Abstract
Over 40 % of eukaryotic proteomes and 15 % of bacterial proteomes are predicted to be intrinsically disordered based on their amino acid sequence. Intrinsically disordered proteins (IDPs) exist as heterogeneous ensembles of interconverting conformations and pose a challenge to the structure-function paradigm by apparently functioning without possessing stable structural elements. IDPs play a prominent role in biological processes involving extensive intermolecular interaction networks and their inherently dynamic nature facilitates their promiscuous interaction with multiple structurally diverse partner molecules. NMR spectroscopy has made pivotal contributions to our understanding of IDPs because of its unique ability to characterize heterogeneity at atomic resolution. NMR methods such as Chemical Exchange Saturation Transfer (CEST) and relaxation dispersion have enabled the detection of ‘invisible’ excited states in biomolecules which are transiently and sparsely populated, yet central for function. Here, we develop a 1Hα CEST pulse sequence which overcomes the resonance overlap problem in the 1Hα-13Cα plane of IDPs by taking advantage of the superior resolution in the 1H-15N correlation spectrum. In this sequence, magnetization is transferred after 1H CEST using a triple resonance coherence transfer pathway from 1Hα (i) to 1HN(i+1) during which the 15N(t1) and 1HN(t2) are frequency labelled. This approach is integrated with spin state-selective CEST for eliminating spurious dips in CEST profiles resulting from dipolar cross-relaxation. We apply this sequence to determine the excited state 1Hα chemical shifts of the intrinsically disordered DNA binding domain (CytRN) of the bacterial cytidine repressor (CytR), which transiently acquires a functional globally folded conformation because of thermal fluctuations. The structure of the excited state, calculated using 1Hα chemical shifts in conjunction with other excited state NMR restraints, is a three-helix bundle incorporating a helix-turn-helix motif that is vital for binding DNA.
Keywords: Intrinsically disordered proteins, nuclear magnetic resonance spectroscopy, chemical exchange saturation transfer, conformational change, alpha proton, protein dynamics
1. Introduction
Intrinsically disordered proteins and protein regions (IDPs) are common in all living organisms and constitute over 40 % of the proteomes of eukaryotes (1). Despite lacking a stable three-dimensional structure, IDPs perform their function in a variety of different ways. Some IDPs fold in the presence of a binding partner (2–5) or upon post-translational modifications such as phosphorylation (6). Other IDPs participate in pathways where a unique structure is not required for function (5) and IDPs can remain disordered in their functional ligand-bound state (7). The amino acid sequences of IDPs are much simpler than those of their folded counterparts, being depleted in large hydrophobic aliphatic and aromatic amino acids like Leu, Ile, Val and Trp that promote order formation. Instead, IDP sequences are enriched in small polar and charged amino acids that include Ser, Gly, Arg and Glu which increase the tendency for disorder in polypeptide chains (8, 9). As a consequence, IDPs exist as structurally heterogeneous ensembles of interconverting conformations. The structural malleability of IDPs and their ability to switch rapidly between conformations enables them to interact with multiple structurally unrelated binding partners (10, 11) and IDPs frequently serve as scaffolds in biological processes such as signal transduction cascades (12, 13) and transcriptional regulation (14, 15). The conformational dynamics of IDPs is central for their function and characterizing the structures populated in the IDP ensemble is a vital step in understanding how IDPs function.
Nuclear magnetic resonance (NMR) spectroscopy is a powerful tool for probing the dynamics of biomolecules such as proteins, nucleic acids and glycans (16–18). The site-specificity afforded by NMR helps in constructing a coherent picture of conformational dynamics across the entire molecule of interest. A number of experiments such as Chemical Exchange and Dark-state Exchange Saturation Transfer (CEST (19–21) and DEST(22)), Carr-Purcell-Meiboom-Gill (CPMG) (23–25), R1ρ Relaxation Dispersion (RD) (26, 27) and Paramagnetic Relaxation Enhancement (PRE) (28, 29) have recently been developed that can detect and structurally characterize transiently (τE > 25 μs) and sparsely (pE > 0.5 %) populated biomolecular conformations (referred to as excited states (E) here). The application of these methods has revealed the importance of rare conformational transitions in protein folding (30–32), Hsp70 chaperone binding (33–35), light sensing by the LOV domain (36), enzyme catalysis by tyrosine phosphatases (37) and dihydrofolate reductase (38, 39), post-translational maturation of superoxide dismutase (40), DNA replication fidelity (41, 42) and transcription termination by the fluoride riboswitch (43), as well as in aberrant protein oligomerization (44, 45) and aggregation (46). However, these methods have found limited applicability in the domain of IDPs (47–50), primarily because IDPs are believed to have flat free energy landscapes devoid of kinetically separated excited state conformations (3, 49, 51).
We recently showed that the intrinsically disordered N-terminal DNA binding domain of the cytidine repressor (CytR), CytRN (CytR(1–66)), is functionally regulated by a conformationally excited state (52). CytR belongs to the LacR family of bacterial transcriptional repressors and regulates the transcription of genes involved in ribonucleotide uptake and degradation (53–56). CytRN has a high charge-to-hydrophobicity ratio as well as a large number of Ala and Lys residues, which cause it to be intrinsically disordered both in the free form and in full-length CytR (52). In the DNA-bound state, CytRN folds into a stable three-helix bundle which interacts with DNA through a helix-turn-helix (HTH) motif formed from the first two helices (H1 and H2) (57). While the conformation of CytRN in the DNA-bound state is very similar to that of its family members such as FruRN and LacRN (58, 59), the latter molecules are folded even in the absence of DNA (60–62). We used multinuclear 15N, 1HN, 13C’ and 13Cα CEST experiments to show that the disordered ensemble of CytRN exists in equilibrium with a globally folded conformation that shares the same three-helix bundle topology found in the CytRN-DNA complex (52). We obtained alpha carbon and carbonyl carbon chemical shifts of the excited state using previously reported triple resonance-based CEST experiments that were critical in defining the backbone fold of the CytRN excited state (63, 64). However, 1Hα excited state chemical shifts could not be obtained because the existing aliphatic 1H-CEST pulse sequence relies on quantifying residue-specific peak intensities in the 2D 1H-13C correlation spectrum (65), where there is severe overlap of resonances because of the disordered nature of native CytRN (Figure 1A). The CPMG relaxation dispersion-based method for measuring 1Hα excited state chemical shifts also suffers from the same overlap problem (66). This is a significant impediment in characterizing millisecond timescale IDP dynamics because 1Hα chemical shifts are sensitive reporters of protein secondary structure (67, 68).
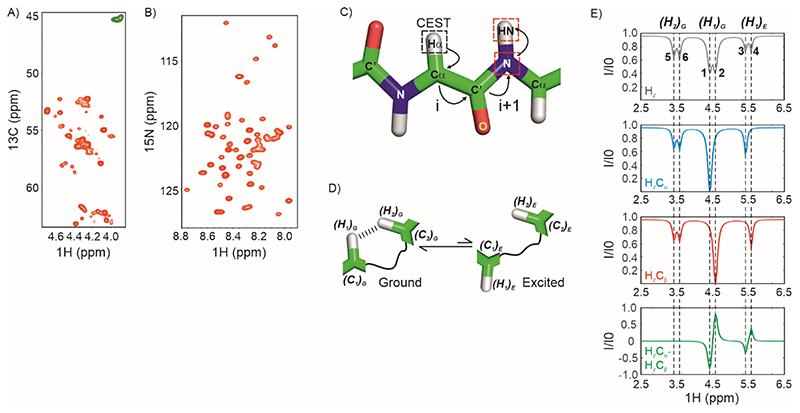
Figure 1. Strategy for acquiring 1Hα CEST data on IDPs.
A) The 1Hα region of a constant-time 1H-13C HSQC spectrum of U-13C,15N labelled CytRN. Green resonances arise from Gly which do not have a scalar coupled sidechain 13Cβ. B) 1H-15N HSQC spectrum of CytRN. C) The coherence transfer pathway in the 1Hα CEST experiment reported here. CEST is carried out on 1Hα (black dashed square) and the magnetization is then transferred from 1Hα(i) through 13Cα(i) and 13CO(i) to 15N (i+1) and then to 1HN(i+1) for detection. Red dashed squares indicate nuclei that are frequency labelled in the experiment. D) Cartoon representation of conformational exchange between ground and excited states of a protein where the alpha proton H1 comes close (< 5 Å) to H2 in the ground state. E) The spin-state-selective approach for acquiring proton CEST without interference from dipolar cross-relaxation. (Top) 1H CEST profile of an alpha proton resonating at 4.5 ppm (major dip), exchanging with a second conformation where the chemical shift of the 1Hα is 5.5 ppm (minor dip). The 1Hα is proximal to a second 1Hα which results in an NOE dip at 3.5 ppm. There is no 13C-decoupling during the CEST period; therefore doublets separated by 1JHαCα are seen for each dip. (Middle) Spin-state-selective CEST data selecting for HzCα (blue) and HzCβ (red). (Bottom) CEST profile of HzCα-HzCβ =2HzCz, where the NOE dip cancels out (green).
Herein, we report a 1Hα CEST pulse sequence (HACACONNH-HA-CEST) that combines spin-state-selective 1Hα CEST acquisition with triple resonance coherence transfer that can be used for determining excited state 1Hα chemical shifts in folded as well as disordered proteins. We validate this experiment on the L24A variant of the FF domain, in which exchange between the folded state and an on-pathway folding intermediate has been extensively characterized in literature (31). We then demonstrate the applicability of this sequence for IDPs by using it to measure 1Hα chemical shifts of 37 residues in natively disordered CytRN. The measurements reinforce the conclusion that disordered CytRN undergoes a disorder-to-order transition to an excited state structure composed of three helices and containing a helix-turn-helix motif.
2. Results and discussion
2.1. Designing the triple resonance 1Hα CEST pulse sequence
The Chemical Exchange Saturation Transfer (CEST) experiment consists of the application of a weak radiofrequency (RF) field of amplitude B1 at a specific chemical shift offset of the nucleus of interest (1Hα in our case) for an exchange duration Tex (69). Longitudinal magnetization (19) or two-spin-order (34) is present at the beginning of the exchange period and the density operator of interest at the end of Tex is quantified through peak intensities in suitable 2D correlation spectra. The RF field is swept across the offset range for the nucleus under study and an array of 2D spectra, one for each offset value, is generated in the CEST experiment. The per-residue CEST profile is a plot of the normalized intensity (I/I0) of the corresponding peak in the 2D spectrum as a function of the offset at which RF irradiation occurs. For a protein in exchange between a major ground state and a minor excited state, the presence of exchange in CEST profiles is visualized as a second minor dip in intensity besides the major saturation dip that occurs at the chemical shift of the nucleus in the excited state.
The chemical exchange saturation transfer (CEST) pulse sequence that is currently used for obtaining excited state aliphatic proton chemical shifts in proteins relies on quantifying peak intensities in a 1H-13C correlation spectrum (65). Figure 1A shows the 1Hα-13Cα region of the HSQC spectrum of the intrinsically disordered DNA binding domain of the Escherichia coli cytidine repressor (CytRN). There is a lot of resonance overlap in this spectrum, which makes it impossible to reliably extract intensities for many of the 1Hα-13Cα correlations. In addition, it can be challenging to obtain the intensities of 1Hα-13Cα correlations because the 1Hα resonances in IDPs cluster near the water resonance. In contrast, resonances are much better resolved in the 1H-15N HSQC spectrum of CytRN (Figure 1B), making this 2D plane an attractive candidate for detecting intensity modulations from CEST.
In order to take advantage of the superior resolution in the 1H-15N 2D plane, our approach for determining excited state 1Hα chemical shifts involves carrying out CEST on 1Hα at the beginning of the pulse sequence and then transferring the magnetization from 1Hα of the ith residue to the 1HN of the i+1 residue through 13Cα(i), 13CO(i) and 15N(i+1) using one-bond scalar couplings (Figure 1C). The 1H CEST profile recorded by directly ‘saturating’ the 1H resonances as described earlier will often contain additional dips due to 1H-1H dipolar interactions (NOEs) with other proximal 1H spins (70, 71). As these additional dips can mask the presence of a minor state dip and can also be accidentally assigned to a minor state resonance it is imperative to record 1H CEST profiles that do not contain these spurious dips. Two approaches that exploit the presence of the attached X-nuclei (13C in this case) have been devised to eliminate the NOE induced spurious dips. The first involves carrying X-spin CEST experiments with weak 1H CW decoupling to indirectly detect the minor state 1H resonance positions (70), while the second involves carrying out (X-) spin-state-selective 1H CEST experiments (71). Here we have adopted the spin-state-selective approach to eliminate spurious NOE dips from 1Hα CEST profiles.
Consider a 1Hα nucleus (H1) exchanging between two environments corresponding to the ground (G) and excited states of a protein (E) (Figure 1D). In addition, H1 is dipolar coupled to a proton H2 which is in close spatial proximity (< ~5 Å) to H1 in the ground state. H1 and H2 are in-turn scalar coupled to their respective covalently bonded carbons C1 and C2. The top panel of Figure 1E (grey) shows the conventional 1Hα CEST profile for this system quantifying the intensity of H1Z in the ground state at the end of Tex. The major dip from the ground state and the minor dip from the excited state are centred at the chemical shifts of (H1)G (4.5 ppm) and (H1)E (5.6 ppm) respectively. Each dip is split by the one-bond scalar coupling of H1 to C1 (1JC1H1) because there is no 13C decoupling during Tex; i.e., the major dip splits into dips 1 and 2, while the minor dip splits into dips 3 and 4. The dip arising due to H2 centred at 3.5 ppm is also split into dips 5 and 6 due to the scalar coupling between H2 and C2. Separating the CEST profiles based on the spin-state (α or β) of (C1)G (blue (α) and red (β) in Figure 1E) results in profiles that contain a single dip arising due to H1 rather than a doublet (dips 2 and 4 for the α spin-state and dips 1 and 3 for the β spin-state). Dips arising due to H2 remain doublets as they arise due to the 1JC2H2 coupling (i.e. the spin-state of C2) rather than the 1JC1H1 coupling. As the dips arising due to H1 are displaced by 1JC1H1 between the two spin-state-selective CEST profiles, subtracting the two profiles will result in antiphase features at the major and minor state H1 shifts while dips 5 and 6 arising due to H2 are subtracted out (Figure 1E, green profile). Following the approach detailed in Yuwen et al. (65), this subtraction is done ‘in-line’ in the HACACONNH-HA-CEST pulse sequence by selecting the 2HZCZ operator at the end of Tex since 2HZCZ = HzCα – HZCβ. This improves the sensitivity of the overall experiment by a factor of compared to acquiring separate CEST profiles for and and subtracting them post-acquisition.
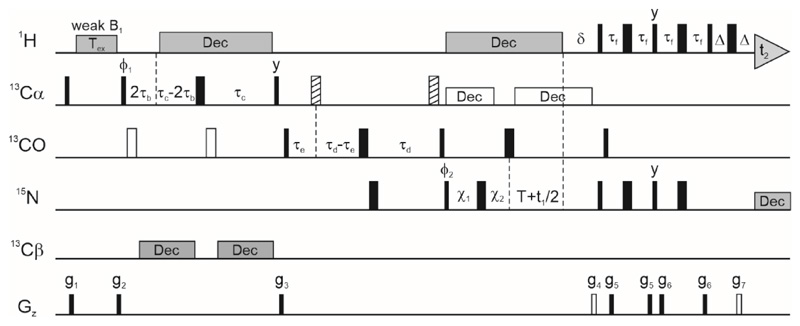
Figure 2 shows the HACACONNH-HA-CEST pulse sequence, in which CEST on 1Hα is carried out at the beginning of the pulse train and 1Hα magnetization is aligned along the z-axis at the beginning of the CEST element. The 2HzCz operator is then selected at the end of Tex and magnetization is transferred to the (i+1)th amide proton using a standard coherence pathway (63, 72–74) taking advantage of scalar couplings 1JCαHα, 1JCαCO, 1JCON and 1JNH in succession. The amide nitrogen and amide proton of the (i+1)th residue are frequency labelled in the indirect and direct dimensions respectively to extract CEST profiles of the ith 1Hα nucleus using the high resolution 1H-15N 2D plane. The weak B1 field (usually 5-50 Hz) is swept through the range of 1Hα chemical shift offsets (typically 2.5 – 6 ppm) and one 2D plane is collected for each offset. The spacing between offsets is approximately the same as the amplitude of the weak RF field (in Hz), though it has been shown recently that significantly larger spacings can be employed without impairing the robustness of the exchange parameters extracted from the CEST data (75). A reference plane is collected for each CEST dataset using a modified version of the pulse sequence lacking the exchange duration (Tex = 0), in which an INEPT pulse train replaces the CEST module for transferring Hz to 2HzCz. Peak intensities in this reference plane (I0) are used to generate the normalized CEST profile.
Figure 2. The pulse sequence for acquiring HACACONNH-based 1Hα CEST data.
The 1H carrier is centred on the water resonance except during the CEST period and the 15N carrier at ~ 118 ppm, while the 13C carrier is placed at 58 ppm till gradient g3 and then jumped to 176 ppm for the rest of the pulse sequence. All pulses are applied along the x-axis unless specified otherwise. Narrow and wide rectangles represent 90° and 180° pulses respectively. Open rectangles correspond to rectangular pulses applied 118 ppm off-resonance while the hashed rectangles are rectangular pulses applied -118 ppm off-resonance. 1H and 15N pulses are applied at the highest power while 13C 90° and 180° pulses are applied at RF field amplitudes of and Hz respectively, where ξ is the frequency difference in Hz between the 13Cα and 13CO spectral bands (90). 1H decoupling is implemented via a ~6 kHz WALTZ-16 (91) composite pulse decoupling pattern. Off-resonance 13Cα decoupling is carried out with a cosine modulated SEDUCE-1 pattern (92, 93) with a pulse width of 315 μs (at 600 MHz B0 field strength), and 15N decoupling during acquisition is carried out with a ~ 1 kHz WALTZ-16 pattern. WURST-2 adiabatic decoupling (94) is used for 13Cβ decoupling in the 16.5-41.5 ppm and 68-72 ppm bands. The phase cycling used in the sequence is: ϕ1 = (-y,y), ϕ2 = (x,x,-x,-x) and receiver = (x,-x,-x,x). The delays used in the experiment are: τb = 1.8 ms, τc = 4.4 ms, τd = 12.4 ms, τe = 5 ms, τf = 2.3 ms, T = 12.4 ms, δ = 5.5 ms and Δ = 0.5 ms. The amplitudes and durations of the various gradients in (G/cm, ms) are: g1: (12000, 0.5), g2: (-8343, 0.5), g3: (10000, 0.8), g4: (30000, 1.25), g5: (4000, 0.3), g6: (2000, 0.4), g7: (30400, 0.125). The two gradients g4 and g7 represented as open rectangles are required for coherence selection. Delays χ1 and χ2 are set to the larger of the two values (T-t1/2, 0) and (0, t1/2-T) respectively, and this allows 15N chemical shift evolution in t1 to occur in a semi-constant manner (72, 95). Quadrature detection in the F1 dimension is implemented using enhanced sensitivity gradient coherence selection (96–98).
Protein CEST profiles on low-γ 13C and 15N nuclei are generally acquired by co-adding scans where the starting magnetization is along the +z and -z directions. This ensures that magnetization decays exponentially to 0 rather than to its equilibrium value of M0, enabling an accurate ‘in situ’ measurement of R1 and eliminating the dependence of the CEST profile on equilibrium magnetization, thereby reducing one fitting variable. It has been previously shown that such an approach is not optimal for 1H CEST and results in smaller dips than if magnetization decays to M0 (76). Therefore, 1Hα magnetization is always aligned along the +z axis at the start of Tex in the current pulse sequence (Figure 2); residue-specific 1Hα longitudinal relaxation rate constants (R1H) are separately estimated by varying Tex using an altered version of the pulse sequence in which an INEPT module is inserted after Tex to transfer magnetization from Hz to 2HzCz, and no B1 field is applied during Tex in this experiment.
2.2. Testing the sequence on a system without measurable millisecond timescale exchange: ubiquitin:
We first tested the HACACONNH-HA-CEST pulse sequence on the folded protein (Figure 3A) ubiquitin that lacks measurable conformational exchange in the millisecond timescale. The signal-to-noise (S/N) of a reference 2D 1H-15N plane from the HACACONNH-HA-CEST experiment for ubiquitin (averaged over 50 isolated peaks) is 1.6 ± 0.5 – fold higher than a constant-time 2D 13C-1H correlation spectrum (averaged over 31 isolated peaks separated by at least 0.4 ppm from water). This is probably because magnetization evolves on the faster relaxing 13Cα nucleus during the 29.4 ms constant time period in the 13C-1H HSQC, compared to evolution for 24.8 ms on the slower relaxing 15N nucleus in the semi-constant period of the HACACONNH-HA-CEST sequence. In addition, the HACACONNH-HA-CEST sequence incorporates a gradient sensitivity enhancement module that improves sensitivity by a factor of The sensitivity of the 1Hα-13Cα 2D correlation map can be further increased by decoupling 13Cβ nuclei with adiabatic decoupling schemes and using a non-constant-time evolution element, as well as by including the sensitivity enhancement scheme for the 1H-13C AX spin pair (77). Nevertheless, it is apparent that the sensitivity disadvantage in using the HACACONNH-HA-CEST experiment is not significant especially for intrinsically disordered proteins that have smaller transverse relaxation rates compared to ordered proteins of the same mass.
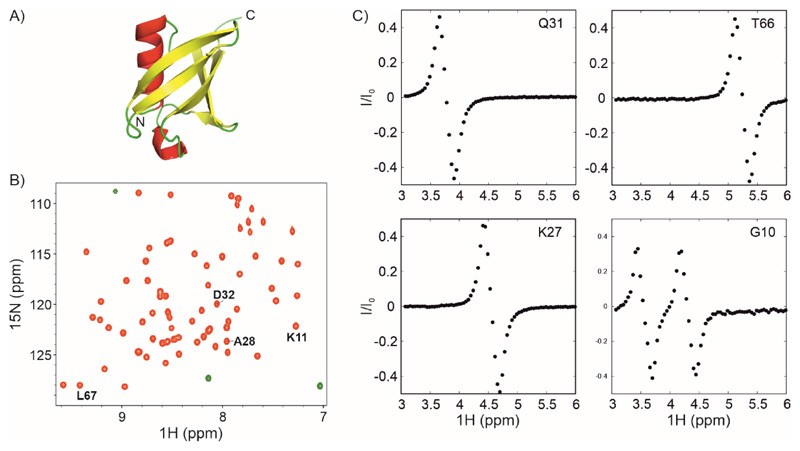
Figure 3. 1Hα CEST on U-13C,15N labelled ubiquitin.
A) Three-dimensional structure of ubiquitin showing helices, sheets and loops in red, yellow and green respectively (PDB ID: 1UBQ (99)). B) 1H-15N HSQC spectrum of ubiquitin. C) 1Hα CEST profiles of selected residues in ubiquitin that were obtained by analyzing the i+1 correlations indicated in the HSQC spectrum in panel B. 1Hα CEST data were acquired using the pulse sequence in Figure 2. The 1Hα CEST profile for Gly10 shows two dips arising from each of the two diastereotopic 1Hα protons of Gly10.
Figure 3B shows the 1H-15N HSQC spectrum of ubiquitin and Figure 3C shows the 1Hα CEST profiles obtained using the HACACONNH-HA-CEST pulse sequence by quantifying the intensity 1H-15N correlations of selected residues as a function of chemical shift offset. The 1Hα CEST profiles of K27, Q31 and T66 appear like the difference in two Lorentzians separated by 1JCαHα, analogous to the spectrum of the antiphase operator 2HzCz. We refer to this as the ‘major dip’ in correspondence with the in-phase major dip in 15N or 13C CEST profiles. While the maximum of the major dip occurs at the chemical shift offset of the TROSY component of the 1Hα-13Cα doublet, the minimum occurs at the chemical shift offset of the anti-TROSY component. The downfield component has negative intensity and the upfield component has positive intensity, unlike in Figure 1E, because the reference and CEST planes are phased opposite to each other in this sequence, with 2HzNz detected in the reference plane and -2HzNz in the CEST planes. Notably, there are no NOEs observable in these three 1Hα CEST profiles, confirming that the effect of dipolar cross-relaxation has been suppressed in this pulse sequence.
The 1Hα CEST profile of G10 (Figure 3C, bottom right) shows the presence of two major dips in intensity at the chemical shifts of the two diastereotopic 1Hα1 and 1Hα2 of G10. Saturation transfer carried out on both 1Hα atoms transfer to the same 1HN of K11 and both major dips are observed in the same CEST profile. Therefore, this pulse sequence cannot be used to obtain excited state 1Hα chemical shifts of Gly residues (78).
2.3. Validating the sequence on the L24A FF domain to characterize N-I exchange
Next, we tested the HACACONNH-HA-CEST pulse sequence on the L24A variant of the FF domain, which is known to exchange between its folded native state (N) and a partially folded on-pathway folding intermediate (I) that has a lifetime of 1.8 ms and a population of 5.4 % (303 K) (Figure 4A, (31, 79)). Since the exchange rate constant between these two states is ~ 580 s-1, N↔I exchange can be conveniently studied with CEST. In addition, there is substantial conformational rearrangement in going from N, which is a four-helix bundle, to I, in which the third helix is longer than in N and the fourth helix is disordered (Figure 4A). A number of residues in H3 and H4 are therefore expected to show large 1Hα chemical shift changes, furnishing a convenient testing system for the HACACONNH-HA-CEST pulse sequence.
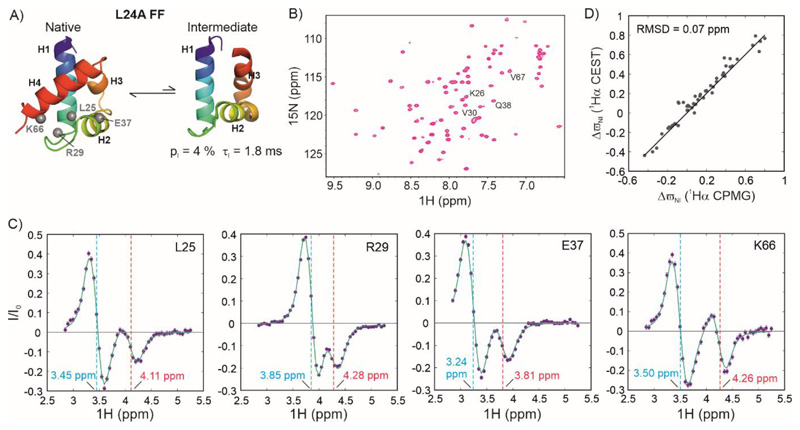
Figure 4. Characterizing exchange in the folded L24A FF domain using 1Hα CEST.
A) Cartoon representation of the native (31) and intermediate states of L24A FF (PDB ID:2L9V (31)) in exchange on the millisecond timescale. B) 1H-15N HSQC spectrum of L24A FF. Resonances whose 1Hα CEST profiles are quantified in panel C are labelled in the spectrum. C) 1Hα CEST profiles of selected resonances of L24A FF. Cyan and red dashed lines indicate the chemical shifts of the native and intermediate states respectively. D) Correlation between the 1Hα chemical shifts of L24A FF intermediate states acquired using 1Hα CPMG (y-axis, (31)) and 1Hα CEST (x-axis). The solid line depicts the y=x function.
Figure 4B shows the 2D 1H-15N correlation spectrum of L24A FF and Figure 4C shows 1Hα CEST profiles of four residues L25, R29, E37 and K66 with large (> 0.4 ppm) chemical shift changes between N and I. In addition to the major dip in each profile, there is a minor dip consisting of two Lorentzians of opposite signs separated by 1JCαHα,E (the J-coupling in the excited state) that arises due to conformational exchange between N and I. Since there is partial overlap between the TROSY and anti-TROSY components of the major and minor dips, all four extrema cannot be visualized separately in these CEST profiles.
In order to extract exchange parameters from the 1Hα CEST profiles, we fit profiles of 22 residues with |ΔϖIN| > 0.35 ppm acquired at 3 B1 field strengths each (15 Hz, 33 Hz and 54 Hz) globally to a two-state model of conformational exchange using the Bloch-McConnell equations (80). The fitting routine results in parameters of kex,IN = 582 ± 6 s-1 and pI = 4.24 ± 0.04 %. While kex,IN matches very well with the previously reported value (580 s-1), pI is significantly lower (5.4 %). This is likely because of the impact of cross-relaxation on the CEST profiles (71). It is therefore preferable to measure kex and pE from 15N CEST experiments and use 1Hα CEST data solely for determining excited state 1Hα chemical shifts.
As the next step, we carried out residue-specific fits of 1Hα CEST profiles of 50 residues to extract chemical shift differences between the native and intermediate states. During this step, kex,IN and pI were fixed to the value obtained from the global analysis of 1Hα CEST data (see Materials and Methods). Figure 4D shows a comparison of these ΔϖIN values with those determined using CPMG relaxation dispersion methods (31). There is very good agreement between the two measurements, with an RMSD over 50 residues of 0.07 ppm, confirming the robustness of the HACACONNH-HA-CEST pulse sequence.
2.4. Measuring 1Hα chemical shifts of the excited state of intrinsically disordered CytRN
Having established the reliability of the HACACONNH-HA-CEST pulse sequence for measuring excited state 1Hα chemical shifts, we applied the methodology to the disorder-to-order transition in CytRN (Figure 5A). The native state of CytRN is intrinsically disordered, as seen from both sequence-based metrics such as the amino acid composition, charge and hydropathy, as well as from circular dichroism and NMR spectroscopy. We recently showed (52, 81) that CytRN transiently populates a higher free energy state in which it adopts a globally folded three-helix bundle structure that resembles the conformation of the DNA-bound protein. We also showed that the folded conformation is responsible for binding DNA and that there is no measurable reactive flux directly from the native disordered state to DNA-bound CytRN (52).
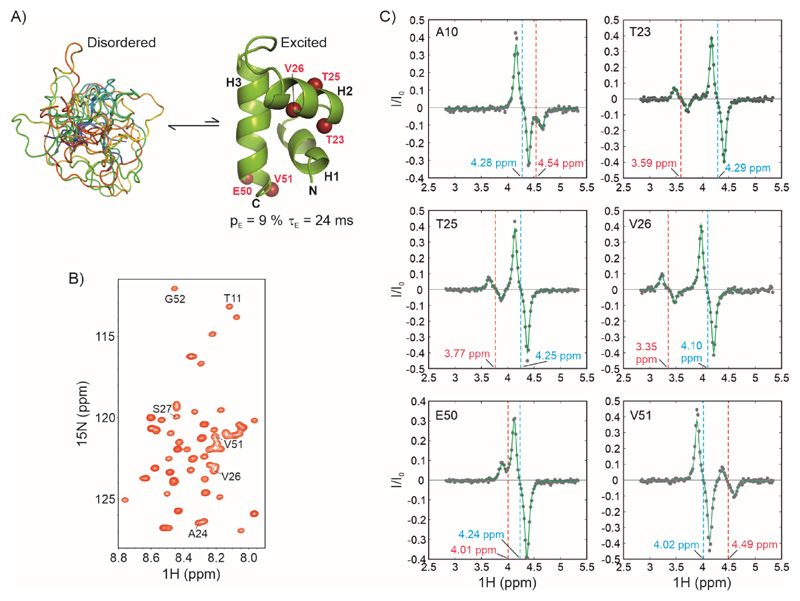
Figure 5. Characterizing exchange in the IDP CytRN using 1Hα CEST.
A) CytRN exchanges between a disordered ensemble and a three-helix bundle folded state (52). B) 1H-15N HSQC spectrum of disordered CytRN showing limited spectral dispersion characteristic of an IDP. Resonances for which 1Hα CEST profiles are quantified in panel C are labelled on the spectrum (except A10, which is not a part of the fragment included in the structure calculation). C) 1Hα CEST profiles of selected residues of CytRN. The chemical shift positions of the ground (disordered) and excited (folded) states are indicated by cyan and red dashed lines respectively.
The structure of the globally folded excited state of CytRN was calculated using backbone 1HN, 15N, 13Cα and 13C’ chemical shifts derived from CEST experiments. 13Cα and 13C’ chemical shifts were determined using previously published triple resonance schemes (63, 64). In order to obtain 1Hα chemical shifts in the folded state of CytRN, we acquired HACACONNH-HA-CEST data at two B1 fields (13 Hz and 36 Hz, see Materials and Methods) on U-13C,15N labelled CytRN at 288 K. The folded conformation of CytRN in exchange with the intrinsically disordered form can be clearly discerned in 1Hα CEST profiles as a minor dip in I/I0, resulting in four extrema (two from the major dip and two from the minor dip) in CEST profiles of T23, T25, V26 and V51 (Figure 5C). 1Hα chemical shifts for 37 non-overlapped residues (Table S1) were extracted using residue-wise fits of 1Hα CEST profiles to the two-state Bloch-McConnell equations. Due to overlap in the 13C-1H plane, minor state 1Hα chemical shifts from only 18 sites are independently quantifiable using the 1H-13C based experiment illustrating the efficacy of the HACACONNH-HA-CEST experiment described here. The 1Hα chemical shift differences between folded and disordered CytRN are substantial and vary between -0.79 and 0.58 ppm. The large changes occurring in these shifts as a result of the helix-coil transition in CytRN underscores the importance of the 1Hα nucleus in determining the structures of locally or globally folded conformations transiently sampled by IDPs.
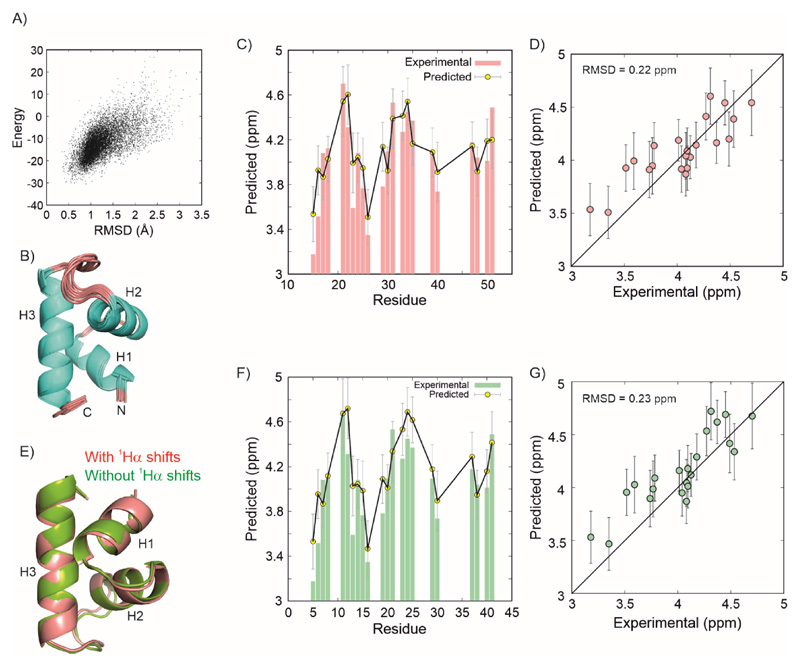
Finally, we assembled 23 1Hα shifts belonging to the structured region of the CytRN excited state (CytRN(11–53)) along with 33 1HN, 34 15N, 25 13Cα and 26 13C’ shifts, as well as 31 1H-15N RDCs from alignment in C8E5/octanol (PEG) and 34 from a 6 % stretched polyacrylamide gel (PAG) to calculate the structure of the excited state using CS-Rosetta (82, 83), as described earlier (52). The structure calculation converges smoothly with a well-defined funnel (Figure 6A) in the energy vs RMSD (to the lowest energy structure) plot and Figure 6B shows the ensemble of 10 lowest energy structures. The structures closely resemble each other with a pairwise all-atom RMSD of 0.97 Å (Cα RMSD = 0.43 Å) and confirm that the excited state adopts a three-helix bundle conformation. Residue-specific 1Hα chemical shifts predicted using Sparta+ (84) for the lowest energy structure match very well with the experimental values (Figure 6C) and the overall RMSD between the predicted and observed chemical shifts is 0.22 ppm (over 22 residues for which predictions are available (Figure 6C, 6D)), well within the prediction accuracy of Sparta+ (0.25 ppm for 1Hα) (84). The structures calculated in the absence and presence of 1Hα chemical shifts (Figure 6E) agree well with each other (RMSD = 0.6 Å over 41 Cα atoms) and have the same helix boundaries for H1 (M12-A19), H2 (T23-M31) and H3 (Q38-V51). The only difference between the two structures is in the small orientations of the H1-H2 and H2-H3 loops. Indeed, the 1Hα chemical shifts predicted by Sparta+ from the CS-Rosetta structure calculated without input 1Hα chemical shifts agree well with the experimental chemical shifts (RMSD = 0.23 ppm over 22 residues, Figure 6F,6G), providing an independent validation of the proposed excited state structure of CytRN. The 1Hα chemical shifts therefore reinforce the conclusion that CytRN undergoes a disorder-to-order switch from its native disordered form to a three-helix bundle conformation compatible with DNA binding. The 1Hα measurements also emphasize the fact that the heterogeneity in the structural ensemble of an IDP can include globally folded conformations with stable secondary and tertiary structure.
Figure 6. Structure of the CytRN excited state calculated chemical shifts and RDCs.
A) Composite chemical shift and Rosetta energy score of structures plotted against the RMSD of each structure to the lowest energy structure. B) Overlay of the cartoon representations of the 10 structures with the lowest energy. Comparison between the experimental (red bars) and predicted 1Hα chemical shifts (yellow circles) for the CytRN excited state shown as a function of residues (C) and as a correlation plot (D). Predictions were done using Sparta+ on the lowest energy structure. Error bars are uncertainties in residue-specific chemical shift predictions from Sparta+. E) Overlay of the CytRN excited state calculated without (green) and with (red) 1Hα chemical shifts. F,G) A comparison of the same experimental data as in panels (C) and (D) with predictions made using Sparta+ (84) on the lowest energy structure calculated without using input 1Hα chemical shifts (52).
3. Conclusions
Intrinsically disordered proteins are structurally malleable polymers in which dynamics is central for function. Here, we report the HACACONNH-HA-CEST NMR pulse sequence tailored towards characterizing millisecond dynamics in IDPs that facilitates the measurement of 1Hα chemical shifts in IDP excited states. In this sequence, we combine spin state-selective 1Hα CEST with triple resonance coherence transfer to the neighbouring 1HN in order to take advantage the superior resolution in the 1H-15N 2D available for IDPs. This sequence adds to the rapidly growing toolbox of NMR methods for elucidating the structure of transiently and sparsely populated protein conformations (excited states) and pinpointing their importance for function and malfunction.
In contrast to the CPMG-based approach for determining excited state 1Hα chemical shifts (66), a specific isotope labelling scheme is not required for measuring HACACONNH-HA-CEST data and the sequence can applied on the same U-13C,15N labelled samples used for acquiring 13Cα, 13Cβ and 13CO CEST. This makes the CEST-based strategy very attractive as it allows us to obtain excited state chemical shifts for all protein backbone nuclei of folded and disordered proteins from a single sample. While CPMG experiments generally require lesser time than CEST, CEST data acquisition can be speeded up using multifrequency irradiation schemes such as DANTE-CEST (85). In addition, the CEST approach automatically provides the sign of the chemical shift difference between the ground and excited states unlike CPMG measurements, where additional experiments must be acquired in order to extract sign information.
4. Materials and methods
4.1. Overexpression and purification of proteins
All proteins were expressed by transforming plasmids containing the gene of interest in BL21(DE3) cells. Ubiquitin was cloned into multiple cloning site of a pET-15b vector while CytRN and L24A FF were cloned into the pET-29b+ vector. Protein expression was carried out in 500 mL 2xM9 minimal media (86) containing the appropriate selection antibiotic. The media was supplemented with 1g/L 15N labelled ammonium chloride and 3g/L U-13C-labelled glucose as the sole nitrogen and carbon sources respectively. Protein expression was induced by adding 1 mM IPTG to the media when the absorbance at 600 nm (OD) reached 0.8-1 for all proteins. Induction was done at 18-20 °C for all proteins typically for 18 hours. Cells were harvested by centrifugation at 7500 rpm for 20 minutes and lysed by sonication in lysis buffer. The purification protocol followed for each protein is described briefly below.
4.1.1. Ubiquitin
Cells were lysed in 50 mM Tris HCl buffer pH 7.6 containing 1 mM EDTA and 0.05% Triton-X100 by sonication followed by incubation at room temperature with 1 mM phenyl methyl sulfonyl fluoride (PMSF) and 7 mg lysozyme per gram of the cell pellet. The lysate was clarified by centrifugation at 15000 rpm for 1 hr and glacial acetic acid was added dropwise to the clear supernatant while stirring until the solution turned milky white. The lysate was stirred for 30 minutes in room temperature and precipitated bacterial proteins were pelleted down by centrifugation for 30 minutes at 15000 rpm. The supernatant was dialyzed twice against 50 mM sodium acetate pH 4.5 at 4 °C. Dialyzed fractions were applied onto a SP-Sepharose cation exchange column with the dialysis buffer for column equilibration and wash, and the same buffer with 0.5 M NaCl (Buffer B) for linear gradient elution. Ubiquitin eluted at ~50 % Buffer B concentration. Elution fractions containing ubiquitin were purified further using gel filtration chromatography on a 120 mL Superdex75 column from which ubiquitin eluted between 82-90 mL. NMR data was acquired in Tris -HCl pH 7.6, 1 mM EDTA, 25 mM NaCl and 10 % D2O.
4.1.2. CytRN
Cells were lysed as described for ubiquitin above and the supernatant after cell lysis was made up to 60 % of the saturation value of ammonium sulphate to precipitate bacterial proteins. The precipitated proteins were removed by centrifugation and the supernatant was dialyzed into 50 mM sodium phosphate buffer containing 30 mM NaCl and 1 mM EDTA (lysis and equilibration buffer) before being applied to a SP-Sepharose cation exchange column. The equilibration buffer containing 1 M NaCl was used for elution (Buffer B). CytRN eluted at ~60 % Buffer B concentration. Fractions containing CytRN were pooled and further purified by gel filtration chromatography on a 120 mL Superdex 75 size exclusion column equilibrated in 150 mM ammonium acetate, from which CytRN eluted at ~70 mL. Pure fractions were checked on SDS-PAGE, pooled and lyophilized. NMR data were acquired in buffer containing 20 mM sodium phosphate buffer (pH 7.0) and 10 % D2O.
4.1.3. L24A FF domain
Cells expressing L24A FF were lysed as described for ubiquitin above. Purification was done using cation exchange chromatography on an SP-Sepharose column as mentioned previously for ubiquitin. The lysis buffer was 50 mM sodium phosphate buffer (pH 5.7) containing 1 mM EDTA, and the same buffer for used for column equilibration. Elution was done with the equilibration buffer containing 500 mM NaCl (Buffer B). L24A FF eluted at ~60 % Buffer B concentration; fractions containing protein were further purified on a 120 mL Superdex 75 column, from which L24A FF eluted at a volume of ~83 mL. NMR samples were prepared in 50 mM sodium acetate buffer pH 5.7, 50 mM NaCl, 1 mM EDTA and 10 % D2O.
4.2. NMR spectroscopy: data acquisition and processing
All NMR experiments were carried out on an Agilent DD2 14.1 T (600 MHz 1H Larmor frequency) spectrometer equipped with a room-temperature (L24A FF) or cryogenically cooled (ubiquitin and CytRN) triple resonance single-axis gradient probe. Measurements on ubiquitin, L24A FF and CytRN were done at 298 K, 303 K and 288 K respectively. All NMR datasets were processed with nmrPipe (87) and visualized with nmrDraw (87) and NMRFAM-Sparky (88).
4.3. 1Hα CEST
All 1Hα CEST data were acquired in an interleaved pseudo-3D manner with the pulse sequence shown in Figure 2. Each dataset contained a reference plane measured as described in Section 2.1 for obtaining normalizing CEST profiles. Peak intensities (I) in each plane of the CEST dataset were extracted by global lineshape analysis using FuDA and plotted as a ratio with the intensity in the reference plane (I0) against the chemical shift offset for that particular plane to generate the CEST profile. Uncertainties in the I/I0 values for each CEST profile were calculated using the scatter in the CEST baselines.
In order to extract ΔϖGE values, 1Hα CEST profiles were fit globally or residue-wise to the two-state Bloch-McConnell equations as described earlier using the software package ChemEx (https://github.com/gbouvignies/ChemEx), which numerically integrates the Bloch-McConnell equations (80). The radiofrequency field used in each 1Hα CEST experiment was calibrated using the method of Guennuegues et al (89). 1Hα R1H values were measured using a modified version of the HACACONNH-HA-CEST pulse sequence as described in the Section 2.1. Nine delays (including two additional repeat points for error analysis) ranging from 1 to 350 ms were used and intensities were fit to a single exponential decay equation as a function of the delay to extract R1H. 1Hα R1H values were then input into the fitting routine for determining ΔϖGE and were not allowed to vary during the fit. It is not essential to measure R1H in order to extract ΔϖGE; fixing R1H to a reasonable value (2 s-1) results in comparable fit quality and very similar ΔϖGE for CytRN (maximum deviation of 18 ppb and average deviation of 2.4 ppb over 37 residues).
4.3.1. Ubiquitin
1Hα CEST profiles were acquired at a B1 field of 28 Hz over a chemical shift range of 3.1 to 6.1 ppm (50 planes) for an exchange duration (Tex) of 250 ms.
4.3.2. L24A FF
1Hα CEST profiles were acquired at B1 fields of 15 Hz (15 Hz spacing, 98 planes), 33 Hz (30 Hz spacing, 50 planes) and 54 Hz (50 Hz spacing, 34 planes) over a chemical shift range of 2.85 to 5.25 ppm (15 Hz and 33 Hz) or 2.71 to 5.38 ppm (54 Hz) for an exchange duration (Tex) of 400 ms. Three B1 field 1Hα CEST profiles for 22 residues (T13, A17, K18, F21, L25, R29, S35, W36, E37, A39, M40, K41, I43, I44, Y49, A51, L55, S56, K58, K59, N63 and K66) with |ΔϖIN| > 0.35 ppm were globally fit to the two-state Bloch-McConnell equations to extract kex,IN (582 ± 6 s-1) and pI (4.24 ± 0.04 %). The ground state in-phase 1Hα transverse relaxation rate constant for the operator H± (R2,N) was allowed to vary, while ΔR2 = R2,I – R2,N was fixed to 0 to enforce the condition that the excited and ground states have the same transverse relaxation rate constants. Here, R2,I is the in-phase transverse relaxation rate constant for the excited state I. Antiphase transverse relaxation rate constants (2Hα±Cαz) of ground and excited state 1Hα were calculated as Ri(2H±Cz) = R2,i – R1,i, while the relaxation rate constant of longitudinal two-spin order 2HZCZ was calculated as Ri(2HzCz) = R1C,i – R1H,i. The longitudinal and transverse cross-correlated relaxation rate constants ηz and ηxy are also varied during the fit within the constraints ηz,G = ηz,E and ηxy,G = ηxy,G. ηz accounts for any small deviation of the 1Hα CEST profile baseline from 0. 1JCαHα was fixed for both the ground and excited states to 143.5 Hz. For residues with small ΔϖIN, the 1Hα CEST profile acquired with a B1 field of 33 Hz was fit residue-wise by fixing the pI and kex,IN to the values obtained from the global fit in order to extract ΔϖIN.
4.3.3. CytRN
1Hα CEST profiles were acquired at B1 fields of 13 Hz (12 Hz spacing, 127 planes) and 36 Hz (30 Hz spacing, 52 planes) over a chemical shift range of 2.83 to 5.33 ppm for an exchange duration (Tex) of 500 ms. Global fitting to the two-state Bloch-McConnell equations was done as described above for L24A FF using 2 B1 field 1Hα CEST profiles of A10, V21, T23, A24, T25, V26, T40, E50, V51, Y53 and P55, all of which have |ΔϖDE| > 0.2 ppm. In this case, ΔR2 was allowed to float as it resulted in a significant improvement in the quality of the fit compared to the case of ΔR2 = 0. Exchange parameters of kex,DE = 38 ± 2 s-1 and pE = 4.5 ± 0.1% were obtained from these global fits. For residues with small |ΔϖDE|, pE and kex,DE were fixed to the values determined from the global fits and the 13 and 36 Hz 1Hα CEST profiles were fit together at the residue level to extract ΔϖDE.
4.4. CS-Rosetta structure calculations
The structure of the CytRN excited state was calculated using 119 chemical shifts (including 23 1Hα shifts) and 65 1H-15N residual dipolar couplings (determined in (52)) as described in detail in (52).
Supplementary Material
5. Acknowledgements
We acknowledge funding for infrastructural support from the following programs of the Government of India: DST-FIST, UGC-CAS, and the DBT-IISc partnership program. K.M. thanks the Council of Scientific and Industrial Research (CSIR) for fellowship support. A.K. thanks DBT/Wellcome Trust India Alliance Early Career Fellowship (grant number IA/E/20/1/505675) for personal support. We thank Lewis E. Kay, Bharathwaj Sathyamoorthy, Siddhartha P. Sarma and Claris Niya Varghese for a critical reading of the manuscript.
6. Funding statement
This work was supported by grants from the DBT/Wellcome Trust India Alliance Fellowship (grant no.: IA/I/18/1/503614 to A.S. and grant no.: IA/E/20/1/505675 to A.K.) and a DST/SERB Core Research grant (no. CRG/2019/003457), as well as a start-up grant from IISc awarded to A.S. and intramural funds from TIFR to P.V.
References
- 1.Uversky VN. Intrinsically disordered proteins and their “mysterious”(meta) physics. Front Phys. 2019;7:10. [Google Scholar]
- 2.Wright PE, Dyson HJ. Linking folding and binding. Curr Opin Struct Biol. 2009;19:31. doi: 10.1016/j.sbi.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gianni S, Dogan J, Jemth P. Coupled binding and folding of intrinsically disordered proteins: what can we learn from kinetics? Curr Opin Struct Biol. 2016;36:18. doi: 10.1016/j.sbi.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Uversky VN. Unusual biophysics of intrinsically disordered proteins. Biochim Biophys Acta: Proteins Proteomics. 2013;1834:932. doi: 10.1016/j.bbapap.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Babu MM. The contribution of intrinsically disordered regions to protein function, cellular complexity, and human disease. Biochem Soc Trans. 2016;44:1185. doi: 10.1042/BST20160172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bah A, et al. Folding of an intrinsically disordered protein by phosphorylation as a regulatory switch. Nature. 2015;519:106. doi: 10.1038/nature13999. [DOI] [PubMed] [Google Scholar]
- 7.Borgia A, et al. Extreme disorder in an ultrahigh-affinity protein complex. Nature. 2018;555:61. doi: 10.1038/nature25762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forman-Kay JD, Mittag T. From sequence and forces to structure, function, and evolution of intrinsically disordered proteins. Structure. 2013;21:1492. doi: 10.1016/j.str.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piovesan D, et al. DisProt 7.0: a major update of the database of disordered proteins. Nucleic Acids Res. 2017;45:D219. doi: 10.1093/nar/gkw1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uversky VN. Multitude of binding modes attainable by intrinsically disordered proteins: a portrait gallery of disorder-based complexes. Chem Soc Rev. 2011;40:1623. doi: 10.1039/c0cs00057d. [DOI] [PubMed] [Google Scholar]
- 11.Fung HYJ, Birol M, Rhoades E. IDPs in macromolecular complexes: the roles of multivalent interactions in diverse assemblies. Curr Opin Struct Biol. 2018;49:36. doi: 10.1016/j.sbi.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright PE, Dyson HJ. Intrinsically disordered proteins in cellular signalling and regulation. Nat Rev Mol Cell Biol. 2015;16:18. doi: 10.1038/nrm3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bondos SE, Dunker AK, Uversky VN. Intrinsically disordered proteins play diverse roles in cell signaling. Cell Communication and Signaling. 2022;20:20. doi: 10.1186/s12964-022-00821-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith NC, Kuravsky M, Shammas SL, Matthews JM. Binding and folding in transcriptional complexes. Curr Opin Struct Biol. 2021;66:156. doi: 10.1016/j.sbi.2020.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Brodsky S, Jana T, Barkai N. Order through disorder: The role of intrinsically disordered regions in transcription factor binding specificity. Curr Opin Struct Biol. 2021;71:110. doi: 10.1016/j.sbi.2021.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Palmer AG., III Chemical exchange in biomacromolecules: past, present, and future. J Magn Reson. 2014;241:3. doi: 10.1016/j.jmr.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anthis NJ, Clore GM. Visualizing transient dark states by NMR spectroscopy. Q Rev Biophys. 2015;48:35. doi: 10.1017/S0033583514000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sekhar A, Kay LE. An NMR view of protein dynamics in health and disease. Annu Rev Biophys. 2019;48:297. doi: 10.1146/annurev-biophys-052118-115647. [DOI] [PubMed] [Google Scholar]
- 19.Vallurupalli P, Bouvignies G, Kay LE. Studying “invisible” excited protein states in slow exchange with a major state conformation. J Am Chem Soc. 2012;134:8148. doi: 10.1021/ja3001419. [DOI] [PubMed] [Google Scholar]
- 20.Forsén S, Hoffman RA. Study of moderately rapid chemical exchange reactions by means of nuclear magnetic double resonance. J Chem Phys. 1963;39:2892. [Google Scholar]
- 21.Ward K, Aletras A, Balaban RS. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST) J Magn Reson. 2000;143:79. doi: 10.1006/jmre.1999.1956. [DOI] [PubMed] [Google Scholar]
- 22.Fawzi NL, Ying J, Ghirlando R, Torchia DA, Clore GM. Atomic-resolution dynamics on the surface of amyloid-[bgr] protofibrils probed by solution NMR. Nature. 2011;480:268. doi: 10.1038/nature10577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mittermaier A, Kay LE. New tools provide new insights in NMR studies of protein dynamics. Science. 2006;312:224. doi: 10.1126/science.1124964. [DOI] [PubMed] [Google Scholar]
- 24.Loria JP, Rance M, Palmer AG. A relaxation-compensated Carr-Purcell-Meiboom-Gill sequence for characterizing chemical exchange by NMR spectroscopy. J Am Chem Soc. 1999;121:2331. [Google Scholar]
- 25.Reddy JG, et al. Simultaneous determination of fast and slow dynamics in molecules using extreme CPMG relaxation dispersion experiments. J Biomol NMR. 2018;70:1. doi: 10.1007/s10858-017-0155-0. [DOI] [PubMed] [Google Scholar]
- 26.Rangadurai A, Szymaski ES, Kimsey IJ, Shi H, Al-Hashimi HM. Characterizing micro-to-millisecond chemical exchange in nucleic acids using off-resonance R1ρ relaxation dispersion. Prog Nucl Magn Reson Spectrosc. 2019;112:55. doi: 10.1016/j.pnmrs.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ban D, Sabo TM, Griesinger C, Lee D. Measuring dynamic and kinetic information in the previously inaccessible supra-τc window of nanoseconds to microseconds by solution NMR spectroscopy. Molecules. 2013;18:11904. doi: 10.3390/molecules181011904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwahara J, Clore GM. Detecting transient intermediates in macromolecular binding by paramagnetic NMR. Nature. 2006;440:1227. doi: 10.1038/nature04673. [DOI] [PubMed] [Google Scholar]
- 29.Clore GM. Exploring sparsely populated states of macromolecules by diamagnetic and paramagnetic NMR relaxation. Protein Sci. 2011;20:229. doi: 10.1002/pro.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korzhnev DM, Religa TL, Banachewicz W, Fersht AR, Kay LE. A transient and low-populated protein-folding intermediate at atomic resolution. Science. 2010;329:1312. doi: 10.1126/science.1191723. [DOI] [PubMed] [Google Scholar]
- 31.Korzhnev DM, et al. Nonnative interactions in the FF domain folding pathway from an atomic resolution structure of a sparsely populated intermediate: An NMR relaxation dispersion study. J Am Chem Soc. 2011;133:10974. doi: 10.1021/ja203686t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tiwari VP, Toyama Y, De D, Kay LE, Vallurupalli P. The A39G FF domain folds on a volcano-shaped free energy surface via separate pathways. Proceedings of the National Academy of Sciences. 2021;118:e2115113118. doi: 10.1073/pnas.2115113118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sekhar A, Rosenzweig R, Bouvignies G, Kay LE. Mapping the conformation of a client protein through the Hsp70 functional cycle. Proc Natl Acad Sci U S A. 2015;112:10395. doi: 10.1073/pnas.1508504112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sekhar A, Rosenzweig R, Bouvignies G, Kay LE. Hsp70 biases the folding pathways of client proteins. Proc Natl Acad Sci U S A. 2016:E2794. doi: 10.1073/pnas.1601846113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sekhar A, et al. Conserved conformational selection mechanism of Hsp70 chaperone-substrate interactions. eLife. 2018;7:e32764. doi: 10.7554/eLife.32764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao X, Rosen MK, Gardner KH. Estimation of the available free energy in a LOV2-Jα photoswitch. Nat Chem Biol. 2008;4:491. doi: 10.1038/nchembio.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whittier SK, Hengge AC, Loria JP. Conformational motions regulate phosphoryl transfer in related protein tyrosine phosphatases. Science. 2013;341:899. doi: 10.1126/science.1241735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boehr DD, McElheny D, Dyson HJ, Wright PE. The dynamic energy landscape of dihydrofolate reductase catalysis. Science. 2006;313:1638. doi: 10.1126/science.1130258. [DOI] [PubMed] [Google Scholar]
- 39.Boehr DD, McElheny D, Dyson HJ, Wright PE. Millisecond timescale fluctuations in dihydrofolate reductase are exquisitely sensitive to the bound ligands. Proceedings of the National Academy of Sciences. 2010;107:1373. doi: 10.1073/pnas.0914163107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Culik RM, et al. Effects of maturation on the conformational free-energy landscape of SOD1. Proc Natl Acad Sci U S A. 2018;115:E2546. doi: 10.1073/pnas.1721022115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kimsey IJ, Petzold K, Sathyamoorthy B, Stein ZW, Al-Hashimi HM. Visualizing transient Watson–Crick-like mispairs in DNA and RNA duplexes. Nature. 2015;519:315. doi: 10.1038/nature14227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimsey IJ, et al. Dynamic basis for dG. dT misincorporation via tautomerization and ionization. Nature. 2018;554:195. doi: 10.1038/nature25487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao B, Guffy SL, Williams B, Zhang Q. An excited state underlies gene regulation of a transcriptional riboswitch. Nat Chem Biol. 2017;13:968. doi: 10.1038/nchembio.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Korzhnev DM, Religa TL, Kay LE. Transiently populated intermediate functions as a branching point of the FF domain folding pathway. Proceedings of the National Academy of Sciences. 2012;109:17777. doi: 10.1073/pnas.1201799109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sekhar A, et al. Thermal fluctuations of immature SOD1 lead to separate folding and misfolding pathways. eLife. 2015;4:e07296. doi: 10.7554/eLife.07296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neudecker P, et al. Structure of an intermediate state in protein folding and aggregation. Science. 2012;336:362. doi: 10.1126/science.1214203. [DOI] [PubMed] [Google Scholar]
- 47.Schneider R, et al. Visualizing the molecular recognition trajectory of an intrinsically disordered protein using multinuclear relaxation dispersion NMR. J Am Chem Soc. 2015;137:1220. doi: 10.1021/ja511066q. [DOI] [PubMed] [Google Scholar]
- 48.Charlier C, et al. Structure and dynamics of an intrinsically disordered protein region that partially folds upon binding by chemical-exchange NMR. J Am Chem Soc. 2017;139:12219. doi: 10.1021/jacs.7b05823. [DOI] [PubMed] [Google Scholar]
- 49.Bolik-Coulon N, Bouvignies G, Carlier L, Ferrage F. Intrinsically Disordered Proteins. Elsevier; 2019. pp. 65–92. [Google Scholar]
- 50.Schneider R, Blackledge M, Jensen MR. Elucidating binding mechanisms and dynamics of intrinsically disordered protein complexes using NMR spectroscopy. Curr Opin Struct Biol. 2019;54:10. doi: 10.1016/j.sbi.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 51.Fisher CK, Stultz CM. Constructing ensembles for intrinsically disordered proteins. Curr Opin Struct Biol. 2011;21:426. doi: 10.1016/j.sbi.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Madhurima K, Nandi B, Munshi S, Naganathan AN, Sekhar A. Functional regulation of an intrinsically disordered protein via a conformationally excited state. Science Advances. 2023 doi: 10.1126/sciadv.adh4591. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weickert MJ, Adhya S. A family of bacterial regulators homologous to Gal and Lac repressors. J Biol Chem. 1992;267:15869. [PubMed] [Google Scholar]
- 54.Barbier CS, Short SA, Senear DF. Allosteric Mechanism of Induction of CytR-regulated Gene Expression: CytR REPRESSOR-CYTIDINE INTERACTION. J Biol Chem. 1997;272:16962. doi: 10.1074/jbc.272.27.16962. [DOI] [PubMed] [Google Scholar]
- 55.Lewis M. The lac repressor. C R Biol. 2005;328:521. doi: 10.1016/j.crvi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 56.Tretyachenko-Ladokhina V, Cocco MJ, Senear DF. Flexibility and adaptability in binding of E. coli cytidine repressor to different operators suggests a role in differential gene regulation. J Mol Biol. 2006;362:271. doi: 10.1016/j.jmb.2006.06.085. [DOI] [PubMed] [Google Scholar]
- 57.Moody CL, Tretyachenko-Ladokhina V, Laue TM, Senear DF, Cocco MJ. Multiple conformations of the cytidine repressor DNA-Binding domain coalesce to one upon recognition of a specific DNA surface. Biochemistry. 2011;50:6622. doi: 10.1021/bi200205v. [DOI] [PubMed] [Google Scholar]
- 58.Bell CE, Lewis M. A closer view of the conformation of the Lac repressor bound to operator. Nat Struct Biol. 2000;7:209. doi: 10.1038/73317. [DOI] [PubMed] [Google Scholar]
- 59.Kalodimos CG, Boelens R, Kaptein R. Toward an Integrated Model of Protein-DNA Recognition as Inferred from NMR Studies on the L ac Repressor System. Chem Rev. 2004;104:3567. doi: 10.1021/cr0304065. [DOI] [PubMed] [Google Scholar]
- 60.Slijper M, Bonvin A, Boelens R, Kaptein R. Refined Structure oflacRepressor Headpiece (1-56) Determined by Relaxation Matrix Calculations from 2D and 3D NOE Data: Change of Tertiary Structure upon Binding to thelacOperator. J Mol Biol. 1996;259:761. doi: 10.1006/jmbi.1996.0356. [DOI] [PubMed] [Google Scholar]
- 61.Nagadoi A, et al. Structural comparison of the free and DNA-bound forms of the purine repressor DNA-binding domain. Structure. 1995;3:1217. doi: 10.1016/s0969-2126(01)00257-x. [DOI] [PubMed] [Google Scholar]
- 62.Penin F, et al. Three-dimensional structure of the DNA-binding domain of the fructose repressor from Escherichia coli by 1H and 15N NMR. J Mol Biol. 1997;270:496. doi: 10.1006/jmbi.1997.1123. [DOI] [PubMed] [Google Scholar]
- 63.Long D, Sekhar A, Kay LE. Triple resonance-based 13Cα and 13Cβ CEST experiments for studies of ms timescale dynamics in proteins. J Biomol NMR. 2014;1 doi: 10.1007/s10858-014-9868-5. [DOI] [PubMed] [Google Scholar]
- 64.Vallurupalli P, Kay LE. Probing slow chemical exchange at carbonyl sites in proteins by chemical exchange saturation transfer NMR spectroscopy. Angew Chem Int Ed. 2013;52:4156. doi: 10.1002/anie.201209118. [DOI] [PubMed] [Google Scholar]
- 65.Yuwen T, Kay LE. A new class of CEST experiment based on selecting different magnetization components at the start and end of the CEST relaxation element: an application to 1H CEST. J Biomol NMR. 2018;70:93. doi: 10.1007/s10858-017-0161-2. [DOI] [PubMed] [Google Scholar]
- 66.Lundström P, Hansen DF, Vallurupalli P, Kay LE. Accurate measurement of alpha proton chemical shifts of excited protein states by relaxation dispersion NMR spectroscopy. J Am Chem Soc. 2009;131:1915. doi: 10.1021/ja807796a. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y, Jardetzky O. Probability-based protein secondary structure identification using combined NMR chemical-shift data. Protein Sci. 2002;11:852. doi: 10.1110/ps.3180102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wishart DS. Interpreting protein chemical shift data. Prog Nucl Magn Reson Spectrosc. 2010;58:62. doi: 10.1016/j.pnmrs.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 69.Vallurupalli P, Sekhar A, Yuwen T, Kay LE. Probing conformational dynamics in biomolecules via chemical exchange saturation transfer: a primer. J Biomol NMR. 2017;67:243. doi: 10.1007/s10858-017-0099-4. [DOI] [PubMed] [Google Scholar]
- 70.Bouvignies G, Kay LE. Measurement of proton chemical shifts in invisible states of slowly exchanging protein systems by chemical exchange saturation transfer. J Phys Chem B. 2012;116:14311. doi: 10.1021/jp311109u. [DOI] [PubMed] [Google Scholar]
- 71.Yuwen T, Sekhar A, Kay LE. Separating Dipolar and Chemical Exchange Magnetization Transfer Processes in 1H-CEST. Angew Chem Int Ed. 2017;56:6122. doi: 10.1002/anie.201610759. [DOI] [PubMed] [Google Scholar]
- 72.Grzesiek S, Bax A. Amino acid type determination in the sequential assignment procedure of uniformly 13 C/15 N-enriched proteins. J Biomol NMR. 1993;3:185. doi: 10.1007/BF00178261. [DOI] [PubMed] [Google Scholar]
- 73.Löhr F, Rüterjans H. A new triple-resonance experiment for the sequential assignment of backbone resonances in proteins. J Biomol NMR. 1995;6:189. doi: 10.1007/BF00211783. [DOI] [PubMed] [Google Scholar]
- 74.Bazzo R, Cicero DO, Barbato G. A new three-dimensional pulse sequence for correlating intraresidue NH, N, and CO chemical shifts in 13C, l5N-labeled proteins. J Magn Reson, Ser B. 1996;110:65. [Google Scholar]
- 75.Bolik-Coulon N, Hansen DF, Kay LE. Optimizing frequency sampling in CEST experiments. J Biomol NMR. 2022;76:167. doi: 10.1007/s10858-022-00403-2. [DOI] [PubMed] [Google Scholar]
- 76.Yuwen T, Kay LE. Longitudinal relaxation optimized amide 1 H-CEST experiments for studying slow chemical exchange processes in fully protonated proteins. J Biomol NMR. 2017;67:295. doi: 10.1007/s10858-017-0104-y. [DOI] [PubMed] [Google Scholar]
- 77.Bouvignies G, Vallurupalli P, Kay LE. Visualizing side chains of invisible protein conformers by solution NMR. J Mol Biol. 2014;426:763. doi: 10.1016/j.jmb.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 78.Tiwari VP, Pandit S, Vallurupalli P. Exchangeable deuterons introduce artifacts in amide 15 N CEST experiments used to study protein conformational exchange. J Biomol NMR. 2019;73:43. doi: 10.1007/s10858-018-00223-3. [DOI] [PubMed] [Google Scholar]
- 79.Sekhar A, Vallurupalli P, Kay LE. Folding of the four-helix bundle FF domain from a compact on-pathway intermediate state is governed predominantly by water motion. Proc Natl Acad Sci U S A. 2012;109:19268. doi: 10.1073/pnas.1212036109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McConnell HM. Reaction rates by nuclear magnetic resonance. J Chem Phys. 1958;28:430. [Google Scholar]
- 81.Munshi S, Rajendran D, Naganathan AN. Entropic control of an excited folded-like conformation in a disordered protein ensemble. J Mol Biol. 2018;430:2688. doi: 10.1016/j.jmb.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shen Y, et al. Consistent blind protein structure generation from NMR chemical shift data. Proc Natl Acad Sci U S A. 2008;105:4685. doi: 10.1073/pnas.0800256105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nerli S, Sgourakis NG. Methods Enzymol. Vol. 614. Elsevier; 2019. pp. 321–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shen Y, Bax A. SPARTA+: a modest improvement in empirical NMR chemical shift prediction by means of an artificial neural network. J Biomol NMR. 2010;48:13. doi: 10.1007/s10858-010-9433-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yuwen T, Kay LE, Bouvignies G. Dramatic Decrease in CEST Measurement Times Using Multi-Site Excitation. ChemPhysChem. 2018;19:1707. doi: 10.1002/cphc.201800249. [DOI] [PubMed] [Google Scholar]
- 86.Azatian SB, Kaur N, Latham MP. Increasing the buffering capacity of minimal media leads to higher protein yield. J Biomol NMR. 2019;73:11. doi: 10.1007/s10858-018-00222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Delaglio F, et al. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 88.Lee W, Tonelli M, Markley JL. NMRFAM-SPARKY: enhanced software for biomolecular NMR spectroscopy. Bioinformatics. 2015;31:1325. doi: 10.1093/bioinformatics/btu830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guenneugues M, Berthault P, Desvaux H. A Method for DeterminingB1Field Inhomogeneity. Are the Biases Assumed in Heteronuclear Relaxation Experiments Usually Underestimated? J Magn Reson. 1999;136:118. doi: 10.1006/jmre.1998.1590. [DOI] [PubMed] [Google Scholar]
- 90.Kay LE, Ikura M, Tschudin R, Bax A. Three-dimensional triple-resonance NMR spectroscopy of isotopically enriched proteins. J Magn Reson. 1990;89:496. doi: 10.1016/j.jmr.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 91.Shaka A, Keeler J, Frenkiel T, Freeman R. An improved sequence for broadband decoupling: WALTZ-16. J Magn Reson. 1983;52:335. [Google Scholar]
- 92.McCoy M, Mueller L. Selective decoupling. Journal of Magnetic Resonance, Series A. 1993;101:122. [Google Scholar]
- 93.McCoy M, Mueller L. Selective shaped pulse decoupling in NMR: homonuclear [carbon-13] carbonyl decoupling. J Am Chem Soc. 1992;114:2108. [Google Scholar]
- 94.Kupce Ē, Freeman R. Optimized adiabatic pulses for wideband spin inversion. Journal of Magnetic Resonance, Series A. 1996;118:299. [Google Scholar]
- 95.Logan TM, Olejniczak ET, Xu RX, Fesik SW. A general method for assigning NMR spectra of denatured proteins using 3D HC (CO) NH-TOCSY triple resonance experiments. J Biomol NMR. 1993;3:225. doi: 10.1007/BF00178264. [DOI] [PubMed] [Google Scholar]
- 96.Kay L, Keifer P, Saarinen T. Pure absorption gradient enhanced heteronuclear single quantum correlation spectroscopy with improved sensitivity. J Am Chem Soc. 1992;114:10663 [Google Scholar]
- 97.Schleucher J, Sattler M, Griesinger C. Coherence selection by gradients without signal attenuation: application to the three-dimensional HNCO experiment. Angewandte Chemie International Edition in English. 1993;32:1489. [Google Scholar]
- 98.Marion D, Ikura M, Tschudin R, Bax A. Rapid recording of 2D NMR spectra without phase cycling. Application to the study of hydrogen exchange in proteins. J Magn Reson. 1989;85:393. [Google Scholar]
- 99.Vijay-Kumar S, Bugg CE, Cook WJ. Structure of ubiquitin refined at 1.8 Åresolution. J Mol Biol. 1987;194:531. doi: 10.1016/0022-2836(87)90679-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.