Abstract
Understanding the effects of plastic pollution in terrestrial ecosystems is a priority in environmental research. A central aspect of this suite of pollutants is that it entails particles, in addition to chemical compounds, and this makes plastic quite different from the vast majority of chemical environmental pollutants. Particles can be habitats for microbial communities, and plastics can be a source of chemical compounds that are released into the surrounding environment. In the aquatic literature, the term ‘plastisphere’ has been coined to refer to the microbial community colonizing plastic debris; here, we use a definition that also includes the immediate soil environment of these particles in order to align the definition with other concepts in soil microbiology (e.g., the rhizosphere). We first highlight major differences in the plastisphere between aquatic and soil ecosystems, then review what is currently known about the soil plastisphere, including which members of the microbial community are enriched, and the possible mechanisms underpinning this selection. We then focus on outlining future prospects for research on the soil plastisphere.
Introduction
Plastic pollution is becoming a central issue in the environmental sciences1–4. Plastic contamination is widespread in different compartments of the Earth, including the ocean4, rivers5, the atmosphere6, and soils7. Plastic, and in particular microplastics, enters ecosystems via many different pathways8 that include atmospheric deposition6 and agricultural activities (mulching, amendments, and irrigation). Since microplastic is a diverse contaminant suite, originating from a wide variety of products, it is typically simply defined as ‘all plastic particles smaller than 5 mm’ 9–11. This definition is somewhat vague, with no agreement on the upper/lower size limits12 (for example the ISO definition uses 1 μm to 1,000 μm), and it covers a wide range of particle types10,13. Plastic pollution is the norm rather than the exception virtually anywhere on Earth, even in remote ecosystems14. Given the generally persistent nature of this contaminant in the environment15 and increasing plastic production16, this problem is likely here to stay, a hallmark of the Anthropocene.
Plastics are a rather unusual type of environmental contaminant, because unlike other chemical pollutants they consist of particles formed by a polymer, and contain a range of additive chemicals, for example those chemicals that give plastics a certain color, flexibility or other desired features17. This is important, because particles have an interior volume and a shape, and the shape of particles has been an important parameter to understand effects in ecosystems18. The fact that plastics are particles is also important for understanding effects of leachates that are released from such particles into the surrounding environment: these leachates (additives or non-intentionally present compounds, such as unincorporated monomers) are gradually released into the environment, often giving rise to toxic effects19. This circumstance has given rise to the hypothesis of a global plastic ‘toxicity debt’20, meaning that we are yet to observe the full scale of microplastic pollution effects, since the leachate release is expected to increase with increasing particle fragmentation. Another aspect related to size is that as particles increasingly fragment in the environment, they are expected to eventually attain nanoparticle size (<1,000 nm)9, at which point they will likely create direct toxic effects, since they can enter biological cells and tissues21. These small-sized plastic particles can be easily taken up in biota and along the food chain (bioaccumulation in organisms and biomagnification by trophic interactions)22.
A major challenge facing plastic pollution research is the seemingly endless diversity of particle types23, which can differ in polymer, chemical additives, shape and size, and also in terms of weathering and degradation24. There is thus a myriad of different particles to consider, each likely differing to some extent in their effects on the environment. This is the reality, but also is the cause of a high degree of context-dependence of effects on a range of these plastic properties. Not only does this diversity of particles make generalizations of microplastic effects from any one study rather difficult, but in the environment plastics typically occur as complex mixtures, not as the single contaminants we typically use in experimental studies, exacerbating the challenge to understand effects and make predictions.
Plastic pollution, especially microplastic, has been proposed as a factor of global change25. This is an important additional perspective, complementing the ecotoxicological vantage point that has been the main focus of plastic pollution research26. This perspective of (micro-) plastic as a global change factor has a number of consequences for how studies are conducted. For example, adopting a global change perspective shifts the focus from current contamination levels to potential future concentrations in the environment, and this readily places the plastic problem in the context of other anthropogenic factors, with which it will interact in all ecosystems27.
While plastic pollution research has started in the ocean28, more recently a major research effort has been underway to understand potential impacts on soils29–32. This is the focus of this Review, highlighting the effects on the soil microbial community, which are now beginning to be unraveled.
Clarifying the definition of the plastisphere in soils
The term ‘plastisphere’ is central to the microbial literature on plastic pollution33. It was initially defined within the context of aquatic environments as the microbial community colonizing plastic particles33,34, and the term plastisphere has been quickly and widely adopted in the soils literature as well35.
However, in soil, there has been a divergent use of this term. Perhaps most authors have directly adopted the original definition (i.e. the plastisphere is the community growing on plastic particles) in their work on soil. But several authors have used ‘plastisphere’ to denote the habitat, the environment, sometimes also called the ‘niche’ on the surface of plastic debris; they then further specified the ‘plastisphere microbial community’ or the ‘plastisphere microbiome’ when they address the biological community inhabiting this environment35–38.
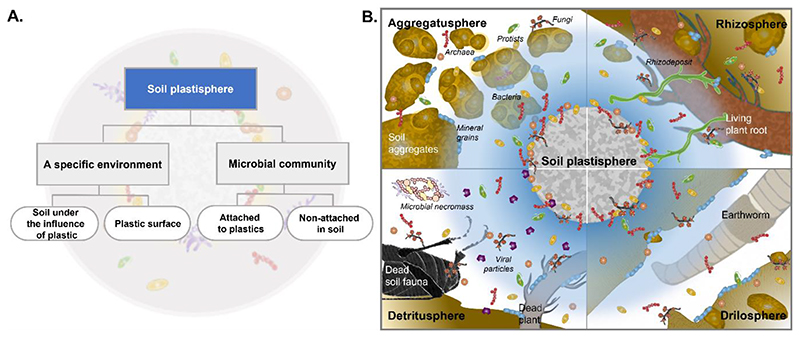
The term ‘sphere’, as in biosphere, really refers to an environment with the community, not just the community that inhabits this environment. Thus, using plastisphere in the sense of a specific environment appears to be the correct one. More importantly, this use also aligns with all other ‘-spheres’ that soil microbiology recognizes39,40 (Fig. 1), the most well-known of which is the rhizosphere41, the soil under immediate influence of roots. This likely explains the intuitive use of plastisphere in terms of denoting a particular soil environment (Fig. 1).
Fig. 1.
The definition of the soil plastisphere (A) and its place in the context of other soil compartments (B). The soil plastisphere consists of a specific environment shaped by the plastic material and the microbial community inhabiting this environment. The microbial community of the plastisphere consists of the attached biofilm (or microbes colonizing the plastic surface), but also of the microbes in the soil under the influence of the plastic particle (A). (B) The soil plastisphere in the context of other important soil features and hotspots39,48, such as the rhizosphere (soil under the influence of roots50), the aggregatusphere (soil aggregates as major building blocks of soil structure101), the drilosphere (soil influenced by earthworms102) and the detritusphere (soil under the influence of dead organic material, detritus103). We here re-define the soil plastisphere as the soil under the direct influence of plastic particles, which includes the biotic community but also the changed physicochemical soil environment itself. The soil plastisphere is envisioned as being embedded within the hierarchy of the other soil compartments.
Therefore, to avoid confusion, we adopt the use of the plastisphere as the environment immediately under the influence of plastic (including the microbial community inhabiting this environment). The community inhabiting the plastisphere is the plastisphere microbial community, which includes microbes attached to the plastic surface, but also microbes in the soil under the influence of plastic (Fig. 1A).
Plastic, for example because of leachates released by these particles, will cause changes in the soil immediately surrounding such particles, including the microbial community that inhabits this soil. This situation differs from the situation in water, where there is less solid matrix (with the exception of the sediments) and particles are generally much more mobile. Even so, also the plastisphere in water will experience such environmental changes, and some studies are now also referring to the plastisphere in the sense of a specific environment in this body of literature42.
This definition highlights the possibility for sampling and analysis approaches (see Box 1) that distinguish the plastic-surface colonizing microbial community specifically, the plastisphere community overall, and the bulk soil (or other soil compartments) for comparison. Work so far has focused on studying the microbial community colonizing the plastic surface35 (typically washing off soil from plastic particles), rather than the entire community inhabiting the plastisphere soil. As a consequence, the work we review here reflects this sampling approach.
Soil and water column differ in heterogeneity and (micro-)plastic mobility
When discussing the soil plastisphere microbial community, it is helpful to appreciate the difference in research focus and general setting, especially input sources, environmental features and transport processes, to the aquatic environment (Fig. 2). As plastic particles enter aquatic environments (ocean, rivers and lakes), they are colonized by microorganisms, including photosynthetically active microbes, depending on a variety of environmental factors33,34,43. In aquatic systems, these plastics are vertically and horizontally transported through the food web or currents33, with transport behavior (e.g. sedimentation rates44) influenced by the plastisphere microbes and their products (Fig. 2A). By comparison, plastic particles are far less mobile in the soil environment, allowing for sufficient time ‘in place’ to influence the plastisphere soil in the first place, and thus microbial colonization is likely more strongly governed by highly localized microscale processes compared to in water (Fig. 2B). Plastic particles do move in the soil, e.g. by bioturbation, ingesting and subsequent excretion, or through pore water flow45 and agricultural activities46. Estimated rates of transport are very low, 5 m in 100 years47. Given that rates are already slow, the plastisphere microbial community is likely less crucial for influencing plastic mobility in soil, and movement is not as important for ‘sampling’ the source microbial community. The second major difference is that soils are extremely heterogeneous39 and particle-rich48, with plastic debris also being incorporated into the main building blocks of soil structure, the soil aggregates49. As a consequence, different plastic particles in a given soil will be exposed to highly diverse soil micro-environments and their respective microbial communities40, from which plastisphere microbial communities will be sourced.
Fig. 2.
Overview of the general difference between plastic in aquatic (A) and terrestrial (B) ecosystems in terms of microplastic behavior, with a focus on comparing movement of the particles. These two contrasting environments differ in sources of microplastic, in properties of the environment, and especially the mobility of the plastic particles. For example, plastic in aquatic systems adds a surface to the water body, whereas soil is already a particle-rich environment25. In soil, particles are substantially less mobile, which means they experience a much more heterogenous set of localized micro-environments (see also Fig. 1). In soils, microplastic can become incorporated into soil aggregates, which makes these particles even less mobile. By contrast, in aquatic environments, plastic debris can be moved by currents until it settles to the sediment at the sea floor.
In soil, microorganisms are spatially (‘hotspots’; small soil volumes with much faster and more intensive microbial processes) and temporally (‘hot moments’; short-term events with accelerated microbial processes) activated by the input of available carbon sources or other environmental factors such as soil moisture content48. Areas in soil that are hotspots of microbial activity include the rhizosphere, the drilosphere (soil under the influence of earthworms) detritusphere (litter layer), and soil aggregates39. These soil compartments are characterized by higher microbial abundance, diversity, and activity48 compared to the ‘bulk’ soil. We view the plastisphere as a component that is embedded within the hierarchy of such soil compartments. Therefore, soil plastispheres and their microbial community are expected to be strongly controlled by location in such hotspots or hot moments. For example, plastic particles located in the rhizosphere will be expected to host microbial communities that are more similar to the rhizosphere than to the bulk soil, since the physicochemical conditions will be co-determined by the plastic material and the overall conditions in the rhizosphere soil, for example in terms of increased labile carbon substrates or pH. However, we currently have very limited data to support this notion. In turn, the plastisphere can also be expected to influence these other micro-environments in soil, for example the rhizosphere, the microbial ecology of which is particularly important for understanding plant growth responses50. The latter is likely a direct function of the abundance of plastic particles, and thus the relative proportion of the plastisphere, in the respective soil compartment.
The soil plastisphere microbial community
Most studies on soil plastisphere microbes have focused on communities colonizing the particles themselves, typically in comparison to the bulk soil; this is in direct analogy to the approach earlier taken earlier in aquatic environments35. The number of reports is increasing rapidly, with more environmental parameters, soil types and microplastics being covered in plastisphere research (see extensive review by Wang et al.35). There are two general properties of the plastisphere microbial community that have emerged so far: the plastisphere community has generally lower diversity than the bulk soil, and it is enriched in microbes with certain traits and genes (Fig. 3A and B).
Fig. 3. The soil plastisphere microbial community differs from that in other soil compartments.
(A) The soil plastisphere is an environmental filter, leading to lower microbial richness and diversity compared to the source community in the bulk soil; and (B) functional composition of microbial communities in the soil plastisphere shifts from that in other soil compartments. Soil plastisphere community composition is influenced by (C) a range of environmental and soil factors and (D) plastic characteristics.
Composition and dynamics
The soil plastisphere generally has lower microbial diversity compared to the surrounding soil37,38,51–54). These data are obtained from direct environmental sampling (observational studies) or dedicated plastic colonization experiments under field or laboratory conditions (reviewed in Wang et al.35) using molecular ecology methods (see Box 1). Laboratory approaches allow more precise assessments of microbial succession patterns and control of parameters influencing the formation of microbial communities, while field observational approaches or long-term incubations permit studying more realistic conditions, including weathering of the plastic material itself. Irrespective of the study design, key comparisons are then typically between the particle surface and the bulk soil, an approach that in the future should be also supplemented by taking into account the plastisphere soil, not just the plastic surface.
Studies typically find differences in taxonomic composition between the plastisphere and bulk soil microbial community. For example, in farmland plastic samples, several bacterial phyla (Actinobacteria, Bacteroidetes, Cyanobacteria, Deinococci, Proteobacteria, and Verrucomicrobia) were enriched in the plastisphere compared to the bulk soil51–53. Likely owing to the high visibility of plastic pollution in agriculture, such studies have been overwhelmingly conducted in agricultural soils. Among these studies, the majority is from China, where plastic-mulching is extensively used in agriculture, and studies focus on bacterial communities. For example, results from a field experiment conducted in maize fields under continental monsoon climate (Loess plateau, China) show that Acidobacteria, Alphaproteobacteria, and Firmicutes are more abundant in the polyethylene plastisphere, compared to other soil environments (bulk soil and rhizosphere)55. Similarly, a study conducted in chili and potato fields (Hebei province, China) using biodegradable plastics (PBAT/PLA) finds clear enrichment in the plastisphere of Actinobacteria and Proteobacteria56. Also working in arable soils, but in Germany, Kublik et al.57 find distinct bacterial communities on polypropylene (PP) and expanded polystyrene (ePS) microplastic particles compared to the bulk soil in an 8-week lab incubation experiment, finding that Acidobacteria rarely colonized microplastic materials. Sampling plastic materials from a landfill site and from a plastic recycling factory in Germany, MacLean et al.37 also document clear differences in plastisphere bacterial communities and those from nearby soil, with Actinobacteria and Proteobacteria highly represented in plastic debris.
Comparing several soil incubation studies, it appears that Proteobacteria are usually enriched in the soil plastisphere37,38,51–55. Although there are several individual studies so far, we still do not have a comprehensive picture of microbial communities in soil plastispheres across different ecosystems, in particular with a large data gap concerning non-arable soils. It is thus unclear if the same type of plastic can enrich unique/core microbial taxa irrespective of soil background. Therefore, networked long-term field incubation studies are needed in the future.
The ecological mechanisms underpinning community assembly in the plastisphere are only beginning to be investigated, but such work is important, as it promises to unravel fundamental insights that could be broadly generalized. For example, initial results documented more negative correlations between bacterial taxa in the soil compared to the plastisphere, and that correlations between bacterial taxa in the plastisphere were predominantly positive54. This could indicate that competition is less prevalent as a force structuring plastisphere microbial communities, and that positive interactions (facilitation) could play a more important role. These community patterns also suggest that the plastisphere environment is more stressful to microbes and potentially represents a strong environmental filter54. Many other parameters await further investigation, such as the role of soil fauna in influencing the plastisphere microbial community58. Such fundamental aspects of community assembly should be addressed more broadly now.
Functional properties
In terms of functional composition, the plastisphere microbial community is selectively enriched in microbial eukaryotes and bacteria, some of which may be potential pathogens, and the latter have been found to carry antibiotic resistance genes (ARGs)54,59. The enrichment of ARGs is of particular concern, and has hence received increasing research attention54,60,61. In particular, the co-location of ARG enrichment and pathogen presence54 raises questions about future environmental risks, if ARGs can spread via horizontal gene transfer. The enrichment of potential pathogens in soil plastispheres deserves greater attention, as this can have significant human health impacts. Many known pathogens, such as staphylococci and Pseudomonas aeruginosa are biofilm-associated through their secretion of extracellular polysaccharides (EPS) and other adhesins, such as proteins62,63. It is therefore likely that bacteria that can produce versatile adhesins will initiate colonization on plastic particles.
Another general feature of traits of microbes inhabiting the soil plastisphere is the enrichment of plastic-degrading microbes37,64,65, which is interesting for obtaining organisms that could be used in bioremediation. For example, in a relatively long-time lab incubation experiment (5 months) using PBAT/PLA plastics and soils (0-10cm) from the Swiss Alps, Rüthi et al.66 found that the plastisphere can be enriched in functional genes related to plastic degradation, carbon and nitrogen cycling, such as nitrogen fixation and the breakdown of organic nitrogen. Using field-collected microplastics (PE and PBAT/PLA plastics for mulching) from an agroecosystem in humid monsoon climate in northern China,
Li et al.56 further demonstrated that biodegradable plastics (i.e. those were the polymer can be decomposed) can enrich bacterial taxa with the potential capability of attacking plastic polymers, such as Proteobacteria and Actinobacteria, even though detailed functional investigations are still needed. Finally, while most attention has been paid by far to bacteria, fungi appear to play a larger role compared to the aquatic plastisphere, with experiments having revealed the presence of fungal assemblages on plastic debris51,67–69. Within the group of eumycotan fungi, there is also evidence of enrichment; for example, Ascomycota were more abundant in the plastisphere compared to the bulk soil36,51,69.
Factors influencing soil plastisphere communities
The soil plastisphere microbial community is broadly determined by the interaction of general soil parameters (largely determining the source community) and the physicochemical properties of the plastic particles themselves35 (Fig. 3C and D). An additional aspect that has not yet been considered is that plastic surfaces will likely already be colonized by microbial communities before they enter soil in the environment (even though in experiments, plastic materials are frequently sterilized prior to addition to soils51,70); this is a situation analogous to the addition of microbial communities that arrive in the soil from compost or sewage sludge additions. Therefore, the addition of exogenous particles may also entail the encounter of entire microbial communities, the one on the plastic and the resident community in the soil. Such interaction and mixing of entire communities has been termed microbial community coalescence71. In general, community coalescence is expected to be a common phenomenon in soils72. Such community coalescence events, and the mechanisms underpinning them, are generally only beginning to be unraveled, and these events are not yet explicitly studied for the plastisphere, but very likely important for a more complete mechanistic understanding of community assembly in the terrestrial plastisphere.
Directly comparing plastic type and soil environment as factors inducing variance in plastisphere bacterial communities, Zhu et al.54 found a roughly equal contribution in their study. Once more data are available, it remains to be seen if this pattern holds, but it is certainly reasonable to expect equivalent importance of these two groups of drivers. Overall, there is still a lack of mechanistic information regarding the assembly of soil plastisphere communities, and this urgently deserves further study.
Soil environmental factors
The full range of soil factors known to influence the bulk microbial community determines the ‘source’ microbial community encountered by plastic particles, and thus all these factors play a key role in explaining plastisphere microbial communities. Soil properties such as soil moisture and pH can determine the features of the soil plastisphere community54,73. For example, Zhu et al.54 and Li et al.73 each compared arable soils that differed (among other parameters) in pH and both studies detected effects of pH on the bacterial community composition in the plastisphere (in the case of Li et al. the effect of pH was stronger than that of arsenic). Soil pH effects on the plastisphere microbial community are unsurprising, since soil pH is a major driver of soil bacterial communities in general, and pH might also change surface properties of microplastics or the availability of other contaminants, for example heavy metals, present in soils74. Given the importance of soil pH in broadly influencing microbial metabolism75, it could be a key factor explaining the variation in metabolic products in the plastisphere; such changes in microbial products could further define plastisphere physicochemical properties. Factors that themselves drive soil properties will also influence plastisphere communities. For example, agricultural activities (e.g., manure addition and fertilization), soil contamination levels (e.g., antibiotics and metals), different source communities (e.g., plant litter and rhizosphere), and different sampling locations (e.g., ecosystem type and contamination levels) have all been found to drive variation in plastisphere microbial community composition53,54,60,73,76–78. A very specific aspect of plastic pollution, compared to many other types of chemical pollution, is that plastic particles often induce physical changes in soil, for example changes in soil aggregation, bulk density or water behavior79–81. These changes to the soil environment will influence the source community composition in the contaminated soils, and thus also in turn the plastisphere itself. Another feedback stems from the effects of microplastics on plants82. Plant growth of different species can be affected by microplastic presence in soil80,83,84, either positively or negatively. Such effects can lead to shifts in plant community composition85. These shifts in plant community composition or productivity will in turn influence the microbial community in the soil and in the plastisphere. In the future, attention should be paid to how and why particularly soil environmental factors affect microbial communities in the plastisphere, and what the ecological consequences are. Such knowledge can then be employed to develop models to predict the long-term impact of plastic pollution in the soil environment.
Plastic properties
Plastic size, shape, polymer composition, biodegradability, additives, surface properties, and degree of weathering are known to generally influence the effects of plastic on soil, and this also extends to the plastisphere community. For example, larger and more weathered plastics had a higher abundance of ARGs in their plastisphere than smaller (1 mm) and less-weathered particles61. Different polymer types can give rise to different bacterial diversity; for instance, LDPE plastispheres had lower bacterial diversity compared to PP and PS70. Community composition, potential pathogens and ARGs in the plastisphere also vary with certain plastic properties, especially surface hydrophobicity54. While several studies have characterized microbial communities in soils containing biodegradable plastics86, the microbial communities associated with their plastisphere are generally less well known.
Bacterial and fungal community composition on biodegradable plastics appear to be different from that in the soil and for non-biodegradable plastics51,69,87.
Plastisphere within the hierarchy of soil compartments
As seen from the sheer number of plastic- and soil environment-related factors that influence plastisphere communities, much more data are needed to achieve a more complete picture. In particular, we believe it will be important to more explicitly consider soil micro-environments39 to align this work on plastispheres with other soil microbiome work. It can be productive to view the plastisphere microbial community as resulting from several hierarchically acting factors. These can be ecosystem type and land use at the broadest level, and the particular soil compartment in which a given plastic particle is located at the smallest scale. Ignoring the compartmentalized, heterogeneous nature of soils is possible if the effects exerted by the plastic material are on average quite strong, overwhelming the effects of the soil compartment source communities (e.g. the rhizosphere effect). However, it will substantially increase our understanding of assembly processes if these properties of the soil compartments are explicitly considered. This work is beginning, with comparisons of plastisphere microbial communities being made not only to bulk soil taxa but also to the rhizosphere compartment55. Future work will need to make effective use of specific experimental approaches (Box 1) that dissect the effects of soil compartment, source communities and plastic material.
Plastic effects in the soil are additionally complicated by the fact that - as particles, not just sources of chemicals - these materials can also affect overall soil physical properties, such as bulk density79 or water holding capacity, in addition to the release of chemicals. Such effects are particularly pronounced for certain shapes of plastic fragments18 that deviate from the population of shapes naturally predominant in soil, such as microplastic fibers. Thus, there are two routes by which plastic particles can affect the bulk soil microbiome: effects of the collection of plastic particles (i.e., their plastispheres) can ‘radiate’ into the soil, and then an effect independent of the plastisphere per se, namely the change of physical properties that arise from an effect at the whole-soil scale. It will be a major challenge to disentangle such effects to be able to more satisfactorily predict consequences of plastic on the soil microbiome.
Conclusions and outlook
The plastisphere in terrestrial ecosystem compartments other than the soil
While this review focuses on soil, the compartment of terrestrial ecosystems currently thought to be most affected by plastic, clearly plastic will also occur in other parts of the ecosystem. Wherever plastics occur, they will also carry a microbial community and the particles might influence the more immediate environment. Clearly, plastics can occur on plant surfaces, since microplastics may land there when deposited from the atmosphere88. The phyllosphere is a relatively harsh microbial environment89,90, and is likely that plastic on the leaf surface will selectively recruit microbes from the leaf surface (similarly to soil), and that plastic adds microbes to the leaf surface in return. There are no studies on this aspect so far. Once taken up into organisms, plastics can reside inside tissues or organs, for example of plants and animals (this includes animals in the soil and aboveground). As in the soil, the expectation is for these plastic particles to carry microbes and to release leachates into the surrounding matrix. It is unclear to what extent the plastisphere (here referring to the environment - including the microbes inhabiting it - inside the organism immediately influenced by the plastic particle) in this case influences effects of plastic, such as retention, movement among tissues, and/or toxicity.
Key questions for future research
The elucidation of the soil plastisphere properties and microbial communities, and the processes these communities influence, is still at an early stage, but the pace of progress is fast35, promising many novel insights in the coming years. We here list key questions that deserve particular attention in this research endeavor (Fig. 4).
Fig. 4. Future developments and outlook.
There are several key questions that need to be addressed to drive progress in our understanding of the soil plastisphere. (A) Overall effects and roles of the soil plastisphere in ecosystem processes and Earth system feedbacks, (B) the spatial extent of the plastisphere in the soil environment, (C) existence of a core microbiome in the soil plastisphere, (D) successional changes of the plastisphere with fate of the plastics in soil environment, (E) interactions between soil plastisphere and other factors of global change.
Given the ubiquitous presence of plastic particles and their associated plastispheres, the question arises what relative importance this volume of soil and its microbial inhabitants have for ecosystem processes, biogeochemical cycles, and potential Earth system feedbacks31. These particles add a novel soil feature, as discussed here, harboring distinct communities and functional gene compositions. We need to estimate potential effects of the plastisphere community on ecosystem biogeochemistry91, including trace gas emissions92, and terrestrial productivity and multifunctionality93, keeping in mind that such effects may unfold only in the long-term, beyond the time scale of many current experimental studies (this highlights a potential role for Long-Term Ecological Research sites). This is particularly crucial, because of key feedback mechanisms that require time to unfold, such as shifts in plant community composition. Studying such effects also requires larger-scale and more complex experimental setups, such as suitably-instrumented mesocosm studies or field experiments. For achieving a mechanistic understanding of any effects of microplastic, we need to partition the contribution of the plastisphere community from that of other soil compartments that may have concurrently changed.
We have introduced the plastisphere as the soil volume immediately influenced by the plastic particles, and so a key question is how far effects radiate into the bulk soil. In other words, how quickly does the effect attenuate with distance from the plastic surface? Taking the rhizosphere for inspiration (which is a few millimeters wide)50, microbial cell numbers can drop very quickly with distance from the root surface. It is reasonable to assume that the plastisphere is of a similar extent, given the slow release of leachates over time, and considering that the plastic surface – as opposed to the root surface – is not enriched in microbial phylotypes, but has lower diversity. However, fungi, including the symbiotic, plant-associated mycorrhizal fungi94, can extend the effect of the rhizosphere by transporting chemicals and organisms along their hyphae into the bulk soil, and a similar effect may well exist for the plastisphere; especially since mycorrhizal fungi have been observed to be stimulated in the presence of microplastic in soil95.
Is there a plastisphere core microbiome? Plastic, as we have explained, is an incredibly diverse contaminant suite, but are there some key features that are conserved across many different types of plastic, such as surface properties, or toxic leachates, that give rise to a core microbiome at some level of phylogenetic resolution35? Such a signal, if sufficiently specific, might be a proxy for the extent of plastic pollution, but might take a longer time to evolve.
Much can be learned by studying the temporal dynamics of plastisphere development58,87. How does the plastisphere environment and microbial community reflect plastic aging? Does the plastisphere become more toxic over time with the release of additional leachates, and with increasing fragmentation and weathering20; or are effects ameliorated over time, because of adjustments in the microbial community and evolutionary adaptation of its members96? This will be a crucial aspect to understand long-term dynamics in ecosystems, clearly a frontier topic.
Crucially, we need to better understand how the plastisphere interacts with other global change factors impinging on terrestrial ecosystems. Ecosystems, and especially soils, will never be affected just by plastic, but by a whole range of other anthropogenic factors27,97–99. How might climate change modify the microbial community of the plastisphere100? How will other chemical pollutants interact with plastisphere? How will the plastisphere react to or modify the rhizosphere of an invasive plant species? Addressing such topics will clearly be the next stage of plastisphere research, since asking such questions will be moving closer to the reality currently unfolding in the environment.
Box 1. Approaches to studying the soil plastisphere and its microbial communities.
There are several possible approaches to studying the soil plastisphere35, including field collection of plastic material, incubation of plastic materials in the field, and laboratory experiments. These approaches are arrayed along a gradient of ecological realism (field sampling) to experimental control (lab experiments), and thus should be chosen with a specific goal in mind.
Soil plastisphere microbial communities can be investigated by several molecular tools in the samples obtained from one of these approaches depending on the purpose of the study, including community composition and functional characterization of both total and active communities35. For example, metagenomic sequencing can be used to study the abundance and composition of antibiotic resistance genes, virulence factors and heavy metal resistance genes in the soil plastisphere104, while proteomics and metabolomics can be employed to study the metabolic pathways of functional microorganisms isolated from the soil plastisphere105. In addition, deep metagenomic and metatranscriptome sequencing can be used to explore the virus information (DNA and RNA) carried by the plastisphere106. As most of the bacteria in the environment are uncultured, single-cell technology provides a means to avoid pure culture while still being able to study environmental microbes from their habitats. Single-cell Raman spectroscopy is an emerging technique capable of deciphering the phenotypic profile of individual bacterial cells in microbial communities in a culture-independent fashion. When further combined with heavy water labelling (Raman-D2O), physiologically active microbes can incorporate deuterium (D) into de novo-synthesized lipids and proteins, generating a new carbon–deuterium (C–D) Raman band.
Characterization of the C–D band of microbes can distinguish antibiotic-tolerant and sensitive cells because of their different activities under antibiotic stress, and this method can be used to investigate other functional traits (i.e., nutrient cycling)107.
For the analysis of soil physicochemical changes, the main challenge is obtaining location-resolved soil samples sufficiently large for analysis pipelines. Here, inspiration can come from rhizosphere analysis108. Rhizosphere soil has traditionally been operationally defined as soil adhering to roots, and plastisphere soil could be similarly delineated (with all the caveats this has in common with collecting rhizosphere soil). For studies using incubation in the field or the lab, additionally a mesh-based sampling approach could be used, as in studies of the rhizosphere108: plastic materials could be enclosed in mesh-compartments, using a different mesh material from the plastics and the mesh size smaller than that of the plastic particles to be examined, and then sampling can proceed at various distances from the mesh surface. In addition, continuous sampling techniques, like involving the use of micro-suction cups, and imaging techniques109 (for example for pH or redox state) can also be applied. Label-free multiphoton imaging techniques110 could be particularly useful for exploring properties of the soil plastisphere. By using a combination of such approaches, the ‘reach’ and spatial organization of the soil plastisphere as it transitions into ‘bulk soil’ could be experimentally explored, while ensuring enough material is present for analysis. This information on the microscale habitat, together with data on plastic (surface) properties could then be used to better understand assembly processes underpinning the soil plastisphere microbial community. Experimentally combining such mesh-compartment designs with other factors, such as the presence of roots or litter, will help disentangle the relative contributions of drivers that govern the microbiome inhabiting these soil compartments (e.g. rhizosphere and detritusphere) and the factors linked to the plastic particles themselves.
Acknowledgements
MCR acknowledges funding from an ERC Advanced Grant, and from the EU projects MINAGRIS and PAPILLIONS, as well as from the BMBF-funded project ‘μPlastic’. YGZ is supported by National Natural Science Foundation of China (42021005).
Footnotes
Competing interests
The authors declare that no competing interests exist.
References
- 1.Thompson RC, Swan SH, Moore CJ, vom Saal FS. Our plastic age. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:1973–1976. doi: 10.1098/rstb.2009.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bank MS, Hansson SV. The Plastic Cycle: A Novel and Holistic Paradigm for the Anthropocene. Environ Sci Technol. 2019;53:7177–7179. doi: 10.1021/acs.est.9b02942. [DOI] [PubMed] [Google Scholar]
- 3.Rochman CM, Hoellein T. The global odyssey of plastic pollution. Science. 2020;368:1184–1185. doi: 10.1126/science.abc4428. [DOI] [PubMed] [Google Scholar]
- 4.Galloway TS, Cole M, Lewis C. Interactions of microplastic debris throughout the marine ecosystem. Nature Ecology Evolution. 2017;1:1–8. doi: 10.1038/s41559-017-0116. [DOI] [PubMed] [Google Scholar]
- 5.Hurley R, Woodward J, Rothwell JJ. Microplastic contamination of river beds significantly reduced by catchment-wide flooding. Nature Geosci. 2018;11:251–257. [Google Scholar]
- 6.Brahney J, Hallerud M, Heim E, Hahnenberger M, Sukumaran S. Plastic rain in protected areas of the United States. Science. 2020;368:1257–1260. doi: 10.1126/science.aaz5819. [DOI] [PubMed] [Google Scholar]
- 7.Nizzetto L, Futter M, Langaas S. Are agricultural soils dumps for microplastics of urban origin? Environ Sci Technol. 2016;50:10777–10779. doi: 10.1021/acs.est.6b04140. [DOI] [PubMed] [Google Scholar]
- 8.Bläsing M, Amelung W. Plastics in soil: Analytical methods and possible sources. Science of The Total Environment. 2018;612:422–435. doi: 10.1016/j.scitotenv.2017.08.086. [DOI] [PubMed] [Google Scholar]
- 9.Hartmann NB, et al. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ Sci Technol. 2019;53:1039–1047. doi: 10.1021/acs.est.8b05297. [DOI] [PubMed] [Google Scholar]
- 10.Kooi M, Koelmans AA. Simplifying microplastic via continuous probability distributions for size, shape, and density. Environ Sci Technol Lett. 2019;6:551–557. [Google Scholar]
- 11.SAPEA. A Scientific Perspective on Microplastics in Nature and Society. 2019 doi: 10.26356/microplastics. [DOI] [Google Scholar]
- 12.Frias JPGL, Nash R. Microplastics: Finding a consensus on the definition. Marine Pollution Bulletin. 2019;138:145–147. doi: 10.1016/j.marpolbul.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 13.Koelmans AA, et al. Risk assessment of microplastic particles. Nat Rev Mater. 2022;7:138–152. [Google Scholar]
- 14.Bergmann M, et al. Plastic pollution in the Arctic. Nat Rev Earth Environ. 2022;3:323–337. [Google Scholar]
- 15.Ward CP, Reddy CM. We need better data about the environmental persistence of plastic goods. Proceedings of the National Academy of Sciences. 2020;117:14618–14621. doi: 10.1073/pnas.2008009117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geyer R, Jambeck JR, Law KL. Production, use, and fate of all plastics ever made. Science Advances. 2017;3:e1700782. doi: 10.1126/sciadv.1700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hahladakis JN, Velis CA, Weber R, Iacovidou E, Purnell P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. Journal of Hazardous Materials. 2018;344:179–199. doi: 10.1016/j.jhazmat.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Rillig MC, Lehmann A, Ryo M, Bergmann J. Shaping up: toward considering the shape and form of pollutants. Environ Sci Technol. 2019;53:7925–7926. doi: 10.1021/acs.est.9b03520. [DOI] [PubMed] [Google Scholar]
- 19.Kim SW, Waldman WR, Kim T-Y, Rillig MC. Effects of different microplastics on nematodes in the soil environment: tracking the extractable additives using an ecotoxicological approach. Environ Sci Technol. 2020;54:13868–13878. doi: 10.1021/acs.est.0c04641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rillig MC, Kim SW, Kim T-Y, Waldman WR. The global plastic toxicity debt. Environ Sci Technol. 2021;55:2717–2719. doi: 10.1021/acs.est.0c07781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehner R, Weder C, Petri-Fink A, Rothen-Rutishauser B. Emergence of nanoplastic in the environment and possible impact on human health. Environ Sci Technol. 2019;53:1748–1765. doi: 10.1021/acs.est.8b05512. [DOI] [PubMed] [Google Scholar]
- 22.Chae Y, Kim D, Kim SW, An Y-J. Trophic transfer and individual impact of nano-sized polystyrene in a four-species freshwater food chain. Sci Rep. 2018;8:284. doi: 10.1038/s41598-017-18849-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rochman CM, et al. Rethinking microplastics as a diverse contaminant suite. Environmental Toxicology and Chemistry. 2019;38:703–711. doi: 10.1002/etc.4371. [DOI] [PubMed] [Google Scholar]
- 24.Waldman WR, Rillig MC. Microplastic research should embrace the complexity of secondary particles. Environ Sci Technol. 2020;54:7751–7753. doi: 10.1021/acs.est.0c02194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Machado AAS, Kloas W, Zarfl C, Hempel S, Rillig MC. Microplastics as an emerging threat to terrestrial ecosystems. Global Change Biology. 2018;24:1405–1416. doi: 10.1111/gcb.14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rillig MC, Leifheit E, Lehmann J. Microplastic effects on carbon cycling processes in soils. PLOS Biology. 2021;19:e3001130. doi: 10.1371/journal.pbio.3001130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rillig MC, et al. The role of multiple global change factors in driving soil functions and microbial biodiversity. Science. 2019;366:886–890. doi: 10.1126/science.aay2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson RC, et al. Lost at sea: where Is all the plastic? Science. 2004;304:838. doi: 10.1126/science.1094559. [DOI] [PubMed] [Google Scholar]
- 29.Rochman CM. Microplastics research—from sink to source. Science. 2018;360:28–29. doi: 10.1126/science.aar7734. [DOI] [PubMed] [Google Scholar]
- 30.Rillig MC. Microplastic in terrestrial ecosystems and the soil? Environ Sci Technol. 2012;46:6453–6454. doi: 10.1021/es302011r. [DOI] [PubMed] [Google Scholar]
- 31.Rillig MC, Lehmann A. Microplastic in terrestrial ecosystems. Science. 2020;368:1430–1431. doi: 10.1126/science.abb5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang W, Ge J, Yu X, Li H. Environmental fate and impacts of microplastics in soil ecosystems: Progress and perspective. Science of The Total Environment. 2020;708:134841. doi: 10.1016/j.scitotenv.2019.134841. [DOI] [PubMed] [Google Scholar]
- 33.Amaral-Zettler LA, Zettler ER, Mincer TJ. Ecology of the plastisphere. Nat Rev Microbiol. 2020;18:139–151. doi: 10.1038/s41579-019-0308-0. [DOI] [PubMed] [Google Scholar]
- 34.Zettler ER, Mincer TJ, Amaral-Zettler LA. Life in the “plastisphere”: microbial communities on plastic marine debris. Environ Sci Technol. 2013;47:7137–7146. doi: 10.1021/es401288x. [DOI] [PubMed] [Google Scholar]
- 35.Wang C, Wang L, Ok YS, Tsang DCW, Hou D. Soil plastisphere: Exploration methods, influencing factors, and ecological insights. Journal of Hazardous Materials. 2022;430:128503. doi: 10.1016/j.jhazmat.2022.128503. [DOI] [PubMed] [Google Scholar]
- 36.Gkoutselis G, et al. Microplastics accumulate fungal pathogens in terrestrial ecosystems. Sci Rep. 2021;11:13214. doi: 10.1038/s41598-021-92405-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacLean J, et al. The terrestrial plastisphere: diversity and polymer-colonizing potential of plastic-associated microbial communities in soil. Microorganisms. 2021;9:1876. doi: 10.3390/microorganisms9091876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Y, Shi J, Wang X, Ding C, Wang J. Deciphering the mechanisms shaping the plastisphere microbiota in soil. mSystems. 2022;7:e00352-22. doi: 10.1128/msystems.00352-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beare MH, Coleman DC, Crossley DA, Hendrix PF, Odum EP. A hierarchical approach to evaluating the significance of soil biodiversity to biogeochemical cycling. Plant and Soil. 1995;170:5–22. [Google Scholar]
- 40.Fierer N. Embracing the unknown: disentangling the complexities of the soil microbiome. Nat Rev Microbiol. 2017;15:579–590. doi: 10.1038/nrmicro.2017.87. [DOI] [PubMed] [Google Scholar]
- 41.Curl EA, Truelove B. The Rhizosphere. Springer Science Business Media; 2012. [Google Scholar]
- 42.Dąbrowska A. A roadmap for a Plastisphere. Marine Pollution Bulletin. 2021;167:112322. doi: 10.1016/j.marpolbul.2021.112322. [DOI] [PubMed] [Google Scholar]
- 43.Cheng J, et al. Relative Influence of Plastic Debris Size and Shape, Chemical Composition and Phytoplankton-Bacteria Interactions in Driving Seawater Plastisphere Abundance, Diversity and Activity. Frontiers in Microbiology. 2021;11 doi: 10.3389/fmicb.2020.610231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Long M, et al. Interactions between microplastics and phytoplankton aggregates: Impact on their respective fates. Marine Chemistry. 2015;175:39–46. [Google Scholar]
- 45.Yu Y, Flury M. Current understanding of subsurface transport of micro- and nanoplastics in soil. Vadose Zone Journal. 2021;20:e20108 [Google Scholar]
- 46.Ren Z, et al. Microplastics in the soil-groundwater environment: Aging, migration, and co-transport of contaminants – A critical review. Journal of Hazardous Materials. 2021;419:126455. doi: 10.1016/j.jhazmat.2021.126455. [DOI] [PubMed] [Google Scholar]
- 47.O’Connor D, et al. Microplastics undergo accelerated vertical migration in sand soil due to small size and wet-dry cycles. Environmental Pollution. 2019;249:527–534. doi: 10.1016/j.envpol.2019.03.092. [DOI] [PubMed] [Google Scholar]
- 48.Kuzyakov Y, Blagodatskaya E. Microbial hotspots and hot moments in soil: Concept review. Soil Biology and Biochemistry. 2015;83:184–199. [Google Scholar]
- 49.Zhang GS, Liu YF. The distribution of microplastics in soil aggregate fractions in southwestern China. Science of The Total Environment. 2018;642:12–20. doi: 10.1016/j.scitotenv.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Philippot L, Raaijmakers JM, Lemanceau P, van der Putten WH. Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol. 2013;11:789–799. doi: 10.1038/nrmicro3109. [DOI] [PubMed] [Google Scholar]
- 51.Bandopadhyay S, et al. Soil microbial communities associated with biodegradable plastic mulch films. Frontiers in Microbiology. 2020;11 doi: 10.3389/fmicb.2020.587074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo G, et al. Deciphering the diversity and functions of plastisphere bacterial communities in plastic-mulching croplands of subtropical China. Journal of Hazardous Materials. 2022;422:126865. doi: 10.1016/j.jhazmat.2021.126865. [DOI] [PubMed] [Google Scholar]
- 53.Zhang M, et al. Microplastics from mulching film is a distinct habitat for bacteria in farmland soil. Science of The Total Environment. 2019;688:470–478. doi: 10.1016/j.scitotenv.2019.06.108. [DOI] [PubMed] [Google Scholar]
- 54.Zhu D, Ma J, Li G, Rillig MC, Zhu Y-G. Soil plastispheres as hotspots of antibiotic resistance genes and potential pathogens. ISME J. 2022;16:521–532. doi: 10.1038/s41396-021-01103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi Z, Xiong L, Liu T, Wu W. Alteration of bacterial communities and co-occurrence networks as a legacy effect upon exposure to polyethylene residues under field environment. Journal of Hazardous Materials. 2022;426:128126. doi: 10.1016/j.jhazmat.2021.128126. [DOI] [PubMed] [Google Scholar]
- 56.Li K, Jia W, Xu L, Zhang M, Huang Y. The plastisphere of biodegradable and conventional microplastics from residues exhibit distinct microbial structure, network and function in plastic-mulching farmland. Journal of Hazardous Materials. 2023;442:130011. doi: 10.1016/j.jhazmat.2022.130011. [DOI] [PubMed] [Google Scholar]
- 57.Kublik S, et al. Microplastics in soil induce a new microbial habitat, with consequences for bulk soil microbiomes. Frontiers in Environmental Science. 2022;10 [Google Scholar]
- 58.Xiang Q, Chen Q-L, Yang X-R, Li G, Zhu D. Soil mesofauna alter the balance between stochastic and deterministic processes in the plastisphere during microbial succession. Science of The Total Environment. 2022;849:157820. doi: 10.1016/j.scitotenv.2022.157820. [DOI] [PubMed] [Google Scholar]
- 59.Li Y, et al. The composition, biotic network, and assembly of plastisphere protistan taxonomic and functional communities in plastic-mulching croplands. Journal of Hazardous Materials. 2022;430:128390. doi: 10.1016/j.jhazmat.2022.128390. [DOI] [PubMed] [Google Scholar]
- 60.Ding J, et al. Exposure to heavy metal and antibiotic enriches antibiotic resistant genes on the tire particles in soil. Science of The Total Environment. 2021;792:148417. doi: 10.1016/j.scitotenv.2021.148417. [DOI] [PubMed] [Google Scholar]
- 61.Lu X-M, Lu P-Z, Liu X-P. Fate and abundance of antibiotic resistance genes on microplastics in facility vegetable soil. Science of The Total Environment. 2020;709:136276. doi: 10.1016/j.scitotenv.2019.136276. [DOI] [PubMed] [Google Scholar]
- 62.Wang L, Wang H, Zhang H, Wu H. Formation of a biofilm matrix network shapes polymicrobial interactions. ISME J. 2023:1–11. doi: 10.1038/s41396-023-01362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Otto M. Staphylococcal Biofilms. Microbiology Spectrum. 2018;6:6.4.27. doi: 10.1128/microbiolspec.gpp3-0023-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ya H, Xing Y, Zhang T, Lv M, Jiang B. LDPE microplastics affect soil microbial community and form a unique plastisphere on microplastics. Applied Soil Ecology. 2022;180:104623 [Google Scholar]
- 65.Puglisi E, et al. Selective bacterial colonization processes on polyethylene waste samples in an abandoned landfill site. Sci Rep. 2019;9:14138. doi: 10.1038/s41598-019-50740-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rüthi J, et al. The plastisphere microbiome in alpine soils alters the microbial genetic potential for plastic degradation and biogeochemical cycling. Journal of Hazardous Materials. 2023;441:129941 [Google Scholar]
- 67.Rüthi J, Bölsterli D, Pardi-Comensoli L, Brunner I, Frey B. The –plastisphere” of biodegradable plastics Is characterized by specific microbial taxa of alpine and arctic Ssoils. Frontiers in Environmental Science. 2020;8 [Google Scholar]
- 68.Sabev HA, Handley PS, Robson GDY. Fungal colonization of soil-buried plasticized polyvinyl chloride (pPVC) and the impact of incorporated biocides. Microbiology. 2006;152:1731–1739. doi: 10.1099/mic.0.28569-0. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y, Ma J, O’Connor P, Zhu Y-G. Microbial communities on biodegradable plastics under different fertilization practices in farmland soil microcosms. Science of The Total Environment. 2022;809:152184. doi: 10.1016/j.scitotenv.2021.152184. [DOI] [PubMed] [Google Scholar]
- 70.Yang Y, Li T, Liu P, Li H, Hu F. The formation of specific bacterial communities contributes to the enrichment of antibiotic resistance genes in the soil plastisphere. Journal of Hazardous Materials. 2022;436:129247. doi: 10.1016/j.jhazmat.2022.129247. [DOI] [PubMed] [Google Scholar]
- 71.Rillig MC, et al. Interchange of entire communities: microbial community coalescence. Trends in Ecology Evolution. 2015;30:470–476. doi: 10.1016/j.tree.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 72.Rillig MC, et al. Soil microbes and community coalescence. Pedobiologia. 2016;59:37–40. [Google Scholar]
- 73.Li H-Q, et al. Soil pH has a stronger effect than arsenic content on shaping plastisphere bacterial communities in soil. Environmental Pollution. 2021;287:117339. doi: 10.1016/j.envpol.2021.117339. [DOI] [PubMed] [Google Scholar]
- 74.Yu H, et al. Decrease in bioavailability of soil heavy metals caused by the presence of microplastics varies across aggregate levels. Journal of Hazardous Materials. 2020;395:122690. doi: 10.1016/j.jhazmat.2020.122690. [DOI] [PubMed] [Google Scholar]
- 75.Luan L, et al. Integrating pH into the metabolic theory of ecology to predict bacterial diversity in soil. Proc Natl Acad Sci USA. 2023;120:e2207832120. doi: 10.1073/pnas.2207832120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Potrykus M, et al. Polypropylene structure alterations after 5 years of natural degradation in a waste landfill. Science of The Total Environment. 2021;758:143649. doi: 10.1016/j.scitotenv.2020.143649. [DOI] [PubMed] [Google Scholar]
- 77.Wang Y, et al. Effects of coexistence of tetracycline, copper and microplastics on the fate of antibiotic resistance genes in manured soil. Science of The Total Environment. 2021;790:148087. doi: 10.1016/j.scitotenv.2021.148087. [DOI] [PubMed] [Google Scholar]
- 78.Xie H, et al. Chemotaxis-selective colonization of mangrove rhizosphere microbes on nine different microplastics. Science of The Total Environment. 2021;752:142223. doi: 10.1016/j.scitotenv.2020.142223. [DOI] [PubMed] [Google Scholar]
- 79.de Souza Machado AA, et al. Impacts of microplastics on the soil biophysical environment. Environ Sci Technol. 2018;52:9656–9665. doi: 10.1021/acs.est.8b02212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Souza Machado AA, et al. Microplastics can change soil properties and affect plant performance. Environ Sci Technol. 2019;53:6044–6052. doi: 10.1021/acs.est.9b01339. [DOI] [PubMed] [Google Scholar]
- 81.Wan Y, Wu C, Xue Q, Hui X. Effects of plastic contamination on water evaporation and desiccation cracking in soil. Science of The Total Environment. 2019;654:576–582. doi: 10.1016/j.scitotenv.2018.11.123. [DOI] [PubMed] [Google Scholar]
- 82.Rillig MC, Lehmann A, de Machado AAS, Yang G. Microplastic effects on plants. New Phytologist. 2019;223:1066–1070. doi: 10.1111/nph.15794. [DOI] [PubMed] [Google Scholar]
- 83.van Kleunen M, Brumer A, Gutbrod L, Zhang Z. A microplastic used as infill material in artificial sport turfs reduces plant growth. Plants, People, Planet. 2020;2:157–166. [Google Scholar]
- 84.Qi Y, et al. Macro-and micro-plastics in soil-plant system: Effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Science of The Total Environment. 2018;645:1048–1056. doi: 10.1016/j.scitotenv.2018.07.229. [DOI] [PubMed] [Google Scholar]
- 85.Lozano YM, Rillig MC. Effects of microplastic fibers and drought on plant communities. Environmental Science Technology. 2020 doi: 10.1021/acs.est.0c01051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Qin M, et al. A review of biodegradable plastics to biodegradable microplastics: Another ecological threat to soil environments? Journal of Cleaner Production. 2021;312:127816 [Google Scholar]
- 87.Ju Z, et al. The succession of bacterial community attached on biodegradable plastic mulches during the degradation in soil. Frontiers in Microbiology. 2021;12 doi: 10.3389/fmicb.2021.785737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bi M, He Q, Chen Y. What roles are terrestrial plants playing in global microplastic cycling? Environ Sci Technol. 2020;54:5325–5327. doi: 10.1021/acs.est.0c01009. [DOI] [PubMed] [Google Scholar]
- 89.Remus-Emsermann MNP, Schlechter RO. Phyllosphere microbiology: at the interface between microbial individuals and the plant host. New Phytologist. 2018;218:1327–1333. doi: 10.1111/nph.15054. [DOI] [PubMed] [Google Scholar]
- 90.Vorholt JA. Microbial life in the phyllosphere. Nat Rev Microbiol. 2012;10:828–840. doi: 10.1038/nrmicro2910. [DOI] [PubMed] [Google Scholar]
- 91.Wang X, et al. Recent advances on the effects of microplastics on elements cycling in the environment. Science of The Total Environment. 2022;849:157884. doi: 10.1016/j.scitotenv.2022.157884. [DOI] [PubMed] [Google Scholar]
- 92.Ren X, Tang J, Liu X, Liu Q. Effects of microplastics on greenhouse gas emissions and the microbial community in fertilized soil. Environmental Pollution. 2020;256:113347. doi: 10.1016/j.envpol.2019.113347. [DOI] [PubMed] [Google Scholar]
- 93.Lozano YM, et al. Effects of microplastics and drought on soil ecosystem functions and multifunctionality. Journal of Applied Ecology. 2021;58:988–996. [Google Scholar]
- 94.Parniske M. Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat Rev Microbiol. 2008;6:763–775. doi: 10.1038/nrmicro1987. [DOI] [PubMed] [Google Scholar]
- 95.Leifheit EF, Lehmann A, Rillig MC. Potential effects of microplastic on arbuscular mycorrhizal fungi. Frontiers in Plant Science. 2021;12 doi: 10.3389/fpls.2021.626709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rillig MC, et al. Evolutionary implications of microplastics for soil biota. Environ Chem. 2018;16:3–7. doi: 10.1071/EN18118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bowler DE, et al. Mapping human pressures on biodiversity across the planet uncovers anthropogenic threat complexes. People and Nature. 2020;2:380–394. [Google Scholar]
- 98.Jackson MC, Pawar S, Woodward G. The temporal dynamics of multiple stressor effects: from individuals to ecosystems. Trends in Ecology Evolution. 2021;36:402–410. doi: 10.1016/j.tree.2021.01.005. [DOI] [PubMed] [Google Scholar]
- 99.Rillig MC, Ryo M, Lehmann A. Classifying human influences on terrestrial ecosystems. Global Change Biology. 2021;27:2273–2278. doi: 10.1111/gcb.15577. [DOI] [PubMed] [Google Scholar]
- 100.Ji L, Tanunchai B, Wahdan SFM, Schädler M, Purahong W. Future climate change enhances the complexity of plastisphere microbial co-occurrence networks, but does not significantly affect the community assembly. Science of The Total Environment. 2022;844:157016. doi: 10.1016/j.scitotenv.2022.157016. [DOI] [PubMed] [Google Scholar]
- 101.Erktan A, Or D, Scheu S. The physical structure of soil: Determinant and consequence of trophic interactions. Soil Biology and Biochemistry. 2020;148:107876 [Google Scholar]
- 102.Andriuzzi WS, Bolger T, Schmidt O. The drilosphere concept: Fine-scale incorporation of surface residue-derived N and C around natural Lumbricus terrestris burrows. Soil Biology and Biochemistry. 2013;64:136–138. [Google Scholar]
- 103.Kögel-Knabner I. The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biology and Biochemistry. 2002;34:139–162. [Google Scholar]
- 104.Wu X, Liu Z, Li M, Bartlam M, Wang Y. Integrated metagenomic and metatranscriptomic analysis reveals actively expressed antibiotic resistomes in the plastisphere. Journal of Hazardous Materials. 2022;430:128418. doi: 10.1016/j.jhazmat.2022.128418. [DOI] [PubMed] [Google Scholar]
- 105.Wright RJ, Bosch R, Gibson MI, Christie-Oleza JA. Plasticizer degradation by marine bacterial isolates: A proteogenomic and metabolomic Characterizationc. Environ Sci Technol. 2020;54:2244–2256. doi: 10.1021/acs.est.9b05228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li R, Zhu L, Cui L, Zhu Y-G. Viral diversity and potential environmental risk in microplastic at watershed scale: Evidence from metagenomic analysis of plastisphere. Environment International. 2022;161:107146. doi: 10.1016/j.envint.2022.107146. [DOI] [PubMed] [Google Scholar]
- 107.Yang K, et al. Temporal dynamics of antibiotic resistome in the plastisphere during microbial colonization. Environ Sci Technol. 2020;54:11322–11332. doi: 10.1021/acs.est.0c04292. [DOI] [PubMed] [Google Scholar]
- 108.Neumann G, George TS, Plassard C. Strategies and methods for studying the rhizosphere—the plant science toolbox. Plant Soil. 2009;321:431–456. [Google Scholar]
- 109.Downie HF, et al. Challenges and opportunities for quantifying roots and rhizosphere interactions through imaging and image analysis. Plant, Cell Environment. 2015;38:1213–1232. doi: 10.1111/pce.12448. [DOI] [PubMed] [Google Scholar]
- 110.Lee J, et al. Label-free multiphoton imaging of microbes in root, mineral, and soil matrices with time-gated coherent Raman and fluorescence lifetime imaging. Environ Sci Technol. 2022;56:1994–2008. doi: 10.1021/acs.est.1c05818. [DOI] [PubMed] [Google Scholar]