inducible factor-1alpha through activation of mitogen-activated protein kinase pathway. J Biol Chem. 2003 Oct 3;278(40):39076–39084. doi: 10.1074/jbc.M305101200. [DOI] [PubMed] [Google Scholar]
- [20].Yang P, Li QJ, Feng Y, Zhang Y, Markowitz GJ, Ning S, Deng Y, Zhao J, Jiang S, Yuan Y, Wang HY, et al. TGF-β-miR-34a-CCL22 signaling-induced Treg cell recruitment promotes venous metastases of HBV-positive hepatocellular carcinoma. Cancer Cell. 2012 Sep 11;22(3):291–303. doi: 10.1016/j.ccr.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tan TL, Chen WN. J Clin Virol. 4. Vol. 33. Elsevier; 2005. A proteomics analysis of cellular proteins associated with HBV genotype-specific HBX: potential in identification of early diagnostic markers for HCC; pp. 293–298. [DOI] [PubMed] [Google Scholar]
- [22].Zhang J, Niu D, Sui J, Ching CB, Chen WN. Proteomics. 10. Vol. 9. Wiley Online Library; 2009. Protein profile in hepatitis B virus replicating rat primary hepatocytes and HepG2 cells by iTRAQ-coupled 2-D LC-MS/MS analysis: insights on liver angiogenesis; pp. 2836–2845. [DOI] [PubMed] [Google Scholar]
- [23].Chen SW, Himeno M, Koui Y, Sugiyama M, Nishitsuji H, Mizokami M, Shimotohno K, Miyajima A, Kido T. Sci Rep. 1. Vol. 10. Nature Publishing Group; 2020. Modulation of hepatitis B virus infection by epidermal growth factor secreted from liver sinusoidal endothelial cells; pp. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Siddiqui ZI, Azam SA, Khan WH, Afroz M, Farooqui SR, Amir F, Azmi MI, Anwer A, Khan S, Mehmankhah M. Front Genet. Vol. 12. Frontiers Media SA; 2021. An in vitro study on the role of hepatitis B virus X protein C-terminal truncation in liver disease development; 633341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Su F, Schneider RJ. Hepatitis B virus HBx protein sensitizes cells to apoptotic killing by tumor necrosis factor α. Proceed Nat Acad Sci Nat Acad Sci. 1997;94(16):8744–8749. doi: 10.1073/pnas.94.16.8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tanaka Y, Kanai F, Kawakami T, Tateishi K, Ijichi H, Kawabe T, Arakawa Y, Kawakami T, Nishimura T, Shirakata Y. Biochem Biophys Res Commun. 2. Vol. 318. Elsevier; 2004. Interaction of the hepatitis B virus X protein (HBx) with heat shock protein 60 enhances HBx-mediated apoptosis; pp. 461–469. [DOI] [PubMed] [Google Scholar]
- [27].Fonseka P, Pathan M, Chitti SV, Kang T, Mathivanan S. FunRich enables enrichment analysis of OMICs datasets. J Mol Biol. 2021 May 28;433(11):166747. doi: 10.1016/j.jmb.2020.166747. [DOI] [PubMed] [Google Scholar]
- [28].Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, et al. STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015 Jan 28;43(D1):D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chin CH, Chen SH, Wu HH, Ho CW, Ko MT, Lin CY. cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. 2014 Dec 8;8(4):S11. doi: 10.1186/1752-0509-8-S4-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003 Nov;13(11):2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG. Primer3—new capabilities and interfaces. Nucleic Acids Res. 2012 Aug;40(15):e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lánczky A, Nagy Á, Bottai G, Munkácsy G, Szabó A, Santarpia L, Győrffy B. miRpower: a web-tool to validate survival-associated miRNAs utilizing expression data from 2178 breast cancer patients. Breast Cancer Res Treat. 2016 Dec 1;160(3):439–446. doi: 10.1007/s10549-016-4013-7. [DOI] [PubMed] [Google Scholar]
- [33].Menyhárt O, Nagy Á, Győrffy B. Determining consistent prognostic biomarkers of overall survival and vascular invasion in hepatocellular carcinoma. R Soc Open Sci. 2018 Dec;5(12):181006. doi: 10.1098/rsos.181006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sookoian S, Flichman D, Garaycoechea ME, Gazzi C, Martino JS, Castaño GO, Pirola CJ. Sci Rep. 1. Vol. 8. Nature Publishing Group; 2018. Mar 23, Lack of evidence supporting a role of TMC4-rs641738 missense variant—MBOAT7-intergenic downstream variant—in the susceptibility to nonalcoholic fatty liver disease; 5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lupberger J, Hildt E. Hepatitis B virus-induced oncogenesis. World J Gastroenterol. 2007 Jan 7;13(1):74–81. doi: 10.3748/wjg.v13.i1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wieland S, Thimme R, Purcell RH, Chisari FV. Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci U S A. 2004 Apr 27;101(17):6669–6674. doi: 10.1073/pnas.0401771101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Iannacone M, Guidotti LG. Nat Rev Immunol. 1. Vol. 22. Nature Publishing Group; 2022. Jan, Immunobiology and pathogenesis of hepatitis B virus infection; pp. 19–32. [DOI] [PubMed] [Google Scholar]
- [38].Guidotti LG, Isogawa M, Chisari FV. Host-virus interactions in hepatitis B virus infection. Curr Opin Immunol. 2015 Oct;36:61–66. doi: 10.1016/j.coi.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bertoletti A, Ferrari C. J Hepatol. 1. Vol. 64. Elsevier; 2016. Apr 1, Adaptive immunity in HBV infection; pp. S71–S83. [DOI] [PubMed] [Google Scholar]
- [40].Lim CJ, Lee YH, Pan L, Lai L, Chua C, Wasser M, Lim TKH, Yeong J, Toh HC, Lee SY, Chan CY, et al. Gut. 5. Vol. 68. BMJ Publishing Group; 2019. May 1, Multidimensional analyses reveal distinct immune microenvironment in hepatitis b virus-related hepatocellular carcinoma; pp. 916–927. [DOI] [PubMed] [Google Scholar]
- [41].Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 2003 Nov;17(14):2115–2117. doi: 10.1096/fj.03-0329fje. [DOI] [PubMed] [Google Scholar]
- [42].Scharte M, Han X, Bertges DJ, Fink MP, Delude RL. American J Physiol Gastrointest Liver Physiol. 3. Vol. 284. American Physiological Society; 2003. Mar, Cytokines induce HIF-1 DNA binding and the expression of HIF-1-dependent genes in cultured rat enterocytes; pp. G373–G384. [DOI] [PubMed] [Google Scholar]
- [43].Wang L, Sun Y, Song X, Wang Z, Zhang Y, Zhao Y, Peng X, Zhang X, Li C, Gao C, Li N, et al. Cell Mol Immunol. 8. Vol. 18. Nature Publishing Group; 2021. Aug, Hepatitis B virus evades immune recognition via RNA adenosine deaminase ADAR1-mediated viral RNA editing in hepatocytes; pp. 1871–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hou ZH, Han QJ, Zhang C, Tian ZG, Zhang J. miR146a impairs the IFN-induced anti-HBV immune response by downregulating STAT1 in hepatocytes. Liver Int. 2014 Jan;34(1):58–68. doi: 10.1111/liv.12244. [DOI] [PubMed] [Google Scholar]
- [45].Hoan NX, Tong HV, Giang DP, Toan NL, Meyer CG, Bock CT, Kremsner PG, Song LH, Velavan TP. Interferon-stimulated gene 15 in hepatitis B-related liver diseases. Oncotarget. 2016 Sep 10;7(42):67777–67787. doi: 10.18632/oncotarget.11955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Li C, Wang J, Zhang H, Zhu M, Chen F, Hu Y, Liu H, Zhu H. Interferon-stimulated gene 15 (ISG15) is a trigger for tumorigenesis and metastasis of hepatocellular carcinoma. Oncotarget. 2014 Aug 6;5(18):8429–8441. doi: 10.18632/oncotarget.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Qiu X, Hong Y, Yang D, Xia M, Zhu H, Li Q, Xie H, Wu Q, Liu C, Zuo C. ISG15 as a novel prognostic biomarker for hepatitis B virus-related hepatocellular carcinoma. Int J Clin Exp Med. 2015 Oct 15;8(10):17140–17150. [PMC free article] [PubMed] [Google Scholar]
- [48].Bhanja Chowdhury J, Shrivastava S, Steele R, Di Bisceglie AM, Ray R, Ray RB. Hepatitis C virus infection modulates expression of interferon stimulatory gene IFITM1 by upregulating miR-130A. J Virol. 2012 Sep;86(18):10221–10225. doi: 10.1128/JVI.00882-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Li T, Ke Z, Liu W, Xiong Y, Zhu Y, Liu Y. Human hepatitis B virus Core protein inhibits IFNα-induced IFITM1 expression by interacting with BAF200. Viruses. 2019 May 9;11(5):427. doi: 10.3390/v11050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wu L, Tang Q, Yin X, Yan D, Tang M, Xin J, Pan Q, Ma C, Yan S. The therapeutic potential of adipose tissue-derived mesenchymal stem cells to enhance radiotherapy effects on hepatocellular carcinoma. [cited 2023 Jan 24];Front Cell Develop Biol. 2019 7 doi: 10.3389/fcell.2019.00267. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Yang G, Xu Y, Chen X, Hu G. IFITM1 plays an essential role in the antiproliferative action of interferon-gamma. Oncogene. 2007;26(4):594–603. doi: 10.1038/sj.onc.1209807. [DOI] [PubMed] [Google Scholar]
- [52].Zhao H, Wang J, Han Y, Huang Z, Ying J, Bi X, Zhao J, Fang Y, Zhou H, Zhou J, Li Z, et al. ARID2: A new tumor suppressor gene in hepatocellular carcinoma. Oncotarget. 2011 Nov 16;2(11):886–891. doi: 10.18632/oncotarget.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Li M, Zhao H, Zhang X, Wood LD, Anders RA, Choti MA, Pawlik TM, Daniel HD, Kannangai R, Offerhaus GJA, Velculescu VE, et al. Nat Genet. 9. Vol. 43. Nature Publishing Group; 2011. Sep, Inactivating mutations of the chromatin remodeling gene ARID2 in hepatocellular carcinoma; pp. 828–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Gao F, Liang H, Lu H, Wang J, Xia M, Yuan Z, Yao Y, Wang T, Tan X, Laurence A, Xu H, et al. Global analysis of DNA methylation in hepatocellular carcinoma by a liquid hybridization capture-based bisulfite sequencing approach. Clin Epigenetics. 2015 Aug 21;7(1):86. doi: 10.1186/s13148-015-0121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Huang WC, Tung SL, Chen YL, Chen PM, Chu PY. BMC Cancer. Vol. 18. BioMed Central Ltd; 2018. May 30, [cited 2023 Jan 24]. IFI44L is a novel tumor suppressor in human hepatocellular carcinoma affecting cancer stemness, metastasis, and drug resistance via regulating met/Src signaling pathway. [Internet] Available from: https://go.gale.com/ps/i.do?p=HRCA&sw=w&issn=147124=07&v=2.1&it=r&id=GALE%7CA546359284&sid=googleScholar&linkaccess=abs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Song G, Shi Y, Zhang M, Goswami S, Afridi S, Meng L, Ma J, Chen Y, Lin Y, Zhang J, Liu Y, et al. Cell Discov. 1. Vol. 6. Nature Publishing Group; 2020. Dec 8, Global immune characterization of HBV/HCV-related hepatocellular carcinoma identifies macrophage and T-cell subsets associated with disease progression; pp. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kao WY, Yang SH, Liu WJ, Yeh MY, Lin CL, Liu CJ, Huang CJ, Lin SM, Lee SD, Chen PJ, Yu MW. Genome-wide identification of blood DNA methylation patterns associated with early-onset hepatocellular carcinoma development in hepatitis B carriers: BLOOD DNA METHYLATION AND EARLY-ONSET HCC WITH HBV. Mol Carcinog. 2017 Feb;56(2):425–435. doi: 10.1002/mc.22505. [DOI] [PubMed] [Google Scholar]
- [58].Wu WJ, Kuo YC, Kao WY, Yang SH, Wu CF, Lin CL, Liu CJ, Huang YW, Yu MW. J Hepatol. Elsevier Science BV; PO BOX 211, 1000 AE Amsterdam, Netherlands: 2019. Influence of HBV genotype on the risk of hepatocellular carcinoma: mediating roles of different phases of viral infection and epigenetic modifications; E271. [Google Scholar]
- [59].Ebrahim M, Mirzaei V, Bidaki R, Shabani Z, Daneshvar H, Karimi-Googheri M, Khaleghinia M, Afrooz MR, Yousefpoor Y, Kazemi Arababadi M. Viral Immunol. 9. Vol. 28. Mary Ann Liebert, Inc., publishers; 2015. Nov, Are RIG-1 and MDA5 Expressions Associated with Chronic HBV Infection? pp. 504–508. [DOI] [PubMed] [Google Scholar]
- [60].Kochs G, Haller O. In: Handbook of Cell Signaling. Second Edition. Bradshaw RA, Dennis EA, editors. Academic Press; San Diego: 2010. [cited 2023 Jan 24]. Chapter 226 - mx proteins: High molecular weight GTPases with antiviral activity; pp. 1855–1864. [Internet] Available from: https://www.sciencedirect.com/science/article/pii/B9780123741455002266. [Google Scholar]
- [61].Chen J, Xu W, Chen Y, Xie X, Zhang Y, Ma C, Yang Q, Han Y, Zhu C, Xiong Y, Wu K, et al. Matrix metalloproteinase 9 facilitates hepatitis B virus replication through binding with type I interferon (IFN) receptor 1 to repress IFN/JAK/STAT signaling. J Virol American Soc Microbiol. 2017 Mar 29;91(8):e01824–16. doi: 10.1128/JVI.01824-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Zav’yalov VP, Hämäläinen-Laanaya H, Korpela TK, Wahlroos T. Interferon-inducible Myxovirus resistance proteins: potential biomarkers for differentiating viral from bacterial infections. Clin Chem. 2019 Jun;65(6):739–750. doi: 10.1373/clinchem.2018.292391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wang YX, Niklasch M, Liu T, Wang Y, Shi B, Yuan W, Baumert TF, Yuan Z, Tong S, Nassal M, Wen YM. Interferon-inducible MX2 is a host restriction factor of hepatitis B virus replication. J Hepatol. 2020 May;72(5):865–876. doi: 10.1016/j.jhep.2019.12.009. [DOI] [PubMed] [Google Scholar]
- [64].Hashemi SMA, Sarvari J, Fattahi MR, Dowran R, Ramezani A, Hosseini SY. Comparison of ISG15, IL28B and USP18 mRNA levels in peripheral blood mononuclear cells of chronic hepatitis B virus infected patients and healthy individuals. Gastroenterol Hepatol Bed Bench. 2019;12(1):38–45. [PMC free article] [PubMed] [Google Scholar]
- [65].Cai J, Liu T, Jiang X, Guo C, Liu A, Xiao X. Downregulation of USP18 inhibits growth and induces apoptosis in hepatitis B virus-related hepatocellular carcinoma cells by suppressing BCL2L1. Exp Cell Res. 2017 Sep 15;358(2):315–322. doi: 10.1016/j.yexcr.2017.07.006. [DOI] [PubMed] [Google Scholar]
- [66].Li L, Lei Q, Song Zhang SJ, Na Kong L, Qin B. PLoS One. 5. Vol. 11. Public Library of Science; 2016. May 26, Suppression of USP18 Potentiates the Anti-HBV Activity of Interferon Alpha in HepG2.2.15 Cells Via JAK/STAT Signaling; e0156496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Li Y, Yao M, Duan X, Ye H, Li S, Chen L, Yang C, Chen Y. The USP18 cysteine protease promotes HBV production independent of its protease activity. Virol J. 2020 Apr 5;17(1):47. doi: 10.1186/s12985-020-01304-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Tong HV, Hoan NX, Binh MT, Quyen DT, Meyer CG, Hang DTT, Hang DTD, Son HA, Van Luong H, Thuan ND, Giang NT, et al. Upregulation of enzymes involved in ISGylation and ubiquitination in patients with hepatocellular carcinoma. Int J Med Sci. 2020 Jan 20;17(3):347–353. doi: 10.7150/ijms.39823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Bao M, Wang Y, Liu Y, Shi P, Lu H, Sha W, Weng L, Hanabuchi S, Qin J, Plumas J, Chaperot L, et al. NFATC3 promotes IRF7 transcriptional activity in plasmacy–toid dendritic cells. J Exp Med. 2016 Oct 17;213(11):2383–2398. doi: 10.1084/jem.20160438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zao X, Cheng J, Shen C, Guan G, Feng X, Zou J, Zhang J, Liu T, Zheng H, Zhang T, Wang J, et al. OncoImmunology. 1. Vol. 10. Taylor & Francis; 2021. Jan 1, NFATc3 inhibits hepatocarcinogenesis and HBV replication via positively regulating RIG-I-mediated interferon transcription; 1869388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Jiang J, Tang H. Protein & Cell. 12. Vol. 1. Springer; 2010. Mechanism of inhibiting type I interferon induction by hepatitis B virus X protein; pp. 1106–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Zannetti C, Roblot G, Charrier E, Ainouze M, Tout I, Briat F, Isorce N, Faure-Dupuy S, Michelet M, Marotel M, Kati S, et al. Characterization of the Inflammasome in human Kupffer cells in response to synthetic agonists and pathogens. J Immunol. 2016 Jul 1;197(1):356–367. doi: 10.4049/jimmunol.1502301. [DOI] [PubMed] [Google Scholar]
- [73].Ho WHJ, Law AMK, Masle-Farquhar E, Castillo LE, Mawson A, O’Bryan MK, Goodnow CC, Gallego-Ortega D, Oakes SR, Ormandy CJ. Activation of the viral sensor oligoadenylate synthetase 2 (Oas2) prevents pregnancy-driven mammary cancer metastases. Breast Cancer Res. 2022 May 3;24(1):31. doi: 10.1186/s13058-022-01525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Liu Y, Wang X, Li S, Hu H, Zhang D, Hu P, Yang Y, Ren H. The role of von Willebrand factor as a biomarker of tumor development in hepatitis B virus-associated human hepatocellular carcinoma: a quantitative proteomic based study. J Proteome. 2014 Jun;25(106):99–112. doi: 10.1016/j.jprot.2014.04.021. [DOI] [PubMed] [Google Scholar]
- [75].Sun S, Li Y, Han S, Jia H, Li X, Li X. A comprehensive genome-wide profiling comparison between HBV and HCV infected hepatocellular carcinoma. BMC Med Genet. 2019 Oct 28;12(1):147. doi: 10.1186/s12920-019-0580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Zhu LM, Shi DM, Dai Q, Cheng XJ, Yao WY, Sun PH, Ding YF, Qiao MM, Wu YL, Jiang SH, Tu SP. Tumor suppressor XAF1 induces apoptosis, inhibits angiogenesis and inhibits tumor growth in hepatocellular carcinoma. Oncotarget. 2014 Jun 18;5(14):5403–5415. doi: 10.18632/oncotarget.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lin Y, Li W. Assessment of XAF1 as a biomarker to differentiate hepatocellular carcinoma from nonneoplastic liver tissues. Chin J Cancer Res. 2012 Mar 1;24(3):201–206. doi: 10.1007/s11670-012-0201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Zhang F, Wu LM, Zhou L, Chen QX, Xie HY, Feng XW, Zheng SS. Predictive value of expression and promoter Hypermethylation of XAF1 in hepatitis B virus-associated hepatocellular carcinoma treated with transplantation. Ann Surg Oncol. 2008 Dec 1;15(12):3494–3502. doi: 10.1245/s10434-008-0146-1. [DOI] [PubMed] [Google Scholar]
- [79].Tsuge M, Fujimoto Y, Hiraga N, Zhang Y, Ohnishi M, Kohno T, Abe H, Miki D, Imamura M, Takahashi S, Ochi H, et al. Hepatitis C virus infection suppresses the interferon response in the liver of the human hepatocyte chimeric mouse. PLoS One. 2011 Aug 23;6(8):e23856. doi: 10.1371/journal.pone.0023856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Chen J, Li Y, Lai F, Wang Y, Sutter K, Dittmer U, Ye J, Zai W, Liu M, Shen F. Functional comparison of IFN-alpha subtypes reveals potent HBV suppression by a concerted action of IFN-alpha and-gamma signaling. Hepatology. 2020;10 doi: 10.1002/hep.31282. [DOI] [PubMed] [Google Scholar]
- [81].Habjan M, Hubel P, Lacerda L, Benda C, Holze C, Eberl CH, Mann A, Kindler E, Gil-Cruz C, Ziebuhr J, Thiel V, et al. PLoS Pathog. 10. Vol. 9. Public Library of Science; 2013. Oct 3, e1003663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Chen Q, Li F, Gao Y, Xu G, Liang L, Xu J. Identification of energy metabolism genes for the prediction of survival in hepatocellular carcinoma. Front Oncol. 2020;10:1210. doi: 10.3389/fonc.2020.01210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Zou L, Chai J, Gao Y, Guan J, Liu Q, Du JJ. Down-regulated PLAC8 promotes hepatocellular carcinoma cell proliferation by enhancing PI3K/Akt/GSK3β/Wnt/β-catenin signaling. Biomed Pharmacother. 2016 Dec;1(84):139–146. doi: 10.1016/j.biopha.2016.09.015. [DOI] [PubMed] [Google Scholar]
- [84].Zhang Y, Pan Q, Shao Z. Extracellular vesicles derived from cancer-associated fibroblasts carry tumor-promotive microRNA-1228-3p to enhance the resistance of hepatocellular carcinoma cells to sorafenib. Hum Cell. 2023 Jan 1;36(1):296–311. doi: 10.1007/s13577-022-00800-7. [DOI] [PubMed] [Google Scholar]
- [85].Slade CD, Reagin KL, Lakshmanan HG, Klonowski KD, Watford WT. Placenta-specific 8 limits IFNγ production by CD4+ T cells in vitro and promotes establishment of influenza-specific CD8+ T cells in vivo. PLoS One. 2020 Jul 8;15(7):e0235706. doi: 10.1371/journal.pone.0235706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Mao M, Chen Y, Yang J, Cheng Y, Xu L, Ji F, Zhou J, Zhang X, Li Z, Chen C, Ju S, et al. Modification of PLAC8 by UFM1 affects tumorous proliferation and immune response by impacting PD-L1 levels in triple-negative breast cancer. J Immunother Cancer BMJ Special J. 2022 Dec 1;10(12):e005668. doi: 10.1136/jitc-2022-005668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Cao N, Mu L, Yang W, Liu L, Liang L, Zhang H. MicroRNA-298 represses hepatocellular carcinoma progression by inhibiting CTNND1-mediated Wnt/β-catenin signaling. Biomed Pharmacother. 2018 Oct 1;106:483–490. doi: 10.1016/j.biopha.2018.06.135. [DOI] [PubMed] [Google Scholar]
- [88].Jiang Y, Han Q, Zhao H, Zhang J. Promotion of epithelial-mesenchymal transformation by hepatocellular carcinoma-educated macrophages through Wnt2b/β-catenin/c-Myc signaling and reprogramming glycolysis. J Exp Clin Cancer Res. 2021 Jan 6;40(1):13. doi: 10.1186/s13046-020-01808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Tang B, Tang F, Wang Z, Qi G, Liang X, Li B, Yuan S, Liu J, Yu S, He S. Overexpression of CTNND1 in hepatocellular carcinoma promotes carcinous characters through activation of Wnt/β-catenin signaling. J Exp Clin Cancer Res. 2016 May 18;35(1):82. doi: 10.1186/s13046-016-0344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Li X, Liu H, Cheng W, Wang J, Zhang H, Lu F, Chen X, Lin W, Junceellolide B. A novel inhibitor of hepatitis B virus. Bioorg Med Chem. 2020;28(16):115603. doi: 10.1016/j.bmc.2020.115603. [DOI] [PubMed] [Google Scholar]
- [91].Iizuka N, Tsunedomi R, Tamesa T, Okada T, Sakamoto K, Hamaguchi T, Yamada-Okabe H, Miyamoto T, Uchimura S, Hamamoto Y, Oka M. Involvement of c-myc-regulated genes in hepatocellular carcinoma related to genotype-C hepatitis B virus. J Cancer Res Clin Oncol. 2006 Jul;132(7):473–481. doi: 10.1007/s00432-006-0094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Mancina RM, Dongiovanni P, Petta S, Pingitore P, Meroni M, Rametta R, Borén J, Montalcini T, Pujia A, Wiklund O, Hindy G, et al. The MBOAT7-TMC4 variant rs641738 increases risk of nonalcoholic fatty liver disease in individuals of European descent. Gastroenterology. 2016 May;150(5):1219–1230.:e6. doi: 10.1053/j.gastro.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Li S. Cells. 5. Vol. 8. Multidisciplinary Digital Publishing Institute; 2019. May, Regulation of Ribosomal Proteins on Viral Infection; p. 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analysed during the current study are publicly available in the Gene Expression Omnibus (GEO) repository, https://www.ncbi.nlm.nih.gov/gds/, RNA sequencing data can be accessed from SRA database using PRJNA960847 BioProject accession number. Information from the datasets analysed during this study are included in this article and its supplementary information files. The raw data supporting the conclusions of this article will be made available on request to the authors.