Abstract
Background
Cognitive symptoms after coronavirus disease 2019 (Covid-19), the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), are well-recognized. Whether objectively measurable cognitive deficits exist and how long they persist are unclear.
Methods
We invited 800,000 adults in a study in England to complete an online assessment of cognitive function. We estimated a global cognitive score across eight tasks. We hypothesized that participants with persistent symptoms (lasting ≥12 weeks) after infection onset would have objectively measurable global cognitive deficits and that impairments in executive functioning and memory would be observed in such participants, especially in those who reported recent poor memory or difficulty thinking or concentrating (“brain fog”).
Results
Of the 141,583 participants who started the online cognitive assessment, 112,964 completed it. In a multiple regression analysis, participants who had recovered from Covid-19 in whom symptoms had resolved in less than 4 weeks or at least 12 weeks had similar small deficits in global cognition as compared with those in the no–Covid-19 group, who had not been infected with SARS-CoV-2 or had unconfirmed infection (−0.23 SD [95% confidence interval {CI}, −0.33 to −0.13] and −0.24 SD [95% CI, −0.36 to −0.12], respectively); larger deficits as compared with the no–Covid-19 group were seen in participants with unresolved persistent symptoms (−0.42 SD; 95% CI, −0.53 to −0.31). Larger deficits were seen in participants who had SARS-CoV-2 infection during periods in which the original virus or the B.1.1.7 variant was predominant than in those infected with later variants (e.g., −0.17 SD for the B.1.1.7 variant vs. the B.1.1.529 variant; 95% CI, −0.20 to −0.13) and in participants who had been hospitalized than in those who had not been hospitalized (e.g., intensive care unit admission, −0.35 SD; 95% CI, −0.49 to −0.20). Results of the analyses were similar to those of propensity-score–matching analyses. In a comparison of the group that had unresolved persistent symptoms with the no–Covid-19 group, memory, reasoning, and executive function tasks were associated with the largest deficits (−0.33 to −0.20 SD); these tasks correlated weakly with recent symptoms, including poor memory and brain fog. No adverse events were reported.
Conclusions
Participants with resolved persistent symptoms after Covid-19 had objectively measured cognitive function similar to that in participants with shorter-duration symptoms, although short-duration Covid-19 was still associated with small cognitive deficits after recovery. Longer-term persistence of cognitive deficits and any clinical implications remain uncertain. (Funded by the National Institute for Health and Care Research and others.)
Poor memory and difficulty thinking or concentrating (commonly referred to as “brain fog”) have been implicated in syndromes occurring after coronavirus disease 2019 (Covid-19) — a situation that has led to suggestions that Covid-19 may have lasting cognitive consequences.1-7 However, objective data on cognitive performance are largely lacking, and how long such deficits may persist and which cognitive functions are most vulnerable are unclear.
In this observational study, our primary hypothesis was that there would be measurable cognitive deficits after Covid-19 that would scale with covariates of illness duration and severity. We secondarily speculated that objective impairments in executive and memory functions would be observable in persons with prolonged symptoms, especially poor memory or brain fog.8-10 We addressed these hypotheses by analyzing cognitive-task performance data9,11 that were obtained in the Real-Time Assessment of Community Transmission (REACT) cohort in England.12-14
Methods
Study Population and Design
In our study cohort, we tracked the prevalence of infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus causing Covid-19, in England from May 1, 2020, to March 31, 2022,12-15 using data from a randomly selected community sample of 3,099,386 adults (≥18 years of age). A total of 2,494,309 participants (80.5%) consented to be recontacted and to allow data linkage with the National Health Service (NHS). Between August 1 and December 30, 2022, we invited a subsample of 800,000 adults (32.1%) to complete a follow-up survey7 and cognitive assessment (Table S1 and Fig. S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). This invited subsample comprised participants who reported positive results on a SARS-CoV-2 test or who suspected that they had had Covid-19 and whose symptoms persisted for at least 12 weeks; participants who, as part of the REACT study, either had a positive result on a polymerase-chain-reaction (PCR) test for SARS-CoV-2 or were unvaccinated and had a positive test for SARS-CoV-2 IgG antibodies on an at-home lateral flow immunoassay device16; and participants who were randomly selected from the remaining REACT study population.
Study Oversight and Implementation
The study was designed by the first, third, penultimate, and last authors. The study team and staff from Ipsos (a market research firm) and H2 Cognitive Designs (a company that develops remote assessment technologies for neurologic disorders and mental health) were responsible for data collection. Data analyses were conducted by the first author. The first draft of the manuscript was prepared by the first and last authors, who vouch for the completeness and accuracy of the data and for the fidelity of the study to the protocol (available at NEJM.org). All the authors contributed to the editing of the manuscript and made the decision to submit the manuscript for publication. Ethics approval for the study was received from the South Central–Berkshire B research ethics committee of the NHS Health Research Authority. Imperial College London and the Department of Health and Social Care served as joint data controllers. A public advisory panel (www.imperial.ac.uk/medicine/research-and-impact/groups/react-study/react_pag/) regularly reviewed the study materials, processes, and results.
Cognitive Assessment
Participants undertook eight computerized online tasks from the Cognitron battery11,17 in a fixed order on their personal devices (e.g., desktop or laptop computer, tablet, or smartphone). The cognitive domains, which have been implicated in post–Covid-19 syndromes,9,18-20 consisted of immediate memory, two-dimensional mental manipulation, spatial working memory, spatial planning, verbal analogical reasoning, word definitions, information sampling, and delayed memory (see the Supplemental Methods section in the Supplementary Appendix). Each task resulted in a primary accuracy-based score as well as in secondary scores (e.g., response times and error types).
History of SARS-CoV-2 Infection and Covid-19
We categorized participants into six groups according to the duration of SARS-CoV-2 infection.7 Categories 2 through 6 required positive results on a PCR test, lateral flow immunoassay, or participant-reported test. Category 1 was defined as “no Covid-19” (i.e., the participant had not had SARS-CoV-2 infection or had an unconfirmed SARS-CoV-2 infection), category 2 as asymptomatic SARS-CoV-2 infection, category 3 as resolved short Covid-19 lasting less than 4 weeks, category 4 as resolved short Covid-19 lasting at least 4 weeks but less than 12 weeks, category 5 as Covid-19 symptoms that resolved at least 12 weeks after infection onset, and category 6 as Covid-19 symptoms that persisted at least 12 weeks after infection onset and had not resolved at the time of the cognitive assessment. Persistent symptoms were defined as 1 or more of 30 specified symptoms that the participants considered to be related to their episodes of Covid-19 (Table S1). Participants who were cognitively assessed within 12 weeks after infection onset were excluded from analyses of symptom duration because the duration was still unknown.
We estimated the date of infection as the date of symptom onset or, for asymptomatic infections, from the date of a positive SARS-CoV-2 test. We used the predominant SARS-CoV-2 strain in the United Kingdom at the time of infection to assign the period when the original virus or a variant was predominant: original virus, before December 1, 2020; the B.1.1.7 (alpha) variant, from December 1, 2020, to April 30, 2021; the B.1.617.2 (delta) variant, from May 1, 2021, to December 15, 2021; and the B.1.1.529 (omicron) variant, from December 16, 2021, onward.21 We considered the participants to be vaccinated against SARS-CoV-2 if they had received the vaccine at least 14 days before infection. We used NHS data linkage to classify three groups with regard to hospitalization: participants who had an emergency department visit, those who were admitted to the hospital, and those who were admitted to the intensive care unit (ICU).
Statistical Analysis
To assess nonresponse bias, we compared the characteristics of participants who had accessed and completed the cognitive assessment with those who had not. We used linear regression to adjust the task-performance scores for age, sex, race and ethnic group, education level, index of multiple deprivation (a national indication of level of deprivation that is based on small geographic areas of residence, assessed in quintiles),7,22 and preexisting health conditions, followed by rank transformation to a normal distribution (Table S2). We used factor analysis to obtain global cognitive scores from the summary scores for the participants who completed all eight tasks.
Using linear regression, we first examined whether the global cognitive score differed according to infection date (assessed in 100-day blocks) among participants with a single episode of Covid-19. We reran this analysis with adjustment for the following time-varying factors: illness duration and hospitalization (emergency department visit or admission to the hospital or ICU) as proxies for severity, variant period (when the original virus or the alpha, delta, or omicron variant was predominant), and the number of vaccine doses (0, 1, or >1) received at least 14 days before infection as potential mediators of severity.
We used multiple linear regression with stepwise selection (unfixed) to identify covariates and two-way interactions that may have contributed to explaining the global cognitive score. The criteria for the inclusion or exclusion of terms (two independent analyses) were based either on frequentist F statistics (add if P<0.001 or remove if P>0.1 for a change in the sum-of-squared errors) or on Bayesian information criteria (BIC; add if BIC <0 or remove if BIC >0.01); these methods selected identical terms.
We used propensity-score matching23 to further account for potential confounding (Table S3). Propensity scores were matched with the use of fixed widths on the probability scale. Widths were adjusted downward until the mean difference in the propensity scores between the compared groups was minimized (<0.1 SD) while the retained group sizes were maximized.
With terms selected in the stepwise regression, we performed linear regression on the summary scores from individual tasks for participants who completed all eight tasks and for those who completed at least one task. Among participants whose illness onset was at least 12 weeks before the cognitive assessment, we compared global cognitive scores according to the presence or absence of each individual symptom that they associated with their having had Covid-19. Among participants with unresolved persistent symptoms, we also compared global cognitive scores according to the presence or absence of each individual recent symptom. Among participants with unresolved persistent symptoms, those in whom symptoms had resolved, and those in the no–Covid-19 group, we estimated the mean differences in scores according to specific cognitive-assessment tasks between participants who reported having poor memory or brain fog during the previous 2 weeks and those who did not.
We conducted sensitivity analyses by including or excluding specified subgroups of participants in order to evaluate their influence on the results. For analyses of global cognitive scores according to infection date, we calculated P values that were unadjusted for multiple testing. Point estimates and 95% confidence intervals are reported for all the other analyses. Statistical analyses were performed with the use of MATLAB software, version R2022a (MathWorks).
Results
Responses to Questionnaire and Cognitive Test
Among the 800,000 participants in the REACT study who were invited, 52,501 had symptoms persisting for at least 12 weeks and either reported a positive test for SARS-CoV-2 or suspected that they had had Covid-19, 13,482 had a positive PCR test for SARS-CoV-2 infection, 85,757 were unvaccinated and tested positive for SARS-CoV-2 IgG antibodies on the basis of an at-home lateral flow immunoassay device, and 648,260 were randomly selected from the remaining REACT study population. Among the 276,840 respondents (34.6% of the 800,000 participants invited) who completed the questionnaire, 141,583 (51.1%) started the cognitive battery (completed at least one task), and 112,964 (79.8%) completed all eight tasks. A total of 58,108 participants had a single SARS-CoV-2 infection. A total of 10,701 participants had symptom onset less than 12 weeks before survey completion and thus were excluded from the analyses of symptom duration.
As compared with the base study population (800,000 participants), the participants who participated in the cognitive assessment were slightly more likely to be women, more likely to be White, and slightly less likely to be from the youngest age groups or from areas with greater levels of multiple deprivation (Table S4A). Participants who reported having poor memory or brain fog were slightly more likely than participants without subjective cognitive symptoms to participate across all the study groups, including the no–Covid-19 group (Table S4B). Participants who had been recruited with a record of persistent symptoms were more likely to complete the assessment (16.6%) than those who were in the random sample (14.3%) or who were invited on the basis of having tested positive for SARS-CoV-2 (11.4%); however, among the responding participants, we observed no differences according to demographic characteristics or other variables between participants who started but did not complete the assessment and those who completed the assessment (Table S4C). Despite these biases, the large sample size in our study meant that adequate numbers were available across demographic groups (e.g., age, sex, race and ethnic group, geographic region, and the index of multiple deprivation [in quintiles]) to provide meaningful data that are broadly generalizable to those groups; the representativeness of the study population is shown in Table S4D.
Primary Analyses
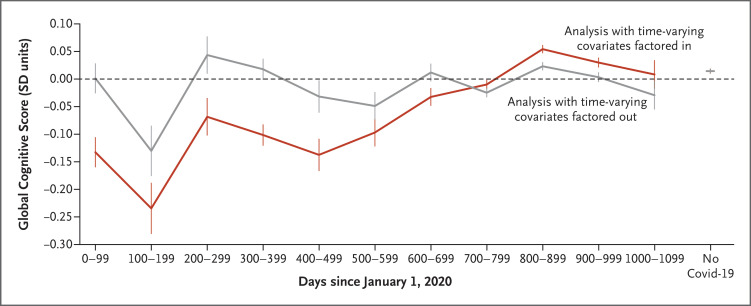
Among participants with a single SARS-CoV-2 infection, those with onset early in the pandemic had greater decrements in the global cognitive score than those with later onset (P<0.001). This association was attenuated after adjustment for proxies and mediators of illness severity, although residual decrements in task performance were observed in participants who had been infected during the first wave of the pandemic, when the original virus was predominant (Figure 1 and Table S5).
Figure 1. Association of Global Cognitive Scores with Infection Date.
Shown are the mean global cognitive scores according to the date of infection (i.e., the number of days since January 1, 2020) among the 58,108 participants who had a single infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The red line shows results before time-varying covariates that are proxies and likely mediators of coronavirus disease 2019 (Covid-19) severity, including illness duration, hospitalization, period when the original virus or variant of SARS-CoV-2 was predominant, and vaccination status, were factored out; the gray line shows results after these covariates were factored out. Results in the no–Covid-19 group (participants who had not had SARS-CoV-2 infection or had unconfirmed infection) are shown on the right side of the graph. Values are point estimates for the linear regression as reported on a standard deviation (SD) scale. Error bars indicate the 95% confidence interval.
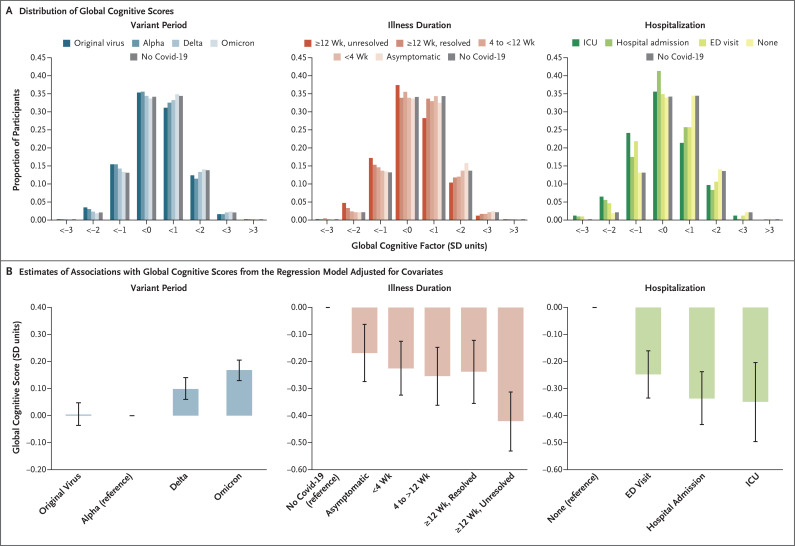
We found a downward shift, as compared with the no–Covid-19 group, in the distribution of global cognitive scores among participants who had been infected early in the pandemic (during periods in which the original virus or alpha variant was predominant), among those with longer illness duration, and among those who had been hospitalized. This finding resulted in elevated probabilities of global cognitive scores less than −2 SD, indicating moderate impairment (probability ratios vs. the no–Covid-19 group: original virus, 1.7; unresolved persistent symptoms, 2.4; and ICU admission, 3.6) (Figure 2 and Table S6).
Figure 2. Association of Global Cognitive Scores with SARS-CoV-2 Variant Period, Illness Duration, and Hospitalization.
Panel A shows the probability distributions of global cognitive scores within discrete ranges for the period of SARS-CoV-2 infection (left), illness duration (middle), and hospitalization (right). As compared with the no–Covid-19 group, there was a shift in distributions to the left, with a higher frequency of moderate impairment (defined as a score below −2 SD) and a lower frequency of superior performance (defined as a score >2 SD). The predominant strain in the United Kingdom at the time of infection was used to assign the period of infection: original virus, before December 1, 2020; the B.1.1.7 (alpha) variant, from December 1, 2020, to April 30, 2021; the B.1.617.2 (delta) variant, from May 1, 2021, to December 15, 2021; and the B.1.1.529 (omicron) variant, from December 16, 2021, onward. Distributions were adjusted for age, other demographic characteristics, and preexisting conditions but not for other covariates. ED denotes emergency department, and ICU intensive care unit. Panel B shows the results of stepwise multiple regression on the global cognitive scores with adjustment for age, other demographic characteristics, and specific preexisting conditions (as separate factors); all selected covariates were included simultaneously in the model and are therefore additive. The reference category in the model is indicated for each covariate. Values are point estimates for the linear regression as reported on a standard deviation scale. 𝙸 bars indicate the 95% confidence interval.
In multiple regression analyses, the stepwise procedure selected the variant period, illness duration, and hospitalization status as covariates to explain variations in the global cognitive score, but no two-way interactions were observed (Tables S7 and S8). The largest deficits in global cognitive scores were observed in the group of participants with SARS-CoV-2 infection during periods in which the original virus or the alpha variant was predominant as compared with those infected with later variants (e.g., −0.17 SD for the alpha variant vs. the omicron variant; 95% confidence interval [CI], −0.20 to −0.13), in the group of participants with unresolved persistent symptoms as compared with the no–Covid-19 group (−0.42 SD; 95% CI, −0.53 to −0.31), and among participants who had been hospitalized for Covid-19 as compared with those who had not been hospitalized (e.g., ICU admission, −0.35 SD; 95% CI, −0.49 to −0.20). The three resolved-symptoms groups had similar small deficits as compared with the no–Covid-19 group (e.g., in the group with resolved symptoms at <4 weeks, −0.23 SD [95% CI, −0.33 to −0.13]; and in the group with resolved symptoms at ≥12 weeks, −0.24 SD [95% CI, −0.36 to −0.12]) (Figure 2).
In analyses that were stratified according to variant period, illness duration was associated, in graded fashion, with deficits in the global cognitive score as compared with the no–Covid-19 group. The mean global cognitive score was lower among participants with unresolved persistent symptoms than among those in the no–Covid-19 group in all the variant periods (original virus, −0.32 SD; alpha variant, −0.33 SD; delta variant, −0.26 SD; and omicron variant, −0.16 SD). Among participants with resolved cases of short duration (<4 weeks), the global cognitive score was lower than among those in the no–Covid-19 group in the early periods of the pandemic (original virus, −0.12 SD; and alpha variant, −0.12 SD) but not in the later periods (delta variant, −0.04 SD; and omicron variant, 0.02 SD) (Fig. S2 and Table S9).
Propensity-Score Matching
In this analysis, we grouped participants according to illness duration and variant period and then performed propensity-score matching of the members of each group with those in the no–Covid-19 group according to demographic characteristics, the number of preexisting conditions, and the presence or absence of recent poor memory or symptoms of brain fog. The results of the cross-group estimates showed effect sizes that were similar to those of the primary regression analyses (Table S10). We also performed propensity-score matching of groups according to hospitalization type (emergency department visit or admission to the hospital or ICU) with either the no–Covid-19 group or the group of participants with SARS-CoV-2 infection who did not seek medical assistance, while also controlling for the variant period. In these analyses, we observed findings similar to those of the main regression analysis, with the greatest deficits observed in the ICU group as compared with the no–Covid-19 group (−0.63 SD) (Table S11A).
In an analysis that matched vaccinated groups with unvaccinated groups with regard to demographic characteristics, number of preexisting conditions, and variant period, we observed a small cognitive advantage among participants who had received multiple vaccinations (one dose, 0.08 SD; and at least two doses, 0.15 SD) (Table S12). An analysis that matched participants who had initially received two doses of the AstraZeneca vaccine to those who had received two doses of the Pfizer vaccine showed a negligible scaled difference in the global cognitive score (−0.07 SD). An analysis in which participants who had had multiple episodes of Covid-19 were matched to those who had had single episodes showed that participants who had had multiple episodes had a small cognitive disadvantage (−0.11 SD), but this result was attenuated (−0.02 SD) in analyses in which the participants were additionally matched for variant period, illness duration, and hospitalization (Table S13).
Secondary Analyses
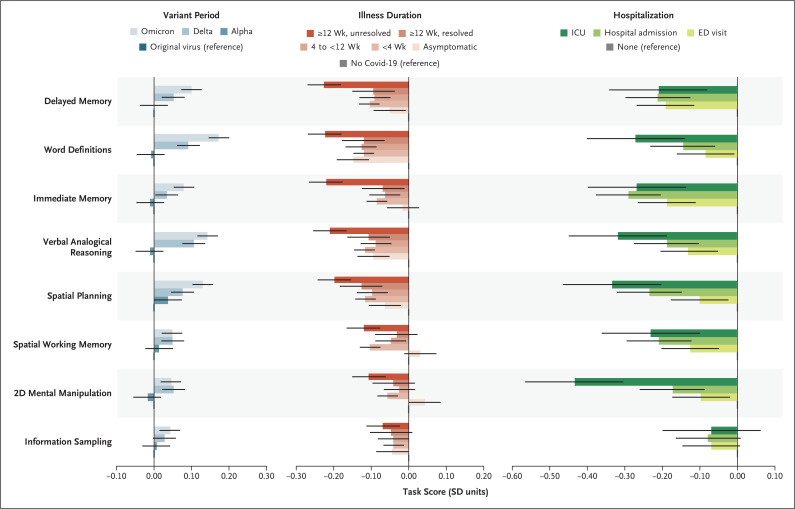
In analyses of individual tasks on the cognitive battery, we observed associations similar to those of the primary regression model, both among the 102,263 participants who completed all the tasks (excluding those within 12 weeks after infection onset) and among the 141,583 participants who completed at least one task. Memory, reasoning, and executive function (i.e., planning) tasks were the most sensitive and had the largest deficits in the group with unresolved persistent symptoms as compared with the no–Covid-19 group (−0.33 to −0.20 SD) (Table S14A and S14B). This pattern was similar with regard to hospitalization but was disproportionately greater for visuospatial deficits (as tested by the two-dimensional mental manipulation task) in the ICU group (Figure 3).
Figure 3. Performance of Specific Tasks According to SARS-CoV-2 Variant Period, Illness Duration, and Hospitalization.
Shown are associations from the multiple regression analyses of the summary scores of the eight individual tasks according to SARS-CoV-2 variant period (left), illness duration (middle), and hospitalization (right). On each task, higher scores, indicated by higher standard deviations, indicate better performance. The reference category in the multiple linear regression model is indicated for each covariate. Error bars indicate the 95% confidence interval. Values are point estimates for the linear regression as reported on a standard deviation scale. The term 2D denotes two-dimensional.
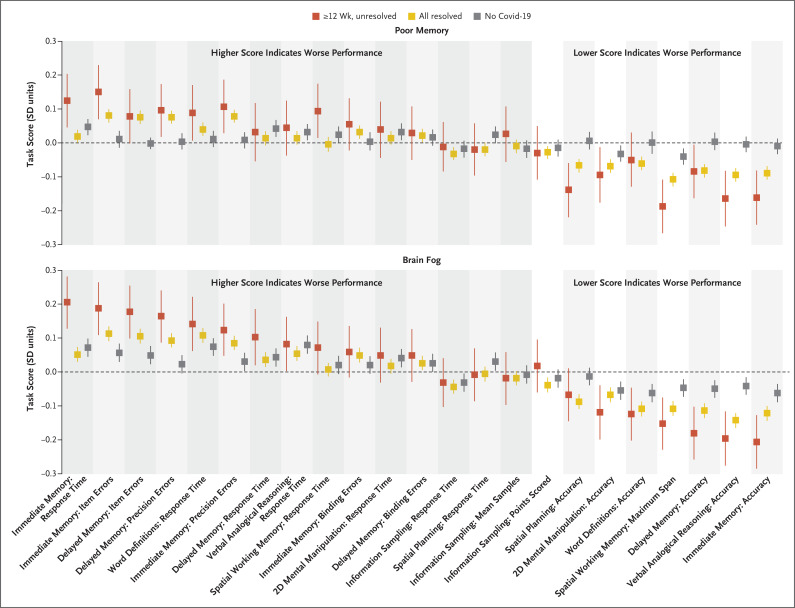
We found small associations between specific task scores and reports of poor memory or brain fog in the previous 2 weeks. Among participants with unresolved persistent symptoms, decrements in specific task scores were observed in verbal analogical reasoning accuracy (−0.20 SD among those reporting poor memory and −0.16 among those reporting brain fog), spatial working memory maximum span (−0.15 SD and −0.19 SD, respectively), and immediate memory accuracy (−0.21 SD and −0.16 SD). Among 53,422 participants with resolved symptoms, the profile was similar to that among participants with unresolved persistent symptoms (correlation of absolute effect sizes between the unresolved-symptoms group and the resolved-symptoms group across tasks: poor memory, r=0.81, and brain fog, r=0.76) but with smaller effect sizes in the resolved-symptoms group (maximum effect size, −0.14 SD among those reporting poor memory and −0.11 SD among those reporting brain fog). These associations were all negligible (<0.1 SD) when we contrasted participants who reported poor memory or brain fog with those who did not report such issues among 46,261 participants in the no–Covid-19 group (Figure 4 and Tables S15 and S16).
Figure 4. Associations of Subjective and Objectively Measured Cognitive Deficits.
Shown are associations of specific cognitive-task performance measures among participants who had poor memory or brain fog in the previous 2 weeks as compared with those who did not. Results are shown for three participant groups: the group with unresolved persistent symptoms (lasting ≥12 weeks), the combined group with resolved symptoms, and the no–Covid-19 group. The direction of the scoring for the individual analyses is shown in the figure. Decrements in performance were greater in the group with unresolved persistent symptoms, with a similar pattern (but smaller decrements) in the group with resolved symptoms. The largest decrements in performance were observed in the memory tasks (immediate and delayed memory and spatial working memory), reasoning tasks (verbal analogical reasoning), and executive tasks (spatial planning) in the group with unresolved persistent symptoms. Definitions of each task are provided in the Supplemental Methods: Cognitive Task Designs section in the Supplementary Appendix. SD indicates the standard-deviation difference in the mean cognitive performance, and error bars indicate the 95% confidence interval.
There were small differences in global cognitive scores according to the presence or absence of individual symptoms. Among the 56,002 participants who were assessed more than 12 weeks since infection, we found associations of global cognitive scores with symptoms from their acute episodes of Covid-19, ranging from −0.43 to −0.24 SD for face swelling, leg swelling, numbness or tingling, feet blisters or sores, and chest pain (Fig. S3A and Table S17A). Among the 2580 participants with unresolved persistent symptoms, we found associations of global cognitive scores with recent symptoms (occurring within the previous 2 weeks), ranging from −0.33 to −0.21 SD for severe fatigue, fever, dizziness, numbness or tingling, poor memory, chest pain, appetite loss, and mood swings (Fig. S3B and Table S17B).
Sensitivity Analyses
In analyses that excluded participants who had been vaccinated before their most severe Covid-19 episode, who were in the no–Covid-19 group, who did or did not report poor memory or brain fog in the previous 2 weeks, or for whom the education level was unknown, we observed findings similar to those of the primary regression analyses. An analysis that placed data from 6643 participants with suspected but unconfirmed Covid-19 in a separate illness-duration category did not materially alter the model estimates. The addition of a covariate for participants who sought medical assistance in a nonhospital setting showed small cognitive deficits as compared with participants who did not seek medical assistance (−0.12 SD). Among participants who did not complete the entire assessment, evaluation of their performance on the first task only (immediate memory) showed associations that were similar to those of the primary regression analysis. The results of these analyses are shown in Tables S11B, S14C, and S18 through S22.
Discussion
In this large community-based study, we found that Covid-19 was associated with longer-term objectively measurable cognitive deficits. The difference of approximately −0.2 SD in the global cognitive score in the groups of participants who had symptoms that had resolved, as compared with the no–Covid-19 group, is classified as “small” according to Cohen’s effect sizes24; this deficit would equate to a difference of −3 points on a typical IQ scale, in which 1 SD equals 15 points. Participants with unresolved persistent symptoms had a greater mean difference of approximately −0.4 SD. This downward shift was most evident at the distribution extreme,25 with a probability of task performance below the cutoff point for moderate impairment (−2 SD) that was 2.4 times as high among these participants as that in the no–Covid-19 group. ICU admission was associated with larger cognitive differences relative to the no–Covid-19 group (−0.63 SD, equivalent to a difference of −9 IQ points), with the probability of a score that was below −2 SD being 3.6 times as high as that in the no–Covid-19 group; this finding aligns with previous findings of medium-to-large-scale cognitive deficits in patients hospitalized in a critical care unit.2,26,27
Multiple findings indicated that the association between Covid-19 and cognitive deficits attenuated as the pandemic progressed. We found smaller cognitive deficits among participants who had been infected during recent variant periods than among those who had been infected with the original virus or the alpha variant. We also found a small cognitive advantage among participants who had received two or more vaccinations and a minimal effect of repeat episodes of Covid-19. Furthermore, the cognitive deficits that were observed in participants who had been infected during the first wave of the pandemic, when the original virus was predominant, coincided with peak strain on health services and a lack of proven effective treatments at that time, and the probability of hospitalization due to Covid-19 has progressively decreased over time.28 The finding that participants with resolved persistent symptoms had global cognitive deficits that were similar to those with shorter-duration symptoms suggests that persons with unresolved persistent symptoms may have some cognitive improvement once symptoms resolve.20
Our assessment comprised tasks that were designed to measure distinct aspects of cognitive performance that are associated with different brain systems.17 The memory, reasoning, and executive function (i.e., planning) tasks were among the most sensitive to Covid-19–related cognitive differences.9,10,26 We found that performance on these tasks differed according to illness duration and hospitalization. Scores on these tasks also correlated (albeit weakly) with recent poor memory or brain fog among participants with resolved symptoms and those with unresolved symptoms but not in the no–Covid-19 group — a finding that highlights the fact that although such symptoms are imprecise, they can reflect objectively measurable deficits. Poorer memory performance was characterized by equivalent reduced accuracy in immediate and delayed recognition rather than by accelerated forgetting — an observation that points to mechanisms of the medial temporal lobe, such as hippocampal neurogenesis,29,30 and functional interactions with frontoparietal attentional systems.31 Increased inflammation in the medial temporal lobe,32,33 accelerated atrophy of functionally associated regions of the brain,30,34 and disturbed functional dynamics have been reported after Covid-19.35,36
Although previous, often underpowered, studies have offered contradictory evidence for associations between mental health and cognitive deficits after Covid-19,5,37,38 our study was powered to detect small associations with high confidence. Our results confirmed associations of cognitive deficits with mood swings and fatigue but also with a variety of other symptoms. Therefore, it is likely that multiple underlying factors contribute to cognitive deficits after Covid-19. This heterogeneity is exemplified by the distinct cross-task profile of impairments in participants who had been admitted to the ICU, who also had cognitive consequences that have been associated with critical care.39
SARS-CoV-2 infection during the period when the delta variant was predominant was associated with better cognitive performance than infection during periods in which the original virus or alpha variant was predominant, a finding that is contrary to some previous findings (e.g., from clinics caring for persons with “long Covid-19” [also called “long Covid” or “post-Covid syndrome,” involving various constellations of symptoms after the acute phase of Covid-19]).40 Of note, the delta variant occurred in a highly vaccinated population. In addition, participants in our study were recruited by means of community-based random sampling, which resulted in the inclusion of persons with more asymptomatic and milder cases than would occur in hospital- or clinic-based studies but which also excluded persons with the most severe cases (e.g., those who died).
This study has certain limitations, including reliance on subjective reporting to identify persons with persistent symptoms. The relationship of our results to the literature about long Covid is complicated owing to a lack of established, defining criteria for post–Covid-19 syndromes. Consequently, we focused on symptoms that had persisted for at least 12 weeks, and we did not depend on a diagnosis of long Covid, which may require clinical assessment. In the absence of baseline cognitive data before infection, we could not assess cognitive change, and the observational nature of the data means that we could not infer causality.
Our calculation of the global cognitive score included the adjustment of raw performance scores for demographic characteristics and specific preexisting health conditions (as separate variables). Given the observational nature of the data, it is possible that some residual confounding remained. Consequently, in addition to standard regression analyses, we applied propensity-score matching23 as an alternative approach to address confounding. In analyses that closely matched selected participants on the basis of potentially confounding variables, we found a highly consistent pattern of results.
Any study that requires active participant engagement has a degree of participant self-selection bias. With regard to our study, persons with the most severe impairment may not have been able or willing to undertake a cognitive assessment. In addition, certain groups, including women and White persons, were slightly overrepresented in our study sample as compared with the base population, whereas younger persons and those from areas with greater levels of multiple deprivation were underrepresented. However, the sample size in our study meant that all sectors of society were represented and contributed meaningful data to the findings.
In this observational study, we found objectively measurable cognitive deficits that may persist for a year or more after Covid-19. We also found that participants with resolved persistent symptoms had small deficits in cognitive scores, as compared with the no–Covid-19 group, that were similar to those in participants with shorter-duration illness. Early periods of the pandemic, longer illness duration, and hospitalization had the strongest associations with global cognitive deficits. The implications of longer-term persistence of cognitive deficits and their clinical relevance remain unclear and warrant ongoing surveillance.
Acknowledgments
We thank all the study participants; Bethan Davies, Rob Elliott, and Graham Blakoe, of Imperial College London; Nicholas Gilby, Sam Clemens, Galini Pantelidou, and Amber Parish, of Ipsos; Agata Czarnecka, Edd Moffett, and Esther Gladstone, of H2 Cognitive Designs (Cognitron); and the members of our public advisory panel (Jo House, Alex Piper, Monique Jackson, Nikki Smith, Karen Cook, Darryl Slack, Caroline Eccles, Rashmi Kumar, and Margaret O’Hara), who helped shape the direction of the study.
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
The views expressed in this article are those of the authors and do not necessarily reflect those of the funders.
This is the New England Journal of Medicine version of record, which includes all Journal editing and enhancements. The Author Accepted Manuscript, which is the author’s version after external peer review and before publication in the Journal, is available at PubMed Central.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported by the National Institute for Health and Care Research and U.K. Research and Innovation, through a grant (MR/V030841/1) for the REACT-GE (Genomics England) study and a grant (COV-LT-0040) for the REACT-LC (Long Covid) study, and by the Department of Health and Social Care in England and the Huo Family Foundation.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Ceban F, Ling S, Lui LMW, et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav Immun 2022;101:93-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crivelli L, Palmer K, Calandri I, et al. Changes in cognitive functioning after COVID-19: a systematic review and meta-analysis. Alzheimers Dement 2022;18:1047-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houben S, Bonnechère B. The impact of COVID-19 infection on cognitive function and the implication for rehabilitation: a systematic review and meta-analysis. Int J Environ Res Public Health 2022;19:7748-7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu E, Xie Y, Al-Aly Z. Long-term neurologic outcomes of COVID-19. Nat Med 2022;28:2406-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie Y, Xu E, Al-Aly Z. Risks of mental health outcomes in people with Covid-19: cohort study. BMJ 2022;376:e068993-e068993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry 2021;8:416-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atchison CJ, Davies B, Cooper E, et al. Long-term health impacts of COVID-19 among 242,712 adults in England. Nat Commun 2023;14:6588-6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He D, Yuan M, Dang W, et al. Long term neuropsychiatric consequences in COVID-19 survivors: cognitive impairment and inflammatory underpinnings fifteen months after discharge. Asian J Psychiatr 2023;80:103409-103409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hampshire A, Trender W, Chamberlain SR, et al. Cognitive deficits in people who have recovered from COVID-19. EClinicalMedicine 2021;39:101044-101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao S, Shibata K, Hellyer PJ, et al. Rapid vigilance and episodic memory decrements in COVID-19 survivors. Brain Commun 2022;4:fcab295-fcab295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Giovane M, Trender WR, Bălăeţ M, et al. Computerised cognitive assessment in patients with traumatic brain injury: an observational study of feasibility and sensitivity relative to established clinical scales. EClinicalMedicine 2023;59:101980-101980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliott P, Whitaker M, Tang D, et al. Design and implementation of a national SARS-CoV-2 monitoring program in England: REACT-1 Study. Am J Public Health 2023;113:545-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riley S, Atchison C, Ashby D, et al. REal-time Assessment of Community Transmission (REACT) of SARS-CoV-2 virus: study protocol. Wellcome Open Res 2021;5:200-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward H, Atchison C, Whitaker M, et al. Design and implementation of a national program to monitor the prevalence of SARS-CoV-2 IgG antibodies in England using self-testing: the REACT-2 Study. Am J Public Health 2023;113:1201-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elliott P, Eales O, Steyn N, et al. Twin peaks: the omicron SARS-CoV-2 BA.1 and BA.2 epidemics in England. Science 2022;376(6600):eabq4411-eabq4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flower B, Brown JC, Simmons B, et al. Clinical and laboratory evaluation of SARS-CoV-2 lateral flow assays for use in a national COVID-19 seroprevalence survey. Thorax 2020;75:1082-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jolly AE, Scott GT, Sharp DJ, Hampshire AH. Distinct patterns of structural damage underlie working memory and reasoning deficits after traumatic brain injury. Brain 2020;143:1158-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akıncı B, Oğul ÖE, Hanoğlu L, et al. Evaluation of cognitive functions in adult individuals with COVID-19. Neurol Sci 2023;44:793-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alemanno F, Houdayer E, Parma A, et al. COVID-19 cognitive deficits after respiratory assistance in the subacute phase: a COVID-rehabilitation unit experience. PLoS One 2021;16(2):e0246590-e0246590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheetham NJ, Penfold R, Giunchiglia V, et al. The effects of COVID-19 on cognitive performance in a community-based cohort: a COVID symptom study biobank prospective cohort study. EClinicalMedicine 2023;62:102086-102086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elliott P, Bodinier B, Eales O, et al. Rapid increase in Omicron infections in England during December 2021: REACT-1 study. Science 2022;375:1406-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National statistics: English indices of deprivation 2019. London: Ministry of Housing, Communities & Local Government, 2019. (https://www.gov.uk/government/statistics/english-indices-of-deprivation-2019).
- 23.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41-55 (https://academic.oup.com/biomet/article/70/1/41/240879). [Google Scholar]
- 24.Sawilowsky S. New effect size rules of thumb. J Mod Appl Stat Methods 2009;8:597-599 (https://jmasm.com/index.php/jmasm/article/view/452/454). [Google Scholar]
- 25.Rose G, Day S. The population mean predicts the number of deviant individuals. BMJ 1990;301:1031-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hampshire A, Chatfield DA, MPhil AM, et al. Multivariate profile and acute-phase correlates of cognitive deficits in a COVID-19 hospitalised cohort. EClinicalMedicine 2022;47:101417-101417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nersesjan V, Fonsmark L, Christensen RHB, et al. Neuropsychiatric and cognitive outcomes in patients 6 months after COVID-19 requiring hospitalization compared with matched control patients hospitalized for non-COVID-19 illness. JAMA Psychiatry 2022;79:486-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet 2022;399:1303-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wixted JT, Squire LR. The medial temporal lobe and the attributes of memory. Trends Cogn Sci 2011;15:210-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Díez-Cirarda M, Yus M, Gómez-Ruiz N, et al. Multimodal neuroimaging in post-COVID syndrome and correlation with cognition. Brain 2023;146:2142-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jenkins LJ, Ranganath C. Prefrontal and medial temporal lobe activity at encoding predicts temporal context memory. J Neurosci 2010;30:15558-15565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanabria-Diaz G, Etter MM, Melie-Garcia L, et al. Brain cortical alterations in COVID-19 patients with neurological symptoms. Front Neurosci 2022;16:992165-992165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis 2020;94:55-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Douaud G, Lee S, Alfaro-Almagro F, et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 2022;604:697-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Churchill NW, Roudaia E, Chen JJ, et al. Effects of post-acute COVID-19 syndrome on the functional brain networks of non-hospitalized individuals. Front Neurol 2023;14:1136408-1136408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Y, Ling Q, Manyande A, Wu D, Xiang B. Brain imaging changes in patients recovered from COVID-19: a narrative review. Front Neurosci 2022;16:855868-855868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marvel CL, Paradiso S. Cognitive and neurological impairment in mood disorders. Psychiatr Clin North Am 2004;27:19-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo P, Benito Ballesteros A, Yeung SP, et al. COVCOG 2: cognitive and memory deficits in long COVID: a second publication from the COVID and Cognition Study. Front Aging Neurosci 2022;14:804937-804937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ollila H, Pihlaja R, Koskinen S, et al. Long-term cognitive functioning is impaired in ICU-treated COVID-19 patients: a comprehensive controlled neuropsychological study. Crit Care 2022;26:223-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agergaard J, Gunst JD, Schiøttz-Christensen B, Østergaard L, Wejse C. Long-term prognosis at 1.5 years after infection with wild-type strain of SARS-CoV-2 and alpha, delta, as well as omicron variants. Int J Infect Dis 2023;137:126-133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.