Abstract
Most cells store metabolic energy in lipid droplets (LDs). LDs are composed of a hydrophobic core, covered by a phospholipid monolayer, and functionalized by a specific set of proteins. Formation of LDs takes place in the endoplasmic reticulum (ER), where neutral lipid biosynthetic enzymes are located. Recent evidence indicate that this process is confined to specific ER subdomains, where proteins meet to initiate LD assembly. The lipodystrophy protein Seipin, is emerging as a major coordinator of LD biogenesis. Seipin forms a large oligomeric toroidal structure, which traps neutral lipids to promote LD nucleation. Here, we discuss the role of LD biogenesis factors that associate with Seipin to assemble functional LDs.
Introduction
Lipid droplets (LDs) constitute an intracellular compartment dedicated for storing metabolic energy in the form of neutral lipids (NLs). The anhydrous core of these droplets is composed of the two most abundant NLs, triacylglycerol (TAG) and steryl esters. This oily drop is shielded from the aqueous environment by a monolayer of phospholipids, which harbors a set of LD-specific proteins, including lipases, acyltransferases and scaffolding proteins [1].
LDs emerge from the ER membrane, in which the enzymes that drive the synthesis of NLs reside [2]. However, LDs do not appear to be formed at random locations throughout the ER. Studies in both yeast and animal cells suggest that the establishment of ER sites from where LDs are being formed requires a delicate interplay between locally enriched LD biogenesis factors, lipid biosynthetic enzymes and their regulators, specific lipids, as well as certain biophysical properties of the membrane to initiate efficient LD formation [3,4].
Over the past years, proteins that play important functions in the earliest steps of LD formation have been identified and their structural and functional characterization is now starting to provide a first glimpse into their mode of action. These include Seipin and its associated protein LDAF1/Promethin, the ER tubulating protein Pex30, the Lipin complex, which regulates production of diacylglycerol (DAG), and NL biosynthetic enzymes, including the diacylglycerol acyltransferases, which promote LD expansion at the ER-LD interface. Droplets emerging from the ER are then stabilized by members of the perilipin (PLIN) family of LD scaffolding proteins (see Table 1 for a description of the respective yeast and mammalian proteins).
Table 1. Overview of key factors of lipid droplet biogenesis.
| Yeast | Mammals | Protein Function | Key Reference |
|---|---|---|---|
| A. Key LD biogenesis proteins from yeast and mammals | |||
| Sei1 | Seipin | Defines sites of LD biogenesis in the ER by sequestering DAG/TAG in its toroidal rings, facilitates flow of TAG between LDs |
[33,37–39,42,43] |
| Ldb16 | – | Yeast-specific subunit of Seipin | [36,41] |
| Ldo16/Ldo45 | LDAF1/Promethin | Seipin partner protein, their association with Seipin is promoted by TAG | [44–47] |
| Pex30 | MCTP2 | Membrane curvature inducing reticulon homology domain containing ER protein, cooperates with Seipin in LD formation |
[6,20,21,24] |
| Pet10 | Perilipins | LD scaffolding proteins that regulate lipase activity | [52] [53] |
| B. Regulators and enzymes of lipid synthesis | |||
| Pahl | Lipin | Converts PA to DAG and gets recruited to Seipin-marked ER sites to regulate LD biogenesis | [5,7,13,14,50,51] |
| Nem1/Spo7 | CTDNEP-1/NEP1-R1 | Heteromeric phosphatase complex that regulates activity of Pah1/Lipin, gets recruited to Seipin sites upon LD induction | [5,13,14] |
| Ice2 | Serinc family members | Promotes ER membrane biogenesis by inhibiting Nem1/Spo7 phosphatase activity | [15–17] |
| Sct1, Gpt2 | GPAT | Catalyzes acylation of glycerol-3-phosphate to lyso-PA, negatively regulated by Seipin | [10,12,48,49] |
| Slc1, Ale1 | AGPAT | Catalyzes acylation of lyso-PA to form PA, interacts with Seipin | [10,49,50] |
| Dga1 | DGAT | Diacylglycerol acyltransferase, catalyzes formation of TAG, colocalizes with sites of LD biogenesis | [5,10,11] |
| Faa1 | ACSL3, FATP1 | Acyl-CoA synthetases, localize to sites of LD formation | [11,54,55] |
The recruitment of these LD biogenesis factors occurs in an ordered manner, thus defining early steps in LD formation. Establishment of these ER sites requires the colocalization of Seipin with the Lipin complex to locally produce DAG. Recruitment of the TAG biosynthetic enzymes then promotes localized synthesis of TAG. LD biogenesis factors interact with DAG and TAG to enhance the local concentration of NLs, and their nucleation into nascent LDs. Thus, the colocalization of Seipin with the Lipin complex is key to activate discrete sites for LD formation [5].
In this review, we discuss recent insights into how the ordered assembly of LDs is restricted to few ER subdomains, effectively preventing the detrimental synthesis and accumulation of TAG throughout the ER membrane [6,7], with an emphasis on the recently described structure and function of Seipin.
The lipin complex controls the branch point between membrane expansion and LD formation
The establishment of subdomains within the ER membrane from where droplets are assembled not only depends on a defined set of proteins, but also the presence of specific lipids, whose biochemical and biophysical properties promote the assembly of LD biogenesis factors [8,9]. In particular, DAG has emerged as a key lipid intermediate that plays a critical function at LD biogenesis sites. DAG serves as an immediate precursor to TAG formation catalyzed by ER residential acyltransferases, which are themselves recruited at active LD biogenesis sites [5,10–12].
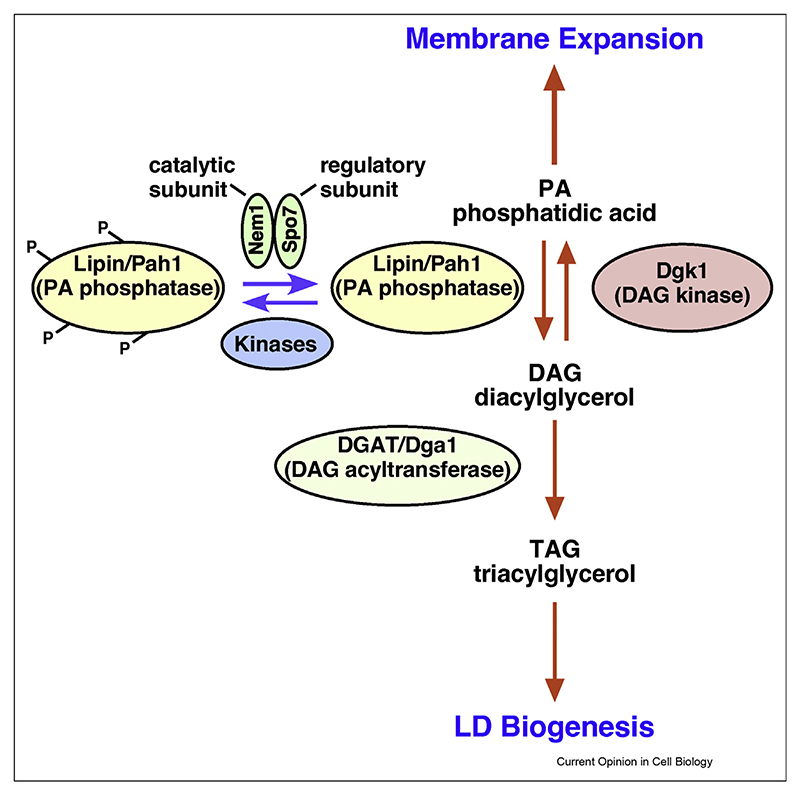
DAG is produced by the dephosphorylation of phosphatidic acid (PA), a reaction that is catalyzed by the Lipin class of lipid phosphatases (Pah1 in yeast) [13,14]. Thereby, Lipin/Pah1 activity controls the crucial bifurcation point between phospholipid synthesis and membrane expansion, on the one hand, and synthesis of the storage lipid TAG, on the other (Fig. 1). Lipin activity is tightly regulated by phosphorylation. The enzyme is activated by a membrane embedded heteromeric phosphatase complex composed of the catalytic subunit Nem1/CTDNEP1, and the regulatory subunit Spo7/NEP1-R1 (Yeast/Mammalian; see Table 1) [13,14]. In addition, this phosphatase complex is directly inhibited by interaction with Ice2, a polytopic ER membrane protein required for the inheritance of the cortical ER [15]. By inhibiting Pah1 activity, Ice2 promotes TAG consumption and thus regulates the switch from neutral lipid storage to consumption [16]. Interestingly, Ice2 belongs to the Serinc (serine incorporator) family of proteins, which contain a lipid-binding groove and restrict HIV infectivity [17,18]. Importantly, lack of Pah1 function causes ER expansion and the accumulation of neutral lipids, particularly steryl esters, throughout the ER membrane [7]. This suggests that DAG has a function in LD formation that is distinct from its role as a direct precursor to TAG formation [7,8,19].
Figure 1. Lipin activity controls membrane expansion and storage lipid synthesis.
The activity of the phosphatidate phosphatase, Lipin (Pah1), which promotes hydrolysis of phosphatidic acid (PA) to diacylglycerol (DAG), is controlled by the ER localized phosphatase complex Nem1/Spo7. While PA acts as precursor for the synthesis of abundant phospholipids and hence for membrane expansion, DAG acts as substrate for production of the storage lipid TAG by acyltransferases, such as DGAT/Dga1, and thus induces LD biogenesis.
The biophysical properties of the ER membrane affect LD formation
Multiple lines of evidence indicate that the biophysical properties of the ER membrane, including its lipid composition, membrane curvature, and surface tension affect the formation of LDs and/or their emergence towards the cytoplasm. This is illustrated by the recent emergence of Pex30 as an important factor in LD formation.
The Pex30 family was originally identified as proteins that affect the number and size of peroxisomes and Pex30 itself regulates the formation of pre-peroxisomal vesicles from the ER in yeast [20]. Pex30 localizes to ER subdomains, where both the biogenesis of peroxisomes and that of LDs occurs [21]. Pex30 and its human homolog, multiple C2 domain containing transmembrane protein (MCTP2), harbor a reticulon homology domain (RHD), and purified Pex30 induces membrane tubulation in vitro indicating that it promotes positive membrane curvature [20]. In addition to the RHD domain, Pex30 contains a C-terminal Dysferlin (DysF) domain [20]. Originally identified in a C. elegans gene required for the maturation of spermatids (FER-1), and then found to be present in human ferlins, including dysferlin and myoferlin, DysF domain containing proteins have been implicated in membrane repair and lipid remodelling [22,23]. The DysF domain of Pex30 is essential for the function of Pex30 in LD formation, suggesting that it controls local membrane properties [24]. Pex30 colocalizes in the ER with Seipin and a double mutant lacking both proteins exhibit a synthetic growth defect, fails to generate proper LDs and accumulates TAG in the ER, indicating that Seipin cooperates with Pex30 in LD biogenesis [6,21]. Consistent with such a collaboration, Pex30 is mislocalized to a single punctum in cells lacking Seipin [6,21]. In cells missing Pex30, on the other hand, Seipin still localizes to discrete ER sites, however, these sites fail to recruit TAG producing enzymes [5]. This suggests that Pex30 plays a crucial role in organizing ER subdomains that are permissive for TAG synthesis and droplet assembly.
Local changes in membrane geometry and/or lipid composition promoted by Pex30 at sites of LD formation might be important to accommodate DAG and/or to facilitate nucleation of TAG within the ER bilayer [6,21,24]. Consistent with this hypothesis, LD formation shows preference towards tubular ER membrane over ER sheets and both Seipin and DAG preferentially enrich at ER tubules to promote droplet nucleation [25]. Moreover, TAG accumulation in ER sheets is energetically favorable compared to ER tubules, thus either promoting outflow of TAG from tubules or their condensation into LDs [25,26]. In agreement with this notion, in vitro LD nucleation can occur by enhancing membrane curvature [25].
In addition to membrane curvature, the structure of lipids and their asymmetric distribution at LD biogenesis sites can alter local surface tension and affect LD emergence [9,27,28]. Negative curvature inducing lipid, such as DAG, will stabilize the embedded state of LDs in the ER membrane, whereas lipids that induce positive curvature, such as lysophospholipids, reduce surface tension and promote emergence of small LDs both in vitro and in vivo [8,9,29]. Recent results indicate that even the acyl chain composition of ER phospholipids affects LD nucleation. Elevated levels of saturated or short chain fatty acids promote accumulation of neutral lipids within the ER membrane and impair NL nucleation, by affecting phase separation of NLs in the membrane [19]. In vitro experiments with giant unilamellar vesicles indicate that LDs bud towards the side of the membrane that has a higher coverage with phospholipids and proteins, resulting in LDs with lower surface tension [28]. Consistent with this, for an LD to emerge towards the cytoplasm, the cytoplasmic leaflet of the ER membrane needs to be replenished with phospholipids, particularly phosphatidylcholine [28]. In the absence of this membrane remodeling, LDs remain exposed to the ER lumen, as observed upon rapid oleate induced expansion of LDs in yeast [28].
Seipin oligomers form a membrane embedded toroidal structure that traps neutral lipids
Seipin is the most extensively studied LD biogenesis protein. It was identified as an ER protein that localizes at the interface between the ER membrane and LDs to control the number, size, and morphology of LDs [30,31]. In the absence of Seipin, LDs are formed stochastically, resulting in many tiny or few supersized LDs, at ectopic sites in the ER. These LDs have an aberrant lipid and protein composition, and are not fully functional [5,32–36]. Expression of the human Seipin BSCL2 (Berardinelli-Seip congenital lipodystrophy type 2) complements a yeast null mutant phenotype indicating that the mode of action of Seipin is conserved [30,31].
Seipin contains short N- and C-terminal domains oriented towards the cytoplasm, two transmembrane domains (TMDs), and a highly conserved large ER luminal domain [30,31]. The two TMDs together with the luminal domain are critical for Seipin function, as these domains are sufficient to rescue the lack of Seipin function [35]. Recent structural characterization of human, fly, and yeast Seipin by cryo-electron microscopy revealed that it forms a large membrane-embedded ring-shaped oligomeric structure composed of 11, 12, and 10 subunits, respectively [37–39] (Fig. 2). Despite the differences in subunit number, Seipin monomers form a toroidal structure of ~15 nm in diameter [37–39]. These structures have provided insights into the mode of action of Seipin. Seipin oligomerization is critical for its function as a point mutant version of Seipin (A212P) associated with lipodystrophy, forms smaller oligomers and fails to rescue Seipin associated LD biogenesis defects [34].
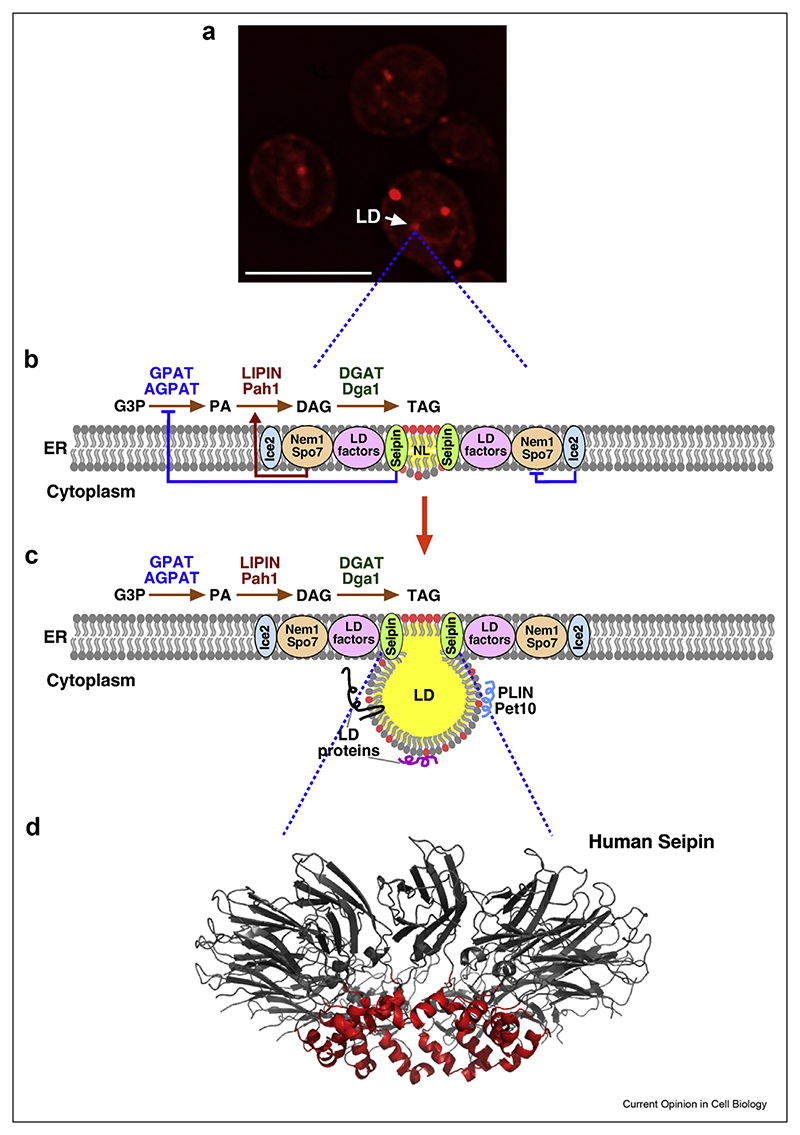
Figure 2. The ordered formation of LDs at discrete sites in the ER membrane.
a) LD biogenesis occurs at discrete ER domains. Fluorescence microscopy image of a yeast strain expressing mCherry-tagged Seipin, induced to form TAG, which drives de novo formation of LDs. Scale bar, 5 μm. b, c) Schematic view of interactions between components needed for LD formation. Seipin is required at the center to promote NL nucleation within the ER membrane. Seipin is assisted by LD factors such as Pex30, and LDAF1/Promethin (see Table 1), and controls the production of PA. The Nem1/Spo7 complex activates Lipin to promote DAG formation, which then serves as substrate for TAG synthesis by DGAT enzymes. Nem1/Spo7 activity is inhibited by Ice2. Upon LD growth and maturation, LD proteins including perilipins (PLIN) localize onto the limiting monolayer. DAG in the ER membrane is depicted by red circles and TAG by the yellow sphere. d) Model of the oligomeric structure of human Seipin. NL nucleation is promoted by the membrane apposed hydrophobic helix (red) within the luminal domain of the Seipin inner ring. The two transmembrane domains of Seipin are not shown.
The Seipin structure revealed two notable features. First, a large ER luminal domain that adopts an eight-stranded beta-sandwich fold, characteristic of lipid-binding proteins such as the sterol-binding Niemann Pick C2 protein (NPC2) [38,39]. This suggests that Seipin may bind lipids in the luminal leaflet of the ER membrane. In agreement with this, in vitro studies with full length and a truncated version of Seipin harboring only the lipid binding domain revealed binding of the anionic phospholipid PA [38]. This lipid-binding domain of Seipin is important for LD formation as mutations in this domain give rise to lipodystrophy [40].
The second interesting feature is the presence of a hydrophobic helix (HH) in the mammalian, and insect Seipin. This HH is apposed to the luminal leaflet of the ER membrane and is sufficient to bind LDs [39]. The yeast protein lacks this membrane apposing hydrophobic helix, but its function is provided by Ldb16, a yeast-specific subunit of the Seipin complex [37]. Ldb16 is an ER membrane protein, with no known human homologs. Lack of either Seipin or Ldb16 results in a similar LD biogenesis defect, that can be rescued by expression of human Seipin [36,41].
Molecular dynamics simulations (MDS) identified key serine residues within the HH of Seipin that directly interact with the carbonyl groups of DAG and TAG within the ER membrane [42,43]. These interactions result in effective nanoscale sequestration of NLs at the inner opening of the Seipin ring, thereby promoting nucleation of NLs, their growth into nascent LDs and possibly even LD emergence [42,43]. Unlike the human and fly Seipin structures, that of yeast Seipin includes regions of the TMDs and MDS indicate that these TMDs contribute to TAG accumulation in the Seipin/Ldb16 complex [37]. Thus, Seipin facilitates clustering of DAG and/or TAG at LD biogenesis sites and its TMDs can contribute to a local membrane-environment that is conductive for proper LD formation, for example, by preventing diffusion of TAG into the bulk of the ER, which may explain why LDs preferentially form at Seipin-defined sites [33,37,42–44]. Consistent with a propensity of Seipin to concentrate DAG, ER subdomains containing Seipin and Nem1 are enriched in DAG as indicated by their colocalization with an ER-DAG sensor [5].
Seipin cooperates with LDAF1/Promethin in nucleation of TAG
Seipin has recently been shown to form a large ~600 kDa hetero-oligomeric complex with Lipid Droplet Assembly Factor 1 (LDAF1) also known as Promethin [44,45]. LDAF1 is upregulated during adipogenesis, localizes to LDs, and copurifies with Seipin [44,45]. LDAF1 is widely conserved across species and shows remote homology to the yeast LD Organization protein Ldo45 and its splice variant Ldo16 [46,47]. Interestingly, LDAF1 interacts with the HH of human Seipin and this association is promoted by TAG [42,44]. This interaction might be promoted by local membrane alterations induced by TAG clustering within the Seipin oligomer, providing a favorable environment for LDAF1 association [42]. Consistent with this proposition, the LDAF1-Seipin complex copurifies with TAG, whereas Seipin alone does not, suggesting that the complex has a higher propensity to trap TAG than Seipin alone [44]. Oligomers of LDAF1 and Seipin form a membrane-embedded complex with as many as 66 transmembrane domains. Such an assembly of hydrophobic helices may serve to promote TAG nucleation. Consistent with this, LD formation is delayed and not as efficient in the absence of LDAF1, with fewer LDs formed for a given amount of TAG. Thus, LDAF1 appears to lower the energy barrier for LD formation, allowing it to occur at lower TAG concentration [42,44]. Upon LD growth and expansion, LDAF1 dissociates from Seipin to move over the LD periphery [44]. LDAF1 has been proposed to adopt a hairpin topology, promoting positive membrane curvature and allowing it to associate with both the ER bilayer and the LD surface monolayer [44].
Does Seipin coordinate lipid synthesis?
Growing evidence suggest that Seipin does not only interact with LD biogenesis factors to control the nucleation of TAG in the ER membrane, but it also regulates lipid synthesis. Lack of Seipin function results in elevated levels of PA and this might inhibit the peroxisome proliferator-activated receptor gamma (PPARg)-dependent transcriptional cascade needed for adipogenesis [30]. Seipin physically interacts with the glycerol-3-phosphate acyltransferase (GPAT) and inhibits its activity to reduce PA synthesis and thereby controls LD expansion [10,48] (Fig. 2). In agreement with this, impaired LD biogenesis in Seipin mutants is partially rescued by inhibiting GPAT activity, whereas overexpression of GPAT blocks adipogenesis and induces supersized LDs [48]. Moreover, Seipin also interacts with the second acyltransferase enzyme needed for PA synthesis, the acylglycerolphosphate acyltransferase (AGPAT), and with Lipin [10,49–51]. Thereby, Seipin might not only control the local production of DAG, but the entire supply chain needed to provide TAG, as well as LD nucleation.
Future directions
Recent advances in defining factors that affect the nucleation, growth and emergence of LDs at specific ER subdomains have provided insights into the mechanisms underlying LD biogenesis. By employing a combination of in vivo, in vitro, and in silico approaches, a broader understanding of this complex process has started to emerge. Impaired assembly of droplets due to lack of key LD biogenesis factors or altered biophysical properties of the ER membrane results in lipid storage disorders. Future work will likely reveal how ER sites specialized for LD formation are selected and how the process is regulated. Elucidation of the function of Seipin partner proteins, such as LDAF1/Promethin in mammals and Ldo16/45 in yeast, and their role in LD assembly is also anticipated. In addition, addressing the function of the ER luminal lipid binding domain of Seipin and how Seipin affects the exchange of proteins and lipids between the ER and LDs will likely improve our understanding of this key protein sitting at the ER-LD junction. What is the role of the Pex30 ER shaping protein in regulating lipid and protein composition at LD biogenesis sites? How does lack of Seipin inhibit adipogenesis and manifests in lipodystrophy? How does Seipin coordinate lipid synthesis? Addressing these outstanding questions is likely to bring novel insights into the mode of action of major players in LD assembly and thereby advance our understanding of lipid storage pathologies.
Acknowledgements
We thank Ola El Atab for providing the Seipin structure, Stéphanie Cottier and Aslihan Ekim Kocabey for helpful comments on the manuscript. This work was supported by the Swiss National Science Foundation (31003A_173003 to RS), an Indo Swiss Joint Research Programme (IZLIZ3_200246 to RS and IC-12044(11)/6/2021-ICD-DBT to VC), an Early Career Intramural Project of the All India Institute of Medical Sciences (AIIMS), New Delhi, and DBT/Wellcome Trust India Alliance Fellowship (Grant IA/I/20/2/505191 awarded to VC).
Footnotes
CRediT author statement
Roger Schneiter: Writing- Reviewing and Editing;
Vineet Choudhary: Writing- Reviewing and Editing.
Conflict of interest statement
Nothing declared.
References
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
* * of outstanding interest
- 1.Olzmann JA, Carvalho P. Dynamics and functions of lipid droplets. Nat Rev Mol Cell Biol. 2018;20:137–155. doi: 10.1038/s41580-018-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walther TC, Chung J, Farese RV. Lipid droplet biogenesis. Annu Rev Cell Dev Biol. 2017;33:491–510. doi: 10.1146/annurev-cellbio-100616-060608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thiam AR, Ikonen E. Lipid droplet nucleation. Trends Cell Biol. 2021;31:108–118. doi: 10.1016/j.tcb.2020.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Henne M, Goodman JM, Hariri H. Spatial compartmentalization of lipid droplet biogenesis. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158499. doi: 10.1016/j.bbalip.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choudhary V, El Atab O, Mizzon G, Prinz WA, Schneiter R. Seipin and Nem1 establish discrete ER subdomains to initiate yeast lipid droplet biogenesis. J Cell Biol. 2020;219:e201910177. doi: 10.1083/jcb.201910177. [** This paper shows that LD biogenesis factors get recruited at Seipin-defined ER subdomains to initiate LD formation in yeast.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang S, Idrissi FZ, Hermansson M, Grippa A, Ejsing CS, Carvalho P. Seipin and the membrane-shaping protein Pex30 cooperate in organelle budding from the endoplasmic reticulum. Nat Commun. 2018;9:2939. doi: 10.1038/s41467-018-05278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adeyo O, Horn PJ, Lee S, Binns DD, Chandrahas A, Chapman KD, Goodman JM. The yeast lipin orthologue Pah1p is important for biogenesis of lipid droplets. J Cell Biol. 2011;192:1043–1055. doi: 10.1083/jcb.201010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choudhary V, Golani G, Joshi AS, Cottier S, Schneiter R, Prinz WA, Kozlov MM. Architecture of lipid droplets in endoplasmic reticulum is determined by phospholipid intrinsic curvature. Curr Biol. 2018;28:915–926.:e9. doi: 10.1016/j.cub.2018.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao M, Huang X, Song BL, Yang H. The biogenesis of lipid droplets: lipids take center stage. Prog Lipid Res. 2019;75:100989. doi: 10.1016/j.plipres.2019.100989. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Airola MV, Reue K. How lipid droplets “TAG” along: glycerolipid synthetic enzymes and lipid storage. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862:1131–1145. doi: 10.1016/j.bbalip.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu N, Zhang SO, Cole RA, McKinney SA, Guo F, Haas JT, Bobba S, Farese RVJ, Mak HY. The FATP1-DGAT2 complex facilitates lipid droplet expansion at the ER-lipid droplet interface. J Cell Biol. 2012;198:895–911. doi: 10.1083/jcb.201201139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marr N, Foglia J, Terebiznik M, Athenstaedt K, Zaremberg V. Controlling lipid fluxes at glycerol-3-phosphate acyltransferase step in yeast: unique contribution of Gat1p to oleic acid-induced lipid particle formation. J Biol Chem. 2012;287:10251–10264. doi: 10.1074/jbc.M111.314112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwiatek JM, Han G-S, Carman GM. Phosphatidate-mediated regulation of lipid synthesis at the nuclear/endoplasmic reticulum membrane. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158434. doi: 10.1016/j.bbalip.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang P, Reue K. Lipin proteins and glycerolipid metabolism: roles at the ER membrane and beyond. Biochim Biophys Acta Biomembr. 2017;1859:1583–1595. doi: 10.1016/j.bbamem.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papagiannidis D, Bircham PW, Lüchtenborg C, Pajonk O, Ruffini G, Brügger B, Schuck S. Ice2 promotes ER membrane biogenesis in yeast by inhibiting the conserved lipin phosphatase complex. EMBO J. 2021:e107958. doi: 10.15252/embj.2021107958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markgraf DF, Klemm RW, Junker M, Hannibal-Bach HK, Ejsing CS, Rapoport TA. An ER protein functionally couples neutral lipid metabolism on lipid droplets to membrane lipid synthesis in the ER. Cell Rep. 2014;6:44–55. doi: 10.1016/j.celrep.2013.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alli-Balogun GO, Levine TP. Fungal Ice2p is in the same superfamily as SERINCs, restriction factors for HIV and other viruses. Proteins. 2021;89:1240–1250. doi: 10.1002/prot.26145. [DOI] [PubMed] [Google Scholar]
- 18.Pye VE, Rosa A, Bertelli C, Struwe WB, Maslen SL, Corey R, Liko I, Hassall M, Mattiuzzo G, Ballandras-Colas A, Nans A, et al. A bipartite structural organization defines the SERINC family of HIV-1 restriction factors. Nat Struct Mol Biol. 2020;27:78–83. doi: 10.1038/s41594-019-0357-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zoni V, Khaddaj R, Campomanes P, Thiam AR, Schneiter R, Vanni S. Pre-existing bilayer stresses modulate triglyceride accumulation in the ER versus lipid droplets. Elife. 2021;10:e62886. doi: 10.7554/eLife.62886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joshi AS, Huang X, Choudhary V, Levine TP, Hu J, Prinz WA. A family of membrane-shaping proteins at ER subdomains regulates pre-peroxisomal vesicle biogenesis. J Cell Biol. 2016;215:515–529. doi: 10.1083/jcb.201602064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joshi AS, Nebenfuehr B, Choudhary V, Satpute-Krishnan P, Levine TP, Golden A, Prinz WA. Lipid droplet and peroxisome biogenesis occur at the same ER subdomains. Nat Commun. 2018;9:2940. doi: 10.1038/s41467-018-05277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bulankina AV, Thoms S. Functions of vertebrate ferlins. Cells. 2020;9:E534. doi: 10.3390/cells9030534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sula A, Cole AR, Yeats C, Orengo C, Keep NH. Crystal structures of the human Dysferlin inner DysF domain. BMC Struct Biol. 2014;14:3. doi: 10.1186/1472-6807-14-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferreira JV, Carvalho P. Pex30-like proteins function as adaptors at distinct ER membrane contact sites. J Cell Biol. 2021;220:e202103176. doi: 10.1083/jcb.202103176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santinho A, Salo VT, Chorlay A, Li S, Zhou X, Omrane M, Ikonen E, Thiam AR. Membrane curvature catalyzes lipid droplet assembly. Curr Biol. 2020;30:2481–2494.:e6. doi: 10.1016/j.cub.2020.04.066. [* This work shows that LDs preferentially form in ER tubules, where Seipin is enriched, and that the presence of TAG in highly curved membrane domains is energetically unfavorable, resulting in its condensation and LD nucleation.] [DOI] [PubMed] [Google Scholar]
- 26.Ganesan S, Tavassoli M, Shabits BN, Zaremberg V. Tubular ER associates with diacylglycerol-rich structures during lipid droplet consumption. Front Cell Dev Biol. 2020;8:700. doi: 10.3389/fcell.2020.00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao Z, Wang X, Huang X, Mak HY. Are endoplasmic reticulum subdomains shaped by asymmetric distribution of phospholipids? Evidence from a C. elegans model system. Bioessays. 2021;43:2000199. doi: 10.1002/bies.202000199. [DOI] [PubMed] [Google Scholar]
- 28.Chorlay A, Monticelli L, Veríssimo Ferreira J, Ben M’barek K, Ajjaji D, Wang S, Johnson E, Beck R, Omrane M, Beller M, Carvalho P, et al. Membrane asymmetry imposes directionality on lipid droplet emergence from the ER. Dev Cell. 2019;50:25–42.:e7. doi: 10.1016/j.devcel.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Ben M’barek K, Ajjaji D, Chorlay A, Vanni S, Forêt L, Thiam AR. ER membrane phospholipids and surface tension control cellular lipid droplet formation. Dev Cell. 2017;41:591–604.:e7. doi: 10.1016/j.devcel.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Rao MJ, Goodman JM. Seipin: harvesting fat and keeping adipocytes healthy. Trends Cell Biol. 2021:S0962–8924, 00119. doi: 10.1016/j.tcb.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salo VT, Hölttä-Vuori M, Ikonen E. Seipin-Mediated contacts as gatekeepers of lipid flux at the endoplasmic reticulum–lipid droplet nexus. Contact. 2020;3:2515256420945820 [Google Scholar]
- 32.Cao Z, Hao Y, Fung CW, Lee YY, Wang P, Li X, Xie K, Lam WJ, Qiu Y, Tang BZ, Shui G, et al. Dietary fatty acids promote lipid droplet diversity through seipin enrichment in an ER subdomain. Nat Commun. 2019;10:2902. doi: 10.1038/s41467-019-10835-4. [* These authors show that human Seipin and its C. elegans ortholog SEIP-1 are targeted to a tubular ER subdomain and that microbial cyclopropane- and polyunsaturated fatty acids are important for proper targeting of SEIP-1 to these ER subdomains.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salo VT, Li S, Vihinen H, Hölttä-Vuori M, Szkalisity A, Horvath P, Belevich I, Peränen J, Thiele C, Somerharju P, Zhao H, et al. Seipin facilitates triglyceride flow to lipid droplet and counteracts droplet ripening via endoplasmic reticulum contact. Dev Cell. 2019;50:478–493.:e9. doi: 10.1016/j.devcel.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 34.Salo VT, Belevich I, Li S, Karhinen L, Vihinen H, Vigouroux C, Magré J, Thiele C, Hölttä-Vuori M, Jokitalo E, Ikonen E. Seipin regulates ER-lipid droplet contacts and cargo delivery. EMBO J. 2016;35:2699–2716. doi: 10.15252/embj.201695170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H, Becuwe M, Housden BE, Chitraju C, Porras AJ, Graham MM, Liu XN, Thiam AR, Savage DB, Agarwal AK, Garg A, et al. Seipin is required for converting nascent to mature lipid droplets. Elife. 2016;5:e16582. doi: 10.7554/eLife.16582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grippa A, Buxó L, Mora G, Funaya C, Idrissi FZ, Mancuso F, Gomez R, Muntanyà J, Sabidó E, Carvalho P. The seipin complex Fld1/Ldb16 stabilizes ER-lipid droplet contact sites. J Cell Biol. 2015;211:829–844. doi: 10.1083/jcb.201502070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klug YA, Deme JC, Corey RA, Renne MF, Stansfeld PJ, Lea SM, Carvalho P. Mechanism of lipid droplet formation by the yeast Sei1/Ldb16 Seipin complex. Nat Commun. 2021;12:5892. doi: 10.1038/s41467-021-26162-6. [** This paper identifies residues within the Sei1/Ldb16 yeast Seipin complex important for the nucleation of TAG within the ER membrane.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan R, Qian H, Lukmantara I, Gao M, Du X, Yan N, Yang H. Human SEIPIN binds anionic phospholipids. Dev Cell. 2018;47:248–256.:e4. doi: 10.1016/j.devcel.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 39.Sui X, Arlt H, Brock KP, Lai ZW, DiMaio F, Marks DS, Liao M, Farese RV, Walther TC. Cryo-electron microscopy structure of the lipid droplet-formation protein seipin. J Cell Biol. 2018;217:4080–4091. doi: 10.1083/jcb.201809067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magré J, Delépine M, Khallouf E, Gedde-Dahl T, Van Maldergem L, Sobel E, Papp J, Meier M, Mégarbané A, Bachy A, Verloes A, et al. BSCL WG: Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nat Genet. 2001;28:365–370. doi: 10.1038/ng585. [DOI] [PubMed] [Google Scholar]
- 41.Wang CW, Miao YH, Chang YS. Control of lipid droplet size in budding yeast requires the collaboration between Fld1 and Ldb16. J Cell Sci. 2014;127:1214–1228. doi: 10.1242/jcs.137737. [DOI] [PubMed] [Google Scholar]
- 42.Prasanna X, Salo VT, Li S, Ven K, Vihinen H, Jokitalo E, Vattulainen I, Ikonen E. Seipin traps triacylglycerols to facilitate their nanoscale clustering in the endoplasmic reticulum membrane. PLoS Biol. 2021;19:e3000998. doi: 10.1371/journal.pbio.3000998. [** These authors together with [43] use molecular dynamics simulations to show that the hydrophobic helix within the luminal domain of Seipin traps TAG, promoting its association with LDAF1/Promethin to support further LD growth.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zoni V, Khaddaj R, Lukmantara I, Shinoda W, Yang H, Schneiter R, Vanni S. Seipin accumulates and traps diacylglycerols and triglycerides in its ring-like structure. Proc Natl Acad Sci U S A. 2021;118:e2017205118. doi: 10.1073/pnas.2017205118. [** This study together with [42] employed molecular dynamics simulations to show that the hydrophobic helix in the Seipin inner ring sequesters TAG and DAG to promote efficient LD formation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chung J, Wu X, Lambert TJ, Lai ZW, Walther TC, Farese RV. LDAF1 and seipin form a lipid droplet assembly complex. Dev Cell. 2019;51:551–563.:e7. doi: 10.1016/j.devcel.2019.10.006. [** This work shows that Seipin forms a complex with LDAF1/Promethin to define sites of LD formation in the ER, and that LDAF1 moves to the LD periphery upon LD growth.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castro IG, Eisenberg-Bord M, Persiani E, Rochford JJ, Schuldiner M, Bohnert M. Promethin is a conserved seipin partner protein. Cells. 2019;8:E268. doi: 10.3390/cells8030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eisenberg-Bord M, Mari M, Weill U, Rosenfeld-Gur E, Moldavski O, Castro IG, Soni KG, Harpaz N, Levine TP, Futerman AH, Reggiori F, et al. Identification of seipin-linked factors that act as determinants of a lipid droplet subpopulation. J Cell Biol. 2018;217:269–282. doi: 10.1083/jcb.201704122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teixeira V, Johnsen L, Martínez-Montañés F, Grippa A, Buxó L, Idrissi FZ, Ejsing CS, Carvalho P. Regulation of lipid droplets by metabolically controlled Ldo isoforms. J Cell Biol. 2018;217:127–138. doi: 10.1083/jcb.201704115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pagac M, Cooper DE, Qi Y, Lukmantara IE, Mak HY, Wu Z, Tian Y, Liu Z, Lei M, Du X, Ferguson C, et al. SEIPIN regulates lipid droplet expansion and adipocyte development by modulating the activity of glycerol-3-phosphate acyltransferase. Cell Rep. 2016;17:1546–1559. doi: 10.1016/j.celrep.2016.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sim MFM, Persiani E, Talukder MMU, Mcilroy GD, Roumane A, Edwardson JM, Rochford JJ. Oligomers of the lipodystrophy protein seipin may co-ordinate GPAT3 and AGPAT2 enzymes to facilitate adipocyte differentiation. Sci Rep. 2020;10:3259. doi: 10.1038/s41598-020-59982-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Talukder MM, Sim MF, O’Rahilly S, Edwardson JM, Rochford JJ. Seipin oligomers can interact directly with AGPAT2 and lipin 1, physically scaffolding critical regulators of adipogenesis. Mol Metabol. 2015;4:199–209. doi: 10.1016/j.molmet.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sim MF, Dennis RJ, Aubry EM, Ramanathan N, Sembongi H, Saudek V, Ito D, O’Rahilly S, Siniossoglou S, Rochford JJ. The human lipodystrophy protein seipin is an ER membrane adaptor for the adipogenic PA phosphatase lipin 1. Mol Metabol. 2012;2:38–46. doi: 10.1016/j.molmet.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao Q, Binns DD, Kinch LN, Grishin NV, Ortiz N, Chen X, Goodman JM. Pet10p is a yeast perilipin that stabilizes lipid droplets and promotes their assembly. J Cell Biol. 2017;216:3199–3217. doi: 10.1083/jcb.201610013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sztalryd C, Brasaemle DL. The perilipin family of lipid droplet proteins: gatekeepers of intracellular lipolysis. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862:1221–1232. doi: 10.1016/j.bbalip.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hariri H, Speer N, Bowerman J, Rogers S, Fu G, Reetz E, Datta S, Feathers JR, Ugrankar R, Nicastro D, Henne WM. Mdm1 maintains endoplasmic reticulum homeostasis by spatially regulating lipid droplet biogenesis. J Cell Biol. 2019;218:1319–1334. doi: 10.1083/jcb.201808119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kassan A, Herms A, Fernandez-Vidal A, Bosch M, Schieber NL, Reddy BJ, Fajardo A, Gelabert-Baldrich M, Tebar F, Enrich C, Gross SP, et al. Acyl-CoA synthetase 3 promotes lipid droplet biogenesis in ER microdomains. J Cell Biol. 2013;203:985–1001. doi: 10.1083/jcb.201305142. [DOI] [PMC free article] [PubMed] [Google Scholar]