Abstract
The induction of teratoma in mice by the transplantation of stem cells into extra-uterine sites has been used as a read-out for cellular pluripotency since the initial description of this phenomenon in 1954. Since then, the teratoma assay has remained the assay of choice to demonstrate pluripotency, gaining prominence during the recent hype surrounding human stem cell research. However, the scientific significance of the teratoma assay has been debated due to the fact that transplanted cells are exposed to a non-physiological environment. Since many mice are used for a result that is heavily questioned, it is time to reconsider the teratoma assay from an ethical point of view. Candidate alternatives to the teratoma assay comprise the directed differentiation of pluripotent stem cells into organotypic cells, differentiation of cells in embryoid bodies, the analysis of pluripotency-associated biomarkers with high correlation to the teratoma forming potential of stem cells, predictive epigenetic footprints, or a combination of these technologies. Each of these assays is capable of addressing one or more aspects of pluripotency, however it is essential that these assays are validated to provide an accepted robust, reproducible alternative. In particular, the rapidly expanding number of human induced pluripotent stem cell lines, requires the development of simple, affordable standardized in vitro and in silico assays to reduce the number of animal experiments performed.
Introduction
The experimental induction of teratoma (for definition of terms see Box 1) in mammals, mostly mice, has been carried out for decades (Evans and Kaufman, 1981; Skreb et al., 1971; Solter et al., 1970; Stevens, 1958, 1970; Stevens & Little, 1954). In stem cell research and banking (ISCBI, 2009) the in vivo teratoma assay can be used to demonstrate the pluripotency of the stem cells in vivo (Gertow et al., 2007; Wesselschmidt, 2011). Basically stem cells, which are considered to be pluripotent, are injected into various anatomical sites e.g. sub-cutaneous, intra-muscular, under the capsule of the kidney, or intra-testicular, of immunocompromised mice potentially developing into an experimental tumor (also see Box 1). The assayed cells are considered pluripotent if the resultant tumor shows characteristics of a teratoma, demonstrating the development of differentiated cells from all three germ layers, namely ectoderm (such as nerve and skin), mesoderm (including bone, cartilage and muscle), and endoderm (liver and gut) (Brivanlou et al., 2003).
Box 1. Explanation of terms used in this paper according to the NIH definitions.
Teratoma — a multi-layered benign tumor that grows from pluripotent cells injected into animals with a dysfunctional immune system. Scientists test whether they have established a human embryonic stem (hES) cell line by injecting putative stem cells into such mice and verifying that the resulting teratoma contains cells derived from all three embryonic germ layers.
Teratocarcinoma — a multi-layered malignant tumor that contains in addition to a teratoma embryonal carcinoma cells, which either give rise to metastases or produce a malignant tumor after re-implantation of the primary tumor mass. The WHO recommended for this type of tumor the more complicated term “mixed embryonal carcinoma and teratoma”. A detailed consideration of the difference between teratoma and teratocarcinoma can be found in a series of comments in Nature Biotechnology Vol. 25, No. 11 (Damjanov and Andrews, 2007). In a teratoma assay development of this type of tumor would immediately lead to the exclusion of the putative pluripotent stem cells from any type of therapeutical application.
Severe combined immunodeficient (SCID) mice — SCID mice are important tools for researching hematopoiesis, innate and adaptive immunity, autoimmunity, infectious diseases, cancer, vaccine development, and regenerative medicine in vivo. So-called because of their severe combined immunodeficiency, SCID mice have reduced ability to reject allogeneic or xenogeneic tissue grafts, and are therefore excellent hosts for human cells and tissues.
Teratoma assay — in this assay putative pluripotent stem cells are implanted into SCID mice where they can proliferate and differentiate to form a teratoma. The pluripotent stem cells grow at the implantation site and are supported by factors of the local milieu and also circulating factors. After a certain time, when the tumor has reached sufficient size, it is removed and subjected to histopathological analysis, immunocytochemistry and gene expression profiling.
Tetraploid complementation assay — an assay that can be used to test a stem cell’s potency. Fusing two 2-cell embryos produces cells with 4 sets of chromosomes (tetraploid cells) that are biased toward developing into extra-embryonic tissues only. The tetraploid cells are not able to generate a developmentally competent embryo itself; however, an embryo can develop properly from “sandwiched” diploid stem cells in case, the injected cells are pluripotent.
Although used regularly and frequently demanded by reviewers of manuscripts as proof of pluripotency, the teratoma assay has never been standardized in terms of graft site, age of mice, number of cells implanted and the cell preparation for a large number of pluripotent cell lines. These factors invariably influence the development of the teratoma (Hentze et al., 2009; Wesselschmidt, 2011). Gropp et al. recently presented a systematic evaluation of some of these factors for two ESC lines (Gropp et al., 2012). In addition to the lack of standardization of the teratoma assay, the assay is also regarded as time, cost and labor intensive. The assay certainly raises ethical concerns, as it may induce pain and suffering of the animals used in the assay. This latter concern impacts on the current legislation on animal welfare and the following section will give an overview on the current status in Europe.
From the animal welfare perspective the teratoma assay raises two major issues: first, the inoculation of genetically manipulated animals with potentially malignant cells that could initiate tumors, and second, the breeding of experimental animals, especially if associated with the suffering and pain of the animal. A classification system, comprising the degree of pain, suffering and distress, was accepted in 1995 and has been in general use throughout Europe. This system is known as the Severity Catalogue of the Swiss Federal Veterinary Office. In the European Union (EU), a binding severity classification system was approved in 2010 as Annex VIII of the new “EU Directive 2010/63/EU for the Protection of Animals used in Scientific Procedures” (European Union, 2010). Accordant regulations and amendments are published by the Canadian Council on Animal Care in Science, the Australian and New Zealand Council for the Care of Animals in Research and Teaching, and in the U.S.A, under the Laboratory Animal Welfare Act.
The Swiss Catalogue considers tumor models as moderately severe (grade 2) and severe (grade 3) procedures. The grade 2 classification represents tumor models, in which the induction or transplantation of tumors does not cause cancerous cachexia or other progressively lethal disease, or models which are discontinued before clinically manifest dysfunctions occur in the animal (e.g. the tumor model in mice and rats). Grade 3 covers tumor models that induce cancerous cachexia or other progressive lethal diseases (Swiss Federal Veterinary Office, 2012). Analogous classifications in the future EU catalogue are moderate and severe. Classification as moderate denotes models of induction of tumors or spontaneous tumors, that are expected to cause moderate pain, distress or moderate interference with normal behavior. Classification as severe refers to models with induction of tumors, that are expected to cause progressive lethal disease associated with long-lasting moderate pain, distress or suffering like tumors causing cachexia, invasive bone tumors, tumors causing metastatic spread, and tumors that are allowed to ulcerate. Taking this classification system into account the actual teratoma assay depends on the implementation of humane endpoints. The growth of one or more tumors to a typical weight of 1–2 g or up to 10% of the body weight would classify as moderate severity. If teratoma were allowed to grow beyond this point severity may increase to grade 3.
In 1959, William Russell and Rex Burch classified humane animal experimental techniques under the headings of replacement, reduction, and refinement – now commonly known as the three Rs. Replacement means the complete substitution of a given animal experiment by one or several alternative tests that, singly or taken together, will supply the needed information, e.g. in vitro experiments, computer modeling, analysis of expression profile, proteome and epigenetic alterations. Reduction refers to animal numbers, which must be kept as low as possible yet still being consistent with the delivery of robust statistical data. Other ways of reducing animal numbers are the avoidance of duplication of experiments performed by other scientists, and the combination of endpoints in toxicology testing. Refinement is the alleviation of experimental severities, e.g. animal-friendly housing and care, use of analgesics, and in general, keeping suffering to a minimum. This includes the setting of humane endpoints (Russell and Burch, 1959). Nowadays, the principles of the 3Rs are widely adopted and the concepts have been incorporated into the legal framework on animal experimentation in several countries, i.e. the German animal welfare act and the Austrian law for animal protection (German Animal Welfare Act, §7 and Animal Protection Act, StF: BGBl. I Nr.80/2010, http://www.ris.bka.gv.at/Dokument.wxe?Abfrage=BgblAuth&Dokumentnummer=BGBLA_2010_I_80 (02.27.2012)). Both the current effective EU Directive on the Protection of Animals Used for Scientific purposes (86/609/EEC) and its revised version (2010/63/EU), repeatedly refer to the 3Rs (2010/63/EU, preamble 11; 27; 31; 38; 39; 48; 49; Arts. 1a; 4; 27; 38; 39; 43; 48; 58; Annexes 5 and 6). In particular, “the use of animals for scientific or educational purposes should […] only be considered where a non-animal alternative is unavailable”, and “when choosing methods, the principles of replacement, reduction and refinement should be implemented through a strict hierarchy of the requirement to use alternative methods” (2010/63/EU, preamble 10; 11; 12 etc.; Art 4 http://ec.europa.eu/foods/fs/aw/aw_legislation/scientific/86-609-eec_en.pdf).
Teratoma assays — when and why?
Safety testing of transplants derived from pluripotent stem cells
Stem cell research has developed in part from tumor research on teratocarcinoma cell lines (Martin and Evans, 1975), therefore, the teratoma assay was originally a tumor assay before it became a useful technique to demonstrate the pluripotency of stem cell lines (Peterson et al., 2011). It is well known that pluripotency and tumorigenicity are closely related phenomena in stem cells (Knoepfler, 2009). The teratoma assay is not only a pluripotency assay but it is also an assay for tumorigenicity. The assay is required for the investigation of the tumor biology of teratoma and it can also be used to address additional questions in developmental or tumor biology (Li et al., 2009). Experimental teratoma can, e.g. provide insights into the in vivo development of human tissues (Gertow et al., 2011; Müller et al., 2010). It is largely unknown which host factors guide the tissue differentiation in teratoma and help to create three-dimensional tissue structures. Further animal studies on these aspects of teratoma growth will likely provide important information for the development of new in vitro tissue differentiation protocols. These protocols might in the end even help to replace the teratoma assay for pluripotency testing by improved in vitro assays.
The teratoma assay as a tumorigenicity assay is of major importance to address safety issues of new stem cell-based therapies or transplantation. The injection of pluripotent stem cell lines into immunodeficient or syngeneic recipients leads usually to growth of benign teratomas (Dressel, 2011), however also the occurrence of teratocarcinomas (see Box 1) that infiltrate tissues and give rise to metastases has been reported after transplantation of some stem cell lines (Erdö et al., 2003). The close link of pluripotency and tumorigenicity is a major challenge for regenerative medicine since it is one important concept of regenerative medicine to generate cells or tissues in vitro from pluripotent stem cells that can be transplanted to replace diseased tissues in patients. Notably, any graft that is derived from pluripotent stem cells is at risk of containing tumorigenic cells. Numbers of pluripotent stem cells as low as 20 for mouse (Lawrenz et al., 2004) and 245 for human embryonic stem cells (Hentze et al., 2009) were reported to form tumors in immunodeficient hosts. The comparison of these numbers illustrates the enormous challenge to provide grafts from pluripotent stem cells that do not contain tumorigenic cells. Importantly, grafts should be free not only of teratoma forming cells but also of other cells leading to tumors of more restricted tissue composition or even only tissue overgrowth (Mauritz et al., 2011). For these reasons, the teratoma assay or transplantation protocols, which could involve the formation of teratoma or other stem cell-derived tumors in experimental animals, will remain important to study the safety of new therapies that are based on stem cells.
Teratoma assays could also identify pluripotent stem cell lines with a lower intrinsic tumorigenic risk compared to others. Notably, the tumorigenic potential of a stem cell line or a transplant derived from stem cells is not sufficiently described by features of these cells. Host factors that support tumor growth need to be identified as well as factors that can reduce the risk of tumor formation after stem cell transplantation therapy. These host factors could include a number and range of factors such as hormones, growth factors, and cytokines, interactions with the extracellular matrix and host cells, paracrine effects of host cells, vascularization, and provision of nutritive factors. The immune system of recipients can contribute to the rejection of tumorigenic (Dressel et al., 2008) but also therapeutically effective cells (Saric et al., 2008). Therefore, how host factors influence tumor risk and the engraftment of stem cell-derived transplants needs to be studied in greater depth.
Some of these questions can be answered in vitro but others will require experiments in animal hosts. As stem cell-based therapies are developed, animal experiments will be required to demonstrate the lack of tumorigenic potential of these grafts before the start of clinical studies. It is a regulatory requirement for any new therapy that involves the transplantation of stem cell-derived grafts to demonstrate convincingly by animal experiments that the grafts are not at a detectable risk of tumor formation (Halme and Kessler, 2006). In conclusion, the teratoma assay or variations of the teratoma assay are used to address research questions that differ from the basic assessment of the pluripotency of stem cells and could not be answered using alternative methods designed to assess the pluripotency of stem cells.
The teratoma assay is used as an in vivo method to test pluripotency of cells, namely the ability of those cells to generate cells/tissue of all three germ layers. If the teratoma assay is really needed to characterize a new cell line, there is an urgent requirement to analyze the teratoma beyond the simple identification of tissues from all three germ layers, as this would provide a tremendous amount of additional information, e.g. embryonic development, differentiation potential, maturation status (Gertow et al., 2011). Although some potent in vitro models exist, it must be stated that the teratoma assays may lead to new insights into the interaction between the host and the injected stem cells and/or their in vivo differentiation products, which would have not been found in an ab initio designed in vitro model (Dressel et al., 2008).
Why are teratoma assays questioned?
Despite the ‘gold standard’ status of this assay, there is little consistency in either the methodology used or the reporting of results. Standardization may aid stem cell researchers to evaluate better and compare results across different reprogramming strategies and differentiation protocols. Unfortunately the methods used for inducing teratoma are poorly documented, frequently only by citing other publications. Screening the literature for more than 1200 original manuscripts that were published between 1998 and 2009 in journals, indexed in the NCBI Medline, describing research on hESCs, as well as 124 original articles between 2007 and 2009 that report on human iPSCs, revealed that the assay certainly lacks standardization. The description of the teratoma assay varied widely and therefore we were not able to classify them in groups. As an example, the number of injected cells varied from clumps of 200–300 cells to 5 million cells in different manuscripts (Müller et al., 2010). As mentioned above, the niche/microenvironment influences the survival and differentiation of the injected pluripotent cells. It was shown that the injection site and the grade of immunodeficiency of the host strongly influence the survival or differentiation of cells (Dressel, 2011).
To standardize this relatively simple assay, parameters such as the strain of mouse used, the number of cells injected, the passage of injected cells, the number of injections per animal, the cell harvest method, the solution for the injection of cells, as well as the time in vivo should be provided. Additionally, the histomorphological analysis and the format of the results vary across the studies. In most cases teratoma is examined by classic histological methods via hematoxylin/eosin stainings, although immunohistochemistry can be helpful and sometimes even indispensable to quantify and definitely identify tissue types. An improvement to the teratoma assay, if required, would be to take biopsies in order to establish a time point at which cells of all three germ layers can be demonstrated. At this point the assay should be terminated to avoid the development of large tumors and prevent suffering to the animal. It may be possible therefore to establish cell growth and differentiation kinetics that ultimately serve to keep the period of tumor growth to a minimum.
Animal welfare and ethical concerns
The greatest disadvantage of the teratoma assay is that it requires the use of experimental animals. According to current legislation animal experiments must be ethically justifiable. Such a justification is generally based on a cost–benefit analysis, in which the suffering of the animals is to be weighed against the potential benefits for research and scientific significance of the results obtained. According to Article 12 (2) of the EU Council Directive 86/609/EEC for the protection of experimental animals the following aspects have to be considered: “where it is planned to subject an animal to an experiment in which it will, or may, experience severe pain which is likely to be prolonged, that experiment must be specifically declared and justified to, or specifically authorized by, the authority.” The following sentence of this article emphasizes that in case of potentially prolonged severe pain the particular experiment, not the overall research goal, must meet high scientific requirements: “The authority shall take appropriate judicial or administrative action if it is not satisfied that the experiment is of sufficient importance for meeting the essential needs of man or animal.” In the future, European legislation will go beyond these demands. Article 15 (2) of EU Directive 2010/63/EU lays down, as a general rule, “that a procedure is not performed if it involves severe pain, suffering or distress that is likely to be long-lasting and cannot be ameliorated.” In most countries of the western hemisphere the teratoma assays, as animal experiments in general, are performed in accord with these regulations. Teratoma is not allowed to grow to an excessive size and experiments are terminated before severe pain, suffering, or distress occurs. Nonetheless, there is a need to find alternatives for the in vivo teratoma assay to reduce the need for animal experimentation.
The second issue regarding animal welfare is the breeding of immune deficient mammals, which leads to highly controversial discussions in both the public and scientific communities. Some ethicists argue that experimental animals have an intrinsic value independent of their use by humans and that their dignity and rights should be respected. An appreciation of the inherent value of animals means that no genetic manipulation should be carried out at all (Vorstenbosch, 1993), unless a basic or very serious human or animal interest is involved, which cannot be met by any other means (Verhoog, 1992). However, many of the immune deficient mice used for teratoma assays do not result from a genetic manipulation but occurred as natural mutations. Nevertheless, all SCID mice are lacking major elements of their immune system. Infections that are not harmful to normal/healthy animals, can cause suffering or death in SCID mice. It should be noted that SCID mice are bred under conditions that usually prevent those infections.
In some countries the generation of genetically modified animals is legally restricted. For example, the German Animal Welfare Act, Article 11b states that it is prohibited to breed vertebrates, or to change them through procedures of biotechnology, if this results in animals or their offspring, lacking parts of the body or organs for species-specific use or if they are unfit or deformed, thereby causing pain, suffering or harm. Although animal experiments are exempted from this restriction, the law still highlights an awareness of the ethical problems of breeding such animals.
Alternatives to the teratoma assay — their advantages and disadvantages
Proof of pluripotency
Stem cells exhibit some unique characteristics such as the ability to self-renew, as well as to differentiate into cell types of all three germ layers. They have been derived from embryos and different sources of postnatal animals. It is logical to classify stem cells based on their developmental potential (Table 1). Embryonic and induced pluripotent stem cells represent the most prominent examples of pluripotent cells, bearing the second highest degree of developmental potential. These cells can give rise to tissue types in vivo as well as in vitro, but they are not able to form the extraembryonic trophoblast lineage (Rossant, 2008).
Table 1. The various levels of cellular developmental potential.
| Totipotency | Potential to give rise to a functional organism with all its cell lineages. In mammals exclusively the zygote and the first four to eight blastomeres are totipotent. |
| Pluripotency | Potential to give rise to all somatic lineages of the body; e.g. embryonic stem cells and induced pluripotent stem cells. |
| Multipotency | Ability of adult stem cells to form multiple cell types of one lineage; e.g. hematopoietic stem cells. |
| Unipotency | Cells form one cell type; e.g. spermatogonial stem cells, which at least under natural conditions, are only able to generate sperms. |
Attributes such as the pluripotentcy and differentiation potential are based on experimental criteria and need to be thoroughly addressed via functional and molecular assays. Therefore, approaches to increase the stringency of results should be applied. From the standpoint of developmental biology many researchers regard in vitro differentiation e.g. in embryoid bodies (EBs) as the least stringent functional test of pluripotency of cultured stem cells. The generation of teratoma is perceived as being the next level of stringency. While these two approaches are suitable for stem cells of animal and human origin, they are limited since they do not test the ability of the cells to undergo normal development. The hESCs used in these assays are regarded as pluripotent since for ethical reasons these cells cannot be tested in primates to demonstrate that they are indeed pluripotent and give rise to all the tissues. So far this functional assay can only be demonstrated for murine and rat stem cells via chimera formation and germ line contribution. The most stringent test for developmental potential is achieved via the aggregation of stem cells with tetraploid host morulae (Eggan et al., 2001; Nagy et al., 1990). This approach results in animals exclusively derived from the donor cells because the 4n host cells will exclusively give rise to the trophectoderm. These “all ES” and the recently described “all iPS” (Boland et al., 2009; Kang et al., 2009; Stadtfeld et al., 2010; Zhao et al., 2009) embryos or animals avoid the formation of a chimera originating from both donor and host cells. For iPS cell lines in vitro tests demonstrating the upregulation of endogenous pluripotency markers as well as the silencing of the transgenes have to be performed. The latter set of testing can be overcome using novel non-genetic approaches for reprogramming such as RNA or protein transfection as well as administration of small molecules (Stadtfeld and Hochedlinger, 2010).
Potential alternatives to teratoma assays are i) the characterization of the expression of pluripotency markers e.g. Oct-4, Nanog, Sox2 (Fong et al., 2008; Mitsui et al., 2003; Pesce and Scholer, 2001), ii) status of the epigenome, iii) in vitro differentiation, either spontaneous or directed, and iv) computer-prediction models, or combinations of these (Table 2). The expression of pluripotency-associated markers may provide a good initial tool to determine the extent of pluripotency. Markers such as TRA-1-60, DNMT3B, and REX1 correlate with the teratoma forming potential of iPS cells (Chan et al., 2009). Yet, teratocarcinoma cell lines, genetically abnormal hESC and iPSC cultures, as well as epigentically irregular, e.g. partially reprogrammed cell lines, do frequently express the same markers at comparable levels (Chan et al., 2009; Müller et al., 2008). Although a selected number of these expression markers may provide a first good predictive value, these criteria are still rather subjective and at the moment it is too early to use these as the sole prediction criterion. Before these markers can be generally applied as a tool to determine pluripotency a thorough validation using many more embryonic stem cell lines and self-renewing somatic stem cells from different species is required. However, together with results derived from more objective experiments such as in vitro differentiation assays, the pluripotency state of stem cells may become predictable.
Table 2. Alternative methods for testing the pluripotency of stem cells.
| System | Assays |
|---|---|
| ES cell culture | Molecular profiling by genomics, epigenomics, proteogenomics and glycomics. |
| Embryoid bodies | Differentiation models assaying spontaneous differentiation, directed differentiation, and special assays such as vascularization or wound healing. |
| In silico models | Computer based models and genome wide data sets obtained from microarrays and next-generation sequencing. |
| Alternative in vivo models | Chicken egg model. |
| Organotypic models | In situ analysis such as skin models, “stripped organ” models, and re-aggregation/integration assays. |
Epigenomic footprints, such as DNA methylation and histone modifications, may be exploited for this as was shown in the same study (Boulting et al., 2011). Loss of chromatin remodeling complex proteins, for example, led to lethality at the blastocyst stage. This supposes that epigenetic rearrangements, to keep pluripotency, need to take place before the formation of the ICM (Cao and Zhang, 2004; Houlard et al., 2006; Klochendler-Yeivin et al., 2000). The dynamic nature of chromatin is specific to pluripotent cells and upon differentiation this changes to a more structured condensed and heterochromatic genome. Basically the euchromatic pluripotent stem cells change from an acetylated histone H3 and H4 environment to increased global levels of trimethylated lysine 9 H3 leading to gene repression, when cells start to differentiate (Atkinson and Armstrong, 2008; Kimura et al., 2004; Lee et al., 2004). Promoter regions of Nanog and Oct3/4 are enriched for acetylation of H4 and trimethylated lysine 4 of H3, where they are active. On the other hand these modifications are absent in the trophectoderm and instead enriched for methylated lysine 9 of H3 to keep them silent (Atkinson and Armstrong, 2008).
In vitro differentiation — directed and spontaneous differentiation
ESCs can be induced to differentiate to most cell types via the aggregation of ESCs in hanging drop cultures, in multi-well plates or in suspension culture (Wobus et al., 1984). Based on this data, more and more examples of directed differentiation emerge, which no longer rely on the plethora of unknown signals, which induce differentiation in EBs. Neurospheres containing neural stem cells can be generated and used to study the neural differentiation program (Ferrari et al., 2010; Studer, 2001). Cardiac bodies generated from isolated cardiac stem cells give rise to cardiomyocytes, endothelial cells and smooth muscle cells (Höbaus et al., 2013; Taubenschmid and Weitzer, 2012). Definitive endoderm can be generated with high efficiency from ESCs in monolayers (Borowiak et al., 2009; Zhu et al., 2009). Murine ESC aggregates resemble the early embryonic development of mouse for 7 to 8 days and spontaneously give rise to cells of ectodermal, endodermal and mesodermal origin. Until day 8, EBs undergo a morphological development resembling early embryogenesis until gastrulation commences (Bader et al., 2001). Later on, differentiation and development of other cell types appear to be chaotic so far (Weitzer, 2006). However cardiomyogenesis seems to follow a morphological program at least until day 8 of differentiation (Fuchs et al., 2012).
Furthermore, in EBs cellular function can be studied by electrophysiology and cell–cell interaction can be studied by immunofluorescence microscopy quite well; however, it seems that in teratoma cell–cell interaction resembles the situation in a tissue much better than in EBs and somatic stem cell aggregates. Likewise, nutrition and blood supply in teratoma reflect physiological conditions better than in EBs and monolayer cultures of stem cells. In teratoma, cells of the host, mainly the blood vessels growing into the tumor, influence the development of the tumor and thus also significantly influence the results obtained from expression analysis etc. In teratoma new blood vessels supply the tissues with nutrition and oxygen, however, in stem cell aggregates thicker than 7 cell layers, no reproducible supply with nutrition and oxygen exists. Thus development of an in vitro angiogenesis model in combination with stem cell aggregation experiments, as a possibility to improve the physiological relevance of this model, is desirable.
In vitro models alone might be sensitive but are not specific enough for the study of the genomics and epigenetics of hESC or hiPSC lines. To address this problem and the gap between in vivo and in vitro models, in silico genome wide methods such as whole genome transcriptome profiles in combination with complex biomarker models can identify deviations from a defined “ideal” phenotype on a global scale (Müller et al., 2008, 2011; Williams et al., 2011).
Computer-based predictive models
Machine-learning based models can identify signatures characteristic of pluripotent stem cells in functional genomic data sets (Brolen et al., 2010; Medine et al., 2010; Müller et al., 2008) and can also highlight deviations from an “ideal” pluripotent stem cell phenotype (Müller et al., 2011; Williams et al., 2011). In silico assays could be a cost effective alternative to teratoma assays and would enable many exploratory bioinformatic downstream applications. Current challenges, in regard to in silico models, are i) standardization issues, ii) acceptance in the field, iii) regulatory issues and iv) most importantly the availability of comparable and multiple datasets of genomic, expression profile, proteomic and epigenetic analysis.
First, pluripotency models can be developed with reasonable funding for one microarray platform (e.g. Illumina) but transfer to other platforms (e.g. RNA-seq) is a resource intensive challenge. Secondly, even as bioinformatics is becoming an important part in pluripotent stem cell research yet most wet stem cell biologists have never received proper training e.g. in using high-level bioinformatic tools such as Bioconductor/R. Hence accessible ways of disseminating bioinformatic assays for pluripotency to a non-expert audience have to be developed. Reliable and standardized ways for the effective communication of such bioinformatic results have to be agreed on by researchers, reviewers and journals, comparable to the Minimum Information About a Microarray Experiment (MIAME) standard required for the reporting of microarray experiments by most peer reviewed journals (Brazma et al., 2001). Finally, global microarray datasets have been widely used in preclinical, exploratory analyses, but rarely as defined outcome measure. Simple signature-based approaches are unable to identify stochastic, random and unexpected events regularly emerging in stem cell cultures, such as epigenetic or even genetic alterations and abnormalities (Williams et al., 2011). As costs for generating high-content datasets are currently dropping below a single day of a postdocs salary due to the next-generation sequencing revolution, a global, genome wide and data driven approach will become more and more attractive and highly desirable for pluripotent stem cell research in spite of possible regulatory challenges.
A first bioinformatic assay for pluripotency in human cells (PluriTest) has been recently developed and published (Müller et al., 2011) and can be used through a simple web interface at www.pluritest.org. Up to date, more than 6200 microarray data sets have been uploaded and analyzed. PluriTest currently supports Illumina gene expression arrays and will be expanded to RNA-seq data in the near future.
Chicken egg model — chorioallantoic membrane
Another option to the study of teratoma formation could be the transplantation of stem cells onto the chorioallantoic membrane (CAM) of avian/chicken embryos. The CAM is situated at the periphery of the chicken embryo as a densely vascularized extraembryonic tissue. It is easily accessible by opening a hole in the egg shell. Experiments have been performed some 100 years ago and this model convinces by the ease of access and the natural immunodeficient environment of the developing embryo. Tumor growth can be observed within days and are of approximately 5–10 mm in size. They have sufficient similarity to teratoma and strongly resemble clinical specimen of patient samples (Durupt et al., 2012; Hagedorn et al., 2005). The CAM model combines the advantages of the in vivo environment with the simplicity of an in vitro experiment, is cheap, fast and without serious ethical issues.
Organotypic models
The development of an in vitro model of teratoma formation, such as a skin model would be of great importance, as it could be cultured in vitro via tissue engineering, where stem cells are injected into skin pads and allowed to develop and differentiate. Another possibility would be to develop strategies for the testing of stem cells for their repopulation potential of cell-free organ templates. These would serve as an ECM-template for directed differentiation. This has already been done with hearts and human trachea, where the organ was completely depleted of cells leaving behind a grid of extracellular material, which can be repopulated by stem cells (Ott and Taylor, 2006; Elliott et al., 2012). Further it is possible to re-aggregate dissociated embryonic kidneys, which were still able to form organotypic renal structures (Unbekandt and Davies, 2010). This method was also used to form chimeric renal structures mixing murine embryonic kidney cells with human amniotic fluid stem cells (hAFSCs). hAFSCs were able to integrate into and contribute to renal structures. Using siRNA knock down technology they showed which genes contributed to renal structure formation (Siegel et al., 2010). This model can be used both as a pluripotency assay and will allow us to gain new insight into putative stem cell therapy in kidney disease models. Adult murine ventricular slices serve as a new in vitro model of adult myocardium with preserved in vivo structure. In the future, these could be used to study functional integration of stem cells transplanted in infarcted hearts in vivo (Halbach et al., 2006).
An example of differential information content of embryoid bodies and teratoma assays
Monitoring the development of cells in EBs allows the analyses of dynamic aspects of cell differentiation and to identify factors which differentially affect consecutive developmental stages of cells or simple tissues. Furthermore, analysis of large numbers of EBs provides the basis for robust statistical analysis of data. This type of information cannot be obtained from histological sections of teratoma because there is only one endpoint per teratoma available and statistical analysis is limited by the rather small number of animals typically used for teratoma assay. The following example will demonstrate that the information obtained from in vitro experiments in EBs may be more informative than that from teratoma assays in mice.
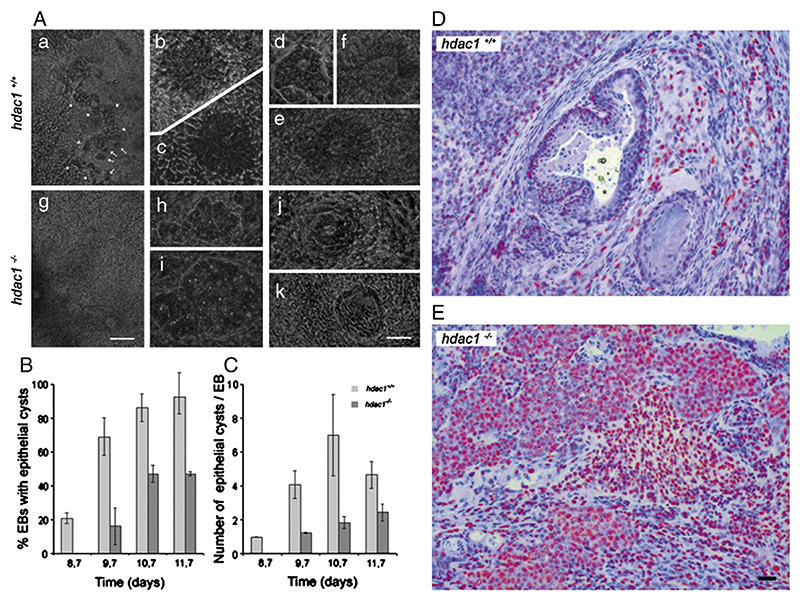
The question, whether the absence of the histone deacetylase 1 in stem cells influences their ability to undergo mesenchymal-to-epithelial transition (Lagger et al., 2010), could be more precisely and quantitatively addressed and answered in EBs than in a mouse teratoma assay (Fig. 1). In hdac1+/+ EBs (Fig. 1A) multiple and well structured columnar epithelial cells develop in cysts, whereas in hdac1−/− EBs only rudimentary structures mainly composed of small cuboidal cells become visible. The data obtained from these experiments have the same informational content than histological sections obtained from teratoma in mice (Figs. 1D and E). In addition, data from several hundred EBs demonstrate both a qualitative and quantitative statistically significant difference in epithelial cyst development, and finally also a delay in time required to obtain epithelial cysts. The latter cannot be obtained from teratoma removed and analyzed only at the end of the experiment.
Figure 1. Development of epithelial cysts in hdac1−/− EBs and teratoma.
(A to C) Wild type (hdac1+/+) and hdac1−/− ESCs were aggregated in hanging drop cultures for 4.7 days and then plated on gelatine coated tissue culture plates. Development of epithelial cysts was monitored between days 5 and 14. First epithelia cysts become visible between days 8 and 8.7. (A) Phase contrast images of hdac1+/+ and hdac1−/− EBs. (a and g) Overview of a typical area adjacent to the center of EBs at day 11. Long arrows, single isolated epithelial cysts. Short arrows, large layers of epithelial cells. (b and c) Primitive epithelial cysts forming between days 8 and 9 in hdac1+/+ EBs. (d to e) Fully developed columnar-epithelial cysts with a clearly visible lumen forming in hdac1+/+ EBs between days 10 and 12. (h and i) Early cysts with non-epithelial small cells forming between days 8 and 9 in hdac1−/− EBs. (j and k) Samples of rarely found cysts with a lumen in hdac1−/− EBs. Note, cells in h to k never develop to a columnar epithelial phenotype. Bars in g (for a and g), 1 mm; in k (for b to e, and h to k), 200 μm. (B) Percentage of EBs with epithelial cysts between days 8.7 and 11.7. (C) Number of epithelial cysts per EB. Data from days 9.7 and 10.7 are from two independent triplicate experiments. Data from days 8.7 and 11.7 are from one triplicate experiment. Mean number of EBs checked per day, 53 +/− 16. Error bars: standard deviation. (D and E) Teratoma were generated from 3 × 106 mouse wild-type (D) and HDAC1-deficient (E) ES cells. Cells were subcutaneously injected in SCID/Balb/c mice and teratoma formation as well as tumor size was monitored every 4 days. Recipient SCID mice were killed after 28 days post-injection and teratoma of both genotypes was removed and analyzed by immunohistochemistry with HDAC2 antibodies (red). Bar in D and E: 170 μm. 3 tumors per cell line were used for statistical analysis and cell counts.
Panels D and E with permission from EMBO Journal adapted from Lagger et al. (2010).
This example shows that experiments with hundreds of EBs have a statistical significance higher than that from a few teratoma and developmental processes can be monitored directly. In contrast, teratoma formation provides only a static view, can only be performed in lower numbers, is less reproducible than stem cell aggregation, and much higher costs accrue.
Conclusions and future perspectives
Due to the extraordinary speed that stem cell research is proceeding, scientists rarely have time to improve or replace assay systems that are working. For industrial and clinical applications the differentiation potential assessed by in vitro differentiation would be sufficient, as often only one specific cell type is needed. Therefore, if proof of pluripotency is not essential, teratoma models should not be used. In fact, directed differentiation of stem cells in monolayers or as EBs, provides much more detailed information on the development and function of those cells. The influence of growth factors, transcription factor expression, and cytokines can be studied on the molecular and cellular levels from the very beginning of differentiation until terminal differentiation of a somatic cell using the EB system. Secondly, the differentiation process based on a standardized EB model is highly reproducible and allows the study of molecular mechanisms guiding the differentiation process (Barbaric et al., 2010). Studying the transcriptional networks regulating differentiation of stem cells in combination with the external signals and their respective signaling pathways will help to define conditions, that will result in the improvement of the model of directed differentiation. This model might indicate that aggregation of stem cells will no longer be a prerequisite for the generation of a specific type of somatic cell. In parallel, models based on cell–cell and cell–matrix interactions need to be established, to provide knowledge from which more complex in vitro models will be developed and validated for clinical, pharmacological, and toxicological applications (Andrews et al., 2010; Laustriat et al., 2010).
To broaden the acceptance of in vitro as well as in silico models as valid pluripotency tests, more research needs to be performed on stem cell aggregates, on directed differentiation of stem cells without prior aggregation, and global correlates of these processes in high content formats such as RNA-seq or Methyl-seq. It is also essential to investigate the fundamental molecular and cellular processes taking place in EBs and somatic stem cell aggregates. It is important to understand these molecular and morphological changes seen in EBs and to correlate them with bona fide embryogenesis.
There are a number of alternative in vitro approaches available to the stem cell community for the assessment of pluripotency. If an objective analysis of the intended use of a cell line suggests that in vivo testing can be excluded, the latter should be dropped altogether. There is no ethical and academic justification to test cell lines universally in a teratoma assay in order to generate results that prove to be less informative than those generated by in vitro approaches.
Footnotes
Disclosures
The central aim of the SET Foundation is to reduce and replace animal experimentation. The Foundation is made up of representatives from industry, animal welfare, science and government. Their role is the transparent, interdisciplinary allocation of funds to eligible projects researching and implementing methods to replace and complement experiments on animals (www.stiftung-set.de).
References
- Andrews PD, Becroft M, Aspegren A, Gilmour J, James MJ, McRae S, Kime R, Allcock RW, Abraham A, Jiang Z, et al. High-content screening of feeder-free human embryonic stem cells to identify pro-survival small molecules. Biochem J. 2010;432:21–33. doi: 10.1042/BJ20101022. [DOI] [PubMed] [Google Scholar]
- Atkinson S, Armstrong L. Epigenetics in embryonic stem cells: regulation of pluripotency and differentiation. Cell Tissue Res. 2008;331:23–29. doi: 10.1007/s00441-007-0536-x. [DOI] [PubMed] [Google Scholar]
- Bader A, Gruss A, Hollrigl A, Al-Dubai H, Capetanaki Y, Weitzer G. Paracrine promotion of cardiomyogenesis in embryoid bodies by LIF modulated endoderm. Differentiation. 2001;68:31–43. doi: 10.1046/j.1432-0436.2001.068001031.x. [DOI] [PubMed] [Google Scholar]
- Barbaric I, Gokhale PJ, Andrews PW. High-content screening of small compounds on human embryonic stem cells. Biochem Soc Trans. 2010;38:1046–1050. doi: 10.1042/BST0381046. [DOI] [PubMed] [Google Scholar]
- Boland MJ, Hazen JL, Nazor KL, Rodriguez AR, Gifford W, Martin G, Kupriyanov S, Baldwin KK. Adult mice generated from induced pluripotent stem cells. Nature. 2009;461:91–94. doi: 10.1038/nature08310. [DOI] [PubMed] [Google Scholar]
- Borowiak M, Maehr R, Chen S, Chen AE, Tang W, Fox JL, Schreiber SL, Melton DA. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell. 2009;4:348–358. doi: 10.1016/j.stem.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulting GL, Kiskinis E, Croft GF, Amoroso MW, Oakley DH, Wainger BJ, Williams DJ, Kahler DJ, Yamaki M, Davidow L, et al. A functionally characterized test set of human induced pluripotent stem cells. Nat Biotechnol. 2011;29:279–286. doi: 10.1038/nbt.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, Stoeckert C, Aach J, Ansorge W, Ball CA, Causton HC, et al. Minimum information about a microarray experiment (MIAME)-toward standards for microarray data. Nat Genet. 2001;29:365–371. doi: 10.1038/ng1201-365. [DOI] [PubMed] [Google Scholar]
- Brivanlou AH, Gage FH, Jaenisch R, Jessell T, Melton D, Rossant J. Stem cells. Setting standards for human embryonic stem cells. Science. 2003;300:913–916. doi: 10.1126/science.1082940. [DOI] [PubMed] [Google Scholar]
- Brolen G, Sivertsson L, Bjorquist P, Eriksson G, Ek M, Semb H, Johansson I, Andersson TB, Ingelman-Sundberg M, Heins N. Hepatocyte-like cells derived from human embryonic stem cells specifically via definitive endoderm and a progenitor stage. J Biotechnol. 2010;145:284–294. doi: 10.1016/j.jbiotec.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Cao R, Zhang Y. The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr Opin Genet Dev. 2004;14:155–164. doi: 10.1016/j.gde.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Chan EM, Ratanasirintrawoot S, Park IH, Manos PD, Loh YH, Huo H, Miller JD, Hartung O, Rho J, Ince TA, et al. Live cell imaging distinguishes bona fide human iPS cells from partially reprogrammed cells. Nat Biotechnol. 2009;27:1033–1037. doi: 10.1038/nbt.1580. [DOI] [PubMed] [Google Scholar]
- Damjanov I, Andrews PW. The terminology of teratocarcinomas and teratomas. Nat Biotechnol. 2007;25:1212. doi: 10.1038/nbt1107-1212a. (discussion 1212) [DOI] [PubMed] [Google Scholar]
- Dressel R. Effects of histocompatibility and host immune responses on the tumorigenicity of pluripotent stem cells. Semin Immunopathol. 2011;33:573–591. doi: 10.1007/s00281-011-0266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressel R, Schindehutte J, Kuhlmann T, Elsner L, Novota P, Baier PC, Schillert A, Bickeboller H, Herrmann T, Trenkwalder C, et al. The tumorigenicity of mouse embryonic stem cells and in vitro differentiated neuronal cells is controlled by the recipients’ immune response. PLoS One. 2008;3:e2622. doi: 10.1371/journal.pone.0002622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durupt F, Koppers-Lalic D, Balme B, Budel L, Terrier O, Lina B, Thomas L, Hoeben RC, Rosa-Calatrava M. The chicken chorioallantoic membrane tumor assay as model for qualitative testing of oncolytic adenoviruses. Cancer Gene Ther. 2012;19:58–68. doi: 10.1038/cgt.2011.68. [DOI] [PubMed] [Google Scholar]
- Eggan K, Akutsu H, Loring J, Jackson-Grusby L, Klemm M, Rideout WM, III, Yanagimachi R, Jaenisch R. Hybrid vigor, fetal overgrowth, and viability of mice derived by nuclear cloning and tetraploid embryo complementation. Proc Natl Acad Sci U S A. 2001;98:6209–6214. doi: 10.1073/pnas.101118898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MJ, De Coppi P, Speggiorin S, Roebuck D, Butler CR, Samuel E, Crowley C, McLaren C, Fierens A, Vondrys D, Cochrane L, et al. Stem-cell-based, tissue engineered tracheal replacement in a child: a 2-year follow-up study. Lancet. 2012;380:994–1000. doi: 10.1016/S0140-6736(12)60737-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdö F, Buhrle C, Blunk J, Hoehn M, Xia Y, Fleischmann B, Focking M, Kustermann E, Kolossov E, Hescheler J, et al. Host-dependent tumorigenesis of embryonic stem cell transplantation in experimental stroke. J Cereb Blood Flow Metab. 2003;23:780–785. doi: 10.1097/01.WCB.0000071886.63724.FB. [DOI] [PubMed] [Google Scholar]
- European Union, T.E.P.a.t.c.o.t. Directive 2010/63/EU on the protection of animals used for scientific purposes. Off J Eur Union. 2010;L276:33–79. [Google Scholar]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Binda E, De Filippis L, Vescovi AL. Isolation of neural stem cells from neural tissues using the neurosphere technique. Curr Protoc Stem Cell Biol. 2010 doi: 10.1002/9780470151808.sc02d06s15. (Chapter 2, Unit 2D 6) [DOI] [PubMed] [Google Scholar]
- Fong H, Hohenstein KA, Donovan PJ. Regulation of self-renewal and pluripotency by Sox2 in human embryonic stem cells. Stem Cells. 2008;26:1931–1938. doi: 10.1634/stemcells.2007-1002. [DOI] [PubMed] [Google Scholar]
- Fuchs C, Scheinast M, Pasteiner W, Lagger S, Hofner M, Hoellrigl A, Schultheis M, Weitzer G. Self-organization phenomena in embryonic stem cell-derived embryoid bodies: axis formation and breaking of symmetry during cardiomyogenesis. Cells Tissues Organs. 2012;195:377–391. doi: 10.1159/000328712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertow K, Przyborski S, Loring JF, Auerbach JM, Epifano O, Otonkoski T, Damjanov I, Ahrlund-Richter L. Isolation of human embryonic stem cell-derived teratomas for the assessment of pluripotency. Curr Protoc Stem Cell Biol. 2007 doi: 10.1002/9780470151808.sc01b04s3. (Chapter 1, Unit 1B 4) [DOI] [PubMed] [Google Scholar]
- Gertow K, Cedervall J, Jamil S, Ali R, Imreh MP, Gulyas M, Sandstedt B, Ahrlund-Richter L. Early events in xenograft development from the human embryonic stem cell line HS181—resemblance with an initial multiple epiblast formation. PLoS One. 2011;6:e27741. doi: 10.1371/journal.pone.0027741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gropp M, Shilo V, Vainer G, Gov M, Gil Y, Khaner H, Matzrafi L, Idelson M, Kopolovic J, Zak NB, et al. Standardization of the teratoma assay for analysis of pluripotency of human ES cells and biosafety of their differentiated progeny. PLoS One. 2012;7:e45532. doi: 10.1371/journal.pone.0045532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn M, Javerzat S, Gilges D, Meyre A, de Lafarge B, Eichmann A, Bikfalvi A. Accessing key steps of human tumor progression in vivo by using an avian embryo model. Proc Natl Acad Sci U S A. 2005;102:1643–1648. doi: 10.1073/pnas.0408622102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbach M, Pillekamp F, Brockmeier K, Hescheler J, Müller-Ehmsen J, Reppel M. Ventricular slices of adult mouse hearts—a new multicellular in vitro model for electrophysiological studies. Cell Physiol Biochem. 2006;18:1–8. doi: 10.1159/000095132. [DOI] [PubMed] [Google Scholar]
- Halme DG, Kessler DA. FDA regulation of stem-cell-based therapies. N Engl J Med. 2006;355:1730–1735. doi: 10.1056/NEJMhpr063086. [DOI] [PubMed] [Google Scholar]
- Hentze H, Soong PL, Wang ST, Phillips BW, Putti TC, Dunn NR. Teratoma formation by human embryonic stem cells: evaluation of essential parameters for future safety studies. Stem Cell Res. 2009;2:198–210. doi: 10.1016/j.scr.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Houlard M, Berlivet S, Probst AV, Quivy JP, Hery P, Almouzni G, Gerard M. CAF-1 is essential for heterochromatin organization in pluripotent embryonic cells. PLoS Genet. 2006;2:e181. doi: 10.1371/journal.pgen.0020181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höbaus J, Heher P, Gottschamel T, Scheinast M, Auner H, Walder D, Wiedner M, Taubenschmid J, Miksch M, Sauer T, et al. Embryonic stem cells facilitate the isolation of persistent clonal cardiovascular progenitor cell lines and leukemia inhibitor factor maintains their self-renewal and myocardial differentiation potential in vitro. Cells Tissues Organs. 2013;197:249–268. doi: 10.1159/000345804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISCBI T.I.S.C.B.I. Consensus guidance for banking and supply of human embryonic stem cell lines for research purposes. Stem Cell Rev. 2009;5:301–314. doi: 10.1007/s12015-009-9085-x. [DOI] [PubMed] [Google Scholar]
- Kang L, Wang J, Zhang Y, Kou Z, Gao S. iPS cells can support full-term development of tetraploid blastocyst-complemented embryos. Cell Stem Cell. 2009;5:135–138. doi: 10.1016/j.stem.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Kimura H, Tada M, Nakatsuji N, Tada T. Histone code modifications on pluripotential nuclei of reprogrammed somatic cells. Mol Cell Biol. 2004;24:5710–5720. doi: 10.1128/MCB.24.13.5710-5720.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klochendler-Yeivin A, Fiette L, Barra J, Muchardt C, Babinet C, Yaniv M. The murine SNF5/INI1 chromatin remodeling factor is essential for embryonic development and tumor suppression. EMBO Rep. 2000;1:500–506. doi: 10.1093/embo-reports/kvd129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoepfler PS. Deconstructing stem cell tumorigenicity: a roadmap to safe regenerative medicine. Stem Cells. 2009;27:1050–1056. doi: 10.1002/stem.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagger S, Meunier D, Mikula M, Brunmeir R, Schlederer M, Artaker M, Pusch O, Egger G, Hagelkruys A, Mikulits W, et al. Crucial function of histone deacetylase 1 for differentiation of teratomas in mice and humans. EMBO J. 2010;29:3992–4007. doi: 10.1038/emboj.2010.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laustriat D, Gide J, Peschanski M. Human pluripotent stem cells in drug discovery and predictive toxicology. Biochem Soc Trans. 2010;38:1051–1057. doi: 10.1042/BST0381051. [DOI] [PubMed] [Google Scholar]
- Lawrenz B, Schiller H, Willbold E, Ruediger M, Muhs A, Esser S. Highly sensitive biosafety model for stem-cell-derived grafts. Cytotherapy. 2004;6:212–222. doi: 10.1080/14653240410006031. [DOI] [PubMed] [Google Scholar]
- Lee JH, Hart SR, Skalnik DG. Histone deacetylase activity is required for embryonic stem cell differentiation. Genesis. 2004;38:32–38. doi: 10.1002/gene.10250. [DOI] [PubMed] [Google Scholar]
- Li Z, Huang H, Boland P, Dominguez MG, Burfeind P, Lai KM, Lin HC, Gale NW, Daly C, Auerbach W, et al. Embryonic stem cell tumor model reveals role of vascular endothelial receptor tyrosine phosphatase in regulating Tie2 pathway in tumor angiogenesis. Proc Natl Acad Sci U S A. 2009;106:22399–22404. doi: 10.1073/pnas.0911189106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GR, Evans MJ. Differentiation of clonal lines of teratocarcinoma cells: formation of embryoid bodies in vitro. Proc Natl Acad Sci U S A. 1975;72:1441–1445. doi: 10.1073/pnas.72.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauritz C, Martens A, Rojas SV, Schnick T, Rathert C, Schecker N, Menke S, Glage S, Zweigerdt R, Haverich A, et al. Induced pluripotent stem cell (iPSC)-derived Flk-1 progenitor cells engraft, differentiate, and improve heart function in a mouse model of acute myocardial infarction. Eur Heart J. 2011;32:2634–2641. doi: 10.1093/eurheartj/ehr166. [DOI] [PubMed] [Google Scholar]
- Medine CN, Greenhough S, Hay DC. Role of stem-cell-derived hepatic endoderm in human drug discovery. Biochem Soc Trans. 2010;38:1033–1036. doi: 10.1042/BST0381033. [DOI] [PubMed] [Google Scholar]
- Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- Müller FJ, Laurent LC, Kostka D, Ulitsky I, Williams R, Lu C, Park IH, Rao MS, Shamir R, Schwartz PH, et al. Regulatory networks define phenotypic classes of human stem cell lines. Nature. 2008;455:401–405. doi: 10.1038/nature07213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller FJ, Goldmann J, Loser P, Loring JF. A call to standardize teratoma assays used to define human pluripotent cell lines. Cell Stem Cell. 2010;6:412–414. doi: 10.1016/j.stem.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Müller FJ, Schuldt BM, Williams R, Mason D, Altun G, Papapetrou EP, Danner S, Goldmann JE, Herbst A, Schmidt NO, et al. A bioinformatic assay for pluripotency in human cells. Nat Methods. 2011;8:315–317. doi: 10.1038/nmeth.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A, Gocza E, Diaz EM, Prideaux VR, Ivanyi E, Markkula M, Rossant J. Embryonic stem cells alone are able to support fetal development in the mouse. Development. 1990;110:815–821. doi: 10.1242/dev.110.3.815. [DOI] [PubMed] [Google Scholar]
- Ott HC, Taylor DA. From cardiac repair to cardiac regeneration—ready to translate? Expert Opin Biol Ther. 2006;6:867–878. doi: 10.1517/14712598.6.9.867. [DOI] [PubMed] [Google Scholar]
- Pesce M, Scholer HR. Oct-4: gatekeeper in the beginnings of mammalian development. Stem Cells. 2001;19:271–278. doi: 10.1634/stemcells.19-4-271. [DOI] [PubMed] [Google Scholar]
- Peterson SE, Tran HT, Garitaonandia I, Han S, Nickey KS, Leonardo T, Laurent LC, Loring JF. Teratoma generation in the testis capsule. J Vis Exp. 2011:e3177. doi: 10.3791/3177. ( http://www.jove.com/video/3177/teratoma-generation-in-the-testis-capsule). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossant J. Stem cells and early lineage development. Cell. 2008;132:527–531. doi: 10.1016/j.cell.2008.01.039. [DOI] [PubMed] [Google Scholar]
- Russell WMS, Burch RL. The Principles of Humane Experimental Technique. Methuen, London: 1959. [Google Scholar]
- Saric T, Frenzel LP, Hescheler J. Immunological barriers to embryonic stem cell-derived therapies. Cells Tissues Organs. 2008;188:78–90. doi: 10.1159/000118784. [DOI] [PubMed] [Google Scholar]
- Siegel N, Rosner M, Unbekandt M, Fuchs C, Slabina N, Dolznig H, Davies JA, Lubec G, Hengstschlager M. Contribution of human amniotic fluid stem cells to renal tissue formation depends on mTOR. Hum Mol Genet. 2010;19:3320–3331. doi: 10.1093/hmg/ddq236. [DOI] [PubMed] [Google Scholar]
- Skreb N, Svajger A, Levak-Svajger B. Growth and differentiation of rat egg-cylinders under the kidney capsule. J Embryol Exp Morphol. 1971;25:47–56. [PubMed] [Google Scholar]
- Solter D, Skreb N, Damjanov I. Extrauterine growth of mouse egg-cylinders results in malignant teratoma. Nature. 1970;227:503–504. doi: 10.1038/227503a0. [DOI] [PubMed] [Google Scholar]
- Stadtfeld M, Hochedlinger K. Induced pluripotency: history, mechanisms, and applications. Genes Dev. 2010;24:2239–2263. doi: 10.1101/gad.1963910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M, Apostolou E, Akutsu H, Fukuda A, Follett P, Natesan S, Kono T, Shioda T, Hochedlinger K. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature. 2010;465:175–181. doi: 10.1038/nature09017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens LC. Studies on transplantable testicular teratomas of strain 129 mice. J Natl Cancer Inst. 1958;20:1257–1275. doi: 10.1093/jnci/20.6.1257. [DOI] [PubMed] [Google Scholar]
- Stevens LC. The development of transplantable teratocarcinomas from intratesticular grafts of pre- and postimplantation mouse embryos. Dev Biol. 1970;21:364–382. doi: 10.1016/0012-1606(70)90130-2. [DOI] [PubMed] [Google Scholar]
- Stevens LC, Little CC. Spontaneous testicular teratomas in an inbred strain of mice. Proc Natl Acad Sci U S A. 1954;40:1080–1087. doi: 10.1073/pnas.40.11.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer L. Stem cells with brainpower. Nat Biotechnol. 2001;19:1117–1118. doi: 10.1038/nbt1201-1117. [DOI] [PubMed] [Google Scholar]
- Swiss Federal Veterinary Office (Hsrg) Classification of Animal Experiments according to Grades of Severity prior to the Experiment (Stress Categories) 2012. ( http://www.tierversuch.ch/show=AWLaw&nav_id4104&lang=en (02.14.2012)).
- Taubenschmid J, Weitzer G. Mechanisms of cardiogenesis in cardiovascular progenitor cells. Int Rev Cell Mol Biol. 2012;293:195–267. doi: 10.1016/B978-0-12-394304-0.00012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unbekandt M, Davies JA. Dissociation of embryonic kidneys followed by reaggregation allows the formation of renal tissues. Kidney Int. 2010;77:407–416. doi: 10.1038/ki.2009.482. [DOI] [PubMed] [Google Scholar]
- Verhoog H. The concept of intrinsic value and transgenic animals. J Agric Ethics. 1992;5:147–160. doi: 10.1007/BF01966357. [DOI] [PubMed] [Google Scholar]
- Vorstenbosch J. The concept of integrity Its significance for the ethical discussion on biotechnology and animals. Livest Prod Sci. 1993;36:109–112. [Google Scholar]
- Weitzer G. Embryonic stem cell-derived embryoid bodies: an in vitro model of eutherian pregastrulation development and early gastrulation. Handb Exp Pharmacol. 2006:21–51. [PubMed] [Google Scholar]
- Wesselschmidt RL. The teratoma assay: an in vivo assessment of pluripotency. Methods Mol Biol. 2011;767:231–241. doi: 10.1007/978-1-61779-201-4_17. [DOI] [PubMed] [Google Scholar]
- Williams R, Schuldt B, Müller FJ. A guide to stem cell identification: progress and challenges in system-wide predictive testing with complex biomarkers. Bioessays. 2011;33:880–890. doi: 10.1002/bies.201100073. [DOI] [PubMed] [Google Scholar]
- Wobus AM, Holzhausen H, Jakel P, Schoneich J. Characterization of a pluripotent stem cell line derived from a mouse embryo. Exp Cell Res. 1984;152:212–219. doi: 10.1016/0014-4827(84)90246-5. [DOI] [PubMed] [Google Scholar]
- Zhao XY, Li W, Lv Z, Liu L, Tong M, Hai T, Hao J, Guo CL, Ma QW, Wang L, et al. iPS cells produce viable mice through tetraploid complementation. Nature. 2009;461:86–90. doi: 10.1038/nature08267. [DOI] [PubMed] [Google Scholar]
- Zhu S, Wurdak H, Wang J, Lyssiotis CA, Peters EC, Cho CY, Wu X, Schultz PG. A small molecule primes embryonic stem cells for differentiation. Cell Stem Cell. 2009;4:416–426. doi: 10.1016/j.stem.2009.04.001. [DOI] [PubMed] [Google Scholar]