Abstract
Background and aims
During fat tolerance tests, plasma triglycerides increase while high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and intermediate-density lipoprotein (IDL) cholesterol decrease. However, it is unknown whether triglyceride content increases and cholesterol content decreases in HDL and LDL+IDL fractions following normal meals in the general population. Therefore, we tested the hypothesis that triglyceride content increases while cholesterol content decreases in HDL and LDL+IDL fractions following normal meals.
Methods
In this cross-sectional study we included 25,656 individuals aged 20–100 years, all without lipid-lowering therapy at examination and selected for metabolomic profiling from the Copenhagen General Population Study. Triglyceride and cholesterol content of 14 lipoprotein fractions were measured using nuclear magnetic resonance (NMR) spectroscopy. Time since last meal was recorded by the examiner immediately before blood sampling.
Results
Following normal meals in age and sex-adjusted analyses and when compared with fasting levels, plasma triglycerides increased for up to 5–6 hours, and triglyceride content increased for up to 6–7 hours in HDL fractions, for up to 6–7 hours in LDL+IDL fractions, and for up to 5–6 hours in very-low-density lipoprotein (VLDL) fractions. Further, plasma cholesterol decreased for up to 1–2 hours, and cholesterol content decreased for up to 2–3 hours in HDL fractions and for up to 5–6 hours in LDL+IDL fractions, while cholesterol content increased for up to 4–5 hours in VLDL fractions.
Conclusions
Following normal meals, triglyceride content increases while cholesterol content decreases in HDL and LDL+IDL fractions.
Introduction
Previously, lipid profiles were recommended measured in the fasting state, although in most individuals, the postprandial period predominates over the fasting state1. However, emerging evidence demonstrates only minimal changes in plasma lipids, lipoproteins, and apolipoproteins following intake of normal meals in the general population2–4. In addition, nonfasting plasma triglycerides have been shown to better predict cardiovascular risk than fasting plasma triglycerides5–7. Accordingly, recent international lipid guidelines now endorse use of non-fasting lipid profiles for cardiovascular risk prediction8–10.
Traditionally, fasting lipid profiles were recommended to limit postprandial increase in plasma triglycerides as observed following oral fat tolerance tests during which individuals typically ingest 1 gram fat per kilogram bodyweight11, the latter typically performed in smaller groups of selected individuals during 6–12 hours. In this design, the postprandial increase in plasma triglycerides were often accompanied by a decrease in low-density lipoproteins (LDL) cholesterol and high-density lipoproteins (HDL) cholesterol12,13. Whereas most such studies on postprandial lipid profiles evaluate traditional lipid measures including plasma triglycerides, HDL cholesterol, and LDL cholesterol, some studies have additionally reported large postprandial changes in triglyceride and cholesterol content of lipoprotein fractions12–17. Notably, in long-term, population-based studies, low HDL cholesterol has been suggested to be a marker of elevated plasma triglycerides and triglyceride-rich remnant lipoproteins18. Importantly, it is unknown whether triglyceride content increases while cholesterol content decreases in HDL and LDL+ intermediate-density lipoprotein (IDL) fractions following normal meals in individuals in the general population.
We tested the hypothesis that triglyceride content increases while cholesterol content decreases in HDL and LDL+IDL fractions following normal meals. To test our hypothesis, we cross-sectionally studied 25,656 individuals aged 20–100 years, all without lipid-lowering therapy at examination and selected for metabolomic profiling from the Copenhagen General Population Study. All included individuals had detailed lipid and lipoprotein profiles measured by nuclear magnetic resonance (NMR) spectroscopy of 14 different lipoprotein fractions within HDL, LDL, IDL and very low-density lipoprotein (VLDL).
Materials and methods
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Danish Data Protection Agency and the Ethics Committee of the Capital Region of Denmark (H-KF-01-144/01). All participants provided written informed consent.
The Copenhagen General Populations Study
The Copenhagen General Population Study is an ongoing prospective cohort study of 109,751 Danish adults aged 20–100 years, recruited in 2003–2015, and with a participation rate of 43% of those invited. Invited individuals were randomly selected from the national Danish Civil Registration System to reflect the adult white Danish population. At examination, participants filled in a questionnaire, had a physical examination, and had blood samples collected for biochemical analyses. A subgroup of 30,335 individuals were selected for metabolomic profiling using NMR spectroscopy, and among these, we included 25,656 individuals not taking lipid-lowering therapy at examination. Time since last meal (hours) was obtained by the examiner immediately before blood sampling.
Triglycerides and cholesterol in lipoprotein fractions
High-throughput NMR spectroscopy19,20 was used to measure triglyceride content, cholesterol content, and particle number of 14 lipoprotein fractions comprising four fractions of HDL including small (S) HDL, medium (M) HDL, large (L) HDL, and extra-large (XL) HDL; three fractions of LDL including S LDL, M LDL, and L LDL; one fraction of intermediate-density lipoprotein (IDL); and six fractions of VLDL including extra small (XS) VLDL, S VLDL, M VLDL, L VLDL, XL VLDL, and chylomicrons and extra extra-large (XXL) VLDL. To preserve lipoprotein composition during long-term storage, serum samples were stored at -80 □ until NMR analysis. The NMR analyses were conducted using the Nightingale assay at the Metabolomic Core Facility at the University of Bristol.
Covariates
Weight (kg) and height (m) were measured by the examiner on the day of examination, and body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Plasma cholesterol, plasma triglycerides, and plasma albumin were measured on fresh blood samples at the day of examination using validated standard biochemical assays at the Department of Clinical Biochemistry at Copenhagen University Hospital in Herlev.
Statistical analyses
Stata/SE 17.0 was used to perform all statistical analyses. Missing information (0.49% of all covariate information) were imputed based on age and sex using single imputation; results were similar without imputation. As is a standard procedure in ultracentrifugation21 and previously done using NMR spectroscopy22–24, triglyceride and cholesterol content of lipoprotein fractions were corrected for recovery relative to plasma triglycerides and plasma cholesterol measured on fresh blood samples at the day of examination to obtain clinically relatable lipid levels. Distribution of plasma triglycerides, triglyceride content in all lipoprotein fractions, cholesterol content in VLDL fractions, and VLDL particle number were skewed and therefore logarithmically transformed in statistical analyses to approach normal distribution.
General linear regression models were used to adjust for age and sex; for age; and for age, sex, and plasma albumin. Student t-tests compared between groups plasma triglycerides, plasma cholesterol, triglyceride content in lipoprotein fractions, cholesterol content in lipoprotein fractions, and particle number according to time since last meal ranging from fasting (≥8 hours), 0–1 hour, 1–2 hours, 2–3 hours, 3–4 hours, 4–5 hours, 5–6 hours, 6–7 hours, or 7–8 hours with fasting (≥8 hours) as the reference. For sensitivity, we added analyses stratified according to BMI and sex, and analyses additionally adjusted for plasma albumin to account for haemodilution following intake of fluid.
In analyses stratified according to BMI, individuals were divided into two groups based on the World Health Organization (WHO) classification of overweight (BMI≥25kg/m2); one group with BMI <25 kg/m2 and another group with BMI≥25kg/m2. To account for multiple comparisons, we used a Bonferroni corrected p-value of < 0.05/8 = 0.00625.
Results
Among 30,335 individuals selected for metabolomic profiling nested within 109,751 individuals from the Copenhagen General Population Study, we studied 25,656 individuals aged 20–100 years and without lipid-lowering therapy at examination (Supplementary Figure 1). Baseline characteristics according to time since last meal are shown in Table 1.
Table 1. Baseline characteristics of 25,656 individuals in the Copenhagen General Population Study according to time since the last meal.
| Time since last meal, hours | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All n=25,656 | Fasting n=377 | 0–1 n=3,113 | 1 –2 n=4,742 | 2–3 n=6,771 | 3–4 n=5,544 | 4–5 n=3,051 | 5–6 n=1,398 | 6–7 n=432 | 7–8 n=228 | |
| Women | 13,543 (53%) | 148 (39%) | 1,831 (59%) | 2,599 (55%) | 3,743 (55%) | 2,938 (53%) | 1,418 (46%) | 575 (41%) | 190 (44%) | 101 (44%) |
| Age, years | 61(50–72) | 58 (49–68) | 55 (47–65) | 59 (49–69) | 63 (53–73) | 64 (53–74) | 59(50–71) | 57 (48–67) | 60 (50–72) | 64 (52–74) |
| Albumin, μmol/L | 605 (566–645) | 628(588–664) | 603 (566–643) | 599 (562–638) | 598 (560–636) | 602 (564–643) | 618 (578–659) | 631 (590–671) | 620 (586–666) | 617 (579–659) |
| BMI, kg/m2 | 26 (23–29) | 26 (24–29) | 26 (23–28) | 26 (23–29) | 26 (23–29) | 26 (23–29) | 26 (24–29) | 26 (23–29) | 26 (23–29) | 27 (24–30) |
Data are n (%) for categorical variables and median (interquartile range) for continuous variables. Baseline characteristics were obtained at the day of examination. Time since last meal (hours) was recorded by the examiner immediately before blood sampling. Time since last meal was obtained once for each participant. BMI = body mass index.
Triglycerides and cholesterol in plasma and main lipoprotein fractions
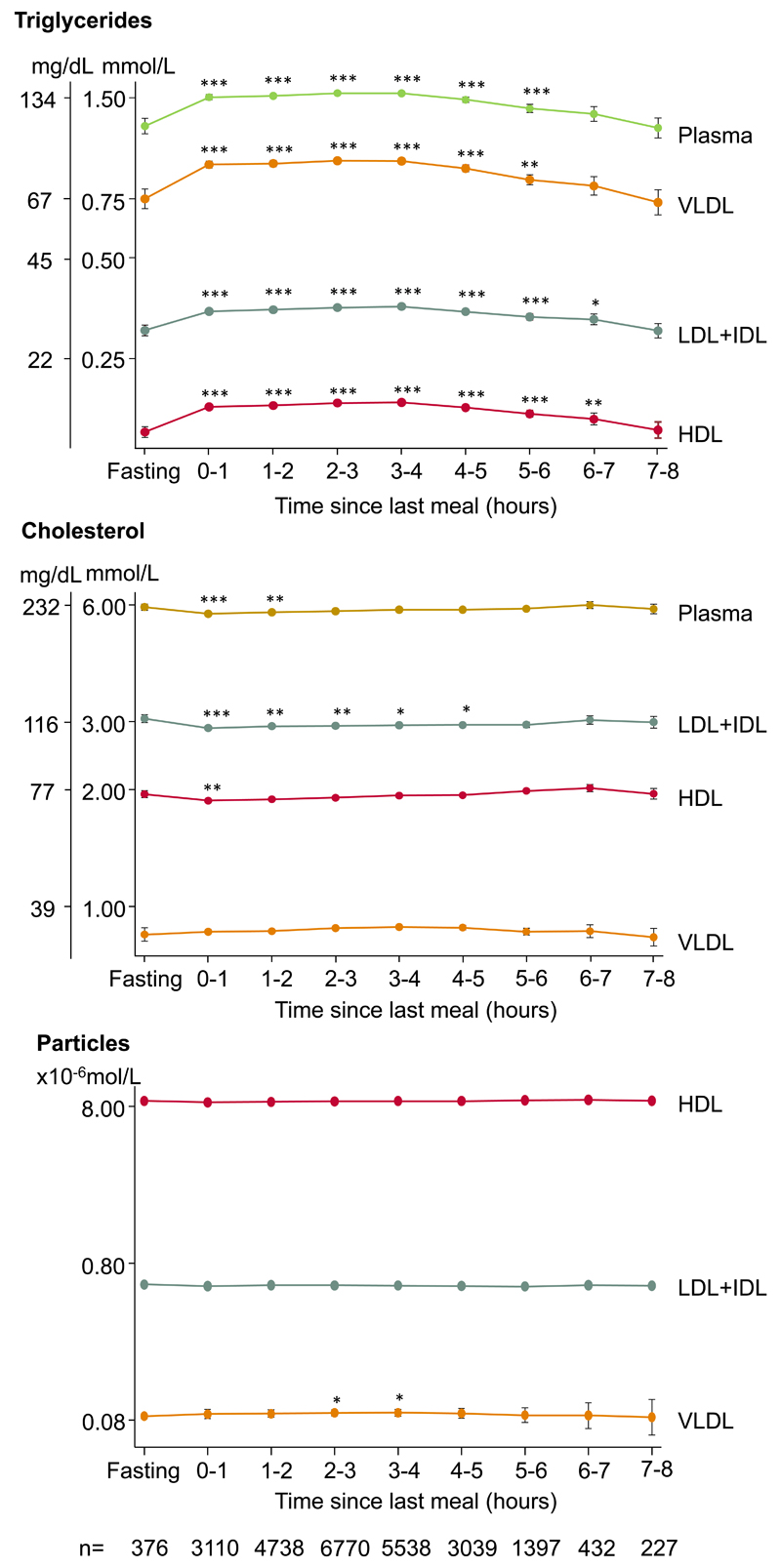
In age and sex-adjusted analyses and when compared with fasting levels, HDL triglycerides increased for up to 6–7 hours, LDL+IDL triglycerides increased for up to 6–7 hours, VLDL triglycerides increased for up to 5–6 hours, and plasma triglycerides increased for up to 5–6 hours (Figure 1, upper panel). Further, HDL cholesterol decreased for up to 0–1 hour, LDL+IDL cholesterol decreased for up to 4–5 hours, and plasma cholesterol decreased for up to 1–2 hours, while no changes in VLDL cholesterol were observed (Figure 1, middle panel). When compared with fasting levels, VLDL particle number increased for up to 3–4 hours (Figure 1, bottom panel). Results were similar in women and men, although most pronounced in men (Supplementary Figure 2).
Figure 1. Triglycerides, cholesterol, and lipoprotein particle number in plasma and in main lipoproteins fractions according to time since the last meal.
Dots represent quadratic means adjusted for age and sex and error bars represent 95% confidence intervals. Plasma triglycerides and plasma cholesterol were measured on fresh blood samples using routine hospital assays. Triglyceride and cholesterol content in lipoprotein fractions were measured using nuclear magnetic resonance spectroscopy and corrected for recovery. The y-axes are on a logarithmic scale. Bonferroni corrected p-values based on 8 parallel tests using unpaired Student t-test versus fasting levels (≥8 hours) are as follows: *p<0.05; **p<0.01; ***p<0.001. Abbreviations: HDL: high-density lipoproteins. IDL: intermediate-density lipoproteins. LDL: low-density lipoproteins. VLDL: very low-density lipoproteins.
A meal often is accompanied by fluid intake resulting in haemodilution, and therefore we added analyses additionally adjusted for plasma albumin (Supplementary Figure 3). In these analyses, plasma cholesterol, HDL cholesterol, and LDL+IDL cholesterol did no longer vary according to time since last meal, while the observed increase in plasma triglycerides, triglyceride content of lipoprotein fractions, and VLDL particle number remained.
Triglycerides in lipoprotein fractions
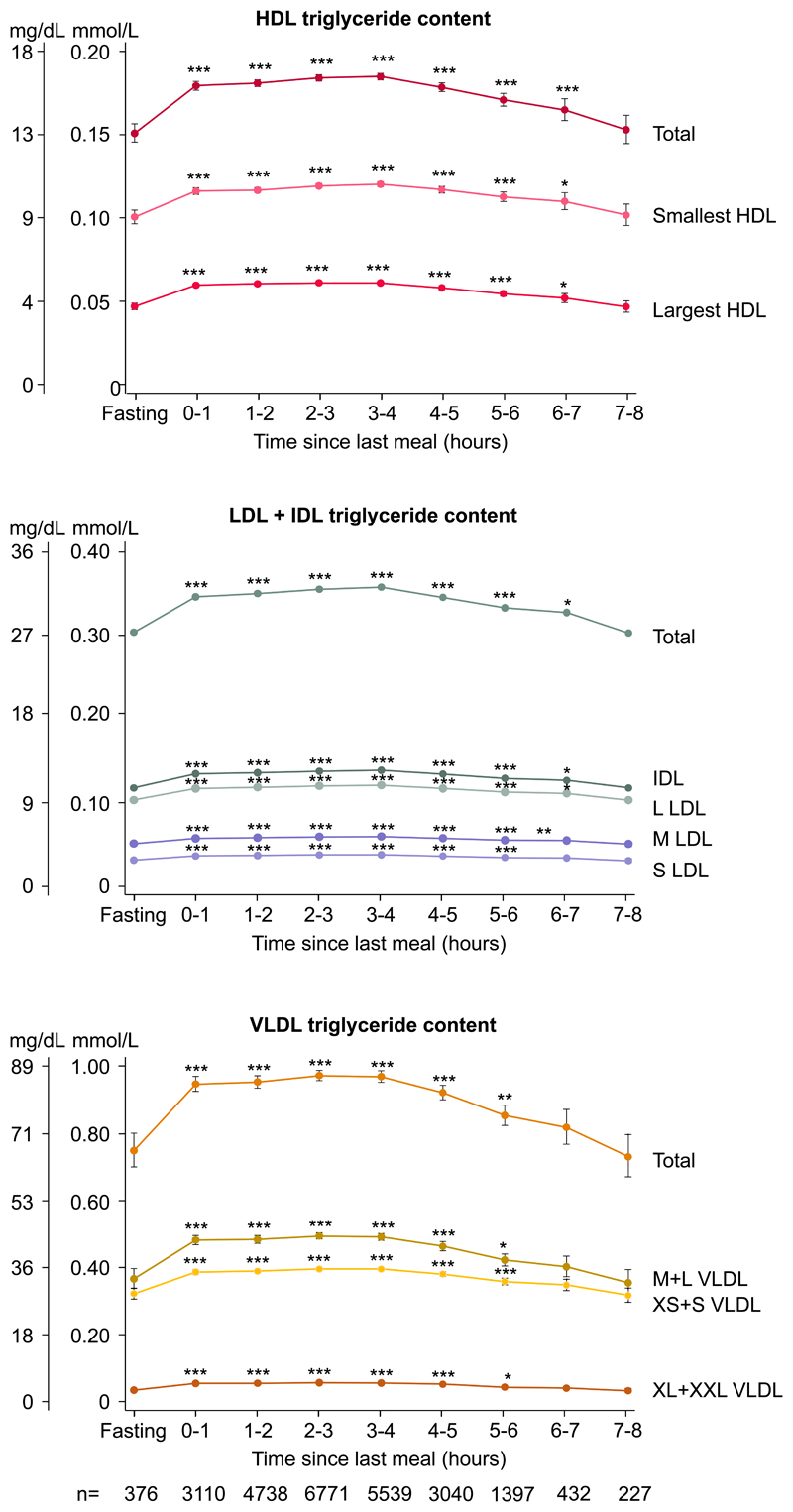
In age and sex-adjusted analyses and when compared with fasting levels, triglyceride content increased for up to 6–7 hours in both the smallest and largest HDL fractions, for up to 5–6 hours in S LDL, for up to 6–7 hours in M LDL, L LDL, and IDL, and for up to 5–6 hours after the last meal in all VLDL fractions (Figure 2).
Figure 2. Concentrations of triglycerides in lipoproteins fractions according to time since last meal.
Dots represent quadratic means adjusted for age and sex and error bars represent 95% confidence intervals. Triglyceride content in lipoprotein fractions were measured using nuclear magnetic resonance spectroscopy and were corrected for recovery. Bonferroni corrected p-values based on 8 parallel tests using unpaired Student t-test versus fasting levels (≥8 hours) are as follows: *p<0.05; **p<0.01; ***p<0.001. Smallest HDL include S+M HDL. Largest HDL include L+XL HDL. Abbreviations: HDL: high-density lipoproteins. IDL: intermediate-density lipoproteins. L: large. LDL: low-density lipoproteins. M: medium. S: small. VLDL: very low-density lipoproteins. XL: extra large. XS: extra small. XXL: extra extra large.
Cholesterol in main lipoprotein fractions according to plasma triglycerides
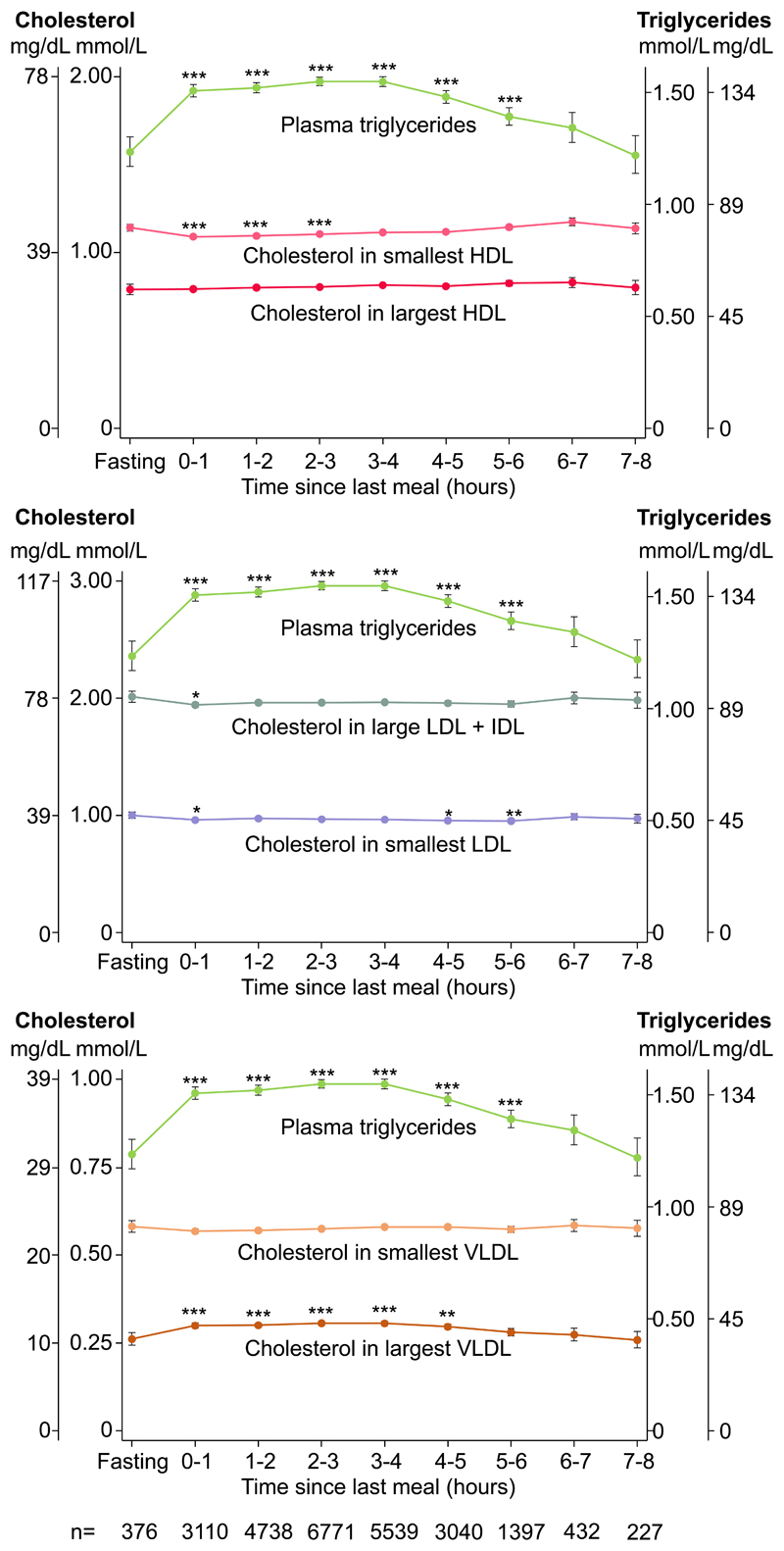
In age, sex, and plasma albumin-adjusted analyses, the observed increase in plasma triglycerides for up to 5–6 hours following the last meal was accompanied by decreased cholesterol content in the smallest HDL fractions for up to 2–3 hours and in LDL+IDL fractions for 0–1 hour, and increased cholesterol content in the four largest fractions of VLDL for up to 4–5 hours after last meal (Figure 3). In contrast, cholesterol content in the two largest subgroups of HDL and in the two smallest subgroups of VLDL remained unaltered.
Figure 3. Cholesterol in main lipoprotein fractions along with plasma triglycerides according to time since last meal.
Dots represent quadratic means adjusted for age and sex and error bars represent 95% confidence intervals. Plasma triglycerides were measured on fresh blood samples using routine hospital assays. Cholesterol content in lipoprotein fractions was measured using nuclear magnetic resonance spectroscopy and was corrected for recovery. Left y-axis shows lipoprotein cholesterol concentration; right y-axis shows plasma triglyceride concentration. Bonferroni corrected p-values based on 8 parallel tests using unpaired Student t-test versus fasting levels (≥8 hours) are as follows: *p<0.05; **p<0.01; ***p<0.001. Smallest HDL include S+M HDL. Largest HDL include L+XL HDL. Smallest LDL include S+ M LDL. Smallest VLDL include XS+ S VLDL. Largest VLDL include M+ L+XL+ XXL VLDL. Abbreviations: HDL: high-density lipoproteins. IDL: intermediate-density lipoproteins. L: large. LDL: low-density lipoproteins. M: medium. S: small. VLDL: very low-density lipoproteins. XL: extra large. XS: extra small. XXL: extra extra large.
Stratification by body mass index
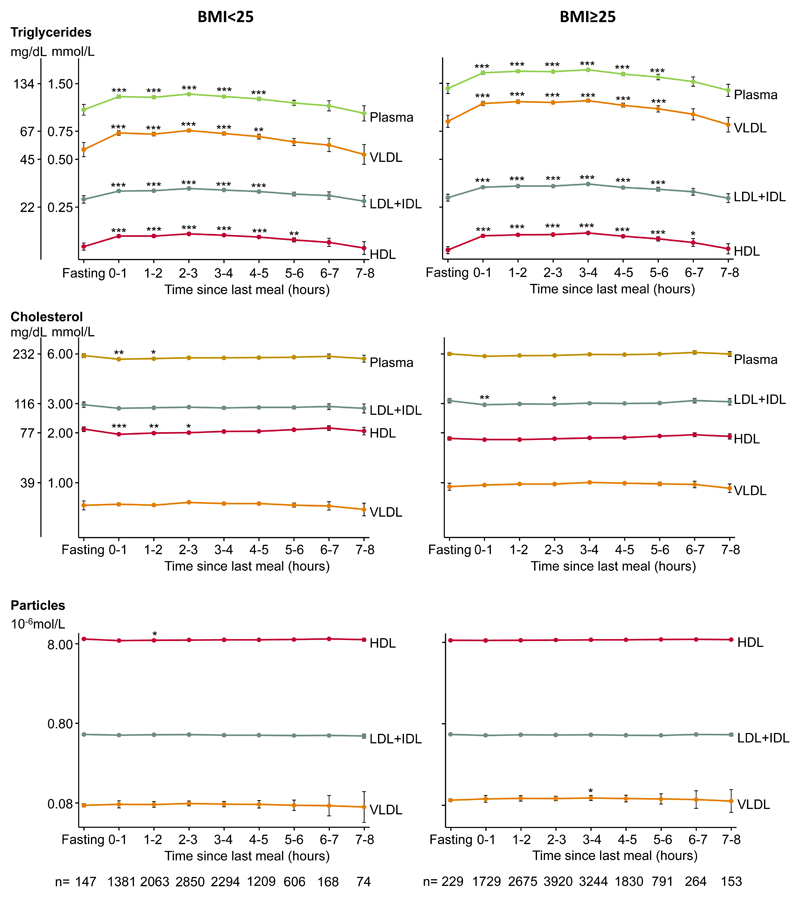
In analyses stratified according to BMI, individuals were divided into two groups based on the WHO classification of overweight (BMI≥25kg/m2); one group with BMI <25 kg/m2 and another group with BMI≥25kg/m2 (Figure 4). Lipid responses following meals were similar in the two BMI groups; however, in general, individuals with BMI≥25kg/m2 had higher levels of plasma triglycerides, VLDL triglycerides, VLDL cholesterol, and VLDL particle number, and lower level of HDL cholesterol.
Figure 4. Triglycerides, cholesterol, and lipoprotein particle number in plasma and in main lipoproteins fractions according to time since the last meal in individuals with normal weight and overweight/obesity separately.
Dots represent quadratic means adjusted for age and sex and error bars represent 95% confidence intervals. Plasma triglycerides and plasma cholesterol were measured on fresh blood samples using routine hospital assays. Triglyceride and cholesterol content in lipoprotein fractions were measured using nuclear magnetic resonance spectroscopy and corrected for recovery. The y-axes are on a logarithmic scale. Bonferroni corrected p-values based on 8 parallel tests using unpaired Student t-test versus fasting levels (≥8 hours) are as follows: *p<0.05; **p<0.01; ***p<0.001. Abbreviations:HDL: high-density lipoproteins. IDL: intermediate-density lipoproteins. LDL: low-density lipoproteins. VLDL: very low-density lipoproteins.
Discussion
In this cross-sectional study of 25,656 individuals from the Copenhagen General Population Study selected for metabolomic profiling, we found that triglyceride content increased while cholesterol content decreased in HDL and LDL+IDL fractions following normal meals in individuals in the general population. These findings are novel.
Mechanistically, these finding can be explained in a simply and straight forward manner. Our results are likely related to the close interaction between HDL, LDL, and triglyceride-rich remnant lipoproteins in their metabolic pathways, which is in part regulated by the action of the cholesteryl ester transfer protein (CETP)25,26. This enzyme facilitates the bidirectional transfer of cholesteryl esters for triglycerides from HDL and cholesterol-rich LDL to triglyceride-rich remnant lipoproteins and triglyceride-rich LDL26–29. Accordingly, even though triglycerides are mainly carried by triglyceride-rich remnant lipoproteins, postprandially, HDL and LDL appear to be enriched with triglycerides likely due to the action of CETP.
Food intake is important when evaluating high plasma triglycerides, and previous epidemiological studies have reported that post-prandial hypertriglyceridemia is an independent risk factor for cardiovascular events5–7. Nevertheless, to our knowledge, this is the first population-based study to investigate the triglyceride and cholesterol content of lipoprotein fractions following normal meals in individuals in the general population. In support of our findings, studies on postprandial lipid response to oral fat tolerance tests in short-term studies of selected groups of individuals or patients have likewise found that triglyceride content increased while cholesterol content decreased in HDL and LDL+IDL fractions14–16. In contrast, one study13 reported decreased LDL triglycerides and another study30 reported decreased triglyceride content and increased cholesterol content in HDL and LDL fractions following oral fat tolerance tests; however, only 22 and 8 healthy individuals were included in the two studies, and therefore, their findings may be different from the overall pattern in the general population. Also, in a long-term population-based study, low high-density lipoprotein (HDL) cholesterol was suggested as a stable marker of elevated plasma triglycerides and triglyceride-rich remnant lipoproteins, like high HbA1c is a stable marker of elevated plasma glucose18; this is in line with the only minimal postprandially changes in HDL cholesterol observed in the present study.
Strengths of the present study include the large sample size representing the Danish adult general population with direct assessment of lipids including triglyceride and cholesterol content of 14 lipoprotein fractions available. Another strength is the fact that time since last meal was recorded by the examiner immediately before blood sampling, simply by a direct question to the participant at this time.
Possible limitations include that total plasma triglycerides and plasma cholesterol measured by NMR spectroscopy were lower than reported by standard hospital assays. To account for this, cholesterol and triglyceride content in the various lipoprotein fractions were corrected for recovery relative to total plasma cholesterol and plasma triglycerides measured by standardized biochemical assays as done in previous studies22–24. This means that the values presented in the present study are directly relatable to values from normal lipid profiles, which could be perceived as a strength. Another potential limitation is that samples were stored at -80 °C before NMR measurements, which could have influenced the composition of lipoprotein fractions; however, we are not aware of any data suggesting this is an issue. Finally, all participants were white people of Danish descent, and accordingly, our findings may not generalize to other ethnic groups, although we are not aware of any data to suggest that these results may not apply to other ethnicities.
Clinically, our findings suggest that triglyceride content increases while cholesterol content decreases in HDL and IDL+LDL fractions following normal meals, and thus indirectly endorse use of non-fasting lipid measurements for risk prediction in the general population as non-fasting measurements better capture the average lipid levels in a person, since the non-fasting state dominates during most of a 24-hour cycle1. Further, non-fasting lipid profile sampling are practically easier for patients, laboratories, and doctors alike1,4. Interestingly, elevated LDL triglycerides are robustly associated with increased risk of atherosclerotic cardiovascular disease31, implying that masking of elevated LDL triglycerides by using fasting lipid profiles makes it more difficult for the physician to evaluate the accurate risk profile in a patient.
In conclusion, in this study of 25,656 individuals from the general population, we observed that triglyceride content increased while cholesterol content decreased in HDL and LDL+IDL fractions following normal meals.
Supplementary Material
Acknowledgments
The authors thank staff and participants of the Copenhagen General Population Study for their important contributions. Parts of the Graphical Abstract were created using Biorender.com.
Financial support
The study was funded by the Independent Research Fund, Denmark [grantno:9039-00360B to BGN], and by Johan Boserup and Lise Boserups Grant [grant no: 20795-24 to MØJ]. George Davey Smith and NMR processing costs were supported by the Medical Research Council Integrative Epidemiology Unit at the University of Bristol MC_UU_00011/1. Funders had no role in study design, data collection, data analysis, data interpretation, writing of the report, or in the decision to submit the paper for publication.
Footnotes
Author contributions
All authors participated in the study design, generation of the hypothesis, and interpretation of the data. MØJ, GDS, and BGN acquired funding. MØJ and JMV conducted statistical analyses. MØJ and JMV wrote the original draft of the report. MB, GSD, and BGN critically reviewed and edited the original draft of the report. All authors had access to all data and BGN had final responsibility for the decision to submit for publication.
Conflict of interest
BGN report consultancies and talks sponsored by AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka Seiken, Amarin, Novartis, Novo Nordisk, Esperion, Abbott, Ultragenix, and Silence Therapeutics. There are no financial or other conflicts of interest for MØJ, JMV, MB, or GDS.
References
- 1.Nordestgaard BG. A Test in Context: Lipid Profile, Fasting Versus Nonfasting. J Am Coll Cardiol. 2017;70(13):1637–1646. doi: 10.1016/j.jacc.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Langsted A, Freiberg JJ, Nordestgaard BG. Fasting and nonfasting lipid levels: influence of normal food intake on lipids, lipoproteins, apolipoproteins, and cardiovascular risk prediction. Circulation. 2008;118(20):2047–56. doi: 10.1161/CIRCULATIONAHA.108.804146. [DOI] [PubMed] [Google Scholar]
- 3.Sidhu D, Naugler C. Fasting time and lipid levels in a community-based population: a cross-sectional study. Arch Intern Med. 2012;172(22):1707–10. doi: 10.1001/archinternmed.2012.3708. [DOI] [PubMed] [Google Scholar]
- 4.Nordestgaard BG, Langsted A, Mora S, et al. Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cut-points-a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur Heart J. 2016;37(25):1944–58. doi: 10.1093/eurheartj/ehw152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298(3):309–16. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 6.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298(3):299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 7.Mora S, Rifai N, Buring JE, Ridker PM. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 2008;118(10):993–1001. doi: 10.1161/CIRCULATIONAHA.108.777334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 9.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–e1143. doi: 10.1161/CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearson GJ, Thanassoulis G, Anderson TJ, et al. 2021 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in Adults. Can J Cardiol. 2021;37(8):1129–1150. doi: 10.1016/j.cjca.2021.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Mihas C, Kolovou GD, Mikhailidis DP, et al. Diagnostic value of postprandial triglyceride testing in healthy subjects: a meta-analysis. Curr Vasc Pharmacol. 2011;9(3):271–80. doi: 10.2174/157016111795495530. [DOI] [PubMed] [Google Scholar]
- 12.Schaefer EJ, Audelin MC, McNamara JR, et al. Comparison of fasting and postprandial plasma lipoproteins in subjects with and without coronary heart disease. Am J Cardiol. 2001;88(10):1129–33. doi: 10.1016/s0002-9149(01)02047-1. [DOI] [PubMed] [Google Scholar]
- 13.Cohn JS, McNamara JR, Schaefer EJ. Lipoprotein cholesterol concentrations in the plasma of human subjects as measured in the fed and fasted states. Clin Chem. 1988;34(12):2456–9. [PubMed] [Google Scholar]
- 14.Blackburn P, Cote M, Lamarche B, et al. Impact of postprandial variation in triglyceridemia on low-density lipoprotein particle size. Metabolism. 2003;52(11):1379–86. doi: 10.1016/s0026-0495(03)00315-9. [DOI] [PubMed] [Google Scholar]
- 15.Hughes TA, Elam MB, Applegate WB, et al. Postprandial lipoprotein responses in hypertriglyceridemic subjects with and without cardiovascular disease. Metabolism. 1995;44(8):1082–98. doi: 10.1016/0026-0495(95)90108-6. [DOI] [PubMed] [Google Scholar]
- 16.Calabresi L, Cassinotti M, Gianfranceschi G, et al. Increased postprandial lipemia in Apo A-IMilano carriers. Arterioscler Thromb. 1993;13(4):521–8. doi: 10.1161/01.atv.13.4.521. [DOI] [PubMed] [Google Scholar]
- 17.Wojczynski MK, Glasser SP, Oberman A, et al. High-fat meal effect on LDL, HDL, and VLDL particle size and number in the Genetics of Lipid-Lowering Drugs and Diet Network (GOLDN): an interventional study. Lipids Health Dis. 2011;10:181. doi: 10.1186/1476-511X-10-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langsted A, Jensen AMR, Varbo A, Nordestgaard BG. Low High-Density Lipoprotein Cholesterol to Monitor Long-Term Average Increased Triglycerides. J Clin Endocrinol Metab. 2020;105(4) doi: 10.1210/clinem/dgz265. [DOI] [PubMed] [Google Scholar]
- 19.Wurtz P, Kangas AJ, Soininen P, Lawlor DA, Davey Smith G, Ala-Korpela M. Quantitative Serum Nuclear Magnetic Resonance Metabolomics in Large-Scale Epidemiology: A Primer on -Omic Technologies. Am J Epidemiol. 2017;186(9):1084–1096. doi: 10.1093/aje/kwx016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soininen P, Kangas AJ, Wurtz P, et al. High-throughput serum NMR metabonomics for cost-effective holistic studies on systemic metabolism. Analyst. 2009;134(9):1781–5. doi: 10.1039/b910205a. [DOI] [PubMed] [Google Scholar]
- 21.Nordestgaard BG, Zilversmit DB. Hyperglycemia in normotriglyceridemic, hypercholesterolemic insulin-treated diabetic rabbits does not accelerate atherogenesis. Atherosclerosis. 1988;72(1):37–47. doi: 10.1016/0021-9150(88)90060-3. [DOI] [PubMed] [Google Scholar]
- 22.Balling M, Langsted A, Afzal S, Varbo A, Davey Smith G, Nordestgaard BG. A third of nonfasting plasma cholesterol is in remnant lipoproteins: Lipoprotein subclass profiling in 9293 individuals. Atherosclerosis. 2019;286:97–104. doi: 10.1016/j.atherosclerosis.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Johansen MO, Vedel-Krogh S, Nielsen SF, Afzal S, Davey Smith G, Nordestgaard BG. Per-Particle Triglyceride-Rich Lipoproteins Imply Higher Myocardial Infarction Risk Than Low-Density Lipoproteins: Copenhagen General Population Study. Arterioscler Thromb Vasc Biol. 2021;41(6):2063–2075. doi: 10.1161/ATVBAHA.120.315639. [DOI] [PubMed] [Google Scholar]
- 24.Balling M, Afzal S, Varbo A, Langsted A, Davey Smith G, Nordestgaard BG. VLDL Cholesterol Accounts for One-Half of the Risk of Myocardial Infarction Associated With apoB-Containing Lipoproteins. J Am Coll Cardiol. 2020;76(23):2725–2735. doi: 10.1016/j.jacc.2020.09.610. [DOI] [PubMed] [Google Scholar]
- 25.von Eckardstein A, Nordestgaard BG, Remaley AT, Catapano AL. High-density lipoprotein revisited: biological functions and clinical relevance. Eur Heart J. 2022 doi: 10.1093/eurheartj/ehac605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shrestha S, Wu BJ, Guiney L, Barter PJ, Rye KA. Cholesteryl ester transfer protein and its inhibitors. J Lipid Res. 2018;59(5):772–783. doi: 10.1194/jlr.R082735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duran EK, Aday AW, Cook NR, Buring JE, Ridker PM, Pradhan AD. Triglyceride-Rich Lipoprotein Cholesterol, Small Dense LDL Cholesterol, and Incident Cardiovascular Disease. J Am Coll Cardiol. 2020;75(17):2122–2135. doi: 10.1016/j.jacc.2020.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller M, Stone NJ, Ballantyne C, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292–333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 29.Patsch JR, Prasad S, Gotto AM, Jr, Bengtsson-Olivecrona G. Postprandial lipemia. A key for the conversion of high density lipoprotein2 into high density lipoprotein3 by hepatic lipase. J Clin Invest. 1984;74(6):2017–23. doi: 10.1172/JCI111624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubois C, Armand M, Azais-Braesco V, et al. Effects of moderate amounts of emulsified dietary fat on postprandial lipemia and lipoproteins in normolipidemic adults. Am J Clin Nutr. 1994;60(3):374–82. doi: 10.1093/ajcn/60.3.374. [DOI] [PubMed] [Google Scholar]
- 31.Balling M, Afzal S, Davey Smith G, et al. Elevated LDL Triglycerides and Atherosclerotic Risk. J Am Coll Cardiol. 2023;81(2):136–152. doi: 10.1016/j.jacc.2022.10.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.