Abstract
Oxidative stress induces neuronal apoptosis and is implicated in cerebral ischemia, head trauma, and age-related neurodegenerative diseases. An early step in this process is the loss of intracellular K+ via K+ channels, and evidence indicates that Kv2.1 is of particular importance in this regard, being rapidly inserted into the plasma membrane in response to apoptotic stimuli. An additional feature of neuronal oxidative stress is the up-regulation of the inducible enzyme heme oxygenase-1 (HO-1), which catabolizes heme to generate biliverdin, Fe2+, and carbon monoxide (CO). CO provides neuronal protection against stresses such as stroke and excitotoxicity, although the underlying mechanisms are not yet elucidated. Here, we demonstrate that CO reversibly inhibits Kv2.1. Channel inhibition by CO involves reactive oxygen species and protein kinase G activity. Overexpression of Kv2.1 in HEK293 cells increases their vulnerability to oxidant-induced apoptosis, and this is reversed by CO. In hippocampal neurons, CO selectively inhibits Kv2.1, reverses the dramatic oxidant-induced increase in K+ current density, and provides marked protection against oxidant-induced apoptosis. Our results provide a novel mechanism to account for the neuroprotective effects of CO against oxidative apoptosis, which has potential for therapeutic exploitation to provide neuronal protection in situations of oxidative stress.
Keywords: potassium channel, hippocampal neuron, heme oxygenase
Oxidative stress is a major contributory factor to neuronal damage and death arising from stroke-associated cerebral ischemia, head trauma, neurodegenerative diseases (such as Alzheimer’s and Parkinson’s diseases), and aging (e.g., refs. 1– 4). Although the pathways leading to neuronal death are complex and varied, loss of neurons through apoptosis is commonly observed (5, 6). An additional feature among many of these age-related conditions is the increased expression of heme oxygenase-1 (HO-1; hsp32) in the brain. Thus, while HO-2 is constitutively expressed in both neurons and astrocytes, HO-1 is inducible in both cell types following oxidative stress or ischemic insult and is also observed in neurodegenerative diseases (7–9). Both oxygenases degrade heme to liberate biliverdin, ferrous iron (Fe2+) and carbon monoxide (CO). This reaction breaks down the prooxidant heme and generates a highly effective antioxidant in bilirubin (produced from biliverdin via bilirubin reductase). In addition, CO is an important signaling molecule, interacting with diverse intracellular signaling pathways (10, 11). Much evidence indicates that heme oxygenases protect the brain against acute glutamate excitotoxicity (12) and in various in vitro and in vivo models of stroke (e.g., refs. 13, 14). It is not yet established how protection is afforded by any specific heme product. However, recent studies suggest that CO inhalation is neuroprotective against experimental stroke (15) and that CO derived from astrocytes in response to hypoxia protects neighboring neurons from apoptosis (16).
Cellular apoptosis is strongly influenced by intracellular K+ levels (17). K+ acts to regulate caspase activation and mitochondrial membrane potential and volume, as well as through its abundance, osmolarity, and, hence, cell volume (17, 18). Intracellular K+ loss is a key early stage in apoptosis (19, 20), and reduced intracellular K+ triggers the apoptotic cascade, including cell shrinkage, mitochondrial cytochrome-c release, and caspase activation (18, 21). K+ efflux occurs via K+ channels, and K+ channel inhibitors can protect against apoptosis triggered by a variety of insults, including oxidative stress (22, 23). Evidence suggests a particularly important role for the delayed rectifier K+ channel Kv2.1 in mediating K+ efflux that leads to neuronal apoptosis: cortical neurons expressing dominant-negative Kv2.1 constructs (therefore lacking functional Kv2.1 channels) are protected against experimentally induced apoptosis, and expression of Kv2.1 in CHO cells increases their susceptibility to apoptosis (24). Furthermore, proapoptotic agents cause a rapid increase in the surface expression of Kv2.1 channels (25). Here, we report that CO inhibits Kv2.1 and, thereby, provides protection against apoptosis triggered by oxidant-induced increases in Kv2.1 surface expression. Our data provide a novel mechanism contributing to the neuroprotective effects of HO-1.
Materials and Methods
HEK293 cell culture
Wild-type and Kv2.1-transfected HEK293 cells (the latter a gift from Dr. James S. Trimmer, University of California, Davis, CA, USA) were maintained at 37°C (5% CO2) in DMEM containing 2 mM glutamine, 10% FBS, 1% glutamax and 1% penicillin/streptomycin. The Kv2.1 gene (KCNB1) was originally cloned from rats. Cells were plated either onto coverslips for electrophysiology or polylysine-coated 96-well plates for MTT and caspase assays (see below).
Primary culture of hippocampal neurons
Hippocampi from 6- to 8-d-old Wistar rats were removed for mechanical and enzymatic dissociation. Tissue was incubated for 15 min at 37°C in PBS containing 0.25 μg/ml trypsin (EC 4.4.21.4, from bovine pancreas; Sigma-Aldrich, Manchester, UK). Trypsin digestion was terminated by the addition of an equal volume of buffer containing 16 μg/ml soybean trypsin inhibitor (SBTI, type I-S; Sigma), 0.5 μg/ml DNaseI (EC 3.1.21.1 type II, from bovine pancreas; 125 kU/ml; Sigma) and 1.5 mM MgSO4. Following centrifugation at 3000 rpm for 5 min, cells were resuspended in minimal Earle’s medium (MEM) with 10% FCS, 19 mM KCl, 13 mM glucose, 50 IU/ml penicillin, and 50 μg/ml streptomycin. Cell suspension (100 μl) was plated onto 10-mm diameter poly-l-lysine-coated coverslips in a 24-well plate for electrophysiology, TUNEL assays, and annexin V binding experiments, or 96-well plates for MTT and caspase activity experiments. Medium was replaced after 24 h with one containing 10% horse serum in place of FCS and 80 μM fluorodexoyurdine (FUDR) to prevent the proliferation of non-neuronal cells. At 48 h, the medium was exchanged for one containing Neurobasal medium, supplemented with 2% B-27, 1% penicillin/streptomycin, 0.5 mM l-glutamine, and 25 μM l-glutamic acid. Cells were maintained in a humidified incubator at 37°C, 95% air:5% CO2 for 14 d, replacing medium every 5–7 d. All experiments were performed from cells cultured between 5 and 14 d.
CO exposure
In most experiments, as in previous studies (26, 27), CO was applied as the donor compound, CORM-2 ([Ru(CO3)Cl2]2, tricarbonyldichlororuthenium (II) dimer; Sigma-Aldrich). CORM-2 was freshly prepared in DMSO and diluted to the required concentration in extracellular solution. As a control, dichlorotetrakis (dimethylsulfoxide) ruthenium (II), the breakdown product (RuCl2(DMSO)4) of CORM-2 (synthesized by the Department of Chemistry, University of Leeds) and referred to as inactive CORM (iCORM), was used. DMSO concentration never exceeded 0.1%. For some experiments, perfusate was bubbled for ≥2 h under positive pressure with 100% CO. This solution was assumed to be saturated with CO and then diluted 1:5 before application to cells (28).
Induction of apoptosis
The oxidant 2,2’-dithiodipyridine (DTDP) was used to trigger apoptosis (24, 25). For electrophysiological experiments, the stimulus consisted of a 5-min exposure to 25 μM DTDP at 37°C. Following DTDP exposure, this solution was replaced with fresh medium containing 10 μM 1,3-boc-aspartyl-(ome)-fluoromethylketone (BAF; a caspase inhibitor). This allowed cells to remain viable for electrophysiological recordings, which were made 1–3 h after the initial DTDP treatment. For measurement of caspase activity, cells were exposed to 100 or 300 μM DTDP for 10 min at 37°C. The cells were thoroughly washed, either 100 μM CORM-2 or iCORM-2 was applied, and cells were incubated for 1 h prior to measuring caspase activity.
Electrophysiology
Fragments of coverslip with attached cells were transferred to a perfused (3–5 ml/min) recording chamber mounted on the stage of an Olympus CK40 inverted microscope (Olympus, Tokyo, Japan). K+ currents were recorded by whole-cell patch clamp; HEK293 cells were held at −90 mV, neurons at −70 mV. The standard perfusate (pH 7.2, 22±1°C) was composed of (in mM): 140 NaCl, 5 KCl, 2 MgCl2, 10 HEPES, 2 CaCl2, and 10 glucose. Patch pipette resistance was 4 – 6 MΩ. Series resistance was monitored after breaking into the whole-cell configuration throughout the duration of experiments. If a significant increase occurred (>20%), the experiment was terminated. Series resistance was monitored and compensated for (60 – 80%) after breaking into the whole-cell configuration throughout the duration of experiments. The pipette solution (pH 7.2) consisted of in (mM): 140 KCl, 5 EGTA, 2 MgCl2, 1 CaCl2, 10 HEPES, and 10 glucose. For some experiments, an anti-Kv2.1 antibody (Neuromab, Davis, CA, USA) was added to the intracellular solution to give a final concentration of 0.5 μg/ml. For HEK293 cells, 2 voltage protocols were adopted: a series of depolarizing steps from −90 to +80 mV in 10-mV increments for 500 ms; and a single step to +50 mV from −90 mV for 100 ms. For hippocampal neurons, similar protocols were used except for the inclusion of a single 30-ms prepulse to −50 mV (29). This protocol inactivates the transient (A-type) K+ currents and effectively isolated the delayed rectifier component of the whole-cell K+ current to which Kv2.1 partly contributes. Signals were sampled at 10 kHz and low-pass filtered at 2 kHz. Voltage-clamp and analysis protocols were performed using an Axopatch 200A amplifier/Digidata 1200 interface (Axon Instruments, Foster City, CA, USA) controlled by Clampex 9.0 software (Molecular Devices, Foster City, CA, USA). Offline analysis was performed using Clampfit 9.0 (Molecular Devices). Conductance values (G) were calculated from the equation G = I/(V − EK), where the Nernst equilibrium potential EK was calculated as −84 mV. Normalized conductance/voltage profiles for the Kv2.1 channel were fitted to a single Boltzmann function with the form G = Gmax/(1 + exp [−(V − V1/2)/k], where Gmax is the maximal conductance, V1/2 is the test potential at which Kv2.1 channels have a half-maximal conductance (G0.5), and k represents the slope of the activation curve. Current densities were calculated by measuring current amplitudes over the last 10 ms of a depolarizing step, when they were observed to have reached a steady-state value, then dividing these measured currents by each cell’s capacitance (determined from calibrated analog offset). Results are presented as means ± SE; statistical analysis was performed using unpaired Student’s t-tests, where P < 0.05 was considered statistically significant. For each experimental condition employed, separate controls were performed, and these control values are pooled in the bar graphs shown.
MTT assay
Cells grown in 96-well plates were exposed to varying concentrations of DTDP for 10 min (37°C, 95% air:5% CO2), after which the medium was replaced with 110 μl of medium containing 0.5 mg/ml thiazolyl blue tetrazolium bromide (MTT) with or without additional drugs and left for 3 h in culture at 37°C. After this incubation period, 111 μl of an acidic isopropanol solution (1 ml 1 M HCl in 24 ml isopropanol) was added to each well. This was triturated so that any formazan crystals present were dissolved. The plate was then scanned using a spectrophotometer at a wavelength of 570 nm. Statistical significance was determined using 1-way ANOVA with Bonferroni multiple-comparison test.
Caspase 3/7 assay
Caspase activity was measured using the fluorimetric Apo-ONE homogeneous caspase 3/7 assay kit (Promega, Southampton, UK). Briefly, 100 μl of Apo-ONE caspase 3/7 reagent containing the rhodamine 110, bis-(N-CBZ-l-aspartyl-l-glutamyl-l-valyl-l-aspartic acid amide; Z-DEVD-R110) was added to a flat-bottomed 96-well plate containing 100 μl of cell culture medium and adhered cells. Contents were gently mixed at 500 rpm for 30 s. The assay was incubated in the dark for 5 h at room temperature to achieve optimal caspase activation. Fluorescence of each well was measured using a Flex station II (Molecular Devices) equipped for an excitation wavelength of 499 nm and an emission wavelength of 521 nm. Data were acquired using Softmax Pro software (Molecular Devices), and data were expressed as mean ± se percentage of control. Statistical significance was determined using 1-way ANOVA with Bonferroni multiple-comparison test.
Annexin V binding
Wells containing coverslips with hippocampal neurons attached were aspirated, medium was replaced with Neurobasal medium supplemented with 0 or 100 μM DTDP (as above), and cells were returned to a humidified incubator (37°C, 95% air:5% CO2) for 10 min. The medium was then aspirated again and replaced with Neurobasal medium supplemented with CORM-2 or iCORM (0 or 100 μM), and the cells were returned to an incubator for 3 h. After this time, coverslips were gently washed with PBS and then with binding buffer consisting of (mM) 10 HEPES, 140 NaCl, and 2.5 CaCl2, pH 7.5. The cells were then stained with annexin V-FITC (Sigma; 2 μg/ml in binding buffer; 10 min at room temperature in the dark). After staining, the cells were washed with binding buffer and observed by fluorescence microscopy (Zeiss LSM 510; Carl Zeiss, Oberkochen, Germany).
TUNEL assay
As an additional assessment of apoptosis, we also performed TdT-mediated dUTP-biotin nick end labeling (TUNEL) assays. Wells containing coverslips with hippocampal neurons attached were aspirated, medium was replaced with Neurobasal medium supplemented with 0 or 100 μM DTDP (as above), and cells were returned to a humidified incubator (37°C, 95% air:5% CO2), for 10 min. The medium was then replaced with Neurobasal medium supplemented with CORM-2 (0 or 100μM). After 3 h, the cells were gently washed with PBS, fixed in 4% paraformaldehyde in PBS for 10 min, washed, and then permeabilized with Neuropore (Trevigen, Gaithersburg, MD, USA) for 20 min. The TUNEL assay was then performed using a commercial assay (TACS 2 TdT-fluor in situ apoptosis detection kit; Trevigen) as per kit instructions. Coverslips were then mounted on microscope slides using Vectashield with DAPI (Vector Laboratories, Burlingame, CA, USA), sealed with nail varnish, and examined on a Zeiss LSM 510 confocal microscope using 488- and 351-nm lasers. At least 20 separate fields containing an average of 30 cells (range 11–66) were acquired at ×40 for each coverslip from 3 different preparations of primary hippocampal neurons. All TUNEL-positive cells (green fluorescence in the nucleus) were counted for each field and expressed as a percentage of the total number of nuclei in that field. Differences between control and treatment groups were assessed using 1-way ANOVA with Bonferroni’s multiple-comparison test.
Immunohistohemistry
Cells were cultured on sterile glass coverslips, washed in Dulbecco’s PBS (DPBS) with Ca2+ and Mg2+, and fixed with cold acetone/methanol (50:50 v/v) for 10 min. Coverslips were washed in DPBS and permeabilized with 0.05% Triton X-100 in 10% normal goat serum (NGS) for 10 min. Cells were incubated overnight at 4°C in 1% NGS containing anti-MAP-2 (mAb HM-2; Abcam, Cambridge, UK; 1:500 dilution) and anti-GFAP, N-terminal (rabbit monoclonal; Epitomics, Burlingame, CA, USA; 1:250). After washing, cells were incubated in 1% NGS with Alexa Fluor 488 goat anti-rabbit and Alexa Fluor 555 goat anti-mouse (Invitrogen Molecular Probes, Paisley, UK; 1:1000) for 1 h at room temperature in the dark. Coverslips were then washed, mounted on microscope slides using Vectashield with DAPI (Vector Laboratories), and sealed with nail varnish. Slides were stored in the dark until used. Cells were examined on a Zeiss laser-scanning confocal microscope (LSM 510). Fluorophores were excited by sequential scanning with argon, helium, and UV lasers, and the composite images were produced using Zeiss AIM software.
Results
CO inhibits recombinant Kv2.1
In light of the central role of Kv2.1 in apoptosis, we speculated that the antiapoptotic effects of CO might arise through modulation of Kv2.1. We therefore monitored whole-cell K+ currents in HEK293 cells stably expressing Kv2.1 while exposing them to CO. This was done initially using the CO-releasing molecule CORM-2 (Fig. 1A), which caused marked suppression of currents without any significant changes in current kinetics. The time course of blockade and recovery was not rapid, reaching equilibrium after ~3–5 min, with a similar time course for recovery (Fig. 1B). The IC50 for CORM-2 was ~30 μM (Fig. 1C), and blockade appeared voltage independent (Fig. 1D), with inhibition observed throughout the range of activating potentials studied. Conductance vs. voltage plots (Fig. 1E) confirmed that the activation profile of Kv2.1 was not affected by CO, and there was no significant difference between the G0.5 voltage values between the control group (15±1.5 mV) and cells exposed to 30 μM CORM-2 (12±1.3 mV).
Figure 1. CO inhibits recombinant Kv2.1.
A) Example families of currents evoked by step depolarizations (−100 to +80 mV, holding potential −90 mV) applied to a representative HEK293 cell stably expressing Kv2.1. Currents were recorded under control conditions, during application of 30 μM CORM-2, and following 10 min washout, as indicated. Scale bars apply to all traces. B) Example time courses of the effects of CORM-2 or the inactive compound iCORM on K+ current amplitudes. Currents were evoked by successive step depolarizations from −90 to +50 mV (100 ms, 0.2 Hz). CORM-2 or iCORM (30 μM) was applied for the period indicated by the shaded region. Current amplitudes have been normalized to those evoked by the first 10 depolarizations, for comparison. C) Concentration-response relationship for the effects of CORM-2 on Kv2.1 currents (determined using step depolarizations to +50 mV). D) Mean ±SE current density vs. voltage relationships, indicating the reversible inhibitory effects of CORM-2 at all activating test potentials (n=21 cells). E) Conductance vs. voltage plots for Kv2.1 determined in the absence and presence of 30 μM CORM-2 (taken from data in D), fitted with a single Boltzmann function as detailed in Materials and Methods.
This inhibitory effect of CORM-2 was attributed to its release of CO, since the inactive form, iCORM, was without significant effect, as was its vehicle, DMSO (Fig. 2A). Further, CO-equilibrated solution (diluted 1:5; see Materials and Methods) also significantly inhibited Kv2.1 (Fig. 2A). CO interacts with diverse cellular signaling pathways (10, 11), and we next probed these pathways in order to understand the mechanism of inhibition of Kv2.1 by CO. Since CO can stimulate generation of mitochondrial ROS, an effect which accounts for CO inhibition of L-type Ca2+ channels (28), we first examined the effects of anti-oxidants. Pretreatment of cells with the antioxidant Mn(III)tetrakis(1-methyl-4-pyridyl)porphyrin pentachloride (MnTMPyP; 100 μM, 30-min preincubation) partially prevented the inhibitory actions of CO, as did the mitochondria-targeted antioxidant, MitoQ (Fig. 2B). This suppression of the inhibitory action of CO suggests that mitochondrial ROS contribute, in part, to the inhibition of Kv2.1.
Figure 2. Probing the mechanism of CO inhibition of Kv2.1.
A) Summary bar graph indicating the mean effects of CORM-2 (30 μM), the solvent DMSO (0.01%), iCORM (30 μM), and CO dissolved as described in Materials and Methods. where ** indicates P < 0.01 (unpaired t test). B) Summary bar graph indicating the mean effects of CORM-2 (30 μM) determined alone (solid bar) or in the presence of the general antioxidant MnTMPyP (100 μM, 30 min pretreatment), the mitochondrial antioxidant, MitoQ (250 nM, 1 h pretreatment), the soluble guanylyl cyclase inhibitor ODQ (10 μM), the activator and inhibitor of cGMP, Sp-, and Rp-cGMPs, respectively (100 nM, 1 h pretreatment), the ERK inhibitor, U0126 (20 μM, 1 h pretreatment), and finally in the presence of both MnTMPyP and U0126. C) Current densities determined in cells pretreated in the experiments reported in B. Data are means ± se taken from the number of cells indicated in parentheses. *P < 0.05, **P < 0.01; unpaired t test.
CO also modulates the soluble gyanylyl cyclase/cGMP/PKG pathway activity via activation of cGMP, and we have shown that this pathway is involved in the activation of leak K+ channels by CO (30). However, this pathway does not appear to be involved in the inhibitory actions of CO on Kv2.1, since the soluble guanylyl cyclase inhibitor 1H-oxadiazole[4,3-a]quinoxalin-1-one (ODQ; 10 μM) did not alter the ability of CO to inhibit Kv2.1 (Fig. 2B). Similarly, CO inhibited Kv2.1 in the presence of the PKG activator, Sp-cGMPS (100 nM, 1 h pretreatment; Fig. 2B), indicating it did not act by activating PKG. However, by contrast, pretreatment of cells with the PKG inhibitor Rp-cGMPs (100 nM, 1 h pretreatment), almost fully prevented channel inhibition by CO (Fig. 2B). PKG inhibition itself caused a partial inhibition of Kv2.1 currents (Fig. 2C), suggesting that PKG influences basal Kv2.1 channel activity and that this influence (potentially via direct phosphorylation) is also required for CO to cause channel inhibition.
Since PKG is integral to several signaling cascades, we also examined candidate downstream kinases, which might mediate the PKG-dependent inhibition of Kv2.1 by CO. Activation of p38 MAP kinase is an important action of CO (11), and this kinase regulates Kv2.1 (31). However, preincubation with the p38 inhibitor SB203580 (20 μM, 30 min) did not alter the ability of CO to inhibit the channel (Fig. 2B). PKG is also known to exert effects via activation of ERK1/2 kinases (32). We found that, as with PKG inhibition, pretreatment of cells with the ERK inhibitor U0126 (20 μM, 1 h) reduced Kv2.1 current density (Fig. 2C) and subsequent application of CORM-2 (30 μM) was significantly less inhibitory (Fig. 2B), suggesting that ERK1/2 kinase activity regulates Kv2.1 channels and their response to CO exposure. Finally, coincubation of cells with both MnTMPyP (100 μM) and U0126 (20 μM) led to a reduction in current density (as observed with U0126 alone; Fig. 2C) and CO was without significant effect following such treatment (Fig. 2B). The effects of all the drugs used to probe the mechanism of CO inhibition of Kv2.1 on evoked current densities are shown in Fig. 2C. Of these, only the PKG and ERK inhibitors reduced current densities.
CO is also known to bind to nitric oxide (NO) synthase and thereby stimulate NO generation. We therefore examined whether NO might mediate the inhibitory actions of CO on Kv2.1. However, we were unable to inhibit Kv2.1 significantly by bath application of the NO donor S-nitroso-N-acetylpenicillamine (SNAP; 10 μM). In addition, pretreatment of cells with the NO inhibitor l-NAME (1 mM, 45 min) did not affect currents and, notably, did not alter the ability of CORM-2 to inhibit Kv2.1 (Fig. 3). In summary, CO inhibition of Kv2.1 does not involve generation of NO and does not require increased PKG activity. Instead, it is mediated, in part, by ROS, and also requires that the channel is tonically regulated by PKG, or its down-stream kinase ERK1/2.
Figure 3.
CO inhibition of Kv2.1 does not involve NO. Bar graph plotting mean ± SE current densities determined in cells before (control) and during exposure to 10 μM SNAP, cells pretreated with 1 mM L-NAME (1 mM, 45 min) and l-NAME-treated cells exposed to 30 μM CORM-2. Numbers of cells are indicated in parentheses; Inset: Kv2.1 currents evoked by step depolarizations to +50 mV before and during exposure to 10 μM SNAP. **P < 0.01; unpaired t test.
CO protects against apoptosis in HEK293 cells expressing Kv2.1
Since Kv2.1 is strongly implicated in the early stages of stress-induced apoptosis (19, 21, 22), we sought to determine whether CO may be protective against apoptosis. Expression of Kv2.1 significantly increased the vulnerability of HEK293 cells to death induced by the oxidant DTDP (Fig. 4A). CO, applied as CORM-2, significantly reduced this vulnerability whereas iCORM at the same concentration did not alter viability (Fig. 4B). Loss of viability was attributed to apoptosis since DTDP exposure increased caspase activation (Fig. 4C), which was also reversed by CO. These findings are consistent with the concept that CO is protective against oxidant-induced apoptosis via suppression of Kv2.1, and in further support of this, we found that brief exposures to DTDP led to a large (2- to 3-fold) increase in K+ current density at all activating voltages, and this enhancement in current density was markedly attenuated by simultaneous exposure to CORM-2 (Fig. 4D).
Figure 4. CO suppresses the Kv2.1-dependent susceptibility to oxidant-induced apoptosis conferred by opposing enhanced channel activity.
A) Cell viability assays (MTT assays as described in Materials and Methods), illustrating the concentration-dependent effects of the oxidant DTDP on viability determined in either wild-type (shaded bars) or Kv2.1-expressing HEK293 cells. B) Viability of Kv2.1-expressing HEK293 cells following exposure to DTDP in the absence of additional drugs, and also in the presence of 100 μM CORM-2 or iCORM, as indicated. Note that viability is partially rescued by CORM-2. C) Caspase 3/7 assay from Kv2.1-expressing HEK293 cells. Cells were exposed to DTDP in the absence of additional drugs, and also in the presence of 100 μM CORM-2 or iCORM, as indicated. D) Left: mean ± SE current density vs. voltage relationships determined in control cells (n=14), following exposure to DTDP (n=6) and following exposure to DTDP in the presence of 30 μM CORM-2 (n=6), as indicated. Right: example families of currents evoked by step depolarizations (−100 to +40 mV) applied to representative HEK293 cells stably expressing Kv2.1. Currents were recorded in a control cell (top traces), a cell exposed to 25 μM DTDP (as described in Materials and Methods; middle traces) or to DTDP in the presence of 30 μM CORM-2 (bottom traces). Scale bars apply to all traces. Bars represent mean ± SE viability determined from 3 experiments, each repeated in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001; 1-way ANOVA with Bonferroni multiple-comparison test.
Hippocampal neurons express CO-sensitive Kv2.1 channels
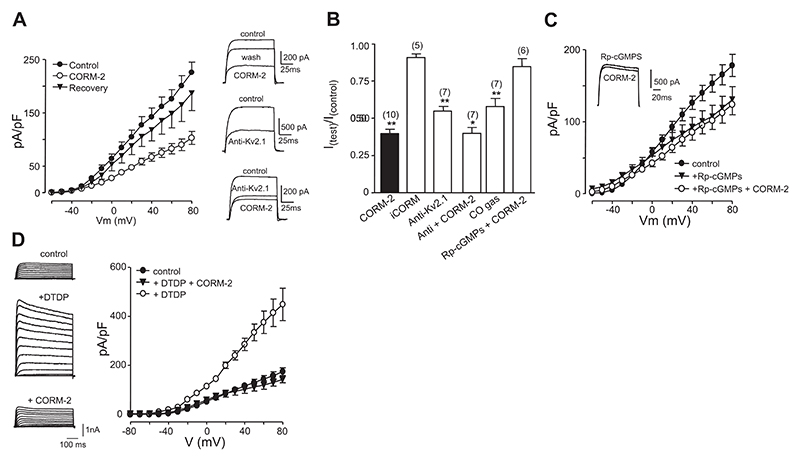
Kv2.1 is a major component of the delayed rectifier K+ current in hippocampal neurons (33). We found that delayed rectifier currents recorded in primary cultures of hippocampal neurons were reversibly inhibited by CORM-2 (30 μM) at all activating test potentials (Fig. 5A), an effect that was not observed when cells were exposed to iCORM, but was mimicked by exposure to CO directly dissolved into solution (Fig. 5A, B). Since hippocampal neurons express numerous K+ channels, we investigated the contribution of Kv2.1 by determining the inhibition of current amplitudes following 10-min intracellular dialysis with an anti-Kv2.1 antibody [time-matched dialysis in the absence of antibody showed little or no (<10%) rundown]. Kv2.1 significantly contributed to these currents, as described previously (33), and following anti-Kv2.1 dialysis, exposure to CO caused a much smaller inhibition of residual K+ currents (Fig. 5A, B). This suggests strongly that CO exerts a high degree of selectivity for Kv2.1 in hippocampal neurons. As was the case for recombinant Kv2.1 (Fig. 1), K+ currents in hippocampal neurons were partially inhibited by the PKG inhibitor Rp-cGMPs (100 nM, 1-h pretreatment; Fig. 5C). More important, during PKG inhibition, CO was unable to modulate K+ currents further (Fig. 5B, C), suggesting that, as for the recombinant channels, CO inhibition of native Kv2.1 required PKG activity. Exposure of neurons to DTDP caused dramatic enhancement of K+ currents, which was fully prevented by CO (Fig. 5D), suggesting, as was the case for HEK293 cells expressing Kv2.1, CO can suppress the apoptotic “surge” of K+ currents.
Figure 5. CO inhibits native Kv2.1 and oxidant-induced K+ current surge in hippocampal neurons.
A) Left: mean ± SE (n=8 cells) current density vs. voltage relationships obtained from hippocampal neurons, indicating the reversible inhibitory effects of CORM-2 throughout the range of activating test potentials. Right: top traces show example currents evoked in a neuron before (control), during (CORM-2) and after exposure (wash) to 30 μM CORM-2. Middle traces exemplify the effects of 10-min intracellular dialysis with an anti-Kv2.1 antibody (0.5 μg/ml). Bottom traces show the modest effects of CORM-2 (30 μM) following 10-min dialysis with anti-Kv2.1 antibody. Currents were obtained by step depolarizations from −70 to +50 mV. B) Summary bar graph showing mean ± SE (taken from number of cells indicated in each case) effects of 30 μM CORM-2 and iCORM. Also shown are the effects of 10-min intracellular dialysis with the anti-Kv2.1 antibody, along with the effects of CORM-2 (30 μM) following dialysis. Last two bars indicate the effects of dissolved CO (diluted 1:5 as described in Materials and Methods) and the lack of effect of CORM-2 in cells pretreated with Rp-cGMPs. In all cases, values were compared to control values except for comparing the anti-Kv2.1 + CORM data, which was compared to the antibody alone. *P < 0.05, **P < 0.01; unpaired t test. C) Mean ± SE current density vs. voltage plots recorded in control cells and following pretreatment of cells for 1 h with Rp-cGMPs (100 nM). Also plotted are the effects of bath application of 30 μM CORM-2 to cells pretreated with Rp-cGMPs. Note the reduction on mean current density in Rp-cGMPs-treated cells and their insensitivity to CORM-2. Inset: example currents evoked by a step depolarization from −90 to +50 mV in the absence (control) and presence (+CORM-2) of 30 μM CORM-2. D) Left: example families of currents evoked by step depolarizations (−100 to +80 mV) applied to representative hippocampal neurons. Currents were recorded in a control cell (top traces), a cell exposed to 25 μM DTDP (as described in Materials and Methods; middle traces) or DTDP in the presence of 30 μM CORM-2 (bottom traces). Scale bars apply to all traces. Right: mean ± SE current density vs. voltage relationships determined in control cells (n=8), following exposure to DTDP (n=8) and following exposure to DTDP in the presence of 30 μM CORM-2 (n=8), as indicated.
The experiments described in Figs. 4D and 5D involved application of CO (in the form of CORM) during the period of oxidant-induced K+ channel “surge.” Thus, it is not clear whether CO can block the enhanced currents arising from newly inserted Kv2.1 channels in the plasma membrane. To investigate this, we stimulated the K+ channel surge as before with DTDP, and following such treatment, examined whether the enhanced currents could then be inhibited by CO. As indicated in Fig. 6, these enhanced currents were similarly inhibited by CO in the recombinant expression system (Fig. 6A) and when studying native channels in hippocampal neurons (Fig. 6B).
Figure 6. CO inhibits currents enhanced by apoptotic oxidant stimulation.
A) Mean ± SE current density vs. voltage relationships determined in Kv2.1-expressing HEK293 cells following exposure to 25 μM DTDP (as described in Materials and Methods) before (open symbols) and during (solid symbols) subsequent bath application of 30 μM CORM-2 (n=7). B) As A, except that currents were recorded in hippocampal neurons following DTDP exposure (n=7).
CO protects hippocampal neurons against oxidant-induced apoptosis
Exposure of hippocampal neurons to DTDP induced a concentration-dependent loss of cell viability (Fig. 7A). CO (applied as 100 μM CORM-2) significantly improved viability, whereas iCORM was without significant effect (Fig. 7A). This loss of viability was attributed to apoptosis, since caspase 3/7 assays indicated a significant increase in activity. This was statistically significant at the higher oxidant concentration tested and was almost completely reversed by CORM-2, but not iCORM (Fig. 7B). Since measurements of viability and caspase activation were made using mixed primary cultures (i.e., containing both neurons and glia), we also performed annexin V binding assays and visualized neurons. Following exposure to DTDP (100 μM), FITC-labeled annexin V binding, indicative of apoptosis initiation, was detected particularly on neuronal soma (Fig. 7C). This binding was dramatically reduced by CORM-2 but not by iCORM. Since annexin V binding is not specific to neurons and could also indicate apoptotic glia (also present in our cultures), we compared the distribution of annexin V binding to the staining patterns in fixed cells when using the neuron-specific marker MAP2 (red, Fig. 7D) and the glial specific marker, GFAP (green, Fig. 7D). Although we cannot discount the possible induction of apoptosis in glia following oxidant stress, it is clear that the vast majority of annexin V binding is observed in the small soma of cells, which have long fine processes; such cells are neuronal rather than glial in nature, as indicated by MAP2 staining. These data further support the concept that CO protects hippocampal neurons against oxidant-induced apoptosis through inhibition of Kv2.1.
Figure 7. CO protects hippocampal neurons from oxidant-induced apoptosis.
A) Normalized viability measurements made from primary hippocampal neuron preparations. Cells were exposed to DTDP in the absence of additional drugs, or in the presence of 100 μM CORM-2 or iCORM, as indicated. Note that viability is partially rescued by CORM-2. B) Caspase 3/7 activity assay was determined in primary hippocampal cultures. Cells were exposed to DTDP in the absence of additional drugs, but in the presence of 100 μM CORM-2 or iCORM, as indicated. Bars represent mean ± SE viability determined from 3 experiments, each repeated in triplicate. *P < 0.05, **P < 0.01; 1-way ANOVA with Bonferroni multiple-comparison test. C) Representative images of hippocampal neurons stained with FITC-labeled annexin V (green) following exposure to oxidant alone, or in the additional presence of 100 μM CORM-2 or iCORM, as indicated. Top panels: superimposed bright-field and fluorescence images. Bottom panels fluorescence images alone. D) Representative image of hippocampal culture, as used for all experiments in the present study, but in this case fixed and immunostained for the neuronal marker MAP-2 (red) and the glial marker, GFAP (green). Also shown are DAPI-stained nuclei (blue). Scale bars = 10 μm.
As an additional assessment of apoptosis and the possibly protective effects of CO, we used TUNEL assays to identify DNA fragmentation, a hallmark feature of apoptosis. As illustrated in Fig. 8A, hippocampal neuron nuclei were largely free of TUNEL staining (indicating low basal apoptotic levels in culture). Exposure to 100 μM DTDP dramatically enhanced DNA fragmentation, as indicated by increased nuclear staining, and this was almost fully prevented by treatment of cells with CORM-2 (100 μM). These findings are quantified in the bar graph of Fig. 8B. Collectively, the data presented in Figs. 7 and 8 indicate clearly that oxidant-induced apoptosis is markedly suppressed by CO in hippocampal neurons.
Figure 8. CO protects hippocampal neurons from oxidantinduced DNA fragmentation.
A) Fluorescent images of DAPI-stained neuronal nuclei (blue) superimposed on which are specks of green, indicative of positive TUNEL staining for DNA fragmentation. Note the increased TUNEL staining following 100 μM DTDP exposure, which is reversed in the presence of 100 μM CORM-2. Scale bar = 4 μm. B) Mean ± SE percentage of TUNEL-positive cells in cultures exposed either to 100 μM CORM-2, 100 μM DTDP or both agents, as indicated (n>20 fields of view). ***P < 0.001; 1-way ANOVA with Bonferroni multiple-comparison test.
Discussion
Oxidative stress-induced apoptotic loss of cells is a common feature of aging and age-related diseases (1, 3, 34), and a key early step in this process is loss of cellular K+ via K+ channels (21). Kv2.1 has been strongly implicated in this regard, mediating amplification of K+ efflux via membrane insertion of new channels (25). This process is tightly regulated by phosphorylation of the channel at Ser-800 under the control of p38 MAP kinase (31). Specific involvement of Kv2.1 in apoptosis has been demonstrated in cortical neurons and introduction of the channel into CHO cells increases apoptosis in response to oxidant stress (18, 21, 24). Our data support these findings and show that expression of Kv2.1 in HEK293 cells increases their vulnerability to oxidant-induced apoptosis (Fig. 3). Unsurprisingly, therefore, K+ channels have been suggested as potential therapeutic targets in disorders related to apoptosis (21, 24, 35). Our data also confirm previous reports that Kv2.1 currents are dramatically enhanced following oxidative stress and, notably, indicate that blockade of Kv2.1 in native (hippocampal neuron) and recombinant systems provides protection against oxidant-induced apoptosis. Most notably, the present study demonstrates for the first time that CO provides protection against such apoptosis by inhibiting Kv2.1.
Ion channels are increasingly being recognized as targets for modulation by CO. Thus, CO can increase activity of BKCa channels directly or via interaction with associated heme (26, 36). CO also augments TREK K+ channels via PKG activation (30) and inhibits L-type Ca2+ channels via mitochondrial ROS production (28). Since antioxidants (including the mitochondria-targeted agent MitoQ) partially reversed the inhibitory effects of CO on Kv2.1 (Fig. 2A), we conclude they contribute to its mechanism of inhibition, but do not fully account for the inhibition. This effect of ROS contrasts with earlier studies of recombinant Kv2.1 (37), but it is in agreement with more recent studies of native Kv2.1 in vascular smooth muscle (38). ROS have been associated with a number of neurological disorders, including Parkinson’s and Alzheimer’s diseases, as well as stroke and normal aging (39). An increase in ROS can be triggered by oxidative stress or indeed CO, and the source of ROS is predominantly mitochondrial (40), although other sources (e.g., NADPH oxidase) exist. Numerous cellular targets for ROS are known, but a growing number of studies highlight a variety of ion channels where they can be beneficial or detrimental (39). Since CO inhibition of Kv2.1 was significantly decreased in the presence of an antioxidant, Kv2.1 can be included in this group of redox-sensing channels.
In addition to the involvement of ROS, we found that CO modulation requires that PKG is active (Fig. 2B) and may regulate the channel tonically, either by direct phosphorylation, or phosphorylation via a downstream kinase, such as ERK1/2, or via regulation of an intermediate protein. Kv2.1 possesses numerous sites for regulation via phosphorylation, particularly in its C-terminal domain, and various kinases can, thereby, regulate its activity (41). It is conceivable, therefore, that PKG may be an important regulatory kinase for Kv2.1, as it has recently been shown for the TASK-1 K+ channel (42). Alternatively, PKG is known to act as an upstream regulator of ERK1/2 in hippocampal neurons (43), and our data suggest that activity of this kinase is also required to increase basal channel activity, and for CO to inhibit the channel. To date, however, most studies indicate that phosphorylation/dephosphorylation of the channel lead to shifts in the voltage dependence of channel activation (41, 44). Our data indicate that PKG modulation does not occur primarily through altered voltage dependence of activation (not shown) and, more important, that CO also does not act to alter activation (Fig. 1E). Regulation of Kv2.1 appears particularly complex; recent studies have indicated that Kv2.1 channels can be present in the plasma membrane as clusters that are nonconducting, but may somehow become activated when they relocate from clusters (45). In addition, small ubiquitin-like modifier (SUMO) proteins can also dramatically alter the activity of Kv2.1 (46). Thus, the actions and potential interactions of ROS, phosphorylation, membrane localization, and SUMOylation to regulate Kv2.1 suggest the activity of this channel is particularly closely regulated, an issue that is perhaps unsurprising given its central role in regulation of excitability and its influence in neuronal apoptosis. Our findings suggest CO can be added to a growing list of physiological regulatory factors, which act at least in part through ROS, and, in so doing, modulate apoptotic vulnerability.
Interestingly, the PKG/ERK signaling cascade is often activated by NO, yet the soluble guanylyl cyclase inhibitor ODQ was without effect on the ability of CO to modulate the channel. Similarly, stimulation of PKG did not occlude the effects of CO (Fig. 2B), and NO did not mimic or mediate the effects of CO (Fig. 3). Finally, p38 MAPK activity is not involved in regulation of the channel by CO. This is perhaps unsurprising, given that p38 phosphorylation of Kv2.1 is required for its insertion into the plasma membrane early in the apoptotic response (31), and so, if CO acted via this mechanism, we would have observed current enhancement, not inhibition.
In summary, the inhibitory actions of CO on Kv2.1 are partly dependent on ROS and, while activation of the guanylyl cyclase/cGMP/PKG pathway does not appear to be involved in CO-mediated inhibition of Kv2.1, tonic channel regulation by PKG or the downstream kinase ERK1/2 is also required for inhibition.
Our data suggest a potentially important protective pathway for neurons in the face of oxidative stress. In this regard, it is noteworthy that increased expression of HO-1 is observed following neuronal oxidative stress and is associated with trauma, stroke, and neurodegenerative diseases (2, 8). However, earlier reports indicate that HO-2 is also protective and, indeed, that CO derived from HO-2 affords protection for neurons (e.g., ref. 47). The present study does not investigate any source of CO, instead focusing on a potential mechanism underlying its protective effects. Interestingly, our proposed mechanism might also account for the fact that astrocytes can generate CO (from HO-1) to provide protection for neighboring neurons against apoptosis (16). In further support of this is the observation that the neuronal Kv2.1 channel clusters in the plasma membrane, specifically opposing astrocytic processes (48).
Our findings also suggest that the inhibition of Kv2.1 by CO may be a pathway for potential therapeutic exploitation to prevent or suppress neurodegeneration. The therapeutic benefit of CO has been demonstrated in animal models of inflammation, transplantation, vascular injury, and liver failure, among other models, and equipment for delivery of CO by inhalation to patients is designed and tested (49). In addition, CO releasing molecules (such as CORM-2 used in the present study) are under intensive development (49). Given that CO inhalation also prevents neuronal damage following stroke (15), the possibility of CO being used to prevent other causes of neuronal damage or death through apoptosis—via its ability to inhibit Kv2.1, as demonstrated here—is real. Furthermore, therapeutic strategies to increase HO-1 induction represent a viable alternative to CO inhalation: several compounds with known beneficial activities can stimulate increased expression of HO-1 (e.g., resveratrol; ref. 50). However, it should also be noted that the overexpression of HO-1 in glia may be deleterious in some models of neurodegeneration (9).
In summary, the present study demonstrates that CO inhibits the proapoptotic K+ channel Kv2.1, expressed either natively in hippocampal neurons or when stably expressed in HEK293 cells, and thereby provides protection against oxidant-induced apoptosis. Since neuronal apoptosis is absolutely dependent on the surge of Kv2.1 channels to the plasma membrane (31), our data suggest a powerful endogenous means by which this process can be regulated, and in so doing provides a potential pathway to exploit in providing neuronal protection in neurodegenerative diseases or following other forms of brain trauma.
Acknowledgments
This work was supported by the Medical Research Council, Wellcome Trust, and British Heart Foundation, and the Department of International Cooperation, Hebei Province Science and Technology Research Council, China (09396427D). The authors are most grateful to Dr. James S. Trimmer (University of California, Davis, CA, USA) for the Kv2.1-expressing HEK293 cell line and to R. Suman for technical assistance.
References
- 1.Droge W, Schipper HM. Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell. 2007;6:361–370. doi: 10.1111/j.1474-9726.2007.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reynolds A, Laurie C, Mosley RL, Gendelman HE. Oxidative stress and the pathogenesis of neurodegenerative disorders. Int Rev Neurobiol. 2007;82:297–325. doi: 10.1016/S0074-7742(07)82016-2. [DOI] [PubMed] [Google Scholar]
- 3.Niizuma K, Endo H, Chan PH. Oxidative stress and mitochondrial dysfunction as determinants of ischemic neuronal death and survival. J Neurochem. 2009;109:133–138. doi: 10.1111/j.1471-4159.2009.05897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson CL, Scafidi S, McKenna MC, Fiskum G. Mitochondrial mechanisms of cell death and neuroprotection in pediatric ischemic and traumatic brain injury. Exp Neurol. 2009;218:371–380. doi: 10.1016/j.expneurol.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hou ST, MacManus JP. Molecular mechanisms of cerebral ischemia-induced neuronal death. Int Rev Cytol. 2002;221:93–148. doi: 10.1016/s0074-7696(02)21011-6. [DOI] [PubMed] [Google Scholar]
- 6.Okouchi M, Ekshyyan O, Maracine M, Aw TY. Neuronal apoptosis in neurodegeneration. Antioxid Redox Signal. 2007;9:1059–1096. doi: 10.1089/ars.2007.1511. [DOI] [PubMed] [Google Scholar]
- 7.Pappolla MA, Chyan YJ, Omar RA, Hsiao K, Perry G, Smith MA, Bozner P. Evidence of oxidative stress and in vivo neurotoxicity of beta-amyloid in a transgenic mouse model of Alzheimer’s disease: a chronic oxidative paradigm for testing antioxidant therapies in vivo. Am J Pathol. 1998;152:871–877. [PMC free article] [PubMed] [Google Scholar]
- 8.Dennery PA. Regulation and role of heme oxygenase in oxidative injury. Curr Top Cell Regul. 2000;36:181–199. doi: 10.1016/s0070-2137(01)80008-x. [DOI] [PubMed] [Google Scholar]
- 9.Schipper HM, Song W, Zukor H, Hascalovici JR, Zeligman D. Heme oxygenase-1 and neurodegeneration: expanding frontiers of engagement. J Neurochem. 2009;110:469–485. doi: 10.1111/j.1471-4159.2009.06160.x. [DOI] [PubMed] [Google Scholar]
- 10.Wu L, Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev. 2005;57:585–630. doi: 10.1124/pr.57.4.3. [DOI] [PubMed] [Google Scholar]
- 11.Kim HP, Ryter SW, Choi AM. CO as a cellular signaling molecule. Annu Rev Pharmacol Toxicol. 2006;46:411–449. doi: 10.1146/annurev.pharmtox.46.120604.141053. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad AS, Zhuang H, Dore S. Heme oxygenase-1 protects brain from acute excitotoxicity. Neuroscience. 2006;141:1703–1708. doi: 10.1016/j.neuroscience.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 13.Dore S, Goto S, Sampei K, Blackshaw S, Hester LD, Ingi T, Sawa A, Traystman RJ, Koehler RC, Snyder SH. Heme oxygenase-2 acts to prevent neuronal death in brain cultures and following transient cerebral ischemia. Neuroscience. 2000;99:587–592. doi: 10.1016/s0306-4522(00)00216-5. [DOI] [PubMed] [Google Scholar]
- 14.Ferris CD, Jaffrey SR, Sawa A, Takahashi M, Brady SD, Barrow RK, Tysoe SA, Wolosker H, Baranano DE, Dore S, Poss KD, et al. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat Cell Biol. 1999;1:152–157. doi: 10.1038/11072. [DOI] [PubMed] [Google Scholar]
- 15.Zeynalov E, Dore S. Low doses of carbon monoxide protect against experimental focal brain ischemia. Neurotox Res. 2009;15:133–137. doi: 10.1007/s12640-009-9014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imuta N, Hori O, Kitao Y, Tabata Y, Yoshimoto T, Matsuyama T, Ogawa S. Hypoxia-mediated induction of heme oxygenase type I and carbon monoxide release from astrocytes protects nearby cerebral neurons from hypoxia-mediated apoptosis. Antioxid Redox Signal. 2007;9:543–552. doi: 10.1089/ars.2006.1519. [DOI] [PubMed] [Google Scholar]
- 17.Yu SP, Canzoniero LM, Choi DW. Ion homeostasis and apoptosis. Curr Opin Cell Biol. 2001;13:405–411. doi: 10.1016/s0955-0674(00)00228-3. [DOI] [PubMed] [Google Scholar]
- 18.Yu SP, Yeh CH, Sensi SL, Gwag BJ, Canzoniero LM, Farhangrazi ZS, Ying HS, Tian M, Dugan LL, Choi DW. Mediation of neuronal apoptosis by enhancement of outward potassium current. Science. 1997;278:114–117. doi: 10.1126/science.278.5335.114. [DOI] [PubMed] [Google Scholar]
- 19.Hughes FM, Bortner CD, Jr, Purdy GD, Cidlowski JA. Intracellular K+ suppresses the activation of apoptosis in lymphocytes. J Biol Chem. 1997;272:30567–30576. doi: 10.1074/jbc.272.48.30567. [DOI] [PubMed] [Google Scholar]
- 20.Bortner CD, Cidlowski JA. Cellular mechanisms for the repression of apoptosis. Annu Rev Pharmacol Toxicol. 2002;42:259–281. doi: 10.1146/annurev.pharmtox.42.083101.143836. [DOI] [PubMed] [Google Scholar]
- 21.Yu SP. Regulation and critical role of potassium homeostasis in apoptosis. Prog Neurobiol. 2003;70:363–386. doi: 10.1016/s0301-0082(03)00090-x. [DOI] [PubMed] [Google Scholar]
- 22.Aizenman E, Stout AK, Hartnett KA, Dineley KE, McLaughlin B, Reynolds IJ. Induction of neuronal apoptosis by thiol oxidation: putative role of intracellular zinc release. J Neurochem. 2000;75:1878–1888. doi: 10.1046/j.1471-4159.2000.0751878.x. [DOI] [PubMed] [Google Scholar]
- 23.Bossy-Wetzel E, Talantova MV, Lee WD, Scholzke MN, Harrop A, Mathews E, Gotz T, Han J, Ellisman MH, Perkins GA, Lipton SA. Crosstalk between nitric oxide and zinc pathways to neuronal cell death involving mitochondrial dysfunction and p38-activated K+ channels. Neuron. 2004;41:351–365. doi: 10.1016/s0896-6273(04)00015-7. [DOI] [PubMed] [Google Scholar]
- 24.Pal S, Hartnett KA, Nerbonne JM, Levitan ES, Aizenman E. Mediation of neuronal apoptosis by Kv2.1-encoded potassium channels. J Neurosci. 2003;23:4798–4802. doi: 10.1523/JNEUROSCI.23-12-04798.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pal SK, Takimoto K, Aizenman E, Levitan ES. Apoptotic surface delivery of K+ channels. Cell Death Differ. 2006;13:661–667. doi: 10.1038/sj.cdd.4401792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams SE, Wootton P, Mason HS, Bould J, Iles DE, Riccardi D, Peers C, Kemp PJ. Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel. Science. 2004;306:2093–2097. doi: 10.1126/science.1105010. [DOI] [PubMed] [Google Scholar]
- 27.Chung SW, Liu X, Macias AA, Baron RM, Perrella MA. Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J Clin Invest. 2008;118:239–247. doi: 10.1172/JCI32730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scragg JL, Dallas ML, Wilkinson JA, Varadi G, Peers C. Carbon monoxide inhibits L-type Ca2+ channels via redox modulation of key cysteine residues by mitochondrial reactive oxygen species. J Biol Chem. 2008;283:24412–24419. doi: 10.1074/jbc.M803037200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plant LD, Webster NJ, Boyle JP, Ramsden M, Freir DB, Peers C, Pearson HA. Amyloid beta peptide as a physiological modulator of neuronal ‘A’-type K+ current. Neurobiol Aging. 2006;27:1673–1683. doi: 10.1016/j.neurobiolaging.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 30.Dallas ML, Scragg JL, Peers C. Modulation of hTREK-1 by carbon monoxide. Neuroreport. 2008;19:345–348. doi: 10.1097/WNR.0b013e3282f51045. [DOI] [PubMed] [Google Scholar]
- 31.Redman PT, He K, Hartnett KA, Jefferson BS, Hu L, Rosenberg PA, Levitan ES, Aizenman E. Apoptotic surge of potassium currents is mediated by p38 phosphorylation of Kv2.1. Proc Natl Acad Sci U S A. 2007;104:3568–3573. doi: 10.1073/pnas.0610159104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ota KT, Pierre VJ, Ploski JE, Queen K, Schafe GE. The NO-cGMP-PKG signaling pathway regulates synaptic plasticity and fear memory consolidation in the lateral amygdala via activation of ERK/MAP kinase. Learn Mem. 2008;15:792–805. doi: 10.1101/lm.1114808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murakoshi H, Trimmer JS. Identification of the Kv2.1 K+ channel as a major component of the delayed rectifier K+ current in rat hippocampal neurons. J Neurosci. 1999;19:1728–1735. doi: 10.1523/JNEUROSCI.19-05-01728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giorgio M, Trinei M, Migliaccio E, Pelicci PG. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat Rev Mol Cell Biol. 2007;8:722–728. doi: 10.1038/nrm2240. [DOI] [PubMed] [Google Scholar]
- 35.Wei L, Yu SP, Gottron F, Snider BJ, Zipfel GJ, Choi DW. Potassium channel blockers attenuate hypoxia- and ischemia-induced neuronal death in vitro and in vivo. Stroke. 2003;34:1281–1286. doi: 10.1161/01.STR.0000065828.18661.FE. [DOI] [PubMed] [Google Scholar]
- 36.Jaggar JH, Li A, Parfenova H, Liu J, Umstot ES, Dopico AM, Leffler CW. Heme is a carbon monoxide receptor for large-conductance Ca2+-activated K+ channels. Circ Res. 2005;97:805–812. doi: 10.1161/01.RES.0000186180.47148.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duprat F, Guillemare E, Romey G, Fink M, Lesage F, Lazdunski M, Honore E. Susceptibility of cloned K+ channels to reactive oxygen species. Proc Natl Acad Sci U S A. 1995;92:11796–11800. doi: 10.1073/pnas.92.25.11796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michelakis ED, Rebeyka I, Wu X, Nsair A, Thebaud B, Hashimoto K, Dyck JR, Haromy A, Harry G, Barr A, Archer SL. O2 sensing in the human ductus arteriosus: regulation of voltage-gated K+ channels in smooth muscle cells by a mitochondrial redox sensor. Circ Res. 2002;91:478–486. doi: 10.1161/01.res.0000035057.63303.d1. [DOI] [PubMed] [Google Scholar]
- 39.Sesti F, Liu S, Cai SQ. Oxidation of potassium channels by ROS: a general mechanism of aging and neurode-generation? Trends Cell Biol. 2010;20:45–51. doi: 10.1016/j.tcb.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 40.Zuckerbraun BS, Chin BY, Bilban M, de Costa dJ, Rao J, Billiar TR, Otterbein LE. Carbon monoxide signals via inhibition of cytochrome-c oxidase and generation of mitochondrial reactive oxygen species. FASEB J. 2007;21:1099–1106. doi: 10.1096/fj.06-6644com. [DOI] [PubMed] [Google Scholar]
- 41.Park KS, Mohapatra DP, Misonou H, Trimmer JS. Graded regulation of the Kv2.1 potassium channel by variable phosphorylation. Science. 2006;313:976–979. doi: 10.1126/science.1124254. [DOI] [PubMed] [Google Scholar]
- 42.Toyoda H, Saito M, Okazawa M, Hirao K, Sato H, Abe H, Takada K, Funabiki K, Takada M, Kaneko T, Kang Y. Protein kinase G dynamically modulates TASK1-mediated leak K+ currents in cholinergic neurons of the basal forebrain. J Neurosci. 2010;30:5677–5689. doi: 10.1523/JNEUROSCI.5407-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chien WL, Liang KC, Teng CM, Kuo SC, Lee FY, Fu WM. Enhancement of long-term potentiation by a potent nitric oxide-guanylyl cyclase activator, 3-(5-hydroxy-methyl-2-furyl)-1-benzyl-indazole. Mol Pharmacol. 2003;63:1322–1328. doi: 10.1124/mol.63.6.1322. [DOI] [PubMed] [Google Scholar]
- 44.Misonou H, Menegola M, Mohapatra DP, Guy LK, Park KS, Trimmer JS. Bidirectional activity-dependent regulation of neuronal ion channel phosphorylation. J Neurosci. 2006;26:13505–13514. doi: 10.1523/JNEUROSCI.3970-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Connell KM, Loftus R, Tamkun MM. Localization-dependent activity of the Kv2.1 delayed-rectifier K+ channel. Proc Natl Acad Sci U S A. 2010;107:12351–12356. doi: 10.1073/pnas.1003028107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dai XQ, Kolic J, Marchi P, Sipione S, Macdonald PE. SUMOylation regulates Kv2.1 and modulates pancreatic beta-cell excitability. J Cell Sci. 2009;122:775–779. doi: 10.1242/jcs.036632. [DOI] [PubMed] [Google Scholar]
- 47.Dore S, Sampei K, Goto S, Alkayed NJ, Guastella D, Blackshaw S, Gallagher M, Traystman RJ, Hurn PD, Koehler RC, Snyder SH. Heme oxygenase-2 is neuroprotective in cerebral ischemia. Mol Med. 1999;5:656–663. [PMC free article] [PubMed] [Google Scholar]
- 48.Du J, Tao-Cheng JH, Zerfas P, McBain CJ. The K+ channel, Kv2.1, is apposed to astrocytic processes and is associated with inhibitory postsynaptic membranes in hippocampal and cortical principal neurons and inhibitory interneurons. Neuroscience. 1998;84:37–48. doi: 10.1016/s0306-4522(97)00519-8. [DOI] [PubMed] [Google Scholar]
- 49.Motterlini R, Otterbein LE. The therapeutic potential of carbon monoxide. Nat Rev Drug Discov. 2010;9:728–743. doi: 10.1038/nrd3228. [DOI] [PubMed] [Google Scholar]
- 50.Chen CY, Jang JH, Li MH, Surh YJ. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem Biophys Res Commun. 2005;331:993–1000. doi: 10.1016/j.bbrc.2005.03.237. [DOI] [PubMed] [Google Scholar]