Abstract
Elevated blood pressure, dyslipidemia, and impaired glycemic control are well-established cardiovascular risk factors in Europeans, but there are comparatively few studies focused on East Asian populations. This study evaluated the potential causal relations between traditional cardiovascular risk factors and disease risk in East Asians through a 2-sample Mendelian randomization approach. We collected summary statistics for blood pressure parameters, lipid subsets, and type 2 diabetes mellitus liability from large genome-wide association study meta-analyses conducted in East Asians and Europeans. These were paired with summary statistics for ischemic heart disease (IHD), ischemic stroke (IS), peripheral vascular disease, heart failure (HF) and atrial fibrillation (AF). We performed univariable Mendelian randomization analyses for each exposure-outcome pair, followed by multivariable analyses for the available lipid subsets. The genetically predicted risk factors associated with IHD and AF were similar between East Asians and Europeans. However, in East Asians only genetically predicted elevated blood pressure was significantly associated with IS (odds ratio 1.05, 95% confidence interval 1.04 to 1.06, p < 0.0001) and HF (odds ratio 1.05, 95% confidence interval 1.04 to 1.06, p < 0.0001), whereas nearly all genetically predicted risk factors were significantly associated with IS and HF in Europeans. In conclusion, this study provides supportive evidence for similar causal relations between traditional cardiovascular risk factors and IHD and AF in both East Asian and European ancestry populations. However, the identified risk factors for IS and HF differed between East Asians and Europeans, potentially highlighting distinct disease etiologies between these populations.
Keywords: lipids, blood pressure, diabetes mellitus, ischemic heart disease, atrial fibrillation, heart failure, peripheral vascular disease, stroke, mendelian randomisation, genetics
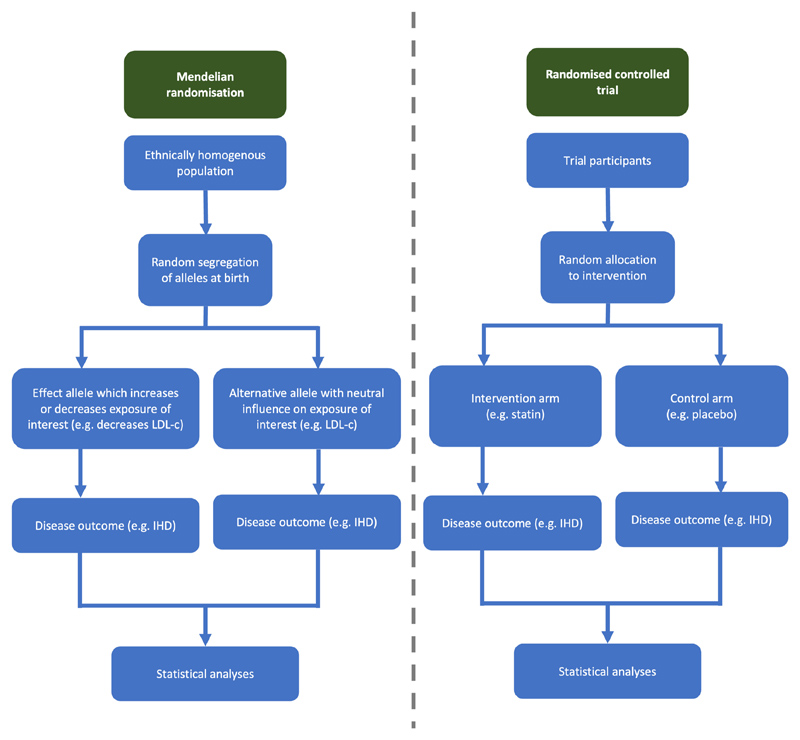
Dyslipidemia, hypertension, and type 2 diabetes mellitus (T2DM) have consistently been identified as common modifiable risk factors for cardiovascular diseases. However, most evidence comes from European ancestry populations,1–3 with comparatively little evidence from subjects of East Asian ancestry despite comprising at least one-fifth of the world population. Several observational studies have demonstrated notable epidemiologic differences in the burden of cardiovascular diseases for East Asians compared with Europeans and North Americans.4–6 However there remains a relative scarcity of randomized trials performed in East Asian populations to investigate these observational findings. Mendelian randomization (MR) is a research methodology that can test potentially causal relations between cardiovascular risk factors and diseases. A proportion of the phenotype of an individual is determined by genetic polymorphisms that are randomly inherited at birth. The random allocation of phenotype-determining genetic polymorphisms is analogous to assignment to a treatment group in a randomized control trial (Figure 1). MR leverages this random and non-modifiable allocation of genetic variants to help eliminate the risk of bias because of reverse causality and minimize bias from confounding, both of which typically hinder interpretation of nonrandomized observational studies. The present study therefore investigates the potentially causal relations between traditional cardiovascular risk factors and common cardiovascular diseases in East Asian ancestry individuals and compares results with those obtained in European ancestry individuals.
Figure 1. Overview of comparison between a Mendelian randomization study and a randomized controlled trial.
Methods
The present study uses a 2-sample MR approach. The exposure variables included low-density lipoprotein cholesterol (LDL-c), triglycerides (TGs), high-density lipoprotein cholesterol (HDL-c), systolic blood pressure (SBP), diastolic blood pressure (DBP) and T2DM liability. Ischemic heart disease (IHD), ischemic stroke (IS), peripheral vascular disease (PVD), heart failure (HF), and atrial fibrillation (AF) were used as the outcome variables. Population homogeneity is a requirement for the samples used in a 2-sample MR analysis, and thus both exposure and outcome data were sourced from either East Asian or European ancestry populations, according to the specified ancestry in the analysis. MR makes 3 further core assumptions which are that the genetic variants should be: (1) strongly associated with the exposure; (2) exclusively associated with the outcome through the exposure; and (3) independent of potential confounders. To address the first criterion, uncorrelated genetic variants were selected that were significantly associated with the exposure subsets at p <5 × 10−8; and to evaluate the latter 2 criteria, sensitivity analyses were performed as described later.
Data for the present study are publicly available. Ethical approval and consent were obtained by the original studies. Preparation of this manuscript was based on the Strengthening the Reporting of Observational Studies in Epidemiology using Mendelian Randomization Guidelines.7
For the East Asian analyses: the data for lipid subsets was extracted from a genome-wide association study (GWAS) meta-analysis performed by the Global Lipids Genetic Consortium for LDL-c, HDL-c and log TG using data from 40 cohorts which included 146,492 East Asian individuals8 (summary statistics available at: http://csg.sph.umich.edu/willer/public/glgc-lipids2021/); BP data were extracted from a GWAS meta-analysis of SBP and DBP from 183,785 East Asian individuals, including 125,778 participants from BioBank Japan (BBJ) (summary statistics for significant SNPs are available from the Supplementary Table of the original manuscript)9; and T2DM liability data were extracted from a GWAS meta-analysis of 433,540 East Asian ancestry individuals from 23 cohorts, including 191,832 individuals from BBJ (summary statistics available at: https://blog.nus.edu.sg/agen/).10
For the European analyses: the LDL-c, HDL-c, and log TG data were similarly extracted from a GWAS meta-analysis performed by the Global Lipids Genetic Consortium using data from up to 146 cohorts and 1,320,016 participants8 (summary statistics available at: http://csg.sph.umich.edu/willer/public/glgc-lipids2021/); BP data were extracted from a GWAS meta-analysis of SBP and DBP including up to 1,006,863 European participants (summary statistics for significant SNPs available from the Supplementary Table of the original manuscript)11; and T2DM liability data were extracted from a GWAS meta-analysis of DIAGRAM, GERA and UK Biobank including up to 659,316 European ancestry individuals (summary statistics available at: https://www.ebi.ac.uk/gwas/studies/GCST006867).12
Single-nucleotide polymorphisms (SNPs) that were associated with each exposure variable at p <5 × 10−8 were extracted. Linkage disequilibrium (LD) clumping was performed (r2 <0.001, 10 megabase distance cutoff, East Asian or European ancestry participants of the 1000 genomes project) and the variants with the smallest p values were selected for further analysis.
GWAS analyses of BBJ data were used for all East Asian outcomes: myocardial infarction (MI),13 IS,13 peripheral artery disease (PAD),13 chronic HF (CHF),13 and AF.14 For each outcome, this included: 14,992 MI cases and 146,212 controls; 22,664 IS cases and 152,022 controls; 4,112 PAD cases and 173,601 controls; 10,540 CHF cases and 169,186 controls; and 8,180 AF cases and 28,612 controls. Cases were defined by past medical history and textmining of electronic medical records. Data for MI, IS, PAD, and CHF are available from: https://pheweb.jp/pheno/MI.13 Data for AF is available from: http://jenger.riken.jp/en/result.14 For the purpose of reporting results later, MI is reported as IHD, PAD as PVD, and CHF as HF.
For the European ancestry analyses: IHD data were extracted from a meta-analysis of 48 studies including 60,801 coronary artery disease cases and 123,504 controls, with 77% of participants of European ancestry (summary statistics available at: http://www.cardiogramplusc4d.org/data-downloads/)15; IS data were extracted from a metaanalysis including 34,217 IS cases and 406,111 controls of European ancestry (summary statistics available at: https://www.ebi.ac.uk/gwas/studies/GCST006908)16; PVD data were extracted from the United Kingdom Biobank GWAS pipeline using the Phenome Scan Analysis Tool, which included 1,456 cases and 461,554 controls (summary statistics available from: https://gwas.mrcieu.ac.uk/datasets/ukb-b-4929/)17; HF data were extracted from a GWAS meta-analysis of 26 studies including 47,309 cases and 930,014 controls of European ancestry (summary statistics available at: https://www.ebi.ac.uk/gwas/studies/GCST009541)18; and AF data were extracted from a GWAS of 6 contributing studies including 60,620 cases and 970,216 controls of European ancestry (summary statistics available at: https://www.ebi.ac.uk/gwas/studies/GCST006414).19
Further details about the genotyping methodology and phenotype definitions can be found in the original GWAS manuscripts (Supplementary Table 9).
The primary MR analysis was an inverse variance weighted (IVW) meta-analysis of the Wald ratios for each genetic variant. Results are expressed as odds of the outcome per SD increase in genetically predicted lipid variables and per mm Hg increase in BP variables. A Bonferroni-corrected p value threshold was set at p = 0.0017 for all primary analyses (0.05 / 30, based on 6 exposure and 5 outcome variables) and p = 0.017 for multi-variable analyses if significant on univariable analysis (0.05 / 3, because 3 lipid subsets were included in each model) (Figures 2 to 6, Supplementary Tables 1 to 4).
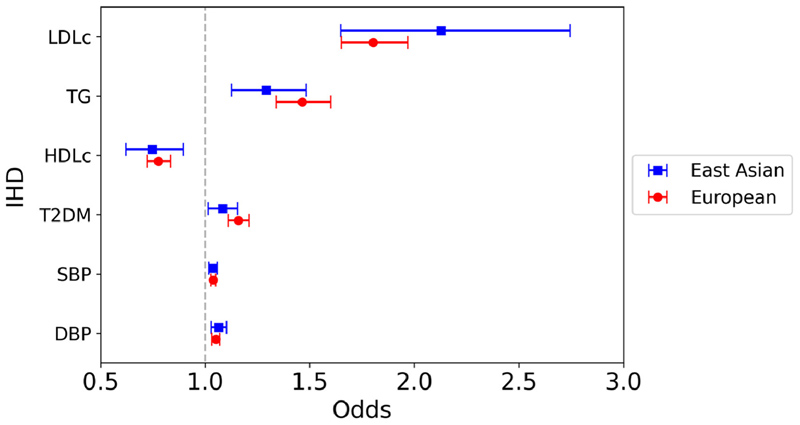
Figure 2. Mendelian randomization inverse variance weighted estimates for the effect of 1 SD increase in genetically predicted elevated LDL-c, TG, and HDL-c, 1 mm Hg increase in SBP, and DBP, and T2DM liability on risk of IHD.
Figure 6. Mendelian randomization inverse variance weighted estimates for the effect of 1 SD increase in genetically predicted elevated LDL-c, TG and HDL-c, 1 mm Hg increase in SBP, and DBP, and T2DM liability on risk of AF.
To evaluate for violation of the main MR assumptions because of horizontal pleiotropy, we performed several sensitivity analyses including weighted-median, weighted-mode, MR-Egger, and MR-RAPS (Supplementary Figure 1, Supplementary Tables 1 and 3). Each method makes different assumptions and thus concordance between methods provides confidence in the conclusion. The weighted-median approach assumes that at least half the instrumental variables are valid. Weighted-mode assumes that the most common causal effect is consistent with the true effect. MR-Egger uses the Instrument Strength Independent of Direct Effect (InSIDE) assumption, which states that the strength of pleiotropic effects from the genetic variants to the outcome is independent of the strength of the association between genetic variants and exposure. Under this assumption, the intercept from MR-Egger analysis estimates the average pleiotropic effect of the genetic variants (Supplementary Table 5). MR-Egger typically has lower power compared with IVW. MR-RAPS is robust to idiosyncratic and systematic pleiotropy, and weak instrument bias. Cochran’s Q statistic was used to test for genetic variant heterogeneity (Supplementary Table 6). Leave-one-out analyses were performed for each exposure-outcome pair (Supplementary Figures 2 and 3). Scatter and funnel plots are presented in Supplementary Figures 4 to 7.
Multivariable MR was subsequently performed to assess whether the effects of the lipid subsets on the outcome were independent of each other (Supplementary Tables 2 and 4, Table 1). Only SNPs that were available in all lipid subsets and outcome summary statistic datasets were considered. Of these, variants associated with at least one lipid trait at p <5 × 10−8 were extracted, clumped after ordering from lowest to highest p value of association with any trait (r2 <0.001, 10 megabase distance cutoff, East Asian, or European ancestry participants of the 1,000 genomes project), and harmonized, before multivariable IVW MR was performed.
Table 1. Multivariable mendelian randomisation estimates for the direct effect of one standard deviation increase in genetically predicted elevated low-density lipoprotein cholesterol, triglycerides and high-density lipoprotein cholesterol respectively, accounting for the other lipid subsets.
| Ischemic heart disease Odds (95% CI) | Ischemic stroke Odds (95% CI) | Peripheral vascular disease Odds (95% CI) | Heart failure Odds (95% CI) | Atrial fibrillation Odds (95% CI) | |
|---|---|---|---|---|---|
| East Asian | |||||
| Low-density lipoprotein cholesterol | 2.06 (1.71-2.48) | 0.94 (0.80-1.13) | 2.28 (1.75-2.96) | 1.17 (0.98-1.40) | 1.13 (0.77-1.66) |
| Triglycerides | 1.21 (0.95-1.55) | 1.40 (1.12-1.77) | 0.81 (0.61-1.08) | 1.01 (0.79-1.28) | 1.37 (0.94-2.00) |
| High-density lipoprotein cholesterol | 0.69 (0.56-0.86) | 1.09 (0.93-1.29) | 0.44 (0.33-0.58) | 0.83 (0.70-0.99) | 0.76 (0.54-1.06) |
| European | |||||
| Low-density lipoprotein cholesterol | 2.01 (1.75-2.31) | 1.32 (1.07-1.63) | 2.40 (1.24-4.63) | 1.34 (1.18-1.52) | 0.95 (0.79-1.15) |
| Triglycerides | 1.27 (1.05-1.52) | 1.15 (0.88-1.51) | 1.31 (0.64-2.69) | 1.21 (0.99-1.46) | 0.69 (0.54-0.87) |
| High-density lipoprotein cholesterol | 0.78 (0.67-0.92) | 0.86 (0.68-1.09) | 0.66 (0.41-1.07) | 0.66 (0.56-0.78) | 0.62 (0.50-0.77) |
Statistical analyses were performed using R version 1.4.1106 with the TwoSampleMR package (The R Foundation for Statistical Computing, Vienna, Austria).
Results
In the main IVW analyses, a significant association was observed between nearly all traditional cardiovascular risk factors and IHD in East Asians (LDL-c: odds ratio [OR] 2.13, 95% confidence interval [CI] 1.65 to 2.75, p <0.0001; TG: OR 1.29, 95% CI 1.13 to 1.48, p = 0.00026; HDL-c: OR 0.75, 95% CI 0.62 to 0.90, p = 0.0016; SBP: OR 1.04, 95% CI 1.02 to 1.06, p = 0.00032; DBP: OR 1.07, 95% CI 1.03 to 1.10, p = 0.00030) (Figure 2). The association between T2DM and IHD did not reach Bonferroni-corrected statistical significance in East Asians on IVW analysis (OR 1.08, 95% CI 1.02 to 1.16, p = 0.016). In the European analyses, all genetically predicted risk factors, including T2DM, were significantly associated with IHD risk. For both the East Asian and European analyses, multivariable MR accounting for all 3 lipid subsets in the same model demonstrated a similar association between genetically predicted higher LDL-c and lower HDL-c with increased risk of IHD, but there was no strong association between genetically predicted TG and IHD risk in the East Asian analysis (Table 1).
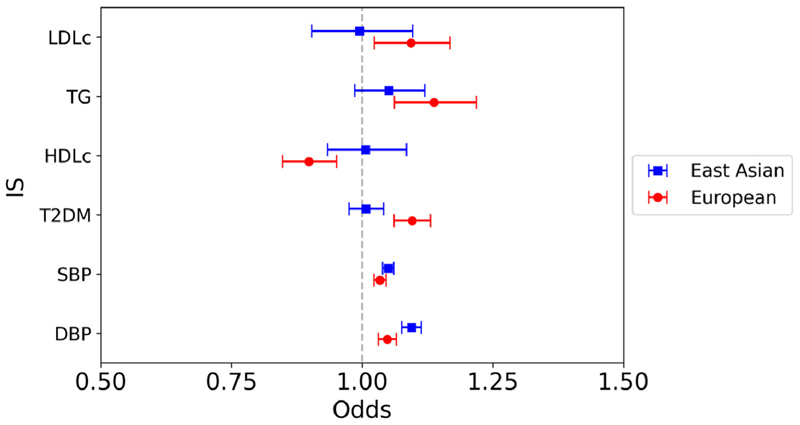
In East Asians, a significant association was observed between the genetically predicted BP parameters and IS on univariable IVW analysis (SBP: OR 1.05, 95% CI 1.04 to 1.06, p <0.0001; DBP: OR 1.09, 95% CI 1.08 to 1.11, p <0.0001), but there was no association between any of the genetically predicted lipid parameters or T2DM on IS risk (Figure 3). This is in contrast to the European analysis, in which significant associations were identified between all risk factor variables, except LDL-c and IS.
Figure 3. Mendelian randomization inverse variance weighted estimates for the effect of 1 SD increase in genetically predicted elevated LDL-c, TG and HDL-c, 1 mm Hg increase in SBP, and DBP, and T2DM liability on risk of IS.
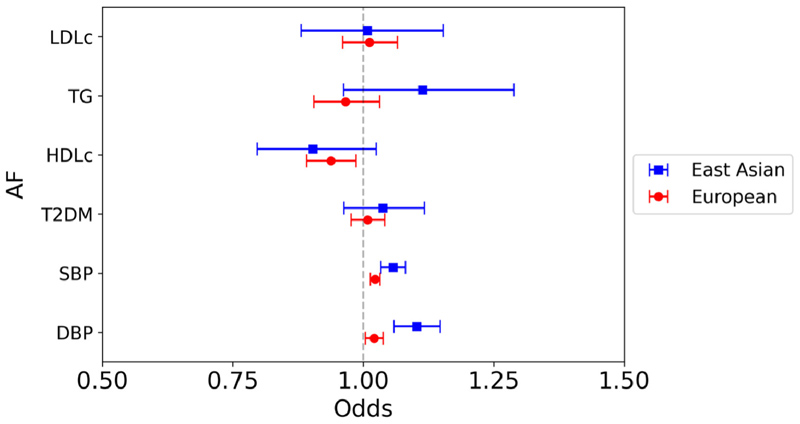
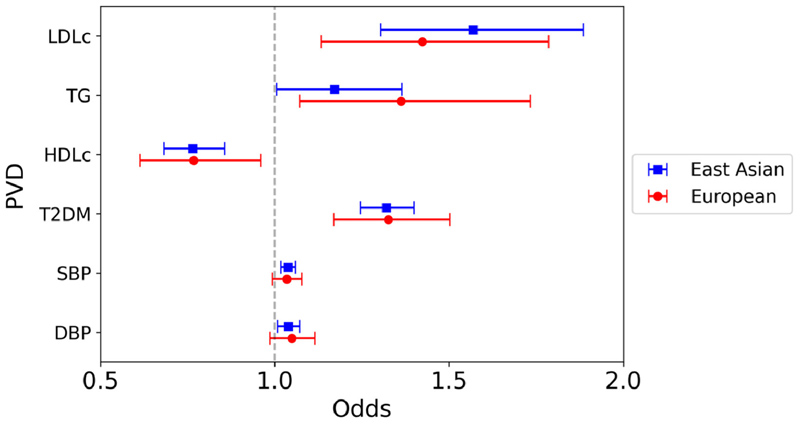
Significant associations were observed between genetically predicted LDL-c, HDL-c, T2DM liability, and SBP with risk of PVD (LDL-c: OR 1.57, 95% CI 1.30 to 1.89, p <0.0001; HDL-c: OR 0.76, 95% CI 0.68 to 0.86, p <0.0001; T2DM: OR 1.32, 95% CI 1.25 to 1.40, p <0.0001; SBP: OR 1.04, 95% CI 1.02 to 1.06, p = 0.00027), but not genetically predicted TG or DBP with PVD risk (TG: OR 1.17, 95% CI 1.01 to 1.36, p = 0.043; DBP: OR 1.04, 95% CI 1.01 to 1.07, p = 0.013) (Figure 4). On multivariable MR, there remained a significant association between both LDL-c and HDL-c with PVD. In contrast, for the European analysis only genetically predicted T2DM liability was significantly associated with increased PVD risk on univariable analysis.
Figure 4. Mendelian randomization inverse variance weighted estimates for the effect of 1 SD increase in genetically predicted elevated LDL-c, TG and HDL-c, 1 mm Hg increase in SBP, and DBP, and T2DM liability on risk of PVD.
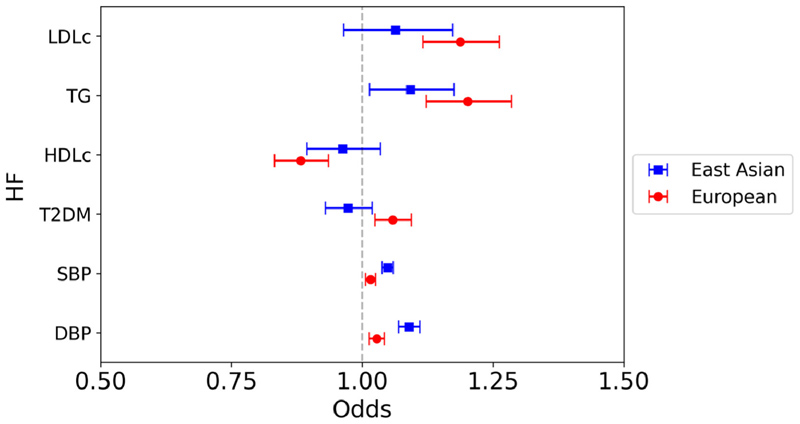
Similar to the results for IS, in East Asians a significant association was observed between the genetically predicted BP parameters and HF on univariable IVW analysis (SBP: OR 1.05, 95% CI 1.04 to 1.06, p <0.0001; DBP: OR 1.09, 95% CI 1.07 to 1.11, p <0.0001), but no association between any of the genetically predicted lipid subsets or T2DM liability on HF risk (Figure 5). This contrasts the European analysis, in which significant associations were identified between all analyzed risk factors and HF.
Figure 5. Mendelian randomization inverse variance weighted estimates for the effect of 1 SD increase in genetically predicted elevated LDL-c, TG, and HDL-c, 1 mm Hg increase in SBP and DBP, and T2DM liability on risk of HF.
In East Asians, genetically predicted higher BP was associated with increased risk of AF (SBP: OR 1.06, 95% CI 1.03 to 1.08; DBP: OR 1.10, 95% CI 1.06 to 1.15), with no association between any of the genetically predicted lipid subsets or T2DM liability on AF (Figure 6). In the European analysis, genetically predicted higher SBP was associated with increased risk of AF, but DBP, the lipid subsets, and T2DM liability were not.
Discussion
Blood pressure, lipid levels and T2DM are traditionally considered to be modifiable risks influenced by both genetic and lifestyle factors. The present study leveraged large-scale GWAS data and the MR approach to evaluate the association between traditional cardiovascular risk factors and diseases in East Asian ancestry individuals. We found similar risk factor profiles for IHD and AF between East Asian and European ancestry populations. However, for East Asians, in contrast to Europeans, only genetically predicted elevated BP was significantly associated with IS and HF.
The increased risk of cardiovascular diseases because of dyslipidemia, hypertension, and T2DM has been established in European populations through large observational and randomized trials1–3 and extrapolated to non-Europeans in clinical practice. The present study supports this approach for IHD and AF, demonstrating largely concordant findings between East Asian and European ancestry populations for the association between traditional cardiovascular risk factors and these outcomes. However, the present study provided evidence for a causal role of genetically predicted elevated BP, but not dyslipidemia or T2DM, as risk factors for IS and HF in East Asians. Previous studies in East Asians have consistently demonstrated that elevated BP contributes to a substantially higher population-attributable risk toward stroke compared with dyslipidemia and diabetes mellitus.20 Existing evidence has additionally demonstrated that East Asians are more frequently diagnosed with masked hypertension, and East Asian populations may experience a higher morning BP surge than Europeans which is predictive of stroke risk.21 Indeed, the relatively increased susceptibility of East Asians to elevated BP was recognized by Kario et al22 in a targeted consensus statement on hypertension management specifically for Asian populations.
The finding that the lipid subsets were significantly associated with IHD and PVD but not IS, HF, or AF in East Asians was unexpected in the context of the results from European ancestry individuals. This may be related to insufficient statistical power, although comparable findings have previously been reported. The Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese (MEGA) trial, which Randomized 7,832 Japanese patients to pravastatin plus diet versus diet alone, showed a significant reduction in risk of MI and overall coronary heart disease but no significant difference in risk of IS in the pravastatin group.23 In the more recent but smaller Japan Statin Treatment Against Recurrent Stroke (J-STARS) trial, in which 1,578 Japanese patients with previous noncardioembolic IS and hypercholesterolemia were randomized to pravastatin or control, the pravastatin group experienced lower rates of atherothrombotic infarct but the overall rate of stroke or transient ischemic attack was not significantly different from the control group.24 Neither study reported outcome rates of PVD, HF, or AF. The inverse association between genetically predicted HDL-c and nearly all the outcomes in the European IVW analyses is also interesting in light of recent studies which have questioned the causal association between HDL-c and cardiovascular events.25,26 This may reflect the larger sample size in the present study compared with previous analyses. Notably, however, these results were not consistently supported by the sensitivity analyses, which is similar to the report by Burgess and Davey Smith27 that demonstrated a significant association in MR analysis between HDL-c and coronary artery disease on IVW but not sensitivity analyses.
Genetic ancestry has previously been shown to modulate the effect of T2DM on risk of diabetic complications. The present study showed an association between genetically predicted T2DM and elevated risk of PVD in East Asians and a trend toward association with IHD that did not reach significance after Bonferroni-correction for multiple testing. Previous studies on the relation between T2DM and cardiovascular diseases in East Asians have not been consistent in their findings. A multiethnic study of 62,432 participants in North America found lower risk of incident MI, congestive HF, stroke and non-traumatic lower extremity amputation, and higher risk of end-stage renal disease in Asian compared with European ancestry individuals.28 In contrast, the Action in Diabetes and Vascular Disease study found that Asian participants with T2DM had higher incidence of stroke and nephropathy, and lower incidence of PVD and coronary disease compared with their Eastern European and Australian counterparts.29 Notably, neither study was specific to East Asians, and it is well-described that within Asia there is heterogeneity regarding cardiovascular disease epidemiology.4
There are several important limitations. Statistical power was limited particularly for the PVD outcome dataset in Europeans, for which there were only 1,456 cases, and consequently the CIs were unsurprisingly wide (Supplementary Table 8). Furthermore, because several of the outcome variables were extracted from biobank data, it is possible that there were inaccuracies in the attribution of participants as a case versus control. Moreover, the outcome definitions between East Asian and European analyses were not identical, as the analyses were limited by the availability of large suitable GWAS datasets. IHD, for example, was defined for East Asians as MI (BBJ) whereas the European dataset included MI, acute coronary syndrome, chronic stable angina, and coronary stenosis >50% (CARDIoGRAM-plusC4D). Importantly, this assumes shared pathogenesis for the spectrum of IHD from mild coronary artery disease to MI. Despite this, the effect of each exposure variable on risk of IHD was comparable between East Asians and Europeans. Furthermore, HF and IS are heterogeneous diseases. IS, for example, has several subtypes with different etiologies that may vary between ancestry groups and this may contribute to the differing risk factor profiles between ancestry groups. Additionally, while there is some overlap between several of the exposure and outcome datasets, the use of strong instruments, achieved by only using SNPs associated with the exposure at p <5 × 10−8 with associated high F-statistics (Supplementary Table 7), is expected to mitigate the potential risk of bias. Although there was evidence of significant heterogeneity in many of the analyses, determined by evaluation of the Q statistic, the concordance between the main IVW and sensitivity analyses suggests that bias is less likely to have substantially impacted the results.
Overall, this study used large-scale GWAS data of East Asian individuals to evaluate the relation between traditional cardiovascular risk factors and diseases in a population that is understudied relative to the proportion of the global population. This analysis demonstrated a similar risk factor profile for IHD and AF between East Asians and Europeans but suggested that elevated BP may be the more pertinent risk factor for IS and HF in East Asians. This study presents an important complementary research approach that may better inform further investigation into population-level cardiovascular disease prevention and treatment strategies in East Asian populations.
Supplementary Material
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjcard.2023.08.007.
Funding
none.
Footnotes
Declaration of Competing Interest
The authors have no competing interests to declare.
References
- 1.Cholesterol Treatment Trialists‘ (CTT) Collaborators. Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, Voysey M, Gray A, Collins R, Baigent C. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blood Pressure Lowering Treatment Trialists‘ Collaboration. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. Lancet. 2021;397:1625–1636. doi: 10.1016/S0140-6736(21)00590-0. [published correction appears in Lancet 2021;397:1884] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffin SJ, Leaver JK, Irving GJ. Impact of metformin on cardiovascular disease: a meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia. 2017;60:1620–1629. doi: 10.1007/s00125-017-4337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao D. Epidemiological features of cardiovascular disease in Asia. JACC Asia. 2021;1:1–13. doi: 10.1016/j.jacasi.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters SAE, Wang X, Lam TH, Kim HC, Ho S, Ninomiya T, Knuiman M, Vaartjes I, Bots ML, Woodward M, Asia Pacific Cohort Studies Collaboration Clustering of risk factors and the risk of incident cardiovascular disease in Asian and Caucasian populations: results from the Asia Pacific Cohort Studies Collaboration. BMJ Open. 2018;8:e019335. doi: 10.1136/bmjopen-2017-019335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park TH, Ko Y, Lee SJ, Lee KB, Lee J, Han MK, Park JM, Cho YJ, Hong KS, Kim DH, Cha JK, et al. Identifying target risk factors using population attributable risks of ischemic stroke by age and sex. J Stroke. 2015;17:302–311. doi: 10.5853/jos.2015.17.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skrivankova VW, Richmond RC, Woolf BAR, Davies NM, Swanson SA, VanderWeele TJ, Timpson NJ, Higgins JPT, Dimou N, Langenberg C, Loder EW, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ. 2021;375:n2233. doi: 10.1136/bmj.n2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham SE, Clarke SL, Wu KH, Kanoni S, Zajac GJM, Ramdas S, Surakka I, Ntalla I, Vedantam S, Winkler TW, Locke AE, et al. The power of genetic diversity in genome-wide association studies of lipids [published correction appears in Nature 2023;618:E19-E20] Nature. 2021;600:675–679. doi: 10.1038/s41586-021-04064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeuchi F, Akiyama M, Matoba N, Katsuya T, Nakatochi M, Tabara Y, Narita A, Saw WY, Moon S, Spracklen CN, Chai JF, et al. Interethnic analyses of blood pressure loci in populations of East Asian and European descent. Nat Commun. 2018;9:5052. doi: 10.1038/s41467-018-07345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spracklen CN, Horikoshi M, Kim YJ, Lin K, Bragg F, Moon S, Suzuki K, Tam CHT, Tabara Y, Kwak SH, Takeuchi F, et al. Identification of type 2 diabetes loci in 433,540 East Asian individuals. Nature. 2020;582:240–245. doi: 10.1038/s41586-020-2263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Million Veteran Program. Evangelou E, Warren HR, Mosen-Ansorena D, Mifsud B, Pazoki R, Gao H, Ntritsos G, Dimou N, Cabrera CP, Karaman I, Ng FL, et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits [published correction appears in Nat Genet 2018;50:1755] Nat Genet. 2018;50:1412–1425. doi: 10.1038/s41588-018-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xue A, Wu Y, Zhu Z, Zhang F, Kemper KE, Zheng Z, Yengo L, Lloyd-Jones LR, Sidorenko J, Wu Y, McRae AF, et al. Genome-wide association analyses identify 143 risk variants and putative regulatory mechanisms for type 2 diabetes. Nat Commun. 2018;9:2941. doi: 10.1038/s41467-018-04951-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakaue S, Kanai M, Tanigawa Y, Karjalainen J, Kurki M, Koshiba S, Narita A, Konuma T, Yamamoto K, Akiyama M, Ishigaki K, et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat Genet. 2021;53:1415–1424. doi: 10.1038/s41588-021-00931-x. [DOI] [PubMed] [Google Scholar]
- 14.Low SK, Takahashi A, Ebana Y, Ozaki K, Christophersen IE, Ellinor PT, Ogishima S, Yamamoto M, Satoh M, Sasaki M, Yamaji T, et al. Identification of six new genetic loci associated with atrial fibrillation in the Japanese population. Nat Genet. 2017;49:953–958. doi: 10.1038/ng.3842. [DOI] [PubMed] [Google Scholar]
- 15.Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S, Saleheen D, Kyriakou T, Nelson CP, Hopewell JC, Webb TR, et al. A comprehensive 1000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47:1121–1130. doi: 10.1038/ng.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, Rutten-Jacobs L, Giese AK, van der Laan SW, Gretarsdottir S, Anderson CD, et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. 2018;50:524–537. doi: 10.1038/s41588-018-0058-3. [published correction appears in Nat Genet 2019;51:1192-1193] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Millard LAC, Davies NM, Gaunt TR, Davey Smith G, Tilling K. Software Application Profile: PHESANT: a tool for performing automated phenome scans in UK Biobank. Int J Epidemiol. 2018;47:29–35. doi: 10.1093/ije/dyx204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah S, Henry A, Roselli C, Lin H, Sveinbjäornsson G, Fatemifar G, Hedman AK, Wilk JB, Morley MP, Chaffin MD, Helgadottir A, et al. Genome-wide association and Mendelian randomisation analysis provide insights into the pathogenesis of heart failure. Nat Commun. 2020;11:163. doi: 10.1038/s41467-019-13690-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nielsen JB, Thorolfsdottir RB, Fritsche LG, Zhou W, Skov MW, Graham SE, Herron TJ, McCarthy S, Schmidt EM, Sveinbjornsson G, Surakka I, et al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat Genet. 2018;50:1234–1239. doi: 10.1038/s41588-018-0171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim YD, Jung YH, Saposnik G. Traditional risk factors for stroke in East Asia. J Stroke. 2016;18:273–285. doi: 10.5853/jos.2016.00885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoshide S, Yano Y, Haimoto H, Yamagiwa K, Uchiba K, Nagasaka S, Matsui Y, Nakamura A, Fukutomi M, Eguchi K, Ishikawa J, et al. Morning and evening home blood pressure and risks of incident stroke and coronary artery disease in the Japanese general practice population: the Japan morning surge-home blood pressure study. Hypertension. 2016;68:54–61. doi: 10.1161/HYPERTENSIONAHA.116.07201. [DOI] [PubMed] [Google Scholar]
- 22.Kario K, Chen CH, Park S, Park CG, Hoshide S, Cheng HM, Huang QF, Wang JG. Consensus document on improving hypertension management in Asian patients, taking into account Asian characteristics. Hypertension. 2018;71:375–382. doi: 10.1161/HYPERTENSIONAHA.117.10238. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto Y, Toyota T, Nakaya N, Nishimoto S, Muranaka M, Yamamoto A, Mizuno K, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368:1155–1163. doi: 10.1016/S0140-6736(06)69472-5. [DOI] [PubMed] [Google Scholar]
- 24.Hosomi N, Nagai Y, Kohriyama T, Ohtsuki T, Aoki S, Nezu T, Maruyama H, Sunami N, Yokota C, Kitagawa K, Terayama Y, et al. The Japan statin treatment against recurrent stroke (J-STARS): a multicenter, randomized, open-label, parallel-group study. EBioMedicine. 2015;2:1071–1078. doi: 10.1016/j.ebiom.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Razavi AC, Jain V, Grandhi GR, Patel P, Karagiannis A, Patel N, Dhindsa DS, Liu C, Desai SR, Almuwaqqat Z, Sun YV, et al. Does elevated high-density lipoprotein cholesterol protect against cardiovascular disease? J Clin Endocrinol Metab. doi: 10.1210/clinem/dgad406. published online July 12, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rohatgi A, Westerterp M, von Eckardstein A, Remaley A, Rye KA. HDL in the 21st century: a multifunctional roadmap for future HDL research. Circulation. 2021;143:2293–2309. doi: 10.1161/CIRCULATIONAHA.120.044221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burgess S, Davey Smith G. Mendelian randomization implicates high-density lipoprotein cholesterol-associated mechanisms in etiology of age-related macular degeneration. Ophthalmology. 2017;124:1165–1174. doi: 10.1016/j.ophtha.2017.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population [published correction appears in JAMA 2002;288:46] JAMA. 2002;287:2519–2527. doi: 10.1001/jama.287.19.2519. [DOI] [PubMed] [Google Scholar]
- 29.Clarke PM, Glasziou P, Patel A, Chalmers J, Woodward M, Harrap SB, Salomon JA. Event rates, hospital utilization, and costs associated with major complications of diabetes: a multicountry comparative analysis. PLoS Med. 2010;7:e1000236. doi: 10.1371/journal.pmed.1000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.