Abstract
Desmin contributes to structural integrity and function of the myocardium but its function seems to be redundant in early cardiomyogenesis in the desmin null mouse model. To test the hypothesis that desmin also plays a supportive role in cardiomyogenic commitment and early differentiation of cardiomyo-cytes we investigated cardiomyogenesis in embryoid bodies expressing different desmin alleles. Constitutive expression of desmin and increased synthesis during mesoderm formation led to the up-regulation of brachy-ury and nkx2.5 genes, accelerated early cardiomyo-genesis and resulted in the development of large, proliferating, highly interconnected, and synchronously beating cardiomyocyte clusters, whereas desmin null cardiomyocytes featured an opposite phenotype. In contrast, constitutive expression of amino-terminally truncated desminΔ1−48 interfered with the beginning of cardiomyogenesis, caused down-regulation of mesodermal and myocardial transcription factors, and hampered myofibrillogenesis and survival of cardiomyocytes. These results provide first evidence that a type III intermediate filament protein takes part in regulating the differentiation of mesoderm to cardiomyocytes at the very beginning of cardiomyogenesis.
Keywords: cardiomyogenesis, desmin, nkx2.5, brachyury, embryoid body
Introduction
Structural integrity and rhythmic excitation of cardiomyocytes is based on a complex cellular system composed of the contractile apparatus, cytoskeleton, membrane skeleton, sarcoplasmic reticulum, and extracellular matrix (Clark et al., 2002). A prominent member of the first three of these compartments is desmin, the subunit of the muscle specific type III intermediate filaments (Capetanaki, 2000; Costa et al., 2004; Paulin and Li, 2004). Expression of desmin becomes visible in murine precardiac mesoderm at day 7.25 (Kuisk et al., 1996), emanating from brachyury and goosecoid expressing mesodermal precursors (Olson, 2004). Additionally, desmin mRNA was detected in embryoid bodies (EBs) at day 5 (Weitzer et al., 1995) suggesting a role in early embryonic development before cardiomyogenesis takes place. Later in development desmin is also expressed in skeletal and smooth muscle cells and influences myogenesis at the transcriptional level by modulating the expression of the skeletal muscle specific bHLH transcription factor genes myogenin, myoD, and myf5 (Li et al., 1994; Weitzer et al., 1995), however, so far appeared to be dispensable for cardiomyogenesis in vitro and in vivo (Weitzer et al., 1995; Li et al., 1996; Milner et al., 1996).
Mice lacking desmin develop lesions in cardiac muscle (Li et al., 1996; Milner et al., 1996) which are accompanied by the disorganization of myofibrils, intercalated discs, and mitochondria (Thornell et al., 1997; Balogh et al., 2002; Capetanaki, 2002). The cardiac phenotype can be partially overcome by the expression of bcl-2, rescuing the mitochondrial phenotype (Weisleder et al., 2004b) and by expression of desmin under the control of the mhcα promoter (Weisleder et al., 2004a). In skeletal muscle, lack of desmin results in lower active force generation, improves fatigue resistance, decreases isometric stress production and reduces vulnerability to mechanical injury (Li et al., 1997; Sam et al., 2000; Balogh et al., 2003; Shah et al., 2004). An amino-terminally deleted desmin rescued myogenesis in skeletal muscle but compromised cardiomyogenesis in EBs (Höllrigl et al., 2002). Finally, in smooth muscle cells desmin has a role in cellular transmission of both active and passive force (Sjuve et al., 1998; Shardonofsky et al., 2006).
Early expression of desmin in precardiac mesoderm (Kuisk et al., 1996) and its influence on transcription of early myogenic transcription factor genes in skeletal muscle (Li et al., 1994; Weitzer et al., 1995) suggests a role for desmin in very early developmental processes such as cardiomyogenic commitment and differentiation. While substantial progress has been made in clarifying desmin’s contribution to function, stability and longevity of the working myocardium, evidence supporting a role in early cardiomyogenesis is lacking. Cardiomyogenesis at its very beginning is supposed to start in the splanchnic mesoderm beneath the developing head fold under the transcriptional control of the T-box, tinman/nkx, and gata families of transcription factors (Hiroi et al., 2001; Masino et al., 2004).
Here, we test the hypothesis that desmin affects very early cardiomyogenesis. To this end we investigated in vitro cardiomyogenesis in EBs, generated from embryonic stem cells (ESCs) constitutively expressing an additional wild-type desmin allele, and from ESCs constitutively expressing two mutant desmin alleles in a null background, giving rise to amino-terminally deleted desminΔ1−48. We found that constitutive expression of desmin indeed promoted early cardiomyogenesis and up-regulates the expression of the brachyury and nkx2.5 genes, whereas absence of desmin delayed cardiomyogenesis, and constitutive expression of desminΔ1−48 negatively affected early cardiomyogenesis and transcription factor gene expression.
Materials and methods
Generation of ESC lines
Des−/− and desΔ1−48/Δ1−48 ESCs were generated as described (Weitzer et al., 1995; Höllrigl et al., 2002). The desmin expression vector was constructed by inserting the murine desmin cDNA (Li and Capetanaki, 1993) into pBK-RSV (Stratagene, La Jolla, CA), and verified by sequencing. Transfection of AB2.2 ESCs with pBK-RSV-Desmin was performed by electroporation and selection for random integration with 180 μg/ml G418.
In vitro differentiation of ESCs and analysis of cardiomyogenesis in EBs
Each 800 ESCs were aggregated in 20 μl medium drops for 4 days, 80 ± 10 EBs of equal size were plated onto gelatinized 10 cm tissue culture dishes and cardiomyogenesis was monitored essentially as described (Weitzer et al., 1995; Bader et al., 2000). Development of cardiomyocytes was compared by calculating the maximal daily increase in the number of beating cardiomyocytes and the maximal percentage of EBs with beating cardiomyocytes at the day of maximum beating activity ± 2 days. Clusters of beating cardiomyocytes in EBs were counted and classified as small, when composed of less than 10, or as large, when composed of more than 10 cardiomyocytes. Number of clusters directly reflects commitment and size of clusters reflects proliferation of cardiomyocytes (Lauss et al., 2005).
Immunofluorescence microscopy
Immunostaining of EBs was performed with desmin antibodies D8281 (Sigma-Aldrich, Vienna, Austria), Y20 (Santa Cruz Biotechnology, Heidelburg, Germany), and with cardiac troponin T (cTnT) antibodies (Neomarkers, Vienna, Austria), followed by FITC- and TRITC-conjugated secondary antibodies (Sigma F4018, T-5268). The annexin V binding assay was performed according to supplier’s protocol (1858777, Roche, Vienna, Austria). For terminal deoxynucleotidyl transferase-mediated dNTP-fluorescein nick end labeling (TUNEL) assays EBs were fixed in 4% para-formaldehyde solution, permeabilized and stained for cTnT, in this case using a Texas red-conjugated secondary antibody (Jackson Immuno research, Suffolk, UK). TUNEL assay was performed according to supplier’s protocol (1684809, Roche) followed by DAPI staining. Photomicrographs were taken from areas where typically cardiomyogenesis takes place in EBs (Weitzer, 2006) on a Leica TCS SP confocal microscope (Wetzlar, Germany) with × 40 and × 100 objectives and on a Zeiss Axiovert LSM 510 microscope (Vienna, Austria) with an × 63 objective. Images were prepared by Adobe Photoshop 7.0.
Fluorescence activated cell sorting analysis
Eighty EBs of each genotype were allowed to develop until day 5, then cells were separated by digestion with trypsin for 10 min at 37°C. 500,000 cells were resuspended in PBS, dead cells were stained with propidium iodide (10 μg/ml) and sorted in a BD Becton&Dickinson LSR1 fluorescence activated cell sorter (Schwechat,Austria).
Western blot analysis
Western blot analysis was performed with intermediate filament preparations from EBs (Weitzer and Wiche, 1987) using anti-desmin (Y20, Santa Cruz Biotechnology), anti-vimentin (ICN, Eschwepe, Germany), and anti-connexin 43 (Sigma-Aldrich) antibodies and secondary alkaline phosphatase conjugated antibodies (Promega, Mannheim, Germany).
Semi-quantitative RT-PCR analysis
mRNA was prepared from ESCs and EBs with the RNeasy Mini Kit (Qiagen, Vienna, Austria) and reverse transcribed with Superscript II polymerase (Invitrogen, Merelbeke, Belgium) in at least three independent experiments. Polymerase chain reaction (PCR) was performed with primer pairs as indicated in Figures 1 and 6. For semi-quantitative PCR samples were diluted 1:0 1:10, 1:100, and 1:1,000. Primer sequences and parameters of amplification are available on request. Number of cycles were carefully determined by several preparatory experiments and chosen so that none of the obtained signals were saturated. RSV promoter driven expression of desmin was determined by using a forward primer situated in the RSV promoter, endogenous expression was detected with a reverse primer situated in the 3′ UTR not contained in the desect allele. Statistical analysis of the brachyury and nkx2.5 expression was performed by measuring the luminosity of ethidiumbromide stained RT-PCR products by Adobe Photoshop 7.0 tools of six independent experiments.
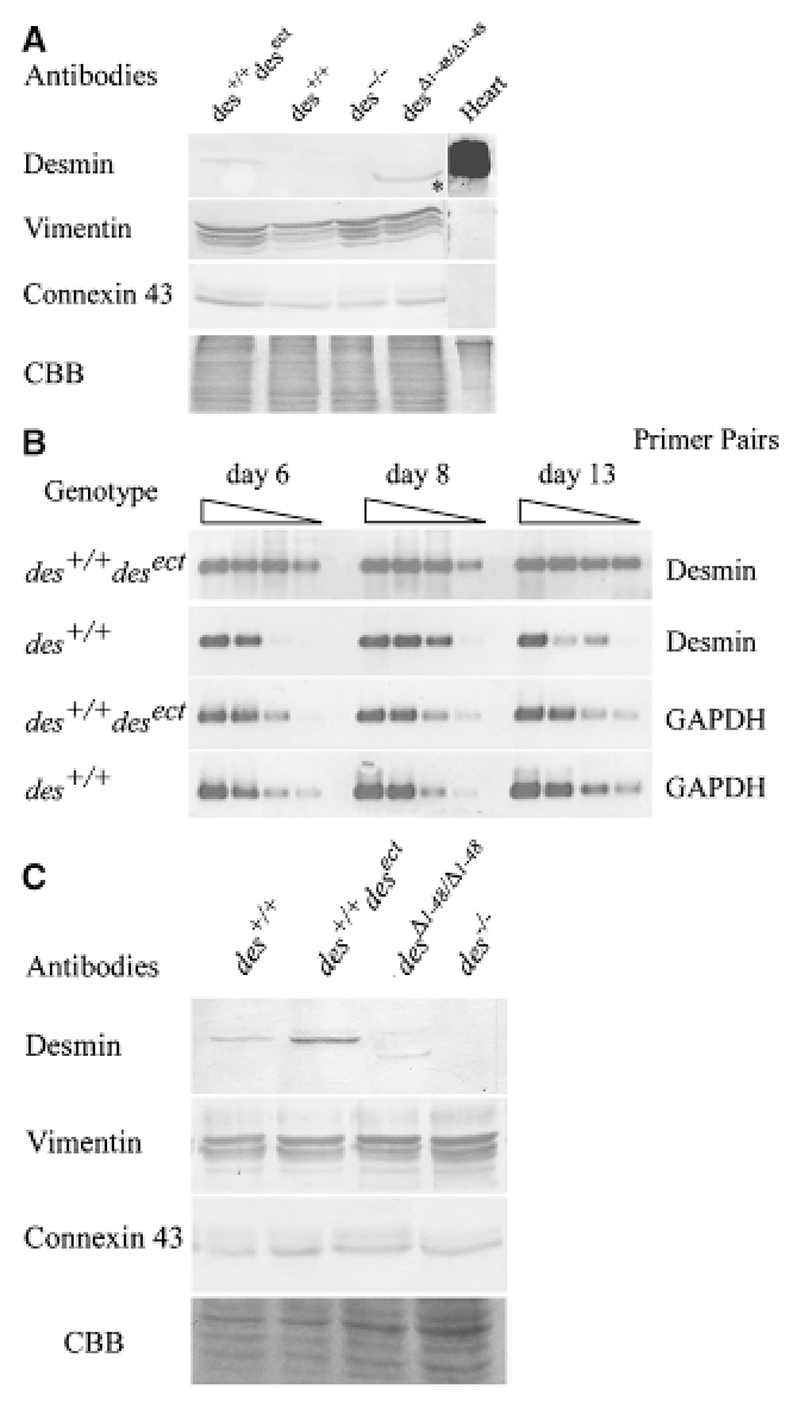
Fig. 1. Expression and synthesis of desmin and desminΔ1−48 in embryonic stem cells and embryoid bodies (EBs).
(A) Western blot analysis of intermediate filament preparations from embryonic stem cells with an additional ectopic allele constitutively expressing desmin (des+/+ desect), two wild-type desmin alleles (des+/+), two null alleles (des−/−), and two constitutively expressed mutant alleles lacking codons 1−48 (desΔ1−48/Δ1−48), with antibodies against desmin, vimentin, and connexin 43. Heart: positive control. Connexin 43 immuno-blot and CBB, Coomassie brilliant blue stained gel, loading controls. *Note, smaller size of the constitutively synthesized desmin lacking amino acids 1−48. (B) Semi-quantitative RT-PCR of des+/+ and des+/+ desect EBs with desmin and GAPDH primer pairs at different stages of EB development. Rows 1−4 represent steps of tenfold dilution of the cDNA for each day. (C) Expression of wild type, ectopic and mutant desmin proteins in EBs. Western blot analysis of intermediate filament preparations from EBs at day 16. Antibodies and genotypes as indicated.
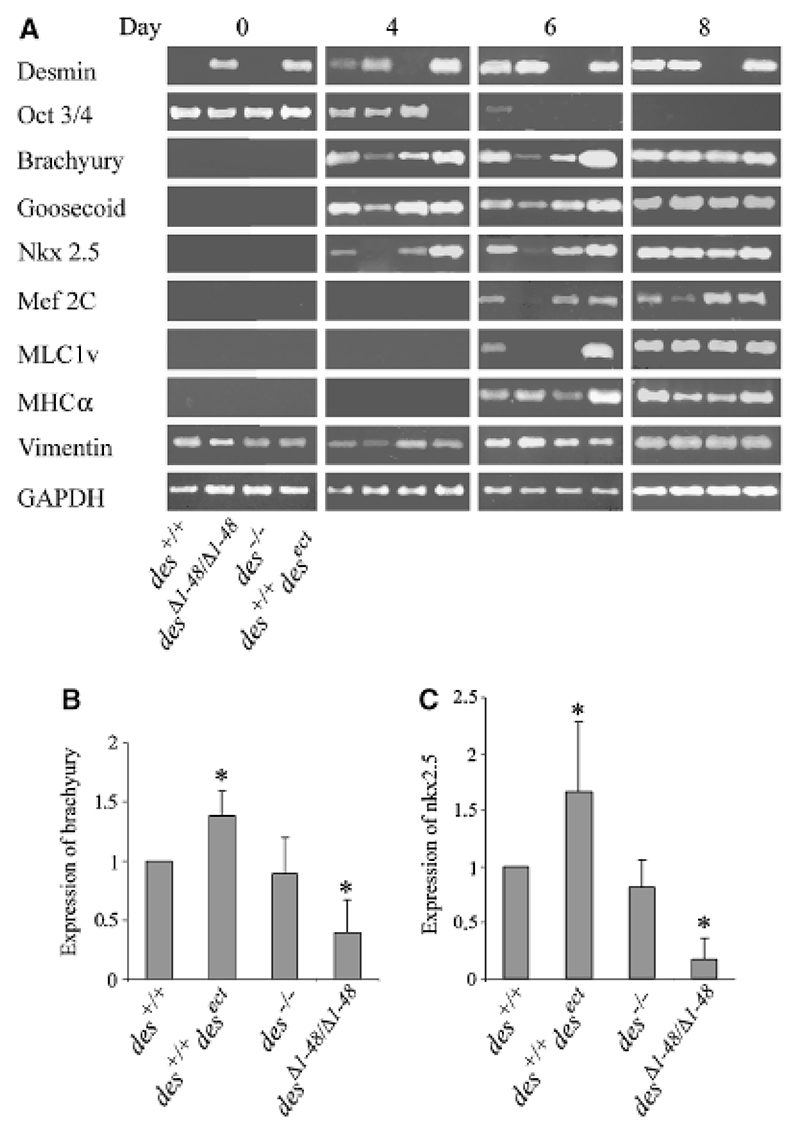
Fig. 6.
Premature synthesis of desmin causes increased expression of mesodermal and myocardial transcription factor genes in embryoid bodies (EBs). mRNA was isolated and reverse transcribed from embryonic stem cells (day 0) and EBs of genotypes as indicated in the lower left corner at days 4, 6, and 8, respectively. (A) Semiquantitative RT-PCR was performed in at least three independent experiments (one typical shown) with primer pairs as indicated. cDNA was normalized by levelling the GAPDH RT-PCR product. Statistical analysis of (B) brachyury and (C) nkx2.5 expression in EBs between days 4 and 6 of six independent experiments. Expression levels normalized to expression in wild-type EBs. *p-values smaller than 0.05 relate to control (des+/+).
Statistical analysis
All data are presented as the arithmetic mean ± standard deviation σx(n−1). Statistical significance was evaluated using one sample and paired samples Student’s t-test, respectively and values of p < 0.05 were considered to indicate statistical significance.
Results
Constitutive expression and synthesis of desmin and desmin Δ1−48 is maintained throughout cardiomyogenesis in EBs
Transfection of ESCs with a desmin expression cassette under the control of the constitutive active RSV promoter resulted in desmin protein synthesis detectable in intermediate filament preparations by Western blot analysis (Fig. 1A, lane 1). Constitutive expression and protein synthesis of desmin Δ1−48 and absence of desmin in des−/− ESCs were previously described (Weitzer et al., 1995; Höllrigl et al., 2002). Constitutive expression of desmin was maintained in EBs throughout the time of cardiomyogenesis (Fig. 1B). Constitutive expression of an additional desmin allele resulted in an increased desmin protein level in des+/+ desect EBs, whereas in des Δ1−48/Δ1−48 EBs the desmin Δ1−48 protein level was similar to that in des+/+ EBs (Fig. 1C). Neither vimentin nor connexin 43 protein levels were affected by the expression of different desmin alleles.
Desmin accelerates differentiation of cardiomyocytes whereas mutant desminΔ1−48 negatively affects cardiomyogenesis in Ebs
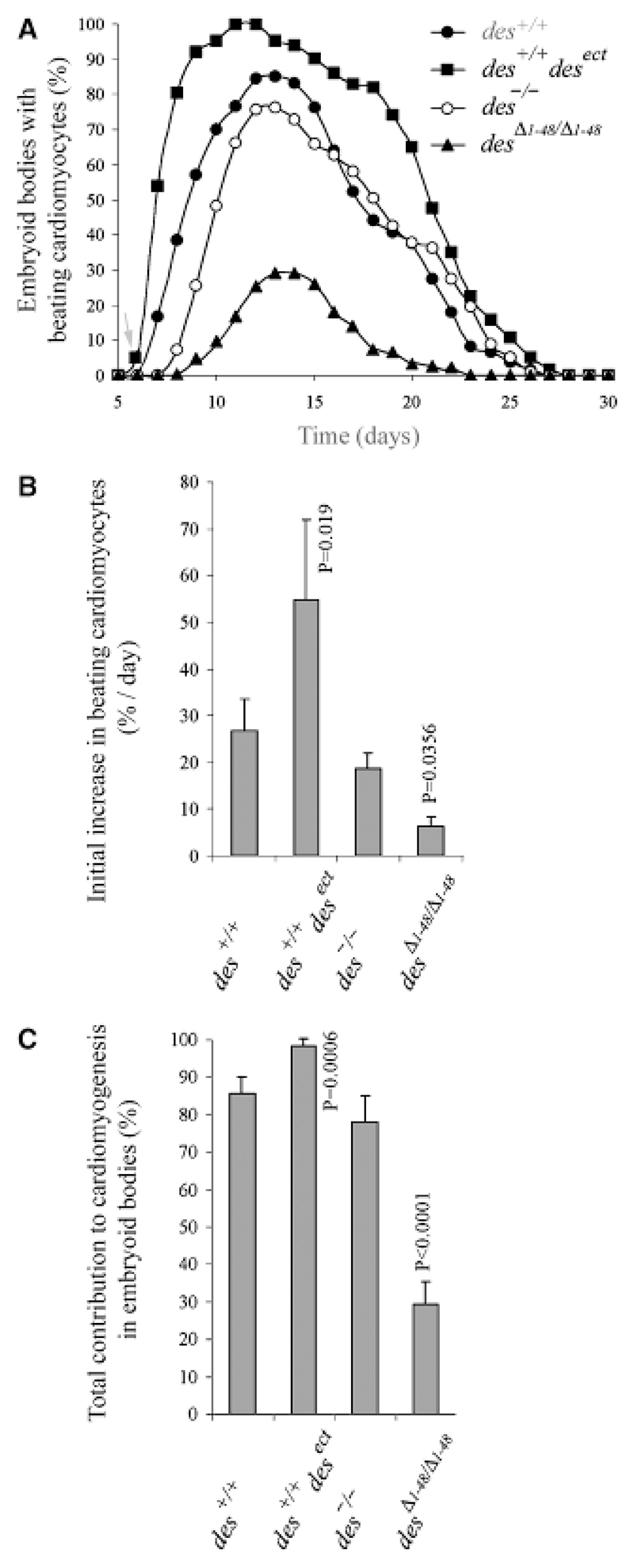
Constitutive synthesis of desmin in differentiating ESCs induced a 24 ± 4 hr earlier onset of cardiomyogenesis in des+/+desect EBs (Fig. 2A, arrow), normally starting at day 7 ± 6 hr in wild-type des+/+ EBs, caused an accelerated development of cardiomyocytes (Fig. 2B), and resulted in large areas of highly proliferating cardiomyocytes in 100% of EBs (Fig. 2C). Absence of any effect of conditioned cell culture supernatant from des+/+desect EBs on cardiomyogenesis in wild-type EBs (data not shown) suggests that desmin does not influence cardiomyogenesis by a paracrine loop via other cell types but acts in a cell autonomous manner. In the absence of desmin, development of cardiomyocytes was delayed by 1 day (Fig. 2A) but rate of differentiation was slightly affected (Fig. 2B). Maximum number of EBs with beating cardiomyocytes was only insignificantly decreased (Fig. 2C), however, the size of cardiomyocyte clusters was much smaller. In order to test the effect of a truncated and potentially dominant-negative mutant molecule in the onset of cardiomyogenesis, differentiation of cardiomyocytes was investigated in ESCs expressing a mutant desmin protein lacking its amino-terminal domain in a null background. Constitutive synthesis of desminΔ1−48 caused a 2 days delay of cardiomyogenesis (Fig. 2A), a drastically reduced differentiation rate (Fig. 2B), and very low number of EBs with small clusters of cardiomyocytes (Fig. 2C). Notably, this phenotype was much worse than that observed in the absence of desmin in des−/− EBs and suggests a functional involvement of desmin in commitment and early differentiation of cardiomyocytes.
Fig. 2. Premature synthesis of desmin causes early onset of cardiomyogenesis in embryoid bodies (EBs), but lack of its amino-terminal domain severely hampers cardiomyogenesis.
(A) Development of beating cardiomyocytes in EBs generated from embryonic stem cells of genotypes as indicated. Note, that only des+/+desect cardiomyocytes started to beat on day 6 (arrow) and onset of cardiomyogenesis in desΔ1−48/Δ1−48 EBs was delayed by 2 days. (B) Acceleration of development to beating cardiomyocytes by desmin. Starting 1 day after the first beating cardiomyocytes were observed, increase of beating cardiomyocytes per day was measured for 3 consecutive days. (C) Influence of desmin on the extent of cardiomyogenesis in EBs. Means were calculated from the day of maximum beating activity ± 2 days. (A−C) Data from experiments with two des+/+desect clones were indistinguishable and thus combined. Data are means of six independent experiments. Number of EBs analyzed in each case: N = 386. Error bars, standard deviation σx(n − 1). Denoted significant p-values relate to control (des+/+).
Desmin facilitates proliferation and maturation of cardiomyocytes
To investigate the influence of desmin and desminΔ1−48 on the morphology of cardiomyocytes, EBs were stained with desmin antibodies and cardiomyocytes were identified with antibodies to cTnT. Compared with wild-type, des+/+ EBs, the size of cardiomyocyte clusters was significantly decreased in des−/− EBs but enormously increased in des+/+desect EBs (Fig. 3A). Frequently the total central area of des+/+desect EBs was covered with an interwoven synchronously beating network of cardiomyocytes, suggesting that overexpression of desmin also increased proliferation of cardiomyocytes. In desΔ1−48/Δ1−48 EBs cluster size was reduced to that observed in des−/− EBs again demonstrating a dominant-negative effect of desminΔ1−48.
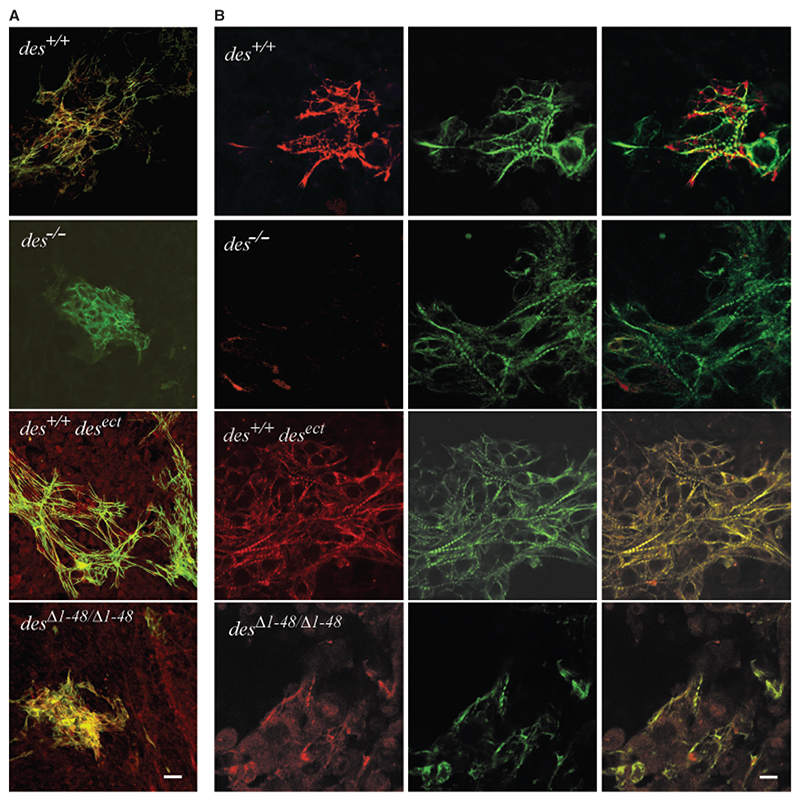
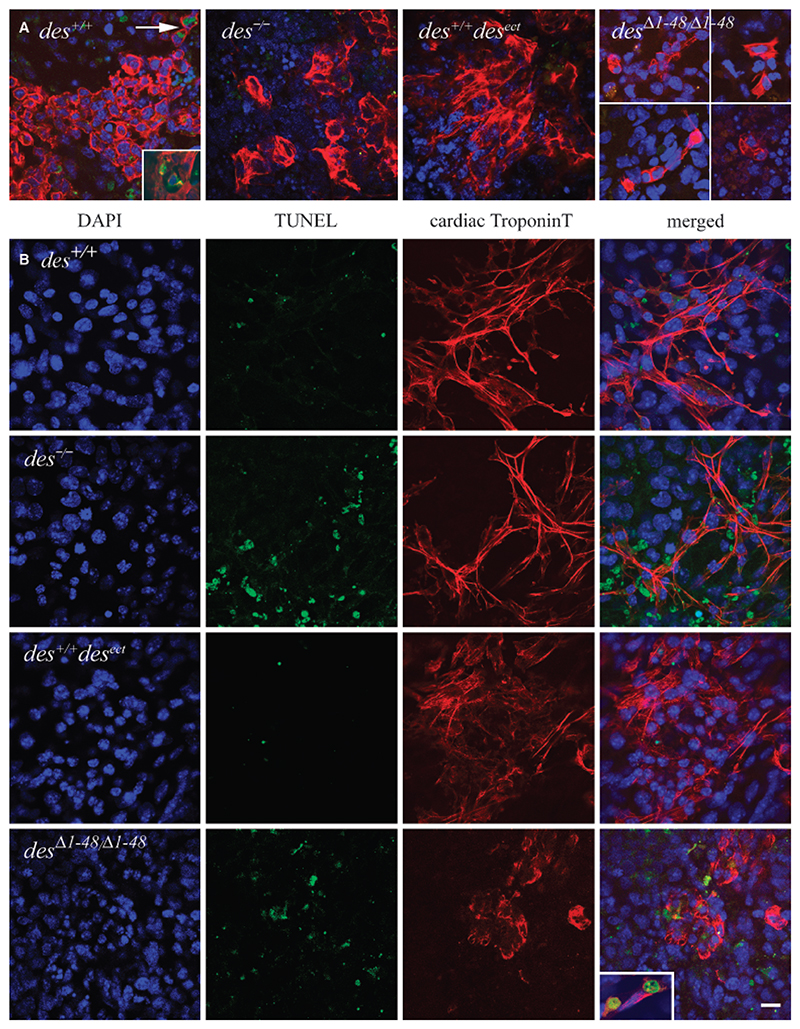
Fig. 3. Desmin increases size and interconnections of cardiomyocyte clusters but desminΔ1−48 causes disorganization of contractile apparatus.
(A) Merged confocal double immunofluorescence micrographs of typical cardiomyocyte clusters in embryoid bodies (EBs) with desmin alleles as indicated. EBs were double stained with a polyclonal antibody to desmin and a secondary TRITC-conjugated antibody (red) and a monoclonal antibody to cTnT and a secondary FITC-conjugated antibody (green) at day 16 after embryonic stem cells aggregation. Note, ectopic synthesis of desmin in all cells of des+/+desect and desΔ1−48/Δ1−48 EBs. Scale bar, 25 μm. (B) High-resolution confocal double immunofluorescence micrographs of cardiomyocytes stained as in (A). Right column, merged images. Scale bar, 10 μm.
At high magnification it became evident that absence of desmin did not significantly alter the number of myofibrils in differentiating cardiomyocytes but expression of desminΔ1−48 drastically inhibited formation of myofibrils with regular sarcomeres and caused a diffuse distribution of cTnT (Fig. 3B). Most desΔ1−48/Δ1−48 cardiomyocytes had an aberrant structure and seemed to be less connected to each other, again demonstrating that truncation of the amino terminus of desmin is worse than the lack of desmin in cardiomyocytes. By contrast, overexpression of desmin resulted in enlarged and very dense array of cardiomyocytes with numerous myofibrils with regularly aligned sarcomeres.
To assess the influence of desmin on early proliferation of still differentiating cardiomyocytes, we measured the number and size of cardiomyocyte clusters shortly before cardiomyogenesis reached maximum levels in EBs. Constitutive synthesis of desmin resulted in increased numbers of cardiomyocyte clusters within all EBs (Fig. 4A), and in an extreme preponderance of large over small clusters of cardiomyocytes (Fig. 4B). Synchronous beating of all cardiomyocytes with significantly increased contraction rates in des+/+desect EBs suggest a physiological interaction of these cardio-myocytes (Fig. 4C). In the absence of desmin cluster size shifted to small not synchronously contracting aggregates (Fig. 4B), with unchanged contraction rates (Fig. 4C). In the presence of desminΔ1−48 number and size of cardiomyocyte clusters was drastically reduced (Figs. 4A,4B), and contraction rates (Fig. 4C) were dramatically lowered. In desΔ1−48/Δ1−48 cardiomyocytes contraction became arrhythmic with increasing age (data not shown).
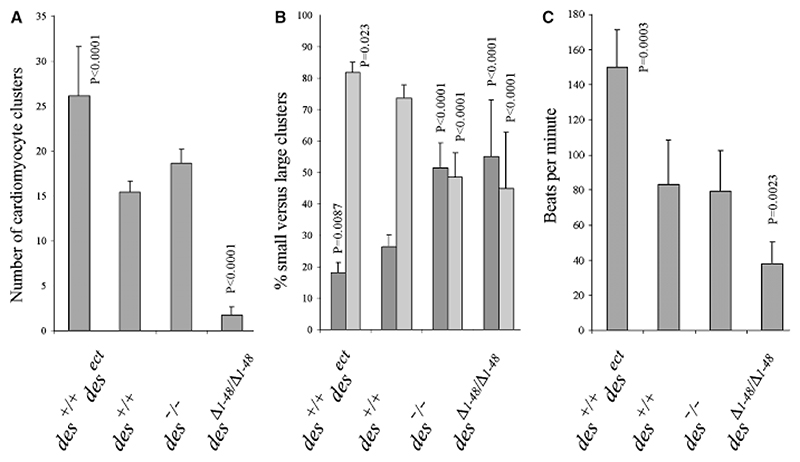
Fig. 4. Desmin promotes commitment and proliferation of cardiomyocytes in embryoid bodies (EBs).
(A) Number of beating cardiomyocyte clusters per EB indicating commitment of cardiomyocytes. Number of EBs analyzed in each case, N = 311; except for des+/+ and des+/+desect, N = 916. (B) Size distribution of cardiomyocyte clusters which is directly proportional to the proliferation of differentiating cardiomyocytes, presented as the relative proportion of small clusters with <10 (dark gray bars) versus large clusters with up to several hundred cardiomyocytes (light gray bars). Number of EBs analyzed in each case: N = 386. (C) Rhythmic contraction of cardiomyocytes was measured on day 16 ± 4. Number of cardiomyocyte clusters analyzed: des+/+, N = 85; des+/+desect, N =109; all others N = 52. Data are means of six independent experiments. Error bars, standard deviation σx(n − 1). Denoted significant p-values relate to control (des+/+).
Low numbers and small size of cardiomyocyte clusters might be also due to increased apoptosis of cardiomyocytes expressing desminΔ1−48. FACS analysis of EBs stained for dying or dead cells with propidium iodide at day 5 when primitive mesoderm forms demonstrated a weak but significant increase in the number of dying cells in desΔ1−48/Δ1−48 EBs (Table 1). This finding was in line with very similar results obtained with EBs expressing a dominant-negative allele of desmin under the control of the desmin promoter (Höllrigl et al., 2007) Thus, it is likely that desminΔ1−48 affects apoptosis of some mesodermal precursors for those un-fortunately still no specific markers exist. To demonstrate a specific influence on cardiomyocytes, we performed TUNEL and annexin V binding assays in EBs on day 8, the first day when cardiomyocytes appear in desΔ1−48/Δ1−48 EBs. Cardiomyocytes of all genotypes had nearly no TUNEL-positive nuclei at day 8 (Fig. 5A). Only two of 1083 cTnT-positive des+/+ cardiomyocyte had TUNEL assay positive nuclei or annexin V bound to its plasma membrane (Fig. 5A, inset). No cTnT-positive desΔ1−48/Δ1−48 cardiomyocytes were TUNEL positive (N = 109). Annexin V binding assay corroborated the very low susceptibility of cTnT-positive cardiomyocytes to apoptosis on day 8 (data not shown). At day 12 again no signs of apoptosis in cardiomyocytes were observed (data not shown). At day 16, however, when desΔ1−48/Δ1−48 cardiomyocytes prematurely seize to contract (see Fig. 2A) a significantly increased number of TUNEL-positive nuclei (25%, N = 60) was observed along with a clearly degenerated morphology (Fig. 5B). Thus, desminΔ1−48 affects most likely pre-myocardial mesoderm and committed and differentiated cardiomyocytes. However, from these data it cannot be excluded that desminΔ1−48 also affects proliferation of committed cardiomyocytes. An increased number of TUNEL-positive nuclei were also found in the neighborhood of cTnT-positive cardiomyocytes in des−/− EBs, however, apoptosis in des+/+ and des+/+desect cardiomyocytes remained low in the range of 5−10% (N = 218 each). Together these data strongly suggest that desmin actively facilitates commitment, proliferation and differentiation of cardiomyocytes.
Table 1. Mutant desmin increases cell death in embryoid bodies at the beginning of mesoderm formation.
| Genotype | % dead cells1 | Standard deviation | p-value | Number of experiments |
|---|---|---|---|---|
| des+/+ | 17.97 | 1.77 | — | 5 |
| des −/− | 17.74 | 0.74 | 0.8123 | 5 |
| des +/+ desect | 19.85 | 1.03 | 0.1797 | 4 |
| des Δ1−48/Δ1−48 | 26.04 | 1.28 | 0.0008 | 5 |
Cells of embryoid bodies were dissociated by mild digestion at day 5 and dying or dead cells were stained with propidium iodide and sorted by FACS analysis.
Fig. 5.
Apoptosis is absent in early differentiating cardiomyocytes but increases with age in des−/− and desminΔ1−48 expressing cardiomyocytes. Embryoid bodies (EBs) fixed on days 8 and 16 after embryonic stem cells aggregation, respectively, and indirectly stained with DAPI for nuclear DNA (blue), for single-strand brakes of DNA by TUNEL assay with fluorescein-labelled dNTPs (green), and for cTnT with anti-cTnT and secondary TRITC-conjugated antibodies (red). Genotypes as indicated. (A) Merged confocal triple immunofluorescence micrographs of typical cardiomyogenic areas in EBs at day 8. In the case of desΔ1−48/Δ1−48 EBs images from different EBs were combined in order to show a larger number of the widely dispersed and rarely found cardiomyocytes. Arrow, two of 1,083 cardiomyocytes positive for TUNEL staining. Insets, examples of rarely found typical apoptotic cells with phosphatidylserine at the outer side of the plasma membranes. FITC-conjugated annexin V (green). (B) Cardiomyocytes in EBs at day 16. Note, that cTnT positive des−/− and desΔ1−48/Δ1−48 cardiomyocytes are surrounded by TUNEL positive cells but very rarely are positive for TUNEL assay and cTnT (inset lower right panel). Scale bar, 25 μm.
Desmin transiently promotes the expression of mesodermal transcription factor brachyury and early myocardial transcription factor nkx2.5
To provide molecular evidence for desmin’s positive influence on the early differentiation of cardiomyocytes we investigated the expression of genes specific for mesoderm and cardiomyocytes by semi-quantitative RT-PCR from days 0 to 8 of in vitro development (Fig. 6A). Changes in the expression pattern of cardiac muscle markers are specific for cardiomyogenesis because skeletal and smooth muscle cells start to differentiate much later in EB development (Weitzer et al., 1995). In wild-type EBs desmin transcripts could be detected at the beginning of mesoderm development at day 4 and absence of desmin in EBs had a weak negative effect on the expression of brachyury, and caused a significant delay in mlc1v expression (Fig. 6, third lanes), indicative of delayed or hampered cardiomyocyte maturation (Trahair et al., 1993), which was also evident from the small size of cardiomyocyte clusters (see Fig. 3A). Constitutive desminΔ1−48 synthesis caused the partial downregulation of mesodermal transcription factor genes brachyury and goosecoid, and the complete lack of early myocardial transcription factor genes nkx2.5 and mef2C expression between days 4 and 6 (Fig. 6A, second lanes). Expression of the cardiomyocyte specific gene mlc1v was delayed by 2 days and mhcα, also expressed in other cell types at later stages of development was not significantly affected. Expression of vimentin, a candidate protein to compensate desmin was not significantly altered in EBs of any genotype. As suggested by the delayed and reduced development of cardiomyocytes in EBs, desminΔ1−48 indeed affects mesodermal precursors of cardiomyocytes and differentiating cardiomyocytes.
Constitutive overexpression and synthesizes of desmin promoted differentiation of ESCs as evident from the earlier down-regulation of the stemness transcription factor gene oct3/4, and significantly promoted the expression of primitive mesoderm markers brachyury and goosecoid. Concomitantly within the limits of resolution, nkx2.5 were transiently up-regulated between days 4 and 6 followed by the transient up-regulation of mlc1v and mhcα on day 6 (Fig. 6A, fourth lanes). Statistical analysis of brachyury and nkx2.5 expression between day 4 and 6 in six independent experiments demonstrates a significant influence of desmin on brachyury expression in precardiac mesoderm and on nkx2.5 expression in early differentiating cardiomyocytes (Figs. 6B, 6C), suggesting that desmin influences cardiomyogenesis already in the primitive mesoderm before cardioblasts differentiate to functional cardiomyocytes.
Discussion
This study demonstrates an involvement of desmin in mechanisms regulating the very beginning of cardiomyogenesis and the expression of transcription factor genes important for mesodermal and early myocardial differentiation.
RSV promoter driven constitutive expression of desmin in ESCs before mesodermal cells become committed to cardiomyogenic lineages induced premature onset of cardiomyogenesis in EBs and resulted in 100% EBs with large and interwoven areas of cardiomyocytes. Mesodermal precursors of cardioblasts differentiated much faster in the presence of desmin. Cardiomyocytes were synchronously beating at twice the rate observed in wild-type EBs and had a perfect differentiated phenotype. Additionally, cardiomyocyte cluster size was significantly increased, which is indicative of increased proliferation of differentiating cardiomyocytes (Lauss et al., 2005), and suggesting that desmin not only fosters commitment but also proliferation of differentiating cardiomyocytes. Absence of any effect of conditioned cell culture supernatant from des+/+desect EBs on cardiomyogenesis in wild-type EBs suggests that desmin influences cardiomyogenesis in a cell autonomous manner. In the absence of desmin exactly the opposite phenotype was observed. Cardiomyogenesis was delayed by 1 day and cardiomyocyte clusters were composed of much less cells which did not beat synchronously and thus seem to be less well connected to each other.
Overexpression of desmin under the control of cardiac specific promoters activated only in committed cardiomyocytes (Wang et al., 2001; Haubold et al., 2003; Weisleder et al., 2004a) had no discernable effects on cardiomyocyte commitment and differentiation. This suggests that indeed it is the expression in mesodermal precursors that triggers increased cardiomyogenesis and subsequent formation of large clusters of cardiomyocytes, and not simply the fact that desmin is expressed from three instead of two alleles in cardiomyocytes.
The influence of desmin on the commitment of mesoderm to cardiomyogenic lineages was further supported by increased expression of mesodermal transcription factor genes brachyury and goosecoid and the faster down-regulation of the stemness gene oct3/4 in the presence of desmin. Concomitantly expression of the gene of the early myocardial transcription factor Nkx2.5 was up-regulated which led to the transient up-regulation of its downstream target gene mef2C (Jamali et al., 2001) and the mlc1 and mhcα genes. In line with these results, the desmin gene was expressed in EBs at day 4, at a time when first primitive mesodermal cells emerge from the inner primitive ectoderm of EBs (Kubo et al., 2004), and vice versa, absence of desmin caused a temporally reduced expression of brachyury and delayed the expression of mlc1v. Notably, at this developmental stage both in vitro and in vivo cardiomyocytes are the only differentiating muscle cells and are also the only cells expressing markers such as nkx2.5 and mef2C. This excludes a contribution to the observed expression patterns by other cell types. T-box transcription factors together with Nkx2.5 are known to synergistically promote cardiogenesis (Hiroi et al., 2001), and desmin has been shown to directly bind to DNA via its amino-terminus (Li et al., 2003; Tolstonog et al., 2005), thus it seems likely that desmin promotes cardiomyogenesis via the up-regulation of brachyury and nkx2.5.
In vivo desmin expression was detected in the premyocardial splanchnic mesoderm (Kuisk et al., 1996) lending physiological relevance to desmin’s influence on the expression of mesodermal and early myocardial transcription factor genes and its supporting role during early cardiomyogenesis in EBs in vitro. Having said that, these data also clearly demonstrate that desmin perse does not drive ESCs into the cardiomyogenic lineage. Pre-gastrulation-like development of extra-embryonic and primitive ectodermal lineages in EBs (Weitzer, 2006) seems to be indispensable for in vitro cardiomyogenesis in providing a still unknown set of specification factors secreted by other cell types.
To further support the hypothesis that desmin influences commitment and very early differentiation of cardioblast, we studied the effects of a mutant desmin protein negatively affecting cardiomyogenesis (Höllrigl et al., 2002). This allows determining whether desmin, not being detectable at this developmental stage in vivo, has to be functional at the time when mesodermal precursors are commitment to the cardiomyogenic lineages.
Indeed, homozygous expression of desminΔ1−48 in the absence of wild-type desmin caused a significant delay and attenuation of cardiomyogenesis in EBs. DesminΔ1−48 likely causes death of some mesodermal cells in day 5 EBs, however, may as well negatively influence proliferation of these cells. In addition, desminΔ1−48 partially inhibited expression of brachyury and goosecoid in mesodermal cells at day 4, and completely inhibited expression of nkx2.5, mef2C and mlc1v in differentiating cardiomyocytes at day 6. This demonstrates that mutant desmin influences developmental processes involved in the differentiation of cardiomyocytes significantly before genes of typical structural proteins like MHCα are expressed. Most importantly, desminΔ1−48 caused a phenotype which was much worse than that observed in des−/− EBs suggesting that this mutant protein actively interferes with the onset of cardiomyogenesis. The amino terminus of desmin, vimentin and glial fibrillary acidic protein has been demonstrated to mediate the interaction of cytoplasmic IF proteins with single-strand and double-strand DNA (dsDNA) (Shoeman et al., 2001; Shoeman et al., 2002; Tolstonog et al., 2005), thus desminΔ1−48 may well lack this function which might be important for differentiation of cardiomyocytes. Integration of too much mutant protein into type III filaments might alter their propensity to disassemble before cytokinesis in cardiomyocytes. However, toxicity of the mutant protein per se has been previously excluded by the fact that cardiomyogenesis and myogenesis was not affected in des+/Δ1−48 EBs (Höllrigl et al., 2002). Deregulation of myocardial differentiation and morphogenesis in desΔ1−48/Δ1−48 EBs may be either a consequence of a pathological function of the desmin protein lacking 48 amino-terminal amino acid residues, or due to the lack of an important function situated in the amino-terminal domain of desmin which is known to interact with several cytoskeletal proteins, such as ankyrin, plectin, and desmoplakin (Costa et al., 2004) and with dsDNA (Tolstonog et al., 2005).
During further development of desΔ1−48/Δ1−48 EBs, however, the number of cardiomyocyte clusters and the number of cells within the clusters was drastically reduced, the later suggesting a negative effect of desminΔ1−48 on proliferation or survival of differentiating cardiomyocytes. Cardiomyocytes had an aberrant morphology featuring reduced numbers of regular myofibrils. Sarcomere length in those myofibrils still forming was significantly reduced from an average of 1.98 ± 0.19 μm (N = 552) in des+/+desect, des+/+, and des−/− cardiomyocytes to 1.46 ± 0.25 mm (N= 225, p = 0.0001) in desΔ1−48/Δ1−48 cardiomyocytes. Although desminΔ1−48 rescued fusion of des−/− myoblasts (Höllrigl et al., 2002), it actively interfered with myofibrillogenesis in cardiomyocytes and caused reduced and arrhythmic contraction. Additionally, desmin formed aggregates, which co-stained for cTnT, suggesting that mutant desmin causes myofibril proteins to aggregate. Aggregation of myofibril proteins has been previously reported to cause apoptosis. Fragments of desmin induce apoptosis in cardiomyocytes (Chen et al., 2003) and desmin aggregation also impairs proteolytic function of cardiomyocytes (Liu et al., 2006). In accordance with these results we observed an increased rate of apoptosis in desΔ1−48/Δ1−48 EBs at day 16 when in vitro cardiomyogenesis comes to a halt and cardiomyocytes slowly stop to contract in wild-type EBs. Only a small number of cTnT-positive cardiomyocytes had TUNEL assay-positive nuclei but a significant number of nuclei in neighboring cells were TUNEL assay positive or showed signs of micronuclei formation and nuclear chromatin aggregation. Because of the defined location of these cells within EBs (Weitzer, 2006) it is very likely that these cells had been cardiomyocytes which had already lost their cTnT protein in the course of apoptosis. Finally, in agreement with findings in desmin null mice (Weisleder et al., 2004b) apoptosis was also increased in matured des−/− cardiomyocytes, supporting the current working model for desmin as an essential protein for structural integrity and function of cardiomyocytes (Capetanaki, 2002; Paulin and Li, 2004) and suggesting that the integrity of the amino-terminal domain which mediates desmin’s interaction with DNA is key to the proper function of desmin in terminally differentiated cardiomyocytes.
In conclusion, we have found that desmin influences early cardiomyogenesis in EBs at the molecular and cellular level. Desmin modulates the expression of mesodermal and early myocardial genes and promotes proliferation of differentiating cardiomyocytes in addition to its well-documented role in the maintenance of the muscle cell phenotype. These data provide evidence to the hypothesis that desmin has a supportive role during commitment and early differentiation of the cardiomyocytes.
Acknowledgments
We thank Karin Habegger and Sabine Enzinger for technical assistance, Thomas Sauer for FACS analysis, Allan Bradley for the AB2.2 ESCs, and Yassemie Capetanaki in whose laboratory the desΔ1−48/Δ1−48 and des−/− ESCs were generated. This work was supported by funds from the Austrian Federal Ministry of Education, Science and Culture (GZ70.078/0002-Pr/472002), the Austrian Fonds zur Förderung der wissenschaftlichen Forschung, grants P11189, P15303 and P18659 and the Hochschuljubiläumsstiftung der Stadt Wien, grant H-933/2003.
References
- Bader A, Al-Dubai H, Weitzer G. Leukemia inhibitory factor modulates cardiogenesis in embryoid bodies in opposite fashions. Circ Res. 2000;86:787–794. doi: 10.1161/01.res.86.7.787. [DOI] [PubMed] [Google Scholar]
- Balogh J, Li Z, Paulin D, Arner A. Lower active force generation and improved fatigue resistance in skeletal muscle from desmin deficient mice. J Muscle Res Cell Motil. 2003;24:453–459. doi: 10.1023/a:1027353930229. [DOI] [PubMed] [Google Scholar]
- Balogh J, Merisckay M, Li Z, Paulin D, Arner A. Hearts from mice lacking desmin have a myopathy with impaired active force generation and unaltered wall compliance. Cardiovasc Res. 2002;53:439–450. doi: 10.1016/s0008-6363(01)00500-4. [DOI] [PubMed] [Google Scholar]
- Capetanaki C. Desmin cytoskeleton in healthy and failing heart. Heart Fail Rev. 2000;5:203–220. doi: 10.1023/A:1009853302447. [DOI] [PubMed] [Google Scholar]
- Capetanaki Y. Desmin cytoskeleton: a potential regulator of muscle mitochondrial behavior and function. Trends Cardiovasc Med. 2002;12:339–348. doi: 10.1016/s1050-1738(02)00184-6. [DOI] [PubMed] [Google Scholar]
- Chen F, Chang R, Trivedi M, Capetanaki Y, Cryns VL. Caspase proteolysis of desmin produces a dominant-negative inhibitor of intermediate filaments and promotes apoptosis. J Biol Chem. 2003;278:6848–6853. doi: 10.1074/jbc.M212021200. [DOI] [PubMed] [Google Scholar]
- Clark KA, McElhinny AS, Beckerle MC, Gregorio CC. Striated muscle cytoarchitecture: an intricate web of form and function. Annu Rev Cell Dev Biol. 2002;18:637–706. doi: 10.1146/annurev.cellbio.18.012502.105840. [DOI] [PubMed] [Google Scholar]
- Costa ML, Escaleira R, Cataldo A, Oliveira F, Mermelstein CS. Desmin: molecular interactions and putative functions of the muscle intermediate filament protein. Braz J Med Biol Res. 2004;37:1819–1830. doi: 10.1590/s0100-879x2004001200007. [DOI] [PubMed] [Google Scholar]
- Haubold K, Herrmann H, Langer SJ, Evans RM, Leinwand LA, Klymkowsky MW. Acute effects of desmin mutations on cytoskeletal and cellular integrity in cardiac myocytes. Cell Motil Cytoskeleton. 2003;54:105–121. doi: 10.1002/cm.10090. [DOI] [PubMed] [Google Scholar]
- Hiroi Y, Kudoh S, Monzen K, Ikeda Y, Yazaki Y, Nagai R, Komuro I. Tbx5 associates with Nkx2-5 and synergistically promotes cardiomyocyte differentiation. Nat Genet. 2001;28:276–280. doi: 10.1038/90123. [DOI] [PubMed] [Google Scholar]
- Höllrigl A, Hofner M, Stary M, Weitzer G. Differentiation of cardiomyocytes requires functional serine residues within the amino-terminal domain of desmin. Differentiation, same issue as this publication. 2007 doi: 10.1111/j.1432-0436.2007.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höllrigl A, Puz S, Al-Dubai H, Kim JU, Capetanaki Y, Weitzer G. Amino-terminally truncated desmin rescues fusion of des(− / −) myoblasts but negatively affects cardiomyogenesis and smooth muscle development. FEBS Lett. 2002;523:229–233. doi: 10.1016/s0014-5793(02)02995-2. [DOI] [PubMed] [Google Scholar]
- Jamali M, Rogerson PJ, Wilton S, Skerjanc IS. Nkx2-5 activity is essential for cardiomyogenesis. J Biol Chem. 2001;276:42252–42258. doi: 10.1074/jbc.M107814200. [DOI] [PubMed] [Google Scholar]
- Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, Fehling HJ, Keller G. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- Kuisk IR, Li H, Tran D, Capetanaki Y. A single MEF2 site governs desmin transcription in both heart and skeletal muscle during mouse embryogenesis. Dev Biol. 1996;174:1–13. doi: 10.1006/dbio.1996.0046. [DOI] [PubMed] [Google Scholar]
- Lauss M, Stary M, Tischler J, Egger G, Puz S, Bader-Allmer A, Seiser C, Weitzer G. Single inner cell masses yield embryonic stem cell lines differing in lifr expression and their developmental potential. Biochem Biophys Res Commun. 2005;331:1577–1586. doi: 10.1016/j.bbrc.2005.04.068. [DOI] [PubMed] [Google Scholar]
- Li G, Tolstonog GV, Traub P. Interaction in vitro of type III intermediate filament proteins with Z-DNA and B−Z-DNA junctions. DNA Cell Biol. 2003;22:141–169. doi: 10.1089/104454903321655783. [DOI] [PubMed] [Google Scholar]
- Li H, Capetanaki Y. Regulation of the mouse desmin gene: transactivated by MyoD, myogenin, MRF4 and Myf5. Nucleic Acids Res. 1993;21:335–343. doi: 10.1093/nar/21.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Choudhary SK, Milner DJ, Munir MI, Kuisk IR, Capetanaki Y. Inhibition of desmin expression blocks myoblast fusion and interferes with the myogenic regulators MyoD and myogenin. J Cell Biol. 1994;124:827–841. doi: 10.1083/jcb.124.5.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Colucci-Guyon E, Pinçon-Raymond M, Mericskay M, Pournin S, Paulin D, Babinet C. Cardiovascular lesions and skeletal myopathy in mice lacking desmin. Dev Biol. 1996;175:362–366. doi: 10.1006/dbio.1996.0122. [DOI] [PubMed] [Google Scholar]
- Li ZL, Mericskay M, Agbulut O, Butlerbrowne G, Carlsson L, Thornell LE, Babinet C, Paulin D. Desmin is essential for the tensile strength and integrity of myofibrils but not for myogenic commitment, differentiation, and fusion of skeletal muscle. J Cell Biol. 1997;139:129–144. doi: 10.1083/jcb.139.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Chen Q, Huang W, Horak KM, Zheng H, Mestril R, Wang X. Impairment of the ubiquitin-proteasome system in desminopathy mouse hearts. FASEB J. 2006;20:362–364. doi: 10.1096/fj.05-4869fje. [DOI] [PubMed] [Google Scholar]
- Masino AM, Gallardo TD, Wilcox CA, Olson EN, Williams RS, Garry DJ. Transcriptional regulation of cardiac progenitor cell populations. Circ Res. 2004;95:389–397. doi: 10.1161/01.RES.0000138302.02691.be. [DOI] [PubMed] [Google Scholar]
- Milner DJ, Weitzer G, Tran D, Bradley A, Capetanaki Y. Disruption of muscle architecture and myocardial degeneration in mice lacking desmin. J Cell Biol. 1996;134:1255–1270. doi: 10.1083/jcb.134.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EN. A decade of discoveries in cardiac biology. Nat Med. 2004;10:467–474. doi: 10.1038/nm0504-467. [DOI] [PubMed] [Google Scholar]
- Paulin D, Li Z. Desmin: a major intermediate filament protein essential for the structural integrity and function of muscle. Exp Cell Res. 2004;301:1–7. doi: 10.1016/j.yexcr.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Sam M, Shah S, Friden J, Milner DJ, Capetanaki Y, Lieber RL. Desmin knockout muscles generate lower stress and are less vulnerable to injury compared with wild-type muscles. Am J Physiol Cell Physiol. 2000;279:C1116–C1122. doi: 10.1152/ajpcell.2000.279.4.C1116. [DOI] [PubMed] [Google Scholar]
- Shah SB, Davis J, Weisleder N, Kostavassili I, McCulloch AD, Ralston E, Capetanaki Y, Lieber RL. Structural and functional roles of desmin in mouse skeletal muscle during passive deformation. Biophys J. 2004;86:2993–3008. doi: 10.1016/S0006-3495(04)74349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shardonofsky FR, Capetanaki Y, Boriek AM. Desmin modulates lung elastic recoil and airway responsiveness. Am J Physiol Lung Cell Mol Physiol. 2006;290:L890–L896. doi: 10.1152/ajplung.00397.2005. [DOI] [PubMed] [Google Scholar]
- Shoeman RL, Hartig R, Berthel M, Traub P. Deletion mutagenesis of the amino-terminal head domain of vimentin reveals dispensability of large internal regions for intermediate filament assembly and stability. Exp Cell Res. 2002;279:344–353. doi: 10.1006/excr.2002.5618. [DOI] [PubMed] [Google Scholar]
- Shoeman RL, Huttermann C, Hartig R, Traub P. Amino-terminal polypeptides of vimentin are responsible for the changes in nuclear architecture associated with human immuno-deficiency virus type 1 protease activity in tissue culture cells. Mol Biol Cell. 2001;12:143–154. doi: 10.1091/mbc.12.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjuve R, Arner A, Li Z, Mies B, Paulin D, Schmittner M, Small JV. Mechanical alterations in smooth muscle from mice lacking desmin. J Muscle Res Cell Motil. 1998;19:415–429. doi: 10.1023/a:1005353805699. [DOI] [PubMed] [Google Scholar]
- Thornell L, Carlsson L, Li Z, Mericskay M, Paulin D. Null mutation in the desmin gene gives rise to a cardio-myopathy. J Mol Cell Cardiol. 1997;29:2107–2124. doi: 10.1006/jmcc.1997.0446. [DOI] [PubMed] [Google Scholar]
- Tolstonog GV, Li G, Shoeman RL, Traub P. Interaction in vitro of type III intermediate filament proteins with higher order structures of single-stranded DNA, particularly with G-quadruplex DNA. DNA Cell Biol. 2005;24:85–110. doi: 10.1089/dna.2005.24.85. [DOI] [PubMed] [Google Scholar]
- Trahair T, Yeoh T, Cartmill T, Keogh A, Spratt P, Chang V, dos Remedios CG, Gunning P. Myosin light chain gene expression associated with disease states of the human heart. J Mol Cell Cardiol. 1993;25:577–585. doi: 10.1006/jmcc.1993.1067. [DOI] [PubMed] [Google Scholar]
- Wang X, Osinska H, Dorn GW, Nieman M, Lorenz JN, Gerdes AM, Witt S, Kimball T, Gulick J, Robbins J. Mouse model of desmin-related cardiomyopathy. Circulation. 2001;103:2402–2407. doi: 10.1161/01.cir.103.19.2402. [DOI] [PubMed] [Google Scholar]
- Weisleder N, Soumaka E, Abbasi S, Taegtmeyer H, Capetanaki Y. Cardiomyocyte-specific desmin rescue of desmin null cardiomyopathy excludes vascular involvement. J Mol Cell Cardiol. 2004a;36:121–128. doi: 10.1016/j.yjmcc.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Weisleder N, Taffet GE, Capetanaki Y. Bcl-2 over-expression corrects mitochondrial defects and ameliorates inherited desmin null cardiomyopathy. Proc Natl Acad Sci USA. 2004b;101:769–774. doi: 10.1073/pnas.0303202101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzer G. Embryonic stem cell-derived embryoid bodies: an in vitro model of eutherian pregastrulation development and early gastrulation. Handbook Exp Pharmacol. 2006;174:21–51. [PubMed] [Google Scholar]
- Weitzer G, Milner DJ, Kim JU, Bradley A, Capetanaki Y. Cytoskeletal control of myogenesis: a desmin null mutation blocks the myogenic pathway during embryonic stem cell differentiation. Dev Biol. 1995;172:422–439. doi: 10.1006/dbio.1995.8070. [DOI] [PubMed] [Google Scholar]
- Weitzer G, Wiche G. Plectin from bovine lenses. Chemical properties, structural analysis and initial identification of interaction partners. Eur J Biochem. 1987;169:41–52. doi: 10.1111/j.1432-1033.1987.tb13578.x. [DOI] [PubMed] [Google Scholar]