Abstract
Although recent structural connectivity studies of traumatic brain injury (TBI) have used graph theory to evaluate alterations in global integration and functional segregation, pooled analysis is needed to examine the robust patterns of change in graph metrics across studies. Following a systematic search, 15 studies met the inclusion criteria for review. Of these, ten studies were included in a random-effects meta-analysis of global graph metrics, and subgroup analyses examined the confounding effects of severity and time since injury. The meta-analysis revealed significantly higher values of normalised clustering coefficient (gö=ö1.445, CI=[0.512, 2.378], pö=ö0.002) and longer characteristic path length (gö=ö0.514, CI=[0.190, 0.838], pö=ö0.002) in TBI patients compared with healthy controls. Our findings suggest that the TBI structural network has shifted away from the balanced small-world network towards a regular lattice. Therefore, these graph metrics may be useful markers of neurocognitive dysfunction in TBI. We conclude that the pattern of change revealed by our analysis should be used to guide hypothesis-driven research into the role of graph metrics as diagnostic and prognostic biomarkers.
Keywords: Traumatic brain injury, Graph theory, Graph metrics, Structural connectomics, Network analysis, Diffusion MRI, Biomarkers, Meta-analysis, Systematic search, Narrative review
1. Introduction
Traumatic Brain Injury (TBI) is one of the leading causes of death and disability in young people, affecting 10 million people worldwide every year (Humphreys et al., 2013; Hyder et al., 2007). The severity of a brain injury is typically described as mild, moderate, or severe, based on time spent unconscious and/or coma rating score, the duration of post-traumatic amnesia, and neuroimaging results. Cognitive deficits (e.g., slow processing speed and poor concentration), motor control deficits (e.g., poor manual dexterity, balance deficits), and behavioural problems (e.g., impulsivity) are particularly common (Rabinowitz and Levin, 2014; Rossi and Sullivan, 1996). Approximately 15–30% of mild TBI cases (Shenton et al., 2012) and up to 65% of moderate-severe cases (Rabinowitz and Levin, 2014; Selassie et al., 2008) report long-term problems. These persistent deficits cause disability and interfere with a patient’s ability to perform day-to-day tasks, for example getting dressed, planning ahead, and preparing food (Rabinowitz and Levin, 2014). Isolating neurological biomarkers holds promise as a means to identify which patients are at risk of long-term disability; which has implications for patient management and development of economically sustainable treatment options.
There is mounting evidence supporting diffusion MRI as a sensitive diagnostic tool in the care of patients with TBI (for reviews, see Delouche et al., 2016; Hulkower et al., 2013; Hutchinson et al., 2018; Xiong et al., 2014). First, changes in white matter organisation following TBI have been demonstrated in several important fibre bundles of the brain (Bendlin et al., 2008), including the superior longitudinal fasciculus (e. g., Farbota et al., 2012; Spitz et al., 2013) and the corpus callosum (e.g., Levin et al., 2008; Mayer et al., 2010; Rutgers et al., 2008). For example, in a meta-analysis of 13 diffusion studies of TBI, significant increases in fractional anisotropy (FA) and decreases in mean diffusivity (MD) were found in the posterior parts of the corpus callosum (Aoki et al., 2012).
Second, decreased white matter organization has been shown to predict poorer outcome in chronic TBI patients of all severity types (Kinnunen et al., 2011; Kraus et al., 2007), and in acute mild TBI patients with persistent symptoms (Niogi et al., 2008). Lower FA in the subregions of the corpus callosum has been associated with poorer bimanual coordination (Caeyenberghs et al., 2011a) and slower processing speed (e.g., Levin et al., 2008; Wilde et al., 2006) in moderate-severe TBI patients. Similarly, lower FA in the cerebellum has been associated with poorer manual dexterity (Caeyenberghs et al., 2011b). Despite multiple reports of altered diffusion metrics, the regional analyses reported in these studies cannot identify how whole brain networks are affected by white matter damage following TBI.
Because TBI may be considered a ‘disconnection syndrome’, where symptoms are accounted for by altered connectivity between regions of the brain, it is important to take global network disruption into account (Catani and Ffytche, 2005; Griffa et al., 2013). Where traditional diffusion approaches such as those outlined above examine isolated brain regions, graph theoretical analysis (GTA) can characterise the global structure of the brain network (or ‘connectome’; Bullmore and Bassett, 2011; Hagmann et al., 2008; Sporns, 2013). Structural GTA represents the brain as a set of ‘edges’ (white matter pathways) that pass between ‘nodes’ (brain regions), using the reconstruction of white matter tracts as weights. This graph is then used to calculate graph metrics, which estimate network properties such as global integration and functional segregation (Rubinov and Sporns, 2010; see also Supplementary Material 1 for definitions, interpretations, and calculations for the graph metrics included in this review).
Connectome analyses have rapidly found applications in the clinical neurosciences because the balance between integration and segregation necessary to support complex function may be affected by disease or injury. In their seminal review, Griffa et al. (2013) propose that graph metrics show promise as biomarkers in neurodevelopmental disorders such as ADHD (e.g., Cao et al., 2013), neurodegenerative diseases like Alzheimer’s disease (e.g., Lo et al., 2010), and psychiatric disorders such as schizophrenia (e.g., Fornito et al., 2012). In one of the first structural GTA studies of TBI, Caeyenberghs et al. (2012) have revealed that young TBI patients have decreased connectivity degree within the brain, which correlated significantly with poor balance. Similarly, Kim et al. (2014) found that longer path length in adults with moderate-severe TBI correlated with poorer higher-order cognitive processes like executive function and verbal learning. Since then, more research has suggested that graph metrics could be ‘biomarkers’ of TBI (e.g., Hellyer et al., 2015; Yuan et al., 2015, 2017b).
With recent growth in the use of structural GTA in all types of TBI, there is a need to conduct a meta-analytical review to probe consistent patterns of change in graph metrics to see which hold promise as biomarkers. In the study presented here, we conduct a narrative review of diffusion MRI papers comparing healthy controls (HCs) using GTA, and the first meta-analysis to date of graph metrics in TBI. Heterogeneity in patient samples is addressed using subgroup analyses. This divides up an already small body of research, and as such the results are for hypothesis generation only. It was also our aim to draw inferences from this data about how graph metrics might be used as biomarkers in TBI, and to provide a framework for hypotheses in future GTA studies.
2. Method
2.1. Search and selection strategy
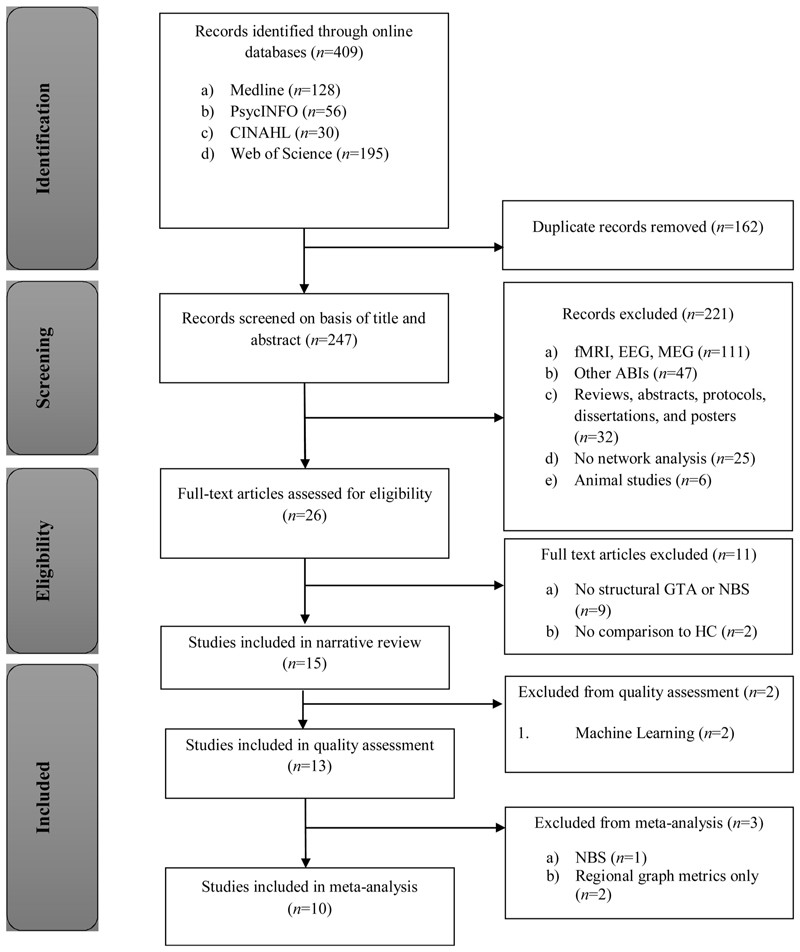
A systematic literature search was conducted using Medline, CINAHL, PsycINFO, and Web of Science for all relevant articles published from 1999 until the last search date (4th of April 2018; see Fig. 1 for PRISMA diagram). The search terms were [((TI OR AB) “traumatic brain injur*” OR TBI)) AND ((TI OR AB) connectom* OR “structural connect*” OR “graph theor*” OR “graph metric*” OR “graph analys*” OR “network analys*”)] (see Supplementary Material 2 for Mesh headings).
Fig. 1. PRISMA flow diagram of the systematic literature search.
Abstracts and titles of 247 unique papers were returned from this search. The reference lists of review papers were searched for additional studies (but none were found). After screening titles and abstracts, we excluded studies of functional MRI, electro-encephalography (EEG) or magneto-encephalography (MEG), animal models of TBI, and other causes of acquired brain injury (such as brain tumours or stroke). Also excluded were studies that did not employ a network analysis (for example, tract-based comparisons of FA), any publications that were not peer-reviewed (e.g., conference abstracts), and review papers.
The remaining 26 articles were examined in full to assess eligibility. Studies that did not compare the structural connectomes between TBI patients and HCs, or that did not calculate graph metrics or run network-based statistics (NBS) were excluded, leaving 15 studies for inclusion in the narrative review. Of these, ten studies were included in the meta-analysis, addressing global graph metrics that directly compared the structural connectomes of TBI patients and HCs. The five studies not included in the meta-analysis were Fagerholm et al. (2015) and Mitra et al. (2016), both of which applied machine learning techniques; Dall’Acqua et al. (2016) which employed Network Based Statistics (NBS) for the group comparisons; and finally Solmaz et al. (2017) and Caeyenberghs et al. (2013), who only investigated group differences in regional graph metrics.
2.2. Quality assessment
Two authors (PI, AC) assessed the methodological quality of each study independently, using a quality checklist for diffusion MRI studies adapted from Strakowski et al. (2000). This checklist has been used to measure methodological quality of papers in previous meta-analyses on schizophrenia (e.g., Baiano et al., 2007; Shepherd et al., 2012), major depressive disorder (e.g., Jiang et al., 2017), and bipolar disorder (Strakowski et al., 2000). As shown in Supplementary Material 3, the checklist included three categories: (i) subjects (items 1–4); (ii) image acquisition methodology and analysis (items 5–10); and (iii) results and conclusions (items 11–13). For each item, scores of 1, 0.5, and 0 were assigned (1 = criteria fully met; 0.5 = criteria partially met; 0 = not met). Total scores vary from 0 to 13. Currently, there are no established cut-off scores for high- and low-quality studies using this tool, however, it was decided by the research team that any study with less than half the total score would be excluded from the analysis for poor methodological quality. Disagreements between reviewers were resolved by a third review from the senior author (KC).
2.3. Data extraction for quantitative synthesis
Global graph metrics estimating global integration (global efficiency, normalised path length, and characteristic path length); functional segregation (normalised clustering coefficient, transitivity, mean local efficiency, modularity); centrality, resilience (betweenness centrality, small-worldness, assortativity); and basic measures (degree, density, and strength) were extracted across studies (see Supplementary Material 1 for comprehensive definitions of these graph metrics). To calculate effect sizes, means and standard deviations were extracted from published articles, supplementary materials, or via email correspondence with the authors (Caeyenberghs et al., 2014; Kim et al., 2014; van der Horn et al., 2016). In one study, p-values and t-scores were used to estimate the effect size (Hellyer et al., 2015). For longitudinal GTA studies (Yuan et al., 2017a, b), only the baseline (‘pretraining’) comparisons between TBI and HCs were included. Two papers reported TBI connectivity data in separate subgroups, one according to severity level (Königs et al., 2017), and the other by post-traumatic complaints (van der Horn et al., 2016). The latter provided pooled data for the purpose of the overall synthesis via email. For Königs et al. (2017) the averages across the TBI group were pooled for the global synthesis in Microsoft Excel (using calculations included in Supplementary Material 4). Graph metrics that were calculated at the local or nodal level were excluded (i.e., local efficiency, eigenvector centrality, and betweenness centrality of singular nodes not averaged across the network) to constrain the scope of the analysis to network-level dysfunction.
2.4. Data analysis for quantitative synthesis
Hedge’s g, the standardised mean difference score between groups, was calculated for each outcome variable (i.e., graph metric) using the Comprehensive Meta-Analysis software, and analysed using a random-effects model (CMA; Biostat, USA, v2.2.064). In basic terms, a separate meta-analysis for each graph metric was run, as each metric should be treated as a separate outcome measure. To calculate the overall effect sizes, mean effects of each metric were pooled across studies and weighted by sample size and the 95% confidence intervals (CI). A positive effect size indicated that the TBI group had a higher mean value of the graph metric compared with the HC group, while a negative value indicated higher mean values in the HC group. Effect sizes were regarded as small if g ≥0.2, medium if g ≥0.5 and large if g ≥0.8 (Cohen, 1988). Also, subgroup analyses on graph metrics were conducted for injury severity (mild, moderate-severe), chronicity (time since injury) (acute:< 6 months post injury; chronic:> 6 months post injury), and age at injury (paediatric : < 18 years old; adult: 18–65 years old). The results of our meta-analysis should be considered as hypothesis generation only, as suggested by the Cochrane guidelines when the number of studies in the analysis is low (Sambunjak et al., 2017).
The I2 statistic was used to index heterogeneity in the data, i.e. the percentage of observed variability that is greater than what would be expected by chance or sampling error alone. High scores (I2 > 75%) suggest heterogeneity due to differences in sample demographics (Higgins et al., 2003). Low I2 scores (I2 < 50%) represent homogenous data, supporting a real effect between HC and TBI groups. Publication bias was assessed using Egger’s test for asymmetry in a funnel plot (Egger et al., 1997).
Finally, false discovery rate (FDR) correction (p < 0.002) was conducted for all analyses in accordance with recommendations by Wang and Ware (2013). Interdependencies between outcomes were accounted for using the Benjamini-Yekutieli procedure on the Bioinformatics toolbox in MATLAB_R2018a (Benjamini and Yekutieli, 2001).
3. Results
3.1. Sample characteristics
The TBI patient pool included 429 participants, and the HC pool 306, with an age range of 8–65 years old. Four studies included mTBI patients only, six studies included moderate-severe TBI patients only, and two studies included both severity types (see Table 1). Chronicity varied widely between studies, with TBI groups ranging from acute (e.g., within 96 h post injury; Yuan et al., 2015) to chronic (e.g., 5.91 years post injury, ± 3.1 years; Yuan et al., 2017a). Six studies recruited paediatric TBI patients, two studies included both children and young adults, and four studies recruited adult TBI patients.
Table 1. Demographics and Processing Methods for Graph Theoretical Studies of Traumatic Brain Injury.
| Participants | Data Acquisition | Processing Pipeline | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample size TBĨ (HC) | Age range at scan (years) or M(SD) | Ave age at injury (years) | Severity | Timea since injury | Number of directions | b-value | Parcellation Scheme | Areas Removed | Number of ROIs | Orientation model | Tractographyd | Weighted Byc | |
| Caeyenberghs et al., 2012 | 12(17) | 8–20 | 10.5 | Moderate-severe | 42 (31.2) | 45 | 800 (1 b0) | Automated Anatomical Labelling | 116 | Principle eigenvector | DT | SD | |
| Caeyenberghs et al., 2013 | 17(16) | 16–34 | 21.2 | Moderate-severe | 51 (29) | 64 | 1000 (1 b0) | Switching Network | 22 | Principle eigenvector | DT | NOS FA | |
| Caeyenberghs et al., 2014 | 21(17) | 9–29 | 21.3 | Moderate-severe | 51 (29) | 64 | 1000 (1 b0) | Automated Anatomical Labelling | 116 | Principle eigenvector | DT | % | |
| Dall’Acqua et al., 2016 | 51(53) | 18–61 | 34.5 | Mild | 0.2 | 64 | 1000 (1 b0) | Automated Anatomical Labelling | Cerebellar regions | 90 | Principle eigenvector | DT | NOS |
| Hellyer et al., 2015 | 63(26) | 37.4 (12.4) | 31.9 | Allb | 5.5 (3.3) | 64 | 1000 (4 b0) | Destreux (Freesurfer) | - | 164 | Principle eigenvector | PT | FA |
| Kim et al., 2014 | 22(18) | 17–57 | 26.0 | Moderate-severe | 40.9 (75.6) | 30 | 1000 (1 b0) | Desikan (Freesurfer) | Cerebellar regions | 95 | Principle eigenvector | PT | SCP |
| Königs et al., 2017 | 36(27) | 8–14 | 7.3 | All | 33.6 (13.2) | 30 | 750 (5 b0) | Automated Anatomical Labelling and FIRST | Cerebellar regions | 84 | Principle eigenvector | PT | SLD FA |
| Solmaz et al., 2017 | 40(35) | 18–64 | NA | Moderate-severe | 3.45 (0.6) | 30 | 1000 (7 b0) | Desikan (Freesurfer) | - | 86 | Principle eigenvector | PT | NOS |
| van der Horn et al., 2016 | 53(20) | 18–65 | 33.4 | Mild | 1 (NA) | 60 | 1000 (7 b0) | Desikan Killianey and subcortical | Cerebellar and ventricle regions | 85 | CSD | PT | NOS |
| Verhelst et al., 2018 | 17(17) | 11–17 | 13.4 | Moderate-severe | 28.3 (13) | 64 | 1200 (1 b0) | Individual parcellation (Freesurfer) | Not known | 82 | CSD | PT (ACT) | NOS |
| Yuan et al., 2015 | 23(20) | 11–16 | 13.7 | Mild | 0.1 (NA) | 61 | 1000 (1 b0) | Automated Anatomical Labelling | Cerebellar regions | 90 | Principle eigenvector | DT | NOS |
| Yuan et al. (2017b) | 17(11) | 9–18 | 7.8 | Moderate-severe | 70.9 (37.2) | 61 | 1000 (1 b0) | Automated Anatomical Labelling | Cerebellar regions | 90 | Principle eigenvector | DT | NOS |
| Yuan et al. (2017b) | 22(20) | 15.45 (1.72) | 15.3 | Mild | 1-4 | 61 | 1000 (7 b0) | Automated Anatomical Labelling | Cerebellar regions | 90 | Principle eigenvector | DT | NOS |
Time is from the injury/onset until MRI scan for TBI patients, described in months; M(SD)). NA = Not Applicable.
55 of the 63 TBI patients were moderate-severe, and as such Hellyer et al. (2015) was included in the moderate-severe subgroup analyses.
NOS = number of streamlines; FA = fractional anisotropy; % = percentage of all streamlines that pass through the node; SD = streamline density (number of fibre connections per unit surface); SLD = the probability of a tract connecting two ROIs; SCP = scaled conditional probability (the number of streamlines from node i to node j, divided by the number of streamlines seeded in node i, scaled by the surface area of the ROI i.
CSD = constrained spherical deconvolution; DT = deterministic tractography; PT = probabilistic Fractography; ACT = anatomically constrained probabilistic Fractography.
3.2. Quality assessment
Table 2 summarises the quality of the 13 papers according to the diffusion MRI checklist categories, ranked according to overall score (maximum score 13). Most papers scored full points for describing parameters of the diffusion scanning sequences. Points were often deducted for poor description of graph metric calculations and failing to correct for multiple comparisons. The ‘subjects’ category of the checklist had the highest average score (3.6/4, 90.5%), followed by ‘methodology’ (5.4/6, 89.7%), and ‘results/conclusions’ (2.5/3, 83.3%). Overall, the total quality score was high, and varied from 9 to 12.5 points out of a possible 13 (average score: 11.5/13, 88.5%). The study of Verhelst et al. (2018) had the highest methodological quality. There was no significant effect of publication bias (Egger’s regression intercept = 1.81, CI: [-1.94, 5.57], p = 0.34), and all studies met the benchmark for inclusion in the meta-analysis, showing that the published studies are a good representation of available evidence.
Table 2. Quality Assessment Results for Graph Theoretical Studies of Traumatic Brain Injury.
| Subjects | Methodology | Results/Conclusions | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | Overall (/4) | T5 | T6 | T7 | T8 | T9 | T10 | Overall (/6) | T11 | T12 | T13 | Overall (/3) | Final Score | |
| Verhelst et al. (2018) | 1 | 1 | 1 | 1 | 4 | 1 | 1 | 1 | 0.5 | 1 | 1 | 5.5 | 1 | 1 | 1 | 3 | 12.5/13 |
| Caeyenberghs et al. (2012) | 1 | 1 | 1 | 0.5 | 3.5 | 1 | 1 | 1 | 1 | 1 | 0.5 | 5.5 | 1 | 1 | 1 | 3 | 12/13 |
| Dall’Acqua et al. (2016) | 1 | 1 | 1 | 1 | 4 | 1 | 0.5 | 0.5 | 1 | 1 | 1 | 5 | 1 | 1 | 1 | 3 | 12/13 |
| van der Horn et al. (2016) | 1 | 1 | 1 | 0.5 | 3.5 | 1 | 1 | 1 | 0.5 | 1 | 1 | 5.5 | 1 | 1 | 1 | 3 | 12/13 |
| Yuan et al. (2015) | 1 | 1 | 1 | 1 | 4 | 1 | 1 | 1 | 0.5 | 0.5 | 1 | 5 | 1 | 1 | 1 | 3 | 12/13 |
| Caeyenberghs et al. (2013) | 1 | 1 | 1 | 1 | 4 | 1 | 1 | 1 | 1 | 1 | 0.5 | 5.5 | 0 | 1 | 1 | 2 | 11.5/13 |
| Caeyenberghs et al. (2014) | 1 | 1 | 1 | 0.5 | 3.5 | 1 | 1 | 1 | 1 | 1 | 0.5 | 5.5 | 1 | 0.5 | 1 | 2.5 | 11.5/13 |
| Königs et al. (2017) | 1 | 1 | 1 | 1 | 3.5 | 1 | 1 | 1 | 0.5 | 1 | 1 | 5.5 | 0 | 1 | 1 | 2 | 11.5/13 |
| Solmaz et al. (2017) | 1 | 0.5 | 0.5 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | 1 | 1 | 1 | 2.5 | 11.5/13 |
| Yuan et al. (2017a) | 1 | 1 | 1 | 1 | 3.5 | 1 | 1 | 1 | 0.5 | 1 | 0.5 | 5 | 0.5 | 1 | 1 | 2.5 | 11.5/13 |
| Hellyer et al. (2015) | 1 | 1 | 0.5 | 1 | 3.5 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | 0 | 0.5 | 1 | 2.5 | 11/13 |
| Kim et al. (2014) | 1 | 1 | 0.5 | 1 | 3.5 | 1 | 1 | 1 | 0.5 | 1 | 1 | 5.5 | 0 | 1 | 1 | 2 | 11/13 |
| Yuan et al. (2017b) | 1 | 1 | 0.5 | 0.5 | 3 | 1 | 1 | 1 | 0.5 | 0.5 | 0.5 | 4.5 | 0 | 1 | 0.5 | 1.5 | 9/13 |
Fagerholm et al. (2015) and Mitra et al. (2016) were excluded from the quality assessment due to incompatibility with the questionnaire (machine learning experiments).
3.3. Meta-analysis
Table 3 summarises the differences in global graph metrics between TBI and HC cohorts across studies. For each graph metric, the direction of significant group differences between TBI and HCs was the same across studies, with the exception of small-worldness and normalised path length. The overall effect sizes for normalised clustering coefficient, global efficiency, density, and characteristic path length were found to be significant (p < 0.05), with moderate to large Hedge’s g effect sizes (g > 0.5) (see Fig. 2, and Supplementary Material 5 for statistics). However, only normalised clustering coefficient and characteristic path length remained significant following FDR correction (p < 0.002). The subgroup analyses revealed longer normalised path length in acute/mild patients; higher small-worldness in chronic patients; higher small-worldness in paediatric TBI patients; and higher normalised clustering coefficient in paediatric TBI patients compared to HCs (FDR corrected, p < 0.001, see Table 4). In the next paragraphs, we will present the results of key overall effects and subgroup analyses for each graph metric that was significant after FDR correction.
Table 3. Graph Metrics in Patients with Traumatic Brain Injury compared to Healthy Controls.
| Graph Metrics | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Segregation | Integration | Centrality/General measures | ||||||||||||
| Cl | γ | Q | Eloc | T | a | Eglob | L | λ | b | σ | k | D | s | |
| Caeyenberghs et al., 2012 | • | ↑ | • | ↓ | • | • | • | • | ↓ | ↑ | ↑ | • | ↓ | • |
| Caeyenberghs et al., 2014 | – | – | • | • | • | • | ↓ | ↑ | – | • | – | • | • | – |
| Hellyer et al., 2015 | ↓ | • | • | • | • | • | • | ↑ | • | • | ↓ | ↓ | • | • |
| Kim et al., 2014 | • | • | – | • | – | • | • | ↑ | • | • | • | • | – | • |
| Königs et al., 2017 | • | • | – | • | – | – | • | – | • | • | • | • | • | • |
| van der Horn et al., 2016 | – | – | – | – | • | • | – | • | • | – | • | • | • | • |
| Verhelst et al., 2018 | • | ↑ | • | • | • | • | • | • | ↑ | • | • | • | ↓ | – |
| Yuan et al., 2015 | • | ↑ | ↑ | – | • | • | ↓ | • | ↑ | – | ↑ | • | • | • |
| Yuan et al. (2017a) | • | – | – | – | • | • | – | • | – | • | ↑ | • | • | • |
| Yuan et al. (2017b) | • | ↑ | – | – | • | • | ↓ | • | ↑ | • | ↑ | • | • | • |
| Total* | 3 | 7 | 6 | 5 | 2 | 1 | 5 | 4 | 6 | 3 | 6 | 1 | 3 | 2 |
↑/ ↓ higher/lower respectively in TBI patients than in HCs; - no significant difference between TBI and HC; • this metric wasn’t measured. *the total number of times this metric was measured.
Cl Clustering coefficient, γ Normalised clustering coefficient, Q Modularity, Eloc Local efficiency, T Transivity, a Assortativity, Eglob Global efficiency, L Characteristic path length, λ Normalised characteristic path length, b Betweenness Centrality, σ Small-worldness, D Density, k Degree, s Strength.
Fig. 2.
Inverted forest plot of the overall effect sizes and 95% confidence intervals for each graph metric, including heterogeneity values (I2). The size of the markers on the I2 graph represent the number of studies in each pooled analysis (range: n = 1 to n = 7), with larger circles indicating a larger n. See subscript of Table 3 for description of graph metric abbreviations.
Table 4. Results of the Subgroup Analyses.
| Outcome | Subgroup | Variable | N | Hedges G | Lower Limit | Upper Limit | Z-Value | P-Value | I-Squared (%) |
|---|---|---|---|---|---|---|---|---|---|
| Global Efficiency (Eglob) | Chronicity/Severity | Acute/mild | 3 | –1.610 | –3.402 | 0.181 | –1.762 | 0.078 | 95.109 |
| Chronic/modsev | 2 | –0.485 | –1.408 | 0.437 | –1.031 | 0.302 | 71.268 | ||
| Age at injury | Adult | 2 | –0.446 | –1.368 | 0.475 | –0.949 | 0.343 | 79.580 | |
| Pediatric | 3 | –1.625 | –3.298 | 0.047 | –1.905 | 0.057 | 92.912 | ||
| Local Efficiency (Eloc) | Chronicity/Severity | Acute/mild | 3 | 0.031 | –0.292 | 0.354 | 0.188 | 0.851 | 0.000 |
| Chronic/modsev | 2 | –0.677 | –2.067 | 0.713 | –0.995 | 0.340 | 89.863 | ||
| Modularity (Q) | Chronicity | Acute/mild | 3 | 0.602 | –0.479 | 1.683 | 1.091 | 0.275 | 89.877 |
| Chronic/modsev/both | 3 | –0.038 | –0.379 | 0.302 | –0.221 | 0.825 | 0.000 | ||
| Age at injury | Adult | 2 | –0.233 | –0.625 | 0.159 | –1.163 | 0.245 | 0.000 | |
| Pediatric | 4 | 0.532 | –0.182 | 1.247 | 1.460 | 0.144 | 81.165 | ||
| Normalised Clustering Coefficient (γ) | Chronicity/Severity | Acute/mild | 3 | 0.915 | –0.379 | 2.209 | 1.386 | 0.166 | 92.389 |
| Chronic/modsev | 4 | 1.924 | 0.382 | 3.465 | 2.446 | 0.014 | 92.440 | ||
| Age at injury | Adult | 2 | 0.150 | –0.571 | 0.871 | 0.408 | 0.683 | 68.072 | |
| Pediatric | 5 | 2.000 | 0.857 | 3.143 | 3.430 | 0.001 | 89.822 | ||
| Normalised Path Length (λ) | Chronicity/Severity | Acute/mild | 2 | 0.965 | 0.523 | 1.408 | 4.274 | * < 0.001 | 0.000 |
| Chronic/modsev | 4 | 0.789 | –0.903 | 2.482 | 0.914 | 0.361 | 94.501 | ||
| Small Worldness (σ) | Chronicity | Acute | 3 | 0.625 | –0.892 | 2.142 | 0.808 | 0.419 | 94.950 |
| Chronic | 3 | 0.950 | 0.402 | 1.499 | 3.396 | *0.001 | 39.536 | ||
| Severity | Mild | 2 | 1.309 | 0.203 | 2.414 | 2.320 | 0.020 | 81.922 | |
| Modsev | 4 | 0.533 | –0.491 | 1.558 | 1.021 | 0.307 | 89.792 | ||
| Age at injury | Adult | 2 | –0.087 | –1.358 | 1.185 | –0.133 | 0.894 | 90.342 | |
| Pediatric | 4 | 1.246 | 0.694 | 1.798 | 4.423 | * < 0.001 | 56.949 |
significant at p < .05.
significant at p < .001.
3.3.1. Global integration
Four of the ten studies investigated characteristic path length. (Caeyenberghs et al., 2014; Hellyer et al., 2015; Kim et al., 2014; Königs et al., 2017). Of the 142 patients in this analysis, 114 were moderate to severe; 63 acute patients were on average 5.5 months post-injury, while 79 chronic patients were on average 3.5 years post-injury; and 101 were adults (average age: ~26.9 years) and 41 were paediatric (average age: ~10.5 years) at injury. Across this entire cohort, characteristic path length was longer in the TBI patients compared with HCs (g = 0.514, p = 0.002, I2 = 28.601%). The heterogeneity value of this graph metric was low, indicating that the dataset was homogenous.
Six studies investigated normalized path length (Caeyenberghs et al., 2012, 2014; Verhelst et al., 2018; Yuan et al., 2017a, 2015; Yuan et al., 2017b) with no overall group effect (g = 0.815, p = 0.129, I2 = 92.1%). Of the 112 patients in this analysis, 67 were moderate to severe; 45 acute patients were between 96 h and 4 months post-injury, while 67 chronic patients were on average 4 years post-injury; and 21 were adults (average age: ~21.3 years) and 91 were paediatric (average age: ~12.1 years) at injury. Subgroup analysis revealed that the acute/ mild TBI group showed significantly increased normalised path length compared with HCs (g = 0.965, p < 0.001, I2 = 0.0%), with a decreased heterogeneity value. The effect size for the chronic/moderate-severe group was not significant.
3.3.2. Functional segregation
Seven studies calculated normalized clustering coefficient (Caeyenberghs et al., 2012, 2014; van der Horn et al., 2016; Verhelst et al., 2018; Yuan et al., 2017a, 2015; Yuan et al., 2017b). Of the 165 patients in this analysis, 67 were moderate to severe; 98 acute patients were between 96 h and 4 months post-injury, while 67 chronic patients were on average 4 years post-injury; and 74 were adults (average age: ~27.4 years) and 91 were paediatric (average age: ~12.1 years) at injury. Normalised clustering coefficient was higher in TBI patients in the overall meta-analysis (g = 1.445, p = 0.002, I2 = 91.484). In the chronicity and severity subgroup-analysis, the effect remained significant in the chronic/moderate-severe patients only (chronic/mod-erate-severe: g = 1.924 p = .014, I2 =92.440%). However, this effect retained a high heterogeneity value. Similarly in the age at injury subgroup analysis, normalised clustering coefficient was significantly higher in the paediatric TBI patients than HCs (g = 2.00, p = 0.001, I2 = 89.82). This effect was not observed for adult TBI patients. However, grouping by age at injury only lowered the observed heterogeneity in normalised clustering coefficient by ~2%.
3.3.3. Small-worldness
Six studies reported on small-worldness differences between TBI and HCs (Caeyenberghs et al., 2012, 2014; Hellyer et al., 2015; Yuan et al., 2017a, 2015; Yuan et al., 2017b), with no significant effect size overall; however, a trend was evident for larger values in TBI patients (g = 0.794, p = 0.06, I2 = 89.736%). Of the 158 patients in this analysis, 105 were moderate to severe; 108 acute patients were between 96 h and 5.5 months post-injury, while 50 chronic patients were on average 4.6 years post-injury; and 84 were adults (average age: ~26.6 years) and 74 were paediatric (average age: ~11.8 years) at injury. Subgroup analysis showed a significant effect size for chronic patients only, with increased small-worldness in chronic TBI patients compared with HCs (g = 0.950, p = .001, I2 = 39.536%). Grouping by chronicity also greatly reduced heterogeneity in the chronic group. Subgroup analysis by severity revealed larger small worldness values for the mild group (g = 1.309, p = .020, I2 = 81.922%); however, heterogeneity remained high and did not survive FDR correction. Finally, small-worldness was significantly higher in the paediatric TBI patients (but not adult TBI patients) compared to HCs (g = 1.25, p < 0.001, I2 = 56.949). Grouping by age at injury reduced the heterogeneity observed in small-worldness, meaning that age at injury could be explaining some of the differences in small-worldness between TBI patients and HCs.
4. Discussion
Our study is the first meta-analysis to assess the consistency of recent graph theoretical studies of TBI. The overall quality of the papers was high, and all met the benchmark for inclusion in the review. Findings suggest that normalized clustering coefficient and characteristic path length may be sensitive diagnostic biomarkers to distinguish TBI patients from HCs, with the former particularly high in chronic/moderate-severe and paediatric TBI patients after subgroup analyses. Furthermore, we suggest that values of normalised path length may be increased in acute/mild patients, and small worldness may be higher in chronic and paediatric TBI patients. In the following sections we will examine the use of graph metrics from a critical view. Specifically, we will discuss the following topics: (4.1) evidence that the TBI network is closer to a regular lattice structure than HCs, and (4.2) the use of graph metrics as diagnostic and prognostic biomarkers in longitudinal studies. In (4.3) we will also point out a number of methodological issues and provide recommendations for the future study of structural connectomics in TBI. Finally, in (4.4) we will address any limitations of this pooled analysis, including heterogeneity in patient samples and parcellation schemes.
4.1. Towards a regular network structure in TBI patients
The hypotheses presented in the research papers reflect the exploratory nature of GTA in TBI studies. Clear rationales and a priori hypotheses regarding the specific choice of graph metrics (together with the expected direction of effect) was omitted in many of the studies analysed. For example, Yuan et al. (2017b) ambiguously predicted that metrics would be “abnormal at baseline but would normalise after training”. Only Yuan et al. (2015) and Königs et al. (2017) justified their choice of each graph metric. While exploratory research is necessary, a clear rationale concerning the selection of graph metrics will advance theoretical reasoning in the field. Furthermore, having a priori hypotheses about the expected direction of effect will minimise multiple comparisons, thereby reducing chance findings that inflate the false positive rate. The findings from our meta-analysis, outlined in the following paragraphs, can serve as a guide in the development of hypotheses for the next generation of GTA studies in TBI.
Small-worldness is the ratio of normalised clustering coefficient to normalised path length, and represents the balance between segregation for local specialization and global integration (Watts and Strogatz, 1998). While all studies found that the TBI connectome is still a smallworld network, there was evidence of a shift towards a regular lattice structure. Small-worldness values were significantly higher for TBI patients greater than 6 months post injury, and for children with TBI. These results suggest a shift in network structure, which is probably due to a secondary process of neurodegeneration and/or is specific to those patients injured during childhood. However, further research is needed to evaluate the neurobiological mechanisms underlying increases in small-worldness. Yuan et al. (2015) and Yuan et al. (2017a) suggested that higher small-worldness is primarily driven by an increase in local clustering. Still, changes in small-worldness alone do not provide insight into the nature of the group differences. Instead, researchers could focus on more specific metrics that can differentiate between alterations in segregation and integration (Fornito et al., 2013; Papo et al., 2016), including measures of clustering and path length as described next.
In line with the observed shift towards a regular network, our review revealed that normalised clustering coefficient was significantly higher in the TBI group compared to HCs. This result indicates that TBI patients have more ‘closed triangles’ in their network graph compared to the controls, denoting greater functional specialisation. We also observed that this effect remained significant in the paediatric group but not the adult group. Yuan et al. (2015) suggested that this finding in paediatric TBI patients reflected an adaptive response to the injury, whereby local connections are increased because they are less vulnerable to damage than long-range connections. However, we argue that this is a costly adaptation, as it would increase the number of steps needed for information to travel between any two regions (Fornito et al., 2016; Sporns, 2011). In fact, our meta-analysis also showed that characteristic path length was significantly longer in the TBI population compared to the HCs, meaning there are a greater number of steps between any two nodes on average in the TBI network than in the HC network. Furthermore, the subgroup analysis demonstrated that normalised path length in the acute mild TBI group (but not the chronic moderate-severe group) was significantly higher than HCs. However due to the paucity of data available, it was impossible to determine whether this effect was driven by chronicity or severity. Despite the lack of data, our findings support the idea that the TBI network topology departs from the economical random-graph (Sporns, 2011).
4.2. Use of graph metrics as diagnostic and prognostic biomarkers
The effects described in Section 4.1 support the use of normalised clustering coefficient and characteristic path length as diagnostic biomarkers to identify group differences between TBI patients and HCs. Graph metrics can also be used to detect the presence or absence of diffuse axonal injuries (DAI) within TBI patients. Two papers included in the review (Fagerholm et al., 2015; Mitra et al., 2016) employed machine learning methods on graph metrics to classify patients. Fagerholm and colleagues were able to classify the presence of DAI in TBI patients with a high accuracy rate of 93.4%, and found that betweenness centrality had the highest ‘feature importance’ when differentiating between patients with microbleeds and HCs. Using a similar machine learning technique, Mitra et al. found that connectivity strength could differentiate mild TBI patients with DAI from HCs with an accuracy rate of 68.16%. These are very promising techniques that clearly demonstrate the use of graph metrics as diagnostic biomarkers.
Another important aspect of evaluating a diagnostic biomarker is the association of the metric with behavioural/clinical outcomes, which was done in all studies apart from one (Hellyer et al., 2015). For example, longer characteristic path length correlated with worse performance on verbal learning task as well as executive dysfunction in moderate-severe TBI patients (Kim et al., 2014). Longer characteristic path length also coincided with lower intelligence scores and shorter working memory span in moderate-severe TBI patients (Königs et al., 2017). Lower normalised clustering coefficient was found to be associated with slower processing speed in mild TBI patients (van der Horn et al., 2016). These significant correlations highlight the potential of normalised clustering coefficient and characteristic path length as biomarkers of behavioural deficits following TBI. However, reminding us of the preliminary nature of this work, a number of studies did not correct for multiple comparisons when running correlations between graph metrics and behavioural tests (Kim et al., 2014; Yuan et al., 2017a). While uncorrected thresholds can be useful for exploratory research, correction for multiple comparisons would strengthen the validity of these findings. Finally, comparison between studies is problematic because different outcome measures were used across studies. We recommend the use of a core set of behavioural tests in the future (e.g., Wefel et al., 2011).
Finally, we wanted to explore whether graph metrics can be used as prognostic biomarkers to predict treatment response. Longitudinal studies are necessary to investigate which graph metrics change in response to training. Only two GTA studies (by the same group, Yuan et al., 2017a, b) so far have conducted longitudinal training studies. Yuan et al. (2017a) found that normalised clustering-coefficient and small-worldness values decreased following 10 weeks of attention and executive function training in TBI patients, but remained the same in the HCs. In an aerobic training study, Yuan et al. (2017b) found that improved Post-Concussion Symptom Inventory scores following 4–16 weeks of training correlated with increased global efficiency and lower normalised path length. However, this study did not investigate the interaction effect between group and time directly. Overall, there is some evidence that network measures can be used as prognostic biomarkers, but further longitudinal analyses are needed to investigate the predictive value of graph metrics.
4.3. Methodological considerations and further recommendations
As a tentative conclusion, our meta-analysis showed that normalized clustering coefficient and characteristic path length are potential diagnostic biomarkers that may be sensitive to group differences between TBI and controls. However, GTA is a mathematical framework that has only recently been applied in neuroscience (for a critical review, see Fornito et al., 2013), and the underlying biological mechanism of change (e.g., increase in axon density, diameter, myelination, sprouting of synapses) is so far unknown. Due to inherent limitations in tractography, we do not know yet whether graph metrics directly reflect white matter integrity (e.g., Jones et al., 2013). Therefore, it is important to refrain from diagnosing ‘abnormal’ graph metrics, when comparing TBI patients to HCs (e.g., Yuan et al., 2017b), until we know the biological mechanisms underpinning graph metrics. Validated neuro-psychometric testing could couple structural connectome measures such as graph metrics (and other diffusion-based measures) to multimodal data with known information processing properties. Until then, structural graph metrics represent the necessary but insufficient properties of the network to function (Sporns, 2012). However, we can get a better understanding if we first obtain reliable patterns of brain connectivity.
There are methodological challenges associated with investigating graph metrics in patients with TBI. These include applying appropriate MRI acquisition and preprocessing techniques, connectome construction, and specifying edge weights (see Table 1 for a summary of the methods used in the studies in this review). Future research should (a) utilise advanced diffusion sequences (e.g., multishell, not used by any studies in the review) with accelerated acquisition speed to accommodate for non-compliance due to poor concentration (e.g., multiband/ compressive sensing); (b) employ robust estimation approaches for diffusion MRI metrics (e.g., Slicewise OutLIer Detection (SOLID; Sairanen et al., 2018)); and (c) apply a model that can resolve crossing fibre orientations (e.g., constrained spherical deconvolution, only used by two papers in the current review). Furthermore, although connection density has a noticeable impact on graph metrics (van Wijk et al., 2010), only six of the thirteen studies in the quality assessment accounted for differences in network density (as suggested byBullmore and Bassett, 2011) when comparing structural networks of TBI and HCs (Caeyenberghs et al., 2012; Hellyer et al., 2015; Königs et al., 2017; Solmaz et al., 2017; van der Horn et al., 2016; Yuan et al., 2015). Similarly, researchers should consider using multiple edge weighting and parcellation schemes to examine the robustness of data (Qi et al., 2015; Sotiropoulos and Zalesky, 2017), as was done by Caeyenberghs et al. (2012, 2013, 2014), Fagerholm et al. (2015), and Königs et al. (2017). Finally, future studies should employ advanced measures of white matter such as fibre density and cross section (Raffelt et al., 2017) as edge weights, because FA (used by three studies) and number of ‘streamlines’ (used by eight studies) lack the microstructural specificity to fully characterise the integrity of the structural network. In summary, by using more advanced MRI acquisition and pre-processing techniques we can get closer to an understanding of the biological underpinnings of the TBI structural connectome.
4.4. Limitations of the pooled analysis
4.4.1. Heterogeneity in parcellation schemes
One limitation of combining different graph analyses is that it inevitably requires pooling data obtained with different parcellation schemes. Differences in the way the cortex is parcellated can significantly impact the results of GTA (Zalesky et al., 2010). As shown in Table 1, five different parcellation schemes (e.g., the Desikan atlas from Freesurfer and the Automated Anatomical Labeling atlas) were used across the papers included in the meta-analysis, each with a different number of regions of interest or ‘nodes’ (range: 82–164). Parcellation schemes with higher resolution (i.e., more nodes) will demonstrate gradual increases in normalised path length and reductions in normalised clustering coefficient (Bassett et al., 2011), while measures of network organisation (e.g., small-worldness) will remain largely the same (Qi et al., 2015). However, because whole brain node templates in this current study were of similar spatial scales, impact on pooled graph metrics should be negligible (Zalesky et al., 2010), and it is therefore likely that this effect is small and does not detract from the overall findings.
4.4.2. Heterogeneity in the TBI samples
Patients with TBI are diverse, and several clinical and demographic factors (such as severity, chronicity, and age at injury) will impact the comparability of patient cohorts across studies. In the present meta-analysis, we attempted to address the issue of heterogeneity in our pooled TBI population by conducting subgroup analyses. However, the heterogeneity values remained above 75% for the majority of the subgroup analyses, indicating that results may still have been driven by differences in sample demographics (Higgins et al., 2003). This is not surprising given the diversity present in the structure of an injured brain, which may include focal lesions, diffuse axonal injury, or both. There were also limited studies that could be included in this review, making some subgroup analyses hard to interpret. For example, there were no studies of moderate-severe TBI patients in the acute phase, or mild TBI patients in the chronic phase that could be included in the normalised path length subgroup analyses (see Table 4). Therefore it is impossible to determine whether normalised path length was increased in the acute/mild group due to the time since injury, or the severity of the injury. Overall, this meta-analysis allows us to see universal trends that are present in the structural connectome of TBI patients; however more research is needed that spans across all TBI subgroups, so that future pooled analyses can better distinguish between all TBI populations.
5. Conclusion
Despite the complexity of applying GTA to the heterogeneous TBI population, our meta-analysis of structural connectivity studies revealed that normalised clustering coefficient and characteristic path length can be regarded as diagnostic biomarkers of TBI. These findings provide an evidentiary framework for future research. The emerging evidence suggests that average path length and clustering is increased in TBI patients, with the overall network more closely resembling a regular lattice. Using graph metrics we are able to differentiate between TBI population and healthy controls on the one hand, and the presence/ absence of DAI on the other hand. Also, there is preliminary evidence that graph metrics predict future response to training. Despite the promising results, the biological mechanisms underlying alterations in graph metrics is unclear. Future research should employ advanced diffusion MRI tools and obtain biologically-validated measures of structural connectivity in longitudinal studies.
Supplementary Material
Funding
Karen Caeyenberghs is supported by a National Health and Medical Research Council Career Development Fellowship and an ACURF Program Grant. Derek K Jones is supported by a Wellcome Trust Investigator Award (096646/Z/11/Z) and a Wellcome Trust Strategic Award (104943/Z/14/Z). Phoebe Imms and Adam Clemente are both supported by the Australian Postgraduate Award under the Australian Research Training Program.
Footnotes
Declarations of interest
None.
Financial disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Adam Clemente, Email: Adam.Clemente@myacu.edu.au.
Mark Cook, Email: markcook@unimelb.edu.au.
Wendyl D’Souza, Email: wendyl1@icloud.com.
Peter H. Wilson, Email: Peter.Wilson@acu.edu.au.
Derek K. Jones, Email: JonesD27@cardiff.ac.uk.
Karen Caeyenberghs, Email: Karen.Caeyenberghs@acu.edu.au.
References
- Baiano M, David A, Versace A, Churchill R, Balestrieri M, Brambilla P. Anterior cingulate volumes in schizophrenia: a systematic review and a meta-analysis of MRI studies. Schizophr Res. 2007;93(1-3):1–12. doi: 10.1016/j.schres.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Brown JA, Deshpande V, Carlson JM, Grafton ST. Conserved and variable architecture of human white matter connectivity. Neuroimage. 2011;54(2):1262–1279. doi: 10.1016/j.neuroimage.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Bendlin BB, Ries ML, Lazar M, Alexander AL, Dempsey RJ, Rowley HA, et al. Longitudinal changes in patients with traumatic brain injury assessed with diffusion-tensor and volumetric imaging. Neuroimage. 2008;42(2):503–514. doi: 10.1016/j.neuroimage.2008.04.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29(4):1165–1188. doi: 10.1214/aos/1013699998. [DOI] [Google Scholar]
- Bullmore ET, Bassett DS. Brain graphs: graphical models of the human brain connectome. Annu Rev Clin Psychol. 2011;7:113–140. doi: 10.1146/annurev-clinpsy-040510-143934. [DOI] [PubMed] [Google Scholar]
- Caeyenberghs K, Leemans A, Coxon J, Leunissen I, Drijkoningen D, Geurts M, et al. Bimanual coordination and corpus callosum microstructure in young adults with traumatic brain injury: a diffusion tensor imaging study. J Neurotrauma. 2011a;28(6):897–913. doi: 10.1089/neu.2010.1721. [DOI] [PubMed] [Google Scholar]
- Caeyenberghs K, Leemans A, Geurts M, Linden CV, Smits-Engelsman BC, Sunaert S, Swinnen SP. Correlations between white matter integrity and motor function in traumatic brain injury patients. Neurorehabil Neural Repair. 2011b;25(6):492–502. doi: 10.1177/1545968310394870. [DOI] [PubMed] [Google Scholar]
- Caeyenberghs K, Leemans A, De Decker C, Heitger M, Drijkoningen D, Linden CV, et al. Brain connectivity and postural control in young traumatic brain injury patients: a diffusion MRI based network analysis. Neuroimage Clin. 2012;1(1):106–115. doi: 10.1016/j.nicl.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caeyenberghs K, Leemans A, Leunissen I, Michiels K, Swinnen SP. Topological correlations of structural and functional networks in patients with traumatic brain injury. Front Hum Neurosci. 2013;7:726. doi: 10.3389/fnhum.2013.00726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caeyenberghs K, Leemans A, Leunissen I, Gooijers J, Michiels K, Sunaert S, Swinnen SP. Altered structural networks and executive deficits in traumatic brain injury patients. Brain Struct Funct. 2014;219(1):193–209. doi: 10.1007/s00429-012-0494-2. [DOI] [PubMed] [Google Scholar]
- Catani M, Ffytche DH. The rises and falls of disconnection syndromes. Brain. 2005;128(10):2224–2239. doi: 10.1093/brain/awh622. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2 ed. Lawrence Earlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Dall’Acqua P, Johannes S, Mica L, Simmen HP, Glaab R, Fandino J, et al. Connectomic and surface-based morphometric correlates of acute mild traumatic brain injury. Front Hum Neurosci. 2016;10:1–15. doi: 10.3389/fnhum.2016.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delouche A, Attye A, Heck O, Grand S, Kastler A, Lamalle L, et al. Diffusion MRI: pitfalls, literature review and future directions of research in mild traumatic brain injury. Eur J Radiol. 2016;85(1):25–30. doi: 10.1016/j.ejrad.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerholm ED, Hellyer PJ, Scott G, Leech R, Sharp DJ. Disconnection of network hubs and cognitive impairment after traumatic brain injury. Brain. 2015;138(6):1696–1709. doi: 10.1093/brain/awv075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farbota KD, Bendlin BB, Alexander AL, Rowley HA, Dempsey RJ, Johnson SC. Longitudinal diffusion tensor imaging and neuropsychological correlates in traumatic brain injury patients. Front Hum Neurosci. 2012;6:160. doi: 10.3389/fnhum.2012.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Zalesky A, Pantelis C, Bullmore ET. Schizophrenia, neuroimaging and connectomics. Neuroimage. 2012;62(4):2296–2314. doi: 10.1016/j.neuroimage.2011.12.090. [DOI] [PubMed] [Google Scholar]
- Fornito A, Zalesky A, Breakspear M. Graph analysis of the human connectome: promise, progress, and pitfalls. Neuroimage. 2013;80:426–444. doi: 10.1016/j.neuroimage.2013.04.087. [DOI] [PubMed] [Google Scholar]
- Fornito A, Zalesky A, Bullmore ET. Fundamentals of Brain Network Analysis. Academic Press; Cambridge, MA: 2016. [Google Scholar]
- Griffa A, Baumann PS, Thiran JP, Hagmann P. Structural connectomics in brain diseases. Neuroimage. 2013;80:515–526. doi: 10.1016/j.neuroimage.2013.04.056. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6(7):e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellyer PJ, Scott G, Shanahan M, Sharp DJ, Leech R. Cognitive flexibility through metastable neural dynamics is disrupted by damage to the structural connectome. J Neurosci. 2015;35(24):9050–9063. doi: 10.1523/JNEUROSCI.4648-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ. Measuring inconsistency in meta-analyses. Br Med J. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulkower MB, Poliak DB, Rosenbaum SB, Zimmerman ME, Lipton ML. A decade of DTI in traumatic brain injury: 10 years and 100 articles later. Am J Neuroradiol. 2013;34(11):2064–2074. doi: 10.3174/ajnr.A3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys I, Wood RL, Phillips CJ, Macey S. The costs of traumatic brain injury: a literature review. Clin Outcomes Res. 2013;5:281–287. doi: 10.2147/CEOR.S44625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson EB, Schwerin SC, Avram AV, Juliano SL, Pierpaoli C. Diffusion MRI and the detection of alterations following traumatic brain injury. J Neurosci Res. 2018;96(4):612–625. doi: 10.1002/jnr.24065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: a global perspective. Neuro Rehabilitation. 2007;22(5):341–353. [PubMed] [Google Scholar]
- Jiang J, Zhao YJ, Hu XY, Du MY, Chen ZQ, Wu M, et al. Microstructural brain abnormalities in medication-free patients with major depressive disorder: a systematic review and meta-analysis of diffusion tensor imaging. J Psychiatry Neurosci. 2017;42(3):150–163. doi: 10.1503/jpn.150341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Knosche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Kim J, Parker D, Whyte J, Hart T, Pluta J, Ingalhalikar M, et al. Disrupted structural connectome is associated with both psychometric and real-world neuropsychological impairment in diffuse traumatic brain injury. J Int Neuropsychol Soc. 2014;20(9):887–896. doi: 10.1017/S1355617714000812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen KM, Greenwood R, Powell JH, Leech R, Hawkins PC, Bonnelle V, et al. White matter damage and cognitive impairment after traumatic brain injury. Brain. 2011;134(2):449–463. doi: 10.1093/brain/awq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Königs M, van Heurn LWE, Bakx R, Vermeulen RJ, Goslings JC, Poll-The BT, et al. The structural connectome of children with traumatic brain injury. Hum Brain Mapp. 2017 doi: 10.1002/hbm.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;130(10):2508–2519. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- Levin HS, Wilde EA, Chu Z, Yallampalli R, Hanten GR, Li X, et al. Diffusion tensor imaging in relation to cognitive and functional outcome of traumatic brain injury in children. J Head Trauma Rehabil. 2008;23(4):197–208. doi: 10.1097/01.HTR.0000327252.54128.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo C-Y, Wang P-N, Chou K-H, Wang J, He Y, Lin C-P. Diffusion tensor tractography reveals abnormal topological organization in structural cortical networks in Alzheimer’s disease. J Neurosci. 2010;30(50):16876–16885. doi: 10.1523/JNEUROSCI.4136-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A, Ling J, Mannell M, Gasparovic C, Phillips J, Doezema D, et al. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology. 2010;74(8):643–650. doi: 10.1212/WNL.0b013e3181d0ccdd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra J, Shen KK, Ghose S, Bourgeat P, Fripp J, Salvado O, et al. Statistical machine learning to identify traumatic brain injury (TBI) from structural disconnections of white matter networks. Neuroimage. 2016;129:247–259. doi: 10.1016/j.neuroimage.2016.01.056. [DOI] [PubMed] [Google Scholar]
- Niogi SN, Mukherjee P, Ghajar J, Johnson C, Kolster RA, Sarkar R, et al. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. Am J Neuroradiol. 2008;29(5):967–973. doi: 10.3174/ajnr.A0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papo D, Zanin M, Martinez JH, Buldu JM. Beware of the small-world neuroscientist! Front Hum Neurosci. 2016;10:96. doi: 10.3389/fnhum.2016.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi S, Meesters S, Nicolay K, Romeny BM, Ossenblok P. The influence of construction methodology on structural brain network measures: a review. J Neurosci Methods. 2015;253:170–182. doi: 10.1016/j.jneumeth.2015.06.016. [DOI] [PubMed] [Google Scholar]
- Rabinowitz AR, Levin HS. Cognitive sequelae of traumatic brain injury. Psychiatr Clin North Am. 2014;37(1):1–11. doi: 10.1016/j.psc.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffelt DA, Tournier JD, Smith RE, Vaughan DN, Jackson G, Ridgway GR, Connelly A. Investigating white matter fibre density and morphology using fixel-based analysis. Neuroimage. 2017;144:58–73. doi: 10.1016/j.neuroimage.2016.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi C, Sullivan J. Motor fitness in children and adolescents with traumatic brain injury. Arch Phys Med Rehabil. 1996;77(10):1062–1065. doi: 10.1016/s0003-9993(96)90069-6. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Rutgers DR, Toulgoat F, Cazejust J, Fillard P, Lasjaunias P, Ducreux D. White matter abnormalities in mild traumatic brain injury: a diffusion tensor imaging study. Am J Neuroradiol. 2008;29(3):514–519. doi: 10.3174/ajnr.A0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairanen V, Leemans A, Tax CM. Robust Estimation of Diffusion MRI Metrics Based on Slicewise Outlier Detection (SOLID) International Society for Magnetic Resonance in Medicine; Paris: 2018. [Google Scholar]
- Sambunjak D, Cumpston M, Watts C. Cochrane Interactive Learning: Conducting an Intervention Review. Cochrane. 2017 Available from https://training.cochrane.org/interactivelearning/module-6-analysing-data. [Google Scholar]
- Selassie AW, Zaloshnja E, Langlois JA, Miller T, Jones P, Steiner C. Incidence of long-term disability following traumatic brain injury hospitalization. J Head Trauma Rehabil. 2008;23(2):123–131. doi: 10.1097/01.HTR.0000314531.30401.39. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Hamoda HM, Schneiderman JS, Bouix S, Pasternak O, Rathi Y, et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imag Behav. 2012;6(2):137–192. doi: 10.1007/s11682-012-9156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd AM, Matheson SL, Laurens KR, Carr VJ, Green MJ. Systematic meta-analysis of insula volume in schizophrenia. Biol Psychiatry. 2012;72(9):775–784. doi: 10.1016/j.biopsych.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Solmaz B, Tunç B, Parker D, Whyte J, Hart T, Rabinowitz A, et al. Assessing connectivity related injury burden in diffuse traumatic brain injury. Hum Brain Mapp. 2017;38(6):2913–2922. doi: 10.1002/hbm.23561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiropoulos SN, Zalesky A. Building connectomes using diffusion MRI: why, how and but. NMR Biomed. 2017 doi: 10.1002/nbm.3752. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz G, Maller JJ, O’Sullivan R, Ponsford JL. White matter integrity following traumatic brain injury: the association with severity of injury and cognitive functioning. Brain Topogr. 2013;26(4):648–660. doi: 10.1007/s10548-013-0283-0. [DOI] [PubMed] [Google Scholar]
- Sporns O. Networks of the Brain. MIT press; Cambridge, MA: 2011. [Google Scholar]
- Sporns O. Discovering the Human Connectome. MIT press; Cambridge, MA: 2012. [Google Scholar]
- Sporns O. Network attributes for segregation and integration in the human brain. Curr Opin Neurobiol. 2013;23(2):162–171. doi: 10.1016/j.conb.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Adler C, Cecil KM, Sax KW. Neuroimaging in bipolar disorder. Bipolar Disord. 2000;2(3):148–164. doi: 10.1034/j.1399-5618.2000.020302.x. [DOI] [PubMed] [Google Scholar]
- van der Horn HJ, Kok JG, de Koning ME, Scheenen ME, Leemans A, Spikman JM, van der Naalt J. Altered wiring of the human structural connectome in adults with mild traumatic brain injury. J Neurotrauma. 2016;34(5):1035–1044. doi: 10.1089/neu.2016.4659. [DOI] [PubMed] [Google Scholar]
- van Wijk BC, Stam CJ, Daffertshofer A. Comparing brain networks of different size and connectivity density using graph theory. PLoS One. 2010;5(10):e13701. doi: 10.1371/journal.pone.0013701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhelst H, Vander Linden C, De Pauw T, Vingerhoets G, Caeyenberghs K. Impaired rich club and increased local connectivity in children with traumatic brain injury: local support for the rich? Hum. Brain Mapp. 2018;39(7):1–12. doi: 10.1002/hbm.24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Ware JH. Detecting moderator effects using subgroup analyses. Prev Sci. 2013;14(2):111–120. doi: 10.1007/s11121-011-0221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH. Collective dynamics of small-world’ networks. Nature. 1998;393:440–442. doi: 10.1038/393440a0. [DOI] [PubMed] [Google Scholar]
- Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12(7):703–708. doi: 10.1016/s1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Chu Z, Bigler ED, Hunter JV, Fearing MA, Hanten GR, et al. Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. J Neurotrauma. 2006;23(10):1412–1426. doi: 10.1089/neu.2006.23.1412. [DOI] [PubMed] [Google Scholar]
- Xiong KL, Zhu YS, Zhang WG. Diffusion tensor imaging and magnetic resonance spectroscopy in traumatic brain injury: A review of recent literature. Brain Imag Behav. 2014;8(4):487–496. doi: 10.1007/s11682-013-9288-2. [DOI] [PubMed] [Google Scholar]
- Yuan W, Wade SL, Babcock L. Structural connectivity abnormality in children with acute mild traumatic brain injury using graph theoretical analysis. Hum Brain Mapp. 2015;36(2):779–792. doi: 10.1002/hbm.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Treble-Barna A, Sohlberg MM, Harn B, Wade SL. Changes in structural connectivity following a cognitive intervention in children with traumatic brain injury. Neurorehabil Neural Repair. 2017a;31(2):190–201. doi: 10.1177/1545968316675430. [DOI] [PubMed] [Google Scholar]
- Yuan W, Wade SL, Quatman-Yates C, Hugentobler JA, Gubanich PJ, Kurowski BG. Structural connectivity related to persistent symptoms after mild TBI in adolescents and response to aerobic training: preliminary investigation. J Head Trauma Rehabil. 2017b doi: 10.1097/HTR.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Harding IH, Cocchi L, Yücel M, Pantelis C, Bullmore ET. Whole-brain anatomical networks: does the choice of nodes matter? Neuroimage. 2010;50(3):970–983. doi: 10.1016/j.neuroimage.2009.12.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.