Abstract
Plant growth, development, and response to the environment are mediated by a group of small signaling molecules named hormones. Plants regulate hormone response pathways at multiple levels, including biosynthesis, metabolism, perception, and signaling. In addition, plants exhibit the unique ability to spatially control hormone distribution. In recent years, multiple transporters have been identified for most of the plant hormones. Here we present an updated snapshot of the known transporters for the hormones abscisic acid, auxin, brassinosteroid, cytokinin, ethylene, gibberellin, jasmonic acid, salicylic acid, and strigolactone. We also describe new findings regarding hormone movement and elaborate on hormone substrate specificity and possible genetic redundancy in hormone transport and distribution. Finally, we discuss subcellular, cell-to-cell and long-distance hormone movement and local hormone sinks that trigger or prevent hormone-mediated responses.
Introduction
Plants are dynamic organisms that respond to the environment and adjust to it. Much of this communication and subsequent responses are mediated by plant hormones, a group of small organic signaling molecules that crosstalk at multiple levels to regulate growth, development, and response to the environment [1]. Hormone homeostasis is integratively regulated by hormone synthesis, metabolism, transport, perception, and signal transduction, which control their related activities in the plant. Hormone perception can be local or distal from the site of the synthesis. Therefore, hormones can be transported to their site of action by active transporters to regulate their distribution, leading to various responses [2–4]. This spatial regulation takes place in the bioactive hormone forms but is also relevant for their intermediates and conjugated forms. Characterization of hormone transporters identified in recent years has revealed a dynamic regulation of hormone distribution and homeostasis [5]. Hormone transporters are divided into different families, each with unique characteristics. Furthermore, other mobile signaling factors, such as small peptides and hormone-like molecules, are also translocated in the plant (reviewed recently by Takahashi et al. 2019 [6]). Here we summarize current knowledge regarding hormone transporters (Figure 1) and discuss the common and unique transport mechanisms in plants (Figure 2).
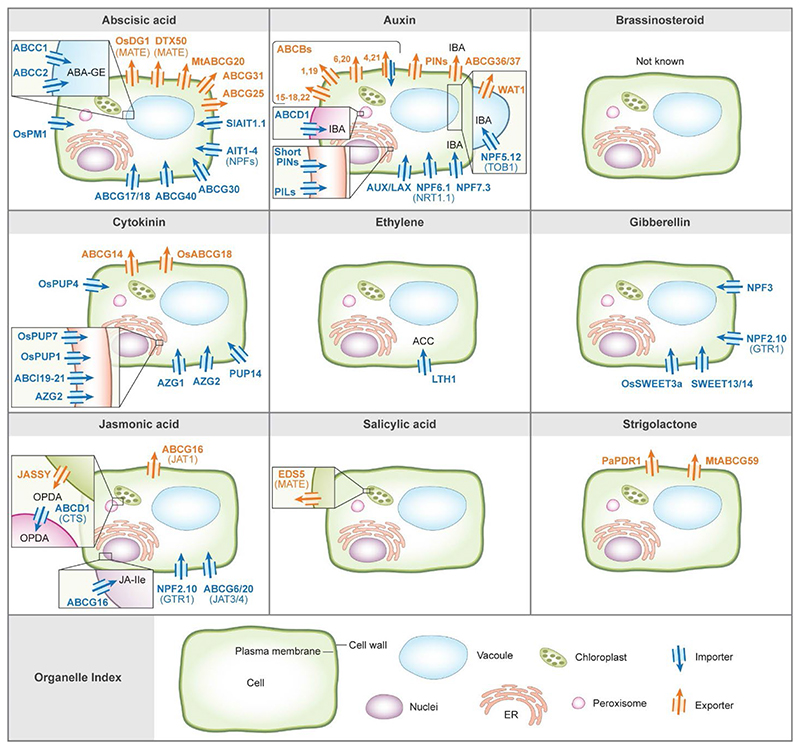
Fig. 1. Overview of plant hormones transporters.
Known transporters for each hormone are shown. Blue arrows represent importers; orange arrows represent exporters. The inset boxes are magnifications of the indicated organelles. The proteins transport the indicated bioactive hormone unless stated otherwise (ABA-GE stands for ABA Glucosyl Ester; IBA for Indole-3-Butyric Acid; ACC for L-aminocyclopropane-1-Carboxylic Acid; JA-Ile for Jasmonoyl-Isoleucine; OPDA for Cis-12-oxophytodienoic Acid). All hormone transporters were characterized in Arabidopsis thaliana unless stated otherwise (Sl, Solanum lycopersicum; Os, Oryza sativa; Mt, Medicago truncatula; Pa, Petunia axillaris). IC stands for isochorismate.
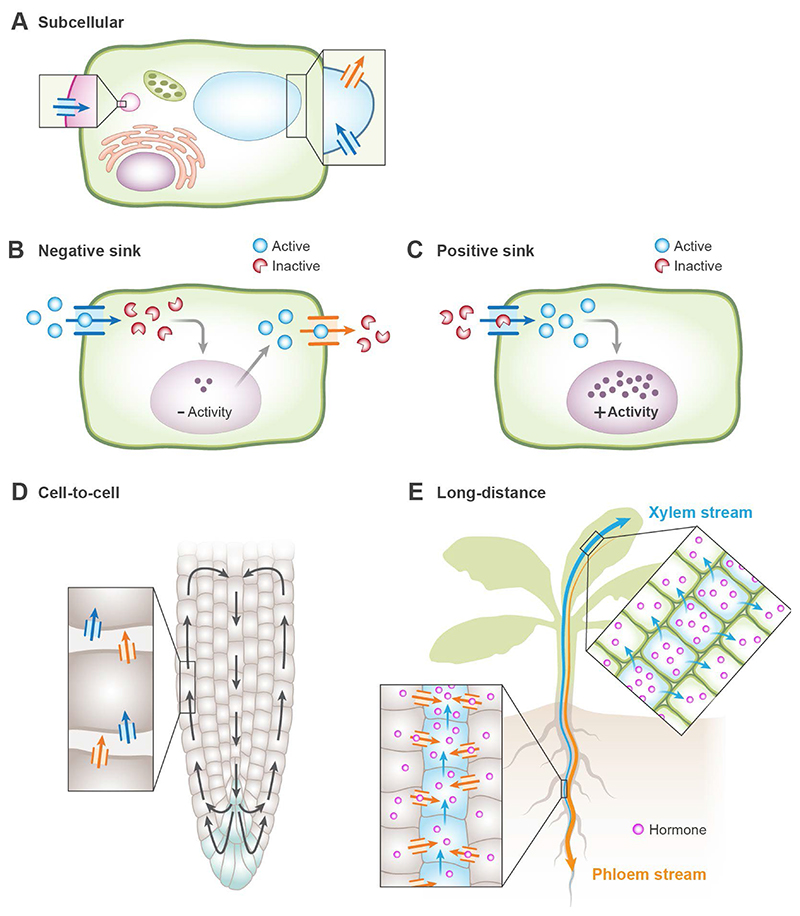
Fig. 2. Overview of hormone transport mechanisms in plants.
Diverse mechanisms of hormone transport are shown. A. Subcellular transport of hormones in or out of organelles (i.e., ABCD1 for peroxisomes and TOB1 for vacuole) leads to activity, storage, or degradation of the bioactive hormone, intermediates, or conjugated hormone forms. B. Negative sinks reduce hormone action. Several scenarios may lead to this phenomenon. First, insufficient hormone activity may result from import of the hormone into the cytosol, when the hormone receptors operate outside the plasma membrane (i.e., PUP14); second, the transporters may import the hormone into the cytosol where conjugation results in inactivity (i.e., ABCG17 and ABCG18); third, hormone export activity may lead to a decrease in the hormone activity in the cell (i.e., possibly ABCBs). C. Positive sinks enhances the activity of hormones by importing hormones or derivatives into the cell (i.e., NPF3 and NPF7.3). D. Cell-to-cell transport of hormones can occur via an exporter or an importer, allowing hormone gradients (i.e., PINs and AUX\LUXs). E. Long-distance transport of the hormone from root to shoot or vice versa occurs through the vasculature. This type of transport includes xylem and phloem loading and unloading (i.e., ABCG14 and DG1).
Abscisic acid
Abscisic acid (ABA) is involved in various processes in plants such as germination, seed dormancy, root development, drought tolerance, stomatal closure, and growth [2,7]. It was long thought that ABA is generated in the root and transported to the shoot to mediate stomatal closure. However, it is now recognized that ABA is also synthesized in the shoot [7–11], primarily in the vasculature [12]. Experiments showed that phloem-specific ABA synthesis reconstitutes ABA activity in stomatal aperture, indicating that ABA can move to regulate distinct responses [7,9,10]. Several ABA transporters have been identified, shedding light on the molecular mechanisms of ABA delivery. Transporters from the ATP binding cassette (ABC) family, such as ABCG25 and ABCG40, were characterized as ABA transporters in Arabidopsis thaliana. ABCG25 is an ABA exporter from the vasculature, and ABCG40 is an ABA importer to guard cells [13,14]. This suggests that there is active transport of ABA from the vasculature to the guard cells. ABCG25 and ABCG31 export ABA out of the endosperm, working in concert with ABCG30 and ABCG40 that import ABA into the embryo [4,15]. The DTX50 (from the MATE family) and AIT1-4 (also known as NPF4.6, 4.5, 4.1, 4.2 respectively), were also identified as ABA transporters in Arabidopsis (notably, AIT2-4 ABA transport activity was shown in yeasts but was not characterized in planta yet) [16–19]. Additional ABA transporters have been characterized in other plants. For instance, SlAIT1.1 was identified as an ABA importer in tomato (Solanum lycopersicum) and was shown to function downstream to the DELLA gibberellin suppressor response to promote stomatal closure [20]. MtABCG20 is an ABA exporter that is present in roots and germinating seeds in Medicago truncatula [21]. Recently, several ABA transporters have also been characterized in rice. The wheat disease resistance gene Lr34res was shown to affect ABA transport in Lr34res-expressing rice plants and yeasts transport assays, but its subcellular localization has not been discovered yet [22]. Furthermore, DG1 is an exporter from the MATE family which facilitates long-distance transport of leaf-derived ABA to control seed development, whereas OsPM1 is an importer involved in stomatal closure and drought responses [23,24].
Moreover, ABA is conjugated with glucose by UDP-glucosyltransferases to generate ABA-glucosyl ester (ABA-GE), a non-active form of ABA [25]. ABCC1 and ABCC2 have shown ABA-GE import activity in a heterologous yeast system and are localized on the plant tonoplast membrane [26]. Recently, ABCG17 and ABCG18 were characterized as plasma membrane ABA transporters that redundantly mediate ABA import, specifically in the leaf mesophyll cells. The ABA import activity mediated by ABCG17 and ABCG18 leads to the formation of conjugated inactive ABA sinks in mesophyll cells and thus restricts stomata closure (short-distance ABA movement) and lateral root emergence (long-distance ABA movement). ABCG17 and ABCG18 are required for ABA homeostasis under normal conditions, whereas abiotic stress conditions repress their expression and therefore release free ABA to promote a rapid ABA response (Unpublished 1). Whether ABA-GE transport is essential for guard cell activity and whether ABA-GE is transported long distance are still unclear.
Auxin
Indole 3-acetic acid (IAA) plays central roles in plant growth and development, controlling cell division, elongation, and differentiation. The combined activities of auxin influx and efflux carrier proteins generate local hormone maxima. Directional auxin gradients are prerequisites for essential developmental processes such as organ development, bending to light (phototropism), and directional root growth (gravitropism and hydrotropism) [27]. Several groups of auxin transporters have been identified and characterized. The best-characterized are the PIN proteins, which facilitate polar cell-to-cell IAA movement. In Arabidopsis, eight PIN proteins differ in the length of their middle hydrophilic loop [28]. The long PIN proteins (PIN1– 4 and 7) are exporters localized to the plasma membrane, and their polar localization determines the direction of auxin flux. Three PIN proteins (PIN5, 6, and 8) have a shorter central hydrophilic domain. PIN5 and PIN8 localize in the endoplasmic reticulum, and PIN6 is detected in both the endoplasmic reticulum and the plasma membrane, suggesting possible roles in regulating intracellular auxin homeostasis [29]. Recent studies have revealed that phosphorylation is essential for PIN activation, so PIN phosphorylation needs to be taken into account in auxin transport studies [30,31].
PIN-driven auxin export is likely ancient and conserved, as the PIN homologue of the green alga Klebsormidium flaccidum function as a plasma membrane-localized auxin exporter. Unlike the canonical PINs, the K. flaccidum does not localize polarly, a crucial mechanism to restrict the direction of auxin flow [32].
Intraspecies and interspecies pin1/3/4/7 knockout complementation experiments revealed that PIN genes underwent three critical evolutionary innovations associated with morphological patterning of shoot/root, inflorescence, and flower, essential architectural organs in Arabidopsis [33]. In addition, systematically swapping of the domains between PIN proteins localized to the endoplasmic reticulum and plasma membrane as well as between apical and basal plasma membrane-localized PINs showed that N- and C-terminal transmembrane domains and the central hydrophilic loop contribute to subcellular localization and cellular polarity [34].
The PIN-like PILS family of transporters reside at the endoplasmic reticulum and seem to limit nuclear auxin response by an auxin sequestration mechanism, defining differential growth rates [35,36]. The AUX/LAX family consists of four functional auxin influx carriers that mediate auxin-related developmental programs in different organs and tissues. AUX1, which is the most studied family member, is required for shoot-wards auxin transport from the root tip to regulate root gravitropism and root hair development [37]. Recent work showed that AUX1 promotes root hair elongation in response to phosphate limitation in Arabidopsis and rice [38,39].
Single-cell nucleus morphokinetic tracking combined with cell-type-specific induction of auxin biosynthesis enabled mapping of directional auxin flow in the root. The experiments showed that auxin flows down the root in a PIN-dependent but AUX1/LAX-independent manner. Similar results were obtained for auxin flux from the epidermis into the vasculature. However, rapid shootward movement of auxin depends on AUX1 and PIN2 proteins. In addition, root skewing requires localized auxin concentrations but is likely not regulated by the activity of PIN2 or AUX1 [40].
Several ABCB family members, ABCB1 (also known as PGP1), 4, 14, 19 (also known as MDR1), and 21, are critical for the polar auxin distribution [41–44]. ABCB6 and 20 are two additional functionally redundant auxin exporter family members reported regulating shoot growth [45]. ABCB15-18 and 22 were recently reported to redundantly allow IAA movement from the lateral root cap, shoot-wards via the epidermis, to regulate lateral root initiation (BioRxiv 2). WAT1 is a tonoplast-localized auxin transporter, shown to export IAA from the vacuole to the cytosol [46]. Several NPF proteins also transport auxins. NRT1.1 (also known as NPF6.1 or CHL1) is a dual-affinity nitrate transporter that mediates nitrogen uptake from the rhizosphere; it also transports IAA [47].
Recent work identified NPF transporters of indole-3-butyric acid (IBA), a precursor of IAA. The first, TOB1 (also known as NPF5.12), promotes IBA sequestration into the vacuole [48]. The second, NPF7.3, moves IBA into columella root cap cells to regulate gravitropic responses [49]. In addition to these two NPF IBA transporters, three additional proteins, PXA1 (also known as ABCD1) [50], ABCG36 [51], ABCG37 [52,53], have been implicated in IBA transport. However, the regulation of IBA uptake carriers and long-distance transport efflux carriers remains unknown [54].
Brassinosteroid
Brassinosteroids (BRs) are steroidal hormones that regulate plant growth and development [55]. It has been controversial whether and how BRs are transported throughout the plant. Experiments conducted in Pisum sativum revealed BR accumulation in different tissues but found no evidence suggesting that BR is transported long-distance [56]. Although BR biosynthesis enzymes are localized to the endoplasmic reticulum, BR receptors are found on the cell surface [55,57]. Therefore, BR must be translocated from the cell’s interior toward the apoplast through passive or active intracellular transport [58–60]. Recent mapping of expression patterns of BR biosynthesis enzymes and cell-type mis-expression studies revealed that BR precursors and possibly the bioactive BRs, brassinolide and castasterone, can move locally within the root over short distances (BioRxiv 3). The movement and tight regulation of the biosynthesis pathway generates a hormone concentration gradient along the root axis (BioRxiv3, 4). Ecdysone, a steroidal hormone from Drosophila melanogaster with structural similarities to BR, is transported by calcium-mediated vesicle exocytosis or by an ABC transporter named Atet [60,61]. Thus, there may be parallel transport mechanisms for BRs [60]. It will be important to obtain evidence for BR export activity and to identify whether there are specific transporters of BR and its precursors.
Cytokinin
Cytokinins (CKs) are involved in numerous developmental and physiological processes such as maintaining shoot and cambial meristem activities and promoting cell division, differentiation and root nodulation [62]. CKs are mobile molecules that have two main active forms. One form is trans-zeatin (t Z), which is synthesized in the roots and translocated acropetally to the shoot through the xylem. The second, N6-(Δ2-isopentenyl) adenine (iP), is produced in the shoot and moves basipetally toward the root by the phloem [63]. An ABCG-type transporter, ABCG14, was identified as a CK transporter involved in long-distance transport from root to shoot [64–66]. abcg14 loss-of-function mutants have a shoot growth repression phenotype that can be rescued by exogenous t Z application. Furthermore, grafting experiments indicated that ABCG14 is required in the root to promote shoot growth [64,65]. In rice, OsABCG18 regulates long-distance transport of root-derived CK, implying that it has similar characteristics to ABCG14 in Arabidopsis [67]. Additionally, it was suggested that members from the equilibrative nucleoside transporter (ENT) family mediate CK transport. Oryza sativa ENT2 and Arabidopsis ENT3, ENT6, ENT7, and ENT8 are involved in CK transport, but the proteins localization and direction of transport are not clear [68–70]. An interesting transport mechanism was proposed for the purine permease (PUP) family in Arabidopsis. PUP14 imports CK from the apoplast to the cytosol, which minimizes the bioactive CK in the apoplast, leading to reduced CK response [71]. Additional PUP family members were characterized as CK importers in rice. OsPUP1 and OsPUP7 import CK into the endoplasmic reticulum, whereas OsPUP4 transports CK from the apoplast into the cytosol. Together, these transporters mediate CK transport to control grain-size and development in rice [72,73]. Furthermore, Arabidopsis PUP1 and PUP2 can uptake t Z and iP in yeast, but their role in plants has not been characterized [74].
Transporters from the AZA-GUANINE RESISTANT (AZG) purine family are CK transporters that are involved in the crosstalk between CK and auxin in Arabidopsis. AZG1 is a CK importer that interacts and co-localizes directly with PIN1 during stress in roots [75]. AZG2 imports CK to regulate lateral root development in an auxin-dependent manner [76]. Moreover, it was suggested that ABCI-type transporter family members ABCI19, ABCI20, and ABCI21 might function together as a CK transporters. Those proteins are localized at the endoplasmic reticulum and are suggested to reduce cytosolic CK levels [77].
CK from the xylem has been argued to be a mobile signal that triggers divisions in the neighboring procambium cells [78]. Recently, it was reported that the transcription factor TMO5 (also known as LHW) can trigger the formation of a mobile CK that increases the density of root hairs [79]. As several of the identified transporters belong to relatively large families (i.e., PUPs), it will be important to examine whether additional CK players exist within these families and to characterize robustness and specificity. This work will likely have to overcome issues of the extensive functional redundancy within the family gene clades.
Ethylene
Ethylene is a gaseous hormone that can diffuse freely both in aqueous and lipid environments of the cell. Ethylene plays multiple roles in plant development and environmental responses with functions in seedling growth, organ development, abscission, ripening, and pathogen responses [80]. Although the gaseous hormone can freely diffuse through membranes and is thus able to move between cells and the intracellular space without the assistance of transporters, evidence suggests that much of its spatiotemporal regulation is due to the localization of its non-gaseous immediate precursor 1-aminocyclopropane-1-carboxylic acid (ACC). Whereas the major transport route of the soluble ACC is likely to be mediated by the xylem, ACC transport via the phloem has also been observed [81]. In 2015, LHT1 was identified as the first ACC transporter; LHT1 mediates ACC movement through the xylem [80]. Alanine and glycine, known as substrates of LHT1, compete with ACC for transport by LHT1. Within the LHT transporter family, ACC transport does not seem to be specific to LHT1. LHT2, but not LHT3 and AAP5, restore ACC responses in lht1 mutant seedlings and suppress the early senescence phenotype of the lht1 mutant [82].
Gibberellin
Gibberellin (GA) promotes diverse plant processes such as seed germination, organ elongation, flowering, and fruit development [83]. GA is present in all vascular plants in many forms, although only a few (e.g., GA1, GA3, GA4, and GA7) were found to be bioactive in plants. The other forms of GA are non-bioactive and exist in plants as precursors or catabolites [84–88]. The biosynthesis steps of the active GAs are complex, and their genes are expressed in different cells, tissues, and developmental stages along the plant [85]. There is evidence for both long- and short-distance transport of GA [84,85,89]. For example, the GA precursor, GA12, is a mobile form that can move from root to shoot [85,90]. The differential fluorescent signal of GA-biosensor GPS1 between the elongation and meristematic zones [91], and of GA biosynthesis genes tissue-specific expression are additional indications for root-transported GA in Arabidopsis [89].
GA molecules are subjected to the ion-trap mechanism, as several other acidic hormones, thus limiting their ability to move out of cells [88]. Therefore, it has been hypothesized that GA efflux transporters are required for GA local movement. However, these exporters, which are possibly masked by functional redundancy, have not been discovered yet [87]. Several GA influx transporters have been identified [87,88]. The NPF proteins are the first GA transporters to be identified. NPF3 promotes GA influx in the elongating endodermal cells of Arabidopsis root. Its expression is repressed by nitrogen and GA but induced by ABA [92,93]. The NPF transporter GTR1 (also known as NPF2.10) appears to be a GA transporter in Arabidopsis [94]. Several other NPF transporters, such as NPF2.5, NPF4.1, and NPF4.6, promote the uptake of GA in Xenopus oocytes [95], although their specific in-planta roles are not clear yet.
A puzzling observation in this field is the nonspecific transport activity of NPF transporters for different GA forms as well as diverse molecules, such as ABA, jasmonic acid, nitrate, glucosinolate, and others [96]. The nonspecific transport activity of NPF members is also relevant to the SWEET family, as several members are sugar transporters as well. SWEET13 and SWEET14 were identified as GA importers in Arabidopsis thaliana, and SWEET3a was characterized as a novel GA transporter in rice [97,98]. Further work is needed to reveal the missing GA exporters and possibly additional importers that regulate GA movement in processes such as germination and flowering time.
Jasmonic acid
Jasmonic acid (JA) is a lipid-derived hormone signal detected during development, abiotic stress such as wounding, and biotic stress leading to immune responses. Such responses trigger an increase in the free JA compound and the bioactive hormone jasmonoyl-L-isoleucine (JA-Ile) not only at the site of damage but also in distal, unharmed tissues.
The first half of JA biosynthesis leading to the synthesis of the intermediate cis-12-oxophytodienoic acid (OPDA) takes place in chloroplasts. It was recently shown that OPDA is exported from the chloroplasts by JASSY, a START protein localized to the chloroplast outer envelope membrane [99]. CTS (also known as ABCD1), localized to the peroxisomal membrane, allows OPDA import into peroxisomes, where further JA biosynthesis occurs [100].
Recent reports using grafting experiments suggest that travel through the phloem by OPDA and its derivatives, but not the bioactive jasmonoyl-isoleucine (JA-Ile) conjugate, is essential to initiate JA signaling in the root [101]. JA-Ile, considered to be the bioactive form of JA, is perceived in the nucleus. Therefore it is interesting that ABCG16 (also known as JAT1) is localized to both the nuclear envelope and plasma membrane and mediates both cellular efflux of the free JA molecule and the nuclear influx of JA-Ile [102]. This was the first demonstration of a nuclear-localized hormone transporter in plants, as far as we are aware.
Recently, Arabidopsis ABCG6 and ABCG20 (also known as JAT3 and JAT4, respectively) were shown to actively regulate leaf-to-leaf translocation of JA produced in response to wounding [103]. Thus, the two phloem-expressed plasma membrane-localized, high-affinity JA and low-affinity JA-Ile importers cooperate in long-distance JA translocation. Notably, yeast assays showed export activity while plant suspension cells showed import-compatible transport activity. In addition, several NPF transporters promote JA and JA-Ile movement in yeast and Xenopus oocytes heterologous systems. NPF2.10 is among the few transporters within the family to be characterized in vivo. The loss of the NPF2.10 function results in reduced JA transport from wounded to un-wounded leaves [104]. However, the transport of JA by the NPFs is not specific as these transporters can promote GA, ABA, and glucosinolates transport [1]. Likely, genetic redundancy is significantly limiting the physiological characterization of these transporters in planta, as they belong to a large protein family.
Salicylic acid
Salicylic acid (SA) regulates multiple plant processes, among them the plant immune response to pathogens. SA has long been known to be essential for systemic acquired resistance (SAR). Following SAR, the bioactive SA hormone is converted to a methylated form, MeSA, which is biologically inactive. MeSA accumulates in the phloem and is transported to the distal tissues, where it is converted back to SA to induce SAR [105]. The process is further regulated by the activity of the uridine diphosphate glycosyltransferase UGT71C3 that catalyzes MeSA glucosylation [106]. Mutations in BSMT1 (SA methyl transferase 1), SABP2 (salicylic acid binding protein 2), or UGT71C3 compromise SAR, but it is not entirely clear which form of SA is mobile.
Several lines of evidence imply that direct SA transport and SA-mediated systemic activity of related signaling molecules influence SAR [107]. Mutants impaired in cuticle establishment are defective in SAR due to alterations in systemic transport of SA [108,109]. Pathogen induced apoplastic accumulation of SA is driven by a pH gradient and deprotonation of SA [108].
An exciting addition to the field comes from the pipecolic acid (PIP) pathway. SA contributes to the induction of synthesis of N-hydroxyl pipecolic acid (NHP) from the PIP precursor Δ1-piperideine-2-carboxylic acid and biosynthesis of PIP upon pathogen infection [110,111]. Importantly, SA signaling and establishment of SAR depends on NHP, highlighting the importance of a new hormone signal during biotic stress responses.
Whereas the cell-to-cell and long- and short-distance SA and MeSA transporters have not yet been found, the MATE family transporter EDS5 was shown to export SA from the chloroplast to the cytosol [112]. The amidotransferase PBS3 catalyzes the conjugation of glutamate to cytosolic isochorismate, and this glutamate conjugate spontaneously decomposes into bioactive SA to control the innate immune response [112,113]. Further experiments are needed to quantitatively map the distribution of SA, MeSA, PIP, and NHP compounds before and after local pathogen infection [107]. Most importantly, the transporters that facilitate the transport of bioactive molecules that activate SAR remains unknown.
Strigolactone
Strigolactones (SLs) are carotenoid-derived phytohormones that have been identified as regulators of lateral root development and bud growth and as triggers for symbiosis between plants and mycorrhizal fungi [114,115]. SL can be synthesized in both roots and shoots. Grafting experiments revealed that root-derived SLs could rescue a shoot SL mutant phenotype, demonstrating that SL can be transported from distant areas. This long-distance transport toward the shoot lateral axils was shown to control lateral bud outgrowth [116– 119]. Moreover, SLs were found to regulate vascular tissue formation and regeneration by inhibiting the auxin PIN-dependent feedback transport [120]. The petunia ABCG-class protein PDR1 was the first characterized SL transporter in plants. It is a plasma membrane-localized exporter, expressed in root cortex and shoot axils [118,121]. Experiments indicate that PDR1 controls short-distance transport in the root tip, hypodermal, and stem axillary cells. Other studies showed that pdr1 mutants are defective in translocation of synthetic SL from root to shoot, implying it also plays a role in long-distance transport of SL [118,119,121]. Nevertheless, long-distance transport of SLs might not be solely dependent on PDR1. It has been suggested that additional transporters and SL precursors remain to be found [119,122]. Recently, ABCG59 was identified as a SL transporter that fine-tunes mycorrhizal symbiosis in Medicago truncatula [123]. Yet, it remains unclear which SL compounds move within plants and whether PDR-mediated transport exists in additional plant species.
Conclusions and future perspectives
In recent years, multiple plant hormone transporters have been characterized in Arabidopsis and other plant species, shedding light on the active mechanisms that drive hormone spatiotemporal regulation (Figure 1) [4,5]. Several distinct mechanisms have been identified (Figure 2): 1) Subcellular hormone transport, which delivers bioactive hormones, intermediates, or conjugated forms into and out of organelles, may occur as part of the biosynthesis processes (i.e., JASSY and ABCD1), during homeostasis (i.e., PILS family members and WAT1), or during the promotion of the response (i.e., ABCG16). 2) A negative hormone sink occurs when transporters import bioactive hormone into the cell and prevent its binding to the receptor at the apoplast (i.e., PUP14). An additional negative hormone sink may result from a plasma membrane exporter that pumps the bioactive hormone out of the cell (i.e., possibly ABCBs). Negative hormone sinks may occur with hormone intermediates and conjugated forms at the tissue or organelle level. 3) Positive hormone sinks result when transporters import bioactive hormones into cells that directly perceive and activate the hormone response (i.e., NPF3 and NPF7.3). Positive hormone sinks may also occur when a hormone exporter delivers the bioactive hormone to the neighboring cell. 4) Cell-to-cell mechanisms are active when the transporters allow directional movement of the hormone. This type of action occurs when transporters are polarized at the subcellular level (i.e., PINs) and are relevant to non-subcellular polarized transporters (i.e., ABCBs that transport auxin). 5) Long-distance transport allows root-to-shoot or shoot-to-root transport of bioactive hormones, their intermediates, or conjugated forms through the xylem and phloem [1]. These transporters can directly load or unload hormones from the vasculature (i.e., ABCG14, NPF4.6, and ABCG25) but also may affect long-distance hormone transport indirectly (i.e., ABCG17 and ABCG18) (Figure 2). We expect that additional transporters for all hormones, from the distinct transport mechanisms discussed above, will be identified in the coming years.
Abbreviations
- ABC
ATP Binding Cassette
- NPF
Nitrate transporter 1(NRT1)/Peptide Transporter family
- AIT
ABA-Importing Transporter
- ABA-GE
ABA Glucosyl Ester
- DG1
Defective Grain-filling 1
- IAA
Indole 3-Acetic Acid
- IBA
Indole-3-Butyric Acid
- PILS
PIN-Likes
- LAX
Like-Aux
- WAT1
Walls Are Thin 1
- TOB1
Transporter of IBA 1
- PXA1
Peroxisomal ABC-Transporter1
- ENT
Equilibrative Nucleoside, Transporter
- PUP
Purine Permease
- AZG
Aza-Guanine Resistant
- GTR1
Glucosinolate Transporter-1
- MATE
DTX/Multidrug and Toxic Compound Extrusion
- ACC
L-aminocyclopropane-1-Carboxylic Acid
- LHT
Lysine–Histidine-like Transporters
- AAP
Amino Acid Permeases
- MeJA
Methyl Jasmonate
- JAT
Jasmonate Transporter
- OPDA
Cis-12-oxophytodienoic Acid
- CTS
ABC Transporter
- JA-Ile
Jasmonoyl-Isoleucine
- SAR
Systemic Acquired Resistance
- MeSA
Methyl Salicylate
- BSMT1
SA methyl transferase 1
- SABP2
salicylic acid binding protein 2
- PIP
Pipecolic acid
- NHP
N-hydroxyl Pipecolic acid
- EDS5
Enhances Disease Susceptibility5
- PBS3
avrPphB Susceptible 3
Acknowledgements
We thank Mary Beth Mudgett for critically reading the article and for helpful suggestions. We apologize to colleagues whose work was not cited due to space limitations. Work in our laboratory is supported by grants from the Israel Science Foundation (2378/19 and 3419/20), the Human Frontier Science Program (HFSP— LIY000540/2020), and the European Research Council (757683-RobustHormoneTrans).
Footnotes
Conflict of interest
The authors declare no competing interests.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Lacombe B, Achard P. Long-distance transport of phytohormones through the plant vascular system. Curr Opin Plant Biol. 2016;34:1–8. doi: 10.1016/j.pbi.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Boursiac Y, Léran S, Corratgé-Faillie C, Gojon A, Krouk G, Lacombe B. ABA transport and transporters. Trends Plant Sci. 2013;18:325–333. doi: 10.1016/j.tplants.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Santner A, Estelle M. Recent advances and emerging trends in plant hormone signalling. Nature. 2009;459:1071–1078. doi: 10.1038/nature08122. [DOI] [PubMed] [Google Scholar]
- 4.Park J, Lee Y, Martinoia E, Geisler M. Plant hormone transporters: What we know and what we would like to know. BMC Biol. 2017;15:1–15. doi: 10.1186/s12915-017-0443-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abualia R, Benkova E, Lacombe B. Transporters and Mechanisms of Hormone Transport in Arabidopsis. Adv Bot Res. 2018;87:115–138. [Google Scholar]
- 6.Takahashi F, Hanada K, Kondo T, Shinozaki K. Hormone-like peptides and small coding genes in plant stress signaling and development. Curr Opin Plant Biol. 2019;51:88–95. doi: 10.1016/j.pbi.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Kuromori T, Sugimoto E, Shinozaki K. Intertissue signal transfer of abscisic acid from vascular cells to guard cells. Plant Physiol. 2014;164:1587–1592. doi: 10.1104/pp.114.235556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christmann A, Hoffmann T, Teplova I, Grill E, Müller A. Generation of active pools of abscisic acid revealed by in vivo imaging of water-stressed arabidopsis. Plant Physiol. 2005;137:209–219. doi: 10.1104/pp.104.053082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merilo E, Jalakas P, Laanemets K, Mohammadi O, Hõrak H, Kollist H, Brosché M. Abscisic acid transport and homeostasis in the context of stomatal regulation. Mol Plant. 2015;8:1321–1333. doi: 10.1016/j.molp.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Merilo E, Yarmolinsky D, Jalakas P, Parik H, Tulva I, Rasulov B, Kilk K, Kollist H. Stomatal VPD response: There is more to the story than ABA. Plant Physiol. 2018;176:851–864. doi: 10.1104/pp.17.00912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuromori T, Seo M, Shinozaki K. ABA Transport and Plant Water Stress Responses. Trends Plant Sci. 2018;23:513–522. doi: 10.1016/j.tplants.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Seo M, Koshiba T. Complex regulation of ABA biosynthesis in plants. Trends Plant Sci. 2002;7:41–48. doi: 10.1016/s1360-1385(01)02187-2. [DOI] [PubMed] [Google Scholar]
- 13.Kang J, Hwang JU, Lee M, Kim YY, Assmann SM, Martinoia E, Lee Y. PDR-type ABC transporter mediates cellular uptake of the phytohormone abscisic acid. Proc Natl Acad Sci U S A. 2010;107:2355–2360. doi: 10.1073/pnas.0909222107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuromori T, Miyaji T, Yabuuchi H, Shimizu H, Sugimoto E, Kamiya A, Moriyama Y, Shinozaki K. ABC transporter AtABCG25 is involved in abscisic acid transport and responses. Proc Natl Acad Sci U S A. 2010;107:2361–2366. doi: 10.1073/pnas.0912516107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang J, Yim S, Choi H, Kim A, Lee KP, Lopez-Molina L, Martinoia E, Lee Y. Abscisic acid transporters cooperate to control seed germination. Nat Commun. 2015;6 doi: 10.1038/ncomms9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanno Y, Hanada A, Chiba Y, Ichikawa T, Nakazawa M, Matsui M, Koshiba T, Kamiya Y, Seo M. Identification of an abscisic acid transporter by functional screening using the receptor complex as a sensor. Proc Natl Acad Sci U S A. 2012;109:9653–9658. doi: 10.1073/pnas.1203567109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiba Y, Shimizu T, Miyakawa S, Kanno Y, Koshiba T, Kamiya Y, Seo M. Identification of Arabidopsis thaliana NRT1/PTR FAMILY (NPF) proteins capable of transporting plant hormones. J Plant Res. 2015;128:679–686. doi: 10.1007/s10265-015-0710-2. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Zhu H, Pan Y, Yu Y, Luan S, Li L. A DTX/MATE-type transporter facilitates abscisic acid efflux and modulates ABA sensitivity and drought tolerance in Arabidopsis. Mol Plant. 2014;7:1522–1532. doi: 10.1093/mp/ssu063. [DOI] [PubMed] [Google Scholar]
- 19.Léran S, Noguero M, Corratgé-Faillie C, Boursiac Y, Brachet C, Lacombe B. Functional Characterization of the Arabidopsis Abscisic Acid Transporters NPF4.5 and NPF4.6 in Xenopus Oocytes. Front Plant Sci. 2020;11:1–6. doi: 10.3389/fpls.2020.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shohat H, Illouz-Eliaz N, Kanno Y, Seo M, Weiss D. The tomato della protein procera promotes abscisic acid responses in guard cells by upregulating an abscisic acid transporter. Plant Physiol. 2020;184:518–528. doi: 10.1104/pp.20.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pawela A, Banasiak J, Biała W, Martinoia E, Jasiński M. MtABCG20 is an ABA exporter influencing root morphology and seed germination of Medicago truncatula. Plant J. 2019;98:511–523. doi: 10.1111/tpj.14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krattinger SG, Kang J, Bräunlich S, Boni R, Chauhan H, Selter LL, Robinson MD, Schmid MW, Wiederhold E, Hensel G, et al. Abscisic acid is a substrate of the ABC transporter encoded by the durable wheat disease resistance gene Lr34. New Phytol. 2019;223:853–866. doi: 10.1111/nph.15815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin P, Zhang G, Hu B, Wu J, Chen W, Ren Z, Liu Y, Xie J, Yuan H, Tu B, et al. Leaf-derived ABA regulates rice seed development via a transporter-mediated and temperature-sensitive mechanism. Sci Adv. 2021;7 doi: 10.1126/sciadv.abc8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao L, Cheng X, Gu Z, Huang W, Li S, Wang L, Wang YF, Xu P, Ma H, Ge X. The AWPM-19 family protein OsPM1 mediates abscisic acid influx and drought response in rice. Plant Cell. 2018;30:1258–1276. doi: 10.1105/tpc.17.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu ZY, Lee KH, Dong T, Jeong JC, Jin JB, Kanno Y, Kim DH, Kim SY, Seo M, Bressan RA, et al. A vacuolar β-Glucosidase homolog that possesses glucose-conjugated abscisic acid hydrolyzing activity plays an important role in osmotic stress responses in Arabidopsis. Plant Cell. 2012;24:2184–2199. doi: 10.1105/tpc.112.095935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burla B, Pfrunder S, Nagy R, Francisco RM, Lee Y, Martinoia E. Vacuolar transport of abscisic acid glucosyl ester is mediated by ATP-binding cassette and proton-antiport mechanisms in Arabidopsis. Plant Physiol. 2013;163:1446–1458. doi: 10.1104/pp.113.222547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enders TA, Strader LC. Auxin activity: Past, present, and future. Am J Bot. 2015;102:180–196. doi: 10.3732/ajb.1400285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J. Local, Efflux-Dependent Auxin Gradients as a Common Module for Plant Organ Formation. Cell. 2003;115:591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- 29.Mravec J, Skůpa P, Bailly A, Hoyerová K, Křeček P, Bielach A, Petrášek J, Zhang J, Gaykova V, Stierhof YD, et al. Subcellular homeostasis of phytohormone auxin is mediated by the ER-localized PIN5 transporter. Nature. 2009;459:1136–1140. doi: 10.1038/nature08066. [DOI] [PubMed] [Google Scholar]
- 30.Barbosa ICR, Hammes UZ, Schwechheimer C. Activation and Polarity Control of PIN-FORMED Auxin Transporters by Phosphorylation. Trends Plant Sci. 2018;23:523–538. doi: 10.1016/j.tplants.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Barbosa ICR, Zourelidou M, Willige BC, Weller B, Schwechheimer C. D6 PROTEIN KINASE Activates Auxin Transport-Dependent Growth and PIN-FORMED Phosphorylation at the Plasma Membrane. Dev Cell. 2014;29:674–685. doi: 10.1016/j.devcel.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Skokan R, Medvecká E, Viaene T, Vosolsob S, Zwiewka M, Müller K, Skůpa P, Karady M, Zhang Y, Janacek DP, et al. PIN-driven auxin transport emerged early in streptophyte evolution. Nat Plants. 2019;5:1114–1119. doi: 10.1038/s41477-019-0542-5. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Rodriguez L, Li L, Zhang X, Friml J. Functional innovations of PIN auxin transporters mark crucial evolutionary transitions during rise of flowering plants. Sci Adv. 2020;6:1–15. doi: 10.1126/sciadv.abc8895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Hartinger C, Wang X, Friml J. Directional auxin fluxes in plants by intramolecular domain-domain coevolution of PIN auxin transporters. New Phytol. 2020;227:1406–1416. doi: 10.1111/nph.16629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barbez E, Kubeš M, Rolčík J, Béziat C, Pinik A, Wang B, Rosquete MR, Zhu J, Dobrev PI, Lee Y, et al. A novel putative auxin carrier family regulates intracellular auxin homeostasis in plants. Nature. 2012;485:119–122. doi: 10.1038/nature11001. [DOI] [PubMed] [Google Scholar]
- 36.Béziat C, Barbez E, Feraru MI, Lucyshyn D, Kleine-Vehn J. Light triggers PILS-dependent reduction in nuclear auxin signalling for growth transition. Nat Plants. 2017;3 doi: 10.1038/nplants.2017.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swarup R, Friml J, Marchant A, Ljung K, Sandberg G, Palme K, Bennett M. Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes Dev. 2001;15:2648–2653. doi: 10.1101/gad.210501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhosale R, Giri J, Pandey BK, Giehl RFH, Hartmann A, Traini R, Truskina J, Leftley N, Hanlon M, Swarup K, et al. A mechanistic framework for auxin dependent Arabidopsis root hair elongation to low external phosphate. Nat Commun. 2018;9:1–9. doi: 10.1038/s41467-018-03851-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giri J, Bhosale R, Huang G, Pandey BK, Parker H, Zappala S, Yang J, Dievart A, Bureau C, Ljung K, et al. Rice auxin influx carrier OsAUX1 facilitates root hair elongation in response to low external phosphate. Nat Commun. 2018;9:1–7. doi: 10.1038/s41467-018-03850-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu Y, Omary M, Hu Y, Doron O, Hoermayer L, Chen Q, Megides O, Chekli O, Ding Z, Friml J, Zhao Y, et al. Cell kinetics of auxin transport and activity in Arabidopsis root growth and skewing. Nat Commun. 2021 doi: 10.1038/s41467-021-21802-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noh B, Murphy AS, Spalding EP. Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell. 2001;13:2441–2454. doi: 10.1105/tpc.010350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kubeš M, Yang H, Richter GL, Cheng Y, Młodzińska E, Wang X, Blakeslee JJ, Carraro N, Petrášek J, Zažímalová E, et al. The Arabidopsis concentration-dependent influx/efflux transporter ABCB4 regulates cellular auxin levels in the root epidermis. Plant J. 2012;69:640–654. doi: 10.1111/j.1365-313X.2011.04818.x. [DOI] [PubMed] [Google Scholar]
- 43.Kaneda M, Schuetz M, Lin BSP, Chanis C, Hamberger B, Western TL, Ehlting J, Samuels AL. ABC transporters coordinately expressed during lignification of Arabidopsis stems include a set of ABCBs associated with auxin transport. J Exp Bot. 2011;62:2063–2077. doi: 10.1093/jxb/erq416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zazinalova E, Murphy AS, Yang H, Hoyerova K, Hosek P. Why So Many?-Auxin Transporters. Cold Spring Harb Perspect Biol. 2010;2:a001552. doi: 10.1101/cshperspect.a001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, Nasser V, Pisanty O, Omary M, Wulff N, Di Donato M, Tal I, Hauser F, Hao P, Roth O, et al. A transportome-scale amiRNA-based screen identifies redundant roles of Arabidopsis ABCB6 and ABCB20 in auxin transport. Nat Commun. 2018;9 doi: 10.1038/s41467-018-06410-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ranocha P, Dima O, Nagy R, Felten J, Corratge-Faillie C, Novak O, Morreel K, Lacombe B, Martinez Y, Pfrunder S, et al. Arabidopsis WAT1 is a vacuolar auxin transport facilitator required for auxin homoeostasis. Nat Commun. 2013;4:1–9. doi: 10.1038/ncomms3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krouk G, Lacombe B, Bielach A, Perrine-Walker F, Malinska K, Mounier E, Hoyerova K, Tillard P, Leon S, Ljung K, et al. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev Cell. 2010;18:927–937. doi: 10.1016/j.devcel.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 48.Michniewicz M, Ho CH, Enders TA, Floro E, Damodaran S, Gunther LK, Powers SK, Frick EM, Topp CN, Frommer WB, et al. TRANSPORTER OF IBA1 Links Auxin and Cytokinin to Influence Root Architecture. Dev Cell. 2019;50:599–609. doi: 10.1016/j.devcel.2019.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watanabe S, Takahashi N, Kanno Y, Suzuki H, Aoi Y, Takeda-Kamiya N, Toyooka K, Kasahara H, Hayashi K, Umeda M, et al. The Arabidopsis NRT1/PTR FAMILY protein NPF7.3/NRT1.5 is an indole-3-butyric acid transporter involved in root gravitropism. Proc Natl Acad Sci. 2020;117:31500–31509. doi: 10.1073/pnas.2013305117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zolman BK, Silva ID, Bartel B. The Arabidopsis pxa1 mutant is defective in an ATP-binding cassette transporter-like protein required for peroxisomal fatty acid β-oxidation. Plant Physiol. 2001;127:1266–1278. [PMC free article] [PubMed] [Google Scholar]
- 51.Strader LC, Bartel B. The arabidopsis PLEIOTROPIC DRUG RESISTANCE8/ABCG36 ATP binding cassette transporter modulates sensitivity to the auxin precursor lndole-3-butyric acid. Plant Cell. 2009;21:1992–2007. doi: 10.1105/tpc.109.065821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Růžička K, Strader LC, Bailly A, Yang H, Blakeslee J, Łangowski Ł, Nejedlá E, Fujita H, Itoh H, Syono K, et al. Arabidopsis PIS1 encodes the ABCG37 transporter of auxinic compounds including the auxin precursor indole-3-butyric acid. Proc Natl Acad Sci U S A. 2010;107:10749–10753. doi: 10.1073/pnas.1005878107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strader LC, Monroe-Augustus M, Rogers KC, Lin GL, Bartel B. Arabidopsis iba response5 suppressors separate responses to various hormones. Genetics. 2008;180:2019–2031. doi: 10.1534/genetics.108.091512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Damodaran S, Strader LC. Indole 3-butyric acid metabolism and transport in Arabidopsis thaliana. Front Plant Sci. 2019;10:1–9. doi: 10.3389/fpls.2019.00851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fujioka S, Yokota T. Biosynthesis and Metabolism of Brassinosteroids. Annu Rev Plant Biol. 2003;54:137–164. doi: 10.1146/annurev.arplant.54.031902.134921. [DOI] [PubMed] [Google Scholar]
- 56.Symons GM, Reid JB. Brassinosteroids do not undergo long-distance transport in pea. Implications for the regulation of endogenous brassinosteroid levels. Plant Physiol. 2004;135:2196–2206. doi: 10.1104/pp.104.043034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He Z, Wang ZY, Li J, Zhu Q, Lamb C, Ronald P, Chory J. Perception of brassinosteroids by the extracellular domain of the receptor kinase BRI1. Science (80-) 2000;288:2360–2363. doi: 10.1126/science.288.5475.2360. [DOI] [PubMed] [Google Scholar]
- 58.Symons GM, Ross JJ, Jager CE, Reid JB. Brassinosteroid transport. J Exp Bot. 2008;59:17–24. doi: 10.1093/jxb/erm098. [DOI] [PubMed] [Google Scholar]
- 59.Tang J, Han Z, Chai J. Q&A: What are brassinosteroids and how do they act in plants. BMC Biol. 2016;14:1–5. doi: 10.1186/s12915-016-0340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vukašinović N, Russinova E. BRexit: Possible Brassinosteroid Export and Transport Routes. Trends Plant Sci. 2018;23:285–292. doi: 10.1016/j.tplants.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 61.Yamanaka N, Marqués G, O’Connor MB. Vesicle-Mediated Steroid Hormone Secretion in Drosophila melanogaster. Cell. 2015;163:907–919. doi: 10.1016/j.cell.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Werner T, Schmülling T. Cytokinin action in plant development. Curr Opin Plant Biol. 2009;12:527–538. doi: 10.1016/j.pbi.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 63.Kudo T, Kiba T, Sakakibara H. Metabolism and long-distance translocation of cytokinins. J Integr Plant Biol. 2010;52:53–60. doi: 10.1111/j.1744-7909.2010.00898.x. [DOI] [PubMed] [Google Scholar]
- 64.Ko D, Kang J, Kiba T, Park J, Kojima M, Do J, Kim KY, Kwon M, Endler A, Song WY, et al. Arabidopsis ABCG14 is essential for the root-to-shoot translocation of cytokinin. Proc Natl Acad Sci U S A. 2014;111:7150–7155. doi: 10.1073/pnas.1321519111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang K, Novak O, Wei Z, Gou M, Zhang X, Yu Y, Yang H, Cai Y, Strnad M, Liu CJ. Arabidopsis ABCG14 protein controls the acropetal translocation of root-synthesized cytokinins. Nat Commun. 2014;5:3274. doi: 10.1038/ncomms4274. [DOI] [PubMed] [Google Scholar]
- 66.Kang J, Lee Y, Sakakibara H, Martinoia E. Cytokinin Transporters: GO and STOP in Signaling. Trends Plant Sci. 2017;22:455–461. doi: 10.1016/j.tplants.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 67.Zhao J, Yu N, Ju M, Fan B, Zhang Y, Zhu E, Zhang M, Zhang K. ABC transporter OsABCG18 controls the shootward transport of cytokinins and grain yield in rice. J Exp Bot. 2019;70:6277–6291. doi: 10.1093/jxb/erz382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hirose N, Makita N, Yamaya T, Sakakibara H. Functional characterization and expression analysis of a gene, OsENT2, encoding an equilibrative nucleoside transporter in rice suggest a function in cytokinin transport. Plant Physiol. 2005;138:196–206. doi: 10.1104/pp.105.060137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hirose N, Takei K, Kuroha T, Kamada-Nobusada T, Hayashi H, Sakakibara H. Regulation of cytokinin biosynthesis, compartmentalization and translocation. J Exp Bot. 2008;59:75–83. doi: 10.1093/jxb/erm157. [DOI] [PubMed] [Google Scholar]
- 70.Sun J, Hirose N, Wang X, Wen P, Xue L, Sakakibara H, Zuo J. Arabidopsis SOI33/AtENT8 gene encodes a putative equilibrative nucleoside transporter that is involved in cytokinin transport in planta. J Integr Plant Biol. 2005;47:588–603. [Google Scholar]
- 71.Zürcher E, Liu J, Di Donato M, Geisler M, Müller B. Plant development regulated by cytokinin sinks. Science (80-) 2016;353:1027–1030. doi: 10.1126/science.aaf7254. [DOI] [PubMed] [Google Scholar]
- 72.Xiao Y, Zhang J, Yu G, Lu X, Mei W, Deng H, Zhang G, Chen G, Chu C, Tong H, et al. Endoplasmic Reticulum-Localized PURINE PERMEASE1 Regulates Plant Height and Grain Weight by Modulating Cytokinin Distribution in Rice. Front Plant Sci. 2020;11:1–12. doi: 10.3389/fpls.2020.618560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xiao Y, Liu D, Zhang G, Gao S, Liu L, Xu F, Che R, Wang Y, Tong H, Chu C. Big Grain3, encoding a purine permease, regulates grain size via modulating cytokinin transport in rice. J Integr Plant Biol. 2019;61:581–597. doi: 10.1111/jipb.12727. [DOI] [PubMed] [Google Scholar]
- 74.Bürkle L, Cedzich A, Döpke C, Stransky H, Okumoto S, Gillissen B, Kühn C, Frommer WB. Transport of cytokinins mediated by purine transporters of the PUP family expressed in phloem, hydathodes, and pollen of Arabidopsis. Plant J. 2003;34:13–26. doi: 10.1046/j.1365-313x.2003.01700.x. [DOI] [PubMed] [Google Scholar]
- 75.Tessi T, Shahriari M, Maurino V, Meissner E, Novak O, Pasternak T, Schumacher B, Flubacher N, Nautscher M, Williams A, et al. The Auxin Transporter PIN1 and the Cytokinin Transporter AZG1 Interact to Regulate the Root Stress Response. Curr Biol. 2020 (sneak peek) [Google Scholar]
- 76.Tessi TM, Brumm S, Winklbauer E, Schumacher B, Pettinari G, Lescano I, González CA, Wanke D, Maurino VG, Harter K, et al. Arabidopsis AZG2 transports cytokinins in vivo and regulates lateral root emergence. New Phytol. 2021;229:979–993. doi: 10.1111/nph.16943. [DOI] [PubMed] [Google Scholar]
- 77.Kim A, Chen J, Khare D, Jin JY, Yamaoka Y, Maeshima M, Zhao Y, Martinoia E, Hwang JU, Lee Y. Non-intrinsic ATP-binding cassette proteins ABCI19, ABCI20 and ABCI21 modulate cytokinin response at the endoplasmic reticulum in Arabidopsis thaliana. Plant Cell Rep. 2020;39:473–487. doi: 10.1007/s00299-019-02503-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ohashi-Ito K, Saegusa M, Iwamoto K, Oda Y, Katayama H, Kojima M, Sakakibara H, Fukuda H. A bHLH complex activates vascular cell division via cytokinin action in root apical meristem. Curr Biol. 2014;24:2053–2058. doi: 10.1016/j.cub.2014.07.050. [DOI] [PubMed] [Google Scholar]
- 79.Wendrich JR, Yang B, Vandamme N, Verstaen K, Smet W, Van de Velde C, Minne M, Wybouw B, Mor E, Arents HE, et al. Vascular transcription factors guide plant epidermal responses to limiting phosphate conditions. Science (80-) 2020;370 doi: 10.1126/science.aay4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shin K, Lee S, Song WY, Lee RA, Lee I, Ha K, Koo JC, Park SK, Nam HG, Lee Y, et al. Genetic identification of ACC-RESISTANT2 reveals involvement of lysine histidine TRANSPORTER1 in the uptake of 1-Aminocyclopropane-1-Carboxylic acid in arabidopsis thaliana. Plant Cell Physiol. 2015;56:572–582. doi: 10.1093/pcp/pcu201. [DOI] [PubMed] [Google Scholar]
- 81.Pattyn J, Vaughan-Hirsch J, Van de Poel B. The regulation of ethylene biosynthesis: a complex multilevel control circuitry. New Phytol. 2021;229:770–782. doi: 10.1111/nph.16873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Choi J, Eom S, Shin K, Lee RA, Choi S, Lee JH, Lee S, Soh MS. Identification of Lysine Histidine Transporter 2 as an 1-Aminocyclopropane Carboxylic Acid Transporter in Arabidopsis thaliana by Transgenic Complementation Approach. Front Plant Sci. 2019;10:1–12. doi: 10.3389/fpls.2019.01092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hedden P, Sponsel V. A Century of Gibberellin Research. J Plant Growth Regul. 2015;34:740–760. doi: 10.1007/s00344-015-9546-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shani E, Weinstain R, Zhang Y, Castillejo C, Kaiserli E, Chory J, Tsien RY, Estelle M. Gibberellins accumulate in the elongating endodermal cells of Arabidopsis root. Proc Natl Acad Sci U S A. 2013;110:4834–4839. doi: 10.1073/pnas.1300436110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Regnault T, Davière J-M, Wild M, Sakvarelidze-Achard L, Heintz D, Carrera Bergua E, Lopez Diaz I, Gong F, Hedden P, Achard P. The gibberellin precursor GA12 acts as a long-distance growth signal in Arabidopsis. Nat Plants. 2015;1:1–6. doi: 10.1038/nplants.2015.73. [DOI] [PubMed] [Google Scholar]
- 86.Yamaguchi S. Gibberellin metabolism and its regulation. Annu Rev Plant Biol. 2008;59:225–251. doi: 10.1146/annurev.arplant.59.032607.092804. [DOI] [PubMed] [Google Scholar]
- 87.Binenbaum J, Weinstain R, Shani E. Gibberellin Localization and Transport in Plants. Trends Plant Sci. 2018;23:410–421. doi: 10.1016/j.tplants.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 88.Rizza A, Jones AM. The makings of a gradient: spatiotemporal distribution of gibberellins in plant development. Curr Opin Plant Biol. 2019;47:9–15. doi: 10.1016/j.pbi.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barker R, Fernandez Garcia MN, Powers SJ, Vaughan S, Bennett MJ, Phillips AL, Thomas SG, Hedden P. Mapping sites of gibberellin biosynthesis in the Arabidopsis root tip. New Phytol. 2021;229:1521–1534. doi: 10.1111/nph.16967. [DOI] [PubMed] [Google Scholar]
- 90.Camut L, Regnault T, Sirlin-Josserand M, Sakvarelidze-Achard L, Carrera E, Zumsteg J, Heintz D, Leonhardt N, Lange MJP, Lange T, et al. Root-derived GA12 contributes to temperature-induced shoot growth in Arabidopsis. Nat Plants. 2019;5:1216–1221. doi: 10.1038/s41477-019-0568-8. [DOI] [PubMed] [Google Scholar]
- 91.Rizza A, Walia A, Lanquar V, Frommer WB, Jones AM. In vivo gibberellin gradients visualized in rapidly elongating tissues. Nat Plants. 2017;3:803–813. doi: 10.1038/s41477-017-0021-9. [DOI] [PubMed] [Google Scholar]
- 92.Tal I, Zhang Y, Jørgensen ME, Pisanty O, Barbosa ICR, Zourelidou M, Regnault T, Crocoll C, Erik Olsen C, Weinstain R, et al. The Arabidopsis NPF3 protein is a GA transporter. Nat Commun. 2016;7 doi: 10.1038/ncomms11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.David LC, Berquin P, Kanno Y, Seo M, Daniel-Vedele F, Ferrario-Méry S. N availability modulates the role of NPF3.1, a gibberellin transporter, in GA-mediated phenotypes in Arabidopsis. Planta. 2016;244:1315–1328. doi: 10.1007/s00425-016-2588-1. [DOI] [PubMed] [Google Scholar]
- 94.Saito H, Oikawa T, Hamamoto S, Ishimaru Y, Kanamori-Sato M, Sasaki-Sekimoto Y, Utsumi T, Chen J, Kanno Y, Masuda S, et al. The jasmonate-responsive GTR1 transporter is required for gibberellin-mediated stamen development in Arabidopsis. Nat Commun. 2015;6 doi: 10.1038/ncomms7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wulff N, Ernst HA, Jørgensen ME, Lambertz S, Maierhofer T, Belew ZM, Crocoll C, Motawia MS, Geiger D, Jørgensen FS, et al. An Optimized Screen Reduces the Number of GA Transporters and Provides Insights Into Nitrate Transporter 1/Peptide Transporter Family Substrate Determinants. Front Plant Sci. 2019;10:1–18. doi: 10.3389/fpls.2019.01106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Corratgé-Faillie C, Lacombe B. Substrate (un)specificity of Arabidopsis NRT1/PTR FAMILY (NPF) proteins. J Exp Bot. 2017;68:3107–3113. doi: 10.1093/jxb/erw499. [DOI] [PubMed] [Google Scholar]
- 97.Kanno Y, Oikawa T, Chiba Y, Ishimaru Y, Shimizu T, Sano N, Koshiba T, Kamiya Y, Ueda M, Seo M. AtSWEET13 and AtSWEET14 regulate gibberellin-mediated physiological processes. Nat Commun. 2016;7:1–11. doi: 10.1038/ncomms13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Morii M, Sugihara A, Takehara S, Kanno Y, Kawai K, Hobo T, Hattori M, Yoshimura H, Seo M, Ueguchi-Tanaka M. The Dual Function of OsSWEET3a as a Gibberellin and Glucose Transporter Is Important for Young Shoot Development in Rice. Plant Cell Physiol. 2020;61:1935–1945. doi: 10.1093/pcp/pcaa130. [DOI] [PubMed] [Google Scholar]
- 99.Guan L, Denkert N, Eisa A, Lehmann M, Sjuts I, Weiberg A, Soll J, Meinecke M, Schwenkert S. JASSY, a chloroplast outer membrane protein required for jasmonate biosynthesis. Proc Natl Acad Sci U S A. 2019;116:10568–10575. doi: 10.1073/pnas.1900482116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Theodoulou FL, Job K, Slocombe SP, Footitt S, Holdsworth M, Baker A, Larson TR, Graham IA. Jasmonic acid levels are reduced in COMATOSE ATP-binding cassette transporter mutants. Implications for transport of jasmonate precursors into peroxisomes. Plant Physiol. 2005;137:835–840. doi: 10.1104/pp.105.059352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schulze A, Zimmer M, Mielke S, Stellmach H, Melnyk CW, Hause B, Gasperini D. Wound-Induced Shoot-to-Root Relocation of JA-Ile Precursors Coordinates Arabidopsis Growth. Mol Plant. 2019;12:1383–1394. doi: 10.1016/j.molp.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 102.Li Q, Zheng J, Li S, Huang G, Skilling SJ, Wang L, Li L, Li M, Yuan L, Liu P. Transporter-Mediated Nuclear Entry of Jasmonoyl-Isoleucine Is Essential for Jasmonate Signaling. Mol Plant. 2017;10:695–708. doi: 10.1016/j.molp.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 103.Li M, Wang F, Li S, Yu G, Wang L, Li Q, Zhu X, Li Z, Yuan L, Liu P. Importers Drive Leaf-to-Leaf Jasmonic Acid Transmission in Wound-Induced Systemic Immunity. Mol Plant. 2020;13:1485–1498. doi: 10.1016/j.molp.2020.08.017. [DOI] [PubMed] [Google Scholar]
- 104.Ishimaru Y, Oikawa T, Suzuki T, Takeishi S, Matsuura H, Takahashi K, Hamamoto S, Uozumi N, Shimizu T, Seo M, et al. GTR1 is a jasmonic acid and jasmonoyl-L-isoleucine transporter in Arabidopsis thaliana. Biosci Biotechnol Biochem. 2017;81:249–255. doi: 10.1080/09168451.2016.1246174. [DOI] [PubMed] [Google Scholar]
- 105.Park SW, Kaimoyo E, Kumar D, Mosher S, Klessig DF. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science (80-) 2007;318:113–116. doi: 10.1126/science.1147113. [DOI] [PubMed] [Google Scholar]
- 106.Chen L, Wang WS, Wang T, Meng XF, Chen TT, Huang XX, Li YJ, Hou BK. Methyl salicylate glucosylation regulates plant defense signaling and systemic acquired resistance. Plant Physiol. 2019;180:2167–2181. doi: 10.1104/pp.19.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kachroo P, Liu H, Kachroo A. Salicylic acid: transport and long-distance immune signaling. Curr Opin Virol. 2020;42:53–57. doi: 10.1016/j.coviro.2020.05.008. [DOI] [PubMed] [Google Scholar]
- 108.Lim GH, Liu H, Yu K, Liu R, Shine MB, Fernandez J, Burch-Smith T, Mobley JK, McLetchie N, Kachroo A, et al. The plant cuticle regulates apoplastic transport of salicylic acid during systemic acquired resistance. Sci Adv. 2020;6 doi: 10.1126/sciadv.aaz0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xia Y, Gao QM, Yu K, Lapchyk L, Navarre DR, Hildebrand D, Kachroo A, Kachroo P. An Intact Cuticle in Distal Tissues Is Essential for the Induction of Systemic Acquired Resistance in Plants. Cell Host Microbe. 2009;5:151–165. doi: 10.1016/j.chom.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 110.Holmes EC, Chen YC, Sattely ES, Mudgett MB. An engineered pathway for N-hydroxy-pipecolic acid synthesis enhances systemic acquired resistance in tomato. Sci Signal. 2019;12:1–11. doi: 10.1126/scisignal.aay3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen YC, Holmes EC, Rajniak J, Kim JG, Tang S, Fischer CR, Mudgett MB, Sattely ES. N-hydroxy-pipecolic acid is a mobile metabolite that induces systemic disease resistance in Arabidopsis. Proc Natl Acad Sci U S A. 2018;115:4920–4929. doi: 10.1073/pnas.1805291115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Serrano M, Wang B, Aryal B, Garcion C, Abou-Mansour E, Heck S, Geisler M, Mauch F, Nawrath C, Métraux JP. Export of salicylic acid from the chloroplast requires the multidrug and toxin extrusion-like transporter EDS5. Plant Physiol. 2013;162:1815–1821. doi: 10.1104/pp.113.218156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rekhter D, Lüdke D, Ding Y, Feussner K, Zienkiewicz K, Lipka V, Wiermer M, Zhang Y, Feussner I. Isochorismate-derived biosynthesis of the plant stress hormone salicylic acid. Science (80-) 2019;365:498–502. doi: 10.1126/science.aaw1720. [DOI] [PubMed] [Google Scholar]
- 114.Al-Babili S, Bouwmeester HJ. Strigolactones, a novel carotenoid-derived plant hormone. Annu Rev Plant Biol. 2015;66:161–186. doi: 10.1146/annurev-arplant-043014-114759. [DOI] [PubMed] [Google Scholar]
- 115.Borghi L, Liu GW, Emonet A, Kretzschmar T, Martinoia E. The importance of strigolactone transport regulation for symbiotic signaling and shoot branching. Planta. 2016;243:1351–1360. doi: 10.1007/s00425-016-2503-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, et al. Strigolactone inhibition of shoot branching. Nature. 2008;455:189–194. doi: 10.1038/nature07271. [DOI] [PubMed] [Google Scholar]
- 117.Domagalska MA, Leyser O. Signal integration in the control of shoot branching. Nat Rev Mol Cell Biol. 2011;12:211–221. doi: 10.1038/nrm3088. [DOI] [PubMed] [Google Scholar]
- 118.Kretzschmar T, Kohlen W, Sasse J, Borghi L, Schlegel M, Bachelier JB, Reinhardt D, Bours R, Bouwmeester HJ, Martinoia E. A petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. Nature. 2012;483:341–344. doi: 10.1038/nature10873. [DOI] [PubMed] [Google Scholar]
- 119.Shiratake K, Notaguchi M, Makino H, Sawai Y, Borghi L. Petunia PLEIOTROPIC DRUG RESISTANCE 1 Is a Strigolactone Short-Distance Transporter with Long-Distance Outcomes. Plant Cell Physiol. 2019;60:1722–1733. doi: 10.1093/pcp/pcz081. [DOI] [PubMed] [Google Scholar]
- 120.Zhang J, Mazur E, Balla J, Gallei M, Kalousek P, Medveová Z, Li Y, Wang Y, Prát T, Vasileva M, et al. Strigolactones inhibit auxin feedback on PIN-dependent auxin transport canalization. Nat Commun. 2020;11 doi: 10.1038/s41467-020-17252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sasse J, Simon S, Gübeli C, Liu GW, Cheng X, Friml J, Bouwmeester H, Martinoia E, Borghi L. Asymmetric localizations of the ABC transporter PaPDR1 trace paths of directional strigolactone transport. Curr Biol. 2015;25:647–655. doi: 10.1016/j.cub.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 122.Liu G, Pfeifer J, de Brito Francisco R, Emonet A, Stirnemann M, Gübeli C, Hutter O, Sasse J, Mattheyer C, Stelzer E, et al. Changes in the allocation of endogenous strigolactone improve plant biomass production on phosphate-poor soils. New Phytol. 2018;217:784–798. doi: 10.1111/nph.14847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Banasiak J, Borghi L, Stec N, Martinoia E, Jasiński M. The Full-Size ABCG Transporter of Medicago truncatula Is Involved in Strigolactone Secretion, Affecting Arbuscular Mycorrhiza. Front Plant Sci. 2020;11:1–14. doi: 10.3389/fpls.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
BioRxiv and unpublished
- 1.Zhang Yuqin, et al. ABA homeostasis and long-distance translocation is regulated by redundant ABCG ABA importers. doi: 10.1126/sciadv.abf6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Jian, et al. ABCB-mediated auxin transport in outer root tissues regulates lateral root spacing in Arabidopsis. bioRxiv. 2020 [Google Scholar]
- 3.Vukašinović Nemanja, Wang Yaowei, Vanhoutte Isabelle, Fendrych Matyáš, Guo Boyu, Kvasnica Miroslav, Jiroutová Petra, Jana Oklestkova MSER. Local brassinosteroid biosynthesis enables optimal root growth Nemanja. bioRxiv. 2020:1–29. doi: 10.1038/s41477-021-00917-x. [DOI] [PubMed] [Google Scholar]
- 4.Ackerman-Lavert M, Fridman Y, Matosevich R, Khandal H, Friedlander L, Vragović K, Ben El R, Horev G, Efroni I, Savaldi-Goldstein S. Auxin requirements for a meristematic state in roots depend on a dual brassinosteroid function. bioRxiv. 2020 doi: 10.1016/j.cub.2021.07.075. [DOI] [PubMed] [Google Scholar]
Recommended reading
- 19.Léran S, Noguero M, Corratgé-Faillie C, Boursiac Y, Brachet C, Lacombe B. Functional Characterization of the Arabidopsis Abscisic Acid Transporters NPF4.5 and NPF4.6 in Xenopus Oocytes. Front Plant Sci. 2020;11:1–6. doi: 10.3389/fpls.2020.00144. [* This study tested ABA transport activity for several NPF4s family members in Xenopus oocyte system. The results show that the ABA transporter activities are pH-dependent and that there is no competitive inhibition of the ABA-analogs pyrabactin and quinabactin on ABA uptake] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shohat H, Illouz-Eliaz N, Kanno Y, Seo M, Weiss D. The tomato della protein procera promotes abscisic acid responses in guard cells by upregulating an abscisic acid transporter. Plant Physiol. 2020;184:518–528. doi: 10.1104/pp.20.00485. [** The authors discuss the negative crosstalk between gibberellin and ABA and demonstrate that PRO (a tomato DELLA protein), promotes guard cell responses. RNA-sequencing analysis of isolated guard cells revealed AIT1.1 as an ABA transporter in Solanum lycopersicum, which is being upregulated by PRO] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pawela A, Banasiak J, Biała W, Martinoia E, Jasiński M. MtABCG20 is an ABA exporter influencing root morphology and seed germination of Medicago truncatula. Plant J. 2019;98:511–523. doi: 10.1111/tpj.14234. [*In this study, MtABCG20 is identified as an ABA exporter involved in seed germination and fine-tunes root morphology. It is proposed that MtABCG20 positively affects lateral root primordium formation and negatively affects the development of root nodulation in Medicago] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin P, Zhang G, Hu B, Wu J, Chen W, Ren Z, Liu Y, Xie J, Yuan H, Tu B, et al. Leaf-derived ABA regulates rice seed development via a transporter-mediated and temperature-sensitive mechanism. Sci Adv. 2021;7 doi: 10.1126/sciadv.abc8873. [** In this study, DG1 was shown to control leaf-to-caryopsis ABA transport for regulating rice seed development. The DG1 dependant ABA translocation is temperature-sensitive as the process’s efficiency increases in high ambient temperature] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skokan R, Medvecká E, Viaene T, Vosolsob S, Zwiewka M, Müller K, Skůpa P, Karady M, Zhang Y, Janacek DP, et al. PIN-driven auxin transport emerged early in streptophyte evolution. Nat Plants. 2019;5:1114–1119. doi: 10.1038/s41477-019-0542-5. [* The study elucidated the evolutionary origins of PINs in auxin mediate directional transport. The authors showed that the single PIN homologue of the green alga Klebsormidium flaccidum functions as a plasma membrane-localized auxin exporter in land plants and heterologous models] [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Rodriguez L, Li L, Zhang X, Friml J. Functional innovations of PIN auxin transporters mark crucial evolutionary transitions during rise of flowering plants. Sci Adv. 2020;6:1–15. doi: 10.1126/sciadv.abc8895. [* The study exposes conserved regions in PINs (auxin transporters) coding sequence and their cis-regulatory that determine the proteins subcellular localization in flowering plants] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Hartinger C, Wang X, Friml J. Directional auxin fluxes in plants by intramolecular domain-domain coevolution of PIN auxin transporters. New Phytol. 2020;227:1406–1416. doi: 10.1111/nph.16629. [* The study provided insights into how different domains of PIN proteins contribute to their differential membrane localization. Swapping between ER-and PM-localized PIN proteins, as well as between apical and basal PM-localized PINs from Arabidopsis showed that the central hydrophilic loops of PIN proteins are the main determinants of PIN’s subcellular localization. The transmembrane domain modules and pairwise matching between the N-and C-trans-membrane-domains also played a crucial role in determining the subcellular localization] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Michniewicz M, Ho CH, Enders TA, Floro E, Damodaran S, Gunther LK, Powers SK, Frick EM, Topp CN, Frommer WB, et al. TRANSPORTER OF IBA1 Links Auxin and Cytokinin to Influence Root Architecture. Dev Cell. 2019;50:599–609. doi: 10.1016/j.devcel.2019.06.010. [** Arabidopsis NPF5.12 (TOB1) is a transporter of IBA (auxin precursor). NPF5.12 is localized to the tonoplast to regulate root architecture] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watanabe S, Takahashi N, Kanno Y, Suzuki H, Aoi Y, Takeda-Kamiya N, Toyooka K, Kasahara H, Hayashi K, Umeda M, et al. The Arabidopsis NRT1/PTR FAMILY protein NPF7.3/NRT1.5 is an indole-3-butyric acid transporter involved in root gravitropism. Proc Natl Acad Sci. 2020;117:31500–31509. doi: 10.1073/pnas.2013305117. [** Arabidopsis NPF7.3 transports IBA (auxin precursor) to regulate root gravitropic response. NPF7.3 is localized to the plasma membrane and expressed in pericycle cells and root cap cells, where the IBA-to-IAA conversion likely occurs] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao J, Yu N, Ju M, Fan B, Zhang Y, Zhu E, Zhang M, Zhang K. ABC transporter OsABCG18 controls the shootward transport of cytokinins and grain yield in rice. J Exp Bot. 2019;70:6277–6291. doi: 10.1093/jxb/erz382. [* OsABCG18 is reported as a transporter regulating long-distance transport of CK in rice, similar to the Arabidopsis ABCG14. OsABCG18 shows plasma membrane localization and is primarily expressed in the root, stem, and leaf vascular tissues. Interestingly, the authors discuss the possibility of increasing grain yield by overexpressing this transporter in the plant] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xiao Y, Zhang J, Yu G, Lu X, Mei W, Deng H, Zhang G, Chen G, Chu C, Tong H, et al. Endoplasmic Reticulum-Localized PURINE PERMEASE1 Regulates Plant Height and Grain Weight by Modulating Cytokinin Distribution in Rice. Front Plant Sci. 2020;11:1–12. doi: 10.3389/fpls.2020.618560. [* OsPUP1, a novel CK transporter in rice, is an ER-localized transporter that facilitates CK cell-to-cell transport. It is suggested that together with [73], this transporter regulates grain weight. This work implies that there might be functional redundancy among the PUP members] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xiao Y, Liu D, Zhang G, Gao S, Liu L, Xu F, Che R, Wang Y, Tong H, Chu C. Big Grain3, encoding a purine permease, regulates grain size via modulating cytokinin transport in rice. J Integr Plant Biol. 2019;61:581–597. doi: 10.1111/jipb.12727. [* This study reports on two novel CK transporters from the PUP family to regulate grain size in rice. OsPUP4 is localized to the plasma membrane and OsPUP7 to the ER. Hormone profiling and qRT-PCR experiments suggested that these transporters are involved in the long-distance transport mechanism of CK] [DOI] [PubMed] [Google Scholar]
- 75.Tessi T, Shahriari M, Maurino V, Meissner E, Novak O, Pasternak T, Schumacher B, Flubacher N, Nautscher M, Williams A, et al. The Auxin Transporter PIN1 and the Cytokinin Transporter AZG1 Interact to Regulate the Root Stress Response. Curr Biol. 2020 [** Together with [76], it is proposed that CK transporters are involved in the crosstalk between CK and auxin in Arabidopsis. This study provides evidence that AZG1 is a CK transporter that directly interacts with PIN1 to regulate lateral root development under stress in increasing salt concentration] [Google Scholar]
- 76.Tessi TM, Brumm S, Winklbauer E, Schumacher B, Pettinari G, Lescano I, González CA, Wanke D, Maurino VG, Harter K, et al. Arabidopsis AZG2 transports cytokinins in vivo and regulates lateral root emergence. New Phytol. 2021;229:979–993. doi: 10.1111/nph.16943. [** Together with [75], it is proposed that CK transporters are involved in the crosstalk between CK and auxin in Arabidopsis. Here, AZG2 is characterized as CK transporter that is induced by auxin to regulate lateral root development] [DOI] [PubMed] [Google Scholar]
- 77.Kim A, Chen J, Khare D, Jin JY, Yamaoka Y, Maeshima M, Zhao Y, Martinoia E, Hwang JU, Lee Y. Non-intrinsic ATP-binding cassette proteins ABCI19, ABCI20 and ABCI21 modulate cytokinin response at the endoplasmic reticulum in Arabidopsis thaliana. Plant Cell Rep. 2020;39:473–487. doi: 10.1007/s00299-019-02503-0. [* This study suggests that ABCI19,20,21 are phylogenetically related proteins that function together as a CK transporter. ABCI19,20,21 are induced by light under the control of the transcription factor HY5 and simultaneously influence CK-related responses] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wendrich JR, Yang B, Vandamme N, Verstaen K, Smet W, Van de Velde C, Minne M, Wybouw B, Mor E, Arents HE, et al. Vascular transcription factors guide plant epidermal responses to limiting phosphate conditions. Science (80-) 2020;370 doi: 10.1126/science.aay4970. [* By employing a single-cell gene expression approach, it has been shown that TMO5/LHW transcription factor triggers the biosynthesis of a mobile CK form in the vascular. In turn, this increases root hair density in a TMO5-and CK-dependent manner] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barker R, Fernandez Garcia MN, Powers SJ, Vaughan S, Bennett MJ, Phillips AL, Thomas SG, Hedden P. Mapping sites of gibberellin biosynthesis in the Arabidopsis root tip. New Phytol. 2021;229:1521–1534. doi: 10.1111/nph.16967. [** Tissue-specific expression of the gibberellin biosynthesis genes in Arabidopsis roots indicated that roots and shoots are autonomous for GA synthesis. The results suggest for bioactive GA or GA intermediates movement between root cell layers] [DOI] [PubMed] [Google Scholar]
- 90.Camut L, Regnault T, Sirlin-Josserand M, Sakvarelidze-Achard L, Carrera E, Zumsteg J, Heintz D, Leonhardt N, Lange MJP, Lange T, et al. Root-derived GA12 contributes to temperature-induced shoot growth in Arabidopsis. Nat Plants. 2019;5:1216–1221. doi: 10.1038/s41477-019-0568-8. [** Grafting wild-type and GA-deficient mutants, combined with GA-content measurements, indicated that root-derived GA12 mediates thermo-induced shoot growth in Arabidopsis. The temperature-dependent root-to-shoot transport of GA12 is an exciting example of how hormones transport mediates the plant response to the environment] [DOI] [PubMed] [Google Scholar]
- 95.Wulff N, Ernst HA, Jørgensen ME, Lambertz S, Maierhofer T, Belew ZM, Crocoll C, Motawia MS, Geiger D, Jørgensen FS, et al. An Optimized Screen Reduces the Number of GA Transporters and Provides Insights Into Nitrate Transporter 1/Peptide Transporter Family Substrate Determinants. Front Plant Sci. 2019;10:1–18. doi: 10.3389/fpls.2019.01106. [** In this study, all Arabidopsis NPF proteins were tested for possible GA transport activity in Xenopus oocytes. The study sheds light on the specificity of the NPF family to different GA forms. In addition, the research shows internal cellular pH can result in altered distribution of membrane-permeable hormones] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Morii M, Sugihara A, Takehara S, Kanno Y, Kawai K, Hobo T, Hattori M, Yoshimura H, Seo M, Ueguchi-Tanaka M. The Dual Function of OsSWEET3a as a Gibberellin and Glucose Transporter Is Important for Young Shoot Development in Rice. Plant Cell Physiol. 2020;61:1935–1945. doi: 10.1093/pcp/pcaa130. [* This study presents OsSWEET3a as a GA transporter involved in the transport of GA20 and glucose to developing leaves. The authors suggest that the GA20 translocation by OsSWEET3a during early plant development allows the later conversion to the bioactive GA1 by OsGA3ox2] [DOI] [PubMed] [Google Scholar]
- 99.Guan L, Denkert N, Eisa A, Lehmann M, Sjuts I, Weiberg A, Soll J, Meinecke M, Schwenkert S. JASSY, a chloroplast outer membrane protein required for jasmonate biosynthesis. Proc Natl Acad Sci U S A. 2019;116:10568–10575. doi: 10.1073/pnas.1900482116. [** The study shows that JASSY, a protein localized to the outer chloroplast envelope, facilitates the export of OPDA from the chloroplast. JASSY regulates plant susceptibility to cold treatment and pathogen attack] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schulze A, Zimmer M, Mielke S, Stellmach H, Melnyk CW, Hause B, Gasperini D. Wound-Induced Shoot-to-Root Relocation of JA-Ile Precursors Coordinates Arabidopsis Growth. Mol Plant. 2019;12:1383–1394. doi: 10.1016/j.molp.2019.05.013. [** Grafting and hormone profiling experiments showed that in Arabidopsis OPDA (precursor of JA-Ile), and its derivatives, but not the bioactive JA-Ile conjugate, translocate from wounded shoots to undamaged roots] [DOI] [PubMed] [Google Scholar]
- 103.Li M, Wang F, Li S, Yu G, Wang L, Li Q, Zhu X, Li Z, Yuan L, Liu P. Importers Drive Leaf-to-Leaf Jasmonic Acid Transmission in Wound-Induced Systemic Immunity. Mol Plant. 2020;13:1485–1498. doi: 10.1016/j.molp.2020.08.017. [** Arabidopsis ABCG6 and ABCG20 (JAT3 and JAT4) are two membrane-localized jasmonate transporters. ABCG6 and ABCG20 are expressed in the phloem to regulate JA translocation as a mobile wound signal in Arabidopsis] [DOI] [PubMed] [Google Scholar]
- 108.Lim GH, Liu H, Yu K, Liu R, Shine MB, Fernandez J, Burch-Smith T, Mobley JK, McLetchie N, Kachroo A, et al. The plant cuticle regulates apoplastic transport of salicylic acid during systemic acquired resistance. Sci Adv. 2020;6 doi: 10.1126/sciadv.aaz0478. [* The study shows that the cuticle regulates the active transport of SA. Cuticle-defective mutants are impaired in long-distance transport of SA and distal accumulation of SAR-induced pipecolic acid] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Holmes EC, Chen YC, Sattely ES, Mudgett MB. An engineered pathway for N-hydroxy-pipecolic acid synthesis enhances systemic acquired resistance in tomato. Sci Signal. 2019;12:1–11. doi: 10.1126/scisignal.aay3066. [** The study shows that tomato produces endogenous NHP in response to a bacterial pathogen. Local expression of NHP biosynthesis enzymes in leaves triggered SAR in distal tissues in the absence of a pathogen] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rekhter D, Lüdke D, Ding Y, Feussner K, Zienkiewicz K, Lipka V, Wiermer M, Zhang Y, Feussner I. Isochorismate-derived biosynthesis of the plant stress hormone salicylic acid. Science (80-) 2019;365:498–502. doi: 10.1126/science.aaw1720. [** The study shows that EDS5 exports isochorismate from the plastid into the cytosol, where PBS3 metabolizes it to isochorismate-9-glutamate. The three proteins (ICS1, EDS5, and PBS3) regulate SA biosynthesis to protect the plant from pathogenic effectors] [DOI] [PubMed] [Google Scholar]
- 119.Shiratake K, Notaguchi M, Makino H, Sawai Y, Borghi L. Petunia PLEIOTROPIC DRUG RESISTANCE 1 Is a Strigolactone Short-Distance Transporter with Long-Distance Outcomes. Plant Cell Physiol. 2019;60:1722–1733. doi: 10.1093/pcp/pcz081. [* The authors provide evidence that PaPDR1 transports SL in a short-distance manner with long-distance effects. This study implies that other transporters or substrates of SL might be responsible for SL longdistance transport in the plant] [DOI] [PubMed] [Google Scholar]
- 120.Zhang J, Mazur E, Balla J, Gallei M, Kalousek P, Medve?ová Z, Li Y, Wang Y, Prát T, Vasileva M, et al. Strigolactones inhibit auxin feedback on PIN-dependent auxin transport canalization. Nat Commun. 2020;11 doi: 10.1038/s41467-020-17252-y. [* This study shows that SLs are essential regulators of vascular tissue formation and regeneration by regulating auxin-mediated processes. The authors suggest that SLs may act downstream of auxin biosynthesis due to inhibition of exogenously applied auxin by SL] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Banasiak J, Borghi L, Stec N, Martinoia E, Jasiński M. The Full-Size ABCG Transporter of Medicago truncatula Is Involved in Strigolactone Secretion, Affecting Arbuscular Mycorrhiza. Front Plant Sci. 2020;11:1–14. doi: 10.3389/fpls.2020.00018. [* This study identifies MtABCG59 (homologue to PaPDR1) as a novel SL transporter. MtABCG59 exhibits root-specific expression and affects arbuscular mycorrhiza in Medicago truncatula. Knockout lines phenotype of the transporter implies that additional unknown SL transporters mediate SL distribution] [DOI] [PMC free article] [PubMed] [Google Scholar]