Abstract
Background
The presence of emphysema is relatively common in patients with fibrotic interstitial lung disease. This has been designated combined pulmonary fibrosis and emphysema (CPFE). The lack of consensus over definitions and diagnostic criteria has limited CPFE research.
Goals
The objectives of this taskforce were to review the terminology, definition, characteristics, pathophysiology, and research priorities of CPFE, and to explore whether CPFE is a syndrome.
Methods
This research statement was developed by a committee including 19 pulmonologists, 5 radiologists, 3 pathologists, 2 methodologists, and 2 patient representatives. The final document was supported by a focused systematic review that identified and summarized all recent publications related to CPFE.
Results
This taskforce identified that patients with CPFE are predominantly male, with history of smoking, severe dyspnea, relatively preserved airflow rates and lung volumes on spirometry, severely impaired diffusion capacity for carbon monoxide, exertional hypoxemia, frequent pulmonary hypertension, and a dismal prognosis. The committee proposes to identify CPFE as a syndrome given the clustering of pulmonary fibrosis and emphysema, shared pathogenetic pathways, unique considerations related to disease progression, increased risk of complications (pulmonary hypertension, lung cancer, mortality), and implications for clinical trial design. There are varying features of interstitial lung disease and emphysema in CPFE. The committee offers a research definition and classification criteria, and proposes that studies on CPFE include a comprehensive description of radiologic and, when available, pathological patterns including some recently described patterns such as smoking-related interstitial fibrosis.
Conclusions
This statement delineates the syndrome of CPFE and highlights research priorities.
Subject category list: 9.23 Interstitial Lung Disease
Keywords: Fibrosis, interstitial lung diseases, emphysema, diagnosis, management
Introduction
Emphysema is relatively common in patients with fibrotic interstitial lung disease (fILD), including idiopathic pulmonary fibrosis (IPF), and is designated “combined pulmonary fibrosis and emphysema” (CPFE)1,2. Despite its clinical significance and a number of published series 3, CPFE remains poorly understood. Imaging features of CPFE vary in both fILD and emphysema, and not all cases correspond to IPF with emphysema. Similarly, the spectrum of pathologic features includes recently described patterns such as airspace enlargement with fibrosis (AEF)4 and smoking-related interstitial fibrosis (SRIF)5. Lack of consensus on criteria for CPFE has limited our ability to compare cohorts and draw consistent conclusions about the features, outcomes, and optimal management of these patients 3. No consensus exists on whether CPFE is a syndrome (i.e. a cluster of clinical and radiologic manifestations with clinically relevant implications and/or major pathogenetic significance)6 or a distinct entity. In essence, CPFE remains relatively understudied, with no specific treatment.
The objectives of this taskforce were: 1) to describe the terminology, definition, etiologies, features, comorbidities, and outcomes of CPFE; and 2) to provide a consensus definition and terminology of CPFE, determine whether it represents a syndrome, describe its management, and identify research priorities.
Methods
This research statement was developed by a committee of experts appointed by the American Thoracic Society (ATS), the European Respiratory Society (ERS), the Japanese Respiratory Society (JRS), and the Asociación Latinoamericana del Tórax (ALAT). The committee included 19 pulmonologists, 5 radiologists, 3 pathologists, 2 methodologists, and 2 patient representatives. Potential conflicts of interest were disclosed and managed in accordance with the ATS policies and procedures. The taskforce communicated during two face-to-face meetings, and via e-mail and teleconferences. Sections of the document were elaborated by subgroups, each with a leader responsible for writing. The final manuscript was approved by all panelists.
The search strategy was published previously and the search was updated on December 1, 2021 3 (online supplement). We searched MEDLINE and EMBASE databases for all original research articles published in English between January 1, 2000 and December 1, 2021, which included patients with both pulmonary fibrosis and emphysema in any distribution (Tables E1 and E2). All forms of original research were included (e.g., randomized control trials and observational studies), apart from case series containing <10 patients. Screening was performed by two reviewers using pre-determined criteria and disagreements were resolved by consensus with a third reviewer (Figure E1).
Historical perspective
The milestones of the description of CPFE are listed in table 1 and described in the online supplement. Since these initial publications, several series cited later in this document have contributed to a more complete description of CPFE, and etiological factors other than smoking have been identified.
Table 1. Milestones in the Description of Combined Pulmonary Fibrosis and Emphysema.
| Year | Author (Reference) | Description |
|---|---|---|
| 1948 | Mallory et al. (7) | First published case report of right heart failure due to CPFE (in a 27-yr-old woman) |
| 1948 | Robbins et al. (8) | Description of areas of fibrosis interspersed with areas of emphysema (thin-walled bullae or blebs) on chest radiograph |
| 1966 | Tourniaire et al. (9) | Report of 4 patients with CPFE who developed PH, with severe alteration of gas transfer and hypoxemia, and coexistence of paraseptal emphysema and perilobular fibrosis at autopsy |
| 1982 | Niewoehner et al. (10) | Hypothesized that pulmonary fibrosis and emphysema represent divergent responses to a common injury (e.g., cigarette smoking) and may share some pathways |
| 1988 | Westcott et al. (11) | Postmortem examination of the lungs of patients with end-stage pulmonary fibrosis showing that traction bronchiectasis was absent or mild in 3 patients with CPFE, ascribed to a reduction in elastic recoil due to emphysema |
| 1990 | Wiggins et al. (12) | Report of 8 patients with CPFE, with preservation of lung volumes and severe reduction in DLco ascribed to the combined impact of the two disease processes |
| 1991 | Schwartz et al. (13) | Increased residual volume and more impaired gas exchange in patients with IPF who smoked cigarettes. IPF reduces the likelihood of developing physiologic correlates of airflow obstruction among cigarette smokers |
| 1993 | Hiwatari et al. (14) | Report of 9 patients with CPFE |
| 1993 | Strickland et al. (15) | Description of areas of honeycombing and bullous emphysematous changes on HRCT in IPF Traction on small airways due to interstitial fibrosis prevents the small airway collapse typical of smoking-related emphysema and results in preserved ventilation in areas of bullous destruction and overall increase in lung volumes |
| 1997 | Doherty et al. (16) | Preserved lung volumes in patients with IPF who smoked |
| 1997 | Wells et al. (17) | Higher lung volumes and lower DLco in presence of emphysema in patients with IPF, after adjusting for the extent of fibrosis on CT |
| 1999 | Hoyle et al. (18) | Overexpression of platelet-derived growth factor in mice provoked lung pathology characterized by emphysematous changes, inflammation, and fibrosis |
| 2003 | Wells et al. (19) | Description of the CPI, which correlates with the extent of fibrosis on HRCT independently of the presence of emphysema and predicts mortality more accurately than DLco and other pulmonary function variables |
| 2005 | Cottin et al. (1) | Retrospective analysis of 61 patients with CPFE. PH is frequent and determines a dismal prognosis. Suggestion to individualize CPFE as an entity |
| 2005 | Lundblad et al. (20) and comment (21) | Morphologic abnormalities consistent with both pulmonary fibrosis and emphysema, in association with generalized lung inflammation, in a mouse model of TNF-α overexpression, supporting a common pathogenetic linkage of both conditions |
| 2006 | Yousem et al. (22) | Pathological description of respiratory bronchiolitis-associated interstitial lung disease with fibrosis |
| 2008 | Kawabata et al. (4) | Pathological description of airspace enlargement with fibrosis |
| 2009 | Cottin et al. (2) | Suggestion that CPFE is a syndrome |
| 2010 | Katzenstein et al. (23) | Pathological description of smoking-related interstitial fibrosis |
Definition of abbreviations: CPFE = combined pulmonary fibrosis and emphysema; CPI = composite physiologic index; CT = computed tomography; HRCT = high-resolution computed tomography; IPF = idiopathic pulmonary fibrosis; PH = pulmonary hypertension; TNF = tumor necrosis factor.
Epidemiology
Emphysema is common in current or former smokers with fILD. Prevalence estimates of CPFE vary depending on the population studied and the definition used, ranging from 8-67% of patients with IPF 24–34. There may be geographical variation in prevalence, with the highest estimates from Asia and Greece, and lower estimates in the United States. These differences may be attributable to differing genetic susceptibility, smoking rates or definitions of CPFE. CPFE is reported in 26-54% of patients with idiopathic interstitial pneumonia 35,36, with higher prevalence in those requiring hospital admission (45-71%)37,38. The prevalence is also higher in patients with lung cancer and idiopathic interstitial pneumonia, including IPF 39,40.
The prevalence of CPFE in the general population is unknown, as most data come from patients with an indication for chest computed tomography (CT). CPFE as previously defined 1 was identified radiographically in 7.3% of males who underwent high-resolution CT (HRCT) of the chest (indication unknown)41 and in 2.8% of all HRCTs done at a single center in Korea 42. In patients with resected lung cancer, CPFE was found in 3-10% of patients 38,43–45(Table 2); however, another lung cancer screening cohort found a much lower prevalence at 0.04% 36.
Table 2. Frequency Estimates of Combined Pulmonary Fibrosis and Emphysema across Different Patient Populations.
| Population | Reported Frequency (%) |
|---|---|
| General population | Unknown |
| Idiopathic pulmonary fibrosis | 8–67 |
| Idiopathic interstitial pneumonia | 26–54 |
| Lung cancer, with underlying idiopathic interstitial pneumonia or idiopathic pulmonary fibrosis | 55–58 |
| Rheumatoid arthritis–interstitial lung disease | 8–58 |
| Systemic sclerosis–interstitial lung disease | 5–12 |
| Lung cancer | 3–10 |
| Lung cancer screening cohort | 0.04 |
| Cohort undergoing chest computed tomography | 3–7 |
Etiologies
Exposures and diseases
Cigarette smoking and male sex are consistently associated with CPFE. CPFE occurs nine times more often in males, and this discrepancy is not wholly attributable to a greater history of smoking in males 46. Almost all patients with CPFE report a history of smoking, with an average exposure of 40 pack-years, with the notable exceptions of some patients with connective tissue disease (CTD) or fibrotic hypersensitivity pneumonitis (fHP)47,48 who on average have less smoking exposure 49–53(Figure E2). A smoking history is more common in CPFE than in isolated IPF 24,32,34,38,43,45,50,54–59 or systemic sclerosis-associated ILD 49. The association between CPFE and number of pack-years suggests a dose response effect 28,55,58,60,61. Emphysema generally precedes fILD when the data are available, although there are some exceptions to this, particularly if considering interstitial lung abnormality as an early form of ILD 1.
CPFE can occur in non-smokers especially in CTD, suggesting CTD itself as a risk factor 28,51,52. In 470 patients with systemic sclerosis, 43 had CPFE on chest CT, including 24 (58%) who had never smoked 62. Approximately 5-10% of patients with systemic sclerosis-associated ILD have radiological findings of CPFE 49,51,63,64. In 116 never smokers with rheumatoid arthritis-associated ILD (RA-ILD), emphysema was present on HRCT in 27% 52. CPFE is also reported in systemic vasculitis, particularly microscopic polyangiitis 65,66. Of 150 consecutive patients with RA, 12 (8%) had both ILD and emphysema 67; however, in patients with rheumatoid lung, the reported prevalence of emphysema is as high as 48% 28,68. Emphysema on HRCT was less extensive in CTD-associated usual interstitial pneumonia (UIP) than in IPF (idiopathic UIP)69. IPF patients with CPFE are more likely to have positive antinuclear antibodies or p-antineutrophil cytoplasmic antibodies than IPF patients without emphysema 1,25.
Multiple occupational and inhalational exposures are associated with CPFE 70–73(Table 3). CPFE is reported in patients with asbestosis and silicosis, occasionally in lifelong non-smokers 74–77. Interestingly, emphysema occurs in 7-23% of patients with fHP 47,78. Occupational exposure to vapors, dusts, gases, and fumes is associated with more extensive radiologic emphysema after adjusting for smoking pack-years 79.
Table 3. Exposures and Etiologies That Are Associated with Combined Pulmonary Fibrosis and Emphysema.
| Variables Associated with CPFE | References |
|---|---|
| Risk factors and demographics | |
| Cigarette smoking | 24, 32, 34, 38, 43, 45, 50, 54–58 |
| Male sex | 1, 32, 38, 43, 45, 46, 55–57, 60, 61 |
| Diseases | |
| Idiopathic pulmonary fibrosis | 24–33 |
| Connective tissue disease | 49–52, 62, 64, 79 |
| ANCA-associated vasculitis | 65, 66, 80, 81 |
| Hypersensitivity pneumonitis | 47, 78, 82, 83 |
| Inhalational exposure | |
| Coal dusts | 46, 70, 71 |
| Asbestos | 74–77 |
| Silica and mineral dust | 84 |
| Other inhalational exposures | 72, 73, 85–87 |
| Rare genetic variants | |
| Telomerase-related genes (TERT, RTEL1) | 88–92 |
| Surfactant-related genes (SFTPC, ABCA3) | 93–98 |
| Other genes (Naf1, PEPD) | 99, 100 |
| Genetic polymorphisms | |
| MMP-9 and TGF-β-1 genes | 101–103 |
| AGER gene | 104 |
| rs2736100 (TERT), rs2076295 GG (DSP) | 105 |
Definition of abbreviations: ANCA = antineutrophil cytoplasmic antibodies; CPFE = combined pulmonary fibrosis and emphysema; MMP = matrix metalloprotease; TGF = transforming growth factor.
Genetic predisposition and aging
Genetic predisposition in combination with risk factors including smoking or exposure to other aero-contaminants, may predispose individuals to develop both fibrosis and emphysema 2, both of which involve aging and cell senescence 107–111. Genetic predilections for CPFE are not well understood, with only a few cases reported of mutations carrying a Mendelian risk of CPFE or IPF. CPFE has been reported in patients carrying mutations in genes associated with surfactant (see online supplement)94–99 or telomeres 89–93. Shorter telomeres are associated with both chronic obstructive pulmonary disease (COPD) and IPF 112, and are, thus, likely associated with CPFE 2,46, although this requires further study 113. If confirmed, CPFE would represent a model of smoking-induced, telomere-related, lung disease. Epigenetic alterations may also be important 114.
Clinical manifestations and comorbidities
Patients with CPFE have a mean age of approximately 65-70 years 1,46 (comparable to IPF and COPD), with 73-100% male predominance 1,24–33,38,43,45,55–57,60,61. Symptoms include exertional dyspnea and cough 1,41. Patients with CPFE and pulmonary hypertension (PH) have significant exertional breathlessness, with the majority having a New York Heart Association functional class of III or IV 115.
In CPFE, the two most prominent comorbidities are lung cancer and PH, discussed in the outcomes section below. Other comorbidities include coronary artery disease, peripheral vascular disease, and diabetes 38,116, although it is not known whether these diseases are more prevalent in CPFE than in IPF without emphysema 42,60. Differences in sample size, study design (retrospective), and methods for identification and documentation of comorbidities contribute to uncertainties. Prospective studies with standardized data collection methods and case definitions are required.
Lung function
Patients with CPFE have limited exercise capacity, severely impaired DLco and transfer coefficient (Kco)1,46,117–119, contrasting with relatively preserved airflow rates and lung volumes. The FVC/DLco ratio is increased in most patients 51.
Compared to isolated IPF, patients with CPFE have higher lung volumes (FVC and TLC), generally comparable FEV1, higher residual volume (RV), lower DLco, lower Kco, and lower PaO2 17,24,26,31–33,37,59,60,119–125, even with adjustment for the extent of fibrosis 17,121(Table 4). The mean FEV1/FVC ratio is usually normal or slightly reduced, may rise with progression of fibrosis, but is typically lower than in isolated IPF where it is usually increased (e.g. > 0.80)26,120. Comparison of physiology between CPFE and isolated IPF may be hampered by differences between studies in the severity of both emphysema and fibrosis, despite attempts to adjust for severity 24.
Table 4. sMain Characteristics of Pulmonary Function in Combined Pulmonary Fibrosis and Emphysema.
| Pulmonary Function Test Measurement | Typical Abnormality Seen in CPFE | Typical Abnormality Seen in fILD without Emphysema |
|---|---|---|
| FVC | Decreased or normal (but preserved compared with idiopathic pulmonary fibrosis alone) | Decreased |
| FEV1 | Decreased or normal | Decreased |
| FEV1/FVC | Variable (normal, decreased, or increased) | Normal or increased |
| TLC | Variable (normal, decreased, or increased) | Decreased |
| FRC | Variable (normal, decreased, or increased) | Decreased |
| Residual volume | Variable (normal, decreased, or increased) | Decreased |
| DLco | Disproportionately decreased | Decreased |
| Transfer coefficient for carbon monoxide | Severely decreased | Normal or decreased |
| Saturation during exercise | Severe desaturation | Desaturation |
| Peak oxygen uptake | Decreased | Decreased |
Definition of abbreviations: CPFE = combined pulmonary fibrosis and emphysema; fILD = fibrotic interstitial lung disease.
Compared to COPD, patients with CPFE have relatively preserved FEV1 and FEV1/FVC, less hyperinflation, and lower DLco 126. A minority of the 132 patients (36% pooled prevalence) from three previous studies had TLC < 80% predicted 1,50,115, while only 41% had FEV1/FVC < 0.70. Of these, 11% had FEV1 > 80% predicted, corresponding to Global initiative for Obstructive Lung Disease stage 1, 37% were classified as stage 0 (FEV1/FVC ≥ 0.70 and FEV1 ≥ 80% of predicted), and 22% were unclassified (with FEV1/FVC ≥ 0.70 and FEV1 < 80% of predicted). In another study, smokers with emphysema were less likely to meet functional criteria for COPD if ILD was present on imaging 127. Thus, the relative preservation of spirometric values may lead to underdiagnosis of chronic lung disease if only spirometry is obtained.
The relative preservation of flow rates and lung volumes is attributed to the counterbalancing effects of the restrictive physiology from pulmonary fibrosis (presumably increased elastic recoil and prevention of expiratory airway collapse by traction forces) and the effects of emphysema on the airways. Thus, in CPFE, FEV1/FVC can actually improve to normal values as fibrotic disease progresses, despite worsening dyspnea and DLco 128, and contrary to COPD 126. TLC correlates positively with emphysema extent on CT, and negatively with fibrosis extent. Conversely, FEV1/FVC correlates negatively with emphysema extent on CT, and positively with fibrosis extent 129. Compared to isolated fILD, patients with CPFE have lower whole-breath inspiratory and expiratory resistance based on analysis of respiratory impedance by multi-frequency forced oscillation technique, further supporting the hypothesis of “normalization” of lung mechanics 130. Conversely, both disease components reduce alveolar capillary gas exchange through either decreased capillary blood volume or alveolar membrane thickening, resulting in greater reductions in DLco.
Severe decrease in arterial oxygen saturation and hypoxemia at exercise is very common in CPFE, especially when complicated by severe PH 1,115,119. Hence, exercise limitation with decrease in oxygen saturation 119, and isolated 131 and/or severe 132 reduction in DLco or Kco, contrasting with a mild ventilatory defect, should raise the suspicion of CPFE and/or PH. Compared to isolated IPF, patients with CPFE have lower exercise capacity despite less extensive fibrosis on HRCT 133,134. Exertional dyspnea is the key limiting factor, related to poor ventilatory efficiency and, presumably, increased dead space in hypoperfused areas 133. Hypercapnia occurs only very late in the disease course. A similar functional profile is observed when CPFE occurs in CTD 49–52 or fHP 47.
Importantly, the presence of significant emphysema impacts on serial lung volume trends, attenuating serial lung volume decline due to progressive fibrosis. Patients with CPFE experience a slower decline in FVC than patients with isolated IPF 26,29,124, whereas decline in DLco and increase in the Composite Physiologic Index (CPI), which quantifies functional impairment due to IPF whilst excluding the functional impact of emphysema, are less affected 29,60. In an analysis of patients with IPF from two randomized controlled trials, emphysema extent ≥15% was associated with reduced FVC decline over 48 weeks compared to those with either no emphysema or emphysema extent <15% 29.
Consequently, no optimal parameter has been validated to monitor disease progression in CPFE. Changes in FVC, commonly used to monitor IPF progression 135, are not reliable indicators of disease progression in patients with CPFE 26,29,124, which has implications for clinical trial design 2,136. Serial change in DLco may be a helpful marker of disease progression but is additionally affected by other factors including vasculopathy, hemoglobin concentration, and measurement variation. Serial change in CPI is not validated for monitoring ILD progression. A FEV1%FVC ratio >1.2 at baseline 137 and a decline in FEV1 by 10% or more at 6 or 12 months 138 were associated with a poor outcome, but these observations warrant confirmation. In clinical practice, a decline in one or several of the above-mentioned functional parameters may be observed in individual patients. In summary, the committee therefore suggests that disease progression in CPFE be monitored using a combination of clinical, imaging, and multiple functional parameters, with less emphasis on FVC trends than in the monitoring of ILD without concurrent emphysema.
Imaging features
Overview
CPFE is characterized by the presence of emphysema and interstitial fibrosis, with a wide variety of appearances on chest HRCT.
Emphysema is identified as a region of low attenuation (also termed density), not bounded by visible walls on CT 139. Emphysematous foci can be categorized as centrilobular, paraseptal, or panacinar 140. Interstitial fibrosis is identified as regions of increased parenchymal attenuation, appearing as reticulation and/or ground glass opacities, variably associated with honeycombing and/or traction bronchiectasis (Table 5). Patterns of emphysema on HRCT in CPFE have been tentatively classified into broad groups 129,141–143(Figures 1-8), however additional work is needed to better define CPFE morphologic subtypes. No studies have formally compared patterns of emphysema in CPFE versus COPD 140.
Table 5. Definitions of High- and Low-Attenuation Parenchymal Features Frequently Visualized on HRCT Imaging in Patients with Combined Pulmonary Fibrosis and Emphysema.
| Attenuation Level of Imaging Feature on CT | Parenchymal Feature | Description |
|---|---|---|
| High attenuation | Smooth ground-glass opacity | Increased-opacity parenchyma occurring on the background of normal-appearing lung |
| Coarse ground-glass opacity | Increased-opacity parenchyma with overlying reticulation or traction bronchiectasis | |
| Reticulation | Linear opacities representing thickened inter- and intralobular septae | |
| Low attenuation | Cysts | A round parenchymal lucency with a well-defined interface with normal lung |
| Honeycomb cysts | Clustered cystic airspaces (3–10 mm in diameter) with well-defined walls that are usually subpleural | |
| Traction bronchiectasis, bronchiolectasis | Irregular bronchial and bronchiolar dilatation caused by surrounding retractile pulmonary fibrosis. Adjacent lung is typically of high attenuation | |
| Air trapping | Parenchymal areas with reduced attenuation that lack volume reduction on expiratory imaging | |
| Emphysema: paraseptal | Subpleural and peribronchovascular regions of low attenuation separated by intact interlobular septa. May be associated with bullae | |
| Emphysema: centrilobular | Centrilobular areas of low attenuation, usually without visible walls. Nonuniform distribution, predominantly located in upper lung zones | |
| Emphysema: panacinar | Generalized decreased attenuation of lung parenchyma with a decrease in blood vessel caliber in the affected lung. Typically lower-zone-predominant location |
Definition of abbreviations: CT = computed tomography; HRCT = high-resolution computed tomography.
Figure 1.
HRCT showing a typical distribution of disease seen in combined pulmonary fibrosis and emphysema (separate emphysema and fibrosis pattern). Paraseptal and centrilobular emphysema is localized to the upper lobes, whilst fibrosis characterized by traction bronchiectasis is localized to the lower lobes.
Figure 8.
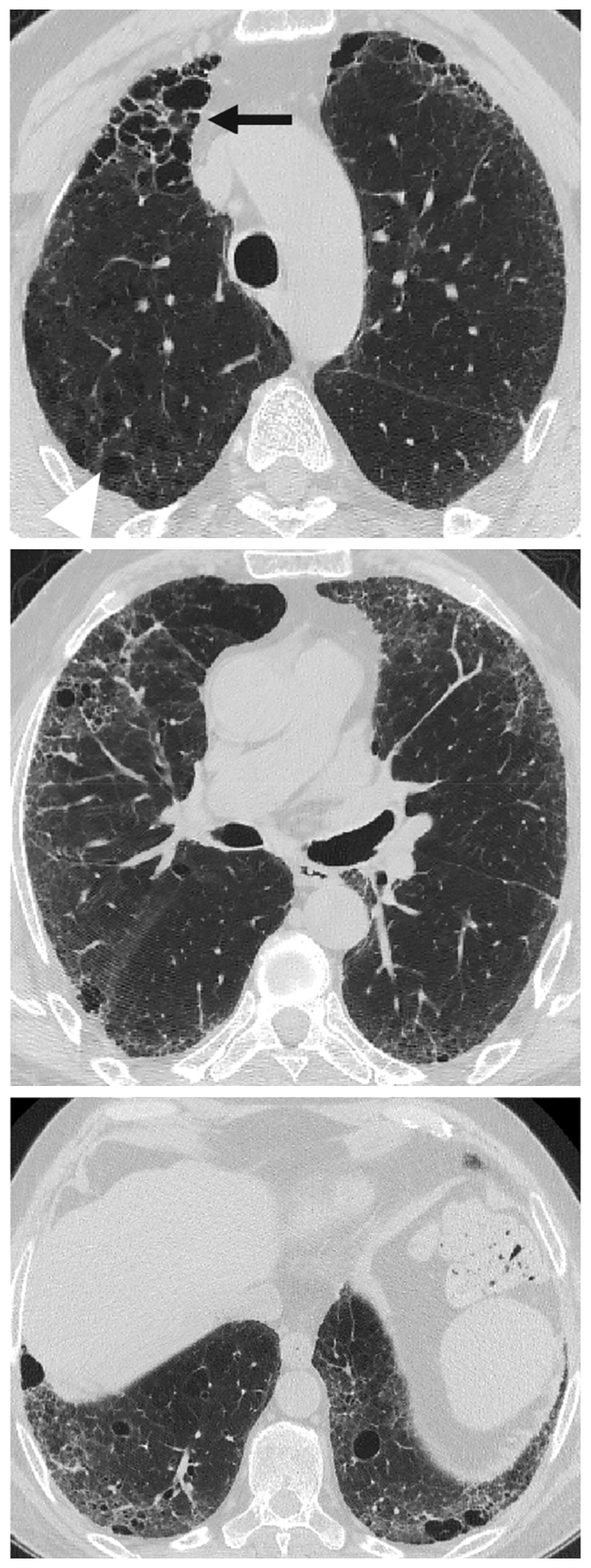
HRCT in a 69-year-old male with idiopathic pulmonary fibrosis, showing diffuse emphysema through the lung zones and lower zone predominant fibrosis (combined pulmonary fibrosis and emphysema, unclassifiable pattern). Emphysema in the right upper lobe is a combination of admixed (black arrow) and isolated emphysema (white arrowhead). Admixed emphysema is visible in the midzones, whilst in the lower zones a mixture of admixed and isolated paraseptal and centrilobular emphysema is apparent.
HRCT scanning parameters for appropriate assessment of ILD can be found elsewhere 144. Classical HRCT patterns may be altered when emphysema and fibrosis are spatially superimposed. For example, expansion of the interlobular septa with collagen fibrosis can make paraseptal emphysema appear as honeycomb cysts. Most studies have focused on patients with IPF and/or a UIP pattern on HRCT imaging 1,24–26,29,30,32,33,38,55,56,58,61,120,121,137,138,141,145–153, although others have included patients with a variety of ILD subtypes and imaging patterns. Given the high proportion of patients with CPFE with UIP pattern on HRCT (Table E3)1,137,145–147, distinguishing admixed emphysema from honeycomb cysts is challenging. The coexistence of emphysema and fibrosis can also create an imaging pattern of thick-walled cystic lesions 141,142, thought to reflect the expansion of emphysema as it is pulled apart by adjacent contracting fibrotic lung. This process, the committee suggests, could be termed traction emphysema given its putative mechanistic similarity to tractionally dilated bronchioles commonly seen within areas of fibrosis. Thick-walled cystic lesions predominating in basal posterior lung zones, consisting of large emphysematous areas surrounded by reticular opacities, have been more frequently described in CPFE than in isolated IPF 141,142. However, it is unknown whether thick-walled cystic lesions are specific for CPFE, and their evolution is yet to be fully described.
New imaging modalities may allow early diagnosis or distinguish IPF from CPFE 154–156. Imaging modalities that combine functional information and anatomic detail such as hyperpolarized Xenon MRI may advance the discrimination of superimposed emphysema and fibrosis 157,158. The reduced red blood cell spectroscopic peak in areas of fibrosis seen with hyperpolarized Xenon MRI could be evaluated alongside the increased apparent diffusion coefficient seen in areas of emphysema where disrupted acinar-airway integrity increases Brownian motion 159,160. However, more work is needed to understand whether aerated honeycomb cysts may mimic similar-sized emphysematous lesions on apparent diffusion coefficient.
All routinely used imaging modalities are constrained by the lack of histopathological definition of damage as emphysematous or fibrotic. Newer ex-vivo imaging techniques like hierarchical phase contrast tomography, able to image entire lungs and focal regions of interest at 2.5μm, may transform our understanding of emphysema-fibrosis interactions by essentially providing three-dimensional histopathological characterization of the lungs 161.
Quantification of HRCT abnormalities
Disease quantification has predominantly relied on semi-quantitative visual HRCT estimation of emphysema and fibrosis extents. However, this approach is limited by several challenges: 1) interobserver variation 30; 2) time constraints for visual scoring; 3) varying methodologies for HRCT scan interrogation (e.g. evaluation of whole CT volumes versus interspaced images); 4) varying HRCT spatial resolution; 5) whether emphysema extent alone or both emphysema and fibrosis extents are quantified 24,29,30,47,52,58,121,138,147,162; and 6) variations in emphysema quantification e.g. total extent of emphysema, versus extents of emphysema lying either within or separate to areas of fibrosis30,31,52,58,163.
Emphysema quantification
The emphysema component of CPFE has been evaluated by imaging rather than lung function tests, given the confounding impact of fibrosis on lung physiology. Reliable estimation of emphysema extent in patients with established pulmonary fibrosis poses significant challenges. Most studies use visual assessment of emphysema by an experienced radiologist, a method that is readily available and has moderate inter-rater agreement. Emphysema thresholds used to characterize a CPFE phenotype on imaging 3(see following section), include: >0% 26,30,58, >5% 121, >10% 24,32,148 and >15% 162 of total lung volume. One study limited assessment of emphysema extent to above the level of the carina 51.
Quantitative methods for scoring emphysema using computer-based measurement of lung density (e.g. density masking) are typically used in studies of chronic obstructive pulmonary disease and remove the problem of observer variability. However, this methodology of emphysema quantification is poorly suited to CPFE despite being attempted in some series 36,42,58,122,123,148,163–165, because it fails to discriminate between low density areas due to emphysema and low density due to honeycomb cysts, traction bronchiectasis or non-emphysematous mosaic attenuation due to small airways disease. Until this limitation can be overcome (possibly by artificial intelligence), visual quantification of emphysema extent remains the method of choice in CPFE.
Differences in morphological patterns of emphysema (subtype: paraseptal vs centrilobular vs mixed vs indeterminate; predominant distribution in the axial plane) have also been used to describe CPFE subtypes 26,120,129,147. Large multicentered studies are required to determine whether these morphological CPFE subtypes correlate with distinct functional or prognostic disease groups. The subtypes identified on HRCT imaging could also be confirmed using histopathological correlative studies 151.
Interstitial lung disease quantification
A minimal threshold extent of lung fibrosis on HRCT imaging has rarely been used in CPFE despite the clinical importance of fILD severity (Figure E3). The concept of a minimal threshold of fibrosis to define CPFE is particular relevant to lung cancer screening populations. Participants in screening studies are typically older with a heavy smoking history, and both emphysema and interstitial lung abnormalities (ILAs) will be frequent 127,166–169. This may result in a high prevalence of combined ILAs 170 and emphysema in screening populations.
In the context of IPF, where ground glass opacification on HRCT largely represents fine fibrosis, fibrosis extent in CPFE has been calculated by summing ground glass opacities, reticulation, and honeycomb cysts 118,171. However, quantitation of fibrosis extent is confounded by volume loss, with lower lobes sometimes greatly contracted to apparently small areas of fibrosis. Yet when considering CTD-related ILD or fHP where ground glass opacities may reflect inflammation rather than fibrosis, there is no consensus on whether ground glass opacities should be considered as part of CPFE fibrosis extent. It has been suggested that to conform to the “fibrosis” element required by a definition of CPFE, ground glass opacities should be quantified only if overlaid by reticular lines or traction bronchiectasis (Figures E4 and 9)171. Agreement on ILD patterns to be quantified in CPFE will be important to harmonize study interpretation in the future, as well as agreement on preferred visual fibrosis quantification methodologies (volumetric lobar scores versus 5- or 6-level HRCT slice scoring; categorical versus continuous scales of fibrosis extent).
Figure 9.
Visual scoring of emphysema. Axial section through the upper lobes in a patient with idiopathic pulmonary fibrosis and emphysema (top). Emphysema is distributed irregularly through the lobe making visual quantification difficult. Visually combining the emphysematous foci together (bottom) and estimating the fraction of the lobe that it comprises (i.e. 50%, 33%, 25%, 20%, 15%, 10%, 5%) can simplify quantitation in challenging cases (online supplement).
Pathology features
CPFE was originally defined based on clinical, physiologic, and HRCT features 2. Histopathologic studies of patients with severe CPFE defined in this way are limited to small series of autopsy cases or explants given the risk of surgical lung biopsy in this population 141,149,151. Overlapping patterns of smoking-related abnormalities are common in lung biopsies from patients undergoing elective lung biopsy for fILD including some for whom a diagnosis of CPFE is uncertain or unanticipated. Here we review patterns of smoking-related abnormalities and pulmonary fibrosis with a focus on features characteristic of CPFE.
Histopathological patterns of smoking-related abnormalities and fibrosis in CPFE
Emphysema is required for a diagnosis of CPFE and is defined as abnormal, permanent enlargement of airspaces distal to the terminal bronchiole, accompanied by the destruction of their walls, without obvious fibrosis (Figure 10)172–175. Morphologic studies of carefully inflated lung specimens from explant pneumonectomies and autopsy lungs provided the basis for an anatomical definition of emphysema and continue to inform our understanding of its pathogenesis. However, the coexistence of emphysema and patterns of fILD can be seen in biopsies, a situation in which pathologists need to document the emphysema as well as the fILD. Centrilobular emphysema is an upper lobe predominant form of emphysema caused by cigarette smoking that is often accompanied by paraseptal emphysema in CPFE patients. Emphysema is common in surgical lung specimens and frequently coexists with other smoking-related abnormalities including respiratory bronchiolitis (RB) and SRIF 5,176.
Figure 10.
Centrilobular emphysema in wedge excision in a heavy smoker with a peripheral small cell carcinoma. An intermediate magnification photomicrograph shows enlarged airspaces with destruction of bronchiolar walls evidenced by detached free-floating connective tissue fragments. Hematoxylin and eosin staining.
RB occurs almost exclusively in cigarette smokers and is defined by the presence of pigmented alveolar macrophages clustered within the lumens of respiratory bronchioles and peribronchiolar air spaces without significant inflammation or fibrosis (Figure 11)177. RB is a common incidental finding in surgical lung specimens, including biopsies in which it may accompany any pattern of pulmonary fibrosis including especially SRIF, desquamative interstitial pneumonia (DIP), UIP and Langerhans cell histiocytosis given the high prevalence of smoking in these populations. RB-ILD is a diagnosis of exclusion reserved for patients in whom RB is thought to explain diffuse ILD after elimination of diagnostic alternatives, a circumstance histologically indistinguishable from incidental RB. RB by itself is not a fibrotic lesion and therefore an insufficient explanation for fibrosis in patients suspected of having CPFE.
Figure 11. Respiratory bronchiolitis (RB) in a patient with RB-ILD. RB is a common finding in patients with CPFE who have concomitant pulmonary fibrosis.
A. Low magnification photomicrograph showing RB characterized by clusters of lightly pigmented macrophages in the lumens of distal bronchioles and peribronchiolar air spaces. B. Higher magnification view showing pigmented intraluminal macrophages in respiratory bronchiole and surrounding air spaces with no significant inflammation or fibrosis. Hematoxylin and eosin staining.
Patterns of fibrosis observed in patients with CPFE are histologically heterogeneous (Table 6)178,179. These patterns include a distinctive form of fibrosis linked to cigarette smoking for which Katzenstein proposed the term SRIF 5,23,61,151. SRIF overlaps with previous descriptions of AEF 4,180,181, RB-associated ILD with fibrosis 22, RB with fibrosis 182, and DIP5. Some cases with a pattern of fibrotic NSIP may also be related to smoking. SRIF is characterized by densely eosinophilic collagen deposited in expanded alveolar septa with preservation of lung architecture and little or no inflammation (Figure 12). SRIF has a distinct predilection for peripheral subpleural and peribronchiolar parenchyma without the variegated “patchwork” distribution more characteristic of UIP. When combined with paraseptal emphysema, SRIF may account for the “thick-walled cystic lesions” that are unique to CPFE and distinct from the honeycomb cysts of UIP (Figure 13)61,141,142. Like RB, SRIF is a common incidental finding in surgical lung specimens, including lung biopsies from patients with other patterns of pulmonary fibrosis 23. Isolated SRIF represents the primary pathological abnormality in a subset of patients with clinical features of ILD in whom it is often combined with RB (Figures E5 and E6) 22,182. SRIF without other patterns of concomitant fibrosis has not been established as a cause of CPFE; therefore, attributing pulmonary fibrosis to SRIF in patients with CPFE requires exclusion of other fibrotic patterns including most importantly UIP.
Table 6. Histopathological Features of Smoking-related Interstitial Fibrosis and Other Patterns of Fibrotic Interstitial Lung Disease in Combined Pulmonary Fibrosis and Emphysema.
| Pattern of Fibrosis | Distribution | Fibroblast Foci | Honeycomb Change | Interstitial Inflammation |
|---|---|---|---|---|
| SRIF (5, 23) | Patchy, subpleural, peribronchiolar | Rare | Rare | Absent |
| DIP (180) | Diffuse | Rare | Absent | Present |
| UIP, probable UIP (144, 180) | Patchy, subpleural, interlobular septa | Present | Present | Patchy, mild (may be more extensive in areas of honeycombing) |
| F-NSIP (180) | Diffuse | Rare | Absent | Present |
| Indeterminate (180, 184) | Patchy or diffuse | ± | ± | ± |
Definition of abbreviations: DIP = desquamative interstitial pneumonia; F-NSIP = fibrotic nonspecific interstitial pneumonia; SRIF = smoking-related interstitial fibrosis; UIP = usual interstitial pneumonia.
Figure 12.
Smoking-related interstitial fibrosis (SRIF) in upper lobe biopsy from 120-pack-year smoker with CPFE characterized by a combination of emphysema and usual interstitial pneumonia in middle and lower lobe biopsies. A. Low magnification photomicrograph showing mild expansion of subpleural parenchyma by paucicellular, densely eosinophilic (“amyloid-like”) collagen with preservation of lung architecture. B. Higher magnification photomicrograph showing subpleural fibrosis without honeycomb change or fibroblast foci. There is mild associated emphysema.
Figure 13. Subpleural cysts of smoking-related interstitial fibrosis (SRIF) contrasted with honeycomb change in usual interstitial pneumonia (UIP).
A. Low magnification photomicrograph of SRIF with associated distal acinar (“paraseptal”) emphysema forming thick-walled cysts. Overall lung architecture is preserved and the paucicellular fibrosis lacks the qualitative variability more characteristic of UIP. The cystic spaces are mainly lined by attenuated pneumocytes. B. Low magnification photomicrograph of honeycomb change in UIP. The fibrosis has a patchy distribution with collapse and distortion of normal lung architecture. The fibrosis includes fibroblast foci (arrow), and a mild, patchy infiltrate of lymphocytes resulting in a variegated appearance that contrasts with the uniform, paucicelluar, densely eosinophilic fibrosis in SRIF. Cystic honeycomb spaces (*) are mainly lined by columnar bronchiolar type epithelium rather than pneumocytes. Hematoxylin and eosin stain.
Langerhans cell histiocytosis (LCH) is a potentially fibrotic form of smoking-related ILD that may occur in combination with other smoking-related abnormalities including emphysema, RB, SRIF, and DIP 5,184. Advanced disease is characterized by cystic change on HRCT that may be difficult to distinguish from emphysema 185, and a pattern of fibrosis in surgical specimens that may mimic other forms of fILD. Histopathological examination of surgical specimens from patients with advanced LCH, whether explants or diagnostic biopsies, is often complicated by the absence of diagnostic Langerhans cells. Microscopic features helpful in separating LCH from other patterns of fibrosis include stellate bronchiolocentric nodules and a characteristic pattern of affiliated paracicatricial airspace enlargement (“scar emphysema”) without subpleural honeycomb change (Figure E7).
There are no criteria for establishing a diagnosis of CPFE on the basis of histopathological findings alone. Supportive features include a combination of emphysema and a pattern of fibrosis other than SRIF or LCH (Figure 12). UIP is the most commonly reported pattern of pulmonary fibrosis in patients with CPFE (Figures E8 and E9)1,37,141,151,186,187. Identifying UIP typical of IPF in the setting of emphysema requires recognition of patchy fibrosis, fibroblast foci, and honeycombing without histologic features to suggest an alternative such as LCH, f-HP, or CTD-associated UIP 48,144,179. Unique to UIP in CPFE is the presence of thick-walled cysts resulting from the combination of emphysema and SRIF (figure 13). Other less commonly described patterns of fibrosis include fibrotic NSIP and DIP 1,188,189. Classifying subtypes of pulmonary fibrosis may be challenging and therefore the histopathological features may remain indeterminate for UIP in the setting of concomitant emphysema 1,151.
Comorbidities identified on basis of histopathological features
The dismal prognosis of CPFE may result from vascular changes that correlate with PH. In a comparison of autopsy findings in patients with CPFE, IPF, and emphysema alone, vascular changes were more extensive in CPFE and IPF compared to those with emphysema alone 149. Vasculopathy was limited to areas of emphysema in those with emphysema alone, but involved emphysematous, fibrotic, and relatively preserved parenchyma in CPFE and IPF. Vascular changes included intimal thickening and medial hypertrophy in small muscular pulmonary arteries as well as intimal thickening in comparably sized small veins. Plexiform lesions were rare and seen only in a small minority of CPFE and IPF patients.
Malignancy is also not uncommon in CPFE, with a higher prevalence of squamous cell carcinomas amongst surgically resected cases 38.
Outcome and complications
There are several important outcomes that have specific relevance in patients with CPFE, with lung cancer and PH being the most clinically relevant. It is currently unknown whether the risk of complications may differ according to different patterns of CPFE (Table 5, Table 6).
Pulmonary hypertension
PH has been reported in 15-55% of patients with CPFE 1,32,49,50,137, with some studies suggesting an increased prevalence in a variety of ILDs 49,120 and others not confirming this association 56,60. Discrepant estimates of PH prevalence may be due to differing methods of PH assessment (e.g. echocardiographic vs. right heart catheterization-defined PH) and differences in statistical modeling 190, and could also be attributable to differing severity of fibrosis and emphysema on HRCT 30. Pathophysiology of PH in CPFE is probably multifactorial 191. Some studies have suggested that the severity of PH is worse among those with CPFE compared to both IPF 24,32 and COPD 192 or emphysema 149 alone. Estimated systolic pulmonary artery pressures are higher in patients with CPFE than in those with isolated IPF 24,119. The additional burden of emphysema, over and above a given extent of fibrosis, increases the risk of PH. However, the likelihood of PH does not differ for matched extents of disease (combined fibrosis and emphysema) on HRCT (or when adjusted for DLco) between patients with CPFE and those with fibrosis alone 30,58.
Lung cancer
Lung cancer has been reported in 2-52% of patients with CPFE 35,37,41,42,57,58,60,137,148,162,163,193, with varying methodology (cross-sectional, longitudinal follow-up). In a meta-analysis 194, patients with CPFE (UIP and emphysema) had a higher risk of lung cancer than those with IPF alone (OR 2.69; 95% CI: 1.78-4.05)194. There were similarly increased risks of lung cancer in patients with CPFE and UIP with the presence of any amount of emphysema (OR 2.93; 95% CI: 1.79-4.79) and with emphysema in ≥10% of the lung volume (OR 2.22; 95% CI: 1.06-4.68), compared to patients who had UIP without emphysema 194.
The most common histopathologic subtypes of lung cancer in CPFE are squamous cell carcinoma and adenocarcinoma 38–40,44,45,116,162,193,195–198. In contrast to the general epidemiology of non-small cell lung cancer with adenocarcinoma accounting for 50% of cases 199, squamous cell carcinomas appear to be more frequent in patients with CPFE 39,40,44,45,162,193,195–198,200. The majority of the lung cancers were located in the lower lobes 39,195. There is greater invasion and the diagnosis is made at a later stage compared to non-small cell lung cancer without CPFE 198,201.
Although individual studies differ in their conclusions 38,44,45,57,145,195,200, a systematic review and meta-analysis concluded that the presence of CPFE is associated with worse survival in patients with non-small cell lung cancer198. 38,44,57,145,195,200Among patients with CPFE and lung cancer, the presence of honeycombing, later cancer stage, and reduced feasibility of surgical resection are predictors of mortality 202. The poor outcome is at least in part related to increased morbidity and mortality of cancer treatments in CPFE, which often limits standard therapy 40,43,45,196,197,200,203,204.
Acute exacerbation
Acute exacerbations of IPF have been reported in patients with CPFE with varying prevalence 50,137,141,195,205–207. Risk factors for acute exacerbation in CPFE may be similar to IPF, including worse gender-age-physiology score and the presence of lung cancer, particularly following surgical resection 43,195,196,204,205. Diffuse ground glass and/or consolidation on chest HRCT help to differentiate exacerbations of fibrosis from exacerbations of emphysema in CPFE 208. The prognosis of acute exacerbation in CPFE might be better than that of isolated IPF 31,207.
Mortality
CPFE is associated with poor survival, with different estimates between series 1,32,35,37,38,55,115,137,163,209, which probably reflect differences in sample size, follow-up time, and comorbidities. Patients with CPFE have worse survival than patients with emphysema alone on HRCT 42. As compared to patients with IPF alone, patients with CPFE were reported to have worse 32,38,49,55,56, comparable 24,26,35,37,39,47,57–59,119,210–212, or better survival 31,120,147. Possible explanations for this discrepancy include diagnostic contamination (with a higher proportion of non-IPF cases in CPFE populations with better survival), attrition bias 26,60, differences in the relative extent of emphysema versus fibrosis in different cohorts 24,59,213, and a ‘healthy smoker’ effect 214. A positive correlation was found in some series between the extent of emphysema and the extent of fibrosis 32, however a negative correlation was found in others 29,129,215. In some series, an attempt was made to examine CPFE specifically in sub-groups mostly or wholly made up of IPF 24,32,36,42,55,120,146,212. However, this goal is complicated by the lack of histologic confirmation of UIP in most CPFE patients with suspected IPF, and difficulties discriminating between true honeycomb change (required for an HRCT pattern of UIP) and the admixture of emphysema and pulmonary fibrosis (“pseudohoneycomb change”) on HRCT 216,217.
Prognostic evaluation of CPFE, with particular reference to comparisons between CPFE and isolated IPF, requires quantification of both pulmonary fibrosis and emphysema. This was conducted in two retrospective cohorts of patients with IPF 58,212, using both visual analysis to the nearest 5% 58,212 and computer-based analysis with the CALIPER software 58. The global disease extent on HRCT (i.e. the combined extent of fibrosis and of emphysema) and the baseline DLco both predicted mortality, reflecting the overall severity of parenchymal lung destruction 58,212. After correction for baseline severity using DLco, the presence or extent of emphysema did not impact on survival 58. 32,212,214
There is no evidence that disease progression, FVC trends apart, differs between patients with IPF who have and do not have emphysema 26,29,60,164. It is likely that the lower rate of FVC decline in CPFE 29 is related to the preservation of volumes by emphysema, especially when admixed with fibrosis 58, rather than to slower progression of fibrosis. Further studies should compare progression of fibrosis using serial HRCT, DLco, CPI, and symptom assessment in patients with or without emphysema.
Predictors of mortality in patients with CPFE include DLco 27,60,115, CPI 119,187, age 27, and the presence of specific co-morbidities such as PH 1,27,32,49,115 and lung cancer 41,57,141,218(Table 7). FVC has not been shown to be a predictor of death among patients with CPFE, unless the FVC is <50% predicted 32 and nor is the smoking history 163. However, predictors of death in CPFE, including the impact of PH 30, are not identified consistently in all studies and further work is needed to determine risk factors for death among patients with CPFE syndrome.
Table 7. Variables Associated with Death among Patients with Combined Pulmonary Fibrosis and Emphysema.
| Variable | Association with Increased Mortality | Reference |
|---|---|---|
| Demographic | Older age | 27 |
| Biologic | Negative antinuclear antibodies | 25 |
| Increased red cell distribution width | 220 | |
| Physiologic | ||
| DLco | Lower DLco %predicted | 27, 41, 115, 203, 220, 221 |
| FVC | <50% predicted | 32 |
| FEV1 | Decline of ⩾10% over 12 mo | 138 |
| CPI | ⩾45 | 119, 188, 220 |
| Oxygen saturation | Oxygen saturation on room air < 90% | 59 |
| Oxygen requirement | Home oxygen use | 203 |
| Complications and comorbidities | ||
| Pulmonary hypertension | Elevated pulmonary artery pressure, right ventricular dysfunction, increased pulmonary vascular resistance, low cardiac index, or main pulmonary artery diameter/ascending aortic diameter ratio (depending on study) | 1, 27, 32, 49, 115, 119, 203, 222 |
| Lung cancer | Presence of lung cancer | 41, 57, 59, 141, 203, 206, 219 |
| Acute exacerbations | Acute exacerbations of fibrosis | 206 |
| Radiologic | Presence of UIP pattern | 27, 188 |
| Presence or extent of honeycombing | 27, 41, 203 | |
| Extent of fibrosis (fibrosis score) | 59, 221 | |
Definition of abbreviations: CPI = composite physiologic index; UIP = usual interstitial pneumonia.
Outcomes in summary
Overall, the data suggest that outcomes are worse for a given extent of fibrosis, when there is emphysema in addition to fibrosis (e.g. outcomes are worse in a patient with 10% fibrosis extent and 20% emphysema extent than in a patient with 10% fibrosis extent and no emphysema). However, the risk of mortality and of developing PH does not differ in patients with both IPF and emphysema compared to those with fibrosis alone when adjusting for severity using baseline DLco or total disease extent on HRCT (e.g. total extent of fibrosis and emphysema)(e.g. outcomes are comparable in a patient with 20% emphysema extent and 10% fibrosis extent, and in a patient with 15% emphysema extent and 15% fibrosis extent)30,58,190.
Pathogenesis and putative mechanisms
The pathogenetic mechanisms leading to the coexistence of emphysema with IPF and other fILDs remain unclear. Likewise, it is uncertain whether IPF and non-IPF/ILD are causally linked with emphysema or if they represent different lung disorders running in parallel and sharing some mechanisms.
Clustering of pulmonary fibrosis and emphysema, i.e. increased risk of emphysema in patients with various fILDs 28,49,51,222, supports the notion of a shared pathophysiology. There is bidirectional interaction between emphysema and fibrosis through mechanical forces 52,223–225. Many pathways and pathogenetic mechanisms are shared between fibrosis and emphysema, including gene expression and pathways, gene variants, telomere dysfunction and shortening, alveolar alterations, epigenomic reprogramming, and enzymatic activity, especially matrix metalloproteinases (Table 8)(detailed description in online supplement). Both emphysema and fibrosis develop in several animal models 18,20,99. However, distinct gene variants and pathways were also identified between emphysema and fibrosis 226–229.
Table 8. Main Features Shared by Pulmonary Fibrosis and Emphysema.
| Domain | Features Shared by Pulmonary Fibrosis and Emphysema |
|---|---|
| Clustering of pulmonary fibrosis and emphysema |
|
| Telomere dysfunction and the accelerated aging processes |
|
| Gene expression and interactome | |
| Environmental exposures, smoking, and epigenomic reprogramming | |
| Mechanical forces | |
| Enzymatic activity | |
| Lung development and lung function trajectories | Hypothesis that abnormal mechanisms early in life may predispose to development of emphysema and fibrosis (as reported for the development of emphysema alone [278, 279]) |
| Experimental models characterized by both emphysema and fibrosis and/or inflammation |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; CPFE = combined pulmonary fibrosis and emphysema; HRCT = high - resolution computed tomography; ILD = interstitial lung disease; IPF = idiopathic pulmonary fibrosis; N.B. = nota bene (note); RA = rheumatoid arthritis; SSc = systemic sclerosis; UIP = usual interstitial pneumonia.
Terminology and definitions
Review of existing terminology and definitions
The contemporary terminology and definition of CPFE was provided in a 2005 publication that described a total of 61 patients who were retrospectively selected from a French multicentric study 1. In this publication, CPFE was described as the presence of upper zone predominant emphysema on HRCT plus a peripheral and basal predominant diffuse parenchymal lung disease with significant fibrosis. Emphysema was not quantified, however “conspicuous” emphysema at visual HRCT inspection was an inclusion criterion. A large number of subsequent studies on CPFE have used similar terminology, but with varying definitions and diagnostic criteria. Despite this somewhat imprecise definition, such criteria identified patient populations with comparable physiology in several studies 1,50,115.
A recent systematic review identified the heterogeneous definitions and diagnostic criteria previously used in 72 previous studies on CPFE 3,279. This systematic review was updated in December 2021 and includes 96 studies, which are summarized in Table 9. CPFE was diagnosed based on criteria proposed by Cottin et al. 1 in 53% (51/96) of all eligible studies 3(Figure E10). A diagnosis of IPF was required in 47 studies (49%), while 49 (51%) included a variety of non-IPF fILD. The extent of fibrosis was determined visually in 89 studies (93%). A minimal extent of 10% fibrosis on chest HRCT was required in three studies 202. The majority of studies (75%) diagnosed CPFE if there was any emphysema present on chest HRCT, while 25% used a specific threshold: >5% 121,124, >10% 24,32,148,202,206, >15% 162, >20% 145, and >25% 151 of total lung volume. Quantitative HRCT was used to evaluate fibrosis extent in 7 studies (e.g., percentage of voxels with mean lung attenuation between 0 and -700 Hounsfield units). Fifty two studies required that emphysema be upper lung predominant, 10 studies included emphysema in all locations, and 34 studies did not specify location criteria. The extent of emphysema was assessed visually in 85/96 studies, with 4 studies using the Goddard method of quantifying emphysema 119,207,211,280, and the remaining 11 studies using quantitative HRCT (e.g., percentage of voxels with mean lung attenuation less than -950 Hounsfield units). Few studies used values from pulmonary function tests to define CPFE.
Table 9. Summary of Various Definitions Used by Studies to Identify Patients with Combined Pulmonary Fibrosis and Emphysema.
| Definition for CPFE | Number of Studies | Total Number of Patients with CPFE |
|---|---|---|
| No clear definition | 3 | 259 |
| Definitions using radiologic features only | ||
Definition proposed by Cottin et al. (1) as outlined below:
|
52 | 3,426 |
| Minimum disease extent for fibrosis or emphysema required | 10 | 975 |
| Presence of any fibrosis and emphysema that is different than criteria by Cottin et al. | 22 | 1,804 |
| Definitions using a combination of domains | ||
| Clinical ILD diagnosis + emphysema on imaging | 5 | 332 |
| Clinical abnormalities (e.g., crackles on auscultation, abnormal gas exchange) + fibrosis and emphysema on imaging | 2 | 55 |
| Airflow obstruction confirmed by spirometry + fibrosis on imaging | 2 | 184 |
Definition of abbreviations: CPFE = combined pulmonary fibrosis and emphysema; ILD = interstitial lung disease.
Limitations of previous definitions and terminology of CPFE
Research in CPFE has primarily been driven by observational studies that have led to an appreciation that CPFE possesses unique clinical, radiologic, and physiologic features. However, a major limitation of previous CPFE research is the heterogeneity of study populations and criteria used to define CPFE, prohibiting direct comparison of different cohorts and validation of key findings.
Both imaging and histopathologic studies indicate that CPFE can encompass a variety of fILDs. IPF and COPD share common risk factors of older age and a history of smoking, resulting in this definition likely capturing the largest and most clinically relevant subgroup of patients with ILD who have concurrent emphysema, while also ensuring a relatively homogeneous population. Allowing CPFE to include a variety of ILD subtypes has the advantage of capturing all patients with these two diseases; however, this approach results in a heterogeneous population that complicates assessment of disease biology that might vary across ILD subtypes. An inclusive definition that encompasses all ILD subtypes can also introduce bias in comparison to control populations given the common risk factors for emphysema (e.g. older age and a history of smoking) that also predispose to some ILD subtypes (most notably IPF). Given its association with smoking, IPF is more frequently associated with emphysema than are CTD-ILD or fHP, even if there is now acceptance that smoking can also cause fibrosis distinct from IPF 242–245. A potential approach to reconcile these conflicting priorities is to carefully and transparently define CPFE in a manner that reflects the clinical setting and/or research objectives. For example, studies evaluating prognosis are likely to require separation of IPF and non-IPF patients, while studies evaluating lung physiology may not require such stratification.
Furthermore, few studies have quantified the extent of fibrosis and of emphysema on chest HRCT. Automated quantification is challenging when both components are present (see section on imaging), hampering the development of imaging criteria and consistency between studies. Hence, the term CPFE does not specify extent thresholds for either pulmonary fibrosis or emphysema, with some previous studies including patients with any amount of each abnormality, and other studies setting higher thresholds based on supposed clinical relevance. When used, specific extent thresholds are more commonly applied to emphysema than to pulmonary fibrosis. It is also debated as to whether disease extent should be quantified by visual or quantitative methods. The designation of CPFE only if certain thresholds for emphysema and/or fibrosis are exceeded has the advantage of excluding subclinical disease that may be of no or minimal clinical consequence, and selecting subjects who are at risk of outcomes typical of CPFE. Using such thresholds increases specificity for CPFE, but at the expense of excluding patients with lesser extent of either component. Decisions regarding the use of specific thresholds have, thus, been partially driven by the purposes of individual studies, with biological studies on disease mechanisms potentially not needing high severity thresholds, but such thresholds viewed as more appropriate for clinical or physiological studies in which trivial disease is unlikely to have a meaningful impact. Studying patients with early disease (e.g., with ILAs)127 offers the best opportunity to learn more about the natural history of CPFE and important biological processes that underlie both of these diseases. Future definitions and diagnostic criteria should allow for identification and study of these patients with early disease, particularly when studying biological mechanisms of disease.
Proposed terminology and definitions
The lack of diagnostic criteria and inability to directly compare study populations has hampered the study of the biology, management, and prognosis of CPFE. There is a need to establish specific criteria for CPFE, including standardized and reproducible methods of quantifying both emphysema and fibrosis. The committee proposes a common terminology (Table 10), a provisional, broad research definition for CPFE that will enable future research, and provisional classification criteria of CPFE clinical syndrome intended to serve clinicians managing patients with CPFE (Table 11). Therefore, the CPFE clinical syndrome was identified based on clinical utility (see below), whilst the research definition of CPFE delineates a larger group of patients that should continue to be studied with the ultimate goal of reviewing the syndrome threshold as further clinical and pathogenetic data emerge.
Table 10. Proposed Terminology to Describe Combined Pulmonary Fibrosis and Emphysema.
| Component | Characteristics | Examples |
|---|---|---|
| Fibrosis | Pattern* |
|
| Assessment* |
|
|
| Emphysema | Predominant pattern on HRCT |
|
| Etiology or diagnostic category of fILD | ILD diagnosis* |
|
| Extent | Extent of fibrosis | % of total lung volume |
| Distribution of fibrosis versus emphysema | Separate entities | Emphysema in the upper zones and fibrosis in the lung bases, with no or little overlap of emphysema and fibrosis in between |
| Progressive transition | Progressive transition from emphysema lesions to fibrosis, with significant overlap or admixture in mid areas | |
| Paraseptal emphysema with fibrosis | Paraseptal emphysema with progressive increase in size toward lung bases where ground glass and reticulation coexist | |
| Thick-walled large cysts | Thick-walled large cysts suggesting AEF or SRIF | |
| Admixed pattern | Admixed emphysema and fibrosis increasing from upper lobes to lower lobes | |
| Comments | Physiology or chest HRCT | Predominance of fibrosis vs. emphysema |
| Echocardiography Right heart catheterization |
|
Definition of abbreviations: AEF = airspace enlargement with fibrosis; CTD = connective tissue disease; fHP = fibrotic hypersensitivity pneumonitis; fILD = fibrotic interstitial lung disease; HRCT = high-resolution computed tomography; NSIP = nonspecific interstitial pneumonia; SRIF = smoking-related interstitial fibrosis; UIP = usual interstitial pneumonia.
It is generally useful to include these items when describing CPFE in the individual patient: a case with UIP on HRCT and emphysema in a smoker may be described as smoking-related “CPFE-radiologic UIP.” A case with fibrotic NSIP on biopsy and emphysema may be described as idiopathic “CPFE-histologic NSIP.” A case of UIP pattern and emphysema on HRCT in a nonsmoker with RA may be described as “RA-associated CPFE-radiologic UIP.”
Table 11. Proposed Research Definition of Combined Pulmonary Fibrosis and Emphysema (to Serve Research Purposes) and Classification Criteria of Combined Pulmonary Fibrosis and Emphysema Clinical Syndrome (Intended to Have Clinical Relevance).
| Research definition of CPFE | Coexistence of both pulmonary fibrosis and emphysema Patients must have both criteria on HRCT: |
| Classification criteria of CPFE clinical syndrome: These additional criteria serve research purposes and may be considered depending on the objective of the study | Patients must have CPFE (see above) and one or more of the following:
|
Definition of abbreviations: CPFE = combined pulmonary fibrosis and emphysema; HRCT = high-resolution computed tomography; ILD = interstitial lung disease.
Emphysema generally predominates in the upper lobes but may be present in other areas of the lung or may be admixed with fibrosis.
Emphysema may be replaced by thick-walled large cysts >2.5 cm in diameter (CPFE, thick-walled large cysts variant).
Surgical lung biopsy is not required if HRCT pattern is diagnostic. However, CPFE is suggested if lung biopsies show emphysema and any pattern of pulmonary fibrosis; emphysema can then be quantified on HRCT.
Emphysema extent is assessed visually by an experienced radiologist (see online supplement). Emphysema extent <5% is unlikely to impact physiology or outcome and is more open to interobserver disagreement.
Signs of fibrosis on HRCT in a patient with ILD include architectural distortion, traction bronchiectasis, honeycombing, and volume loss. Caution must be exerted for the identification of honeycombing in patients with associated emphysema. Ground-glass attenuation may be present. Interstitial lung abnormalities (170) are not sufficient to consider CPFE.
Emphysema extent >15% extent is associated with relatively stable FVC over time. Several studies have used a 10% threshold; however, association with outcome in FVC has not been demonstrated.
The committee acknowledged the absence of clear justification to deviate from the entrenched historical term of “CPFE”, recognizing that this is the simplest and broadest label for this group of patients. Similarly, the committee proposes retention of the literal definition of CPFE as the coexistence of both pulmonary fibrosis and emphysema. All subtypes of fILD and emphysema are thus included in the overall CPFE population, but with an important requirement that the fILD subtype be clearly described given the potential biases that can be introduced by including multiple ILDs in this definition. However, it was proposed that studies on CPFE include a comprehensive description of the radiologic and, when available, of the pathological patterns (e.g. “CPFE – IPF”, or “CPFE – radiologic UIP”, or “CPFE - histologic nonspecific interstitial pneumonia”, or “CPFE - radiologic SRIF”) or the underlying disease when known (e.g. CPFE – fHP or CPFE – RA). This would facilitate comparison between studies through a common terminology, and emphasize the heterogeneity of what can be grouped under the umbrella of CPFE.
The most common definition of CPFE is the presence of lung fibrosis and upper-lobe predominant emphysema. The requirement for emphysema in many studies to be upper-lobe predominant minimizes potential confounding by the presence of honeycombing, which is typically lower-lobe predominant and can be difficult to distinguish from paraseptal emphysema. However, the committee proposed that in CPFE, emphysema may be present in other areas of the lung, may be admixed with fibrosis, or may be replaced by thick-walled large cysts greater than 2.5-cm in diameter (“CPFE, thick-walled large cysts variant”).
Some studies have required a specific extent of emphysema, with 15% predicting a distinct outcome for patients with more than this threshold 29, and 10% being a more commonly used threshold 24,32,124,148. For research purposes, the committee proposed to define CPFE based on emphysema extent ≥ 5% of total lung volume (Table 11, Figure 9, and online supplement). For clinicians managing patients with CPFE, the committee proposed classification of CPFE clinical syndrome based on emphysema extent ≥ 15% of total lung volume, and/or in cases of disproportionately decreased DLco or precapillary PH not related to the sole presence of emphysema, fibrosis, or etiological context. The committee acknowledged that further research is needed to refine criteria of CPFE clinical syndrome. For example, studies aiming to evaluate the clinical or functional outcome of patients with CPFE clinical syndrome should assess what specific extents of both pulmonary fibrosis and emphysema ensure clinical relevance of each component. Despite physiological differences compared to isolated ILD and COPD, lung function and especially FEV1/FVC is not sufficiently sensitive or specific to be useful in defining CPFE 29; more studies are needed to assess the potential value of Kco or FVC/DLco.
The committee did not recommend a minimal extent of fibrosis on HRCT, however acknowledged that fILD (not ILAs) is required to defined CPFE. The committee, however, recommended that fibrosis extent and emphysema extent should both be assessed in future studies, using visual assessment, and that the association of the study end points with the presence of emphysema above and below thresholds of emphysema extent should be analyzed, as well as their association with patterns of fILD. The committee also emphasized that there should be generally no restriction on the cause of emphysema (e.g., smoking cigarettes, cannabis, biomass fuel exposure) or of fILD (smoking cigarettes, CTD, idiopathic, etc) unless a study is focused on emphysema of a particular etiology. Future research is required to determine the reproducibility and relevance for research of the CPFE research definition; and the clinical utility of the classification criteria of CPFE clinical syndrome, which in the future may be refined based on physiologic or imaging predictors of outcome that are yet to be identified.
Is CPFE a syndrome?
Background and hypothesis
Current management and future study of CPFE will be facilitated by a clearer understanding of whether this entity has clinical relevance (clinical utility) or if it is biologically unique (pathogenic utility). In early descriptions 12,17,19, CPFE had been viewed as the coincidental coexistence of IPF and emphysema, with a common linkage to smoking. In 2005, the description of the characteristic functional profile of CPFE in a series of 61 patients 1, taken together with the observation of a high prevalence of PH, provided support for “the individualization of CPFE as a discrete clinical entity apart from both IPF and pulmonary emphysema”. The authors considered that “CPFE was not just a distinct phenotype of IPF, but deserved the terminology of syndrome as a result of the association of symptoms and clinical manifestations, each with a probability of being present increased by the presence of the other” 2. However, no consensus exists on whether CPFE is a syndrome or distinct entity.
The committee considered the following options for CPFE: 1) coexistence of two diseases with no clinically relevant implications or major pathogenetic significance (two coincident diseases); 2) coexistence of two diseases with clinically relevant implications and/or major pathogenetic significance (a syndrome); 3) a single biologically unique entity distinct from both IPF and emphysema (one distinct disease).
Definition of a syndrome
In a seminal article 6, Dr. Scadding described a clinical syndrome as one of the four main classes of characteristics by which diseases could be defined : "Patients with a recognizably similar pattern of symptoms and signs were said to be suffering from the same disease. A recognizable pattern of this sort is called a syndrome" 6. A syndrome, therefore, consists of a disease or disorder that involves a particular group of signs and/or symptoms. However, the contemporary definition of a syndrome requires greater provenance than the mere recognition of an association, be it between clinical variables or underlying disease processes. A proposed syndrome generally provides either clinical utility (e.g. serves as an aid to diagnosis, prognostic evaluation, or management) and/or pathogenetic utility (e.g. underlying pathogenetic mechanisms unique to the syndrome are present, providing an avenue for the development of new therapies). In 2005, Cottin et al proposed CPFE as a discrete entity, arguing that “it deserves the terminology of syndrome as a result of the association of symptoms and clinical manifestations, each with a probability of being present increased by the presence of the other” 2.
The main arguments in favor and against CPFE being a syndrome are summarized in Table 12. The Committee favored the term of syndrome based on the following arguments:
Table 12. Main Arguments in Favor of and Against Combined Pulmonary Fibrosis and Emphysema Being a Syndrome.
| In Favor of a Syndrome | Against a Syndrome | |
|---|---|---|
| Pathogeny | Clustering of emphysema and fILD to a greater extent than would be expected from the pack-year smoking history (in patients with IPF, idiopathic nonspecific interstitial pneumonia, rheumatoid arthritis-ILD, and systemic sclerosis-ILD) | No primary pathogenetic pathways unique to CPFE identified |
| Presentation | High prevalence of thick-walled large cysts on HRCT. High prevalence of airspace enlargement with fibrosis on histopathology | No histopathological or radiologic feature specific for CPFE |
| Complications and comorbidities | Increased risk of PH compared with IPF for a given extent of fibrosis Increased risk of lung cancer compared with IPF or emphysema alone | The risk of PH is dependent on total extent of fibrosis and emphysema, the quantification of which is challenging |
| Mortality | Increased mortality compared with IPF for a given extent of fibrosis | The mortality risk is dependent on total extent of fibrosis and emphysema, the quantification of which is challenging |
| Monitoring | FVC alone is not appropriate to monitor disease progression and a primary endpoint in clinical trials. Consider screening and/or monitoring for PH and lung cancer | — |
| Diagnosis | Identification of honeycombing and of the UIP pattern is challenging in patients with concurrent emphysema | — |
Definition of abbreviations: CPFE = combined pulmonary fibrosis and emphysema; fILD = fibrotic interstitial lung diseases; HRCT = high-resolution computed tomography; IPF = idopathic pulmonary fibrosis; PH = pulmonary hypertension; UIP = usual interstitial pneumonia.
Pathogenetic utility
There are multiple pathways common to both pulmonary fibrosis and emphysema; however, no primary pathogenetic pathways unique to CPFE have been identified. One argument in favor of CPFE being a syndrome is the clustering of pulmonary fibrosis and emphysema, e.g. that the presence of emphysema on HRCT is more prevalent than expected in several fILDs (see section on pathogenesis). Taken together, these observations suggest that CPFE may result from involvement of shared pathways in at least some patients.
However, if CPFE represents a biologically distinct syndrome, it is questionable whether it will be applicable to all patients with CPFE. Despite the phenomenon of clustering of emphysema with pulmonary fibrosis, the two diseases will inevitably co-exist in some patients as coincidental smoking-related processes. The definition of a patient group with a unique pathogenetic pathway, if it exists, is likely to require careful morphologic evaluation of histopathologic and HRCT features. Thick-walled cystic lesions (with emphysematous destruction and surrounding dense wall fibrosis) may represent a unique imaging pattern of CPFE, as they were present histologically in an autopsy study in over 70% of patients with CPFE, but never in patients with either isolated pulmonary fibrosis or isolated emphysema 141. The pattern of SRIF or AEF may also represent a unique histopathologic pattern of CPFE 4,180. Much work therefore remains to define CPFE morphologic subtypes and potential identification of signature pathogenetic pathways.
Clinical utility
For the present, the acceptance of CPFE as a syndrome is mostly dependent on its perceived clinical utility. The strongest argument is that monitoring of disease progression cannot be reliably based on FVC in patients with CPFE: serial FVC trends, generally viewed as the cardinal monitoring measure in IPF, are less reliable in CPFE-IPF, with a lower prognostic significance than in the remaining IPF patients without emphysema. The high prevalence of lung cancer and PH further supports the designation of CPFE as a syndrome, especially with the perspective of therapeutic consequences 281.
Another approach to address whether the syndrome of CPFE is a distinct condition would be to demonstrate that its outcome differs from that of IPF alone. However, challenges in the diagnosis and quantification of CPFE hamper prognostic evaluation. As discussed above, difficulties comparing outcomes between patients with CPFE and those with fibrosis alone stem from the heterogeneity of CPFE, both for the emphysema and the fILD components, and from the need to quantify both components to adjust for severity of disease when studying outcome. However, in general, additional emphysema alerts the clinician of a greater likelihood of PH and greater mortality than might be expected for a given extent of fILD 30,58,190. In addition, patients with CPFE have a higher risk of lung cancer than those with IPF alone 194,198.
CPFE as a discrete syndrome
Taken in their entirety, the considerations summarized above indicate that CPFE should be considered a syndrome based on distinct clinical features and pathogenetic considerations and to facilitate further potentially crucial pathogenetic research. Whether it might correspond to a single biologically unique entity in a proportion of cases warrants further study.
Management
General measures
There is a paucity of controlled data and no clinical practice guidelines to inform treatment decisions in patients with CPFE 282. Although some have advocated management based on a “treatable traits” approach (e.g. identifying disease phenotypes and possibly endotypes important for management in the individual patient) 283,284, there are no high-quality data indicating that treatment of emphysema or PH in the context of CPFE improves health outcomes of these patients. Management of CPFE as summarized in Table 13 is therefore typically extrapolated from approaches used in isolated COPD and from data in IPF trials in which patient sub-groups with CPFE have been explored.
Table 13. Key Points of Current Practice Management in Patients with Combined Pulmonary Fibrosis and Emphysema.
| General measures | |
| Pulmonary fibrosis |
|
| Pulmonary emphysema |
|
| Complications and comorbidities |
|
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; COVID-19 = coronavirus disease; CPFE = combined pulmonary fibrosis and emphysema; fILD = fibrotic interstitial lung disease; IPF = idiopathic pulmonary fibrosis; PH = pulmonary hypertension.
Smoking cessation is appropriate in all patients who continue to smoke, as well as avoidance of any other potential inhalational exposures. Supplemental oxygen therapy is recommended in the context of resting hypoxemia 285, and may also have benefits when prescribed only for hypoxemia that occurs during exercise and nocturnally, even in those patients who are normoxemic at rest 285,286. Regular exercise and pulmonary rehabilitation are recommended for most patients with CPFE 192. Although no studies have evaluated pulmonary rehabilitation in CPFE, pulmonary rehabilitation and regular exercise are a cornerstone of management of patients with emphysema and are increasingly used in patients with fILD. As most exacerbations of both COPD and fILD are thought to be triggered by a respiratory tract infection (either from a virus or bacteria), influenza, pneumococcal, and COVID-19 vaccination are also recommended as per standard intervals, unless contraindicated 287,288. Referral for consideration of lung transplantation should be made early in the disease course for appropriate patients due to the progressive natural history of CPFE 289, particularly when complicated by PH.
Treatment of pulmonary fibrosis
Decisions about pharmacologic treatment are guided by the underlying diagnosis of fILD 288. Management of pulmonary fibrosis in the setting of CPFE is informed by the landmark clinical trials of nintedanib and pirfenidone 291–296. Both antifibrotic medications slow progression of mild-to-moderate IPF and other subtypes of progressive pulmonary fibrosis by approximately 50% at 12 months. While patients with significant emphysema (greater than the volume of fibrosis on HRCT) and those with significant airflow obstruction have generally been excluded from these studies, the presence of emphysema in a proportion of patients might have contributed to slow decline in FVC in the placebo arm in CAPACITY 1 291. A subgroup analysis of the IPF INPULSIS trials with nintedanib found no difference in the magnitude of the treatment effect with regards to the presence of mild-to-moderate emphysema 297. Importantly, in the INBUILD trial of nintedanib in fibrotic lung disease other than IPF, progressing despite management 294, the treatment effects were uniform across individual ILDs 298. Therefore, antifibrotic medications may have benefit in IPF patients with CPFE, and in other forms of pulmonary fibrosis with CPFE, progressing despite management. In patients with fILD other than IPF, combined with emphysema, including fHP and CTD-ILD, glucocorticoids and/or immunosuppressive therapy may be beneficial 288. However, there is a need to specifically study CPFE in future trials given its unique physiology. Specifically, the preserved FVC 24 and slower rates of FVC progression 29 indicate that FVC, the traditional endpoint for IPF trials, may be seriously flawed as a primary endpoint in CPFE, as discussed earlier.
Treatment of pulmonary emphysema
Recognition of the individual phenotype of each patient is recommended given the lack of controlled data specific to the treatment of CPFE 35. Inhaled bronchodilators may have benefit in select patients with CPFE who have significant airflow limitation (ie. COPD)299, and one uncontrolled cohort study has suggested a possible improvement in FEV1 following the use of a combination of inhaled corticosteroid and long-acting bronchodilator 286,299. Further studies of inhaled bronchodilators with/without corticosteroids are needed in patients with CPFE due to the relatively well-preserved spirometric values 24.
Surgical or bronchoscopic lung volume reduction therapy removes emphysematous tissue, enabling relatively normal tissue to expand; however, most patients with CPFE would be precluded from such procedure given the frequently severe reduction in DLco 300. Bronchoscopic approach with endobronchial valves is generally safer, although no direct comparison with surgery was performed 301. It is uncertain, however, whether removal of emphysematous tissue will lead to improvements or worsening of lung mechanics in those with CPFE.
Treatment of pulmonary hypertension
Management of PH in the presence of CPFE is based upon managing the underlying respiratory disorder, treating hypoxemia with supplemental oxygen, and ensuring optimal timing for lung transplant referral 115. Controlled data do not support the use of oral PH specific therapies 191,302, including endothelin receptor antagonists (bosentan, ambrisentan), phosphodiesterase-5 inhibitors (sildenafil, tadalafil), or stimulator of soluble guanylate cyclase (riociguat)303, although uncontrolled observational studies show possible benefit from PH therapies 304,305, and there are encouraging secondary endpoint trends in trials using sildenafil in IPF 295,306,307. Particular caution should be exercised, as treatment with ambrisentan and riociguat may be detrimental in patients with fILD 308,309 and especially those with CPFE 310. Recently, nebulized treprostinil improved 6-minute walk distance, decreased NT-pro-brain natriuretic peptide levels, improved FVC, and reduced the risk of clinical worsening compared to placebo in patients with ILD and group 3 precapillary PH confirmed by right heart catheterization 281,311; however, clinical implementation remains limited due to multiple challenges. To date, retrospective data have not demonstrated any survival benefit of PH therapy in patients with CPFE, and further research is required to specifically evaluate these therapies, particularly in those patients with preserved spirometry and “out of proportion PH”.
Treatment of lung cancer
The overall approach to management of lung cancer in CPFE is similar to other populations, with prioritization of surgical resection where possible (e.g., stage I and II non-small cell lung cancer), multiple additional options considered in other situations (e.g., chemotherapy, targeted medications, radiotherapy), and palliation appropriate for many patients 312. Unfortunately, relatively more patients with CPFE are not candidates for various forms of treatment and complication rates are generally higher for those who are treated, with harm likely driven by the combined severity of emphysema and underlying fILD. For example, standard of care cancer treatment could not be instituted in 17% of patients with CPFE and lung cancer due to limitations in treatment directly attributable to CPFE 116.
CPFE is a risk factor for post-surgical morbidity and mortality compared to lung cancer without CPFE 40,45,197,203, with high rates of acute lung injury 200, acute disease exacerbations 43,196,204, and tumor recurrence 201. The risk of treatment-associated acute exacerbation of ILD is of particular concern in patients with CPFE, with increased rates of exacerbation following surgical resection, radiation, and many forms of chemotherapy. Lung-preserving resection options, improved anesthetic considerations, targeted medications, and stereotactic ablative radiotherapy may conceivably all reduce this risk to some extent 312, although there are currently limited direct data to guide risk estimation. Additional studies will continue to test the safety and efficacy of these treatment options in patients with fILD, with these results likely to be generalizable to patients with CPFE.
Clinical trial perspectives
Choice of endpoint
There have been a limited number of clinical trials on CPFE, in part due to its complicated pathophysiology and the lack of a standardized definition. The potential impact of emphysema (CPFE) on commonly used outcomes in COPD and ILD and the change of these variables over time is uncertain and presents difficulty when considering how to include and study these patients in clinical trials. In particular, the use of FVC as an endpoint is hampered in CPFE by its relative stability 29, despite disease progression and a high risk of mortality. The use of DLco is limited by the general functional severity of disease (i.e. floor effect), variability of measurement, and its multiple determinants 313. CPI is not validated as an endpoint. Mortality has been considered impracticable as a primary endpoint 314. Consideration could be given to a composite endpoint (e.g. death, respiratory hospitalization, or categorical FVC decline). However, composite endpoints are usually driven by, and are only as meaningful as, their least severe component 315. HRCT analysis of fibrosis either using visual methods, or future quantitative computer tools that can discriminate emphysema accurately, and/or blood biomarkers may be particularly useful if validated as endpoints.
One retrospective series suggesting that change in FEV1 (decline in FEV1 > 10% over 12 months) was the best physiologic predictor of increased risk of mortality in patients with at least moderate CPFE 138. Although further study is needed, these limited data may have important implications during the design and execution of future clinical trials.
Patients with CPFE in idiopathic pulmonary fibrosis trials
The observation that serial change in FVC, now the favored primary endpoint in IPF treatment trials 316, is confounded by concurrent emphysema, has major implications for future IPF trial design 29. In future IPF trials, patients with a significant functional impact from concurrent emphysema are likely to be excluded. The approach taken in the CAPACITY and ASCEND trials of pirfenidone was to exclude patients with obstructive lung disease based on FEV1/FVC ratio < 0.7 or < 0.8, respectively 291,293. Thus, the effect of pirfenidone on patients with IPF and airflow obstruction is unknown. However, physiology variables are insensitive in excluding patients with emphysema in the setting of IPF 29, and imaging criteria such as extent of emphysema on HRCT may be more appropriate.
In the INPULSIS trials of nintedanib, patients with a FEV1/FVC ratio of < 0.7 were also excluded 292. A post-hoc analysis found that 39.6% of patients had emphysema (scored yes/no at baseline) and 38.8% had a FEV1/FVC ratio > 0.7 and ≤ 0.8 297. The treatment effect of nintedanib versus placebo was similar between patients with and without emphysema, and when comparing different thresholds of FEV1/FVC (0.7 < FEV1/FVC < 0.8 or FEV1/FVC > 0.8 297. Further study is needed to better understand the impact of presence and severity of CPFE and effect of treatment with pirfenidone or nintedanib.
Relevance of CPFE for the non-specialist
Whilst most non-ILD pulmonary specialists and general practitioners have an appreciation for COPD, many will be less familiar with the diagnosis and treatment of CPFE.
In patients with clinical diagnoses of COPD, severely reduced DLco in the setting of minimal to moderate airflow obstruction indicates that additional investigations may be useful and especially chest HRCT. While emphysema alone may present with a disproportionate reduction in DLco, CPFE is considered, particularly given the high prevalence of ILAs on HRCT imaging (in ~8% of smokers aged over 60) and their association with restrictive lung deficits that can obscure features of airflow obstruction by spirometry 127. Although HRCT imaging is not currently considered standard of care in patients with COPD, it has been recently proposed in the diagnosis of COPD 317 and an increasing number of patients undergo imaging, either for lung cancer screening or as additional diagnostic workup for advanced treatments such as endobronchial valve placement. In such instances, HRCT findings may be the first clinical clue that fibrosis is also present. In COPD cohort studies, patients with ILAs have worse clinical outcomes than those without ILAs, including reduced exercise capacity 318 and increased all-cause mortality 319.
After identifying CPFE, additional history and diagnostic testing may be warranted as outlined in this document, similar to what is appropriate in patients with isolated ILD 144,282. Consultation with, or referral to, an ILD specialist may be helpful to determine if the patient is a candidate for ILD specific therapy, although further research is needed to better understand the optimal treatment of this patient population. In general, the presence of emphysema is associated with a worse outcome and a greater likelihood of PH than might be expected for a given extent of ILD. Future research is also needed for the evaluation of lung cancer risk in this population. While both IPF and COPD increase the risk for lung cancer compared to the general population, lung cancer risk for patients with CPFE (or ILAs and emphysema) may be elevated beyond emphysema or IPF alone 148,194,198. Such patients also have generally poor prognosis 198.
Research priorities
The CPFE taskforce committee identified several gaps in our knowledge that need to be addressed, including: 1) to understand the pathogenetic mechanisms in CPFE; 2) to understand the pathobiology, disease behavior, and natural history of CPFE; 3) to improve methods that allow an early diagnosis; and 4) to evaluate potential therapeutic opportunities. Questions and statements identifying some of the topics that were considered important for research are listed in Table 14.
Table 14. Research Priorities in Combined Pulmonary Fibrosis and Emphysema.
| Epidemiology |
|
| Biopathology |
|
| Diagnosis |
|
| Outcome |
|
| Management |
|
| Clinical trials |
|
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; CPFE = combined pulmonary fibrosis and emphysema; fHP = fibrotic hypersensitivity pneumonitis; HRCT = high-resolution computed tomography; ILD = interstitial lung disease; IPF = idiopathic pulmonary fibrosis; UIP = usual interstitial pneumonia.
Conclusions
CPFE is characterized by a wide variety of appearances and patterns on chest HRCT and when available on histopathology. Clustering of pulmonary fibrosis and emphysema (regardless of the type of fILD), the frequency of associated comorbidities and complications especially PH and lung cancer, the relevance for disease progression monitoring, and the involvement of pathogenetic pathways shared by both components, suggest that CPFE should be considered a syndrome. Despite numerous case series and studies, many important questions remain unanswered. This ATS/ERS/JRS/ALAT Research Statement offers research definition and classification criteria and identifies major research priorities that will better delineate this entity, understand its pathogenesis, and guide its management.
Supplementary Material
Figure 2.
HRCT showing a typical distribution of disease seen in combined pulmonary fibrosis and emphysema (progressive transition pattern). A predominant pattern of centrilobular emphysema is seen in the upper lobes, extending to the midzones of the lungs in a 72-year-old patient with idiopathic pulmonary fibrosis. No emphysema is seen in the lower zones. The appearances are in keeping with a progressive transition pattern of combined pulmonary fibrosis and emphysema
Figure 3.
HRCT showing a typical distribution of disease seen in combined pulmonary fibrosis and emphysema (paraseptal emphysema pattern). In this 67 year-old male diagnosed with idiopathic pulmonary fibrosis, extensive isolated paraseptal and centrilobular emphysema is present in the upper zones. Whilst the centrilobular emphysema is mostly isolated in the midzones, paraseptal emphysema is increasingly admixed resembling honeycomb cysts in the left lung. Within the left lower lobe, paraseptal emphysema mimicking honeycomb cysts lies adjacent to more centrally placed irregularly shaped centrilobular emphysema (arrow).
Figure 4.
Chest HRCT showing worsening of admixed destructive emphysema (combined pulmonary fibrosis and emphysema, admixed pattern). In the pair of axial images of the upper (A, B) and lower zones (C, D), taken 2 years apart (A, C: baseline; B, D: follow-up) in a 66-year old male patient with idiopathic pulmonary fibrosis, isolated emphysema in the right upper lobe becomes admixed with fibrosis over time. In the left lower lobe, centrally placed emphysema becomes pulled apart (‘traction emphysema’) and expands as the surrounding fibrosis evolves.
Figure 5.
Chest HRCT showing emphysema admixed with desquamative interstitial pneumonia confirmed by lung biopsy (combined pulmonary fibrosis and emphysema, admixed pattern at HRCT). Areas of low attenuation are admixed with ground glass opacities (high attenuation) and thickening of peri-emphysematous areas.
Figure 6.
Chest HRCT showing admixed emphysema and fibrosis with thick-walled large cysts (combined pulmonary fibrosis and emphysema, thick-walled large cysts pattern). Histopathology demonstrated predominantly smoking-related interstitial fibrosis (SRIF).
Figure 7.
Sagittal CT images of the lungs performed over the course of 4 years in a 66-year-old male ex-smoker diagnosed with idiopathic pulmonary fibrosis and emphysema (combined pulmonary fibrosis and emphysema, thick-walled large cysts pattern). In the top left image low-attenuation lesions without clearly visible walls in keeping with emphysema (arrow) are visible adjacent to the diaphragm and lie within fibrotic regions of lung. Over the next 4 years, as the fibrosis matures, the low-attenuation lesions coalesce (arrow) and enlarge in size forming a thick-walled cystic lesion.
Figure 14. Desquamative interstitial pneumonia (DIP).
A. Low magnification photomicrograph showing a relatively uniform interstitial pneumonia compounded by prominent clusters of pigments alveolar macrophages. B. Higher magnification photomicrograph shows the interstitial inflammation that distinguishes DIP from SRIF (compare to Figure 14B). Hematoxylin and eosin stain.
Acknowledgements
This research statement was funded by the ATS and ERS, and developed by an ad hoc task force from ATS, ERJ, JRS, ALAT:
Funding
Joint ATS/ERS Assembly/Committee Project Application 2019.
Footnotes
Chair
Vincent Cottin
Co-chairs
Yoshikazu Inoue (JRS)
Moises Selman (ALAT) (writing group lead for pathogenesis and research priorities)
Christopher J. Ryerson (ATS) (writing group lead for terminology and classification criteria)
Athol U. Wells (ERS) (writing group lead for lung function and syndrome definition)
Methodologists
Rebecca L. Morgan (ATS)
Thomy Tonia (ERS)
ATS experts
Tamera J. Corte (writing group lead for management)
Kevin R. Flaherty (writing group lead for clinical trials)
MeiLan K. Han (writing group lead for relevance for non-specialists)
Kerri A. Johannson (writing group lead for epidemiology and etiologies)
Joyce S. Lee (writing group lead for clinical manifestations and comorbidities, and outcome)
Fernando J. Martinez
Jeffrey L. Myers
Margaret L. Salisbury
Don D. Sin
Alyson W. Wong (writing group lead for systematic review)
ERS experts
Alvar Agusti
Katerina M. Antoniou
Joseph Jacob writing (group lead for imaging)
Andrew G. Nicholson
Mariaelena Occhipinti
Venerino Poletti
Nicola Sverzellati
Claudia Valenzuela
JRS experts
Tae Iwasawa
Masanori Kitaichi (group lead for pathology)
ALAT experts
Fabian Caro
Matias Florenzano
Patient representatives
Liam Galvin
Pauline Bianchi
This article has an online data supplement, which is accessible from this issue's table of content online at www.atsjournals.org.
Contributor Information
Moises Selman, Email: mselmanl@yahoo.com.mx.
Yoshikazu Inoue, Email: giichiyi@me.com.
Alyson W. Wong, Email: awong@providencehealth.bc.ca.
Tamera J. Corte, Email: tameracorte@mac.com.
Kevin R. Flaherty, Email: flaherty@umich.edu.
MeiLan K. Han, Email: mrking@umich.edu.
Joseph Jacob, Email: j.jacob@ucl.ac.uk.
Kerri A. Johannson, Email: kerri.johannson@ahs.ca.
Masanori Kitaichi, Email: kitaichi.masanori.fq@mail.hosp.go.jp.
Joyce S. Lee, Email: JOYCE.LEE@CUANSCHUTZ.EDU.
Alvar Agusti, Email: aagusti@clinic.cat.
Katerina M. Antoniou, Email: kantoniou@uoc.gr.
Pauline Bianchi, Email: paulinebianchi@yahoo.com.
Fabian Caro, Email: fabiancarodoc@gmail.com.
Matias Florenzano, Email: mflorenzano@gmail.com.
Liam Galvin, Email: liam.galvin@eu-ipff.org.
Tae Iwasawa, Email: tae_i_md@wb3.so-net.ne.jp.
Fernando J. Martinez, Email: fjm2003@med.cornell.edu.
Rebecca L. Morgan, Email: morganrl@mcmaster.ca.
Jeffrey L. Myers, Email: myerjeff@med.umich.edu.
Andrew G. Nicholson, Email: a.nicholson@rbht.nhs.uk.
Mariaelena Occhipinti, Email: mariaelena.occhipinti@gmail.com.
Venerino Poletti, Email: venerino.poletti@gmail.com.
Margaret L. Salisbury, Email: margaret.salisbury@vumc.org.
Don D. Sin, Email: Don.Sin@hli.ubc.ca.
Nicola Sverzellati, Email: nicola.sverzellati@unipr.it.
Thomy Tonia, Email: thomy.tonia@ersnet.org.
Claudia Valenzuela, Email: claudiavale@hotmail.com.
Christopher J. Ryerson, Email: Chris.Ryerson@hli.ubc.ca.
Athol U. Wells, Email: RBHILD@rbht.nhs.uk.
References
- 1.Cottin V, Nunes H, Brillet PY, et al. Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur Respir J. 2005;26:586–93. doi: 10.1183/09031936.05.00021005. [DOI] [PubMed] [Google Scholar]
- 2.Cottin V, Cordier JF. The syndrome of combined pulmonary fibrosis and emphysema. Chest. 2009;136:1–2. doi: 10.1378/chest.09-0538. [DOI] [PubMed] [Google Scholar]
- 3.Wong AW, Liang J, Cottin V, Ryerson CJ. Diagnostic Features in Combined Pulmonary Fibrosis and Emphysema: A Systematic Review. Ann Am Thorac Soc. 2020;17:1333–6. doi: 10.1513/AnnalsATS.202002-122RL. [DOI] [PubMed] [Google Scholar]
- 4.Kawabata Y, Hoshi E, Murai K, et al. Smoking-related changes in the background lung of specimens resected for lung cancer: a semiquantitative study with correlation to postoperative course. Histopathology. 2008;53:707–14. doi: 10.1111/j.1365-2559.2008.03183.x. [DOI] [PubMed] [Google Scholar]
- 5.Katzenstein AL. Smoking-related interstitial fibrosis (SRIF): pathologic findings and distinction from other chronic fibrosing lung diseases. J Clin Pathol. 2013;66:882–7. doi: 10.1136/jclinpath-2012-201338. [DOI] [PubMed] [Google Scholar]
- 6.Scadding JG. Health and disease: what can medicine do for philosophy? J Med Ethics. 1988;14:118–24. doi: 10.1136/jme.14.3.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mallory TB, Castleman B, Parris EE. Case records of the Massachusetts general hospital. Case 34191. New Engl J Med. 1948;238:667–71. [Google Scholar]
- 8.Robbins LL. Idiopathic pulmonary fibrosis: roentgenologic findings. Radiology. 1948;51:459–67. doi: 10.1148/51.4.459. [DOI] [PubMed] [Google Scholar]
- 9.Tourniaire A, Tartulier M, Blum J, Deyrieux F. [Early detection of pulmonary circulatory sound in pure fibrosis and in fibrosis complicated by emphysema] Presse Med. 1966;74:2139–44. [PubMed] [Google Scholar]
- 10.Niewoehner DE, Kleinerman J, Rice DB. Pathologic changes in the peripheral airways of young cigarette smoker’s. N Engl J Med. 1974;291:755–8. doi: 10.1056/NEJM197410102911503. [DOI] [PubMed] [Google Scholar]
- 11.Westcott JL, Cole SR. Traction bronchiectasis in end-stage pulmonary fibrosis. Radiology. 1986;161:665–9. doi: 10.1148/radiology.161.3.3786716. [DOI] [PubMed] [Google Scholar]
- 12.Wiggins J, Strickland B, Turner-Warwick M. Combined cryptogenic fibrosing alveolitis and emphysema: the value of high resolution computed tomography in assessment. Respir Med. 1990;84:365–9. doi: 10.1016/s0954-6111(08)80070-4. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz DA, Merchant RK, Helmers RA, Gilbert SR, Dayton CS, Hunninghake GW. The influence of cigarette smoking on lung function in patients with idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1991;144:504–6. doi: 10.1164/ajrccm/144.3_Pt_1.504. [DOI] [PubMed] [Google Scholar]
- 14.Hiwatari N, Shimura S, Takishima T. Pulmonary emphysema followed by pulmonary fibrosis of undetermined cause. Respiration. 1993;60:354–8. doi: 10.1159/000196235. [DOI] [PubMed] [Google Scholar]
- 15.Strickland NH, Hughes JM, Hart DA, Myers MJ, Lavender JP. Cause of regional ventilation-perfusion mismatching in patients with idiopathic pulmonary fibrosis: a combined CT and scintigraphic study. AJR. 1993;161:719–25. doi: 10.2214/ajr.161.4.8372745. [DOI] [PubMed] [Google Scholar]
- 16.Doherty MJ, Pearson MG, O’Grady EA, Pellegrini V, Calverley PM. Cryptogenic fibrosing alveolitis with preserved lung volumes. Thorax. 1997;52:998–1002. doi: 10.1136/thx.52.11.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells AU, King AD, Rubens MB, Cramer D, du Bois RM, Hansell DM. Lone cryptogenic fibrosing alveolitis: a functional-morphologic correlation based on extent of disease on thin-section computed tomography. Am J Respir Crit Care Med. 1997;155:1367–75. doi: 10.1164/ajrccm.155.4.9105081. [DOI] [PubMed] [Google Scholar]
- 18.Hoyle GW, Li J, Finkelstein JB, et al. Emphysematous lesions, inflammation, and fibrosis in the lungs of transgenic mice overexpressing platelet-derived growth factor. Am J Pathol. 1999;154:1763–75. doi: 10.1016/S0002-9440(10)65432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wells AU, Desai SR, Rubens MB, et al. Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. Am J Respir Crit Care Med. 2003;167:962–9. doi: 10.1164/rccm.2111053. [DOI] [PubMed] [Google Scholar]
- 20.Lundblad LK, Thompson-Figueroa J, Leclair T, et al. Tumor necrosis factor-alpha overexpression in lung disease: a single cause behind a complex phenotype. Am J Respir Crit Care Med. 2005;171:1363–70. doi: 10.1164/rccm.200410-1349OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cottin V, Cordier JF. Combined pulmonary fibrosis and emphysema: an experimental and clinically relevant phenotype. Am J Respir Crit Care Med. 2005;172:1605. doi: 10.1164/ajrccm.172.12.1605a. author reply - 6. [DOI] [PubMed] [Google Scholar]
- 22.Yousem SA. Respiratory bronchiolitis-associated interstitial lung disease with fibrosis is a lesion distinct from fibrotic nonspecific interstitial pneumonia: a proposal. Mod Pathol. 2006;19:1474–9. doi: 10.1038/modpathol.3800671. [DOI] [PubMed] [Google Scholar]
- 23.Katzenstein AL, Mukhopadhyay S, Zanardi C, Dexter E. Clinically occult interstitial fibrosis in smokers: classification and significance of a surprisingly common finding in lobectomy specimens. Hum Pathol. 2010;41:316–25. doi: 10.1016/j.humpath.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Ryerson CJ, Hartman T, Elicker BM, et al. Clinical features and outcomes in combined pulmonary fibrosis and emphysema in idiopathic pulmonary fibrosis. Chest. 2013;144:234–40. doi: 10.1378/chest.12-2403. [DOI] [PubMed] [Google Scholar]
- 25.Tzouvelekis A, Zacharis G, Oikonomou A, et al. Increased incidence of autoimmune markers in patients with combined pulmonary fibrosis and emphysema. BMC Pulm Med. 2013;13:31. doi: 10.1186/1471-2466-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akagi T, Matsumoto T, Harada T, et al. Coexistent emphysema delays the decrease of vital capacity in idiopathic pulmonary fibrosis. Respiratory Medicine. 2009;103:1209–15. doi: 10.1016/j.rmed.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Alsumrain M, De Giacomi F, Nasim F, et al. Combined pulmonary fibrosis and emphysema as a clinicoradiologic entity: Characterization of presenting lung fibrosis and implications for survival. Respir Med. 2019;146:106–12. doi: 10.1016/j.rmed.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Antoniou KM, Walsh SL, Hansell DM, et al. Smoking-related emphysema is associated with idiopathic pulmonary fibrosis and rheumatoid lung. Respirology. 2013;18:1191–6. doi: 10.1111/resp.12154. [DOI] [PubMed] [Google Scholar]
- 29.Cottin V, Hansell DM, Sverzellati N, et al. Effect of Emphysema Extent on Serial Lung Function in Patients with Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2017;196:1162–71. doi: 10.1164/rccm.201612-2492OC. [DOI] [PubMed] [Google Scholar]
- 30.Jacob J, Bartholmai BJ, Rajagopalan S, et al. Likelihood of pulmonary hypertension in patients with idiopathic pulmonary fibrosis and emphysema. Respirology. 2018;23:593–9. doi: 10.1111/resp.13231. [DOI] [PubMed] [Google Scholar]
- 31.Kurashima K, Takayanagi N, Tsuchiya N, et al. The effect of emphysema on lung function and survival in patients with idiopathic pulmonary fibrosis. Respirology. 2010;15:843–8. doi: 10.1111/j.1440-1843.2010.01778.x. [DOI] [PubMed] [Google Scholar]
- 32.Mejia M, Carrillo G, Rojas-Serrano J, et al. Idiopathic pulmonary fibrosis and emphysema: decreased survival associated with severe pulmonary arterial hypertension. Chest. 2009;136:10–5. doi: 10.1378/chest.08-2306. [DOI] [PubMed] [Google Scholar]
- 33.Tasaka S, Mizoguchi K, Funatsu Y, et al. Cytokine profile of bronchoalveolar lavage fluid in patients with combined pulmonary fibrosis and emphysema. Respirology. 2012 doi: 10.1111/j.1440-1843.2012.02182.x. [DOI] [PubMed] [Google Scholar]
- 34.Sangani R, Ghio A, Culp S, Patel Z, Sharma S. Combined Pulmonary Fibrosis Emphysema: Role of Cigarette Smoking and Pulmonary Hypertension in a Rural Cohort. Int J Chron Obstruct Pulmon Dis. 2021;16:1873–85. doi: 10.2147/COPD.S307192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato S, Tanino Y, Misa K, et al. Identification of Clinical Phenotypes in Idiopathic Interstitial Pneumonia with Pulmonary Emphysema. Intern Med. 2016;55:1529–35. doi: 10.2169/internalmedicine.55.6009. [DOI] [PubMed] [Google Scholar]
- 36.Choi SH, Lee HY, Lee KS, et al. The value of CT for disease detection and prognosis determination in combined pulmonary fibrosis and emphysema (CPFE) PLoS One. 2014;9:e107476. doi: 10.1371/journal.pone.0107476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jankowich MD, Rounds S. Combined pulmonary fibrosis and emphysema alters physiology but has similar mortality to pulmonary fibrosis without emphysema. Lung. 2010;188:365–73. doi: 10.1007/s00408-010-9251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang M, Yoshizawa A, Kawakami S, et al. The histological characteristics and clinical outcomes of lung cancer in patients with combined pulmonary fibrosis and emphysema. Cancer Med. 2016;5:2721–30. doi: 10.1002/cam4.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao L, Xie S, Liu H, et al. Lung cancer in patients with combined pulmonary fibrosis and emphysema revisited with the 2015 World Health Organization classification of lung tumors. Clin Respir J. 2018;12:652–8. doi: 10.1111/crj.12575. [DOI] [PubMed] [Google Scholar]
- 40.Sato S, Koike T, Hashimoto T, et al. Surgical Outcomes of Lung Cancer Patients with Combined Pulmonary Fibrosis and Emphysema and Those with Idiopathic Pulmonary Fibrosis without Emphysema. Annals of thoracic and cardiovascular surgery : official journal of the Association of Thoracic and Cardiovascular Surgeons of Asia. 2016;22:216–23. doi: 10.5761/atcs.oa.15-00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim YS, Jin GY, Chae KJ, et al. Visually stratified CT honeycombing as a survival predictor in combined pulmonary fibrosis and emphysema. Br J Radiol. 2015;88:20150545. doi: 10.1259/bjr.20150545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee CH, Kim HJ, Park CM, et al. The impact of combined pulmonary fibrosis and emphysema on mortality. Int J Tuberc Lung Dis. 2011;15:1111–6. doi: 10.5588/ijtld.10.0491. [DOI] [PubMed] [Google Scholar]
- 43.Otsuka H, Sugino K, Hata Y, et al. Clinical features and outcomes of patients with lung cancer as well as combined pulmonary fibrosis and emphysema. Molecular and clinical oncology. 2016;5:273–8. doi: 10.3892/mco.2016.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyamoto A, Kurosaki A, Moriguchi S, et al. Reduced area of the normal lung on high-resolution computed tomography predicts poor survival in patients with lung cancer and combined pulmonary fibrosis and emphysema. Respir Investig. 2019;57:140–9. doi: 10.1016/j.resinv.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 45.Fukui M, Suzuki K, Matsunaga T, Oh S, Takamochi K. Outcomes of lung cancer resection for patients with combined pulmonary fibrosis and emphysema. Surg Today. 2016;46:341–7. doi: 10.1007/s00595-015-1234-z. [DOI] [PubMed] [Google Scholar]
- 46.Jankowich MD, Rounds SIS. Combined pulmonary fibrosis and emphysema syndrome. A review. Chest. 2012;141:222–31. doi: 10.1378/chest.11-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jacob J, Odink A, Brun AL, et al. Functional associations of pleuroparenchymal fibroelastosis and emphysema with hypersensitivity pneumonitis. Respir Med. 2018;138:95–101. doi: 10.1016/j.rmed.2018.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raghu G, Remy-Jardin M, Ryerson CJ, et al. Diagnosis of Hypersensitivity Pneumonitis in Adults. An Official ATS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202:e36–e69. doi: 10.1164/rccm.202005-2032ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Champtiaux N, Cottin V, Chassagnon G, et al. Groupe d’Etudes et de Recherche sur les Maladies Orphelines Pulmonaires. Combined pulmonary fibrosis and emphysema in systemic sclerosis: A syndrome associated with heavy morbidity and mortality. Semin Arthritis Rheum. 2019;49:98–104. doi: 10.1016/j.semarthrit.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 50.Cottin V, Nunes H, Mouthon L, et al. Combined pulmonary fibrosis and emphysema syndrome in connective tissue disease. Arthritis Rheum. 2011;63:295–304. doi: 10.1002/art.30077. [DOI] [PubMed] [Google Scholar]
- 51.Antoniou KM, Margaritopoulos GA, Goh NS, et al. Combined Pulmonary Fibrosis and Emphysema in Scleroderma-Related Lung Disease Has a Major Confounding Effect on Lung Physiology and Screening for Pulmonary Hypertension. Arthritis Rheumatol. 2016;68:1004–12. doi: 10.1002/art.39528. [DOI] [PubMed] [Google Scholar]
- 52.Jacob J, Song JW, Yoon HY, et al. Prevalence and Effects of Emphysema in Never-Smokers with Rheumatoid Arthritis Interstitial Lung Disease. EBioMedicine. 2018;28:303–10. doi: 10.1016/j.ebiom.2018.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cottin V, Freymond N, Cabane J, Cordier JF. Combined pulmonary fibrosis and emphysema syndrome in a patient age 28 years with severe systemic sclerosis. J Rheumatol. 2011;38:2082–3. doi: 10.3899/jrheum.101365. [DOI] [PubMed] [Google Scholar]
- 54.Silva DR, Gazzana MB, Barreto SSM, Knorst MM. Idiopathic pulmonary fibrosis and emphysema in smokers. J Bras Pneumol. 2008;34:779–86. doi: 10.1590/s1806-37132008001000005. [DOI] [PubMed] [Google Scholar]
- 55.Ye Q, Huang K, Ding Y, et al. Cigarette smoking contributes to idiopathic pulmonary fibrosis associated with emphysema. Chin Med J (Engl) 2014;127:469–74. [PubMed] [Google Scholar]
- 56.Tokgoz Akyil F, Sevim T, Akman C, et al. The predictors of mortality in IPF - Does emphysema change the prognosis? Sarcoidosis Vasc Diffuse Lung Dis. 2016;33:267–74. [PubMed] [Google Scholar]
- 57.Portillo K, Perez-Rodas N, Garcia-Olive I, et al. Lung Cancer in Patients With Combined Pulmonary Fibrosis and Emphysema and Idiopathic Pulmonary Fibrosis. A Descriptive Study in a Spanish Series. Arch Bronconeumol. 2017;53:304–10. doi: 10.1016/j.arbres.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 58.Jacob J, Bartholmai BJ, Rajagopalan S, et al. Functional and prognostic effects when emphysema complicates idiopathic pulmonary fibrosis. Eur Respir J. 2017;50:1700379. doi: 10.1183/13993003.00379-2017. [DOI] [PubMed] [Google Scholar]
- 59.Lai RS, Chen CF, Chu KA, Lin MH. The effect of emphysema on survival in patients with idiopathic pulmonary fibrosis: A retrospective study in Taiwan. Journal of the Chinese Medical Association : JCMA. 2019;82:922–8. doi: 10.1097/JCMA.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 60.Kim YJ, Shin SH, Park JW, et al. Annual Change in Pulmonary Function and Clinical Characteristics of Combined Pulmonary Fibrosis and Emphysema and Idiopathic Pulmonary Fibrosis: Over a 3-Year Follow-up. Tuberculosis and respiratory diseases. 2014;77:18–23. doi: 10.4046/trd.2014.77.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chae KJ, Jin GY, Jung HN, et al. Differentiating Smoking-Related Interstitial Fibrosis (SRIF) from Usual Interstitial Pneumonia (UIP) with Emphysema Using CT Features Based on Pathologically Proven Cases. PLoS One. 2016;11:e0162231. doi: 10.1371/journal.pone.0162231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ariani A, Silva M, Bravi E, et al. Overall mortality in combined pulmonary fibrosis and emphysema related to systemic sclerosis. RMD Open. 2019;5:e000820. doi: 10.1136/rmdopen-2018-000820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamakawa H, Takemura T, Iwasawa T, et al. Emphysematous change with scleroderma-associated interstitial lung disease: the potential contribution of vasculopathy? BMC Pulm Med. 2018;18:25. doi: 10.1186/s12890-018-0591-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bae SS, Pourzand L, Kim GH, et al. The disconnect between visual assessment of air trapping and lung physiology for assessment of small airway disease in scleroderma-related interstitial lung disease: An observation from the Scleroderma Lung Study II Cohort. J Scleroderma Related Disorders. 2021 doi: 10.1177/23971983211047160. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tzouvelekis A, Zacharis G, Oikonomou A, et al. Combined pulmonary fibrosis and emphysema associated with microscopic polyangiitis. Eur Respir J. 2012;40:505–7. doi: 10.1183/09031936.00216311. [DOI] [PubMed] [Google Scholar]
- 66.Gocho K, Sugino K, Sato K, Hasegawa C, Uekusa T, Homma S. Microscopic polyangiitis preceded by combined pulmonary fibrosis and emphysema. Respir Med Case Rep. 2015;15:128–32. doi: 10.1016/j.rmcr.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dawson JK, Fewins HE, Desmond J, Lynch MP, Graham DR. Fibrosing alveolitis in patients with rheumatoid arthritis as assessed by high resolution computed tomography, chest radiography, and pulmonary function tests. Thorax. 2001;56:622–7. doi: 10.1136/thorax.56.8.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim HC, Lee JS, Lee EY, et al. Risk prediction model in rheumatoid arthritis-associated interstitial lung disease. Respirology. 2020;25:1257–64. doi: 10.1111/resp.13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Song JW, Do K-H, Kim M-Y, Jang SJ, Colby TV, Kim DS. Pathologic and radiologic differences between idiopathic and collagen vascular disease-related usual interstitial pneumonia. Chest. 2009;136:23–30. doi: 10.1378/chest.08-2572. [DOI] [PubMed] [Google Scholar]
- 70.Leigh J, Driscoll TR, Cole BD, Beck RW, Hull BP, Yang J. Quantitative relation between emphysema and lung mineral content in coalworkers. Occup Environ Med. 1994;51:400–7. doi: 10.1136/oem.51.6.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heppleston AG. The pathological recognition and pathogenesis of emphysema and fibrocystic disease of the lung with special reference to coal workers. Ann N Y Acad Sci. 1972;200:347–69. doi: 10.1111/j.1749-6632.1972.tb40201.x. [DOI] [PubMed] [Google Scholar]
- 72.Marchiori E, Lourenco S, Gasparetto TD, Zanetti G, Mano CM, Nobre LF. Pulmonary talcosis: imaging findings. Lung. 2010;188:165–71. doi: 10.1007/s00408-010-9230-y. [DOI] [PubMed] [Google Scholar]
- 73.Daniil Z, Koutsokera A, Gourgoulianis K. Combined pulmonary fibrosis and emphysema in patients exposed to agrochemical compounds. Eur Respir J. 2006;27:434. doi: 10.1183/09031936.06.00124505. [DOI] [PubMed] [Google Scholar]
- 74.Akira M, Yamamoto S, Inoue Y, Sakatani M. High-resolution CT of asbestosis and idiopathic pulmonary fibrosis. AJR Am J Roentgenol. 2003;181:163–9. doi: 10.2214/ajr.181.1.1810163. [DOI] [PubMed] [Google Scholar]
- 75.Copley SJ, Wells AU, Sivakumaran P, et al. Asbestosis and idiopathic pulmonary fibrosis: comparison of thin-section CT features. Radiology. 2003;229:731–6. doi: 10.1148/radiol.2293020668. [DOI] [PubMed] [Google Scholar]
- 76.Copley SJ, Lee YC, Hansell DM, et al. Asbestos-induced and smoking-related disease: apportioning pulmonary function deficit by using thin-section CT. Radiology. 2007;242:258–66. doi: 10.1148/radiol.2421051167. [DOI] [PubMed] [Google Scholar]
- 77.Huuskonen O, Kivisaari L, Zitting A, Kaleva S, Vehmas T. Emphysema findings associated with heavy asbestos-exposure in high resolution computed tomography of finnish construction workers. J Occup Health. 2004;46:266–71. doi: 10.1539/joh.46.266. [DOI] [PubMed] [Google Scholar]
- 78.Cormier Y, Brown M, Worthy S, Racine G, Muller NL. High-resolution computed tomographic characteristics in acute farmer’s lung and in its follow-up. Eur Respir J. 2000;16:56–60. doi: 10.1034/j.1399-3003.2000.16a10.x. [DOI] [PubMed] [Google Scholar]
- 79.Marchetti N, Garshick E, Kinney GL, et al. Association between occupational exposure and lung function, respiratory symptoms, and high-resolution computed tomography imaging in COPDGene. Am J Respir Crit Care Med. 2014;190:756–62. doi: 10.1164/rccm.201403-0493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lakhanpal S, Lie JT, Conn DL, Martin WJ., 2nd Pulmonary disease in polymyositis/dermatomyositis: a clinicopathological analysis of 65 autopsy cases. Ann Rheum Dis. 1987;46:23–9. doi: 10.1136/ard.46.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nemoto M, Noma S, Otsuki A, et al. Combined pulmonary fibrosis and emphysema with myeloperoxidase-antineutrophil cytoplasmic antibody positivity that resolved upon smoking cessation. Respir Med Case Rep. 2018;25:165–9. doi: 10.1016/j.rmcr.2018.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alba DB, Suarez SR, Morillo SG. Relationship Between Combination of Pulmonary Fibrosis and Emphysema, ANCA, and Vasculitis. Chest. 2020;158:1288–9. doi: 10.1016/j.chest.2020.02.080. [DOI] [PubMed] [Google Scholar]
- 83.Erkinjuntti-Pekkanen R, Rytkonen H, Kokkarinen JI, Tukiainen HO, Partanen K, Terho EO. Long term risk of emphysema in patients with farmer’s lung and matched control farmers. Am J Respir Crit Care Med. 1998;158:662–5. doi: 10.1164/ajrccm.158.2.9710012. [DOI] [PubMed] [Google Scholar]
- 84.Soumagne T, Pana-Katatali H, Degano B, Dalphin JC. Combined pulmonary fibrosis and emphysema in hypersensitivity pneumonitis. BMJ case reports. 2015;2015 doi: 10.1136/bcr-2015-211560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Begin R, Filion R, Ostiguy G. Emphysema in silica- and asbestos-exposed workers seeking compensation. A CT scan study. Chest. 1995;108:647–55. doi: 10.1378/chest.108.3.647. [DOI] [PubMed] [Google Scholar]
- 86.Karkhanis VS, Joshi JM. Combined pulmonary fibrosis and emphysema in a tyre industry worker. Lung India. 2012;29:273–6. doi: 10.4103/0970-2113.99116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Porru S, Placidi D, Quarta C, Sabbioni E, Pietra R, Fortaner S. The potencial role of rare earths in the pathogenesis of interstitial lung disease: a case report of movie projectionist as investigated by neutron activation analysis. J Trace Elem Med Biol. 2001;14:232–6. doi: 10.1016/S0946-672X(01)80008-0. [DOI] [PubMed] [Google Scholar]
- 88.Roshan R, Guptal M, Kulshrestha R, Menon B, Chhabra SK. Combined pulmonary fibrosis and emphysema in a welder. Monaldi Arch Chest Dis. 2012;77:26–8. doi: 10.4081/monaldi.2012.164. [DOI] [PubMed] [Google Scholar]
- 89.Alder JK, Guo N, Kembou F, et al. Telomere length is a determinant of emphysema susceptibility. Am J Respir Crit Care Med. 2011;184:904–12. doi: 10.1164/rccm.201103-0520OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hisata S, Sakaguchi H, Kanegane H, et al. A Novel Missense Mutation of DKC1 In Dyskeratosis Congenita With Pulmonary Fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2013;30:221–5. [PubMed] [Google Scholar]
- 91.Stanley SE, Chen JJ, Podlevsky JD, et al. Telomerase mutations in smokers with severe emphysema. J Clin Invest. 2015;125:563–70. doi: 10.1172/JCI78554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Newton CA, Batra K, Torrealba J, et al. Telomere-related lung fibrosis is diagnostically heterogeneous but uniformly progressive. Eur Respir J. 2016;48:1710–20. doi: 10.1183/13993003.00308-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nunes H, Monnet I, Kannengiesser C, et al. Is telomeropathy the explanation for combined pulmonary fibrosis and emphysema syndrome?: report of a family with TERT mutation. Am J Respir Crit Care Med. 2014;189:753–4. doi: 10.1164/rccm.201309-1724LE. [DOI] [PubMed] [Google Scholar]
- 94.Cottin V, Reix P, Khouatra C, Thivolet-Bejui F, Feldmann D, Cordier JF. Combined pulmonary fibrosis and emphysema syndrome associated with familial SFTPC mutation. Thorax. 2011;66:918–9. doi: 10.1136/thx.2010.151407. [DOI] [PubMed] [Google Scholar]
- 95.Epaud R, Delestrain C, Louha M, Simon S, Fanen P, Tazi A. Combined pulmonary fibrosis and emphysema syndrome associated with ABCA3 mutations. Eur Respir J. 2014;43:638–41. doi: 10.1183/09031936.00145213. [DOI] [PubMed] [Google Scholar]
- 96.Ota C, Kimura M, Kure S. ABCA3 mutations led to pulmonary fibrosis and emphysema with pulmonary hypertension in an 8-year-old girl. Pediatr Pulmonol. 2016;51:E21–3. doi: 10.1002/ppul.23379. [DOI] [PubMed] [Google Scholar]
- 97.Cottin V, Cordier JF. SFTPC mutations in patients with familial pulmonary fibrosis: combined with emphysema? Am J Respir Crit Care Med. 2011;183:1113. doi: 10.1164/ajrccm.183.8.1113a. author reply -4. [DOI] [PubMed] [Google Scholar]
- 98.van Moorsel CH, van Oosterhout MF, Barlo NP, et al. Surfactant protein C mutations are the basis of a significant portion of adult familial pulmonary fibrosis in a dutch cohort. Am J Respir Crit Care Med. 2010;182:1419–25. doi: 10.1164/rccm.200906-0953OC. [DOI] [PubMed] [Google Scholar]
- 99.Glasser SW, Detmer EA, Ikegami M, Na CL, Stahlman MT, Whitsett JA. Pneumonitis and emphysema in sp-C gene targeted mice. J Biol Chem. 2003;278:14291–8. doi: 10.1074/jbc.M210909200. [DOI] [PubMed] [Google Scholar]
- 100.Stanley SE, Gable DL, Wagner CL, et al. Loss-of-function mutations in the RNA biogenesis factor NAF1 predispose to pulmonary fibrosis-emphysema. Sci Transl Med. 2016;8:351ra107. doi: 10.1126/scitranslmed.aaf7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cottin V, Nasser M, Traclet J, et al. Prolidase deficiency: a new genetic cause of combined pulmonary fibrosis and emphysema syndrome in the adult. Eur Respir J. 2020 doi: 10.1183/13993003.01952-2019. [DOI] [PubMed] [Google Scholar]
- 102.Kukkonen MK, Tiili E, Vehmas T, Oksa P, Piirila P, Hirvonen A. Association of genes of protease-antiprotease balance pathway to lung function and emphysema subtypes. BMC Pulm Med. 2013;13:36. doi: 10.1186/1471-2466-13-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gu H, You H, Ning W, et al. Internal migration and maternal health service utilisation in Jiangsu, China. Trop Med Int Health. 2017;22:124–32. doi: 10.1111/tmi.12806. [DOI] [PubMed] [Google Scholar]
- 104.Xu L, Bian W, Gu XH, Shen C. Genetic polymorphism in matrix metalloproteinase-9 and transforming growth factor-beta1 and susceptibility to combined pulmonary fibrosis and emphysema in a Chinese population. Kaohsiung J Med Sci. 2017;33:124–9. doi: 10.1016/j.kjms.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kinjo T, Kitaguchi Y, Droma Y, et al. The Gly82Ser mutation in AGER contributes to pathogenesis of pulmonary fibrosis in combined pulmonary fibrosis and emphysema (CPFE) in Japanese patients. Scientific reports. 2020;10:12811. doi: 10.1038/s41598-020-69184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guzman-Vargas J, Ambrocio-Ortiz E, Pérez-Rubio G, et al. Differential Genomic Profile in TERT, DSP, and FAM13A Between COPD Patients With Emphysema, IPF, and CPFE Syndrome. Front Med (Lausanne) 2021;8:725144. doi: 10.3389/fmed.2021.725144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chilosi M, Poletti V, Rossi A. The pathogenesis of COPD and IPF: distinct horns of the same devil? Respir Res. 2012;13:3. doi: 10.1186/1465-9921-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Faner R, Rojas M, Macnee W, Agusti A. Abnormal lung aging in chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;186:306–13. doi: 10.1164/rccm.201202-0282PP. [DOI] [PubMed] [Google Scholar]
- 109.Chilosi M, Carloni A, Rossi A, Poletti V. Premature lung aging and cellular senescence in the pathogenesis of idiopathic pulmonary fibrosis and COPD/emphysema. Translational research : the journal of laboratory and clinical medicine. 2013;162:156–73. doi: 10.1016/j.trsl.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 110.Selman M, Pardo A. Revealing the Pathogenic and Aging-related Mechanisms of the Enigmatic Idiopathic Pulmonary Fibrosis. An Integral Model. Am J Respir Crit Care Med. 2014;189:1161–72. doi: 10.1164/rccm.201312-2221PP. [DOI] [PubMed] [Google Scholar]
- 111.Selman M, Martinez FJ, Pardo A. Why an Aging Smoker Lung Develops IPF and Not COPD? Am J Respir Crit Care Med. 2018 doi: 10.1164/rccm.201806-1166PP. [DOI] [PubMed] [Google Scholar]
- 112.Duckworth A, Gibbons MA, Allen RJ, et al. Telomere length and risk of idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease: a mendelian randomisation study. Lancet Respir Med. 2021;9:285–94. doi: 10.1016/S2213-2600(20)30364-7. [DOI] [PubMed] [Google Scholar]
- 113.Armanios M. Telomerase makes connections between pulmonary fibrosis and emphysema. Am J Respir Crit Care Med. 2014;189:754–5. doi: 10.1164/rccm.201401-0019LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Warburton D, Shi W, Xu B. TGF-beta-Smad3 signaling in emphysema and pulmonary fibrosis: an epigenetic aberration of normal development? Am J Physiol Lung Cell Mol Physiol. 2013;304:L83–5. doi: 10.1152/ajplung.00258.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cottin V, Le Pavec J, Prevot G, et al. Pulmonary hypertension in patients with combined pulmonary fibrosis and emphysema syndrome. Eur Respir J. 2010;35:105–11. doi: 10.1183/09031936.00038709. [DOI] [PubMed] [Google Scholar]
- 116.Girard N, Marchand Adam S, Naccache JM, et al. Lung cancer in combined pulmonary fibrosis and emphysema: a series of 47 Western patients. J Thorac Oncol. 2014;9:1162–70. doi: 10.1097/JTO.0000000000000209. [DOI] [PubMed] [Google Scholar]
- 117.Kitaguchi Y, Fujimoto K, Hanaoka M, Honda T, Hotta J, Hirayama J. Pulmonary function impairment in patients with combined pulmonary fibrosis and emphysema with and without airflow obstruction. Int J Chron Obstruct Pulmon Dis. 2014;9:805–11. doi: 10.2147/COPD.S65621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wells AU, Margaritopoulos GA, Antoniou KM, Nunes H, Costabel U. CPFE: distinctive and non-distinctive features. Eur Respir Mon. 2016;71:175–85. [Google Scholar]
- 119.Çiftci F, Gülpinar B, Atasoy Ç, Kayacan O, Saryal S. Combined pulmonary fibrosis and emphysema: How does cohabitation affect respiratory functions? Advances in medical sciences. 2019;64:285–91. doi: 10.1016/j.advms.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 120.Bodlet A, Maury G, Jamart J, Dahlqvist C. Influence of radiological emphysema on lung function test in idiopathic pulmonary fibrosis. Respir Med. 2013;107:1781–8. doi: 10.1016/j.rmed.2013.08.039. [DOI] [PubMed] [Google Scholar]
- 121.Mura M, Zompatori M, Pacilli AM, Fasano L, Schiavina M, Fabbri M. The presence of emphysema further impairs physiologic function in patients with idiopathic pulmonary fibrosis. Respir Care. 2006;51:257–65. [PubMed] [Google Scholar]
- 122.Matsuoka S, Yamashiro T, Matsushita S, et al. Quantitative CT evaluation in patients with combined pulmonary fibrosis and emphysema: correlation with pulmonary function. Acad Radiol. 2015;22:626–31. doi: 10.1016/j.acra.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 123.Ando K, Sekiya M, Tobino K, Takahashi K. Relationship between quantitative CT metrics and pulmonary function in combined pulmonary fibrosis and emphysema. Lung. 2013;191:585–91. doi: 10.1007/s00408-013-9513-1. [DOI] [PubMed] [Google Scholar]
- 124.Yoon HY, Kim TH, Seo JB, et al. Effects of emphysema on physiological and prognostic characteristics of lung function in idiopathic pulmonary fibrosis. Respirology. 2019;24:55–62. doi: 10.1111/resp.13387. [DOI] [PubMed] [Google Scholar]
- 125.Ash SY, Harmouche R, Ross JC, et al. Interstitial Features at Chest CT Enhance the Deleterious Effects of Emphysema in the COPDGene Cohort. Radiology. 2018;288:600–9. doi: 10.1148/radiol.2018172688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kitaguchi Y, Fujimoto K, Hayashi R, Hanaoka M, Honda T, Kubo K. Annual changes in pulmonary function in combined pulmonary fibrosis and emphysema: over a 5-year followup. Respir Med. 2013;107:1986–92. doi: 10.1016/j.rmed.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 127.Washko GR, Hunninghake GM, Fernandez IE, et al. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364:897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ponçot-Mongars R, Zysman M, Regent D, Gomez E, Chaouat A, Chabot F. Syndrome emphysème - fibrose pulmonaire: histoire naturelle de la maladie. Evolution chronologique clinique, fonctionnelle respiratoire et scanographique [Combined pulmonary fibrosis and emphysema: the natural history of the disease. The chronological evolution of clinical features, respiratory function and the CT scan] Rev Mal Respir. 2013;30:222–6. doi: 10.1016/j.rmr.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 129.Brillet P, Cottin V, Letoumelin P, et al. Syndrome emphysème des sommets et fibrose pulmonaire des bases combinés (syndrome emphysème/fibrose): aspects tomodensitométriques et fonctionnels [Combined apical emphysema and basal fibrosis syndrome (emphysema/fibrosis syndrome): CT imaging features and pulmonary function tests] J Radiol. 2009;90:43–51. doi: 10.1016/s0221-0363(09)70077-0. [DOI] [PubMed] [Google Scholar]
- 130.Mori K, Shirai T, Mikamo M, et al. Respiratory mechanics measured by forced oscillation technique in combined pulmonary fibrosis and emphysema. Respiratory physiology & neurobiology. 2013;185:235–40. doi: 10.1016/j.resp.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 131.Aduen JF, Zisman DA, Mobin SI, et al. Retrospective study of pulmonary function tests in patients presenting with isolated reduction in single-breath diffusion capacity: implications for the diagnosis of combined obstructive and restrictive lung disease. Mayo Clin Proc. 2007;82:48–54. doi: 10.4065/82.1.48. [DOI] [PubMed] [Google Scholar]
- 132.Kiakouama L, Cottin V, Glerant JC, Bayle JY, Mornex JF, Cordier JF. Conditions associated with severe carbon monoxide diffusion coefficient reduction. Respir Med. 2011;105:1248–56. doi: 10.1016/j.rmed.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 133.Costa CM, Neder JA, Verrastro CG, et al. Uncovering the mechanisms of exertional dyspnoea in combined pulmonary fibrosis and emphysema. European Respiratory Journal. 2020;55:1901319. doi: 10.1183/13993003.01319-2019. [DOI] [PubMed] [Google Scholar]
- 134.Westhoff M, Litterst P, Ewert R. Cardiopulmonary Exercise Testing in Combined Pulmonary Fibrosis and Emphysema. Respiration. 2021;100:395–403. doi: 10.1159/000513848. [DOI] [PubMed] [Google Scholar]
- 135.du Bois RM, Weycker D, Albera C, et al. Ascertainment of individual risk of mortality for patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:459–66. doi: 10.1164/rccm.201011-1790OC. [DOI] [PubMed] [Google Scholar]
- 136.Behr J. Disease Progression in Idiopathic Pulmonary Fibrosis. FVC Is Not Enough. Am J Respir Crit Care Med. 2017;196:1094–5. doi: 10.1164/rccm.201706-1246ED. [DOI] [PubMed] [Google Scholar]
- 137.Kishaba T, Shimaoka Y, Fukuyama H, et al. A cohort study of mortality predictors and characteristics of patients with combined pulmonary fibrosis and emphysema. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-000988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schmidt SL, Nambiar AM, Tayob N, et al. Pulmonary function measures predict mortality differently in IPF versus combined pulmonary fibrosis and emphysema. Eur Respir J. 2011;38:176–83. doi: 10.1183/09031936.00114010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hansell DM, Bankier AA, Macmahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: Glossary of terms for thoracic imaging. Radiology. 2008 doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 140.Lynch DA, Austin JH, Hogg JC, et al. CT-Definable Subtypes of Chronic Obstructive Pulmonary Disease: A Statement of the Fleischner Society. Radiology. 2015;277:192–205. doi: 10.1148/radiol.2015141579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Inomata M, Ikushima S, Awano N, et al. An autopsy study of combined pulmonary fibrosis and emphysema: correlations among clinical, radiological, and pathological features. BMC Pulm Med. 2014;14:104. doi: 10.1186/1471-2466-14-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cottin V, Cordier JF. Combined pulmonary fibrosis and emphysema in connective tissue disease. Curr Opin Pulm Med. 2012;18:418–27. doi: 10.1097/MCP.0b013e328356803b. [DOI] [PubMed] [Google Scholar]
- 143.Bak SH, Park HY, Nam JH, et al. Predicting clinical outcome with phenotypic clusters using quantitative CT fibrosis and emphysema features in patients with idiopathic pulmonary fibrosis. PLoS One. 2019;14:e0215303. doi: 10.1371/journal.pone.0215303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 2018;198:e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 145.Minegishi Y, Kokuho N, Miura Y, et al. Clinical features, anti-cancer treatments and outcomes of lung cancer patients with combined pulmonary fibrosis and emphysema. Lung Cancer. 2014;85:258–63. doi: 10.1016/j.lungcan.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 146.Sugino K, Ishida F, Kikuchi N, et al. Comparison of clinical characteristics and prognostic factors of combined pulmonary fibrosis and emphysema versus idiopathic pulmonary fibrosis alone. Respirology. 2014;19:239–45. doi: 10.1111/resp.12207. [DOI] [PubMed] [Google Scholar]
- 147.Todd NW, Jeudy J, Lavania S, et al. Centrilobular emphysema combined with pulmonary fibrosis results in improved survival. Fibrogenesis Tissue Repair. 2011;4:6. doi: 10.1186/1755-1536-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kwak N, Park CM, Lee J, et al. Lung cancer risk among patients with combined pulmonary fibrosis and emphysema. Respir Med. 2014;108:524–30. doi: 10.1016/j.rmed.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 149.Awano N, Inomata M, Ikushima S, et al. Histological analysis of vasculopathy associated with pulmonary hypertension in combined pulmonary fibrosis and emphysema: comparison with idiopathic pulmonary fibrosis or emphysema alone. Histopathology. 2017;70:896–905. doi: 10.1111/his.13153. [DOI] [PubMed] [Google Scholar]
- 150.Cottin V, Fabien N, Khouatra C, Moreira A, Cordier JF. Anti-elastin autoantibodies are not present in combined pulmonary fibrosis and emphysema. Eur Respir J. 2009;33:219–21. doi: 10.1183/09031936.00140208. [DOI] [PubMed] [Google Scholar]
- 151.Kinoshita Y, Watanabe K, Ishii H, Kushima H, Fujita M, Nabeshima K. Distribution of emphysema and fibrosis in idiopathic pulmonary fibrosis with coexisting emphysema. Histopathology. 2019;74:1103–8. doi: 10.1111/his.13831. [DOI] [PubMed] [Google Scholar]
- 152.Mitchell PD, Das JP, Murphy DJ, et al. Idiopathic pulmonary fibrosis with emphysema: evidence of synergy among emphysema and idiopathic pulmonary fibrosis in smokers. Respir Care. 2015;60:259–68. doi: 10.4187/respcare.03389. [DOI] [PubMed] [Google Scholar]
- 153.Zhao Y, Cui A, Wang F, et al. Characteristics of pulmonary inflammation in combined pulmonary fibrosis and emphysema. Chin Med J (Engl) 2012;125:3015–21. [PubMed] [Google Scholar]
- 154.Nandy S, Raphaely RA, Muniappan A, et al. Diagnostic Accuracy of Endobronchial Optical Coherence Tomography for the Microscopic Diagnosis of Usual Interstitial Pneumonia. Am J Respir Crit Care Med. 2021;204:1164–79. doi: 10.1164/rccm.202104-0847OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Montesi SB, Zhou IY, Liang LL, et al. Dynamic contrast-enhanced magnetic resonance imaging of the lung reveals important pathobiology in idiopathic pulmonary fibrosis. ERJ open research. 2021;7 doi: 10.1183/23120541.00907-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Myc LA, Shim YM, Laubach VE, Dimastromatteo J. Role of medical and molecular imaging in COPD. Clinical and translational medicine. 2019;8:12. doi: 10.1186/s40169-019-0231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sergiacomi G, Bolacchi F, Cadioli M, et al. Combined pulmonary fibrosis and emphysema: 3D time-resolved MR angiographic evaluation of pulmonary arterial mean transit time and time to peak enhancement. Radiology. 2010;254:601–8. doi: 10.1148/radiol.09081546. [DOI] [PubMed] [Google Scholar]
- 158.Fleming H, Clifford SM, Haughey A, et al. Differentiating combined pulmonary fibrosis and emphysema from pure emphysema: utility of late gadolinium-enhanced MRI. European radiology experimental. 2020;4:61. doi: 10.1186/s41747-020-00187-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chan HF, Weatherley ND, Johns CS, et al. Airway Microstructure in Idiopathic Pulmonary Fibrosis: Assessment at Hyperpolarized (3)He Diffusion-weighted MRI. Radiology. 2019;291:223–9. doi: 10.1148/radiol.2019181714. [DOI] [PubMed] [Google Scholar]
- 160.Weatherley ND, Eaden JA, Stewart NJ, et al. Experimental and quantitative imaging techniques in interstitial lung disease. Thorax. 2019;74:611–9. doi: 10.1136/thoraxjnl-2018-211779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Walsh CL, Tafforeau P, Wagner WL, et al. Imaging intact human organs with local resolution of cellular structures using hierarchical phase-contrast tomography. Nature methods. 2021;18:1532–41. doi: 10.1038/s41592-021-01317-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kitaguchi Y, Fujimoto K, Hanaoka M, Kawakami S, Honda T, Kubo K. Clinical characteristics of combined pulmonary fibrosis and emphysema. Respirology. 2010;15:265–71. doi: 10.1111/j.1440-1843.2009.01676.x. [DOI] [PubMed] [Google Scholar]
- 163.Chae KJ, Jin GY, Han YM, et al. Prevalence and progression of combined pulmonary fibrosis and emphysema in asymptomatic smokers: A case-control study. Eur Radiol. 2015;25:2326–34. doi: 10.1007/s00330-015-3617-3. [DOI] [PubMed] [Google Scholar]
- 164.Matsuoka S, Yamashiro T, Matsushita S, et al. Morphological disease progression of combined pulmonary fibrosis and emphysema: comparison with emphysema alone and pulmonary fibrosis alone. J Comput Assist Tomogr. 2015;39:153–9. doi: 10.1097/RCT.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 165.Nemoto M, Nei Y, Bartholmai B, et al. Automated computed tomography quantification of fibrosis predicts prognosis in combined pulmonary fibrosis and emphysema in a real-world setting: a single-centre, retrospective study. Respir Res. 2020;21:275. doi: 10.1186/s12931-020-01545-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hunninghake GM. Interstitial lung abnormalities: erecting fences in the path towards advanced pulmonary fibrosis. Thorax. 2019;74:506–11. doi: 10.1136/thoraxjnl-2018-212446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sverzellati N, Guerci L, Randi G, et al. Interstitial lung diseases in a lung cancer screening trial. Eur Respir J. 2011;38:392–400. doi: 10.1183/09031936.00201809. [DOI] [PubMed] [Google Scholar]
- 168.Jin GY, Lynch D, Chawla A, et al. Interstitial lung abnormalities in a CT lung cancer screening population: prevalence and progression rate. Radiology. 2013;268:563–71. doi: 10.1148/radiol.13120816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lederer DJ, Enright PL, Kawut SM, et al. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-lung study. Am J Respir Crit Care Med. 2009;180:407–14. doi: 10.1164/rccm.200812-1966OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hatabu H, Hunninghake GM, Richeldi L, et al. Interstitial lung abnormalities detected incidentally on CT: a Position Paper from the Fleischner Society. Lancet Respir Med. 2020;8:726–37. doi: 10.1016/S2213-2600(20)30168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Adeloye D, Chua S, Lee C, et al. Global and regional estimates of COPD prevalence: Systematic review and meta-analysis. Journal of global health. 2015;5:020415. doi: 10.7189/jogh.05-020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pratt PC, Kilburn KH. A modern concept of the emphysemas based on correlations of structure and function. Hum Pathol. 1970;1:443–63. doi: 10.1016/s0046-8177(70)80077-6. [DOI] [PubMed] [Google Scholar]
- 173.Alli AF. Pulmonary emphysema in Ibadan: a pathological study of unselected necropsy lungs. Trop Geogr Med. 1972;24:28–38. [PubMed] [Google Scholar]
- 174.Snider GL, Kleinerman J, Thurlbeck WM, Bengali ZH. The definition of emphysema. Report of a National Heart, Lung, and Blood Institute, Division of Lung Diseases workshop. Am Rev Respir Dis. 1985;132:182–5. doi: 10.1164/arrd.1985.132.1.182. [DOI] [PubMed] [Google Scholar]
- 175.Snider GL. Chronic obstructive pulmonary disease: a definition and implications of structural determinants of airflow obstruction for epidemiology. Am Rev Respir Dis. 1989;140:S3–8. doi: 10.1164/ajrccm/140.3_Pt_2.S3. [DOI] [PubMed] [Google Scholar]
- 176.Katzenstein AL. Smoking-related interstitial fibrosis (SRIF), pathogenesis and treatment of usual interstitial pneumonia (UIP), and transbronchial biopsy in UIP. Mod Pathol. 2012;25(Suppl 1):S68–78. doi: 10.1038/modpathol.2011.154. [DOI] [PubMed] [Google Scholar]
- 177.Fraig M, Shreesha U, Savici D, Katzenstein A-LA. Respiratory bronchiolitis: a clinicopathologic study in current smokers, ex-smokers, and never-smokers. Am J Surg Pathology. 2002;26:647–53. doi: 10.1097/00000478-200205000-00011. [DOI] [PubMed] [Google Scholar]
- 178.American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 179.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–48. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yamada T, Nakanishi Y, Homma T, et al. Airspace enlargement with fibrosis shows characteristic histology and immunohistology different from usual interstitial pneumonia, nonspecific interstitial pneumonia and centrilobular emphysema. Pathol Int. 2013;63:206–13. doi: 10.1111/pin.12054. [DOI] [PubMed] [Google Scholar]
- 181.Rabeyrin M, Thivolet F, Ferretti GR, et al. Usual interstitial pneumonia end-stage features from explants with radiologic and pathological correlations. Ann Diagn Pathol. 2015;19:269–76. doi: 10.1016/j.anndiagpath.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 182.Reddy TL, Mayo J, Churg A. Respiratory bronchiolitis with fibrosis. High-resolution computed tomography findings and correlation with pathology. Ann Am Thorac Soc. 2013;10:590–601. doi: 10.1513/AnnalsATS.201304-088OC. [DOI] [PubMed] [Google Scholar]
- 183.Katzenstein AL, Mukhopadhyay S, Myers JL. Diagnosis of usual interstitial pneumonia and distinction from other fibrosing interstitial lung diseases. HumPathol. 2008;39:1275–94. doi: 10.1016/j.humpath.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 184.Vassallo R, Jensen EA, Colby TV, et al. The overlap between respiratory bronchiolitis and desquamative interstitial pneumonia in pulmonary Langerhans cell histiocytosis: highresolution CT, histologic, and functional correlations. Chest. 2003;124:1199–205. doi: 10.1378/chest.124.4.1199. [DOI] [PubMed] [Google Scholar]
- 185.Akira M. Radiographic Differentiation of Advanced Fibrocystic Lung Diseases. Ann Am Thorac Soc. 2017;14:432–40. doi: 10.1513/AnnalsATS.201611-883PS. [DOI] [PubMed] [Google Scholar]
- 186.Rogliani P, Mura M, Mattia P, et al. HRCT and histopathological evaluation of fibrosis and tissue destruction in IPF associated with pulmonary emphysema. Respir Med. 2008;102:1753–61. doi: 10.1016/j.rmed.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 187.Sugino K, Nakamura Y, Ito T, Isshiki T, Sakamoto S, Homma S. Comparison of clinical characteristics and outcomes between combined pulmonary fibrosis and emphysema associated with usual interstitial pneumonia pattern and non-usual interstitial pneumonia. Sarcoidosis Vasc Diffuse Lung Dis. 2015;32:129–37. [PubMed] [Google Scholar]
- 188.Sugino K, Ota H, Fukasawa Y, Uekusa T, Homma S. Pathological characteristics in idiopathic nonspecific interstitial pneumonia with emphysema and pulmonary hypertension. Respirol Case Rep. 2013;1:39–42. doi: 10.1002/rcr2.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Jankowich MD, Polsky M, Klein M, Rounds S. Heterogeneity in combined pulmonary fibrosis and emphysema. Respiration. 2008;75:411–7. doi: 10.1159/000107048. [DOI] [PubMed] [Google Scholar]
- 190.Cottin V. Combined pulmonary fibrosis and emphysema: bad and ugly all the same? Eur Respir J. 2017;50 doi: 10.1183/13993003.00846-2017. [DOI] [PubMed] [Google Scholar]
- 191.Nathan SD, Barbera JA, Gaine SP, et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J. 2019;53:1801914. doi: 10.1183/13993003.01914-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tomioka H, Mamesaya N, Yamashita S, Kida Y, Kaneko M, Sakai H. Combined pulmonary fibrosis and emphysema: effect of pulmonary rehabilitation in comparison with chronic obstructive pulmonary disease. BMJ open respiratory research. 2016;3:e000099. doi: 10.1136/bmjresp-2015-000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chiba S, Ohta H, Abe K, et al. The diagnostic value of the interstitial biomarkers KL-6 and SP-D for the degree of fibrosis in combined pulmonary fibrosis and emphysema. Pulmonary medicine. 2012;2012:492960. doi: 10.1155/2012/492960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chen Q, Liu P, Zhou H, Kong H, Xie W. An increased risk of lung cancer in combined pulmonary fibrosis and emphysema patients with usual interstitial pneumonia compared with patients with idiopathic pulmonary fibrosis alone: a systematic review and meta-analysis. Therapeutic advances in respiratory disease. 2021;15:17534666211017050. doi: 10.1177/17534666211017050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Usui K, Tanai C, Tanaka Y, Noda H, Ishihara T. The prevalence of pulmonary fibrosis combined with emphysema in patients with lung cancer. Respirology. 2011;16:326–31. doi: 10.1111/j.1440-1843.2010.01907.x. [DOI] [PubMed] [Google Scholar]
- 196.Mimae T, Suzuki K, Tsuboi M, et al. Surgical Outcomes of Lung Cancer in Patients with Combined Pulmonary Fibrosis and Emphysema. Annals of surgical oncology. 2015;22(Suppl 3):S1371–9. doi: 10.1245/s10434-015-4577-1. [DOI] [PubMed] [Google Scholar]
- 197.Hata A, Sekine Y, Kota O, Koh E, Yoshino I. Impact of combined pulmonary fibrosis and emphysema on surgical complications and long-term survival in patients undergoing surgery for non-small-cell lung cancer. Int J Chron Obstruct Pulmon Dis. 2016;11:1261–8. doi: 10.2147/COPD.S94119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Koo HJ, Do KH, Lee JB, Alblushi S, Lee SM. Lung Cancer in Combined Pulmonary Fibrosis and Emphysema: A Systematic Review and Meta-Analysis. PLoS One. 2016;11:e0161437. doi: 10.1371/journal.pone.0161437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Travis WD, Brambilla E, Noguchi M, et al. The New IASLC/ATS/ERS International Multidisciplinary Lung Adenocarcinoma Classification. JThoracic Oncol. 2011;6:244–85. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hashimoto N, Iwano S, Kawaguchi K, et al. Impact of Thin-Section Computed Tomography-Determined Combined Pulmonary Fibrosis and Emphysema on Outcomes Among Patients With Resected Lung Cancer. Ann Thorac Surg. 2016;102:440–7. doi: 10.1016/j.athoracsur.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 201.Maeda R, Funasaki A, Motono N, Sekimura A, Usuda K, Uramoto H. Combined pulmonary fibrosis and emphysema predicts recurrence following surgery in patients with stage I non-small cell lung cancer. Med Oncol. 2018;35:31. doi: 10.1007/s12032-018-1091-x. [DOI] [PubMed] [Google Scholar]
- 202.Nasim F, Moua T. Lung cancer in combined pulmonary fibrosis and emphysema: a large retrospective cohort analysis. ERJ open research. 2020;6 doi: 10.1183/23120541.00521-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kumagai S, Marumo S, Yamanashi K, et al. Prognostic significance of combined pulmonary fibrosis and emphysema in patients with resected non-small-cell lung cancer: a retrospective cohort study. Eur J Cardiothorac Surg. 2014;46:e113–9. doi: 10.1093/ejcts/ezu384. [DOI] [PubMed] [Google Scholar]
- 204.Takenaka T, Furuya K, Yamazaki K, Miura N, Tsutsui K, Takeo S. The prognostic impact of combined pulmonary fibrosis and emphysema in patients with clinical stage IA non-small cell lung cancer. Surg Today. 2018;48:229–35. doi: 10.1007/s00595-017-1577-8. [DOI] [PubMed] [Google Scholar]
- 205.Oh JY, Lee YS, Min KH, et al. Presence of lung cancer and high gender, age, and physiology score as predictors of acute exacerbation in combined pulmonary fibrosis and emphysema: A retrospective study. Medicine (Baltimore) 2018;97:e11683. doi: 10.1097/MD.0000000000011683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Moon SW, Park MS, Kim YS, et al. Combined pulmonary fibrosis and emphysema and idiopathic pulmonary fibrosis in non-small cell lung cancer: impact on survival and acute exacerbation. BMC polm. 2019;19:177. doi: 10.1186/s12890-019-0951-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ikuyama Y, Ushiki A, Kosaka M, et al. Prognosis of patients with acute exacerbation of combined pulmonary fibrosis and emphysema: a retrospective single-centre study. BMC polm. 2020;20:144. doi: 10.1186/s12890-020-01185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zantah M, Dotan Y, Dass C, Zhao H, Marchetti N, Criner GJ. Acute exacerbations of COPD versus IPF in patients with combined pulmonary fibrosis and emphysema. Respir Res. 2020;21:164. doi: 10.1186/s12931-020-01432-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zheng H, Yu X, Chen P, et al. Clinical features and prognosis differences of advanced pulmonary fibrosis and combined advanced pulmonary fibrosis and emphysema syndrome in the elderly. Int J Clin Exp Med. 2017;10:13808–12. [Google Scholar]
- 210.Lee SH, Park JS, Kim SY, et al. Clinical features and prognosis of patients with idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2019;23:678–84. doi: 10.5588/ijtld.18.0194. [DOI] [PubMed] [Google Scholar]
- 211.Tsutani Y, Kagimoto A, Handa Y, Mimae T, Miyata Y, Okada M. Prognostic role of interstitial pneumonia with or without emphysema in patients with clinical stage I lung cancer. Jpn J Clin Oncol. 2021;51:1123–31. doi: 10.1093/jjco/hyab073. [DOI] [PubMed] [Google Scholar]
- 212.Zhao A, Gudmundsson E, Mogulkoc N, et al. Mortality in combined pulmonary fibrosis and emphysema patients is determined by the sum of pulmonary fibrosis and emphysema. ERJ open research. 2021;7 doi: 10.1183/23120541.00316-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Cottin V, Cordier JF, Wells AU. Centrilobular emphysema combined with pulmonary fibrosis results in improved survival: a response. Fibrogenesis Tissue Repair. 2011;4:16. doi: 10.1186/1755-1536-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Antoniou KM, Hansell DM, Rubens MB, et al. Idiopathic pulmonary fibrosis: outcome in relation to smoking status. Am J Respir Crit Care Med. 2008;177:190–4. doi: 10.1164/rccm.200612-1759OC. [DOI] [PubMed] [Google Scholar]
- 215.Desai SR, Wells AU, Rubens MB, du Bois RM, Hansell DM. Traction bronchiectasis in cryptogenic fibrosing alveolitis: associated computed tomographic features and physiological significance. Eur Radiol. 2003;13:1801–8. doi: 10.1007/s00330-002-1779-2. [DOI] [PubMed] [Google Scholar]
- 216.Akira M, Inoue Y, Kitaichi M, Yamamoto S, Arai T, Toyokawa K. Usual interstitial pneumonia and nonspecific interstitial pneumonia with and without concurrent emphysema: thin-section CT findings. Radiology. 2009;251:271–9. doi: 10.1148/radiol.2511080917. [DOI] [PubMed] [Google Scholar]
- 217.Walsh SL, Calandriello L, Sverzellati N, Wells AU, Hansell DM. Interobserver agreement for the ATS/ERS/JRS/ALAT criteria for a UIP pattern on CT. Thorax. 2016;71:45–51. doi: 10.1136/thoraxjnl-2015-207252. [DOI] [PubMed] [Google Scholar]
- 218.Lee G, Kim KU, Lee JW, Suh YJ, Jeong YJ. Serial changes and prognostic implications of CT findings in combined pulmonary fibrosis and emphysema: comparison with fibrotic idiopathic interstitial pneumonias alone. Acta Radiol. 2017;58:550–7. doi: 10.1177/0284185116664227. [DOI] [PubMed] [Google Scholar]
- 219.Gürün Kaya A, Özyürek BA, Şahin Özdemirel T, Öz M, Erdogan Y. Prognostic Significance of Red Cell Distribution Width in Idiopathic Pulmonary Fibrosis and Combined Pulmonary Fibrosis Emphysema. Medical principles and practice : international journal of the Kuwait University, Health Science Centre. 2021;30:154–9. doi: 10.1159/000511106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Malli F, Papakosta D, Antoniou K, et al. Combined pulmonary fibrosis and emphysema characteristics in a Greek cohort. ERJ open research. 2019;5 doi: 10.1183/23120541.00014-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zeba F, Yanning W, Melek J, et al. Prognostic Significance of Pulmonary Artery to Aorta Ratio and Other CT Markers in Pulmonary Fibrosis With and Without Emphysema. Lung. 2021;199:677–80. doi: 10.1007/s00408-021-00490-2. [DOI] [PubMed] [Google Scholar]
- 222.Marten K, Milne D, Antoniou KM, et al. Non-specific interstitial pneumonia in cigarette smokers: a CT study. Eur Radiol. 2009 doi: 10.1007/s00330-009-1308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Walsh SL, Wells AU, Sverzellati N, et al. Relationship between fibroblastic foci profusion and high resolution CT morphology in fibrotic lung disease. BMC medicine. 2015;13:241. doi: 10.1186/s12916-015-0479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Salvatore M, Singh A, Yip R, et al. Progression of probable UIP and UIP on HRCT. Clin Imaging. 2019;58:140–4. doi: 10.1016/j.clinimag.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 225.Oikonomou A, Mintzopoulou P, Tzouvelekis A, et al. Pulmonary fibrosis and emphysema: Is the emphysema type associated with the pattern of fibrosis? World J Radiol. 2015;7:294–305. doi: 10.4329/wjr.v7.i9.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hobbs BD, de Jong K, Lamontagne M, et al. Genetic loci associated with chronic obstructive pulmonary disease overlap with loci for lung function and pulmonary fibrosis. Nat Genet. 2017;49:426–32. doi: 10.1038/ng.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sakornsakolpat P, Prokopenko D, Lamontagne M, et al. Genetic landscape of chronic obstructive pulmonary disease identifies heterogeneous cell-type and phenotype associations. Nat Genet. 2019;51:494–505. doi: 10.1038/s41588-018-0342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Fingerlin TE, Murphy E, Zhang W, et al. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet. 2013;45:613–20. doi: 10.1038/ng.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hanaoka M, Ito M, Droma Y, et al. Comparison of gene expression profiling between lung fibrotic and emphysematous tissues sampled from patients with combined pulmonary fibrosis and emphysema. Fibrogenesis Tissue Repair. 2012;5:17. doi: 10.1186/1755-1536-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Armanios M. Telomerase and idiopathic pulmonary fibrosis. Mutation research. 2012;730:52–8. doi: 10.1016/j.mrfmmm.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Petrovski S, Todd JL, Durheim MT, et al. An Exome Sequencing Study to Assess the Role of Rare Genetic Variation in Pulmonary Fibrosis. Am J Respir Crit Care Med. 2017;196:82–93. doi: 10.1164/rccm.201610-2088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Snetselaar R, van Moorsel CHM, Kazemier KM, et al. Telomere length in interstitial lung diseases. Chest. 2015;148:1011–8. doi: 10.1378/chest.14-3078. [DOI] [PubMed] [Google Scholar]
- 233.Arish N, Petukhov D, Wallach-Dayan SB. The Role of Telomerase and Telomeres in Interstitial Lung Diseases: From Molecules to Clinical Implications. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20122996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hoffman TW, van Moorsel CHM, Borie R, Crestani B. Pulmonary phenotypes associated with genetic variation in telomere-related genes. Curr Opin Pulm Med. 2018;24:269–80. doi: 10.1097/MCP.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 235.Ley B, Torgerson DG, Oldham JM, et al. Rare Protein-Altering Telomere-related Gene Variants in Patients with Chronic Hypersensitivity Pneumonitis. Am J Respir Crit Care Med. 2019;200:1154–63. doi: 10.1164/rccm.201902-0360OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Juge PA, Borie R, Kannengiesser C, et al. Shared genetic predisposition in rheumatoid arthritis-interstitial lung disease and familial pulmonary fibrosis. Eur Respir J. 2017;49 doi: 10.1183/13993003.02314-2016. [DOI] [PubMed] [Google Scholar]
- 237.Alder JK, Chen JJL, Lancaster L, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci U S A. 2008;105:13051–6. doi: 10.1073/pnas.0804280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence in patients with pulmonary emphysema. Am J Respir Crit Care Med. 2006;174:886–93. doi: 10.1164/rccm.200509-1374OC. [DOI] [PubMed] [Google Scholar]
- 239.Povedano JM, Martinez P, Flores JM, Mulero F, Blasco MA. Mice with Pulmonary Fibrosis Driven by Telomere Dysfunction. Cell reports. 2015;12:286–99. doi: 10.1016/j.celrep.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 240.Kusko RL, Brothers JF, 2nd, Tedrow J, et al. Integrated Genomics Reveals Convergent Transcriptomic Networks Underlying Chronic Obstructive Pulmonary Disease and Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2016;194:948–60. doi: 10.1164/rccm.201510-2026OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Halu A, Liu S, Baek SH, et al. Exploring the cross-phenotype network region of disease modules reveals concordant and discordant pathways between chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Hum Mol Genet. 2019;28:2352–64. doi: 10.1093/hmg/ddz069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Baumgartner KB, Samet JM, Stidley CA, Colby TV, Waldron JA. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1997;155:242–8. doi: 10.1164/ajrccm.155.1.9001319. [DOI] [PubMed] [Google Scholar]
- 243.Ekstrom M, Gustafson T, Boman K, et al. Effects of smoking, gender and occupational exposure on the risk of severe pulmonary fibrosis: a population-based casecontrol study. BMJ Open. 2014;4:e004018. doi: 10.1136/bmjopen-2013-004018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Abramson MJ, Murambadoro T, Alif SM, et al. Occupational and environmental risk factors for idiopathic pulmonary fibrosis in Australia: case-control study. Thorax. 2020;75:864–9. doi: 10.1136/thoraxjnl-2019-214478. [DOI] [PubMed] [Google Scholar]
- 245.Bellou V, Belbasis L, Evangelou E. Tobacco Smoking and Risk for Pulmonary Fibrosis: A Prospective Cohort Study From the UK Biobank. Chest. 2021;160:983–93. doi: 10.1016/j.chest.2021.04.035. [DOI] [PubMed] [Google Scholar]
- 246.Murphy SE, Park SL, Balbo S, et al. Tobacco biomarkers and genetic/epigenetic analysis to investigate ethnic/racial differences in lung cancer risk among smokers. NPJ precision oncology. 2018;2:17. doi: 10.1038/s41698-018-0057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Sundar IK, Rahman I. Gene expression profiling of epigenetic chromatin modification enzymes and histone marks by cigarette smoke: implications for COPD and lung cancer. Am J Physiol Lung Cell Mol Physiol. 2016;311:L1245–l58. doi: 10.1152/ajplung.00253.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ikezoe K, Hackett TL, Peterson S, et al. Small Airway Reduction and Fibrosis Is an Early Pathologic Feature of Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2021;204:1048–59. doi: 10.1164/rccm.202103-0585OC. [DOI] [PubMed] [Google Scholar]
- 249.Pardo A, Cabrera S, Maldonado M, Selman M. Role of matrix metalloproteinases in the pathogenesis of idiopathic pulmonary fibrosis. Respir Res. 2016;17:23. doi: 10.1186/s12931-016-0343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Segura-Valdez L, Pardo A, Gaxiola M, Uhal BD, Becerril C, Selman M. Upregulation of gelatinases A and B, collagenases 1 and 2, and increased parenchymal cell death in COPD. Chest. 2000;117:684–94. doi: 10.1378/chest.117.3.684. [DOI] [PubMed] [Google Scholar]
- 251.Houghton AM. Matrix metalloproteinases in destructive lung disease. Matrix biology : journal of the International Society for Matrix Biology. 2015;44-46:167–74. doi: 10.1016/j.matbio.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 252.Manicone AM, Gharib SA, Gong KQ, et al. Matrix Metalloproteinase-28 Is a Key Contributor to Emphysema Pathogenesis. Am J Pathol. 2017;187:1288–300. doi: 10.1016/j.ajpath.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Maldonado M, Salgado-Aguayo A, Herrera I, et al. Upregulation and Nuclear Location of MMP28 in Alveolar Epithelium of Idiopathic Pulmonary Fibrosis. Am J Respir Cell Mol Biol. 2018;59:77–86. doi: 10.1165/rcmb.2017-0223OC. [DOI] [PubMed] [Google Scholar]
- 254.Maldonado M, Buendia-Roldan I, Vicens-Zygmunt V, et al. Identification of MMP28 as a biomarker for the differential diagnosis of idiopathic pulmonary fibrosis. PLoS One. 2018;13:e0203779. doi: 10.1371/journal.pone.0203779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Selman M, Ruiz V, Cabrera S, et al. TIMP-1, -2, -3, and -4 in idiopathic pulmonary fibrosis. A prevailing nondegradative lung microenvironment? Am J Physiol Lung Cell Mol Physiol. 2000;279:L562–74. doi: 10.1152/ajplung.2000.279.3.L562. [DOI] [PubMed] [Google Scholar]
- 256.Pardo A, Barrios R, Gaxiola M, et al. Increase of lung neutrophils in hypersensitivity pneumonitis is associated with lung fibrosis. Am J Respir Crit Care Med. 2000;161:1698–704. doi: 10.1164/ajrccm.161.5.9907065. [DOI] [PubMed] [Google Scholar]
- 257.Zuo F, Kaminski N, Eugui E, et al. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci U S A. 2002;99:6292–7. doi: 10.1073/pnas.092134099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Herrera I, Cisneros J, Maldonado M, et al. Matrix metalloproteinase (MMP)-1 induces lung alveolar epithelial cell migration and proliferation, protects from apoptosis, and represses mitochondrial oxygen consumption. J Biol Chem. 2013;288:25964–75. doi: 10.1074/jbc.M113.459784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Mattey DL, Nixon NB, Dawes PT. Association of circulating levels of MMP-8 with mortality from respiratory disease in patients with rheumatoid arthritis. Arthritis Res Ther. 2012;14:R204. doi: 10.1186/ar4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Gilligan DM, O’Connor CM, Ward K, Moloney D, Bresnihan B, FitzGerald MX. Bronchoalveolar lavage in patients with mild and severe rheumatoid lung disease. Thorax. 1990;45:591–6. doi: 10.1136/thx.45.8.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Doyle TJ, Patel AS, Hatabu H, et al. Detection of Rheumatoid Arthritis-Interstitial Lung Disease Is Enhanced by Serum Biomarkers. Am J Respir Crit Care Med. 2015;191:1403–12. doi: 10.1164/rccm.201411-1950OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Moinzadeh P, Krieg T, Hellmich M, et al. Elevated MMP-7 levels in patients with systemic sclerosis: correlation with pulmonary involvement. Exp Dermatol. 2011;20:770–3. doi: 10.1111/j.1600-0625.2011.01321.x. [DOI] [PubMed] [Google Scholar]
- 263.Andersen GN, Nilsson K, Pourazar J, et al. Bronchoalveolar matrix metalloproteinase 9 relates to restrictive lung function impairment in systemic sclerosis. Respir Med. 2007;101:2199–206. doi: 10.1016/j.rmed.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 264.Kennedy B, Branagan P, Moloney F, et al. Biomarkers to identify ILD and predict lung function decline in scleroderma lung disease or idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2015;32:228–36. [PubMed] [Google Scholar]
- 265.Manetti M, Guiducci S, Romano E, et al. Increased serum levels and tissue expression of matrix metalloproteinase-12 in patients with systemic sclerosis: correlation with severity of skin and pulmonary fibrosis and vascular damage. Ann Rheum Dis. 2012;71:1064–72. doi: 10.1136/annrheumdis-2011-200837. [DOI] [PubMed] [Google Scholar]
- 266.Vuorinen K, Myllärniemi M, Lammi L, et al. Elevated matrilysin levels in bronchoalveolar lavage fluid do not distinguish idiopathic pulmonary fibrosis from other interstitial lung diseases. Apmis. 2007;115:969–75. doi: 10.1111/j.1600-0463.2007.apm_697.x. [DOI] [PubMed] [Google Scholar]
- 267.Rosas IO, Richards TJ, Konishi K, et al. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med. 2008;5:e93. doi: 10.1371/journal.pmed.0050093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.García-de-Alba C, Becerril C, Ruiz V, et al. Expression of matrix metalloproteases by fibrocytes: possible role in migration and homing. Am J Respir Crit Care Med. 2010;182:1144–52. doi: 10.1164/rccm.201001-0028OC. [DOI] [PubMed] [Google Scholar]
- 269.García de Alba C, Buendia-Roldân I, Salgado A, et al. Fibrocytes contribute to inflammation and fibrosis in chronic hypersensitivity pneumonitis through paracrine effects. Am J Respir Crit Care Med. 2015;191:427–36. doi: 10.1164/rccm.201407-1334OC. [DOI] [PubMed] [Google Scholar]
- 270.Andersson-Sjoland A, de Alba CG, Nihlberg K, et al. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40:2129–40. doi: 10.1016/j.biocel.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 271.Dupin I, Thumerel M, Maurat E, et al. Fibrocyte accumulation in the airway walls of COPD patients. Eur Respir J. 2019;54 doi: 10.1183/13993003.02173-2018. [DOI] [PubMed] [Google Scholar]
- 272.de Brouwer B, Drent M, van den Ouweland JMW, et al. Increased circulating desmosine and age-dependent elastinolysis in idiopathic pulmonary fibrosis. Respir Res. 2018;19:45. doi: 10.1186/s12931-018-0747-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Sato T, Kajikuri T, Saito Y, Chikuma M, Nagai S. Determination of desmosine in bronchoalveolar lavage fluids by time-resolved fluoroimmunoassay. Clin Chim Acta. 2008;387:113–9. doi: 10.1016/j.cca.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 274.Kim C, Ko Y, Kim SH, et al. Urinary desmosine is associated with emphysema severity and frequent exacerbation in patients with COPD. Respirology. 2018;23:176–81. doi: 10.1111/resp.13170. [DOI] [PubMed] [Google Scholar]
- 275.Turino GM. Chronic Obstructive Pulmonary Disease. A Biomarker and a Potential Therapy. Ann Am Thorac Soc. 2018;15:S15–s7. doi: 10.1513/AnnalsATS.201707-568KV. [DOI] [PubMed] [Google Scholar]
- 276.Gregory AD, Kliment CR, Metz HE, et al. Neutrophil elastase promotes myofibroblast differentiation in lung fibrosis. J Leukoc Biol. 2015;98:143–52. doi: 10.1189/jlb.3HI1014-493R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Agusti A, Faner R. Lung function trajectories in health and disease. Lancet Respir Med. 2019;7:358–64. doi: 10.1016/S2213-2600(18)30529-0. [DOI] [PubMed] [Google Scholar]
- 278.Agusti A, Hogg JC. Update on the Pathogenesis of Chronic Obstructive Pulmonary Disease. N Engl J Med. 2019;381:1248–56. doi: 10.1056/NEJMra1900475. [DOI] [PubMed] [Google Scholar]
- 279.Wong A, Ryerson CJ, Liang C, Cottin V. Combined pulmonary fibrosis and emphysema: a systematic review and meta-analysis. PROSPERO; 2019. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=126108 . [DOI] [PubMed] [Google Scholar]
- 280.Goddard PR, Nicholson EM, Laszlo G, Watt I. Computed tomography in pulmonary emphysema. Clin Radiol. 1982;33:379–87. doi: 10.1016/s0009-9260(82)80301-2. [DOI] [PubMed] [Google Scholar]
- 281.Waxman A, Restrepo-Jaramillo R, Thenappan T, et al. Inhaled Treprostinil in Pulmonary Hypertension Due to Interstitial Lung Disease. N Engl J Med. 2021;384:325–34. doi: 10.1056/NEJMoa2008470. [DOI] [PubMed] [Google Scholar]
- 282.Raghu G, Collard HR, Egan JJ, et al. An Official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Agusti A, Bel E, Thomas M, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J. 2016;47:410–9. doi: 10.1183/13993003.01359-2015. [DOI] [PubMed] [Google Scholar]
- 284.Wongkarnjana A, Scallan C, Kolb MRJ. Progressive fibrosing interstitial lung disease: treatable traits and therapeutic strategies. Curr Opin Pulm Med. 2020;26:436–42. doi: 10.1097/MCP.0000000000000712. [DOI] [PubMed] [Google Scholar]
- 285.Jacobs SS, Krishnan JA, Lederer DJ, et al. Home Oxygen Therapy for Adults with Chronic Lung Disease. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202:e121–e41. doi: 10.1164/rccm.202009-3608ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Zhang L, Zhang C, Dong F, et al. Combined pulmonary fibrosis and emphysema: a retrospective analysis of clinical characteristics, treatment and prognosis. BMC Pulm Med. 2016;16:137. doi: 10.1186/s12890-016-0300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Cottin V, Crestani B, Cadranel J, et al. Recommandations pratiques pour le diagnostic et la prise en charge de la fibrose pulmonaire idiopathique – Actualisation 2017. Version longue [French practical guidelines for the diagnosis and management of idiopathic pulmonary fibrosis - 2017 update. Full-length version] Rev Mal Respir. 2017;34:900–68. doi: 10.1016/j.rmr.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 288.Wijsenbeek M, Cottin V. Spectrum of Fibrotic Lung Diseases. N Engl J Med. 2020;383:958–68. doi: 10.1056/NEJMra2005230. [DOI] [PubMed] [Google Scholar]
- 289.Takahashi T, Terada Y, Pasque MK, et al. Clinical Features and Outcomes of Combined Pulmonary Fibrosis and Emphysema After Lung Transplantation. Chest. 2021 doi: 10.1016/j.chest.2021.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Cottin V, Bonniaud P, Cadranel J, et al. [French practical guidelines for the diagnosis and management of IPF - 2021 update, short version] Rev Mal Respir. 2022 [Google Scholar]
- 291.Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377:1760–9. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 292.Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–82. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 293.King TE, Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–92. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 294.Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N Engl J Med. 2019;381:1718–27. doi: 10.1056/NEJMoa1908681. [DOI] [PubMed] [Google Scholar]
- 295.Behr J, Prasse A, Kreuter M, et al. Pirfenidone in patients with progressive fibrotic interstitial lung diseases other than idiopathic pulmonary fibrosis (RELIEF): a double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir Med. 2021;9:476–86. doi: 10.1016/S2213-2600(20)30554-3. [DOI] [PubMed] [Google Scholar]
- 296.Maher TM, Corte TJ, Fischer A, et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 2020;8:147–57. doi: 10.1016/S2213-2600(19)30341-8. [DOI] [PubMed] [Google Scholar]
- 297.Cottin V, Azuma A, Raghu G, et al. Therapeutic effects of nintedanib are not influenced by emphysema in the INPULSIS trials. Eur Respir J. 2019;53 doi: 10.1183/13993003.01655-2018. [DOI] [PubMed] [Google Scholar]
- 298.Wells AU, Flaherty KR, Brown KK, et al. Nintedanib in patients with progressive fibrosing interstitial lung diseases-subgroup analyses by interstitial lung disease diagnosis in the INBUILD trial: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Respir Med. 2020;8:453–60. doi: 10.1016/S2213-2600(20)30036-9. [DOI] [PubMed] [Google Scholar]
- 299.Dong F, Zhang Y, Chi F, et al. Clinical efficacy and safety of ICS/LABA in patients with combined idiopathic pulmonary fibrosis and emphysema. Int J Clin Exp Med. 2015;8:8617–25. [PMC free article] [PubMed] [Google Scholar]
- 300.Fishman A, Fessler H, Martinez F, et al. Patients at high risk of death after lungvolume-reduction surgery. N Engl J Med. 2001;345:1075–83. doi: 10.1056/NEJMoa11798. [DOI] [PubMed] [Google Scholar]
- 301.van Geffen WH, Slebos DJ, Herth FJ, Kemp SV, Weder W, Shah PL. Surgical and endoscopic interventions that reduce lung volume for emphysema: a systemic review and meta-analysis. Lancet Respir Med. 2019;7:313–24. doi: 10.1016/S2213-2600(18)30431-4. [DOI] [PubMed] [Google Scholar]
- 302.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J. 2015;46:903–75. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 303.Shioleno AM, Ruopp NF. Group 3 Pulmonary Hypertension: A Review of Diagnostics and Clinical Trials. Clin Chest Med. 2021;42:59–70. doi: 10.1016/j.ccm.2020.11.006. [DOI] [PubMed] [Google Scholar]
- 304.Hoeper MM, Behr J, Held M, et al. Pulmonary Hypertension in Patients with Chronic Fibrosing Idiopathic Interstitial Pneumonias. PLoS One. 2015;10:e0141911. doi: 10.1371/journal.pone.0141911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Zimmermann GS, von Wulffen W, Huppmann P, et al. Haemodynamic changes in pulmonary hypertension in patients with interstitial lung disease treated with PDE-5 inhibitors. Respirology. 2014;19:700–6. doi: 10.1111/resp.12294. [DOI] [PubMed] [Google Scholar]
- 306.Zisman DA, Schwarz M, Anstrom KJ, Collard HR, Flaherty KR, Hunninghake GW. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med. 2010;363:620–8. doi: 10.1056/NEJMoa1002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Kolb M, Raghu G, Wells AU, et al. Nintedanib plus Sildenafil in Patients with Idiopathic Pulmonary Fibrosis. N Engl J Med. 2018;379:1722–31. doi: 10.1056/NEJMoa1811737. [DOI] [PubMed] [Google Scholar]
- 308.Raghu G, Behr J, Brown KK, et al. Treatment of idiopathic pulmonary fibrosis with ambrisentan: a parallel, randomized trial. Ann Intern Med. 2013;158:641–9. doi: 10.7326/0003-4819-158-9-201305070-00003. [DOI] [PubMed] [Google Scholar]
- 309.Nathan SD, Behr J, Collard HR, et al. Riociguat for idiopathic interstitial pneumonia-associated pulmonary hypertension (RISE-IIP): a randomised, placebo-controlled phase 2b study. Lancet Respir Med. 2019;7:780–90. doi: 10.1016/S2213-2600(19)30250-4. [DOI] [PubMed] [Google Scholar]
- 310.Nathan SD, Cottin V, Behr J, et al. Impact of lung morphology on clinical outcomes with riociguat in patients with pulmonary hypertension and idiopathic interstitial pneumonia: A post hoc subgroup analysis of the RISE-IIP study. J Heart Lung Transplant. 2021;40:494–503. doi: 10.1016/j.healun.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 311.Nathan SD, Waxman A, Rajagopal S, et al. Inhaled treprostinil and forced vital capacity in patients with interstitial lung disease and associated pulmonary hypertension: a post-hoc analysis of the INCREASE study. Lancet Respir Med. 2021;9:1266–74. doi: 10.1016/S2213-2600(21)00165-X. [DOI] [PubMed] [Google Scholar]
- 312.Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. Lancet. 2021;398:535–54. doi: 10.1016/S0140-6736(21)00312-3. [DOI] [PubMed] [Google Scholar]
- 313.Punjabi NM, Shade D, Patel AM, Wise RA. Measurement variability in single-breath diffusing capacity of the lung. Chest. 2003;123:1082–9. doi: 10.1378/chest.123.4.1082. [DOI] [PubMed] [Google Scholar]
- 314.Kaner RJ, Bajwa EK, El-Amine M, et al. Design of Idiopathic Pulmonary Fibrosis Clinical Trials in the Era of Approved Therapies. Am J Respir Crit Care Med. 2019;200:133–9. doi: 10.1164/rccm.201903-0592PP. [DOI] [PubMed] [Google Scholar]
- 315.Spagnolo P, Cocconcelli E, Cottin V. In: Idiopathic pulmonary fibrosis. Second Edition. Meyer KC, Nathan SD, editors. Springer Nature; Switzerland: 2019. Clinical trials in IPF: What are the best endpoints? pp. 433–53. [Google Scholar]
- 316.Karimi-Shah BA, Chowdhury BA. Forced vital capacity in idiopathic pulmonary fibrosis--FDA review of pirfenidone and nintedanib. N Engl J Med. 2015;372:1189–91. doi: 10.1056/NEJMp1500526. [DOI] [PubMed] [Google Scholar]
- 317.Lowe KE, Regan EA, Anzueto A, et al. COPDGene(®) 2019: Redefining the Diagnosis of Chronic Obstructive Pulmonary Disease. Chronic obstructive pulmonary diseases (Miami, Fla) 2019;6:384–99. doi: 10.15326/jcopdf.6.5.2019.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Doyle TJ, Washko GR, Fernandez IE, et al. Interstitial lung abnormalities and reduced exercise capacity. Am J Respir Crit Care Med. 2012;185:756–62. doi: 10.1164/rccm.201109-1618OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Putman RK, Hatabu H, Araki T, et al. Association Between Interstitial Lung Abnormalities and All-Cause Mortality. Jama. 2016;315:672–81. doi: 10.1001/jama.2016.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.