Abstract
Cell plasticity represents the ability of cells to be reprogrammed and to change their fate and identity, enabling homeostasis restoration and tissue regeneration following damage. Cell plasticity also contributes to pathological conditions, such as cancer, enabling cells to acquire new phenotypic and functional features by transiting across distinct cell states that contribute to tumor initiation, progression, metastasis, and resistance to therapy. Here, we review the intrinsic and extrinsic mechanisms driving cell plasticity that promote tumor growth and proliferation, as well as metastasis and drug tolerance. Finally, we discuss how cell plasticity could be exploited for anti-cancer therapy.
Introduction
Although lineage specification and differentiation were long assumed to be unidirectional and irreversible, cell identity is currently recognized to be less rigid and more plastic than previously thought. Cell plasticity refers to the reprograming of a cell towards a different fate in response to intrinsic or extrinsic factors1,2. Stem cells are plastic and have the capacity to self-renew and differentiate into one or more cell lineages. The capacity of terminally differentiated cells, such as fibroblasts, to be reprogrammed back to a pluripotent state shows that plasticity is not only a stem-cell feature3,4. Cells can display plasticity through dedifferentiation (the reversion of a differentiated cell into an undifferentiated state within the same lineage), transdifferentiation (the conversion of a differentiated cell into another differentiated cell lineage, forming the basis of metaplasia)5 (Figure 1A) and epithelial-to- mesenchymal transition (EMT), a process through which epithelial cells lose epithelial characteristics, such as cell-cell junctions and polarity, and acquire a mesenchymal phenotype6.
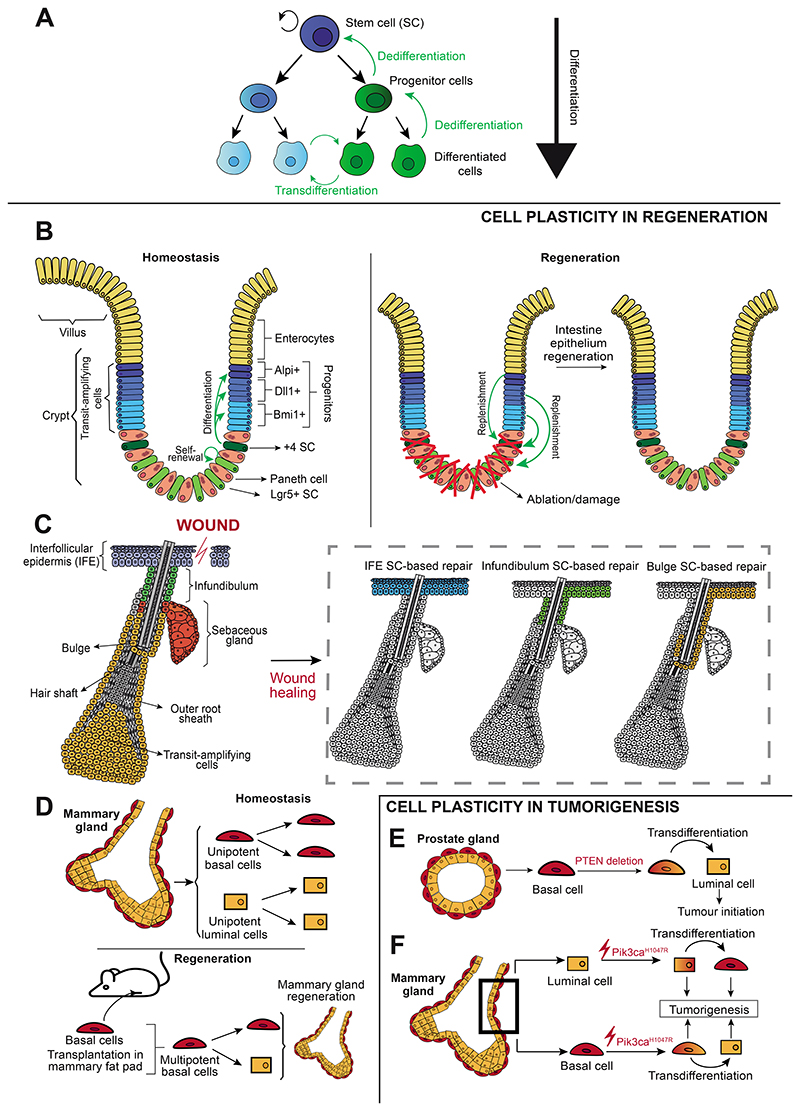
Figure 1. Cell plasticity during homeostasis, regeneration and tumorigenesis.
(A) Stem cell differentiation, dedifferentiation and transdifferentiation occurring during cell plasticity. (B) Lgr5+ intestinal stem cells self-renew and give rise to the distinct intestinal lineages during homeostasis. Following stem cell lineage ablation, more committed progenitors can replenish the pool of stem cells, enabling epithelium regeneration. (C) During homeostasis, the different epidermal compartments are sustained by distinct pools of unipotent SCs whereas during wound healing, interfollicular epidermis stem cells contribute to skin repair but also stem cells from the infundibulum and bulge can migrate upwards, proliferate, and be reprogrammed into interfollicular epidermis stem cells to contribute to regeneration. (D) Under homeostatic conditions, basal and luminal cells in the mammary gland are unipotent. Following transplantation into the mammary fat pad, basal cells become multipotent and can give rise to luminal cells, enabling the generation of a functional mammary gland. (E) PTEN deletion in basal cells of the prostate gland promotes basal-to-luminal transdifferentiation and leads to tumor initiation. (F) Pik3caH1047R expression in basal cells in the mammary gland leads to a transdifferentiation into luminal cells, while its expression in luminal cells enables a transdifferentiation into basal cells. Both basal and luminal cells expressing Pik3caH1047R can initiate tumorigenesis. IFE, interfollicular epidermis; SC, stem cell.
Plasticity is essential to restore homeostasis after tissue damage, inflammation, or senescence, but can also contribute to tumorigenesis. During cancer progression, tumor cells can switch between cell states –a process primarily mediated by cell plasticity— to overcome selective pressures. Thus, cell plasticity largely fuels intra-tumor heterogeneity2,7,8 (as well as other sources such as DNA mutations9,10) and fitness, increasing the adaptability of tumor cells9, and contributes substantially to tumor growth, metastasis, and resistance to therapy.
Cell Plasticity from Homeostasis to Tumorigenesis
Under physiological conditions in adult tissues, replenishment of differentiated cells is ensured by multipotent or lineage restricted stem cells. During wound healing and tissue regeneration, the latter can become plastic and expand their differentiation potential to replace other cell types and promote tissue repair8.
The intestinal epithelium is one of the most rapidly self-renewing tissues in mammals. Lgr5 marks the stem cells in the small intestine and colon11 that initiate the formation of crypt-villus self-organizing mouse organoids12. Intestinal crypts contain stem cells and transit amplifying progenitors that can revert to a multipotent state under regenerative conditions13. Following Lgr5+ stem cell lineage ablation in mice, committed Bmi1-expressing cells can sustain homeostasis and replenish the pool of Lgr5+ stem cells14. Even more differentiated Alpi+ enterocyte progenitors can revert into Lgr5+ cells15. Following damage, committed precursors, such as secretory Dll1+ progenitors or Paneth cells, which are derived from Lgr5+ cells, can revert to the latter to replenish the stem cell pool and enable regeneration in mice16,17 (Figure 1B).
In response to ionizing irradiation in the mouse intestine, YAP, the transcriptional activator of the Hippo pathway, promotes cell survival and a regenerative state required for tumor formation18. Colon regeneration following dextran sulphate sodium-induced colitis in mouse models activates the YAP/TAZ pathway to reprogram adult cells into a fetal-like state required for regeneration19. Parasitic helminth infection in mice suppresses the normal adult stem cell program and promotes a similar state20. The YAP1-dependent stem cell state has been associated with intestinal regeneration also by single-cell transcriptomics21. However, YAP has also been proposed to antagonize stemness during regeneration and act as a tumor suppressor gene in a mouse model of colorectal cancer, possibly reflecting differences in the models employed22. In intestinal tumors, different populations have been identified resembling Lgr5+ crypt-base columnar stem cells and Lgr5- regenerative stem cells expressing the fetal-like state, whose respective abundance is regulated by intrinsic and extrinsic stimuli23.
The skin epidermis is composed by a pilosebaceous unit containing one hair follicle, its associated sebaceous gland and surrounding interfollicular epidermis8. During homeostasis, these different regions are maintained by their own pool of unipotent stem cells. During wound healing, different interfollicular epidermis stem and progenitor cells are recruited. Hair follicle and infundibulum stem cells migrate upwards towards the interfollicular epidermis, are progressively reprogrammed into interfollicular epidermis stem cells, proliferate, and contribute to skin repair8,24–26. The niche is important for this reprograming: when mouse hair follicle stem cells are ablated, the empty niche can recruit more committed cells that revert to a stem-like state and stably replenish the stem cell pool27 (Figure 1C).
Many glandular epithelia are composed of an inner luminal layer surrounded by an outer layer of myoepithelial and/or basal cells, and develop from multipotent progenitors, which are progressively replaced by unipotent stem cells during adult tissue homeostasis8. When taken out of their natural environment in absence of luminal cells, basal stem cells exhibit a greater differentiation potential, giving rise to luminal cells, and generate functional mammary glands in mice28–30 (Figure 1D). In prostate, the existence of multipotent basal progenitors during postnatal development contrasts with the distinct pools of unipotent basal and luminal stem cells that mediate adult regeneration31–33. Luminal cell depletion by infection, E-cadherin knock-out or genetic ablation can stimulate basal cell multipotency in glandular epithelia to replenish luminal cells34–36.
The ability of differentiated cells to revert to a stem-like state has major implications for tumorigenesis, with some oncogenic drivers influencing plasticity during tumor initiation. Tumor suppressors such as TP53, RB1 or PTEN regulate developmental differentiation programs, and when dysregulated are associated with cancer5. In glandular epithelia, unipotent basal and luminal stem cells can reacquire multipotency during tumor initiation. During mouse prostate tumor initiation, PTEN deletion in basal cells promotes basal-to-luminal transdifferentiation33,37 (Figure 1E). Combined TP53 and RB1 loss-of-function mutations promote transdifferentiation from adenocarcinoma to neuroendocrine carcinoma in mouse prostate cancer38,39. Similarly, in the mouse mammary gland, BRCA1 inactivation in luminal progenitors leads to basal-like breast cancer, displaying heterogeneous expression of basal and luminal markers40. Oncogenic Pik3caH1047R expression induces multipotency in mammary gland lineage-restricted progenitors early during tumor initiation, setting the basis for intra-tumor heterogeneity41,42 (Figure 1F).
Inflammation also regulates plasticity during regeneration and tumor initiation43. In the mouse small intestine, inflammation is followed by a loss of Lgr5+ stem cells, thereby inducing Paneth cells to re-enter the cell cycle, acquire stem-like properties and contribute to tissue regeneration44. In absence of inflammation, only intestinal stem cells can induce tumor formation following APC deletion. Co-deletion of APC and IκBα, which activates NF-kB signaling, induces tumor formation by non-stem cells, showing that inflammatory signals can expand their tumor-initiating capacities45. In the mouse prostate gland, bacterial infection-induced inflammation promotes basal-to-luminal transdifferentiation and accelerates tumor initiation from basal cells34. Inflammation promotes cell plasticity in the pancreas, by triggering acinar-to-ductal metaplasia46. When oncogenic Kras is expressed in the presence of inflammation, metaplasia progresses to neoplasia47,48. Tissue regeneration in the presence of oncogenic Kras induces a unique chromatin state essential for tumor formation49. In Nr5a2+/- mice, an AP1-dependent transcriptional switch from differentiation to inflammation potentially explains why mutations around the human NR5A2 gene promote pancreatic cancer50.
Tumor Growth and Proliferation
Tumors are composed by tumor cells of different states, accomplishing distinct functions. In this section, we discuss the extensively studied concept that tumor growth is sustained by cancer stem cells (CSCs).
Cancer Stem Cells and Intrinsic Regulation of Proliferative States
CSCs express a stem-like program, are able to self-renew, sustain tumor growth, and give rise to tumor cells with more restricted proliferative potential51. For example, colorectal CSCs express a gene signature reminiscent of normal intestinal stem cells52,53.
Whereas the xenotransplantation assay was the main method initially used to define CSCs, other approaches including lineage tracing, barcoding and lineage ablation were developed54 (Box 1; Figure 2A). These efforts showed that CSCs might not be a unique population but might instead represent several subpopulations. In a strict hierarchical organization, CSCs would give rise to subpopulations with more limited growth and differentiation potential, which could never revert to a CSC state55,56. However, evidence suggests that both CSCs and non-CSCs are plastic and might undergo phenotypic transitions under certain conditions (e.g., therapy)54. For example, JARID1B expression is essential for continuous tumor growth in melanoma, with this phenotype being dynamic – JARID1B- cells can become JARID1B+ and vice versa-, suggesting that melanoma maintenance is a dynamic process mediated by a temporarily distinct subpopulation57. Differentiated colon cancer cells can revert to a CSC state to compensate the CSC loss and replenish the CSC population58,59. Genetic ablation of Lgr5+ CSCs in xenografted mouse colorectal cancer organoids restricts tumor growth without leading to regression. Tumors are then maintained by proliferative Lgr5- cells that replenish the CSC pool. Lgr5+ CSCs reappear when ablation is discontinued, leading to rapid tumor regrowth and indicating plasticity of more differentiated tumor cells following CSC ablation58. This finding is supported by patient-derived organoids. Following Lgr5+ CSC ablation in xenografted human colorectal cancer organoids, Lgr5− cells replenish the Lgr5+ CSC pool, mediating tumor relapse59, and suggesting that therapies targeting CSCs without preventing cell plasticity would be insufficient.
Box 1. Functional strategies to identify cancer stem cells.
In classical xenotransplantation experiments, the capacity of a subpopulation to initiate a tumor following transplantation into immunodeficient mice over serial passages is interpreted as evidence of CSC presence54,271 (Figure 2A). These studies identified CD34+ CD38+ CSCs in acute myeloid leukemia272, CD44+ CD24-/low in breast cancer273, EpCAMhigh/CD44+ in colorectal cancer274, and CD133+ in brain275, pancreas276 and colon tumors277–279.
Xenotransplantation experiments enable the study of the tumor-propagating capacity of a specific tumor subpopulation in patient-derived samples. However, this technique has inherent technical and biological limitations, such as the lack of native architecture and stroma54,271. Xenotransplantation might not consider clonal cooperation or competition and can present clonal selection, leading to the formation of dominant clones with low frequency in the primary tumor, and different degrees of mouse immunodeficiency might lead to variable results280. Xenotransplantation reveals the potential of certain subpopulations to form tumors, which might not be representative of the fate of the tumor cells within their native microenvironment.
Lineage tracing is the gold standard method for defining cell fate in vivo and has been used to study CSCs within their native microenvironment and the hierarchical organization of tumor growth62,281 (Figure 2A). Conventional lineage tracing was largely restricted to genetic mouse models, but CRISPR-Cas9 gene editing technology enables to perform lineage tracing in patient-derived tumor organoids, as shown by colorectal cancer studies59,282. Emerging lineage tracing approaches combined with single-cell sequencing rely on naturally occurring molecular barcodes, such as somatic nuclear mutations and copy-number variations to conduct longitudinal studies along disease progression283. Mitochondrial DNA mutations can also be used as phylogenetic barcodes to study clonal dynamics.
Laser- or genetic-induced lineage ablation is another powerful approach to assess the importance of a subpopulation for tumor growth, maintaining the natural microenvironment of the tumor54,271. In tumors maintained by CSCs, CSC ablation will result in tumor regression, such as it occurs when ablating Nestin+ cells in mouse glioblastoma285, Sox2+ cells in mouse skin squamous cell carcinoma286, Dclk1+ cells in mouse intestinal tumors287 or Lgr5+ cells in human colorectal cancer59 (Figure 2A).
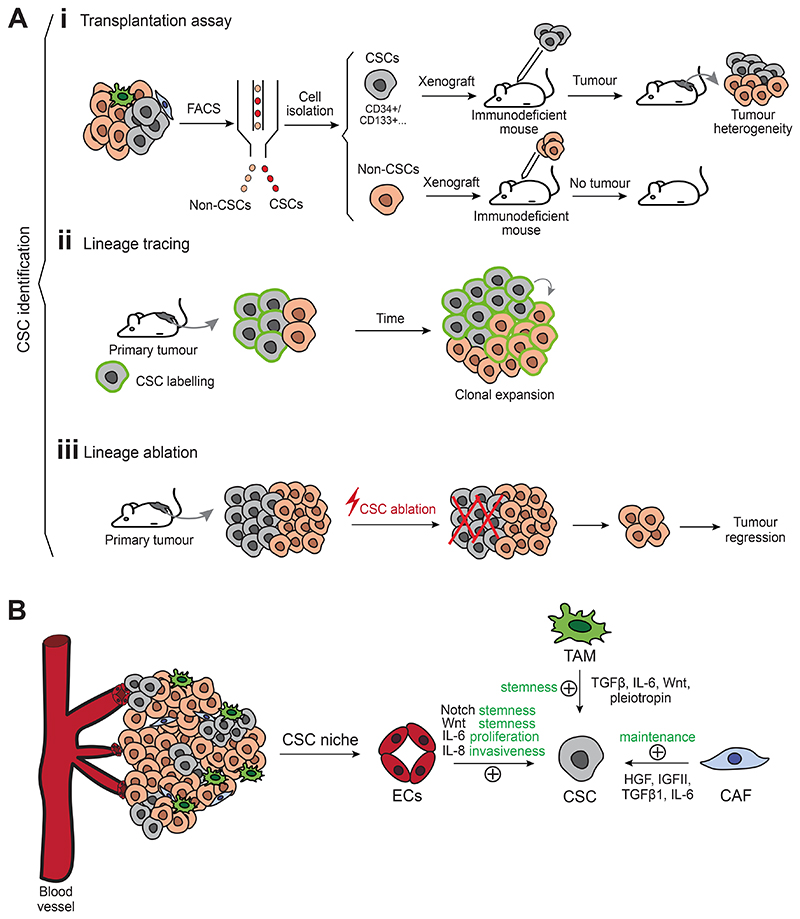
Figure 2. Defining cancer stem cells and their niche.
(A) Functional strategies to identify CSCs include: (i) transplantation assays (tumor subpopulations isolated by fluorescence-activated cell sorting are transplanted into immunodeficient mice. If CSCs are grafted, a tumor will appear and will recapitulate tumor heterogeneity, while non-CSCs will be less efficient to propagate the tumor following transplantation), (ii) lineage tracing of CSCs (which allows to follow their fate during tumor progression and to assess clonal expansion) and (iii) lineage ablation (which allows the elimination of a specific subpopulation. If CSCs are eliminated, the remaining subpopulations will not be able to sustain tumor growth, and tumor regression will occur). (B) A crosstalk between CSCs and their microenvironment is essential to sustain tumor growth. CSCs are supported by a niche composed by cancer-associated fibroblasts, endothelial cells and immune cells, which extrinsically promote tumor stemness. CAF, cancer-associated fibroblast; CSC, cancer stem cell; EC, endothelial cell; FACS, fluorescence-activated cell sorting; TAM, tumor-associated macrophage.
Clonal analysis combined with lineage tracing helped define the evolutionary dynamics of tumor growth, supporting in some cases a neutral drift of tumor evolution with the emergence of subclones. In mouse skin tumors, neutral competition of tumor cells in benign papilloma indicates that tumor growth is mediated by stochastic cell fate decisions, reminiscent of the clonal dynamics of normal stem cells60,61, further suggesting that tumor heterogeneity can be sometimes explained by neutral drift rather than selective pressures62,63. Barcoding human glioblastoma cells shows that clonal dynamics during tumor growth is consistent with neutral evolution fueled by glioblastoma stem cells64. The notion that tumors can evolve through neutral drift implies that non-genetic cancer cell plasticity, rather than the sole process of genetic selection driven by selective pressures and gain of fitness, contributes to tumor growth and adaptation in some cancers.
Proliferative states have been reported by single cell transcriptomics in multiple cancer types, including mouse hepatocellular carcinoma65 and human breast cancer66, oligodendroglioma67, glioblastoma68,69 and lung cancer70, supporting that tumors present proliferative states corresponding to cells that fuel tumor growth and likely reflect CSCs.
The Cancer Stem Cell Niche
The niche describes the microenvironment that sustains renewal and restricts premature differentiation of the stem cell pool71. The CSC niche is composed of heterogeneous and interacting cell populations and plays a major role in tumorigenesis, being essential for CSC regulation and promoting cancer cell plasticity (Figure 2B)7. Lineage tracing in human colon cancer xenografts reveals that functional colorectal CSCs that give rise to dominant clones driving tumor expansion, predominantly reside at the leading edge, close to cancer-associated fibroblasts (CAFs), which produce osteopontin, a factor that drives in situ clonogenicity72. Similarly, osteopontin arising from the vascular niche enhances CSC phenotypes and promotes tumor growth in mouse glioma73. In physiological situations, stem cells or their differentiated progeny can participate in the niche formation74,75. In cancer, some tumor subpopulations can contribute to the formation of the niche by a Wnt-dependent mechanism76.
The vascular niche refers to a specialized highly vascularized region composed of endothelial cells, pericytes, smooth muscle cells and immune cells, which creates a tumor-permissive microenvironment by influencing stemness, chemoresistance, invasion and metastasis77. Endothelial cells maintain stemness in CSCs by secreting Wnt and Notch ligands and direct cell-cell interactions, as shown in human pancreatic ductal adenocarcinoma organoids and breast cancer mouse models78,79. Endothelial cells also increase invasiveness and proliferation through IL880 and IL6 secretion in skin squamous cell carcinoma81 (Figure 2B). In melanoma, the CSC pool localizes near the vasculature and endothelial cells stimulate tumor cell dedifferentiation, promoting growth through NOTCH3-dependent cell-cell communication82. CSCs can induce vascular niche formation through VEGF secretion, which subsequently regulates CSC renewal. VEGF secretion by CSCs promotes stemness in a cell autonomous manner by an autocrine Flt1/Nrp1 signaling loop in mouse skin cancer83,84.
Apart from attracting and reprograming endothelial cells during tumorigenesis, CSCs can transdifferentiate into endothelial-like cells through vascular mimicry. Low oxygen levels within the tumor might promote stemness and the acquisition of endothelial features by CSCs85. Human glioblastoma CSCs cultured under endothelial conditions can differentiate into endothelial cells, with a significant proportion of them arising from tumor cell differentiation following xenotransplantation86. Transdifferentiation of tumor cells into endothelial cells has been shown in different human and murine cancers87,88, but its biological relevance remains unclear. In mouse breast cancer, vascular mimicry occurs in a tumor subpopulation secreting Serpine2 and Slp1 independently from endothelial-mediated neovascularization, and is thus resistant to classical anti-angiogenic therapy85,89.
CAFs participate in CSC maintenance through cytokine secretion, including HGF, IGFII, TGFβ1, IL6 and multiple CC-chemokine ligands, and matrix remodeling through matrix metalloproteinase secretion and deposition of collagen and hyaluronan90,91 (Figure 2B). Only specific fibroblast subsets can promote tumor stemness. In breast and lung cancer patients, a fibroblast subpopulation expressing CD10 and GPR77 promotes stemness through IL6 and IL8 secretion, localizes near CSCs and is characterized by sustained NF-κB pathway activation, dependent on GPR77-induced p65 phosphorylation. Anti-GPR77 treatment reduces tumor growth in patient-derived xenografts92. In mouse hepatocellular carcinoma, HGF secretion by myofibroblasts regulates CSC plasticity through c-MET/FRA1/HEY1 signaling93. Additionally, HGF promotes resistance to BRAF inhibitors in mouse and human melanoma and lung cancer94,95. In colon cancer, HGF-producing myofibroblasts activate Wnt, stimulate CSC features at the tumor edges and promote invasion, suggesting that CSC identity is partly regulated by the microenvironment96. Tumor-cell-intrinsic Wnt signaling can regulate fibroblast plasticity and induce a myofibroblast phenotype that promotes tumor growth and inhibits EMT97. However, CAFs are a heterogeneous population and specific subtypes present antitumoral properties. In a murine model of metastatic colorectal cancer, myofibroblasts exert tumor-restraining functions through BMP4 secretion, which inhibits stemness in intestinal stem cells. Myofibroblast depletion results in an increased CSC pool98. CAF plasticity has been also suggested to occur in human solid tumors99.
Immune cells are key components of the CSC niche71. Depletion of tumor-associated macrophages or inflammatory monocytes by inhibiting the myeloid cell receptors CCR2 or CSF1R decreases CSC features in pancreatic cancer100. CSCs and macrophage communication occurs through direct interaction, as in breast cancer, where the macrophage-created CSC niche fuels EMT, inducing EphA4 expression in CSCs, which in turn promotes cytokine secretion and sustains CSC stemness101. Cytokine secretion by macrophages (e.g., TGFβ, IL-6, Wnt ligands and pleiotropin) promotes stemness in tumor cells, primarily through STAT3 signaling102,103 (Figure 2B).
CSC localization inside tumors is key for their functional properties. Gradients of cytokines, availability of nutrients and cell-cell interactions differ if cells are close to the tumor migration front, blood vessels, or in the necrotic hypoxic tumor core. Hypoxic regions are associated with acidity and necrosis, promoting tumor aggressiveness, with hypoxia being an inducer of stemness56 through hypoxia-induced factors 1 and 2 (HIF1 and HIF2), which are expressed in acute- and long-term hypoxia, respectively104. Transplantation of breast cancer cell lines in a hypoxic mouse model increases the CSC population within the hypoxic regions, which remains stable across serial transplantation and is maintained by PI3K/AKT pathway105. In human pancreatic cancer, hypoxia-mediated production of L-2 hydroxyglutarate through LDHA activation results in histone H3 hypermethylation and increased stemness, by altering the transcription of differentiation genes and inducing CD133 and Sox2106.
Plasticity Along the Metastatic Cascade
Metastasis occurs through a multistep cascade, which includes the detachment of cancer cells from the primary tumor, local invasion into the surrounding tissue, intravasation into the blood or lymphatic vessels, extravasation, colonization of a secondary organ and growth of a secondary tumor. Growing evidence indicates that only certain subpopulations of tumor cells, termed metastasis-initiating cells (MICs), are able to form metastases107. In contrast to tumor initiation, which is linked to mutations in cancer drivers, no metastasis-specific mutations have been identified108,109, although certain mutations might predispose to metastasis110,111. MICs are highly plastic, displaying different degrees of stemness, EMT and metabolic plasticity along the entire metastatic cascade (Figure 3).
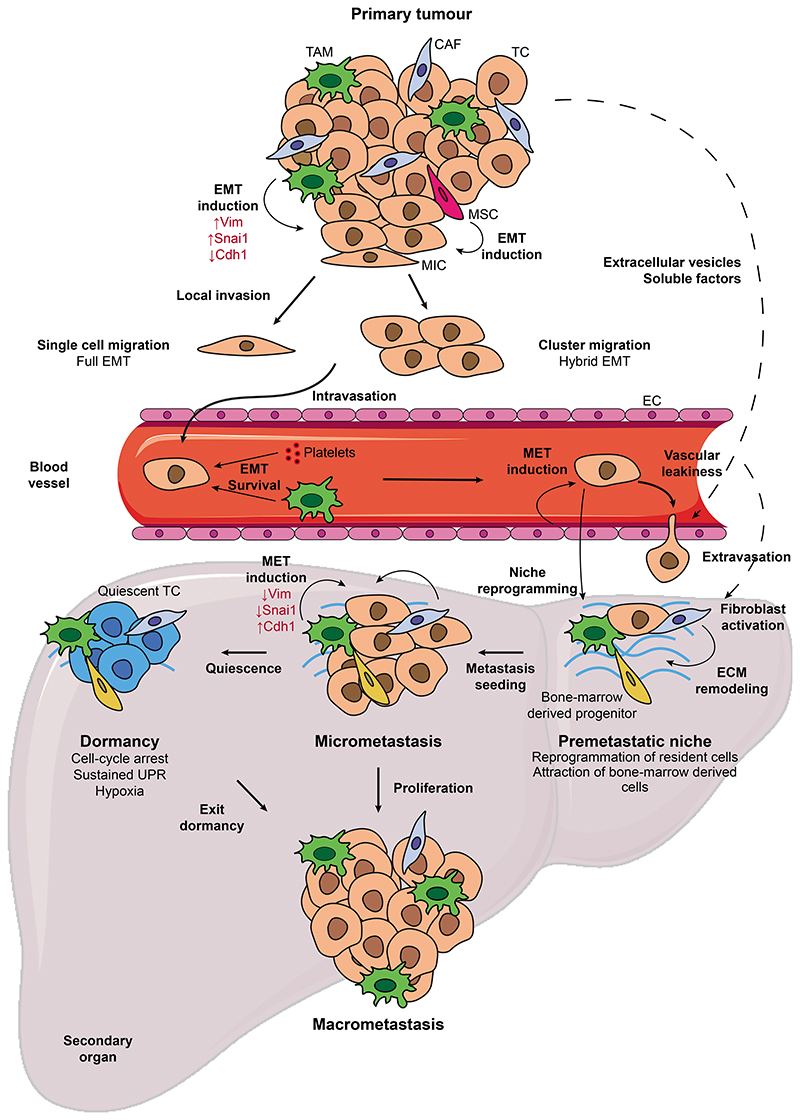
Figure 3. Cell plasticity along the metastatic cascade.
T umor cells can acquire metastasis-initiating properties through the induction of EMT by intrinsic and extrinsic stimuli. EMT allows MICs to detach from the primary tumor and the vascular niche facilitates MIC intravasation into the bloodstream, where single or clustered CTCs exhibit high plasticity and hybrid EMT. Interaction of CTCs with platelets and macrophages can promote plasticity, while platelet coating protects CTCs from the shredding force. The secondary organ is prepared by the primary tumor through the secretion of extracellular vesicles and soluble factors which create a permissive microenvironment. Colonizing the metastatic site involves the reversion of tumor cells to the epithelial state in response to signals coming from the metastatic niche. Following seeding, tumor cells can enter dormancy, which confers them with immune evasion traits and resistance to therapy, or proliferate and give rise to macroscopic metastases. CAF, cancer-associated fibroblast; CTC, circulating tumor cell; EC, endothelial cell; ECM: extracellular matrix; EMT, epithelial-to-mesenchymal transition; MET, mesenchymal-to-epithelial transition; MIC, metastasis-initiating cell; MSC: mesenchymal stem cell; SC, stem cell; TAM, tumor-associated macrophage; TC, tumor cell.
Intrinsic Regulation of Cancer Cell Plasticity
Metastasis initiation
The importance of EMT for metastasis was first demonstrated by seminal work showing that Twist1 was essential for metastasis in breast cancer cell lines112. The deletion of other EMT transcription factors also impairs metastasis, as shown with Zeb1 deletion in pancreatic cancer models113.
EMT can be triggered by different transcription factors, with Snai1, Snai2, Twist1, Zeb1 and Zeb2 being considered core EMT transcription factors that can induce the classic EMT program and are often co-expressed. Their redundancy and compensatory mechanisms might explain why the loss of one is not always sufficient to block metastasis. Nevertheless, these factors can have non-redundant functions involving stemness and survival and besides these core factors, a growing number of factors can induce EMT, such as FOXC2, SOX4 and PRRX1113.
EMT was long considered a binary switch, but recent studies have demonstrated that EMT tumor cells present intermediate, partial or hybrid states that can transit from one to another while co-express epithelial and mesenchymal markers. In mouse skin squamous cell carcinoma and mammary tumors, distinct EMT subpopulations exhibit different plasticity, invasive and metastatic potential. Early hybrid EMT includes the most metastatic states, while late EMT states are the most invasive114,115. Early and late EMT are relatively stable in comparison to other intermediate states, which are highly plastic116,117. Single-cell transcriptomics has identified hybrid EMT states in mouse skin squamous cell carcinoma and mammary tumors114, and in human nasopharyngeal carcinoma118, glioblastoma68, melanoma119, and head and neck squamous cell carcinoma120. Hybrid EMT has been associated with poor patient outcome in 32 cancer types121. Partial EMT states are located at the tumor leading edge in human oral squamous cell carcinoma, suggesting an association with local invasion120.
EMT promotes stemness, allowing MICs to give rise to secondary tumors122–125 (Figure 3). Lineage tracing has identified MICs within primary tumors and tracked tumor cells undergoing partial (expressing N-cadherin) and complete (expressing vimentin) EMT in mammary tumors 126,127. N-cadherin, but not vimentin, labels MICs, supporting that partial EMT is required for metastasis initiation126,127. An inducible CRISPR-Cas9-based lineage reporter approach combined with single cell transcriptomics confirmed the high metastatic potential of hybrid EMT states in a pancreatic cancer mouse model128. In several human cancers, L1CAM is expressed by MICs and enhances metastatic spreading, extravasation, and outgrowth129. L1CAM+ MICs emerge after the loss of epithelial integrity in a subset of cells mimicking the intestinal repair program130,131.
During tumorigenesis, the metabolic phenotype of cancer cells can be modified depending on nutrient availability, proliferative rate, and tumor mutational burden. The metastatic cascade imposes important adaptations for metastatic cells to overcome nutrient variations and oxidative stress132. MICs often present increased anaerobic glycolysis (also known as the Warburg effect)133. The dysregulation of oxidative phosphorylation is associated with poor prognosis and correlated with EMT in multiple cancers134. In human oral squamous cell carcinoma, tumor cells with low levels of mitochondrial tRNAMet with m5C modification at position 34, which promotes translation of mitochondrial genes, are unable to transit from glycolysis to oxidative phosphorylation, displaying impaired metastatic capacity135. Lactate and pyruvate metabolism can induce signaling pathways that promote migration and invasion136. Moreover, a metabolic switch in the primary tumor can induce a pro-metastatic cancer cell phenotype. In breast cancer, downregulation of phosphoglycerate dehydrogenase (PHGDH) and activation of the hexosamine–sialic acid pathway potentiates metastatic dissemination through a proliferative-to-invasive phenotypic switch137.
Whereas metastatic dissemination was considered a late event during tumor progression, increasing evidence suggests that it can occur relatively early during tumorigenesis138. In a breast cancer mouse model, metastatic spread occurs at the early stage of tumor formation, driven by progesterone and HER2 signaling. First, progesterone signaling promotes migration and dissemination, and at later stages increased cell density downregulates the progesterone receptor, switching migration towards proliferation139. Cell plasticity regulated by the transcription factor ZP281 induces a mesenchymal-like state that promotes early dissemination and dormancy in early metastatic lesions, by preventing the switch to an epithelial-like proliferative state140.
Local invasion and dissemination of tumor cells
Tumor cells in a full EMT state invade their surrounding tissue as mesenchymal single cells, whereas hybrid EMT states promote collective migration, with tumor cells at the leading edge presenting a more pronounced EMT phenotype compared to follower cells141 (Figure 3). Hybrid EMT cells migrating collectively are associated with plasticity, stemness, invasion, and increased metastatic ability114,127. Next, tumor cells intravasate blood vessels as circulating tumor cells (CTCs) with some of these surviving to extravasate into a secondary organ, in which they will either proliferate to enable metastatic outgrowth or undergo dormancy142 (Figure 3). Xenografts revealed MIC markers among human luminal breast cancer CTCs that give rise to bone, lung, and liver metastases. MIC-containing CTC subpopulations express EpCAM, CD44, CD47 and MET143.
Whereas most CTCs are single cells in circulation, a less prevalent fraction is shed and travels in clusters, showing an increased metastatic potential and associating with poor outcomes144–146. Both single and clustered CTCs exhibit shifts in epithelial and mesenchymal marker expression, displaying plasticity during tumor progression. Whereas epithelial cells that lose adhesion-dependent survival signals and intravasate into blood vessels normally undergo anoikis, EMT enables single tumor cells to change their fate towards a mesenchymal phenotype, in which adherence-independent survival signals prevent cell death144,147. Rare primary tumor cells simultaneously express mesenchymal and epithelial markers, whereas CTC clusters in breast cancer patients are positive for mesenchymal markers and weakly positive for epithelial markers, supporting a role of EMT in CTC dissemination148. CTCs detected in the blood of mice with skin squamous cell carcinoma are EpCAM− and enriched in hybrid EMT states, demonstrating that hybrid phenotypes exhibit increased colonization potential and intravasate more efficiently114,149. Hybrid EMT has been detected in CTCs from patients with non-small cell lung cancer150, prostate151, colorectal152, pancreatic153, breast,liver, gastric, and nasopharyngeal cancers115. The sodium channel NALCN regulates CTC dissemination, with its loss of function in a mouse model increasing the proportion of CTCs and the blood trafficking of normal non-mutated cells154.
Plasticity within distinct CTC phenotypes has been shown to contribute to cancer progression and chemoresistance. Analysis of CTCs from women with ER+/HER2− breast tumors reveals that 84% of CTCs acquire HER2 expression without genetic amplification. Cultured HER2+ and HER2− CTCs interconvert spontaneously, with oxidative stress and chemotherapy enhancing a transition towards the HER2− phenotype whereas HER2+ state is the most proliferative155. While in circulation, the oxidative stress of CTCs increases and to prevent ROS-mediated cell death, tumor cells increase antioxidant production156. In melanoma patient-derived xenografts and mouse models, metastatic cells increasingly depend on NADPH-generating enzymes from the folate pathway to regenerate glutathione and withstand oxidative stress157. Efficiently, metastatic cells increase lactate uptake through MCT1 upregulation, preventing oxidative stress158. Metabolic changes depend on the path by which tumor cells reach the secondary organ. In melanoma, CTCs migrating through blood vessels are subjected to higher oxidative stress and ferroptosis than CTCs in lymphatic vessels, and become dependent on the ferroptosis inhibitor GPX4 to survive, whereas CTCs migrating through lymphatic vessels rely on the antioxidant-like oleic acid and glutathione159. CTC clustering protects from ROS production through Hif1α induction and mitophagy, switching energy production towards glycolysis. Blocking metabolic rewiring following CTC clustering inhibits metastasis160.
Metastatic colonization
EMT reversion by mesenchymal-to-epithelial transition (MET) can promote metastasis (Figure 3). Loss of E-cadherin increases invasiveness, but its expression protects cells from oxidative stress during dissemination and seeding, promoting metastatic colonization161. Tumor cells can form heterotypic junctions using E-cadherin and N-cadherin expressed by stromal cells in the metastatic niche, promoting survival and growth162. Some MICs display hybrid EMT, maintaining E-cadherin expression and mesenchymal traits163.
Whereas metastasis is associated with EMT in mouse skin squamous cell carcinoma, most metastases do not display EMT features, suggesting that MET can be important for colonization149. Evidence shows that metastases can reacquire an epithelial phenotype, but whether this is a cause or consequence of the metastatic cascade remains unknown164. Several studies highlight the need of downregulating EMT factors for metastasis formation. Twist1-mediated EMT in squamous cell carcinoma promotes invasion and CTC circulation, whereas Twist1 downregulation promotes metastatic colonization165. Prrx1 promotes EMT and invasion in pancreatic ductal adenocarcinoma but needs to be repressed for metastatic colonization166. Prrx1’s action was later shown to be mediated by two distinct isoforms: Prrx1b promoting EMT, invasion and migration and Prrx1a stimulating liver metastatic outgrowth, tumor differentiation, and MET. Thus metastatic dissemination needs a switch from Prrx1b at the first step of the metastatic cascade to Prrx1a at its end167.
MICs can arise from CSCs or be generated by the dedifferentiation of non-CSCs. In mouse models of colorectal cancer, disseminated cells do not express the stem cell marker Lgr5. However, a fraction of the disseminated cells re-express Lgr5 during macro-metastasis formation168, explaining why Lgr5 lineage ablation inhibits liver metastasis formation in colorectal cancer58. Recently, metastatic recurrence in colorectal cancer has been shown to arise from residual EMP1-expressing cells, a subset of Lgr5- tumor cells endowed with migratory properties. The ablation of EMP1+ cells in vivo during primary colorectal cancer growth prevents metastatic dissemination, whereas ablation after primary tumor resection does not affect metastatic progression. Therefore, EMP1+ cells can be considered the cell of origin of metastasis in colorectal cancer, whereas the Lgr5+ stem cell and proliferation programs are necessary for metastatic outgrowth, demonstrating the importance of cell plasticity in metastasis formation169. Additionally, the organotropism of metastatic cells is partially dictated by the conjunction of their metabolic needs and the nutrients available in the secondary organ. Metastatic breast cancer cells preferentially metastasize to the lung because they use the local pyruvate to boost collagen hydroxylation, leading to the establishment of a metastatic niche170.
Extrinsic Regulation Of Cancer Cell Plasticity
Metastasis initiation and the tumor niche
The niche is crucial for EMT induction and metastasis initiation (Figure 3). Fibroblasts support tumor cells by secreting extracellular matrix and matrix metalloproteinases, promoting migration, invasion, and angiogenesis, and favoring tumor cell plasticity. TGFβ secretion by tumor cells is essential for fibroblast recruitment and activation during the first steps of tumorigenesis. Activated fibroblasts then activate autocrine and paracrine secretion of TGFβ, inducing EMT in tumor cells and promoting immune escape171,172 (Figure 4). Co-transplantation experiments of CSCs and fibroblasts with high TGFβ expression show increased lung metastasis in a TGFβ-dependent manner in squamous cell carcinoma173. Fibroblasts can indirectly induce EMT by promoting increased extracellular matrix stiffness leading to mechanotransduction signals174,175 (Figure 4).
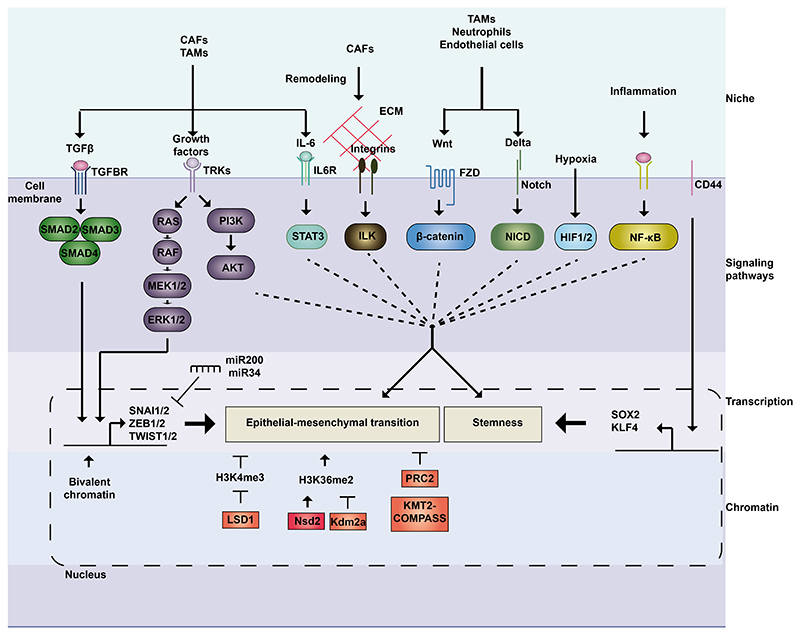
Figure 4. Molecular mechanisms regulating cancer cell plasticity.
Cancer cell plasticity is regulated extracellularly, by signals coming from the microenvironment, and intrinsically, through signaling pathways, transcriptional programs, and chromatin remodeling. TFGβ and RAS-MAPK pathways can act jointly to induce EMT. CD44 and Wnt regulate stemness, while Notch, JAK-STAT and integrins act on stemness and EMT in a context-dependent manner. Hypoxia induces stemness, while NF-κB is involved in plasticity by its role in inflammation. These pathways activate transcriptional programs regulated by key transcription factors involved in EMT (e.g., SNAI1/2, ZEB1/2, TWIST1/2) and stemness (e.g., SOX2, KLF4). Their action can be modulated by negative feedback loops involving miRNAs (e.g., ZEB/miR-200 and SNAI1/- miR-34) and depends on the chromatin landscape. LSD1 can remove the transcriptionally active H3K4me3 histone mark and collaborate with Snai1 to silence epithelial genes. Nsd2 and Kdm2a exhibit antagonist actions, as writer and eraser of H3K36me2, histone mark increased during EMT. PRC2 and KMT2-COMPASS are critical to regulate the epithelial state. CAF, cancer-associated fibroblast; ECM: extracellular matrix; FZD, frizzled; HIF, Hypoxia-inducible factor; IL6R, interleukin-6 receptor; TAM, tumor-associated macrophage; TGFBR, Transforming Growth Factor Receptor; TRK, Tyrosine receptor kinase.
The abundance of blood vessels within the vascular niche of the primary tumor increases the bloodstream accessibility of tumor cells. Stromal and tumor cells secrete cytokines and chemokines to recruit immunosuppressive and pro-tumoral macrophages and tumor-associated neutrophils that promote invasiveness by secreting EGF and modulating the extracellular matrix through cathepsins and matrix metalloproteinase-9, and can increase MIC survival176 (Figure 3). Mesenchymal stem-like cells in tumor niches arise from the bone marrow and other perivascular regions (e.g., adipose tissue), and interact with tumor and stromal cells to promote vascularization, immune modulation and extracellular matrix remodeling177. They can induce EMT through exosome communication, TGFβ secretion and extracellular matrix remodeling, especially through hyaluronan secretion, activating CD44 and upregulating LOX and TWIST1 in breast cancer cells178,179 (Figure 3). Macrophages also influence EMT and tumor cell plasticity. In glioblastoma, macrophages induce EMT through oncostatin-M secretion, activating STAT3 pathway in tumor cells180 (Figure 4). In both mouse and human non-small cell lung cancer, resident macrophages promote EMT and invasion during early metastatic dissemination and protect tumor cells from immune destruction by inducing a regulatory T-cell response (Figure 3). In skin cancer, macrophage infiltration increases in hybrid or full EMT tumor areas, as compared to epithelial regions. Macrophage depletion increases epithelial states and decreases EMT, showing the importance of macrophage-tumor cell communication in regulating EMT114.
Dissemination of tumor cells and crosstalk with the tumor microenvironment
Tumor cells survive in the bloodstream by being coated with platelets and interacting with white-blood cells, fibroblasts, macrophages, and endothelial cells147. Crosstalk between tumor cells and macrophages is required for CTC-mediated colorectal cancer metastasis and promotes EMT-related plasticity182 (Figure 3). Neutrophil-tumor cell clusters seem to be more metastatic than tumor cell clusters alone, due to a neutrophil-mediated increased cell cycle progression in tumor cells183. Interaction with platelets provides resistance to the bloodstream shredding force and induce EMT through TGFβ and NF-κB pathway activation184 (Figure 4).
Metastatic niche
The metastatic niche is the specific microenvironment generated by stromal cells, the extracellular matrix and diffusing signals that stimulate metastasis formation. Perivascular niches create excellent metastatic niches. Although the crosstalk between the metastatic perivascular niche and tumor cells is not fully understood, several mechanisms have been identified. In breast-to-lung cancer metastasis, tumor cells secrete tenascin C, which activates macrophages through TLF4 receptor. Macrophages activate endothelial cells through TNFα and nitric oxide secretion, supporting metastasis formation185. Therapy might favor metastatic niche formation. Lung radiotherapy can create a pro-metastatic microenvironment through neutrophil activation, which then activate Notch signaling, inducing tumor stemness and enhancing metastasis186 (Figure 4). The metastatic niche promotes metastatic outgrowth but can favor further dissemination. For instance, the bone microenvironment promotes multi-organ metastases through epigenetic reprogramming of tumor cells, mediated by enhanced EZH2 activity, promoting disseminated tumor cell stemness in the bone187.
The mechanisms of MET induction in MICs are not fully understood but involve signals from the metastatic niche. E-selectin secretion in the metastatic niche induces a specific form of MET in the bone through Wnt pathway activation188. LIF secretion by bone mesenchymal stem cells induces MET through the activation of LIFR, ERK and STAT3 in early disseminated CSCs189. In liver metastasis from colon cancer, MET can be induced through Src and EGFR pathway inhibition190. In lung metastasis, versican secretion by bone-marrow derived myeloid progenitors recruited to the lung inhibits Smad2 phosphorylation and Snai1 expression in MICs, resulting in MET and increased proliferation191. In breast cancer-derived lung metastasis, MET can be induced by fibroblasts through TGFβ pathway inhibition and BMP activation192 (Figure 3). Fibroblast activation occurs through MIC-secreted thrombospondin-2, which depends on MIC mesenchymal features, showing that MET is not required in the first step of colonization but needs to be induced through microenvironment reprogramming192. MET induction can occur through PKA activation in human breast cancer but blocks tumor initiating properties and decreases metastasis by promoting differentiation193.
Increasing evidence suggests that tumor cells prepare their niche prior to colonization. Premetastatic niche conditioning involves vascular leakiness, reprogramming of resident cells and attraction of bone-marrow derived cells194 (Figure 3). Some mechanisms are induced by disseminated cells at the metastatic site but distant reprogramming by the primary tumor through secretion of soluble molecules and exosomes also occurs. MiR-25-3p-containing exosomes secreted by colorectal cancer can induce angiogenesis and vascular leakiness through Klf2 and Klf4 inhibition in endothelial cells. In vivo treatment with these exosomes leads to increased vascular permeability in lung and liver, whereas depleting miR-25-3p reduces metastasis in both organs195. A phenotypic switch in pericytes and vascular smooth muscle cells of the premetastatic niche towards a more undifferentiated state is mediated by increased Klf4 expression due to tumor-derived factors and exosomes. Reprogrammed perivascular cells exhibit increased proliferation and expression of extracellular matrix components, creating a permissive soil for metastasis196.
Tumor Dormancy
Disseminated cells can enter dormancy at the metastatic site (Figure 3). This growth arrest occurs by a balance between proliferation and apoptosis due to poor vascularization, immune destruction, lack of nutrients and growth factors, or through inhibitory signals from the microenvironment (e.g., TGFβ)197–199. Dormant cells are characterized by activated survival pathways, cell-cycle arrest and sustained unfolded protein response and hypoxia200 (Figure 3). Quiescence allows cells to evade immune responses and chemotherapy, remaining undetectable by imaging techniques but being responsible for relapse even years after clinical remission200.
Mechanisms by which tumor cells enter and exit dormancy are not fully understood (Figure 3). Dormant cells display plasticity to transit between states, but whether EMT or MET promote reactivation and awakening from dormancy remains unclear. EMT induced by inflammation in a Zeb1-dependent manner awakes dormant tumor cells in xenografting experiments124,201. However, in breast cancer, TGFβ exhibits cytostatic effects, impairs the cell cycle, and promotes dormancy, whereas the TGFβ antagonist Coco promotes the reactivation of dormant cells in the lung199,202. Additionally, mesenchymal CSCs need to undergo MET and express E-cadherin to enable contact between tumor cells and promote survival and proliferation203.
Dormancy is tightly controlled by the microenvironment. Secretion of collagen-III by tumor cells at the metastatic site favors dormancy, whereas disruption of the collagen-III enriched matrix induces awakening and proliferation of dormant cells through DDR1-mediated STAT1 signaling204. In the lung, inflammation induces the formation of neutrophil extracellular traps, which favor the awakening of tumor cells through laminin cleavage and integrin α3β1 activation205. Cancer cells can be primed by the primary tumor to become dormant. In breast cancer and head and neck squamous cell carcinoma, tumor cells exposed to hypoxia are prone to becoming dormant206. Modifications of the microenvironment during aging also play a role in entering or exiting dormancy. Age-related changes in fibroblasts have been linked to increased metastasis in melanoma. Aged dermal fibroblasts show increased secretion of the Wnt antagonist sFRP2, which induces resistance to ROS-mediated DNA damage response in melanoma cells, conferring resistance to therapy and increased metastasis. Aged fibroblasts in the lung secrete more sFRP1 and block Wnt5a-mediated induction of dormancy, stimulating metastatic growth207,208. Age-related changes affecting the microenvironment might explain the resurgence of metastatic lesions years after treatment.
Cell Plasticity and Cancer Therapy
Drug tolerance constitutes a major obstacle for therapy. In the following section, we discuss the roles of plasticity in therapy resistance.
Drug Tolerance Mechanisms
Although therapeutic resistance was thought to be exclusively a consequence of genetic alterations in tumor cells (Figure 5A; Figure 5B), accumulating evidence suggests that drug tolerant states exist in absence of mutations. Drug-tolerant persistent (DTP) cells display four hallmarks: slow proliferation, metabolic flexibility, adaptation to the microenvironment and phenotypic plasticity. The major difference between mutations conferring resistance and DTP states is the absence of reversibility or plasticity in mutations, whereas DTP cells survive but do not proliferate under treatment and their progeny remains sensitive to treatment after drug withdrawal209,210.
Figure 5. Genetic induced drug resistance and non-genetic drug tolerance in anti-cancer therapy.
Pre-existing (A) or acquired (B) mutations can confer intrinsic genetic drug resistance, by which mutated tumor cells can display a clonal selection, survive, and proliferate under a particular therapeutic regimen. (C) Non-genetic drug tolerance can occur through transcriptional selection of primed cells that acquire a DTP dormant state during therapy and can lead to tumor relapse after therapy. (D) Non-genetic drug tolerance can occur through an adaptation to the therapeutic pressure, by which plastic tumor cells acquire a DTP quiescent state following therapy and can lead to tumor relapse after therapy. (E) Targeting the signaling pathways activated in the DTP state enables its eradication. The DTP state induced upon BRAFi/MEKi treatment in melanoma relies on FAK signaling and the transcriptional program of this state is largely driven by the nuclear receptor RXR. Consistently, the DTP state can be targeted by FAK inhibition and RXR antagonism. However, de novo mutations could still lead to genetic resistance and tumor relapse221,222. DTP, drug tolerant persister; RAR, retinoic acid receptor; RXR, retinoid X receptor; SC, stem cell.
Primed DTP cells might exist prior to treatment, with expression of a particular transcriptional program providing them with intrinsic tolerance to a drug and leading to their selection under treatment (Figure 5C). In other cases, DTP cells become induced upon treatment, as tumor cells adapt to therapeutic pressures and activate a transcriptional program that provides a selective advantage to escape209,210 (Figure 5D). The acquired DTP state exploits plasticity, as tumor cells undergo a phenotypic switch and adopt a reversible quiescent state to survive. The DTP state can manifest as transient or stable. Transient DTP cells regenerate the initial tumor heterogeneity after drug withdrawal, with the tumor remaining sensitive to therapy. By contrast, in a stable tolerance situation, the tumor adapts to therapy, becoming insensitive to it. The therapy-evasive traits of DTP cells are mediated by epigenetic, transcriptional, translational regulatory processes and complex interactions between tumor cells and within their microenvironment10,209,210. Tumor cells employ a developmentally conserved mechanism similar to diapause to drive the DTP state, as observed in organoids, patient-derived xenografts and patient samples211,212.
EMT promotes drug tolerant states and EMT tumor cells are highly resistant to anti-cancer therapy209. A recent study has demonstrated that Rhoj, a small GTPase, controls the resistance of EMT tumor cells to a wide range of chemotherapeutic agents by promoting DNA repair through the regulation of nuclear actin213. Primed DTP cells have been described in melanoma and breast cancer. In vitro studies in BRAF-mutant melanoma identify a DTP state upon BRAF inhibition that arises through a multistep process214. Before therapy, rare subpopulations display a transient primed state with high expression of resistance markers (e.g., EGFR), with this state becoming stable through epigenetic reprogramming following treatment. Genetic factors such as SOX10 and MITF affect fate decisions, revealing a plasticity model of resistance to BRAF inhibition that pushes cells towards differentiation214,215. Single-cell sequencing of triple negative breast cancers treated with chemotherapy shows resistant genotypes to be pre-existing, but also reveals the existence of a small fraction of primed DTPs, whereas chemotherapy induces an acquired DTP state through transcriptional reprogramming216.
Emerging evidence indicates that tolerance can be acquired by switching to a phenotypically distinct DTP state. In prostate cancer, DTP cell plasticity is promoted by combined loss-of-function mutations of TP53, RB1 or PTEN39. Both mouse and human models demonstrate that tumors develop resistance to androgen deprivation therapy by enzalutamide by a phenotypic shift from androgen receptor-dependent luminal epithelial cells to androgen receptor-independent basal-like cells, enabled by the loss of TP53 and RB1 functions and mediated by increased SOX2 and EZH2 expression39,217. Single-cell transcriptomics of patient samples with prostate cancer reveals that resistant adenocarcinoma cells upregulate EMT and TGFβ signaling gene programs, whereas small cell carcinoma exhibits higher activity of NANOG, SOX2 and EZH2218. Mouse and human organoids and genetically engineered mouse models of prostate cancer show the emergence of a DTP state in an epithelial population by JAK/STAT signaling following androgen receptor inhibition219,220.
In BRAF-mutant melanoma patient-derived xenografts, dedifferentiation into a reversible neural crest stem-like state driven by RXRG and FAK signaling contributes to the development of resistance to RAF/MEK inhibitors221,222 (Figure 5E). In basal cell carcinoma, Hedgehog pathway inhibition by vismodegid leads to differentiation towards squamous and sebaceous identities, but some tumor cells enter a quiescent Lgr5-expressing state characterized by Wnt signaling223,224. In resistant non-small cell lung cancer patients with EGFR mutations, transformation to small cell lung cancer is observed histologically following EGFR inhibition. DTP cells present RB loss and transdifferentiate into a different epigenetic state that does not require EGFR signaling225. Single-cell transcriptomics of non-small cell lung cancer patient biopsies before and after targeted therapy reveals the existence of a slow proliferating population with alveolar traits226. Induction of a slow-cycling DTP state seems to be a common survival mechanism. Despite most cells remaining quiescent, recent work in lung cancer reveals DTP lineages that can maintain their proliferative capacity in presence of drugs227.
Epigenetic reprogramming mechanisms also drive DTP state plasticity in vitro and in vivo. A DTP state maintained by an altered chromatin state that requires histone demethylase KDM5A/JARID1 was identified in EGFR mutant non-small cell lung cancer following TKI treatment228,229. Upon RTK inhibition, glioblastoma stem cells transit to a DTP state characterized by upregulation of neurodevelopmental programs, dependency on Notch signaling, redistribution of repressive histone methylation and dependency on histone demethylases KDM6A/B230. In breast basal-like cancer, the DTP state upon treatment with MEK and/or PI3K/mTOR inhibitors is EMT-related and driven by changes in BRD4, KDM5B and EZH2231. Following γ-secretase inhibition in T-cell acute lymphoblastic leukemia, pre-existing DTP cells adopt an altered chromatin state and are BRD4 dependent232.
The importance of EMT in therapy resistance has been shown in different contexts6,113. Snail determines the response to mTOR kinase inhibitors by transcriptional repression of 4E-BP1 in human breast, colon, and lung cancer cell lines233. A mesenchymal undifferentiated DTP state that often expresses ZEB1, and depends on a druggable lipid-peroxidase pathway that protects against ferroptosis has been observed in human tumors and cell lines under multiple treatment modalities across cancer lineages 234.
WNT signaling is the major oncogenic driver of colorectal cancer. Whereas in most cases, constitutive activation is mediated by mutations of downstream pathway components, such as APC or beta-catenin, a fraction of colorectal cancers is mediated by a fusion protein between the Wnt co-receptors Rspo3 and Ptprk235, which render tumor cells sensitive to Wnt signaling inhibition. A blocking antibody against Rspo3 inhibits tumor growth and induces the switch from a stemness state towards a differentiated state236. YAP signaling can promote WNT independence in these tumors by lineage reversion to a fetal-like state237. In colorectal cancer patient-derived xenografts, minimal residual disease following EGFR blockade is associated with the acquisition of a DTP state that displays a Paneth cell-like phenotype characterized by high WNT signaling and regulated by YAP inactivation238. Colorectal cancer patient-derived organoids show that chemotherapy induces quiescence in TP53-wildtype tumor cells, linked to the acquisition of the fetal-like state, with Mex3a marking a latent Lgr5+ DTP state, which persists by downregulating Wnt after chemotherapy and adopts a transient state reminiscent to YAP+ intestinal progenitors239,240. Lgr5+ CSCs that display a dormant behavior express p27. Lgr5+p27+ cells wake from dormancy through FAK-YAP activation241.
Elimination of Drug Tolerant Cells
Multiple plasticity mechanisms can promote a DTP state acquisition. Although some mechanisms could be tumor-specific, altering cell fate decisions by targeting hallmarks of DTP cells across cancers, including slow proliferation, signaling pathway activation, adapted metabolism, or microenvironment regulators, could help eliminate minimal residual disease and avoid relapse209,210.
A first approach to eradicate DTP cells relies on targeting their slow proliferation by incorporating epigenetic modulators to existing therapies. Disrupting the repressed chromatin state that maintains resistance to EGFR TKIs in non-small cell lung cancer by HDAC inhibition or by IGF-1 receptor inhibition, is lethal to DTP cells in vitro228,229. Several clinical studies examine the combination of a HDAC inhibitor with a TKI, which appears to be well tolerated and present clinical benefits in non-small cell lung cancer progression (NCT01302808)242. Similarly, co-treatment with the PI3K/mTOR inhibitor BEZ235 and the BET/BRD4 inhibitor JQ1 in basal-like breast cancer prevents chromatin remodeling, inhibiting the acquisition of the DTP state and resulting in cell death in vitro and xenograft regression in vivo231. JQ1 induces DTP cell apoptosis in vitro in T-cell acute lymphoblastic leukemia following γ-secretase inhibition, whereas combined therapy with JQ1 is effective in vivo232.
Targeting signaling pathways activated in tumor cells could eliminate DTP cells. The stem-like state acquired following RAF/MEK-inhibition in melanoma can be targeted by a combination of FAK inhibition and RXR antagonism221,222. Although eliminating the DTP subpopulation is sufficient to avoid non-genetic tolerance, resistance can occur through the acquisition of de novo mutations221,222 (Figure 5E). In basal cell carcinoma, targeting the Wnt and Hedgehog pathways together leads to DTP state eradication in vivo223,224. Inhibition of JAK/STAT signaling in mouse and human prostate organoids re-sensitizes tumors to androgen receptor-targeted therapy219. Targeting YAP/TAZ might prevent or reverse WNT-inhibitor resistance in intestinal cancer and eliminate quiescent cells in colorectal cancer237,239,241. TGFβ inhibition increases squamous cell carcinoma susceptibility to chemotherapy, preventing entry into a quiescent state243. Blocking TGFβ signaling reduces stemness and attenuates metastasis upon chemotherapy in breast cancer244. In EMT cells, the DTP state depends on GPX4, the loss of which results in ferroptotic death in vitro and prevents relapse in vivo234,245.
Targeting microenvironment regulators could contribute to eliminating DTP cells. The microenvironment elicits innate resistance to RAF inhibitors through the expression of HGF, while dual inhibition of BRAF and the HGF receptor MET prevents drug resistance in BRAF-mutant melanoma246. Chemotherapy induces JNK pathway activation in breast cancer patients, enhancing the expression of the extracellular matrix and stem-cell niche components osteopontin, SPP1 and TNC, and conferring chemoresistance. JNK or SPP1 inhibition sensitizes mouse tumors and metastases to chemotherapy247. Inflammatory fibroblasts control the response to therapy in rectal cancer248. IL-1 dependent signaling elevates DNA damage in inflammatory fibroblasts, promoting senescence and resulting in therapy resistance, which could be overcome by IL-1R inhibition, leading to a clinical trial testing the combination of chemoradiotherapy with IL-1R antagonist in rectal cancer (NCT04942626)248.
The highly dynamic, heterogeneous, and plastic properties of the DTP state are a major challenge. Transcriptional profiling by single cell sequencing to measure phenotypic changes along clinical evolution could enable individualized therapies to overcome drug tolerance.
Targeting Cell Plasticity
Strategies to inhibit CSC self-renewing capacities or to promote their differentiation can lead to CSC exhaustion and tumor regression. Anti-CSC therapy was first shown for acute promyelocytic leukemia, with all-trans retinoic acid promoting leukemic cell differentiation into terminally differentiated myeloid cells249. Today, combination of retinoic acid, arsenic trioxide and/or chemotherapy cures more than 90% patients with this type of leukemia249.
LSD1 is required to sustain the tumorigenic program of CSCs in several cancer types, and is important for maintaining plasticity and proliferation in Merkel cell carcinoma in vivo250. H3K4 methylation is required for retinoic acid-driven differentiation, but this methylation mark is lost in acute myeloid leukemia due to LSD1 overexpression. A phase I trial (NCT02273102) recently demonstrated that responsiveness to retinoic acid can be potentiated by LSD1 inhibition251. Epigenetic therapy also relies on HDAC and JAK/STAT inhibitors. The JAK1/2 inhibitor ruxolitinib and the HDAC inhibitor belinostat independently enhance dependence on BCL-2 for survival, sensitizing leukemic cells to the BCL-2 inhibitor venetoclax252. Other epigenetic drugs include DNMT inhibitors (e.g., azacytidine and decitabine, approved for myelodysplastic syndromes), and EZH2 and BET inhibitors, which are in clinical studies for hematologic malignancies253. A better understanding of sensitive tumor cells and the effect of epigenetic inhibitors on normal cells would improve the rationale of using epigenetic therapy to target plasticity and avoid toxic side effects.
Markers defining the stemness tumor state have been considered unlikely candidates for antibody therapy, as they are expressed by healthy stem cells. Accordingly, an antibody-drug conjugate directed against CD33+ CSCs in acute myeloid leukemia received FDA approval but was withdrawn due to toxicity54. A bivalent antibody against EGFR and LGR5 inhibits EGFR in CSCs, suppressing tumor growth in epithelial tumors and blocking metastasis initiation254.
An alternative approach relies on inhibiting CSC signaling pathways. In preclinical glioblastoma studies, combined therapy with Notch/γ-secretase inhibitor, radiotherapy and temozolomide reduces stemness markers and tumor growth while prolonging survival255. Notch inhibition has been assessed in clinical trials for more malignancies, such as breast and lung cancer, failing to meet expectations due to dose-limiting gastrointestinal toxicity256,257. Most signaling pathways involved in plasticity are key developmental pathways, targeting of which commonly leads to off-tumor toxicities due to effects on normal cells. Resistance to therapy targeting CSC due to plasticity of non-CSCs, which can replenish the CSC pool, limits its efficacy54,258. Combined treatment with molecules preventing plasticity of non-CSCs would be required for successful clinical outcomes. Dormancy remains a major challenge for therapy and awakening this subpopulation to increase its susceptibility to chemotherapy (e.g., by activating IFNα pathway) is being considered259. Maintaining the quiescent state to prevent metastatic outgrowth is an alternative, although it would require lifelong treatment.
Intra-tumor heterogeneity and cell plasticity also pose persisting challenges. Impairing plasticity as a therapeutic approach to limit the degree of heterogeneity and restrain the capacity of tumor cells to resist therapy seems promising, as blocking the mechanisms inducing plasticity in DTP cells might lead to therapeutic benefits. However, these mechanisms might differ among tumors and multiple adaptation mechanisms may act redundantly to sustain the DTP state. Further efforts would be needed to develop clinically relevant treatments targeting plasticity in solid cancers260.
As tumor cell plasticity is often mediated by the microenvironment, targeting it to sensitize tumor cells might be a promising therapeutic approach. WNT16B could become an attractive target for increasing responsiveness to chemotherapy in prostate cancer, as WNT16B expression in the microenvironment attenuates the effects of chemotherapy in vivo261.
Immune Escape
Cell plasticity and stemness play an important role in immune evasion. CSCs appear to be the first tumor subpopulation to escape immune surveillance, due to their slow cycling traits and their abilities to downregulate the expression of antigen presenting machinery262. In squamous cell carcinoma, CSCs responding to TGFβ resist immunotherapy based on adoptive cytotoxic T-cell transfer. These CSCs express the immune marker CD80 and inhibit cytotoxic activity of T-cells by exhaustion, following CTLA-4 engagement. Immunotherapy blocking CTLA-4 or TGFB1 sensitizes CSCs to adoptive cytotoxic T-cell transfer in mouse and human tumors263.
Metastatic cells escape immune surveillance through quiescence. Metastases from breast cancer expressing Sox2 and Sox9 and displaying CSCs features can escape NK-mediated clearance by entering a slow-cycling state through downregulation of Wnt signaling in vivo264. EMT induction in tumor cells has been associated with immune evasion and resistance to cytotoxic T-cells and NK cells265. Mechanisms driving resistance are not fully understood but include perturbation of the immune synapse, induction of autophagy and PD-L1 expression266,267.
Combined therapy to reduce the immunosuppressive microenvironment and cell plasticity by targeting cytokines, such as TGFβ, has the potential to increase the efficacy of immune checkpoint blockade. The presence of TGFβ in the microenvironment blocks the acquisition of the CD4+ Th1 phenotype268. Moreover, TGFβ signaling in fibroblasts restricts the localization of CD8+ T-cells in the peritumoral stroma rich in fibroblasts and collagen, whereas TGFβ inhibition allows T-cell infiltration into the tumor268,269. However, a bifunctional antibody targeting both TGFβ ligand and PD-L1, has recently failed in a clinical trial for metastatic colorectal cancer (NCT03436563) and substantial tumor progression in the first four patients led to premature discontinuation of the study270.
Preclinical mouse findings would need to be highly reproducible and rigorously validated with human biospecimens to be considered for patient selection criteria in clinical trials. Improving the drug optimization and lead selection process would improve the success of a given drug candidate targeting plasticity.
Concluding Remarks
This review presents the importance of cell plasticity in cancer initiation and progression, metastasis, and resistance to therapy. Distinct modes of plasticity are involved in maintaining tumor growth through proliferative states and CSCs, which are also essential in the metastatic cascade. Plasticity also allows tumor cells to evade selective pressures and overcome therapy. A better understanding of tumor-cell intrinsic and extrinsic mechanisms that regulate plasticity could open the road to novel therapeutic strategies and improve patient survival in the near future.
Acknowledgements
C.B. is supported by WELBIO, FNRS, TELEVIE, Fond Erasme, Fondation Contre le Cancer, ULB Foundation, FNRS/FWO EOS and the European Research Council advanced grant TTTS. A.P.G. is supported by the ITN network EVOMET (No 955951) of the EU Horizon 2020 research and innovation program. K.B. is supported by TELEVIE.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Mills JC, Stanger BZ, Sander M. Nomenclature for cellular plasticity: are the terms as plastic as the cells themselves? EMBO J. 2019;38:e103148. doi: 10.15252/embj.2019103148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan S, Norgard RJ, Stanger BZ. Cellular Plasticity in Cancer. Cancer Discov. 2019;9:837–851. doi: 10.1158/2159-8290.CD-19-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gurdon JB. The Developmental Capacity of Nuclei taken from Intestinal Epithelium Cells of Feeding Tadpoles. Development. 1962;10:622–640. [PubMed] [Google Scholar]
- 4.Takahashi K, Yamanaka S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Le Magnen C, Shen MM, Abate-Shen C. Lineage Plasticity in Cancer Progression and Treatment. Annu Rev Cancer Biol. 2018;2:271–289. doi: 10.1146/annurev-cancerbio-030617-050224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nieto MA, Huang RY-J, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 8.Blanpain C, Fuchs E. Plasticity of epithelial stem cells in tissue regeneration. Science. 2014;344:1242281. doi: 10.1126/science.1242281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer. 2012;12:323–334. doi: 10.1038/nrc3261. [DOI] [PubMed] [Google Scholar]
- 10.Hinohara K, Polyak K. Intratumoral Heterogeneity: More Than Just Mutations. Trends Cell Biol. 2019;29:569–579. doi: 10.1016/j.tcb.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 12.Sato T, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 13.Metcalfe C, Kljavin NM, Ybarra R, de Sauvage FJ. Lgr5+ Stem Cells Are Indispensable for Radiation-Induced Intestinal Regeneration. Cell Stem Cell. 2014;14:149–159. doi: 10.1016/j.stem.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Tian H, et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tetteh PW, et al. Replacement of Lost Lgr5-Positive Stem Cells through Plasticity of Their Enterocyte-Lineage Daughters. Cell Stem Cell. 2016;18:203–213. doi: 10.1016/j.stem.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 16.van Es JH, et al. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol. 2012;14:1099–1104. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buczacki SJA, et al. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature. 2013;495:65–69. doi: 10.1038/nature11965. [DOI] [PubMed] [Google Scholar]
- 18.Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, Wrana JL. Yap-dependent reprogramming of Lgr5+ stem cells drives intestinal regeneration and cancer. Nature. 2015;526:715–718. doi: 10.1038/nature15382. [DOI] [PubMed] [Google Scholar]
- 19.Yui S, et al. YAP/TAZ-Dependent Reprogramming of Colonic Epithelium Links ECM Remodeling to Tissue Regeneration. Cell Stem Cell. 2018;22:35–49.:e7. doi: 10.1016/j.stem.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nusse YM, et al. Parasitic helminthes induce fetal-like reversion in the intestinal stem cell niche. Nature. 2018;559:109–113. doi: 10.1038/s41586-018-0257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayyaz A, et al. Single-cell transcriptomes of the regenerating intestine reveal a revival stem cell. Nature. 2019;569:121–125. doi: 10.1038/s41586-019-1154-y. [DOI] [PubMed] [Google Scholar]
- 22.Cheung P, et al. Regenerative Reprogramming of the Intestinal Stem Cell State via Hippo Signaling Suppresses Metastatic Colorectal Cancer. Cell Stem Cell. 2020;27:590–604.:e9. doi: 10.1016/j.stem.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gil Vazquez E, et al. Dynamic and adaptive cancer stem cell population admixture in colorectal neoplasia. Cell Stem Cell. 2022;29:1213–1228.:e8. doi: 10.1016/j.stem.2022.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Page ME, Lombard P, Ng F, Göttgens B, Jensen KB. The Epidermis Comprises Autonomous Compartments Maintained by Distinct Stem Cell Populations. Cell Stem Cell. 2013;13:471–482. doi: 10.1016/j.stem.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaks V, et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- 26.Ito M, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 27.Rompolas P, Mesa KR, Greco V. Spatial organization within a niche as a determinant of stem-cell fate. Nature. 2013;502:513–518. doi: 10.1038/nature12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shackleton M, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 29.Stingl J, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 30.Van Keymeulen A, et al. Distinct stem cells contribute to mammary gland development and maintenance. Nature. 2011;479:189–193. doi: 10.1038/nature10573. [DOI] [PubMed] [Google Scholar]
- 31.Ousset M, et al. Multipotent and unipotent progenitors contribute to prostate postnatal development. Nat Cell Biol. 2012;14:1131–1138. doi: 10.1038/ncb2600. [DOI] [PubMed] [Google Scholar]
- 32.Tika E, Ousset M, Dannau A, Blanpain C. Spatiotemporal regulation of multipotency during prostate development. Development. 2019;146:dev.180224. doi: 10.1242/dev.180224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi N, Zhang B, Zhang L, Ittmann M, Xin L. Adult Murine Prostate Basal and Luminal Cells Are Self-Sustained Lineages that Can Both Serve as Targets for Prostate Cancer Initiation. Cancer Cell. 2012;21:253–265. doi: 10.1016/j.ccr.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwon O-J, Zhang L, Ittmann MM, Xin L. Prostatic inflammation enhances basal-to-luminal differentiation and accelerates initiation of prostate cancer with a basal cell origin. Proc Natl Acad Sci. 2014;111 doi: 10.1073/pnas.1318157111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toivanen R, Mohan A, Shen MM. Basal Progenitors Contribute to Repair of the Prostate Epithelium Following Induced Luminal Anoikis. Stem Cell Rep. 2016;6:660–667. doi: 10.1016/j.stemcr.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centonze A, et al. Heterotypic cell–cell communication regulates glandular stem cell multipotency. Nature. 2020;584:608–613. doi: 10.1038/s41586-020-2632-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, et al. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature. 2009;461:495–500. doi: 10.1038/nature08361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Z, et al. Synergy of p53 and Rb Deficiency in a Conditional Mouse Model for Metastatic Prostate Cancer. Cancer Res. 2006;66:7889–7898. doi: 10.1158/0008-5472.CAN-06-0486. [DOI] [PubMed] [Google Scholar]
- 39.Ku SY, et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science. 2017;355:78–83. doi: 10.1126/science.aah4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molyneux G, et al. BRCA1 Basal-like Breast Cancers Originate from Luminal Epithelial Progenitors and Not from Basal Stem Cells. Cell Stem Cell. 2010;7:403–417. doi: 10.1016/j.stem.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Van Keymeulen A, et al. Reactivation of multipotency by oncogenic PIK3CA induces breast tumour heterogeneity. Nature. 2015;525:119–123. doi: 10.1038/nature14665. [DOI] [PubMed] [Google Scholar]
- 42.Koren S, et al. PIK3CAH1047R induces multipotency and multi-lineage mammary tumours. Nature. 2015;525:114–118. doi: 10.1038/nature14669. [DOI] [PubMed] [Google Scholar]
- 43.Greten FR, et al. IKKβ Links Inflammation and Tumorigenesis in a Mouse Model of Colitis-Associated Cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 44.Schmitt M, et al. Paneth Cells Respond to Inflammation and Contribute to Tissue Regeneration by Acquiring Stem-like Features through SCF/c-Kit Signaling. Cell Rep. 2018;24:2312–2328.:e7. doi: 10.1016/j.celrep.2018.07.085. [DOI] [PubMed] [Google Scholar]
- 45.Schwitalla S, et al. Intestinal Tumorigenesis Initiated by Dedifferentiation and Acquisition of Stem-Cell-like Properties. Cell. 2013;152:25–38. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 46.Strobel O, et al. In Vivo Lineage Tracing Defines the Role of Acinar-to-Ductal Transdifferentiation in Inflammatory Ductal Metaplasia. Gastroenterology. 2007;133:1999–2009. doi: 10.1053/j.gastro.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morris JP, Cano DA, Sekine S, Wang SC, Hebrok M. β-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J Clin Invest. 2010;120:508–520. doi: 10.1172/JCI40045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kopp JL, et al. Identification of Sox9-Dependent Acinar-to-Ductal Reprogramming as the Principal Mechanism for Initiation of Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2012;22:737–750. doi: 10.1016/j.ccr.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alonso-Curbelo D, et al. A gene–environment-induced epigenetic program initiates tumorigenesis. Nature. 2021;590:642–648. doi: 10.1038/s41586-020-03147-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cobo I, et al. Transcriptional regulation by NR5A2 links differentiation and inflammation in the pancreas. Nature. 2018;554:533–537. doi: 10.1038/nature25751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kreso A, Dick JE. Evolution of the Cancer Stem Cell Model. Cell Stem Cell. 2014;14:275–291. doi: 10.1016/j.stem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 52.Merlos-Suárez A, et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell. 2011;8:511–524. doi: 10.1016/j.stem.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 53.Matano M, et al. Modeling colorectal cancer using CRISPR-Cas9–mediated engineering of human intestinal organoids. Nat Med. 2015;21:256–262. doi: 10.1038/nm.3802. [DOI] [PubMed] [Google Scholar]
- 54.Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23:1124–1134. doi: 10.1038/nm.4409. [DOI] [PubMed] [Google Scholar]
- 55.Gupta PB, Pastushenko I, Skibinski A, Blanpain C, Kuperwasser C. Phenotypic Plasticity: Driver of Cancer Initiation, Progression, and Therapy Resistance. Cell Stem Cell. 2019;24:65–78. doi: 10.1016/j.stem.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prager BC, Xie Q, Bao S, Rich JN. Cancer Stem Cells: The Architects of the Tumor Ecosystem. Cell Stem Cell. 2019;24:41–53. doi: 10.1016/j.stem.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roesch A, et al. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell. 2010;141:583–594. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Sousa e Melo F, et al. A distinct role for Lgr5+ stem cells in primary and metastatic colon cancer. Nature. 2017;543:676–680. doi: 10.1038/nature21713. [DOI] [PubMed] [Google Scholar]
- 59.Shimokawa M, et al. Visualization and targeting of LGR5+ human colon cancer stem cells. Nature. 2017;545:187–192. doi: 10.1038/nature22081. [DOI] [PubMed] [Google Scholar]
- 60.Clayton E, et al. A single type of progenitor cell maintains normal epidermis. Nature. 2007;446:185–189. doi: 10.1038/nature05574. [DOI] [PubMed] [Google Scholar]
- 61.Mascré G, et al. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature. 2012;489:257–262. doi: 10.1038/nature11393. [DOI] [PubMed] [Google Scholar]
- 62.Driessens G, Beck B, Caauwe A, Simons BD, Blanpain C. Defining the mode of tumour growth by clonal analysis. Nature. 2012;488:527–530. doi: 10.1038/nature11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williams MJ, Werner B, Barnes CP, Graham TA, Sottoriva A. Identification of neutral tumor evolution across cancer types. Nat Genet. 2016;48:238–244. doi: 10.1038/ng.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lan X, et al. Fate mapping of human glioblastoma reveals an invariant stem cell hierarchy. Nature. 2017;549:227–232. doi: 10.1038/nature23666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou L, et al. Lineage tracing and single-cell analysis reveal proliferative Prom1+ tumour-propagating cells and their dynamic cellular transition during liver cancer progression. Gut. 2021;71:1656–1668. doi: 10.1136/gutjnl-2021-324321. [DOI] [PubMed] [Google Scholar]
- 66.Pal B, et al. A single-cell RNA expression atlas of normal, preneoplastic and tumorigenic states in the human breast. EMBO J. 2021;40 doi: 10.15252/embj.2020107333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tirosh I, et al. Single-cell RNA-seq supports a developmental hierarchy in human oligodendroglioma. Nature. 2016;539:309–313. doi: 10.1038/nature20123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Neftel C, et al. An Integrative Model of Cellular States, Plasticity, and Genetics for Glioblastoma. Cell. 2019;178:835–849.:e21. doi: 10.1016/j.cell.2019.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Couturier CP, et al. Single-cell RNA-seq reveals that glioblastoma recapitulates a normal neurodevelopmental hierarchy. Nat Commun. 2020;11:3406. doi: 10.1038/s41467-020-17186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marjanovic ND, et al. Emergence of a High-Plasticity Cell State during Lung Cancer Evolution. Cancer Cell. 2020;38:229–246.:e13. doi: 10.1016/j.ccell.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Plaks V, Kong N, Werb Z. The Cancer Stem Cell Niche: How Essential Is the Niche in Regulating Stemness of Tumor Cells? Cell Stem Cell. 2015;16:225–238. doi: 10.1016/j.stem.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lenos KJ, et al. Stem cell functionality is microenvironmentally defined during tumour expansion and therapy response in colon cancer. Nat Cell Biol. 2018;20:1193–1202. doi: 10.1038/s41556-018-0179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pietras A, et al. Osteopontin-CD44 Signaling in the Glioma Perivascular Niche Enhances Cancer Stem Cell Phenotypes and Promotes Aggressive Tumor Growth. Cell Stem Cell. 2014;14:357–369. doi: 10.1016/j.stem.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tumbar T, et al. Defining the Epithelial Stem Cell Niche in Skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pardo-Saganta A, et al. Parent stem cells can serve as niches for their daughter cells. Nature. 2015;523:597–601. doi: 10.1038/nature14553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tammela T, et al. A Wnt-producing niche drives proliferative potential and progression in lung adenocarcinoma. Nature. 2017;545:355–359. doi: 10.1038/nature22334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ping Y-F, Zhang X, Bian X-W. Cancer stem cells and their vascular niche: Do they benefit from each other? Cancer Lett. 2016;380:561–567. doi: 10.1016/j.canlet.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 78.Choi J-I, et al. Cancer-initiating cells in human pancreatic cancer organoids are maintained by interactions with endothelial cells. Cancer Lett. 2021;498:42–53. doi: 10.1016/j.canlet.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 79.Jiang H, et al. Jagged1-Notch1-deployed tumor perivascular niche promotes breast cancer stem cell phenotype through Zeb1. Nat Commun. 2020;11:5129. doi: 10.1038/s41467-020-18860-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McCoy MG, et al. Endothelial cells promote 3D invasion of GBM by IL-8-dependent induction of cancer stem cell properties. Sci Rep. 2019;9:9069. doi: 10.1038/s41598-019-45535-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Van de Velde M, et al. Tumor exposed-lymphatic endothelial cells promote primary tumor growth via IL6. Cancer Lett. 2021;497:154–164. doi: 10.1016/j.canlet.2020.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Karras P, et al. A cellular hierarchy in melanoma uncouples growth and metastasis. Nature. 2022;610:190–198. doi: 10.1038/s41586-022-05242-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beck B, et al. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature. 2011;478:399–403. doi: 10.1038/nature10525. [DOI] [PubMed] [Google Scholar]
- 84.Lichtenberger BM, et al. Autocrine VEGF Signaling Synergizes with EGFR in Tumor Cells to Promote Epithelial Cancer Development. Cell. 2010;140:268–279. doi: 10.1016/j.cell.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 85.Wei X, et al. Mechanisms of vasculogenic mimicry in hypoxic tumor microenvironments. Mol Cancer. 2021;20:7. doi: 10.1186/s12943-020-01288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ricci-Vitiani L, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–828. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 87.Wang R, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–833. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 88.Soda Y, et al. Transdifferentiation of glioblastoma cells into vascular endothelial cells. Proc Natl Acad Sci. 2011;108:4274–4280. doi: 10.1073/pnas.1016030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wagenblast E, et al. A model of breast cancer heterogeneity reveals vascular mimicry as a driver of metastasis. Nature. 2015;520:358–362. doi: 10.1038/nature14403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Loh JJ, Ma S. The Role of Cancer-Associated Fibroblast as a Dynamic Player in Mediating Cancer Stemness in the Tumor Microenvironment. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.727640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Saw PE, Chen J, Song E. Targeting CAFs to overcome anticancer therapeutic resistance. Trends Cancer. 2022;8:527–555. doi: 10.1016/j.trecan.2022.03.001. [DOI] [PubMed] [Google Scholar]
- 92.Su S, et al. CD10+GPR77+ Cancer-Associated Fibroblasts Promote Cancer Formation and Chemoresistance by Sustaining Cancer Stemness. Cell. 2018;172:841–856.:e16. doi: 10.1016/j.cell.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 93.Lau EYT, et al. Cancer-Associated Fibroblasts Regulate Tumor-Initiating Cell Plasticity in Hepatocellular Carcinoma through c-Met/FRA1/HEY1 Signaling. Cell Rep. 2016;15:1175–1189. doi: 10.1016/j.celrep.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 94.Wang W, et al. Crosstalk to stromal fibroblasts induces resistance of lung cancer to epidermal growth factor receptor tyrosine kinase inhibitors. Clin Cancer Res. 2009;15:6630–6638. doi: 10.1158/1078-0432.CCR-09-1001. [DOI] [PubMed] [Google Scholar]
- 95.Wilson TR, et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature. 2012;487:505–509. doi: 10.1038/nature11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vermeulen L, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 97.Mosa MH, et al. A Wnt-Induced Phenotypic Switch in Cancer-Associated Fibroblasts Inhibits EMT in Colorectal Cancer. Cancer Res. 2020;80:5569–5582. doi: 10.1158/0008-5472.CAN-20-0263. [DOI] [PubMed] [Google Scholar]
- 98.McAndrews KM, et al. αSMA+ fibroblasts suppress Lgr5+ cancer stem cells and restrain colorectal cancer progression. Oncogene. 2021;40:4440–4452. doi: 10.1038/s41388-021-01866-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Luo H, et al. Pan-cancer single-cell analysis reveals the heterogeneity and plasticity of cancer-associated fibroblasts in the tumor microenvironment. Nat Commun. 2022;13:6619. doi: 10.1038/s41467-022-34395-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mitchem JB, et al. Targeting Tumor-Infiltrating Macrophages Decreases Tumor-Initiating Cells, Relieves Immunosuppression, and Improves Chemotherapeutic Responses. Cancer Res. 2013;73:1128–1141. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lu H, et al. A Breast Cancer Stem Cell Niche Supported by Juxtacrine Signaling from Monocytes and Macrophages. Nat Cell Biol. 2014;16:1105. doi: 10.1038/ncb3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jinushi M, et al. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc Natl Acad Sci U S A. 2011;108:12425–12430. doi: 10.1073/pnas.1106645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wan S, et al. Tumor-Associated Macrophages Produce Interleukin 6 and Signal via STAT3 to Promote Expansion of Human Hepatocellular Carcinoma Stem Cells. Gastroenterology. 2014;147:1393–1404. doi: 10.1053/j.gastro.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mathieu J, et al. HIF Induces Human Embryonic Stem Cell Markers in Cancer Cells. Cancer Res. 2011;71:4640–4652. doi: 10.1158/0008-5472.CAN-10-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim H, Lin Q, Glazer PM, Yun Z. The hypoxic tumor microenvironment in vivo selects the cancer stem cell fate of breast cancer cells. Breast Cancer Res BCR. 2018;20:16. doi: 10.1186/s13058-018-0944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gupta VK, et al. Hypoxia-driven oncometabolite L-2HG maintains stemness-differentiation balance and facilitates immune evasion in pancreatic cancer. Cancer Res. 2021;81:4001–4013. doi: 10.1158/0008-5472.CAN-20-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gkountela S, Aceto N. Stem-like features of cancer cells on their way to metastasis. Biol Direct. 2016;11:33. doi: 10.1186/s13062-016-0135-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Birkbak NJ, McGranahan N. Cancer Genome Evolutionary Trajectories in Metastasis. Cancer Cell. 2020;37:8–19. doi: 10.1016/j.ccell.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 109.Priestley P, et al. Pan-cancer whole-genome analyses of metastatic solid tumours. Nature. 2019;575:210–216. doi: 10.1038/s41586-019-1689-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pierce SE, et al. LKB1 inactivation modulates chromatin accessibility to drive metastatic progression. Nat Cell Biol. 2021;23:915–924. doi: 10.1038/s41556-021-00728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yaeger R, et al. RAS mutations affect pattern of metastatic spread and increase propensity for brain metastasis in colorectal cancer. Cancer. 2015;121:1195–1203. doi: 10.1002/cncr.29196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang J, et al. Twist, a Master Regulator of Morphogenesis, Plays an Essential Role in Tumor Metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 113.Stemmler MP, Eccles RL, Brabletz S, Brabletz T. Non-redundant functions of EMT transcription factors. Nat Cell Biol. 2019;21:102–112. doi: 10.1038/s41556-018-0196-y. [DOI] [PubMed] [Google Scholar]
- 114.Pastushenko I, et al. Identification of the tumour transition states occurring during EMT. Nature. 2018;556:463–468. doi: 10.1038/s41586-018-0040-3. [DOI] [PubMed] [Google Scholar]
- 115.Pastushenko I, Blanpain C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019;29:212–226. doi: 10.1016/j.tcb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 116.Kröger C, et al. Acquisition of a hybrid E/M state is essential for tumorigenicity of basal breast cancer cells. Proc Natl Acad Sci U S A. 2019;116:7353–7362. doi: 10.1073/pnas.1812876116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bierie B, et al. Integrin-β4 identifies cancer stem cell-enriched populations of partially mesenchymal carcinoma cells. Proc Natl Acad Sci U S A. 2017;114:E2337–E2346. doi: 10.1073/pnas.1618298114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhao J, et al. Single cell RNA-seq reveals the landscape of tumor and infiltrating immune cells in nasopharyngeal carcinoma. Cancer Lett. 2020;477:131–143. doi: 10.1016/j.canlet.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 119.Wouters J, et al. Robust gene expression programs underlie recurrent cell states and phenotype switching in melanoma. Nat Cell Biol. 2020;22:986–998. doi: 10.1038/s41556-020-0547-3. [DOI] [PubMed] [Google Scholar]
- 120.Puram SV, et al. Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Head and Neck Cancer. Cell. 2017;171:1611–1624.:e24. doi: 10.1016/j.cell.2017.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Deshmukh AP, et al. Identification of EMT signaling cross-talk and gene regulatory networks by single-cell RNA sequencing. Proc Natl Acad Sci U S A. 2021;118:e2102050118. doi: 10.1073/pnas.2102050118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jang G-B, et al. Blockade of Wnt/β-catenin signaling suppresses breast cancer metastasis by inhibiting CSC-like phenotype. Sci Rep. 2015;5:12465. doi: 10.1038/srep12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lawson DA, et al. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature. 2015;526:131–135. doi: 10.1038/nature15260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Morel A-P, et al. Generation of Breast Cancer Stem Cells through Epithelial-Mesenchymal Transition. PLoS ONE. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li Y, et al. Genetic Fate Mapping of Transient Cell Fate Reveals N-Cadherin Activity and Function in Tumor Metastasis. Dev Cell. 2020;54:593–607.:e5. doi: 10.1016/j.devcel.2020.06.021. [DOI] [PubMed] [Google Scholar]
- 127.Lüönd F, et al. Distinct contributions of partial and full EMT to breast cancer malignancy. Dev Cell. 2021;56:3203–3221.:e11. doi: 10.1016/j.devcel.2021.11.006. [DOI] [PubMed] [Google Scholar]
- 128.Simeonov KP, et al. Single-cell lineage tracing of metastatic cancer reveals selection of hybrid EMT states. Cancer Cell. 2021;39:1150–1162.:e9. doi: 10.1016/j.ccell.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Er EE, et al. Pericyte-like spreading by disseminated cancer cells activates YAP and MRTF for metastatic colonization. Nat Cell Biol. 2018;20:966–978. doi: 10.1038/s41556-018-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ganesh K, et al. L1CAM defines the regenerative origin of metastasis-initiating cells in colorectal cancer. Nat Cancer. 2020;1:28–45. doi: 10.1038/s43018-019-0006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Valiente M, et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell. 2014;156:1002–1016. doi: 10.1016/j.cell.2014.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Faubert B, Solmonson A, DeBerardinis RJ. Metabolic reprogramming and cancer progression. Science. 2020;368:eaaw5473. doi: 10.1126/science.aaw5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lu J, Tan M, Cai Q. The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett. 2015;356:156–164. doi: 10.1016/j.canlet.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gaude E, Frezza C. Tissue-specific and convergent metabolic transformation of cancer correlates with metastatic potential and patient survival. Nat Commun. 2016;7:13041. doi: 10.1038/ncomms13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Delaunay S, et al. Mitochondrial RNA modifications shape metabolic plasticity in metastasis. Nature. 2022;607:593–603. doi: 10.1038/s41586-022-04898-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bergers G, Fendt S-M. The metabolism of cancer cells during metastasis. Nat Rev Cancer. 2021;21:162–180. doi: 10.1038/s41568-020-00320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rossi M, et al. PHGDH heterogeneity potentiates cancer cell dissemination and metastasis. Nature. 2022;605:747–753. doi: 10.1038/s41586-022-04758-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Klein CA. Cancer progression and the invisible phase of metastatic colonization. Nat Rev Cancer. 2020;20:681–694. doi: 10.1038/s41568-020-00300-6. [DOI] [PubMed] [Google Scholar]
- 139.Hosseini H, et al. Early dissemination seeds metastasis in breast cancer. Nature. 2016;540:552–558. doi: 10.1038/nature20785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nobre AR, et al. ZFP281 drives a mesenchymal-like dormancy program in early disseminated breast cancer cells that prevents metastatic outgrowth in the lung. Nat Cancer. 2022;3:1165–1180. doi: 10.1038/s43018-022-00424-8. [DOI] [PubMed] [Google Scholar]
- 141.Aiello NM, et al. EMT Subtype Influences Epithelial Plasticity and Mode of Cell Migration. Dev Cell. 2018;45:681–695.:e4. doi: 10.1016/j.devcel.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Majidpoor J, Mortezaee K. Steps in metastasis: an updated review. Med Oncol. 2021;38:3. doi: 10.1007/s12032-020-01447-w. [DOI] [PubMed] [Google Scholar]
- 143.Baccelli I, et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol. 2013;31:539–544. doi: 10.1038/nbt.2576. [DOI] [PubMed] [Google Scholar]
- 144.Aceto N, Toner M, Maheswaran S, Haber DA. En Route to Metastasis: Circulating Tumor Cell Clusters and Epithelial-to-Mesenchymal Transition. Trends Cancer. 2015;1:44–52. doi: 10.1016/j.trecan.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 145.Wang C, et al. Longitudinally collected CTCs and CTC-clusters and clinical outcomes of metastatic breast cancer. Breast Cancer Res Treat. 2017;161:83–94. doi: 10.1007/s10549-016-4026-2. [DOI] [PubMed] [Google Scholar]
- 146.Costa C, et al. Analysis of a Real-World Cohort of Metastatic Breast Cancer Patients Shows Circulating Tumor Cell Clusters (CTC-clusters) as Predictors of Patient Outcomes. Cancers. 2020;12:1111. doi: 10.3390/cancers12051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Castro-Giner F, Aceto N. Tracking cancer progression: from circulating tumor cells to metastasis. Genome Med. 2020;12:31. doi: 10.1186/s13073-020-00728-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yu M, et al. Circulating Breast Tumor Cells Exhibit Dynamic Changes in Epithelial and Mesenchymal Composition. Science. 2013;339:580–584. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Revenco T, et al. Context Dependency of Epithelial-to-Mesenchymal Transition for Metastasis. Cell Rep. 2019;29:1458–1468.:e3. doi: 10.1016/j.celrep.2019.09.081. [DOI] [PubMed] [Google Scholar]
- 150.Lecharpentier A, et al. Detection of circulating tumour cells with a hybrid (epithelial/mesenchymal) phenotype in patients with metastatic non-small cell lung cancer. Br J Cancer. 2011;105:1338–1341. doi: 10.1038/bjc.2011.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Armstrong AJ, et al. Circulating Tumor Cells from Patients with Advanced Prostate and Breast Cancer Display Both Epithelial and Mesenchymal Markers. Mol Cancer Res. 2011;9:997–1007. doi: 10.1158/1541-7786.MCR-10-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Balcik-Ercin P, Cayrefourcq L, Soundararajan R, Mani SA, Alix-Panabières C. Epithelial-to-Mesenchymal Plasticity in Circulating Tumor Cell Lines Sequentially Derived from a Patient with Colorectal Cancer. Cancers. 2021;13:5408. doi: 10.3390/cancers13215408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ting DT, et al. Single-cell RNA sequencing identifies extracellular matrix gene expression by pancreatic circulating tumor cells. Cell Rep. 2014;8:1905–1918. doi: 10.1016/j.celrep.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rahrmann EP, et al. The NALCN channel regulates metastasis and nonmalignant cell dissemination. Nat Genet. 2022;54:1827–1838. doi: 10.1038/s41588-022-01182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jordan NV, et al. HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature. 2016;537:102–106. doi: 10.1038/nature19328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tasdogan A, Ubellacker JM, Morrison SJ. Redox Regulation in Cancer Cells during Metastasis. Cancer Discov. 2021;11:2682–2692. doi: 10.1158/2159-8290.CD-21-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Piskounova E, et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015;527:186–191. doi: 10.1038/nature15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tasdogan A, et al. Metabolic heterogeneity confers differences in melanoma metastatic potential. Nature. 2020;577:115–120. doi: 10.1038/s41586-019-1847-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ubellacker JM, et al. Lymph protects metastasizing melanoma cells from ferroptosis. Nature. 2020;585:113–118. doi: 10.1038/s41586-020-2623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Labuschagne CF, Cheung EC, Blagih J, Domart M-C, Vousden KH. Cell Clustering Promotes a Metabolic Switch that Supports Metastatic Colonization. Cell Metab. 2019;30:720–734.:e5. doi: 10.1016/j.cmet.2019.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Padmanaban V, et al. E-cadherin is required for metastasis in multiple models of breast cancer. Nature. 2019;573:439–444. doi: 10.1038/s41586-019-1526-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang H, et al. The Osteogenic Niche Promotes Early-Stage Bone Colonization of Disseminated Breast Cancer Cells. Cancer Cell. 2015;27:193–210. doi: 10.1016/j.ccell.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bakir B, Chiarella AM, Pitarresi JR, Rustgi AK. EMT, MET, Plasticity, and Tumor Metastasis. Trends Cell Biol. 2020;30:764–776. doi: 10.1016/j.tcb.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kowalski PJ, Rubin MA, Kleer CG. E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res. 2003;5:R217–R222. doi: 10.1186/bcr651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. 2012;22:725–736. doi: 10.1016/j.ccr.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ocaña OH, et al. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell. 2012;22:709–724. doi: 10.1016/j.ccr.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 167.Takano S, et al. Prrx1 isoform switching regulates pancreatic cancer invasion and metastatic colonization. Genes Dev. 2016;30:233–247. doi: 10.1101/gad.263327.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fumagalli A, et al. Plasticity of Lgr5-Negative Cancer Cells Drives Metastasis in Colorectal Cancer. Cell Stem Cell. 2020;26:569–578.:e7. doi: 10.1016/j.stem.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cañellas-Socias A, et al. Metastatic recurrence in colorectal cancer arises from residual EMP1+ cells. Nature. 2022;611:603–613. doi: 10.1038/s41586-022-05402-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Elia I, et al. Breast cancer cells rely on environmental pyruvate to shape the metastatic niche. Nature. 2019;568:117–121. doi: 10.1038/s41586-019-0977-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Derynck R, Turley SJ, Akhurst RJ. TGFβ biology in cancer progression and immunotherapy. Nat Rev Clin Oncol. 2021;18:9–34. doi: 10.1038/s41571-020-0403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 173.Shi X, et al. Cancer-Associated Fibroblasts Facilitate Squamous Cell Carcinoma Lung Metastasis in Mice by Providing TGFβ-Mediated Cancer Stem Cell Niche. Front Cell Dev Biol. 2021;9:668164. doi: 10.3389/fcell.2021.668164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wei SC, et al. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat Cell Biol. 2015;17:678–688. doi: 10.1038/ncb3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Fattet L, et al. Matrix Rigidity Controls Epithelial-Mesenchymal Plasticity and Tumor Metastasis via a Mechanoresponsive EPHA2/LYN Complex. Dev Cell. 2020;54:302–316.:e7. doi: 10.1016/j.devcel.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang H, Yung MMH, Ngan HYS, Chan KKL, Chan DW. The Impact of the Tumor Microenvironment on Macrophage Polarization in Cancer Metastatic Progression. Int J Mol Sci. 2021;22:6560. doi: 10.3390/ijms22126560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hass R. Role of MSC in the Tumor Microenvironment. Cancers. 2020;12:2107. doi: 10.3390/cancers12082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kletukhina S, Neustroeva O, James V, Rizvanov A, Gomzikova M. Role of Mesenchymal Stem Cell-Derived Extracellular Vesicles in Epithelial–Mesenchymal Transition. Int J Mol Sci. 2019;20:4813. doi: 10.3390/ijms20194813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.El-Haibi CP, et al. Critical role for lysyl oxidase in mesenchymal stem cell-driven breast cancer malignancy. Proc Natl Acad Sci U S A. 2012;109:17460–17465. doi: 10.1073/pnas.1206653109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hara T, et al. Interactions between cancer cells and immune cells drive transitions to mesenchymal-like states in glioblastoma. Cancer Cell. 2021;39:779–792.:e11. doi: 10.1016/j.ccell.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Casanova-Acebes M, et al. Tissue-resident macrophages provide a pro-tumorigenic niche to early NSCLC cells. Nature. 2021;595:578–584. doi: 10.1038/s41586-021-03651-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wei C, et al. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol Cancer. 2019;18:64. doi: 10.1186/s12943-019-0976-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Szczerba BM, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019;566:553–557. doi: 10.1038/s41586-019-0915-y. [DOI] [PubMed] [Google Scholar]
- 184.Labelle M, Begum S, Hynes RO. Direct Signaling Between Platelets and Cancer Cells Induces an Epithelial-Mesenchymal-Like Transition and Promotes Metastasis. Cancer Cell. 2011;20:576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hongu T, et al. Perivascular tenascin C triggers sequential activation of macrophages and endothelial cells to generate a pro-metastatic vascular niche in the lungs. Nat Cancer. 2022;3:486–504. doi: 10.1038/s43018-022-00353-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nolan E, et al. Radiation exposure elicits a neutrophil-driven response in healthy lung tissue that enhances metastatic colonization. Nat Cancer. 2022;3:173–187. doi: 10.1038/s43018-022-00336-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhang W, et al. The bone microenvironment invigorates metastatic seeds for further dissemination. Cell. 2021;184:2471–2486.:e20. doi: 10.1016/j.cell.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Esposito M, et al. Bone Vascular Niche E-selectin Induces Mesenchymal-Epithelial Transition and Wnt Activation in Cancer Cells to Promote Bone Metastasis. Nat Cell Biol. 2019;21:627–639. doi: 10.1038/s41556-019-0309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lin W-H, et al. STAT3 phosphorylation at Ser727 and Tyr705 differentially regulates the EMT-MET switch and cancer metastasis. Oncogene. 2021;40:791–805. doi: 10.1038/s41388-020-01566-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Xu H, et al. The mechanisms of colorectal cancer cell mesenchymal-epithelial transition induced by hepatocyte exosome-derived miR-203a-3p. BMC Cancer. 2021;21:718. doi: 10.1186/s12885-021-08419-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gao D, et al. Myeloid progenitor cells in the premetastatic lung promote metastases by inducing mesenchymal to epithelial transition. Cancer Res. 2012;72:1384–1394. doi: 10.1158/0008-5472.CAN-11-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.del Pozo Martin Y, et al. Mesenchymal Cancer Cell-Stroma Crosstalk Promotes Niche Activation, Epithelial Reversion, and Metastatic Colonization. Cell Rep. 2015;13:2456–2469. doi: 10.1016/j.celrep.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ognjenovic NB, et al. Limiting Self-Renewal of the Basal Compartment by PKA Activation Induces Differentiation and Alters the Evolution of Mammary Tumors. Dev Cell. 2020;55:544–557.:e6. doi: 10.1016/j.devcel.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Peinado H, et al. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. 2017;17:302–317. doi: 10.1038/nrc.2017.6. [DOI] [PubMed] [Google Scholar]
- 195.Zeng Z, et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun. 2018;9:5395. doi: 10.1038/s41467-018-07810-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Murgai M, et al. KLF4-dependent perivascular cell plasticity mediates pre-metastatic niche formation and metastasis. Nat Med. 2017;23:1176–1190. doi: 10.1038/nm.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Holmgren L, O’Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1:149–153. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- 198.Koebel CM, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 199.Bragado P, et al. TGFβ2 dictates disseminated tumour cell fate in target organs through TGFβ-RIII and p38α/β signalling. Nat Cell Biol. 2013;15:1351–1361. doi: 10.1038/ncb2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Massagué J, Ganesh K. Metastasis-Initiating Cells and Ecosystems. Cancer Discov. 2021;11:971–994. doi: 10.1158/2159-8290.CD-21-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.De Cock JM, et al. Inflammation triggers Zeb1-dependent escape from tumor latency. Cancer Res. 2016;76:6778–6784. doi: 10.1158/0008-5472.CAN-16-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bui AT, Laurent F, Havard M, Dautry F, Tchénio T. SMAD signaling and redox imbalance cooperate to induce prostate cancer cell dormancy. Cell Cycle Georget Tex. 2015;14:1218–1231. doi: 10.1080/15384101.2015.1014145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Giancotti FG. Mechanisms governing metastatic dormancy and reactivation. Cell. 2013;155:750–764. doi: 10.1016/j.cell.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Di Martino JS, et al. A tumor-derived type III collagen-rich ECM niche regulates tumor cell dormancy. Nat Cancer. 2021;3:90–107. doi: 10.1038/s43018-021-00291-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Albrengues J, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361:eaao4227. doi: 10.1126/science.aao4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Fluegen G, et al. Phenotypic heterogeneity of disseminated tumour cells is preset by primary tumour hypoxic microenvironments. Nat Cell Biol. 2017;19:120–132. doi: 10.1038/ncb3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kaur A, et al. sFRP2 in the aged microenvironment drives melanoma metastasis and therapy resistance. Nature. 2016;532:250–254. doi: 10.1038/nature17392. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 208.Fane ME, et al. Stromal changes in the aged lung induce an emergence from melanoma dormancy. Nature. 2022;606:396–405. doi: 10.1038/s41586-022-04774-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Shen S, Vagner S, Robert C. Persistent Cancer Cells: The Deadly Survivors. Cell. 2020;183:860–874. doi: 10.1016/j.cell.2020.10.027. [DOI] [PubMed] [Google Scholar]
- 210.Marine J-C, Dawson S-J, Dawson MA. Non-genetic mechanisms of therapeutic resistance in cancer. Nat Rev Cancer. 2020;20:743–756. doi: 10.1038/s41568-020-00302-4. [DOI] [PubMed] [Google Scholar]
- 211.Rehman SK, et al. Colorectal Cancer Cells Enter a Diapause-like DTP State to Survive Chemotherapy. Cell. 2021;184:226–242.:e21. doi: 10.1016/j.cell.2020.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Dhimolea E, et al. An Embryonic Diapause-like Adaptation with Suppressed Myc Activity Enables Tumor Treatment Persistence. Cancer Cell. 2021;39:240–256.:e11. doi: 10.1016/j.ccell.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Debaugnies M, et al. RHOJ controls EMT-associated resistance to chemotherapy. Nature. 2023;616:168–175. doi: 10.1038/s41586-023-05838-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Shaffer SM, et al. Rare cell variability and drug-induced reprogramming as a mode of cancer drug resistance. Nature. 2017;546:431–435. doi: 10.1038/nature22794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Torre EA, et al. Genetic screening for single-cell variability modulators driving therapy resistance. Nat Genet. 2021;53:76–85. doi: 10.1038/s41588-020-00749-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kim C, et al. Chemoresistance Evolution in Triple-Negative Breast Cancer Delineated by Single-Cell Sequencing. Cell. 2018;173:879–893.:e13. doi: 10.1016/j.cell.2018.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Mu P, et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53 - and RB1 -deficient prostate cancer. Science. 2017;355:84–88. doi: 10.1126/science.aah4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.He MX, et al. Transcriptional mediators of treatment resistance in lethal prostate cancer. Nat Med. 2021;27:426–433. doi: 10.1038/s41591-021-01244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Deng S, et al. Ectopic JAK–STAT activation enables the transition to a stem-like and multilineage state conferring AR-targeted therapy resistance. Nat Cancer. 2022;3:1071–1087. doi: 10.1038/s43018-022-00431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Chan JM, et al. Lineage plasticity in prostate cancer depends on JAK/STAT inflammatory signaling. Science. 2022;377:1180–1191. doi: 10.1126/science.abn0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Rambow F, et al. Toward Minimal Residual Disease-Directed Therapy in Melanoma. Cell. 2018;174:843–855.:e19. doi: 10.1016/j.cell.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 222.Marin-Bejar O, et al. Evolutionary predictability of genetic versus nongenetic resistance to anticancer drugs in melanoma. Cancer Cell. 2021;39:1135–1149.:e8. doi: 10.1016/j.ccell.2021.05.015. [DOI] [PubMed] [Google Scholar]
- 223.Biehs B, et al. A cell identity switch allows residual BCC to survive Hedgehog pathway inhibition. Nature. 2018;562:429–433. doi: 10.1038/s41586-018-0596-y. [DOI] [PubMed] [Google Scholar]
- 224.Sánchez-Danés A, et al. A slow-cycling LGR5 tumour population mediates basal cell carcinoma relapse after therapy. Nature. 2018;562:434–438. doi: 10.1038/s41586-018-0603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Niederst MJ, et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat Commun. 2015;6:6377. doi: 10.1038/ncomms7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Maynard A, et al. Therapy-Induced Evolution of Human Lung Cancer Revealed by Single-Cell RNA Sequencing. Cell. 2020;182:1232–1251.:e22. doi: 10.1016/j.cell.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Oren Y, et al. Cycling cancer persister cells arise from lineages with distinct programs. Nature. 2021;596:576–582. doi: 10.1038/s41586-021-03796-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Sharma SV, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141:69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Guler GD, et al. Repression of Stress-Induced LINE-1 Expression Protects Cancer Cell Subpopulations from Lethal Drug Exposure. Cancer Cell. 2017;32:221–237.:e13. doi: 10.1016/j.ccell.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 230.Liau BB, et al. Adaptive Chromatin Remodeling Drives Glioblastoma Stem Cell Plasticity and Drug Tolerance. Cell Stem Cell. 2017;20:233–246.:e7. doi: 10.1016/j.stem.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Risom T, et al. Differentiation-state plasticity is a targetable resistance mechanism in basal-like breast cancer. Nat Commun. 2018;9:3815. doi: 10.1038/s41467-018-05729-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Knoechel B, et al. An epigenetic mechanism of resistance to targeted therapy in T cell acute lymphoblastic leukemia. Nat Genet. 2014;46:364–370. doi: 10.1038/ng.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wang J, et al. Snail determines the therapeutic response to mTOR kinase inhibitors by transcriptional repression of 4E-BP1. Nat Commun. 2017;8:2207. doi: 10.1038/s41467-017-02243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Viswanathan VS, et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature. 2017;547:453–457. doi: 10.1038/nature23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Seshagiri S, et al. Recurrent R-spondin fusions in colon cancer. Nature. 2012;488:660–664. doi: 10.1038/nature11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Storm EE, et al. Targeting PTPRK-RSPO3 colon tumours promotes differentiation and loss of stem-cell function. Nature. 2016;529:97–100. doi: 10.1038/nature16466. [DOI] [PubMed] [Google Scholar]
- 237.Han T, et al. Lineage Reversion Drives WNT Independence in Intestinal Cancer. Cancer Discov. 2020;10:1590–1609. doi: 10.1158/2159-8290.CD-19-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Lupo B, et al. Colorectal cancer residual disease at maximal response to EGFR blockade displays a druggable Paneth cell-like phenotype. Sci Transl Med. 2020;12:eaax8313. doi: 10.1126/scitranslmed.aax8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Solé L, et al. p53 wild-type colorectal cancer cells that express a fetal gene signature are associated with metastasis and poor prognosis. Nat Commun. 2022;13:2866. doi: 10.1038/s41467-022-30382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Álvarez-Varela A, et al. Mex3a marks drug-tolerant persister colorectal cancer cells that mediate relapse after chemotherapy. Nat Cancer. 2022;3:1052–1070. doi: 10.1038/s43018-022-00402-0. [DOI] [PubMed] [Google Scholar]
- 241.Ohta Y, et al. Cell-matrix interface regulates dormancy in human colon cancer stem cells. Nature. 2022;608:784–794. doi: 10.1038/s41586-022-05043-y. [DOI] [PubMed] [Google Scholar]
- 242.Gerber DE, et al. Phase 1 study of romidepsin plus erlotinib in advanced non-small cell lung cancer. Lung Cancer. 2015;90:534–541. doi: 10.1016/j.lungcan.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Brown JA, et al. TGF-β-Induced Quiescence Mediates Chemoresistance of Tumor-Propagating Cells in Squamous Cell Carcinoma. Cell Stem Cell. 2017;21:650–664.:e8. doi: 10.1016/j.stem.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Park S-Y, et al. Combinatorial TGF-β attenuation with paclitaxel inhibits the epithelial-to-mesenchymal transition and breast cancer stem-like cells. Oncotarget. 2015;6:37526–37543. doi: 10.18632/oncotarget.6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Hangauer MJ, et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature. 2017;551:247–250. doi: 10.1038/nature24297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Straussman R, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–504. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Insua-Rodríguez J, et al. Stress signaling in breast cancer cells induces matrix components that promote chemoresistant metastasis. EMBO Mol Med. 2018;10 doi: 10.15252/emmm.201809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Nicolas AM, et al. Inflammatory fibroblasts mediate resistance to neoadjuvant therapy in rectal cancer. Cancer Cell. 2022;40:168–184.:e13. doi: 10.1016/j.ccell.2022.01.004. [DOI] [PubMed] [Google Scholar]
- 249.de Thé H. Differentiation therapy revisited. Nat Rev Cancer. 2018;18:117–127. doi: 10.1038/nrc.2017.103. [DOI] [PubMed] [Google Scholar]
- 250.Leiendecker L, et al. LSD1 inhibition induces differentiation and cell death in Merkel cell carcinoma. EMBO Mol Med. 2020;12:e12525. doi: 10.15252/emmm.202012525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Tayari MM, et al. Clinical Responsiveness to All-trans Retinoic Acid Is Potentiated by LSD1 Inhibition and Associated with a Quiescent Transcriptome in Myeloid Malignancies. Clin Cancer Res. 2021;27:1893–1903. doi: 10.1158/1078-0432.CCR-20-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Herbaux C, et al. BH3 profiling identifies ruxolitinib as a promising partner for venetoclax to treat T-cell prolymphocytic leukemia. Blood. 2021;137:3495–3506. doi: 10.1182/blood.2020007303. [DOI] [PubMed] [Google Scholar]
- 253.Nervi C, De Marinis E, Codacci-Pisanelli G. Epigenetic treatment of solid tumours: a review of clinical trials. Clin Epigenetics. 2015;7:127. doi: 10.1186/s13148-015-0157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Herpers B, et al. Functional patient-derived organoid screenings identify MCLA-158 as a therapeutic EGFR × LGR5 bispecific antibody with efficacy in epithelial tumors. Nat Cancer. 2022;3:418–436. doi: 10.1038/s43018-022-00359-0. [DOI] [PubMed] [Google Scholar]
- 255.Yahyanejad S, Theys J, Vooijs M. Targeting Notch to overcome radiation resistance. Oncotarget. 2016;7:7610–7628. doi: 10.18632/oncotarget.6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zhou B, et al. Notch signaling pathway: architecture, disease, and therapeutics. Signal Transduct Target Ther. 2022;7:95. doi: 10.1038/s41392-022-00934-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Takebe N, et al. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol. 2015;12:445–464. doi: 10.1038/nrclinonc.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Ganesh K, Massagué J. Targeting metastatic cancer. Nat Med. 2021;27:34–44. doi: 10.1038/s41591-020-01195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Essers MAG, et al. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–908. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- 260.Cazet AS, et al. Targeting stromal remodeling and cancer stem cell plasticity overcomes chemoresistance in triple negative breast cancer. Nat Commun. 2018;9:2897. doi: 10.1038/s41467-018-05220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Sun Y, et al. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nat Med. 2012;18:1359–1368. doi: 10.1038/nm.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Agudo J, et al. Quiescent tissue stem cells evade immune surveillance. Immunity. 2018;48:271–285.:e5. doi: 10.1016/j.immuni.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Miao Y, et al. Adaptive Immune Resistance Emerges from Tumor-Initiating Stem Cells. Cell. 2019;177:1172–1186.:e14. doi: 10.1016/j.cell.2019.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Malladi S, et al. Metastatic Latency and Immune Evasion through Autocrine Inhibition of WNT. Cell. 2016;165:45–60. doi: 10.1016/j.cell.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Terry S, et al. New insights into the role of EMT in tumor immune escape. Mol Oncol. 2017;11:824–846. doi: 10.1002/1878-0261.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Akalay I, et al. Epithelial-to-mesenchymal transition and autophagy induction in breast carcinoma promote escape from T-cell-mediated lysis. Cancer Res. 2013;73:2418–2427. doi: 10.1158/0008-5472.CAN-12-2432. [DOI] [PubMed] [Google Scholar]
- 267.Jiang X, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer. 2019;18:10. doi: 10.1186/s12943-018-0928-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Tauriello DVF, et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554:538–543. doi: 10.1038/nature25492. [DOI] [PubMed] [Google Scholar]
- 269.Mariathasan S, et al. TGF-β attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–548. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Morris VK, et al. Bintrafusp Alfa, an Anti-PD-L1:TGFβ Trap Fusion Protein, in Patients with ctDNA-positive, Liver-limited Metastatic Colorectal Cancer. Cancer Res Commun. 2022;2:979–986. doi: 10.1158/2767-9764.CRC-22-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Nassar D, Blanpain C. Cancer Stem Cells: Basic Concepts and Therapeutic Implications. Annu Rev Pathol Mech Dis. 2016;11:47–76. doi: 10.1146/annurev-pathol-012615-044438. [DOI] [PubMed] [Google Scholar]
- 272.Lapidot T, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 273.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Dalerba P, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Singh SK, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 276.Hermann PC, et al. Distinct Populations of Cancer Stem Cells Determine Tumor Growth and Metastatic Activity in Human Pancreatic Cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 277.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 278.Ricci-Vitiani L, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 279.Vermeulen L, et al. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc Natl Acad Sci. 2008;105:13427–13432. doi: 10.1073/pnas.0805706105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Quintana E, et al. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Schepers AG, et al. Lineage Tracing Reveals Lgr5+Stem Cell Activity in Mouse Intestinal Adenomas. Science. 2012;337:730–735. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- 282.Cortina C, et al. A genome editing approach to study cancer stem cells in human tumors. EMBO Mol Med. 2017;9:869–879. doi: 10.15252/emmm.201707550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Penter L, Gohil SH, Wu CJ. Natural Barcodes for Longitudinal Single Cell Tracking of Leukemic and Immune Cell Dynamics. Front Immunol. 2022;12:788891. doi: 10.3389/fimmu.2021.788891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Ludwig LS, et al. Lineage Tracing in Humans Enabled by Mitochondrial Mutations and Single-Cell Genomics. Cell. 2019;176:1325–1339.:e22. doi: 10.1016/j.cell.2019.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Chen J, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Boumahdi S, et al. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature. 2014;511:246–250. doi: 10.1038/nature13305. [DOI] [PubMed] [Google Scholar]
- 287.Nakanishi Y, et al. Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat Genet. 2013;45:98–103. doi: 10.1038/ng.2481. [DOI] [PubMed] [Google Scholar]