Abstract
Objective
The natural trajectory of clinical arthritis progression at the tissue level remains elusive. We hypothesized that subclinical inflammation in different joint tissues (synovitis, tenosynovitis, osteitis) increases in a distinct temporal order in patients with clinically suspect arthralgia (CSA) who develop rheumatoid arthritis (RA) and subsides in a different sequence when CSA spontaneously resolves.
Methods
We studied 185 serial magnetic resonance images (MRIs) from CSA patients with subclinical joint inflammation from the placebo arm of the TREAT EARLIER trial: 52 MRIs from 21 RA progressors (MRIs conducted at 1 year before, at 4 months before, and upon RA development), and 133 MRIs from 35 patients with spontaneous resolution of pain (MRIs conducted at baseline and at 4, 12, and 24 months). MRIs were scored for osteitis, synovitis, and tenosynovitis. We used cross-lagged models to evaluate 2 types of time patterns between pairs of inflamed tissues: a simultaneous pattern (coinciding changes) and a subsequent pattern (inflammatory changes in 1 tissue preceding changes in another tissue).
Results
In patients who developed RA, synovitis, tenosynovitis, and osteitis increased simultaneously. Increasing osteitis occurred in the final 4 months before RA diagnosis, following incremental tenosynovitis and synovitis changes during the 1 year to 4 months before diagnosis (P < 0.01). In anti–citrullinated protein antibody (ACPA)-positive and ACPA-negative patients who progressed to RA, osteitis increased just before RA development. In patients with pain resolution, simultaneous decreases in synovitis, tenosynovitis, and osteitis occurred, with tenosynovitis decreasing in the first 4 months after CSA onset preceding decreasing synovitis and osteitis during 4–12 months (P = 0.02 and P < 0.01).
Conclusion
We identified natural sequences of subclinical inflammation in different joint tissues, which deepens our understanding of clinical arthritis and RA development. During RA progression, increasing osteitis followed previous increases in tenosynovitis and synovitis. During pain resolution, tenosynovitis decreased first, followed by decreasing synovitis and osteitis.
Introduction
The presence of clinically apparent arthritis in 1 or several joints is a prerequisite for diagnosis of rheumatoid arthritis (RA) (1). Correspondingly, the presence of clinical arthritis in hand and foot joints is the hallmark of RA. During the past decade, research on “pre-RA” has yielded important discoveries on the development of autoimmunity (2). However, the natural processes in which the systemic immune response passes on to the joint and the trajectory through which clinical arthritis develops remain elusive.
RA is classically characterized by synovitis. Notwithstanding, magnetic resonance images (MRIs) of hands and feet of RA patients have shown that the tenosynovium and bone are also often inflamed. Subclinical tenosynovitis, synovitis, and osteitis can also already occur in the at-risk stage of clinically suspect arthralgia (CSA) (3). So far, the natural sequence in the development of subclinical inflammation during the CSA phase in these different joint tissues (synovitis, tenosynovitis, osteitis) has not been determined. Hypothetically, this process does not occur randomly but occurs in specific time sequences. Knowledge of such natural time sequences may increase our pathophysiologic understanding of clinical arthritis and RA development. This knowledge may ultimately allow the substages of increasing RA risk in the trajectory from CSA to RA to be defined and allow the risk stratification of arthralgia patients to be performed using time order information, which may be helpful in daily practice.
The CSA phase is suitable for the study of time sequences in subclinical joint inflammation, since some CSA patients develop RA, whereas others have a spontaneous resolution of their symptoms without therapeutic intervention (3,4). MRI studies can also detect subtle subclinical “inflammation” in the healthy population, especially in older populations, mostly with regard to synovitis and bone marrow edema (5). Thus, a reference to normality should be included when MRI studies are used for prognostic purposes in CSA patients, in order to prevent false-positive findings (6).
To our knowledge, longitudinal MRI studies in CSA are scarce. Studies have shown that MRI-detected joint inflammation scores are greater at RA diagnosis than at CSA presentation but are lower in CSA patients whose symptoms have resolved compared with at CSA onset (4,7). However, this earlier research only had the availability of 2 MRIs per CSA patient. It remains to be elucidated in what order synovitis, tenosynovitis, and osteitis naturally increase during RA development or subside in CSA patients who have spontaneous symptom resolution.
We hypothesized that distinct natural time orders can be identified for subclinical inflammation progression of joint tissues (synovium, bone, tendon sheath) in CSA patients who develop RA, as well as for subclinical inflammation resolution in CSA patients who achieve a spontaneous resolution of symptoms. We aimed to address these 2 questions by taking advantage of a unique data set of serial MRIs of hands and feet in CSA patients enrolled in the placebo group of the TREAT EARLIER trial and studying the natural “trajectory of local tissue inflammation” in patients who progressed to RA and patients who had symptom resolution.
Patients and Methods
Study design
Because we aimed to unravel the natural course of inflammation in serial MRIs, we studied patients who participated in the placebo group of the TREAT EARLIER trial. This randomized, double-blind, 2-year proof-of-concept trial has been previously described (8). Briefly, the trial included adults with CSA and MRI-detected subclinical joint inflammation. Patients were recruited from all rheumatology out-patient clinics in southwest Netherlands between April 2015 and September 2019.
CSA was characterized as recent-onset (<1 year) arthralgia of the small joints suspicious for progression to RA according to the rheumatologist, regardless of autoantibody status. Per definition, clinical arthritis or another explanation for arthralgia was absent. Subclinical inflammation was defined as present if at least 1 joint tissue (synovitis, tenosynovitis, osteitis) showed inflammation, as scored by both readers, and if present in fewer than 5% of age-matched symptom-free volunteers at the same location.
After inclusion in the trial, participants were randomly assigned (1:1) to 1 year of active treatment or placebo. All participants were then followed for a second year without treatment. During follow-up, concomitant treatment with analgesics, such as acetaminophen or nonsteroidal antiinflammatory drugs, was allowed, except within 24 hours before MRI scans. Treatment with any other disease-modifying antirheumatic drug (DMARD) or glucocorticoid (systemic or intraarticular) was prohibited during the trial. Participants only proceeded to open-label DMARD therapy in routine clinical practice if they reached the primary endpoint. Patients had study visits every 4 months, which included assessment of clinical arthritis development (the primary trial endpoint) by the treating rheumatologists and collection of clinical data (including pain).
The presence of clinical arthritis was based on the physical evaluation of the patient’s joints by 2 rheumatologists. When clinical arthritis was detected, patients had an additional study visit 2 weeks later to determine if the arthritis persisted. When a participant had an increase in symptoms between 2 study visits, an immediate additional visit was scheduled.
Ethics and consent
This study was conducted in accordance with the Declaration of Helsinki, and all patients provided written, informed consent. The study was approved by the medical ethical committee of the Leiden University Medical Centre. The TREAT EARLIER trial was registered with the Netherlands Trials Registry (NTR4853-trial-NL4599).
Study groups of patients who progressed to RA or achieved symptom resolution
The 2 groups of interest were CSA patients in the placebo group who progressed to RA and patients who had a spontaneous resolution of pain. These patients had clearly defined outcomes that are clinically relevant. Selection of groups with these distinguishable outcomes also permitted the study of homogeneous subgroups from the total CSA population. RA was defined as persistent clinical arthritis with a clinical diagnosis of RA involving at least 2 joints or fulfillment of the American College of Rheumatology/EULAR 2010 criteria for RA (1). Spontaneous resolution of pain was achieved if a patient did not develop clinical arthritis and had a score of ≤20 on a numeric rating scale (0–100) of pain at the last study visit. This cut-off for absence of joint pain was chosen in agreement with the literature (9). Patients who did not fulfill these definitions were characterized as having CSA complaints that improved partially or persisted while not developing persistent clinical arthritis and were not evaluated in our study (see flowchart in Supplementary Figure 1, available on the Arthritis & Rheumatology website at https://onlinelibrary.wiley.com/doi/10.1002/art.42527).
Serial MRI scans and scores
According to the trial protocol, MRIs were obtained at baseline and after 4, 12, and 24 months. If persistent clinical arthritis was detected, an additional and final MRI was performed. Consequently, among patients who developed RA within 12 months after inclusion, fewer than 4 MRIs were performed. Metacarpophalangeal joints 2–5, the wrist, and metatarsophalangeal joints 1–5 on the most painful side at baseline (or dominant side in case of symmetric symptoms) were imaged. Follow-up MRIs were performed at the side of the baseline MRI.
Two readers independently scored synovitis and osteitis MRIs in line with the Rheumatoid Arthritis Magnetic Resonance Imaging Scoring system and scored tenosynovitis MRIs as described by Haavardsholm et al. Readers had knowledge of MRI time order but were blinded for any clinical data (including RA development and pain) (10,11). Interreader reliability was excellent (intraclass correlation coefficient 0.94). We used the mean scores of the 2 readers in our analyses and summed the scores on osteitis, synovitis, and tenosynovitis in all imaged joints. Total inflammation scores summed the scores on osteitis, synovitis, and tenosynovitis at the patient level. Supplementary Methods 1 provides details on the scanning and scoring protocol (available on the Arthritis & Rheumatology website at https://onlinelibrary.wiley.com/doi/10.1002/art.42527).
Statistical analyses
We examined the course of subclinical inflammation in different joint tissues over successive time intervals between the MRIs of the CSA phase. The studied intervals differed between the pain resolution group and the RA progression group. For patients in the pain resolution group, data from 3 time intervals during 2 years of follow-up were evaluated: 1) MRI interval from trial inclusion (baseline) to the 4-month follow-up, 2) MRI interval from the 4-month to the 12-month follow-up, and 3) MRI interval from the 12-month to the 24-month follow-up.
Naturally, patients progressed to RA at different moments during follow-up. Because our aim was to study pathophysiologic processes, we considered the moment of RA diagnosis as the reference in our analysis, since this moment is the best comparable time point between patients in disease development. In accordance with the trial protocol, MRIs were performed at trial inclusion, at the 4-month follow-up, at the 12-month follow-up, and at the 24-month follow-up, as long as patients had not developed RA until that moment. An additional and final MRI was performed upon RA development. We assigned the 2 MRIs performed before RA development as 4 months prior to or 1 year prior to RA development, depending on which was the closest to the actual time interval between the MRI and RA development, as also illustrated in the example timeline (Supplementary Methods 4, available on the Arthritis & Rheumatology website at https://onlinelibrary.wiley.com/doi/10.1002/art.42527). This allowed evaluation of 2 time intervals in the RA progression group: 1) from 1 year to 4 months before RA diagnosis and 2) from 4 months before RA to RA diagnosis.
The course over time of inflammation in the synovium, the tenosynovium, and bone in both groups was plotted using unmodeled mean values. Using cross-lagged models, we further evaluated 2 time patterns in 1 model: a simultaneous pattern (inflammatory change in 1 tissue is associated with concurring change in another tissue) and a time order (inflammatory change in 1 tissue precedes change in another tissue in the next time interval) (12). We expressed estimates as standardized regression coefficients, which are independent of scale. Higher values indicate that a higher proportion of change in inflammation in 1 tissue is explained by change in another tissue, and a value of 0 indicates no explanation. Further explanations of these analyses are shown in the Supplementary Methods 2, available on the Arthritis & Rheumatology website at https://onlinelibrary.wiley.com/doi/10.1002/art.42527.
Cross-lagged models were fitted with full-information likelihood, appropriate for missingness at random (13). Patients who developed RA more quickly after first presentation with CSA may only have had 2 MRIs (at RA development and 4 months before). This would result in missingness in the “1 year prior to RA diagnosis” time point. We assumed missingness at random on this point, as this implies that missingness is explained by variables included in the model, and we know from previous research that MRI inflammation is associated with RA development (3,7).
In a subanalysis, we evaluated unmodeled MRI data from the RA progression group stratified for anti–citrullinated protein antibody (ACPA) status. This was done because of presumed underlying differences in etiopathology between the ACPA subtypes, although it is so far unclear whether the trajectory between CSA to RA is also different for the ACPA subtypes (14). Cross-lagged models could not be used on this point as patient numbers became too low after stratification.
We performed 2 sensitivity analyses for the RA progressors. In the first, MRI-detected inflammation was evaluated only in the joints in which clinical arthritis had developed. The second sensitivity analysis concerned the time distribution. In the primary analysis for RA progressors, we retrospectively assigned MRIs to the best applicable time point before RA development. This method is compelling from a pathophysiologic perspective because the moment of RA development is best comparable within the disease progression of different patients. Nevertheless, it might have had statistical implications, as it led to larger variations in MRI intervals between patients. Therefore, a sensitivity analysis was performed to assess whether this choice had any influence on the results. In this sensitivity analysis, we ensured similar time intervals between the MRIs in all patients who progressed to RA. We did this by using the time intervals starting from trial inclusion, as originally outlined in the protocol: baseline to the 4-month follow-up and 4 months to 12 months (see example timeline A in Supplementary Methods 4, available at https://onlinelibrary.wiley.com/doi/10.1002/art.42527). Of note, in this analysis, RA development could have occurred at any of the follow-up time points. Moreover, to further homogenize time intervals, we only analyzed MRIs if performed within 4 weeks of the MRI time points in the protocol.
We used R3.6.1, RStudio1.2.5042, Onyx 1.0–101, OpenMx 2.14.11, and SPSS version 16 for analyses. P values (2-sided) less than 0.05 were considered significant.
Data sharing
Requests for data collected (such as deidentified participant data) can be made to the corresponding author; requests will be considered on an individual basis.
Results
Patient characteristics
Baseline characteristics of both patient groups are shown in Supplementary Table 1, available on the Arthritis & Rheumatology website at https://onlinelibrary.wiley.com/doi/10.1002/art.42527. In agreement with the inclusion criteria of the trial, all patients had an MRI that was positive for subclinical inflammation at the study start (i.e., synovitis/tenosynovitis/osteitis in ≥1 location, with adjustment for presence in the general population). Among patients in the RA progression group, 57% were female, mean age was 48 years, and 52% were ACPA positive. Among patients in the pain resolution group, 63% were female, mean age was 50 years, and 11% were ACPA positive (Supplementary Table 1). Baseline characteristics were fairly comparable between the patient groups, but positivity results for ACPA and rheumatoid factor were higher in patients who progressed to RA than in patients who had pain resolution.
Time orders of subclinical joint inflammation during progression from CSA to RA
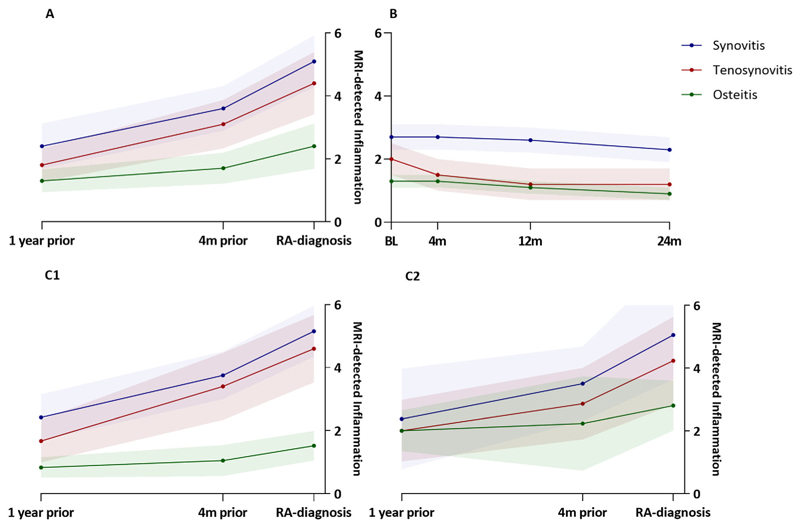
An average number of 2.5 MRIs per patient were performed in the RA progression group during a mean duration to RA development of 11 months. First, we depicted the unmodeled data during progression from CSA to RA. As expected, synovitis, tenosynovitis, and osteitis all increased. Around 1 year before RA development, the mean MRI scores were 2.4 for synovitis, 1.8 for tenosynovitis, and 1.3 for osteitis. Synovitis increased further to a mean score of 5.1 at RA diagnosis, with mean increase of 0.15 points/month in the first interval between 1 year and 4 months before RA diagnosis and a 0.38 points/month increase in the last 4 months before RA diagnosis.
Similarly, the mean MRI score for tenosynovitis increased to 4.4 at RA diagnosis (from mean 1.8 around 1 year before RA diagnosis); the monthly increase was 0.16 points/month between 1 year and 4 months before RA diagnosis and a 0.33 points/month in the last 4 months before RA diagnosis. The mean score for osteitis increased from 1.3 around 1 year before RA diagnosis to 2.4 at RA diagnosis, with 0.05 points/month between 1 year and 4 months before RA diagnosis and 0.18 points/month in the last 4 months before RA diagnosis. From the graphical depiction, the increase in inflammation in these 3 tissues during the last 4 months before diagnosis appeared to be stronger than in the preceding interval (Figure 1A).
Figure 1.
Inflammation scores as detected by magnetic resonance imaging (MRI) in different joint tissues (synovitis, tenosynovitis, and osteitis) over time in patients with clinically suspect arthralgia (CSA) who progressed to rheumatoid arthritis (RA) (A), patients who achieved spontaneous pain resolution (B), and CSA patients who progressed to RA stratified as negative (C1) or positive (C2) for anti–citrullinated protein antibody (ACPA). Scores are the mean ± SE (unmodeled data). The RA progression group had an average of 2.5 MRIs per patient (all patients had ≥2 MRIs, 10 patients had 3 MRIs) during a mean duration to RA development of 11 months. The median interval between RA diagnosis and the MRI conducted 4 months (4m) prior was 133 days (interquartile range 38–172); the median interval between RA diagnosis and the MRI 12 months (12m) prior was 392 days (interquartile range 268–573). BL = baseline; 24m = 24 months.
To statistically test a time order of incrementing inflammation in the different tissues, we used cross-lagged models, evaluating both simultaneous and subsequent changes in inflamed tissues. Because cross-lagged models assessed inflammation in 2 tissues at the same time, we performed these analyses 3 times (i.e., once for every possible pair within the 3 tissues). In our evaluation of simultaneous joint inflammation changes, we found that, during the last 4 months before RA diagnosis, all 3 pairs of synovitis, tenosynovitis, and osteitis increased simultaneously (Figure 2 shows a graphical representation and Table 1 lists the relevant estimates).
Figure 2.
Simultaneous and subsequent increases (Δ) of inflammation scores in pairs of different joint tissues during the 2 intervals in the year before RA development in which a time order was found. Vertical arrows represent a simultaneous inflammatory change in the 2 joint tissues. Diagonal arrows represent time orders. Estimates are the proportion of change in inflammation score in 2 tissues not explained by other patterns and are independent of scale. Higher values indicate that a higher proportion of change in inflammation in 1 tissue is explained by change in another tissue, and a value of 0 indicates no explanation. Significant effects are shown in black with asterisk; non-significant effects are shown in gray. See Figure 1 for definitions.
Table 1. Estimates of simultaneous changes and time order changes in inflammation in joint tissue pairs in patients who progressed to rheumatoid arthritis*.
| Tissue pair and time period | Simultaneous change (95% CI) |
Tissue order based on time period |
Time order change (95% CI) |
|---|---|---|---|
| Synovitis and tenosynovitis | |||
| From the 12 to 4 months before RA diagnosis | 0.11 (−0.10, 0.33) | Synovitis precedes tenosynovitis | 0.03 (–0.50, 0.56) |
| From 4 months before to RA diagnosis | 0.18 (0.05, 0.32)† | Tenosynovitis precedes synovitis | 0.29 (–0.10, 0.68) |
| Synovitis and osteitis | |||
| From the 12 to 4 months before RA diagnosis | 0.04 (−0.07, 0.16) | Synovitis precedes osteitis | 0.62 (0.08, 1.17)† |
| From 4 months before to RA diagnosis | 0.38 (0.17, 0.59)† | Osteitis precedes synovitis | 0.07 (−0.29, 0.43) |
| Tenosynovitis and osteitis | |||
| From the 12 to 4 months before RA diagnosis | −0.01 (−0.10, 0.08) | Tenosynovitis precedes osteitis | 0.55 (0.23, 0.88)† |
| From 4 months before to RA diagnosis | 0.24 (0, 0.48)† | Osteitis precedes tenosynovitis | 0.06 (−0.27, 0.39) |
Simultaneous change estimates represent the correlation of the proportion of change in inflammation, based on magnetic resonance imaging scores, of 2 joint tissues that is not explained by the subsequent pattern and previous values of inflammation in those tissues. Time order change estimates represent the standardized regression coefficients of change in inflammation in 1 tissue to subsequent change in inflammation in another tissue, corrected for the simultaneous pattern and previous values of inflammation in those tissues. Standardized regression coefficients are independent of scale. Higher values indicate higher explanation of inflammatory change in 1 tissue by change in the previous period of inflammation in another tissue; value of 0 indicates no explanation. Positive estimates indicate that an increase in the first period is associated with more increase in the subsequent period; negative estimates indicate that more increase in the first period is associated with less increase in the subsequent period.
P < 0.05 (significant).
With respect to time orders, an increase in osteitis in the last 4 months before RA development was associated with an increase in synovitis during the preceding interval (from 1 year before to 4 months before) (standardized β 0.62 [95% confidence interval 0.08–1.17]). Similarly, an increase in osteitis in the last 4 months was preceded by an earlier increase in tenosynovitis (β 0.55 [95% confidence interval 0.23–0.88]) (Figure 2 and Table 1). Thus, an increase in osteitis followed earlier increases in synovitis and tenosynovitis. An example of serial MRIs within a patient who progressed to RA that illustrates this time order is shown in Figure 3.
Figure 3.
Serial wrist MRIs of a CSA patient who progressed to RA at 1 year before RA development (A), at 4 months before RA development (B), and on RA development (C). Synovitis, tenosynovitis (both indicated by single arrows), and osteitis (indicated by double arrows) all increased over the 3 time points. The largest increases in tenosynovitis and synovitis are seen between A and B, which is followed by the largest increase in osteitis between B and C. MRIs show mild synovitis of the radiocarpal joint (A); increased synovitis of the radioulnar, radiocarpal, and intercarpal synovium, tenosynovitis of the extensor carpi ulnaris, and some osteitis of the lunatum (B); and increased osteitis in several wrist bones (C). To enhance readability, not every single inflammatory change was indicated by arrows. See Figure 1 for definitions.
Time orders of subclinical joint inflammation in the pain resolution group
In the patients who achieved pain resolution, an average number of 3.8 MRIs per patient were performed during a mean follow-up duration of 24 months. At the 4-month visit, 43% of patients in the pain resolution group were pain free; an equally dispersed number of the remaining patients became pain free over the other visits (Supplementary Figure 2, available on the Arthritis & Rheumatology website at https://onlinelibrary.wiley.com/doi/10.1002/art.42527). In the unmodeled data, we observed a decrease in synovitis, tenosynovitis, and osteitis over the 2-year follow-up. The mean score for tenosynovitis decreased by 0.5 in the first 4 months and a further 0.3 between 4 months and 1 year after inclusion. Mean scores for synovitis and osteitis remained stable in the first 4 months, with 0.4 decreases from 4 months to 2 years after study inclusion. Thus, only the score for tenosynovitis decreases in the first 4 months after presentation in the CSA patients who ultimately developed spontaneous pain resolution (Figure 1B).
When we used cross-lagged analyses to study the time sequences, we observed that synovitis, tenosynovitis, and osteitis decreased simultaneously in the 2 intervals during the first year of follow-up (Figure 4 and Table 2). In addition, we observed time orders involving tenosynovitis. A decrease in tenosynovitis in the first 4 months after CSA onset preceded a decrease in synovitis (β 0.46 [95% confidence interval 0.16–0.76]) and osteitis (β 0.44 [95% confidence interval 0.14–0.75]) in the time interval between the 4- and 12-month follow-up (Figure 4, Table 2). Thus, in patients who achieved pain resolution, inflammation in the tenosynovium appeared to be the first to decrease.
Figure 4.
Simultaneous and subsequent decreases (Δ) in inflammation in pairs of different joint tissues during the 3 intervals in the year before resolution of pain in which a time order was found. Vertical arrows represent a simultaneous inflammatory change in 2 joint tissues. Diagonal arrows represent time orders. Estimates are the proportion of change in inflammation score in 2 tissues not explained by other patterns and are independent of scale. Higher values indicate that a higher proportion of change in inflammation in 1 tissue is explained by change in another tissue, and a value of 0 indicates no explanation. Significant effects are shown in black with asterisk; nonsignificant effects are shown in gray. See Figure 1 for definitions.
Table 2. Estimates of simultaneous changes and time order changes in inflammation in joint tissue pairs in patients who had pain resolution*.
| Tissue pair and time period after study inclusion |
Simultaneous change (95% CI) |
Tissue order and time period | Time order change (95% CI) |
|---|---|---|---|
| Synovitis and tenosynovitis | Synovitis precedes tenosynovitis | ||
| From BL to 4 months | 0.25 (0.13, 0.37)† | From BL to 4 months to 4 to 12 months | 0.24 (−0.02, 0.5) |
| From 4 to 12 months | 0.12 (0.04, 0.21)† | From 4 to 12 months to 12 to 24 months | −0.15 (−0.43, 0.13) |
| From 12 to 24 months | 0.07 (0, 0.14)† | Tenosynovitis precedes synovitis | |
| From BL to 4 months to 4 to 12 months | 0.46 (0.16, 0.76)† | ||
| From 4 to 12 months to 12 to 24 months | −0.41 (−0.89, 0.08) | ||
| Synovitis and osteitis | Synovitis precedes osteitis | ||
| From BL to 4 months | 0.31 (0.06, 0.55)† | From BL to 4 months to 4 to 12 months | 0.28 (−0.03, 0.59) |
| From 4 to 12 months | 0.12 (0.02, 0.23)† | From 4 to 12 months to 12 to 24 months | 0.24 (−0.02, 0.5) |
| From 12 to 24 months | −0.01 (−0.08, 0.05) | Osteitis precedes synovitis | |
| From BL to 4 months to 4 to 12 months | 0.30 (−0.05, 0.56) | ||
| From 4 to 12 months to 12 to 24 months | −0.30 (−0.91, 0.31) | ||
| Tenosynovitis and osteitis | Tenosynovitis precedes osteitis | ||
| From BL to 4 months | 0.27 (0.09, 0.45)† | From BL to 4 months to 4 to 12 months | 0.44 (0.14, 0.75)† |
| From 4 to 12 months | 0.10 (0.01, 0.18)† | From 4 to 12 months to 12 to 24 months | 0.05 (−0.21, 0.32) |
| From 12 to 24 months | −0.02 (−0.06, 0.02) | Osteitis precedes tenosynovitis | |
| From BL to 4 months to 4 to 12 months | 0.19 (−0.04, 0.41) | ||
| From 4 to 12 months to 12 to 24 months | −0.30 (−0.70, 0.09) |
Simultaneous change estimates represent the correlation of the proportion of change in inflammation, based on magnetic resonance imaging scores, of 2 joint tissues that is not explained by the subsequent pattern and previous values of inflammation in those tissues. Time order changes estimates represent the standardized regression coefficients of change in inflammation in 1 tissue to subsequent change in inflammation in another tissue, corrected for the simultaneous pattern and previous values of inflammation in those tissues. Standardized regression coefficients are independent of scale. Higher values indicate higher explanation of inflammatory change in 1 tissue by change in the previous period of inflammation in another tissue; value of 0 indicates no explanation. Positive estimates indicate that an increase in the first period is associated with more increase in the subsequent period; negative estimates indicate that more increase in the first period is associated with less increase in the subsequent period. BL = baseline.
P < 0.05 (significant).
ACPA stratification
Because of previous research that revealed differences in etiopathology of ACPA-positive and ACPA-negative RA, we stratified for ACPA status (14). In the graphical depiction of unmodeled data of CSA patients who progressed to RA, we observed that osteitis scores were overall higher in ACPA-positive patients than in ACPA-negative patients. Similar to the main analyses, in both of the ACPA groups, the increase in synovitis and tenosynovitis seemed to be stronger than the increase in osteitis in the earliest interval (1 year before to 4 months before RA diagnosis) (Figure 1C). We could not use cross-lagged models to confirm that these time orders within the ACPA subgroups because of the low number of patients per stratum.
Sensitivity analysis
We performed a sensitivity analysis to evaluate the trajectories of MRI-detected inflammation in the joints in which clinical arthritis had developed. This sensitivity analysis revealed patterns of simultaneous change and time orders similar to the main analyses (Supplementary Table 2, available at https://onlinelibrary.wiley.com/doi/10.1002/art.42527).
Finally, we performed a sensitivity analysis for the RA progression group with cross-lagged models, after ensuring equal time intervals between the MRIs by using time since baseline and not analyzing the 3 MRIs that were performed more than 4 weeks from the protocol time point. This yielded comparable conclusions on time orders and simultaneous change as the primary analysis in this group (Supplementary Table 3, available at https://onlinelibrary.wiley.com/doi/10.1002/art.42527).
Discussion
Although clinical arthritis is the key feature of RA and is required for its diagnosis, the trajectory through which clinical arthritis develops remains elusive. With the ultimate aim to better understand how clinical arthritis develops and with the benefit of having a unique data set of serial MRIs in CSA patients, we studied whether natural time sequences exist in which subclinical inflammation evolves in different joint tissues during RA development and spontaneous resolution of arthralgia without intervention. For the first time, we observed distinct natural time orders in the development of subclinical inflammation in different joint tissues within these 2 different at-risk subgroups. During progression to RA, osteitis was the last to expand, and, during pain resolution, tenosynovitis resolved first. These findings increase our understanding of the processes in the joint during the trajectory of clinical arthritis development in RA. Knowledge of time sequences may eventually become clinically relevant by developing a staging method for CSA patients; incorporation of time sequences of subclinical joint inflammation in risk stratification could improve prognostication of arthralgia patients who are at risk of RA.
In CSA patients who progressed to RA, the largest increase in osteitis was preceded by initial increases in synovitis and tenosynovitis. Our determination of these time orders for the first time, to our knowledge, is a step forward in understanding how clinical arthritis develops in the joint. This time order can be further supported by comparing MRI scores observed in symptom-free controls in a previous study with scores of progressors at CSA onset in our study (on average 11 months before RA development) (5). This comparison shows that osteitis scores in RA progressors at CSA onset in our present study have an interquartile range from 0.3 to 2.0, which is similar to the interquartile range in symptom free-controls from a general population of people age 40–60 years (0.3–2.0) (5). On the other hand, synovitis and tenosynovitis scores at CSA onset in patients who ultimately developed RA had interquartile ranges of 1–4.5 and 0.5–4.5, respectively, which are higher than in symptom-free controls, who had interquartile ranges synovitis and tenosynovitis scores of 0.5–1.5 and 0–0.125, respectively (5).
Although our serial MRI study started in CSA patients with a positive MRI and not in a healthy state, these comparisons support the conclusions of our present study by suggesting that especially synovitis and tenosynovitis were increased at the time of the first MRI, in contrast to osteitis. Our present study also aligns with findings from preclinical animal studies that suggested that tenosynovitis precipitates osteoclast invasion of the adjacent juxtaarticular bone in very early stages of arthritis; however, synovitis was not evaluated in this previous study (15).
Interestingly, the graphical depictions stratified for ACPA suggested that a “final” increase in osteitis was present in both ACPA-positive and ACPA-negative patients who developed RA. Although we know from earlier research that differences in genetic and environmental risk factors and underlying autoimmune responses exist between the ACPA subgroups, our present study might suggest that similarities exist at the joint level in both subgroups, perhaps indicating a final common pathway in the development of clinical arthritis (14). The similar clinical presentation on diagnosis of patients with ACPA-positive and ACPA-negative RA may also be indicative of this (16). Unfortunately, patient numbers were too low to perform cross-lagged model analyses separately for the ACPA stratification groups. Future research is needed for this subject. Because osteitis cannot be detected with ultrasound, future research on this topic should also be performed with MRI as the imaging modality.
In our study, CSA patients with subclinical joint inflammation who experienced spontaneous resolution of joint pain showed decreasing tenosynovitis that preceded decreasing synovitis and osteitis. In earlier cross-sectional research in CSA, tenosynovitis was shown to be strongly associated with joint symptoms and functioning (17). Although the attention of longitudinal studies is generally focused on progression to RA, understanding the processes related to spontaneous resolution may be equally informative. An understanding of the first changes of inflamed tissues may be helpful to guide future studies. For instance, molecular studies of local mechanisms critical to resolution of subclinical joint inflammation can focus first on tenosynovitis and take biopsies from this tissue.
In our present study, participants were included if they had subclinical inflammation in at least 1 location, as this was a trial inclusion criterium. Therefore, a first development of subclinical inflammation could not be captured within the follow-up of this study, which could be considered a limitation. In addition, the earliest time interval spanned 8 months (1 year to 4 months before diagnosis). Hence, the earliest changes may not have been captured. An ideal subsequent serial MRI study design to distinguish time orders in the development of tenosynovitis and synovitis during progression to RA would encompass more repeated MRIs with smaller time intervals. In that way, patients who develop RA quickly would have multiple MRIs before diagnosis. Such a study is impossible with the current Rheumatoid Arthritis Magnetic Resonance Imaging Scoring protocol because of the high costs related to the long scan times and because contrast agent administration is required to depict synovitis and tenosynovitis and high frequent administration of intravenous contrast agent may increase the risk of gadolinium depositions (18). Advanced MRI protocols however require less scanning time (<10 minutes) and do not need intravenous contrast administration (19). These advances may allow for frequently repeated MRIs in the near future and make it possible to discern more specifically in which joint tissue inflammation starts during the development of RA.
In the pain resolution group, MRI was performed upon inclusion, at 4 months, at 12 months, and at 24 months. Because part of the patients in this group already achieved pain resolution within 4 months, some patients only had an MRI before and after but not during pain resolution. In addition, the final interval spanned 12 months. Both factors could contribute to the explanation of why we could not differentiate a later sequence in descending synovitis and osteitis.
We used cross-lagged models to evaluate the influence of both simultaneous changes and time orders of inflammation in pairs of tissues at the same time, while adjusting for the other pattern. Associations found using statistical modeling cannot formally prove causation. Nevertheless, the time patterns observed here can be regarded as an approximation of the trajectory of inflamed tissues.
A strength of our work is that both the patients and the rheumatologists were blinded for serial MRI data. Outcomes of RA development and pain resolution were therefore not influenced by the MRI results.
At the time of our study, several clinical trials have been performed in which individuals at risk of RA were treated before arthritis development with various pharmacologic interventions. So far, among the trials published in peer-reviewed journals, the treatments at most delayed RA development but did not prevent the development of this burdensome chronic disease (8,20,21). This highlights the urgency to unravel the mechanisms that are driving the development of clinical arthritis, of which we have barely scratched the surface. Ultimately, molecular research of joint tissues is needed to understand these crucial mechanisms, for which it is essential to know the sequences in which different joint tissues become inflamed or in which inflammation resolves.
Another possible implication of our present work is related to prognostication of patients with CSA, which is of clinical relevance. So far, research on prognostication has used a single measurement, frequently measured when patients first present with arthralgia or CSA, to predict an outcome such as RA development (3,22). We now show that inflammation of different joint tissues evolves in a specific order. A further understanding of the trajectories and temporal orders may propose an emerging opportunity to advance prognostication toward staging, as used, for example, for different types of malignancies or chronic kidney disease. The development of a staging system in CSA, using the earlier mentioned MRI techniques that allow serial MRIs at shorter intervals, is a subject for further research (19). Once a staging system for the trajectory from CSA to RA is developed, possible future treatment of arthralgia patients might be different for various substages, with an aim of inducing recovery toward a better stage.
In conclusion, our work, for the first time to our knowledge, showed time orders in the development of subclinical inflammation in CSA patients during progression to RA and resolution of pain. This knowledge increases our understanding of RA development and hence could fuel better prognostication and targeted therapeutic interventions in the future for patients at risk of RA.
Supplementary Material
Acknowledgments
We thank G. Kracht for assistance in preparing the MRIs for Figure 3.
Footnotes
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Krijbolder had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Krijbolder, Matthijssen, van der Helmvan Mil.
Acquisition of data. Krijbolder, Matthijssen, van Dijk, van Steenbergen, Boeters, Willemze, Schouffoer, van der Helm-van Mil.
Analysis and interpretation of data. Krijbolder, Matthijssen, van Dijk, van Steenbergen, Boeters, Willemze, Schouffoer, van der Helm-van Mil.
References
- 1.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580–8. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- 2.Scherer HU, Häupl T, Burmester GR. The etiology of rheumatoid arthritis. J Autoimmun. 2020;110:102400. doi: 10.1016/j.jaut.2019.102400. [DOI] [PubMed] [Google Scholar]
- 3.Van Steenbergen HW, Mangnus L, Reijnierse M, et al. Clinical factors, anticitrullinated peptide antibodies and MRI-detected subclinical inflammation in relation to progression from clinically suspect arthralgia to arthritis. Ann Rheum Dis. 2016;75:1824–30. doi: 10.1136/annrheumdis-2015-208138. [DOI] [PubMed] [Google Scholar]
- 4.Brinck RM, Boeters DM, van Steenbergen HW, et al. Improvement of symptoms in clinically suspect arthralgia and resolution of subclinical joint inflammation: a longitudinal study in patients that did not progress to clinical arthritis. Arthritis Res Ther. 2020;22:11. doi: 10.1186/s13075-020-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mangnus L, van Steenbergen HW, Reijnierse M, et al. Magnetic resonance imaging-detected features of inflammation and erosions in symptom-free persons from the general population. Arthritis Rheumatol. 2016;68:2593–602. doi: 10.1002/art.39749. [DOI] [PubMed] [Google Scholar]
- 6.Boer AC, Burgers LE, Mangnus L, et al. Using a reference when defining an abnormal MRI reduces false-positive MRI results-a longitudinal study in two cohorts at risk for rheumatoid arthritis. Rheumatology (Oxford) 2017;56:1700–6. doi: 10.1093/rheumatology/kex235. [DOI] [PubMed] [Google Scholar]
- 7.Brinck RM, van Steenbergen HW, van der Helm-van Mil AH. Sequence of joint tissue inflammation during rheumatoid arthritis development. Arthritis Res Ther. 2018;20:260. doi: 10.1186/s13075-018-1756-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krijbolder DI, Verstappen M, van Dijk BT, et al. Intervention with methotrexate in patients with arthralgia at risk of rheumatoid arthritis to reduce the development of persistent arthritis and its disease burden (TREAT EARLIER): a randomised, double-blind, placebo-controlled, proof-of-concept trial. Lancet. 2022;400:283–94. doi: 10.1016/S0140-6736(22)01193-X. [DOI] [PubMed] [Google Scholar]
- 9.Wolfe F, Michaud K. Assessment of pain in rheumatoid arthritis: minimal clinically significant difference, predictors, and the effect of anti-tumor necrosis factor therapy. J Rheumatol. 2007;34:1674–83. [PubMed] [Google Scholar]
- 10.Ostergaard M, Peterfy C, Conaghan P, et al. OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging Studies: core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. J Rheumatol. 2003;30:1385–6. [PubMed] [Google Scholar]
- 11.Haavardsholm EA, Ostergaard M, Ejbjerg BJ, et al. Introduction of a novel magnetic resonance imaging tenosynovitis score for rheumatoid arthritis: reliability in a multireader longitudinal study. Ann Rheum Dis. 2007;66:1216–20. doi: 10.1136/ard.2006.068361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selig J, Little T. In: Handbook of developmental research methods. Laursen B, Little TD, Card NA, editors. Guilford; New York: 2012. Autoregressive and cross-lagged panel analysis for longitudinal data; pp. 265–78. [Google Scholar]
- 13.Enders CK, Bandalos DL. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Struct Equ Modeling. 2001;8:430–57. [Google Scholar]
- 14.Van der Woude D, van der Helm-van Mil AH. The target of ACPA. Rheumatology (Oxford) 2016;55:1711–3. doi: 10.1093/rheumatology/kew270. [DOI] [PubMed] [Google Scholar]
- 15.Hayer S, Redlich K, Korb A, et al. Tenosynovitis and osteoclast formation as the initial preclinical changes in a murine model of inflammatory arthritis. Arthritis Rheum. 2007;56:79–88. doi: 10.1002/art.22313. [DOI] [PubMed] [Google Scholar]
- 16.Burgers LE, van Steenbergen HW, ten Brinck RM, et al. Differences in the symptomatic phase preceding ACPA-positive and ACPA-negative RA: a longitudinal study in arthralgia during progression to clinical arthritis. Ann Rheum Dis. 2017;76:1751–4. doi: 10.1136/annrheumdis-2017-211325. [DOI] [PubMed] [Google Scholar]
- 17.Rogier C, Hayer S, van der Helm-van Mil A. Not only synovitis but also tenosynovitis needs to be considered: why it is time to update textbook images of rheumatoid arthritis. Ann Rheum Dis. 2020;79:546–7. doi: 10.1136/annrheumdis-2019-216350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology. 2015;275:772–82. doi: 10.1148/radiol.15150025. [DOI] [PubMed] [Google Scholar]
- 19.Boeren AM, Niemantsverdriet E, Verstappen M, et al. Towards a simplified fluid-sensitive MRI protocol in small joints of the hand in early arthritis patients: reliability between modified Dixon and regular gadolinium enhanced TSE fat saturated MRI-sequences. Skeletal Radiol. 2023;52:1193–202. doi: 10.1007/s00256-022-04238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bos WH, Dijkmans BA, Boers M, et al. Effect of dexamethasone on autoantibody levels and arthritis development in patients with arthralgia: a randomised trial. Ann Rheum Dis. 2010;69:571–4. doi: 10.1136/ard.2008.105767. [DOI] [PubMed] [Google Scholar]
- 21.Gerlag DM, Safy M, Maijer KI, et al. Effects of B-cell directed therapy on the preclinical stage of rheumatoid arthritis: the PRAIRI study. Ann Rheum Dis. 2019;78:179–85. doi: 10.1136/annrheumdis-2017-212763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthijssen X, Van Dijk B, Wouters F, et al. FRI0542 obtaining high positive predictive values for the development of clinically apparent arthritis in patients presenting with clinically suspect arthralgia; is it feasible? Ann Rheum Dis. 2020;79(Suppl):872. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.