Abstract
Ageing populations and improved survival, have contributed to a rise in the number of people living with multimorbidity, raising issues related to polypharmacy, treatment burden, competing priorities and poor coordination of care. Self-management programs are increasingly included as an essential component of interventions to improve outcomes in this population. However, an overview of how interventions supporting self-management in patients with multimorbidity is missing. This scoping review focused on mapping the literature on patient-centered interventions for people living with multimorbidity. We searched several databases, clinical registries, and grey literature for RCTs published between 1990–2019 describing interventions that supported self-management in people with multimorbidity. We included 72 studies that were found to be very heterogeneous when it comes to the population, delivery modes and modalities, intervention elements and facilitators. The results pointed to an extensive use of cognitive behavioral therapy as a basis for interventions, as well as behavior change theories and disease management frameworks. The most coded behavior change techniques stemmed from the categories Social Support, Feedback and monitoring and Goals and Planning. To allow for implementation of effective interventions in clinical practice, improved reporting of intervention mechanisms in RCTs is warranted.
Keywords: Multimorbidity, self-management, scoping review, behavior change techniques
Introduction
Multimorbidity, conceptualized as the co-existence of two or more chronic diseases in an individual is linked with decreased quality of life, functional decline, and increased healthcare utilization (Wallace et al., 2015).People living with multimorbidity commonly experience mental health difficulties, treatment burden, polypharmacy, and functional decline (Bayliss, Bayliss, Ware, & Steiner, 2004; Fortin et al., 2014; May, Montori, & Mair, 2009; Tinetti, Bogardus, & Agostini, 2004). This may have profound implications for research, treatment, and health policy (Fortin, Soubhi, Hudon, Bayliss, & Akker, 2007; Multimorbidity: A Priority for Global Health Research, 2018). Evidence highlights that treating one condition at a time is inconvenient, inefficient, and unsatisfactory for people with multimorbidity and their health care provider (Bower et al., 2011; Noël et al., 2007; Smith, O’Kelly, & O’Dowd, 2010). A more holistic approach is warranted that considers people’s social context, chronic conditions, and daily life (van der Aa, van den Broeke, Stronks, & Plochg, 2017).
A broad consensus is that multimorbidity is best addressed by a patient-centered approach (Multiple Chronic Conditions: A Strategic Framework, 2010., (Farmer et al., 2016). While there is no single definition of patient-centered care (or person-centered care), Bauman and colleagues regard it as the partnership between health practitioner and patient, based on communication and a focus that goes beyond specific conditions to emphasize health promotion and healthy lifestyles (Bauman et al., 2003). McWilliam highlighted that empowering patients to self-manage is central to patient-centered approaches (McWilliam, 2009). Chronic condition self-management and patient-centeredness are closely linked through the emphasis on shared responsibility and decision-making to achieve better health and wellbeing as defined by the person and the acknowledgement of the social, psychological, biological, and spiritual aspects that impact on self–management ability, placed within a context that respects the beliefs and values of the person (Lawn & Pols, 2005).
Self-management programs are increasingly recognized as an essential component of patientcentered interventions to improve outcomes and considered high-quality care for patients living with multimorbidity (Smith et al., 2016), (Bayliss et al., 2003). Some core elements for this population include providing education about multiple chronic conditions, psychological strategies to support the adjustment to life with chronic conditions, strategies to support adherence to multiple treatments, support around activities of daily living and physical functioning and providing social support (Taylor et al., 2014). There is a strong consensus that multiple causes of mortality and morbidity are linked with the behavior of individuals (Conner & Norman, 1996). Previous studies have emphasized the importance of unhealthy behavior (e.g., smoking, diet, alcohol use, physical inactivity) in the development and progression of multimorbidity (Wikström et al., 2015), (Fortin et al., 2014), (Dhalwani et al., 2016). Given the fact that behavior is modifiable, considerable efforts have been devoted to research, campaigns and interventions targeting health behaviors (e.g., weight control, smoking cessation, and increased physical activity) to improve health and thereby deter the development of chronic conditions and multimorbidity (Suls & Green, 2019). Defined as ‘overt behavioural patterns, actions and habits that relate to health maintenance, health restoration and health improvement’ (Gochman, 1997), health behaviors have received considerable attention from researchers, who explored the factors influencing how and why people engage in these behaviors (Conner et al., 2002). Understanding how (patient-centered) interventions might support selfmanagement in patients with multimorbidity is pivotal in promoting health in this population (e.g., by preventing or reversing physical inactivity). However, an overview of the literature in this area is missing.
A scoping review is ideally suited to determine the scope or coverage of a body of literature on a given topic as well as an overview of its volume and focus. In addition, it can be a useful tool to develop a theoretical understanding of the likely process of change by drawing on existing evidence and theory. This scoping review was part of the process of informing the development of an exercise and self-management intervention designed for people living with multimorbidity (see https://www.mobilize-project.dk/). Conducting a scoping review of the literature is a very good strategy to get an overview of the field.
Furthermore, despite the wide recognition of the biopsychosocial model and the connection between physical and psychological disorders, multimorbidity has not been a major focus in health psychology to date. This scoping review will be the first to contribute to a detailed understanding of the interventions evaluated in people with multiple chronic conditions by mapping the characteristics, types, duration, targets, delivery modes and modalities, impact, facilitators, behavior change techniques and frameworks/models used in the interventions. Classifying behavior change techniques used in interventions to promote self-management in people with multimorbidity will also be a first step in identifying potential mechanisms of change.
Objectives
This scoping review focused on mapping the literature on patient-centered interventions for people living with multimorbidity that support self-management. In accordance with the definition of selfmanagement by Lorig and Holman (2003) (i.e., medical, or behavioral management of the disease, role management, and emotional management), we included a broad range of interventions focusing on health behavior change and/or providing psychological or social support or other approaches. These interventions were classified according to the underlying theory and behavior change techniques (BCTs) and strategies utilized.
Materials and methods
This scoping review conformed to the framework for conducting scoping reviews developed by the Joanna Briggs Institute (Peters et al., 2015). The reporting followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR)(Tricco et al., 2018). The protocol for the scoping review was made public a priori and is available on the Open Science Framework (https://osf.io/eszb7).
Eligibility criteria
The following eligibility criteria were formulated to identify relevant studies. The studies were randomized controlled trials published between January 2000 and November 2019 as multimorbidity is a newer term (Tugwell & Knottnerus, 2019). The participants were adults (18 + years old) living with multimorbidity, defined for this review, as having at least two of the following conditions: osteoarthritis (OA), ischemic heart disease or heart failure, hypertension, type 2 diabetes mellitus (T2DM), chronic obstructive pulmonary disease (COPD) and depression.1 Studies that targeted multimorbidity (two or more chronic conditions) where at least two of the conditions of interest for this scoping review were present were also included. Further, the interventions had to incorporate self-management elements directed at individuals. We focused on self-management elements that included patient education, support for decision-making, self-monitoring, and psychological and social support. We excluded interventions focusing solely on medical service usage (e.g., physician visits, hospital stays etc.) or merely evaluating professional and organizational interventions. In addition, one or more behavior change techniques (BCTs) had to be present for the study to be included. Studies focusing solely on medical service usage (e.g., physician visits, hospital stays etc.) or merely evaluating professional and organizational interventions were excluded. We did not apply any restriction to the type of comparator groups, outcome domains, country of origin or the language of the papers.
Searching for the evidence
Information sources
The search strategy (see Supplementary file 1) was adapted from two previous reviews (Bricca et al., 2020), (Willett et al., 2019). The final search strategy was tailored for use in the different databases.
The following electronic databases were searched: Cochrane Database of Systematic Reviews, Joanna Briggs Library, MEDLINE via PubMed, EMBASE via Ovid, CINAHL via Ovid, Scopus, and Psychinfo via Ovid. In addition, a search for grey literature was performed to identify unpublished trials.The search was done in the following databases: World Health Organization International Clinical Trials Registry Platform, Agency for Healthcare Research and Quality, ClinicalTrials.gov, Open-Grey.eu, and WorldCat.org. Citation tracking was be performed using Web of Science (WoS). Finally, the reference lists of reviews and trials found through the searches were hand-searched for additional references to ensure that relevant articles were not missed.
Selecting the studies
The identified citations were uploaded to EndNote X9, and duplicates were removed. Due to the search size, four reviewers participated in the screening (MS, GZ, AB & MD). The identified studies were divided into two, and the two teams (AB & MD and MS & GZ) screened titles and abstracts independently and applied the eligibility criteria accordingly. Eligibility uncertainties were resolved by screening the full-text article and discuss until a consensus was reached. The qualified studies were then read in full text and evaluated against the eligibility criteria for the final decision by two reviewers (MS & GZ). Any disagreement was discussed until consensus. If information was missing (e.g., distribution of included conditions), the corresponding author was contacted via email. They were given two weeks to reply and sent a second email if they did not respond to the first one.
Data charting process
Key variables were extracted using an a priori developed extraction template compiled in Microsoft Excel (2019) in duplicates by two reviewers (MJ & GZ). Study information on identifiers of the target population, purpose, context, intervention details, outcome(s), theoretical framework or theory, and the target behavior(s)/outcomes was extracted. The two reviewers checked the data and uncertainties were resolved by revisiting the full-text, supplementary materials or requesting additional information from the authors.
BCT coding process
Each intervention was coded for BCTs using Michie et al. V1 behavior change taxonomy (Michie et al., 2013). BCTs have been conceptualized as replicable components of an intervention designed to alter or redirect causal processes that regulate behavior (e.g., Social support, Providing feedback, Goal setting, Information about health consequences). All the intervention elements (stated either in the manuscript, protocol, or supplementary material) that contained a specific BCT were coded. Two researchers (MJ & GZ) performed the coding independently after being trained in using the taxonomy. After coding the interventions, the two researchers discussed coding related issues and consulted a third researcher (ZS) for advice on these issues. Coding related disagreements were resolved through discussion. The final agreed coding was summarized in a grid presenting each study and the coded BCTs. The interventions were clustered by type, target behavior(s)/outcomes and combinations of chronic conditions they address. These elements were reported in the original studies or deduced from the content of the interventions. SPSS 26 (IBM SPSS Statistics for Windows [Internet], 2019) and Microsoft Excel (2019) were used to categorize and summarize the data. A table presenting the BCTs used in each study was developed. The total number of BCTs (individually and hierarchal groups) used across trials was calculated. A breakdown of the frequency and specific combinations of BCTs utilized in the interventions was compiled. The results were presented visually (using tables, charts, and diagrams) and in a descriptive format (narrative synthesis).
Synthesis of results
The results are presented both in a visual form (e.g., using tables, and charts, as appropriate) and in a descriptive format (narrative synthesis) and include frequencies and proportions according to the intervention characteristics (type, duration, targets, delivery modes and modalities, impact, facilitators, behavior change techniques and frameworks/models utilized).
Results
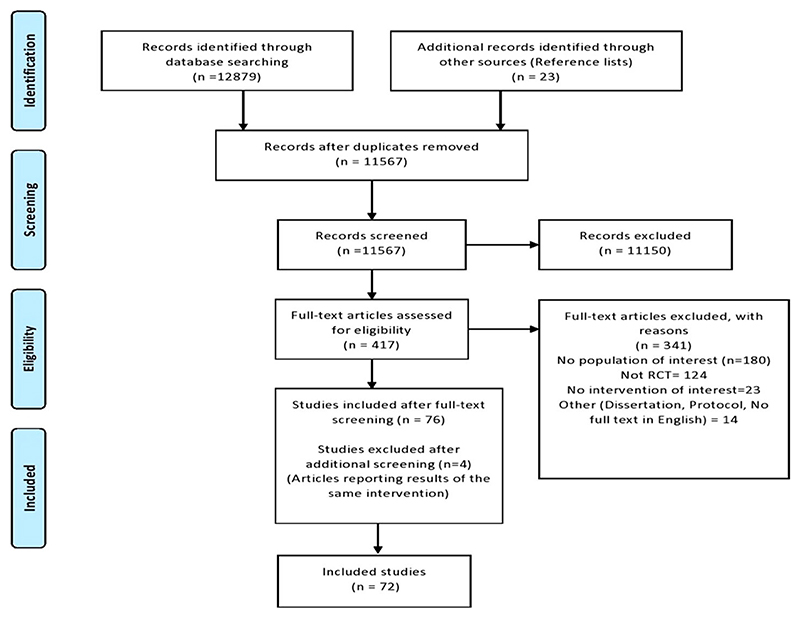
The initial search yielded a total of 12,879 studies and 23 additional studies from other sources. After checking for full-text availability, removing duplicates and screening articles against the inclusion and exclusion criteria, a total of 72 RCT studies were identified for inclusion and data extraction (Figure 1). Table 1 presents the participants, intervention characteristics and target of interventions of the included RCT studies.
Figure 1. Prisma diagram illustrating the flow of information through the different phases.
Table 1. Characteristics of included studies.
| Author, Year, Country | Participants | Type of intervention | Target of intervention | Comparator3 | Theory mentioned in text |
|---|---|---|---|---|---|
| (Ahmadpanah et al., 2016), Iran | Hypertension & Depression N = 45 | Metacognitive detached mindfulness therapy (MDM) or Stress management training (SMT) | Reducing high blood pressure, depression, and anxiety | UC + | Metacognitive detached mindfulness therapy (MDM), stress management principles (SMT), systematic desensitization (Wolpe 1958) attention training techniques (ATT) (Kang, Gruber, & Gray, 2013) |
| (Alexopoulos et al., 2013) United States | COPD & Depression N = 138 | Personalised intervention for depression and COPD (PID-C) | Depression, adherence to rehabilitation and treatment | UC | Theory of reasoned action (TRA) |
| (Alexopoulos et al., 2016), United States | COPD & Depression N = 101 | Problem Solving-Adherence (PSA) and personalized intervention (PID-C) | Depression, adherence to rehabilitation and treatment | PID-C vs. PSA | Problem- solving therapy (PST) |
| (Barker et al., 2018), Australia | MM (COPD, bronchiectasis, heart failure, coronary artery disease or ischemic heart disease and at least one other chronic condition, such as diabetes, hypertension, and cancer) N = 17 | A multimorbidity rehabilitation program (exercise and education) | Improving exercise capacity, self-management, quality of life and reducing hospitalizations | UC | Multimorbidity rehabilitation |
| (Barley et al., 2014), United Kingdom | Heart Disease & Depression N = 81 | Personalised care (PC) | Improving Selfmanagement, reducing depression | UC | Self-efficacy (goal setting and action planning) |
| (Beckie et al., 2011), United Kingdom | Heart Disease & Depression N = 252 | Behavior change plan (Informed by TTM) | Reducing depression | UC | Transtheoretical model (TTM) and Motivational Interviewing (MI) |
| (Behnammoghadam et al., 2015), Iran | Heart Disease & Depression N = 60 | EMDR therapy | Reducing depression | UC + | EMDR therapy |
| (Berkman et al., 2003) United States | Heart Disease & Depression N = 2481 | Psychoeducation and medication | Reducing depression and increasing social support | UC + | CBT, Social Learning Theory |
| (Bogner & de Vries, 2008), United States | Hypertension & Depression N =64 | Integrated care | Improving adherence to medication, increase BP control, and reduce depression | UC | Theory of reasoned action (TRA) |
| (Bogner & de Vries, 2010), United States | Diabetes & Depression N = 58 | Integrated care | Improve medication adherence, glycaemic control and reduce depression | UC | Theory of reasoned action (TRA) |
| (Bogner et al., 2012), United States | Diabetes & Depression N = 180 | Integrated care | Improve adherence to medication, glycaemic control and depression. | UC | Theory of reasoned action (TRA) |
| (Bogner et al., 2013) United States | Hypertension & Depression N =60 | Integrated care | Improve BP control and depressive symptoms. | UC | Theory of reasoned action (TRA) |
| Diabetes & Depression N = 360 | Self-management support | UC + | Self-management support | ||
| (Cherrington et al., 2018), United States | Improving self-management (T2DM control, exercise, diet, stress management), reducing depressive symptoms and healthcare utilization) | ||||
| (Clarke et al., 2019), Australia | Diabetes & Depression N = 723 | Self-guided CBT | Improving work and social functioning (in T2DM), reducing depression | CG + Placebo control program (Healthy Lifestyles) with health and lifestyle information | CBT |
| (Coventry et al., 2015), United Kingdom | MM (diabetes and/or coronary heart disease, Depression) N = 387 | Collaborative care | Reduce depressive symptoms and anxiety, increase self-management | UC | Collaborative care model, ABC model |
| (de Godoy & de Godoy, 2003), Brazil | COPD & Depression N = 30 | Exercise, psychotherapy and education | Reduce depression and anxiety | CG + PT, Exercise, Education | Exercise, psychotherapy, and education |
| (de Groot et al., 2019), United States | Diabetes & Depression N = 140 | Psychotherapy, exercise, and education | Reduce depression | UC or EX (Exercise only) or CBT + EX all offered the Dining with Diabetes nutrition education program | Beck’s model of cognitive therapy |
| (Dejesus et al., 2009) United States | Diabetes & Hypertension N = 54 | Education and home BP selfmonitoring | Improve self-management | UC | Chronic disease model |
| (Dekker et al., 2012), United States | Heart Disease & Depression N = 41 | Brief cognitive therapy | Reduce depressive symptoms and negative thinking, improve QoL | UC | Beck’s model of cognitive therapy (CT) |
| (Denver et al., 2003), United Kingdom | Diabetes & Hypertension N = 120 | Nurse-led HT clinic group | Improve management of uncontrolled HT | UC | Nurse-led HT clinic group |
| (Doyle et al., 2017), Australia | COPD & Depression N=110 | Telephone CBT | Reducing depression and anxiety and improving self-efficacy | UC + Active social control (befriending) | CBT, Befriending |
| (Dunbar et al., 2014), United States | Diabetes & Heart Disease N = 65 | Education, counseling, selfmanagement | Self-care and selfmanagement (diet, medication, symptom monitoring, physical activity, and recognition of the interaction between self-management strategies for HF and DM) | UC + | Integrated theoretical framework to guide HF and DM self-care |
| (Dunbar et al., 2014), United States | Diabetes & Heart Disease N = 134 | Integrated HF-T2DM Self-Care | Integrate and improve HF and DM self-care (diet, medications, selfmonitoring, symptoms, and PA) | UC + | Integrated theoretical framework to guide HF and DM self-care |
| (Edelman et al., 2010) United States | Diabetes & Hypertension N = 239 | Self-management and medical clinic education groups | Improve self-care and selfmanagement | UC | Self-management model, Group medical clinics |
| (Edelman et al., 2015), United States | Diabetes & Hypertension N = 377 | Tailored behavioral intervention | Improve self-management of comorbid T2DM and HT | UC + | Nurse led behavioral management |
| (Egede et al., 2018), United States | Diabetes & Depression N = 90 | Reducing depression and anxiety and improving glycaemic control | Reducing depression and anxiety and improving glycaemic control | BAT via telemed at home vs. BAT in same room | Behavioral activation |
| (Fortin et al., 2016), Canada | MM (Diabetes, cardiovascular disease, COPD, asthma, tobacco smoking, obesity, hyperlipidaemia, Prediabetes) N = 332 | Self-management support and health education | Improve chronic disease prevention and selfmanagement of multimorbidity | CG + 3 months delayed intervention | Chronic disease prevention and management (CDPM) |
| (Freedland et al., 2009), United States | Heart Disease & Depression N = 123 | CBT or Supportive stress management and medication | Reducing depression and improving HF self-care after surgery | UC | Cognitive behavior therapy (CBT), Stress management |
| (Freedland et al., 2015), United States | Heart Disease & Depression N = 158 | CBT and tailored education (for HF) | Reduce depression and improve self-care in HF patients | UC | CBT and tailored education (for HF) |
| (Gary et al., 2010), United States | Heart Disease & Depression N = 74 | CBT, exercise, CBT and exercise | Improving physical activity, reducing depression, and enhancing quality of life | CBT + Exercise vs CBT only vs Exercise only vs. UC | CBT (Beck’s model of depression) |
| (Garvey et al., 2015), Ireland | MM (two or more chronic conditions, 43 conditions identified, most common: arthritis, congestive cardiac failure, diabetes, depression and hypertension) N = 50 | Multimodal program: Fatigue management; healthy eating; maintaining physical activity, maintaining mental health; managing medications and communicating effectively with health professional | Improving activity participation, self-efficacy, self-management and quality of life | UC + Waiting list (invited to attend an OPTIMAL course following trial completion) | Adapted Stanford Chronic Disease Self-Management Program, Bandura’s model of Self efficacy |
| (Glasgow et al., 2006), United States | Diabetes & Depression N = 335 | Computer-assisted Selfmanagement Intervention (focused on healthy eating and physical activity) | Improving diabetes selfmanagement (by improving healthy eating and exercise) | UC | Chronic care model selfmanagement framework, Motivational interviewing (MI) (Miller & Rollnick) |
| (Hochhalter et al., 2010), United States | MM (treated for at least two of following: arthritis, lung disease, heart disease, diabetes, hypertension, depression, and osteoporosis) N = 79 | Patient engagement intervention or safety Group | Improve patients’ selfefficacy for managing multimorbidity | Group A: Intervention (Appointment - workshop + phone call) Group B: Safety (attention control - workshop + phone call) Group C: UC | Self-Determination Theory (SDT) |
| (Huang et al., 2016), Taiwan | Diabetes & Depression N = 61 | Motivational enhancement therapy (MET) and CBT | Reduce depressive symptoms and increase quality of life | UC + | Theory of stress and coping (Lazarus and Folkman) modified by Miller & Rollnick’s motivational principles |
| (Hynninen et al., 2010), Norway | COPD & Depression N = 51 | CBT | Reduce anxiety and depression | UC + | CBT adapted by Stanley et al.) |
| (Jang et al., 2018), Korea | Heart Disease & Depression N =44 | Mindfulness-based art therapy (MBAT) | Reduce depression, trait anxiety, anger and anger expression | UC [Waiting list control (received MBAT afterwards)] | Kabat-Zinn’s mindfulness meditation (MBSR,) Monti’s Mindfulness-based art therapy (MBAT) and Selfregulation theory |
| (Kasteleyn et al.,2016), Netherlands | Diabetes & Heart Disease N = 161 | Self-management support, motivational interviewing | Improve self-management, self-efficacy and wellbeing, reducing distress | UC + Attention control, 1 telephone consultation | Leventhal’s Common-Sense Model of self-regulation (CSM), Bandura’s Social Cognitive Theory, Motivational interviewing |
| (Kunik et al., 2008), United States | COPD & Depression N = 238 | CBT | Reduce anxiety and depression | Education group | CBT |
| (Lamers et al., 2010), Netherlands | COPD & Depression N = 187 | Minimal Psychological Intervention (MPI) | Reducing depression and anxiety, and improving quality of life | UC | CBT and self-management |
| (Lamers et al., 2011), Netherlands | Diabetes & Depression N = 208 | Minimal Psychological Intervention (MPI) | Reducing depression and improving quality of life | UC | CBT and self-management |
| (Landman et al.,2013), Netherlands | Diabetes & Hypertension N = 48 | Biofeedback | Lowering blood pressure | CG [visually identical device guiding users to a breathing frequency of approximately 14 breaths/ min] | Biofeedback |
| (Liu et al.,2019), China | COPD & Depression N = 60 | Reduce depression and improve the quality of life | Reduce depression and improve the quality of life | UC + | Music Therapy |
| (Logan et al., 2012), Canada | Diabetes & Hypertension N = 110 | Telemonitoring Self-Care Support System | Improve blood pressure control and self-care | UC | Telemonitoring Self-Care Support System |
| (Logtenberg et al., 2007), Netherlands | Diabetes & Hypertension N = 30 | Device-guided breathing (Resperate) | Change in mean daytime ambulatory BP | UC + | Device-guided breathing (Resperate) |
| (Long et al., 2015), China | Diabetes & Depression N = 100 | Group counseling | Reduce depression, improve treatment compliance and blood sugar control | UC + | Cognitive-behavioral group counseling, group dynamics |
| (Ludman et al., 2013), United States | MM (Depression, Diabetes and/or coronary heart disease) N = 214 | TEAM care (self-management support, monitoring disease and pharmacotherapy) | Improving self-care-efficacy and confidence to maintain lifestyle changes (diet and exercise), reducing depression | UC | Collaborative care, Social Cognitive Theory, Self-management support |
| (Lundgren et al., 2016), Sweden | Heart Disease & Depression N = 50 | Internet-Based Cognitive Behavioral Therapy (ICBT) | Reduce depression and anxiety and improve quality of life | CG + | CBT |
| (Lutes et al., 2018), United States | Diabetes & Depression N = 139 | Collaborative care | Reducing depression and diabetes distress, improving self-care (diet, physical activity, smoking, foot care) | UC + educational materials | CBT, Problem-solving Therapy (PST), Collaborative care model |
| (Lynch et al., 2014), United States | Diabetes & Hypertension N = 61 | Self-management intervention-Lifestyle Improvement through Food and Exercise (LIFE) | Improve self-management (diet, physical activity, and glycaemic control) | CG | Information processing model for food choice, self-management |
| (Moral et al., 2015), Spain | MM (mean of 5 chronic conditions/patient: hypertension and diabetes -the most prevalent) N = 154 | Motivational Interviewing | To promote adherence to treatment in people treated by polypharmacy | UC | Motivational Interviewing (MI) |
| (Nie et al., 2019) China | Heart Disease & Depression N = 284 | Education and lifestyle improving program (IPEL) | Reducing anxiety and depression, improving lifestyle (healthy diet, physical activity, and smoking cessation) | UC | Multicare intervention |
| (Norlund et al., 2018), Sweden | Heart Disease & Depression N = 239 | Internet-Based Cognitive Behavioral Therapy (ICBT) |
Reducing depression and anxiety | TAU | CBT |
| (O’Moore et al., 2018), Australia | Osteoarthritis & Depression N = 69 | ICBT Sadness Program | Reducing depression | UC | CBT |
| (O’Neil et al., 2014), Australia | Heart Disease & Depression N = 121 | Reducing depression, improving self-management | Reducing depression, improving selfmanagement | UC | CBT |
| (Onyechi et al., 2016), Nigeria | Diabetes & Depression N = 80 | Cognitive behavioral coaching | Reducing depression | UC | Cognitive Behavioral Coaching for Depression Manual (CBCDM) |
| (Ose et al., 2019), Germany | MM (Diabetes and at least two other severe chronic comorbidities) N = 495 | Care management program | Improving self-management and self-care (diet, exercise, self-monitoring of blood glucose and foot care) | UC | Care management, Primary Care Network (PCN) |
| (Parswani et al., 2013), New Zealand | Heart Disease & Depression N =30 | MBSR | Reduce anxiety and depression | TAU + health education session | Mindfulness meditation (Jon Kabat-Zinn, Segal et al.) |
| (Penckofer et al., 2012), United States | Diabetes & Depression N = 74 | Psychoeducation (SWEEP program) CBT and education | Reduce depression, anxiety and anger and improve quality of life | UC + | CBT, ‘Reality Management Approach for Persons with Depression’ (Munoz et al.) |
| (Pibernik-Okanovic et al., 2015), Croatia | Diabetes & Depression N = 209 | Psychoeducation, Physical exercise, enhanced UC | Reducing depression, improving diabetes distress, self-management and quality of life | Group A: CBT + Self-help manual. Group B: Exercise (same interval and duration as CBT) Group C: Enhanced treatment as usual | CBT |
| Diabetes & Depression N = 291 | UC + | CBT | |||
| (Piette et al., 2011), United States | Telephone-delivered CBT program | Improve management of depressive symptoms, physical activity levels, and diabetes-related outcomes | |||
| (Rachmani et al., 2002), Israel | Diabetes & Hypertension N = 141 | Patient Participation Program | Improve self-management, self-monitoring, and disease control | UC | Intensive therapy, self-management |
| (Renn et al., 2018), United States | COPD & Depression N=175 | Brief CBT (Psychoeducation, chronic disease selfmanagement, goal setting, behavioral activation, cognitive restructuring, relaxation, and coping with physical health symptoms) | Reduce illness intrusiveness and improve selfmanagement | EUC (enhanced usual care) | CBT |
| (Richards et al., 2018), United Kingdom | Heart Disease & Depression N = 29 | EPC (enhanced psychological care) | Reduce depression and anxiety, mortality and morbidity and improve quality of life | UC | Enhanced psychological care, rehabilitation |
| (Safren et al., 2014), United States | Diabetes & Depression N = 87 | Enhanced treatment as usual, CBT | Reduce depression, improve adherence and glycaemic control | UC + | CBT, Motivational interviewing (MI) |
| (Salisbury et al., 2018), United Kingdom | MM (at least three types of chronic condition) N = 1546 | Patient-centred care model (3D approach) | Improve patient-cantered care, self-care, and quality of life | UC | Patient-centred care model (3D approach) |
| (Schneider et al., 2016), United States | Diabetes & Depression N = 29 | Behavioral Activation and Exercise | Increasing exercise level and exercise enjoyment | Enhanced Usual Care + Depression treatment referrals were also provided. Participants received information available from the ADA on nutrition, exercise, and glucose monitoring |
Behavioral Activation (BA) |
| (Shen et al., 2018), China | Heart Disease & Depression N = 60 | Psychological Intervention Program | Improving mental state, reducing stress and negative coping styles | UC | Comprehensive psychological intervention (cognitive therapy; relaxation therapy, emotional support) |
| (Taveira et al., 2011), United States | Diabetes & Depression N = 88 | Psychoeducation (Pharmacist- Led Group Medical Appointments), Shared medical appointments (SMAs) | Reducing depression, improving self-care and self-care competence | UC | Psychoeducation (Pharmacist-Led Group Medical Appointments), Shared medical appointments (SMAs) |
| ICBT | UC | ||||
| (Turner et al., 2014), Australia | Heart Disease & Depression N = 42 | Decreasing depression, increasing self- efficacy, improve health perceptions and behavior change (physical activity, diet, medication adherence, and smoking cessation) | CBT and motivational interviewing (MI) | ||
| (Wakefield et al., 2011), United States | Diabetes & Hypertension N = 302 | Home telehealth device and nurse care management | Improve self-care (diet, exercise, smoking cessation, foot care, advice for sick days, medication, weight management, preventive care) | High-intensity vs low- intensity vs UC | Care management |
| (Wu et al., 2012) Australia | Heart Disease & Depression N = 30 | Peer CDSMP (self-management) | Improving knowledge, selfefficacy, and self-care behavior | UC + | Bandura’s theory of selfmanagement (Bandura, 1977, 2004) |
| (Yoo et al., 2009), Korea, Republic of | Diabetes & Hypertension N = 123 | Ubiquitous Chronic Disease Care (UCDC) system (self-management) | Improving self-management | UC | Chronic Disease Care |
Intervention characteristics
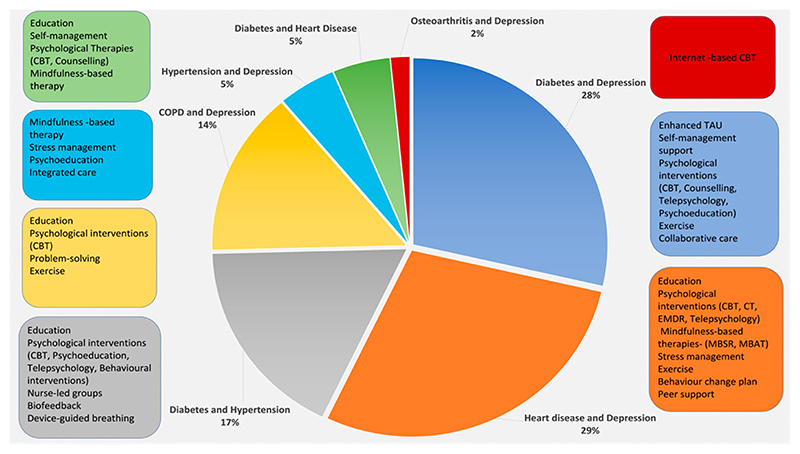
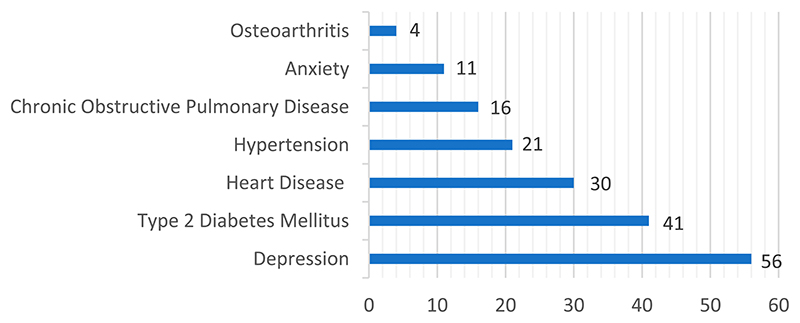
The 72 studies included a total of 13,866 participants. Age was giving in 65 of the 72 included studies, and the participants had a combined mean age of 61.1 years (SD 5.8). In seven studies, age was reported as a median or percentage, with a median age of 60.6 years. In the 70 studies that reported gender, 51% of the participants were females. In the 37 studies where ethnicity was stated, the majority was given as white (30%), other (10%), or African American (6%). The 72 studies were conducted in 20 different countries, with the USA being the most represented (44%), followed by Australia (10%), the United Kingdom (7%) and the Netherlands (7%). Depression was the most prevalent condition (56 studies), while osteoarthritis was the least occurring (4 studies). Regarding clusters of conditions, the most frequent combinations were heart disease and depression and Type 2 Diabetes and depression (both were present in 18 studies) (see Figure 3). Although not an inclusion criteri anxiety was present in 11 studies, either as an inclusion criterion for the single studies or as a comorbidity co-occurring with depression (see Figure 2).
Figure 3. Types of interventions according to the most common clusters of conditions.
Figure 2. Presence of single conditions in the 72 studies.
Intervention duration and target
Intervention duration was reported in 70 studies. In 41 of the studies (59%), the intervention duration was short (1-3 months), while 24% had a medium duration (4-6 months) and only 17% were of longer duration (6-12 months). Most interventions targeted depression (46 out of 72). Other interventions aimed to improve self-monitoring (18), self-care (14) and quality of life (14) and reduce anxiety (15).
Intervention type and comparator
The 72 studies reported a diversity of interventions with different combinations of elements (see Figure 3). Most of the interventions (37) had a psychotherapeutic component (e.g., cognitive behavioral therapy (CBT) or internet-based CBT, Cognitive Therapy, Counseling, Mindfulness Therapy, Motivational Interviewing, Telepsychology) consistent with the fact that depression was the most prevalent single condition. Patient education, psychoeducation, self-management, and self-care were also frequently included (n = 31) in addition. Other elements less frequently encountered were music therapy, biofeedback, stress management and exercise therapy. We included studies utilizing different types of comparators, from usual care to active interventions. The most common comparators were usual care (n = 38) and enhanced usual care (n = 23).
Delivery modes and modalities
Most of the interventions were delivered either face-to-face (n = 36) or both face-to-face and via telephone (n = 25). Only eight interventions had a web-based element, while two were delivered exclusively via telephone and one employed audiotape. Most of the interventions (n = 40) included were delivered one-to-one. Seventeen interventions were delivered in a group, ten were mixed, and five were self-guided.
Primary outcomes and impact of interventions on outcomes
The most frequently encountered primary outcome in the included studies was Depression (see Figure 4). Other commonly reported primary outcomes were blood pressure (17 studies), and quality of life (15 studies). Regarding intervention effectiveness (on primary outcomes), a large percentage of studies reported significant differences favoring the intervention group(s) (54 studies).
Figure 4. Frequency of primary outcomes.
Sixteen studies reported that there was no significant difference in outcomes between the intervention and the control group, while two studies were reported as being underpowered.
Professionals facilitating the interventions
Various professionals (i.e., diverse backgrounds and training) delivered the interventions either alone on their own or in a team. 28 interventions were delivered by nurses, followed by psychotherapists and psychologists (ten and eight, respectively). Other facilitators were physicians (n = 6), social workers (n = 3), occupational therapist (n = 1), pharmacists (n = 3), or physiotherapist (n = 1). Volunteers and research coordinators were also involved in the delivery of interventions.
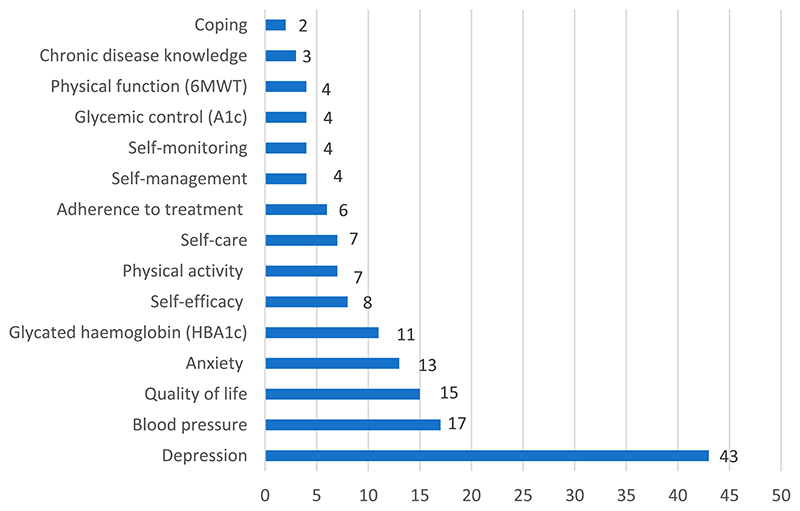
BCTs identified in the interventions
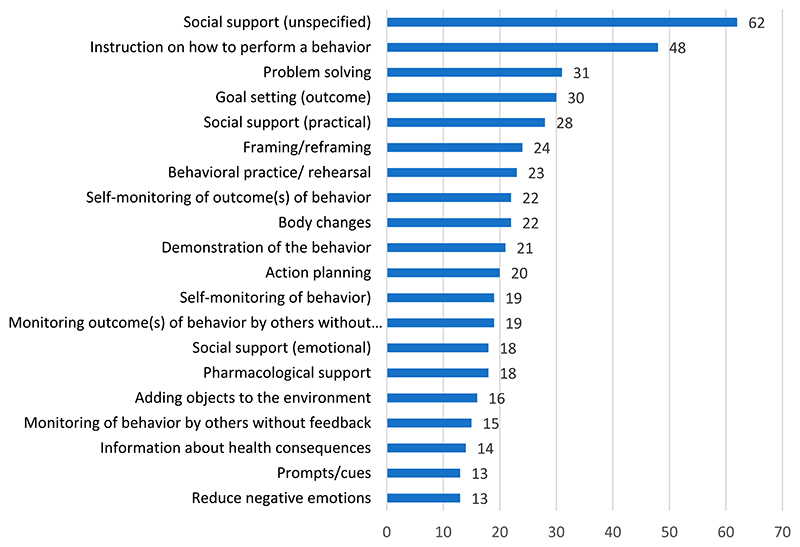
A total of 582 different BCTs were coded 2 (see Supplementary file 2). The highest number of BCTs used in a single study was 16, while the lowest was two. The most frequently identified BCTs were Social support (unspecified) (coded 62 times), Instruction on how to perform a behaviour (n = 48), Problem-solving (n = 31) and Goal setting (outcome) (n = 30) (see Figure 5). In line with this, the clusters with the most coded BCTs were Social support (n = 108), Feedback and monitoring (n = 99) and Goals and planning (n = 96). The least coded BCTs were Vicarious consequences, Self-talk, Rewarding completion, Valued self-identity and Incompatible beliefs all coded one time. These BCTs correspond to the clusters: Associations, Scheduled consequences, Self-belief, Covert learning and Reward and threat.
Figure 5. BCTs sorted by frequency.
Challenges arose when faced with coding BCTs present in interventions delivered to the comparator groups due to the lack of information describing their content. This was also because the most frequently encountered comparator was usual care or enhanced usual care, without providing details about usual care.
Frameworks/models used in interventions
All 72 studies included either a framework, model, or theory to inform the development of the intervention (based on information from the articles and supplementary materials).
Specific information about how the theory/model was used was in most cases deemed insufficient or lacking.
Discussion
To our knowledge, this is the first study to compile a comprehensive map of the interventional components and behavior change techniques used in patient-centered interventions that target self-management in people living with multimorbidity. We included 72 studies that reported interventions that were heterogeneous in terms of targeted populations, delivery modes and modalities, intervention elements, and facilitators (see Table 1 & Figure 5). The results pointed to extensive use of CBT as a basis for interventions, as well as behavior change theories and chronic disease management frameworks. In addition, the most coded BCTs stemmed from the categories of Social Support, Feedback and monitoring, and Goals and planning.
Very few studies have explored behavior change strategies utilized in patient-centered interventions for people living with chronic conditions. Findings from a previous review identifying BCTs and intervention features of dietary and physical activity interventions for patients with type 2 diabetes revealed that four BCTs were associated with a > 0.3% reduction in HbA1c: Instruction on how to perform a behaviour, Behavioural practice/rehearsal, Demonstration of the behaviour and Action planning (Cradock et al., 2017). However, contrary to our results that showed extensive use of theoretical models and frameworks as a basis for the interventions, this previous review by (Cradock et al., 2017) found that only three out of the thirteen RCTs included used a theory or model. Another similar systematic review focusing on the effects of BCTs for physical activity and healthy eating in overweight and obese adults found support for the use of goal setting and self-monitoring of behavior (Samdal et al., 2017). The authors of this review highlighted the importance of using a person-centered approach in maintaining healthy eating and physical activity long-term. The findings of our review are in line with previous literature focusing on the essential role of social support in improving outcomes for people with multimorbidity. Olaya and colleagues (Olaya et al., 2017) conducted a longitudinal survey including 2113 participants aged 60 + and confirmed the hypothesis that having two chronic physical conditions increased the risk of mortality over a 3-year follow-up period among people with low social support, compared with participants with no chronic illnesses. The authors emphasized that designing interventions that aim to increase social support can help improve the health status and survival of people who suffer from multimorbidity. Similarly, Vogel et al. explored the impact of perceived social support on health-related quality of life in people living with multimorbidity and found that higher perceived social support was associated with higher health-related quality of life scores (Vogel et al., 2012). Given the important role that social support (both emotional and practical) plays in the adaptation to chronic conditions (Eriksson & Rosenqvist, 1993), (King et al., 2018) future interventions designed for people experiencing multimorbidity should consider.
incorporating elements of social support (e.g., emotional support from family or friends, practical support from healthcare professionals). This may potentially act as a buffer to the limitations that multimorbidity imposes on people’s social lives (Sells et al., 2009). Finally, the findings of this review are in line with a Cochrane systematic review looking at interventions for improving outcomes in patients with multimorbidity in the primary care (Smith et al., 2021) in those interventions designed for this population are complex and multifaceted, reflecting the different needs of the individuals.
Limitations
One limitation is related to the poor reporting and vague description of interventions, which contributed to difficulties in coding the BCTs utilized in the studies. Furthermore, we cannot judge the quality of the trials included due to the absence of a quality assessment, which was beyond the scope of this review, that aimed to map the literature on interventions in this specific area. Another important limitation is the short duration of interventions, most of which (60%) were short (1-3 months). At best, the studies provide an indication of the characteristics of short-term interventions supporting self-management, lacking a focus on the maintenance of behavior change which is fundamental in self-management long-term.
While we included studies published until 2019 we believe that given the large number of studies included (k = 72) and the level of details of our analyses, including the BCT assessment, our results provide an unbiased and comprehensive overview of the topic.
Finally, defining multimorbidity as living with two or more of six specific chronic conditions as well as the period searched (2000-2019) may have limited the volume of literature selected for this review.
Implications
The findings presented here provide a starting point for further investigation of patient-centered interventions that include self-management designed for people living with multiple chronic conditions. Within the context of multimorbidity, there are some populations or clusters that could benefit from more focus. For example, while a vast volume of research has concentrated on people living with diabetes and depression or heart disease and depression, only a restricted number of trials were designed for people living with multimorbidity involving COPD or osteoarthritis.
There is a need for a better understanding of the emergence and persistence of multiple chronic conditions as well as an awareness of the relationship between psychological, behavioral, social, and environmental factors and multiple chronic conditions. Despite that the focus of health psychology is studying the connection between physical and psychological disorders, including the psychological determinants of health behavior, multimorbidity has not been a primary concern (Suls & Green, 2019). Nevertheless, health psychology has demonstrated the adoption of a biopsychosocial approach concerning the co-occurrence of psychological and medical conditions. Interventions are needed to promote health behavior adherence among people with chronic conditions that also address the challenges of living with multimorbidity. Recent developments in the design of health behavior interventions have highlighted the importance of theory and classifying intervention components (BCTs) (Michie et al., 2013) and mapping these intervention components onto mechanisms of change. Clear and accurate reporting of interventions is needed (Michie et al., 2009). Both CONSORT and TIDieR guidelines are useful in providing recommendations for reporting specific characteristics such as intervention content and delivery (Moher et al., 2001), (Hoffmann et al., 2014). To further develop effective programs for people with multiple chronic conditions, researchers should adhere to MRC guidelines by incorporating behavior change theory into interventions (Craig et al., 2008) and adequately report the relevant active components or BCTs (Painter et al., 2008). This will contribute to increased transparency and improved reporting as well as provide clinicians with sufficient information to implement effective interventions, also facilitating the understanding of potential intervention mechanisms.
We suggest that future research should focus on enriching the existing evidence base regarding the effectiveness of interventions for people living with multimorbidity. This aligns well with Smith et al. (2021) who highlighted that there are remaining gaps in our knowledge about the effectiveness of interventions for people with multimorbidity despite the increased number of RCTs in this area. One of the challenges is that the interventions tested so far for people with multimorbidity were not developed and tailored to this population (but for people with single chronic conditions). This might have contributed to the small effects observed in systematic reviews (Smith et al., 2021). Moreover, in addition to effectiveness, it is also important to consider the potential for implementation of interventions and the contextual factors relevant in their implementation (MRC Framework, 2021).
Finally, intervention designers should consider a full range of different components when developing patient-centered programs for people living with multimorbidity, using previous research evidence and recommendations, and based on a good understanding of patients’ needs and preferences, preferably also involving these when designing interventions. Not only will this enhance the acceptability of the interventions, but it will potentially lead to higher effectiveness.
Conclusions
This review highlighted the complexity and diversity of patient-centered interventions designed for people living with multiple chronic conditions. Even though all the interventions included in this review were informed either by a chronic disease management framework, psychotherapeutic approach, behavior change model, or theory, reporting needs to improve to allow adequate coding of BCTs and evaluation and implementation of effective interventions in clinical practice.
Acknowledgements
We want to acknowledge the support of Prof. Marie Johnston and Zoe Swithenbank, who was instrumental in coding the behavior change techniques. We are grateful to Mathias Constantin Lindhardt for his help, particularly in contacting authors. In addition, we would like to thank the authors who provided information and supplementary materials (Kathryn Barker, Martin Fortin, Russel E. Glasgow, Roger Luiz Moral).
Funding
European Research Council (ERC) under MOBILIZE project, European Research Council (ERC) Horizon 2020 research and innovation program, grant agreement No 801790, Næstved, Slagelse and Ringsted Hospitals’ Research Fund and The Association of Danish Physiotherapists Research Fund. Furthermore, Dr. Skou is currently funded by a program grant from Region Zealand [grant no Exercise First] and another grant from the European Union’s Horizon 2020 research and innovation program [grant no 945377 (ESCAPE)].
Footnotes
The reason why we focus on six specific conditions is that these conditions share a common risk factor (physical inactivity) and pathogenesis (systemic inflammation). Previous research has shown that interventions targeting specific related conditions, e.g., via risk factors, or with a shared focus on improving function, may be more beneficial than interventions targeting multimorbidity in general (Smith et al., 2016). In addition, these six conditions have been selected as the focus of our overall project (Mobilize- see https://www.mobilize-project.dk/).
BCTs range from 1.1 to 16.3 (See Behaviour change taxonomy V1), ✓ means the specific BCT is present
UC- Usual Care; UC +- Enhanced Usual Care; CG- Control group; TAU-Treatment as Usual; CBT- Cognitive Behavioural Therapy; EX- Exercise, BAT-Behavioural Activation Therapy; MBAT- Mindfulness Based Art Therapy; MBSR-Mindfulness Based Stress Reduction; MM-Multimorbidity
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Academy of Medical Sciences Report. Multimorbidity: A priority for global health research. 2018. Retrieved from https://acmedsci.ac.uk/policy/policy-projects/multimorbidity.
- Ahmadpanah M, Paghale SJ, Bakhtyari A, Kaikhavani S, Aghaei E, Nazaribadie M, Holsboer-Trachsler E, Brand S. Effects of psychotherapy in combination with pharmacotherapy, when compared to pharmacotherapy only on blood pressure, depression, and anxiety in female patients with hypertension. Journal of Health Psychology. 2016;21(7):1216–1227. doi: 10.1177/1359105314550350. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Kiosses DN, Sirey JA, Kanellopoulos D, Novitch RS, Ghosh S, Seirup JK, Raue PJ. Personalised intervention for people with depression and severe COPD. British Journal of Psychiatry. 2013;202(3):235–236. doi: 10.11192/bjp.bp.112.120139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Sirey JA, Banerjee S, Kiosses DN, Pollari C, Novitch RS, Artis A, Raue PJ. Two behavioral interventions for patients with major depression and severe COPD. The American Journal of Geriatric Psychiatry. 2016;24(11):964–974. doi: 10.1016/j.jagp.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy: Toward a unifying theory of behavioral change. Psychological Review. 1977;84(2):191–215. doi: 10.1037/0033-295X.84.2.191. [DOI] [PubMed] [Google Scholar]
- Bandura A. Swimming against the mainstream: The early years from chilly tributary to transformative mainstream. Behaviour Research and therapy. 2004;42(6):613–630. doi: 10.1016/j.brat.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Barker K, Holland AE, Lee AL, Haines T, Ritchie K, Boote C, Saliba J, Lowe S, Pazsa F, Thomas L, Turczyniak M, et al. Multimorbidity rehabilitation versus disease-specific rehabilitation in people with chronic diseases: a pilot randomized controlled trial. Pilot and Feasibility Studies. 2018;4(1):181. doi: 10.1186/s40814-018-0369-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barley EA, Walters P, Haddad M, Phillips R, Achilla E, McCrone P, Van Marwijk H, Mann A, Tylee A. The UPBEAT nurse-delivered personalized care intervention for people with coronary heart disease who report current chest pain and depression: A randomised controlled pilot study. PloS one. 2014;9(6):e98704. doi: 10.1371/journal.pone.0098704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman AE, Fardy HJ, Harris PG. Getting it right: Why bother with patient-centred care? Medical Journal of Australia. 2003;179(5):253–256. doi: 10.5694/j.1326-5377.2003.tb05532.x. [DOI] [PubMed] [Google Scholar]
- Bayliss EA, Bayliss MS, Ware JE, Steiner JF. Predicting declines in physical function in persons with multiple chronic medical conditions: What we can learn from the medical problem list. Health and Quality of Life Outcomes. 2004;2(1):1473. doi: 10.1186/1477-7525-2-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss EA, Steiner JF, Fernald DH, Crane LA, Main DS. Descriptions of barriers to self-care by persons with comorbid chronic diseases. The Annals of Family Medicine. 2003;1(1):15–21. doi: 10.1370/afm.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckie TM, Beckstead JW, Schocken DD, Evans ME, Fletcher GF. The effects of a tailored cardiac rehabilitation program on depressive symptoms in women: A randomized clinical trial. International Journal of Nursing Studies. 2011;48(1):3–12. doi: 10.1016/j.ijnurstu.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnammoghadam M, Alamdari AK, Behnammoghadam A, Darban F. Effect of Eye movement desensitization and reprocessing (EMDR) on depression in patients With myocardial infarction (MI) Global Journal of Health Science. 2015;7(6):258–262. doi: 10.5539/gjhs.v7n6p258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, Czajkowski SM, DeBusk R, Hosking J, Jaffe A, Kaufmann PG, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: The enhancing recovery in coronary heart disease patients (ENRICHD) Randomized Trial Jama. 2003;289(23):3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- Bogner HR, de Vries HF. Integration of depression and hypertension treatment: A pilot, randomized controlled trial. The Annals of Family Medicine. 2008;6(4):295–301. doi: 10.1370/afm.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogner HR, de Vries HF. Integrating type 2 diabetes mellitus and depression treatment among African Americans: A randomized controlled pilot trial. The Diabetes Educator. 2010;36(2):284–292. doi: 10.1177/0145721709356115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogner HR, de Vries HF, Kaye EM, Morales KH. Pilot trial of a licensed practical nurse intervention for hypertension and depression. Family Medicine. 2013;45(5):323–329. [PMC free article] [PubMed] [Google Scholar]
- Bogner HR, Morales KH, de Vries HF, Cappola AR. Integrated management of type 2 diabetes mellitus and depression treatment to improve medication adherence: A randomized controlled trial. The Annals of Family Medicine. 2012;10(1):15–22. doi: 10.1370/afm.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower P, Macdonald W, Harkness E, Gask L, Kendrick T, Valderas JM, Dickens C, Blakeman T, Sibbald B. Multimorbidity, service organization and clinical decision making in primary care: a qualitative study. Family Practice. 2011;28(5):579–587. doi: 10.1093/fampra/cmr018. [DOI] [PubMed] [Google Scholar]
- Bricca A, Harris LK, Jager M, Smith SM, Juhl CB, Skou ST. Benefits and harms of exercise therapy in people with multimorbidity: A systematic review and meta-analysis of randomised controlled trials. Ageing Research Reviews. 2020;63:101166. doi: 10.1016/j.arr.2020.101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrington AL, Khodneva Y, Richman JS, Andreae SJ, Gamboa C, Safford MM. Impact of peer support on acute care visits and hospitalizations for individuals With diabetes and depressive symptoms: A cluster-randomized controlled trial. Diabetes Care. 2018;41(12):2463–2470. doi: 10.2337/dc18-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke J, Sanatkar S, Baldwin PA, Fletcher S, Gunn J, Wilhelm K, Campbell L, Zwar N, Harris M, Lapsley H, Hadzi-Pavlovic D, et al. A Web-based cognitive behavior therapy intervention to improve social and occupational functioning in adults With type 2 diabetes (The SpringboarD trial): Randomized controlled trial. Journal of Medical Internet Research. 2019;21(5):e12246. doi: 10.2196/12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner M, Norman P. Predicting health behaviour: Research and practice with social cognition models (Reprint ed) Open University Press; 1996. https://go.exlibris.link/LzrybTty . [Google Scholar]
- Conner M, Norman P, Bell R. The theory of planned behavior and healthy eating. Health Psychology. 2002;21(2):194–201. doi: 10.1037/0278-6133.21.2.194. [DOI] [PubMed] [Google Scholar]
- Coventry P, Lovell K, Dickens C, Bower P, Chew-Graham C, McElvenny D, Hann M, Cherrington A, Garrett C, Gibbons CJ, Baguley C, et al. Integrated primary care for patients with mental and physical multimorbidity: Cluster randomised controlled trial of collaborative care for patients with depression comorbid with diabetes or cardiovascular disease. BMJ. 2015;350:h638. doi: 10.1136/bmj.h638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cradock KA, ÓLaighin G, Finucane FM, Gainforth HL, Quinlan LR, Ginis KAM. Behaviour change techniques targeting both diet and physical activity in type 2 diabetes: A systematic review and meta-analysis. International Journal of Behavioral Nutrition and Physical Activity. 2017;14(1):18. doi: 10.1186/s12966-016-0436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M, Medical Research Council G Developing and evaluating complex interventions: The new Medical Research Council guidance. Bmj. 2008;337:a1655. doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Godoy DV, de Godoy RF. A randomized controlled trial of the effect of psychotherapy on anxiety and depression in chronic obstructive pulmonary disease11No commercial party having a direct financial interest in the results of the research supporting this article has or willconfer a benefit upon the author(s)or upon any organization with which the author(s) is/are associated. Archives of Physical Medicine and Rehabilitation. 2003;84(8):1154–1157. doi: 10.1016/S0003-9993(03)00239-9. [DOI] [PubMed] [Google Scholar]
- de Groot M, Shubrook JH, Hornsby WG, Pillay Y, Mather KJ, Fitzpatrick K, Yang Z, Saha C. Program ACTIVE II: Outcomes from a randomized, multistate community-based depression treatment for rural and urban adults With type 2 diabetes. Diabetes Care. 2019;42(7):1185–1193. doi: 10.2337/dc18-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejesus RS, Chaudhry R, Leutink DJ, Hinton MA, Cha SS, Stroebel RJ. Effects of efforts to intensify management on blood pressure control among patients with type 2 diabetes mellitus and hypertension: A pilot study. Vascular Health and Risk Management. 2009;5:705–711. doi: 10.2147/VHRM.S5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker RL, Moser DK, Peden AR, Lennie TA. Cognitive therapy improves three-month outcomes in hospitalized patients with heart failure. Journal of Cardiac Failure. 2012;18(1):10–20. doi: 10.1016/j.cardfail.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denver EA, Barnard M, Woolfson RG, Earle KA. Management of uncontrolled hypertension ina nurse-led clinic compared with conventional care for patients with type2 diabetes. Diabetes Care. 2003;26(8):2256–2260. doi: 10.2337/diacare.26.8.2256. [DOI] [PubMed] [Google Scholar]
- Dhalwani NN, O’Donovan G, Zaccardi F, Hamer M, Yates T, Davies M, Khunti K. Long terms trends of multimorbidity and association with physical activity in older English population. International Journal of Behavioral Nutrition and Physical Activity. 2016;13(1):8. doi: 10.1186/s12966-016-0330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle C, Bhar S, Fearn M, Ames D, Osborne D, You E, Gorelik A, Dunt D. The impact of telephone-delivered cognitive behaviour therapy and befriending on mood disorders in people with chronic obstructive pulmonary disease: A randomized controlled trial. British Journal of Health Psychology. 2017;22(3):542–556. doi: 10.1111/bjhp.12245. [DOI] [PubMed] [Google Scholar]
- Dunbar SB, Butts B, Reilly CM, Gary RA, Higgins MK, Ferranti EP, Culler SD, Butler J. A pilot test of an integrated self-care intervention for persons with heart failure and concomitant diabetes. Nursing Outlook. 2014;62(2):97–111. doi: 10.1016/j.outlook.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman D, Dolor RJ, Coffman CJ, Pereira KC, Granger BB, Lindquist JH, Neary AM, Harris AJ, Bosworth HB. Nurse-led behavioral management of diabetes and hypertension in community practices: A randomized trial. Journal of General Internal Medicine. 2015;30(5):626–633. doi: 10.1007/s11606-014-3154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman D, Fredrickson SK, Melnyk SD, Coffman CJ, Jeffreys AS, Datta S, Jackson GL, Harris AC, Hamilton NS, Stewart H, Stein J, et al. Medical clinics versus usual care for patients with both diabetes and hypertension: A randomized trial. Annals of Internal Medicine. 2010;152(11):689–696. doi: 10.7326/0003-4819-152-11-201006010-00001. [DOI] [PubMed] [Google Scholar]
- Egede LE, Walker RJ, Payne EH, Knapp RG, Acierno R, Frueh BC. Effect of psychotherapy for depression via home telehealth on glycemic control in adults with type 2 diabetes: Subgroup analysis of a randomized clinical trial. Journal of Telemedicine and Telecare. 2018;24(9):596–602. doi: 10.1177/1357633X17730419. [DOI] [PubMed] [Google Scholar]
- Eriksson BS, Rosenqvist U. Social support and glycemic control in non-insulin dependent diabetes mellitus patients: Gender differences. Women Health. 1993;20(4):59–70. doi: 10.1300/J013v20n04_04. [DOI] [PubMed] [Google Scholar]
- Farmer C, Fenu E, O’Flynn N, Guthrie B. Clinical assessment and management of multimorbidity: Summary of NICE guidance. Bmj. 2016;354:ii4843. doi: 10.1136/bmj.i4843. [DOI] [PubMed] [Google Scholar]
- Fortin M, Chouinard MC, Dubois MF, Belanger M, Almirall J, Bouhali T, Sasseville M. Integration of chronic disease prevention and management services into primary care: A pragmatic randomized controlled trial (PR1MaC) CMAJ Open. 2016;4(4):E588–E598. doi: 10.9778/cmajo.20160031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin M, Haggerty J, Almirall J, Bouhali T, Sasseville M, Lemieux M. Lifestyle factors and multimorbidity: A cross sectional study. BMC Public Health. 2014;14(1):686. doi: 10.1186/1471-2458-14-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin M, Haggerty J, Almirall J, Bouhali T, Sasseville M, Lemieux M. Lifestyle factors and multimorbidity: a cross sectional study. BMC Public Health. 2014;14(1) doi: 10.1186/1471-2458-14-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin M, Soubhi H, Hudon C, Bayliss EA, Akker Mvd. Multimorbidity’s many challenges. BMJ. 2007;334(7602):1016–1017. doi: 10.1136/bmj.39201.463819.2C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedland KE, Carney RM, Rich MW, Steinmeyer BC, Rubin EH. Cognitive Behavior Therapy for Depression and Self-Care in Heart Failure Patients: A Randomized Clinical Trial. JAMA Internal Medicine. 2015;175(11):1773–1782. doi: 10.1001/jamainternmed.2015.5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedland KE, Skala JA, Carney RM, Rubin EH, Lustman PJ, Davila-Roman VG, Steinmeyer BC, Hogue CW. Treatment of depression after coronary artery bypass surgery: A randomized controlled trial. Archives of General Psychiatry. 2009;66(4):387–396. doi: 10.1001/archgenpsychiatry.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey J, Connolly D, Boland F, Smith SM. OPTIMAL, an occupational therapy led self-management support programme for people with multimorbidity in primary care: A randomized controlled trial. BMC Family Practice. 2015;16(1):59. doi: 10.1186/s12875-015-0267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary RA, Dunbar SB, Higgins MK, Musselman DL, Smith AL. Combined exercise and cognitive behavioral therapy improves outcomes in patients with heart failure. Journal of Psychosomatic Research. 2010;69(2):119–131. doi: 10.1016/j.jpsychores.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow RE, Nutting PA, Toobert DJ, King DK, Strycker LA, Jex M, O’Neill C, Whitesides H, Merenich J. Effects of a brief computer-assisted diabetes self-management intervention on dietary, biological and quality-of-life outcomes. Chronic Illness. 2006;2(1):27–38. doi: 10.1179/174592006(93851. [DOI] [PubMed] [Google Scholar]
- Gochman DS. Handbook of health behaviour research. Plenum; 1997. [Google Scholar]
- Hochhalter AK, Song J, Rush J, Sklar L, Stevens A. Making the Most of Your Healthcare intervention for older adults with multiple chronic illnesses. Patient Education and Counseling. 2010;81(2):207–213. doi: 10.1016/j.pec.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, Altman DG, Barbour V, Macdonald H, Johnston M, Lamb SE, et al. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. Bmj. 2014;348:g1687. doi: 10.1136/bmj.g1687. [DOI] [PubMed] [Google Scholar]
- Huang CY, Lai HL, Chen CI, Lu YC, Li SC, Wang LW, Su Y. Effects of motivational enhancement therapy plus cognitive behaviour therapy on depressive symptoms and health-related quality of life in adults with type II diabetes mellitus: A randomised controlled trial. Quality of Life Research. 2016;25(5):1275–1283. doi: 10.1007/s11136-015-1165-6. [DOI] [PubMed] [Google Scholar]
- Hynninen MJ, Bjerke N, Pallesen S, Bakke PS, Nordhus IH. A randomized controlled trial of cognitive behavioral therapy for anxiety and depression in COPD. Respiratory Medicine. 2010;104(7):986–994. doi: 10.1016/j.rmed.2010.02.020. [DOI] [PubMed] [Google Scholar]
- IBM SPSS Statistics for Windows. IBM Corp; 2019. [Internet] [Google Scholar]
- Jang SH, Lee JH, Lee HJ, Lee SY. Effects of mindfulness-based Art therapy on psychological symptoms in patients with coronary artery disease. Journal of Korean Medical Science. 2018;33(12):e88. doi: 10.3346/jkms.2018.33.e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Gruber J, Gray JR. Mindfulness and de-automatization. Emotion Review. 2013;5(2):192–201. [Google Scholar]
- Kasteleyn MJ, Vos RC, Rijken M, Schellevis FG, Rutten GE. Effectiveness of tailored support for people with Type 2 diabetes after a first acute coronary event: A multicentre randomized controlled trial (the Diacourse-ACE study) Diabetic Medicine. 2016;33(1):125–133. doi: 10.1111/dme.12816. [DOI] [PubMed] [Google Scholar]
- King DE, Xiang J, Pilkerton CS. Multimorbidity trends in United States adults, 1988-2014. The Journal of the American Board of Family Medicine. 2018;31(4):503–513. doi: 10.3122/jabfm.2018.04.180008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunik ME, Veazey C, Cully JA, Souchek J, Graham DP, Hopko D, Carter R, Sharafkhaneh A, Goepfert EJ, Wray N, Stanley MA. COPD education and cognitive behavioral therapy group treatment for clinically significant symptoms of depression and anxiety in COPD patients: A randomized controlled trial. Psychological Medicine. 2008;38(3):385–396. doi: 10.1017/S0033291707001687. [DOI] [PubMed] [Google Scholar]
- Lamers F, Jonkers CC, Bosma H, Chavannes NH, Knottnerus JA, van Eijk JT. Improving quality of life in depressed COPD patients: Effectiveness of a minimal psychological intervention. COPD: Journal of Chronic Obstructive Pulmonary Disease. 2010;7(5):315–322. doi: 10.3109/15412555.2010.510156. [DOI] [PubMed] [Google Scholar]
- Lamers F, Jonkers CC, Bosma H, Knottnerus JA, van Eijk JT. Treating depression in diabetes patients: Does a nurse-administered minimal psychological intervention affect diabetes-specific quality of life and glycaemic control? A randomized controlled trial. Journal of Advanced Nursing. 2011;67(4):788–799. doi: 10.1111/j.1365-2648.2010.05540.x. [DOI] [PubMed] [Google Scholar]
- Landman GW, Drion I, van Hateren KJ, van Dijk PR, Logtenberg SJ, Lambert J, Groenier KH, Bilo HJ, Kleefstra N. Device-guided breathing as treatment for hypertension in type 2 diabetes mellitus: A randomized, double-blind, sham-controlled trial. JAMA Internal Medicine. 2013;173(14):1346–1350. doi: 10.1001/jamainternmed.2013.6883. [DOI] [PubMed] [Google Scholar]
- Lawn SBM, Pols R. National Chronic Disease Strategy-Self-Management: Report to the Australian Government Department of Health and Ageing. 2005
- Liu H, Song M, Zhai ZH, Shi RJ, Zhou XL. Group singing improves depression and life quality in patients with stable COPD: A randomized community-based trial in China. Quality of Life Research. 2019;28(3):725–735. doi: 10.1007/s11136-018-2063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan AG, Irvine MJ, McIsaac WJ, Tisler A, Rossos PG, Easty A, Feig DS, Cafazzo JA. Effect of home blood pressure telemonitoring with self-care support on uncontrolled systolic hypertension in diabetics. Hypertension. 2012;60(1):51–57. doi: 10.1161/HYPERTENSIONAHA.111.188409. [DOI] [PubMed] [Google Scholar]
- Logtenberg SJ, Kleefstra N, Houweling ST, Groenier KH, Bilo HJ. Effect of device-guided breathing exercises on blood pressure in hypertensive patients with type 2 diabetes mellitus: A randomized controlled trial. Journal of Hypertension. 2007;25(1):241–246. doi: 10.1097/HJH.0b013e32801040d5. [DOI] [PubMed] [Google Scholar]
- Long F, Yan J, Hu P, Xia M, Liu H, Gu C. Effect of group counseling on depression, compliance and blood sugar level in diabetic patients. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2015;40(8):879–885. doi: 10.11817/j.issn.1672-7347.2015.08.009. [DOI] [PubMed] [Google Scholar]
- Lorig KR, Holman HR. Self-management education: History, definition, outcomes, and mechanisms. Annals of Behavioral Medicine. 2003;26(1):1–7. doi: 10.1207/S15324796ABM2601_01. [DOI] [PubMed] [Google Scholar]
- Ludman EJ, Peterson D, Katon WJ, Lin EH, Von Korff M, Ciechanowski P, Young B, Gensichen J. Improving confidence for self care in patients with depression and chronic illnesses. Behavioral Medicine. 2013;39(1):1–6. doi: 10.1080/08964289.2012.708682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren JG, Dahlstrom O, Andersson G, Jaarsma T, Karner Kohler A, Johansson P. The effect of guided Web-based cognitive behavioral therapy on patients with depressive symptoms and heart failure: A pilot randomized controlled trial. Journal of Medical Internet Research. 2016;18(8):e194. doi: 10.2196/jmir.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutes LD, Cummings DM, Littlewood K, Solar C, Carraway M, Kirian K, Patil S, Adams A, Ciszewski S, Hambidge B. COMRADE: A randomized trial of an individually tailored integrated care intervention for uncontrolled type 2 diabetes with depression and/or distress in the rural southeastern US. Contemporary Clinical Trials. 2018;70:8–14. doi: 10.1016/j.cct.2018.04.007. [DOI] [PubMed] [Google Scholar]
- Lynch EB, Liebman R, Ventrelle J, Avery EF, Richardson D. A self-management intervention for African Americans with comorbid diabetes and hypertension: A pilot randomized controlled trial. Preventing Chronic Disease. 2014;11 doi: 10.5888/pcd11.130349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May C, Montori VM, Mair FS. We need minimally disruptive medicine. BMJ. 2009;339:b2803. doi: 10.1136/bmj.b2803. aug 11 2. [DOI] [PubMed] [Google Scholar]
- McWilliam CL. Patients, persons or partners? Involving those with chronic disease in their care. Chronic Illness. 2009;5(4):277–292. doi: 10.1177/1742395309349315. [DOI] [PubMed] [Google Scholar]
- Michie S, Fixsen D, Grimshaw JM, Eccles MP. Specifying and reporting complex behaviour change interventions: The need for a scientific method. Implementation Science. 2009;4(1):40. doi: 10.1186/1748-5908-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, Eccles MP, Cane J, Wood CE. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: Building an international consensus for the reporting of behavior change interventions. Annals of Behavioral Medicine. 2013;46(1):81–95. doi: 10.1007/s12160-013-9486-6. [DOI] [PubMed] [Google Scholar]
- Moher D, Schulz KF, Altman DG. The CONSORT statement: Revised recommendations for improving the quality of reports of parallel-group randomised trials. The Lancet. 2001;357(9263):1191–1194. doi: 10.1016/S0140-6736(00)04337-3. [DOI] [PubMed] [Google Scholar]
- Moral RR, Torres LA, Ortega LP, Larumbe MC, Villalobos AR, Garcia JA, Rejano JM, PS A-A, Collaborative Group, A.-A. P. S. Effectiveness of motivational interviewing to improve therapeutic adherence in patients over 65 years old with chronic diseases: A cluster randomized clinical trial in primary care. Patient Education and Counseling. 2015;98(8):977–983. doi: 10.1016/j.pec.2015.03.008. [DOI] [PubMed] [Google Scholar]
- Nie C, Li T, Guo X. Intensive patients’ education and lifestyle improving program in CAD patients. Western Journal of Nursing Research. 2019;41(9):1254–1269. doi: 10.1177/0193945918810205. [DOI] [PubMed] [Google Scholar]
- Noël PH, Parchman ML, Williams JW, Cornell JE, Shuko L, Zeber JE, Kazis LE, Lee AFS, Pugh JA. The challenges of multimorbidity from the patient perspective. Journal of General Internal Medicine. 2007;22(S3):419–424. doi: 10.1007/s11606-007-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norlund F, Wallin E, Olsson EMG, Wallert J, Burell G, von Essen L, Held C. Internet-Based cognitive behavioral therapy for symptoms of depression and anxiety Among patients With a recent myocardial infarction: The U-CARE heart randomized controlled trial. Journal of Medical Internet Research. 2018;20(3):e88. doi: 10.2196/jmir.9710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaya B, Domenech-Abella J, Moneta MV, Lara E, Caballero FF, Rico-Uribe LA, Haro JM. All-cause mortality and multimorbidity in older adults: The role of social support and loneliness. Experimental Gerontology. 2017;99:120–126. doi: 10.1016/j.exger.2017.10.001. [DOI] [PubMed] [Google Scholar]
- O’Moore K, Newby A, Andrews JM, Hunter G, Bennell DJ, Smith K, Williams J, D A. Internet cognitive-behavioral therapy for depression in older adults With knee osteoarthritis: A randomized controlled trial. Arthritis Care Research. 2018;70(1):61–70. doi: 10.1002/acr.23257. [DOI] [PubMed] [Google Scholar]
- O’Neil A, Taylor B, Sanderson K, Cyril S, Chan B, Hawkes AL, Hare DL, Jelinek M, Venugopal K, Atherton JJ, Amerena J, et al. Efficacy and feasibility of a tele-health intervention for acute coronary syndrome patients with depression: Results of the “MoodCare” randomized controlled trial. Annals of Behavioral Medicine. 2014;48(2):163–174. doi: 10.1007/s12160-014-9592-0. [DOI] [PubMed] [Google Scholar]
- Onyechi KCN, Eseadi C, Okere AU, Onuigbo LN, Umoke PCI, Anyaegbunam NJ, Otu MS, Ugorji NJ. Effects of cognitive behavioral coaching on depressive symptoms in a sample of type 2 diabetic inpatients in Nigeria. Medicine. 2016;95(31):e4444. doi: 10.1097/MD.0000000000004444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ose D, Kamradt M, Kiel M, Freund T, Besier W, Mayer M, Krisam J, Wensing M, Salize HJ, Szecsenyi J. Care management intervention to strengthen self-care of multimorbid patients with type 2 diabetes in a German primary care network: A randomized controlled trial. PloS one. 2019;14(6):e0214056. doi: 10.1371/journal.pone.0214056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter JE, Borba CP, Hynes M, Mays D, Glanz K. The use of theory in health behavior research from 2000 to 2005: A systematic review. Annals of Behavioral Medicine. 2008;35(3):358–362. doi: 10.1007/s12160-008-9042-y. [DOI] [PubMed] [Google Scholar]
- Parswani MJ, Sharma MP, Iyengar S. Mindfulness-based stress reduction program in coronary heart disease: A randomized control trial. International Journal of Yoga. 2013;6(2):111–117. doi: 10.4103/0973-6131.113405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penckofer SM, Ferrans C, Mumby P, Byrn M, Emanuele MA, Harrison PR, Durazo-Arvizu RA, Lustman P. A psychoeducational intervention (SWEEP) for depressed women with diabetes. Annals of Behavioral Medicine. 2012;44(2):192–206. doi: 10.1007/s12160-012-9377-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. International Journal of Evidence-Based Healthcare. 2015;13(3):141–146. doi: 10.1097/XEB.0000000000000050. [DOI] [PubMed] [Google Scholar]
- Pibernik-Okanovic M, Hermanns N, Ajdukovic D, Kos J, Prasek M, Sekerija M, Lovrencic MV. Does treatment of subsyndromal depression improve depression-related and diabetes-related outcomes? A randomised controlled comparison of psychoeducation, physical exercise and enhanced treatment as usual. Trials. 2015;16(1):305. doi: 10.1186/s13063-015-0833-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette JD, Richardson C, Himle J, Duffy S, Torres T, Vogel M, Barber K, Valenstein M. A randomized trial of telephonic counseling plus walking for depressed diabetes patients. Medical Care. 2011;49(7):641–648. doi: 10.1097/MLR.0b013e318215d0c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachmani R, Levi Z, Slavachevski I, Avin M, Ravid M. Teaching patients to monitor their risk factors retards the progression of vascular complications in high-risk patients with Type 2 diabetes mellitus-a randomized prospective study. Diabetic Medicine. 2002;19(5):385–392. doi: 10.1046/j.1464-5491.2002.00701.x. [DOI] [PubMed] [Google Scholar]
- Renn BN, Hundt NE, Sansgiry S, Petersen NJ, Kauth MR, Kunik ME, Cully JA. Integrated brief cognitive behavioral therapy improves illness intrusiveness in veterans With chronic obstructive pulmonary disease. Annals of Behavioral Medicine. 2018;52(8):686–696. doi: 10.1093/abm/kax045. [DOI] [PubMed] [Google Scholar]
- Richards SH, Campbell JL, Dickens C, Anderson R, Gandhi M, Gibson A, Kessler D, Knight L, Kuyken W, Richards DA, Taylor RS, et al. Enhanced psychological care in cardiac rehabilitation services for patients with new-onset depression: The CADENCE feasibility study and pilot RCT. Health Technology Assessment. 2018;22(30):1–220. doi: 10.3310/hta22300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safren SA, Gonzalez JS, Wexler DJ, Psaros C, Delahanty LM, Blashill AJ, Margolina AI, Cagliero E. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in patients with uncontrolled type 2 diabetes. Diabetes Care. 2014;37(3):625–633. doi: 10.2337/dc13-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury C, Man M-S, Bower P, Guthrie B, Chaplin K, Gaunt DM, Brookes S, Fitzpatrick B, Gardner C, Hollinghurst S, Lee V, et al. Management of multimorbidity using a patient-centred care model: A pragmatic cluster-randomised trial of the 3D approach. The Lancet. 2018;392(10141):41–50. doi: 10.1016/S0140-6736(18)31308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samdal GB, Eide GE, Barth T, Williams G, Meland E. Effective behaviour change techniques for physical activity and healthy eating in overweight and obese adults; systematic review and meta-regression analyses. International Journal of Behavioral Nutrition and Physical Activity. 2017;14(1):42. doi: 10.1186/s12966-017-0494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider KL, Panza E, Handschin B, Ma Y, Busch AM, Waring ME, Appelhans BM, Whited MC, Keeney J, Kern D, Blendea M, et al. Feasibility of pairing behavioral activation With exercise for women With type 2 diabetes and depression: The Get It study pilot randomized controlled trial. Behavior Therapy. 2016;47(2):198–212. doi: 10.1016/j.beth.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sells D, Sledge WH, Wieland M, Walden D, Flanagan E, Miller R, Davidson L. Cascading crises, resilience and social support within the onset and development of multiple chronic conditions. Chronic Illness. 2009;5(2):92–102. doi: 10.1177/1742395309104166. [DOI] [PubMed] [Google Scholar]
- Shen X, Zhu X, Wu Y, Zhou Y, Yang L, Wang Y, Zheng Q, Liu Y, Cong S, Xiao N, Zhao Q. Effects of a psychological intervention programme on mental stress, coping style and immune function in percutaneous coronary intervention patients. PloS one. 2018;13(1):e0187745. doi: 10.1371/journal.pone.0187745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, O’Kelly S, O’Dowd T. GPs’ and pharmacists’ experiences of managing multimorbidity: a ‘Pandora’s box’. British Journal of General Practice. 2010;60(576):e285–e294. doi: 10.3399/bjgp10X514756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Wallace E, O’Dowd T, Fortin M. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database of Systematic Reviews. 2016;3:CD006560. doi: 10.1002/14651858.CD006560.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Wallace E, O’Dowd T, Fortin M. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database of Systematic Reviews. 2021;2021:CD006560. doi: 10.1002/14651858.CD006560.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suls J, Green PA. Multimorbidity in health psychology and behavioral medicine. Health Psychology. 2019;38(9):769–771. doi: 10.1037/hea0000783. [DOI] [PubMed] [Google Scholar]
- Taveira TH, Dooley AG, Cohen LB, Khatana SA, Wu WC. Pharmacist-led group medical appointments for the management of type 2 diabetes with comorbid depression in older adults. Annals of Pharmacotherapy. 2011;45(11):1346–1355. doi: 10.1345/aph.1Q212. [DOI] [PubMed] [Google Scholar]
- Taylor SJC, Pinnock H, Epiphaniou E, Pearce G, Parke HL, Schwappach A, Purushotham N, Jacob S, Griffiths CJ, Greenhalgh T, Sheikh A. A rapid synthesis of the evidence on interventions supporting self-management for people with long-term conditions: PRISMS-Practical systematic Review of Self-Management Support for long-term conditions. Vol. 2. NIHR Journals Library; 2014. Health Services and Delivery Research; pp. 1–580. [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Bogardus ST, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. New England Journal of Medicine. 2004;351(27):2870–2874. doi: 10.1056/NEJMsb042458. [DOI] [PubMed] [Google Scholar]
- Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, Hempel S, et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Annals of Internal Medicine. 2018;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- Tugwell P, Knottnerus JA. Multimorbidity and Comorbidity are now separate MESH headings. Journal of Clinical Epidemiology. 2019;105:vi–viii. doi: 10.1016/j.jclinepi.2018.11.019. [DOI] [PubMed] [Google Scholar]
- Turner A, Murphy BM, Higgins RO, Elliott PC, Le Grande MR, Goble AJ, Worcester MU. An integrated secondary prevention group programme reduces depression in cardiac patients. European Journal of Preventive Cardiology. 2014;21(2):153–162. doi: 10.1177/2047487312467747. [DOI] [PubMed] [Google Scholar]
- van der Aa MJ, van den Broeke JR, Stronks K, Plochg T. Patients with multimorbidity and their experiences with the healthcare process: A scoping review. Journal of Comorbidity. 2017;7(1):11–21. doi: 10.15256/joc.2017.7.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel I, Miksch A, Goetz K, Ose D, Szecsenyi J, Freund T. The impact of perceived social support and sense of coherence on health-related quality of life in multimorbid primary care patients. Chronic Illness. 2012;8(4):296–307. doi: 10.1177/1742395312445935. [DOI] [PubMed] [Google Scholar]
- Wakefield BJ, Holman JE, Ray A, Scherubel M, Adams MR, Hillis SL, Rosenthal GE. Effectiveness of home telehealth in comorbid diabetes and hypertension: A randomized, controlled trial. Telemedicine and e-Health. 2011;17(4):254–261. doi: 10.1089/tmj.2010.0176. [DOI] [PubMed] [Google Scholar]
- Wallace E, Salisbury C, Guthrie B, Lewis C, Fahey T, Smith SM. Managing patients with multimorbidity in primary care. BMJ. 2015;350:h176. doi: 10.1136/bmj.h176. jan20 2. [DOI] [PubMed] [Google Scholar]
- Wikström K, Lindström J, Harald K, Peltonen M, Laatikainen T. Clinical and lifestyle-related risk factors for incident multimorbidity: 10-year follow-up of Finnish population-based cohorts 1982-2012. European Journal of Internal Medicine. 2015;26(3):211–216. doi: 10.1016/j.ejim.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Willett M, Duda J, Fenton S, Gautrey C, Greig C, Rushton A. Effectiveness of behaviour change techniques in physiotherapy interventions to promote physical activity adherence in lower limb osteoarthritis patients: A systematic review. PloS one. 2019;14(7):e0219482. doi: 10.1371/journal.pone.0219482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpe J. Psychotherapy by reciprocal inhibition. Stanford University Press; 1958. [Google Scholar]
- Wu CJ, Chang AM, Courtney M, Kostner K. Peer supporters for cardiac patients with diabetes: A randomized controlled trial. International Nursing Review. 2012;59(3):345–352. doi: 10.1111/j.1466-7657.2012.00998.x. [DOI] [PubMed] [Google Scholar]
- Yoo HJ, Park MS, Kim TN, Yang SJ, Cho GJ, Hwang TG, Baik SH, Choi DS, Park GH, Choi KM. A Ubiquitous Chronic Disease Care system using cellular phones and the internet. Diabetic Medicine. 2009;26(6):628–635. doi: 10.1111/j.1464-5491.2009.02732.x. [DOI] [PubMed] [Google Scholar]