Abstract
First identified in 1975, tau was implicated in Alzheimer’s disease 10 years later. Filamentous tangle inclusions were known to be made of hyperphosphorylated tau by 1991, with similar inclusions gaining recognition for being associated with other neurodegenerative diseases. In 1998, mutations in MAPT, the gene that encodes tau, were identified as the cause of a dominantly inherited form of frontotemporal dementia with abundant filamentous tau inclusions. While this result indicated that assembly of tau into aberrant filaments is sufficient to drive neurodegeneration and dementia, most cases of tauopathy are sporadic. More recent work in experimental systems showed that filamentous assemblies of tau may first form in one brain area, and then spread to others in a prion-like fashion. Beginning in 2017, work on human brains using high-resolution techniques has led to a structure-based classification of tauopathies, which has opened the door to a better understanding of the significance of tau filament formation.
Keywords: tau filaments, tau gene mutations, tau isoforms, tauopathies
1. Introduction
Publication of this article marks the 50th anniversary of the description and naming of tau (Tubulin-Associated Unit) by Weingarten et al. (1975). At the time of its discovery, one could not have predicted that interest in tau would increase so dramatically; this happened because of the finding that tau is the main component of the filamentous inclusions that characterize many human neurodegenerative diseases that have been dubbed tauopathies. The most common of these diseases is Alzheimer’s disease (AD), with others including chronic traumatic encephalopathy (CTE), Pick’s disease (PiD), progressive supranuclear palsy (PSP), globular glial tauopathy (GGT), corticobasal degeneration (CBD), and argyrophilic grain disease (AGD). A great deal of work was needed between 1985 and 1991 to firmly establish that tau is the main component of the aberrant filamentous tangles of AD (Brion et al., 1985; Delacourte et al., 1986; Goedert et al., 1988; Grundke-Iqbal, Iqbal, Quinlan, et al., 1986; Grundke-Iqbal, Iqbal, Tung, et al., 1986; Kondo et al., 1988; Kosik et al., 1986, 1988; Lee et al., 1991; Nukina & Ihara, 1986; Wischik, Novak, Edwards, et al., 1988; Wischik, Novak, Thøgersen, et al., 1988; Wood et al., 1986).
Tangle pathology was identified by Alois Alzheimer via light microscopy (Alzheimer, 1907), after which Michael Kidd used electron microscopy to show that the tangles are composed of abnormal filaments, which he named paired helical filaments (PHFs; Kidd, 1963). Identified as minority species were straight filaments (SFs), which do not exhibit the modulation in width shown by PHFs. Tau was first reported to have a role in neurodegeneration by Brion et al., who showed labelling of the tangle pathology of AD by a tau-specific antibody (Brion et al., 1985).
2. Tau Isoforms
Martin Roth brought the tangle problem to the “MRC Laboratory of Molecular Biology” where, under the direction of Aaron Klug, biochemical, immunological, structural, and molecular biological techniques were used, to find out what PHFs are made of. In conducting this work, we also characterized human tau isoforms.
Since the PHF is defined by its ultrastructure, electron microscopy alone is insufficient to reveal its component parts. Required was a label for individual filaments using microscopy, as well as for the protein bands revealed by gel electrophoresis from successively purified tangle preparations. The protein bands could then be partially sequenced, so that cDNA clones could be isolated and sequenced.
Claude Wischik and Tony Crowther used proteases to break down the insoluble tangles, in order to study the structural organization of PHFs (Crowther & Wischik, 1985; Wischik et al., 1985). To obtain a label for purified PHF components, Michal Novak and Cesar Milstein produced monoclonal antibodies, one of which (6–423) decorated individual PHFs isolated from tangle fragments in electron microscopy and also labelled a 12 kDa protein gel band extracted from purified PHF preparations. The partial amino acid sequence of this band was then determined by John Walker, after which Michel Goedert isolated and sequenced cDNAs from a human brain library. A striking repeat pattern was revealed by the deduced amino acid sequences that was unrelated to any known sequence. A major 6 kb and a minor 2 kb band were observed by RNA blotting, and these were identical to the pattern obtained using a cDNA clone encoding murine tau (Drubin et al., 1984), kindly provided by Gloria Lee and Marc Kirschner.
Lee and Kirschner had deduced the amino acid sequence of a form of murine tau after sequencing this and other cDNA clones; when Klug read part of our sequence over the telephone to Kirschner, it was clear that we had established tau as an integral component of PHFs (Goedert et al., 1988; Wischik, Novak, Edwards, et al., 1988; Wischik, Novak, Thøgersen, et al., 1988). Following this work, studies using electron microscopy and image reconstruction demonstrated that PHFs and SFs are each composed of two identical C-shaped subunits of tau that are linked differently, giving rise to their characteristic morphologies (Crowther, 1991).
January 1988 saw reporting of the first sequence of an isoform of murine tau with three repeats in the microtubule-binding region (0N3R; Lee et al., 1988), followed by that of the sequence of an isoform of human tau (0N3R) in June of that year (Goedert et al., 1988). In the following year, we identified a four-repeat isoform of human tau (0N4R; Goedert, Spillantini, Potier, et al., 1989), as well as the tau isoforms that are expressed in adult human brains (0N3R, 1N3R, 2N3R, 0N4R, 1N4R, and 2N4R, Figure 1; Goedert, Spillantini, Jakes, et al., 1989). Six tau isoforms expressed by alternative mRNA splicing in adult bovine brains were described in parallel studies (Himmler, 1989; Himmler et al., 1989). The expression of human tau isoforms from their cDNAs and alignment with dephosphorylated adult brain tau established the identification by our group of the major tau isoforms (Goedert & Jakes, 1990). Two years later, big tau, an isoform that nearly doubles the molecular mass of the brain isoforms, was described as the predominant tau isoform in the peripheral nervous system (Couchie et al., 1992; Goedert, Spillantini, & Crowther, 1992).
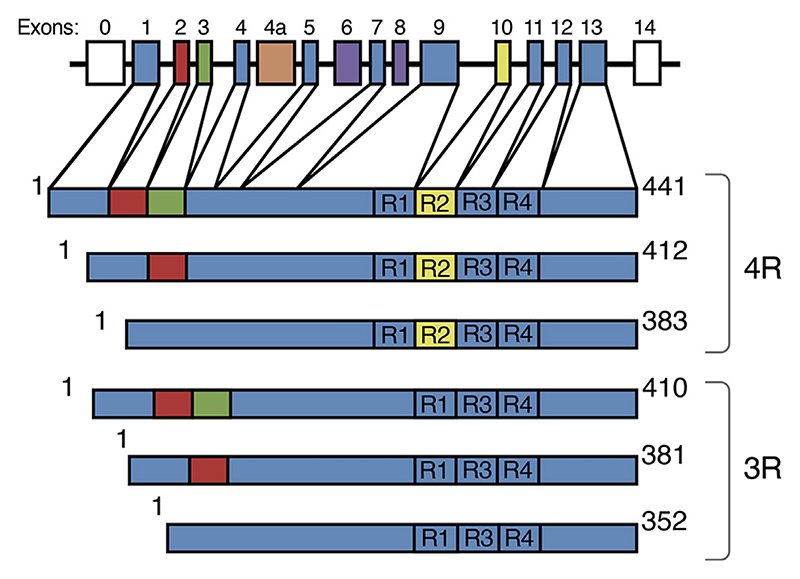
Figure 1.
Human brain tau isoforms. MAPT and the six tau isoforms expressed in adult human brains. MAPT consists of 14 exons (E). Alternative mRNA splicing of E2 (red), E3 (green) and E10 (yellow) gives rise to six tau isoforms (352–441 amino acids). The constitutively spliced exons (E1, E4, E5, E7, E9, E11, E12, and E13) are shown in blue. E6 and E8 (violet) are not transcribed in human brains. E4a (orange) is only expressed in the peripheral nervous system. The repeats (R1-R4) are shown, with three isoforms having four repeats (4R) and the other three isoforms having three repeats (3R).
The presence or absence of three inserts defines the six isoforms of brain tau that range from 352 to 441 amino acids, and these are generated through alternative mRNA splicing of the MAPT gene (Figure 1). Inserts of 29 or 58 amino acids (1N and 2N) are located near the N-terminus and one insert is in the C-terminal half. The latter consists of repeat R2 in the three isoforms with four repeats (4R), whereas the other three isoforms have three repeats (3R). Exons 9–12 of MAPT encode repeats 1–4 (Figure 1). 3R and 4R tau are present at similar levels in adult human brains (Goedert & Jakes, 1990). Only the shortest tau isoform (0N3R) is present in developing human brains. Together with some adjoining sequences, the repeats constitute the microtubule-binding domains of tau (reviewed in Wang & Mandelkow, 2015), and also the cores of filamentous tau in neurodegenerative diseases; this suggests that physiological function and pathological assembly are mutually exclusive. Although often said to stabilize axonal microtubules and promote their assembly, tau is in fact enriched in their more labile domains, where it promotes assembly (Qiang et al., 2018).
The isoform composition of tau is not conserved across species. 3R, 4R, and 5R tau isoforms are expressed in adult chicken brains (Yoshida & Goedert, 2002), while most adult rodents express only 4R tau (Götz et al., 1995). The Caenorhabditis elegans and Drosophila melanogaster genomes each encode one protein with tau-like repeats (Goedert, Baur, et al., 1996; Heidary & Fortini, 2001). The high-molecular weight proteins MAP2 and MAP4 display similar repeats (Aizawa et al., 1990; Lewis et al., 1988). MAP2 and tau likely shared a recent common ancestor, whereas MAP4 probably derives from a nonvertebrate ancestor (Sündermann et al., 2016).
3. Tau Assemblies
Tau research was made difficult for many years by the insolubility of PHFs in tangle fragments. A method based on sarkosyl solubility (Greenberg & Davies, 1990), which enriches for less insoluble PHFs, mainly made of full-length tau, was a pivotal step forward. Antibodies specific for the N- and C-termini of tau decorated these filaments in negative stain immunoelectron microscopy (Goedert, Spillantini, Cairns, & Crowther, 1992), but this was not the case for antibodies against repeats R3 or R4 of tau, because their epitopes are occluded in the filaments. This work, together with the results of biochemical studies, supported the view that tau filaments are made of an ordered core and a protease-sensitive fuzzy coat (this distinction was first made by Wischik, Novak, Edwards, et al., 1988). The core is required for a filament to look like a filament, whereas the fuzzy coat makes up the rest of the tau molecule. Tau filaments have the biophysical characteristics of amyloid (Berriman et al., 2003).
Tau’s ability to interact with microtubules is negatively regulated by phosphorylation (Lindwall & Cole, 1984) and filamentous tau is aberrantly hyperphosphorylated (Grundke-Iqbal, Iqbal, Tung, et al., 1986). It is unknown if phosphorylation is necessary and/or sufficient for the assembly of tau into filaments in the brain. In addition to phosphorylation, filamentous tau is also known to undergo acetylation, glycation, isomerization, O-GlcNAcylation, nitration, sumoylation, ubiquitination, and truncation (reviewed in Goedert et al., 2017). Acetylation of lysine residues (21 of which are located between residues 244 and 380) reduces charge, which may play a role in the filament assembly of tau.
All six tau isoforms are present in disease filaments in AD, CTE, familial British dementia (FBD), familial Danish dementia (FDD), primary age-related tauopathy (PART), and some other diseases, whereas Pick bodies are only made of 3R tau. 4R tau makes up the filaments in CBD, AGD, PSP, and GGT. Tau filament morphologies vary in different diseases, even when comprised of the same isoforms (reviewed in Goedert et al., 2017).
Attempts to assemble recombinant tau into filaments began in the early 1990s. This was possible with a fragment containing the repeat region (Crowther et al., 1992; Wille et al., 1992), but full-length tau resisted assembly. Only when negatively charged substances, such as sulfated glycosaminoglycans, were used, did full-length tau also assemble into filaments (Goedert, Jakes, et al., 1996; Pérez et al., 1996). These filaments were decorated by antibodies against the N- and C-termini of tau, but not by an antibody specific for the repeats.
In vitro (von Bergen et al., 2000), in cells (Falcon et al., 2015), and in transgenic mice (Macdonald et al., 2019), a hexapeptide sequence in R3 (VQIVYK, amino acids 306–311) is needed for filament assembly. Steric zippers are formed by microcrystals of residues 306–311(Sawaya et al., 2007). Residues 310–313 in tau (YKPV) differ from the equivalent residues in MAP2 (TKKI). When the latter were changed to YKPV, MAP2c also assembled (Xie et al., 2015).
4. Tau Genetics
Human genetics established the link between tau dysfunction and neurodegeneration. A dominantly inherited form of frontotemporal dementia and parkinsonism was found to be associated with chromosome 17q21–22 (Wilhelmsen et al., 1994), the region where MAPT resides (Neve et al., 1986). Then, in June 1998, mutations in MAPT were linked to a type of frontotemporal dementia associated with parkinsonism (Hutton et al., 1998; Poorkaj et al., 1998; Spillantini et al., 1998). Filamentous inclusions composed of 3R, 4R, or 3R + 4R tau were found in neurons or in both neurons and glia (reviewed in Goedert et al., 2017). Deposits of Aβ outside of cells, a defining characteristic of AD, were not present.
Now, it was known that a mechanism going from monomeric to filamentous tau is sufficient to cause neurodegeneration and associated cognitive deficits. By December 2023, 65 pathogenic MAPT mutations had been identified (Figure 2). Behavioral symptoms are the most common clinical manifestation, but in some cases, MAPT mutations are also associated with parkinsonism. Neurological syndromes like those of PiD, PSP, GGT, CBD, and motor neuron disease have been described. Onset of disease symptoms is variable but can be as early as in the third decade.
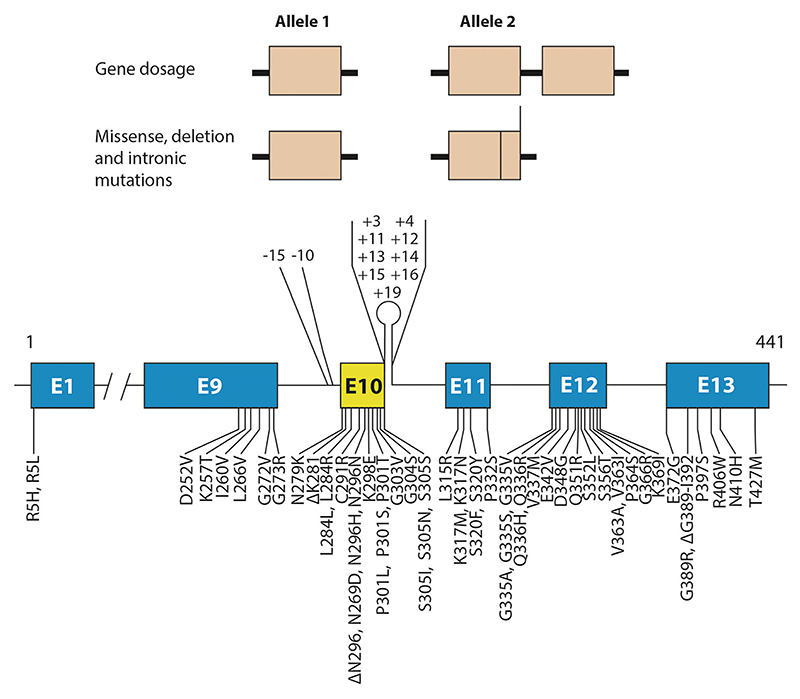
Figure 2.
Mutations in MAPT in FTDP-17 T. Gene dosage mutations, where one allele of MAPT is doubled, as well as missense, deletion and intronic mutations in MAPT are dominantly inherited. (Fifty-five coding region and eleven intronic mutations are shown.) They give rise to 65 different forms of FTDP-17 T (pathogenic intronic mutation –15/+4 is compound heterozygous).
Most MAPT mutations are concentrated in exons 9–12 (encoding repeats R1–R4) and the introns flanking exon 10, with a smaller number in exon 13. Exon 1 is where the R5H and R5L mutations are located. Gene dosage mutations can also give rise to FTDP-17T, indicating that the overexpression of wild-type tau is sufficient to cause disease (Wallon et al., 2021).
Some mutations have a primary effect at the protein level, whereas others impact the alternative splicing of tau pre-mRNA. The latter can be intronic or exonic, with 3R or 4R tau isoforms being overexpressed and assembling into disease filaments. These findings indicate that the healthy ratio of 3R:4R tau in adult human brains is pivotal for preventing neurodegeneration and dementia.
Pathogenic mutations in MAPT led to the production of transgenic rodent lines that show neurodegeneration and abundant tau filaments (Allen et al., 2002; Götz et al., 2001; Lewis et al., 2000; Yoshiyama et al., 2007). Tau aggregation correlates with neurodegeneration (Macdonald et al., 2019).
Transgenic mouse lines led in turn to the identification of the prion-like properties of assembled tau (Clavaguera et al., 2009), which parallel the staging of tau pathology in AD (Braak & Braak, 1991). Intracerebral injection of brain extracts from a mouse line transgenic for human mutant tau with abundant tau inclusions into a line transgenic for wild-type human tau without inclusions led to the assembly of wild-type human tau and the propagation over time of inclusions to distant brain areas. Short filaments from brain extracts of mice transgenic for human P301S tau were then shown to have the greatest seeding activity (Jackson et al., 2016). Producing “tauopathy in a dish” is an ongoing goal, with work on induced pluripotent stem cell-derived cortical neurons from patients with MAPT splicing mutation N279K showing earlier expression of 4R tau than controls (Iovino et al., 2015).
5. Tau Filament Structures From Human Brains
The high-resolution structures of tau inclusions remained unknown, even decades after the presence of inclusions had been shown in human brains. This changed in 2017, when electron cryo-microscopy (cryo-EM) made it possible (He & Scheres, 2017) to determine the structures of amyloid filaments from human brains (Fitzpatrick et al., 2017).
Cryo-EM structures of PHFs and SFs that were extracted from the frontal cortex of an individual with AD showed that each filament type comprises two identical protofilaments with a C-shaped ordered core, the Alzheimer tau fold (Fitzpatrick et al., 2017). PHFs and SFs are distinguished by different packings of protofilaments. The ordered core, which forms a β-sheet-rich structure that is characteristic of amyloids, comprises amino acids 306–378 (in the numbering of the 441 amino acid tau isoform). The tau filament core thus consists of the whole of R3 and R4, and 10–13 amino acids after R4. Tau monomers can be incorporated into the filaments, regardless of whether they contain R2, accounting for the presence of all six tau isoforms in PHFs and SFs in AD (Fitzpatrick et al., 2017; Goedert, Spillantini, Cairns, & Crowther, 1992). The remaining 80% of tau forms the fuzzy coat. Other AD cases displayed the same tau filament structures in frontal cortex (Falcon, Zhang, Schweighauser, et al., 2018), and this was true also of tau filaments from different brain regions of individuals with AD. Unknown is what connections may exist between intracellular inclusions of tau and extracellular deposits of Aβ (Bloom, 2014).
In subsequent years, structures of tau filaments from PiD (Falcon, Zhang, Murzin, et al., 2018), CTE (Falcon et al., 2019), CBD (Arakhamia et al., 2020; Zhang et al., 2020), PART (Shi, Murzin, et al., 2021), AGD, PSP and GGT (Shi, Zhang, et al., 2021) were determined, giving rise to a structure-based classification of tauopathies (Figure 3; Shi, Zhang, et al., 2021). Tau filament structures from CTE brains are similar to those of AD, but differ by a larger cavity in the β-helix region and the presence there of a density of unknown identity. In PiD, the tau filament core consists of the C-terminal two thirds of R1, the whole of R3 and R4, and 10–13 amino acids after R4.
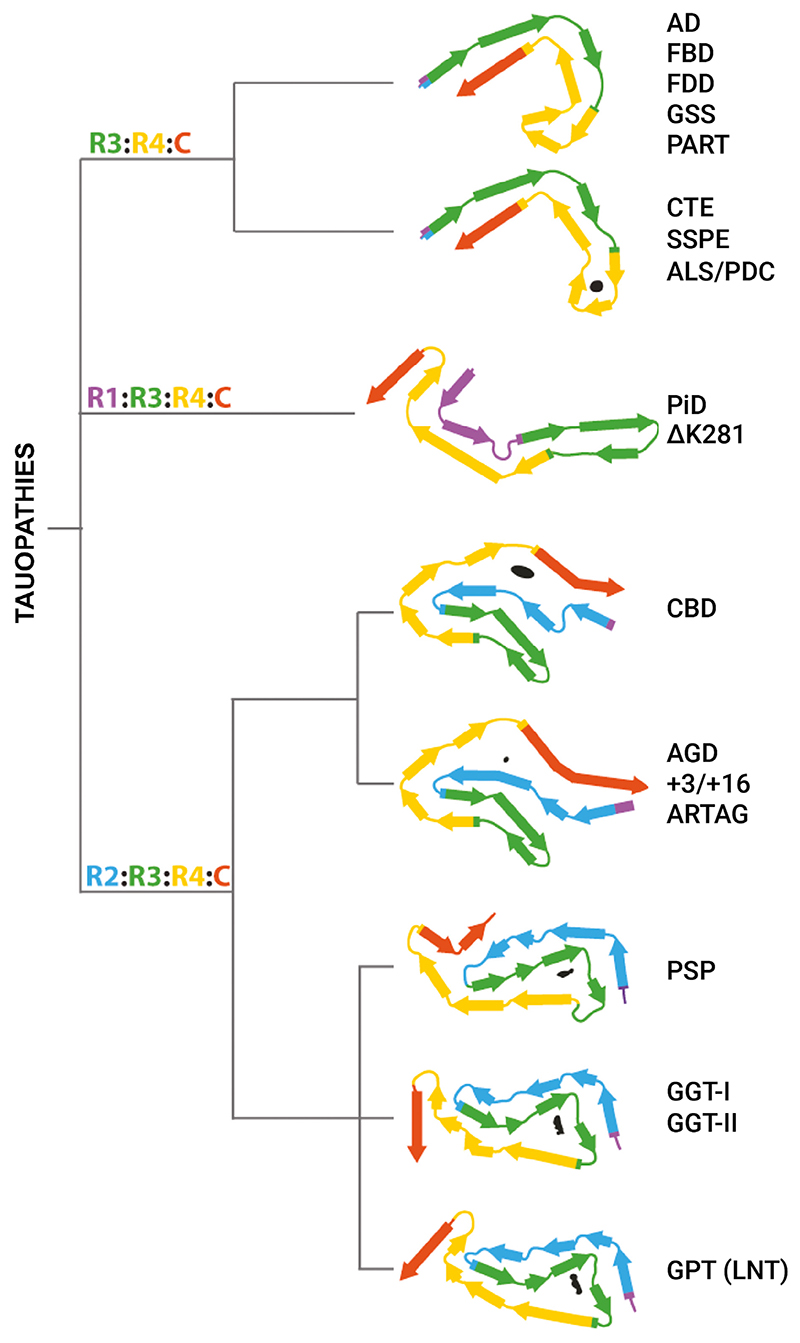
Figure 3.
Structure-based classification of tauopathies. The dendrogram shows the proposed classification, with the corresponding folds displayed with the first β-strand in R3 oriented approximately horizontally, except for the globular glial tauopathy (GGT) and globular glial tauopathy-progressive supranuclear palsy-tau (GPT) folds, which are aligned to the progressive supranuclear palsy (PSP) fold. Internal, nonproteinaceous densities are shown in black. AD, Alzheimer’s disease; AGD, argyrophilic grain disease; ALS/PDC, amyotrophic lateral sclerosis/parkinsonism–dementia complex; ARTAG, age-related tau astrogliopathy; CBD, corticobasal degeneration; CTE, chronic traumatic encephalopathy; FBD, familial British dementia; FDD, familial Danish dementia; GSS, Gerstmann-Sträussler-Scheinker disease; LNT, limbic-predominant neuronal inclusion body 4R tauopathy; PART, primary age-related tauopathy; PiD, Pick’s disease; SSPE, subacute sclerosing panencephalitis.
In CBD, AGD, PSP, and GGT, the cores of tau filaments consist of the whole of R2, R3, and R4, and 10–13 amino acids after R4. CBD and AGD folds are four layered, whereas PSP and GGT folds are three layered. We also identified a three layered fold intermediate between those of GGT and PSP in a case of atypical PSP. We suggested that this globular glial tauopathy-progressive supranuclear palsy-tau (GPT) fold gives rise to a new clinicopathological entity that we named limbic-predominant neuronal inclusion body 4R tauopathy (LNT) (Shi, Zhang, et al., 2021).
Specific tau folds characterize different diseases, but several conditions share a fold. FBD, FDD, PART, and cases of Gerstmann–Sträussler–Scheinker disease have the Alzheimer tau fold in common (Hallinan et al., 2021; Shi, Murzin, et al., 2021; Shi, Zhang, et al., 2021). The CTE tau fold is also found in subacute sclerosing panencephalitis (SSPE) (Qi, Hasegawa, et al., 2023) and the amyotrophic lateral sclerosis/parkinsonism–dementia complex (ALS/PDC) of the island of Guam and the Kii peninsula of Japan (Qi, Verheijen, et al., 2023). A familial form of PiD is characteristic of cases with mutation ΔK281 in MAPT (Schweighauser, Garringer, et al., 2023). Moreover, tau filaments from AGD, aging-related tau astrogliopathy, and cases of mutations in intron 10 of MAPT share a common fold (Shi, Zhang, et al., 2021). The structures of filaments from these cases with MAPT mutations indicate that the relative overproduction of wild-type 4R tau can give rise to the AGD fold, whereas that of wild-type 3R tau generates the Pick fold.
Postmortem human tau filament structures display additional densities. Many densities face outwards into the surrounding solvent or the fuzzy coat, whereas others are buried within the ordered filament cores. These densities (which may correspond to posttranslational modifications of tau or noncovalently bound cofactors) may play an important role in giving rise to a particular tau fold, but their identities remain to be discovered.
6. Conclusion
The assembly of amyloid filaments using purified recombinant proteins has been used extensively to study mechanisms underlying amyloid formation. Cryo-EM has yielded structures of in vitro assembled tau filaments (Abskharon et al., 2022; Zhang et al., 2019), but none were identical to those of filaments extracted from human brains. Tau filaments from transgenic mouse models also have a different appearance from those in human diseases (Schweighauser, Murzin, et al., 2023).
What is needed, then, are methods by which one can form tau filaments with structures like those from human brains. So far, this has only been possible for fragments of tau (Lövestam et al., 2022). In this system, N- and C-terminal truncations were shown to be critical for forming PHFs. The addition of 100–200 mM sodium chloride led to the formation of tau filaments with the CTE fold.
The ordered assembly of tau is believed to be the gain of toxic function that causes human tauopathies (reviewed in Goedert, 2016). Downstream, propagation of assembled tau and neurodegeneration take place. Short tau filaments are the major species responsible for propagation, at least in transgenic mice (Jackson et al., 2016). The tau species that lead to neurodegeneration remain to be identified. Mechanisms of propagation and neurodegeneration are probably linked and may be influenced by the structural differences between tau filaments that have been identified by cryo-EM (reviewed in Scheres et al., 2023). Elucidating these mechanisms will be key for developing safe and effective therapies for these diseases.
References
- Abskharon R, Sawaya MR, Boyer DR, Cao Q, Nguyen BA, Cascio D, Eisenberg DS. Cryo-EM structure of RNA-induced tau fibrils reveals a small C-terminal core that may nucleate fibril formation. Proceedings of the National Academy of Sciences of the United States of America. 2022;119:e2119952119. doi: 10.1073/pnas.2119952119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa H, Emori Y, Murofushi H, Kawasaki H, Sakai H, Suzuki K. Molecular cloning of a ubiquitously distributed microtubule-associated protein with Mr 190,000. Journal of Biological Chemistry. 1990;265:13489–13855. [PubMed] [Google Scholar]
- Allen B, Ingram E, Takao M, Smith MJ, Jakes R, Virdee K, Yoshida H, Holzer M, Craxton M, Emson PC, Atzori C, et al. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. Journal of Neuroscience. 2002;22:9340–9351. doi: 10.1523/JNEUROSCI.22-21-09340.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer A. Über eine eigenartige Erkrankung der Hirnrinde. All-gemeine Zeitschrift für Psychiatrie. 1907;64:146–148. [Google Scholar]
- Arakhamia T, Lee CE, Carlomagno Y, Kumar M, Duong DM, Wesseling H, Kundinger SR, Wang K, Williams D, DeTure M, Dickson DW, et al. Posttranslational modifications mediate the structural diversity of tauopathy strains. Cell. 2020;180:633–644. doi: 10.1016/j.cell.2020.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berriman J, Serpell LC, Oberg KA, Fink AL, Goedert M, Crowther RA. Tau filaments from human brain and from in vitro assembly of recombinant tau show cross-β structure. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9034–9038. doi: 10.1073/pnas.1530287100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom G. Amyloid-β and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurology. 2014;71:505–508. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brion JP, Passareiro H, Nunez J, Flament-Durand J. Mise en évidence immunologique de la protéine tau au niveau des lésions de dégénérescence neurofibrillaire de la maladie d’Alzheimer. Archives de Biologie (Bruxelles) 1985;95:229–235. [Google Scholar]
- Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M, Jucker M, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nature Cell Biology. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couchie D, Mavilia C, Georgieff IS, Liem RKH, Shelanski ML, Nunez J. Primary structure of high molecular weight tau present in the peripheral nervous system. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:4378–4381. doi: 10.1073/pnas.89.10.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther RA. Straight and paired helical filaments in Alzheimer disease have a common structural unit. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:2288–2292. doi: 10.1073/pnas.88.6.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther RA, Olesen OF, Jakes R, Goedert M. The microtubule binding repeats of tau protein assemble into filaments like those found in Alzheimer’s disease. FEBS Letters. 1992;309:199–202. doi: 10.1016/0014-5793(92)81094-3. [DOI] [PubMed] [Google Scholar]
- Crowther RA, Wischik CM. Image reconstruction of the Alzheimer paired helical filament. EMBO Journal. 1985;4:3661–3665. doi: 10.1002/j.1460-2075.1985.tb04132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacourte A, Défossez A. Alzheimer’s disease; tau proteins, the promoting factors of microtubule assembly, are major components of paired helical filaments. Journal of Neurological Sciences. 1986;76:173–186. doi: 10.1016/0022-510x(86)90167-x. [DOI] [PubMed] [Google Scholar]
- Drubin DG, Caput D, Kirschner MW. Studies on the expression of the microtubule-associated protein tau, during mouse brain development, with newly isolated complementary DNA probes. Journal of Cell Biology. 1984;98:1090–1097. doi: 10.1083/jcb.98.3.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon B, Cavallini A, Angers R, Glover S, Murray TK, Barnham L, Jackson S, O’Neill M, Isaacs AM, Hutton ML, Szekeres PG, et al. Conformation determines the seeding potencies of native and recombinant tau aggregates. Journal of Biological Chemistry. 2015;290:1049–1065. doi: 10.1074/jbc.M114.589309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon B, Zhang W, Murzin AG, Murshudov G, Garringer HJ, Vidal R, Crowther RA, Ghetti B, Scheres SHW, Goedert M. Structures of filaments from Pick’s disease reveal a novel tau protein fold. Nature. 2018;561:137–140. doi: 10.1038/s41586-018-0454-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon B, Zhang W, Schweighauser M, Murzin AG, Vidal R, Garringer HJ, Ghetti B, Scheres SHW, Goedert M. Tau filaments from multiple cases of sporadic and inherited Alzheimer’s disease adopt a common fold. Acta Neuropathologica. 2018;136:699–708. doi: 10.1007/s00401-018-1914-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon B, Zivanov J, Zhang W, Murzin AG, Garringer HJ, Vidal R, Crowther RA, Newell KL, Ghetti B, Goedert M, Scheres SHW. Novel tau filament fold in chronic traumatic encephalopathy encloses hydrophobic molecules. Nature. 2019;568:420–423. doi: 10.1038/s41586-019-1026-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick AWP, Falcon B, He S, Murzin AG, Murshudov G, Garringer HJ, Crowther RA, Ghetti B, Goedert M, Scheres SHW. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature. 2017;547:185–190. doi: 10.1038/nature23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M. The ordered assembly of tau is the gain-of-toxic function that causes human tauopathies. Alzheimer’s & Dementia. 2016;12:1040–1050. doi: 10.1016/j.jalz.2016.09.001. [DOI] [PubMed] [Google Scholar]
- Goedert M, Baur CP, Ahringer J, Jakes R, Hasegawa M, Spillantini MG, Smith MJ, Hills F. PTL-1, a microtubule-associated protein with tau-like repeats from the nematode Caenorhabditis elegans. Journal of Cell Science. 1996;109:2661–2672. doi: 10.1242/jcs.109.11.2661. [DOI] [PubMed] [Google Scholar]
- Goedert M, Eisenberg DS, Crowther RA. Propagation of tau aggregates and neurodegeneration. Annual Review of Neuroscience. 2017;40:189–210. doi: 10.1146/annurev-neuro-072116-031153. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R. Expression of separate isoforms of human tau protein; correlation with the tau pattern in brain and effects on tubulin polymerization. EMBO Journal. 1990;9:4225–4230. doi: 10.1002/j.1460-2075.1990.tb07870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Jakes R, Spillantini MG, Hasegawa M, Smith MJ, Crowther RA. Assembly of microtubule-associated protein tau into Alzheimer-like filaments induced by sulphated glycosaminoglycans. Nature. 1996;383:550–553. doi: 10.1038/383550a0. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Cairns NJ, Crowther RA. Tau proteins of Alzheimer paired helical filaments; abnormal phosphorylation of all six brain isoforms. Neuron. 1992;8:159–168. doi: 10.1016/0896-6273(92)90117-v. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Crowther RA. Cloning of a big tau microtubule-associated protein characteristic of the peripheral nervous system. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:1983–1987. doi: 10.1073/pnas.89.5.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: Sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron. 1989;3:519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Potier MC, Ulrich J, Crowther RA. Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats; differential expression of tau protein mRNAs in human brain. EMBO Journal. 1989;8:393–399. doi: 10.1002/j.1460-2075.1989.tb03390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Wischik CM, Crowther RA, Walker JE, Klug A. Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: Identification as the microtubule-associated protein tau. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:4051–4055. doi: 10.1073/pnas.85.11.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz J, Chen F, Barmettler R, Nitsch RM. Tau filament formation in transgenic mice expressing P301L tau. Journal of Biological Chemistry. 2001;276:529–534. doi: 10.1074/jbc.M006531200. [DOI] [PubMed] [Google Scholar]
- Götz J, Probst A, Spillantini MG, Schäfer T, Jakes R, Bürki K, Goedert M. Somatodendritic localization and hyperphosphorylation of tau protein in transgenic mice expressing the longest human brain tau isoform. EMBO Journal. 1995;14:1304–1313. doi: 10.1002/j.1460-2075.1995.tb07116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg SG, Davies P. A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:5827–5831. doi: 10.1073/pnas.87.15.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. Journal of Biological Chemistry. 1986;261:6084–6089. [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Tung Y-C, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau in Alzheimer cytoskeletal pathology. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallinan GI, Hoq MR, Ghosh M, Vago FS, Fernandez A, Garringer HJ, Vidal R, Jiang W, Ghetti B. Structure of tau filaments in prion protein amyloidoses. Acta Neuropathologica. 2021;142:227–241. doi: 10.1007/s00401-021-02336-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Scheres SHW. Helical reconstruction in RELION. Journal of Structural Biology. 2017;198:163–176. doi: 10.1016/j.jsb.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidary G, Fortini ME. Identification and characterization of the Drosophila tau homolog. Mechanisms of Development. 2001;108:171–178. doi: 10.1016/s0925-4773(01)00487-7. [DOI] [PubMed] [Google Scholar]
- Himmler A. Structure of the bovine tau gene: Alternatively spliced transcripts generate a protein family. Molecular and Cellular Biology. 1989;9:1389–1396. doi: 10.1128/mcb.9.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmler A, Drechsel D, Kirschner MW, Martin DW. Tau consists of a set of proteins with repeated C-terminal microtubule-binding domains and variable N-terminal domains. Molecular and Cellular Biology. 1989;9:1381–1388. doi: 10.1128/mcb.9.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, et al. Association of missense and 50-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Iovino M, Agathou S, González-Rueda A, Del—Castillo Velasco-Herrera M, Borroni B, Alberrici A, Lynch T, O’Dowd S, Geti I, Gaffney D, Vallier L, et al. Early maturation and distinct tau pathology in induced pluripotent stem cell-derived neurons from patients with MAPT mutations. Brain. 2015;138:3345–3359. doi: 10.1093/brain/awv222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SJ, Kerridge C, Cooper J, Cavallini A, Falcon B, Cella CV, Landi A, Szekeres PG, Murray TK, Ahmed Z, Goedert M, et al. Short fibrils constitute the major species of seed-competent tau in the brains of mice transgenic for human P301S tau. Journal of Neuroscience. 2016;36:762–772. doi: 10.1523/JNEUROSCI.3542-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd M. Paired helical filaments in electron microscopy of Alzheimer’s disease. Nature. 1963;197:192–193. doi: 10.1038/197192b0. [DOI] [PubMed] [Google Scholar]
- Kondo J, Honda T, Mori H, Hamada Y, Miura R, Ogawara M, Ihara Y. The carboxyl third of tau is tightly bound to paired helical filaments. Neuron. 1988;1:827–834. doi: 10.1016/0896-6273(88)90130-4. [DOI] [PubMed] [Google Scholar]
- Kosik KS, Joachim CL, Selkoe DJ. Microtubule-associated protein tau is a major antigenic component of paired helical filaments in Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik KS, Orecchio LD, Binder L, Trojanowski JQ, Lee VM-Y, Lee G. Epitopes that span the tau molecule are shared with paired helical filaments. Neuron. 1988;1:819–827. doi: 10.1016/0896-6273(88)90129-8. [DOI] [PubMed] [Google Scholar]
- Lee G, Cowan N, Kirschner MW. The primary structure and heterogeneity of tau protein from mouse brain. Science. 1988;239:285–288. doi: 10.1126/science.3122323. [DOI] [PubMed] [Google Scholar]
- Lee VM-Y, Balin BJ, Otvos L, Trojanowski JQ. A68 – A major subunit of paired helical filaments and derivatized forms of normal tau. Science. 1991;251:675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, van Slegtenhorst M, Gwinn-Hardy K, Murphy MP, Baker M, Yu X, Duff K, et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nature Genetics. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- Lewis SA, Wang DH, Cowan NJ. Microtubule-associated protein MAP2 shares a microtubule binding motif with tau protein. Science. 1988;242:936–939. doi: 10.1126/science.3142041. [DOI] [PubMed] [Google Scholar]
- Lindwall G, Cole RD. Phosphorylation affects the ability of tau protein to promote microtubule-assembly. Journal of Biological Chemistry. 1984;259:5301–5305. [PubMed] [Google Scholar]
- Lövestam S, Koh FA, van Knippenberg B, Kotecha A, Murzin AG, Goedert M, Scheres SHW. Assembly of recombinant tau into filaments identical to those of Alzheimer’s disease and chronic traumatic encephalopathy. eLife. 2022;11:e76494. doi: 10.7554/eLife.76494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald JA, Bronner IF, Drynan L, Fan J, Curry A, Fraser G, Lavenir I, Goedert M. Assembly of transgenic human P301S tau is necessary for neurodegeneration in murine spinal cord. Acta Neuropathologica Communications. 2019;7:44. doi: 10.1186/s40478-019-0695-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve RL, Harris P, Kosik KS, Kurnit DM, Donlon TA. Identification of cDNA clones for the human microtubule-associated protein tau and chromosomal localization of the genes for tau and microtubule-associated protein 2. Brain Research. 1986;387:271–280. doi: 10.1016/0169-328x(86)90033-1. [DOI] [PubMed] [Google Scholar]
- Nukina N, Ihara Y. One of the antigenic determinants of paired helical filaments is related to tau protein. Journal of Biochemistry. 1986;99:1541–1544. doi: 10.1093/oxfordjournals.jbchem.a135625. [DOI] [PubMed] [Google Scholar]
- Pérez M, Valpuesta JM, Medina M, Montejo de Garcini E, Avila J. Polymerization of tau in the presence of heparin: The minimal sequence required for tau-tau interaction. Journal of Neurochemistry. 1996;67:1183–1190. doi: 10.1046/j.1471-4159.1996.67031183.x. [DOI] [PubMed] [Google Scholar]
- Poorkaj P, Bird TD, Wijsman E, Nemens E, Garruto RM, Anderson L, Andreadis A, Wiederholt WC, Raskind M, Schellenberg GD. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Annals of Neurology. 1998;43:815–825. doi: 10.1002/ana.410430617. [DOI] [PubMed] [Google Scholar]
- Qi C, Hasegawa M, Takao M, Sakai M, Sasaki M, Mizutani M, Akagi A, Iwasaki Y, Miyahara H, Yoshida M, Scheres SHW, et al. Identical tau filaments in subacute sclerosing panencephalitis and chronic traumatic encephalopathy. Acta Neuropathologica Communications. 2023;11:74. doi: 10.1186/s40478-023-01565-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi C, Verheijen BM, Kokubo Y, Shi Y, Tetter S, Murzin AG, Nakahara A, Morimoto S, Vermultst M, Sasaki R, Aronica E, et al. Tau filaments from amyotrophic lateral sclerosis/parkinsonism-dementia complex (ALS/PDC) adopt the CTE fold. Proceedings of the National Academy of Sciences of the United States of America. 2023;120:e2306767120. doi: 10.1073/pnas.2306767120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang L, Sun X, Austin TO, Muralidharan H, Jean DC, Liu M, Yu W, Baas PW. Tau does not stabilize axonal microtubules but rather enables them to have long labile domains. Current Biology. 2018;28:2181–2189. doi: 10.1016/j.cub.2018.05.045. [DOI] [PubMed] [Google Scholar]
- Sawaya MR, Sambashivan S, Nelson R, Ivanova MI, Sievers SA, Apostol MI, Thompson MJ, Balbirnie M, Wiltzius JJW, McFarlane HT, Madsen AØ, et al. Atomic structures of amyloid cross-beta spines reveal varied steric zippers. Nature. 2007;447:453–457. doi: 10.1038/nature05695. [DOI] [PubMed] [Google Scholar]
- Scheres SHW, Ryskeldi-Falcon B, Goedert M. Molecular pathology of neurodegenerative diseases by cryo-EM of amyloids. Nature. 2023;621:701–710. doi: 10.1038/s41586-023-06437-2. [DOI] [PubMed] [Google Scholar]
- Schweighauser M, Garringer HJ, Klingstedt T, Nilsson KPR, Masuda-Suzukake M, Murrell JR, Risacher DL, Vidal R, Scheres SHW, Goedert M, Ghetti B, et al. Mutation ΔK281 in MAPT causes Pick’s disease. Acta Neuropathologica. 2023;146:211–226. doi: 10.1007/s00401-023-02598-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighauser M, Murzin AG, Macdonald J, Lavenir I, Crowther RA, Scheres SHW, Goedert M. Cryo-EM structures of tau filaments from the brains of mice transgenic for human mutant P301S tau. Acta Neuropathologica Communications. 2023;11:160. doi: 10.1186/s40478-023-01658-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Murzin AG, Falcon B, Epstein A, Machin J, Tempest P, Newell KL, Vidal R, Garringer HJ, Sahara N, Higuchi M, et al. Cryo-EM structures of tau filaments from Alzheimer’s disease with PET ligand APN-1607. Acta Neuropathologica. 2021;141:697–708. doi: 10.1007/s00401-021-02294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Zhang W, Yang Y, Murzin AG, Falcon B, Kotecha A, van Beers M, Tarutani A, Kametani F, Garringer HJ, Vidal R, et al. Structure-based classification of tauopathies. Nature. 2021;598:359–363. doi: 10.1038/s41586-021-03911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:7737–7741. doi: 10.1073/pnas.95.13.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sündermann F, Fernandez M-P, Morgan RO. An evolutionary roadmap to the microtubule-associated MAP tau. BMC Genomics. 2016;17:264. doi: 10.1186/s12864-016-2590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Bergen M, Friedhoff P, Biernat J, Heberle J, Mandelkow EM, Mandelkow E. Assembly of tau protein into Alzheimer paired helical filaments depends on a local sequence motif ((306)VQIVYK (311)) forming beta structure. Proceedings of the National Academy of Sciences USA. 2000;97:5129–5134. doi: 10.1073/pnas.97.10.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallon D, Bolida S, Rovelet-Lecrux A, Thierry M, Lagarde J, Miguel L, Lecourtois M, Bonnevalle A, Sarazin M, Bottlaender M, Mula M, et al. Clinical and neuropathological diversity of tauopathy in MAPT duplication carriers. Acta Neuropathologica. 2021;142:259–278. doi: 10.1007/s00401-021-02320-4. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mandelkow E. Tau in physiology and pathology. Nature Reviews Neuroscience. 2015;17:22–35. doi: 10.1038/nrn.2015.1. [DOI] [PubMed] [Google Scholar]
- Weingarten MD, Lockwood AR, Hwo S-Y, Kirschner MW. A protein factor essential for microtubule assembly. Proceedings of the National Academy of Sciences of the United States of America. 1975;72:1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsen KC, Lynch T, Pavlou E, Higgins M, Nygaard TG. Localization of disinhibition-dementia-parkinsonism-amyotrophy complex to 17q21-22. American Journal of Human Genetics. 1994;55:1159–1165. [PMC free article] [PubMed] [Google Scholar]
- Wille H, Drewes G, Biernat J, Mandelkow EM, Mandelkow E. Paired helical filaments and antiparallel dimers formed from microtubule-associated protein tau in vitro. Journal of Cell Biology. 1992;118:573–584. doi: 10.1083/jcb.118.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischik CM, Crowther RA, Stewart M, Roth M. Subunit structure of paired helical filaments in Alzheimer’s disease. Journal of Cell Biology. 1985;100:1905–1912. doi: 10.1083/jcb.100.6.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischik CM, Novak M, Edwards PC, Klug A, Tichelaar W, Crowther RA. Structural characterization of the core of the paired helical filament of Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:4884–4888. doi: 10.1073/pnas.85.13.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischik CM, Novak M, Thøgersen HC, Edwards PC, Runswick MJ, Jakes R, Walker JE, Milstein C, Roth M, Klug A. Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:4506–4510. doi: 10.1073/pnas.85.12.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JG, Mirra SS, Pollock NJ, Binder LI. Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:4040–4043. doi: 10.1073/pnas.83.11.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, Soeda Y, Shinzaki Y, In Y, Tomoo K, Ihara Y, Miyasaka T. Identification of key amino acids responsible for the distinct aggregation properties of microtubule-associated protein 2 and tau. Journal of Neurochemistry. 2015;135:19–26. doi: 10.1111/jnc.13228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Goedert M. Molecular cloning and functional characterization of chicken brain tau: Isoforms with up to five tandem repeats. Biochemistry. 2002;41:15203–15211. doi: 10.1021/bi026464m. [DOI] [PubMed] [Google Scholar]
- Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski JQ, Lee VM-Y. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Zhang W, Falcon B, Murzin AG, Fan J, Crowther RA, Goedert M, Scheres SHW. Heparin-induced tau filaments are polymorphic and differ from those in Alzheimer’s and Pick’s diseases. eLife. 2019;8:e43584. doi: 10.7554/eLife.43584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Tarutani A, Newell KL, Murzin AG, Matsubara T, Falcon B, Vidal R, Garringer HJ, Shi Y, Ikeuchi T, Murayama S, et al. Novel tau filament fold in corticobasal degeneration. Nature. 2020;580:283–287. doi: 10.1038/s41586-020-2043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]