Abstract
Vascular tissues serve a dual function in plants providing both physical support as well as controlling the transport of nutrients, water, hormones and other small signaling molecules. Xylem tissues transport water from root to shoot; phloem tissues transfer photosynthates from shoot to root; while divisions of the (pro)cambium increase the number of xylem and phloem cells. Although vascular development constitutes a continuous process from primary growth in the early embryo and meristem regions to secondary growth in the mature plant organs, it can be artificially separated into distinct processes including cell type specification, proliferation, patterning and differentiation. In this review, we focus our attention to how hormonal signals orchestrate the molecular regulation of vascular development in the Arabidopsis thaliana primary root meristem. Although auxin and cytokinin have taken center stage in this aspect since their discovery, other hormones including brassinosteroids, abscisic acid and jasmonic acid are also taking up leading roles during vascular development. All these hormonal cues synergistically or antagonistically participate in the development of vascular tissues, forming a complex hormonal control network.
Keywords: abscisic acid, auxin, brassinosteroids, cytokinin, jasmonic acid, vascular development
Introduction
Over the course of hundreds of millions of years, multicellular land plants evolved from a green algal ancestor (Strother and Foster, 2021; Woudenberg et al., 2022). During this evolutionary process, the acquisition of a long distance transporting system contributed to the ability of land plants to escape the constraints of their aquatic environment and grow taller than ever before. This transporting system consisting of water- and food-conducting cells is best studied in the vascular plant lineage (tracheophytes) where these tissues are known as xylem and phloem tissues respectively. Despite the enormous diversity in shape and size of transporting tissues in tracheophytes, most of our knowledge on the molecular pathways that control vascular tissue development comes from the study of Arabidopsis thaliana. Here, vascular tissues are initially formed during the early globular stage of embryogenesis from four inner procambium precursor cells (Scheres et al., 1994). Additional rounds of oriented divisions eventually generate all the cell types of xylem, phloem and (pro)cambium in both the root and hypocotyl. Vascular tissues in the shoot however, originate from the shoot apical meristem (Weigel and Jürgens, 2002). A characteristic diarch patterning is found in the primary and lateral root meristems, characterized by a central xylem axis flanked by two phloem poles and intervening procambium (Figure 1). This well described organization is however rather specific to root meristems, as the patterning in other organs or even the same organ but in a different development stage varies dramatically. For example, in roots undergoing secondary growth, concentric rings are observed of xylem, cambium and phloem (Baum et al., 2002; Ragni and Greb, 2018). Leaf vascular tissues are organized with xylem on the adaxial side and phloem on the abaxial side (De Rybel et al., 2016; Scarpella and Meijer, 2004).
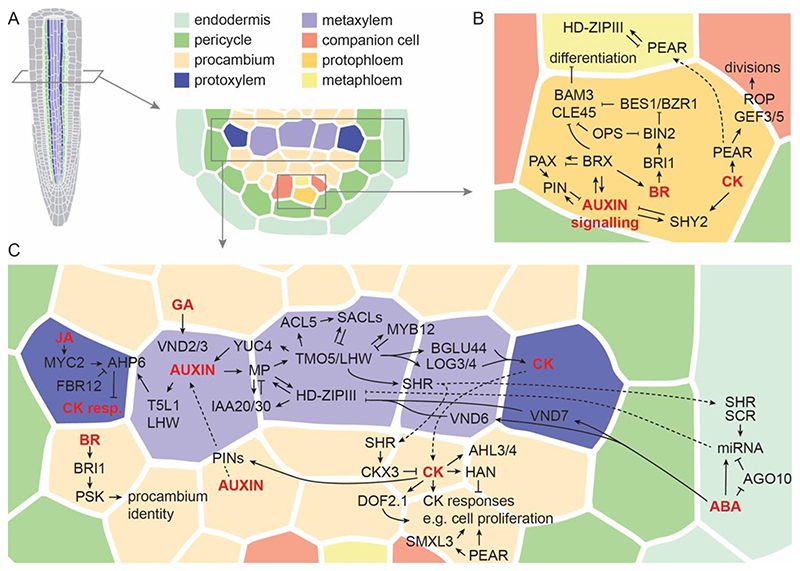
Figure1. Hormones control the molecular regulation of vascular development in the primary root meristem of Arabidopsis.
A cross-section through a primary root meristem is shown (A) illustrating the known hormones, molecular regulators and their interactions in the phloem (B) and xylem (C) regions. Hormones are indicated in red; dotted lines illustrate mobility of the hormone or molecular regulator. Please note that the presence and locations of some regulators and hormones on the realistic tissue template are not exact to keep the image orderly and due to space limitations. This image also does not represent accurate hormone levels and distributions across the tissue for the same reasons.
Over the past decades, our understanding of the key molecular regulators that control vascular development has been steadily increasing by applying biochemical, genetic and genomic strategies. In many cases, connections to hormonal signals as indispensable regulatory factors in vascular development have been revealed (reviewed in (Cho et al., 2017; Dettmer et al., 2009; Ramachandran et al., 2020)). Although a role for auxin and cytokinin in virtually all aspects of vascular development has been clear from the moment these hormones were identified and described (Agusti and Blazquez, 2020; De Rybel et al., 2016; Smet and De Rybel, 2016), important roles for other hormones in vascular development are also being discovered. These hormones also rarely act alone and their signaling pathways intersect in an intricate hormonal network controlling vascular development. Given the complex nature of these interactions, we will focus our attention to the hormonal control of vascular development during primary root meristem development in Arabidopsis thaliana.
Auxin and cytokinin: the yin and yang of vascular development
The role of auxin and cytokinin is intimately connected to virtually all developmental processes. It is thus no surprise that most of the literature dealing with hormonal control of vascular development implicates these two main phytohormones. Here, we will discuss the most important events during the development of vascular tissues in a chronological order from specification of vascular identity, touching upon proliferation and patterning, and ending with differentiation of specialized structures to fulfil both the transporting and structural support function of these tissues.
Specification of vascular identity during early embryogenesis
Cells with a vascular identity are first specified during the globular stage of embryogenesis. The four provascular initial cells each undergo an oriented cell division which generates the ground tissue layer (which will later split up into cortex and endodermis) and the pre-provascular tissues. Another round of oriented divisions generates the pericycle and the provascular cells (De Rybel et al., 2013; Scheres et al., 1994; Yoshida et al., 2014). Mutations in MONOPTEROS/AUXIN RESPONSE FACTOR 5 (MP/ARF5) and its inhibitor BODENLOS/INDOLE-3-ACETIC ACID INDUCIBLE 12 (BDL/IAA12) lead to abnormal division orientation in the provascular initial cells (Berleth and Jurgens, 1993; Hardtke and Berleth, 1998). A similar phenotype is observed in PIN-FORMED (PIN) family mutants, which fail to form a correct auxin gradient during early embryo development (Friml et al., 2003). As such, auxin signaling and transport are critically required to overwrite the default division rule, thus allowing asymmetric divisions which create the provascular initial cells. An initially broad MP expression domain gets gradually confined to the vascular region, which is consistent with the auxin response maximum produced by PIN mediated auxin concentration (Friml et al., 2003; Hardtke and Berleth, 1998). Moreover, MP expression can be activated by auxin and PIN1 expression is reduced in mp, suggest a positive feedback loop between auxin, PIN and MP to create a stable auxin maximum in the provascular tissues during embryogenesis (Schlereth et al., 2010; Weijers et al., 2006; Wenzel et al., 2007).
Although defects in vascular specification are mostly associated with auxin-related mutants, it is to be expected that similar effects will be found in cytokinin-related mutants given the tight interaction between auxin and cytokinin during vascular development.
An auxin/cytokinin interplay controls vascular cell proliferation and patterning
Cytokinin perception and signaling play key roles during cell proliferation and patterning. The wooden leg (wol) mutant defective in the gene encoding for the cytokinin receptor ARABIDOPSIS HISTIDINE KINASE 4 (AHK4, also known as CYTOKININ RESPONSE 1(CRE1)), exhibits a strong reduction in the number of vascular cell files which all differentiate as protoxylem (Mahonen et al., 2000; Scheres et al., 1995). Similar phenotypes are found in arabidopsis response regulator (arr1, 10, 12) triple mutants (Argyros et al., 2008; Yokoyama et al., 2007) involved in cytokinin signaling, and higher order mutations in LONELY GUY (LOG) family members defective in the final step of cytokinin biosynthesis (De Rybel et al., 2014). Auxin interacts with these factors via inducing the MP-dependent basic helix–loop–helix (bHLH) transcription factor TARGET OF MONOPTEROS 5 (TMO5) (Schlereth et al., 2010). TMO5 is first expressed in the four provascular initial cells (De Rybel et al., 2014; Schlereth et al., 2010), where it is sufficient to rescue the provascular defects found in mp (De Rybel et al., 2013). TMO5 and its close homologs form heterodimeric complexes with members from another bHLH subclade consisting of LONESOME HIGHWAY (LHW) and its close homologs. The TMO5/LHW dimer is both required and sufficient to trigger periclinal and radial cell division activity, and as such controls meristem width (De Rybel et al., 2013; Ohashi-Ito et al., 2013; Ohashi-Ito et al., 2014). The TMO5/LHW dimers directly bind to the promoter regions of LOG3, LOG4 and BETA GLUCOSIDASE 44 (BGLU44) (De Rybel et al., 2014; Ohashi-Ito et al., 2014), thereby increasing the levels of active cytokinin in the xylem axis (Figure 1). This xylem-derived cytokinin was proposed to influence the root hair responses in the epidermis upon phosphate limiting conditions (Wendrich et al., 2020). Moreover, different TMO5/LHW heterodimer complex variations are suggested to perform the cytokinin biosynthesis inducing activity throughout the plant body in all meristem regions, not just in the root apical meristem (Mor et al., 2022). In the primary root meristem, TMO5/LHW is restricted to the xylem axis (De Rybel et al., 2013). As such, the active cytokinin produced in these xylem cells is thought to diffuse to the neighboring procambium cells. Here, it triggers cell proliferation together with cytokinin delivered to these cells by the phloem tissues (Bishopp et al., 2011b). Auxin and cytokinin thus establish a mutually excluding negative feedback loop forming distinct domains of hormone responses with high cytokinin signaling in the procambium cells and high auxin signaling in the central xylem axis in order to correctly pattern the vascular cylinder (Bishopp et al., 2011a; De Rybel et al., 2014; Ohashi-Ito et al., 2014). This auxin response maximum in the xylem axis is maintained by cytokinin-mediated effects on PIN expression and their subcellular localization. Indeed, PIN expression is reduced and PIN1 polarity is affected in cytokinin receptor mutants (Bishopp et al., 2011a; Marhavy et al., 2011; Marhavy et al., 2014) (Figure 1). Moreover, the class III homeodomain-leucine zipper (HD-ZIP III) transcription factor REVOLUTA (REV), was found to directly promote the expression of auxin influx carriers AUX1, LAX2, and LAX3 (Baima et al., 2014). The triple aux1 lax1 lax2 mutant displayed aberrant protoxylem formation (el-Showk et al., 2015), indicating that both auxin efflux and influx are required to maintain the auxin maximum in the xylem axis. Additionally, the auxin-induced TMO5-LIKE1/LHW (T5L1/LHW) heterodimer induces expression of AHP6 in the protoxylem and adjacent pericycle cells, where it antagonizes cytokinin signaling and maintains protoxylem identity (Mähönen et al., 2006; Ohashi-Ito et al., 2014). Conversely, AHP6 expression was suppressed by cytokinin signaling via FUMONISIN B1-RESISTANT12 (FBR12), which encodes a eukaryotic translation initiation factor 5A (Ren et al., 2013) (Figure 1).
TMO5/LHW finetunes its own activity by inducing expression of repressors of the dimer activity. As a first example, SUPPRESSOR OF ACAULIS 51(SAC51) and SAC51-LIKE (SACL) homologs form alternative heterodimers with LHW, thereby competing with TMO5 for the binding to LHW and repressing TMO5/LHW dimer activity (Katayama et al., 2015; Vera-Sirera et al., 2015). The SACL factors themselves are controlled by the MP-dependent thermospermine synthase ACAULIS5 (ACL5) which represses the inhibitory action of upstream Open Reading Frames (uORFs) onto the main ORF of SACL genes (Katayama et al., 2015; Vera-Sirera et al., 2015). MYB12 is another example where TMO5/LHW induces expression of its own repressors. The cytokinin-dependent MYB12 interacts with TMO5 and reduces expression of TMO5/LHW target genes, resulting in a second negative feed-back loop to ensure optimal TMO5/LHW levels during vascular proliferation (Wybouw et al., 2023). Finally, T5L1/LHW induces expression of YUCCA4 (YUC4), a key auxin biosynthesis gene. Conversely, auxin biosynthesis is required for maintaining the expression levels of T5L1 and LHW in order to initiate xylem development (Ohashi-Ito et al., 2019) (Figure 1). As such, local auxin biosynthesis also forms a positive feedback loop for fine-tuning the level of T5L1/LHW dimers.
Downstream of TMO5/LHW, the cytokinin inducible DOF-type transcription factor DOF2.1 is also sufficient to trigger ectopic cell proliferation. Loss of function of the non-mobile DOF2.1 and its two close homologs TMO6 and DOF6 reduces the number of a specific subset of outer procambium vascular cell files (Smet et al., 2019). Expression of related mobile DOF-type transcription factors collectively called PHLOEM EARLY DOF (PEAR) genes is induced by cytokinin and the respective proteins diffuse through plasmodesmata forming a short-range concentration gradient that peaks at the protophloem sieve elements (SE) and induces periclinal divisions in and around the phloem pole SE (Miyashima et al., 2019) (Figure 1). SUPPRESSOR OF MAX2 1-LIKE3 (SMXL3) was found to be a direct target of PEAR and is expressed in the phloem pole and surrounding procambium cells. Ectopic overexpression of SMXL3 was also able to induce periclinal cell division in the vascular bundle (Miyashima et al., 2019). Besides controlling proliferation, PEAR proteins also seem to regulate division orientation to promote protophloem SE lineage bifurcation via RHO OF PLANTS (ROP) GTPase signaling by inducing two protophloem expressed ROP Guanine nucleotide Exchange Factors, ROPGEF3 and ROPGEF5 (Roszak et al., 2021).
In addition, both expression and movement of PEAR proteins are antagonized by members of the HD-ZIP III proteins: PHABULOSA (PHB), PHAVOLUTA (PHV), REV, CORONA (CNA)/ATHB15 and ARABIDOPSIS THALIANA HOMEOBOX 8 (ATHB8) (Baima et al., 1995; Carlsbecker et al., 2010). Triple mutants show ectopic protoxylem partly replacing metaxylem, and mutants lacking all five HD-ZIP III transcription factors fail to differentiate xylem (Carlsbecker et al., 2010). HD-ZIP III expression in the phloem pole SE is promoted by PEAR proteins, thus creating a negative-feedback loop which instructs robust boundaries between dividing an non-dividing cells in the phloem pole (Miyashima et al., 2019) (Figure 1). As such, cytokinin induces members of the DOF-type transcription factor family, which control cell proliferation in distinct subdomains of the vascular bundle in a cell autonomous (DOF2.1, (Smet et al., 2019)) and cell non-autonomous (PEARs, (Miyashima et al., 2019)) manner.
Furthermore, HD-ZIP III expression is concentrated in the central vascular domain by activity of miRNA165/166 (Carlsbecker et al., 2010; Muller et al., 2016; Muraro et al., 2014; Schlereth et al., 2010; Ursache et al., 2014). Vascular expressed SHORT ROOT (SHR) moves towards the endodermis, where it is sequestered into the nucleus by binding to SCRAECROW (SCR). This activates expression of miRNA165/166 (Carlsbecker et al., 2010; Cui et al., 2007; Helariutta et al., 2000; Nakajima et al., 2001). The endodermis synthesized miRNA165/166 diffuses to create an inward gradient, resulting in high miRNA concentrations at the periphery of the xylem axis in the protoxylem positions and low level in the central metaxylem position. These miRNAs degrade the HD-ZIP III family transcripts and as such define protoxylem and metaxylem positions (Carlsbecker et al., 2010; Lee et al., 2006) (Figure 1). Auxin biosynthesis is required for HD-ZIP III-mediated xylem patterning, as defects in metaxylem development were observed in auxin biosynthesis mutants (Ursache et al., 2014). Furthermore, PHB induces expression of MP and its inhibitors IAA20 and IAA30 (Muller et al., 2016). Since MP also directly regulates expression of IAA20 (Krogan et al., 2014), this forms a feed-forward loop that stabilizes the auxin response during vascular patterning and the differentiation of xylem cell types (Muller et al., 2016). SHR was more recently also shown to be a direct target of TMO5/LHW (Yang et al., 2021). SHR is hypothesized to move to the neighboring procambium cells, where it binds to the promoter region of CYTOKININ OXIDASE 3 (CKX3) and activates its expression (Cui et al., 2011; Hao and Cui, 2012). This cytokinin degrading enzyme counteracts the increase in active cytokinin levels via the LOG3/4 and BGLU44 enzymes which are also under TMO5/LHW control in order to achieve optimal levels of active cytokinin in the vascular cells, allowing normal cell proliferation (Yang et al., 2021) (Figure 1).
A pair of transcription factors, AT-HOOK MOTIF NUCLEAR LOCALIZED PROTEIN 3 (AHL3) and AHL4, have been reported to be involved in the regulation of vascular tissue boundaries in Arabidopsis root. Mutants show a misspecification of tissue boundaries with ectopic xylem being formed in the procambium domain. As AHL4 was showed to be cytokinin inducible and exogenous application of cytokinin abolished extra protoxylem in ahl4-1 mutants, these results suggest that cytokinin plays an important role in controlling tissue boundaries partially via, or parallel to, these AT-HOOK mediated pathway (Zhou et al., 2013). In addition, the cytokinin-induced GATA-type transcription factor HANABA TARANU (HAN) was shown to finetune the proliferation position and frequency in the vascular bundle together with B-type ARRs. It is suggested that this activity shapes the mechanical stresses in the vascular bundle and help to control tissue boundaries (Fujiwara et al., 2023).
Auxin and cytokinin regulate vascular differentiation
In order to become functional as transporting tissues, xylem and phloem cells undergo drastic morphological changes accompanied with the formation of tissue-specific secondary cell walls. The process in which xylem cells differentiate into tracheary elements (TE) and phloem cells into SE is highly controlled by molecular networks and peptide-receptor interactions (reviewed in (Blob et al., 2018; Heo et al., 2017; Ruonala et al., 2017; Sun et al., 2022)). The typical feature of TE differentiation is programmed cell death, which occurs after secondary cell wall deposition, when unknown signals induce rupture of the tonoplast membrane and cessation of cytoplasmic flow (Escamez and Tuominen, 2014). During SE differentiation, cells enucleate and lose most of their organelles while they interconnect through generating sieve plates (Heo et al., 2017). SE remain alive by establishing multiple cytoplasmic connections through plasmodesmata with the neighboring companion cells (Lee and Frank, 2018). Although the transcriptional networks controlling the different steps of xylem differentiation are well known, the link to auxin control remains sparse. As one example, plants overexpressing the auxin-inducible T5L1/LHW heterodimer show ectopic TE differentiation in various root tissues, similar to that of the acl5 mutant (Katayama et al., 2015; Vera-Sirera et al., 2015). ACL5 was found to negatively regulate TE differentiation by inhibiting programmed cell death (Muniz et al., 2008).
Recently, the role of auxin and cytokinin in SE differentiation have become more clear and we will thus focus our attention to this topic. The MYB-type transcription factor ALTERED PHLOEM DEVELOPMENT (APL) plays an important role in promoting phloem differentiation and inhibiting xylem differentiation during vascular development (Bonke et al., 2003) (Figure 1). The cytokinin dependent PEAR proteins were recently shown to directly promote APL expression, while PLETHORA (PLT) transcription factors repress APL expression (Roszak et al., 2021). The auxin-dependent PLT1 shows a gradient with highest expression in the stem cell niche. As such, the auxin gradient in the root apical meristem instructs PLT and PEAR transcription factors to antagonistically control the zone of APL expression leading to SE differentiation (Roszak et al., 2021).
Another important hormonal controlled regulator in SE is BREVIS RADIX (BRX), which co-localizes with PIN proteins at the rootward end of protophloem SE cells (Marhava et al., 2018; Scacchi et al., 2009; Scacchi et al., 2010). BRX-mediated protophloem differentiation antagonizes the pathway regulated by CLAVATA 3/EMBRYO SURROUNDING REGION 45 (CLE45) and its receptor BARELY ANY MERISTEM3 (BAM3) (Breda et al., 2019; Depuydt et al., 2013; Rodriguez-Villalon et al., 2014). Auxin induces BRX transcription via MP, but negatively regulates BRX protein abundance and plasma-membrane localization (Mouchel et al., 2006; Scacchi et al., 2009). The D6PK/D6PKL-related kinase PROTEIN KINASE ASSOCIATED WITH BRX (PAX) was identified as interactor of BRX (Marhava et al., 2018). D6PK-family kinases can activate auxin efflux by mediating PIN phosphorylation (Weller et al., 2017; Zourelidou et al., 2014). This activity is dampened by BRX (Marhava et al., 2018). PAX is required for efficient BRX plasma-membrane localization, but auxin negatively regulates this process and promote PAX activity in a dynamic steady state equilibrium. This ensures a fine-tuned PIN activity to modulate auxin flux through the developing protophloem cell file which is required for protophloem SE differentiation (Marhava et al., 2018). Fitting with this, brx and pax mutants display discontinuous protophloem strand (Marhava et al., 2018; Moret et al., 2020). These “gap” cells created by discontinuous differentiation of protophloem are not random and can be explained by a fate-determining bi-stability generated by auxin competition between neighboring cells (Moret et al., 2020). Cytokinin antagonizes the auxin effect on BRX activity in developing protophloem cells. Both cytokinin and auxin induce SHORT HYPOCOTYL 2 (SHY2) expression (Abel et al., 1995; Ioio et al., 2008), an AUXIN/INDOLE-ACETIC ACID (AUX/IAA) that negatively regulates the activity of AUXIN RESPONSE FACTORS (ARFs). Interestingly, BRX is required for proper SHY2 expression, reflecting a feedback mechanism on auxin response. As such, this cross-regulatory antagonism between BRX and SHY2 might determine ARF activity in protophloem cells (Scacchi et al., 2010).
Brassinosteroids complete the holy trinity of hormonal control
Brassinosteroids (BR) have also emerged as important hormonal regulators of vascular patterning and differentiation. This notion has been fueled by the development of an ectopic induction system for phloem differentiation named Vascular cell Induction culture System Using Arabidopsis Leaves (VISUAL) (Kondo et al., 2015). In this system, Arabidopsis leaf mesophyll cells are reprogrammed into vascular procambium cells, and then differentiate into xylem TE and phloem SE (Kondo et al., 2015). In the VISUAL system, auxin and cytokinin are applied together with a specific GLYCOGEN SYNTHASE KINASE 3 (GSK3) inhibitor which activates BR signaling called bikinin (De Rybel et al., 2009) to trigger vascular differentiation. Although the results obtained using the artificial VISUAL system need to be validated in the respective endogenous developmental and tissue contexts, as just one example, NAC20 was identified as a regulator of the master transcription factor APL (Kondo et al., 2016). Furthermore, BRI1-EMS-SUPPRESSOR1(BES1) and its closest homolog BRASSINAZOLE RESISTANT1 (BZR1) were shown to act as key regulators that, redundantly and positively, regulate both xylem and phloem cell differentiation from vascular stem cells (Saito et al., 2018). By modifying the VISUAL method, a specific system for inducing companion cell (CC)-like cell differentiation named VISUAL-CC was developed (Tamaki et al., 2020). A comprehensive gene expression analysis revealed that GSK3 kinase activity plays an important role in determining the SE/CC ratio, which may define a cell fate switch during phloem development (Tamaki et al., 2020).
Mutations in BRASSINOSTEROID INSENSITIVE1 (BRI1), one of the BR receptors, result in ectopic xylem formation in procambial positions. This abnormal vascular patterning is independent from the canonical BR signaling and involves the BRI1 interacting partner RECEPTOR-LIKE PROTEIN 44 (RLP44). RLP44 is itself required for expression of the peptide hormone phytosulfokine (PSK) receptor (Figure 1). PSK signaling is required for maintenance of procambial cell identity, as mutants in this pathway phenocopy the ectopic xylem phenotype seen in the bri1 mutant. However, the exact mechanisms that BR signaling uses to mediate vascular patterning in the Arabidopsis root meristem remains obscure (Holzwart et al., 2018). Arabidopsis plants carrying mutations in three of the BR receptor kinases BRI1, BRI1-LIKE 1 (BRL1) and BRL3 (bri triple) display severe patterning and differentiation defects, including disturbances in protophloem SE differentiation. This phenotype can however be partially rescued by protophloem-specific BRI1 expression, suggesting BR perception in the protophloem is required for normal phloem development (Kang et al., 2017). Interestingly, this rescued phenotype by phloem specific expressed BRI1 is related to the antagonism of CLE45 peptide signaling in the protophloem. Knockout of CLE45 perception in the bri triple mutant background can rescue proper phloem development, although the dwarf phenotype is still retained in such lines, indicating BR-regulated protophloem differentiation is depended on CLE45/BAM3 signal pathway (Graeff et al., 2020)
Loss-of-function mutants in OCTOPUS (OPS) show similar vascular defects as brx (Bauby et al., 2007; Nagawa et al., 2006). OPS has been suggested to act as an “insulator” to antagonize CLE45 signals through physical interactions with BAM3, thus promoting differentiation of developing protophloem (Breda et al., 2019) (Figure 1). In another aspect, OPS is also a positive regulator of the BR signaling pathway upstream of the key transcription factors BES1 and BZR1, which accumulate with unphosphorylated forms in the nucleus to induce BR responses. BRASSINOSTEROID-INSENSITIVE 2 (BIN2), a GSK3 that phosphorylates BES1 and BZR1 to induce their degradation, can directly interact with OPS and be restricted in the plasma membrane. In addition, treatment of bikinin as well as downstream dominant mutants bes1-D and bzr1-D can rescue the phloem defects of ops mutants, indicating that OPS antagonizes BIN2 to promote phloem differentiation (Anne et al., 2015).
The brx mutant root phenotype is due to a root specific deficiency of BR caused by decreased expression of the BRX-dependent rate-limiting enzyme CONSTITUTIVE PHOTOMORPHOGENESIS AND DWARF (CPD). Moreover, the application of BR during embryonic or post-embryonic growth could rescue the brx phenotype (Mouchel et al., 2006). Further, auxin responsiveness is globally reduced in brx mutants, demonstrating that BRX-mediated BR levels are rate-limiting for auxin response. Moreover, brx mutants show enhanced ABA-mediated inhibition of root growth (Rodrigues et al., 2009), BRX transcription is induced by auxin and suppressed by BR, suggesting that BRX acts at nexus of multiple hormones, including a feedback loop that controls BR levels to allow for an optimal auxin response in phloem cells (Mouchel et al., 2006; Rodrigues et al., 2009).
Abscisic acid regulates xylem patterning and differentiation
Exogenous abscisic acid (ABA) application induces supernumerary protoxylem and metaxylem strands in wild type Arabidopsis roots, whereas this abnormal patterning was not observed when ABA signaling is compromised. Although the endogenous role of ABA in vascular development needs more examination, ABA is likely required for proper xylem patterning (Ramachandran et al., 2018). ABA acts through non-cell-autonomous signaling as inhibition of ABA signaling in the endodermis cell layer displayed defects in xylem continuity (Ramachandran et al., 2018). In the endodermis cell layer, ABA induces miRNA165/166 biosynthesis and at the same time inhibits expression of ZWILLE/ARGONAUTE 10 (ZLL/AGO10), a suppressor of miR165/166 (Bloch et al., 2019) (Figure 1). As described previously, miRNA165/166 degrades HD-ZIPIII transcripts, forming a gradient that defines protoxylem and metaxylem positions (Carlsbecker et al., 2010). In addition, ABA triggers excessive protoxylem in the metaxylem position via inducing expression of the transcription factor VASCULAR-RELATED NAC DOMAIN7 (VND7) (Ramachandran et al., 2021). Together with its close homolog, VND6, these plant-specific NAC-type transcription factors were identified as key transcriptional switches for proto- and metaxylem cell fate (Kubo et al., 2005). Additionally, expression of HD-ZIP III genes REV and PHB is negatively regulated by VND7 during protoxylem TE differentiation (Taylor-Teeples et al., 2015).
In addition to VND6 and VND7, other members of this TF family, including VND1-5, are also involved in regulating TE differentiation by inducing genes related to secondary cell wall formation and programmed cell death (Tan et al., 2018; Zhou et al., 2014). Moreover, VND2 and VND3 were identified as positive regulators of metaxylem differentiation in response to ABA and drought stress (Augstein, 2022; Ramachandran et al., 2021). ABA treatment and water limitation not only promote xylem cell fate changes but also accelerate the xylem differentiation rate (Ramachandran et al., 2021).
Jasmonic acid at the nexus of hormone cross-talk
Despite the fact that the role of endogenous JA in xylem development remains poorly studied, exogenous jasmonic acid (JA) application is able to trigger formation of extra protoxylem strands in the root of Arabidopsis wild type and JA biosynthetic mutants. However, this effect is abolished in JA signaling mutants, suggesting that JA responses play a positive role in regulating xylem development (Jang et al., 2017). This effect depends on Arabidopsis amine oxidase (AtAO1) dependent H2O2 production (Ghuge et al., 2015). Intriguingly, JA treatment reduces cytokinin responses in the vasculature tissues and exogenous cytokinin application counteracts the xylem induction effect of JA. At a molecular level, the JA responsive transcription factor MYC2 regulates expression of ARABIDOPSIS HISTIDINE PHOSPHO-TRANSFER PROTEIN 6 (AHP6), which suppresses cytokinin-regulated xylem formation (Jang et al., 2017) . As such, JA and cytokinin signaling act as antagonists in regulating xylem development (Figure 1). Because drought increases JA and decreases cytokinin responses, drought stress-induced xylem formation may involve in this antagonistic pathway (Jang and Choi, 2018). Besides its interaction with the cytokinin signaling pathway, JA reduces PIN7 expression and overexpression of PIN7 suppressed the formation of extra xylem in response to JA. This suggests that JA regulation of xylem development also requires polar auxin transport (Jang et al., 2019). Given the auxin response maximum in the xylem axis is maintained by cytokinin-mediated effects on PIN expression and their subcellular localization (Bishopp et al., 2011a; Marhavy et al., 2011; Marhavy et al., 2014), xylem development clearly involves cross-talk between multiple hormones.
Wait, what about the others?
Although clear roles for (gibberellic acid) GA, strigolactones (SL) and ethylene have been reported for vascular development during secondary growth in Arabidopsis (Agusti et al., 2011; Ben-Targem et al., 2021; Carlsbecker and Augstein, 2021; Etchells et al., 2012; Hu et al., 2022; Mäkilä et al., 2022; Ragni et al., 2011; Yang et al., 2020), very little has been shown for primary vascular development in the Arabidopsis root meristem. In one of the few reports, xylem development has been shown to be affected by high concentration of salt, including the formation of additional protoxylem cells; protoxylem gaps and early differentiation of the inner metaxylem (Augstein and Carlsbecker, 2022). The additional protoxylem and early inner metaxylem differentiation phenotype are ABA-dependent, whereas the protoxylem gaps are regulated by GA signaling (Augstein and Carlsbecker, 2022; Ramachandran et al., 2021). As such, GA is a negative regulator of protoxylem differentiation under high-salt conditions. GA has also been reported as a positive regulator of procambium cell formation. Light is required for procambium cell formation in the VISUAL system, but this can be replaced by GA application in dark conditions (Yamazaki et al., 2018). Although light did not elevate the endogenous GA content, both GA and light could reduce the accumulation of DELLA proteins, which suppresses procambium cell formation during vascular development in the VISUAL system (Yamazaki et al., 2018).
Outlook
In this review, we have summarised the current knowledge of how plant hormones impinge on the molecular regulators of vascular development. Although auxin and cytokinin have been the classically described as the main players, recent literature highlights an increasingly important role for other hormones as well. Our understanding of vascular tissue development is thus likely to develop into an intricate network of hormonal cross-talk influencing the molecular players depending on the developmental stage and environmental conditions. This connects to one of the major unresolved issues in vascular development: the fragmentation of available information in specific developmental stages, tissues and organs. Given that vascular tissues form one connected network throughout the plant body and vascular development is in reality a continuous process, this fragmented data on the function of molecular regulators and the hormonal control mechanisms will need to connected at some point into a holistic model. Untangling this complex regulatory system over multiple tissue types will require technological advancements in the form of high resolution spatiotemporal information provided by emerging single cell applications such as single cell and spatial transcriptomics, metabolomics and proteomics (Seyfferth et al., 2021).
The plant vascular system transports water, sugars and nutrients throughout the plant body and generates almost all of the material that makes up wood in trees. Therefore, modifying the number or type of vascular cells in plants can improve source to sink transport, biomassproduction to sequester atmospheric CO2, or plant characteristics for producing bio-fuels. Moreover, specifically modulating xylem characteristics, such as cell number and size, have been shown to assist plants in coping with drought and other stresses (Arend and Fromm, 2007; Henry et al., 2012; Prince et al., 2017; Richards and Passioura, 1989; Tang et al., 2018). As such, vascular characteristics are promising, yet underutilised, targets for crop breeding or editing efforts. Considering the complex hormonal cross-talk, the mobility of hormones and molecular players, cell-to-cell communications and multiple feed-back/feed-forward connections between the molecular regulators underlying these processes; a multidisciplinary systems biology approach will be required to first understand the system and then modulate it to create, select or breed plants with optimal characteristics to thrive in a changing climate.
Highlight.
Hormones control the molecular regulation of primary root vascular development in Arabidopsis thaliana
Funding
This work was funded by The Research Foundation - Flanders (FWO post-doc fellowship 1215820N); the European Research Council (ERC Starting Grant TORPEDO; 714055); and the China Scholarship Council (PhD fellowship 202009350010).
Footnotes
Author Contributions:
Y.S. and B.D.R. wrote the paper with input from B.Y.
Conflict of interest:
The authors declare no conflict of interest.
Contributor Information
Yanbiao Sun, Email: yanbiao.sun@psb.vib-ugent.be.
Baojun Yang, Email: bjyang@genetics.ac.cn.
References
- Abel S, Nguyen MD, Theologis A. ThePS-IAA4/5-like Family of Early Auxin-inducible mRNAs inArabidopsis thaliana. Journal of Molecular Biology. 1995;251:533–549. doi: 10.1006/jmbi.1995.0454. [DOI] [PubMed] [Google Scholar]
- Agusti J, Blazquez MA. Plant vascular development: mechanisms and environmental regulation. Cellular and Molecular Life Sciences. 2020;77:3711–3728. doi: 10.1007/s00018-020-03496-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agusti J, Herold S, Schwarz M, Sanchez P, Ljung K, Dun EA, Brewer PB, Beveridge CA, Sieberer T, Sehr EM, Greb T. Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. Proceedings of the National Academy of Sciences, USA. 2011;108:20242–20247. doi: 10.1073/pnas.1111902108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anne P, Azzopardi M, Gissot L, Beaubiat S, Hematy K, Palauqui JC. OCTOPUS Negatively Regulates BIN2 to Control Phloem Differentiation in Arabidopsis thaliana. Current Biology. 2015;25:2584–2590. doi: 10.1016/j.cub.2015.08.033. [DOI] [PubMed] [Google Scholar]
- Arend M, Fromm J. Seasonal change in the drought response of wood cell development in poplar. Tree Physiology. 2007;27:985–992. doi: 10.1093/treephys/27.7.985. [DOI] [PubMed] [Google Scholar]
- Argyros RD, Mathews DE, Chiang YH, Palmer CM, Thibault DM, Etheridge N, Argyros DA, Mason MG, Kieber JJ, Schaller GE. Type B response regulators of Arabidopsis play key roles in cytokinin signaling and plant development. The Plant Cell. 2008;20:2102–2116. doi: 10.1105/tpc.108.059584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augstein F. Mechanisms of plant root xylem developmental plasticity in response to water deficiency and salt. Acta Universitatis Upsaliensis. 2022 [Google Scholar]
- Augstein F, Carlsbecker A. Salinity induces discontinuous protoxylem via a DELLA-dependent mechanism promoting salt tolerance in Arabidopsis seedlings. New Phytologist. 2022;236:195–209. doi: 10.1111/nph.18339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baima S, Forte V, Possenti M, Penalosa A, Leoni G, Salvi S, Felici B, Ruberti I, Morelli G. Negative feedback regulation of auxin signaling by ATHB8/ACL5-BUD2 transcription module. Molecular Plant. 2014;7:1006–1025. doi: 10.1093/mp/ssu051. [DOI] [PubMed] [Google Scholar]
- Baima S, Nobili F, Sessa G, Lucchetti S, Ruberti I, Morelli G. The expression of the Athb-8 homeobox gene is restricted to provascular cells in Arabidopsis thaliana. Development. 1995;121:4171–4182. doi: 10.1242/dev.121.12.4171. [DOI] [PubMed] [Google Scholar]
- Bauby H, Divol F, Truernit E, Grandjean O, Palauqui JC. Protophloem differentiation in early Arabidopsis thaliana development. Plant Cell Physiology. 2007;48:97–109. doi: 10.1093/pcp/pcl045. [DOI] [PubMed] [Google Scholar]
- Baum SF, Dubrovsky JG, Rost TL. Apical organization and maturation of the cortex and vascular cylinder in Arabidopsis thaliana (Brassicaceae) roots. American Journal of Botany. 2002;89:908–920. doi: 10.3732/ajb.89.6.908. [DOI] [PubMed] [Google Scholar]
- Ben-Targem M, Ripper D, Bayer M, Ragni L. Auxin and gibberellin signaling cross-talk promotes hypocotyl xylem expansion and cambium homeostasis. Journal of Experimental Botany. 2021;72:3647–3660. doi: 10.1093/jxb/erab089. [DOI] [PubMed] [Google Scholar]
- Berleth T, Jurgens G. The role of the monopteros gene in organising the basal body region of the Arabidopsis embryo. Development. 1993;118:575–587. [Google Scholar]
- Bishopp A, Help H, El-Showk S, Weijers D, Scheres B, Friml J, Benkova E, Mahonen AP, Helariutta Y. A mutually inhibitory interaction between auxin and cytokinin specifies vascular pattern in roots. Current Biology. 2011a;21:917–926. doi: 10.1016/j.cub.2011.04.017. [DOI] [PubMed] [Google Scholar]
- Bishopp A, Lehesranta S, Vaten A, Help H, El-Showk S, Scheres B, Helariutta K, Mahonen AP, Sakakibara H, Helariutta Y. Phloem-transported cytokinin regulates polar auxin transport and maintains vascular pattern in the root meristem. Current Biology. 2011b;21:927–932. doi: 10.1016/j.cub.2011.04.049. [DOI] [PubMed] [Google Scholar]
- Blob B, Heo JO, Helariutta Y. Phloem differentiation: an integrative model for cell specification. Journal of Plant Research. 2018;131:31–36. doi: 10.1007/s10265-017-0999-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch D, Puli MR, Mosquna A, Yalovsky S. Abiotic stress modulates root patterning via ABA-regulated microRNA expression in the endodermis initials. Development. 2019;146 doi: 10.1242/dev.177097. [DOI] [PubMed] [Google Scholar]
- Bonke M, Thitamadee S, Mähönen AP, Hauser M-T, Helariutta Y. APL regulates vascular tissue identity in Arabidopsis. Nature. 2003;426:181–186. doi: 10.1038/nature02100. [DOI] [PubMed] [Google Scholar]
- Breda AS, Hazak O, Schultz P, Anne P, Graeff M, Simon R, Hardtke CS. A Cellular Insulator against CLE45 Peptide Signaling. Current Biology. 2019;29:2501–2508.:e2503. doi: 10.1016/j.cub.2019.06.037. [DOI] [PubMed] [Google Scholar]
- Carlsbecker A, Augstein F. Xylem versus phloem in secondary growth: a balancing act mediated by gibberellins. Journal of Experimental Botany. 2021;72:3489–3492. doi: 10.1093/jxb/erab148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsbecker A, Lee JY, Roberts CJ, Dettmer J, Lehesranta S, Zhou J, Lindgren O, Moreno-Risueno MA, Vaten A, Thitamadee S, Campilho A, et al. Cell signalling by microRNA165/6 directs gene dose-dependent root cell fate. Nature. 2010;465:316–321. doi: 10.1038/nature08977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Dang TV, Hwang I. Emergence of plant vascular system: roles of hormonal and non-hormonal regulatory networks. Current Opinion in Plant Biology. 2017;35:91–97. doi: 10.1016/j.pbi.2016.11.013. [DOI] [PubMed] [Google Scholar]
- Cui H, Hao Y, Kovtun M, Stolc V, Deng XW, Sakakibara H, Kojima M. Genome-wide direct target analysis reveals a role for SHORT-ROOT in root vascular patterning through cytokinin homeostasis. Plant Physiology. 2011;157:1221–1231. doi: 10.1104/pp.111.183178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Levesque MP, Vernoux T, Jung JW, Paquette AJ, Gallagher KL, Wang JY, Blilou I, Scheres B, Benfey PN. An Evolutionarily Conserved Mechanism Delimiting SHR Movement Defines a Single Layer of Endodermis in Plants. Science. 2007;316:421–425. doi: 10.1126/science.1139531. [DOI] [PubMed] [Google Scholar]
- De Rybel B, Adibi M, Breda AS, Wendrich JR, Smit ME, Novak O, Yamaguchi N, Yoshida S, Van Isterdael G, Palovaara J, Nijsse B, et al. Plant development. Integration of growth and patterning during vascular tissue formation in Arabidopsis. Science. 2014;345:1255215. doi: 10.1126/science.1255215. [DOI] [PubMed] [Google Scholar]
- De Rybel B, Audenaert D, Vert G, Rozhon W, Mayerhofer J, Peelman F, Coutuer S, Denayer T, Jansen L, Nguyen L, Vanhoutte I, et al. Chemical inhibition of a subset of Arabidopsis thaliana GSK3-like kinases activates brassinosteroid signaling. Chemistry Biology. 2009;16:594–604. doi: 10.1016/j.chembiol.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rybel B, Mahonen AP, Helariutta Y, Weijers D. Plant vascular development: from early specification to differentiation. Nature Reviews: Molecular Cell Biology. 2016;17:30–40. doi: 10.1038/nrm.2015.6. [DOI] [PubMed] [Google Scholar]
- De Rybel B, Moller B, Yoshida S, Grabowicz I, Barbier de Reuille P, Boeren S, Smith RS, Borst JW, Weijers D. A bHLH complex controls embryonic vascular tissue establishment and indeterminate growth in Arabidopsis. Developmental Cell. 2013;24:426–437. doi: 10.1016/j.devcel.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Depuydt S, Rodriguez-Villalon A, Santuari L, Wyser-Rmili C, Ragni L, Hardtke CS. Suppression of Arabidopsis protophloem differentiation and root meristem growth by CLE45 requires the receptor-like kinase BAM3. Proceedings of the National Academy of Sciences, USA. 2013;110:7074–7079. doi: 10.1073/pnas.1222314110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer J, Elo A, Helariutta Y. Hormone interactions during vascular development. Plant Molecular Biology. 2009;69:347–360. doi: 10.1007/s11103-008-9374-9. [DOI] [PubMed] [Google Scholar]
- el-Showk S, Help-Rinta-Rahko H, Blomster T, Siligato R, Maree AF, Mahonen AP, Grieneisen VA. Parsimonious Model of Vascular Patterning Links Transverse Hormone Fluxes to Lateral Root Initiation: Auxin Leads the Way, while Cytokinin Levels Out. PLoS Computational Biology. 2015;11:e1004450. doi: 10.1371/journal.pcbi.1004450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escamez S, Tuominen H. Programmes of cell death and autolysis in tracheary elements: when a suicidal cell arranges its own corpse removal. Journal of Experimental Botany. 2014;65:1313–1321. doi: 10.1093/jxb/eru057. [DOI] [PubMed] [Google Scholar]
- Etchells JP, Provost CM, Turner SR. Plant vascular cell division is maintained by an interaction between PXY and ethylene signalling. PLoS Genetics. 2012;8:e1002997. doi: 10.1371/journal.pgen.1002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jürgens G. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature. 2003;426:147–153. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- Fujiwara M, Imamura M, Matsushita K, Roszak P, Yamashino T, Hosokawa Y, Nakajima K, Fujimoto K, Miyashima S. Patterned proliferation orients tissue-wide stress to control root vascular symmetry in Arabidopsis. Current Biology. 2023 doi: 10.1016/j.cub.2023.01.036. [DOI] [PubMed] [Google Scholar]
- Ghuge SA, Carucci A, Rodrigues-Pousada RA, Tisi A, Franchi S, Tavladoraki P, Angelini R, Cona A. The Apoplastic Copper AMINE OXIDASE1 Mediates Jasmonic Acid-Induced Protoxylem Differentiation in Arabidopsis Roots. Plant Physiology. 2015;168:690–707. doi: 10.1104/pp.15.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeff M, Rana S, Marhava P, Moret B, Hardtke CS. Local and Systemic Effects of Brassinosteroid Perception in Developing Phloem. Current Biology. 2020;30:1626–1638.:e1623. doi: 10.1016/j.cub.2020.02.029. [DOI] [PubMed] [Google Scholar]
- Hao Y, Cui H. SHORT-ROOT regulates vascular patterning, but not apical meristematic activity in the Arabidopsis root through cytokinin homeostasis. Plant Signaling Behavior. 2012;7:314–317. doi: 10.4161/psb.19118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardtke CS, Berleth T. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. The EMBO Journal. 1998;17:1405–1411. doi: 10.1093/emboj/17.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helariutta Y, Fukaki H, Wysocka-Diller J, Nakajima K, Jung J, Sena G, Hauser M-T, Benfey PN. The SHORT-ROOT Gene Controls Radial Patterning of the Arabidopsis Root through Radial Signaling. Cell. 2000;101:555–567. doi: 10.1016/s0092-8674(00)80865-x. [DOI] [PubMed] [Google Scholar]
- Henry A, Cal AJ, Batoto TC, Torres RO, Serraj R. Root attributes affecting water uptake of rice (Oryza sativa) under drought. Journal of Experimental Botany. 2012;63:4751–4763. doi: 10.1093/jxb/ers150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo JO, Blob B, Helariutta Y. Differentiation of conductive cells: a matter of life and death. Current Opinion in Plant Biology. 2017;35:23–29. doi: 10.1016/j.pbi.2016.10.007. [DOI] [PubMed] [Google Scholar]
- Holzwart E, Huerta AI, Glockner N, Garnelo Gomez B, Wanke F, Augustin S, Askani JC, Schurholz AK, Harter K, Wolf S. BRI1 controls vascular cell fate in the Arabidopsis root through RLP44 and phytosulfokine signaling. Proceedings of the National Academy of Sciences, USA. 2018;115:11838–11843. doi: 10.1073/pnas.1814434115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Hu X, Yang Y, He C, Hu J, Wang X. Strigolactone signaling regulates cambial activity through repression of WOX4 by transcription factor BES1. Plant Physiology. 2022;188:255–267. doi: 10.1093/plphys/kiab487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioio RD, Nakamura K, Moubayidin L, Perilli S, Taniguchi M, Morita MT, Aoyama T, Costantino P, Sabatini S. A Genetic Framework for the Control of Cell Division and Differentiation in the Root Meristem. Science. 2008;322:1380–1384. doi: 10.1126/science.1164147. [DOI] [PubMed] [Google Scholar]
- Jang G, Chang SH, Um TY, Lee S, Kim JK, Choi YD. Antagonistic interaction between jasmonic acid and cytokinin in xylem development. Scientific Reports. 2017;7:10212. doi: 10.1038/s41598-017-10634-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang G, Choi YD. Drought stress promotes xylem differentiation by modulating the interaction between cytokinin and jasmonic acid. Plant Signaling Behavior. 2018;13:e1451707. doi: 10.1080/15592324.2018.1451707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang G, Yoon Y, Choi YD. Jasmonic Acid Modulates Xylem Development by Controlling Expression of PIN-FORMED 7. Plant Signaling Behavior. 2019;14:1637664. doi: 10.1080/15592324.2019.1637664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YH, Breda A, Hardtke CS. Brassinosteroid signaling directs formative cell divisions and protophloem differentiation in Arabidopsis root meristems. Development. 2017;144:272–280. doi: 10.1242/dev.145623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama H, Iwamoto K, Kariya Y, Asakawa T, Kan T, Fukuda H, Ohashi-Ito K. A Negative Feedback Loop Controlling bHLH Complexes Is Involved in Vascular Cell Division and Differentiation in the Root Apical Meristem. Current Biology. 2015;25:3144–3150. doi: 10.1016/j.cub.2015.10.051. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Fujita T, Sugiyama M, Fukuda H. A novel system for xylem cell differentiation in Arabidopsis thaliana. Molecular Plant. 2015;8:612–621. doi: 10.1016/j.molp.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Nurani AM, Saito C, Ichihashi Y, Saito M, Yamazaki K, Mitsuda N, Ohme-Takagi M, Fukuda H. Vascular Cell Induction Culture System Using Arabidopsis Leaves (VISUAL) Reveals the Sequential Differentiation of Sieve Element-Like Cells. The Plant Cell. 2016;28:1250–1262. doi: 10.1105/tpc.16.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogan NT, Yin X, Ckurshumova W, Berleth T. Distinct subclades of Aux/IAA genes are direct targets of ARF5/MP transcriptional regulation. New Phytologist. 2014;204:474–483. doi: 10.1111/nph.12994. [DOI] [PubMed] [Google Scholar]
- Kubo M, Udagawa M, Nishikubo N, Horiguchi G, Yamaguchi M, Ito J, Mimura T, Fukuda H, Demura T. Transcription switches for protoxylem and metaxylem vessel formation. Genes Development. 2005;19:1855–1860. doi: 10.1101/gad.1331305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Colinas J, Wang JY, Mace D, Ohler U, Benfey PN. Transcriptional and posttranscriptional regulation of transcription factor expression in Arabidopsis roots. Proceedings of the National Academy of Sciences, USA. 2006;103:6055–6060. doi: 10.1073/pnas.0510607103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Frank M. Plasmodesmata in phloem: different gateways for different cargoes. Current Opinion in Plant Biology. 2018;43:119–124. doi: 10.1016/j.pbi.2018.04.014. [DOI] [PubMed] [Google Scholar]
- Mähönen AP, Bishopp A, Higuchi M, Nieminen KM, Kinoshita K, Törmäkangas K, Ikeda Y, Oka A, Kakimoto T, Helariutta Y. Cytokinin Signaling and Its Inhibitor AHP6 Regulate Cell Fate During Vascular Development. Science. 2006;311:94–98. doi: 10.1126/science.1118875. [DOI] [PubMed] [Google Scholar]
- Mahonen AP, Bonke M, Kauppinen L, Riikonen M, Benfey PN, Helariutta Y. A novel two-component hybrid molecule regulates vascular morphogenesis of the Arabidopsis root. Genes Development. 2000;14:2938–2943. doi: 10.1101/gad.189200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkilä R, Wybouw B, Smetana O, Vainio L, Solé-Gil A, Lyu M, Ye L, Wang X, Siligato R, Jenness MK, Murphy AS, et al. Gibberellins promote polar auxin transport to regulate stem cell fate decisions in cambium. bioRxiv. 2022:2022.2007.2015.500224. doi: 10.1038/s41477-023-01360-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhava P, Bassukas AEL, Zourelidou M, Kolb M, Moret B, Fastner A, Schulze WX, Cattaneo P, Hammes UZ, Schwechheimer C, Hardtke CS. A molecular rheostat adjusts auxin flux to promote root protophloem differentiation. Nature. 2018;558:297–300. doi: 10.1038/s41586-018-0186-z. [DOI] [PubMed] [Google Scholar]
- Marhavy P, Bielach A, Abas L, Abuzeineh A, Duclercq J, Tanaka H, Parezova M, Petrasek J, Friml J, Kleine-Vehn J, Benkova E. Cytokinin modulates endocytic trafficking of PIN1 auxin efflux carrier to control plant organogenesis. Developmental Cell. 2011;21:796–804. doi: 10.1016/j.devcel.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Marhavy P, Duclercq J, Weller B, Feraru E, Bielach A, Offringa R, Friml J, Schwechheimer C, Murphy A, Benkova E. Cytokinin controls polarity of PIN1-dependent auxin transport during lateral root organogenesis. Current Biology. 2014;24:1031–1037. doi: 10.1016/j.cub.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Miyashima S, Roszak P, Sevilem I, Toyokura K, Blob B, Heo J-o, Mellor N, Help-Rinta-Rahko H, Otero S, Smet W, Boekschoten M, Hooiveld G, et al. Mobile PEAR transcription factors integrate positional cues to prime cambial growth. Nature. 2019;565:490–494. doi: 10.1038/s41586-018-0839-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor E, Pernisova M, Minne M, Cerutti G, Ripper D, Nolf J, Andres J, Ragni L, Zurbriggen MD, De Rybel B, Vernoux T. bHLH heterodimer complex variations regulate cell proliferation activity in the meristems of Arabidopsis thaliana. iScience. 2022;25:105364. doi: 10.1016/j.isci.2022.105364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moret B, Marhava P, Aliaga Fandino AC, Hardtke CS, Ten Tusscher KHW. Local auxin competition explains fragmented differentiation patterns. Nature Communications. 2020;11:2965. doi: 10.1038/s41467-020-16803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchel CF, Osmont KS, Hardtke CS. BRX mediates feedback between brassinosteroid levels and auxin signalling in root growth. Nature. 2006;443:458–461. doi: 10.1038/nature05130. [DOI] [PubMed] [Google Scholar]
- Muller CJ, Valdes AE, Wang G, Ramachandran P, Beste L, Uddenberg D, Carlsbecker A. PHABULOSA Mediates an Auxin Signaling Loop to Regulate Vascular Patterning in Arabidopsis. Plant Physiology. 2016;170:956–970. doi: 10.1104/pp.15.01204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniz L, Minguet EG, Singh SK, Pesquet E, Vera-Sirera F, Moreau-Courtois CL, Carbonell J, Blazquez MA, Tuominen H. ACAULIS5 controls Arabidopsis xylem specification through the prevention of premature cell death. Development. 2008;135:2573–2582. doi: 10.1242/dev.019349. [DOI] [PubMed] [Google Scholar]
- Muraro D, Mellor N, Pound MP, Help H, Lucas M, Chopard J, Byrne HM, Godin C, Hodgman TC, King JR, Pridmore TP, et al. Integration of hormonal signaling networks and mobile microRNAs is required for vascular patterning in Arabidopsis roots. Proceedings of the National Academy of Sciences, USA. 2014;111:857–862. doi: 10.1073/pnas.1221766111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagawa S, Sawa S, Sato S, Kato T, Tabata S, Fukuda H. Gene trapping in Arabidopsis reveals genes involved in vascular development. Plant Cell Physiology. 2006;47:1394–1405. doi: 10.1093/pcp/pcl009. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Sena G, Nawy T, Benfey PN. Intercellular movement of the putative transcription factor SHR in root patterning. Nature. 2001;413:307–311. doi: 10.1038/35095061. [DOI] [PubMed] [Google Scholar]
- Ohashi-Ito K, Iwamoto K, Nagashima Y, Kojima M, Sakakibara H, Fukuda H. A Positive Feedback Loop Comprising LHW-TMO5 and Local Auxin Biosynthesis Regulates Initial Vascular Development in Arabidopsis Roots. Plant Cell Physiology. 2019;60:2684–2691. doi: 10.1093/pcp/pcz156. [DOI] [PubMed] [Google Scholar]
- Ohashi-Ito K, Matsukawa M, Fukuda H. An atypical bHLH transcription factor regulates early xylem development downstream of auxin. Plant Cell Physiology. 2013;54:398–405. doi: 10.1093/pcp/pct013. [DOI] [PubMed] [Google Scholar]
- Ohashi-Ito K, Saegusa M, Iwamoto K, Oda Y, Katayama H, Kojima M, Sakakibara H, Fukuda H. A bHLH Complex Activates Vascular Cell Division via Cytokinin Action in Root Apical Meristem. Current Biology. 2014;24:2053–2058. doi: 10.1016/j.cub.2014.07.050. [DOI] [PubMed] [Google Scholar]
- Prince SJ, Murphy M, Mutava RN, Durnell LA, Valliyodan B, Shannon JG, Nguyen HT. Root xylem plasticity to improve water use and yield in water-stressed soybean. Journal of Experimental Botany. 2017;68:2027–2036. doi: 10.1093/jxb/erw472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragni L, Greb T. Secondary growth as a determinant of plant shape and form. Seminars in Cell Developmental Biology. 2018;79:58–67. doi: 10.1016/j.semcdb.2017.08.050. [DOI] [PubMed] [Google Scholar]
- Ragni L, Nieminen K, Pacheco-Villalobos D, Sibout R, Schwechheimer C, Hardtke CS. Mobile gibberellin directly stimulates Arabidopsis hypocotyl xylem expansion. The Plant Cell. 2011;23:1322–1336. doi: 10.1105/tpc.111.084020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran P, Augstein F, Mazumdar S, Nguyen TV, Minina EA, Melnyk CW, Carlsbecker A. Abscisic acid signaling activates distinct VND transcription factors to promote xylem differentiation in Arabidopsis. Current Biology. 2021;31:3153–3161.:e3155. doi: 10.1016/j.cub.2021.04.057. [DOI] [PubMed] [Google Scholar]
- Ramachandran P, Augstein F, Nguyen V, Carlsbecker A. Coping With Water Limitation: Hormones That Modify Plant Root Xylem Development. Frontiers in Plant Science. 2020;11:570. doi: 10.3389/fpls.2020.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran P, Wang G, Augstein F, de Vries J, Carlsbecker A. Continuous root xylem formation and vascular acclimation to water deficit involves endodermal ABA signalling via miR165. Development. 2018;145 doi: 10.1242/dev.159202. [DOI] [PubMed] [Google Scholar]
- Ren B, Chen Q, Hong S, Zhao W, Feng J, Feng H, Zuo J. The Arabidopsis eukaryotic translation initiation factor eIF5A-2 regulates root protoxylem development by modulating cytokinin signaling. The Plant Cell. 2013;25:3841–3857. doi: 10.1105/tpc.113.116236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards RA, Passioura JB. A breeding program to reduce the diameter of the major xylem vessel in the seminal roots of wheat and its effect on grain yield in rain-fed environments. Australian Journal of Agricultural Research. 1989;40:943–950. [Google Scholar]
- Rodrigues A, Santiago J, Rubio S, Saez A, Osmont KS, Gadea J, Hardtke CS, Rodriguez PL. The short-rooted phenotype of the brevis radix mutant partly reflects root abscisic acid hypersensitivity. Plant Physiology. 2009;149:1917–1928. doi: 10.1104/pp.108.133819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Villalon A, Gujas B, Kang YH, Breda AS, Cattaneo P, Depuydt S, Hardtke CS. Molecular genetic framework for protophloem formation. Proceedings of the National Academy of Sciences, USA. 2014;111:11551–11556. doi: 10.1073/pnas.1407337111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak P, Heo JO, Blob B, Toyokura K, Sugiyama Y, de Luis Balaguer MA, Lau WWY, Hamey F, Cirrone J, Madej E, Bouatta AM, et al. Cell-by-cell dissection of phloem development links a maturation gradient to cell specialization. Science. 2021;374:eaba5531. doi: 10.1126/science.aba5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruonala R, Ko D, Helariutta Y. Genetic Networks in Plant Vascular Development. Annual Review of Genetics. 2017;51:335–359. doi: 10.1146/annurev-genet-120116-024525. [DOI] [PubMed] [Google Scholar]
- Saito M, Kondo Y, Fukuda H. BES1 and BZR1 Redundantly Promote Phloem and Xylem Differentiation. Plant Cell Physiology. 2018;59:590–600. doi: 10.1093/pcp/pcy012. [DOI] [PubMed] [Google Scholar]
- Scacchi E, Osmont KS, Beuchat J, Salinas P, Navarrete-Gomez M, Trigueros M, Ferrandiz C, Hardtke CS. Dynamic, auxin-responsive plasma membrane-to-nucleus movement of Arabidopsis BRX. Development. 2009;136:2059–2067. doi: 10.1242/dev.035444. [DOI] [PubMed] [Google Scholar]
- Scacchi E, Salinas P, Gujas B, Santuari L, Krogan N, Ragni L, Berleth T, Hardtke CS. Spatio-temporal sequence of cross-regulatory events in root meristem growth. Proceedings of the National Academy of Sciences, USA. 2010;107:22734–22739. doi: 10.1073/pnas.1014716108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpella E, Meijer AH. Pattern formation in the vascular system of monocot and dicot plant species. New Phytologist. 2004;164:209–242. doi: 10.1111/j.1469-8137.2004.01191.x. [DOI] [PubMed] [Google Scholar]
- Scheres B, Di Laurenzio L, Willemsen V, Hauser MT, Janmaat K, Weisbeek P, Benfey PN. Mutations affecting the radial organisation of the Arabidopsis root display specific defects throughout the embryonic axis. Development. 1995;121:53–62. [Google Scholar]
- Scheres B, Wolkenfelt H, Willemsen V, Terlouw M, Lawson E, Dean C, Weisbeek P. Embryonic origin of the Arabidopsis primary root and root meristem initials. Development. 1994;120:2475–2487. [Google Scholar]
- Schlereth A, Moller B, Liu W, Kientz M, Flipse J, Rademacher EH, Schmid M, Jurgens G, Weijers D. MONOPTEROS controls embryonic root initiation by regulating a mobile transcription factor. Nature. 2010;464:913–916. doi: 10.1038/nature08836. [DOI] [PubMed] [Google Scholar]
- Seyfferth C, Renema J, Wendrich JR, Eekhout T, Seurinck R, Vandamme N, Blob B, Saeys Y, Helariutta Y, Birnbaum KD, De Rybel B. Advances and Opportunities in Single-Cell Transcriptomics for Plant Research. Annual Review of Plant Biology. 2021;72:847–866. doi: 10.1146/annurev-arplant-081720-010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smet W, De Rybel B. Genetic and hormonal control of vascular tissue proliferation. Current Opinion in Plant Biology. 2016;29:50–56. doi: 10.1016/j.pbi.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Smet W, Sevilem I, de Luis Balaguer MA, Wybouw B, Mor E, Miyashima S, Blob B, Roszak P, Jacobs TB, Boekschoten M, Hooiveld G, et al. DOF2.1 Controls Cytokinin-Dependent Vascular Cell Proliferation Downstream of TMO5/LHW. Current Biology. 2019;29:520–529.:e526. doi: 10.1016/j.cub.2018.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strother PK, Foster C. A fossil record of land plant origins from charophyte algae. Science. 2021;373:792–796. doi: 10.1126/science.abj2927. [DOI] [PubMed] [Google Scholar]
- Sun P, Wang H, Zhao P, Yu Q, He Y, Deng W, Guo H. The Regulation of Xylem Development by Transcription Factors and Their Upstream MicroRNAs. International Journal of Molecular Sciences. 2022;23 doi: 10.3390/ijms231710134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki T, Oya S, Naito M, Ozawa Y, Furuya T, Saito M, Sato M, Wakazaki M, Toyooka K, Fukuda H, Helariutta Y, et al. VISUAL-CC system uncovers the role of GSK3 as an orchestrator of vascular cell type ratio in plants. Communications Biology. 2020;3:184. doi: 10.1038/s42003-020-0907-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan TT, Endo H, Sano R, Kurata T, Yamaguchi M, Ohtani M, Demura T. Transcription Factors VND1-VND3 Contribute to Cotyledon Xylem Vessel Formation. Plant Physiology. 2018;176:773–789. doi: 10.1104/pp.17.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N, Shahzad Z, Lonjon F, Loudet O, Vailleau F, Maurel C. Natural variation at XND1 impacts root hydraulics and trade-off for stress responses in Arabidopsis. Nature Communications. 2018;9:3884. doi: 10.1038/s41467-018-06430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Teeples M, Lin L, de Lucas M, Turco G, Toal TW, Gaudinier A, Young NF, Trabucco GM, Veling MT, Lamothe R, Handakumbura PP, et al. An Arabidopsis gene regulatory network for secondary cell wall synthesis. Nature. 2015;517:571–575. doi: 10.1038/nature14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursache R, Miyashima S, Chen Q, Vaten A, Nakajima K, Carlsbecker A, Zhao Y, Helariutta Y, Dettmer J. Tryptophan-dependent auxin biosynthesis is required for HD-ZIP III-mediated xylem patterning. Development. 2014;141:1250–1259. doi: 10.1242/dev.103473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera-Sirera F, De Rybel B, Urbez C, Kouklas E, Pesquera M, Alvarez-Mahecha JC, Minguet EG, Tuominen H, Carbonell J, Borst JW, Weijers D, et al. A bHLH-Based Feedback Loop Restricts Vascular Cell Proliferation in Plants. Developmental Cell. 2015;35:432–443. doi: 10.1016/j.devcel.2015.10.022. [DOI] [PubMed] [Google Scholar]
- Weigel D, Jürgens G. Stem cells that make stems. Nature. 2002;415:751–754. doi: 10.1038/415751a. [DOI] [PubMed] [Google Scholar]
- Weijers D, Schlereth A, Ehrismann JS, Schwank G, Kientz M, Jurgens G. Auxin triggers transient local signaling for cell specification in Arabidopsis embryogenesis. Developmental Cell. 2006;10:265–270. doi: 10.1016/j.devcel.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Weller B, Zourelidou M, Frank L, Barbosa IC, Fastner A, Richter S, Jurgens G, Hammes UZ, Schwechheimer C. Dynamic PIN-FORMED auxin efflux carrier phosphorylation at the plasma membrane controls auxin efflux-dependent growth. Proceedings of the National Academy of Sciences, USA. 2017;114:E887-E896. doi: 10.1073/pnas.1614380114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendrich JR, Yang B, Vandamme N, Verstaen K, Smet W, Van de Velde C, Minne M, Wybouw B, Mor E, Arents HE, Nolf J, et al. Vascular transcription factors guide plant epidermal responses to limiting phosphate conditions. Science. 2020;370 doi: 10.1126/science.aay4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel CL, Schuetz M, Yu Q, Mattsson J. Dynamics of MONOPTEROS and PIN-FORMED1 expression during leaf vein pattern formation in Arabidopsis thaliana. Plant Journal. 2007;49:387–398. doi: 10.1111/j.1365-313X.2006.02977.x. [DOI] [PubMed] [Google Scholar]
- Woudenberg S, Renema J, Tomescu AMF, De Rybel B, Weijers D. Deep origin and gradual evolution of transporting tissues: Perspectives from across the land plants. Plant Physiology. 2022;190:85–99. doi: 10.1093/plphys/kiac304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wybouw B, Arents HE, Yang B, Nolf J, Smet W, Vandorpe M, Minne M, Luo X, De Clercq I, Van Damme D, Glanc M, et al. MYB12 is part of a feedback loop regulating cell division orientation in the root meristem vasculature. Journal of Experimental Botany. 2023 doi: 10.1093/jxb/erad020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K, Kondo Y, Kojima M, Takebayashi Y, Sakakibara H, Fukuda H. Suppression of DELLA signaling induces procambial cell formation in culture. Plant Journal. 2018;94:48–59. doi: 10.1111/tpj.13840. [DOI] [PubMed] [Google Scholar]
- Yang B, Minne M, Brunoni F, Plackova L, Petrik I, Sun Y, Nolf J, Smet W, Verstaen K, Wendrich JR, Eekhout T, et al. Non-cell autonomous and spatiotemporal signalling from a tissue organizer orchestrates root vascular development. Nature Plants. 2021;7:1485–1494. doi: 10.1038/s41477-021-01017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Wang S, Li S, Du Q, Qi L, Wang W, Chen J, Wang H. Activation of ACS7 in Arabidopsis affects vascular development and demonstrates a link between ethylene synthesis and cambial activity. Journal of Experimental Botany. 2020;71:7160–7170. doi: 10.1093/jxb/eraa423. [DOI] [PubMed] [Google Scholar]
- Yokoyama A, Yamashino T, Amano Y, Tajima Y, Imamura A, Sakakibara H, Mizuno T. Type-B ARR transcription factors, ARR10 and ARR12, are implicated in cytokinin-mediated regulation of protoxylem differentiation in roots of Arabidopsis thaliana. Plant Cell Physiology. 2007;48:84–96. doi: 10.1093/pcp/pcl040. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Barbier de Reuille P, Lane B, Bassel GW, Prusinkiewicz P, Smith RS, Weijers D. Genetic control of plant development by overriding a geometric division rule. Developmental Cell. 2014;29:75–87. doi: 10.1016/j.devcel.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Zhou J, Wang X, Lee JY, Lee JY. Cell-to-cell movement of two interacting AT-hook factors in Arabidopsis root vascular tissue patterning. The Plant Cell. 2013;25:187–201. doi: 10.1105/tpc.112.102210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Zhong R, Ye ZH. Arabidopsis NAC domain proteins, VND1 to VND5, are transcriptional regulators of secondary wall biosynthesis in vessels. PLoS One. 2014;9:e105726. doi: 10.1371/journal.pone.0105726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zourelidou M, Absmanner B, Weller B, Barbosa IC, Willige BC, Fastner A, Streit V, Port SA, Colcombet J, de la Fuente van Bentem S, Hirt H, et al. Auxin efflux by PIN-FORMED proteins is activated by two different protein kinases, D6 Protein Kinase and Pinoid. Elife. 2014;3 doi: 10.7554/eLife.02860. [DOI] [PMC free article] [PubMed] [Google Scholar]