Abstract
Introduction
Patients with interstitial lung disease (ILD) may develop pulmonary hypertension (PH), often disproportionate to ILD severity. Right ventricle to left ventricle diameter ratio (RV:LV) measured at CT pulmonary angiography (CTPA), has been shown to provide valuable information in pulmonary arterial hypertension patients and to predict death or deterioration in acute pulmonary embolism.
Methods
Demographics, ILD subtype, echocardiography and detailed CTPA measurements were collected in consecutive patients undergoing both CTPA and right heart catheterisation (RHC) at the Royal Brompton Hospital between 2005 and 2015. Fibrosis severity was formally scored using CT criteria. RV:LV ratio at CTPA was evaluated by three different methods. Cox-proportional hazard analysis was used to assess the relation of CTPA-derived parameters to predict death or lung transplantation.
Results
92 patients were included: 64% male, mean age 65±11 years, with FVC 57±20% (predicted), TLCOc 22±8% (predicted) and KCOc 51±17% (predicted). PH was confirmed at RHC in 78%. Of all CTPA-derived measures, an RV:LV ratio ≥1.0 strongly predicted mortality or transplantation at univariate analysis (HR 3.26, 95%CI:1.49-7.13, p=0.003), whereas invasive haemodynamic data did not. The RV:LV ratio remained an independent predictor at multivariate analysis (HR: 3.19, CI:1.44-7.10, p=0.004), adjusting for an ILD diagnosis of IPF and CT derived ILD severity.
Conclusion
An increased RV:LV ratio measured at CTPA provides a simple, non-invasive method of risk stratification in patients with suspected ILD-PH. This should prompt closer follow up, more aggressive treatment and consideration of lung transplantation.
Introduction
Pulmonary hypertension (PH) is common in interstitial lung disease (ILD) and impacts adversely on outcome, which is independent of the subtype of interstitial lung disease1. Clinical signs of PH are difficult to detect, and physicians rely on the integration of pre-test probability and non-invasive investigations, such as echocardiography, brain natriuretic peptide (BNP), six-minute walk data and ancillary signs afforded by CT evaluation. Invasive right heart catheterisation (RHC) remains essential for confirming the diagnosis of PH2. In patients with PH due to lung disease and/or hypoxia (group 3 PH), RHC is usually reserved for patients worked-up for lung transplantation or in whom PH appears disproportionate to the severity of the ILD. Patients considered to have a “pulmonary vascular phenotype”, should be investigated further and may be enrolled in studies using pulmonary arterial hypertension therapies2.
Echocardiographic variables such as right ventricular systolic pressure (RVSP) predict both the presence of PH and mortality with varying degrees of accuracy3–7. However, suitable echocardiographic windows are often difficult to attain in ILD patients. Several studies have evaluated the ability of the CT-derived main pulmonary artery (MPA) diameter and MPA to aorta (Ao) diameter ratio (MPA:Ao) to predict the presence of PH in ILD. Although useful predictors of PH in other conditions8, studies differ in patients with ILD, with some demonstrating that MPA dilatation occurs in the absence of PH9,10. However a recent study found that MPA diameter was reliable in detecting PH in patients with ILD11. Recently, the MPA:Ao was shown to be an independent predictor of survival or transplantation in a large cohort of unselected patients with idiopathic pulmonary fibrosis (IPF)12.
Right ventricular (RV) to left ventricular (LV) ratio measured on CT (RV:LV) has been shown to predict the presence of PH in patients with pulmonary arterial hypertension13,14. In addition, studies have shown that the CT-derived RV:LV ratio predicts 30-day mortality in patients following acute pulmonary embolism15. We hypothesized that patients with ILD associated PH (ILD-PH) would have a larger RV:LV ratio, and an increased RV:LV ratio would predict PH and be associated with a worse prognosis.
Methods
Consecutive ILD patients with suspected PH referred to the Royal Brompton Hospital National Pulmonary Hypertension Service between 2005 and 2015 were reviewed. Patients were included if a CTPA had been performed within 6 months of the baseline diagnostic right heart catheterisation (RHC) study. To reflect a pure ‘group 3’ PH cohort, those with an ILD diagnosis of an idiopathic interstitial pneumonia or chronic hypersensitivity pneumonitis were included16,17, whereas those with sarcoidosis or a connective tissue disease were excluded, as were those with co-existent acute and/or chronic pulmonary thromboembolism detected on CTPA. This study had institutional review board approval (Royal Brompton, Harefield reference 2016PH002B).
Right heart catheterisation
RHC was performed using standard techniques2 with haemodynamic measurements obtained at rest. PH was defined as a mean pulmonary arterial pressure (mPAP) ≥25mmHg. Cardiac output (CO) was measured using the indirect Fick method with oxygen consumption estimated using the LaFarge equation. Pulmonary vascular resistance (PVR) was calculated as PVR= (mPAP–pulmonary capillary wedge pressure)/CO.
CTPA acquisition
CT was performed at full inspiration. A continuous scale of ILD severity was produced using volumetric HRCT images scored independently by an experienced radiologist (JJ). Lobar extents of reticulation or honeycombing were scored to the nearest 5% to create a lobar fibrosis score. Lobar scores of fibrosis were summed and divided by 6 to create an overall fibrosis score per patient18. All CTPA examinations were performed at the discretion of the PH team at the time of the PH assessment. Intravenous administration of contrast medium was performed with standard intravenous access, using automated administrator injection equipment. Bolus tracking was used to trigger the start of the acquisition of images. Electrocardiogram gating of image acquisition was not performed. We analysed the RV:LV ratio using a threshold of 1.0 to define RV dilatation.
CTPA measurements
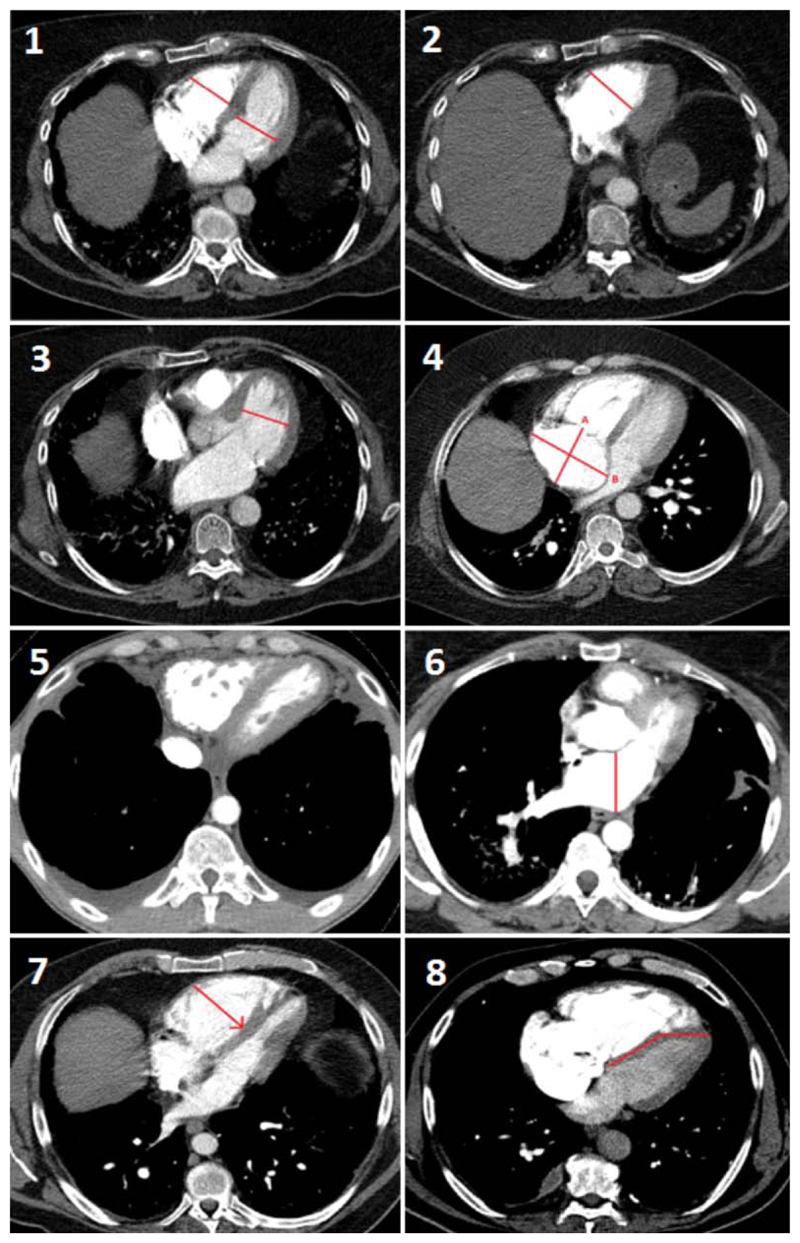
All measurements were performed on standard axial imaging. All scans were anonymised, and the reviewer blinded to all clinical and haemodynamic data (SB). The following measurements (for full details of measurements see Figure 1 and supplementary material) were performed:
The main pulmonary artery (MPA) and aortic (Ao) diameter were measured and the MPA:Ao ratio calculated19.
The RV was said to be “larger” or “smaller” than the LV using a subjective evaluation of RV and LV size where no measurements were performed, and the reviewer could evaluate the entire scan20.
RV and LV diameter were measured at their widest point21 at the mid-ventricular level, (Figure 1, panel 1), on the same CT axial image, and the RV:LV ratio calculated (RV:LVaxial).
The RV and LV diameters were also measured at their widest point22 at mid ventricular level (Figure 1, panel 2 and 3), and the RV:LV ratio calculated (RV:LVlargest).
The right atrium (RA) diameter was measured (Figure 1, panel 4) in the longitudinal plane (RAlongitudinal) and in the transverse plane (RAtransverse).
Reflux of contrast media into the inferior vena cava was scored as absent or present (Figure 1, panel 5).
The left atrium (LA) diameter was measured (Figure 1, panel 6).
Ventricular septal bowing was scored as present or absent (Figure 1, panel 7 and 8).
Figure 1. CTPA measurements performed.
(1) The largest diameter of the right ventricle (RV) and left ventricle (LV) were measured at the mid-ventricular level at the level which most closely resembled a four-chamber view (and the RV:LV ratio calculated RV:LVaxial), the largest RV diameter (2) and LV diameter (3) were measured at the mid-ventricular level where it was largest (i.e. on different axial CT slices), and the RV:LV ratio calculated RV:LVlargest. The right atrium (RA) was measured (4) on both the longitudinal (A, delineated as the posterior border of the RA to the tricuspid annulus), and transverse planes (B, the widest point between RA walls). Reflux of contrast was graded as 0 where no reflux into the IVC was seen, or 1 where reflux into the IVC was present (5). The left atrium was measured (6) from its posterior to anterior border. The septum was said to be “bowed” if either it was deviated into the LV (7), or if the interventricular septum was deviated from its normal orientation (8).
Echocardiography
Images were acquired using a 3MHz frequency harmonic phased-array transducer. Doppler echocardiography was performed as per the American Society of Echocardiography recommendations23,24. The 2D-echo datasets were interpreted by a cardiologist with advanced echocardiography training.
Statistical analysis
All statistical analyses were performed using R version 3.3.1 (R Foundation for Statistical Computing). Data were summarised as number (percentage) for categorical variables and mean±SD or median [interquartile range] for continuous variables as appropriate. Continuous variables were compared using t-test, Wilcoxon Rank-Sum test and categorical data was compared with Chi-squared test. Continuous measurements were compared using Bland and Altman analysis. Survival analysis was performed using Cox-proportional hazard modelling, with the date of the CTPA as the start of follow up and patients followed over 5 years. Kaplan Meier plots were used to estimate outcome. Receiver operating characteristic analysis was performed to evaluate the ability of the RV:LV ratio to detect PH. The primary end-point was death or lung transplantation, and all other patients were censored at the last date of clinical contact. Backwards selection of variables in multivariate models was used, including severity of fibrosis measured at CT, ILD subtype (IPF versus non-IPF), and the RV:LVlargest ratio.
Results
Patient demographics
92 patients were included in the study, with a mean age of 62±11 years; 64% male. The time between RHC and CTPA was 0.1±1.1 months. Most patients had IPF (n=58, 63%, Table 1), with FVC 57±20% predicted, TLCOc 22±8% predicted and KCOc 51±17% predicted. Pulmonary hypertension (mPAP≥25mmHg) was confirmed at RHC in 72/92 (78%) patients; 31/92 (34%) had severe PH (mPAP≥35mmHg) (Table 2).
Table 1. Interstitial lung disease diagnoses.
| ILD diagnosis | CTPA Cohort (n=92) |
|---|---|
| Idiopathic pulmonary fibrosis | 58 |
| Chronic hypersensitivity pneumonitis | 13 |
| Idiopathic non-specific interstitial pneumonitis | 13 |
| Smoking related ILD | 3 |
| Unclassifiable ILD | 3 |
| Fibrotic cryptogenic organising pneumonia | 1 |
| Pleuro-parenchymal fibroelastosis | 1 |
Abbreviations: ILD Interstitial lung disease
Table 2. Invasive and non-invasive variables stratified by RV:LVlargest ratio.
Patients were stratified by the RV:LVlargest ratio of 1.0.
| Entire Cohort (n=92) | RV:LV <1.0 (n=13) | RV:LV ≥1.0 (n=79) | p value | |
|---|---|---|---|---|
| Age | 65±11 | 63±8 | 66±12 | 0.6 |
| Male gender n (%) | 59 (64) | 6 (46) | 53 (67) | 0.2 |
| Functional class, (II/III/IV) (%) | (2/80/18) | (0/85/15) | (3/78/19) | 0.8 |
| Long term oxygen therapy n (%) | 77 (83%) | 11 (85) | 66 (83) | 0.9 |
| Treatment with PH therapies, n (%) | 40 (43) | 3 (23) | 37 (47) | 0.2 |
| Pulmonary function tests | ||||
| FEV1 (% predicted) | 58±18 | 51±17 | 59±18 | 0.1 |
| FVC (% predicted) | 57±20 | 52±18 | 58±20 | 0.3 |
| TLcoc (% predicted) | 22±8 | 29±8 | 21±7 | 0.01 |
| Kcoc (% predicted) | 51±17 | 67±17 | 48±16 | 0.003 |
| Composite physiological Index | 66±9 | 62±9 | 66±9 | 0.1 |
| Echocardiography | ||||
| Tricuspid regurgitant velocity (m/s) | 3.79±0.6 | 3.57±0.4 | 3.82±0.6 | 0.1 |
| RVSP (mmHg) | 67±19 | 59±11 | 68±20 | 0.04 |
| Right atrial area (cm2) | 20±7 | 15±6 | 20±7 | 0.03 |
| Pulmonary acceleration time (ms) | 76±17 | 81±20 | 75±16 | 0.3 |
| RV:LVecho | 0.77[0.6-1.1] | 0.58[0.5-0.6] | 0.8[0.6-1.0] | 0.01 |
| TAPSE (mm) | 1.8±0.5 | 1.9±0.5 | 1.8±0.5 | 0.5 |
| RV Fractional area change (%) | 37±8 | 37±6 | 37±8 | 0.9 |
| BNP (ng/L) | 82[42-270] | 48[29-84] | 90[44-355] | 0.03 |
| Right heart catheter haemodynamics | ||||
| mPAP (mmHg) | 31±9 | 28±7 | 32±9 | 0.06 |
| mPAP ≥25mmHg, n (%) | 72 (78) | 9 (69) | 63 (80) | 0.6 |
| PVR (Wood units) | 5.5±3.2 | 3.9±2.0 | 5.8±3.3 | 0.01 |
| Cardiac Output (litres/minute) | 4.4±1.3 | 4.3±0.9 | 4.4±1.3 | 0.7 |
| CT Variables | ||||
| Fibrosis score (%) | 46±14 | 43±12 | 46±14 | 0.3 |
| Main pulmonary artery diameter (mm) | 34±5 | 30±5 | 35±4 | 0.01 |
| MPADiameter:Aorta ratio | 1.1[0.9-1.2] | 1.0[0.9-1.1] | 1.1[1.0-1.2] | 0.2 |
| RAlongitudinal diameter (mm) | 50±9 | 45±9 | 50±9 | 0.05 |
| RAtransverse diameter (mm) | 60±12 | 49±6 | 62±12 | <0.001 |
| LA diameter (mm) | 38±9 | 38±10 | 38±9 | 0.9 |
| Ventricular septal bowing n (%) | 35 (38) | 0 (0) | 35 (44) | 0.002 |
| IVC diameter (mm) | 26±6 | 21±6 | 27±6 | 0.02 |
| IVC reflux, n (%) | 62 (67) | 7 (54) | 55 (69) | 0.3 |
Abbreviations: PH pulmonary hypertension, FEV1 Forced expiratory volume in one second, FVC Forced vital capacity, TLCOc corrected transfer factor, KCOc corrected transfer coefficient, RVSP Right ventricular systolic pressure, RV Right ventricle, LV Left ventricle, TAPSE Transannular plane systolic excursion, BNP Brain natriuretic peptide, mPAP mean pulmonary pressure at right heart catheterisation, PVR pulmonary vascular resistance, ILD, Interstitial lung disease, MPA Main pulmonary artery, RA Right atrium, LA Left atrium, IVC Inferior vena cava.
Data are mean±standard deviation or median [interquartile range].
CTPA measured RV:LV measurements
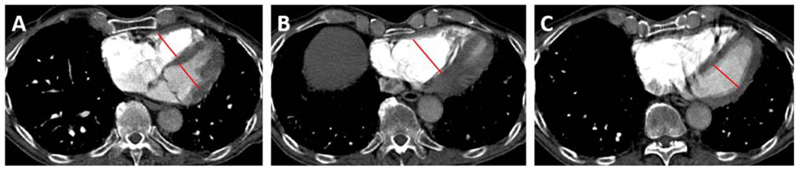
The mean RVaxial diameter was 45.3±9.0mm, and mean RVlargest diameter was 52.1±8.7mm. The RV was deemed to be subjectively larger than the LV in n=71 (77%) patients. The RV:LVlargest method produced larger RV (52±0.8mm versus 45±0.9mm, p<0.001) and LV values (35±0.8mm versus 39±0.7mm, p<0.001) compared to the RV:LVaxial method (Figure 2). Bland-Altman analysis showed that the mean RVlargest diameter was 7.0 [5.8-8.1mm] larger than the RVaxial method. The RV:LVaxial ratio (1.38±0.5) did not differ when compared to the RV:LVlargest ratio (1.39±0.4, p=0.9, although, RV enlargement frequently occurred inferiorly and was missed by the RV:LVaxial method (figure 2). Use of the RV:LVlargest method resulted in the reclassification of 6 (32%) patients with a RV:LVaxial ratio of <1.0 into the ≥1.0 category.
Figure 2. Comparison of RV:LV measurement methodologies.
Panel A shows the RV:LVaxial measurements. Panel B shows the same patient with the RV measured at the mid-ventricular level at its widest point. Panel C shows the same patient with the LV measured at the mid-ventricular level at its widest point. In our cohort the use of the RV:LVlargest method resulted in the reclassification of n=7 (37%) of patients with a RV:LVaxial ratio of <1.0 into the ≥1.0 category.
Use of RV:LV ratio at CT to predict the presence of PH
RV:LVaxial ratio predicted the presence of PH with an area under the curve (AUC) of 69.4%. An RVaxial >1.0 identified PH with a sensitivity of 83.9%, and specificity of 50.0%. The AUC of RV:LVlargest ratio for predicting the presence of PH was 59.3%. An RV:LVlargest >1.0 identified PH with a sensitivity of 90.4%, and specificity of 34.8%.
Comparison of patients stratified by the RV:LVIargest ratio
PH was present in n=9 (69%) patients with an RV:LVlargest <1.0, and in n=63 (80%) of patients with an RV:LVlargest ≥1.0. PVR was significantly higher in patients with an RV:LVlargest ≥1.0 (5.8±3.3 versus 3.9±2.0 Wood units, p=0.01, Table 2). Patients with an RV:LVlargest ≥1.0 had a larger MPA diameter (p=0.01), larger transverse (p<0.001) and longitudinal RA (p=0.05) diameter measured at CT, larger RA area measured at echocardiogram (p=0.03), higher RVSP (p=0.04), higher BNP (p=0.03) and larger inferior vena cava diameter (p=0.02). Ventricular septal bowing only occurred when the RV:LVlargest ratio was >1.0. Spirometry was not different between groups, although measures of gas transfer and gas transfer co-efficient were lower in patients with an RV:LVlargest ≥1.0 (p=0.01 for TLcoc and p=0.003 for Kcoc) (Table 2). The fibrosis score measured at CT was not different between groups (p=0.3).
Univariate and Multivariate predictors of mortality
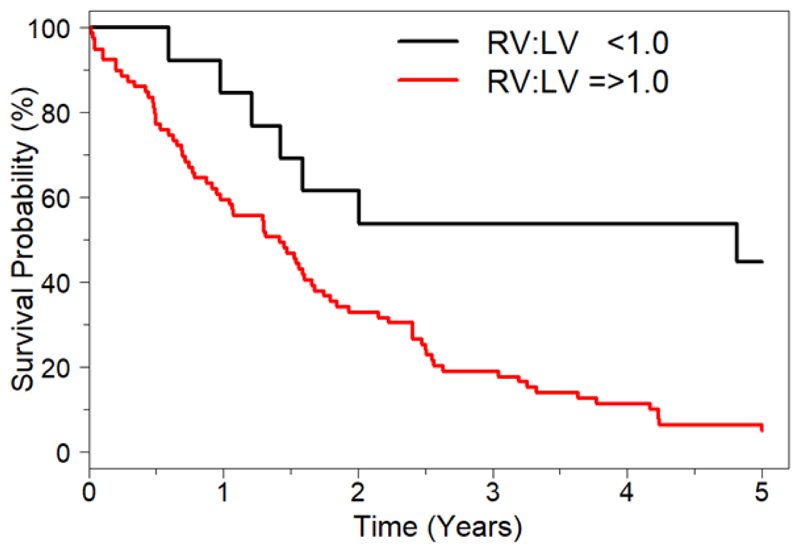
Median follow up was 18.8 months [8.3 – 31.6 months]; 74 patients died (80.4%) and 6 (6.5%) underwent transplantation over the 5-year follow up period. Univariate Cox regression analysis results are shown in Table 3. At univariate assessment, the following were associated with an increased risk of mortality: a diagnosis of IPF (hazard ratio (HR) 1.94, 95% confidence interval (CI) 1.21-3.10, p=0.006); subjectively larger RV than LV at CT (HR: 2.08, CI: 1.16-3.74, p=0.01); an RV:LVaxial ≥1.0 (HR: 2.17, CI:1.19-3.97,p=0.01) and an RV:LVlargest ratio ≥1.0 (HR: 3.26, CI: 1.49-7.15, p=0.003) (Figure 3). Fibrosis score measured at CT was associated with mortality (HR: 1.37, CI: 1.15-1.63, p<0.001) per 10% increase in fibrosis score. Neither haemodynamic or echocardiographic variables predicted mortality at univariate assessment. Neither MPA diameter nor MPA:Ao diameter predicted mortality (Table 3).
Table 3. Univariate assessment of invasive and non-invasive variables.
| Hazard ratio (95% CI) | Univariate p-value | |||
|---|---|---|---|---|
| Age | 1.01 (0.98-1.03) | 0.6 | ||
| Composite physiological index | 1.06 (1.03-1.09) | <0.001 | ||
| Male gender | 1.51 (0.95-2.38) | 0.08 | ||
| Diagnosis of Idiopathic pulmonary fibrosis | 1.94 (1.21-3.10) | 0.006 | ||
| Fibrosis score (Increase by 10%) | 1.37 (1.15-1.63) | <0.001 | ||
| Vasodilator treatment | 0.62 (0.40-0.97) | 0.04 | ||
| Right heart catheter haemodynamics | ||||
| Mean pulmonary artery pressure | 1.00 (0.98-1.03) | 0.7 | ||
| Mean pulmonary artery pressure ≥25mmHg | 1.15 (0.68-1.97) | 0.6 | ||
| Pulmonary vascular resistance | 1.03 (0.96-1.10) | 0.4 | ||
| Cardiac Output | 0.88 (0.73-1.01) | 0.2 | ||
| Echocardiography | ||||
| Right ventricular systolic pressure | 1.01 (0.99-1.01) | 0.3 | ||
| Right atrial area | 1.00 (0.97-1.04) | 0.8 | ||
| Pulmonary acceleration time | 1.00 (0.99-1.02) | 0.7 | ||
| RV:LVecho | 1.07 (0.71-1.60) | 0.7 | ||
| Transannular plane systolic excursion | 0.97 (0.93-1.02) | 0.3 | ||
| RV Fractional area change | 1.01 (0.98-1.04) | 0.5 | ||
| CT Pulmonary Angiography | ||||
| MPA diameter (per 1mm increase) | 1.01 (0.96-1.06) | 0.7 | ||
| MPA diameter (>32mm) | 1.50 (0.93-2.43) | 0.1 | ||
| MPADiameter:Aorta ratio | 0.97 (0.84-1.13) | 0.7 | ||
| RV subjectively larger than LV | 2.08 (1.16-3.74) | 0.01 | ||
| RV:LVaxial ratio | 1.04 (0.99-1.07) | 0.1 | ||
| RV:LVaxial ratio ≥ 1.0 | 2.17 (1.19-3.97) | 0.01 | ||
| RV:LVlargest ratio | 1.06 (1.01-1.11) | 0.02 | ||
| RV:LVlargest ratio ≥ 1.0 | 3.26 (1.49-7.15) | 0.003 | ||
| Ventricular septal bowing | 1.30 (0.81-1.96) | 0.3 | ||
| RAtransverse | 1.01 (0.99-1.03) | 0.3 | ||
| RAlongitudinal | 1.02 (0.99-1.05) | 0.09 | ||
Abbreviations: As per table 2.
Figure 3. Kaplan Meier survival estimates of patients with ILD stratified by right ventricle to left ventricle ratio (RV:LV) using the RV:LVlargest method RV:LV<1.0 (n=13), RV:LV≥1.0 (n=80).
An RV:LVlargest ≥1.0 was an adverse predictor of mortality. Hazard ratio=3.26 (CI 1.49-7.15) p=0.003.
Abbreviations: RV:LV right ventricle to left ventricle ratio
At multivariate analysis, after adjustment for the fibrosis score at HRCT and a diagnosis of IPF, an RV:LVlargest ≥1.0 remained an independent predictor of mortality / lung transplant (HR: 3.19, CI: 1.44-7.10, p=0.004) (Table 4).
Table 4. Multivariate adjustment of the RV:LV ratio.
| Hazard ratio | Confidence interval | P value | |
|---|---|---|---|
| Fibrosis score at CT (per 10% increase) | 1.32 | 1.11-1.56 | 0.004‡ |
| IPF diagnosis | 1.91 | 1.17-3.14 | 0.001‡ |
| RV:LVlargest ratio >1.0 | 3.19 | 1.44-7.10 | 0.004‡ |
Abbreviations: IPF idiopathic pulmonary fibrosis, RV Right ventricle, LV Left ventricle.
Remained independent after adjustment for PH treatment status. ≠
Discussion
This study demonstrates that CTPA is a useful method of risk stratification in patients with ILD who are suspected of having PH. The RV:LVlargest ratio was superior to invasive haemodynamics and echocardiography in terms of predicting outcome. An RV:LVlargest ratio ≥1.0 (HR: 3.26, CI: 1.49-7.15, p=0.003) was strongly associated with mortality, as well as higher PVR, and remained an independent predictor of mortality, after adjusting for ILD severity and a diagnosis of IPF. The RV:LV ratio had poor specificity in detecting PH at RHC however, suggesting that the RV:LV ratio at CT cannot be relied upon to exclude PH.
In our cohort, a high proportion of patients had PH (78%). Of 80 patients with an RV:LVlargest ≥1.0, 16 (20%) had borderline PH (mPAP of 21[16-23] mmHg, and PVR 2.8[1.8-3.1] Wood units). This may relate to the impact of exercise on the RV in these patients, or the time taken to develop PH and the relative compliance of the pulmonary circulation. PH can progress rapidly in IPF patients awaiting transplant: of 44 patients included in the study by Nathan et al., 38.6% had PH at initial transplant workup RHC, which rapidly increased to 86.4% at the time of transplantation25. The factors leading to the development of PH in patients with ILD remain poorly understood and include fibrosis-induced destruction of pulmonary vessels, and excessive pulmonary vascular remodelling. It is likely that patients with borderline haemodynamics progress and develop PH, in part related to acute exacerbations, which are more common in those awaiting lung transplant26. A sub-group of patients seem to develop RV dilatation even without PH at RHC, and are at an increased risk of mortality, hence haemodynamic assessment following exercise is likely to be an important future component of assessment27. It seems probable in ILD patients that RV diameter and the MPAD serve somewhat as barometers for current and prior disease trends being influenced by progressive interstitial lung disease (be it either slow progression or dramatic deterioration as occurs during acute exacerbations), pulmonary vascular remodelling, and hypoxia. An RV:LV>1 may be seen as a tipping point in favour of worse prognosis. The only other study in patients with PH, evaluating the prognostic role of RV/LV ratio at CTPA, is in patients with chronic thromboembolic PH and supports our findings28.
Previous studies have differed in their findings that haemodynamics predict outcome in ILD-PH. For example, studies that evaluated PH at initial IPF diagnosis using RHC (when the prevalence of PH = 8.1%) found that the best mPAP threshold to predict outcome was 17mmHg29. Another study again in early stage IPF (PH prevalence = 14.9%) found that a mPAP of 20mmHg was the best threshold to predict mortality30. In contrast, in 135 patients with IPF undergoing lung transplant evaluation (PH prevalence = 29%), mPAP did not predict mortality, however PVR did4. Similarly, in a mixed ILD cohort of 66 patients with a high prevalence of PH (75.7%), mPAP did not predict mortality, however PVR predicted short-term mortality31. Another factor to consider is pulmonary vasodilator treatment (HR:0.62, CI:0.40-0.97, p=0.04) which suggests a beneficial effect in our cohort. Although, the decision to treat was closely linked to ILD subtype and PH severity and strongly limits inference of vasodilator benefits.
The finding that RV:LV ratio measured at CTPA predicted mortality, whereas haemodynamic assessment did not, challenges previously held beliefs regarding the diagnosis of ILD-PH and risk stratification. Perhaps it is time to re-evaluate whether RHC is the best way to predict risk in ILD-PH, and whether the standard definition of PH (mPAP ≥25mmHg) should be used which disregards patients with borderline PH in whom important changes in RV morphology may occur. Indeed, a positive treatment effect with sildenafil has been suggested in IPF patients with right ventricular dysfunction on echocardiography32, reinforcing the importance of RV assessment in this setting.
Limitations
The studies retrospective design leads to selection bias. All patients studied had a high pretest probability of PH or were being assessed for lung transplantation. This bias may overestimate the prevalence of RV dilatation in ILD but is unlikely to have influenced its relation to outcome. CTPA was performed at PH assessment and therefore did not factor in the decision to refer to PH services. In addition, the lack of electrocardiographic gating at CTPA acquisition may reduce the accuracy of RV:LV measurement but makes the findings of this study reproducible in everyday clinical practice. Finally, it was not possible to adjust for treatment of the underlying ILD or the use of advanced pulmonary vasodilator therapies in this analysis due to the heterogeneity of the treatment regimens.
We used an “ILD fibrosis score” to record the extent of disease. However, our results were unchanged if we substituted FVC or CPI as measures of disease severity (supplementary material). The ILD fibrosis score was not different between patients with dilated and non-dilated RV at CTPA, which replicates previous study findings suggesting that ILD severity is not the sole cause of PH / RV dilatation in this group of patients33,34.
Conclusion
The RV:LV ratio measured at CTPA is a useful non-invasive screening tool to identify high risk patients with suspected ILD-PH including the impact of borderline PH on the RV. It is a strong prognostic marker in this population and is superior to invasive haemodynamic assessment.
Acknowledgments
SB performed all CTPA measurements and had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
JJ scored ILD severity and contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
CB undertook echocardiographic measurements and contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
KD, AK, MK, GK, ER, PM, FC, VK, PG, CM, MW, AD and AW contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
SW and LP (joint final authors) had full access to all the data and contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript
Abbreviations list
- Ao
Aorta
- AUC
Area under the curve
- BNP
Brain natriuretic peptide
- CI
Confidence interval
- CO
Cardiac output
- CT
Computerised tomography
- CTPA
Computerised tomography pulmonary angiogram
- FVC
Forced vital capacity
- HR
Hazard ratio
- HRCT
High resolution computerised tomography
- ILD
Interstitial lung disease
- IPF
Idiopathic pulmonary fibrosis
- ILD-PH
Interstitial lung disease associated pulmonary hypertension
- KCOc
Gas transfer co-efficient
- LA
Left atrium
- LV
Left ventricle
- MPA
Main pulmonary artery
- MPA:Ao
Main pulmonary artery to Aorta ratio
- mPAP
Mean pulmonary arterial pressure
- PH
Pulmonary Hypertension
- PVR
Pulmonary vascular resistance
- RHC
Right heart catheter
- RA
Right atrium
- RV
Right ventricle
- RVSP
Right ventricular systolic pressure
- TLCOc
Gas transfer
- RV:LV
Right ventricle to left ventricle ratio
Footnotes
Conflict of Interest
Simon Bax - No conflict of interest
Joseph Jacob – reports receiving personal fees from Boehringer Ingelheim and Roche, outside the submitted work, and was supported by a Wellcome Trust Clinical Research Career Development Fellowship (209553/Z/17/Z).
Riaz Ahmed - No conflict of interest
Charlene Bredy - No conflict of interest
Konstantinos Dimopoulos - reports receiving unrestricted educational grants and has acted as a consultant for Actelion, GSK, Pfizer and Bayer, outside the submitted work.
Aleksander Kempny - reports receiving grants from Actelion Global, outside the submitted work.
Maria Kokosi - No conflict of interest
Gregory Kier - No conflict of interest
Elisabetta Renzoni - reports personal fees from Roche, Boehringer Ingelheim outside the submitted work.
Philip L Molyneaux - reports personal fees from Roche and Boehringer Ingelheim, outside the submitted work.
Felix Chua - reports personal fees from Roche and Boehringer Ingelheim, outside the submitted work.
Vasilis Kouranos - No conflict of interest
Peter George - reports personal fees from Roche, Boehringer Ingelheim and Teva outside the submitted work.
Colm McCabe - No conflict of interest
Michael Wilde - No conflict of interest
Anand Devaraj - reports personal fees from Roche, GSK and Boehringer Ingelheim, outside the submitted work.
Athol Wells - reports receiving personal fees from Intermune, Boehringer Ingelheim, Gilead, MSD, Roche, Bayer and Chiesi, outside the submitted work.
S John Wort - reports receiving grants from Actelion, GSK, Pfizer and Bayer, outside the submitted work.
Laura Price - reports educational grants from Actelion and GSK, during the conduct of the study.
Contributor Information
Joseph Jacob, Email: j.jacob@ucl.ac.uk.
Riaz Ahmed, Email: Riazeahmed@nhs.net.
Charlene Bredy, Email: Charlenebredy@hotmail.com.
Konstantinos Dimopoulos, Email: k.dimopoulos02@gmail.com.
Aleksander Kempny, Email: A.Kempny@rbht.nhs.uk.
Maria Kokosi, Email: M. Kokosi@rbht.nhs.uk.
Gregory Kier, Email: Gregory.Keir@health.qld.gov.au.
Elisabetta Renzoni, Email: E.Renzoni@rbht.nhs.uk.
Philip L Molyneaux, Email: p.molyneaux@imperial.ac.uk.
Felix Chua, Email: F.Chua@rbht.nhs.uk.
Vasilis Kouranos, Email: V.Kouranos@rbht.nhs.uk.
Peter George, Email: P.George@rbht.nhs.uk.
Colm McCabe, Email: C.McCabe2@rbht.nhs.uk.
Michael Wilde, Email: Michael.Wilde@SASH.nhs.uk.
Anand Devaraj, Email: A. Devaraj@rbht.nhs.uk.
Athol Wells, Email: RBHILD@rbht.nhs.uk.
S John Wort, Email: S.Wort@rbht.nhs.uk.
Laura C Price, Email: Laura.Price@rbht.nhs.uk.
References
- 1.Lettieri CJ, Nathan SD, Barnett SD, Ahmad S, Shorr AF. Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest. 2006;129(3):746–752. doi: 10.1378/chest.129.3.746. [DOI] [PubMed] [Google Scholar]
- 2.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37(1):67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 3.Arcasoy SM, Christie JD, Ferrari VA, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med. 2003;167(5):735–740. doi: 10.1164/rccm.200210-1130OC. [DOI] [PubMed] [Google Scholar]
- 4.Rivera-Lebron BN, Forfia PR, Kreider M, Lee JC, Holmes JH, Kawut SM. Echocardiographic and hemodynamic predictors of mortality in idiopathic pulmonary fibrosis. Chest. 2013;144(2):564–570. doi: 10.1378/chest.12-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nathan SD, Shlobin OA, Barnett SD, et al. Right ventricular systolic pressure by echocardiography as a predictor of pulmonary hypertension in idiopathic pulmonary fibrosis. Respir Med. 2008;102(9):1305–1310. doi: 10.1016/j.rmed.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amsallem M, Boulate D, Kooreman Z, et al. Investigating the value of right heart echocardiographic metrics for detection of pulmonary hypertension in patients with advanced lung disease. Int J Cardiovasc Imaging. 2017 doi: 10.1007/s10554-017-1069-3. [DOI] [PubMed] [Google Scholar]
- 7.Bax S, Bredy C, Kempny A, et al. A stepwise composite echocardiographic score predicts severe pulmonary hypertension in patients with interstitial lung disease. ERJ Open Res. 2018;4(2) doi: 10.1183/23120541.00124-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen Y, Wan C, Tian P, et al. CT-base pulmonary artery measurement in the detection of pulmonary hypertension: a meta-analysis and systematic review. Medicine. 2014;93(27):e256. doi: 10.1097/MD.0000000000000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alhamad EH, Al-Boukai AA, Al-Kassimi FA, et al. Prediction of pulmonary hypertension in patients with or without interstitial lung disease: reliability of CT findings. Radiology. 2011;260(3):875–883. doi: 10.1148/radiol.11103532. [DOI] [PubMed] [Google Scholar]
- 10.Devaraj A, Wells AU, Meister MG, Corte TJ, Hansell DM. The effect of diffuse pulmonary fibrosis on the reliability of CT signs of pulmonary hypertension. Radiology. 2008;249(3):1042–1049. doi: 10.1148/radiol.2492080269. [DOI] [PubMed] [Google Scholar]
- 11.Chin M, Johns C, Currie BJ, et al. Pulmonary Artery Size in Interstitial Lung Disease and Pulmonary Hypertension: Association with Interstitial Lung Disease Severity and Diagnostic Utility. Front Cardiovasc Med. 2018;5:53. doi: 10.3389/fcvm.2018.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin S, King CS, Puri N, et al. Pulmonary artery size as a predictor of outcomes in idiopathic pulmonary fibrosis. Eur Respir J. 2016;47(5):1445–1451. doi: 10.1183/13993003.01532-2015. [DOI] [PubMed] [Google Scholar]
- 13.Spruijt OA, Bogaard HJ, Heijmans MW, et al. Predicting pulmonary hypertension with standard computed tomography pulmonary angiography. Int J Cardiovasc Imaging. 2015;31(4):871–879. doi: 10.1007/s10554-015-0618-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan AL, Juarez MM, Shelton DK, et al. Novel computed tomographic chest metrics to detect pulmonary hypertension. BMC Med Imaging. 2011;11:7. doi: 10.1186/1471-2342-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becattini C, Agnelli G, Germini F, Vedovati MC. Computed tomography to assess risk of death in acute pulmonary embolism: a meta-analysis. Eur Respir J. 2014;43(6):1678–1690. doi: 10.1183/09031936.00147813. [DOI] [PubMed] [Google Scholar]
- 16.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacob J, Bartholmai BJ, Rajagopalan S, et al. Mortality prediction in idiopathic pulmonary fibrosis: evaluation of computer-based CT analysis with conventional severity measures. Eur Respir J. 2017;49(1) doi: 10.1183/13993003.01011-2016. [DOI] [PubMed] [Google Scholar]
- 19.Devaraj AWA, Meister MG, Corte TJ, Wort SJ, Hansell DM. Detection of pulmonary hypertension with multidetector CT and echocardiography alone and in combination. Radiology. 2010;254(2):609–616. doi: 10.1148/radiol.09090548. [DOI] [PubMed] [Google Scholar]
- 20.Kumamaru KK, Hunsaker AR, Bedayat A, et al. Subjective assessment of right ventricle enlargement from computed tomography pulmonary angiography images. The International Journal of Cardiovascular Imaging. 2012;28(4):965–973. doi: 10.1007/s10554-011-9903-5. [DOI] [PubMed] [Google Scholar]
- 21.Contractor S, Maldjian PD, Sharma VK, Gor DM. Role of helical CT in detecting right ventricular dysfunction secondary to acute pulmonary embolism. Journal of computer assisted tomography. 2002;26(4):587–591. doi: 10.1097/00004728-200207000-00020. [DOI] [PubMed] [Google Scholar]
- 22.Reid JH, Murchison JT. Acute right ventricular dilatation: a new helical CT sign of massive pulmonary embolism. Clinical radiology. 1998;53(9):694–698. doi: 10.1016/s0009-9260(98)80297-3. [DOI] [PubMed] [Google Scholar]
- 23.Douglas PS, DeCara JM, Devereux RB, et al. Echocardiographic imaging in clinical trials: American Society of Echocardiography Standards for echocardiography core laboratories: endorsed by the American College of Cardiology Foundation. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2009;22(7):755–765. doi: 10.1016/j.echo.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 24.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2010;23(7):685–713. doi: 10.1016/j.echo.2010.05.010. quiz 786-688. [DOI] [PubMed] [Google Scholar]
- 25.Nathan SD, Shlobin OA, Ahmad S, et al. Serial development of pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Respiration; international review of thoracic diseases. 2008;76(3):288–294. doi: 10.1159/000114246. [DOI] [PubMed] [Google Scholar]
- 26.Judge EP, Fabre A, Adamali HI, Egan JJ. Acute exacerbations and pulmonary hypertension in advanced idiopathic pulmonary fibrosis. Eur Respir J. 2012;40(1):93–100. doi: 10.1183/09031936.00115511. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong HF, Schulze PC, Bacchetta M, Thirapatarapong W, Bartels MN. Impact of pulmonary hypertension on exercise performance in patients with interstitial lung disease undergoing evaluation for lung transplantation. Respirology. 2014;19(5):675–682. doi: 10.1111/resp.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ema R, Sugiura T, Kawata N, et al. The dilatation of main pulmonary artery and right ventricle observed by enhanced chest computed tomography predict poor outcome in inoperable chronic thromboembolic pulmonary hypertension. Eur J Radiol. 2017;94:70–77. doi: 10.1016/j.ejrad.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Hamada K, Nagai S, Tanaka S, et al. Significance of pulmonary arterial pressure and diffusion capacity of the lung as prognosticator in patients with idiopathic pulmonary fibrosis. Chest. 2007;131(3):650–656. doi: 10.1378/chest.06-1466. [DOI] [PubMed] [Google Scholar]
- 30.Kimura M, Taniguchi H, Kondoh Y, et al. Pulmonary hypertension as a prognostic indicator at the initial evaluation in idiopathic pulmonary fibrosis. Respiration; international review of thoracic diseases. 2013;85(6):456–463. doi: 10.1159/000345221. [DOI] [PubMed] [Google Scholar]
- 31.Corte TJ, Wort SJ, Gatzoulis MA, Macdonald P, Hansell DM, Wells AU. Pulmonary vascular resistance predicts early mortality in patients with diffuse fibrotic lung disease and suspected pulmonary hypertension. Thorax. 2009;64(10):883–888. doi: 10.1136/thx.2008.112847. [DOI] [PubMed] [Google Scholar]
- 32.Han MK, Bach DS, Hagan PG, et al. Sildenafil preserves exercise capacity in patients with idiopathic pulmonary fibrosis and right-sided ventricular dysfunction. Chest. 2013;143(6):1699–1708. doi: 10.1378/chest.12-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zisman DA, Karlamangla AS, Ross DJ, et al. High-Resolution Chest Computed Tomography Findings Do Not Predict The Presence of Pulmonary Hypertension in Advanced Idiopathic Pulmonary Fibrosis. Chest. 2007;132(3):773–779. doi: 10.1378/chest.07-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fischer A, Swigris JJ, Bolster MB, et al. Pulmonary hypertension and interstitial lung disease within PHAROS: impact of extent of fibrosis and pulmonary physiology on cardiac haemodynamic parameters. Clinical and experimental rheumatology. 2014;32(6 Suppl 86):S-109-114. [PubMed] [Google Scholar]