Abstract
Primary aldosteronism is the most common single cause of hypertension and is potentially curable when only one adrenal gland is the culprit. The importance of primary aldosteronism to public health derives from its high prevalence but huge under-diagnosis (estimated to be <1% of all affected individuals), despite the consequences of poor blood pressure control by conventional therapy and enhanced cardiovascular risk. This state of affairs is attributable to the fact that the tools used for diagnosis or treatment are still those that originated in the 1970–1990s. Conversely, molecular discoveries have transformed our understanding of adrenal physiology and pathology. Many molecules and processes associated with constant adrenocortical renewal and interzonal metamorphosis also feature in aldosterone-producing adenomas and aldosterone-producing micronodules. The adrenal gland has one of the most significant rates of non-silent somatic mutations, with frequent selection of those driving autonomous aldosterone production, and distinct clinical presentations and outcomes for most genotypes. The disappearance of aldosterone synthesis and cells from most of the adult human zona glomerulosa is the likely driver of the mutational success that causes aldosterone-producing adenomas, but insights into the pathways that lead to constitutive aldosterone production and cell survival may open up opportunities for novel therapies.
Introduction
Within a year of the discovery of aldosterone in 1953, a description of the first patient with primary aldosteronism (PA) signalled a dual trajectory for clinical and research interest in the hormone1,2. Over the next 70 years, many of its intertwined physiological and pathological roles have become clear (Fig. 1). For much of this period, the clinical syndrome of PA has held the status of an endocrine curiosity. Perhaps because of the dramatic clinical features of the index case described by Jerome Conn (sustained, severe hypertension, marked hypokalaemia and an adrenal tumour large enough to be seen by adrenal venography)2, PA was for many decades after Conn’s death considered a rare, relatively benign form of hypertension only to be suspected if the patient was profoundly hypokalaemic3–7. However, the characterization of PA as a rare, benign disease with a requirement for hypokalaemia is now acknowledged to be incorrect.
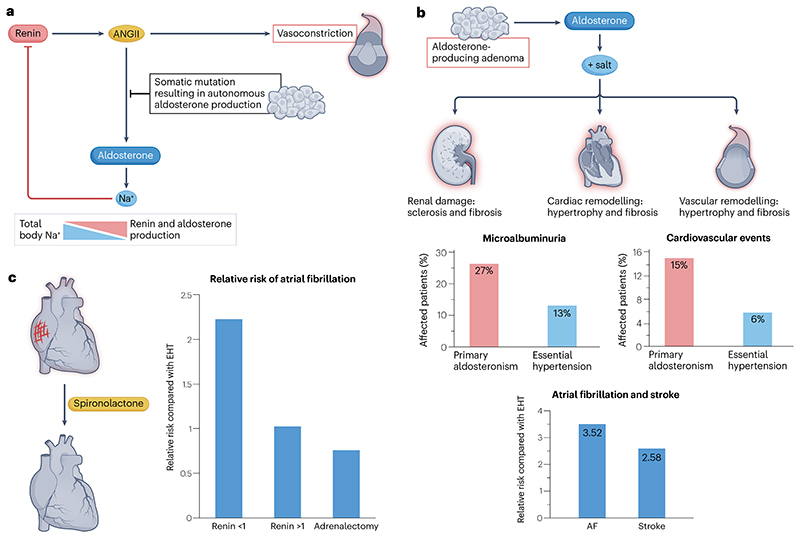
Fig. 1. The cause, impact, and reversal with treatment, of sporadic primary aldosteronism.
a, In sporadic primary aldosteronism, somatic mutations result in autonomous aldosterone production, which leads to excess Na+retention by the kidneys. These in turn detect a high Na+flux through the Na+-K+−2Cl- transporter in juxtaglomerular macula densa cells, which signal to the adjacent renin-producing cells of the afferent arteriole to switch off renin secretion. The net consequence is a sustained elevation of plasma aldosterone and suppression of plasma renin and angiotensin II (ANGII). b, Autonomous aldosterone production in the presence of salt is associated with continuous renal Na+ reabsorption despite the suppression of ANGII and renin. Such inappropriate sodium retention leads to renal damage, cardiac remodelling, and vascular remodelling10,12. Patients with primary aldosteronism are at a higher risk than patients with essential hypertension of kidney and cardiovascular damage, detected as events such as atrial fibrillation and stroke. Relative risk data for atrial fibrillation and stroke are taken from Monticone et al.12. c, The effects of aldosterone can be reversed with the use of competitive mineralocorticoid receptor antagonists (MRAs), such as spironolactone, which inhibit the reabsorption of Na+ in the distal convoluted tubule and collecting duct. The inhibition of Na+ retention can be detected as a rise in plasma renin. In patients whose renin is adequately de-suppressed with use of MRAs, tissue damage by aldosterone and salt is prevented or reversed, attenuating the increased risks of cardiovascular and renal events11,191. Relative risk data for atrial fibrillation following MRA treatment or adrenalectomy are taken from Hundemer et al.191. AT1R, ANGII type 1 receptor. Graphs showing the percentage of patients affected by microalbuminuria and cardiovascular events in part b are adapted with permission from ref. 10, Elsevier.
Over the past decade, clinical and molecular studies have catapulted PA onto the centre stage of cardiometabolic research. First, several rigorous clinical studies that used plasma aldosterone-to-renin ratio (ARR), rather than absolute thresholds of plasma aldosterone itself, indicated prevalence rates for PA of 5–11% in unselected hypertensive populations – a range that is uncannily similar to Conn’s prediction of 10%8–10. On top of these prevalence figures, the observation that the cardiometabolic consequences of PA (including stroke, atrial fibrillation, renal impairment and ischaemic heart disease) exceeded that of essential hypertension by a ratio of at least 2:1, provides an impetus to advance screening strategies and targeted therapies for PA11,12. We and others have estimated that fewer than 1% of cases of PA are detected and treated13,14; this underdiagnosis combined with the excess morbidity attributed to PA makes it difficult to exaggerate the public health consequences of this shortfall in clinical care.
Through molecular studies, it became clear that a substantial proportion of unilateral aldosterone-producing adenomas (APAs), which account for 30–50% of all cases of PA, harbour a gain-of-function somatic mutation15–17. The landmark report in 2011 of mutations in the K+-channel gene, KCNJ5 (ref. 18), heralded a rich vein of molecular research. Recurrent somatic mutations in several additional genes were soon discovered, along with a larger number of upregulated genes in APAs or in the aldosterone-producing cells of the adrenal zona glomerulosa (ZG) from which APAs were presumed to arise. Understanding of the molecular signature of most APAs was enhanced by the development of selective and specific antisera for the steroidogenic enzymes19, and through the discovery of gene expression patterns that were unique to subsets of APAs20–23. These genotype–phenotype studies, combined with the finding that APA size correlates inversely with the expression of CYP11B2 (which encodes aldosterone synthase)24, led to the realization that the ‘classic Conn’s tumour’ manifestation of PA is the exception rather than the rule. This realization was reinforced by the paradox that the cellular appearance of these ‘classic’ APAs resembles the cortisol-producing zona fasciculata (ZF) rather than ZG17,18,21,25.
These discoveries herald a new era in PA research. The clinical studies highlight the public health challenge posed by PA – that is, how to recognize the >99% of patients who currently elude diagnosis. The molecular studies suggest a potential means with which to meet this challenge. Clinically, little has changed in the 30, 40 and 50 years since the advent of laparoscopic surgery, CT scans and adrenal vein sampling (AVS), respectively26–29 (Fig. 2). The entire adrenal gland is still removed for the sake of a 1-cm benign lesion. The contrast of this approach with that of another such lesion – the peptic ulcer – could hardly be more striking, where over a similar period of 50 years, curative therapy progressed from gastrectomy to a week of drug therapy. If science can identify the ‘helicobacter’ of PA, we can surely look for its ‘omeprazole’ as a medical cure.
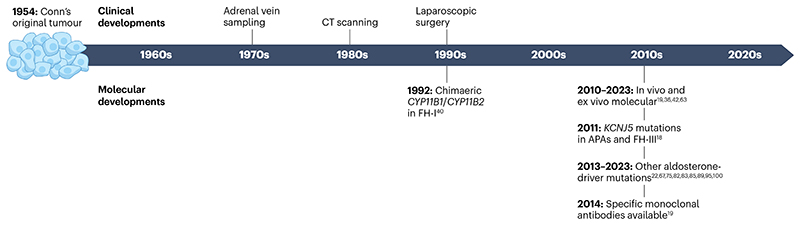
Fig. 2. Timeline of clinical and molecular advances in primary aldosteronism.
Seventy years after the first description of Conn’s syndrome, now known as primary aldosteronism, standard clinical practice for its management remains the removal of the entire adrenal, regardless of lesion size. Standard techniques include adrenal vein sampling, CT scanning and laparoscopic surgery. However, a number of key molecular scientific discoveries in the last 30 years have provided insights into the molecular pathogenesis of primary aldosteronism, and may identify new therapeutic approaches18,19,22,36,40,42,63,67,75,81–83,85,89,95,100. APA, aldosterone-producing adenoma; FH, familial hyperaldosteronism
In this Review, we focus on molecular discoveries and developments that have the potential to transform the clinical management of patients with PA. We describe emerging innovations in diagnosis and therapy that may – in the future – be tailored to permit optimal management of each patient. We appreciate that many of the innovations and discoveries have been made in patients undergoing investigation or treatment for unilateral PA, who are a fraction of the total and are furthest in line from primary care, where the main bottleneck of diagnosing PA resides. However, interest in them is justified partly because they are the patients in whom links between molecular pathogenesis and outcomes are most readily studied, and partly because the current arduous course of diagnosing and managing their disease discourages physicians from seeking such patients14. We culminate in a hypothesis that draws on public health and molecular medicine to bridge the physiological and pathological links between Na+ and aldosterone by suggesting that PA arises – at least in part – as a maladaptive response to the chronic over-ingestion of salt.
Defining primary aldosteronism
PA is the most common cause of endocrine arterial hypertension and is characterized by the autonomous production of aldosterone from one or both adrenal glands. The autonomy of this disorder contrasts with normal physiological processes, and with secondary aldosteronism, in which aldosterone secretion is regulated largely by angiotensin II (ANGII). As the production of ANGII is dependent on the secretion of the enzyme renin, the practical definition of PA refers to the ratio of aldosterone and renin measured in blood30. Like many other medical conditions defined by a threshold value within a quantitative continuum, the application of this definition is straightforward only for the minority of patients who reside near the extreme end of the continuum. When the continuum is a ratio, in this case the ARR, in which the culprit hormone (aldosterone) is the arithmetically distributed numerator, and the denominator a log-normally distributed sodium sensor (renin), the problems of diagnosis are multiplied: almost any measurable value of aldosterone in the presence of an undetectable plasma renin creates an ARR that is apparently diagnostic of PA. In practice, however, ARR frequently fails as a standalone measure of PA, and the autonomy of aldosterone secretion needs to be confirmed by showing that aldosterone is not suppressed (below another threshold value) by administration of salt or captopril29.
The pathogenesis of hypertension in PA can be ascribed to a combination of inappropriate sodium retention by epithelial cells of the kidneys (and consequent extracellular fluid volume expansion), together with the vasoconstricting actions of aldosterone31. The word ‘inappropriate’ is important; high levels of aldosterone are homeostatically logical in states of sodium depletion, but in the context of sodium excess can cause tissue injury, especially in the heart and kidney, as has been reviewed elsewhere32. In addition to ANGII, other determinants of aldosterone secretion under physiological conditions are extracellular potassium and adrenocorticotrophic hormone (ACTH)33.
The definition of PA as outlined by the Endocrine Society emphasizes the inappropriateness of aldosterone production for total body sodium status and for the prevailing levels of ANGII and blood potassium29. In practice it is not easy or routine to measure total body sodium status or ANGII concentrations, and blood potassium concentrations are not always a reliable indicator of total body potassium levels. Indeed, the measurement of aldosterone itself is also not without controversy, and much higher prevalence figures for PA might be obtained if 24-h urine measurements were routinely used to avoid diurnal fluctuations in secretion34. However, the definition is pertinent to our hypothesis that the pathogenesis and frequency of PA relates – at least in part – to a maladaptive response to high sodium intake.
Prior to the availability of molecular and genetic insights, PA was believed to result from either a single, unilateral, benign APA or a bilateral idiopathic adrenal hyperplasia (IAH). Clinical subtyping investigations, cross-sectional imaging and AVS, were used in an attempt to distinguish these two pathological conditions, with a view to applying laparoscopic surgery for the treatment of APA and medical management for IAH (as reviewed elsewhere35). However, in reality, no clinical pathological criteria define either APA or IAH. The standard, historical approach to the diagnosis of APA was to perform classic haematoxylin and eosin (H&E) staining of the resected adrenal gland to detect the presence of one or more nodules (thereby raising suspicion of an APA) and then to identify the predominant cell type. Some nodules were reported to have small, compact cells; some to have large, foamy, lipid-rich cells; and some to have a mixture of the two. The development of highly specific antibodies to the enzymes responsible for the final step of cortisol and aldosterone synthesis (11-β hydroxylase and aldosterone synthase, encoded by CYP11B1 and CYP11B2, respectively) facilitated a link between descriptive pathology and functional activity, and uncovered a spectrum of unilateral PA manifestation that included unilateral micro- or macronodular hyperplasia19,36,37. In patients with bilateral PA, in whom conventional pathology reported gross hyperplasia, immunohistochemistry showed mainly focal rather than diffuse expression of aldosterone synthase38. However, a decade on, only a few hospitals perform routine CYP11B2 immunohistochemistry of adrenal glands removed from patients with PA.
Germline mutations
PA can be categorized on the basis of its heritability. The vast majority of cases of PA are sporadic but, rarely, the condition affects multiple members of the same family and is termed familial hyperaldosteronism (FH). Various forms of FH exist, distinguished by their clinical features and reported genetic causes. The characteristic early onset and heritability of FH makes it an attractive target for understanding the more common sporadic PA; conversely, large-scale next-generation sequencing studies of sporadic cases has greatly informed the FH field. All of the Mendelian causes of PA are rare, and specialists within different regions often find that different members of a handful of families are referred to them. Although familial cases represent a tiny proportion of PA, investigations of these patients have shown that PA can be caused by mutations that induce constitutive aldosterone production (Table 1).
Table 1. Mutations associated with primary aldosteronism.
| Gene | Occurrence | Mechanism of action | Refs. |
|---|---|---|---|
| ATP1A1 | Somatic in APAs | Impaired pump function and pathologically channel like-permeabilities for Na+, H+ and Ca2+ | 22,67 |
| ATP2B3 | Somatic in APAs | Channel like-permeabilities for Na+, H+ and Ca2+ | 67 |
| CACNA1D | Germline in PASNA and somatic in APAs | Increases Ca2+ permeability | 22,75 |
| CACNA1H | Germline in FH-IV and somatic in APAs | Increases Ca2+ permeability | 81,82 |
| CADM1 | Somatic in APAs | Inhibition of gap junctions and modulation of biological rhythms | 95 |
| CLCN2 | Germline in FH-II and somatic in APAs | Increases Cl- efflux | 85 |
| CTNNB1 | Somatic in APAs and adrenocortical carcinomas | Prevents β-catenin degradation, which increases signalling via the TCF/LEF family | 190 |
| CYP11B1/2 hybrid gene | Germline in FH-I | Pathologically activated by ACTH via MC2R and cAMP signalling | 40 |
| GNA11 and GNAQ | Somatic in APAs | Prevents termination of G protein signalling downstream of the AT1R, leading to increased calcium release from intracellular stores Co-occurs with CTNNB1 mutations | 89 |
| KCNJ5 | Germline in FH-III and somatic in APAs | Abnormal Na+ influx | 18 |
| SLC30A1 | Somatic in APAs | Increases Zn2+ permeability | 100 |
Many of the mutations cause membrane depolarization, activation of voltage-gated calcium channels, calcium influx and increased calcium signalling, which stimulates CYP11B2 expression and aldosterone production. ACTH, adrenocorticotropic hormone; APAs, aldosterone-producing adenomas; AT1R, angiotensin II type 1 receptor; FH, familial hyperaldosteronism; MC2R, melanocortin 2 receptor; PASNA, primary aldosteronism with seizures and neurological abnormalities; TCF/LEF, T cell factor/lymphoid enhancer factor.
Somatic mutations
In contrast to germline mutations, which are rare and unlikely to be seen by most hypertension specialists, somatic mutations that cause PA are among the most common recurrent mutations seen in medicine. Indeed, a systematic analysis of non-silent somatic mutation rates across organs found a significant excess in the adrenal gland, similar to that of skin39. Most of these mutations are likely to be non-functional – for example, representing products of DNA damage by reactive oxygen species generated during incomplete mitochondrial steroid oxidations. However, in some instances, gain-of-function mutations that drive constitutive aldosterone production might be beneficial to the mutated cells, and thus increase their frequency through the evolutionary selection of such mutations. In the following sections we describe some of the key findings of recurrent somatic mutations that have been linked to PA (Box 1).
KCNJ5
The field of molecular genetics in PA started with Lifton’s discovery of the CYP11B1/CYP11B2 chimaeric gene in families with GRA40 (Table 1); however, this finding did not impact our understanding of sporadic PA until his landmark identification, 19 years later, of somatic mutations in KCNJ5 (ref. 18). These mutations were found by whole exome sequencing of four relatively large APAs, each containing a Gly151Arg or Leu168Arg mutation. These findings were replicated and, in all, 8 out of 22 APAs were found to have one of the mutations18. KCNJ5 encodes the Kir3.4 potassium channel and the two mutations both affect residues at or near its K+-selectivity channel (Fig. 3). An important link between Lifton’s 1992 and 2011 discoveries are his report, published shortly before KCNJ5 identification, of a family with Mendelian ‘non-glucocorticoid remediable aldosteronism’41. A germline mutation (Thr158Ala) in KCNJ5 was subsequently found in the father and two daughters, whose huge adrenals with bilateral hyperplasia required surgical removal to control severe hypertension. A feature of this family was that not only aldosterone but also cortisol was unsuppressed by dexamethasone, despite the absence of clinical features of hypercortisolism. In common with GRA families, the sisters demonstrated high excretion of the hybrid steroids, 18-oxocortisol and 18-hydroxycortisol. Subsequent investigations of patients with sporadic PA have similarly revealed that a subset demonstrates incomplete suppression of serum cortisol in response to dexamethasone pre-adrenalectomy, and that the APAs in this subset of patients have a KCNJ5 mutation21,41–44.
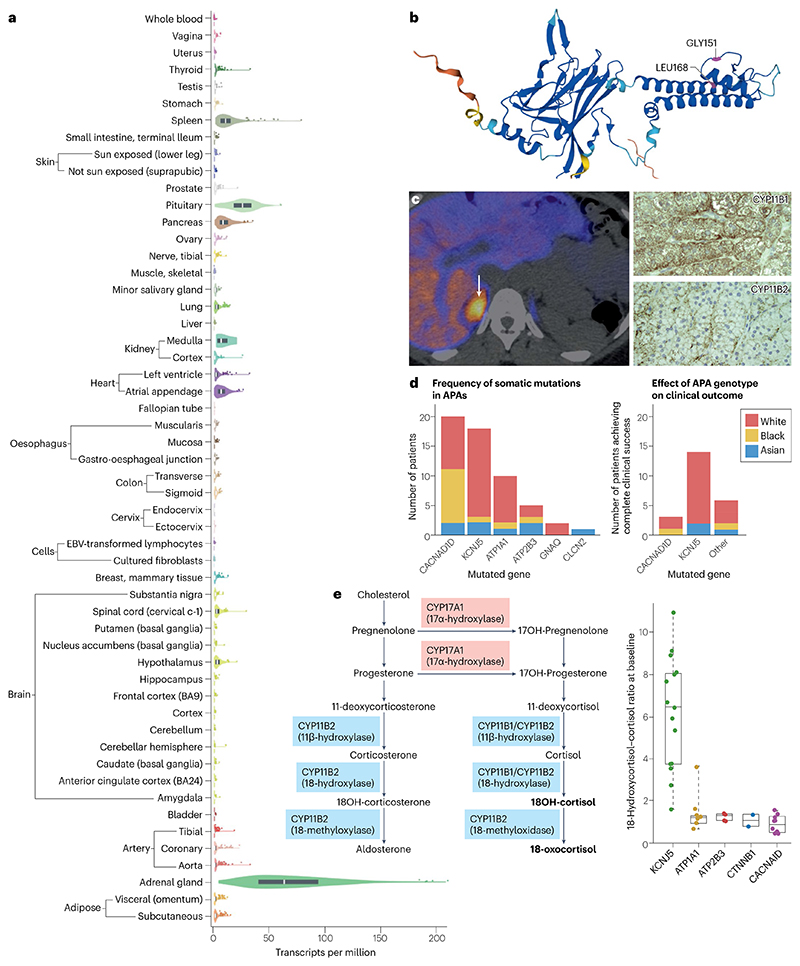
Fig. 3. The contribution of KCNJ5 mutations to the pathogenesis of primary aldosteronism.
a Search for KCNJ5 (ENSG00000120457.11) in the GTEx portal shows that the adrenal gland expresses the highest levels of this gene. Data are shown as transcripts per million, calculated from a gene model with isoforms collapsed to a single gene. No other normalization steps have been applied. Box plots are shown as median and 25th and 75th percentiles; points are displayed as outliers if they are above or below 1.5 times the interquartile range140. b, Structural analysis of the potassium channel Kir 3.4, which is encoded by KCNJ5, shows the originally described mutations (Gly151Arg and Leu168Arg) within or close to the selectivity pore of the channel18. c, A typical 11C-metomidate PET-CT scan in a patient with a KCNJ5-mutant APA (arrow). Immunohistochemistry of tissue sections from mutant APAs showed them to more closely resemble cortisol-producing (zona fasciculata; ZF) than aldosterone-producing (zona glomerulosa; ZG) adrenal cells, with more striking staining for CYP11B1 than CYP11B2. d, Data from the MATCH study indicate that KCNJ5 mutations are the most common mutations found in APAs from self-identified white individuals63. Data from MATCH also indicate that APA genotype is indicative of complete clinical success following adrenalectomy, with removal of KCNJ5-mutated APAs most likely to result in complete clinical success. The colours represent self-reported ethnicity. e, The presence of ZF- and ZG-specific enzymes (CYP17A1 and CYP11B2, respectively) in the same cell of a KCNJ5-mutant APA indicates that such cells can synthesize the ‘hybrid’ steroids 18-oxocortisol and 18-hydroxy-cortisol (18-OH-cortisol) that a normal ZG or ZF cell alone would not be able to produce. Data from the MATCH study have demonstrated the potential diagnostic utility of hybrid steroids as a metabolic marker of a KCNJ5-mutated APA63. Part a reproduced from ref. 192, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). Part b reproduced from ref. 193, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). Parts d and e adapted from ref. 63, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/).
The Kir 3.4 potassium channel is highly expressed in aldosterone-producing cells of the ZG and contributes to hyperpolarization of the ZG cell membrane45. Indeed, had transcriptome data been as widely available in 2011 as it is now, KCNJ5 might have been an obvious candidate gene to test for mutations, given its strikingly high and selective expression in the human adrenal gland (Fig. 3). Its absence from the adrenal glands of mice, in which models of PA had centred on the potassium leak channels KCNK3 and KCNK9, are an illustration of why human datasets have largely proven more valuable than rodent studies for clues to the pathogenesis of hypertension46. The occasional patients with germline mutations or mosaicism of KCNJ5 highlight the potential impact of non-adrenal mutant KCNJ5 expression, particularly in the heart and pituitary gland, where even low-level expression could contribute to arrhythmias and reduced dexamethasone suppression of cortisol, respectively41,47. Cells transfected in vitro with the Gly151Arg or Leu168Arg Kir 3.4 mutants demonstrated loss of selectivity for potassium, increased permeability to sodium and ZG membrane depolarization18. The resultant influx of calcium activates aldosterone synthesis and secretion. Further KCNJ5 mutations have subsequently been documented within or near to the potassium selectivity filter (between Glu145 and Leu168), with very few exceptions, but the originally described mutations account for the vast majority of mutant KCNJ5 (refs. 18,48–53).
A set of somatic KCNJ5 mutations, which also occasionally appear in the germline – for example, Thr158Ala, Gly151Arg and Glu145Gln – cause a florid Mendelian syndrome with macroscopic adrenal hyperplasia, early-onset hypertension and hypokalaemia18,54,55. By contrast, mutations that are unique to the germline (for example, Gly151Glu and Tyr152Cys) cause a milder clinical phenotype that responds readily to treatment with mineralocorticoid antagonists54,56,57. Functional studies suggest that these germline-only variants cause more profound sodium permeability than other variants, which may result in cell death and hence prevent the development of hyperplasia54.
Genotype-phenotype subtyping of KCNJ5 mutant APAs
As the first gene found in sporadic PA, KCNJ5 mutant APAs became the most extensively characterized genotype15,58. The estimated prevalence of KCNJ5 mutations in resected APAs is 43%; however, the reported range is broad (12–80%), with higher frequencies reported in Japan and China than in European countries, and a lower frequency in one study of African Americans58–60. Whether these reported variations reflect true differences in prevalence or selection bias remains unclear. Somatic KCNJ5 mutations in APAs are more frequent in women than in men, except possibly in Asian cohorts, and present at a younger age than other APA genotypes58,59,61,62 (Fig. 3). In our own analysis, we identified KCNJ5 mutations in 43% of APAs, and we found a strong genotype–phenotype pattern, not only in age and gender but also in several aspects of pathology and gene expression21. On H&E-stained sections, the cells resembled those of adrenal ZF rather than ZG, and on both quantitative (q)PCR and semi-quantitative immunohistochemistry, we observed higher expression of CYP11B1 than CYP11B2, and high levels of CYP17A1, which encodes the obligatory enzyme in cortisol synthesis, 17-α-hydroxylase. The overall picture led us to describe the KCNJ5-mutant APAs as ZF-like, and most of the non-KCNJ5-mutant APAs as ZG-like21. In retrospect, it seems remarkable that the ‘classic Conn’s tumour’, which is typically 1.5–2.5 cm in diameter, is composed of cells that resemble the lipid-rich, cortisol-secreting cells of ZF, rather than the compact cells of ZG. We now realize that such a ZF phenotype is uncommon except in KCNJ5 mutant APAs21,63. KCNJ5-wild type APAs most commonly have compact cells which, except for their rounded nuclei, resemble ZG cells. The ZG-type of APA is often small (0.5–1.5 cm in diameter, and hence commonly missed on CT), but stains more densely for aldosterone synthase than the KCNJ5-mutant, ZF-like APAs. KCNJ5-mutant APAs also have a lower density of KCNJ5 itself than ZG-like APAs; this finding may sound paradoxical, but it is consistent with the lower expression of KCNJ5 in the ZF than the ZG and the overall ZF-like phenotype of KCNJ5-mutant APAs.
We initially hypothesized that the phenotype of KCNJ5-mutant APAs pointed to an origin in the outer ZF rather than the ZG, and that such mutations in a ZG cell with abundant KCNJ5 would mimic the outcome of HEK293T cells transfected with Kir 3.4 containing the electrophysiologically strongest (Gly151Glu) mutation; that is, rapid cell death by Ca2+ overload54. However, extensive transcriptome analyses have revealed more similarities than differences between ZF-type and ZG-type APAs22,64. The likely inference from these findings is that the ZF-like cells in KCNJ5-mutant APAs reflect the consequence of, rather than origin of the mutation, and that the mutation of KCNJ5 in a ZG cell recapitulates the changes that occur spontaneously during ZG cell transition or migration into the ZF64,65. Primary ZG cells are difficult to transfect, but in one experiment we found that transfection of mutant KCNJ5 switched off the gene NEFM, which encodes neurofilament M – a key marker of ZG cells, supporting the notion that the ZF-like phenotype of KCNJ5 mutant APAs is a consequence of its mutation, as opposed to the mutation originally arising in ZF cells66.
ATP1A1 and ATP2B3
The identification of a KCNJ5 genotype–phenotype correlation prompted a search for further mutations in archived APA tissue. Comparisons of whole exome sequencing data from APAs without the KCNJ5 mutation and/or with ZG morphology, with adjacent normal adrenal tissue, led to the discovery of somatic mutations in ATP1A1 and ATP2B3 (encoding the α-1 subunits of Na+/K+-ATPase and Ca2+-ATPase3, respectively)22,67 (Fig. 4). Mutations in these two genes account for 5–20% of all APAs and are more common in white people than in other populations16,17.
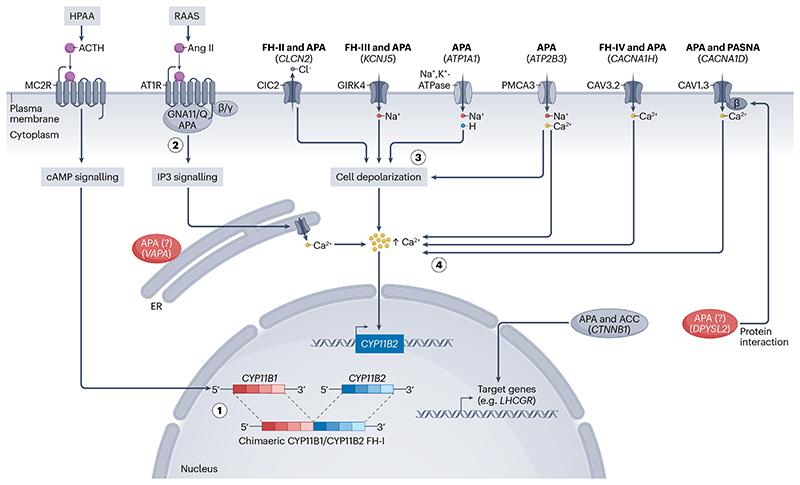
Fig. 4. Germline and somatic mutations associated with primary aldosteronism.
Pathological autonomous aldosterone production in a cell can occur by a number of different mechanisms. (1) In familial hyperaldosteronism (FH)-I, the CYP11B1/CYP11B2 hybrid gene is activated by cAMP signalling, which occurs when adrenocorticotropic hormone (ACTH) binds to the melanocortin 2 receptor (MC2R) in response to hypothalamic–pituitary–adrenal axis (HPAA) activation. (2) GNA11 and GNAQ mutations in aldosterone-producing adenomas (APAs) prevent termination of G protein signalling downstream of the renin–angiotensin–aldosterone system, which is activated when angiotensin II (ANGII) binds to the angiotensin 1 receptor (AT1R)), leading to increased calcium release from intracellular stores via the inositol trisphosphate (IP3) signalling pathway. These mutations co-occur with CTNNB1 mutations, which are found in APAs and adrenocarcinomas, and prevent β-catenin degradation, leading to increased transcription of target genes such as LHCGR. (3) CLCN2 mutations in FH-II and APAs, KCNJ5 mutations in FH-III and APAs, and ATP1A1 and ATP2B3 mutations in APAs lead to abnormal permeabilities for Cl- Na+, H+, and Ca2+. These abnormalities cause cell depolarization, which increases intracellular calcium concentrations, thereby stimulating CYP11B2 expression and aldosterone production. Acidification and an impaired pump function may also have a pathological role (not shown). (4) CACNA1H mutations in FH-IV and APAs and CACNA1D in primary aldosteronism with seizures and neurological abnormalities (PASNA) and APAs increase calcium permeability by stimulating the calcium signalling pathway directly. High-probability pathogenic somatic mutations have also been found in DPYSL2 and VAPA. DPYSL2 is highly abundant in the zona glomerulosa and has previously been linked to ion-channel trafficking. Based on sequence prediction, we hypothesize that it may bind to the β-subunit binding site of Cav1.3 (refs. 63,171). VAPA is also linked to protein trafficking and is thought to be expressed on the endoplasmic reticulum where IP3 signalling acts on the calcium channel to release calcium from intracellular stores.
The ATP1A1-encoded Na+/K+-ATPase is a member of the P-type ATPase family. The catalysed exchange of three cytoplasmic sodium ions for two potassium ions by this ATPase generates electrochemical gradients that maintain resting membrane potential and cellular excitability. The α-subunit consists of ten transmembrane domains (M1–M10) with intracellular N- and C-tails. Various point mutations and deletions in ATPA1A have been described, most involving disruption of a leucine at position 104, or a glutamic acid at position 334 – regions that juxtapose to form the key area for binding of sodium and potassium. It was initially thought that alterations in the affinity of the pump for sodium and potassium resulted in a loss of function leading to abnormal depolarization, opening of voltage-gated calcium channels and augmented aldosterone synthesis following increases in intracellular calcium concentrations67. However, ATP1A1 mutations have now been demonstrated to increase aldosterone production through a variety of mechanisms including a gain of function22. This suggests that some genes need to achieve both gain and loss of function in order to overcome pathways that inhibit aldosterone production68,69. To date, no germline mutations in ATP1A1 have been reported to cause PA, although rare cases have been described of germline ATP1A1 mutations causing magnesium wasting and abnormalities of the central nervous system (for example, spastic paraplegia or the Charcot–Marie–Tooth-like disorder)70,71.
The ATP2B3-encoded plasma membrane calcium ATPase (also known as PMCA3) belongs to the same family as the Na+/K+-ATPase and is similarly composed of ten transmembrane segments. PMCA3 removes one cytosolic Ca2+ in exchange for two H+ and has a key role in the maintenance of intracellular calcium homeostasis. Several mutations in ATP2B3 have been described: all involve deletions of a least 2 amino acid residues in an area that is essential for calcium ion binding within the M4 domain16,17,60,67,72. The deletions result in membrane depolarization; abnormally increased permeability to cations, notably sodium and calcium; opening of voltage-gated calcium channels; and augmented CYP11B2 activity73. Germline mutations in ATP2B3 cause X-linked congenital cerebellar ataxia, but not in association with PA74. The widespread, fundamental role of ATPases makes them an unattractive target for pharmacological modification in the context of PA.
CACNA1D
Whole-exome sequencing of ZG-like and/or KCNJ5 wild type APAs also led to the identification of gain-of-function somatic mutations in CACNA1D, which encodes the voltage-gated L-type Ca2+ channel, Cav1.3 (refs. 22,75). This channel consists of repeat domains, numbered I–IV, each of which has six transmembrane segments (S1–S6) and a membrane-associated loop between the S5 and S6 segments76. Of importance to channel activation are segments S4–S6, as S4 is involved in voltage sensing, whereas S5, S6 and the membrane-associated loop form the pore lining77. More than 70 mutations have been described in CACNA1D so far, and their overall reported prevalence in APAs in most reports has been around 9%38,78 (Fig. 5). However, our own experience, using PET CT to visualize smaller APAs, showed 9% to be a marked under-estimate, and below we discuss how CACNA1D mutations pre-dominate among somatic mutations found in patients with PA and non-adenomatous ZG.
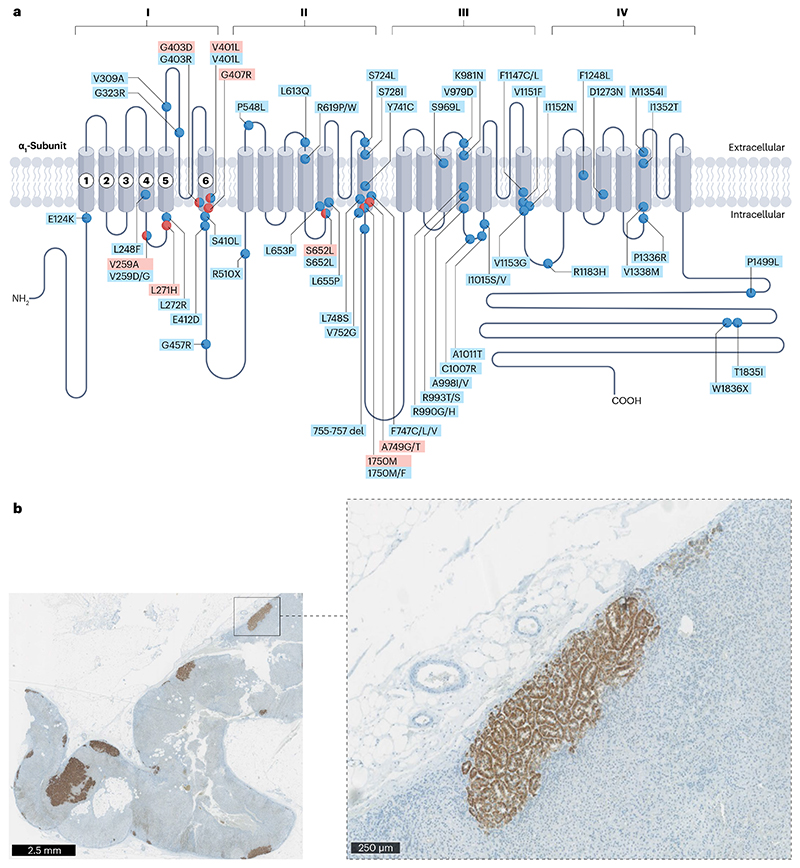
Fig. 5. The plethora of CACNA1D mutations associated with aldosterone-producing adenomas and aldosterone-producing micronodules.
a, CACNA1D, which encodes the pore-forming α1 subunit of a voltage-gated calcium channel (Cav1.3), is the most commonly mutated gene in aldosterone-producing adenomas (APAs) and aldosterone-producing micronodules (APMs) from patients with primary aldosteronism who self-identify as Black or who have bilateral adrenal hyperplasia. The Cav1.3 α1-subunit comprises four homologous repeats (I–IV), each comprising six transmembrane segments (S1–S6). Germline mutations (red circles) and somatic mutations (blue circles) have been identified throughout regions I–IV. More than 70 mutations have been described, mostly in small APAs or APMs194. The majority of mutations in CACNA1D with evidence of pathological function are in conserved sites within functional domains, such as the voltage-sensing domain, the channel-activation gate and the cytoplasmic S4-S5 linker, which couples the voltage-sensing domain to the pore. b, The identification of APMs (and small zona glomerulosa (ZG)-like APAs) was facilitated by the development of a specific monoclonal antibody for CYP11B2, which discriminated it from the highly abundant and homologous CYP11B1 (ref. 19). These APMs and small ZG-like APAs commonly harbour a CACNA1D somatic mutation22,107.
The majority of mutations in CACNA1D occur in segments S4–S6, where they affect the function of the voltage-sensing domain, the channel activation gate and coupling of the voltage-sensing domain to the pore75. Patch clamping of cells transfected with mutant Cav1.3 channels show that calcium influx is increased by various mechanisms, including a shift in voltage-dependent activation towards more negative voltages, a deceleration in channel inactivation and/or an increase in current through a higher open channel probability. Two of the originally identified mutations (that is, p.Gly403Arg and p.Ile770Met) were simultaneously reported in paediatric patients presenting with an extreme phenotype of PA, seizures and neurological abnormalities75. In both of these patients, antagonism of the calcium channels resulted in remission of hypertension and PA79.
Rare recurrent aldosterone-driver mutations
CACNA1H and CLCN2
A small subset of unilateral APAs have been reported to harbour recurrent somatic mutations in CACNA1H (Ile1430Thr, Val1937Met)80,81, in the same region as the germline mutations that cause FH type IV (CACNA1H Met1549Val and Met1549Ile82; Table 1). CACNA1H encodes the pore-forming α1H subunit of the voltage-dependent T-type calcium channel Cav3.2, which, similar to CACNA1D, is expressed in the adrenal ZG82. Also like CACNA1D, mutations in CACNA1H induce calcium influx, which increases CYP11B2 mRNA levels and thus enhances aldosterone secretion. Yet, the prevalence of somatic CACNA1H mutations in APAs seems to be low, more similar to that of ATP2B3 mutations81. However, most studies have only sequenced selected exons or hotspot regions of CACNA1H owing to its large size, and thus may have not thoroughly investigated the prevalence of pathogenic somatic mutations in this gene.
A similarly rare cause of sporadic PA is somatic mutations in CLCN2. These mutations occur in the same regions in which a germline mutation (Gly24Asp or Asn356_Leu359del) is associated with FH type II83–86 (Table 1). CLCN2 encodes the inwardly rectifying chloride channel, CIC-2. The expression of mutant CLCN2 leads to depolarization of the adrenal cell membrane and activates the voltage-gated calcium channel, leading to Ca2+ influx, which in turn activates CYP11B2 transcription83,85.
CTNNB1, GNA11/Q and CADM1
Although most somatic mutations in APAs are in genes that encode ion channels or transporters, whole-exome sequencing of 10 ZG-like APAs identified one APA with an exon 3 mutation in CTNNB1 (ref. 22), which encodes β-catenin – a protein that is important for a number of processes, including cell differentiation. The index patient harbouring this mutant APA, and her first replicate both presented with PA in early pregnancy22,87. The putative relevance became apparent from scrutiny of their APA transcriptomes, which revealed an >100-fold increased expression of the gene that encodes the receptor for the pregnancy hormone, LHCGR, compared with levels in other APAs87. However, pregnancy and high LHCGR expression have not been consistently seen in subsequent reports88.
Whole-exome sequencing of an APA from a boy who presented with PA at puberty identified a somatic mutation in CTNNB1 that co-existed with a mutation at Gln209 in GNA11. This combination was coincidentally also found in 2 of 40 consecutive APAs that we analysed at the same time89, and in retrospect, was also reported in our index CTNNB1-mutant patient22. Further genotyping has identified a total of 16 patients with a double mutation of a phosphorylation site in exon-3 of CTNNB1 (which results in β-catenin entering the nucleus and activating transcription) and the Gln209 residue of GNA11 or the closely homologous GNAQ – the G-proteins that mediate aldosterone production in response to ANGII stimulation89. 15 of these 16 patients are women, most of whom presented in the first trimester of pregnancy; all patients achieved 100% complete clinical success following adrenalectomy. Exon 3 mutations in CTNNB1 are frequent in malignant tumours, including adrenocarcinomas. Somatic p.Gln209 mutations in GNAQ cause most uveal melanomas, and, as mosaic mutations of GNA11 or GNAQ, cause Sturge–Weber and other cutaneous syndromes90–92. Elevations in the expression of LHCGR were observed in 15 of the 16 women with double-mutant APAs, but not in any of the 8 women APAs with solitary mutations of CTNNB1. LHCGR is coupled to adenylate cyclase, and mediates a rapid onset of hyperaldosteronism when stimulated in aldosterone-producing cells87,93. The p.Gln209 residue of GNA11 and GNAQ is analogous to the p.Gln227 residue of GNAS, which is mosaically mutated in McCune–Albright syndrome, defined by the presence of different genotypic variants among cells arising from a single zygote94. Some of the adrenal tissue adjacent to the double-mutant APAs was similarly mosaic. Our hypothesis for the strikingly high complete success rate of adrenalectomy in these patients is that these mutations arise embryogenically after the right or left adrenal primordium develops, such that there is no contralateral abnormality.
Whole-exome sequencing of the 40 consecutive APAs also identified another gene, CADM1, with a deleterious p.Val380Asp mutation within its single intramembranous domain95. CADM1 (previously known as SynCAM) encodes a cell adhesion molecule, which is most highly expressed in the central nervous system, especially the cerebellum, where it has a role in synaptogenesis96. Five further patients, with either p.Gly379Asp or p.Val380Asp somatic mutations, were subsequently identified by whole exome or targeted sequencing of ~550 APAs95. Transfection of the mutations into adrenocortical cells led to upregulation of pathways linked to biological rhythm, with upregulation and downregulation, respectively, of the out-of-phase E-box and RORE clock genes. One of the RORE clock genes, NPAS2, which was upregulated in the index CADM1-mutant tumour, is a paralog of CLOCK. Like CLOCK, it dimerizes with genes involved in circadian rhythm, such as BMAL1, to drive the transcription of other clock genes (such as CRY1–2 and PER1–3)97,98. Of interest, the two index cases (P1 and P2) with p.Gly379Asp or p.Val380Asp mutations in CADM1 had apparently periodic secretion of aldosterone. This finding prompts the question of whether CADM1 mutations may be more frequent (than our estimated 1%) among patients who elude diagnosis by conventional, random blood sampling. An unrelated clinical study coincidentally reported that measurement of 24-h urinary aldosterone secretion rather than a ‘snapshot’ measurement of ARR in blood, may reveal the prevalence of PA to be twice as high as the 5–11% estimate34. Serial blood measurements in PA, and microdialysis measurements of interstitial aldosterone, also point to great variability in aldosterone secretion, possibly following diurnal or ultradian rhythms99. The two patients with CADM1-mutant APAs who had documented periodicity of aldosterone secretion were the two who achieved complete clinical success. The explanation for this observation might again be related to true lateralization, with rare mutations being unlikely to arise bilaterally. An alternative explanation is that cumulative exposure of target tissue to aldosterone is lower in patients with variable than in those with sustained aldosterone excess.
Somatic deletion in a zinc transporter SLC30A1.
Discovery of recurrent somatic mutations in SLC30A1 (ref. 100), which encodes the zinc transporter ZnT1, extends the number of ion channels or transporters linked to PA. The 51_57del variant caused abnormal Na+ influx, depolarization of the resting membrane potential and opening of voltage-gated calcium channels. As with other somatic mutations in APAs, the resultant increase in cytosolic Ca2+ signalling stimulated CYP11B2 expression and aldosterone production.
Insights from transcriptome analyses
The rank of the genes upregulated in APAs, compared with the adjacent adrenal, varies among studies, but marked upregulation of CYP11B2, VSNL1 (encoding a putative transmembrane calcium-sensor), and PCP4 (a Purkinje-cell gene), are consistent findings20,101,102. All three of these genes are also expressed, diffusely or in clusters, at higher levels in the ZG than in the ZF102,103.
Our own microarray of seven APAs that we considered to be ZF-like (all with KCNJ5 mutations), seven ZG-like APAs and regions (ZG and ZF) adjacent to all 14 APAs (isolated using laser capture microdissection), identified striking differences between the transcriptomes of the ZF-like and ZG-like APAs64,65,104–106, which are corroborated by a similar laser capture microdissection-based microarray107 and by later RNA sequencing-based comparisons of APA transcriptomes63,88. Notably, levels of CYP11B1 and CYP17A1 were higher in the ZF-like APAs than in the ZG-like APAs. Second, a much larger number of genes were several-fold up-regulated in ZG-like APAs than the converse. Genes that were more upregulated in ZG-like APAs (that is the ATP1A1 and CACNA1D mutants) compared with ZF-like APAs (the KCNJ5 mutants) included NPNT (encoding nephronectin), MYOM1 (encoding the heart-muscle protein, myomesin) and KIAA1549L (also known as C11orf41, encoding a neuronal gene of unknown function)22,64. Although NPNT is selectively expressed in the ZG, where its encoded protein binds to an integrin (α8, β1) around the glomeruli106, MYOM1 and KIAA1549L are barely expressed in normal adrenal tissue. However, differences between ZF-like and ZG-like APAs were greatly out-numbered by similarities in their transcriptomes.
We have identified 40 genes with expression at least 4-fold higher in the ZG than in the ZF; indeed, 10–25-fold higher in the case of the top six genes (Supplementary Table 1)104. With the exception of VSNL1, none of the other ‘top genes’ in ZG were previously associated with aldosterone production65,104,105,108. Several have functional pedigrees in other tissues. For instance, LGR5 and its ligand RSPO3 provide Wnt-directed signals that control the migration of gut stem cells from the crypt to the intestinal surface109. In mice, Rspo3 is required for adrenal development and adrenocortical cell replenishment from the capsule110, suggesting that LGR5 could potentially have a role in controlling the centripetal migration of the adrenal cortex. However, the absence of CYP11B2 from this list (Supplementary Table 1) corresponds to an almost complete absence of the protein from the ZG of adult adrenal tissue19,111,112. Interestingly, ex vivo expression of CYP11B2 is inhibited by LGR5 transfection, and its expression in vivo is inversely related to that of LGR5 (ref. 105). Systematic pathway analysis of genes differentially regulated in ZG compared with ZF also indicated negative regulation of steroid and lipid synthesis, and of Wnt signalling64,113. These findings suggest that suppression of aldosterone secretion in adult ZG is, in part, an active process rather than a consequence of ANGII withdrawal due to excess of salt in the modern diet.
Differences in gene expression between ZF-like and ZG-like APAs were much smaller than differences between ZF and ZG22,63,64. Above, we inferred that ‘ZF-like’ refers to the pathology and biochemistry, but not the origins of the KCNJ5-mutant APAs. This inference is supported by the finding that several genes, such as VAT1L and VCAN, which are selectively expressed in the ZG (Table 1), were more highly expressed in the ZF-like than in the ZG-like APAs104. The converse set of genes (that is, those that were more highly expressed in ZG-like than in ZF-like APAs) were poorly expressed in adjacent adrenal tissue, and/or showed small differences in gene expression between normal ZF and ZG. This apparent anomaly was subsequently explained by the discovery of aldosterone-producing cell clusters (now known as aldosterone-producing micronodules, APMs).
Aldosterone-producing micronodules
The vanishing CYP11B2 paradox – that is, the disappearance of the enzyme required to synthesize aldosterone from the gland that makes the hormone – was largely resolved by the tour-de-force discovery of specific epitopes within the two highly homologous enzymes, 11-β hydroxylase and aldosterone synthase, which generated highly specific monoclonal antibodies for immunohistochemistry19. Numerous subsequent studies demonstrated that the diffuse expression of aldosterone synthase observed throughout the ZG of the paediatric adrenal gland was usually absent in adult ZG114. Instead, CYP11B2-positive cells were limited to discrete ~1–3-mm areas, which are now known as APMs, located beneath the adrenal capsule36,115,116 (Fig. 5b). Like the cells of most APAs they have rounded nuclei, unlike the dentate nuclei of physiological ZG cells.
Whether APMs should be considered a common precursor of ZG-like APAs, or a coincidental, physiological or pathological response to chronic salt excess is unknown. Excess salt, common in our modern diet, switches off the physiological production of aldosterone by suppressing the renin-angiotensin-aldosterone system, leading to decreased expression of aldosterone synthase (Fig. 1). If salt is responsible for the suppression of aldosterone synthase outside of APMs, there may be evolutionary advantage in the existence of a non-suppressible reserve of aldosterone synthase that enables constitutive aldosterone production, for example, to enable the production of transient ACTH-mediated increases in aldosterone in response to stress or periods of profound salt depletion117–120. It is also likely that APMs retain sensitivity to potassium, with aldosterone release providing essential protection against hyperkalaemia. However, studies of laser-dissected APMs have found CACNA1D mutations in 50–60%, as well as mutations in other genes linked to aldosterone production17,38,107. The CACNA1D mutations found in APMs are not always the same as those found in the adjacent APA17. However, transition of an APM into an APA has been captured in adrenal sections121,122, and it is likely that at least some APMs are a pathological precursor of mutant CACNA1D-containing APAs.
Unlike transcriptome analyses of APAs, which can be readily performed owing to their abundance, whole-transcriptome analyses of APMs are constrained by the technical challenge of extracting small amounts of RNA from laser-based microscopic sample dissection and collection107,123. A 2015 microarray analysis of RNA from APMs and three normal adrenocortical zones corroborated or anticipated many of the genes upregulated in APAs that were not found in the non-CYP11B2-expressing ZG107,124. For example, MC2R, which encodes the ACTH receptor, and CLRN1, which has been linked to hearing and vision, were upregulated in the APM samples and are upregulated in most APAs. Other genes, including DFNB31 (encoding WHRN), which, like CLRN1, has been linked to deafness125–127, are also increased in APAs, especially in ZG-like APAs. The mechanisms that underlie hearing and sight in the inner ear and retina, respectively, may have some commonalities with those involved in aldosterone regulation. For example, CACNA1D is expressed in the retina and inner hair cells of the ear128, and Cαcnα1d-deficient mice and humans with homozygous loss-of-function, are deaf129. Another group of genes, including NPHP1 and CFAP221, that are upregulated in APMs, are associated with primary cilia function, suggesting a possible role in sensing of the ionic environment130. Many of the genes upregulated in APMs compared with the ZG are also abundant in the ZF and/or zona reticularis (ZR), inferring that it is their down-regulation in ZG that is notable, and presumably related to the remarkable suppression of aldosterone synthase in the majority of the adult human ZG. Evidence that we should indeed focus as much on the genes that are down-regulated in ZG adjacent to an APA (presumably due to negative feedback from aldosterone and/or Na+ excess) as on the genes upregulated in APMs, comes from our microarray analysis in which we compared 14 pairs of ZG and ZF samples adjacent to an APA with 7 pairs of ZG and ZF samples adjacent to a phaeochromocytoma. Although the latter pairs of adrenal samples are an imperfect ‘normal control’, phaeochromocytoma has the merit of causing Na+ loss (through pressure natriuresis)131. Several ‘APM-associated genes’, including CYP11B2 itself, as well as the ACTH receptor gene MC2R, the neuronal genes NETO2 and BEX1, and TSPAN12, had 2–8-fold lower expression in ZG samples adjacent to an APA than in ZG samples adjacent to a phaeochromocytoma, for example20,107,115,132–139.
Our 2015 microarray study also identified several genes that were upregulated in APMs (compared with their expression in the three physiological adrenocortical zones), but were not previously associated with aldosterone regulation. For example, SLC35F1 encodes a neuronal decamembrane-spanning protein from the SLC35F family of nucleotide sugar transporters. TMEM266 is one of several genes that is restricted to the ZG and cerebellum140. Its ability to undergo rapid structural changes (that is, within milliseconds) in response to voltage changes could explain the pacemaker activity of ZG cells141–144. The expression of TMEM266 in APMs, and SLC30A1 in APAs (discussed earlier), shed light on roles of zinc in aldosterone regulation. Coincidentally, CASZ1 encodes a zinc finger transcription factor that may function as a tumour suppressor. It was one of only two positive loci in a large genome-wide association study of PA, providing evidence that at least some APMs are causally linked to PA145.
One caveat to the interpretation of findings from transcriptome analyses of APMs is the frequent need to pool RNA from several APMs, given their small size. This limitation has made it difficult to investigate heterogeneity in an adrenal – for example, to assess whether some APMs function physiologically as an emergency reserve of aldosterone production and others represent a pre-tumorous pathology. Developments in single-cell technology enabling in situ analyses, may resolve such questions. However, findings from one study that used in situ metabolomics indicates that two types of APM are likely to exist, one of which demonstrates similarity to APAs117.
Translation of molecular pathogenesis
The discovery, in APAs, of recurrent somatic mutations in ion channels and transporters was consistent with earlier studies of adrenocortical cell function and rodent models of PA. Functional studies of adrenocortical cells had shown that the hyperpolarization of ZG cells explained their exquisite sensitivity to elevations of extracellular K+, whereas knockout models of K+-channels demonstrated the potential for depolarization to cause PA146,147. Paradoxically, this consistency with prior studies limited the initial impact of the molecular findings, and their potential for clinical translation29. However, three key insights from studies published since 2020 may have changed this trajectory, leading to the validation of non-invasive molecular probes as an alternative to AVS; demonstrating translatable correlations of somatic genotype with clinical presentation and outcomes following adrenalectomy; and yielding proof-of-concept in a prospective evaluation of a minimally invasive alternative to adrenalectomy.
Molecular imaging of APAs
The MATCH study was a prospective, randomized controlled study that used a within-patient design to compare the accuracy of a non-invasive test – 11C-metomidate PET CT (MTO) – with AVS in predicting the outcome from adrenalectomy in patients with PA63. Complete or partial biochemical success was achieved in 93% of the 78 patients predicted by MTO and/or AVS to have unilateral PA. Statistically, MTO was non-inferior to AVS for predicting biochemical and clinical success following adrenalectomy. However, its requirement for on-site synthesis, and for dexamethasone pre-treatment, means that MTO is unlikely to remain the PET ligand of choice, and will be replaced by longer-acting, and/or more CYP11B2-selective ligands148–152. Nevertheless, MATCH established the principle of molecular imaging as a non-invasive, operator-independent alternative to AVS.
Somatic genotype explains and predicts clinical outcome
The MATCH trial also identified correlations between somatic genotype and clinical presentation and outcomes following adrenalectomy. Logistic regression of the effect of age, gender, blood pressure and APA genotype on surgical outcome found that APA genotype was the strongest predictor. 14 of 18 patients with KCNJ5 mutations, compared with 1 of 20 patients with CACNA1D mutations, achieved complete success, which was sustained for at least 2 years63. Although somatic genotype was a post-operative finding, KCNJ5 mutations correlated strongly with pre-operative measurements of urinary hybrid steroids (that is, the 18-hydroxy cortisol-to-cortisol ratio) (Fig. 3).
Circumstantial evidence suggests that CACNA1D mutations are frequently bilateral38. Not only are they the most common mutation in APMs, but they probably also underlie the pathology of bilateral ‘hyperplasia’. This proposal is supported by the finding in one study that 15 of 15 adrenals with bilateral hyperplasia had APMs, compared with 4 of 15 with diffuse CYP11B2 positivity; of 99 APMs isolated from these adrenals, 57 (58%) had mutations in CACNA1D whereas only one had a mutation in KCNJ5 (ref. 38). Of note, the same adrenal gland may have up to eight different CACNA1D mutations in different APMs17. The multiplicity of spontaneous CACNA1D mutations, and their finding in the adrenals of patients with proven bilateral disease, suggests that mutations in this gene commonly occur in both adrenals. The converse is true of KCNJ5 mutations, which may explain why KCNJ5-mutant tumours are usually solitary, and hypertension is usually cured by their removal. Moreover, CACNA1D mutations are also more common than KCNJ5 mutations in Black patients60,63; in the MATCH study, it was the self-identified Black patients with CACNA1D mutations who were least likely to achieve either biochemical or clinical success.
Candidate surrogates for CACNA1D mutations, which would have the potential to identify patients, likely to achieve as much benefit from medical as surgical therapy, have been suggested by transcriptome analyses of APAs88. These studies have identified neuronal proteins, such as neurexins, as potential candidates, because their RNA expression was highest in CACNA1D-mutant samples. The neurexins are cleaved into soluble ectodomains, which are anticipated to have low background levels because of the blood–brain barrier153.
Molecular imaging guides minimally invasive alternative to surgery
Although molecular imaging was developed to replace invasive AVS, it also has a potential role in facilitating alternatives to surgery. Radiofrequency ablation may provide an alternative to adrenalectomy, particularly for small adenomas; however, studies to date, mainly by the percutaneous route, suggest that a trade-off exists between safety and efficacy, as the electrical field from the centre of a non-spherical adenoma may encroach on adjacent adrenal medulla, stimulating adrenaline release and a peri-procedural pressor crisis154–157. Given the proximity of the left adrenal gland to the stomach, we have performed a proof-of-concept study of endoscopic ultrasound-guided transgastric radiofrequency ablation of PET-imaged APAs in 28 patients. The adenoma, in this approach, is both seen and approached from the stomach, and ablation is achieved by multiple short bursts as the catheter is continually re-positioned. Preliminary findings158 suggest that this approach is feasible and safe. Both percutaneous and endoscopic approaches need to be formally compared, with each other and with adrenalectomy, and a prospective RCT is now in progress159.
Therapeutic implications
Existing calcium-channel blockers (CCBs) are non-selective and block both the vascular Cav1.2 and 75% identical Cav1.3, which is expressed mainly in parts of the nervous system (such as the cerebellum and inner ear cells) and endocrine organs. CCBs probably reduce aldosterone secretion in PA, and their use is often regarded as a ‘confounding medicine’ to be withdrawn during diagnostic tests. However, evidence of their ability to reduce autonomous aldosterone secretion is anecdotal160, and even less is known about the efficacy of CCBs to reduce aldosterone secretion by CACNA1D-mutant compared with other APAs. In mice, heterozygous deletion of Cyp11b2 does not induce a phenotype, suggesting that a >50% reduction in aldosterone secretion is required for a clinical effect161. The dose of CCBs is effectively limited by their Cav1.2 (vascular) effects, particularly peripheral oedema; hence, a selective Cav1.3 inhibitor used at maximal dose is in theory attractive. In studies of an adrenal cell line transfected with two separate, electrophysiologically distinct CACNA1D mutants, excess aldosterone secretion consequent upon augmented calcium influx was inhibited by a selective antagonist of Cav1.3, 1-(3-chlorophenethyl)–3-cyclopentylpyrimidine-2,4,6-(1H,3H,5H)-trione162, and by the maximum soluble concentration of a non-selective antagonist of L-type calcium channels, dihydropyridine calcium channel antagonist, nifedipine163. Targeting of Cav1.3 may have particular value in two common groups of patients in whom CACNA1D mutations are suspected to predominate. As discussed earlier, these are patients of African ancestry, even if investigations suggest unilateral disease, and any patient in whom investigations have suggested bilateral disease17,38,107.
Loss of potassium selectivity in the two most common KCNJ5 mutants combined with a loss of sensitivity to the potassium channel blockers barium and tertiapin-Q led to the suggestion that molecules could potentially be identified that block ion passage through the mutant, but not the wild type, channel. Indeed, in an adrenal cell line expressing the mutant KCNJ5, the macrolide antibiotic roxithromycin markedly inhibited CYP11B2 expression and aldosterone production164. Similar effects were observed with idremcinal, a macrolide motilin receptor agonist, and with synthesized macrolide derivatives that lacked antibiotic or motilide activity. Macrolide-derived selective inhibitors of mutant KCNJ5 thus have the potential to advance the diagnosis and treatment of KCNJ5-mutant APAs164.
Future perspectives
The finding that the ontogeny of adrenal glands in primates is fundamentally different from that of rodents should prompt the exploration of alternative or additional explanations for the physiological and pathological regulation of aldosterone165. The current centripetal migration model of adrenocortical development describes a common origin for gonadal and adrenocortical cells, with the latter constantly renewed from stem cells in the outer adrenal capsule, which metamorphose sequentially into ZG, ZF and ZR cells166. However, new research has demonstrated that primate adrenal tissue does not originate from an adrenogenital ridge165. Testing whether centripetal migration is the correct model for human adrenal gland development is difficult but becoming feasible through the application of single-cell analyses, which use somatic DNA variants as barcodes for cell ancestry167,168. Questions about cell ontogeny in human adrenal are raised by the finding of juxta-medullary ZG cells, by the presence of adrenomedullary cells in the ZG169,170 and by our finding that RSPO3-LGR5 signalling inhibits aldosterone production in the human adrenal gland105,169,170. This observation seems contrary to the role of RSPO3 in rodent adrenal, where it stimulates replenishment of ZG cells that have migrated centripetally110.
The distinctive clinical and molecular phenotypes of the APAs with GNA11, GNAQ or CADM1 mutations suggest that further insights might be gained from the identification of additional genes that are recurrently mutated, especially if they cause presentations of PA that are currently overlooked. Although the 2023 discoveries of CADM1 and SLC30A1 mutations indicate that recurrent somatic variants of unexpected genes may still await discovery, realistically, most new mutations are likely now to be private – meaning that they are rare and occur in single families or patients. As experiments of nature, we can still learn from them, but it will be difficult to be confident that they are causally related to PA in the absence of functional studies. We have identified one such somatic mutation in DPYSL2 (also known as CRMP2) – this gene is enriched in the ZG, is associated with ion-channel trafficking and is likely to bind to the β-subunit binding site of Cav1.363,171,172. Another mutation was identified in VAPA, which has also been linked to protein trafficking63,173 (Fig. 4). Moreover, the most upregulated gene in double-mutant APAs (that is, APAs with a double mutation in GNA11 or GNAQ and CTNNB1), the cerebellar gene TMEM132E, also has a role in trafficking a family of cholinergic receptors that are ion channels89,174. These findings suggest that we should investigate accessory proteins – such as those involved in channel trafficking – for their pathogenic role in disease onset. If we think of the molecular causes of PA as the potential targets of curative therapy, then such accessory proteins may provide as many opportunities for therapeutic intervention as the aldosterone synthetic pathway itself.
Of greater interest now than the search for individual new mutations is the question of why functional somatic mutations in the adrenal cortex are overall so common, and whether a therapy that reverses the consequence of these mutations would cause an involution of the APAs. Since our first report of somatic mutations a decade ago22, we have speculated that the driver is the reported apoptosis of CYP11B2- expressing ZG cells when CYP11B2 itself is inhibited or genetically deleted120,161,166. The complete suppression of aldosterone synthase expression in most adult ZG cells outside the APMs – which we attribute to excess salt intake – is associated with the appearance of apoptotic cells within the ZG itself, rather than at the ZR–medullary boundary105 (Fig. 6). An inference from these observations is that a therapy that suppresses aldosterone synthesis may cure PA. Therapeutically, the most direct route to switching off aldosterone production is inhibition of aldosterone synthase. After many years during which candidate drugs demonstrated insufficient selectivity to avoid inhibiting 11β-hydroxylase – which is responsible for cortisol synthesis and exhibits close homology to aldosterone synthase – a 12-week phase II trial of baxdrostat, which exhibits 100:1 selectivity for aldosterone synthase, demonstrated a comparable blood pressure reduction in patients with resistant hypertension to that achieved previously in similar patients using spironolactone120,175.
Fig. 6. A proposal for the evolutionary selection of aldosterone-producing adenomas and aldosterone-producing micronodules.
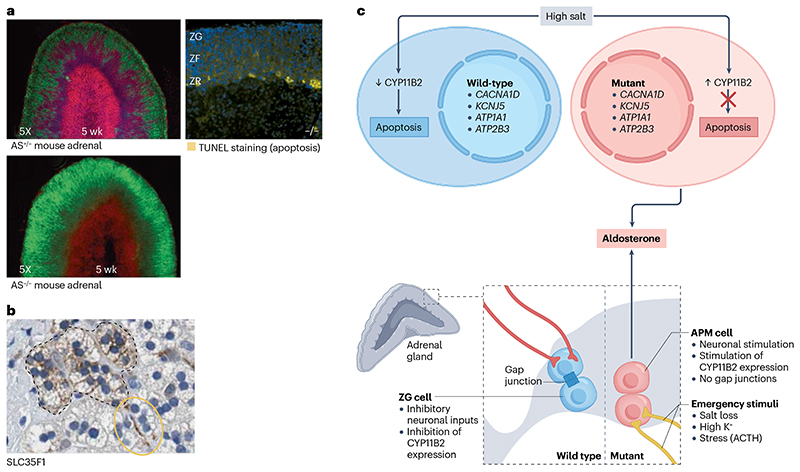
a, Confocal analysis of adrenal tissue from mice with heterozygous (A+/−) and homozygous (AS−/−) knockout of the gene that encodes aldosterone synthase (CYP11B2) shows that homozygous CYP11B2 deletion induces centripetal migration of outer zona glomerulosa (ZG; green) cells towards the inner zona fasciculata (ZF) layer by 5 weeks of age166. At 6 months, ZG cells that have migrated to the zona reticularis (ZR)–medulla boundary in AS−/− mice expresses markers for apoptosis, terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) staining (yellow)161. b, The neuronal gene, SLC35F1 is highly expressed both in APM cells (encapsulated by the dashed brown lines) and in nerve-like structures adjacent to these (yellow circle)195. c, We propose that salt-induced suppression of aldosterone synthesis in the ZG is a scenario analogous to that induced by genetic CYP11B2 deletion. We also propose that the presence of mutations in aldosterone-driver genes such as CACNA1D may facilitate the evolutionary selection of APMs, by imparting a survival advantage. The presence of gap junctions between normal cells of the ZG may enable propagation of neuronal signals and thereby also contribute to the inhibition of aldosterone production in this region. By contrast, the main gapjunction proteins are largely absent from APMs. We also propose that neuronal excitation may over-ride negative feedback regulation in APMs and contribute to autonomous CYP11B2 production. Note that this model in which APMs arise from clonal selection is unproven. Part a adapted from ref. 161, Oxford Academic Press, and ref. 166, Elsevier. Part b is from The Human Protein Atlas196, image available at https://www.proteinatlas.org/ENSG00000196376-SLC35F1/tissue/adrenal+gland#img.
The long-term effects of baxdrostat, both in hypertension and in PA, will be of interest. Selective inhibition of Cav1.3 may also hold promise. Although no such selective inhibitor currently exists, structural modelling has identified that the antihistamine, cinnarizine, can bind and block the central pore cavity of Cav1.3176.
There are obvious public health implications if the change in aldosterone synthase expression, from diffuse to almost absent, between children and adults is salt-induced. We do not yet know whether salt is the sole culprit or whether suppression of renin–angiotensin is the sole mediator of aldosterone suppression. The renin status of patients at the time of adrenalectomy is not usually published. However, our prospective comparison of surgically and medically treated patients, in which all patients were force-titrated to spironolactone 100 mg or a maximum tolerated equivalent, showed that this dose lifted the suppression of renin in most patients63. These findings support our suspicion that withdrawal of ANGII (the downstream product of renin secretion) is not the sole factor in the disappearance of CYP11B2 from most adult human adrenals. Moreover, many of the genes that are upregulated in the ZG region in humans, such as LGR5, inhibit aldosterone production, contrary to the expectation for prominent genes in an endocrine gland, and cause apoptosis of transfected adrenocortical cells105. In addition, the presence of gap junctions between cells of the ZG may enable propagation of neuronal signals and thereby also contribute to the inhibition of aldosterone production in this region95.
LGR5 and GJA1 (the main gap junction protein) are largely absent from APMs, along with the ZG-specific transcripts for the inhibitory GABA receptor B (GABBR2) and neuronal cadherin (CDH12). What is unknown, however, is whether APMs are a mutation-driven, pre-tumorous pathology or whether there is a key cell or process (for example, an excitatory and inhibitory neuronal input), that is extrinsic to APMs and non-aldosterone producing ZG-glandular cells, and determines the delineation between these. The most up-regulated gene in APMs, after CYP11B2 itself, is a neuronal gene, SLC35F1, that is highly expressed both in APM cells and in nerves innervating these (Fig. 6). Indeed, several of the most upregulated transcripts in APMs and/or APAs are either neuronal (PCP4, KIAA1549L, KCND3, NRXN2, PPP1R17, SV2B), with highest expression typically in the cerebellum, or neuroendocrine (UNC79, CADM1, VAT1L), with high expression in the adrenal medulla and pituitary20,63,107. Most of the ‘neuronal genes’ are enriched in APAs of particular genotypes, namely CACNA1D-, ATP1A1- and ATP2B3-mutant APAs, but are virtually absent from KCNJ5- and double-mutant APAs63. Whether individual neurotransmitters are exciting different cells-of-origin for each APA genotype and thereby over-riding negative feedback regulation or whether cerebellar-type synapses between ZG cells are critical to the oscillation of currents around the glomeruli is unknown, but may soon be addressed by the emergence of new techniques for in situ transcriptomic analyses.
Neuronal input does not necessarily explain the ectopic expression of neuronal genes. An additional clue to the mechanisms underlying ZG pathophysiology may be the high abundance in ZG and certain APAs of three molecules – VPREB3, SHOC1 and KIAA1210 – that have no obvious reason to be expressed in adrenal tissue. The only known function of VPREB3 is in pro-B lymphocytes, where its encoded protein acts as part of a proxy B cell receptor for testing permutations of recombined immunoglobulin genes; however, it is also richly expressed in cerebellar Purkinje cells177. SHOC1 functions in meiosis, by promoting and protecting sites of DNA recombination; it is also highly expressed in the XY body178. KIAA1210, also has a specific function in gonads, being found in cell bridges between spermatogonia and (like SHOC1) the XY body. KIAA1210 regulates the dynamic changes in chromatin structures that occur during spermiogenesis179,180. Microarray studies and the Human Protein Atlas show high ZG and capsular expression of these three genes64. One suggestion is that the expression of all three molecules might be invoked in adrenal tissue by cell fusion and its sequelae. Such cell fusion is used by various cell types, including trophoblasts, myoblasts, osteoclasts, hepatocytes (in animal studies) and cancer cells181–184, as an alternative to stem cell differentiation for the replenishment of tissue181,182,185,186. Evidence of cell fusion within adrenal tissue is suggested by the finding of occasional hybrid adrenocortical and adrenomedullary cells on electron microscopy, and should be readily confirmed by single-cell sequencing analyses187. Cell fusion would not only allow ZG cells to synapse with each other but may also offer an opportunity for a form of meiotic recombination to occur during the subsequent ploidy reduction and establishment of synkaryons. These hybrid cells, with restored diploidy, would likely reproduce normally and transcribe two copies of each gene. However, their fitness to regulate and produce essential adrenal steroids may be increased by the merger of two types of ‘cell machinery’ and by selection of cells with two copies of the fittest parental gene (rather than one of each). The high adrenal expression of APOBEC3C, a single-strand DNA editing gene associated with hypermutation in immune cells, and UNG, a DNA-repair gene, indicate that, as in immune cells, gene ‘improvement’ may not be limited to shuffling (recombination) but may also involve somatic mutation. Selection of ‘improved’ genes arises, we propose, through the need of adrenocortical cells to survive oxidative steroidogenesis, migration and transformation, which successively generates two essential steroids. However, under conditions of salt excess, ZG steroidogenesis becomes a futile release of reactive oxygen species, as no steroid precursor is offered as a co-substrate with molecular oxygen to the steroidogenic electron transport chain188,189. Under such circumstances, the process of mutation and recombination promotes, we propose, the emergence of cells that constitutively produce aldosterone, restoring oxygen usage, and protecting against the apoptosis seen in models of aldosterone synthase deletion161,166.
Conclusions
Earlier we mentioned peptic ulcers as an historical analogy and challenge. Two obvious differences with the stomach may explain why adrenal pathology has received far less attention. One is the relative sizes of the adrenal gland and stomach, and the other is that we have two of the former. These anatomical differences have dulled the need to find treatments less brutal than the removal of one of the adrenal glands, while justifying the use of complex tests to answer a question that may be misleading – that is, whether the PA is unilateral or bilateral. In the MATCH trial, two thirds of patients had unilateral disease, which improved after surgery, but only one third of these patients achieved a sustained, complete cure. Shifting priorities to the 99% of patients with undiagnosed PA presents two clear questions: first, how to identify patients who have a high likelihood of complete cure following non-pharmacological intervention, and second, how can PA be cured in the remainder.
A growing body of literature suggests that distinct genotype–phenotype patterns and clinical surrogates for genotype will facilitate diagnosis and enable prediction of individual patient outcomes. The hope is that at least one of the gene mutations, proteins, cells or pathways discovered will be sufficiently unique to autonomous aldosterone production that it is targeted to switch off autonomous aldosterone production through the delivery of a small drug, RNA or gene-editor. Notably, the adrenal cortex, in its hunger for cholesterol as precursor to steroidogenesis, has a far higher density than any other tissue of receptors for LDL and HDL. Lipid nanoparticles incorporate one of these lipids to deliver RNA-based therapies; and receptors for both lipids are enriched in cells with autonomous aldosterone production. Suicide therapy surely beckons.
Supplementary Material
Box 1.
Key findings from studies of somatic mutations in APAs
80-95% of aldosterone-producing adenomas (APAs) have a somatic mutation within key regions of the transporters encoded by KCNJ5, ATP1A1 and ATP2B3, or near the Ca2+ pores of the calcium channel encoded by CACNA1D17,63.
The highest numbers of mutations have been identified in CYP11B2-positive regions of APAs, representing the areas that express aldosterone synthase17.
In adult human adrenals, CYP11B2 is largely confined to microscopic clusters, termed aldosterone-producing micronodules; a mutated aldosterone-driver gene can be found in 30-60% of these clusters, mainly involving CACNA1D36.
The phenotype of KCNJ5-mutant APAs differs from that of other APAs: clinically, KCNJ5-mutant APAs present more frequently in younger individuals and women; macroscopically, they are larger; and microscopically, they comprise large, foamy cells; they also differ in their gene expression (demonstrating co-expression of zona fasciculata (ZF) enzymes, CYP11B1 and CYP17A1)21,58,63.
APAs with mutations in CACNA1D, ATP1A1 or ATP2B3 are more similar to each other than to KCNJ5-mutant APAs, and more similar than KCNJ5-mutant APAs to the microscopic aldosterone-producing micronodules22,63,106,107.
The translational questions arising from these findings are:
Whether clinical outcomes or management should differ between genotypes, and if so, whether these differences can be predicted prior to surgical removal of the APA?
Whether the high prevalence of APAs is explained by their pathogenesis and links between this and mechanisms of aldosterone regulation in adult humans?
Answers to these questions may come from our understanding of the ZF phenotype of KCNJ5-mutant APAs, and the inferred co-existence of CYP11B1 and CYP11B2 (encoding 11-β hydroxylase and aldosterone synthase) within the same cell197. The consequences of coexistent CYP11B1 and CYP11B2 can be inferred from studies of patients with familial glucocorticoid remediable aldosteronism (GRA). GRA is caused by a rare germline chimaera between the two genes, which results in production of the hybrid steroids 18-hydroxycortisol and 18-oxocortisol, as has also been shown for patients with KCNJ5 mutations41,197,198. Steroid profiling using liquid chromatography with tandem mass spectrometry (LC-MS/MS) has enabled measurement of these hybrid steroids199 and have demonstrated levels of the hybrid steroids to be higher in APAs than in bilateral adrenal hyperplasia200,201. Steroid profiling of peripheral blood using LC-MS/MS has potential value particularly for the identification of KCNJ5-mutant APAs, and may enable the identification of candidates for whom invasive adrenal vein sampling (AVS) can be avoided and substituted with findings of solitary adenoma on CT or MRI, or in whom complete remission of hypertension post-adrenalectomy is most likely29,202. However, steroid profiling using LS-MS/MS requires expensive instrumentation and a high level of expertise. More widely available at present is the overnight dexamethasone suppression test based on the often-overlooked finding that dexamethasone failed to suppress either cortisol or aldosterone in siblings first described to have germline KCNJ5 mutations41.
Key points.
Primary aldosteronism (PA) is a common cause of hypertension; it is potentially curable when caused by a unilateral aldosterone-producing adenoma (APA), but it can lead to resistant hypertension and a high cardiovascular risk in cases in which PA is undiagnosed.
Current clinical guidelines and practice centres on risky tests and procedures, such as saline suppression, adrenal sampling and surgery, and poorly tolerated drugs such as spironolactone; these practices have remained largely unchanged over the past 30 years.
By contrast, the discovery of somatic mutations over the past decade has identified these as the cause of autonomous aldosterone production in most patients with PA, including 80–90% of APAs; the identified mutations most commonly affect the ion-channel genes KCNJ5 and CACNA1D.
Several distinct genotype–phenotype patterns and emerging clinical surrogates for genotype suggest the potential for molecular discovery to predict individual patient outcomes, permitting rational stratification of interventions.
In vivo molecular ligands for aldosterone synthase have been validated for non-invasive PET CT detection of APAs; on the left side, ultrasound-guided endoscopic radiofrequency ablation of PET-imaged APAs seems to be a safe alternative to surgery.
Genetic deletion models suggest curative potential for the suppression, rather than blockade, of aldosterone synthesis.
Acknowledgements
E.A.B.A.’s work is supported by the Ministry of Higher Education (MOHE) Malaysia, a Long-term Research Grant Scheme-Jejak Sarjana Ulung (LRGS-JSU), LRGS/1/2021/SKK15/UKM/02/2 (mentored by M.J.B); the Royal Society-Newton Advanced Fellowship, NA170257/FF-2018–033 (co-applicant with M.J.B.); and the UK-MY Joint Partnership on Noncommunicable Diseases 2019 program, NEWTON-MRC/2020/002 (co-applicant with M.J.B.). W.M.D.’s and M.J.B.’s work is supported by the National Institute for Health Research (NIHR, Efficacy and Mechanisms Evaluation project 14/145/09), Barts Charity (Project MGU0360), NIHR Integrated Academic Training Clinical Lectureship, the Medical Research Council (MR/S006869/1) and the British Heart Foundation (PG/16/40/32137). M.J.B. is also supported by the British Heart Foundation (Project MCPG1P3R and PhD studentship FS/14/75/31134) and NIHR Barts Biomedical Research Centre (BRC-1215–20022).
Footnotes
Author contributions
The authors contributed equally to all aspects of the article.
Competing interests
The authors declare no competing interests.
Peer review information Nature Reviews Nephrology thanks Celso Gomez-Sanchez and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
References
- 1.Simpson SA, et al. [Isolation from the adrenals of a new crystalline hormone with especially high effectiveness on mineral metabolism] Experientia. 1953;9:333–335. doi: 10.1007/BF02155834. [DOI] [PubMed] [Google Scholar]
- 2.Conn JW, Louis LH. Primary aldosteronism, a new clinical entity. Ann Intern Med. 1956;44:1–15. doi: 10.7326/0003-4819-44-1-1. [DOI] [PubMed] [Google Scholar]
- 3.Andersen GS, Toftdahl DB, Lund JO, Strandgaard S, Nielsen PE. The incidence rate of phaeochromocytoma and Conn’s syndrome in Denmark, 1977-1981. J Hum Hypertens. 1988;2:187–189. [PubMed] [Google Scholar]
- 4.Grell GA, Hanchard B, Fletcher PR, Clarke WF, Williams W. Conn’s syndrome. Case report and a review of the syndrome of primary aldosteronism (SOPA) West Indian Med J. 1984;33:48–54. [PubMed] [Google Scholar]
- 5.Simpson F, Barnett A. Primary aldosteronism (Conn’s syndrome): report of a case, and an analysis of published cases. Med J Aust. 1961;1:729–735. [Google Scholar]
- 6.Merrell RC. Aldosterone-producing tumors (Conn’s syndrome) Semin Surg Oncol. 1990;6:66–70. doi: 10.1002/ssu.2980060203. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan NM. Hypokalemia in the hypertensive patient, with observations on the incidence of primary aldosteronism. Ann Intern Med. 1967;66:1079–1090. doi: 10.7326/0003-4819-66-6-1079. [DOI] [PubMed] [Google Scholar]
- 8.Conn JW, Rovner DR, Cohen EL, Nesbit RM. Normokalemic primary aldosteronism: its masquerade as essential hypertension. JAMA. 1966;195:21–26. doi: 10.1001/jama.1965.03090030022005. [DOI] [PubMed] [Google Scholar]
- 9.Rossi GP, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293–2300. doi: 10.1016/j.jacc.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 10.Monticone S, et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol. 2017;69:1811–1820. doi: 10.1016/j.jacc.2017.01.052. [DOI] [PubMed] [Google Scholar]
- 11.Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6:51–59. doi: 10.1016/S2213-8587(17)30367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monticone S, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6:41–50. doi: 10.1016/S2213-8587(17)30319-4. [DOI] [PubMed] [Google Scholar]
- 13.Williams B, Brown MJ. Investigation of primary aldosteronism in patients with resistant hypertension — Authors’ reply. Lancet Diabetes Endocrinol. 2018;6:600–601. doi: 10.1016/S2213-8587(18)30174-8. [DOI] [PubMed] [Google Scholar]
- 14.Mulatero P, et al. Guidelines for primary aldosteronism: uptake by primary care physicians in Europe. J Hypertens. 2016;34:2253–2257. doi: 10.1097/HJH.0000000000001088. [DOI] [PubMed] [Google Scholar]
- 15.Fernandes-Rosa FL, Boulkroun S, Zennaro MC. Somatic and inherited mutations in primary aldosteronism. J Mol Endocrinol. 2017;59:R47–R63. doi: 10.1530/JME-17-0035. [DOI] [PubMed] [Google Scholar]
- 16.Nanba K, et al. Targeted molecular characterization of aldosterone-producing adenomas in white Americans. J Clin Endocrinol Metab. 2018;103:3869–3876. doi: 10.1210/jc.2018-01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Sousa K, et al. Genetic, cellular, and molecular heterogeneity in adrenals with aldosterone-producing adenoma. Hypertension. 2020;75:1034–1044. doi: 10.1161/HYPERTENSIONAHA.119.14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi M, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331:768–772. doi: 10.1126/science.1198785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez-Sanchez CE, et al. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol. Cell Endocrinol. 2014;383:111–117. doi: 10.1016/j.mce.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang T, et al. Gene expression profiles in aldosterone-producing adenomas and adjacent adrenal glands. Eur J Endocrinol. 2011;164:613–619. doi: 10.1530/EJE-10-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azizan EA, et al. Microarray, qPCR, and KCNJ5 sequencing of aldosterone-producing adenomas reveal differences in genotype and phenotype between zona glomerulosa-and zona fasciculata-like tumors. J Clin Endocrinol Metab. 2012;97:E819–E829. doi: 10.1210/jc.2011-2965. [DOI] [PubMed] [Google Scholar]
- 22.Azizan EA, et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet. 2013;45:1055–1060. doi: 10.1038/ng.2716. [DOI] [PubMed] [Google Scholar]
- 23.Kobuke K, et al. Calneuron 1 Increased Ca2+ in the endoplasmic reticulum and aldosterone production in aldosterone-producing adenoma. Hypertension. 2018;71:125–133. doi: 10.1161/HYPERTENSIONAHA.117.10205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monticone S, et al. Immunohistochemical, genetic and clinical characterization of sporadic aldosterone-producing adenomas. Mol Cell Endocrinol. 2015;411:146–154. doi: 10.1016/j.mce.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Åkerström T, et al. Novel somatic mutations and distinct molecular signature in aldosterone-producing adenomas. Endocr Relat Cancer. 2015;22:735–744. doi: 10.1530/ERC-15-0321. [DOI] [PubMed] [Google Scholar]
- 26.Takeda M, Go H, Imai T, Nishiyama T, Morishita H. Laparoscopic adrenalectomy for primary aldosteronism: report of initial ten cases. Surgery. 1994;115:621–625. [PubMed] [Google Scholar]
- 27.Dunnick NR, et al. CT in the diagnosis of primary aldosteronism: sensitivity in 29 patients. AJR Am J Roentgenol. 1993;160:321–324. doi: 10.2214/ajr.160.2.8424342. [DOI] [PubMed] [Google Scholar]
- 28.Young WF, et al. Role for adrenal venous sampling in primary aldosteronism. Surgery. 2004;136:1227–1235. doi: 10.1016/j.surg.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 29.Funder JW, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889–1916. doi: 10.1210/jc.2015-4061. [DOI] [PubMed] [Google Scholar]
- 30.Hiramatsu K, et al. A screening test to identify aldosterone-producing adenoma by measuring plasma renin activity. Results in hypertensive patients Arch Intern Med. 1981;141:1589–1593. [PubMed] [Google Scholar]
- 31.Gros R, Ding Q, Liu B, Chorazyczewski J, Feldman RD. Aldosterone mediates its rapid effects in vascular endothelial cells through GPER activation. Am J Physiol Cell Physiol. 2013;304:C532–C540. doi: 10.1152/ajpcell.00203.2012. [DOI] [PubMed] [Google Scholar]
- 32.Funder JW. Primary aldosteronism and salt. Pflugers Arch. 2015;467:587–594. doi: 10.1007/s00424-014-1658-0. [DOI] [PubMed] [Google Scholar]
- 33.Funder J. Stress: Neuroendocrinology and Neurobiology. Academic Press; 2017. pp. 221–225. [Google Scholar]
- 34.Brown JM, et al. The unrecognized prevalence of primary aldosteronism: a cross-sectional study. Ann Intern Med. 2020;173:10–20. doi: 10.7326/M20-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drake WM, Brown MJ. In: Oxford Textbook of Endocrinology and Diabetes. 3rd. Wass JAH, et al., editors. Oxford University Press; 2022. [Google Scholar]
- 36.Nishimoto K, et al. Adrenocortical zonation in humans under normal and pathological conditions. J Clin Endocrinol Metab. 2010;95:2296–2305. doi: 10.1210/jc.2009-2010. [DOI] [PubMed] [Google Scholar]
- 37.Gomez-Sanchez CE, Gomez-Sanchez EP. Immunohistochemistry of the adrenal in primary aldosteronism. Curr Opin Endocrinol Diabetes Obes. 2016;23:242–248. doi: 10.1097/MED.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Omata K, et al. Cellular and genetic causes of idiopathic hyperaldosteronism. Hypertension. 2018;72:874–880. doi: 10.1161/HYPERTENSIONAHA.118.11086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yizhak K, et al. RNA sequence analysis reveals macroscopic somatic clonal expansion across normal tissues. Science. 2019;364:eaaw0726. doi: 10.1126/science.aaw0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lifton RP, et al. A chimaeric 11 beta-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature. 1992;355:262–265. doi: 10.1038/355262a0. [DOI] [PubMed] [Google Scholar]
- 41.Geller DS, et al. A novel form of human mendelian hypertension featuring nonglucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab. 2008;93:3117–3123. doi: 10.1210/jc.2008-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burton TJ, et al. Evaluation of the sensitivity and specificity of (11)C-metomidate positron emission tomography (PET)-CT for lateralizing aldosterone secretion by Conn’s adenomas. J Clin Endocrinol Metab. 2012;97:100–109. doi: 10.1210/jc.2011-1537. [DOI] [PubMed] [Google Scholar]
- 43.Laycock K, et al. ‘Pseudo-failure’ of adrenal vein sampling due to cortisol co-secretion by KCNJ5-mutant adenoma, and prediction of complete clinical success by urine hybrid steroid assay. Endocrine Abstracts. 2023 doi: 10.1530/endoabs.91.OC1. [DOI] [Google Scholar]
- 44.Yamada M, et al. KCNJ5 mutations in aldosterone-and cortisol-co-secreting adrenal adenomas. Endocr J. 2012;59:735–741. doi: 10.1507/endocrj.ej12-0247. [DOI] [PubMed] [Google Scholar]
- 45.Gomez-Sanchez CE, Oki K. Minireview: potassium channels and aldosterone dysregulation: is primary aldosteronism a potassium channelopathy? Endocrinology. 2014;155:47–55. doi: 10.1210/en.2013-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen AX, Nishimoto K, Nanba K, Rainey WE. Potassium channels related to primary aldosteronism: expression similarities and differences between human and rat adrenals. Mol Cell Endocrinol. 2015;417:141–148. doi: 10.1016/j.mce.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maria AG, et al. Mosaicism for KCNJ5 causing early-onset primary aldosteronism due to bilateral adrenocortical hyperplasia. Am J Hypertens. 2020;33:124–130. doi: 10.1093/ajh/hpz172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng CJ, et al. Novel KCNJ5 mutations in sporadic aldosterone-producing adenoma reduce Kir3.4 membrane abundance. J Clin Endocrinol Metab. 2015;100:E155–E163. doi: 10.1210/jc.2014-3009. [DOI] [PubMed] [Google Scholar]
- 49.Murthy M, Azizan EA, Brown MJ, O’Shaughnessy KM. Characterization of a novel somatic KCNJ5 mutation delI157 in an aldosterone-producing adenoma. J Hypertens. 2012;30:1827–1833. doi: 10.1097/HJH.0b013e328356139f. [DOI] [PubMed] [Google Scholar]
- 50.Charmandari E, et al. A novel point mutation in the KCNJ5 gene causing primary hyperaldosteronism and early-onset autosomal dominant hypertension. J Clin Endocrinol Metab. 2012;97:E1532–E1539. doi: 10.1210/jc.2012-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng FF, et al. A novel somatic mutation 145-147delETEinsK in KCNJ5 increases aldosterone production. J Hum Hypertens. 2017;31:756–759. doi: 10.1038/jhh.2017.50. [DOI] [PubMed] [Google Scholar]
- 52.Kuppusamy M, et al. A novel KCNJ5-insT149 somatic mutation close to, but outside, the selectivity filter causes resistant hypertension by loss of selectivity for potassium. J Clin Endocrinol Metab. 2014;99:E1765–E1773. doi: 10.1210/jc.2014-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rege J, Turcu AF, Rainey WE. Primary aldosteronism diagnostics: KCNJ5 mutations and hybrid steroid synthesis in aldosterone-producing adenomas. Gland Surg. 2020;9:3–13. doi: 10.21037/gs.2019.10.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scholl UI, et al. Hypertension with or without adrenal hyperplasia due to different inherited mutations in the potassium channel KCNJ5. Proc Natl Acad Sci USA. 2012;109:2533–2538. doi: 10.1073/pnas.1121407109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Monticone S, et al. A case of severe hyperaldosteronism caused by a de novo mutation affecting a critical salt bridge Kir3.4 residue. J Clin Endocrinol Metab. 2015;100:E114–E118. doi: 10.1210/jc.2014-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mulatero P, et al. KCNJ5 mutations in European families with nonglucocorticoid remediable familial hyperaldosteronism. Hypertension. 2012;59:235–240. doi: 10.1161/HYPERTENSIONAHA.111.183996. [DOI] [PubMed] [Google Scholar]
- 57.Monticone S, et al. A novel Y152C KCNJ5 mutation responsible for familial hyperaldosteronism type III. J Clin Endocrinol Metab. 2013;98:E1861–E1865. doi: 10.1210/jc.2013-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lenzini L, et al. A meta-analysis of somatic KCNJ5 K+ channel mutations in 1636 patients with an aldosterone-producing adenoma. J Clin Endocrinol Metab. 2015;100:E1089–E1095. doi: 10.1210/jc.2015-2149. [DOI] [PubMed] [Google Scholar]
- 59.Wang B, et al. Prevalence and characterization of somatic mutations in Chinese aldosterone-producing adenoma patients. Medicine. 2015;94:e708. doi: 10.1097/MD.0000000000000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nanba K, et al. Genetic characteristics of aldosterone-producing adenomas in Blacks. Hypertension. 2019;73:885–892. doi: 10.1161/HYPERTENSIONAHA.118.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Azizan E, et al. Half of an unselected series of aldosterone-producing adenomas have somatic mutation of the KCNJ5 channel and a zona fasciculata profile. J Hum Hypertens. 2011;25:627. [Google Scholar]
- 62.Okamura T, et al. Characteristics of Japanese aldosterone-producing adenomas with KCNJ5 mutations. Endocr J. 2017;64:39–47. doi: 10.1507/endocrj.EJ16-0243. [DOI] [PubMed] [Google Scholar]
- 63.Wu X, et al. [(11)C]metomidate PET-CT versus adrenal vein sampling for diagnosing surgically curable primary aldosteronism: a prospective, within-patient trial. Nat Med. 2023;29:190–202. doi: 10.1038/s41591-022-02114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou J, et al. Transcriptome pathway analysis of pathological and physiological aldosterone-producing human tissues. Hypertension. 2016;68:1424–1431. doi: 10.1161/HYPERTENSIONAHA.116.08033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maniero C, et al. NEFM (Neurofilament Medium) Polypeptide, a marker for zona glomerulosa cells in human adrenal, inhibits D1R (Dopamine D1 Receptor)-mediated secretion of aldosterone. Hypertension. 2017;70:357–364. doi: 10.1161/HYPERTENSIONAHA.117.09231. [DOI] [PubMed] [Google Scholar]
- 66.Maniero C. A functional study on novel genes involved in regulating aldosterone secretion in normal human zona glomerulosa and in aldosterone-producing adenomas. Doctoral dissertation, University of Cambridge; 2017. [Google Scholar]
- 67.Beuschlein F, et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet. 2013;45:440–444.:444e1–2. doi: 10.1038/ng.2550. [DOI] [PubMed] [Google Scholar]
- 68.Stindl J, et al. Pathogenesis of adrenal aldosterone-producing adenomas carrying mutations of the Na+/K+-ATPase. Endocrinology. 2015;156:4582–4591. doi: 10.1210/en.2015-1466. [DOI] [PubMed] [Google Scholar]
- 69.Meyer DJ, Gatto C, Artigas P. On the effect of hyperaldosteronism-inducing mutations in Na/K pumps. J Gen Physiol. 2017;149:1009–1028. doi: 10.1085/jgp.201711827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schlingmann KP, et al. Germline de novo mutations in ATP1A1 cause renal hypomagnesemia, refractory seizures, and intellectual disability. Am J Hum Genet. 2018;103:808–816. doi: 10.1016/j.ajhg.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stregapede F, et al. Hereditary spastic paraplegia is a novel phenotype for germline de novo ATP1A1 mutation. Clin Genet. 2020;97:521–526. doi: 10.1111/cge.13668. [DOI] [PubMed] [Google Scholar]
- 72.Williams TA, et al. Somatic ATP1A1, ATP2B3, and KCNJ5 mutations in aldosterone-producing adenomas. Hypertension. 2014;63:188–195. doi: 10.1161/HYPERTENSIONAHA.113.01733. [DOI] [PubMed] [Google Scholar]
- 73.Tauber P, et al. Cellular pathophysiology of an adrenal adenoma-associated mutant of the plasma membrane Ca2+-ATPase ATP2B3. Endocrinology. 2016;157:2489–2499. doi: 10.1210/en.2015-2029. [DOI] [PubMed] [Google Scholar]
- 74.Zanni G, et al. Mutation of plasma membrane Ca2+ ATPase isoform 3 in a family with X-linked congenital cerebellar ataxia impairs Ca2+ homeostasis. Proc Natl Acad Sci USA. 2012;109:14514–14519. doi: 10.1073/pnas.1207488109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scholl UI, et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet. 2013;45:1050–1054. doi: 10.1038/ng.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Catterall WA. Signaling complexes of voltage-gated sodium and calcium channels. Neurosci Lett. 2010;486:107–116. doi: 10.1016/j.neulet.2010.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dutta RK, Söderkvist P, Gimm O. Genetics of primary hyperaldosteronism. Endocr Relat Cancer. 2016;23:R437–R454. doi: 10.1530/ERC-16-0055. [DOI] [PubMed] [Google Scholar]
- 78.Azizan EA, Brown MJ. Novel genetic determinants of adrenal aldosterone regulation. Curr Opin Endocrinol Diabetes Obes. 2016;23:209–217. doi: 10.1097/MED.0000000000000255. [DOI] [PubMed] [Google Scholar]
- 79.Korah HE, Scholl UI. an update on familial hyperaldosteronism. Horm Metab Res. 2015;47:941–946. doi: 10.1055/s-0035-1564166. [DOI] [PubMed] [Google Scholar]
- 80.Tseng CS, et al. A novel somatic mutation of CACNA1H p.V1937M in unilateral primary hyperaldosteronism. Front Endocrinol. 2022;13:816476. doi: 10.3389/fendo.2022.816476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nanba K, et al. Somatic CACNA1H mutation as a cause of aldosterone-producing adenoma. Hypertension. 2020;75:645–649. doi: 10.1161/HYPERTENSIONAHA.119.14349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scholl UI, et al. Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. Elife. 2015;4:e06315. doi: 10.7554/eLife.06315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Scholl UI, et al. CLCN2 chloride channel mutations in familial hyperaldosteronism type II. Nat Genet. 2018;50:349–354. doi: 10.1038/s41588-018-0048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rege J, et al. Identification of somatic mutations in CLCN2 in aldosterone-producing adenomas. J Endocr Soc. 2020;4:bvaa123. doi: 10.1210/jendso/bvaa123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fernandes-Rosa FL, et al. A gain-of-function mutation in the CLCN2 chloride channel gene causes primary aldosteronism. Nat Genet. 2018;50:355–361. doi: 10.1038/s41588-018-0053-8. [DOI] [PubMed] [Google Scholar]
- 86.Dutta RK, et al. A somatic mutation in CLCN2 identified in a sporadic aldosterone-producing adenoma. Eur J Endocrinol. 2019;181:K37–K41. doi: 10.1530/EJE-19-0377. [DOI] [PubMed] [Google Scholar]
- 87.Teo AE, et al. Pregnancy, primary aldosteronism, and adrenal CTNNB1 mutations. N Engl J Med. 2015;373:1429–1436. doi: 10.1056/NEJMoa1504869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Backman S, et al. RNA sequencing provides novel insights into the transcriptome of aldosterone producing adenomas. Sci Rep. 2019;9:6269. doi: 10.1038/s41598-019-41525-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou J, et al. Somatic mutations of GNA11 and GNAQ in CTNNB1-mutant aldosterone-producing adenomas presenting in puberty, pregnancy or menopause. Nat Genet. 2021;53:1360–1372. doi: 10.1038/s41588-021-00906-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ayturk UM, et al. Somatic activating mutations in GNAQ and GNA11 are associated with congenital hemangioma. Am J Hum Genet. 2016;98:1271. doi: 10.1016/j.ajhg.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Van Raamsdonk CD, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010;363:2191–2199. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shirley MD, et al. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N Engl J Med. 2013;368:1971–1979. doi: 10.1056/NEJMoa1213507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saner-Amigh K, et al. Elevated expression of luteinizing hormone receptor in aldosterone-producing adenomas. J Clin Endocrinol Metab. 2006;91:1136–1142. doi: 10.1210/jc.2005-1298. [DOI] [PubMed] [Google Scholar]
- 94.Rey RA, et al. Unexpected mosaicism of R201H-GNAS1 mutant-bearing cells in the testes underlie macro-orchidism without sexual precocity in McCune-Albright syndrome. Hum Mol Genet. 2006;15:3538–3543. doi: 10.1093/hmg/ddl430. [DOI] [PubMed] [Google Scholar]
- 95.Wu X, et al. Somatic intramembranous mutations of CADM1 in aldosterone-producing adenomas, and gap junction-dependent regulation of aldosterone production. Nat Genet. 2023 doi: 10.1038/s41588-023-01403-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fujita E, Urase K, Soyama A, Kouroku Y, Momoi T. Distribution of RA175/TSLC1/ SynCAM, a member of the immunoglobulin superfamily, in the developing nervous system. Brain Res Dev Brain Res. 2005;154:199–209. doi: 10.1016/j.devbrainres.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 97.Crumbley C, Wang Y, Kojetin DJ, Burris TP. Characterization of the core mammalian clock component, NPAS2, as a REV-ERBα/RORα target gene. J Biol Chem. 2010;285:35386–35392. doi: 10.1074/jbc.M110.129288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Laposky A, Turek F. Encyclopedia of Neuroscience. Elsevier; 2009. pp. 909–914. [Google Scholar]
- 99.Upton TJ. U-RHYTHM microdialysis: towards ambulatory metabolodynamics. Doctoral dissertation, University of Otago; 2021. [Google Scholar]
- 100.Rege J, et al. Zinc transporter somatic gene mutations cause primary aldosteronism. Preprint at bioRxiv. 2022 doi: 10.1101/2022.07.25.501443. [DOI] [Google Scholar]
- 101.Williams TA, et al. Visinin-like 1 is upregulated in aldosterone-producing adenomas with KCNJ5 mutations and protects from calcium-induced apoptosis. Hypertension. 2012;59:833–839. doi: 10.1161/HYPERTENSIONAHA.111.188532. [DOI] [PubMed] [Google Scholar]
- 102.Felizola SJ, et al. PCP4: a regulator of aldosterone synthesis in human adrenocortical tissues. J Mol Endocrinol. 2014;52:159–167. doi: 10.1530/JME-13-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Trejter M, et al. Visinin-like peptide 1 in adrenal gland of the rat. Gene expression and its hormonal control. Peptides. 2015;63:22–29. doi: 10.1016/j.peptides.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 104.Zhou J, et al. DACH1, a zona glomerulosa selective gene in the human adrenal, activates transforming growth factor-β signaling and suppresses aldosterone secretion. Hypertension. 2015;65:1103–1110. doi: 10.1161/HYP.0000000000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shaikh LH, et al. LGR5 activates noncanonical wnt signaling and inhibits aldosterone production in the human adrenal. J Clin Endocrinol Metab. 2015;100:E836–E844. doi: 10.1210/jc.2015-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Teo AE, et al. Physiological and pathological roles in human adrenal of the glomeruli-defining matrix protein NPNT (Nephronectin) Hypertension. 2017;69:1207–1216. doi: 10.1161/HYPERTENSIONAHA.117.09156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nishimoto K, et al. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc Natl Acad Sci USA. 2015;112:E4591–E4599. doi: 10.1073/pnas.1505529112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maniero C, et al. ANO4 (Anoctamin 4) is a novel marker of zona glomerulosa that regulates stimulated aldosterone secretion. Hypertension. 2019;74:1152–1159. doi: 10.1161/HYPERTENSIONAHA.119.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 110.Vidal V, et al. The adrenal capsule is a signaling center controlling cell renewal and zonation through Rspo3. Genes Dev. 2016;30:1389–1394. doi: 10.1101/gad.277756.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nanba K, et al. Age-related autonomous aldosteronism. Circulation. 2017;136:347–355. doi: 10.1161/CIRCULATIONAHA.117.028201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.van de Wiel E, et al. Changes of the CYP11B2 expressing zona glomerulosa in human adrenals from birth to 40 years of age. Hypertension. 2022;79:2565–2572. doi: 10.1161/HYPERTENSIONAHA.122.19052. [DOI] [PubMed] [Google Scholar]
- 113.Lucas C, et al. Loss of LGR4/GPR48 causes severe neonatal salt wasting due to disrupted WNT signaling altering adrenal zonation. J Clin Invest. 2023;133:e164915. doi: 10.1172/JCI164915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hayashi T, et al. Expression of aldosterone synthase CYP11B2 was inversely correlated with longevity. J Steroid Biochem Mol Biol. 2019;191:105361. doi: 10.1016/j.jsbmb.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Boulkroun S, et al. Adrenal cortex remodeling and functional zona glomerulosa hyperplasia in primary aldosteronism. Hypertension. 2010;56:885–892. doi: 10.1161/HYPERTENSIONAHA.110.158543. [DOI] [PubMed] [Google Scholar]
- 116.Williams TA, et al. International histopathology consensus for unilateral primary aldosteronism. J Clin Endocrinol Metab. 2021;106:42–54. doi: 10.1210/clinem/dgaa484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sun N, et al. Mass spectrometry imaging establishes 2 distinct metabolic phenotypes of aldosterone-producing cell clusters in primary aldosteronism. Hypertension. 2020;75:634–644. doi: 10.1161/HYPERTENSIONAHA.119.14041. [DOI] [PubMed] [Google Scholar]
- 118.Goodchild E, Stoetaert J, Drake W, Linton K, Brown MJ. OR09-05 expression of SLC35F1 in the plasma membrane of cells of aldosterone producing cell clusters (APCCs) and its possible role in aldosterone synthesis. J Endocr Soc. 2020;4:OR09-05 [Google Scholar]
- 119.Markou A, et al. Stress-induced aldosterone hyper-secretion in a substantial subset of patients with essential hypertension. J Clin Endocrinol Metab. 2015;100:2857–2864. doi: 10.1210/jc.2015-1268. [DOI] [PubMed] [Google Scholar]
- 120.Bogman K, et al. Preclinical and early clinical profile of a highly selective and potent oral inhibitor of aldosterone synthase (CYP11B2) Hypertension. 2017;69:189–196. doi: 10.1161/HYPERTENSIONAHA.116.07716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nishimoto K, et al. Case report: nodule development from subcapsular aldosterone-producing cell clusters causes hyperaldosteronism. J Clin Endocrinol Metab. 2016;101:6–9. doi: 10.1210/jc.2015-3285. [DOI] [PubMed] [Google Scholar]
- 122.Nishimoto K, et al. Human adrenocortical remodeling leading to aldosterone-producing cell cluster generation. Int J Endocrinol. 2016;2016:7834356. doi: 10.1155/2016/7834356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Baker JE, et al. Targeted RNA sequencing of adrenal zones using immunohistochemistry-guided capture of formalin-fixed paraffin-embedded tissue. Mol Cell Endocrinol. 2021;530:111296. doi: 10.1016/j.mce.2021.111296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gene Expression Omnibus. Series GSE68889. 2023. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE68889 .
- 125.Ebermann I, et al. Two truncating USH3A mutations, including one novel, in a German family with Usher syndrome. Mol Vis. 2007;13:1539–1547. [PubMed] [Google Scholar]
- 126.Khan MI, et al. CLRN1 mutations cause nonsyndromic retinitis pigmentosa. Ophthalmology. 2011;118:1444–1448. doi: 10.1016/j.ophtha.2010.10.047. [DOI] [PubMed] [Google Scholar]
- 127.Audo I, et al. A novel DFNB31 mutation associated with Usher type 2 syndrome showing variable degrees of auditory loss in a consanguineous Portuguese family. Mol Vis. 2011;17:1598–1606. [PMC free article] [PubMed] [Google Scholar]
- 128.Kersten FF, et al. Association of whirlin with Cav1.3 (α1Dchannels in photoreceptors, defining a novel member of the usher protein network. Invest Ophthalmol Vis Sci. 2010;51:2338–2346. doi: 10.1167/iovs.09-4650. [DOI] [PubMed] [Google Scholar]
- 129.Platzer J, et al. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 130.Bai Y, et al. Primary cilium in kidney development, function and disease. Front Endocrinol. 2022;13:952055. doi: 10.3389/fendo.2022.952055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mackenzie IS, Brown MJ. Molecular and clinical investigations in patients with low-renin hypertension. Clin Exp Nephrol. 2009;13:1–8. doi: 10.1007/s10157-008-0071-4. [DOI] [PubMed] [Google Scholar]
- 132.Arnaldi G, et al. ACTH receptor mRNA in human adrenocortical tumors: overexpression in aldosteronomas. Endocr Res. 1998;24:845–849. doi: 10.3109/07435809809032695. [DOI] [PubMed] [Google Scholar]
- 133.Assié G, et al. Steroidogenesis in aldosterone-producing adenoma revisited by transcriptome analysis. J Clin Endocrinol Metab. 2005;90:6638–6649. doi: 10.1210/jc.2005-1309. [DOI] [PubMed] [Google Scholar]
- 134.Bassett MH, et al. Expression profiles for steroidogenic enzymes in adrenocortical disease. J Clin Endocrinol Metab. 2005;90:5446–5455. doi: 10.1210/jc.2005-0836. [DOI] [PubMed] [Google Scholar]
- 135.Fallo F, et al. Quantitative assessment of CYP11B1 and CYP11B2 expression in aldosterone-producing adenomas. Eur J Endocrinol. 2002;147:795–802. doi: 10.1530/eje.0.1470795. [DOI] [PubMed] [Google Scholar]
- 136.Schubert B, et al. Angiotensin II type 1 receptor and ACTH receptor expression in human adrenocortical neoplasms. Clin Endocrinol. 2001;54:627–632. doi: 10.1046/j.1365-2265.2001.01253.x. [DOI] [PubMed] [Google Scholar]
- 137.Williams TA, et al. Teratocarcinoma-derived growth factor-1 is upregulated in aldosterone-producing adenomas and increases aldosterone secretion and inhibits apoptosis in vitro. Hypertension. 2010;55:1468–1475. doi: 10.1161/HYPERTENSIONAHA.110.150318. [DOI] [PubMed] [Google Scholar]
- 138.Ye P, Mariniello B, Mantero F, Shibata H, Rainey WE. G-protein-coupled receptors in aldosterone-producing adenomas: a potential cause of hyperaldosteronism. J Endocrinol. 2007;195:39–48. doi: 10.1677/JOE-07-0037. [DOI] [PubMed] [Google Scholar]
- 139.Yang Y, et al. BEX1 is differentially expressed in aldosterone-producing adenomas and protects human adrenocortical cells from ferroptosis. Hypertension. 2021;77:1647–1658. doi: 10.1161/HYPERTENSIONAHA.120.16774. [DOI] [PubMed] [Google Scholar]
- 140.GTEx Portal. GTEx Analysis Release V8 (dbGaP Accession phs000424v8p2. 2023. https://gtexportal.org/home/gene/KCNJ5 .
- 141.Papp F, et al. TMEM266 is a functional voltage sensor regulated by extracellular Zn(2) Elife. 2019;8 doi: 10.7554/elife.42372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kawai T, et al. Insight into the function of a unique voltage-sensor protein (TMEM266) and its short form in mouse cerebellum. Biochem J. 2022;479:1127–1145. doi: 10.1042/BCJ20220033. [DOI] [PubMed] [Google Scholar]
- 143.Guagliardo NA, et al. TASK-3 channel deletion in mice recapitulates low-renin essential hypertension. Hypertension. 2012;59:999–1005. doi: 10.1161/HYPERTENSIONAHA.111.189662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Barrett PQ, et al. Role of voltage-gated calcium channels in the regulation of aldosterone production from zona glomerulosa cells of the adrenal cortex. J Physiol. 2016;594:5851–5860. doi: 10.1113/JP271896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Le Floch E, et al. Identification of risk loci for primary aldosteronism in genome-wide association studies. Nat Commun. 2022;13:5198. doi: 10.1038/s41467-022-32896-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Spät A, Rohács T, Horváth A, Szabadkai G, Enyedi P. The role of voltage-dependent calcium channels in angiotensin-stimulated glomerulosa cells. Endocr Res. 1996;22:569–576. doi: 10.1080/07435809609043748. [DOI] [PubMed] [Google Scholar]
- 147.Davies LA, et al. TASK channel deletion in mice causes primary hyperaldosteronism. Proc Natl Acad Sci USA. 2008;105:2203–2208. doi: 10.1073/pnas.0712000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wannachalee T, Lieberman L, Turcu AF. High prevalence of autonomous aldosterone production in hypertension: how to identify and treat it. Curr Hypertens Rep. 2022;24:123–132. doi: 10.1007/s11906-022-01176-7. [DOI] [PubMed] [Google Scholar]
- 149.Silins I, et al. First-in-human evaluation of [18F]CETO: a novel tracer for adrenocortical tumours. Eur J Nucl Med Mol Imaging. 2023;50:398–409. doi: 10.1007/s00259-022-05957-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Goodchild E, et al. Endocrine Abstracts. Vol. 86 Bioscientifica; 2022. [Google Scholar]
- 151.Sander K, et al. Development of [18F]aldoview as the first highly selective aldosterone synthase pet tracer for imaging of primary hyperaldosteronism. J Med Chem. 2021;64:9321–9329. doi: 10.1021/acs.jmedchem.1c00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hu J, et al. Accuracy of gallium-68 pentixafor positron emission tomography-computed tomography for subtyping diagnosis of primary aldosteronism. JAMA Netw Open. 2023;6:e2255609. doi: 10.1001/jamanetworkopen.2022.55609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Martín-de-Saavedra MD, Santos MD, Penzes P. Intercellular signaling by ectodomain shedding at the synapse. Trends Neurosci. 2022;45:483–498. doi: 10.1016/j.tins.2022.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Guo RQ, Li YM, Li XG. Comparison of the radiofrequency ablation versus laparoscopic adrenalectomy for aldosterone-producing adenoma: a meta-analysis of perioperative outcomes and safety. Updates Surg. 2021;73:1477–1485. doi: 10.1007/s13304-021-01069-5. [DOI] [PubMed] [Google Scholar]
- 155.Fintelmann FJ, et al. Catecholamine surge during image-guided ablation of adrenal gland metastases: predictors, consequences, and recommendations for management. J Vasc Interv Radiol. 2016;27:395–402. doi: 10.1016/j.jvir.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 156.Oguro S, et al. Safety and feasibility of radiofrequency ablation using bipolar electrodes for aldosterone-producing adenoma: a multicentric prospective clinical study. Sci Rep. 2022;12:14090. doi: 10.1038/s41598-022-18136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhao Z, et al. Catheter-based adrenal ablation remits primary aldosteronism: a randomized medication-controlled trial. Circulation. 2021;144:580–582. doi: 10.1161/CIRCULATIONAHA.121.054318. [DOI] [PubMed] [Google Scholar]
- 158.Argentesi G, et al. Endocrine Abstracts. Vol. 86 Bioscientifica; 2022. [Google Scholar]
- 159.US National Library of Medicine. ClinicalTrialsgov. 2023. https://clinicaltrials.gov/ct2/show/NCT05405101 .
- 160.Brown MJ, Hopper RV. Calcium-channel blockade can mask the diagnosis of Conn’s syndrome. Postgrad Med J. 1999;75:235–236. doi: 10.1136/pgmj.75.882.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee G, et al. Homeostatic responses in the adrenal cortex to the absence of aldosterone in mice. Endocrinology. 2005;146:2650–2656. doi: 10.1210/en.2004-1102. [DOI] [PubMed] [Google Scholar]
- 162.Kang S, et al. CaV1.3-selective L-type calcium channel antagonists as potential new therapeutics for Parkinson’s disease. Nat Commun. 2012;3:1146. doi: 10.1038/ncomms2149. [DOI] [PubMed] [Google Scholar]
- 163.Xie CB, et al. Regulation of aldosterone secretion by Cav1.3. Sci Rep. 2016;6:24697. doi: 10.1038/srep24697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Scholl UI, et al. Macrolides selectively inhibit mutant KCNJ5 potassium channels that cause aldosterone-producing adenoma. J Clin Invest. 2017;127:2739–2750. doi: 10.1172/JCI91733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cheng K, et al. The developmental origin and the specification of the adrenal cortex in humans and cynomolgus monkeys. Sci Adv. 2022;8:eabn8485. doi: 10.1126/sciadv.abn8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Freedman BD, et al. Adrenocortical zonation results from lineage conversion of differentiated zona glomerulosa cells. Dev Cell. 2013;26:666–673. doi: 10.1016/j.devcel.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ludwig LS, et al. Lineage tracing in humans enabled by mitochondrial mutations and single-cell genomics. Cell. 2019;176:1325–1339.:e22. doi: 10.1016/j.cell.2019.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.N MP, et al. Improved SNV discovery in barcode-stratified scRNA-seq alignments. Genes. 2021;12:1558. doi: 10.3390/genes12101558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bechmann N, Berger I, Bornstein SR, Steenblock C. Adrenal medulla development and medullary-cortical interactions. Mol Cell Endocrinol. 2021;528:111258. doi: 10.1016/j.mce.2021.111258. [DOI] [PubMed] [Google Scholar]
- 170.Bornstein SR, Ehrhart-Bornstein M, Scherbaum WA. Morphological and functional studies of the paracrine interaction between cortex and medulla in the adrenal gland. Microsc Res Tech. 1997;36:520–533. doi: 10.1002/(SICI)1097-0029(19970315)36:6<520::AID-JEMT9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 171.Brittain JM, et al. An atypical role for collapsin response mediator protein 2 (CRMP-2) in neurotransmitter release via interaction with presynaptic voltage-gated calcium channels. J Biol Chem. 2009;284:31375–31390. doi: 10.1074/jbc.M109.009951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Simms BA, Zamponi GW. Trafficking and stability of voltage-gated calcium channels. Cell Mol Life Sci. 2012;69:843–856. doi: 10.1007/s00018-011-0843-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Di Mattia T, et al. FFAT motif phosphorylation controls formation and lipid transfer function of inter-organelle contacts. EMBO J. 2020;39:e104369. doi: 10.15252/embj.2019104369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gu S, et al. Hair cell α9α10 nicotinic acetylcholine receptor functional expression regulated by ligand binding and deafness gene products. Proc Natl Acad Sci USA. 2020;117:24534–24544. doi: 10.1073/pnas.2013762117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Freeman MW, et al. Phase 2 trial of baxdrostat for treatment-resistant hypertension. N Engl J Med. 2022;388:395–405. doi: 10.1056/NEJMoa2213169. [DOI] [PubMed] [Google Scholar]
- 176.Yao X, Gao S, Yan N. Structural basis for pore blockade of human voltage-gated calcium channel Ca(v)1.3 by motion sickness drug cinnarizine. Cell Res. 2022;32:946–948. doi: 10.1038/s41422-022-00663-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Felizola SJ, et al. Pre-B lymphocyte protein 3 (VPREB3) expression in the adrenal cortex: precedent for non-immunological roles in normal and neoplastic human tissues. Endocr Pathol. 2015;26:119–128. doi: 10.1007/s12022-015-9366-7. [DOI] [PubMed] [Google Scholar]
- 178.Guiraldelli MF, et al. SHOC1 is a ERCC4-(HhH)2-like protein, integral to the formation of crossover recombination intermediates during mammalian meiosis. PLoS Genet. 2018;14:e1007381. doi: 10.1371/journal.pgen.1007381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Iwamori T, Iwamori N, Matsumoto M, Ono E, Matzuk MM. Identification of KIAA1210 as a novel X-chromosome-linked protein that localizes to the acrosome and associates with the ectoplasmic specialization in testes. Biol Reprod. 2017;96:469–477. doi: 10.1095/biolreprod.116.145458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Iwamori T, Iwamori N, Matsumoto M, Imai H, Ono E. Novel localizations and interactions of intercellular bridge proteins revealed by proteomic profiling1- Biol Reprod. 2020;102:1134–1144. doi: 10.1093/biolre/ioaa017. [DOI] [PubMed] [Google Scholar]
- 181.Ogle BM, Cascalho M, Platt JL. Biological implications of cell fusion. Nat Rev Mol Cell Biol. 2005;6:567–575. doi: 10.1038/nrm1678. [DOI] [PubMed] [Google Scholar]
- 182.Sottile F, Aulicino F, Theka I, Cosma MP. Mesenchymal stem cells generate distinct functional hybrids in vitro via cell fusion or entosis. Sci Rep. 2016;6:36863. doi: 10.1038/srep36863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jacobsen BM, et al. Spontaneous fusion with, and transformation of mouse stroma by, malignant human breast cancer epithelium. Cancer Res. 2006;66:8274–8279. doi: 10.1158/0008-5472.CAN-06-1456. [DOI] [PubMed] [Google Scholar]
- 184.Mortensen K, Lichtenberg J, Thomsen PD, Larsson LI. Spontaneous fusion between cancer cells and endothelial cells. Cell Mol Life Sci. 2004;61:2125–2131. doi: 10.1007/s00018-004-4200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pfannkuche K. Preface in Cell Fusion: Overviews and Methods. Humana: 2015. [DOI] [PubMed] [Google Scholar]
- 186.Dörnen J, Sieler M, Weiler J, Keil S, Dittmar T. Cell fusion-mediated tissue regeneration as an inducer of polyploidy and aneuploidy. Int J Mol Sci. 2020;21:1811. doi: 10.3390/ijms21051811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bornstein SR, Ehrhart-Bornstein M, Scherbaum WA. Ultrastructural evidence for cortico-chromaffin hybrid cells in rat adrenals? Endocrinology. 1991;129:1113–1115. doi: 10.1210/endo-129-2-1113. [DOI] [PubMed] [Google Scholar]
- 188.Hanukoglu I. Antioxidant protective mechanisms against reactive oxygen species (ROS) generated by mitochondrial P450 systems in steroidogenic cells. Drug Metab Rev. 2006;38:171–196. doi: 10.1080/03602530600570040. [DOI] [PubMed] [Google Scholar]
- 189.Ziegler GA, Vonrhein C, Hanukoglu I, Schulz GE. The structure of adrenodoxin reductase of mitochondrial P450 systems: electron transfer for steroid biosynthesis. J Mol Biol. 1999;289:981–990. doi: 10.1006/jmbi.1999.2807. [DOI] [PubMed] [Google Scholar]
- 190.Tadjine M, Lampron A, Ouadi L, Bourdeau I. Frequent mutations of beta-catenin gene in sporadic secreting adrenocortical adenomas. Clin Endocrinol. 2008;68:264–270. doi: 10.1111/j.1365-2265.2007.03033.x. [DOI] [PubMed] [Google Scholar]
- 191.Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Incidence of atrial fibrillation and mineralocorticoid receptor activity in patients with medically and surgically treated primary aldosteronism. JAMA Cardiol. 2018;3:768–774. doi: 10.1001/jamacardio.2018.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.GTEx Portal. GTEx analysis release V8 (dbGaP accession phs000424v8p2) [accessed 10 April 2023]. https://gtexportal.org/home/gene/KCNJ5 .
- 193.RCSB Protein Data Bank. Crystal structure of the G protein-gated inward rectifier K” channel GIRK2 (Kir32) in complex with sodium and PIP2. 2023. https://www.rcsb.org/structure/3SYA .
- 194.Ortner NJ, et al. De novo CACAN1D Ca2+ channelopathies: clinical phenotypes and molecular mechanism. Pflugers Arch. 2020;472:755–773. doi: 10.1007/s00424-020-02418-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.The Human Protein Atlas. SLC35F1. 2023. https://www.proteinatlas.org/ENSG00000196376-SLC35F1/tissue/adrenal+gland#img .
- 196.Uhlén M, et al. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 197.Williams TA, et al. Genotype-specific steroid profiles associated with aldosterone-producing adenomas. Hypertension. 2016;67:139–145. doi: 10.1161/HYPERTENSIONAHA.115.06186. [DOI] [PubMed] [Google Scholar]
- 198.Seccia TM, Caroccia B, Gomez-Sanchez EP, Gomez-Sanchez CE, Rossi GP. The biology of normal zona glomerulosa and aldosterone-producing adenoma: pathological implications. Endocr Rev. 2018;39:1029–1056. doi: 10.1210/er.2018-00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lenders JWM, Eisenhofer G, Reincke M. Subtyping of patients with primary aldosteronism: an update. Horm Metab Res. 2017;49:922–928. doi: 10.1055/s-0043-122602. [DOI] [PubMed] [Google Scholar]
- 200.Eisenhofer G, et al. Mass spectrometry-based adrenal and peripheral venous steroid profiling for subtyping primary aldosteronism. Clin Chem. 2016;62:514–524. doi: 10.1373/clinchem.2015.251199. [DOI] [PubMed] [Google Scholar]
- 201.Satoh F, et al. Measurement of peripheral plasma 18-oxocortisol can discriminate unilateral adenoma from bilateral diseases in patients with primary aldosteronism. Hypertension. 2015;65:1096–1102. doi: 10.1161/HYPERTENSIONAHA.114.04453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Vilela LAP, et al. KCNJ5 somatic mutation is a predictor of hypertension remission after adrenalectomy for unilateral primary aldosteronism. J Clin Endocrinol Metab. 2019;104:4695–4702. doi: 10.1210/jc.2019-00531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.