Abstract
Although most cancer drugs modulate the activities of cellular pathways by changing post-translational modifications (PTMs), surprisingly little is known regarding the extent and the time- and dose-response characteristics of drug-regulated PTMs. Here, we introduce a proteomic assay termed decryptM that quantifies drug-PTM modulation for thousands of PTMs in cells to shed light on target engagement and drug mechanism of action (MoA). Examples range from detecting DNA damage by chemotherapeutics, to identifying drug-specific PTM signatures of kinase inhibitors, to demonstrating that rituximab kills CD20-positive B-cells by over-activating B-cell receptor signaling. DecryptM profiling of 31 cancer drugs in 13 cell lines demonstrates the broad applicability of the approach. The resulting 1.8 million dose-response curves are provided as an interactive molecular resource in ProteomicsDB.
Most drugs act on proteins, are proteins themselves, lead to the production or degradation of proteins, or otherwise use the protein machinery of a cell to exert their therapeutic effects. Drug-regulated protein post-translational modifications (PTMs) can constitute molecular target engagement markers, can identify drug-modulated pathways, and can illuminate downstream phenotypic responses of the cell. PTM-specific antibodies are most frequently employed for this purpose. However, only a few exist, and those few yield semi-quantitative information only, biasing the investigation of drug mechanism of action (MoA) to well-characterized proteins and pathways. Because desired or undesired polypharmacology is common and because drug responses often differ between cell types, it is important to characterize drugs on a proteome-wide scale in order to understand their MoAs. Consequently, proteomics has become an important tool in drug discovery and chemical biology (1–4). Yet, little systematic information is available about drug action at the level of PTMs, although many drugs modulate the activity of enzymes that regulate PTMs, such as kinases or lysine deacetylases (KDACs). Even more surprising is the lack of literature characterizing drugs at the level of proteins and PTMs in a dose- and time-dependent fashion, arguably among the most important characteristics of drug action in any biological context (5).
DecryptM: proteome-wide drug dose-response profiling of PTMs
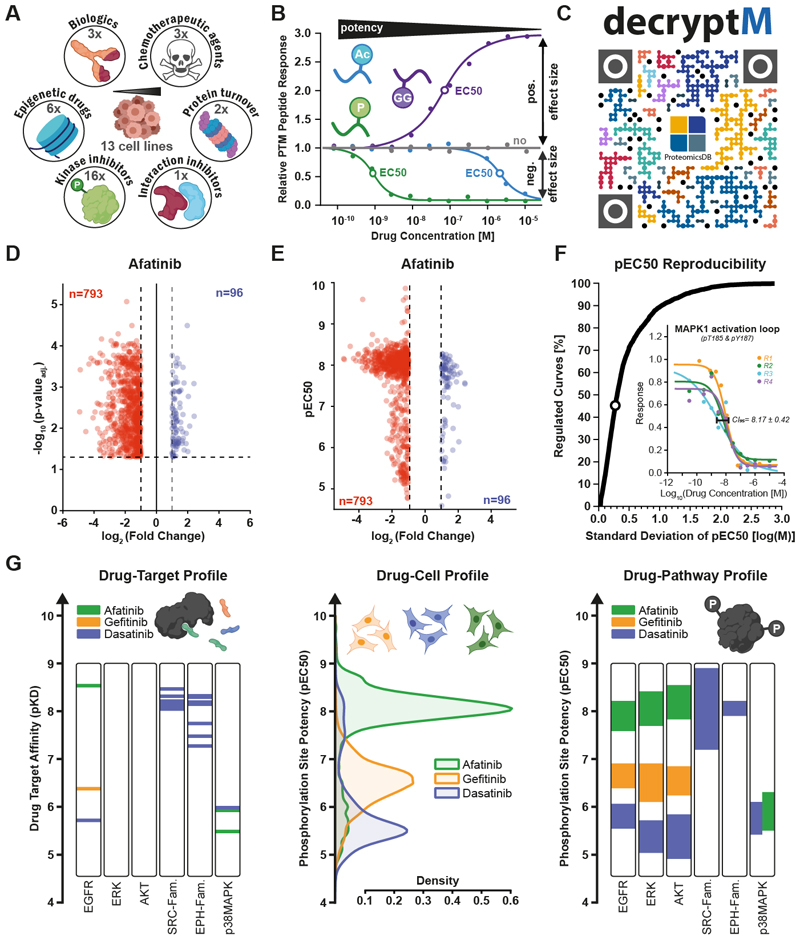
Here, we present a quantitative proteomic approach termed decryptM, able to assess target and pathway engagement as well as the MoA of diverse cancer drugs by systematically measuring their dose- (and time-) resolved modulation of PTMs in cells (Fig. 1AB). Briefly, cells are treated with increasing concentrations of a drug and multiplexed using stable isotopes (tandem mass tags; TMT; except for ubiquitinylation) (6). Tryptic peptides are fractionated by off-line chromatography for proteome expression profiling, and peptides bearing PTMs are enriched by immunoprecipitation (acetylation, ubiquitinylation) or immobilized metal affinity chromatography (IMAC; phosphorylation). Following liquid chromatography tandem mass spectrometry (LC-MS/MS) analysis, we derived dose-response characteristics for each peptide from the TMT reporter ion intensities by fitting and filtering sigmoidal curves using a four-parameter logistic regression (Fig. S1A) (see Methods for details). Data collected for 31 drugs representing six drug classes in 13 human cancer cell line models (Fig. 1A) demonstrate that the decryptM approach is broadly applicable. The body of data represents 1.8 million quantitative cellular drug assays (dose-response curves) including 47,502 regulated phosphopeptides (124,660 detected on 11,982 proteins), 7,316 regulated ubiquitinylated peptides (9,173 detected on 3,006 proteins), and 546 regulated acetylated peptides (2,478 detected on 1,377 proteins;Fig. S1BC). Most PTMs were not regulated by most drugs which is valuable for understanding which pathways are addressed (or not) by each drug in cells. Only few examples can be highlighted in this report, but all primary mass spectrometry, peptide and protein identification, and quantification data, as well as dose-response curves are available from PRIDE (PXD037285). In addition, the decryptM data have been incorporated into ProteomicsDB (proteomicsdb.org/decryptm; Fig. 1C) (7), where it can be interactively explored via a web browser or mobile device (Fig. S2A). Among the new functionalities is a drug-centric view allowing searching and filtering dose-response curves for EC50 values (half-maximal effective concentration), curve quality metrics, sequence motifs, and other parameters and cross-references to UniProt and PhosphoSitePlus (8, 9). Multiple PTM peptides or a range of EC50 values can be compared to discover similarities that may place functionally unknown sites into the same pathway. In a protein-centric visualization, the position of drug-regulated PTM-sites can be shown within the protein sequence and its 3D structure (Fig. S2BC).
Fig. 1. DecryptM profiling of therapeutic drugs reveals essential details of their molecular mechanism of action.
(A) Schematic representation of the data set comprising 31 drugs from six different classes and 14 cellular models. (B) Schematic representation of the 1.8 million drug dose-response curves obtained for proteins or post-translationally modified peptides by LC-MS/MS (P, phosphorylation; GG, ubiquitinylation; Ac, acetylation). For each peptide, PTM site, or protein, the half-maximal effective concentration (EC50) and the cellular effect size was determined. (C) QR code linking to ProteomicsDB where all dose-response curves can be interactively explored. (D) Volcano plot summarizing replicate (n=4) single-dose afatinib treatments of A-431 cells (10 μM drug concentration, t-test p-value multiple testing corrected at 1% FDR, fold change cut-off of 2). Each dot is one phosphopeptide (blue: upregulated by drug, red: down-regulated). Volcano plots for dasatinib and gefitinib can be found in Fig. S4A. (E) Same as panel D, but providing potency information for each regulated phosphopeptide (pEC50 = -logEC50). Not-regulated phosphopeptides were omitted for clarity. Potency plots for Dasatinib and Gefitinib can be found inFig. S4B. (F) Cumulative distribution plot shows the reproducibility of pEC50 estimations from replicate dose-response analysis (n=4) of afatinib, gefitinib, and dasatinib. Seventy-two percent of all pEC50 estimates were reproducible within ½ log step of drug concentration. The inset shows replicate dose response curves (along with the confidence interval at 95%) for the activation loop peptide of MAPK1 as a representative example, indicated by the dot in the cumulative distribution. (G) Left panel: in-vitro drug-target affinities of afatinib, gefitinib and dasatinib measured by Kinobeads assays (pKD = -logKD) (2) for selected kinases. Middle panel: in-cellulo potency (pEC50) distributions of phosphorylation sites regulated by the same drugs in A-431 cells. Right panel: same as middle panel but grouping regulated phosphopeptides by kinase substrate sites in annotated pathways (PhosphoSitePlus; Table S1).
Decrypting drugs and PTMs
DecryptM drug profiles are more informative than the conventional, differential approach of measuring drug effect sizes in replicates at one (typically high) drug concentration. This is because dose-response curves yield EC50 values, effect sizes, and additional curve fit parameters (Fig. 1B). These advantages of decryptM are illustrated by analyzing the phosphoproteome of A431 epidermoid carcinoma cells (dependent on EGFR expression) in response to the EGFR inhibitor afatinib (Fig. 1D, Fig. S3). While replicates of single-dose treatments identified a large number of statistically significantly regulated phosphopeptides, only the dose-response data discriminates effects by potency (Fig. 1E; Table S1). For afatinib, most phosphopeptides produced a coherent drug response at a pEC50 of ~8 (10 nM; pEC50 = -log EC50). Further phosphopeptides were regulated with weaker potency and smaller effect sizes, rendering these less interesting for primary MoA elucidation. Neither fold-changes nor p-values of single-dose replicates are indicative of such differences in afatinib potency (Fig. S4). DecryptM assays are reproducible with 70% and 90% of all determined EC50 values within half a log or one log of drug concentration, respectively (Fig. 1F).
The ability to assign potency to each regulated phosphopeptide has important implications for the interpretation of drug perturbation experiments. To illustrate this, we extended the experiment to include the lesser potent EGFR inhibitor gefitinib and the multi-kinase inhibitor dasatinib, which has a weak affinity for EGFR. Target deconvolution using Kinobeads (a proteome-wide quantitative drug binding assay using immobilized kinase inhibitors) (2) showed that none of the three drugs interacts with ERK (MAPK1/3) or AKT, dasatinib interacts with members of the SRC and EPH families with high potency, and that dasatinib and afatinib only weakly bind p38 MAPK (Fig 1G). DecryptM profiling revealed that most regulated phosphopeptides, and the pathways they are involved in, closely mirrored the potency for their targets (Fig. 1G; Table S2). This close coherence between drug-target affinity (pKD) and in-cell drug-PTM potency (pEC50) raises the exciting possibility that functionally uncharacterized PTM sites may be placed into known pathways and thus be ‘decrypted’ on the grounds of guilt-by-association. In the following, examples representing six drug classes are discussed to highlight how decryptM profiling may be used to characterize the MoA of drugs in cells and to assign functional context to PTMs.
Chemotherapeutic drugs
DecryptM profiles were generated for the microtubule stabilizer paclitaxel and the anti-metabolites cytarabine and methotrexate in K562 chronic myelogenous leukemia cells. Only two phosphopeptides showed regulation by methotrexate within the timeframe (30 min drug treatment) of the experiment, and these could not be linked to anti-folate related biology. For paclitaxel, 7 out of 7,438 measured phosphopeptides were upregulated with EC50 values of about 1μM. The corresponding proteins, MAP7, MAP7D3, and ARHGEF2, all function in microtubule stabilization. This result demonstrates the extreme specificity with which drug-regulation can be called by the dose-dependent measurements and shows that the molecular MoA of paclitaxel is detectable already after 30 min (Fig. S5A). For cytarabine, 23 phosphopeptides were potently regulated (EC50 <100 nM), and many of the underlying proteins are involved in the DNA damage response (e. g. NBM, NIPBL, TIPIN; Fig. S5B). Most of these phosphopeptides are annotated to respond to ionizing radiation or UV light in PhosphoSitePlus (e. g. HMGA1, PSMD4, ZMYM4, RFC1). Eight are phosphorylated at SQ sites, the substrate motif of the DNA damage response kinases ATM and ATR, and several have indeed been shown to be substrates of these kinases. This data shows that decryptM profiling can detect the cellular response to DNA damage as early as 30 min, long before any phenotypic changes can be observed.
Protein interaction inhibitors
SHP099 is an allosteric inhibitor of the non-receptor tyrosine phosphatase SHP-2 (PTPN11). It inhibits the growth of cancer cells by stabilizing the inactive form of SHP-2, thereby blocking the activation of the MAPK pathway by receptor tyrosine kinases (RTK) (10). This MoA is reflected in the 30 min decryptM profile of SHP099 in the EGFR overexpressing esophageal squamous cell carcinoma cell line KYSE-520 (Fig. S5C). Although SHP099 does not inhibit phosphorylation sites indicating EGFR activity, phosphorylation of the EGFR substrate GAB1 is diminished in a dose-dependent fashion. This is because GAB1 can no longer associate with EGFR via SHP2, and this uncoupling prevents MAPK1/3 activation. Phosphorylation sites on proteins downstream of MAPK1/3 were inhibited with similar potencies (~700 nM; e.g. the kinases STK10 and RPS6KA1, the adaptor GAB2, the phosphatase DUSP16, and the transcription factor ELK1). About 30 further proteins, including many transcriptional regulators, also showed EC50 -values between 100-500 nM. The current analysis places these proteins and phosphopeptides into the functional context of the RTK-MAPK axis and illustrates how pharmacological SHP-2 inactivation leads to a rapid breakdown of oncogenic signaling and transcriptional activity.
Proteasome inhibitors
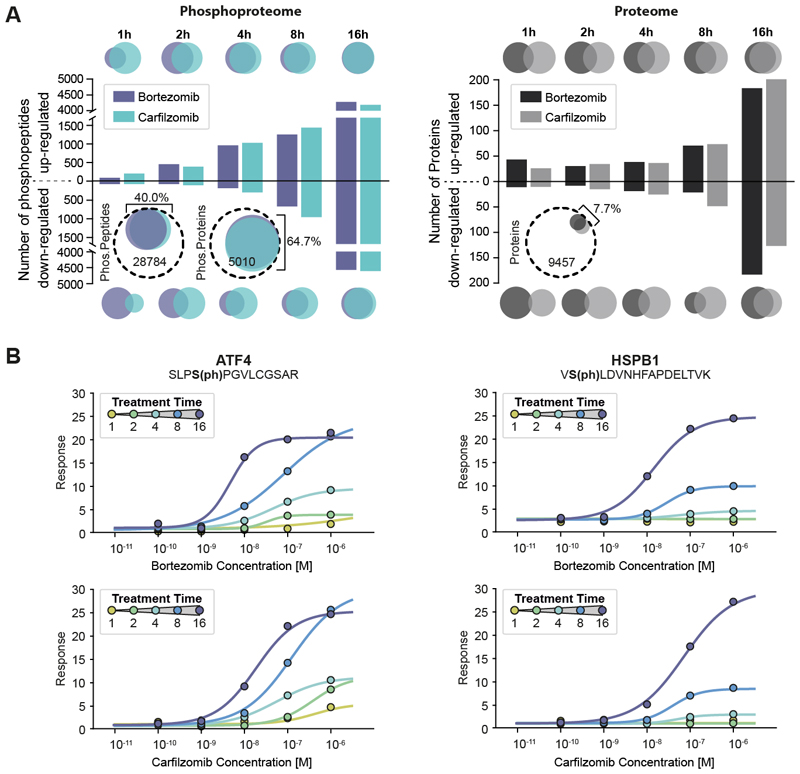
The proteasome processes proteins that are marked for degradation by phosphorylation and ubiquitinylation. Bortezomib (BTZ) and carfilzomib (CFZ) have become the therapeutic backbone for treating multiple myeloma. Both drugs inhibit the proteasome by reversible covalent (BTZ) or irreversible (CFZ) binding to the protease PSMB5. Two-dimensional decryptM profiling (i.e. dose-response data collected at five different time points) in the multiple myeloma cell line RPMI 8226 showed broadly similar effects for both drugs (Fig. 2A; Fig. S6). The number of regulated phosphorylation sites drastically increased over time and included very strong activation of cellular stress (Table S6). Among these changes is the potent (EC50 <100 nM) and strong (20-fold) upregulation of phosphorylation at S248 of ATF4, the master transcription factor of the integrated stress response (11), and at S98 of the small heat shock protein HSPB1, both an activator of the proteasome and an inhibitor of apoptosis (Fig. 2B) (12, 13). The two-dimensional decryptM data also revealed that drug effects became more potent over time (Fig 2B; Fig. S6AB). This may be explained by the drugs’ slow binding mode (14) or their covalent nature, which may reduce the number of functional proteasomes over time.
Fig. 2. Two-dimensional (2D) decryptM analysis of covalent proteasome inhibitors highlighting how cells mount a massive stress response.
(A) Left panel: Bar plot showing the number of dose-dependently up- or down-regulated phosphopeptides at a given time of treatment with bortezomib and carfilzomib in RPMI8226 cells. The Venn diagrams at the top and bottom illustrate the number (size) and overlap of phosphopeptides between the two drugs at a given time point. The circled Venn diagrams illustrate the total (dashed line) and regulated fraction (colored circles) of phosphopeptides and proteins detected for the two drugs. Right panel: same as left panel but for protein expression. (B) Examples for 2D decryptM profiles for bortezomib (top row) and carfilzomib (bottom row) for two exemplary phosphopeptides on proteins involved in cellular stress responses (AFT4, HSPB1).
In stark contrast to the phosphorylation data, very few proteins showed expression changes (Fig. 2A). Most of these are stress response-related transcription factors and chaperones (Fig. S6C) which can be rationalized as follows: upon inhibition of the proteasome, misfolded proteins accumulate in the cell, reflected in a strong increase in ubiquitin levels (Fig. S6D) and the detection of numerous known phospho-degrons (Table S3). Consequently, the cell increases the production of stress-resolving proteins. At the same time, the decryptM data showed reduction in phosphorylation on many members of the transcription and translation machineries of the cell, indicating the shutdown of these processes in response to the drugs. This way, the cell may be able to sustain proteostasis for some time before eventually succumbing to the loss of the ability to degrade proteins.
Epigenetic drugs
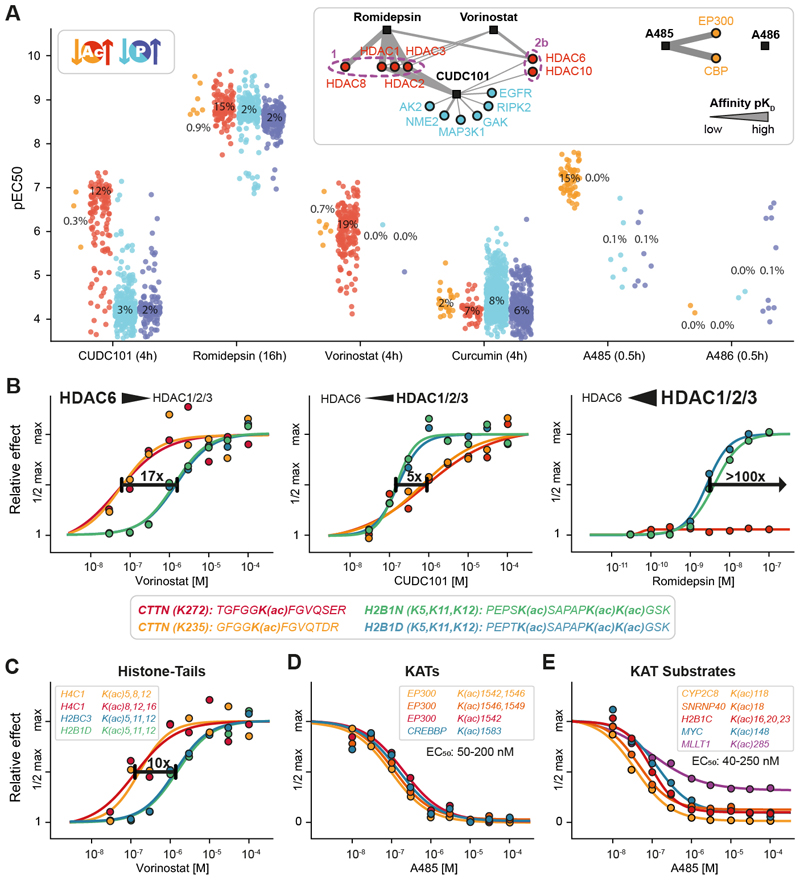
Cancer cell proliferation requires frequent chromatin remodeling. Therefore, lysine acetyltransferases (KATs) and lysine deacetylases (KDACs) have become drug targets. To learn more about their cellular MoAs, we extended the decryptM approach to protein acetylation in HeLa cells. CUDC-101, a dual multi-kinase and HDAC1/2/3 inhibitor, upregulated 139 of the 1,133 measured acetylated peptides with nanomolar potency (Fig. 3A). The potency for phosphopeptides was much weaker, indicating that CUDC-101 engages its HDAC targets with 100-fold higher affinity than its kinase targets in cells. This questions the pharmacological relevance of kinase inhibition at least in this model system. The HDAC inhibitor vorinostat also had no noticeable effect on protein phosphorylation within 4 hours. In contrast, the natural compound romidepsin triggered a broad and potent response of the phosphoproteome after 16 hours, which may be related to HDAC inhibition but may also result from the extended incubation time, which was chosen because of the slow on-rate of this peptidic drug (15).
Fig. 3. Phosphorylation and acetylation decryptM signatures of epigenetic drugs delineate substrate preferences of KDACs and KATs.
(A) Strip plots of the cellular potency (pEC50) of drug-regulated acetylated (red) and phosphorylated peptides (blue). The insets show the affinity networks of targets engaged by the drugs determined by Kinobeads, KDAC-beads, or recombinant KAT assays. The strength of the line indicates the potency of drug:target interactions (apparent dissociation constant Kdapp;Table S2) (2, 16, 21). The treatment time is indicated in parenthesis next to drug names and percentages indicate the proportion of regulated PTM peptides vs. all PTM peptides measured in the assay. (B) Examples of cellular dose-response curves for three HDAC inhibitors illustrating the different potencies by which these drugs regulate cellular acetylation levels on cortactin or histones. (C) Same as panel B, but for several acetylation sites on histone tails. (D) Same as panel B, but for auto-acetylation sites on KATs after KAT inhibitor A485 treatment. (E) Same as panel B, but for known and putative novel KAT substrate acetylation sites.
DecryptM profiles of KDAC inhibitors mirrored their target selectivity (Fig. 3A). For instance, vorinostat binds much more potently to HDAC6 than HDAC1/2/3 (15, 16). Consequently, the drug potently enhanced acetylation of the HDAC6 substrate cortactin (CTTN, EC50 50-90 nM) (17) but not of the HDAC1/2/3 substrates H2B1N and H2B1D (EC50 1.0-1.5 μM). The weak HDAC6 inhibitor CUDC-101 increased CTTN acetylation only at substantially higher concentrations and romidepsin had no effect on CTTN acetylation within the tested dose range (Fig. 3B). This confirms that CTTN acetylation at K235 and K272 are markers for HDAC6 engagement in cells. Other acetylated peptides with the same characteristics may thus be considered potential HDAC6 substrates. Examples include the cytoskeletal proteins PARVA and DBNL or proteins that play a regulatory role in autophagy, including DAP and PLAA (Fig. S7A). These processes are regulated by HDAC6 (18), and a previous study indeed showed increased acetylation of DBNL, PARVA, and PLAA in response to the HDAC6-selective inhibitor tubacin (19).
The decryptM data also uncovered details regarding the acetylation of histone tails. For instance, the N-terminal tri-acetylated peptide of H4C1 showed a 10-fold more potent effect than the corresponding peptide of H2BC3 in response to vorinostat (Fig. 3C) and romidepsin (Fig. S7B). We propose that this reflects different drug-target affinities for different HDAC1/2/3 isoforms and their complexes with defined substrate preferences. These drugs may have a distinct affinity to HDAC2 compared to HDAC1/3, as HDAC2 has recently been shown to deacetylate these sites on H2B, while HDAC1/3 primarily act on H4 (20).
The development of the KATs inhibitor A485 marked a breakthrough because it was the first compound that selectively and potently inhibited transcriptional co-activators CBP and p300 (21). In a seminal report, Weinert et al. used proteomics and a single dose of A485 to identify a large number of potential CBP/p300 substrates and their temporal dynamics (22). DecryptM profiles of A485 in HeLa cells showed that A485 downregulated relatively few (n=59) acetylated peptides and did not have much impact on the phosphoproteome (Fig. 3A). Several (auto-) acetylation sites on CBP and p300 were inhibited with 50-200 nM EC50, demonstrating target engagement in cells (Fig. 3D). Numerous histones and other nuclear proteins showed effects in the same dose range, including MYC K185, confirming their specificity as substrates (Fig. 3E). In addition, many new drug-responsive acetylation sites on functionally very diverse proteins were identified. Among these were MLLT1 (EC50 100 nM), a chromatin reader that recognizes protein acetylation, SNRNP40 (EC50 50 nM), a spliceosomal protein, and the P450 enzyme CYP2C8 (EC50 38 nM). CYP2C8 typically resides in the ER and detoxifies xenobiotics such as the cancer drug Paclitaxel (23). Because the protein is likely a CBP/p300 substrate, CYP2C8 may have additional functions in the nucleus akin to other oxygenases (24). The structurally similar but inactive analog A486 was ~350-fold less potent for CBP acetylation, illustrating the potential value of decryptM profiles for understanding structure-activity relationships (Fig. S7C). The natural compound curcumin has been proposed to inhibit KDACs and KATs (25). Because it is chemically reactive and among the strongest pan-assay interference compounds (PAINS) (26), the large number of regulated PTM peptides observed in the decryptM analysis (Fig. 3A) likely reflects unselective polypharmacology rather than any particular molecular mechanism.
Kinase inhibitors
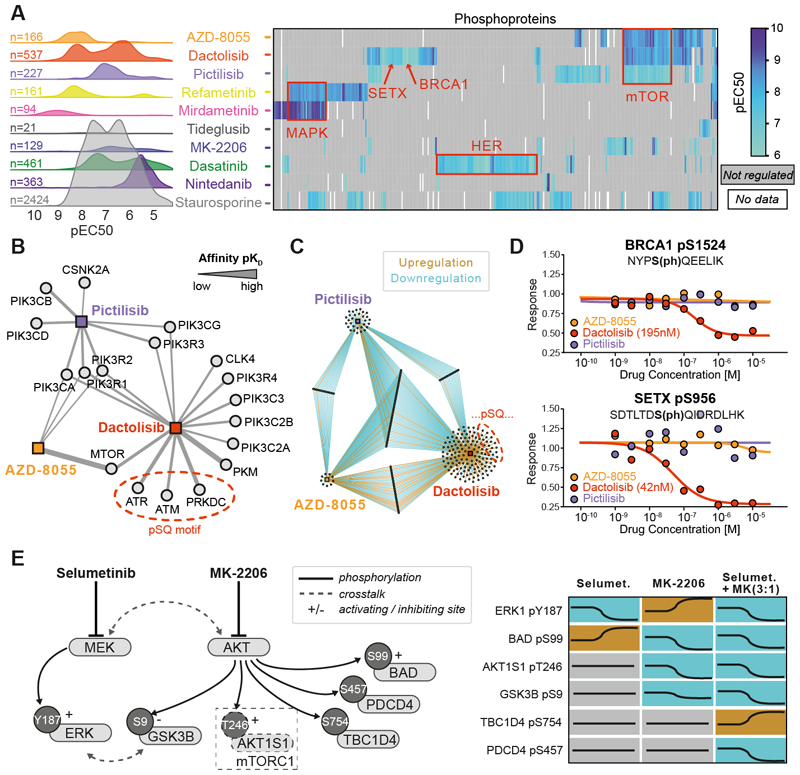
Kinase inhibitors (KI) have become important medicines, particularly in oncology. Although their target profiles and selectivity have been extensively studied, far less is known about which PTMs these drugs affect in cells. To begin to address this systematically, we performed decryptM for 10 KIs with a diverse target spectrum in A549 lung cancer cells. This survey uncovered drug-regulated phosphopeptides (Fig. 4A) in structured domains as well as disordered regions of proteins (Fig. S8AB). The pan-kinase inhibitor staurosporine regulated nearly 2500 phosphopeptides over a broad range of potencies. All other drugs showed fewer regulated phosphopeptides (typically 10s to 100s) and had narrower pEC50 distributions because of fewer targeted kinases. However, many KIs showed bi-modal pEC50 distributions (Fig. S9), implying engagement of different targets and pathways with distinct potencies or unspecific cellular toxicity at very high concentrations (Fig. S10).
Fig. 4. DecryptM analysis of kinase inhibitors identifies drug-specific signatures and place phosphorylation sites into functional contexts.
(A) Left panel: decryptM- derived potency distribution plots of the number of phosphopeptides regulated by 10 kinase inhibitors in A549 cells. Right panel: pEC50 heatmap summarizing drug effects on annotated substrates of kinases or pathway members (red boxes). Not-regulated or missing values are shown in gray or white, respectively. (B) Affinity network (based on Kinobeads assays in pKD) of kinases inhibited by the three designated PI3K/mTOR inhibitors Pictilisib, AZD8055, and dactolisib. The strength of the line indicates the affinity of drug:target interactions (C) Network of phosphopeptides regulated by the same three drugs as in panel B. (D) Example dose-response curves for pSQ-sites on BRCA1 and SETX that were uniquely regulated by dactolisib. (E) Schematic representation of cross-talk between the MAPK and AKT pathways and dose-dependent phosphorylation changes by selumetinib and MK-2206 alone or in combination (concentration ratio of 3:1).
We next asked to what extent KIs regulate the same phosphopeptides and signaling pathways. Each drug appeared to leave a particular decryptM signature that may constitute a pharmacodynamic marker for target and pathway engagement, identify points of conversion of several pathways, or distinguish closely related compounds. Evidence for these aspects could be delineated from clustering proteins with drug-regulated phosphorylation sites (Fig. 4A). For instance, the two MEK inhibitors refametinib and mirdametinib shared regulated phosphopeptides in the MAPK pathway. Similarly, the designated mTOR inhibitor AZD-8055, the dual mTOR/PI3K inhibitor dactolisib, and the PI3K inhibitor pictilisib shared regulated phosphopeptides of mTOR pathway members. Extending the pathway analysis to all drugs portrayed a similar picture that can be rationalized by shared targets between drugs, but drug-pathway networks also highlighted connections beyond linear signal propagation that are the result of pathway crosstalk or feedback loops (Fig. S11).
Other parts of the decryptM signatures appeared compound-specific. Dactolisib is the only drug among the three mentioned above that potently targets the DNA damage sensor kinase PRKDC (KD 42 nM), as well as the DNA damage-activated kinases ATR (KD 91 nM) and ATM (KD 477 nM), as determined by the Kinobeads assay (Fig. 4B; Table S2). These three kinases phosphorylate substrates with SQ/TQ motifs. The decryptM profile of dactolisib contained 17 regulated SQ/TQ phosphorylation sites with EC50 -values of 25-190 nM on 15 proteins not shared with the other two drugs, ruling them out as mTOR substrates (Fig. 4C). Among them is the BRCA1 oncogene, a known ATM/ATR substrate (Fig. 4D). The kinases for the other phosphorylation sites have not yet been delineated, but four respond to ionizing radiation (PhosphoSitePlus). It may, therefore, be reasoned that some of these sites could be targets of ATM, ATR, or PRKDC. Among these is the functionally orphan site pS956 of SETX (47 nM), a protein that has been reported to be involved in the cellular response to DNA damage (Fig. 4D; PhosphoSitePlus).
In an attempt to functionalize phosphorylation sites more systematically, we adopted a published machine learning approach that assigns functional scores to phosphorylation sites (27). The analysis revealed a small but statistically significant difference between drug regulated and not regulated phosphorylation sites for all 13 KIs tested (Fig. S12). Most of the top scoring cases were already functionally annotated in PhosphositePlus but also contained sites without an ascribed function. Distinctive cases identified from decryptM data may therefore be prioritized for functional follow-up studies based on their computed functional scores. The data generated here may also aid in further developing such machine learning tools.
Cancer cells often develop resistance to single KIs, unfortunately. For instance, resistance to MEK or AKT inhibitors can be conveyed by cross-talk of the two pathways (28). DecryptM profiling in A549 lung cancer cells (Fig. 4E) confirmed that the AKT inhibitor MK-2206 reduced phosphorylation of the AKT substrates AKT1S1 (T2469) and GSK3B (S9) but induced ERK1 activity via phosphorylation at Y187. Conversely, the MEK inhibitor selumetinib abrogated ERK phosphorylation but induced phosphorylation of the AKT substrate BAD at S99. Drug combinations have proven effective in tackling such resistance mechanisms, and combining MK-2206 and selumetinib led to the desired inhibition of both pathways as well as to synergistic cell killing (Fig. S13AB). The decryptM profiles also uncovered surprising changes in protein phosphorylation. For instance, phosphorylation of the Rab GTPase activating protein TBC1D4 at S754 only increased, and phosphorylation of the tumor suppressor PDCD4 at S457 only decreased when combining both drugs (Fig. 4E; Fig. S13C). These differing outcomes imply the presence of further players in the pathway cross-talk that have yet to be discovered.
The molecular wiring of signaling pathways in different cancer cells can diverge strongly, and decryptM profiling can uncover such cell-line specific signatures for a given drug. We chose the exquisitely selective EGFR/HER2 inhibitor lapatinib as an example and profiled three common cell line models that recapitulate important aspects of breast cancer biology: BT-474 (HR-positive, HER2-positive), SK-BR-3 (HR-negative, HER2-positive), and MDA-MB-175 (HR-negative, HER2-negative, HER3-positive) (29). Full proteome measurements confirmed that BT-474 cells express high levels of EGFR and HER2 (both inhibited by lapatinib), that SK-BR-3 cells express EGFR and HER2 at lower levels, and that MDA-MB-175 cells only express traces of EGFR and HER2 but high levels of HER3 (no functional kinase domain; Fig. S14AB; Table S4). Lapatinib reduced growth in all cell lines (158 nM, 234 nM, and 695 nM, respectively; Fig. S14C; Table S5). The drug regulated hundreds of phosphopeptides in BT-474 and MDA-MB-175 cells but merely five in SK-BR-3 cells (Fig. S14D). Interestingly, these five included potent inhibition of pY427 on SHC1 (18 nM) and pT246 on AKT1S1 (11 nM), verifying drug engagement of the RTK-SHC1-PI3K-AKT axis. In contrast to the other cell lines, SK-BR-3 cells showed no changes in SOS1, RAF, MEK, and ERK phosphorylation. This indicates that the MAPK pathway is decoupled from EGFR/HER2 signaling in SK-BR-3 cells and may explain why stronger phosphorylation changes were observed in BT-474 and MDA-MB-175 cells (Fig. S14E). Their response to lapatinib resembled each other but differed in extent and detail. BT-474 cells showed a more prominent gene ontology signature for ERBB/MAPK than MDA-MB-175 cells. The opposite was observed for mTOR signaling (Table S6). Both cells scored high for the spliceosome, which indicates cell stress. This is in line with live cell imaging data of BT-474 spheroids, which detected cytotoxicity within 12-24 hours of lapatinib treatment (Movie S1).
Antibodies
Therapeutic antibodies mark cells for recognition by the immune system, block receptor-ligand interactions, abrogate intracellular survival signaling, or promote death-inducing pathways. Interestingly, the intracellular MoAs of some of even the most established therapeutic antibodies are still rather unclear. As an example, we applied decryptM analysis to the breast cancer therapeutics trastuzumab (presumed to block signaling by preventing HER2 dimerization) and pertuzumab (blocking signaling by preventing HER2-HER3 dimerization) (30, 31) in the same three breast cancer cell line used for lapatinib above (Fig. S15A). In stark contrast to lapatinib, trastuzumab had no potent impact on the cellular phosphoproteomes of any of the three cell lines (Fig. S15B). Live cell imaging of BT-474 spheroids showed that lapatinib led to their rapid disintegration, but spheroids continued to grow over the course of seven days under trastuzumab treatment (Fig. S15C, Movie S1). The decryptM data concurs with other reports in that the MoA of trastuzumab does not include a clear element of blocking mitogenic signaling (30, 31). DecryptM analysis of pertuzumab resulted in ~30 regulated phosphopeptides in SK-BR-3 cells (no HER3 expression) and ~150 in BT-474 cells (low HER3 expression), but there was virtually no overlap with phosphopeptides regulated by lapatinib. In contrast, pertuzumab also regulated ~150 phosphopeptides in MDA-MB-175 cells (high HER3 expression), including downregulated phosphopeptides shared with lapatinib such as on SHC1, MAPK1/3, and AKT1S1 (Fig. S15D). This result shows that pertuzumab cuts off the HER3-MAPK and HER3-PI3K/AKT signaling axis and explains how pertuzumab can retard cell cycle progression in breast cancer (32). We note that, in this context, among many other sites, pertuzumab reduced UCK2 phosphorylation at S254. This site is annotated to respond to EGF and nocodazole (PhosphoSitePlus). The former connects it to HER3 and the latter points to arresting cells in G2/M of the cell cycle. Similarly, the antibody led to the downregulation of pS202 on OXR1. This site also responds to EGF (PhosphoSitePlus), and depletion of OXR1 in cells has been shown to lead to G2/M cell cycle arrest (33). Both sites are also regulated by lapatinib. This makes a case for placing UCK2 and OXR1 into a functional context with HER2/HER3 signaling and cell cycle arrest in MDA-MB-175 cells.
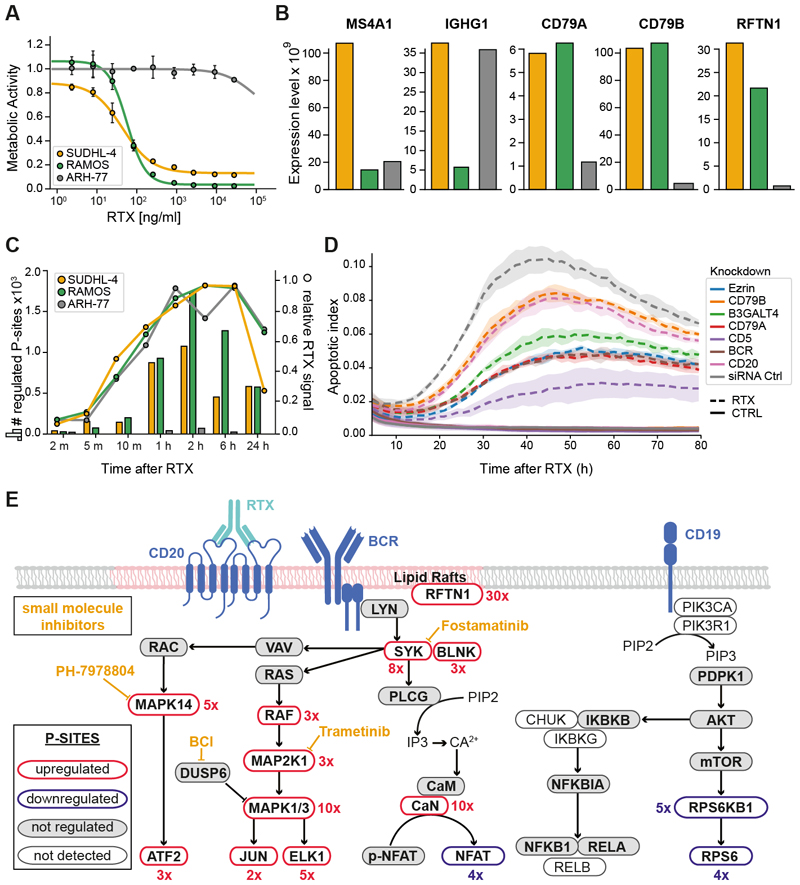
Rituximab (RTX) is used for the treatment of B-cell malignancies by way of targeting MS4A1 (CD20). Despite decades of research, many molecular aspects of how RTX leads to B-cell depletion have remained elusive, and the relevance of the different response mechanisms in patients is still largely undetermined (34, 35). To investigate to what extent phosphorylation-dependent signaling pathways are involved in the MoA of RTX, we chose the RTX-sensitive cell lines SU-DHL-4 (B-cell lymphoma) and Ramos (Burkitt's Lymphoma) as well as the RTX-insensitive plasma leukemia line ARH-77 (Fig. 5A; Table S5) for decryptM analysis. Time-resolved full proteome analysis showed, that all cell lines express MS4A1 and components of the B-cell receptor (Fig. 5B), that all cell lines rapidly bind RTX (Fig. 5C), and that all cells do not undergo substantial proteome expression changes over the course of the experiment. In stark contrast, two-dimensional decryptM analysis of the phosphoproteome (RTX dose-response at eight time points between 0 min and 24 h) showed massive responses in RTX-sensitive SU-DHL-4 and Ramos cells, while RTX-insensitive ARH-77 cells remained ‘silent’ (Fig. 5C). Within 1-10 min of RTX treatment, GO analysis revealed a dominant signature for activation of B-cell receptor (BCR) signaling (p=3.2 E-6 in SU-DHL-4 cells; p=2.0 E-9 in Ramos cells; Table S6). In addition, a number of proteins involved in cytoskeleton remodeling and lipid raft formation were also strongly regulated. The response of SU-DHL-4 cells to RTX could be attenuated by siRNA-mediated knockdown (2 days) of its target MS4A1/CD20, components of the B-cell receptor (IGHG1, CD79A, CD79B, CD5), the lipid raft regulator EZR (36), and B3GT4, an enzyme involved in the biosynthesis of the ganglioside GM1, which is a major component of lipid rafts (Fig. 5D). At later time points (2-24 hours), the B-cell receptor signaling signature persisted (p=1.4 E-8) and was accompanied by a strong signal for the spliceosome (p=1.0 E-8), cellular senescence (p=1.2 E-3) and apoptosis (p=1.3 E-2; Table S6), reflective of increasing cellular stress responses and shifting cells towards apoptotic cell death (Fig. S16). This interpretation is backed by multiple lines of evidence, including a >10x downregulation of phosphorylation on the proliferation marker MKI67, live cell imaging data of SU-DHL-4 cells showing rapid aggregation of cells akin to other B-cell stimuli (37), rapid Annexin V staining indicating apoptosis induction, and cell death as measured by cytotox green (Fig. S17A). Similar GO signatures were also observed in Ramos cells (Table S6).
Fig. 5. DecryptM analysis of rituximab reveals antibody-based killing of B-cells via activation of the BCR-MAPK signalling axis.
(A) Viability assays of rituximab (RTX) sensitive and resistant cell lines. (B) Protein expression of B-cell receptor and lipid raft components in the same cell lines. (C) Number of dose-response regulated phosphopeptides at different times of RTX treatment for the same cell lines (left axis, bars) and the kinetics of RTX binding to these cells (right axis, lines). (D) Temporal dynamics of apoptosis induction upon addition of RTX to SUDHL-4 cells with and without prior siRNA-mediated knock-down of B-cell receptor and lipid raft components. Induction of apoptosis was monitored by annexin V-labeling using live cell imaging. Shaded areas indicate the standard deviation of replicate experiments (n=3). (E) Summary representation of the major five pathways involved in BCR-signaling and working model based on time- and dose-resolved decryptM profiles as well as pharmacological inhibition of certain proteins (yellow) of how engagement of these pathways (or lack thereof) leads to RTX-mediated cell death. Figures in red indicate the fold change of RTX-induced phosphorylation regulation.
Regulating the survival (immune response) or death (tolerance induction) of B cells is essential to maintain the integrity of the immune system. The right balance is achieved by a concert of activated signaling networks, the details of which are not fully understood, cell type specific and highly dynamic. Depending on the antigen bound by the BCR and other co-stimulating factors, MAPK, JNK, and PI3K-AKT-mTOR signaling, as well as translocation of the transcription factors NF-kB and NFAT are induced upon BCR engagement. All four pathways are covered by the decryptM data of SU-DHL-4 cells, but very different levels of engagement following RTX treatment were detected (Fig. 5E). For instance, phosphorylation of the SYK (a BCR-proximal kinase of key importance in BCR signaling into the MAPK and p38 pathways) was up >10-fold. An even stronger upregulation of the activation loop phosphorylation sites on MAPK1/3 and on many of their substrates was observed within minutes of RTX treatment. Robust induction of phosphorylation of calcineurin (PPP3CB/C) and, consequently, rapid and potent de-phosphorylation of several sites on NFAT proteins was also observed (e. g. NFATC2-S110; Fig. S17B). In contrast, engagement of the survival-promoting PI3K/AKT and NFkB pathways could not be detected. Downregulated phosphorylation sites on the mTORC1 substrate RPS6KB1 and its substrate RPS6 may even hint toward reduced mTORC1 activity upon RTX treatment (Fig. S17B). Comparing protein expression across the three cell lines showed that ARH-77 cells express very low levels of the BCR complex partners CD79A/B, which are necessary for the initiation of BCR signaling, and RFTN1 (raftlin), a major component of lipid rafts responsible for their integrity and required for BCR signaling (Fig. 5B) (38). This suggests that ARH-77 cells failed to respond to RTX because they are unable to form stable rafts and a functional BCR signalosome. We note that RFTN1 phosphorylation at S467 increased 10-fold within 2 min of RTX treatment, 30-fold within 5 min, and remained high for at least two hours in SU-DHL-4 cells (Fig. S17C). Although the upstream kinase is not yet known, the decryptM data places this phosphorylation site (as well as many others not discussed here) into the functional context of BCR signaling. A recent report has also linked RTX to B-cell death via engaging BCR-signaling in SU-DHL-4 cells (39), and our study concurs with many of their observations. The time- and dose-dependent data provided here enabled a more fine-grained analysis of the events eventually leading to cell death.
Adding to the siRNA experiments presented above, we performed time-resolved live cell imaging experiments to measure cytotoxicity in SU-DHL-4 cells treated with RTX alone or pre-treated with small molecule inhibitors directed against targets involved in B-cell receptor signaling and the MAPK pathway (Fig. S18AB). Inhibition of SYK by fostamatinib and of MAP2K1 (the kinase upstream of MAPK1/3) by trametinib led to a clear reduction of RTX-mediated cytotoxicity. The same was observed, to a lesser extent, for inhibition of MAPK1/3 by ulixertinib and of p38 MAPK by PH-7978804. We then used FACS to analyze SU-DHL-4 cells treated with rituximab alone or in combination with the SYK inhibitor fostamatinib or the DUSP1/6 inhibitor BCI (Fig. S18CD). Also in this assay, SYK inhibition diminished the cytotoxic effect of RTX. BCI alone was effective in killing cells (by indirectly activating MAPK1/3 via DUSP1/6 inhibition) and has been shown to induce cell death in patient-derived pre-B ALL cells via MAPK1/3 activation (40). Interestingly, the combination of BCI with RTX was even more effective in inducing cytotoxicity in SU-DHL-4 cells.
The collective evidence supports the following model of RTX-mediated killing of CD20-positive B-cells: RTX binds to CD20 located in lipid rafts alongside the B-cell receptor complex leading to a strong activation of the MAPK pathway and the calcineurin-NFAT axis. Unlike in physiological BCR activation, the balancing activity of PI3K-AKT and NFkB signaling appears to be missing in SU-DHL-4 cells. Consequently, RTX likely tips the balance towards tolerance/apoptotic cell death (Fig. 5E) (41–44). Our data may even suggest that the detection of MS4A1 in B-cell cancer patients alone is insufficient to stratify them for RTX treatment (35) and that components such as BCR and RFTN1 should be considered in addition. To what extent the above preliminary model may be generalized remains to be investigated by extending the analysis to further cell lines and patient derived models.
Conclusions
The examples above were meant to illustrate the substantial potential of decryptM to characterize the MoA of drugs, generate drug-specific PTM signatures, study resistance mechanisms, and place drug-regulated PTM sites of unclear significance into a functional context. Given the streamlined workflow developed here, decryptM can now be performed at scale. The method should be extendable to any molecule that modulates cellular activity by affecting PTMs or protein expression, including GPCR ligands, cytokines, chemokines, co-factors, metabolites, biologics, peptides, or hormones—to name a few.
There are also limitations of the approach in its current form. For instance, if a drug hits multiple targets with similar potency, it will still be difficult to attribute the decryptM data to one or the other target. This highlights the continuing need for more selective chemical probes to study PTM biology. At present, only bulk material has been analyzed. Hence, effects specific to certain cellular states (or even single cells) are likely missed. The analysis is not fully comprehensive because standard MS-based PTM proteomics still misses PTM sites for which specific antibodies are available today. Another technical limitation is that the analysis of acetylation and ubiquitinylation requires rather large quantities of starting material. As with other profiling technologies, decryptM profiles are powerful starting points but may not fully stand alone. In other words, the profiles generate many hypotheses that will have to be followed up by further orthogonal experimentation.
In the future, decryptM profiles may serve to monitor and perhaps eventually predict drug responses in-vivo once sufficient drugs and cell systems have been analyzed. Looking beyond, we envision that matching decryptM profiles of cancer drugs with cancer patient PTM profiles indicative of oncogenic signaling will become an important tool in molecular tumor boards for personalized and evidence-based treatment recommendations.
Materials and Methods Summary
Full details of the decryptM methodology are described in the supplementary materials and are summarized here as follows: Cell lines were treated with increasing concentrations of a drug (dose-dependent) or for increasing times at a fixed drug concentration (time-dependent). Cells were then lysed, proteins extracted, and peptides generated by trypsin digestion. The peptides were stable-isotope labeled using tandem mass tags (TMT) (45). Post-translationally modified peptides (PTMs) were enriched by immunoprecipitation (IP; acetylation, ubiquitinylation) or immobilized metal affinity chromatography (IMAC; phosphorylation) (46). Full proteome or PTM samples were analyzed by LC-MS/MS, and peptides and proteins identified and quantified using MaxQuant/Andromeda (47). Dose-response curves for each PTM peptide were fitted to a 4-parameter sigmoidal curve on the basis of TMT reporter ion intensities, yielding drug EC50 values for each protein or PTM peptide, the size of each effect as well as curve-fitting quality metrics. A set of filters was applied to extract regulated dose-response curves from each experiment. The dose- or time-dependent regulated PTMs (or protein expression values) were further analyzed to explore the mode of action of the respective drugs and the specific responses of different cell lines. Regulated curves were functionally annotated based on the PhosphoSitePlus (PSP) database (8). Drug-target affinities were obtained from competition pulldown experiments using Kinobeads (2) and HDAC beads as described (16).
Supplementary Material
One Sentence Summary.
Measuring dose-dependent effects of drugs on post-translational modifications on a proteome-wide scale reveals how these drugs work in cells.
Acknowledgements
The authors are grateful to Andrea Hubauer, Michaele Krötz-Fahning, Nicolas Goldbach, Michael Lorenz, Wassim Gabriel, and Elias Kahl for technical assistance. Some aspects of figures were created with BioRender.com.
Funding
This work was partly funded by the Federal Ministry of Education and Research (CLINSPECT-M, FKZ161L0214A; DIAS, FKZ 031L0168), the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation); SFB1309 – 325871075; SFB 1335 – 360372040; GR 4575/1-1; SA 1374/4-2), the European Research Council (ERC AdG grant no. 833710 and no. 834154), the European Union’s Horizon 2020 Program (grant agreement 823839 - H2020-INFRAIA-2018-1, EPIC-XS), the Wilhelm Sander-Foundation (2020.174.1) and the German Cancer Aid (Deutsche Krebshilfe, Max Eder Grant, Project ID: 70114720).
Footnotes
Author contributions:
JZ, BR and BK conceived the decryptM approach. JZ, FPB, SvW, JW, NB, AS, MAG, KK, LC, FMH, SL, SE, MR, FMH, PP, LV, CF, MD, LR, AyS, AV, performed laboratory experiments. FPB, JM, TH, PS, LL, GS, MT, JZ, SvW, JW, NB, AS, KK, LC, FMH, SL, SE, MR, PP, LV, CF, MD, LR, AyS, AV, BK performed data analysis. JRu, JZ, StW, BMG, DS, GM, MB, HH, MT, MW, BK directed and supervised experiments and data analysis. FPB, AS, MT and BK wrote manuscript with input from all authors.
Competing interests:
BK and MW are founders and shareholders of OmicScouts and MSAID. They have no operational role in either company. HH is co-founder, shareholder and CEO of OmicScouts. TH, LR, AyS, and GS are present or past employees of OmicScouts. JZ is currently an employee of AstraZeneca, SvW an employee of Novartis, and FMH an employee of OmicEra Diagnostics GmbH, but all of the work in this study has been performed while at TUM. All other authors declare no competing interests.
Data and materials availability
The data supporting the findings of this study are available within the paper, the supplementary information and public repositories. The raw mass spectrometry proteomics data, protein identification and quantification results (Table S7) as well as time- and dose-response curves have been deposited with the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD037285. From this data, all analysis presented in this study can be reproduced. In addition, dose-response data is also available in ProteomicsDB (https://www.proteomicsdb.org/decryptm). The code of the decryptM data processing pipeline can be found at Github (kusterlab/decryptM), and is also archived at Zenodo (48). There are no restrictions on materials other than those imposed by the commercial availability of cell lines, antibodies, drugs and other reagents used in this study.
References
- 1.Benns HJ, Wincott CJ, Tate EW, Child MA. Activity- and reactivity-based proteomics: Recent technological advances and applications in drug discovery. Curr Opin Chem Biol. 2021;60:20–29. doi: 10.1016/j.cbpa.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Klaeger S, et al. The target landscape of clinical kinase drugs. Science. 2017;358 doi: 10.1126/science.aan4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lill JR, Mathews WR, Rose CM, Schirle M. Proteomics in the pharmaceutical and biotechnology industry: a look to the next decade. Expert Rev Proteomics. 2021;18:503–526. doi: 10.1080/14789450.2021.1962300. [DOI] [PubMed] [Google Scholar]
- 4.Savitski MM, et al. Tracking cancer drugs in living cells by thermal profiling of the proteome. Science. 2014;346:1255784. doi: 10.1126/science.1255784. [DOI] [PubMed] [Google Scholar]
- 5.Budayeva HG, et al. Phosphoproteome Profiling of the Receptor Tyrosine Kinase MuSK Identifies Tyrosine Phosphorylation of Rab GTPases. Mol Cell Proteomics. 2022;21:100221. doi: 10.1016/j.mcpro.2022.100221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson A, et al. Tandem mass tags: a novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Anal Chem. 2003;75:1895–1904. doi: 10.1021/ac0262560. [DOI] [PubMed] [Google Scholar]
- 7.Lautenbacher L, et al. ProteomicsDB: toward a FAIR open-source resource for life-science research. Nucleic Acids Res. 2022;50:D1541–D1552. doi: 10.1093/nar/gkab1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hornbeck PV, et al. PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res. 2015;43:D512–520. doi: 10.1093/nar/gku1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.UniProt C. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 2021;49:D480–D489. doi: 10.1093/nar/gkaa1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YN, et al. Allosteric inhibition of SHP2 phosphatase inhibits cancers driven by receptor tyrosine kinases. Nature. 2016;535:148–152. doi: 10.1038/nature18621. [DOI] [PubMed] [Google Scholar]
- 11.Pakos-Zebrucka K, et al. The integrated stress response. EMBO Rep. 2016;17:1374–1395. doi: 10.15252/embr.201642195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy D, et al. HSPB1 facilitates ERK-mediated phosphorylation and degradation of BIM to attenuate endoplasmic reticulum stress-induced apoptosis. Cell Death Dis. 2017;8:e3026. doi: 10.1038/cddis.2017.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parcellier A, et al. HSP27 is a ubiquitin-binding protein involved in I-kappaBalpha proteasomal degradation. Mol Cell Biol. 2003;23:5790–5802. doi: 10.1128/MCB.23.16.5790-5802.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasinoff BB. Progress curve analysis of the kinetics of slow-binding anticancer drug inhibitors of the 20S proteasome. Arch Biochem Biophys. 2018;639:52–58. doi: 10.1016/j.abb.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 15.Robers MB, et al. Target engagement and drug residence time can be observed in living cells with BRET. Nat Commun. 2015;6:10091. doi: 10.1038/ncomms10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lechner S, et al. Target deconvolution of HDAC pharmacopoeia reveals MBLAC2 as common off-target. Nat Chem Biol. 2022 doi: 10.1038/s41589-022-01015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, et al. HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol Cell. 2007;27:197–213. doi: 10.1016/j.molcel.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JY, et al. HDAC6 controls autophagosome maturation essential for ubiquitin-selective quality-control autophagy. EMBO J. 2010;29:969–980. doi: 10.1038/emboj.2009.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scholz C, et al. Acetylation site specificities of lysine deacetylase inhibitors in human cells. Nat Biotechnol. 2015;33:415–423. doi: 10.1038/nbt.3130. [DOI] [PubMed] [Google Scholar]
- 20.Moreno-Yruela C, et al. Hydroxamic acid-modified peptide microarrays for profiling isozyme-selective interactions and inhibition of histone deacetylases. Nat Commun. 2021;12:62. doi: 10.1038/s41467-020-20250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lasko LM, et al. Discovery of a selective catalytic p300/CBP inhibitor that targets lineage-specific tumours. Nature. 2017;550:128–132. doi: 10.1038/nature24028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinert BT, et al. Time-Resolved Analysis Reveals Rapid Dynamics and Broad Scope of the CBP/p300 Acetylome. Cell. 2018;174:231–244.:e212. doi: 10.1016/j.cell.2018.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsukada C, et al. Functional characterization of 12 allelic variants of CYP2C8 by assessment of paclitaxel 6alpha-hydroxylation and amodiaquine N-deethylation. Drug Metab Pharmacokinet. 2015;30:366–373. doi: 10.1016/j.dmpk.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Wu J, et al. The non-canonical effects of heme oxygenase-1, a classical fighter against oxidative stress. Redox Biol. 2021;47:102170. doi: 10.1016/j.redox.2021.102170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassan FU, et al. Curcumin as an Alternative Epigenetic Modulator: Mechanism of Action and Potential Effects. Front Genet. 2019;10:514. doi: 10.3389/fgene.2019.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baell JB, Holloway GA. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J Med Chem. 2010;53:2719–2740. doi: 10.1021/jm901137j. [DOI] [PubMed] [Google Scholar]
- 27.Ochoa D, et al. The functional landscape of the human phosphoproteome. Nat Biotechnol. 2020;38:365–373. doi: 10.1038/s41587-019-0344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kun E, Tsang YTM, Ng CW, Gershenson DM, Wong KK. MEK inhibitor resistance mechanisms and recent developments in combination trials. Cancer Treat Rev. 2021;92:102137. doi: 10.1016/j.ctrv.2020.102137. [DOI] [PubMed] [Google Scholar]
- 29.Dai X, Cheng H, Bai Z, Li J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J Cancer. 2017;8:3131–3141. doi: 10.7150/jca.18457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 31.Nami B, Maadi H, Wang Z. Mechanisms Underlying the Action and Synergism of Trastuzumab and Pertuzumab in Targeting HER2-Positive Breast Cancer. Cancers (Basel) 2018;10 doi: 10.3390/cancers10100342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brockhoff G, et al. Differential impact of Cetuximab, Pertuzumab and Trastuzumab on BT474 and SK-BR-3 breast cancer cell proliferation. Cell Prolif. 2007;40:488–507. doi: 10.1111/j.1365-2184.2007.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang M, et al. Transcriptome analysis of human OXR1 depleted cells reveals its role in regulating the p53 signaling pathway. Sci Rep. 2015;5:17409. doi: 10.1038/srep17409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Felberg A, et al. Monitoring of the Complement System Status in Patients With B-Cell Malignancies Treated With Rituximab. Front Immunol. 2020;11:584509. doi: 10.3389/fimmu.2020.584509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pierpont TM, Limper CB, Richards KL. Past, Present and Future of Rituximab-The World’s First Oncology Monoclonal Antibody Therapy. Front Oncol. 2018;8:163. doi: 10.3389/fonc.2018.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta N, et al. Quantitative proteomic analysis of B cell lipid rafts reveals that ezrin regulates antigen receptor-mediated lipid raft dynamics. Nat Immunol. 2006;7:625–633. doi: 10.1038/ni1337. [DOI] [PubMed] [Google Scholar]
- 37.Zhou J, et al. A kinetic investigation of interacting, stimulated T cells identifies conditions for rapid functional enhancement, minimal phenotype differentiation, and improved adoptive cell transfer tumor eradication. PLoS One. 2018;13:e0191634. doi: 10.1371/journal.pone.0191634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saeki K, Miura Y, Aki D, Kurosaki T, Yoshimura A. The B cell-specific major raft protein, Raftlin, is necessary for the integrity of lipid raft and BCR signal transduction. EMBO J. 2003;22:3015–3026. doi: 10.1093/emboj/cdg293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edelmann J, et al. Rituximab and obinutuzumab differentially hijack the B cell receptor and NOTCH1 signaling pathways. iScience. 2021;24:102089. doi: 10.1016/j.isci.2021.102089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shojaee S, et al. Erk Negative Feedback Control Enables Pre-B Cell Transformation and Represents a Therapeutic Target in Acute Lymphoblastic Leukemia. Cancer Cell. 2015;28:114–128. doi: 10.1016/j.ccell.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barnabei L, Laplantine E, Mbongo W, Rieux-Laucat F, Weil R. NF-kappaB: At the Borders of Autoimmunity and Inflammation. Front Immunol. 2021;12:716469. doi: 10.3389/fimmu.2021.716469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buhl AM, Pleiman CM, Rickert RC, Cambier JC. Qualitative regulation of B cell antigen receptor signaling by CD19: selective requirement for PI3-kinase activation, inositol-1-4-5-trisphosphate, production and Ca2+ mobilization. J Exp Med. 1997;186:1897–1910. doi: 10.1084/jem.186.11.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Healy JI, et al. Different nuclear signals are activated by the B cell receptor during positive versus negative signaling. Immunity. 1997;6:419–428. doi: 10.1016/s1074-7613(00)80285-x. [DOI] [PubMed] [Google Scholar]
- 44.Schulze-Luehrmann J, Ghosh S. Antigen-receptor signaling to nuclear factor kappa B. Immunity. 2006;25:701–715. doi: 10.1016/j.immuni.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 45.Zecha J, et al. TMT Labeling for the Masses: A Robust and Cost-efficient, In-solution Labeling Approach. Mol Cell Proteomics. 2019;18:1468–1478. doi: 10.1074/mcp.TIR119.001385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruprecht B, et al. Comprehensive and reproducible phosphopeptide enrichment using iron immobilized metal ion affinity chromatography (Fe-IMAC) columns. Mol Cell Proteomics. 2015;14:205–215. doi: 10.1074/mcp.M114.043109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 48.Bayer FP. Code for “Decrypting drug actions and protein modifications by dose- and time-resolved proteomics. zenodo. 2023 doi: 10.5281/zenodo.7647751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perez-Riverol Y, et al. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res. 2019;47:D442–D450. doi: 10.1093/nar/gky1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hughes CS, et al. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat Protoc. 2019;14:68–85. doi: 10.1038/s41596-018-0082-x. [DOI] [PubMed] [Google Scholar]
- 51.Bian Y, et al. Robust, reproducible and quantitative analysis of thousands of proteomes by micro-flow LC-MS/MS. Nat Commun. 2020;11:157. doi: 10.1038/s41467-019-13973-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruprecht B, Zecha J, Zolg DP, Kuster B. High pH Reversed-Phase Micro-Columns for Simple, Sensitive, and Efficient Fractionation of Proteome and (TMT labeled) Phosphoproteome Digests. Methods Mol Biol. 2017;1550:83–98. doi: 10.1007/978-1-4939-6747-6_8. [DOI] [PubMed] [Google Scholar]
- 53.Ting L, Rad R, Gygi SP, Haas W. MS3 eliminates ratio distortion in isobaric multiplexed quantitative proteomics. Nat Methods. 2011;8:937–940. doi: 10.1038/nmeth.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dinkel H, et al. Phospho.ELM: a database of phosphorylation sites--update 2011. Nucleic Acids Res. 2011;39:D261–267. doi: 10.1093/nar/gkq1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raudvere U, et al. g:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update) Nucleic Acids Res. 2019;47:W191–W198. doi: 10.1093/nar/gkz369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sugiyama N, Imamura H, Ishihama Y. Large-scale Discovery of Substrates of the Human Kinome. Sci Rep. 2019;9:10503. doi: 10.1038/s41598-019-46385-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shannon P, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 59.Reinecke M, et al. Chemoproteomic Selectivity Profiling of PIKK and PI3K Kinase Inhibitors. ACS Chem Biol. 2019;14:655–664. doi: 10.1021/acschembio.8b01020. [DOI] [PubMed] [Google Scholar]
- 60.Ritz C, Baty F, Streibig JC, Gerhard D. Dose-Response Analysis Using R. PLoS One. 2015;10:e0146021. doi: 10.1371/journal.pone.0146021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available within the paper, the supplementary information and public repositories. The raw mass spectrometry proteomics data, protein identification and quantification results (Table S7) as well as time- and dose-response curves have been deposited with the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD037285. From this data, all analysis presented in this study can be reproduced. In addition, dose-response data is also available in ProteomicsDB (https://www.proteomicsdb.org/decryptm). The code of the decryptM data processing pipeline can be found at Github (kusterlab/decryptM), and is also archived at Zenodo (48). There are no restrictions on materials other than those imposed by the commercial availability of cell lines, antibodies, drugs and other reagents used in this study.