Abstract
Background
The prevalence of type 2 diabetes is increasing rapidly, particularly among younger adults. It is estimated that people with diabetes die, on average, six years earlier than people without diabetes. Our aim was to provide reliable estimates of the associations of age at diagnosis of diabetes with all-cause and cause-specific mortality and reductions in life expectancy.
Methods
We conducted a combined analysis of individual-participant-data from two large-scale data sources in 19 high-income countries, Emerging Risk Factors Collaboration (96 cohorts, baseline years 1961-2020, latest follow up years 1980-2020) and UK Biobank (baseline year 2006, latest follow up year 2020). We calculated age- and sex-adjusted hazard ratios (HRs) for all-cause mortality according to age at diagnosis of diabetes in 1,515,718 participants, in whom deaths were recorded during 23.1 million person-years of follow-up. We estimated cumulative survival by applying age-specific HRs to contemporary age-specific death rates in US and Europe.
Findings
We observed a log-linear dose-response association between earlier age at diagnosis of diabetes and higher risk of all-cause mortality as compared to concurrent participants without diabetes. HRs were 2.69 (95% CI: 2.43-2.97) at 30-39 years, 2.26 (2.08-2.45) at 40-49 years, 1.84 (1.72-1.97) at 50-59 years, 1.57 (1.47-1.67) at 60-69 years, and 1.39 (1.25-1.51) at age ≥70 years. HRs per decade earlier diagnosis were similar for men and women. Using US death rates, a 50-year-old with diabetes, diagnosed at age 30, 40, or 50 years died on average 14, 10, or 6 years earlier, respectively, than an individual without diabetes. Corresponding estimates were 13, 9, or 5 years earlier using EU death rates.
Interpretation
Every decade of earlier diagnosis of diabetes was associated with about three to four years of lower life expectancy, highlighting the potential value of early interventions that delay or prevent diabetes.
Funding
BHF, MRC, NIHR, HDRUK
Introduction
The prevalence of type 2 diabetes is rising globally, driven mainly by behavioural and societal factors related to obesity, nutrition and physical activity.1-3 In 2021, 537 million adults were estimated to have diabetes worldwide, with an increasing numbers diagnosed at younger ages.3,4
Previous estimates have suggested that adults with type 2 diabetes die, on average, six years earlier than counterparts without diabetes.5-7 There is uncertainty, however, about how this average reduction in life expectancy varies according to age at diagnosis.8-19 Valid characterization of this association requires prospective comparison of outcomes within the same cohorts of people with diabetes diagnosed at varying ages. However, few population cohorts have had sufficient statistical power, detail, and duration of follow-up to enable secure estimation.20-25 Moreover, existing modelling studies have only considered diabetes as a binary condition in estimating the impact on life expectancy using state-transition models and life tables that rely on inputs from aggregated data.7,26-29 Thus few of published studies have directly analysed the associations of age at diagnosis of diabetes per se with mortality and life expectancy.
Here we aim to provide reliable estimates of the associations of age at diagnosis of diabetes with all-cause and cause-specific mortality and reductions in life expectancy in high-income countries. We analysed individual records from 97 long-term prospective cohorts involving 1,515,718 participants followed-up for a total of 23.1 million person-years.
Methods
Study design, data sources, and participants
We conducted a combined analysis of individual-participant data from two large-scale data sources, each constituting prospective population cohort studies with information on age at diagnosis of diabetes (appendix pp 2-3 and p 24). First, the Emerging Risk Factors Collaboration (ERFC) is a collaboration of prospective cohort studies with information about a variety of risk factors, cardiovascular disease outcomes, and mortality.30 Prospective cohort studies contributing to the ERFC were included in this analysis if they met all of the following criteria: had recruited participants on the basis of informed consent; did not select participants on the basis of having previous chronic disease (including cardiovascular disease and diabetes); had recorded information on diabetes status, and age at diagnosis of diabetes; had recorded cause-specific deaths; and had accrued more than 1 year of follow-up. The second data source was the UK Biobank (UKBB), a single large prospective study in which participants were recruited from 22 centres throughout the UK.31 After giving consent, participants provided biological samples and completed a touch-screen questionnaire, a computer-assisted interview, and a physical examination (appendix p 26). Data from participants in the UKBB have been linked with death records of the UK Office for National Statistics through National Health Service identification numbers. For all studies, written informed consent was obtained from participants and approval was obtained from relevant ethics committees.
We ascertained baseline diabetes status on the basis of self-report information, medical records, medication usage, or a combination of these factors (appendix p 4).5,32 To calculate age at diagnosis of diabetes, we used information recorded at the “baseline” enrolment survey in prospective cohort studies, supplemented, when available, by information on new-onset incident type 2 diabetes recorded during follow-up (appendix p 27). For 37,513 of 47,404 (79%) new-onset incident cases, age at diagnosis of diabetes was calculated using date of diagnosis provided by the contributing cohorts. For the remaining 9891 (21%) of the new-onset incident cases of diabetes, for whom information was provided as diabetes status (yes/no) at date-stamped resurveys, we estimated the age at diagnosis as the participant’s age at the midpoint of two consecutive surveys in which the participant developed diabetes (appendix pp 2-3). We also computed an accuracy indicator as half-width of the time interval between the two surveys, and the average was ±2.4 (SD 0.9) years (appendix pp 2-3).
We classified mortality according to the primary cause (or, in its absence, the underlying cause) on the basis of coding from the International Classification of Diseases, revisions 8 through 10, to at least 3 digits, or according to study-specific classification systems. Classification of deaths was based on death certificates, supplemented in 76 studies by medical records, findings on autopsy, and other sources in the ERFC. The date of latest mortality follow-up was October 2014 in the ERFC and November 2020 in UKBB.
Statistical analysis
To be eligible for the analysis, participants had to have information recorded about their history of diabetes plus age and sex. To focus analysis on individuals with type 2 diabetes, we excluded 3695 participants diagnosed with diabetes at age <30 years, who would be more likely to have type 1 diabetes. To assess “dose-response” relationships, we categorized participants according to their history of diabetes (yes vs no) and their age at diagnosis into 10-year groups: i.e., 30 to <40 years, 40 to <50 years, 50 to <60 years, 60 to <70 years, and ≥70 years. We also assessed the continuous shape of associations using fractional polynomials. We then assessed adjusted associations, guided by the dose-response analyses results and prior evidence for other continuous covariates. The primary outcome was all-cause mortality, with additional outcomes including deaths from vascular disease, cancer, and nonvascular conditions not attributed to cancer (appendix pp 5-6). Hazard ratios (HRs) for age at diagnosis of diabetes were calculated separately within each study using time-dependent Cox proportional hazards regression models (i.e., allowing diabetes status, age at diagnosis, and other covariates to change during follow up, when reassessed). The timescale for the survival analysis was duration (in years) since entry to the study at baseline. Participants were included in analyses of mortality outcomes irrespective of previous non-fatal events. For each specific cause of death, participants’ data were censored if a participant was lost to follow-up, died from other causes, or reached the end of follow-up period. HRs calculated in this manner for each cause of death are aetiologically interpretable and provide reliable assessments of the marginal cause-specific associations, including in the case of competing risks with low to moderate correlations of failure times, that would be typical of most practical circumstances.33-35 Sensitivity analyses were conducted for cause-specific mortality considering death from other causes as competing risks using the Fine and Gray regression model. Study-specific estimates (i.e. log HRs) were then pooled across studies by multivariate random-effects meta-analysis due to expected heterogeneity with diverse data sources analysed.36 To avoid model overfitting, studies with fewer than 10 deaths for any outcome (i.e. all cause and cause specific death) were excluded from the main analyses for relevant outcomes. Further sensitivity analyses excluded studies with fewer than 80 deaths (i.e. applying stricter 10 events per variable rule at the study level). The proportional hazards assumption, assessed by meta-analysis of study-specific interaction of coded exposure variable (indicators or continuous) and the survival analysis time in years, was met (P>0.05).
Because the principal objective of our study was to estimate reductions in life expectancy according to age at diagnosis of diabetes, the main analysis calculated HRs stratified by sex and adjusted for age only. A secondary objective was to explore the extent to which the age-specific relevance of diabetes could be accounted by other known factors associated with mortality risk. Hence, HRs were sequentially adjusted for several variables recorded after diagnosis of diabetes, including smoking status, body-mass index (BMI), systolic blood pressure, total cholesterol, measures of glycemia, measures of renal function, measures of inflammation, level of education, and self-reported use of medications. These variables were selected considering subject matter knowledge and data availability. The order of sequential adjustment reflected prioritisation of a variable as a confounder, mediator, or indicator of severity of diabetes, consistent with principles of the modified disjunctive cause criterion reasoning.37 We investigated effect modification with tests for interaction for individual characteristics (age, sex, smoking, history of CVD) and by meta-regression of study-specific log HRs (i.e. outcome) on study-level characteristics (diabetes diagnosis information available, median year of baseline, median year of follow up) assuming normal error terms36 and using a 0.001 significance threshold to make some allowance for multiple testing (i.e. 0.01/7 for seven interactions assessed at 0.01 nominal significance each). Between-study heterogeneity of log HRs was assessed by the I2 statistic.38
Appendix (p28) provides details of the methods used to estimate reductions in life expectancy by age at diagnosis of diabetes. Briefly, estimates of cumulative survival from 40 years of age onward according to age at diagnosis of diabetes were calculated by applying the HRs for cause-specific mortality (specific to age at risk and sex) to respective mortality rates obtained from the detailed mortality component of the US Centers for Disease Control and Prevention’s CDC WONDER database,39 which recorded 2.7 million deaths among more than 320 million individuals during year 2015.9,13 This method does not rely on the survival estimates from the cohort data; instead, it makes inferences by estimating age-at-risk specific HRs from the cohort data, which are then combined with external population age-specific mortality rates.11 Supplementary analyses used European Union (EU) death rates during 2015. Analyses involved Stata version 15.1 (StataCorp), 2-sided P-values, and used a significance level of P<0.05 unless stated otherwise.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
A total of 1,515,718 participants from 97 prospective cohorts had sufficient information for inclusion in this analysis (comprising 1,017,695 participants in 96 ERFC cohorts and 498,023 participants in UKBB, Table 1 and appendix pp 2-3). In ERFC the median year of recruitment was 1990 (range 1961-2020) and the median of year of latest follow up was 2015 (range 1980-2020). Corresponding values in UKBB were 2009 and 2020. In the ERFC, the large majority of participants were enrolled in Europe (50%) or North America (42%). Overall, the mean (SD) age of participants at baseline was 55.0 (9.2) years and 690,596 participants (46%) were male. In ERFC, age at diagnosis of prevalent diabetes was available for 23,335 participants (57% of 41,160 participants who had prevalent diabetes) and a further 45,585 participants had diabetes diagnosed during follow-up (i.e., new-onset disease). In UKBB, age at diagnosis of prevalent diabetes was available for 24,981 participants (98% of 25,416 participants who had prevalent diabetes), and a further 1819 participants had diabetes diagnosed during follow-up. The mean (SD) age at diagnosis of diabetes was 54 (9) years for the prevalent cases and 65 (9) years for the incident cases. Over a median follow-up of 12.5 years (5th-95th percentile: 5.0-32.1 years; 23.1 million person-years at risk), there were 246,670 deaths recorded, including 84,443 deaths due to vascular diseases, 85,014 deaths due to cancer, and 61,516 deaths due to non-vascular, non-cancer causes (appendix p 5).
Table 1. Baseline participant characteristics by age at diagnosis of diabetes.
| Overall | Age at diagnosis of diabetes* | |||||||
|---|---|---|---|---|---|---|---|---|
| ERFC (1017695 participants, 96 cohorts) | N | Mean (SD) or n (%) | No Diabetes | 30 to <40 yrs | 40 to <50 yrs | 50 to <60 yrs | 60 to <70yrs | ≥70 yrs |
| No. of participants (Row %) | 1017695 | 1017695 (100.0%) | 948775 (93.2%) | 3253 (0.3%) | 8763 (0.9%) | 18605 (1.8%) | 21527 (2.1%) | 16772 (1.6%) |
| Prevalent diabetes, No. (Col %) | 1017695 | 23335 (2%) | - | 2835 (87%) | 5602 (64%) | 7047 (38%) | 5632 (26%) | 2219 (13%) |
| Incident diabetes, No. (Col %) | 1017695 | 45585 (4%) | - | 418 (13%) | 3161 (36%) | 11558 (62%) | 15895 (74%) | 14553 (87%) |
| Age at baseline, mean (SD), yrs | 1017695 | 54.6 (9.7) | 54.4 (9.8) | 49.4 (8.0) | 51.0 (7.4) | 55.5 (6.8) | 60.6 (6.0) | 67.6 (4.9) |
| Male sex, No. (%) | 1017695 | 463920 (46%) | 436576 (46%) | 1187 (36%) | 4150 (47%) | 8246 (44%) | 7796 (36%) | 5965 (36%) |
| Female sex, No. (%) | 1017695 | 553775 (54%) | 512199 (54%) | 2066 (64%) | 4613 (53%) | 10359 (56%) | 13731 (64%) | 10807 (64%) |
| Current smoker, No. (%) | 945856 | 267678 (28%) | 252507 (29%) | 914 (29%) | 2583 (31%) | 4763 (27%) | 4422 (21%) | 2489 (15%) |
| Systolic blood pressure, mean (SD), mmHg | 753256 | 135 (19) | 134 (19) | 135 (19) | 138 (19) | 142 (19) | 143 (19) | 146 (20) |
| Body mass index, mean (SD), kg/m2 | 890293 | 26.0 (4.3) | 25.8 (4.1) | 27.9 (6.0) | 28.6 (5.7) | 28.9 (5.5) | 28.5 (5.2) | 27.8 (4.7) |
| Total cholesterol, mean (SD), mmol/l | 594679 | 5.80 (1.11) | 5.79 (1.11) | 5.55 (1.15) | 5.69 (1.15) | 5.86 (1.12) | 5.87 (1.14) | 5.93 (1.15) |
| Random glucose, mean (SD), mmol/l | 398521 | 5.39 (1.23) | 5.25 (0.87) | 8.20 (4.04) | 7.98 (3.58) | 7.51 (2.96) | 6.94 (2.57) | 6.42 (2.31) |
| Fasting glucose, mean (SD), mmol/l | 196159 | 5.30 (1.09) | 5.15 (0.64) | 7.88 (3.77) | 7.27 (3.38) | 6.94 (2.66) | 6.48 (2.03) | 5.93 (1.33) |
| HbA1c, mean (SD), % | 96802 | 5.42 (0.79) | 5.25 (0.48) | 7.37 (1.89) | 7.14 (1.79) | 6.71 (1.59) | 6.30 (1.22) | 6.07 (0.95) |
| History of CVD, No. (%) | 1017695 | 73091 (7%) | 63734 (7%) | 422 (13%) | 1097 (13%) | 2256 (12%) | 2820 (13%) | 2762 (16%) |
| Medication usage, No. (%)$ | 614036 | 84056 (14%) | 66186 (12%) | 1060 (53%) | 2610 (47%) | 4777 (41%) | 5261 (38%) | 4162 (33%) |
| Vocational/University education level, No. (%) | 389924 | 99850 (26%) | 94710 (26%) | 313 (23%) | 775 (23%) | 1578 (23%) | 1583 (21%) | 891 (16%) |
| Non-white ethnic group, No. (%) | 534131 | 71348 (13%) | 61154 (12%) | 617 (35%) | 1781 (33%) | 3378 (30%) | 2817 (22%) | 1601 (15%) |
| UK Biobank (498023 participants) | N | Mean (SD) or n (%) | No Diabetes | 30 to <40 yrs | 40 to <50 yrs | 50 to <60 yrs | 60 to <70yrs | ≥70 yrs |
| No. of participants (Row %) | 498023 | 498023 (100.0%) | 471223 (94.6%) | 1550 (0.3%) | 5859 (1.2%) | 11041 (2.2%) | 7406 (1.5%) | 944 (0.2%) |
| Prevalent diabetes, No. (Col %) | 498023 | 24981 (5%) | - | 1550 (100%) | 5810 (99%) | 10798 (98%) | 6818 (92%) | 5 (1%) |
| Incident diabetes, No. (Col %) | 498023 | 1819 (0%) | - | 0 (0%) | 49 (1%) | 243 (2%) | 588 (8%) | 939 (99%) |
| Age at baseline, mean (SD), yrs | 498023 | 57.0 (8.1) | 56.8 (8.1) | 52.8 (8.5) | 54.0 (7.1) | 60.8 (4.9) | 65.4 (4.3) | 62.0 (5.3) |
| Male sex, No. (%) | 498023 | 226676 (46%) | 210224 (45%) | 882 (57%) | 3560 (61%) | 6889 (62%) | 4508 (61%) | 613 (65%) |
| Female sex, No. (%) | 498023 | 271347 (54%) | 260999 (55%) | 668 (43%) | 2299 (39%) | 4152 (38%) | 2898 (39%) | 331 (35%) |
| Current smoker, No. (%) | 497789 | 52494 (11%) | 49497 (11%) | 243 (16%) | 815 (14%) | 1193 (11%) | 665 (9%) | 81 (9%) |
| Systolic blood pressure, mean (SD), mmHg | 497112 | 138 (19) | 137 (19) | 137 (17) | 138 (17) | 141 (17) | 144 (17) | 145 (18) |
| Body mass index, mean (SD), kg/m2 | 495426 | 27.5 (4.8) | 27.2 (4.6) | 31.3 (7.0) | 32.1 (6.5) | 31.7 (5.7) | 30.8 (5.2) | 30.3 (4.8) |
| Total cholesterol, mean (SD), mmol/l | 465833 | 5.70 (1.14) | 5.76 (1.11) | 4.53 (1.10) | 4.53 (1.09) | 4.52 (1.05) | 4.63 (1.09) | 5.60 (1.16) |
| Random glucose, mean (SD), mmol/l | 426110 | 5.10 (1.19) | 4.97 (0.78) | 8.93 (4.54) | 7.92 (3.85) | 7.35 (3.07) | 6.67 (2.53) | 5.66 (1.75) |
| Fasting glucose, mean (SD), mmol/l | 17712 | 5.15 (1.12) | 5.04 (0.70) | 7.42 (4.23) | 7.38 (3.59) | 7.00 (2.99) | 6.23 (2.28) | 5.29 (0.81) |
| HbA1c, mean (SD), % | 462791 | 4.88 (0.89) | 4.76 (0.61) | 8.20 (2.27) | 7.37 (2.20) | 6.98 (1.76) | 6.53 (1.53) | 5.66 (1.20) |
| History of CVD, No. (%) | 498023 | 69693 (14%) | 62346 (13%) | 363 (23%) | 1316 (22%) | 3122 (28%) | 2344 (32%) | 202 (21%) |
| Medication usage, No. (%)$ | 490758 | 135849 (28%) | 113681 (24%) | 1364 (90%) | 4707 (82%) | 9453 (87%) | 6173 (85%) | 471 (51%) |
| Vocational/University education level, No. (%) | 494242 | 298113 (60%) | 284262 (61 %) | 875 (58%) | 3237 (57%) | 5811 (54%) | 3362 (46%) | 566 (60%) |
| Non-white ethnic group, No. (%) | 496231 | 26390 (5%) | 23052 (5%) | 363 (24%) | 1167 (20%) | 1234 (11%) | 522 (7%) | 52 (6%) |
Includes people with a history of diabetes at the baseline survey and people with incident diabetes.
Includes use of lipid-lowering, anti-hypertensive, or anti-diabetic medication at baseline.
Differences in characteristics across categories of age at diagnosis of diabetes were all statistically significant (p < 0.001 adjusted for age and sex) based on Wald tests.
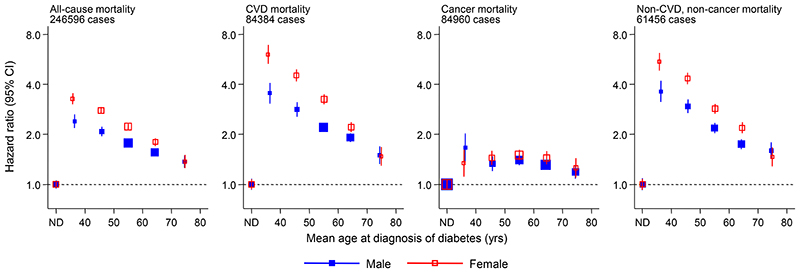
In analyses adjusted for age we observed a log-linear dose-response association between earlier age at diagnosis of diabetes and higher risk of all-cause, CVD and “non-CVD, non-cancer” mortality for each sex (Figure 1), with broadly similar findings in combined analyses adjusted for sex and continuous modelling with fractional polynomials (appendix p 13). Further adjusted analyses used data from 92 cohorts and 1,132,277 participants with complete information on the following factors: age at diagnosis of diabetes, age, sex, smoking, body mass index, systolic blood pressure and total cholesterol. Compared to participants without a history of diabetes, HRs for all-cause mortality, adjusted for age and sex only, were: 2.69 (95% CI: 2.43-2.97) for those diagnosed at 30-39 years, 2.26 (2.08-2.45) at 40-49 years, 1.84 (1.72-1.97) at 50-59 years, 1.57 (1.47-1.67) at 60-69 years, and 1.39 (1.29-1.51) at age ≥70 years (Table 2). For participants diagnosed with diabetes at age 30-39 years, HRs were 4.20 (3.57-4.94) for vascular mortality, 1.55 (1.30-1.85) for cancer mortality, and 3.99 (3.50-4.55) for non-vascular non-cancer mortality (mainly comprising diseases of the respiratory system, nervous system, infections, and external causes). Across all ages, HRs per decade of earlier diagnosis of diabetes were 1.14 (1.08-1.19) for all-cause mortality, 1.19 (1.11-1.27) for vascular mortality, 0.95 (0.88-1.02) for cancer mortality, and 1.18 (1.10-1.27) for non-vascular non-cancer mortality (Table 2).
Figure 1. Sex-specific hazard ratios for all-cause and cause-specific mortality according to age at diagnosis of diabetes.
ND, No diabetes. The 6 categories of age at diagnosis correspond to: ND, 30 to <40 yrs, 40 to <50 yrs, 50 to <60 yrs, 60 to <70yrs, and ≥70 yrs. Hazard ratios adjusted for age. The reference category is no diabetes. Studies with fewer than 10 events of any outcome were excluded from the analysis of that outcome. Sizes of the boxes are proportional to the inverse of the variance of the log-transformed hazard ratios. Vertical lines represent 95% CIs.
Table 1. Hazard ratios for all-cause and cause-specific mortality according to age at diagnosis of diabetes with adjustment for conventional risk factors.*.
| HR (95% CI) adjusted for... | ||||
|---|---|---|---|---|
| No. of events | Age and sex | Age, sex, and smoking | Age, sex, smoking, and other risk factors** | |
| All-cause mortality | ||||
| No Diabetes | 153068 | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| 30 to <40 yrs | 676 | 2.69 (2.43, 2.97) | 2.74 (2.49, 3.02) | 2.64 (2.41,2.90) |
| 40 to <50 yrs | 2070 | 2.26 (2.08, 2.45) | 2.33 (2.14, 2.53) | 2.24 (2.06, 2.43) |
| 50 to <60 yrs | 4197 | 1.84 (1.72, 1.97) | 1.87 (1.75, 1.99) | 1.79 (1.69, 1.90) |
| 60 to <70 yrs | 4125 | 1.57 (1.47, 1.67) | 1.60 (1.51,1.70) | 1.55 (1.46, 1.64) |
| ≥70 yrs | 3026 | 1.39 (1.29, 1.51) | 1.43 (1.33, 1.55) | 1.41 (1.31,1.53) |
| Per decade earlier | 167162 | 1.14 (1.08, 1.19) | 1.13 (1.08, 1.19) | 1.13 (1.07, 1.19) |
| P-value*** | <0.0001 | <0.0001 | <0.0001 | |
| I2 (95% CI) | 67 (59, 74) | 68 (60, 74) | 67 (60, 74) | |
| CVD mortality | ||||
| No Diabetes | 53857 | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| 30 to <40 yrs | 278 | 4.20 (3.57, 4.94) | 4.26 (3.65, 4.99) | 3.93 (3.41,4.53) |
| 40 to <50 yrs | 821 | 3.19 (2.80, 3.64) | 3.31 (2.90, 3.76) | 2.93 (2.60, 3.30) |
| 50 to <60 yrs | 1564 | 2.31 (2.10, 2.53) | 2.36 (2.16, 2.58) | 2.10 (1.94, 2.27) |
| 60 to <70 yrs | 1580 | 1.95 (1.78, 2.14) | 1.98 (1.82, 2.17) | 1.81 (1.67, 1.97) |
| ≥70 yrs | 1251 | 1.50 (1.35, 1.67) | 1.54 (1.38, 1.71) | 1.48 (1.33, 1.65) |
| Per decade earlier | 59351 | 1.19 (1.11, 1.27) | 1.19 (1.11, 1.27) | 1.19 (1.11, 1.28) |
| P-value*** | <0.0001 | <0.0001 | <0.0001 | |
| I2 (95% CI) | 58 (47, 67) | 59 (48, 67) | 59 (48, 67) | |
| Cancer mortality | ||||
| No Diabetes | 53217 | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| 30 to <40 yrs | 124 | 1.55 (1.30, 1.85) | 1.56 (1.31,1.86) | 1.48 (1.24, 1.76) |
| 40 to <50 yrs | 433 | 1.28 (1.16, 1.42) | 1.32 (1.19, 1.45) | 1.25 (1.13, 1.37) |
| 50 to <60 yrs | 1211 | 1.33 (1.22, 1.46) | 1.37 (1.25, 1.49) | 1.35 (1.26, 1.44) |
| 60 to <70 yrs | 1178 | 1.27 (1.17, 1.38) | 1.29 (1.19, 1.40) | 1.28 (1.19, 1.37) |
| ≥70 yrs | 593 | 1.29 (1.18, 1.42) | 1.32 (1.20, 1.44) | 1.31 (1.20, 1.42) |
| Per decade earlier | 56756 | 0.95 (0.88, 1.02) | 0.95 (0.88, 1.02) | 0.94 (0.88, 1.02) |
| P-value*** | 0.176 | 0.160 | 0.128 | |
| I2 (95% CI) | 26 (2, 44) | 24 (0, 43) | 24 (0, 43) | |
| Non-CVD, non-cancer mortality | ||||
| No Diabetes | 35986 | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| 30 to <40 yrs | 250 | 3.99 (3.50, 4.55) | 4.04 (3.54, 4.60) | 3.90 (3.42, 4.45) |
| 40 to <50 yrs | 701 | 3.24 (2.88, 3.64) | 3.34 (2.96, 3.76) | 3.31 (2.95, 3.71) |
| 50 to <60 yrs | 1224 | 2.31 (2.09, 2.54) | 2.37 (2.15, 2.60) | 2.38 (2.16, 2.64) |
| 60 to <70 yrs | 1137 | 1.84 (1.67, 2.02) | 1.87 (1.70, 2.05) | 1.88 (1.71,2.06) |
| ≥70 yrs | 861 | 1.66 (1.48, 1.87) | 1.71 (1.52, 1.93) | 1.76 (1.56, 1.98) |
| Per decade earlier | 40159 | 1.18 (1.10, 1.27) | 1.18 (1.09, 1.27) | 1.16 (1.08, 1.25) |
| P-value*** | <0.0001 | <0.0001 | <0.0001 | |
| I2 (95% CI) | 50 (35, 61) | 51 (36, 62) | 50 (36, 62) | |
Analyses based on ERFC and UK Biobank, including 92 cohorts and 1,132,277 participants with complete information on age at diagnosis of diabetes, age, sex, smoking and other risk factors.
Other risk factors were body mass index, systolic blood pressure and total cholesterol.
P-value for log-linear analyses per decade earlier.
HRs for all-cause mortality changed little after additional adjustment for other risk factors (Table 2). However, HRs were attenuated substantially after further adjustment for measures of glycemia (i.e., fasting glucose or HbA1c), a pattern also observed for the cause-specific mortality that we studied (appendix p 7). There was little change in HRs after adjustment for measures of renal function (i.e., estimated glomerular filtration rate), inflammation (i.e., C-reactive protein), or lipids (i.e., non-HDL, HDL, triglycerides; appendix p 7).
Broadly similar HRs to those noted above were observed in sensitivity analyses that compared results by: diabetes defined using prevalent disease, incident disease, or both; participant characteristics (eg, smoking status; appendix p 14). HRs differed somewhat by calendar time of study enrolment, or follow-up period (appendix pp 14-15), and by data source (ie, ERFC and UKBB; appendix p 16). Tests for interactions on additive scale were generally confirmative of positive interactions of female sex, current smoking, older age, and history of CVD with diabetes status categorised according to age at diagnosis (appendix p 8). Associations were broadly similar also in analyses that estimated HRs for all-cause and cause-specific mortality according to duration of diabetes (ie, time since diagnosis), rather than age at diagnosis (appendix pp 9-10 and 17). Supplementary analyses according to detailed components of non-CVD mortality suggested broadly similar associations for cancer mortality components, but potentially notable variations in the magnitude of associations for non-CVD non-cancer mortality components (appendix p 11), such as HRs per decade earlier diagnosis of diabetes of 1.46 (1.16, 1.84) for renal disease mortality, 1.28 (1.07, 1.53) for infection related mortality, 1.21 (1.04, 1.42) for external causes of mortality, 1.20 (1.03, 1.40) for digestive system disease mortality, and 1.07 (0.96, 1.19) for respiratory system disease mortality, among others. Results of cause-specific mortality were broadly similar when using competing risks adjusted analyses (appendix p 12). Loss to follow up was less than 10% in majority of studies but the percentage of right censored participants and cause-specific deaths somewhat varied across cohorts (appendix pp 18-19). Sensitivity analyses excluding studies with fewer than 80 cause-specific deaths showed similar findings as in the main analyses excluding studies with fewer than 10 deaths (appendix p 12).
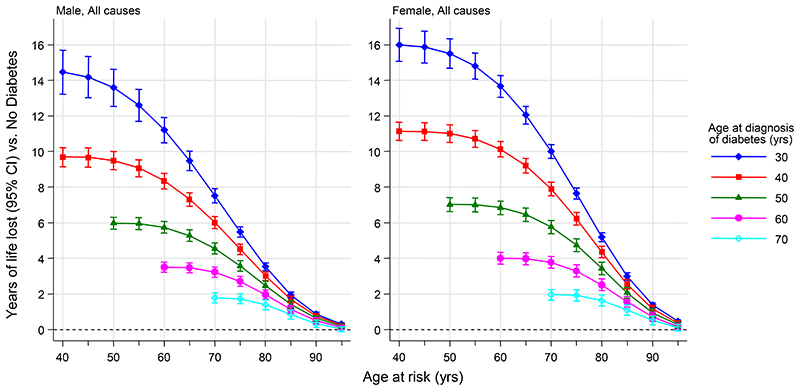
Life expectancy
Compared to absence of diabetes at different attained ages, earlier age at diagnosis of diabetes was associated with greater reductions in life expectancy using US death rates (Figure 2). For example, at age 50 years, individuals with diabetes diagnosed at ages 30, 40, and 50 years on average died about 14, 10, and 6 years earlier, respectively, than individuals without diabetes (Figure 2). These estimates were slightly higher in women (16, 11, and 7 years, respectively) than men (14, 9, and 5 years, respectively; Figure 2). Depending on age and sex, vascular deaths accounted for about 30% to 45% of the reduction in life expectancy associated with diabetes, with the remaining percentage being largely due to non-CVD, non-cancer deaths (appendix p 20). Findings were broadly similar in analyses using EU 2015 death rates, with corresponding estimates being about 13, 9, or 5 years earlier death on average (appendix p 21). In supplementary analyses including people with diabetes diagnosed before age 30 years, we found similar patterns in estimated reductions in life expectancy with highest estimated reductions in those diagnosed in childhood and more notably higher in women than men (appendix pp 22-23). At age 50 years, the estimates corresponded to about two to three years reduction per decade earlier diagnosis.
Figure 2. Estimated years of life lost by age at diagnosis of diabetes compared with those without diabetes.
The estimates of cumulative survival from 40 years of age onwards according to age at diagnosis of diabetes were calculated by applying hazard ratios (specific to age at risk) for all-cause mortality associated with age at diagnosis of diabetes to US 2015 death rates at the age of 40 years or older.
Discussion
We analysed over 23 million person-years of longitudinal data from population cohorts in 19 high-income countries. We found a steep log-linear dose-response association between earlier age at diagnosis of diabetes and higher risk of all-cause mortality. Overall, every decade of earlier diagnosis of diabetes was associated with about four years of reduced life expectancy. Our modelling has suggested that, for individuals surviving to age 50, those with diabetes diagnosed at age 30, 40, and 50 years died, on average, 14, 10, and 6 years earlier, respectively, than individuals without diabetes. The strongest associations of earlier age at diagnosis of diabetes were for vascular causes of death (e.g., myocardial infarction and stroke), and other non-neoplastic causes of death, mainly respiratory, neurological, and infectious diseases as well as external causes. Our estimates of reduced life expectancy associated with diabetes were somewhat greater for women than men. A key implication of our results is the high priority that should be given to developing and implementing interventions that prevent or delay onset of diabetes, especially as the prevalence of diabetes among younger adults is rising globally3.
Our observation of higher HRs for mortality with earlier age at diagnosis of diabetes suggests that the relative impact of diabetes is greatest at ages when the underlying risk of mortality in the general population is lowest. The same phenomenon has been previously observed for other vascular risk factors, including blood pressure40 and LDL-cholesterol41. Conversely, in older adults, in whom the underlying mortality risk is high, the proportional relevance of diabetes is smaller. It has been previously suggested that individuals who develop type 2 diabetes at younger ages may have more aggressive “phenotypes”42 (characterised by higher BMI, blood pressure, and pro-atherogenic lipids,43 as well as faster deterioration in glycemic control24,44) than individuals who develop diabetes at older ages, potentially leading to premature mortality.45 Our findings are consistent with this hypothesis, suggesting the large excess mortality associated with diabetes at younger ages may, in part, reflect cumulative exposure to worsened metabolic profiles. Furthermore, we observed substantial attenuation of excess mortality associated with diabetes after adjustment for glycemic markers, suggesting that early detection of diabetes by screening and intensive glucose management are relevant to prevention of long-term complications in adults with type 2 diabetes.46,47,48
Our study had several strengths and it is distinctive and complimentary to previously reported studies.7-19,26-29 Our focus on age at diagnosis of diabetes avoided inherent difficulties in defining age at onset of diabetes (which may require near continuous assessment of glycemic status),49 and in defining duration of diabetes (which may be confounded by the timing and duration of participants’ entry into prospective cohort studies). Furthermore, our study estimated age at diagnosis of diabetes using information from people diagnosed with prevalent diabetes as well as those diagnosed with incident diabetes. Our study’s access to individual-participant data avoided limitations of previous literature-based reviews, allowing extensive sensitivity analyses to assess potential sources of heterogeneity and interactions according to study-level (including calendar time) and individual-level characteristics. Our estimation of reductions in life expectancy relied on age-specific HRs directly estimated from individual-level data and applied to contemporary population-specific mortality rates. This was desirable because HRs are often less variable across similar populations and time and can be more precisely estimated in combined data synthesis as in our study. Generalisability of the findings was enhanced by inclusion of data from 97 prospective studies based in many different Western populations recruited between 1964 and 2009 and latest follow up between 1980 and 2020.
Our study also had potential limitations. Contributing prospective studies defined diabetes in varying ways. There were, however, no major differences in results across studies due to such variation. Between-study heterogeneity of associations was moderate to high, and not explained by the characteristics assessed in subgroup analyses. We did not have information as to the pathophysiological subtype of diabetes. However, given that we excluded participants diagnosed with diabetes at age <30 years, it may be reasonable to infer that the large majority of participants had type 2 diabetes.50 We did not have information on whether individuals with diabetes were differentially treated and/or followed up depending on age at diagnosis or duration of diabetes (e.g. specific type of medication, dose or intensity of treatment), factors which are likely to have had an impact on long-term disease outcomes. Residual confounding due to measurement error in variables considered for adjustment (e.g. smoking) has not been addressed. We also did not have information on other co-morbidities (e.g. mental health) and socio-economic variables that would have been useful to adjust for. The present analysis involved participants who were mostly of European continental ancestry; future studies should seek to evaluate these results in other ethnic and racial groups. Finally, while we found broadly similar results for cause-specific mortality using competing and non-competing adjusted models, the aetiological interpretation is limited for models adjusted for competing risk.51 However, non-competing risk adjusted models may be subject to selection bias as HRs are calculated conditional on those who have survived.52
In conclusion, our study has suggested that every decade of earlier diagnosis of diabetes is associated with about three to four years of lower life expectancy, highlighting the potential value of early interventions that delay or prevent diabetes.
Supplementary Material
Research in Context.
Evidence before this study
We searched articles published in Medline from inception to November 30, 2022 that reported on associations of age at diagnosis or duration of diabetes with all-cause mortality, and on years of life lost according to age at diagnosis of diabetes. Few population cohorts have had sufficient statistical power, detail, and duration of follow-up to enable reliable estimation, or have directly analysed the associations of age at diagnosis of diabetes per se with mortality and life expectancy.
Added value of this study
Using data from large-scale data sources in 19 high-income countries involving individual records on 1,515,718 participants in whom deaths were recorded during 23.1 million person-years of follow-up, we calculated age- and sex-adjusted hazard ratios for all-cause mortality according to age at diagnosis of diabetes and estimated cumulative survival by applying the age-specific estimates to contemporary age-specific death rates. We found a steep log-linear dose-response association between earlier age at diagnosis of diabetes and higher risk of all-cause mortality. Every decade of earlier diagnosis of diabetes was associated with about four years of reduced life expectancy. Our public health modelling has suggested that, for individuals surviving to age 50 years, those with diabetes diagnosed at age 30, 40, and 50 years died, on average, 14, 10, and 6 years earlier, respectively, than individuals without diabetes.
Implications of all the available evidence
As earlier diagnosis of diabetes is associated with shorter life expectancy, high priority should be given to developing and implementing interventions that prevent or delay onset of diabetes, especially as the prevalence of diabetes among younger adults is rising globally. In addition, the evidence highlights the need for intensive treatment of risk factors for premature mortality among young adults diagnosed with diabetes.
Acknowledgements
A full list of Emerging Risk Factors Collaboration investigators is provided at the end of the manuscript. For the purpose of open access, the author has applied a CC-BY public copyright licence to any Author Accepted Manuscript version arising from this submission. This research has been conducted using the UK Biobank Resource under Application Number 13784.
Sources of funding
No specific funding was obtained for this manuscript. The coordinating centre was supported by core funding from the: UK Medical Research Council (G0800270; MR/L003120/1), British Heart Foundation (SP/09/002; RG/13/13/30194; RG/18/13/33946) and NIHR Cambridge Biomedical Research Centre (BRC-1215-20014). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
JD holds a British Heart Foundation Professorship and a NIHR Senior Investigator Award. AMW is supported by a BHF-Turing Cardiovascular Data Science Award and by the EC-Innovative Medicines Initiative (BigData@Heart). EDA holds a NIHR Senior Investigator Award.
Footnotes
joint senior authors
Deceased
Authors and contributors:
Conception and design: SK, NS, JD EDA;
Acquisition and interpretation of data: all authors;
Performed analyses: SK, EDA;
Drafted the manuscript: SK, EDA, LS, NS, JD;
Critically revised paper: all authors;
Approved of final submission: all authors.
SK and EDA have verified the underlying data. SK, JD and EDA are responsible for the decision to submit the manuscript. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declaration of interest:
The authors declare the following conflicts of interest: ASB reports grants outside of this work from AstraZeneca, Bayer, Biogen, BioMarin, Merck, Novartis, and Sanofi. BGN reports consulting fees form AstraZeneca, Sanofi, Regeneron, Ionis, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Esperion, Silence Therapeutics, Ultragenyx, payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Sanofi, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Abbott, Mankind, and participation on a data safety monitoring board or advisory board for AstraZeneca, Ionis, Kowa, Novartis, Esperion. BBY reports grants from the National Health and Medical Research Council paid to his Institution. CK report grants from NIH, grants or contracts from Grifols and Diagnostica Stago, consulting feed from BMS Pfizer, participation on a data safety monitoring board or advisory board for BMS Pfizer, leadership or fiduciary role in RECOVER-CDC Steering Committee, and stock or stock options for Insera. CC reports consulting fees from Alliance for Better Bone Health, Amgen, Eli Lilly, GSK, Medtronic, Merck, Novartis, Pfizer, Roche, Servier, Takeda, and UCB. DAL reports grants from UK Medical Research Council, National Institute of Health & Research, British Heart Foundation, European Research Council, US National Institute of Health, and Diabetes UK paid to her Institution, and participation on a data safety monitoring board or advisory board for UK Biobank, Bradford Institute Health Research, and NIHR-BHF. DN reports grants outside of this work from GSK and participation on a data safety monitoring board or advisory board for GSK. EDA reports grants from British Heart Foundation, NIHR, and NIHR Senior Investigator Award, and participation on a data safety monitoring board or advisory board from Our Future Health, and EURAC Research. HMK reports grants outside of this work from National Institutes of Health Agency for Healthcare Research and Quality, Foundation for a Smoke-Free World, State of CT Department of Public Health, US Food and Drug Administration, Johnson & Johnson, American Heart Association, Centers for Medicare & Medicaid Services, Google, and Pfizer, consultant fees from Massachusetts Medical Society, Eyedentifeye, and F-Prime, participation on a data safety monitoring board or advisory board for Aetna, Reality Labs, Element Science, and United Health, stock or stock options for Element Science and Eyedentifeye, and other financial or non-financial interests Hugo Health, and Refactor Health. JSu reports stock or stock options from Anagram kommunikation AB, and Symptoms Europe AB. JD reports support from British Heart Foundation Professorship and NIHR Senior Investigator Award, and grants or contracts from any entity from Merck Sharp & Dohme, Novartis, Pfizer, and AstraZeneca outside the submitted work. JG report grants or contracts from any entity from MRC, and ADDI, payment or honoraria for lecture and support for attending meetings and/or travel from Yonsei University, and leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid for Dementias Platform UK and BrainWaves study. JSh reports grants or contracts from any entity from AstraZeneca, consulting fees from AstraZeneca, Sanofi, Novo Nordisk, MSD, Eli Lilly, Pfizer, and payment or honoraria for lecture and support for attending meetings and/or travel from Astra Zeneca, Mylan, Sanofi, Boehringer Ingelheim, Zuellig, and Abbott. KJM reports grants National Institutes of Health (US). LS is now a full-time employee at Regeneron Genetics Center, LLC. LGB reports grants. LEW reports grants from NIH/NHLBI. NS reports grants paid to institution from AstraZeneca, Boehringer Ingelheim, Novartis and Roche Diagnostics, consulting fees from Abbott Laboratories, Afimmune, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Hanmi Pharmaceuticals, Merck, Shatp & Dohme, Novartis, Novo Nordisk, Pfizer, Roche Diagnostics and Sanofi, and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Abbott Laboratories, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen and Novo Nordisk. PJN reports grants from NIH. PMR has received institutional research grant support from Kowa, Novartis, Amarin, Pfizer, Esperion, NovoNordisk, and the NHLBI, has served as a consultant to Novartis, AstraZeneca, Kowa, and Novo Nordisk, and receives compensation for service on the Peter Munk Advisory Board (University of Toronto), the Leducq Foundation, Paris FR, and the Baim Institute (Boston, MA). RTdJ reports grants from Takeda, and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Amgen. SGT has received institutional research grant support from British Heart Foundation.
SJG reports to be a trustee of the Novo Nordisk UK research Foundation medical research charity. SKi reports receiving grants or contract from VASCage (Research Centre on Vascular Ageing and Stroke, project number 868624) of the Austrian Research Promotion Agency FFG (COMET program-Competence Centers for Excellent Technologies) funded by the Federal Ministry for Climate Protection, Environment, Energy, Transport, Innovation and Technology; the Federal Ministry for Labour and Economy; and the federal states Tyrol (via Standortagentur), Salzburg, and Vienna (via Vienna Business Agency).
SK has received institutional research grant support from British Heart Foundation, UK Medical Research Council, UK National Institute for Health and Care Research, and Health Data Research UK. SB has received institutional research grant support Wellcome Trust and Medical Research Council. SWS reports receiving institutional grants from National Institutes of Health, royalties from Springer Publishing, and consulting fees from Fred Hutchinson Cancer Institute and State University of NY at Buffalo. TS reports consulting form Amarin, Amgen, Novartis, Orion Pharma, Raisio Group, Sankyo, and leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid for the Finnish guidelines for dyslipidemia. VS reports receiving institutional grants from Juho Vainio Foundation and Bayer Ltd.
Investigators
AFTCAPS: Robert W Tipping; ARIC: Stuart Russell, Michelle Johansen, Michael P Bancks, Morgana Mongraw-Chaffin; AUSDIAB: Dianna Magliano, Elizabeth LM Barr, Paul Z Zimmet; BHS: Matthew W Knuiman; BRHS: Peter H Whincup; BRUN: Johann Willeit, Peter Willeit, Christoph Leitner; BWHHS: Debbie A Lawlor; CAPS: Yoav Ben-Shlomo, Peter Elwood; CHARL: Susan E Sutherland, Kelly J Hunt; CHS: Mary Cushamn; CONOR: Randi M Selmer, Lise Lund Håheim, Inger Ariansen; COPEN: Anne Tybjærg-Hansen, Ruth Frikke-Schmidt, Anne Langsted; CUORE: Chiara Donfrancesco, Cinzia Lo Noce; DESIR: Beverley Balkau, Fabrice Bonnet, Frederic Fumeron; DRECE: David Lora Pablos, Carmen Romero Ferrero, Teresa García Morales; EAS: Stela Mclachlan; EPESEBOS: Jack Guralnik; EPESEIOW: Jack Guralnik; EPESENCA: Jack Guralnik; EPESENHA: Jack Guralnik; EPICNOR: Kay-Tee Khaw; ESTHER: Hermann Brenner, Bernd Holleczek, Hannah Stocker; FINE_FIN: Aulikki Nissinen; FINE_IT: Luigi Palmieri, Cinzia Lo Noce; FNRSK92: Erkki Vartiainen, Pekka Jousilahti, Kennet Harald; FNRSK97: Erkki Vartiainen, Pekka Jousilahti, Kennet Harald; FRAMOFF: Joseph M Massaro, Michael Pencina, Asya Lyass; FUNAGATA: Shinji Susa, Toshihide Oizumi, Takamasa Kayama; GOH: Angela Chetrit, Jesse Roth, Liat Orenstein; GOTO13: Lennart Welin, Kurt Svärdsudd; GOTO33: Lennart Welin, Kurt Svärdsudd; GOTO43: Lennart Welin, Kurt Svärdsudd; GOTOW: Lauren Lissner, Dominique Hange, Kirsten Mehlig; HBS: Veikko Salomaa, Reijo S Tilvis; HCS: Elaine Dennison, Cyrus Cooper, Leo Westbury; HIMS: Paul E Norman, Osvaldo P Almeida, Graeme J Hankey; HISAYAMA: Jun Hata, Mao Shibata, Yoshihiko Furuta; HOORN: Marieke T Blom, Femke Rutters, Mirthe Muilwijk; HPFS: Peter Kraft, Sara Lindstrom, Constance Turman; IKNS: Masahiko Kiyama, Akihiko Kitamura, Kazumasa Yamagishi; ISRAEL: Yariv Gerber; KAREL72: Tiina Laatikainen; KIHD: Jukka T Salonen; LASA Natasja van Schoor, E M van Zutphen; MCVDRFP: W M Monique Verschuren; MDCS: Gunnar Engström, Olle Melander; MESA: Bruce M Psaty, Michael Blaha, Ian H de Boer, Richard A Kronmal; MIDSPAN: Naveed Sattar; MORGEN: WM Monique Verschuren; MOSWEGOT: Annika Rosengren; MRCOLD: Dorothea Nitsch; MRFIT: Greg Grandits; NCS: Aage Tverdal; NHANES1: Hee-Choon Shin, Juan R Albertorio, Richard F Gillum; NHS: Frank B Hu; NPHSI: Jackie A Cooper, Steve Humphries; NSHS: Felicia Hill-Briggs, Elizabeth Vrany, Mark Butler, Joseph E Schwartz; OSAKA: Masahiko Kiyama, Akihiko Kitamura, Hiroyasu Iso; PARIS1: Pierre Amouyel, Dominique Arveiler, Jean Ferrieres; PREVEND: Ron T Gansevoort, Rudolf de Boer, Lyanne Kieneker; PRHHP: Carlos J Crespo; PROCAM: Gerd Assmann; PROSPER: Stella Trompet, Naveed Sattar, Patricia Kearney; QUEBEC: Bernard Cantin, Jean-Pierre Despres, Bernard Lamarche; RANCHO: Gail Laughlin, Linda McEvoy; REYK: Thor Aspelund, Bolli Thorsson, Gunnar Sigurdsson; ROTT: Martijn Tilly, M Arfan Ikram; ROTTII: Martijn Tilly, M Arfan Ikram; ROTTIII: Martijn Tilly, M Arfan Ikram; SHIP: Marcus Dörr, Sabine Schipf, Henry Völzke; SHS: Amanda M Fretts, Jason G Umans, Tauqeer Ali, Nawar Shara; SPEED: George Davey-Smith; TARFS: Günay Can, Hüsniye Yüksel, Uğur Özkan; TOYAMA: Hidaeki Nakagawa, Yuko Morikawa, Masao Ishizaki; TROMSØ: Inger Njølstad, Tom Wilsgaard, Ellisiv Mathiesen; ULSAM: Johan Sundström; USPHS2: Julie Buring, Nancy Cook; WCWC: Volker Arndt, Dietrich Rothenbacher; WHIOS: JoAnn Manson, Lesley Tinker; WHITE2: Martin Shipley, Adam G Tabak, Mika Kivimaki; WHS: Nancy Cook, Julie Buring; WOSCOPS: Chris Packard, Naveed Sattar, Michele Robertson; ZUTE: Edith Feskens, Marianne Geleijnse, Daan Kromhout
Contributor Information
Emerging Risk Factors Collaboration:
Stephen Kaptoge, Sreenivasa Rao Kondapally Seshasai, Luanluan Sun, Matthew Walker, Thomas Bolton, Sarah Spackman, Feven Ataklte, Peter Willeit, Steven Bell, Steven Burgess, Lisa Pennells, Servet Altay, Gerd Assmann, Yoav Ben-Shlomo, Lyle G Best, Cecilia Björkelund, Dan G Blazer, Hermann Brenner, Eric J Brunner, Gilles R Dagenais, Jackie A Cooper, Cyrus Cooper, Carlos J Crespo, Mary Cushman, Ralph B D’Agostino Sr, Makoto Daimon, Lori B Daniels, Rachel Dankner, Karina W Davidson, Renate T de Jongh, Chiara Donfrancesco, Pierre Ducimetiere, Petra J M Elders, Gunnar Engström, Ian Ford, John Gallacher, Stephan J L Bakker, Uri Goldbourt, Agustin Gómez de la Cámara, Sameline Grimsgaard, Vilmundur Gudnason, Per-Olof Hansson, Hironori Imano, J Wouter Jukema, Christopher Kabrhel, Jussi Kauhanen, Maryam Kavousi, Stefan Kiechl, Matthew W Knuiman, Daan Kromhout, Harlan M Krumholz, Lewis H Kuller, Tiina Laatikainen, Debbie A Lawlor, Haakon E Meyer, Kenneth Mukamal, Paul J Nietert, Toshiharu Ninomiya, Dorothea Nitsch, Børge G Nordestgaard, Luigi Palmieri, Jackie F Price, Paul M Ridker, Qi Sun, Annika Rosengren, Ronan Roussel, Masaru Sakurai, Veikko Salomaa, Ben Schöttker, Jonathan E Shaw, Timo E Strandberg, Johan Sundström, Hanna Tolonen, Aage Tverdal, WM Monique Verschuren, Henry Völzke, Lynne Wagenknecht, Robert B Wallace, S Goya Wannamethee, Nicholas J Wareham, Sylvia Wassertheil-Smoller, Kazumasa Yamagishi, Bu B Yeap, Seamus Harrison, Michael Inouye, Simon Griffin, Adam S Butterworth, Angela M Wood, Simon G Thompson, Naveed Sattar, John Danesh, and Emanuele Di Angelantonio
Data sharing
Data from UK Biobank is available to any bona fide scientific research on application. Data from the Emerging Risk Factors Collaboration is available at the discretion of the principal investigators of the individual studies.
References
- 1.Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378(9785):31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 2.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4o4 million participants. The Lancet. 2016;387(10027):1513–30. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geiss LS, Wang J, Cheng YJ, et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980-2012. JAMA. 2014;312(12):1218–26. doi: 10.1001/jama.2014.11494. [DOI] [PubMed] [Google Scholar]
- 4.International Diabetes Federation. IDF Diabetes Atlas. 10th. International Diabetes Federation; Brussels: [accessed July, 20223]. https://diabetesatlas.orgl . [Google Scholar]
- 5.Emerging Risk Factors Collaboration. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–41. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gregg EW, Zhuo X, Cheng YJ, Albright AL, Narayan KMV, Thompson TJ. Trends in lifetime risk and years of life lost due to diabetes in the USA, 1985-2011: a modelling study. The Lancet Diabetes Endocrinology. 2014;2(11):867–74. doi: 10.1016/S2213-8587(14)70161-5. [DOI] [PubMed] [Google Scholar]
- 7.Tomic D, Morton JI, Chen L, et al. Lifetime risk, life expectancy, and years of life lost to type 2 diabetes in 23 high-income jurisdictions: a multinational, population-based study. The Lancet Diabetes Endocrinology. 2022;10(11):795–803. doi: 10.1016/S2213-8587(22)00252-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pavkov ME, Bennett PH, Knowler WC, Krakoff J, Sievers ML, Nelson RG. Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. JAMA. 2006;296(4):421–6. doi: 10.1001/jama.296.4.421. [DOI] [PubMed] [Google Scholar]
- 9.Barnett KN, Ogston SA, McMurdo ME, Morris AD, Evans JM. A 12-year follow-up study of all-cause and cardiovascular mortality among 10,532 people newly diagnosed with Type 2 diabetes in Tayside, Scotland. Diabet Med. 2010;27(10):1124–9. doi: 10.1111/j.1464-5491.2010.03075.x. [DOI] [PubMed] [Google Scholar]
- 10.Gulliford MC, Charlton J. Is relative mortality of type 2 diabetes mellitus decreasing? Am J Epidemiol. 2009;169(4):455–61. doi: 10.1093/aje/kwn342. [DOI] [PubMed] [Google Scholar]
- 11.Emerging Risk Factors Collaboration. Association of Cardiometabolic Multimorbidity With Mortality. JAMA. 2015;314(1):52–60. doi: 10.1001/jama.2015.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Saeed AH, Constantino MI, Molyneaux L, et al. An Inverse Relationship Between Age of Type 2 Diabetes Onset and Complication Risk and Mortality: The Impact of Youth-Onset Type 2 Diabetes. Diabetes Care. 2016;39(5):823–9. doi: 10.2337/dc15-0991. [DOI] [PubMed] [Google Scholar]
- 13.Brun E, Nelson RG, Bennett PH, et al. Diabetes duration and cause-specific mortality in the Verona Diabetes Study. Diabetes Care. 2000;23(8):1119–23. doi: 10.2337/diacare.23.8.1119. [DOI] [PubMed] [Google Scholar]
- 14.Silbernagel G, Rosinger S, Grammer TB, et al. Duration of type 2 diabetes strongly predicts all-cause and cardiovascular mortality in people referred for coronary angiography. Atherosclerosis. 2012;221(2):551–7. doi: 10.1016/j.atherosclerosis.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Herrington WG, Alegre-Diaz J, Wade R, et al. Effect of diabetes duration and glycaemic control on 14-year cause-specific mortality in Mexican adults: a blood-based prospective cohort study. Lancet Diabetes Endocrinol. 2018;6(6):455–463. doi: 10.1016/S2213-8587(18)30050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wannamethee SG, Shaper AG, Whincup PH, Lennon L, Sattar N. Impact of diabetes on cardiovascular disease risk and all-cause mortality in older men: influence of age at onset, diabetes duration, and established and novel risk factors. Arch Intern Med. 2011;171(5):404–10. doi: 10.1001/archinternmed.2011.2. [DOI] [PubMed] [Google Scholar]
- 17.Huo L, Magliano DJ, Rancière F, et al. Impact of age at diagnosis and duration of type 2 diabetes on mortality in Australia 1997-2011. Diabetologia. 2018;61(5):1055–63. doi: 10.1007/s00125-018-4544-z. [DOI] [PubMed] [Google Scholar]
- 18.Faerch K, Carstensen B, Almdal TP, Jorgensen ME. Improved survival among patients with complicated type 2 diabetes in Denmark: a prospective study (2002-2010) J Clin Endocrinol Metab. 2014;99(4):E642–6. doi: 10.1210/jc.2013-3210. [DOI] [PubMed] [Google Scholar]
- 19.Knuiman MW, Welbom TA, Whittall DE. An Analysis of Excess Mortality Rates for Persons with Non-Insulin-dependent Diabetes Mellitus in Western Australia using the Cox Proportional Hazards Regression Model. American Journal of Epidemiology. 1992;135(6):638–48. doi: 10.1093/oxfordjournals.aje.a116343. [DOI] [PubMed] [Google Scholar]
- 20.Li HY, Jiang YD, Chang CH, Chung CH, Lin BJ, Chuang LM. Mortality trends in patients with diabetes in Taiwan: a nationwide survey in 2000-2009. J Formos Med Assoc. 2012;111(11):645–50. doi: 10.1016/j.jfma.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Holden SH, Barnett AH, Peters JR, et al. The incidence of type 2 diabetes in the United Kingdom from 1991 to 2010. Diabetes Obes Metab. 2013;15(9):844–52. doi: 10.1111/dom.12123. [DOI] [PubMed] [Google Scholar]
- 22.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. Jama. 2003;290(14):1884–90. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 23.Nwaneri C, Bowen-Jones D, Cooper H, Chikkaveerappa K, Afolabi BA. Falling mortality rates in Type 2 diabetes mellitus in the Wirral Peninsula: a longitudinal and retrospective cohort population-based study. Postgrad Med J. 2012;88(1046):679–83. doi: 10.1136/postgradmedj-2012-130877. [DOI] [PubMed] [Google Scholar]
- 24.Huo X, Gao L, Guo L, et al. Risk of non-fatal cardiovascular diseases in early-onset versus late-onset type 2 diabetes in China: a cross-sectional study. Lancet Diabetes Endocrinol. 2016;4(2):115–24. doi: 10.1016/S2213-8587(15)00508-2. [DOI] [PubMed] [Google Scholar]
- 25.Sattar N, Rawshani A, Franzen S, et al. Age at Diagnosis of Type 2 Diabetes Mellitus and Associations With Cardiovascular and Mortality Risks. Circulation. 2019;139(19):2228–37. doi: 10.1161/CIRCULATIONAHA.118.037885. [DOI] [PubMed] [Google Scholar]
- 26.Alva ML, Hoerger TJ, Zhang P, Cheng YJ. State-level diabetes-attributable mortality and years of life lost in the United States. Annals of Epidemiology. 2018;28(11):790–5. doi: 10.1016/j.annepidem.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 27.Tönnies T, Baumert J, Heidemann C, von der Lippe E, Brinks R, Hoyer A. Diabetes free life expectancy and years of life lost associated with type 2 diabetes: projected trends in Germany between 2015 and 2040. Population Health Metrics. 2021;19(1):38. doi: 10.1186/s12963-021-00266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koyama AK, Cheng YJ, Brinks R, et al. Trends in lifetime risk and years of potential life lost from diabetes in the United States, 1997-2018. PLOS ONE. 2022;17(5):e0268805. doi: 10.1371/journal.pone.0268805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leung M-YM, Pollack LM, Colditz GA, Chang S-H. Life Years Lost and Lifetime Health Care Expenditures Associated With Diabetes in the U.S., National Health Interview Survey, 19972000. Diabetes Care. 2014;38(3):460–8. doi: 10.2337/dc14-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emerging Risk Factors Collaboration. The Emerging Risk Factors Collaboration: analysis of individual data on lipid, inflammatory and other markers in over 1.1 million participants in 104 prospective studies of cardiovascular diseases. Eur J Epidemiol. 2007;22(12):839–69. doi: 10.1007/s10654-007-9165-7. [DOI] [PubMed] [Google Scholar]
- 31.Sudlow C, Gallacher J, Allen N, et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLOS Medicine. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–22. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pintilie M. Analysing and interpreting competing risk data. Stat Med. 2007;26(6):1360–7. doi: 10.1002/sim.2655. [DOI] [PubMed] [Google Scholar]
- 34.Freidlin B, Korn EL. Testing treatment effects in the presence of competing risks. Stat Med. 2005;24(11):1703–12. doi: 10.1002/sim.2054. [DOI] [PubMed] [Google Scholar]
- 35.Tai BC, De Stavola BL, de Gruttola V, Gebski V, Machin D. First-event or marginal estimation of cause-specific hazards for analysing correlated multivariate failure-time data? Stat Med. 2008;27(6):922–36. doi: 10.1002/sim.2944. [DOI] [PubMed] [Google Scholar]
- 36.Thompson S, Kaptoge S, White I, Wood A, Perry P, Danesh J. Statistical methods for the time-to-event analysis of individual participant data from multiple epidemiological studies. Int J Epidemiol. 2010;39(5):1345–59. doi: 10.1093/ije/dyq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.VanderWeele TJ. Principles of confounder selection. European Journal of Epidemiology. 2019;34(3):211–9. doi: 10.1007/s10654-019-00494-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention. National Center for Health Statistics Underlying cause of death 1999-2015 on CDC WONDER Online Database (released Dec, 2016) [accessed April, 2023]. https://wonder.cdc.gov/ucd-icd10.html .
- 40.Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. The Lancet. 2002;360(9349):1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 41.Emerging Risk Factors Collaboration. Major Lipids, Apolipoproteins, and Risk of Vascular Disease. JAMA. 2009;302(18):1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hillier TA, Pedula KL. Characteristics of an adult population with newly diagnosed type 2 diabetes: the relation of obesity and age of onset. Diabetes Care. 2001;24(9):1522–7. doi: 10.2337/diacare.24.9.1522. [DOI] [PubMed] [Google Scholar]
- 43.Wright AK, Welsh P, Gill JMR, et al. Age-, sex-and ethnicity-related differences in body weight, blood pressure, HbA1c and lipid levels at the diagnosis of type 2 diabetes relative to people without diabetes. Diabetologia. 2020;63(8):1542–53. doi: 10.1007/s00125-020-05169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinarsson AO, Rawshani A, Gudbjörnsdottir S, Franzén S, Svensson A-M, Sattar N. Short-term progression of cardiometabolic risk factors in relation to age at type 2 diabetes diagnosis: a longitudinal observational study of 100,606 individuals from the Swedish National Diabetes Register. Diabetologia. 2018;61(3):599–606. doi: 10.1007/s00125-017-4532-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zoungas S, Woodward M, Li Q, et al. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia. 2014;57(12):2465–74. doi: 10.1007/s00125-014-3369-7. [DOI] [PubMed] [Google Scholar]
- 46.Zoungas S, Arima H, Gerstein HC, et al. Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta-analysis of individual participant data from randomised controlled trials. The Lancet Diabetes Endocrinology. 2017;5(6):431–7. doi: 10.1016/S2213-8587(17)30104-3. [DOI] [PubMed] [Google Scholar]
- 47.Turnbull FM, Abraira C, Anderson RJ, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;52(11):2288–98. doi: 10.1007/s00125-009-1470-0. [DOI] [PubMed] [Google Scholar]
- 48.Simmons RK, Griffin SJ, Lauritzen T, Sandbæk A. Effect of screening for type 2 diabetes on risk of cardiovascular disease and mortality: a controlled trial among 139,075 individuals diagnosed with diabetes in Denmark between 2001 and 2009. Diabetologia. 2017;60(11):2192–9. doi: 10.1007/s00125-017-4299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Porta M, Curletto G, Cipullo D, et al. Estimating the delay between onset and diagnosis of type 2 diabetes from the time course of retinopathy prevalence. Diabetes Care. 2014;37(6):1668–74. doi: 10.2337/dc13-2101. [DOI] [PubMed] [Google Scholar]
- 50.Thomas NJ, Jones SE, Weedon MN, Shields BM, Oram RA, Hattersley AT. Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified survival analysis from UK Biobank. The Lancet Diabetes Endocrinology. 2018;6(2):122–9. doi: 10.1016/S2213-8587(17)30362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mansournia MA, Nazemipour M, Etminan M. A practical guide to handling competing events in etiologic time-to-event studies. Global Epidemiology. 2022;4:100080. doi: 10.1016/j.gloepi.2022.100080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hernán MA. The Hazards of Hazard Ratios. Epidemiology. 2010;21(1) doi: 10.1097/EDE.0b013e3181c1ea43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from UK Biobank is available to any bona fide scientific research on application. Data from the Emerging Risk Factors Collaboration is available at the discretion of the principal investigators of the individual studies.