Abstract
Introduction
Many studies of the Richardson’s syndrome phenotype of progressive supranuclear palsy (PSP) have elucidated regions of progressive atrophy and neural correlates of clinical severity. However, the neural correlates of survival and how these differ according to variant phenotypes are poorly understood. We set out to identify structural changes that predict severity and survival from scanning date to death.
Methods
Structural magnetic resonance imaging data from 112 deceased people with clinically defined ‘probable’ or ‘possible’ PSP were analysed. Neuroanatomical regions of interest volumes, thickness and area were correlated with ‘temporal stage’, defined as the ratio of time from symptom onset to death, time from scan to death (‘survival from scan’), and in a subset of patients, clinical severity, adjusting for age and total intracranial volume. Forty-nine participants had post mortem confirmation of the diagnosis.
Results
Using T1-weighted magnetic resonance imaging, we confirmed the midbrain, and bilateral cortical structural correlates of contemporary disease severity. Atrophy of the striatum, cerebellum and frontotemporal cortex correlate with temporal stage and survival from scan, even after adjusting for severity. Subcortical structure-survival relationships were stronger in Richardson’s syndrome than variant phenotypes.
Conclusions
Although the duration of PSP varies widely between people, an individual’s progress from disease onset to death (their temporal stage) reflects atrophy in striatal, cerebellar and frontotemporal cortical regions. Our findings suggest magnetic resonance imaging may contribute to prognostication and stratification of patients with heterogenous clinical trajectories and clarify the processes that confer mortality risk in PSP.
Introduction
Survival in the Richardson’s syndrome phenotype of progressive supranuclear palsy (PSP) is typically 5 to 7 years from symptom onset.1–6 Midbrain and frontal lobe atrophy are characteristically associated with the presence of PSP7–13 and imaging changes can differentiate Richardson’s syndrome from other ‘parkinsonian’ syndromes as well as correlating with clinical features and severity.10,14–18 There is also extensive evidence from imaging14,15,19–23 and post mortem studies24,25 for the importance of white matter pathology in PSP. Progressive atrophy has been demonstrated and linked to disease progression and severity of clinical features.26,27
Whilst several clinical features have been correlated with survival,2,6,28–31 the neuroanatomical predictors or determinants of survival time after a scan remain poorly understood. As proposed in the Neuroimaging Biomarker Utility System for PSP,32 the correlates of survival may differ from the imaging hallmarks of PSP (‘diagnosis’), or the correlates of motor and cognitive severity (‘severity’), or those regions with progressive atrophy (‘progression’). If, for example, clinical feature A is particularly associated with higher risk of death (e.g., bulbar failure), but clinical features B and C contribute strongly to a clinical severity rating scale (e.g., limb and ocular signs), then the anatomical correlates of survival (mediated by A) would differ from the anatomical correlates of severity (represented by B and C). Individual clinical measures may also vary in their dynamic range, with floor or ceiling effects that obscure progression of disease. The survival following an assessment is usually considered in terms of absolute time, for example, months between diagnosis/symptom onset and death. However, survival time varies greatly between people with PSP. To compare across individuals, progression through the illness can also be considered as a proportion of the duration of the disease to its full course, providing a normalised measure of survival. We will refer to this proportion of time from onset to end of illness as the ‘temporal stage’ (see Figure 1, panel A). This ‘temporal stage’ differs from a clinical severity-based staging of PSP, or the identification of progressive atrophy, which underlie many former studies. Improved understanding of the mechanisms underlying progression and survival, and predicting them, is important for stratification in clinical trials design, and for targeting factors that mediate survival.
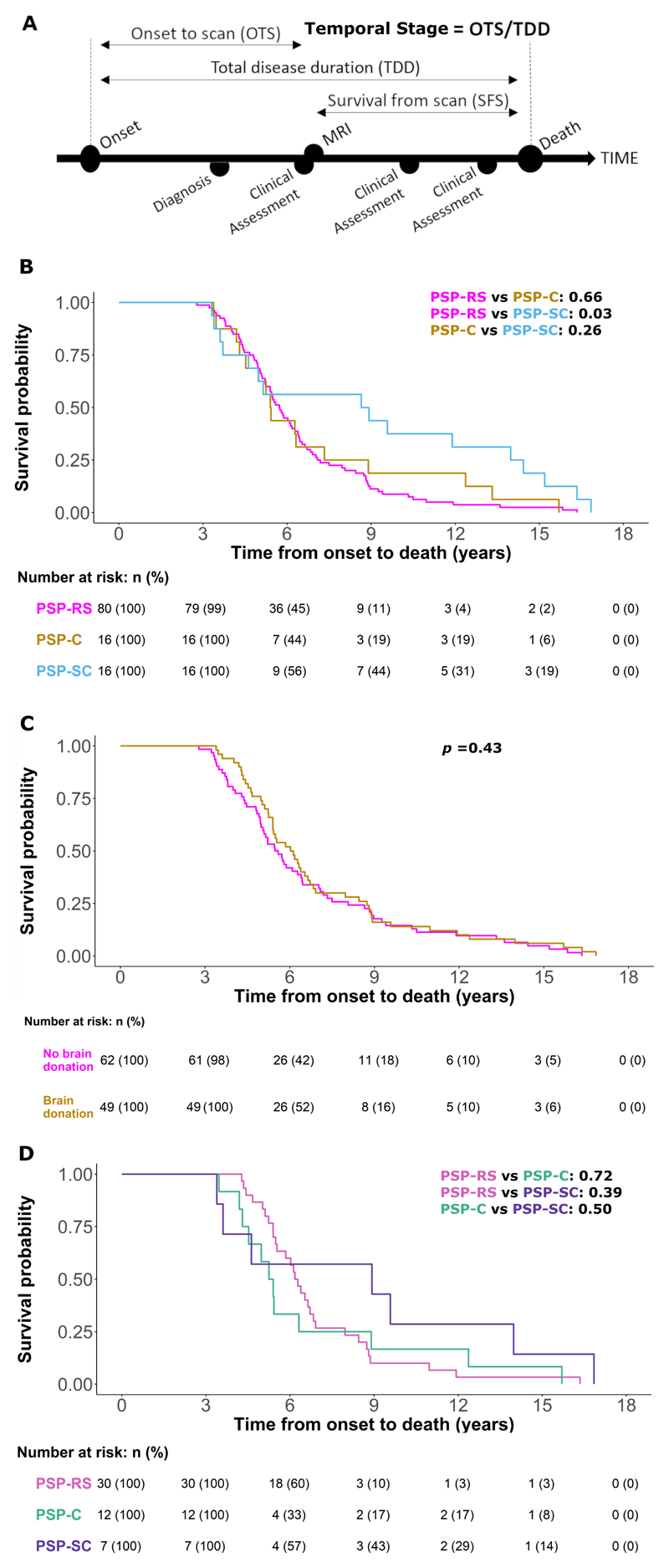
Figure 1.
(A): Study schematic and definitions of key variables. Temporal stage (‘stage’) is defined as the time from symptom onset to MRI (time to MRI, TTM) divided by the time from symptom onset to death (total disease duration, TDD) (B, C & D): Survival analysis from symptom onset to death. Analysis was performed using a Cox regression model split according to diagnostic groups with associated number at risk tables below each plot. Displayed p-values represent pairwise log-rank comparisons with correction for multiple comparisons. (B) PSP-Richardson’s syndrome (PSP-RS), PSP-Cortical (PSP-C) and PSP-Subcortical (PSP-SC) groups. (C) Groups split according to those who donated their brain to the Cambridge Brain Bank (‘Brain donation’) and those who did not (‘No brain donation’). PSP = progressive supranuclear palsy, MRI = magnetic resonance imaging. (D) PSP-Richardson’s syndrome (PSP-RS), PSP-Cortical (PSP-C) and PSP-Subcortical (PSP-SC) groups in the pathologically confirmed subset
In recent years, a focus of phenotypic variation within PSP neuroimaging has been the identification of brainstem derived biomarkers. Compared to Richardson’s syndrome, PSP-parkinsonism (PSP-P) has been found to have a greater midbrain area,33–35 decreased pons to midbrain area ratio,33,35–37 increased superior cerebellar peduncle width33,34,38 and decreased magnetic resonance parkinsonism index score.37,39,40 Similarly, midbrain area and pons to midbrain area ratio have been found to differ between Richardson’s syndrome and PSP-progressive gait freezing (PSP-PGF),35,41 PSP- speech/language (PSP-SL),41 PSP-corticobasal syndrome (PSP-CBS)35 and grouped variant diagnoses.42 When considering non-brainstem markers, frontal cortical atrophy was more marked in PSP-CBS, PSP-frontal (PSP-F) and PSP-SL variants than Richardson’s syndrome, while dentatorubrothalamic tract involvement was only observed in PSP-CBS, PSP-F and Richardson’s syndrome in the largest study of its type using 2017 Movement Disorders Society (MDS) diagnostic criteria.43
It is unknown how structural variation predicts prognosis and survival. Our primary hypothesis was that structural change (atrophy) predicts survival, at least in terms of the ‘temporal stage’ from onset to death when the scan was undertaken. Our second hypothesis was that although there are structural correlates of disease severity (e.g., PSP-Rating Scale, PSPRS), they differ from those associated with survival. Our third hypothesis was that neuroanatomical correlates of severity and survival differ by disease phenotype. Specifically, we predicted stronger subcortical regional correlations in PSP-Subcortical phenotypes (i.e., PSP-P and PSP-PGF) than PSP-Cortical phenotypes (i.e., PSP-CBS, PSP-SL and PSP-F).
Methods
Participants
Between June 2007 and June 2019, 122 people with a contemporary clinical diagnosis of probable or possible PSP underwent standardised structural imaging. They were retrospectively classified according to 2017 MDS criteria for a diagnosis of ‘probable’ or ‘possible’ PSP.44 Cases of multiple PSP diagnoses at presentation were resolved under the hierarchical multiple allocations extinction criteria (MAX rules).45 All participants had died by the census date of 31st August 2020 and neuropathological confirmation was available for 49 patients who donated their brain to the Cambridge Brain Bank. ‘Onset to scan’ (OTS) was defined as time from the first recognised symptom onset to MRI scan, ‘total disease duration’ (TDD) defined as time from the first symptom onset to death and ‘survival from scan’ (SFS) defined as time from MRI scan to death. The ‘temporal stage’ was defined as the fraction OTS/TDD, for each participant (Figure 1, panel A).
The study was conducted in accordance with the 1964 Helsinki declaration. Participants gave written, informed consent. Ethical approval was granted in October 2015 for the ‘Pick’s Disease and Progressive Supranuclear Palsy Prevalence and Incidence’ protocol (12/EE/0475) by the East of England Cambridge Central Research Ethics Committee and in March 2007 for the ‘Diagnosis and prognosis in Progressive Supranuclear Palsy and Corticobasal Degeneration’ protocol (07/Q0102/3) by the East of England Essex Research Ethics Committee. Neuropathological data were obtained with ethical approval from the Health Research Authority, NHS England (IRAS—202 802, ‘Neurodegeneration Research in Dementia’).
Acquisition of MRI
Participants underwent T1-weighted magnetic resonance imaging between 2007 and 2019 at the Wolfson Brain Imaging Centre, University of Cambridge, UK, on various Siemens scanners, all with 3T field strength. Visual inspection was performed to identify and label participants with large ventricles and ten individuals were excluded on grounds of image quality or lesion, leaving 86 TrioTim, 15 PRISMA, 6 Skyra and 5 Verio scans. The T1-weighted magnetization-prepared rapid acquisition gradient-echo sequence (MPRAGE) was closely matched across scanners based on the international Genetic Frontotemporal Dementia Initiative protocols (repetition times 2000ms, echo time 2.93ms, Flip angle 8°, 1.1mm isotropic).46 Where participants had more than one scan during the study period, the scan chosen for analysis was selected according to (i) the highest image quality, with least motion artefact, and (ii) the shortest interval between scan and PSPRS evaluation.
The Volumetric Analysis
Pre-processing of the MPRAGE images used automated scripts in the Statistical Parametric Mapping software (SPM12) (www.fil.ion.ucl.ac.uk/spm/software/spm12) in Matlab 2019b (mathworks.com). Cortical reconstruction and volumetric parcellation were performed using the standard recon-all pipeline of FreeSurfer 6.0.0 (Massachusetts General Hospital, Harvard Medical School; http://surfer.nmr.mgh.harvard.edu/) with adjustments for large ventricles where appropriate, and additional brainstem structures parcellation. Regional analyses were performed using cortical grey matter volume measures from 68 Desikan-Killiany Atlas cortical regions and 38 subcortical volume measures from the segmentation were applied.47,48 Total intracranial volume (TIV) was calculated separately using Statistical Parametric Mapping (SPM12).
Output from the Freesurfer pipeline was then imported to R Studio (version 4.0.3) to determine group differences and assess the relationship of outcome measures with regional brain parameter. Regional parameters of interest were cortical thickness, cortical area, cortical volume, and subcortical volume. Generalised linear mixed models were constructed including temporal stage, phenotype (e.g., Richardson’s syndrome, PSP-Cortical and PSP-Subcortical) and survival from scan separately as covariates of interest, while age, TIV and PSPRS were included as covariates of no interest across all models. Sex differences were subsumed under the TIV covariate. Pearson’s partial correlations were used to explore relationships between PSPRS, temporal stage and survival from scan. Correlation coefficients were converted using Fisher’s r to z transformation and compared via calculation of the observed z test statistic. Following these analyses, we constructed a simple exploratory multiple regression model constituting the top 5 imaging region-of-interest parameters and baseline characteristics to estimate the predictive accuracy of consequent survival (i.e., the difference between actual date of death and the date predicted by the model in key brain regions). Regional z-values were calculated for each patient using region-specific mean and standard deviation. For all regional analyses, significance levels were assessed using false discovery rate (FDR) correction. An FDR adjusted p-value <0.05 was considered significant.
Results
Clinical results
Clinical and demographic features are summarised in Table 1A. A Kaplan-Meier survival curve for the cohort by clinical diagnosis (median survival 5.73 years) is illustrated in Figure 1, panel B. As expected, ‘temporal stage’ and ‘survival from scan’ were significantly correlated (Pearson r = -0.689, p<10-8). The PSPRS was significantly correlated with ‘temporal stage’ (Pearson r = 0.538, p<10-6) and ‘survival from scan’ (Pearson r = -0.319, p = 0.004). The Subcortical group were diagnosed over two years later on average after symptom onset than the Richardson’s syndrome group whereas the Cortical group were diagnosed after a similar interval.
Table 1A. Clinical and demographic characteristics of the study population split by disease phenotype.
| PSP-RS | PSP-C | PSP-SC | PSP-C vs PSP-RS | PSP-C vs PSP-SC | PSP-RS vs PSP-SC | |
|---|---|---|---|---|---|---|
| N=80 | N=16 | N=16 | p-value | p-value | p-value | |
| Sex, Male/Female (%) | 0.60 (0.49) | 0.56 (0.51) | 0.62 (0.50) | 0.959 | 0.933 | 0.982 |
| Age at symptom onset | 67.3 (7.22) | 67.0 (9.11) | 64.6 (7.73) | 0.986 | 0.642 | 0.387 |
| Age at diagnosis | 70.6 (6.76) | 70.4 (8.28) | 70.5 (6.29) | 0.995 | 1.000 | 0.998 |
| Age at scan | 71.2 (6.75) | 71.6 (7.96) | 70.6 (6.43) | 0.968 | 0.902 | 0.947 |
| Age at death | 73.7 (6.54) | 73.9 (7.25) | 73.6 (6.67) | 0.991 | 0.990 | 0.999 |
| Duration from symptom onset to diagnosis | 3.29 (2.34) | 3.44 (2.34) | 5.92 (4.24) | 0.701 | 0.171 | 0.019 |
| Duration from symptom onset to scan | 3.86 (2.39) | 4.65 (2.63) | 6.01 (4.20) | 0.150 | 0.670 | 0.140 |
| Total disease duration (symptom onset to death) | 6.38 (2.63) | 6.94 (3.73) | 9.04 (5.09) | 0.980 | 0.420 | 0.210 |
| Duration from diagnosis to death | 3.09 (1.56) | 3.50 (2.65) | 3.12 (1.77) | 0.960 | 0.870 | 0.760 |
| Survival from scan | 2.52 (1.53) | 2.29 (1.73) | 3.03 (1.94) | 0.450 | 0.420 | 0.470 |
| Temporal Stage | 0.60 (0.20) | 0.68 (0.19) | 0.63 (0.14) | 0.289 | 0.758 | 0.823 |
| PSP Rating Scale | 37.1 (13.1) | 32.9 (17.3) | 39.0 (10.8) | 0.704 | 0.603 | 0.895 |
| Scan to PSP Rating Scale (days) | 26.7 (28.8) | 29.3 (31.5) | 29.6 (25.4) | 0.738 | 0.854 | 0.626 |
| Phenotype | ||||||
| PSP-CBS | 0 | 7 (44%) | 0 | |||
| PSP-F | 0 | 6 (37%) | 0 | |||
| PSP-P | 0 | 0 | 7 (44%) | |||
| PSP-PGF | 0 | 0 | 3 (19%) | |||
| PSP-PI | 0 | 0 | 6 (37%) | |||
| PSP-RS | 80 (100%) | 0 | 0 | |||
| PSP-SL | 0 | 3 (19%) | 0 |
Abbreviations: PSP = progressive supranuclear palsy, PSP-C = PSP-Cortical, PSP-CBS = PSP corticobasal syndrome, PSP-F = PSP frontal, PSP-P = PSP parkinsonism, PSP-PGF = PSP progressive gait freezing, PSP-PI = PSP postural instability, PSP-RS = PSP-Richardson’s syndrome, PSP-SC = PSP-Subcortical, PSP-SL = speech and language.
Neuropathology
Neuropathology was examined in forty-nine participants in the study. Forty-six (46/49 = 93.9%) had typical PSP pathology. Two (2/49 = 4.1%) had tau pathology that was intermediate between PSP and corticobasal degeneration, in anatomical distribution and neuroglial involvement (but lacking ballooned achromatic neurons). One case had dual pathology with the combination of typical PSP tauopathy plus cortical Lewy bodies. Demographic and clinical information for this sub-group is provided in Table 1B. The Kaplan-Meier survival curve for the 49 participants who donated to the brain bank was not different from that who did not donate (Log rank 0.43) (Figure 1, panel C). No significant differences in survival probability according to phenotype were observed in the pathologically confirmed group (Figure 1, panel D).
Table 1B. Clinical and demographic characteristics of the study population with pathological confirmation of diagnosis split by disease phenotype.
| PSP-RS | PSP-C | PSP-SC | PSP-C vs PSP-RS | PSP-C vs PSP-SC | PSP-RS vs PSP-SC | |
|---|---|---|---|---|---|---|
| N=30 | N=12 | N=7 | p-value | p-value | p-value | |
| Sex, Male/Female (%) | 0.53 (0.51) | 0.50 (0.52) | 0.43 (0.53) | 1.000 | 1.000 | 1.000 |
| Age at symptom onset | 65.8 (6.85) | 67.8 (9.52) | 62.7 (8.29) | 0.735 | 0.362 | 0.613 |
| Age at diagnosis | 69.4 (6.97) | 70.8 (9.09) | 68.7 (6.74) | 0.843 | 0.824 | 0.974 |
| Age at scan | 70.0 (6.95) | 72.2 (8.64) | 69.1 (6.96) | 0.657 | 0.657 | 0.958 |
| Age at death | 72.7 (6.44) | 74.5 (7.77) | 71.4 (6.34) | 0.715 | 0.602 | 0.892 |
| Duration from symptom onset to diagnosis | 3.62 (2.53) | 3.06 (1.77) | 6.02 (4.96) | 0.630 | 0.260 | 0.220 |
| Duration from symptom onset to scan | 4.22 (2.79) | 4.45 (2.34) | 6.44 (5.31) | 0.520 | 0.540 | 0.530 |
| Total disease duration (symptom onset to death) | 6.91 (2.57) | 6.73 (3.74) | 8.71 (5.26) | 0.210 | 0.650 | 0.690 |
| Duration from diagnosis to death | 3.29 (1.66) | 3.68 (3.01) | 2.68 (1.48) | 0.750 | 0.650 | 0.340 |
| Survival from scan | 2.69 (1.59) | 2.29 (1.84) | 2.26 (1.34) | 0.280 | 1.000 | 0.490 |
| Temporal Stage | 0.60 (0.20) | 0.67 (0.18) | 0.67 (0.17) | 0.566 | 0.998 | 0.642 |
| PSP Rating Scale | 39.0 (15.2) | 38.4 (17.5) | 43.0 (14.9) | 0.997 | 0.914 | 0.910 |
| Scan to PSP Rating Scale (days) | 21.3 (22.8) | 35.8 (34.9) | 35.0 (35.0) | 0.336 | 0.881 | 0.517 |
| Phenotype | ||||||
| PSP-CBS | 0 | 6 (50%) | 0 | |||
| PSP-F | 0 | 3 (25%) | 0 | |||
| PSP-P | 0 | 0 | 2 (29%) | |||
| PSP-PGF | 0 | 0 | 1 (14%) | |||
| PSP-PI | 0 | 0 | 4 (57%) | |||
| PSP-RS | 30 (100%) | 0 | 0 | |||
| PSP-SL | 0 | 3 (25%) | 0 |
Abbreviations: PSP = progressive supranuclear palsy, PSP-C = PSP-Cortical, PSP-CBS = PSP corticobasal syndrome, PSP-F = PSP frontal, PSP-P = PSP parkinsonism, PSP-PGF = PSP progressive gait freezing, PSP-PI = PSP postural instability, PSP-RS = PSP-Richardson’s syndrome, PSP-SC = PSP-Subcortical, PSP-SL = speech and language.
Imaging results
TrioTim scans constituted the majority in each phenotypic group: 63/80 (78.8%) in Richardson’s syndrome, 12/16 (75.0%) in the Cortical group and 11/16 (68.8%) in the Subcortical group. The same pattern was observed in the pathologically confirmed subset: 24/30 (80.0%) in Richardson’s syndrome, 10/12 (83.3%) in the Cortical group and 5/7 (71.4%) in the Subcortical group. Across all analyses no significant differences were observed between scanner type, in age, sex, clinical severity (as measured by PSP Rating Scale) or phenotypic group.
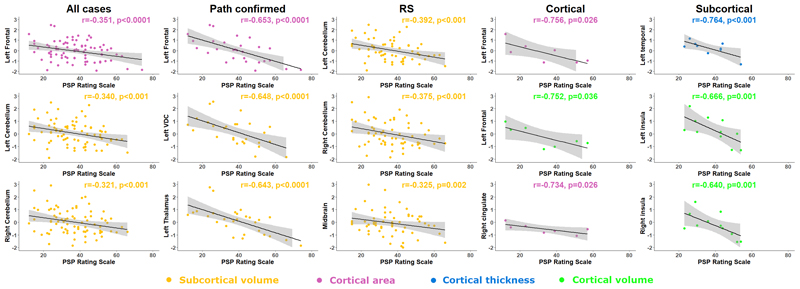
Results from partial correlation analyses between regional parameters and total PSPRS score are displayed in Figure 2, Table 2, Supplementary Figure 1, and Supplementary Tables 1, 4 and 5. Midbrain volume correlated with PSPRS in all participants (r= -0.286, n=80, p=0.002) and in the Richardson’s syndrome group (r= -0.325, n= 62, p=0.002), with a similar but stronger relationship observed in the pathologically confirmed group (r= -0.587, n=28, p<0.001). Pons volume also correlated with PSPRS in all participants (r= -0.194, n=80, p=0.039), the pathologically confirmed group (r= -0.451, n=28, p=0.001) and the Richardson’s syndrome group (r= -0.221, n=62, p=0.044). Outside the brainstem, broadly symmetrical patterns of volumetric correlation with PSPRS were observed in the ventral diencephalon, cerebellum, frontal, temporal, occipital and cingulate cortex of the whole group (combined phenotypes), and the ventral diencephalon and cerebellum of the Richardson’s syndrome group. Left frontal cortical area showed strong correlation with PSPRS in the whole group (r = -0.351, n=80, p<0.0001) and the pathologically confirmed group (r = -0.653, n=28, p<0.0001). Left temporal thickness showed strong correlation in the Subcortical group (r = -0.764, n=11, p<0.001) and frontal, temporal and cingulate volumes also showed broadly symmetrical correlation with PSPRS in the Subcortical group, and these differed from the Richardson’s syndrome group. Left frontal cortical area (r = -0.756, n=7, p=0.026) and right cingulate cortical area (r = -0.734, n=7, p=0.026) showed strong correlation with PSPRS in the Cortical group but no significant correlations were observed between subcortical regional volumes and PSPRS in the separate Cortical or Subcortical subgroups analyses.
Figure 2. Structural correlates of severity.
Pearson’s r partial correlation coefficient analysis between Progressive Supranuclear Palsy Rating Scale and region-of-interest z-values according to disease phenotype. RS – Richardson’s syndrome.
Table 2. Within group summary table of top 5 strongest Pearson’s r correlations between region-of-interest parameters and Progressive Supranuclear Palsy Rating Scale (PSP-RS), temporal stage and survival from scan.
| All (n=80) | Path (n=28) | Richardson’s Syndrome (n=62) | Cortical (n=7) | Subcortical (n=11) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marker | Region | r | p-value | Region | r | p-value | Region | r | p-value | Region | r | p-value | Region | r | p-value |
| PSP-RS | |||||||||||||||
| Left frontal area | -0.351 | <0.001 | Left frontal area | -0.653 | <0.001 | Left cerebellum volume | -0.392 | <0.001 | Left frontal area | -0.756 | 0.026 | Left temporal thickness | -0.764 | <0.001 | |
| Left cerebellum volume | -0.340 | <0.001 | Left ventral diencephalon volume | -0.648 | <0.001 | Right cerebellum volume | -0.375 | <0.001 | Left frontal volume | -0.752 | 0.036 | Left insula volume | -0.666 | 0.009 | |
| Right cerebellum volume | -0.321 | <0.001 | Left thalamus volume | -0.643 | <0.001 | Midbrain volume | -0.325 | 0.002 | Right cingulate area | -0.734 | 0.026 | Right cingulate volume | -0.640 | 0.009 | |
| Right cingulate area | -0.316 | <0.001 | Left temporal area | -0.625 | <0.001 | Right cingulate area | -0.319 | 0.004 | Left temporal area | -0.679 | 0.043 | Right frontal volume | -0.629 | 0.009 | |
| Right frontal area | -0.314 | <0.001 | Left putamen volume | -0.618 | <0.001 | Superior cerebellar peduncle volume | -0.294 | 0.006 | Right frontal volume | -0.629 | 0.088 | Left frontal volume | -0.613 | 0.009 | |
| Temporal Stage | |||||||||||||||
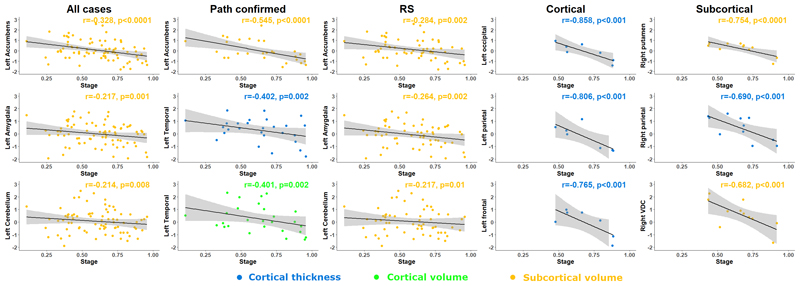
| Left accumbens volume | -0.328 | <0.001 | Left accumbens volume | -0.545 | <0.001 | Left accumbens volume | -0.284 | 0.002 | Left occipital thickness | -0.858 | <0.001 | Right putamen volume | -0.754 | <0.001 | |
| Left amygdala volume | -0.217 | 0.009 | Left temporal thickness | -0.402 | 0.002 | Left amygdala volume | -0.264 | 0.002 | Left parietal thickness | -0.806 | <0.001 | Right parietal thickness | -0.690 | <0.001 | |
| Left cerebellum volume | -0.214 | 0.008 | Left temporal volume | -0.401 | 0.002 | Left cerebellum volume | -0.217 | 0.012 | Left frontal thickness | -0.765 | <0.001 | Right ventral diencephalon volume | -0.682 | <0.001 | |
| Right putamen volume | -0.200 | 0.012 | Right temporal thickness | -0.386 | 0.002 | Right putamen volume | -0.202 | 0.020 | Right frontal thickness | -0.705 | 0.001 | Left occipital volume | -0.648 | <0.001 | |
| Left putamen volume | -0.194 | 0.012 | Left putamen volume | -0.381 | 0.003 | Left putamen volume | -0.191 | 0.029 | Right occipital thickness | -0.700 | 0.001 | Right accumbens volume | -0.625 | 0.002 | |
| Survival from Scan | |||||||||||||||
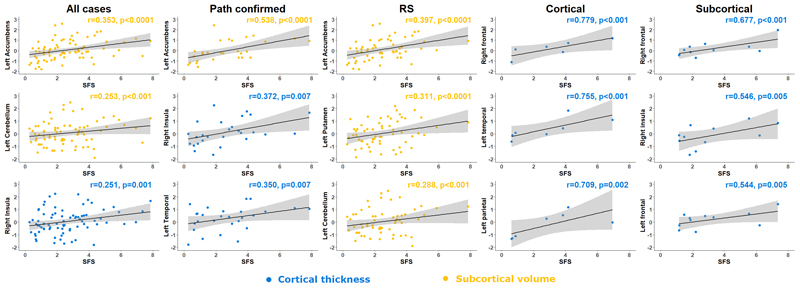
| Left accumbens volume | 0.353 | <0.001 | Left accumbensvolume | 0.538 | <0.001 | Left accumbens volume | 0.397 | <0.001 | Right frontal thickness | 0.779 | <0.001 | Right frontal thickness | 0.677 | <0.001 | |
| Left cerebellum volume | 0.253 | <0.001 | Right insula thickness | 0.372 | 0.007 | Left putamen volume | 0.311 | <0.001 | Left temporal thickness | 0.755 | <0.001 | Right insula thickness | 0.546 | 0.005 | |
| Right insula thickness | 0.251 | 0.001 | Left temporal thickness | 0.350 | 0.007 | Left cerebellum volume | 0.288 | <0.001 | Left parietal thickness | 0.709 | 0.002 | Left frontal thickness | 0.544 | 0.005 | |
| Left putamen volume | 0.247 | 0.001 | Left putamen volume | 0.305 | 0.024 | Left amygdala volume | 0.276 | <0.001 | Left frontal thickness | 0.690 | 0.002 | Left insula thickness | 0.398 | 0.072 | |
| Right putamen volume | 0.210 | 0.005 | Right temporal thickness | 0.290 | 0.031 | Left ventral diencephalon volume | 0.266 | <0.001 | Right insula thickness | 0.655 | 0.004 | Left parietal thickness | 0.366 | 0.081 | |
Regional partial correlation analyses with ‘temporal stage’ are displayed in Figure 3, Table 2, Supplementary Figure 2 and 3, and Supplementary Tables 6 and 7. The putamen, accumbens, ventral diencephalon, frontal and temporal cortex volumes showed strong correlation bilaterally with proximity to the end of the illness (i.e., temporal stage) in both the Subcortical and pathologically confirmed groups. The putamen and cerebellum showed similar but weaker negative correlations with more advanced temporal stage in the Richardson’s syndrome group. The Cortical and Subcortical groups showed strong bilateral negative correlations with multi-region cortical thickness and temporal stage but did not display any symmetrical volumetric correlates with temporal stage. No significant symmetrical correlations between cortical area and temporal stage were observed in any group. Inter group differences in correlation coefficients of temporal stage were most marked between Richardson’s syndrome and Subcortical groups. No differences in correlation coefficients of regional volumes and temporal stage were observed between Richardson’s syndrome and Cortical groups.
Figure 3. Structural correlates of survival in terms of ‘temporal stage’ (ratio from onset to death).
Pearson’s r partial correlation coefficient analysis between ‘temporal stage’ and region of interest z-values according to disease phenotype. RS – Richardson’s syndrome.
Regional partial correlation analyses with ‘survival from scan’ are displayed in Figure 4, Table 2, Supplementary Figures 2 and 3 and Supplementary Tables 3, 8 and 9. The putamen, hippocampus and accumbens regional volumes showed strong negative correlation bilaterally with shorter survival from scan in all participants, while the pallidum and accumbens also negatively correlated symmetrically with shorter survival from scan in the Richardson’s syndrome group. In other words, more atrophy was associated with shorter survival, even after adjusting for clinical severity at the time of the scan (as measured by the PSPRS). The Cortical group showed strong bilateral positive correlations with multi-region cortical thickness and survival from scan but did not display symmetrical volumetric correlates with survival from scan. The Subcortical group showed strong positive bilateral frontal correlation between cortical thickness and survival from scan but did not display volumetric correlates with survival from scan. No significant symmetrical correlations between cortical area and survival from scan were observed in any group.
Figure 4. Structural correlates of survival in terms of survival from scan.
Pearson’s r partial correlation coefficient analysis between survival from scan and region of interest z-values according to disease phenotype. RS – Richardson’s syndrome.
The multiple regression analysis of the predictive accuracy of survival from scan according to baseline characteristics and the 5 regions of interest is displayed in Supplementary Figure 4. In summary, the combination of left accumbens volume, left cerebellum volume, right insula thickness, left putamen volume and right putamen volume predicted 36% of the variance in survival time, from scan to death (R2 = 0.364, F(8, 71) = 5.08, p<0.0001).
Discussion
The principal result of this study is that atrophy in cortical and subcortical regions is related to how far ‘through’ the illness a patient with PSP is, from their symptom onset to death. We refer to this normalised survival as the ‘temporal stage’ of their PSP. These regional volume correlates differ from the baseline midbrain correlates of clinical severity (i.e., correlation with PSP Rating Scale). The structure-survival relationships also vary by clinical phenotype, with subcortical phenotypes demonstrating the strongest structure-survival relationships. Further, a multiple regression model constructed with just five regions of interest predicted the survival time from scan to death. Such an approach could in principle be used in clinical settings to support discussions about likely disease trajectories and prognostic estimates. Taken together, these findings suggest that despite the expected relationship between ‘temporal stage’ and clinical severity, their neuroanatomical correlates are distinct.
The concept of ‘temporal stage’ we use here warrants further discussion. We do not mean clinical severity staging, but the percentage in time of the disease course from onset to death. Unlike the absolute survival from scan date to date of death, this temporal staging normalizes the time of the imaging to the individual’s whole duration of symptomatic disease. This staging corrects for the marked variability in progression in PSP and allows severity of brain atrophy at a discrete timepoint to be compared with the elapsed fraction of the disease course at that point. We propose that temporal staging better accounts for heterogeneity than absolute time; indeed, ‘temporal stage’ had stronger correlations with regional volumes than ‘survival from scan’ in years. However, we recognise that ‘temporal stage’ is dependent on an estimate of the onset taken from the reported onset of symptoms, which may be biased with hindsight, and prone to error in patient and carer reports. It is likely that neuropathology onset precedes reported symptom onset by several years as it does in other forms of frontotemporal lobar degeneration.46,49 Similarly, the timing of death itself may be influenced by many factors, and not only reflect the extent of brain pathology. For example, environmental factors, home environment, daily care, medical care, and other comorbidities may also have an impact on survival from scan. In the UK, the National Health Service provides universal healthcare that is free at the point of care, including palliative care services which would be available in principle to all patients in our cohort, without dependence on personal wealth or insurance. In the UK, euthanasia and assisting access to euthanasia is illegal, and we are not aware of euthanasia in any of the cohort.
Note that we did not examine longitudinal changes in atrophy. Our outcome measure relates to the survival after imaging, not the volumetric change during the following year of life. Longitudinal imaging studies have shown significant progression of atrophy during one year, in regions cross-sectionally associated with PSP (e.g., the midbrain volume and medial frontal cortex), and at rates which differ from Parkinson’s disease.17,27,50,51 Decline in midbrain volumes correlate with changes in clinical measures.27 However, these studies address a very different question to that of the current study. Here we tested the relationship between brain structure and future survival, in other words, prediction. The anatomical correlates of survival are not the typical regions with most significant progressive atrophy or related to clinical severity.52 This disparity might arise from differential non-linear changes in volume between regions, or the specific range of measures included in the PSPRS.
The Richardson’s syndrome phenotype of PSP disrupts the circuits between orbitofrontal cortex, striatum and thalamus.16,19,53–56 Atrophy in these regions, and changes to white matter connections between them are well described in PSP, and likely underlie the functional breakdown in networks which support motor and cognitive functions.52 Our findings suggest that regional volume loss progresses in a largely symmetrical and linear pattern throughout the disease course in Richardson’s syndrome, with subcortical regions demonstrating a stronger linear relationship than cortical regions. In typical Richardson’s syndrome, subcortical regions develop neuroglial pathology first, and may therefore have more advanced degeneration compared to cortical areas at mid and later stages of disease.57 This may increase their predictive signal-to-noise. The globular rather than sheet-like morphology of many subcortical regions (e.g., thalamus, pallidum), may also increase the volumetric signal-to-noise ratio. Changes in the intrinsic grey matter signal, and juxta-cortical white matter, from PSP pathology might in principle change the signal intensities that affect the volume estimations, thus contributing to the findings in our study.
Subcortical phenotypes demonstrated stronger linear relationships between temporal stage and regional volumes than Richardson’s syndrome while consistent, symmetrical findings were not demonstrated in Cortical phenotypes. It may be that the heterogeneity of the Cortical phenotypes, and low numbers in comparison to the Richardson’s syndrome group, contributed to reduced power and thus Type II error. If the null result is true, rather than Type II error, it would suggest (but not in itself prove) that the PSPRS is a function of cortical pathology. This is plausible, as many of the actions and tasks referred to in the PSPRS are based on cortical systems (e.g., cognition, social engagement, behaviour, praxis, volitional motor and oculomotor control), and change in PSPRS over a year of PSP disease progression correlate with cortical synaptic loss.58 Encouragingly, the strongest relationships between temporal stage and regional volumes were shown in the group with the highest degree of diagnostic certainty; those with neuropathological confirmation. This study was not designed to identify the mediating factors in survival, but the structural data raise hypotheses about the determinants and predictors of survival which require further testing in larger and prospective cohorts.
This study has several limitations. The date of symptom onset is challenging to estimate, especially for Cortical phenotypes: the onset of motor symptoms or a first fall may be clearly recalled but subtler changes in behaviour or personality may not be. Indeed, common cognitive and behavioural changes such as apathy, impulsivity, language and executive dysfunction are frequently missed or underestimated in early stages of the disease59–64 while the MAX rules45 are intrinsically based towards Richardson’s syndrome’s motor features as the illness progresses from variant phenotypes. This lack of clarity will affect the estimation of survival from onset to death and hence the calculation of temporal ‘stage’. Retrospective allocation of phenotypes is also subject to error, especially when reliant on contemporaneous clinical notes. A clinician practicing in 2007, for example, may have been more likely to have documented features integral to the diagnostic criteria of the time, rather than highlighting characteristics suggestive of a variant phenotype by today’s standards. Whilst the cross-sectional nature of the study allows correlation with survival, it lacks the longitudinal imaging necessary for formal mediation analysis. In part this reflects the long timespan of the current study (2007-2019), during which time there have been marked changes in diagnosis, phenotyping and MRI sequences and scanners. Our analysis utilised T1-weighted volumetric imaging building on the established literature of grey and white matter changes in PSP, but our inferences about networks are based on overlapping spatial distributions rather than functional imaging which would provide further valuable information.
In summary, atrophy of a symmetric network of subcortical regions in the striatum, cerebellum and frontotemporal cortex is related to survival risk in PSP, manifest as a correlation with the temporal stage from symptom onset to death. The relationship between atrophy and survival may be useful to identify and treat the mechanisms of poor survival, and to stratify heterogeneous patient cohorts in clinical trials with optimised outcome measures for disease-modifying approaches.
Supplementary Material
Funding
This study was co-funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at Cambridge University Hospitals NHS Foundation Trust and the University of Cambridge including the Cambridge Brain Bank (BRC-1215-20014; NIHR203312); the Wellcome Trust (103838; 220258); the Holt Fellowship (RG86564); the Medical Research Council (MR/P01271X/1; SUAG/092 G116768 and SUAG/051 G101400); and the Cambridge Centre for Parkinson-plus (RG95450). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Financial Disclosure
Dr Street, Dr Bevan-Jones, Dr Malpetti, Mr Jones, Dr Passamonti, Dr Rittman, Dr Coyle-Gilchrist, Dr Allison and Mrs Dawson report no disclosures. Dr Ghosh provides consultancy to NICE, UCB and Biogen and sits on the research committee of the PSP Association (UK). Professor Rowe serves as editor to Brain and is a non-remunerated trustee of the Guarantors of Brain and the PSP Association (UK). He provides consultancy to Asceneuron, Biogen, UCB, SVHealth and Wave, and has research grants from AZ-Medimmune, Janssen, Lilly as industry partners in the Dementias Platform UK.
Footnotes
Author contribution statement:
D Street was responsible for the final design, acquisition, analysis, interpretation, drafting and final approval of the submitted version of the manuscript.
WRB-J was responsible for initial design, acquisition, analysis, interpretation, drafting and final approval of the submitted version of the manuscript.
MM and PSJ substantially contributed to the design, analysis and interpretation of the work and drafting and final approval of the submitted version of the manuscript.
LP, BCPG, TR, ITSC-G, KA and CED substantially contributed to the acquisition and interpretation of data and drafting and final approval of the submitted version of the manuscript.
JBR was responsible for the final design, acquisition, analysis, interpretation, drafting and final approval of the submitted version of the manuscript.
Conflicts of interest: The authors have no conflicts of interest.
References
- 1.Coyle-Gilchrist ITS, Dick KM, Patterson K, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86(18):1736–1743. doi: 10.1212/WNL.0000000000002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arena JE, Weigand SD, Whitwell JL, et al. Progressive supranuclear palsy: progression and survival. J Neurol. 2016;263(2):380–389. doi: 10.1007/s00415-015-7990-2. [DOI] [PubMed] [Google Scholar]
- 3.Litvan I, Mangone CA, McKee A, et al. Natural history of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome) and clinical predictors of survival: a clinicopathological study. J Neurol Neurosurg Psychiatry. 1996;60(6):615–620. doi: 10.1136/jnnp.60.6.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Respondek G, Stamelou M, Kurz C, et al. The phenotypic spectrum of progressive supranuclear palsy: A retrospective multicenter study of 100 definite cases. Mov Disord. 2014;29(14):1758–1766. doi: 10.1002/mds.26054. [DOI] [PubMed] [Google Scholar]
- 5.Golbe LI, Davis PH, Schoenberg BS, Duvoisin RC. Prevalence and natural history of progressive supranuclear palsy. Neurology. 1988;38(7):1031. doi: 10.1212/WNL.38.7.1031. [DOI] [PubMed] [Google Scholar]
- 6.Chiu WZ, Kaat LD, Seelaar H, et al. Survival in progressive supranuclear palsy and frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2010;81(4):441–445. doi: 10.1136/jnnp.2009.195719. [DOI] [PubMed] [Google Scholar]
- 7.Brenneis C, Seppi K, Schocke M, Benke T, Wenning GK, Poewe W. Voxel based morphometry reveals a distinct pattern of frontal atrophy in progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 2004;75(2):246–249. doi: 10.1136/jnnp.2003.015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price S, Paviour D, Scahill R, et al. Voxel-based morphometry detects patterns of atrophy that help differentiate progressive supranuclear palsy and Parkinson’s disease. Neuroimage. 2004;23(2):663–669. doi: 10.1016/j.neuroimage.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Padovani A, Borroni B, Brambati SM, et al. Diffusion tensor imaging and voxel based morphometry study in early progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 2006;77(4):457–464. doi: 10.1136/jnnp.2005.075713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paviour D, Price SL, Jahanshahi M, Lees AJ, Fox NC. Regional brain volumes distinguish PSP, MSA-P, and PD: MRI-based clinico-radiological correlations. Mov Disord. 2006;21(7):989–996. doi: 10.1002/mds.20877. [DOI] [PubMed] [Google Scholar]
- 11.Josephs KA, Whitwell JL, Dickson DW, et al. Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiol Aging. 2008;29(2):280–289. doi: 10.1016/j.neurobiolaging.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizzo G, Martinelli P, Manners D, et al. Diffusion-weighted brain imaging study of patients with clinical diagnosis of corticobasal degeneration, progressive supranuclear palsy and Parkinson’s disease. Brain. 2008;131(10):2690–2700. doi: 10.1093/brain/awn195. [DOI] [PubMed] [Google Scholar]
- 13.Huppertz H-J, Moller L, Martin S, et al. Differentiation of Neurodegenerative Parkinsonian Syndromes by Volumetric Magnetic Resonance Imaging Analysis and Support Vector Machine Classification. Mov Disord. 2016;31(10):1506–1517. doi: 10.1002/mds.26715. [DOI] [PubMed] [Google Scholar]
- 14.Whitwell JL, Master AV, Avula R, et al. Clinical Correlates of White Matter Tract Degeneration in Progressive Supranuclear Palsy. Arch Neurol. 2011;68(6):753–760. doi: 10.1001/archneurol.2011.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang J, Shao N, Li J, Shang H. Voxelwise meta-analysis of white matter abnormalities in progressive supranuclear palsy. Neurol Sci. 2014;35(1):7–14. doi: 10.1007/s10072-013-1512-8. [DOI] [PubMed] [Google Scholar]
- 16.Gardner RC, Boxer AL, Trujillo A, et al. Intrinsic connectivity network disruption in progressive supranuclear palsy. Ann Neurol. 2013;73(5):603–616. doi: 10.1002/ana.23844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dutt S, Binney RJ, Heuer HW, et al. Progression of brain atrophy in PSP and CBS over 6 months and 1 year. Neurology. 2016;87(19):2016–2025. doi: 10.1212/WNL.0000000000003305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Wai Y, Lin W-Y, et al. Microstructural changes in patients with progressive supranuclear palsy: a diffusion tensor imaging study. J Magn Reson Imaging. 2010;32(1):69–75. doi: 10.1002/jmri.22229. [DOI] [PubMed] [Google Scholar]
- 19.Whitwell JL, Avula R, Master A, et al. Disrupted thalamocortical connectivity in PSP : A resting-state fMRI, DTI, and VBM study. Park Relat Disord. 2011;17(8):599–605. doi: 10.1016/j.parkreldis.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seppi K, Schocke MFH, Esterhammer R, et al. Diffusion-weighted imaging discriminates progressive supranuclear palsy from PD, but not from the parkinson variant of. Neurology. 2003;60:1031–1034. doi: 10.1212/01.wnl.0000049911.91657.9d. [DOI] [PubMed] [Google Scholar]
- 21.Paviour DC, Thornton JS, Lees AJ, Jäger HR. Diffusion-weighted magnetic resonance imaging differentiates parkinsonian variant of multiple-system atrophy from progressive supranuclear palsy. Mov Disord. 2007;22(1):68–74. doi: 10.1002/mds.21204. [DOI] [PubMed] [Google Scholar]
- 22.Focke NK, Helms G, Scheewe S, et al. Individual Voxel-Based Subtype Prediction can Differentiate Progressive Supranuclear Palsy from Idiopathic Parkinson Syndrome and Healthy Controls. Hum Brain Mapp. 2011;32(11):1905–1915. doi: 10.1002/hbm.21161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsukamoto K, Matsusue E, Kanasaki Y, et al. Significance of apparent diffusion coefficient measurement for the differential diagnosis of multiple system atrophy, progressive supranuclear palsy, and Parkinson’s disease: evaluation by 3.0-T MR imaging. Neuroradiology. 2012;54:947–955. doi: 10.1007/s00234-012-1009-9. [DOI] [PubMed] [Google Scholar]
- 24.Dickson DW, Ahmed Z, Algom AA, Tsuboi Y, Josephs KA. Neuropathology of variants of progressive supranuclear palsy. Curr Opin Neurol. 2010;23(4):394–400. doi: 10.1097/WCO.0b013e32833be924. [DOI] [PubMed] [Google Scholar]
- 25.Dickson DW, Kouri N, Murray ME, Josephs KA. Neuropathology of frontotemporal lobar degeneration-Tau (FTLD-Tau) J Mol Neurosci. 2011;45(3):384–389. doi: 10.1007/s12031-011-9589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Höglinger GU, Schöpe J, Stamelou M, et al. Longitudinal magnetic resonance imaging in progressive supranuclear palsy: A new combined score for clinical trials. Mov Disord. 2017;32(6):842–852. doi: 10.1002/mds.26973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Josephs KA, Xia R, Mandrekar J, et al. Modeling trajectories of regional volume loss in progressive supranuclear palsy. Mov Disord. 2013;28(8):1117–1124. doi: 10.1002/mds.25437. [DOI] [PubMed] [Google Scholar]
- 28.Dell’Aquila C, Zoccolella S, Cardinali V, et al. Predictors of survival in a series of clinically diagnosed progressive supranuclear palsy patients. Park Relat Disord. 2013;19(11):980–985. doi: 10.1016/j.parkreldis.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Josephs KA, Petersen RC, Knopman DS, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology. 2006;66(1):41–48. doi: 10.1212/01.wnl.0000191307.69661.c3. [DOI] [PubMed] [Google Scholar]
- 30.Borroni B, Grassi M, Agosti C, et al. Survival in Frontotemporal Lobar Degeneration and Related Disorders: Latent Class Predictors and Brain Functional Correlates. Rejuvenation Res. 2009;12(1):33–44. doi: 10.1089/rej.2008.0812. [DOI] [PubMed] [Google Scholar]
- 31.Ghosh BCP, Carpenter RHS, Rowe JB. A Longitudinal Study of Motor, Oculomotor and Cognitive Function in Progressive Supranuclear Palsy. PLoS One. 2013;8(9):1–14. doi: 10.1371/journal.pone.0074486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Eimeren T, Antonini A, Berg D, et al. Neuroimaging biomarkers for clinical trials in atypical parkinsonian disorders: Proposal for a Neuroimaging Biomarker Utility System. Alzheimer’s Dement Diagnosis, Assess Dis Monit. 2019;11:301–309. doi: 10.1016/j.dadm.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Longoni G, Agosta F, Kostić VS, et al. MRI measurements of brainstem structures in patients with Richardson’s syndrome, progressive supranuclear palsyparkinsonism, and Parkinson’s disease. Mov Disord. 2011;26(2):247–255. doi: 10.1002/mds.23293. [DOI] [PubMed] [Google Scholar]
- 34.Quattrone A, Caligiuri ME, Morelli M, et al. Imaging counterpart of postural instability and vertical ocular dysfunction in patients with PSP: A multimodal MRI study. Parkinsonism Relat Disord. 2019;63:124–130. doi: 10.1016/j.parkreldis.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 35.Picillo M, Tepedino MF, Abate F, et al. Midbrain MRI assessments in progressive supranuclear palsy subtypes. J Neurol Neurosurg Psychiatry. 2020;91(1):98–103. doi: 10.1136/jnnp-2019-321354. [DOI] [PubMed] [Google Scholar]
- 36.Hwang M, Yang H, Kim Y, et al. Differential Progression of Midbrain Atrophy in Parkinsonism: Longitudinal MRI Study. Neurodegener Dis. 2017;17(1):31–37. doi: 10.1159/000448174. [DOI] [PubMed] [Google Scholar]
- 37.Quattrone A, Morelli M, Nigro S, et al. A new MR imaging index for differentiation of progressive supranuclear palsy-parkinsonism from Parkinson’s disease. Parkinsonism Relat Disord. 2018;54:3–8. doi: 10.1016/j.parkreldis.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 38.Agosta F, Kostić VS, Galantucci S, et al. The in vivo distribution of brain tissue loss in Richardson’s syndrome and PSP-parkinsonism: a VBM-DARTEL study. Eur J Neurosci. 2010;32(4):640–647. doi: 10.1111/j.1460-9568.2010.07304.x. [DOI] [PubMed] [Google Scholar]
- 39.Quattrone A, Nicoletti G, Messina D, et al. MR imaging index for differentiation of progressive supranuclear palsy from Parkinson disease and the Parkinson variant of multiple system atrophy. Radiology. 2008;246(1):214–221. doi: 10.1148/radiol.2453061703. [DOI] [PubMed] [Google Scholar]
- 40.Quattrone A, Morelli M, Quattrone A, et al. Magnetic Resonance Parkinsonism Index for evaluating disease progression rate in progressive supranuclear palsy: A longitudinal 2-year study. Parkinsonism Relat Disord. 2020;72:1–6. doi: 10.1016/j.parkreldis.2020.01.019. [DOI] [PubMed] [Google Scholar]
- 41.Grijalva RM, Pham NTT, Huang Q, et al. Brainstem Biomarkers of Clinical Variant and Pathology in Progressive Supranuclear Palsy. Mov Disord. doi: 10.1002/mds.28901. Published online December 31, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Picillo M, Abate F, Ponticorvo S, et al. Association of MRI Measures With Disease Severity and Progression in Progressive Supranuclear Palsy. Front Neurol. 2020;11:603161. doi: 10.3389/fneur.2020.603161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitwell JL, Tosakulwong N, Botha H, et al. Brain volume and flortaucipir analysis of progressive supranuclear palsy clinical variants. NeuroImage Clin. 2020;25:102152. doi: 10.1016/j.nicl.2019.102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Höglinger GU, Respondek G, Stamelou M, et al. Clinical Diagnosis of Progressive Supranuclear Palsy - The Movement Disorder Society Criteria. Mov Disord. 2017;00(00):1–12. doi: 10.1002/mds.26987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grimm MJ, Respondek G, Stamelou M, et al. How to apply the movement disorder society criteria for diagnosis of progressive supranuclear palsy. Mov Disord. 2019;34(8):1228–1232. doi: 10.1002/mds.27666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rohrer JD, Nicholas JM, Cash DM, et al. Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the Genetic Frontotemporal dementia Initiative (GENFI) study: A cross-sectional analysis. Lancet Neurol. 2015;14(3):253–262. doi: 10.1016/S1474-4422(14)70324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/S0896-6273(02)00569-X. [DOI] [PubMed] [Google Scholar]
- 48.Iglesias JE, Van Leemput K, Bhatt P, et al. Bayesian segmentation of brainstem structures in MRI. Neuroimage. 2015;113:184–195. doi: 10.1016/j.neuroimage.2015.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Street D, Whiteside D, Rittman T, Rowe JB. Prediagnostic Progressive Supranuclear Palsy - Insights from the UK Biobank. Park Relat Disord. 2022 July;95:59–64. doi: 10.1016/j.parkreldis.2022.01.004. 2021. [DOI] [PubMed] [Google Scholar]
- 50.Tsai RM, Lobach I, Bang J, et al. Clinical correlates of longitudinal brain atrophy in progressive supranuclear palsy. Park Relat Disord. 2016;28:29–35. doi: 10.1016/j.parkreldis.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guevara C, Bulatova K, Barker GJ, Gonzalez G, Crossley N, Kempton MJ. Whole-Brain Atrophy Rate in Idiopathic Parkinson ’ s Disease, Multiple System Atrophy, and Progressive Supranuclear Palsy. Parkinsons Dis. 2016;2016 doi: 10.1155/2016/9631041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whitwell JL, Höglinger GU, Antonini A, et al. Radiological biomarkers for diagnosis in PSP: Where are we and where do we need to be? Mov Disord. 2017;32(7):955–971. doi: 10.1002/mds.27038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Erbetta A, Mandelli ML, Grisoli M, et al. Topographic Involvement of the Thalamus in Progressive Supranuclear Palsy and Corticobasal. Am J Neuroradiol. 2009;30(8):1482–1487. doi: 10.3174/ajnr.A1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Upadhyay N, Suppa A, Piattella MC, et al. Functional disconnection of thalamic and cerebellar dentate nucleus networks in progressive supranuclear palsy and corticobasal syndrome. Park Relat Disord. 2016;39:52–57. doi: 10.1016/j.parkreldis.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 55.Piattella MC, Tona F, Bologna M, et al. Disrupted resting-state functional connectivity in progressive supranuclear palsy. Am J Neuroradiol. 2015;36(5):915–921. doi: 10.3174/ajnr.A4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burciu RG, Ofori E, Shukla P, et al. Distinct patterns of brain activity in progressive supranuclear palsy and Parkinson’s disease. Mov Disord. 2015;30(9):1248–1258. doi: 10.1002/mds.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kovacs GG, Ghetti B, Goedert M. Classification of diseases with accumulation of Tau protein. Neuropathol Appl Neurobiol. 2022;48(3):1–10. doi: 10.1111/nan.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holland N, Jones PS, Savulich G, et al. Longitudinal Synaptic Loss in Primary Tauopathies: An In Vivo [11 C]UCB-J Positron Emission Tomography Study. Mov Disord. 2023;38(7):1316–1326. doi: 10.1002/mds.29421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brown RG, Lacomblez L, Landwehrmeyer BG, et al. Cognitive impairment in patients with multiple system atrophy and progressive supranuclear palsy. Brain. 2010;133(8):2382–2393. doi: 10.1093/brain/awq158. [DOI] [PubMed] [Google Scholar]
- 60.Chaithra SP, Prasad S, Holla VV, et al. The Non-Motor Symptom Profile of Progressive Supranuclear Palsy. J Mov Disord. 2020;13(2):118–126. doi: 10.14802/jmd.19066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gerstenecker A, Duff K, Mast B, Litvan I. Behavioral abnormalities in progressive supranuclear palsy. Psychiatry Res. 2013;210(3):1205–1210. doi: 10.1016/j.psychres.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pellicano C, Assogna F, Cellupica N, et al. Neuropsychiatric and cognitive profile of early Richardson’s syndrome, Progressive Supranuclear Palsy-parkinsonism and Parkinson’s disease. Park Relat Disord. 2017;45:50–56. doi: 10.1016/j.parkreldis.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 63.Peterson KA, Patterson K, Rowe JB. Language impairment in progressive supranuclear palsy and corticobasal syndrome. J Neurol. 2019:0123456789. doi: 10.1007/s00415-019-09463-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rittman T, Ghosh BC, McColgan P, et al. The Addenbrooke’s Cognitive Examination for the differential diagnosis and longitudinal assessment of patients with parkinsonian disorders. J Neurol Neurosurg Psychiatry. 2013;84(5):544–551. doi: 10.1136/jnnp-2012-303618. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.