Abstract
Disorders of Gut-Brain interaction (DGBI) are frequently encountered in clinical practice, and recommendations for diagnosis and management are well established. In a large subset of patients, more than one DGBI diagnosis is present, and in this overlap group symptom severity and impact is higher and the management approach is not well established. This review aims to guide clinicians to understand, recognize and manage overlapping DGBI by identifying causes and pitfalls of overlap conditions, and presenting potential practical approaches to diagnosis, treatment and follow-up. A number of clinical factors may contribute to finding overlapping DGBI, including the anatomical basis of the Rome classification, the potential confusion of symptom descriptors, and patients biases towards higher symptom intensity ratings. Overlapping DGBI may also be caused by mechanistic factors such as pathophysiological mechanisms involving multiple gastrointestinal segments, and the impact of disorders in one segment on sensorimotor function in remote gastrointestinal parts, through neural or hormonal signaling. In terms of management, detailed history taking, which can be facilitated using pictograms, as well as careful assessment of relative timing and cohesion of different symptoms and recognition of associated psychosocial dysfunction are key initial steps. Unnecessary technical investigations and complex combination treatment schedules should be avoided. Based on identification of the dominant symptom pattern and putative underlying pathophysiological mechanisms a single treatment modality is preferably initiated, taking into account the efficacy spectrum of different therapies. Follow-up of the patient’s condition allows to adjust the therapeutic approach as needed, while avoiding unnecessary additional technical investigations.
Introduction
In up to 50% of patients seen in gastroenterology clinical practice, routine diagnostic investigations fail to identify an abnormality that readily explains the symptoms (1). In these patients, who are referred to as having functional gastrointestinal disorders or disorders of gut-brain interaction (DGBI), it is hypothesized that alterations of gastrointestinal sensorimotor function underlie symptom generation (2). The Rome process, updated most recently in the 2016 Rome IV consensus, classifies these patients in different diagnostic categories based on anatomical regions (esophageal, gastroduodenal, biliopancreatic, bowel and anorectal disorders) and symptom groupings (2). The aspiration and underlying assumption is to identify homogeneous patient groups in terms of symptom presentation and underlying pathophysiology, which require specific management and respond more predictably to particular therapeutic approaches.
However, the occurrence of overlap between different categories of DGBI hampers the concept of separate symptom-based diagnostic entities with a particular management and therapeutic approach (3). The fact that large overlap between entities exists has been one of the major points of criticism of the Rome approach (4,5). Managing patients with overlapping DGBI also constitutes a major challenge for clinicians, as currently available diagnostic and management algorithms target single disease entities (6). To some extent, the Rome process has aimed at decreasing overlap through the definitions of entities. For instance, the Rome III and IV functional (FC) constipation diagnostic criteria require that criteria for irritable bowel syndrome (IBS) are not fulfilled (7). When the Rome III requirement that patients meeting IBS criteria cannot be given a diagnosis of FC is suspended, most patients fulfill criteria for both (8).
Our aim was to provide guidance on the clinical management of patients with overlapping DGBI. Relevant literature was assessed through a Pubmed search using “Rome criteria, functional gastrointestinal disorders, overlapping disorders, irritable bowel syndrome or functional dyspepsia or chronic constipation or heartburn” in English language, since 1990. Given the lack of diagnostic and interventional studies published on overlapping disorders, the yield of a systematic review for the practicing clinician would be low. The manuscript is a narrative review of the literature which reflects extensive experience in dealing with complex patients in a referral center for DGBI, where overlap is the norm rather than the exception.
Prevalence and relevance of overlapping conditions
The literature has provided extensive evidence for the occurrence of overlapping DGBI, both at the epidemiological and at the clinical care level (3, 9–12). The Rome Global Epidemiology Study showed that of the 40% of the general population meeting Rome IV DGBI criteria, more than 30% fulfill criteria for DGBI in 2 or more anatomical regions (3). Symptom severity scores, psychosocial co-morbidity and healthcare utilization increase with the number of overlapping conditions while quality of life decreases (3). In the advanced care clinical setting, patients presenting with overlapping disorders are the biggest group and symptom severity and impact are highest in those with overlapping conditions (11,12). Hence, in clinical practice, patients with overlapping conditions represent a common challenge.
Mechanisms underlying the occurrence of overlapping conditions
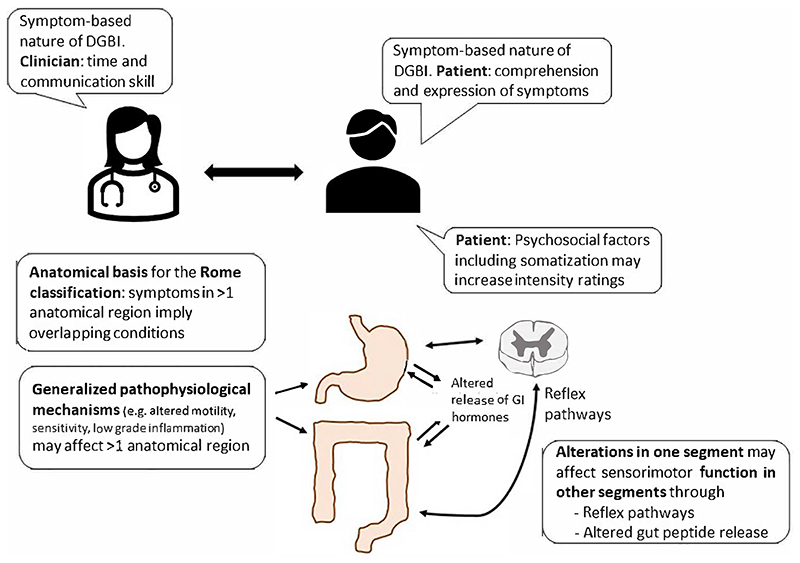
A number of mechanisms, clinical as well as pathophysiological, may contribute to the high prevalence of overlapping DGBI in the general population and in the clinical setting (Figure 1).
Figure 1. Factors contributing to overlapping of Disorders of Gut-Brain Interaction.
1. The anatomical basis of the Rome diagnostic criteria
The Rome diagnostic category scheme is based on the presumed anatomical site of origin of the symptoms to classify patients with DGBI into esophageal, gastroduodenal, biliopancreatic, bowel and anorectal disorder categories (2). A factor analysis of the symptom groupings in a population-based sample of 5931 respondents from 3 countries who filled out the Rome diagnostic questionnaire provided objective support for the Rome symptom groupings (13). On the other hand, a study in 1805 DGBI patients from 11 Asian countries who filled out a more extensive questionnaire identified 3 symptom clusters that involved more than one anatomical region, which are consequently not considered in the Rome classification scheme (14). In the Rome classification approach, any entity involving more than one anatomical region would be categorized as at least two different Rome diagnostic entities. It is conceivable that cultural and linguistic factors contribute to the different cluster findings in Asia versus other continents such as Europe and North America (the West), although a role for the questionnaire design used cannot be excluded (13–16). Whether the same clusters can also be identified in a Western population is the topic of an ongoing international study using a comprehensive integrated questionnaire (17).
2. Symptom descriptors and intensity ratings
Diagnostic categorization of DGBI depends on accurate assessment of the presenting symptom pattern and severity, as revealed by the patient during history taking (2). Indeed, as currently no suitable biomarkers are available, the symptom pattern is the main determinant of individual DGBI diagnosis (18) This requires sufficient time and skill from the clinician and also ability of the patient to understand and express individual symptoms and their distinctions (19). Additionally, diagnostic categorization of DGBI as defined by the Rome consensus is not only driven by the presence of specific symptoms, but also their level of intensity and frequency, which need to exceed specific diagnostic threshold values (2,6,7). DGBI patients, for instance those with IBS, have a lowered objective response threshold for using negative affective terms to label bodily experiences and for using higher bothersome or intensity ratings (20,21). Such bias towards higher intensity ratings increases the likelihood of reaching diagnostic thresholds for DGBI, and hence of overlapping conditions. Several studies on DGBI have confirmed the relevance of psychosocial co-morbidities, especially anxiety and somatization, as determinants of the presence and impact of DGBI, including their overlap (3,22–24). Somatization - used here in the descriptive sense of “widespread somatic symptoms” in line with the Rome IV consensus and with the use of the word in DGBI literature - is potentially the most important mechanism generating a higher intensity rating bias (22–24). A similar mechanism may underlie the reported instability of DGBI diagnoses over time, where prospectively followed patients may change questionnaire-based diagnoses over time (25). It remains to be established whether a true shift in symptom pattern occurs, or whether limited differences in symptom intensity and frequency rating result in different diagnostic categorisation.
3. The role of common pathophysiological mechanisms
While the pathophysiological basis of DGBI remains incompletely understood, some of the plausible candidate mechanisms may involve several anatomical sites and hence be relevant to a number of Rome DGBI diagnostic entities. Visceral hypersensitivity, often driven by central sensitization of signals from visceral afferents in the gut-brain axis, is a key factor determining symptom severity both in upper and lower gastrointestinal DGBI, and if present is likely to contribute to the presence of overlapping conditions (26).
Hypocontractility of the gastrointestinal tract is another pathophysiological finding which often involves several anatomical regions (27). Alterations in immune cell composition – and function have been shown in IBS and functional dyspepsia (FD) patients at both the mucosal and systemic level, as recently reviewed (28). Most recently, atypical allergic reactions to food have been reported both in IBS and in FD, in the duodenum and the rectum possibly contributing to overlap of these DGBI (29–31).
4. (Alterations in one segment may affect sensorimotor function in other segments of the gastrointestinal tract)
Besides the often wider presence of hypocontractility in the gastrointestinal tract, disordered motility in one part of the gastrointestinal tract may impact on the function of other parts, and this is presumably mediated through gut peptide or prevertebral neural reflex pathways (32). Delayed gastric emptying, for instance, is commonly observed in patients with slow transit constipation, but inflating a balloon in the rectum is able to significantly delay gastric emptying in healthy controls (33). Possibly through a similar mechanism, slow transit constipation is also associated with impaired gastric accommodation, a key pathophysiological mechanism in FD (34). Impaired gastric accommodation has also been identified as a key trigger for transient lower esophageal sphincter relaxations, the main mechanism underlying gastroesophageal reflux events (35). Fermentation and the presence of short chain fatty acids in the colon also decreases postprandial pressure in the lower esophageal sphincter and is associated with enhanced transient lower esophageal sphincter relaxation occurrence (36).
Clinical approach and management of overlapping conditions
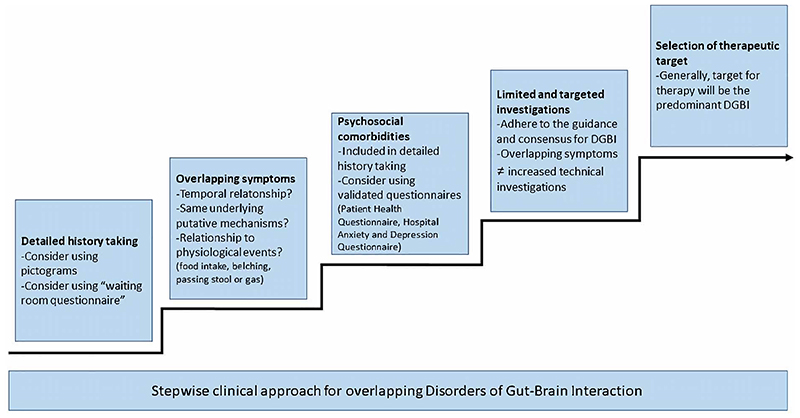
This section aims at providing guidance to clinicans managing patients with overlapping DGBI, and provides some potential solutions to the issues outlined above. The stepwise approach is summarized in Figure 2.
Figure 2. Stepwise clinical approach in the management of patients with overlapping Disorders of Gut-Brain Interaction.
1. Detailed history taking to evaluate symptoms suggestive of (overlapping) DGBI and consideration of underlying pathophysiological mechanisms
In view of the relevance of symptom criteria for DGBI diagnoses, a systematic assessment of the symptom pattern is mandatory to accurately identify the presence of (overlapping) DGBI (3). The quality of the history taking and interaction with the patient is determined by the physician’s and patient’s communication skills (19). Moreover, there my be a discrepancy in anatomical understanding and description of a perceived symptom between the patient and clinician (37). Hence, even a dedicated history taking by an experienced clinician may not allow all patients to fully express the individual and multidimensional nature of their symptoms (37). In these cases, pictograms accompanying verbal descriptors are able to significantly improve symptom descriptors by patients and may help clarify overlap, as well as identify the most bothersome symptom (37,38). An integrated “waiting room questionnaire” with symptom descriptors and pictograms for the main DGBI showed promise as a tool to facilitate accurately diagnosing DGBI (39).
The presence of DGBI symptoms from different anatomical locations already implies that overlapping conditions are present (2). Overlapping DGBI can also be present within the same anatomical region: postprandial distress syndrome with nausea and vomiting for instance comprises two distinct gastroduodenal disorders (40,41).
In case of overlapping conditions, it is important to determine the temporal relationship between symptoms belonging to different DGBI or anatomical regions. The history taking should establish whether these symptoms usually occur, worsen and improve together, have a fixed timing towards each other, or whether they evolve separately.In addition, the relationship of symptoms belonging to different DGBI or anatomical regions to physiological events such as food intake, belching or passing stools or gas, is an important clinical indicator. For instance, when epigastric pain and diarrhea are present in the same patient, it is relevant to question whether they do occur simultaneously or not, with food-induced reactions as a common mechanism in mind (29–31). Similarly, with the associations of colonic stasis with delayed emptying and impaired accommodation outlined above (33,34), it is relevant in a patient expressing constipation as well as postprandial fullness or early satiation, to evaluate whether the latter symptoms also improve after a bowel movement or worsen during constipation episodes.
2. Evaluation of the presence of psychosocial co-morbidities that enhance symptom intensity reporting
As outlined above, psychosocial factors such as anxiety, depression and especially somatization may lead to amplified symptom reporting, thereby increasing the likelihood of overlapping DGBI diagnoses (20–24). Psychosocial co-morbidities are a key part of the Multi-Dimensional Clinical Profile, which summarizes factors besides the categorical DGBI diagnosis that are relevant to consider in clinical care of DGBI patients (43). The presence of these amplifying and confounding factors can be evaluated through history taking or aided by validated questionnaires such as the Patient Health Questionnaires and the Hospital Anxiety and Depression questionnaire (22,41). More in-depth and practical guidance on using these questionnaires in clinical practice are provided elsewhere (42).
3. Selection of limited and targeted additional technical investigations
While overlapping DGBI are highly prevalent in clinical practice, diagnostic guidelines have focused on patients with single DGBI diagnoses. There is a need for systematically collected diagnoscticand outcome studies in DGBI overlap patients. Besides the symptom assessment, as outlined in the previous sections, the presence of risk or alarm factors should also be evaluated. The presence of these symptoms or findings, such as weight loss, blood in the stools, the family history (of inflammatory bowel disease, coeliac disease or abdominal cancer), or age above the threshold for upper or lower gastrointestinal screening endoscopy should determine the extent of the technical investigations as indicated by international consensus (7,40). The presence of multiple symptoms should not lower the thresholds for additional technical investigations.
In patients with multiple symptoms, for instance in case of overlapping DGBI diagnosis, diagnostic uncertainty and likelihood of incomplete response to therapy is larger. However, the yield of additional technical examinations such as repeat endoscopy, radiological imaging or more sophisticated function testing remains low (7,44–48). Clinicians should thus positively diagnose the respective (overlap of) DGBI if the criteria are fulfilled and limited diagnostic workup has ruled out organic disease as recommended by current guidelines pertaining to the relevant DGBI (49,50). Of note, in clinical practice patients may also be diagnosed with DGBI before symptom duration reaches the diagnostic threshold (51).
4. Determination of the therapeutic target
The presence of overlapping DGBI does not implicate combination therapy from the onset. Based on the symptom pattern assessment and identification of potential underling pathophysiological features, the primary DGBI entity or symptom to target needs to be determined (3,9–12). The therapeutic target will often be driven by the predominant symptom, as indicated by the patient (37,52). Based on presumed underlying pathophysiology, another target may be chosen, for instance targeting colonic transit in case of overlapping postprandial distress syndrome with chronic constipation, in patients whose symptoms evolve in parallel (34). However, there is a lack of studies that investigates whether this type of clinical markers is able to predict therapeutic outcome.
The available literature shows that associated symptoms, which are not necessarily a part of the cardinal symptom pattern may also improve with therapy, even when not directly targeted by the mode of action of the chosen pharmacotherapeutic agent (53–55). Furthermore, for a number of therapeutic approaches, efficacy on symptoms outside their primary indication has been demonstrated, either in treatment trials, or can be expected based on their pharmacological effects on gastrointestinal (patho-) physiology (53–73). Knowledge of the spectrum of symptom improvement with these approaches, summarized in Table 1, can help to choose the optimal first-line therapy choice. For instance, as shown in Table 1, the 5-HT3 receptor antagonist ondansetron may improve nausea as well as diarrhea and urgency, and the 5-HT1A agonist buspirone may improve dysphagia, PDS as well as rectal urgency (53,60–62,70). Patients should be made aware of the triggered symptom pattern and that improvement of overlapping symptoms might not occur simultaneously but sequentially in order to appropriately manage expectations. While the preceding statements and Table 1 favor a single treatment choice, there is a clear need to compare the efficacy of single treatment initiation based on the predominant symptom to the use of combination therapies as initial approach.
Table.
Guidance for selecting therapeutic choices In the management of patients with overlapping Disorders of Gut-Brain Interaction. Brain-gut behavioural therapies and the use of neuromodulators for chronic visceral pain are addressed in the main text.
| Oesophageal symptoms | Gastroduodenal symptoms | Bowel symptoms | |
|---|---|---|---|
| Proton pump inhibitors (42,45) | Efficacy for heartburn shown in clinical trial | Efficacy for postprandial distress syndrome shown in clinical trial | Efficacy not evaluated |
| Baclofen (46,47) | Efficacy for heartburn and belching shown in clinical trial | Efficacy not evaluated | Efficacy not evaluated |
| Pyridostigmine (48) | Efficacy for dysphagia suggested by mechanistic evaluation | Efficacy not evaluated | Efficacy for constipation suggested by mechanistic evaluation |
| Buspirone (48,49,60,63) | Efficacy for dysphagia suggested by mechanistic evaluation | Efficacy for postprandial distress syndrome shown in clinical trial | Efficacy for faecal urgency suggested by mechanistic evaluation |
| Itopride (43,50) | Efficacy for heartburn suggested by mechanistic evaluation | Efficacy for postprandial distress syndrome, epigastric pain, and nausea shown in clinical trial | Efficacy not evaluated |
| Ondansetron (51,52) | Efficacy not evaluated | Efficacy for nausea shown in clinical trial | Efficacy for diarrhoea and faecal urgency shown in clinical trial |
| Mirtazapine (53,60,63) | Efficacy not evaluated | Efficacy for postprandial distress syndrome and nausea shown in clinical trial | Efficacy not evaluated |
| Peppermint oil (54,55) | Efficacy not evaluated | Efficacy for postprandial distress syndrome and epigastric pain shown in clinical trial | Efficacy for abdominal pain shown in clinical trial |
| Prucalopride (56,57,58) | Efficacy for heartburn shown in clinical trial | Efficacy for postprandial distress syndrome and nausea shown in clinical trial | Efficacy for bloating and constipation shown in clinical trial |
| Tricyclic agent (44,61,63–65) | Efficacy not evaluated | Efficacy for epigastric pain shown in clinical trial | Efficacy for abdominal pain and diarrhoea shown in clinical trial |
| Low FODMAP diet (59,60) | Efficacy for heartburn suggested by mechanistic evaluation | Efficacy for postprandial distress syndrome shown in clinical trial | Efficacy for abdominal pain, bloating, constipation, diarrhoea, and faecal urgency shown in clinical trial |
Table 1 does not address the use of brain-gut behavioral therapies, which have a potential to offer improvement throughout the entire spectrum of DGBI (74). Psychological therapies such as cognitive behavioural therapies can be considered early on in patients that recognize the relationship of psychological factors and fluctuation of symptoms, especially if they are motivated to take on an active role in self-management (74). Brain-gut behavioral therapies are especially valuable to address hypervigilance, gastrointestinal fear conditioning and visceral anxiety which aggravate symptom severity and impact, especially in patients with overlapping DGBI (3,74). However, specific research is needed to establish whether the overlapping DGBI population has a superior response to brain-directed therapies. Neuromodulators should be considered depending on the predominant symptom profile and additional psychosocial factors as presented in table 1 and explained in detail elsewhere (70). Major psychological co-morbidity should be recognized and treated separately if clinically relevant and severe enough (70). They are especially appropriate in case of overlapping painful conditions where neuromodulators can restore defective anti-nociceptive processes leading to visceral hypersensitivity and allodynia (70). In patients with a high somatization score, a long-term goal of gradual symptom improvement, rather than elimination, is probably most realistic and should be discussed (9,23,24,74–77).
5. Follow-up
The long-term prognosis of patients with overlapping compared to those with single DGBI still needs to be studied. After starting the first-line therapy, the timing of follow-up will depend on the response profile of the treatment, and may vary from 4 to 12 weeks (53–73). Currently available patient reported outcome measures mainly focus single diagnostic entities, and may need to be combined or specifically developed for this group. Adjustment of therapy will depend on the magnitude of the initial response. In case of insufficient improvement, therapy can be switched to another choice. In case of incomplete improvement, combination therapy can be considered depending on the nature of the residual non-responding symptom(s). Adding technical investigations and referral of the patient to other specialists in case of insufficient treatment response should be carefully considered, especially in patients with widespread somatic symptoms (gastrointestinal and extra-intestinal) (76,77).
Conclusion
In epidemiological studies as well as in clinical practice, DGBI commonly overlap in the same subject. The presence of overlapping DGBI is associated with higher symptom severity and impact. Currently available guidelines only address the management of patients with a single DGBI, and algorithms are lacking for the overlap group, in spite of the high clinical burden and need. Multiple factors contribute to the frequent occurrence of overlapping disorders, including both clinical characteristics and evaluations, as well as common pathophysiological pathways.
While overlapping DGBI are highly prevalent in clinical practice, research and management guidelines have focused on patients with single DGBI diagnoses. There is a need for systematically collected phenotyping and outcome studies in DGBI overlap patients. Patient reported outcome measures may need to be combined or specifically developed for this group. The efficacy of single treatment initiation based on the predominant symptom needs to be compared to the use of combination therapies as initial approach. Peripherally acting pharmacotherapeutic or dietary approaches need to be compared to neuromodulator therapies and to brain-gut behavioral therapies, to establish whether the overlapping DGBI population has a superior response to brain-directed therapies and whether clinical markers are able to predict therapeutic outcome. Finally, the long-term prognosis of single versus overlapping DGBI needs to be compared.
In terms of current management recommendations, detailed history taking, which can be aided by pictogram containing symptom questionnaires, as well as careful assessment of relative timing and cohesion of different symptoms is key to understanding the patients’ DGBI diagnoses and detailed symptom pattern. The presence of psychosocial dysfunction, which aggravate symptom reporting, should be considered, and only targeted additional technical investigations should be used. A single treatment modality is preferably initiated, based on identification of the dominant symptom pattern and taking into account putative underlying pathophysiological mechanism and the efficacy spectrum of different therapies. Follow-up visits allow to adjust or change the therapeutic approach, while avoiding unnecessary repeat or additional technical investigations for the broad symptom spectrum in overlapping DGBI patients.
Funding
Supported by Methusalem grant METH/21/04 from Leuven University to Jan Tack.
Footnotes
Author contributions:
LMB: input on bowel disorders, data collection, critical review and editing of manuscript.
FC: input on gastroduodenal disorders, critical review and editing of manuscript.
KR: input on esophageal disorders, critical review and editing of manuscript.
ES: input on pharmacological treatment, critical review and editing of manuscript.
JT: conceptualisation, data collection, original draft of manuscript and final editing.
Disclosures: Jan Tack has given Scientific advice to Adare, AlfaWassermann, Arena, Bayer, Christian Hansen, Clasado, Danone, Devintec, Falk, FitForMe, Grünenthal, Ironwood, Janssen, Kiowa Kirin, Menarini, Mylan, Neurogastrx, Neutec, Novartis, Nutricia, Reckitt Benckiser, Ricordati, Shionogi, Takeda, Truvion, Tsumura, Zealand and Zeria pharmaceuticals, has received research support from Biohit, Shire, Sofar and Takeda, and has served on the Speaker bureau for Abbott, Allergan, AstraZeneca, FitForMe, Janssen, Kyowa Kirin, Mayoly, Menarini, Mylan, Novartis, Schwabe Parmaceuticals, Takeda, Wellspect and Zeria. The other authors have no conflict of interest to declare.
Declaration of originality:
This paper has not been submitted to another journal, and has not been published in whole or in part elsewhere previously.
References
- 1.Talley NJ. Functional gastrointestinal disorders as a public health problem. Neurogastroenterol Motil. 2008 May;20(Suppl 1):121–9. doi: 10.1111/j.1365-2982.2008.01097.x. [DOI] [PubMed] [Google Scholar]
- 2.Drossman DA, Hasler WL. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology. 2016 May;150(6):1257–61. doi: 10.1053/j.gastro.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 3.Sperber AD, Freud T, Aziz I, Palsson OS, Drossman DA, Dumitrascu DL, Fang X, Fukudo S, Ghoshal UC, Kellow J, Khatun R, et al. Greater Overlap of Rome IV Disorders of Gut-Brain Interactions Leads to Increased Disease Severity and Poorer Quality of Life. Clin Gastroenterol Hepatol. 2022 May;20(5):e945–e956. doi: 10.1016/j.cgh.2021.05.042. [DOI] [PubMed] [Google Scholar]
- 4.Holtmann GJ, Talley NJ. Inconsistent symptom clusters for functional gastrointestinal disorders in Asia: is Rome burning? Gut. 2018 Jun 19;:pii: gutjnl-2017-314775. doi: 10.1136/gutjnl-2017-314775. [DOI] [PubMed] [Google Scholar]
- 5.Siah KTH, Gong X, Yang XJ, Whitehead WE, Chen M, Hou X, Pratap N, Ghoshal UC, Syam AF, Abdullah M, Choi MG, et al. Rome Foundation-Asian working team report: Asian functional gastrointestinal disorder symptom clusters. Gut. 2018 Jun;67(6):1071–1077. doi: 10.1136/gutjnl-2016-312852. [DOI] [PubMed] [Google Scholar]
- 6.Drossman DA. Rome Foundation Diagnostic Algorithms. Am J Gastroenterol. 2010 Apr;105(4):741–2. doi: 10.1038/ajg.2010.63. [DOI] [PubMed] [Google Scholar]
- 7.Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R. Bowel Disorders. Gastroenterology. 2016;150:1393–1407. doi: 10.1053/j.gastro.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 8.Siah KT, Wong RK, Whitehead WE. Chronic Constipation and Constipation-Predominant IBS: Separate and Distinct Disorders or a Spectrum of Disease? Gastroenterol Hepatol (N Y) 2016 Mar;12(3):171–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Aziz I, Palsson OS, Törnblom H, Sperber AD, Whitehead WE, Simrén M. The Prevalence and Impact of Overlapping Rome IV-Diagnosed Functional Gastrointestinal Disorders on Somatization, Quality of Life, and Healthcare Utilization: A Cross-Sectional General Population Study in Three Countries. Am J Gastroenterol. 2018 Jan;113(1):86–96. doi: 10.1038/ajg.2017.421. [DOI] [PubMed] [Google Scholar]
- 10.Vakil N, Stelwagon M, Shea EP, Miller S. Symptom burden and consulting behavior in patients with overlapping functional disorders in the US population. United European Gastroenterol J. 2016 Jun;4(3):413–22. doi: 10.1177/2050640615600114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Wulffen M, Talley NJ, Hammer J, McMaster J, Rich G, Shah A, Koloski N, Kendall BJ, Jones M, Holtmann G. Overlap of Irritable Bowel Syndrome and Functional Dyspepsia in the Clinical Setting: Prevalence and Risk Factors. Dig Dis Sci. 2019 Feb;64(2):480–486. doi: 10.1007/s10620-018-5343-6. [DOI] [PubMed] [Google Scholar]
- 12.de Bortoli N, Tolone S, Frazzoni M, Martinucci I, Sgherri G, Albano E, Ceccarelli L, Stasi C, Bellini M, Savarino V, Savarino EV, et al. Gastroesophageal reflux disease, functional dyspepsia and irritable bowel syndrome: common overlapping gastrointestinal disorders. Ann Gastroenterol. 2018 Nov-Dec;31(6):639–648. doi: 10.20524/aog.2018.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clevers E, Whitehead WE, Palsson OS, Sperber AD, Törnblom H, Van Oudenhove L, Tack J, Simrén M. Factor Analysis Defines Distinct Upper and Lower Gastrointestinal Symptom Groups Compatible With Rome IV Criteria in a Population-based Study. Clin Gastroenterol Hepatol. 2018 Aug;16(8):1252–1259. doi: 10.1016/j.cgh.2018.02.042. [DOI] [PubMed] [Google Scholar]
- 14.Siah KTH, Gong X, Yang XJ, Whitehead WE, Chen M, Hou X, Pratap N, Ghoshal UC, Syam AF, Abdullah M, Choi MG, et al. Rome Foundation-Asian working team report: Asian functional gastrointestinal disorder symptom clusters. Gut. 2017 Jun 7;:pii: gutjnl-2016-312852. doi: 10.1136/gutjnl-2016-312852. [DOI] [PubMed] [Google Scholar]
- 15.Chuah KH, Mahadeva S. Cultural Factors Influencing Functional Gastrointestinal Disorders in the East. J Neurogastroenterol Motil. 2018 Oct 1;24(4):536–543. doi: 10.5056/jnm18064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahadeva S, Ford AC. Clinical and epidemiological differences in functional dyspepsia between the east and the west. Neurogastroenterol Motil. 2016;28:167–174. doi: 10.1111/nmo.12657. [DOI] [PubMed] [Google Scholar]
- 17.Colomier E, Holvoet L, Carbone F, Chuah KH, Mahaveda S, Siah K, Holtmann G, Suzuki H, Ueda T, Simren M, Gwee KA, et al. Symptom clusters outisde the Rome IV consensus can be identified in both eastern and western patients with a disorder of gut-brain interaction: preliminary results of an ongoing international multicenter study. Gastroenterolgoy. 2022;162(suppl 7):S5934 [Google Scholar]
- 18.Simrén M, Tack J. Combining symptoms and biomarkers: The future diagnostic approach for disorders of gut-brain interaction? Neurogastroenterol Motil. 2020 Nov;32(11):e14019. doi: 10.1111/nmo.14019. [DOI] [PubMed] [Google Scholar]
- 19.Drossman DA, Chang L, Deutsch JK, Ford AC, Halpert A, Kroenke K, Nurko S, Ruddy J, Snyder J, Sperber A. A Review of the Evidence and Recommendations on Communication Skills and the Patient-Provider Relationship: A Rome Foundation Working Team Report. Gastroenterology. 2021 Nov;161(5):1670–1688.:e7. doi: 10.1053/j.gastro.2021.07.037. [DOI] [PubMed] [Google Scholar]
- 20.Naliboff BD, Munakata J, Fullerton S, Gracely RH, Kodner A, Harraf F, Mayer EA. Evidence for two distinct perceptual alterations in irritable bowel syndrome. Gut. 1997 Oct;41(4):505–12. doi: 10.1136/gut.41.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitehead WE, Palsson OS. Is rectal pain sensitivity a biological marker for irritable bowel syndrome: psychological influences on pain perception. Gastroenterology. 1998 Nov;115(5):1263–71. doi: 10.1016/s0016-5085(98)70099-x. [DOI] [PubMed] [Google Scholar]
- 22.Van Oudenhove L, Vandenberghe J, Geeraerts B, Vos R, Persoons P, Fischler B, Demyttenaere K, Tack J. Determinants of symptoms in functional dyspepsia: gastric sensorimotor function, psychosocial factors or somatisation? Gut. 2008 Dec;57(12):1666–73. doi: 10.1136/gut.2008.158162. [DOI] [PubMed] [Google Scholar]
- 23.Van Oudenhove L, Törnblom H, Störsrud S, Tack J, Simrén M. Depression and Somatization Are Associated With Increased Postprandial Symptoms in Patients With Irritable Bowel Syndrome. Gastroenterology. 2016 Apr;150(4):866–74. doi: 10.1053/j.gastro.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Choung RS, et al. Multiple functional gastrointestinal disorders linked to gastroesophageal reflux and somatization: A population-based study. Neurogastroenterol Motil. 2017 doi: 10.1111/nmo.13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halder SL, Locke GR, 3rd, Schleck CD, Zinsmeister AR, Melton LJ, 3rd, Talley NJ. Natural history of functional gastrointestinal disorders: a 12-year longitudinal population-based study. Gastroenterology. 2007;133:799–807. doi: 10.1053/j.gastro.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Simrén M, Törnblom H, Palsson OS, van Tilburg MAL, Van Oudenhove L, Tack J, Whitehead WE. Visceral hypersensitivity is associated with GI symptom severity in functional GI disorders: consistent findings from five different patient cohorts. Gut. 2018 Feb;67(2):255–262. doi: 10.1136/gutjnl-2016-312361. [DOI] [PubMed] [Google Scholar]
- 27.Keller J, Bassotti G, Clarke J, Dinning P, Fox M, Grover M, Hellström PM, Ke M, Layer P, Malagelada C, Parkman HP, et al. Expert consensus document: Advances in the diagnosis and classification of gastric and intestinal motility disorders. Nat Rev Gastroenterol Hepatol. 2018 May;15(5):291–308. doi: 10.1038/nrgastro.2018.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burns G, Carroll G, Mathe A, Horvat J, Foster P, Walker MM, Talley NJ, Keely S. Evidence for Local and Systemic Immune Activation in Functional Dyspepsia and the Irritable Bowel Syndrome: A Systematic Review. Am J Gastroenterol. 2019 Mar;114(3):429–436. doi: 10.1038/s41395-018-0377-0. [DOI] [PubMed] [Google Scholar]
- 29.Fritscher-Ravens A, Pflaum T, Mösinger M, Ruchay Z, Röcken C, Milla PJ, Das M, Böttner M, Wedel T, Schuppan D. Many Patients With Irritable Bowel Syndrome Have Atypical Food Allergies Not Associated With Immunoglobulin E. Gastroenterology. 2019 Jul;157(1):109–118.:e5. doi: 10.1053/j.gastro.2019.03.046. [DOI] [PubMed] [Google Scholar]
- 30.Aguilera-Lizarraga J, Florens MV, Viola MF, Jain P, Decraecker L, Appeltans I, Cuende-Estevez M, Fabre N, Van Beek K, Perna E, Balemans D, et al. Local immune response to food antigens drives meal-induced abdominal pain. Nature. 2021 Feb;590(7844):151–156. doi: 10.1038/s41586-020-03118-2. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van den Houte K, Bercik P, Simren M, Tack J, Vanner S. Mechanisms Underlying Food-Triggered Symptoms in Disorders of Gut-Brain Interactions. Am J Gastroenterol. 2022 Jun 1;117(6):937–946. doi: 10.14309/ajg.0000000000001812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azpiroz F, Feinle-Bisset C, Grundy D, Tack J. Gastric sensitivity and reflexes: basic mechanisms underlying clinical problems. J Gastroenterol. 2014 Feb;49(2):206–18. doi: 10.1007/s00535-013-0917-8. [DOI] [PubMed] [Google Scholar]
- 33.Coremans G, Geypens B, Vos R, Tack J, Margaritis V, Ghoos Y, Janssens J. Influence of continuous isobaric rectal distension on gastric emptying and small bowel transit in young healthy women. Neurogastroenterol Motil. 2004 Feb;16(1):107–11. doi: 10.1046/j.1365-2982.2003.00463.x. [DOI] [PubMed] [Google Scholar]
- 34.Penning C, Vu MK, Delemarre JB, Masclee AA. Proximal gastric motor and sensory function in slow transit constipation. Scand J Gastroenterol. 2001 Dec;36(12):1267–73. doi: 10.1080/003655201317097100. [DOI] [PubMed] [Google Scholar]
- 35.Pauwels A, Altan E, Tack J. The gastric accommodation response to meal intake determines the occurrence of transient lower esophageal sphincter relaxations and reflux events in patients with gastro-esophageal reflux disease. Neurogastroenterol Motil. 2014 Apr;26(4):581–8. doi: 10.1111/nmo.12305. [DOI] [PubMed] [Google Scholar]
- 36.Piche T, Zerbib F, Varannes SB, Cherbut C, Anini Y, Roze C, le Quellec A, Galmiche JP. Modulation by colonic fermentation of LES function in humans. Am J Physiol Gastrointest Liver Physiol. 2000 Apr;278(4):G578–84. doi: 10.1152/ajpgi.2000.278.4.G578. [DOI] [PubMed] [Google Scholar]
- 37.Tack J, Carbone F, Holvoet L, Vanheel H, Vanuytsel T, Vandenberghe A. The use of pictograms improves symptom evaluation by patients with functional dyspepsia. Aliment Pharmacol Ther. 2014 Sep;40(5):523–30. doi: 10.1111/apt.12855. [DOI] [PubMed] [Google Scholar]
- 38.Rábago R, Bonilla A, Escamilla-Diego E, Higuera de la Tijera MF, Schmulson M. Pictograms are more effective than verbal descriptors in Spanish for bloating and distension. Neurogastroenterol Motil. 2022 Apr 8;:e14364. doi: 10.1111/nmo.14364. [DOI] [PubMed] [Google Scholar]
- 39.Goelen N, Carbone F, Holvoet L, Vandenberghe A, Arts J, Caenepeel P, Tack J. The waiting room questionnaire: validation of a novel patient reported outcome questionnaire for the diagnosis of functional gastrointestinal disorders. Gastroenterology. 2017;152(Suppl. 1):S744–5. [Google Scholar]
- 40.Stanghellini V, Chan FK, Hasler WL, Malagelada JR, Suzuki H, Tack J, Talley NJ. Gastroduodenal Disorders. Gastroenterology. 2016 May;150(6):1380–92. doi: 10.1053/j.gastro.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 41.Sperber AD, Bangdiwala SI, Drossman DA, Ghoshal UC, Simren M, Tack J, Whitehead WE, Dumitrascu DL, Fang X, Fukudo S, Kellow J, et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology. 2021 Jan;160(1):99–114.:e3. doi: 10.1053/j.gastro.2020.04.014. [DOI] [PubMed] [Google Scholar]
- 42.Luo Y, Keefer L. Role of psychological questionnaires in clinical practice and research within functional gastrointestinal disorders. Neurogastroenterology & Motility. 2021;33:e14297. doi: 10.1111/nmo.14297. [DOI] [PubMed] [Google Scholar]
- 43.Drossman DA. Improving the Treatment of Irritable Bowel Syndrome With the Rome IV Multidimensional Clinical Profile. Gastroenterol Hepatol (N Y) 2017 Nov;13(11):694–696. [PMC free article] [PubMed] [Google Scholar]
- 44.Sahai AV, Penman ID, Mishra G, Williams D, Pearson A, Wallace MB, van Velse A, Hoffman BJ, Hawes RH. An assessment of the potential value of endoscopic ultrasound as a cost-minimizing tool in dyspeptic patients with persistent symptoms. Endoscopy. 2001 Aug;33(8):662–7. doi: 10.1055/s-2001-16223. [DOI] [PubMed] [Google Scholar]
- 45.Pironti A, Tadeu V, Pedroni A, Porcu A, Manca A, Massarelli G, Realdi G, Dore MP. Role of routine small intestinal biopsy in adult patient with irritable bowel syndrome-like symptoms. Minerva Med. 2010 Jun;101(3):129–34. [PubMed] [Google Scholar]
- 46.Furman DL, Cash BD. The role of diagnostic testing in irritable bowel syndrome. Gastroenterol Clin North Am. 2011 Mar;40(1):105–19. doi: 10.1016/j.gtc.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 47.Lariño-Noia J, de la Iglesia D, Iglesias-García J, Macías F, Nieto L, Bastón I, Villalba C, Domínguez-Muñoz JE. Morphological and functional changes of chronic pancreatitis in patients with dyspepsia: A prospective, observational, cross-sectional study. Pancreatology. 2018 Apr;18(3):280–285. doi: 10.1016/j.pan.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 48.Sabaté JM, Rivière S, Jouet P, Gastaldi-Menager C, Fagot-Campagna A, Tuppin P. Healthcare use by 30,000 patients with irritable bowel syndrome (IBS) in France: a 5-year retrospective and one-year prospective national observational study. BMC Gastroenterol. 2019 Jun 27;19(1):111. doi: 10.1186/s12876-019-1031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lacy BE, Pimentel M, Brenner DM, Chey WD, Keefer LA, Long MD, Moshiree B. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am J Gastroenterol. 2021 Jan 1;116(1):17–44. doi: 10.14309/ajg.0000000000001036. [DOI] [PubMed] [Google Scholar]
- 50.Wauters L, Dickman R, Drug V, Mulak A, Serra J, Enck P, Tack J, ESNM FD Consensus Group. Accarino A, Barbara G, Bor S, et al. United European Gastroenterology (UEG) and European Society for Neurogastroenterology and Motility (ESNM) consensus on functional dyspepsia. United European Gastroenterol J. 2021 Apr;9(3):307–331. doi: 10.1002/ueg2.12061. PMID. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Drossman DA, Tack J. Rome Foundation Clinical Diagnostic Criteria for Disorders of Gut-Brain Interaction. Gastroenterology. 2022 Mar;162(3):675–679. doi: 10.1053/j.gastro.2021.11.019. [DOI] [PubMed] [Google Scholar]
- 52.Karamanolis G, Caenepeel P, Arts J, Tack J. Association of the predominant symptom with clinical characteristics and pathophysiological mechanisms in functional dyspepsia. Gastroenterology. 2006 Feb;130(2):296–303. doi: 10.1053/j.gastro.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 53.Simrén M, Törnblom H, Palsson OS, Whitehead WE. Management of the multiple symptoms of irritable bowel syndrome. Lancet Gastroenterol Hepatol. 2017 Feb;2(2):112–122. doi: 10.1016/S2468-1253(16)30116-9. [DOI] [PubMed] [Google Scholar]
- 54.Bardhan KD, Stanghellini V, Armstrong D, Berghöfer P, Gatz G, Mönnikes H. Evaluation of GERD symptoms during therapy. Part I Development of the new GERD questionnaire ReQuest. Digestion. 2004;69(4):229–37. doi: 10.1159/000079707. [DOI] [PubMed] [Google Scholar]
- 55.Carbone F, Vandenberghe A, Holvoet L, Piessevaux H, Arts J, Caenepeel P, Staessen D, Vergauwe P, Maldague P, De Ronde T, Wuestenberghs F, et al. A double-blind randomized, multicenter, placebo-controlled study of itopride in functional dyspepsia postprandial distress syndrome. Neurogastroenterol Motil. 2022 Aug;34(8):e14337. doi: 10.1111/nmo.14337. [DOI] [PubMed] [Google Scholar]
- 56.Braak B, Klooker TK, Wouters MM, Lei A, van den Wijngaard RM, Boeckxstaens GE. Randomised clinical trial: the effects of amitriptyline on drinking capacity and symptoms in patients with functional dyspepsia, a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2011 Sep;34(6):638–48. doi: 10.1111/j.1365-2036.2011.04775.x. [DOI] [PubMed] [Google Scholar]
- 57.Wauters L, Ceulemans M, Frings D, Lambaerts M, Accarie A, Toth J, Mols R, Augustijns P, De Hertogh G, Van Oudenhove L, Tack J, et al. Proton Pump Inhibitors Reduce Duodenal Eosinophilia, Mast Cells, and Permeability in Patients With Functional Dyspepsia. Gastroenterology. 2021 Apr;160(5):1521–1531. doi: 10.1053/j.gastro.2020.12.016. [DOI] [PubMed] [Google Scholar]
- 58.Blondeau K, Boecxstaens V, Rommel N, Farré R, Depeyper S, Holvoet L, Boeckxstaens G, Tack JF. Baclofen improves symptoms and reduces postprandial flow events in patients with rumination and supragastric belching. Clin Gastroenterol Hepatol. 2012 Apr;10(4):379–84. doi: 10.1016/j.cgh.2011.10.042. [DOI] [PubMed] [Google Scholar]
- 59.Pauwels A, Raymenants K, Geeraerts A, Boecxstaens V, Masuy I, Broers C, Vanuytsel T, Tack J. Clinical trial: a controlled trial of baclofen add-on therapy in PPI-refractory gastro-oesophageal reflux symptoms. Aliment Pharmacol Ther. 2022 Jul;56(2):231–239. doi: 10.1111/apt.17068. [DOI] [PubMed] [Google Scholar]
- 60.Jandee S, Geeraerts A, Geysen H, Rommel N, Tack J, Vanuytsel T. Management of Ineffective Esophageal Hypomotility. Front Pharmacol. 2021 May 26;12:638915. doi: 10.3389/fphar.2021.638915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maxton DG, Morris J, Whorwell PJ. Selective 5-hydroxytryptamine antagonism: a role in irritable bowel syndrome and functional dyspepsia? Aliment Pharmacol Ther. 1996 Aug;10(4):595–9. doi: 10.1046/j.1365-2036.1996.30172000.x. [DOI] [PubMed] [Google Scholar]
- 62.Garsed K, Chernova J, Hastings M, Lam C, Marciani L, Singh G, Henry A, Hall I, Whorwell P, Spiller R. A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut. 2014 Oct;63(10):1617–25. doi: 10.1136/gutjnl-2013-305989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tack J, Ly HG, Carbone F, Vanheel H, Vanuytsel T, Holvoet L, Boeckxstaens G, Caenepeel P, Arts J, Van Oudenhove L. Efficacy of Mirtazapine in Patients With Functional Dyspepsia and Weight Loss. Clin Gastroenterol Hepatol. 2016 Mar;14(3):385–392. doi: 10.1016/j.cgh.2015.09.043. [DOI] [PubMed] [Google Scholar]
- 64.Li J, Lv L, Zhang J, Xu L, Zeng E, Zhang Z, Wang F, Tang X. A Combination of Peppermint Oil and Caraway Oil for the Treatment of Functional Dyspepsia: A Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med. 2019 Nov 14;2019:7654947. doi: 10.1155/2019/7654947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ingrosso MR, Ianiro G, Nee J, Lembo AJ, Moayyedi P, Black CJ, Ford AC. Systematic review and meta-analysis: efficacy of peppermint oil in irritable bowel syndrome. Aliment Pharmacol Ther. 2022 Aug 9; doi: 10.1111/apt.17179. [DOI] [PubMed] [Google Scholar]
- 66.Tack J, Stanghellini V, Dubois D, Joseph A, Vandeplassche L, Kerstens R. Effect of prucalopride on symptoms of chronic constipation. Neurogastroenterol Motil. 2014 Jan;26(1):21–7. doi: 10.1111/nmo.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carbone F, Van den Houte K, Clevers E, Andrews CN, Papathanasopoulos A, Holvoet L, Van Oudenhove L, Caenepeel P, Arts J, Vanuytsel T, Tack J. Prucalopride in Gastroparesis: A Randomized Placebo-Controlled Crossover Study. Am J Gastroenterol. 2019 Aug;114(8):1265–1274. doi: 10.14309/ajg.0000000000000304. [DOI] [PubMed] [Google Scholar]
- 68.Black CJ, Staudacher HM, Ford AC. Efficacy of a low FODMAP diet in irritable bowel syndrome: systematic review and network meta-analysis. Gut. 2022 Jun;71(6):1117–1126. doi: 10.1136/gutjnl-2021-325214. [DOI] [PubMed] [Google Scholar]
- 69.Van den Houte K, Carbone F, Tothe J, Mariën Z, Schol J, Colomier E, Van den Bergh J, Vanderstappen J, Pauwels N, Matthys C, Vanuytsel T, et al. Symptoms and duodenal mucosal integrity are improved by a dietary intervention in functional dyspepsia. UEG journal. 2021;9(S8):10. (abstract. [Google Scholar]
- 70.Drossman DA, Tack J, Ford AC, Szigethy E, Törnblom H, Van Oudenhove L. Neuromodulators for Functional Gastrointestinal Disorders (Disorders of Gut-Brain Interaction): A Rome Foundation Working Team Report. Gastroenterology. 2018 Mar;154(4):1140–1171.:e1. doi: 10.1053/j.gastro.2017.11.279. [DOI] [PubMed] [Google Scholar]
- 71.Cannon RO, 3rd 1, Quyyumi AA, Mincemoyer R, Stine AM, Gracely RH, Smith WB, Geraci MF, Black BC, Uhde TW, Waclawiw MA, et al. Imipramine in patients with chest pain despite normal coronary angiograms. Engl J Med. 1994;330:1411–9. doi: 10.1056/NEJM199405193302003. [DOI] [PubMed] [Google Scholar]
- 72.Peghini PL, Katz PO, Castell DO. Imipramine decreases oesophageal pain perception in human male volunteers. Gut. 1998;42(6):807–13. doi: 10.1136/gut.42.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ostovaneh MR, Saeidi B, Hajifathalian K, Farrokhi-Khajeh-Pasha Y, Fotouhi A, Mirbagheri SS, et al. Comparing omeprazole with fluoxetine for treatment of patients with heartburn and normal endoscopy who failed once daily proton pump inhibitors: Double-blind placebo-controlled trial. Neurogastroenterol Motil. 2014;26(5):670–8. doi: 10.1111/nmo.12313. [DOI] [PubMed] [Google Scholar]
- 74.Keefer L, Ballou SK, Drossman DA, Ringstrom G, Elsenbruch S, Ljótsson B. A Rome Working Team Report on Brain-Gut Behavior Therapies for Disorders of Gut-Brain Interaction. Gastroenterology. 2022 Jan;162(1):300–315. doi: 10.1053/j.gastro.2021.09.015. [DOI] [PubMed] [Google Scholar]
- 75.Shiha MG, Aziz I. Review article: Physical and psychological comorbidities associated with irritable bowel syndrome. Aliment Pharmacol Ther. 2021 Dec;54(Suppl 1):S12–S23. doi: 10.1111/apt.16589. [DOI] [PubMed] [Google Scholar]
- 76.Sayuk GS, Elwing JE, Lustman PJ, Clouse RE. Predictors of premature antidepressant discontinuation in functional gastrointestinal disorders. Psychosom Med. 2007 Feb-Mar;69(2):173–81. doi: 10.1097/PSY.0b013e318031391d. [DOI] [PubMed] [Google Scholar]
- 77.Cassell B, Gyawali CP, Kushnir VM, Gott BM, Nix BD, Sayuk GS. Beliefs about GI medications and adherence to pharmacotherapy in functional GI disorder outpatients. Am J Gastroenterol. 2015 Oct;110(10):1382–7. doi: 10.1038/ajg.2015.132. [DOI] [PMC free article] [PubMed] [Google Scholar]