Abstract
Benzodiazepine site agonists or inverse agonists enhance or reduce γ-aminobutyric acidA (GABAA) receptor-mediated inhibition of neurons, respectively. Recently, it was demonstrated that the point mutation γ2F77I causes a drastic change in the affinity of a variety of benzodiazepine agonists or inverse agonists in receptor binding studies. Here we investigated the potency and efficacy of 10 benzodiazepine site ligands from 6 structural classes in wild-type and γ2F77I point mutated recombinant GABAA receptors composed of α1β3γ2, α2β3γ2, α3β3γ2, α4β3γ2, α5β3γ2, and α6β3γ2 subunits. Results indicate that the effects of the benzodiazepine site ligands zolpidem, zopiclone, Cl218872, L-655,708 and DMCM were nearly completely eliminated in all mutated receptors up to a 1 μM concentration. The effects of bretazenil, Ro15-1788 or abecarnil were eliminated in some, but not all mutated receptors, suggesting that the γ2F77I mutation differentially influences the actions of these ligands in different receptor subtypes. In addition, this point mutation also influences the efficacy of diazepam for enhancing GABA-induced chloride flux, suggesting that the amino acid residue γ2F77 might also be involved in the transduction of the effect of benzodiazepines from binding to gating. The application of these drugs in a novel mouse model is discussed.
Keywords: GABAA receptors, Benzodiazepine site ligands, Recombinant receptors, Potency, Efficacy, γ2F77I mutation
1. Introduction
GABAA receptors are chloride ion channels that can be opened by GABA. These receptors are composed of five subunits that can belong to different subunit classes. 19 different subunits (6α, 3β, 3γ, δ, ε, π, θ, 3ρ) have been identified and could give rise to a large variety of different GABAA receptor subtypes with distinct subunit composition. The majority of GABAA receptors found in the brain, however, are composed of 1γ and 2α and 2β subunits (Olsen and Sieghart, 2008).
GABAA receptors can be modulated by a large variety of pharmacologically and clinically important drugs, such as benzodiazepines, barbiturates, neuroactive steroids, anesthetics and convulsants. A variety of evidence indicates that these compounds exert their action via distinct allosteric binding sites on these receptors (Sieghart, 1995). The benzodiazepine binding site of GABAA receptors so far has been most thoroughly investigated. It is located in the extracellular domain of GABAA receptors at the interface formed by α and γ subunits (Ernst et al., 2003; Sigel and Buhr, 1997). The currently prescribed benzodiazepines and most of the structurally unrelated compounds interacting with the benzodiazepine binding site of GABAA receptors mediate their effects predominantly by interacting with GABAA receptors composed of α1βγ2, α2βγ2, α3βγ2 or α5βγ2 subunits (Sieghart, 1995).
The point mutation γ2F77I has been demonstrated previously to drastically reduce the affinity of some but not all benzodiazepine site ligands for the mutated receptors (Buhr et al., 1997; Ogris et al., 2004; Wingrove et al., 1997). This led to its application in the recently developed “loxγ2F77I-swap mouse model” (Wulff et al., 2007). This model uses the strategy to first eliminate the interaction of certain drugs with GABAA receptors all over the brain using transgenic mice containing the point mutation γ2F77I in the γ2 subunit gene (Cope et al., 2005, 2004) and then to replace the γ2F77I subunit by the wild-type γ2 subunit in specific neurons, only (Wulff et al., 2007). By a systemic application of benzodiazepine site ligands that cannot interact with the point mutated GABAA receptors only the re-introduced wild-type receptors are modulated, allowing the function of the respective neurons in the brain to be investigated.
In a previous study we identified several benzodiazepine site ligands of different structural classes that exhibit a drastic reduction in their affinity for GABAA receptors containing the point mutation γ2F77I (Ogris et al., 2004). The effect of this mutation on the potency and efficacy of most of these compounds in different GABAA receptor subtypes so far is not known. Here we investigated the effect of this point mutation on the action of ligands that showed the strongest reduction in the affinity for the mutated receptors in various recombinant GABAA receptors. Results indicate that potency and efficacy of these compounds is distinct for each receptor subtype and that the point mutation γ2F77I more or less completely eliminates the action of some of these compounds over a wide concentration range.
2. Materials and methods
2.1. Chemicals
Compounds were obtained from the following sources: diazepam (7-chloro-1,3-dihydro-1-methyl-5-phenyl-2H-1,4-benzodiazepin-2-one), Ro15-1788 (ethyl-8-fluoro-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylate), bretazenil (t-butyl(s)-8-bromo-11,12,13,13a-tetrahydro-9-oxo-9H-imidazo[1,5-a] pyrrolo[2,1-c][1,4]benzodiazepine-1-carboxylate) (Hoffmann La Roche, Basle, Switzerland); L-655,708 (ethyl-7-methoxy-11,12,13,13a-tetrahydro-9-oxo-9H-imidazo[1,5-a]pyrrolo[2,1-c][1,4] benzodiazepine-1-carboxylate) was purchased from Tocris Cookson Ltd. UK; methyl-6,7-dimethoxy-4-ethyl-β-carboline-3-carboxylate (DMCM), (Ferrosan, Soeborg, Denmark); zopiclone (4-methyl-1-piperazinecarboxylic acid-6-(5-chloro-2-pyridinyl)-6,7-dihydro-7-oxo-5H-pyrrolo[3,4-b]pyrazin-5-yl ester) (Rhone-Poulenc, Paris, France); FG7142 (N-methyl-β-carboline-3-carboxamide) was purchased from Tocris bioscience UK; abecarnil (Isopropyl-6-benzyloxy-4-methoxymethyl-β-carboline-3-carboxylate) was a gift of Dr. Schneider, Schering AG, Germany; Cl218872 (3-methyl-6-[3-trifluoromethyl-phenyl]-1,2,4-triazolo[4,3-b]pyridazine) (American Cyana-mide Comp., Wayne, N.J., U.S.A.); zolpidem (N,N,6-trimethyl-2-(4-methylphenyl)imidazo[1,2-a]-pyridine-3-acetamide) (Synthelabo Recherche, Bagneux, France).
2.2. Two-electrode voltage clamp
cDNAs of rat GABAA receptor subunits α1, α4, β3, and γ2S were cloned as described (Ebert et al., 1996). cDNAs of the rat subunits α2, α3, and α5 were gifts from P. Malherbe and that of α6 subunits was a gift from P. Seeburg. After linearizing the cDNA vectors with appropriate restriction endonucleases, capped transcripts were produced using the mMESSAGE mMACHINE® T7 transcription kit (Ambion, TX). The capped transcripts were polyadenylated using yeast poly(A) polymerase (USB, OH) and were diluted and stored in diethylpyrocarbonate-treated water at −70 °C.
The methods used for isolating, culturing, injecting and defolliculating of oocytes were identical with those described by Sigel et al., (1990) and were described elsewhere (Li et al., 2003). Mature female Xenopus laevis (Nasco, WI) were anesthetized in a bath of ice-cold 0.17% Tricain (Ethyl-m-aminobenzoat, Sigma, MO) before decapitation and removal of the frogs' ovary. Stage 5 to 6 oocytes with the follicle cell layer around them were singled out of the ovary using a platinum wire loop. Oocytes were stored and incubated at 18 °C in modified Barths' Medium (88 mM NaCl, 10 mM HEPES–NaOH (pH 7.4), 2.4 mM NaHCO3, 1 mM KCl, 0.82 mM MgSO4, 0.41 mM CaCl2, 0.34 mM Ca(NO3)2) that was supplemented with 100 U/ml penicillin and 100 µg/ml streptomycin. Oocytes with follicle cell layers still around them were injected with an aqueous solution of cRNA. A total of 2.5 ng of cRNA per oocyte was injected. Subunit ratio was 1:1:5 for αxβ3γ2. After injection of cRNA, oocytes were incubated for at least 36 h before the enveloping follicle cell layers were removed. Collagenase-treatment (type IA, Sigma, MO) and mechanically defolliculating of the oocytes was described elsewhere (Li et al., 2003).
For electrophysiological recordings, oocytes were placed on a nylon-grid in a bath of Xenopus Ringer solution (XR solution, containing 90 mM NaCl, 5 mM HEPES–NaOH (pH 7.4), 1 mM MgCl2, 1 mM KCl and 1 mM CaCl2). The oocytes were constantly washed by a flow of 6 ml/min XR solution which could be switched to XR solution containing GABA and/or drugs. For current measurements the oocytes were impaled with two microelectrodes (2–3 MΩ) which were filled with 2 M KCl. Maximum currents measured in cRNA injected oocytes were in the microampere range for all subtypes of GABAA receptors.
Drugs were diluted into XR solution from DMSO-solutions resulting in a final concentration of 0.1% DMSO perfusing the oocytes. Drugs were pre-applied for 30 s before the addition of GABA, which was then co-applied with the drugs until a peak response was observed. Between two applications, oocytes were washed in XR solution for up to 15 min to ensure full recovery from desensitization. To test for modulation of GABA-induced currents by drugs a concentration of GABA that was titrated to trigger 3% of the respective maximum GABA-elicited current of the individual oocyte (EC3) was applied to the cell together with various concentrations of drugs. Such a low GABA concentration corresponds with that occurring at extrasynaptic receptors, that represent the majority of GABAA receptors in the brain (Farrant and Nusser, 2005) and results in a situation where probably only one of the two GABA binding sites of the receptors is occupied (Walters et al., 2000). In addition, this low GABA concentration is in the flat part of the dose–response curve, and thus, the data are not as much dependent on slight variations in the GABA concentration. At this GABA concentration benzodiazepine site agonists are producing stronger effects, whereas inverse agonists sometimes (but not always) are showing weaker effects compared to higher GABA concentrations. To use comparable conditions for positive and negative allosteric modulators, we decided to perform all measurements at GABA EC3. All recordings were performed at room temperature at a holding potential of −60 mV using a Warner OC-725C two-electrode voltage clamp (Warner Instruments, Hamden, CT) or a Dagan CA-1B Oocyte Clamp (Dagan Corporation, Minneapolis, MN). Data were digitized, recorded and measured using a Digidata 1322A data acquisition system (Axon Instruments, Union City, CA).
3. Results
3.1. Potency and efficacy of benzodiazepine site ligands for various GABAA receptor subtypes
To determine the effect of the γ2F77I point mutation on the potency and efficacy of GABAA receptor subtypes, it was necessary to compare these parameters with the respective wild-type receptors. On screening the available literature concerning the action of benzodiazepine site ligands at different receptor subtypes it is evident that complete dose–response curves for α1–6βγ2 receptors only rarely have been published. In many cases only the maximum efficacy of a drug at only a few receptor subtypes has been reported although recently it became clear that the spectrum of in vivo actions of a compound depends on its relative potency and efficacy at the various receptor subtypes especially at low drug concentrations (Rivas et al., 2009; Savic et al., 2008). In addition, the few data available have been generated in different heterologous expression systems (Xenopus oocytes, HEK cells, mouse fibroblast L(tk−) cells, etc.), using two-electrode voltage clamp or patch clamp techniques, using GABAA receptor subunits from different species (rat, mouse, human) and sometimes even using a mixture of subunits from different species (Petroski et al., 2006). Furthermore, different β subunits were used in combination with α and γ2 subunits, the buffer solution, perfusion velocity, electrophysiological conditions (voltage clamped between −60 and −80 mV) and the concentration of GABA applied in these experiments differed in different publications (EC3–EC50) as did the experimental protocol (rapidity and duration of GABA or drug application, washing conditions in between measurements). The data, thus, in most cases cannot be directly compared (Hevers and Luddens, 1998; Olsen and Sieghart, 2008). To investigate possible changes in potency and efficacy of benzodiazepine site ligands induced by the γ2F77I point mutation, we thus also had to investigate the effects of these ligands on the wild-type receptors.
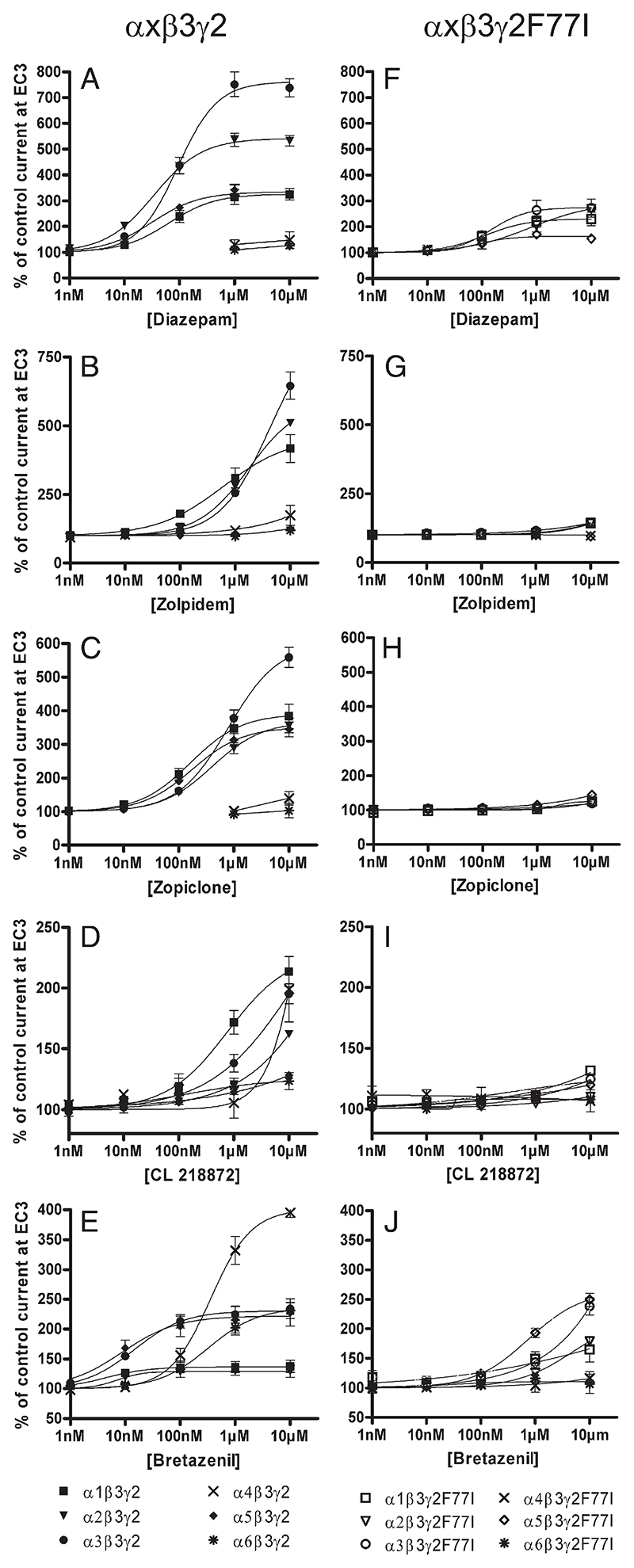
Here we used the two-electrode voltage clamp method to determine the complete dose–response curves of 10 different benzodiazepines site ligands from 6 different structural classes in recombinant GABAA receptors expressed in Xenopus oocytes and composed of α1β3γ2, α2β3γ2, α3β3γ2, α4β3γ2, α5β3γ2, or α6β3γ2 subunits. None of the compounds investigated (see structural formula in Fig. 1) was able to elicit a chloride current in the absence of GABA in the concentration range investigated, but all of them were able to modulate GABA-induced chloride flux. As indicated in Figs. 2A and 3A, diazepam dose-dependently enhanced currents elicited by a GABA concentration generating 3% of the maximum GABA current of the α1β3γ2, α2β3γ2, α3β3γ2, or α5β3γ2 receptor (GABA EC3) with a potency (EC50) of 63 ± 11 nM, 34 ± 2 nM, 93 ± 7 nM or 32 ± 4 nM, respectively (Table 1). Under our conditions, diazepam exhibited its highest efficacy for α3βγ2 receptors. At this receptor subtype GABA EC3 control current (100%) was stimulated up to 738 ± 36% by diazepam. GABA EC3 currents of α2β3γ2 receptors were stimulated to 532 ± 20% and those of α1β3γ2 or α5β3γ2 receptors were stimulated to 324 ± 22% or 321 ± 14%, respectively. Diazepam did not stimulate GABA-induced chloride flux in α4β3γ2 or in α6β3γ2 receptors. These data are consistent with results published previously (Dawson et al., 2006; Puia et al., 1991; Smith et al., 2001).
Fig. 1. Structures of compounds used in this study.
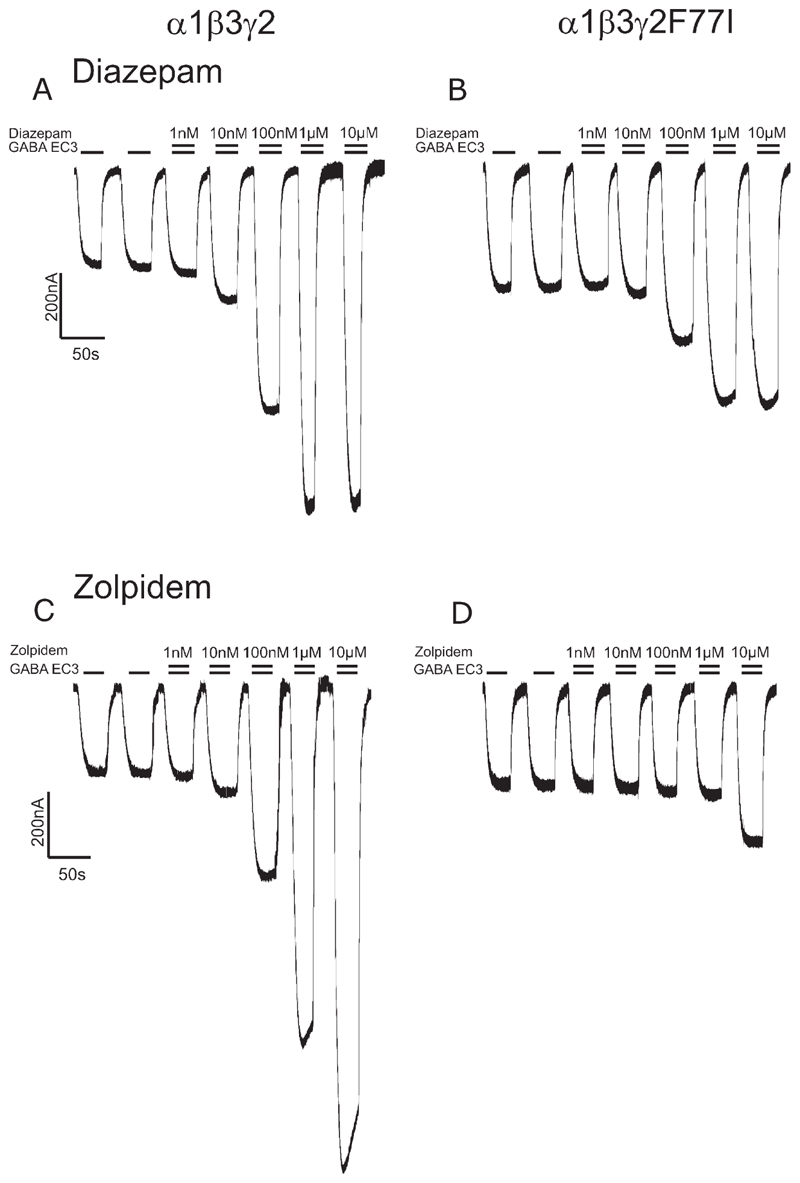
Fig. 2.
Modulation of GABA EC3-currents in recombinant α1β3γ2 (A,C) and α1β3γ2F77I (B,D) receptors by (A,B) diazepam and (C,D) zolpidem. The higher GABA EC3 currents in B and D reflect a stronger expression of α1β3γ2F77 receptors.
Fig. 3.
Concentration–effect curves for A) and F) diazepam, B) and G) zolpidem, C) and H) zopiclone, D) and I) CL 218872 and E) and J) bretazenil on α1β3γ2 (■), α2β3γ2(▾), α3β3γ2(●), α4β3γ2(X), α5β3γ2(♦), α6β3γ2(*), α1β3γ2F77I(□), α2β3γ2F77I(▽), α3β3γ2F77I(○), α4β3γ2F77I(X), α5β3γ2F77I(◊) and α6β3γ2F77I(*) receptors. Data are normalized to a control GABA current at EC3. Data points represent means ± SEM from at least 3 oocytes derived from ≥ 2 batches.
Table 1.
Potency (EC50) and efficacy (% GABA EC3) of various benzodiazepine site ligands for recombinant rat αxβ3γ2 or αxβ3γ2F77I receptors.
| α1β3γ2 | α1β3γ2F77I | α2β3γ2 | α2β3γ2F77I | α3β3γ2 | α3β3γ2F77I | α4β3γ2 | α4β3γ2F77I | α5β3γ2 | α5β3γ2F77I | α6β3γ2 | α6β3γ2F77I | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EC50 [nM] | 63 ± 11 | 110 ± 11 | 34 ± 2 | 120 ± 8 | 93 ± 7 | 143 ± 31 | n.d. | n.d. | 32 ± 4 | 78 ± 20 | n.d. | n.d. | |
| Diazepam | 100 nM | 239 ± 23 | 162 ± 17 | 426 ± 22 | 181 ± 1 | 437 ± 32 | 166 ± 17 | n.d. | n.d. | 274 ± 16 | 135 ± 7 | n.d. | n.d. |
| 1 μM | 314 ± 29 | 220 ± 21 | 536 ± 26 | 265 ± 4 | 752 ± 48 | 263 ± 39 | ns | n.d. | 342 ± 21 | 170 ± 4 | ns | n.d. | |
| 10 μM | 324 ± 22 | 229 ± 26 | 532 ± 20 | 278 ± 6 | 738 ± 36 | 272 ± 36 | ns | n.d. | 321 ± 14 | 154 ± 2 | ns | n.d. | |
| EC50 [nM] | n.d | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Zolpidem | 100 nM | 180 ± 14 | ns | 132 ± 4 | ns | 121 ± 3 | ns | ns | n.d. | ns | ns | n.d. | n.d. |
| 1 μM | 310 ± 36 | ns | 280 ± 19 | ns | 255 ± 14 | ns | ns | n.d. | ns | ns | n.d. | n.d. | |
| 10 μM | 417 ± 51 | 142 ± 8 | 511 ± 9 | 145 ± 3 | 645 ± 50 | 145 ± 5 | ns | n.d. | ns | n.d. | ns | n.d. | |
| EC50 [nM] | 163 ± 19 | n.d. | 400 ± 64 | n.d. | >793 | n.d. | n.d. | n.d. | 176 ± 1 | n.d. | n.d. | n.d. | |
| Zopiclone | 100 nM | 211 ± 17 | ns | 157 ± 9 | ns | 161 ± 10 | ns | n.d. | n.d. | 191 ± 8 | ns | n.d. | n.d. |
| 1 μM | 347 ± 35 | ns | 289 ± 16 | ns | 377 ± 24 | 107 ± 1 | ns | n.d. | 313 ± 18 | 114 ± 2 | ns | n.d. | |
| 10 μM | 383 ± 36 | 125 ± 3 | 356 ± 22 | 121 ± 2 | 559 ± 30 | 119 ± 4 | ns | n.d. | 345 ± 22 | 144 ± 1 | ns | n.d. | |
| EC50 [nM] | >776 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| CL 218872 | 100 nM | 119 ± 7 | ns | ns | ns | 114 ± 2 | ns | ns | n.d. | ns | ns | ns | n.d. |
| 1 μM | 172 ± 10 | ns | ns | ns | 138 ± 7 | ns | ns | n.d. | 114 ± 1 | ns | ns | n.d. | |
| 10 μM | 214 ± 13 | 132 ± 4 | 162 ± 3 | ns | 195 ± 8 | 125 ± 3 | 199 ± 27 | n.d. | 128 ± 2 | 120 ± 3 | ns | n.d. | |
| EC50 [nM] | 4 ± 1 | n.d. | 7 ± 1 | n.d. | 15 ± 2 | n.d. | >354 | n.d. | 9 ± 2 | >748 | >322 | n.d. | |
| Bretazenil | 10 nM | 126 ± 5 | ns | 119 ± 4 | ns | 154 ± 6 | ns | ns | ns | 168 ± 13 | ns | ns | ns |
| 100 nM | 134 ± 7 | 120 ± 6 | 128 ± 5 | 104 ± l | 213 ± 11 | 117 ± 3 | 156 ± 11 | ns | 205 ± 17 | 124 ± 3 | ns | ns | |
| 1 μM | 136 ± 9 | 149 ± 12 | 130 ± 7 | 125 ± 3 | 224 ± 14 | 143 ± 7 | 332 ± 23 | ns | 216 ± 22 | 193 ± 8 | 202 ± 12 | ns | |
| 10 μM | 138 ± 10 | 164 ± 21 | 129 ± 10 | 179 ± 8 | 234 ± 17 | 238 ± 15 | 395 ± 8 | ns | 228 ± 23 | 249 ± 11 | 231 ± 13 | ns | |
| EC50 [nM] | n.d. | n.d. | 5 ± 1 | n.d. | 11 ± 5 | n.d. | >232 | n.d. | n.d. | n.d. | >204 | n.d. | |
| Ro15–1788 | 10 nM | ns | ns | 121 ± 2 | ns | 128 ± 4 | ns | ns | ns | ns | ns | ns | ns |
| 100 nM | ns | ns | 128 ± 4 | ns | 148 ± 7 | 106 ± 1 | 132 ± 8 | ns | ns | ns | 128 ± 18 | ns | |
| 1 μM | ns | ns | 130 ± 5 | ns | 156 ± 8 | 118 ± 2 | 162 ± 19 | ns | ns | ns | 166 ± 9 | ns | |
| 10 μM | ns | ns | 133 ± 7 | ns | 157 ± 8 | 139 ± 4 | 181 ± 10 | ns | ns | ns | 179 ± 6 | ns | |
| EC50 [nM] | n.d. | n.d. | n.d. | n.d. | 10 ± 3 | n.d. | 168 ± 71 | n.d. | n.d. | n.d. | >470 | n.d. | |
| L-655,708 | 10 nM | ns | ns | ns | ns | 113 ± 1 | ns | ns | ns | ns | ns | 118 ± 4 | ns |
| 100 nM | ns | ns | ns | ns | 126 ± 1 | ns | 143 ± 12 | ns | 91 ± 1 | ns | 131 ± 12 | ns | |
| 1 μM | ns | ns | 93 ± 1 | ns | 131 ± 4 | ns | 215 ± 4 | ns | 84 ± 5 | ns | 176 ± 25 | ns | |
| 10 μM | 90 ± 4 | ns | 88 ± 1 | ns | 126 ± 4 | ns | 224 ± 15 | ns | 66 ± 7 | ns | 199 ± 31 | ns | |
| EC50 [nM] | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| DMCM | 10 nM | 87 ± 8 | ns | 71 ± 7 | 103 ± 1 | 78 ± 1 | ns | ns | n.d. | 53 ± 4 | ns | ns | n.d. |
| 100 nM | 79 ± 11 | ns | 54 ± 5 | 111 ± 2 | 69 ± 1 | ns | 77 ± 1 | n.d. | 52 ± 5 | ns | 118 ± 9 | n.d. | |
| 1 μM | ns | 123 ± 3 | 54 ± 3 | 123 ± 1 | 75 ± 3 | 126 ± 5 | 88 ± 2 | n.d. | 56 ± 6 | ns | 189 ± 21 | n.d. | |
| 10 μM | 232 ± 54 | 269 ± 3 | ns | 283 ± 17 | 185 ± 15 | 281 ± 26 | 155 ± 18 | 155 ± 9 | 145 ± 14 | 211 ± 40 | 353 ± 22 | n.d. | |
| EC50 [nM] | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| FG7142 | 100 nM | ns | 107 ± 1 | ns | ns | ns | ns | ns | n.d. | ns | 111 ± 2 | ns | n.d. |
| 1 μM | 88 ± 5 | 111 ± 1 | ns | ns | 95 ± 2 | 115 ± 5 | ns | n.d. | ns | 112 ± 4 | ns | n.d. | |
| 10 μM | 92 ± 4 | 124 ± 3 | ns | 117 ± 4 | 91 ± 2 | 126 ± 6 | ns | n.d. | 84 ± 5 | 122 ± 4 | ns | n.d. | |
| EC50 [μM] | >9 | n.d. | >1 | n.d. | >2 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Abecarnil | 10 nM | 192 ± 31 | ns | ns | ns | ns | ns | n.d. | n.d. | ns | ns | n.d. | n.d. |
| 100 nM | 330 ± 58 | ns | ns | ns | 216 ± 44 | ns | n.d. | n.d. | ns | ns | n.d. | n.d. | |
| 1 μM | 407 ± 83 | 179 ± 25 | 261 ± 45 | 206 ± 16 | 320 ± 71 | 192 ± 4 | ns | n.d. | 169 ± 23 | 177 ± 11 | ns | n.d. | |
| 10 μM | 640 ± 157 | 279 ± 42 | 353 ± 62 | 420 ± 29 | 480 ± 100 | 384 ± 24 | 279 ± 81 | n.d. | 232 ± 38 | 283 ± 7 | 218 ± 13 | n.d. |
All efficacy values given in the table are significantly different from GABA EC3. Significance is at least P<0.05, calculated by a Student’s t-test. (ns) not significant, (n.d.) not determined.
In agreement with previous results investigating receptors containing β2 subunits (Baur and Sigel, 2007; Petroski et al., 2006; Sanna et al., 2002) the imidazopyridine zolpidem exhibited the highest potency for receptors containing α1 subunits (Fig. 3B). An approximately 5–8-fold higher zolpidem concentration is needed to generate a comparable enhancement of GABA-induced chloride flux in α2β3γ2 or α3β3γ2 receptors. Due to the relatively low potency of zolpidem, no saturating stimulation could be reached up to 10 μM (Fig. 2B), and thus, correct EC50 values cannot be given. At 1 or 10 μM concentrations, however, zolpidem stimulated GABA-induced chloride flux to 310 ± 36% or 417 ± 51%, 280 ± 19% or 511 ± 9%, and 255 ± 14% or 645 ± 50% in α1β3γ2, α2β3γ2, or α3β3γ2 receptors, respectively (Table 1). At these concentrations, therefore, due to its higher efficacy for α2β3γ2 or α3β3γ2 receptors, zolpidem has lost its α1 subtype selectivity. As expected, zolpidem, a compound that exhibits a very low affinity for α5β3γ2 receptors (Sieghart, 1995), was unable to modulate these receptors up to a concentration of 10 μM (Baur and Sigel, 2007; Petroski et al., 2006; Sanna et al., 2002). Zolpidem also did not significantly enhance GABA-induced chloride flux in α4β3γ2 and α6β3γ2 receptors.
The cyclopyrrolone zopiclone dose-dependently enhanced GABA EC3 in receptors composed of α1β3γ2, α2β3γ2, α3β3γ2 or α5β3γ2 subunits with an EC50 of 163 ± 19 nM, 400 ± 64 nM, > 793 nM, or 176 ± 1 nM and a maximal stimulation to 383 ± 36%, 356 ± 22%, 559 ± 30% or 345 ± 22% of control current, respectively (Fig. 3C, Table 1). Zopiclone did not significantly stimulate GABA-induced chloride flux in α4β3γ2 and α6β3γ2 receptors up to a 10 μM concentration. These data confirm and extend previous results (Fleck, 2002; Petroski et al., 2006) and again indicate that EC50 values cannot be used to predict a differential action of drugs on different receptor subtypes.
The triazolopyridazine Cl218872 dose-dependently enhanced GABA EC3 in α1β3γ2, and with an approximately 3-fold reduced potency in α3β3γ2 receptors (Fig. 3D). The potency for enhancing GABA current in α2β3γ2 was further reduced about 3-fold, whereas only very weak stimulation (up to 128 ± 2%) of GABA current was obtained for α5β3γ2 receptors. At 1 μM concentration this compound seemed to exhibit no effect at α4β3γ2 receptors, whereas at 10 μM concentration it stimulated GABA-induced chloride flux. However, no significant stimulation was obtained for α6β3γ2 receptors up to a 10 μM concentration. Due to the low potency of this compound, no EC50 values can be given, but 10 μM Cl218872 stimulated GABA-induced chloride current in α1β3γ2, α2β3γ2, α3β3γ2, α4β3γ2 or α5β3γ2 receptors to 214 ± 13%, 162 ± 3%, 195 ± 8%, 199 ± 27%, or 128 ± 2%, respectively (Table 1). These data confirm and extend previous results (Wafford et al., 1993a,b).
In this study we investigated three different imidazobenzodiazepines. The imidazobenzodiazepine bretazenil (Fig. 3E) in agreement with previous results (Atack, 2003; Knoflach et al., 1996; Puia et al., 1992) weakly stimulated GABA-induced chloride flux with EC50's of 4 ± 1 nM, 7 ± 1 nM, 15 ± 2 nM, or 9 ± 2 nM and a maximal stimulation of 138 ± 10%, 129 ± 10, 234 ± 17% or 228 ± 23%, for α1β3γ2, α2β3γ2, α3β3γ2 or α5β3γ2 receptors, respectively (Table 1). This compound thus preferentially activates α3β3γ2 and α5β3γ2 receptors. Interestingly, bretazenil exhibited a lower potency (EC50 of > 354 nM) but a much stronger efficacy (stimulation to 395 ± 8%) at α4β3γ2 receptors. For α6β3γ2 receptors, EC50 and maximal stimulation of this compound was > 322 nM and 231 ± 13%, respectively (Knoflach et al., 1996).
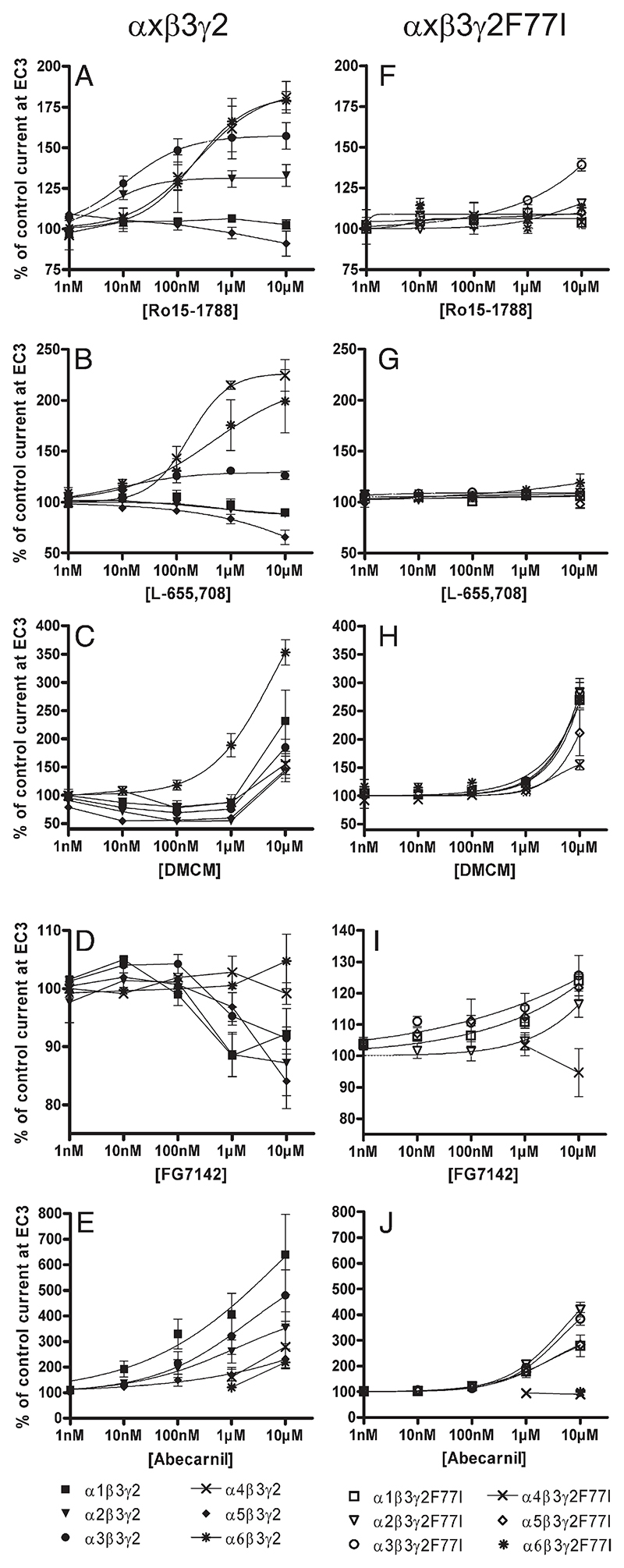
The imidazobenzodiazepine Ro15-1788 (flumazenil) exhibited no significant effects on GABA-induced chloride currents in α1β3γ2 and α5β3γ2 receptors (Fig. 4A), but weakly stimulated GABA EC3 in α2β3γ2 and α3β3γ2 receptors with an EC50 of 5 ± 1 nM and 11 ± 5 nM and a maximal stimulation of GABA-induced chloride flux to 133 ± 7% and 157 ± 8%, respectively. This compound, however, stimulated GABA-induced chloride flux in α4β3γ2 receptors with an EC50 of > 232 nM up to 181 ± 10% and in α6β3γ2 with an EC50 of > 204 nM up to 179 ± 6% (Table 1). These data confirm and extend previous results (Hadingham et al., 1996; June et al., 2003; Whittemore et al., 1996).
Fig. 4.
Concentration–effect curves for A) and F) Ro15-1788, B) and G) L-655,708, C) and H) DMCM, D) and I) FG7142 and E) and J) abecarnil on α1β3γ2 (■), α2β3γ2(▾), α3β3γ2(•), α4β3γ2(X), α5β3γ2(♦), α6β3γ2(*), α1β3γ2F77I(□), α2β3γ2F77I(▽), α3β3γ2F77I(○), α4β3γ2F77I(X), α5β3γ2F77I(◊) and α6β3γ2F77I(*) receptors. Data are normalized to a control GABA current at EC3. Data points represent means ± SEM from at least 3 oocytes derived from ≥ 2 batches.
The imidazobenzodiazepine L-655,708 (Fig. 4B) in receptor binding assays exhibits a 30–50-fold selectivity for α5 receptors (Atack et al., 2006; Quirk et al., 1996). In electrophysiological studies it behaved as an inverse agonist at α5β3γ2 receptors (reduction of chloride current to 84 ± 5 or 66 ± 7% at 1 μM or 10 μM concentration, respectively; Table 1), and as a low potency weak partial inverse agonist at α1β3γ2 and α2β3γ2 receptors (reduction of GABA-induced chloride flux to 90% at 10 μM concentration). This compound for these receptors thus exhibited properties comparable to those published previously (Atack et al., 2006). The slightly smaller efficacy as well as the smaller potency observed for α5β3γ2 receptors in our study probably were due to the fact that our two-electrode voltage clamp measurements, in contrast to the patch clamp measurements used previously, could not reliably resolve current changes below 10%. In our hands, however, L-655,708 was a highly potent but very weak partial agonist at α3β3γ2 receptors (EC50 10 ± 3 nM, stimulation to 126 ± 4% of control current), in contrast to previous data (Atack et al., 2006) where this compound exhibited a very weak inverse agonist activity (reduction of GABA-induced chloride flux to 90% at 10 μM concentration). Different experimental conditions might have contributed to this discrepancy. Interestingly, there is also a clear and previously noted (Atack et al., 2006) discrepancy between the affinities of this compound in receptor binding assays and its potencies in electrophysiological studies. This is a frequently observed phenomenon probably due to different experimental conditions between binding assays and electrophysiological measurements. Thus, a different measuring temperature, equilibrium binding conditions vs. acute effects on receptors, affinity for the benzodiazepine binding site vs. measuring multiple effects of the drug possibly caused by additional interactions with other sites could have contributed to this discrepancy. The latter conclusion is supported by the very flat dose–response curve of this compound (Fig. 4B, Atack et al., 2006) suggesting interaction with several binding sites. Finally, in extension of previous results we here demonstrated that this compound enhanced GABA-induced chloride flux in α4β3γ2 and α6β3γ2 receptors up to 224 ± 15% and 199 ± 31% with EC50's of 168 ± 71 nM and >470 nM, respectively.
We also investigated three different β-carbolines. The β-carboline inverse agonist DMCM exhibited a biphasic effect (Fig. 4C). At concentrations up to 1 μM it reduced GABA-induced chloride flux in α1β3γ2, α2β3γ2, α3β3γ2, α4β3γ2, or α5β3γ2 receptors (maximal inhibition to 79 ± 11%, 54 ± 3%, 69 ± 1%, 88 ± 2%, or 52 ± 5%, respectively, Table 1) (Dawson et al., 2006). At 10 μM, however, it stimulated GABA-induced chloride flux in these receptors (Dawson et al., 2006; Whittemore et al., 1996). In α6β3γ2 receptors, however, DMCM did not reduce GABA-induced currents but strongly enhanced the chloride flux to 189 ± 21% at 1 µM and to 353 ± 22% at 10 µM concentrations.
In contrast, the β-carboline inverse agonist FG7142 (Fig. 4D) inhibited GABA-induced chloride flux at 1 μM and 10 μM concentration (strongest effect: 88 ± 5%, 91 ± 2% or 84 ± 1% of control current, for α1β3γ2, α3β3γ2 or α5β3γ2 receptors, respectively; Table 1). Although there seemed also to be an inhibition at α2β3γ2 receptors, the reduction was not significant. This compound did not significantly influence α4β3γ2 or α6β3γ2 receptors. The stronger effects seen in previous reports probably were due to the different experimental conditions used (Dawson et al., 2006; Taylor et al., 1988).
Finally, the β-carboline abecarnil (Fig. 4E) dose-dependently stimulated GABA EC3 ineach receptor investigated. The rank order of stimulation was α1β3γ2>α3β3γ2>α2β3γ2>α4β3γ2>α5β3γ2>α6β3γ2 receptors achieving 640 ± 157%, 480 ± 100%, 353 ± 62%, 279 ± 81%, 232 ± 38% and 218 ± 13% of control currents at 10 μM concentrations, respectively (Table 1). The dose–response curves were flat, indicating an interaction with more than one binding site at GABAA receptors. To the best of our knowledge (Atack, 2003; Hadingham et al., 1996) no systematic investigation on the effects of this compound on various receptor subtypes seems to have been published.
3.2. The point mutation γ2F77I changes the potency of GABA to stimulate chloride flux in different receptor subtypes
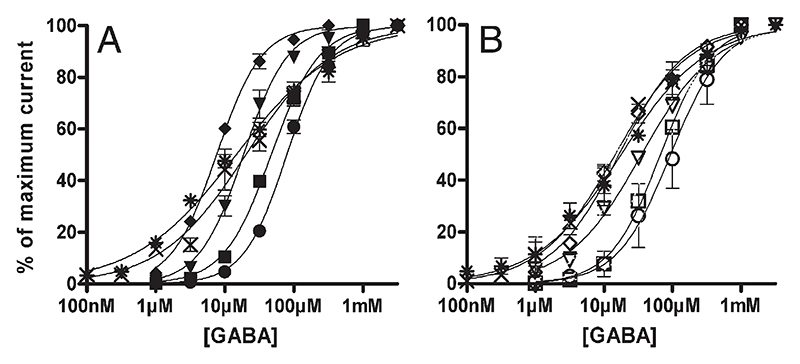
To investigate the influence of the γ2F77I mutation on the potency of GABA for enhancing chloride ion flux, recombinant receptors composed of α1, α2, α3, α4, α5, or α6, plus β3 subunits and either wt-γ2 or γ2F77I subunits were investigated for GABA-induced currents. As shown in Fig. 5, GABA dose-dependently stimulated chloride ion flux in all receptors investigated. GABA was most potent for stimulating chloride flux in receptors composed of α5β3γ2 (EC50 of 8 ± 0.4 μM) followed by receptors composed of α6β3γ2 (EC50 of 15 ± 2 µM), α2β3γ2 (EC50 of 19 ± 3 μM), α4β3γ2 (EC50 of 20 ± 5 µM), α1β3γ2 (EC50 of 47 ± 5 μM) and α3β3γ2 (EC50 of 79 ± 5 μM) (Fig. 5A).
Fig. 5.
GABA dose–response curves of α1β3γ2(■), α2β3γ2(▾), α3β3γ2(●), α4β3γ2(X), α5β3γ2(♦), α6β3γ2(*), receptors (Fig. 5A) and α1β3γ2F77I(□), α2β3γ2F77I(▽), α3β3γ2F77I(○), α4β3γ2F77I(X), α5β3γ2F77I(◊), and α6β3γ2F77I(*) receptors (Fig. 5B). Data are normalized to maximum GABA current. Data points represent means ± SEM from at least 3 oocytes derived from ≥ 2 batches. The maximum GABA-induced currents were 6 ± 0.4 µA (n = 43), 4 ± 0.4 µA (n = 21), 6 ± 0.7 µA (n =14), 1 ± 0.1 µA (n = 22), 8 ± 0.9 µA (n =14) and 1 ± 0.1 µA (n = 20) for α1β3γ2, α2β3γ2, α3β3γ2, α4β3γ2, α5β3γ2 and α6β3γ2 receptors and 5 ± 0.7 µA (n = 21), 7 ± 0.9 µA (n = 22), 3 ± 0.3 µA (n =19), 2 ± 0.2 µA (n =19), 7 ± 0.7 µA (n =18) and 1 ± 0.1 µA (n = 14) for α1β3γ2F77I, α2β3γ2F77I, α3β3γ2F77I, α4β3γ2F77I, α5β3γ2F77I and α6β3γ2F77I receptors (data represent means ± SEM, number of experiments is given in parenthesis). The EC50 values were 47 ± 5 µM, 19 ± 3 µM, 79 ± 5 µM, 20 ± 5 µM, 8 ± 0.4 µM and 15 ± 2 µM for α1β3γ2, α2β3γ2, α3β3γ2, α4β3γ2, α5β3γ2 and α6β3γ2 receptors and 67 ± 6 µM, 35 ± 3 µM, 94 ± 4 µM, 15 ± 2 µM, 17 ± 3 µM and 18 ± 6 µM for α1β3γ2F77I, α2β3γ2F77I, α3β3γ2F77I, α4β3γ2F77I, α5β3γ2F77I and α6β3γ2F77I receptors. The values for the Hill coefficient were 1.3, 1.4, 1.4, 0.7, 1.3 and 0.6 for α1β3γ2, α2β3γ2, α3β3γ2, α4β3γ2, α5β3γ2 and α6β3γ2 receptors and 1.2, 0.8, 1.1, 0.8, 0.9 and 0.7 for α1β3γ2F77I, α2β3γ2F77I, α3β3γ2F77I, α4β3γ2F77I, α5β3γ2F77I and α6β3γ2F77I receptors.
In the presence of the point mutations γ2F77I the EC50 of GABA was changed from 8 ± 0.4 μM to 17 ± 3 μM, from 15 ± 2 μM to 18 ± 6 μM, from 19 ± 3 μM to 35 ± 3 μM, from 20 ± 5 μM to 15 ± 2 μM, from 47 ± 5 μM to 67 ± 6 μM, from 79 ± 5 μM to 94 ± 4 μM, for α5β3γ2F77I, α6β3γ2F77I, α2β3γ2F77I, α4β3γ2F77I, α1β3γ2F77I, or α3β3γ2F77I receptors, respectively (Fig. 5B).
Interestingly, the GABA dose–response curve for α4β3γ2, α6β3γ2, and α2β3γ2F77I, α4β3γ2F77I, α5β3γ2F77I and α6β3γ2F77I receptors were more flat than the other curves (see Hill coefficients in legend to Fig. 5). This possibly indicates that the γ2 subunit in α4β3γ2 or α6β3γ2 receptors, or the γ2F77I subunit in α2β3γ2F77I, α4β3γ2F77I, α5β3γ2F77I and α6β3γ2F77I receptors might have differentially influenced the two GABA binding sites of the respective receptors (Baumann et al., 2003; Baur and Sigel, 2005; Hadley and Amin, 2007). Presumably, the GABA site involving the α subunit neighbouring the γ2 or γ2F77I subunit is more strongly influenced by these subunits than the other GABA binding site with a more distant location to the γ subunit.
3.3. The point mutation γ2F77I eliminates the action of several benzodiazepine binding site ligands on different receptor subtypes over a wide concentration range
In GABAA receptors containing the γ2F77I mutation the potency of diazepam for stimulation of GABA-induced chloride flux was reduced (Fig. 3F). Thus, the EC50 of diazepam increased from 63 ± 11 nM to 110 ± 11 nM, from 34 ± 2 nM to 120 ± 8 nM, from 93 ± 7 nM to 143 ± 31 nM or from 32 ± 4 nM to 78 ± 20 nM for α1β3γ2, α2β3γ2, α3β3γ2 or α5β3γ2 receptors, respectively (Table 1). Similarly, the efficacy of diazepam for enhancing GABA EC3 was significantly reduced in receptors containing the γ2F77I mutation (Figs. 2B, 3F). It changed from 324 ± 22% to 229 ± 26%, from 532 ± 20% to 278 ± 6%, from 738 ± 36% to 272 ± 36% and from 321 ± 14% to 154 ± 2%, for α1β3γ2, α2β3γ2, α3β3γ2 or α5β3γ2 receptors, respectively (Table 1).
The effects of the γ2F77I mutation were even more extreme for zolpidem (Fig. 3G), zopiclone (Fig. 3H), Cl218872 (Fig. 3I), and the imidazobenzodiazepine L-655,708 (Fig. 4G). Here this point mutation completely eliminated the ability of these drugs to modulate GABA EC3 up to a concentration of 1 μM in all receptors investigated. At a 10 μM concentration, zolpidem, zopiclone and Cl218872 exhibited a quite weak 20–40% stimulation of GABA-induced chloride flux in some receptors. The effects of the two β-carboline inverse agonists DMCM (Fig. 4H) and FG7142 (Fig. 4I) on the various receptor subtypes were also drastically changed. The inverse agonist effect of DMCM was completely eliminated in all receptors investigated up to a concentration of 1 μM, whereas the strong agonist effect of DMCM above 1 μM was not much influenced in receptors containing the γ2F77I subunit. The partial inverse agonist effect of FG7142 was converted into a very weak partial agonist effect in the respective mutated receptors.
The effects of this point mutation on the action of the imidazobenzodiazepines bretazenil (Fig. 3J) and Ro15-1788 (Fig. 4F) and the β-carboline abecarnil (Fig. 4J) were more complex. Whereas these compounds were inactive in the mutated receptors up to a concentration of 100 nM, at higher concentrations these drugs could stimulate GABA EC3 in at least some of the receptor subtypes investigated.
4. Discussion
In the present study we for the first time provide dose–response curves under comparable conditions for 10 benzodiazepine site ligands from 6 different structural classes for modulation of GABA-induced chloride flux in recombinant α1β3γ2, α2β3γ2, α3β3γ2, α4β3γ2, α5β3γ2 and α6β3γ2 receptors. In addition, we evaluated the effect of the point mutation γ2F77I on the dose–response curves of these drugs in individual GABAA receptor subtypes.
4.1. Changes in GABA potency indicate the formation of receptors containing the γ2F77I subunit
Most of the receptors containing the γ2F77I subunit exhibited a reduced potency of GABA for activation of chloride currents as compared to their respective wild-type receptor. Since GABA is mediating its effect by binding to the two β + α-interfaces (Ernst et al., 2003) it is surprising that GABA potency is changed by a mutation in the γ subunit. But this effect is in line with the previous observation that the presence of a γ subunit reduced the potency of GABA for stimulating chloride flux in all receptor subtypes investigated (Baburin et al., 2008; Hadley and Amin, 2007; Whittemore et al., 1996; Ramerstorfer and Sieghart, unpublished). A change in the structure of the γ2 subunit thus might have further reduced the potency of GABA.
The lower potency of GABA at receptors containing the γ2F77I mutation as opposed to the higher potency of GABA in GABAA receptors containing no γ subunit, indicate that receptors containing the mutated γ2 subunit were actually formed under the experimental conditions used. This conclusion is supported by the finding that the maximal GABA-induced currents measured in α1–6β3γ2F77I injected Xenopus oocytes were in the range of the respective wild-type receptors and of GABA-induced currents published previously (see legend to Fig. 5) (Hadley and Amin, 2007; Whittemore et al., 1996) and were significantly larger than those measured in receptors composed of α and β subunits, only (Ramerstorfer and Sieghart, unpublished results). Furthermore, 10 μM of Zn2+ reduced GABA-induced chloride flux in α1β3 receptors to 5.5 ± 1%, whereas this concentration reduced GABA-induced chloride flux in α1β3γ2 or α1β3γ2F77I receptors to 70.3 ± 3% or 75.4 ± 3%, respectively (means ± SEM, n=4; experiments not shown). Changes in the efficacy of benzodiazepine site ligands in receptors containing this mutation thus cannot be explained by a reduced formation of receptors containing the γ2F77I subunit.
4.2. The point mutation γ2F77I reduces potency and efficacy of several benzodiazepine site ligands
The point mutation γ2F77I caused only a relatively small change in the potency of diazepam for stimulation of GABA-induced chloride flux. This is in agreement with receptor binding studies that indicate only a small change in the affinity of diazepam for these receptors (Ogris et al., 2004). The maximal stimulation of the GABA-induced chloride flux by diazepam, however, was drastically reduced in the mutated receptors, supporting the conclusion that the residue γ2F77 is at least as important for the transduction of the diazepam effect as for binding of this compound to the benzodiazepine binding site.
In contrast, the point mutation γ2F77I nearly completely eliminated the effect of zolpidem on each receptor investigated, as expected from the absence of electrophysiological and behavioural effects of this compound in mice containing the point mutation γ2F77I (Cope et al., 2005, 2004; Wulff et al., 2007).
As with zolpidem, the effects of the sedative–hypnotic compound zopiclone, the triazolopyridazine Cl218872, or the imidazobenzodiazepine L-655,708 were more or less completely eliminated in all mutated receptor subtypes investigated, in agreement with an approximately 300-fold, 100-fold, 900-fold and > 1000-fold shift in affinity of these compounds, respectively, for GABAA receptors of mice containing the point mutation γ2F77I (Ogris et al., 2004). The effects of some of these compounds at 10 μM concentration are probably too weak to be of importance in behavioural studies.
Similarly, the inverse agonist effect of DMCM was completely eliminated, whereas the agonistic effect at concentrations above 1 μM was not drastically changed in GABAA receptors containing the γ2F77I mutation. The very weak agonistic effect of DMCM at 1 μM and the stronger agonistic effect at 10 μM concentration explain the in vivo finding that DMCM did not produce convulsions but produced even modest anxiolytic effects in γ2F77I mice (Leppa et al., 2005). In GABAA receptors containing the γ2F77I mutation the inverse agonist effects of FG7142 were converted to very weak partial agonistic effects resulting in a maximum stimulation of the GABA-induced current to 126 ± 6%. In contrast, the effects of the imidazobenzodiazepines Ro15-1788 or bretazenil or of the β-carboline abecarnil were not completely eliminated in some receptors above a concentration of 1 μM, suggesting a differential effect of the γ2F77I mutation for these compounds in different receptor subtypes.
4.3. Use of benzodiazepine site ligands in the γ2F77I-swap mouse model
The point mutation γ2F77I, thus, completely eliminated the ability of zolpidem, zopiclone, Cl218872, or the imidazobenzodiazepine L-655,708, to enhance GABA EC3 up to a concentration of 1 μM in all receptors investigated. Similarly, the inverse agonistic effects of the β-carbolines DMCM and FG7142 were completely eliminated by this mutation. Although recombinant receptors from rat have been used in the present study, the benzodiazepine binding site of GABAA receptors seems to be highly conserved in different species as indicated by similar affinities and efficacies of various ligands in different species and different recombinant receptors (Atack et al., 2009; Sieghart et al., 1985). Although we cannot exclude differences in the efficacies of benzodiazepine site ligands in individual GABAA receptor subtypes in rat and mouse, this seems not very likely. These compounds therefore are prime candidates to be used in the loxγ2F77I-swap mouse model, in which the γ2F77I subunit is replaced by the EGFP-tagged wild-type γ2 subunit in certain neurons in specific brain regions, only (Wulff et al., 2007). These compounds will have lost their wild-type efficacy in the brain of the loxγ2F77I mice as has been demonstrated for zolpidem (Cope et al., 2005, 2004) and DMCM (Leppa et al., 2005) already, and will have restored this efficacy only in neurons in which the point mutated γ2 subunit was replaced by the EGFP-tagged wild-type γ2 subunit. Zopiclone is a strong agonist at α1β3γ2, α2β3γ2, α3β3γ2 and α5β3γ2 receptors, whereas DMCM is an inverse agonist at these receptors. Although these compounds are not subtype selective, they can be used to reduce or enhance the electrical activity of neurons in specific brain areas in our loxγ2F77I-swap mouse model, respectively, by modulating the main receptors involved in the actions of classical benzodiazepines. This will allow to study the function of these cells in various behavioural parameters.
Whereas zopiclone at 1 μM concentration is an equally strong agonist at α1β3γ2, α2β3γ2, α3β3γ2 and α5β3γ2 receptors, zolpidem is only an agonist at α1β3γ2, α2β3γ2, and α3β3γ2 receptors. These compounds do not modulate α4β3γ2 and α6β3γ2 receptors. Electrophysiological or behavioural effects that can be elicited by zopiclone but not by zolpidem will thus provide information on effects mediated via α5β3γ2 receptors.
The imidazobenzodiazepine Ro15-1788 (flumazenil) is an antagonist at α1β3γ2 and α5β3γ2 receptors, but is a weak partial agonist, at α2β3γ2, α3β3γ2, α4β3γ2, and α6β3γ2 receptors. So this compound is not a pure antagonist at all GABAA receptors, as widely assumed, explaining previous reports on some effects of this drug in animals and man (Nutt, 1983; Skerritt and Macdonald, 1983; Vellucci and Webster, 1983). At 100 nM concentrations, this compound will more or less exclusively stimulate the action of GABA at α2β3γ2, α3β3γ2 and α4β3γ2 receptors in the forebrain. Although flumazenil exhibits only a weak efficacy, any effect observed with this drug can thus be contributed to these receptor subtypes.
Although the receptor subtype-selectivity of the compounds investigated is limited, there is the hope that other compounds from the structural classes of imidazobenzodiazepines, imidazopyridines, β-carbolines, triazolopyridazines or cyclopyrrolones could be developed with a more receptor subtype-selective profile. These compounds then not only could be used to investigate the function of the respective receptor subtypes in the brain of wild-type mice, but also in individual cell types in our loxγ2F77I-swap mouse model. Such compounds also will have an interesting spectrum of action in man and can be developed for a possible clinical application.
Acknowledgement
Financial support by project S10203-B13 of the Austrian Science Fund is gratefully acknowledged.
References
- Atack JR. Anxioselective compounds acting at the GABA(A) receptor benzodiazepine binding site. Curr Drug Targets. 2003;2:213–232. doi: 10.2174/1568007033482841. [DOI] [PubMed] [Google Scholar]
- Atack JR, Bayley PJ, Seabrook GR, Wafford KA, McKernan RM, Dawson GR. L-655,708 enhances cognition in rats but is not proconvulsant at a dose selective for alpha5-containing GABAA receptors. Neuropharmacology. 2006;51:1023–1029. doi: 10.1016/j.neuropharm.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Atack JR, Maubach KA, Wafford KA, O’Connor D, Rodrigues AD, Evans DC, Tattersall FD, Chambers MS, MacLeod AM, Eng WS, Ryan C, et al. In vitro and in vivo properties of 3-tert-butyl-7-(5-methylisoxazol-3-yl)-2-(1-methyl-1H-1,2,4-triazol-5-ylme thoxy)-pyrazolo[1,5-d]-[1,2,4]triazine (MRK-016), a GABAA receptor alpha5 subtype-selective inverse agonist. J Pharmacol Exp Ther. 2009;331:470–484. doi: 10.1124/jpet.109.157636. [DOI] [PubMed] [Google Scholar]
- Baburin I, Khom S, Timin E, Hohaus A, Sieghart W, Hering S. Estimating the efficiency of benzodiazepines on GABA(A) receptors comprising gamma1 or gamma2 subunits. Br J Pharmacol. 2008;155:424–433. doi: 10.1038/bjp.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E. Individual properties of the two functional agonist sites in GABA(A) receptors. J Neurosci. 2003;23:11158–11166. doi: 10.1523/JNEUROSCI.23-35-11158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur R, Sigel E. Benzodiazepines affect channel opening of GABA A receptors induced by either agonist binding site. Mol Pharmacol. 2005;67:1005–1008. doi: 10.1124/mol.104.008151. [DOI] [PubMed] [Google Scholar]
- Baur R, Sigel E. Replacement of histidine in position 105 in the alpha(5) subunit by cysteine stimulates zolpidem sensitivity of alpha(5)beta(2)gamma(2) GABA(A) receptors. J Neurochem. 2007;103:2556–2564. doi: 10.1111/j.1471-4159.2007.04982.x. [DOI] [PubMed] [Google Scholar]
- Buhr A, Baur R, Sigel E. Subtle changes in residue 77 of the γ subunit of α1β2γ2 GABAA receptors drastically alter the affinity for ligands of the benzodiazepine binding site. J Biol Chem. 1997;272:11799–11804. doi: 10.1074/jbc.272.18.11799. [DOI] [PubMed] [Google Scholar]
- Cope DW, Wulff P, Oberto A, Aller MI, Capogna M, Ferraguti F, Halbsguth C, Hoeger H, Jolin HE, Jones A, Mckenzie ANJ, et al. Abolition of zolpidem sensitivity in mice with a point mutation in the GABAA receptor γ2 subunit. Neuropharmacology. 2004;47:17–34. doi: 10.1016/j.neuropharm.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Cope DW, Halbsguth C, Karayannis T, Wulff P, Ferraguti F, Hoeger H, Leppa E, Linden AM, Oberto A, Ogris W, Korpi ER, et al. Loss of zolpidem efficacy in the hippocampus of mice with the GABAA receptor gamma2 F77I point mutation. Eur J Neurosci. 2005;21:3002–3016. doi: 10.1111/j.1460-9568.2005.04127.x. [DOI] [PubMed] [Google Scholar]
- Dawson GR, Maubach KA, Collinson N, Cobain M, Everitt BJ, MacLeod AM, Choudhury HI, McDonald LM, Pillai G, Rycroft W, Smith AJ, et al. An inverse agonist selective for alpha5 subunit-containing GABAA receptors enhances cognition. J Pharmacol Exp Ther. 2006;316:1335–1345. doi: 10.1124/jpet.105.092320. [DOI] [PubMed] [Google Scholar]
- Ebert V, Scholze P, Sieghart W. Extensive heterogeneity of recombinant gamma-aminobutyric acidA receptors expressed in alpha 4 beta 3 gamma 2-transfected human embryonic kidney 293 cells. Neuropharmacology. 1996;35:1323–1330. doi: 10.1016/s0028-3908(96)00062-7. [DOI] [PubMed] [Google Scholar]
- Ernst M, Brauchart D, Boresch S, Sieghart W. Comparative modeling of GABAA receptors: limits, insights, future developments. Neuroscience. 2003;119:933–943. doi: 10.1016/s0306-4522(03)00288-4. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fleck MW. Molecular actions of (S)-desmethylzopiclone (SEP-174559), an anxiolytic metabolite of zopiclone. J Pharmacol Exp Ther. 2002;302:612–618. doi: 10.1124/jpet.102.033886. [DOI] [PubMed] [Google Scholar]
- Hadingham KL, Garrett EM, Wafford KA, Bain C, Heavens RP, Sirinathsinghji DJ, Whiting PJ. Cloning of cDNAs encoding the human gamma-aminobutyric acid type A receptor alpha 6 subunit and characterization of the pharmacology of alpha 6-containing receptors. Mol Pharmacol. 1996;49:253–259. [PubMed] [Google Scholar]
- Hadley SH, Amin J. Rat alpha6beta2delta GABAA receptors exhibit two distinct and separable agonist affinities. J Physiol. 2007;581:1001–1018. doi: 10.1113/jphysiol.2007.132886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevers W, Luddens H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- June HL, Foster KL, McKay PF, Seyoum R, Woods JE, Harvey SC, Eiler WJ, Grey C, Carroll MR, McCane S, Jones CM, et al. The reinforcing properties of alcohol are mediated by GABA(A1) receptors in the ventral pallidum. Neuropsychopharmacology. 2003;28:2124–2137. doi: 10.1038/sj.npp.1300239. [DOI] [PubMed] [Google Scholar]
- Knoflach F, Benke D, Wang Y, Scheurer L, Luddens H, Hamilton BJ, Carter DB, Mohler H, Benson JA. Pharmacological modulation of the diazepam-insensitive recombinant gamma-aminobutyric acidA receptors alpha 4 beta 2 gamma 2 and alpha 6 beta 2 gamma 2. Mol Pharmacol. 1996;50:1253–1261. [PubMed] [Google Scholar]
- Leppa E, Vekovischeva OY, Linden AM, Wulff P, Oberto A, Wisden W, Korpi ER. Agonistic effects of the beta-carboline DMCM revealed in GABA(A) receptor gamma 2 subunit F77I point-mutated mice. Neuropharmacology. 2005;48:469–478. doi: 10.1016/j.neuropharm.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Li X, Cao H, Zhang C, Furtmueller R, Fuchs K, Huck S, Sieghart W, Deschamps J, Cook JM. Synthesis, in vitro affinity, and efficacy of a bis 8-ethynyl-4H-imidazo[1, 5a]-[1, 4]benzodiazepine analogue, the first bivalent alpha5 subtype selective BzR/GABA(A) antagonist. J Med Chem. 2003;46:5567–5570. doi: 10.1021/jm034164c. [DOI] [PubMed] [Google Scholar]
- Nutt D. Pharmacological and behavioural studies of benzodiazepine antagonists and contragonists. Adv Biochem Psychopharmacol. 1983;38:153–173. [PubMed] [Google Scholar]
- Ogris W, Poltl A, Hauer B, Ernst M, Oberto A, Wulff P, Hoger H, Wisden W, Sieghart W. Affinity of various benzodiazepine site ligands in mice with a point mutation in the GABA(A) receptor gamma2 subunit. Biochem Pharmacol. 2004;68:1621–1629. doi: 10.1016/j.bcp.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroski RE, Pomeroy JE, Das R, Bowman H, Yang W, Chen AP, Foster AC. Indiplon is a high-affinity positive allosteric modulator with selectivity for alpha1 subunit-containing GABAA receptors. J Pharmacol Exp Ther. 2006;317:369–377. doi: 10.1124/jpet.105.096701. [DOI] [PubMed] [Google Scholar]
- Puia G, Vicini S, Seeburg PH, Costa E. Influence of recombinant gamma-aminobutyric acid-A receptor subunit composition on the action of allosteric modulators of gamma-aminobutyric acid-gated Cl− currents. Mol Pharmacol. 1991;39:691–696. [PubMed] [Google Scholar]
- Puia G, Ducic I, Vicini S, Costa E. Molecular mechanisms of the partial allosteric modulatory effects of bretazenil at gamma-aminobutyric acid type A receptor. Proc Natl Acad Sci U S A. 1992;89:3620–3624. doi: 10.1073/pnas.89.8.3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk K, Blurton P, Fletcher S, Leeson P, Tang F, Mellilo D, Ragan CI, McKernan RM. [3H]L-655,708, a novel ligand selective for the benzodiazepine site of GABAA receptors which contain the α5 subunit. Neuropharmacology. 1996;35:1331–1335. doi: 10.1016/s0028-3908(96)00061-5. [DOI] [PubMed] [Google Scholar]
- Rivas FM, Stables JP, Murphree L, Edwankar RV, Edwankar CR, Huang S, Jain HD, Zhou H, Majumder S, Sankar S, Roth BL, et al. Antiseizure activity of novel gamma-aminobutyric acid (A) receptor subtype-selective benzodiazepine analogues in mice and rat models. J Med Chem. 2009;52:1795–1798. doi: 10.1021/jm801652d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E, Busonero F, Talani G, Carta M, Massa F, Peis M, Maciocco E, Biggio G. Comparison of the effects of zaleplon, zolpidem, and triazolam at various GABA(A) receptor subtypes. Eur J Pharmacol. 2002;451:103–110. doi: 10.1016/s0014-2999(02)02191-x. [DOI] [PubMed] [Google Scholar]
- Savic MM, Huang S, Furtmuller R, Clayton T, Huck S, Obradovic DI, Ugresic ND, Sieghart W, Bokonjic DR, Cook JM. Are GABAA receptors containing alpha5 subunits contributing to the sedative properties of benzodiazepine site agonists? Neuropsychopharmacology. 2008;33:332–339. doi: 10.1038/sj.npp.1301403. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of γ-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- Sieghart W, Eichinger A, Riederer P, Jellinger K. Comparison of benzodiazepine receptor binding in membranes from human or rat brain. Neuropharmacology. 1985;24:751–759. doi: 10.1016/0028-3908(85)90009-7. [DOI] [PubMed] [Google Scholar]
- Sigel E, Buhr A. The benzodiazepine binding site of GABAA receptors. Trends Pharmacol Sci. 1997;18:425–429. doi: 10.1016/s0165-6147(97)01118-8. [DOI] [PubMed] [Google Scholar]
- Sigel E, Baur R, Trube G, Mohler H, Malherbe P. The effect of subunit composition of rat brain GABAA receptors on channel function. Neuron. 1990;5:703–711. doi: 10.1016/0896-6273(90)90224-4. [DOI] [PubMed] [Google Scholar]
- Skerritt JH, Macdonald RL. Benzodiazepine Ro 15-1788: electrophysiological evidence for partial agonist activity. Neurosci Lett. 1983;43:321–326. doi: 10.1016/0304-3940(83)90208-2. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Alder L, Silk J, Adkins C, Fletcher AE, Scales T, Kerby J, Marshall G, Wafford KA, McKernan RM, Atack JR. Effect of alpha subunit on allosteric modulation of ion channel function in stably expressed human recombinant gamma-aminobutyric acid(A) receptors determined using (36)Cl ion flux. Mol Pharmacol. 2001;59:1108–1118. doi: 10.1124/mol.59.5.1108. [DOI] [PubMed] [Google Scholar]
- Taylor SC, Johnston AL, Wilks LJ, Nicholass JM, File SE, Little HJ. Kindling with the beta-carboline FG7142 suggests separation between changes in seizure threshold and anxiety-related behaviour. Neuropsychobiology. 1988;19:195–201. doi: 10.1159/000118460. [DOI] [PubMed] [Google Scholar]
- Vellucci SV, Webster RA. Is Ro15-1788 a partial agonist at benzodiazepine receptors? Eur J Pharmacol. 1983;90:263–268. doi: 10.1016/0014-2999(83)90247-9. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Bain CJ, Whiting PJ, Kemp JA. Functional comparison of the role of gamma subunits in recombinant human gamma-aminobutyric acidA/benzodiazepine receptors. Mol Pharmacol. 1993a;44:437–442. [PubMed] [Google Scholar]
- Wafford KA, Whiting PJ, Kemp JA. Differences in affinity and efficacy of benzodiazepine receptor ligands at recombinant gamma-aminobutyric acidA receptor subtypes. Mol Pharmacol. 1993b;43:240–244. [PubMed] [Google Scholar]
- Walters RJ, Hadley SH, Morris KD, Amin J. Benzodiazepines act on GABAA receptors via two distinct and separable mechanisms. Nat Neurosci. 2000;3:1274–1281. doi: 10.1038/81800. [DOI] [PubMed] [Google Scholar]
- Whittemore ER, Yang W, Drewe JA, Woodward RM. Pharmacology of the human gamma-aminobutyric acidA receptor alpha 4 subunit expressed in Xenopus laevis oocytes. Mol Pharmacol. 1996;50:1364–1375. [PubMed] [Google Scholar]
- Wingrove PB, Thompson SA, Wafford KA, Whiting PJ. Key amino acids in the γ subunit of the γ-aminobutyric acidA receptor that determine ligand binding and modulation at the benzodiazepine site. Mol Pharmacol. 1997;52:874–881. doi: 10.1124/mol.52.5.874. [DOI] [PubMed] [Google Scholar]
- Wulff P, Goetz T, Leppa E, Linden AM, Renzi M, Swinny JD, Vekovischeva OY, Sieghart W, Somogyi P, Korpi ER, Farrant M, et al. From synapse to behavior: rapid modulation of defined neuronal types with engineered GABAA receptors. Nat Neurosci. 2007;10:923–929. doi: 10.1038/nn1927. [DOI] [PMC free article] [PubMed] [Google Scholar]