Abstract
Autoreactive B cells mediate autoimmune pathology, but exactly how remains unknown. A hallmark of rheumatoid arthritis (RA), a common autoimmune disease, is the presence of disease-specific anticitrullinated protein antibodies (ACPAs). Here, we showed that ACPA-positive B cells in patients with RA strongly expressed T cell–stimulating ligands, produced abundant proinflammatory cytokines, and were proliferative while escaping inhibitory signals. This activated state was found at different degrees in different stages of disease: highest in patients with recent-onset RA, moderate in patients with established RA, and far less pronounced in ACPA-positive individuals “at risk” for developing disease. The activated autoreactive B cell response persisted in patients who achieved clinical remission with conventional treatment. ACPA-positive B cells in blood and synovial fluid secreted increased amounts of the chemoattractant interleukin-8, which attracted neutrophils, the most abundant immune cell in arthritic joints. Tetanus toxoid–specific B cells from the same patients exhibited properties of memory B cells without the activation and proliferation phenotype, but these cells transiently acquired a similar proliferative phenotype upon booster vaccination. Together, these data indicated that continuous antigenic triggering of autoreactive B cells occurs in human autoimmune disease and support the emerging concept of immunological activity that persists under treatment even in clinical remission, which may revise our current concept of treatment targets for future therapeutic interventions. In addition, our data pointed to a pathogenic role of ACPA-positive B cells in the inflammatory disease process underlying RA and favor approaches that aim at their antigen-specific inactivation or depletion.
Introduction
The role of autoreactive B cells in the pathogenesis of human auto-immune diseases is poorly understood. Autoantibodies are frequently detectable before the onset of clinical symptoms. This predisease phase of clinically “silent” autoimmunity can last for years, and the triggers that promote the transition to autoimmune disease are usually unknown. Although autoantibodies themselves may or may not be pathogenic, it is remarkable that many autoimmune diseases are responsive to CD20+ B cell depletion. This indicates that B cells, and in particular, the autoreactive subset(s), are crucially involved in disease-relevant pathogenic processes. Hence, it is of considerable importance to understand the phenotypic and functional characteristics of these cells in various phases of autoimmunity and in established disease to decipher both the initiating triggers and the contribution of B cells to human disease.
Rheumatoid arthritis (RA) is a common autoimmune disease that causes inflammation and destruction of joints. Most patients harbor anticitrullinated protein antibodies (ACPAs), which are antibodies that recognize proteins in which arginine residues have been posttranslationally modified to citrulline. ACPA-positive RA is thought to evolve as a multistep process in which the break of immunological tolerance to citrullinated antigens precedes the onset of clinical disease (1). ACPAs are considered risk factors for disease development and, in established RA, prognosticate erosive joint destruction (2). The transition from ACPA-positive autoimmunity to autoimmune disease is associated with the development of inflammatory joint pain (arthralgia) (3) and an expansion of the ACPA response (1). The latter is marked by rising ACPA serum titers, spreading of epitope recognition, and an expanding number of detectable ACPA isotypes and likely driven by T cells under the influence of human leukocyte antigen (HLA) susceptibility molecules (4–7). How this predisease expansion of the humoral autoimmune response relates to the initiation of joint inflammation remains unclear. Potentially, early cartilage damage, fibroblast activation, the generation of citrullinated antigens in the synovium, and the migration of ACPA-positive B cells or plasmablasts toward these triggers could be involved.
Established RA is remarkably responsive to CD20+ B cell–depleting therapy, a feature shared with several other autoimmune diseases (8). The efficacy of B cell depletion on clinical measures of disease activity is greater in patients with ACPA-positive RA than in ACPA-negative patients (9). Clinical improvement upon anti-CD20 therapy precedes the reduction of ACPA serum concentrations (10), and patients can achieve long-term remission despite the persistence of high-titer ACPA. These observations suggest that CD20+ B cells—in particular autoreactive, ACPA-positive B cells—could play a central role in driving and maintaining the inflammatory disease process. We previously identified ACPA-positive B cells in peripheral blood of patients with RA using differentially labeled streptavidin tetramers bound to second-generation cyclic citrullinated peptides (CCP2) (11). ACPA-positive B cells circulate in peripheral blood in low frequency as class-switched cells of which most are positive for CD20 and CD27, suggesting a memory phenotype (12). In addition, ACPA-secreting plasmablasts and plasma cells are present in the inflamed joint in which they encounter an inflammatory environment favoring long-term survival (13).
Here, we investigated the molecular characteristics of these cells and defined their pathogenic effector functions. We analyzed protective tetanus toxoid (TT)–specific and autoreactive ACPA-positive B cells directly ex vivo from individual patients with RA and from ACPA-positive individuals at risk for developing disease. We found that ACPA-positive B cells in patients with established RA display an activated, proliferative memory phenotype and reduced abundance of the inhibitory receptor CD32. This phenotype was most prominent in patients with recent-onset disease, whereas in the at-risk phase of ACPA-positive arthralgia preceding RA [a phase in which synovial inflammation is absent (14, 15)], ACPA-positive B cells showed signs of proliferation with low abundance of other activation markers. ACPA-positive B cells isolated from the inflamed synovial compartment during established disease produced proinflammatory cytokines spontaneously ex vivo, in particular the functionally active neutrophil chemoattractant interleukin 8 (IL-8). These data suggested that the onset of RA associates with a strongly activated phenotype of ACPA-positive B cells. These B cells were ideally equipped to communicate with T cells and remained constantly activated throughout the established phase of disease, proliferating and capable of attracting neutrophils to joints through the secretion of IL-8.
Results
Identification of autoreactive B cells directed against citrullinated antigens in RA
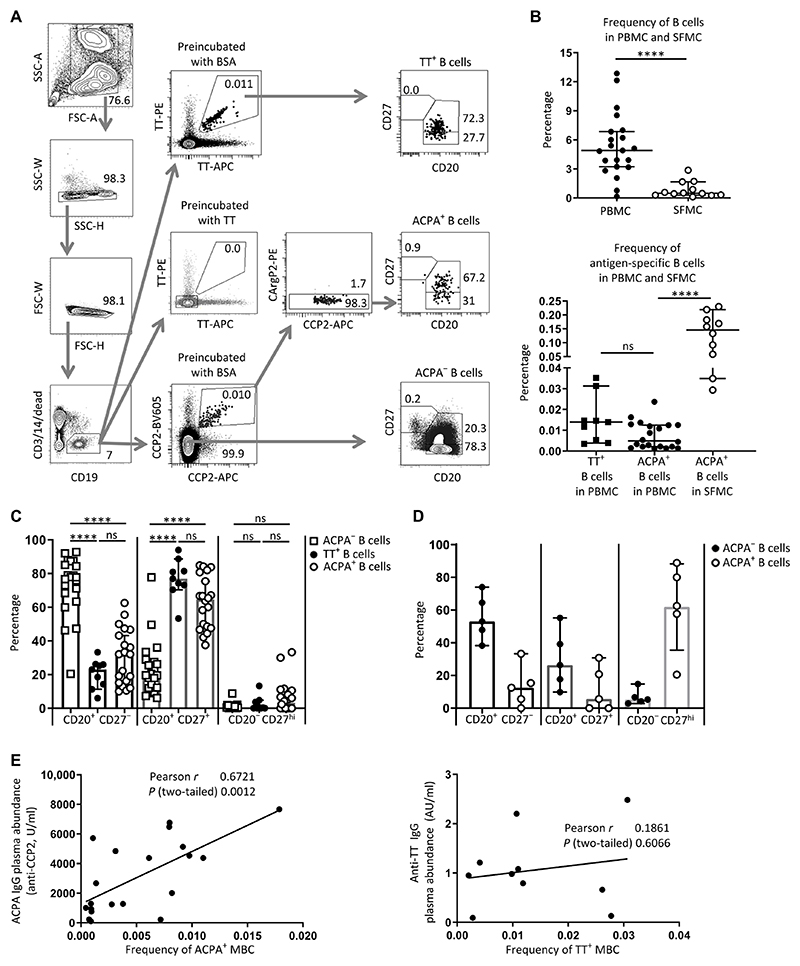
The very low frequency of antigen-specific B cell populations in the circulation challenges their reliable phenotypic characterization by flow cytometric assessment of individual surface markers. Therefore, we opted to compare the phenotype of ACPA-positive B cells not only to the large pool of B cells of undefined specificity but also to a circulating, antigen-specific B cell population that occurs at a similar frequency in patients. We simultaneously evaluated ACPA-positive and TT-specific B cells from individual patients. We used a dual-staining approach for both specificities using differentially labeled antigens to increase and ensure the elimination of nonspecific background signals (Fig. 1A) (11, 16). Blocking experiments were used as additional measure of specificity. Analysis of peripheral blood mononuclear cells (PBMCs) from ACPA-positive RA donors revealed that TT-specific B cells circulated in patients with RA at a frequency comparable to that of ACPA-positive B cells (0.015% for TT-specific B cells and 0.01% for ACPA-positive B cells) (Fig. 1B). Moreover, >70% of TT-specific B cells were positive for CD20 and CD27, indicative of memory B cells (MBCs); 65% of ACPA-positive B cells displayed this phenotype (Fig. 1C). Unlike ACPA-positive B cells, CD20−CD27hi plasmablasts or plasma cells were generally absent from the TT-specific compartment, although the differences in these B cell populations were not statistically significant. These data indicated that CD20+CD27+ B cells, hereafter referred to as MBCs, of both antigen-specific immune responses circulate at similar frequency in the peripheral blood of individual patients with RA, which enables direct phenotypic comparison.
Fig. 1. Identification and subset characterization of ACPA-positive and tetanus toxoid–specific B cells in RA.
(A) Gating strategy. Peripheral blood mononuclear cells (PBMCs) from single donors (patients with RA, n = 21) were divided into three fractions and stained with either CCP2 and CArgP2 streptavidin tetramers to identify ACPA+ B cells or with directly labeled tetanus toxoid (TT) to identify TT+ B cells. Preincubation with unlabeled TT or bovine serum albumin (BSA) was used to demonstrate specificity of the TT staining. Subsets of B cells were delineated by the presence of CD20 and CD27. SSC, side scatter; FSC, forward scatter. (B) Top: Frequency of CD19+ B cells in PBMC and synovial fluid mononuclear cells (SFMCs). Bottom: Frequency of TT+ B cells and ACPA+ B cells in PBMCs versus ACPA+ B cells in SFMCs. (C) Subset distribution of ACPA-negative (ACPA−), TT+, and ACPA+ B cells in PBMCs based on CD20 and CD27 abundance (n = 21). ns, not significant. (D) Subset distribution of ACPA+ and ACPA− B cells in SFMCs (n = 5). (E) Correlation between the frequency of ACPA+ memory B cells (MBCs) (defined as CD20+CD27+) in PBMC and plasma ACPA IgG concentrations (left, n = 20) and correlation between the frequency of TT+CD20+CD27+ B cells and plasma anti-TT IgG antibodies (right, n = 10). The correlation between ACPA+ MBCs and plasma ACPA IgG remains upon removal of the data point with the highest ACPA plasma concentration (r = 0.57; P = 0.01). In (B) to (E), each dot represents one patient sample. ****P ≤ 0.0001. Two-tailed Mann-Whitney test in upper panel of (B), one-way ANOVA with Dunn’s multiple comparison test in lower panel of (B) and in (C) and (D); Pearson correlation in (E). All data represent median ± 95% confidence interval; n = number of donors. AU, arbitrary units.
We also isolated cells from the synovial fluid of inflamed joints and found that ACPA-positive B cells were present at enhanced frequency (Fig. 1B) compared with their frequency in PBMCs. Furthermore, >50% of cells were CD20−CD27hi, indicative of plasmablasts or plasma cells (Fig. 1D and fig. S1). The frequency of TT-specific B cells was not analyzed in this compartment because of the low number of total B cells, which precluded simultaneous use of the material for concurrent antigen-specific B cell analysis.
The frequency of ACPA-positive MBCs in PBMC correlated with ACPA–immunoglobulin G (IgG) plasma concentrations in the samples analyzed (Fig. 1E), confirming previous results (11). In the same patients, no correlation was observed between the frequency of TT-specific MBCs and anti-TT IgG in plasma (Fig. 1E).
Circulating ACPA-positive B cells in established RA display an activated and proliferative phenotype
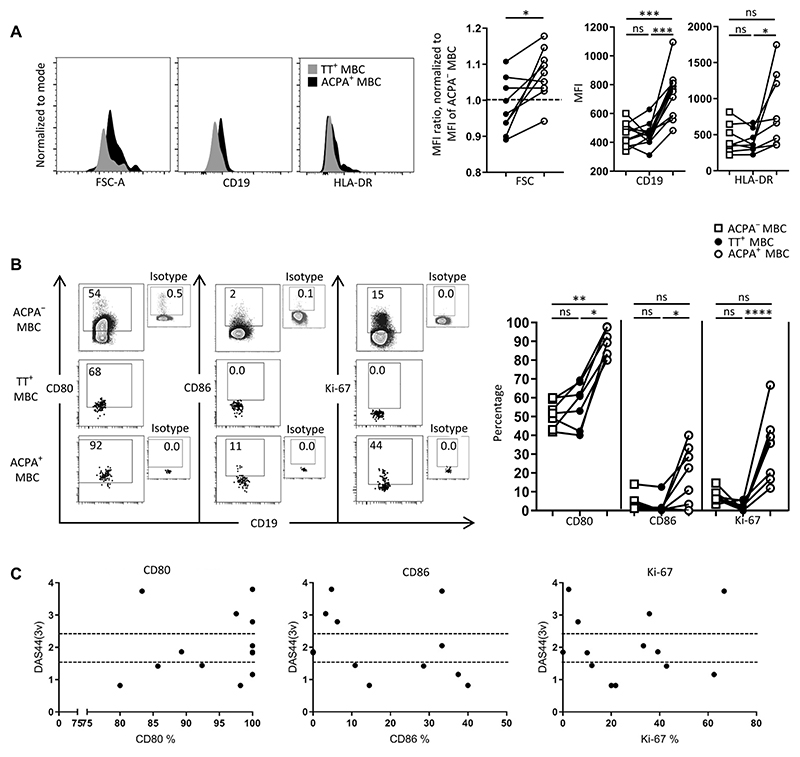
We evaluated the abundance of selected markers on ACPA-positive and TT-specific B cells in peripheral blood. In patients with established RA, ACPA-positive MBCs were larger in size and had higher intensity staining for the stimulatory receptor and mature B cell marker CD19 and for the antigen-presenting molecule HLA-DR than did TT-specific counterparts from the same individual (Fig. 2A). Moreover, more ACPA-positive B cells were also positive for the ligands CD80 and CD86, which are involved in stimulating T cells, than were TT-specific MBCs or ACPA-negative MBCs. In addition, TT-specific MBCs were negative for the proliferation marker Ki-67, whereas an average of 36% (range, 17 to 67%) of ACPA-positive MBCs were positive for this marker (Fig. 2B and fig. S2). Gene expression analysis by reverse transcription polymerase chain reaction (RT-PCR) confirmed the enhanced transcript abundance for CD19, CD86, and CD80 between circulating ACPA-positive MBCs and ACPA-negative MBCs (fig. S3).
Fig. 2. Phenotypic characteristics of memory (CD20+CD27+) ACPA−, TT+, and ACPA+ B cells in RA patient–derived PBMCs.
(A and B) Forward scatter and proportion of MBCs positive for activation markers (CD19, HLA-DR, CD80, CD86, and Ki-67) in the respective cell populations in PBMCs of individual donors in which both ACPA-positive (ACPA+) and TT-specific (TT+) B cells were assessed in parallel (n = 19). Median fluorescence intensity (MFI) was analyzed for markers present in the entire cell population (FSC, CD19, and HLA-DR). Subsets of cells were positive for CD80, CD86, and Ki-67. These data are depicted as percentage positive cells within the respective cell population (CD80, CD86, HLA-DR, and Ki-67 were assessed in n = 8 of 19 donors). FSC data were compiled from samples that had not undergone permeabilization to avoid influencing cell size (n = 9 of 19). Connected dots depict data from individual patients. (C) Correlation between clinical disease activity parameters and the characteristics of ACPA+ B cells [n = 20; see fig. S4 for data on erythrocyte sedimentation rate (ESR)]. Data are presented as percentage of ACPA+ MBCs positive for the respective marker in relation to the disease activity score (DAS). The DAS was calculated on the basis of three variables {3v: ESR and an evaluation of 44 joints for signs of pain (tender joint count) and swelling (swollen joint count) [DAS44(3v)]}. The dashed lines represent the category of disease activity, with a score >2.4 being high disease activity, >1.6 and <2.4 being moderate disease activity, and <1.6 low disease activity or remission. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001. Two-tailed Wilcoxon signed-rank test in (A) for the FSC graph, one-way ANOVA with Dunn’s multiple comparisons test in (A) for the CD19 and HLA-DR graphs and (B). n = number of donors.
Thus, these ACPA-positive B cells had markers of an “activated” phenotype and of a memory phenotype. Therefore, we refer to them as activated MBCs (aMBCs). The ACPA-positive aMBCs had functionally important phenotypic differences when compared with protective (TT-specific) MBCs in the steady state in patients with established RA.
The frequency of these ACPA-positive aMBCs did not correlate with measures of systemic inflammation (erythrocyte sedimentation rate, fig. S4) or composite disease activity scores (Fig. 2C). Together, these results suggested continuous immune activation of the auto-reactive B cell response in patients with established RA, irrespective of disease control by conventional synthetic disease-modifying antirheumatic drugs (csDMARDs).
ACPA-positive B cells in individuals with joint pain who are “at risk” for developing RA
In most patients with RA, the presence and expansion of ACPA-positive B cells precedes clinically detectable arthritis (1). Given the activated, proliferative phenotype of ACPA-positive B cells within the CD20+CD27+ compartment in established disease, we investigated the properties of ACPA-positive B cells in ACPA-positive individuals with joint pain (arthralgia) who had, so far, shown no clinical signs of arthritis (Table 1).
Table 1. Characteristics of patients with ACPA-positive arthralgia and established RA included in the study.
| At-risk arthralgia | Established RA | |
|---|---|---|
| Number of patients | 8 | 20 |
| ACPA-IgG plasma concentrations | ||
| Median (AU/ml) | 420 | 2343 |
| Range (AU/ml) | 31–2563 | 101–7667 |
| Frequency of ACPA-positive B cells in total PBMCs |
||
| Median (%) | 0.0017 | 0.0049 |
These individuals harbored similar frequencies of circulating ACPA-positive B cells compared to patients with RA with matched ACPA-IgG plasma abundance (fig. S5, A and B). A correlation between the frequency of ACPA-positive B cells and ACPA IgG serum abundance was not established in this population, possibly as a consequence of one outlying donor with a high frequency of ACPA-positive B cells (fig. S5C). Upon removal of this donor from the analysis, however, a correlation was noted (Pearson r = 0.81, P = 0.02; fig. S5C, dotted line) similar to the one observed in patients with RA (Fig. 1E). Furthermore, most ACPA-positive B cells in these individuals were of the MBC (CD20+CD27+) phenotype (fig. S5D), whereas lower numbers of differentiated plasmablasts or plasma cells (CD20−CD27hi) were detectable compared to patients with established disease (median 0% in arthralgia versus 4% in RA for total ACPA-positive B cells).
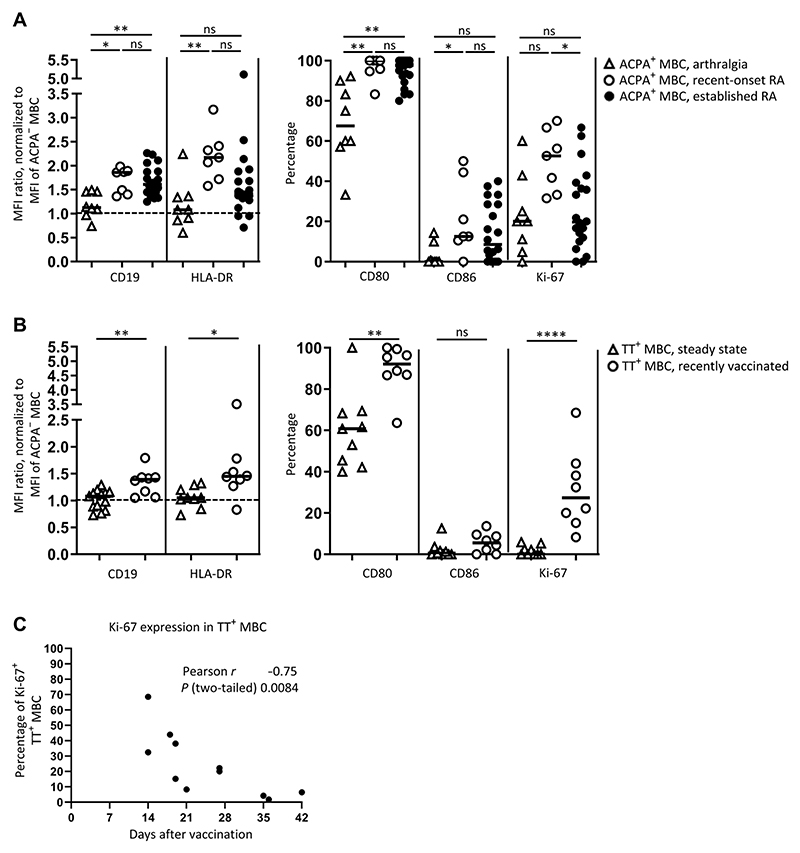
We focused on the memory cell compartment and compared the phenotype of these cells in the at-risk individuals and those with established RA. We observed similar frequencies of ACPA-positive MBCs that were positive for Ki-67 (median, 20%; range, 0 to 60%) from individuals with arthralgia and those with established RA (Fig. 3A). The other markers indicative of activation, however, were not increased (Fig. 3A). Instead, the abundance of CD19 and HLA-DR expressed by ACPA-positive MBCs was comparable to the steady-state TT-specific MBC compartment of patients with RA, as was the frequency of cells expressing CD80 and CD86 (Fig. 3B and fig. S6). Thus, despite the increased proportion of cells positive for Ki-67, we observed a far less activated phenotype of ACPA-positive MBCs in individuals with joint pain (arthralgia) that so far lacked the clinical signs of arthritis.
Fig. 3. Comparison of the characteristics of ACPA-positive MBCs in different phases of disease.
ACPA-positive MBCs were analyzed from patients with arthralgia (n = 8), recent-onset, untreated RA (n = 7), and established, treated RA (n = 20) and compared to TT-specific B cells in the steady state (assessed in n = 13 patients with established, treated RA) and in recently vaccinated patients with RA (n = 11). (A) The abundance and percentage of cells positive for CD19, HLA-DR, CD80, CD86, or Ki-67 within the ACPA+ MBCs from the indicated RA disease stages. (B) The abundance and percentage of cells positive for CD19, HLA-DR, CD80, CD86, or Ki-67 within the TT-specific (TT+) MBCs from patients with RA in the steady state (n = 13) and in patients upon recent vaccination (assessed within 30 days after booster immunization, n = 8 of 11). (C) Correlation of percent of Ki-67-positive, TT-specific (TT+) MBCs with time after vaccination with TT in untreated patients with RA (includes three patients assessed >30 days after booster immunization, n = 11). Every dot represents an individual patient; lines represent median values. *P ≤ 0.05, **P ≤ 0.01, and ****P ≤ 0.0001. One-way ANOVA with Dunn’s multiple comparisons test in (A); two-tailed Mann-Whitney test in (B); Pearson correlation in (C). n = number of donors.
ACPA-positive B cells at the onset of clinical disease
Given the differential phenotype of ACPA-positive MBCs in patients with established disease compared to individuals with joint pain (arthralgia), we questioned whether the onset of RA associates with autoreactive B cells displaying phenotypic characteristics of aMBC. We evaluated cells from patients with recent-onset, ACPA-positive RA who had not yet started immunosuppressive medication. In this early, untreated phase of disease, we noted a high abundance and proportion of ACPA-positive MBCs positive for the activation markers that we had found in these cells in patients with established disease (Fig. 3A). The intensity of HLA-DR staining and the proportion of cells positive for Ki-67 exceeded those observed under treatment.
Although longitudinal samples would be required to firmly establish the differences observed between the phases of arthralgia, early-onset disease, and established RA in individual donors, our data indicated that early-onset, ACPA-positive RA is characterized by a highly activated and proliferative phenotype of ACPA-positive MBCs. The data further suggested that therapeutic intervention partially reduces the activated phenotype of ACPA-positive MBCs. However, in the patients studied, treatment failed to “silence” these cells to a resting state comparable to that of a “conventional” memory recall response, in this case directed against TT.
Vaccine-induced TT-specific B cells display a phenotype similar to ACPA-positive B cells
We sought to understand triggers that induce an aMBC phenotype in ACPA-positive B cells. We hypothesized that ACPA-positive aMBC resemble memory cells that have recently left the germinal center in response to detection of antigen. Such “recent germinal center emigrant” MBCs exhibit the markers Ki-67, CD27, and elevated expression of CD19, among others (17). To test our hypothesis in the human setting, we administered TT booster vaccination to 11 patients with RA and assessed the phenotype of circulating TT-specific MBC in 8 of 11 patients within 28 days after vaccination and in the remaining 3 at a later time. Upon vaccination, the intensity of staining for CD19 and HLA-DR, along with the percent of cells positive for CD80 and Ki-67, was higher on TT-specific MBCs compared with that of TT-specific MBCs from patients with RA in the steady state (Fig. 3B). The abundance of expression of these activation markers in TT-specific MBCs in the recently vaccinated patients with RA was similar to those of ACPA-positive aMBCs (Fig. 3 and fig. S6). However, with increasing time after vaccination, the proliferative MBC phenotype of TT-specific MBCs ceased; the proportion of TT-specific MBCs positive for Ki-67 decreased with time after tetanus booster vaccination (Fig. 3C). Although the other activation markers also decreased, the change was not significant over the 42 days that the vaccinated individuals were monitored (fig. S7). These observations suggested that the phenotype of ACPA-positive aMBCs results from continuous, antigen-driven activation of ACPA-positive MBCs. These cells fail to reach the state of quiescence.
ACPA-positive B cells in established RA down-regulate the inhibitory receptor CD32
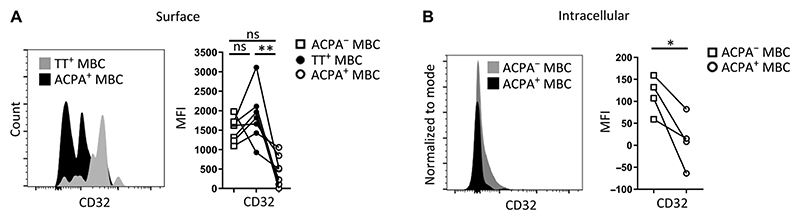
B cell activation during conventional immune responses is counter-balanced by inhibitory signals that enable controlled regulation of the immune response. Immune complexes, for example, can engage the inhibitory Fcγ-receptor IIb (CD32b), thereby providing negative feedback signals that dampen the activation of antigen-specific B cells. To study whether ACPA-positive aMBCs are susceptible to these control mechanisms, we evaluated the abundance of CD32 on these and TT-specific MBCs. Although TT-specific MBCs in the steady state had similar amounts of CD32 at the cell surface as was present on the total MBC pool (ACPA-negative MBCs), circulating ACPA-positive aMBCs had reduced abundance of this receptor at the cell surface (Fig. 4A). We also detected reduced amounts of intracellular CD32, indicating that down-regulation rather than internalization of CD32 is responsible for the reduction at the cell surface (Fig. 4B). These data suggested that ACPA-positive aMBCs are not only continuously activated and driven to proliferate but also escape inhibitory control mechanisms.
Fig. 4. Presence of the inhibitory receptor CD32 on ACPA+ MBCs and TT-specific MBCs from patients with established RA.
(A) Abundance of CD32 on the surface of ACPA+ or TT-specific MBCs (n = 7). (B) Abundance of intracellular CD32 in ACPA+ or ACPA− MBCs (n = 4). Connected dots depict data from individual patient samples. *P ≤ 0.05, **P ≤ 0.01. One-way ANOVA with Dunn’s multiple comparisons test in (A), two-tailed Wilcoxon signed-rank test in (B). n = number of donors.
Inflammatory cytokine production by ACPA-positive B cells
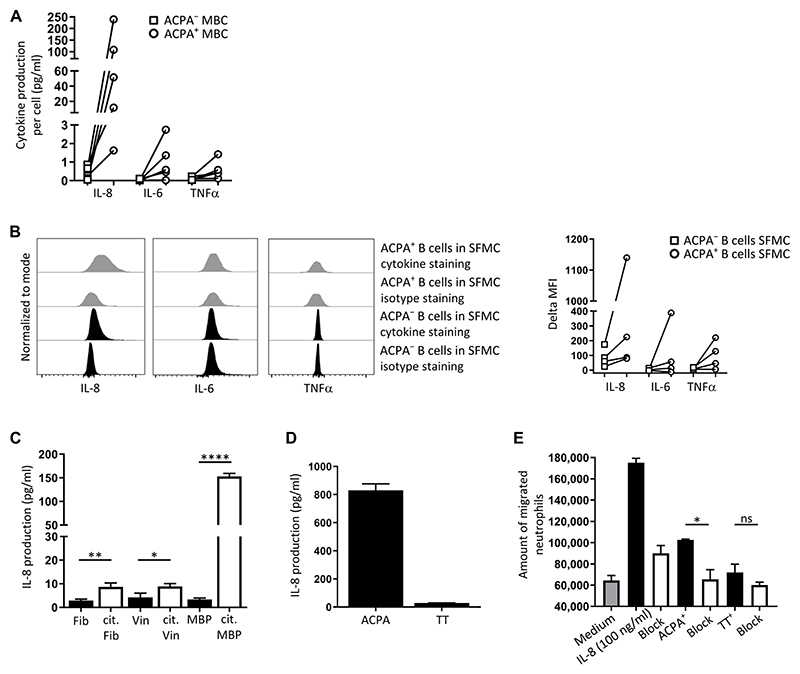
B cells can exert multiple effector functions by differentiating into antibody-secreting cells, by presenting antigen to T cells, and by secreting soluble mediators (18, 19). We assessed a range of cytokines produced by ACPA-positive aMBCs to understand how the activated phenotype contributes to disease-relevant processes. ACPA-positive CD20+CD27+ B cells from individual donors were sorted and cultured for 7 days in the presence of irradiated CD40 ligand (CD40L)–positive cells and anti-IgM and anti-IgG Fab2 fragments to stimulate the B cell receptor (BCR). An equal number of ACPA-negative MBC from the same donors were cultured under the same conditions as controls. Supernatants at day 7 were analyzed for the presence of 11 cytokines [IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12 p70, granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-γ (IFN-γ), tumor necrosis factor–α (TNFα), and vascular endothelial growth factor (VEGF)] using Luminex technology (fig. S8). Compared to ACPA-negative MBCs, ACPA-positive B cells of all donors secreted remarkable amounts of IL-8 (single-cell median, 51.5; range, 1.2 to 239 pg/ml) and, to a lesser extent, IL-6 (single-cell median, 0.6; range, 0.01 to 2.7 pg/ml) and TNFα (single-cell median, 0.4; range, 0.01 to 1.2 pg/ml) (Fig. 5A). This secretory capacity was observed despite a lower abundance of surface IgG on ACPA-positive CD20+CD27+ B cells compared to that on ACPA-negative CD20+CD27+ B cells from the same donors (fig. S9).
Fig. 5. Functional properties of ACPA+B cells.
(A) Cumulative secretion of IL-8, IL-6, or TNFα by PBMC-derived ACPA+ or ACPA− MBCs upon BCR and CD40 stimulation for 7 days (n = 5) (see fig. S8 for additional cytokine measurements). Equal numbers of ACPA+ and ACPA− B cells were cultured per donor. Results are shown as cytokine secretion per sorted cell. (B) Production of IL-8, IL-6, or TNFα by ACPA+ B cells from RA synovial fluid in the absence of ex vivo stimulation (n = 4). SFMCs were cultured overnight in medium in the presence of brefeldin A (2 μg/ml) to block release of the cytokines into the medium. Representative flow cytometry data (left) and collated results (right) are shown. To compare production, the difference between median fluorescence intensities of isotype staining and those of cytokine staining for the ACPA− and ACPA+ B cells was plotted. (C) IL-8 secretion by an immortalized ACPA+ B cell clone upon stimulation with the indicated citrullinated proteins and irradiated CD40L-positive cells. Immortalized ACPA B cells were cultured with citrullinated (cit) fibrinogen (Fib), vinculin (Vin), or myelin basic protein (MBP) (white bars) or their mock-citrullinated counterparts (black bars) in the presence of irradiated CD40L-positive cells for 3 days (one of three representative experiments is shown; n = 3). Supernatants were collected and tested for the presence of IL-8 by ELISA. (D) IL-8 secretion by the ACPA+ B cell clone and a TT-specific B cell clone upon stimulation with CCP2-avidin and soluble CD40L oligomers (n = 3, N = 3). Results from one representative experiment are shown. (E) Neutrophil migration induced by supernatants from CCP2-stimulated ACPA+ B cells. Healthy donor neutrophils were incubated for 90 min with supernatants from ACPA+ B cells stimulated with CCP2 tetramer for 3 days (n = 3, N = 3). Results from one representative experiment are shown. *P ≤ 0.05, **P ≤ 0.01, and ****P ≤ 0.0001. Two-tailed, unpaired Student’s t test in (C); two-tailed paired t test in (E). In (A) and (B), n = number of donors. In (C) to (E), data represent means ± SD from one representative experiment of three experiments (N = 3) with three technical replicates (n = 3).
To assess the relevance of our findings for the inflammatory environment at the site of inflammation, we isolated mononuclear cells directly from synovial fluid (SFMCs). SFMCs were cultured in medium without any stimulation in the presence of brefeldin A to block protein secretion. After 20 hours, cells were harvested and stained for the presence of dead cells and for the presence of surface markers [CD3, a T cell marker; CD14, a monocyte marker (both used to exclude non-B mononuclear cells); CD19; CD20; and CD27]; in addition, cells were fixed and permeabilized, followed by intracellular staining with CCP2 streptavidin tetramers and antibodies recognizing IL-8, IL-6, or TNFα. We detected ACPA-positive B cells that were positive for IL-8 in these cultures without stimulation (Fig. 5B). In all samples, the signal for IL-8, but not for IL-6 and TNFα, from ACPA-positive B cells exceeded that obtained from ACPA-negative B cells from the same compartment.
These results with the circulating ACPA-positive B cells and the ACPA-positive B cells in the synovial fluid showed that these populations have different properties. When stimulated, the ACPA-positive aMBCs from peripheral blood of patients with RA produced inflammatory cytokines, especially IL-8 and, to a lower extent, IL-6 and TNFα. Cells from the inflamed synovial fluid produced IL-8 ex vivo without additional stimulation.
IL-8 produced by ACPA-positive B cells induces neutrophil migration
Given the low frequency of ACPA-positive B cells in peripheral blood, which impedes functional analyses, we studied an immortalized, ACPA-positive human B cell clone (20, 21) to assess the functionality of IL-8 produced by ACPA-positive B cells. A TT-specific B cell clone, generated using identical technology, was included as control. ACPA-positive, clonal B cells stimulated with any one of the citrullinated proteins, fibrinogen, vinculin, or myelin basic protein (MBP), which are three well-established citrullinated antigens (22), produced higher amounts of IL-8 compared with cells cultured in the presence of the noncitrullinated versions of the same proteins (Fig. 5C). Differences were observed as to the amount of IL-8 secretion induced, possibly because of differential avidity of the clone for the individual, citrullinated antigens. The TT-specific clone did not react to stimulation with citrullinated proteins (fig. S10). Instead, these cells secreted IL-8 upon BCR stimulation with anti-IgG and anti-IgM or in response to stimulation with both CD40L and anti-IgG and anti-IgM (fig. S10), as did primary human B cells upon BCR ligation in combination with CD40 stimulation (fig. S11).
IL-8 is a chemoattractant for neutrophils (23). Moreover, neutrophils are the most prevalent immune cells in RA joints and contribute to joint damage in RA (24). Therefore, the neutrophil chemoattractant capacity of IL-8 produced by the ACPA-positive B cell clone was assessed. The clonal B cells or the TT-specific B cell clone (as a negative control) were stimulated with CCP2 streptavidin tetramers and soluble CD40L oligomers. At day 3, supernatants from these cultures were tested for IL-8 production by enzyme-linked immunosorbent assay (ELISA) (Fig. 5D) and used in a neutrophil migration assay. Supernatant of the stimulated, ACPA-positive B cell clone, but not supernatants from the TT-specific B cell clone, enhanced neutrophil migration, which was inhibited by adding a neutralizing mouse anti-human IL-8 antibody (Fig. 5E). Together, these data showed that ACPA-positive B cells can produce functional IL-8, which induced neutrophil migration in vitro.
Discussion
The functional role of autoreactive B cells in the immune pathogenesis of human autoimmune disease is largely unclear. Hypotheses on the pathogenic involvement of such cells in disease-relevant processes have mainly been generated from murine studies and extrapolated from the study of (secreted) autoantibodies. Moreover, the relevance of these cells in disease pathology is supported by the clinical efficacy of broadly B cell–depleting interventions. In-depth analysis of the autoreactive B cell compartment itself on the cellular level, however, has been hampered by difficulties in reliably identifying and isolating these rare, antigen-specific human B cell populations at high purity from patients. Using well-defined clinical phenotypes of a prototypic human autoimmune disease, RA, and its prearthritic, at-risk phase of arthralgia, we here studied the characteristics of autoreactive B cells circulating in the peripheral blood and synovial fluid of individual patients. We used stringent, multimeric, antigen-specific multicolor flow cytometry and cell sorting. We studied B cells directed against citrullinated antigens in this context, because the secreted ACPA repertoire represents the most specific, autoreactive humoral immune response in this disease (2), with numerous in vitro and epidemiologic studies pointing toward its immune-pathogenic involvement in the inflammatory disease process (1, 25–30). We hypothesized that the phenotypic and functional characterization of these cells could lead to better insights into their potential role in the initiation and maintenance of inflammation; in addition, it could indicate whether these cells are attractive targets for antigen-specific interventions.
TT-specific B cells, representative of a prototypic human MBC response to a foreign antigen, were chosen as a suitable comparator because of their similar frequency and differentiation state in peripheral blood. We found that, in patients with RA, TT-specific MBCs circulate in a quiescent, resting state, whereas >30% (on average) of ACPA-positive B cells actively proliferate and show additional signs of activation. The phenotype of ACPA-positive B cells resembled the phenotype of TT-specific B cells shortly after TT-booster vaccination. These data are important because they provide evidence that the autoreactive B cell response in a prominent human autoimmune disease, RA, is continuously activated and does not reach a quiescent or steady state that is observed for recall responses after infection or vaccination (17). Similar to our observations on the protein and mRNA level for selected markers in the autoreactive MBCs, a distinct transcriptional profile has been described for vaccination-induced B cells (17). This profile contrasts with that of conventional MBCs, suggesting that such cells may undergo differential developmental pathways during germinal center responses. Whether this is also the case for autoreactive aMBC that we identified here remains to be studied.
Furthermore, we found that ACPA-positive B cells had reduced amounts of the inhibitory receptor CD32 and secreted functionally active, proinflammatory cytokines upon stimulation, in particular IL-8. In the inflamed joint, we additionally observed B cells that produced such cytokines without ex vivo stimulation. The frequency of ACPA-positive MBCs in the synovial compartment was very low, limiting detailed phenotypic analyses. However, our data suggested that aMBCs could differentiate toward plasmablasts or plasma cells at this site. Together, these results provided evidence for a mechanism of how autoreactive, in this case ACPA-positive, B cells contribute to inflammatory disease processes in RA. The frequency of ACPA-positive aMBCs strongly correlated with ACPA titer in plasma, whereas the frequency of circulating TT-specific MBCs and plasma anti-TT antibodies were disconnected [our data and other’s (31)]. Furthermore, we detected ACPA-positive B cells in the memory and the plasmablast or plasma cell populations in circulation, in contrast to TT-specific B cells that circulated primarily as MBCs. These data indicated that the ACPA response is dynamic and suggested that some of the ACPA-positive aMBCs in the circulation originate from recent stimulation events, conceivably from antigen encounter in germinal centers.
In our phenotypic analysis, ACPA-positive aMBC were also highly positive for costimulatory molecules CD80 and CD86 and had abundant HLA-DR. These features are compatible with a close interaction between these B cells and T cells, most likely in germinal centers or similar structures at extrafollicular sites (32, 33). BCRs of ACPA-positive B cells are heavily mutated, presumably because of extensive T cell help under the influence of certain HLA susceptibility molecules (1, 34). Consequently, HLA association, extensive somatic hypermutation, and the presence of class-switched ACPA have been taken as indicators that T cell help drives the maturation of the ACPA response (1). This maturation occurs in the prearthritic at-risk phase of RA and precedes the clinical transition from arthralgia to overt arthritis (5). Therefore, we hypothesized that the activation status of ACPA-positive B cells would be less pronounced or absent from individuals who had not yet presented with established RA. Although ACPA-positive B cells from these ACPA+ arthralgia individuals were readily detectable in the circulation and a fraction of them were positive for Ki-67, they closely resembled the resting MBC phenotype observed for TT-specific B cells in patients with established RA, at least with regard to the markers tested. In line, no ACPA-positive plasmablasts or plasma cells were observed in the circulation at this stage. We deliberately chose individuals who had maintained a status of arthralgia for at least 2 years to enable a clear discrimination between immunological phenotypes. During additional follow-up of 2 years after the analyses, none of the individuals studied here progressed to RA. This could indicate that the degree to which ACPA-positive B cells have the markers described associates with or even precedes disease precipitation. In this context, it is intriguing that at the stage of early onset RA, a proxy for recent progression from arthralgia to arthritis, ACPA-positive MBCs were highly positive for these markers of activation and a marker of proliferation. Thus, we propose that the acquisition of the activated phenotype by ACPA-positive MBC associates with the onset of disease. Hence, it will be important for subsequent work to closely follow the phenotype of ACPA-positive B cells over time in individuals with arthralgia who transition to RA versus those that do not.
In human autoimmunity, it remains important to understand why and how autoreactive B cells escape from control mechanisms that should normally prevent their emergence and persistence. B cell activation requires balanced signals from activating receptors, such as CD19, and from inhibitory receptors, such as CD32b. Both excess CD19 (35, 36) and CD32b deficiency (37) in mice facilitate autoantibody production, supporting the essential roles of CD19 and CD32b in regulating and maintaining peripheral B cell tolerance. We found that ACPA-positive aMBCs had increased amounts of CD19 and reduced amounts of CD32 compared to TT-specific MBCs or ACPA-negative MBCs. This would result in desensitizing ACPA-positive aMBCs from CD32-mediated inhibition and, hence, confer resistance to a key regulator that could limit autoreactivity. A mechanism for the reduction of CD32 on ACPA-positive aMBC is CD40 stimulation by T cells (38).
Several results supported the concept of interactions between T cells and B cells as driving the ACPA response. Not only does CD40 stimulation of B cells by T cells reduce the abundance of CD32, which we observed on the ACPA-positive aMBCs, but a fraction of the ACPA-positive aMBCs were also positive for the T cell–stimulating proteins CD80 and CD86, which activate CD28 on T cells. CD80/86-CD28 interaction in the cross-talk between B and T cells is essential for the activation of autoreactive T cells and the induction of arthritis in the proteoglycan-induced arthritis mouse model (39). Therefore, it is possible that ACPA-positive B cells partly exert their pathogenic effects in RA by presenting antigens to T cells, thereby recruiting the help required for their own expansion and further differentiation.
Activation and continuous antigenic triggering of B cells can induce the secretion of various cytokines and inflammatory mediators. Upon stimulation, we observed that ACPA-positive aMBCs secreted remarkable amounts of IL-8, along with lesser amounts of IL-6 and TNFα. ACPA-positive B cells from the synovial fluid also produced IL-8 without additional stimulation ex vivo. The IL-8 secreted by these cells is likely functional in vivo, because IL-8 derived from stimulated, ACPA-positive immortalized human B cells induced neutrophil migration in vitro. Neutrophil migration was mostly but not completely blocked upon inhibition of IL-8, indicating that not only IL-8 but also other factors secreted by these B cells can induce neutrophil migration. Neutrophils are the most abundant cell type in the inflamed synovial compartment of affected joints in established RA (24). By local production in the early phases of arthritis development, activation of ACPA-positive B cells could be highly relevant; IL-8 secretion could stimulate the attraction and influx of neutrophils. In addition, the influx and activation of other innate immune cells, such as macrophages, could also be stimulated, and it is possible that cytokines derived from ACPA-positive B cells could contribute to the local activation of fibroblasts. Expanded B cell clones of unknown specificity have been observed in the peripheral blood of at-risk individuals with arthralgia, and such expanded clones predicted the transition to overt RA (40). Upon arthritis development, these clones disappeared from peripheral blood and were found in synovial tissue. Thus, it is intriguing to speculate that activated, autoreactive B cell clones, whether citrulline specific or not, migrate to joints and actively contribute to the initiation of arthritis through the secretion of IL-8.
Last, we observed the activated phenotype of ACPA-positive B cells in all patients with established RA. Most of these received csDMARDs. There was no association between the phenotype of ACPA-positive aMBCs and measures of disease activity. Thus, patients in clinical remission had activated, autoreactive B cells in their circulation. This suggests that, in RA, clinical remission induced by antirheumatic treatment reflects suppression of inflammation rather than quiescence of the underlying immune response. Hence, patients in clinical remission are not necessarily in a state of “immunological remission,” which could explain why disease frequently flares in patients upon treatment discontinuation (41). Future studies should therefore evaluate whether the phenotype of the ACPA+ B cell response reflects “immunological disease activity” and whether immunological remission as defined by a quiescent phenotype of ACPA-positive B cells may prognosticate the chance to successfully taper medication.
We acknowledge limitations to our study. First, the cross-sectional nature of our analyses impedes us from concluding about the dynamics of the activated phenotype in individual patients. Because the patients with arthralgia analyzed did not progress to RA within the time of follow-up, longitudinal analysis in a prospective setting is necessary to delineate whether the activated phenotype of ACPA-positive B cells precedes or coincides with the onset of RA. B cells in synovial fluid were limited; consequently, we could not perform full phenotypic characterization of ACPA-positive B cells and a comparison with TT-specific B cells in this compartment. We only evaluated a small number of markers. Thus, in-depth transcriptomic profiling or proteomic analyses will be necessary to delineate the full pathogenic potential of ACPA-positive B cells to identify potential targets for therapeutic interventions.
In summary, we refined the conceptual understanding of human autoreactive B cells in different phases of a common autoimmune disease, RA. We provided evidence that ACPA-positive autoreactive B cells have phenotypic and functional characteristics that fit well with continuous antigen-driven activation and an immune-pathogenic role in this disease. ACPA-positive B cells were proliferative, showed markers that facilitate the activation of T cells, down-regulated the inhibitory receptor CD32, up-regulated CD19, and actively differentiated into IL-8 and ACPA-producing plasmablasts or plasma cells. This phenotype was most prominent at the onset of disease. In addition, it persisted in patients who reached treatment-induced clinical remission, which provides evidence for the concept of immunological remission as a relevant treatment target in RA. Therefore, specific depletion or silencing of this activated B cell compartment is a relevant and testable treatment strategy for future therapeutic interventions.
Materials and Methods
Study design
The study was designed as a cross-sectional analysis of peripheral blood and synovial fluid samples of individual patients with ACPA+ RA or ACPA+ clinically suspect arthralgia [CSA (42)] or of healthy individuals. No therapeutic intervention was administered to patients for the purpose of the study. Patients who had received prior treatment with biologic agents were excluded. The researcher performing sample workup and analysis was aware of the diagnosis but blinded to the clinical parameters of the patients. Sample numbers and experimental replicates are provided in the figure legends.
Patients and healthy individuals
Peripheral blood and synovial fluid from patients with ACPA+ RA were obtained at the outpatient clinic of the Department of Rheumatology at Leiden University Medical Center (LUMC). All patients with RA met the 2010 American College of Rheumatology/European League against Rheumatism (ACR/EULAR) criteria for RA at the time of diagnosis. Treatment regimens included csDMARDs and glucocorticoids. All patients were naïve for biologic agents and had never received B cell–depleting therapy. Individuals with arthralgia were recruited from the CSA cohort (43) of the LUMC on the basis of their ACPA positivity. None of these had, so far, developed clinical signs of arthritis and, hence, did not meet the 2010 ACR/EULAR criteria for RA at the time of sampling. Selected patients with RA received TT booster vaccination in the untreated phase of recent-onset disease; peripheral blood was sampled within a maximum of 42 days after vaccination. All donors gave written informed consent. Permission for conduct of the study was obtained from the ethical review board of LUMC.
Cell isolation and culture
To determine the concentration of CCP2 tetramers for flow cytometry experiments, human embryonic kidney (HEK) 293 T cells expressing cell-surface ACPA (HEKACPA-TM) were generated by transducing HEK 293T cells with lentiviral vectors encoding the transmembrane antibody against CitFib1.1, as previously described (11). These cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 8% heat-inactivated fetal calf serum (FCS), penicillin/streptomycin (100 U/ml), 2 mM GlutaMAX, and l puromycin (1 μg/ml) (from InvivoGen).
To assess the abundance or presence of activation and inhibitory markers, PBMCs were isolated from 50 ml of heparinized peripheral blood using Ficoll-Paque gradient centrifugation and stored over-night at 4°C in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 8% FCS, penicillin/streptomycin (100 U/ml), and 2 mM GlutaMAX (the combination is referred to as culture medium). For the same purpose, synovial fluid from swollen joints was obtained by arthrocentesis and centrifuged; the pellet was harvested and suspended in phosphate-buffered saline (pH 7.4) at room temperature. SFMCs were isolated and stored at 4°C in culture medium.
To determine cytokine secretion by Luminex assay, sorted ACPA-positive or control cells were cultured in IMDM supplemented with 10% FCS, penicillin/streptomycin (100 U/ml), 2 mM GlutaMAX, and gentamicin (50 μg/ml) in a 96-well flat-bottom plate on a layer of 7000 gray–irradiated murine fibroblasts positive for human CD40L (5 × 103 cells per well) in the presence of anti-IgM (5 μg/ml) and IgG F(ab′)2 fragments (Jackson ImmunoResearch Laboratories). Supernatants were harvested after 7 days of incubation.
To assess the production of cytokines from SFMCs, synovial fluid was treated with hyaluronidase (100 μg/ml; Sigma-Aldrich) for 30 min at 37°C before SFMC isolation as described above. SFMCs were then cultured with culture medium and brefeldin A (2 μg/ml) for 20 hours before staining.
Immortalized B cells recognizing CCP2 or TT were generated by transducing antigen-specific human MBCs with retroviruses encoding Bcl-6 and Bcl-xL. Transduced cells can be visualized by their expression of green fluorescent protein. Immortalized clones were cultured in the presence of irradiated CD40L-positive cells and mouse IL-21–Fc (mIL21-Fc), as previously described (20, 21).
To assess IL-8 production by immortalized ACPA B cells, 1 × 105 immortalized ACPA+ or TT+ B cells were cultured with 1 × 104 irradiated CD40L-positive cells and citrullinated MBP (cit-MBP) (30 μg/ml), cit-vinculin (100 μg/ml), cit-fibrinogen (60 μg/ml), or their mock citrullinated proteins. Supernatants were collected after 3 days of culture.
To assess neutrophil migration, neutrophils were isolated from peripheral blood of healthy volunteers using dextran sedimentation and Ficoll-Paque gradient centrifugation, as previously described (44). The purity of such isolations was >90%. Supernatants were collected from cultured, immortalized ACPA+ and TT+ B cells (1 × 105 each), which were stimulated with CCP2-avidin (100 μg/ml) and soluble MEGA-CD40L (100 ng/ml) (Enzo) for 3 days.
Antigen labeling
To identify B cells specific for citrullinated antigens by flow cytometry, biotinylated CCP2 or its arginine control variant (CArgP2) were conjugated with Brilliant Violet 605 (BV605) or allophycocyanin (APC)–labeled streptavidin or phycoerythrin (PE)–labeled extravidin (Sigma-Aldrich, Life Technologies, and BioLegend), as previously described (11). To identify TT-specific B cells, TT (Statens Serum Institut) was labeled with APC or PE using AnaTag labeling kit, according to the manufacturer’s instruction (AnaSpec). Optimal concentrations of labeled CCP2 or CArgP2 tetramers and labeled TT were obtained by titration on HEKACPA-TM and wild-type HEK 293T cells or on immortalized TT-specific B cells, respectively.
Flow cytometry
To stain for activation markers on both ACPA-positive and TT-specific B cells, PBMCs were divided into three equal fractions and stained for dead cells using Fixable Violet Dead Cell Stain Kit (Molecular Probes). Two fractions were subsequently stained with CCP2-APC, CCP2-BV605 and CArgP2-PE tetramers, anti-CD3 Pacific Blue (PB, clone UCHT1), CD14 PB (clone M5E2), CD19 APC-Cy7 (clone Sj25C1), CD20 Alexa Fluor 700 (clone 2H7, BioLegend), CD27 PE-Cy7 (clone M-T271), anti-CD80 Brilliant Blue 515 (clone L307.4), CD86 BV510 (clone 2331), HLA-DR PE-CF594 (clone G46-6), or the respective isotype control antibodies for anti-CD80, anti-CD86, and HLA-DR (except anti-CD20, all BD Biosciences). To stain for the inhibitory marker CD32, anti-CD32 PE-CF594 (clone FLI8.26, BD Biosciences) or its isotype control antibody were used in fraction 2. The third fraction was stained with TT-APC, TT-PE, and the same labeled antibodies as in fraction 1. Subsequently, all fractions were fixed, permeabilized (Fixation/Permeabilization Concentrate, Diluent, and Buffer by eBioscience) and stained with anti–Ki-67 PerCP-eFluor710 (clone 20Raj1, eBioscience) or an appropriate isotype control. To test the staining specificity of TT-specific B cells, cells were preincubated with 10× excess of TT or bovine serum albumin before staining with labeled TT.
To stain for intracellular cytokines, unstimulated SFMCs (see Cell Isolation and Culture) were divided into two fractions. Both were stained for dead cells, CD3, CD14, CD19, CD20, and CD27. Subsequently, cells were fixed, permeabilized (BD Perm/Wash, BD Biosciences), and stained intracellularly with CCP2-BV605 and CCP2-APC tetramers. One fraction was additionally stained with anti–IL-8 PE (clone G265-8), anti–IL-6 fluorescein isothiocyanate (clone AS12), and anti-TNFα PE-CF594 (clone Mab11) (all from BD Bio-sciences). The other fraction was additionally stained with the iso-type controls of these antibodies. All flow cytometry measurements were conducted using a BD LSRFortessa (BD Biosciences).
Cell sorting
PBMCs were stained using markers for dead cells, CD3, CD14, CD19, CD20, CD27, CCP2-APC, CCP2-BV605, and CArgP2-PE. ACPA-positive MBCs (dead cell staining negative, CD3-, CD14−, CD19+, CD20+, CD27+, CCP2+/+, and CArgP−) and tetramer-negative MBCs (CCP2−/−) were sorted using BD FACSAria III (BD Biosciences).
Protein citrullination
Citrullinated vinculin, fibrinogen, and MBP were generated by incubating overnight vinculin435–739 (1 mg/ml; provided by S. van Kasteren, Leiden Institute of Chemistry), fibrinogen (1 mg/ml; Sigma-Aldrich), and MBP (1 mg/ml; Sigma-Aldrich) with 0.1 M tris-HCl, 10 mM CaCl2, and human recombinant Peptidyl Arginine Deiminase Type 4 (Sigma-Aldrich), 5 U for vinculin, 5 U for fibrinogen, and 3 U for MBP. Unmodified proteins were generated by incubating the proteins with PAD4 and tris-HCl without CaCl2.
Enzyme-linked immunosorbent assay
The presence of ACPA-IgG and anti-TT IgG in patient’s plasma was assessed by ELISA on the basis of reactivity against biotinylated CCP2 or TT, respectively. Biotinylated CCP2 was coupled to streptavidin-coated ELISA plates, and TT was directly coated to C96 Maxisorp Nunc-Immuno plates (Thermo Fisher Scientific). Plasma-heparin samples were tested at a 1:50 dilution and higher. Bound ACPA and anti-TT IgG were detected by polyclonal rabbit human IgG horseradish peroxidase (Dako) and stained with ABTS.H2O2 (Sigma-Aldrich). The presence of secreted IL-8 by stimulated immortalized B cells was assessed by Human IL-8 ELISA (Ready-SET-Go! 2nd Generation, Affymetrix eBioscience) according to the manufacturer’s instruction.
Luminex assay
To assess the cytokine production by ACPA-positive MBCs, supernatants of cultured ACPA-positive and tetramer-negative MBCs were analyzed using high-sensitivity Luminex assay (R&D Systems Magnetic Luminex Performance Assay) for the production of human IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12 p70, GM-CSF, IFN-γ, TNFα, and VEGF, according to the manufacturer’s instruction.
Neutrophil migration assay
The capacity of IL-8 produced by immortalized ACPA-positive B cells to induce neutrophil migration was assessed by adding the supernatant of stimulated cells to the bottom of a ChemoTx disposable chemotaxis 96-well system (3-μm-diameter filter; Neuro Probe). Neutrophils (2 × 105) were added on top of the filter for 90 min according to the manufacturer’s instruction. As a positive control, rhL-8 (100 ng/ml; BioLegend) was added to the culture medium. To test for specificity of the effect of IL-8, the supernatant was incubated with mouse anti–IL-8 blocking antibody (50 μg/ml; clone 807, Abcam) for 1 hour at 37°C before the assay. The number of migrated neutrophils was assessed by flow cytometry. To correct for technical variations in cell counts obtained by flow cytometry, 10 μl of cell counting beads (±10,000 beads; Invitrogen) were added to fixed neutrophils and mixed thoroughly before measurement on a BD LSRFortessa (BD Biosciences). The number of migrated neutrophils was normalized to the number of beads added to the sample.
Real-time PCR of isolated B cell populations
Pools of 8 to 30 CD19+CD20+CD27+ CCP2+/+CArgP2− B cells and respective CCP2− control B cells were obtained by fluorescence-activated cell sorting from patients with ACPA+ RA. Cells were subjected to mRNA isolation and complementary DNA (cDNA) synthesis according to Picelli et al. (SmartSeq2 protocol) (45). cDNA was preamplified (18 to 23 cycles) using KAPA HiFi HotStart ReadyMix, followed by purification with Ampure XP beads. RT-PCRs were performed using 1:20 diluted cDNA and SYBR Green. Negative controls included H20 in PCRs and “empty wells” included from the sorting procedure. Primer sequences are provided in Table 2.
Table 2. List of primers used for real-time PCR of isolated B cell populations.
| Gene | Forward | Reverse |
|---|---|---|
| B2M | 5′ GATCGAGACATGTAAGCAGC ′3 | 5′ TCAAACATGGAGACAGCAC ′3 |
| CD19 | 5′ TGGAGACGGGTCTGTTGTTG ′3 | 5′ CAGCAGCCAGTGCCATAGTA ′3 |
| CD20 | 5′ CGTGCTCCAGACCCAAATCT ′3 | 5′ TCAGTTAGCCCAACCACTTCT ′3 |
| CD80 | 5′ CATCACCATCCAAGTGTCCA ′3 | 5′ TGCCAGTAGATGCGAGTTTG ′3 |
Statistical analysis
Statistical analyses were performed using GraphPad Prism 6.02. Correlations and comparisons were assessed using nonparametric tests. Mann-Whitney test was used to compare data from unpaired samples (for example, healthy donors versus patients with RA); Wilcoxon signed-rank test was used to compare paired data from individual patients with RA. In case of multiple comparisons, one-way analysis of variance (ANOVA) was used, followed by Dunn’s test to correct for multiple testing. Specific information is provided in the figure legends. Individual subject-level data are provided in data file S1.
Supplementary Material
Acknowledgments
We thank J. W. Drijfhout (LUMC, Leiden) for providing the CCP2 peptide. Vinculin435-739 was provided by S. van Kasteren, Leiden Institute of Chemistry. Editorial services were provided by N. R. Gough (BioSerendipity LLC).
Funding
This work was supported by the Netherlands Organization for Scientific Research (NWO) Gravitation Program Subgrant 00019 from the Institute for Chemical Immunology, by NWO projects 435000033 and 91214031, the EU/EFPIA Innovative Medicines Initiative Joint Undertaking (IMI-JU) BeTheCure grant (contract no.1151422) and the IMI2-JU RTCure grant (contract no. 777357). H.U.S. is the recipient of a NWO-ZonMW clinical fellowship (project 90714509), a NWO-ZonMW VENI grant (project 91617107), and a ZonMW Enabling Technology Hotels grant (project 435002030) and received support from the Dutch Arthritis Foundation (projects 15-2-402 and 18-1-205). A.H.M.v.d.H.-v.M. received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (Starting grant, agreement no. 714312). D.L.B. is recipient of a NWO-ZonMW VICI grant and received funding from the ERC (Consolidator grant).
Footnotes
Author contributions: H.K., E.I.H.v.d.V., N.J.B., L.M.S., A.B., and P.F.K. designed and performed the experiments and analyzed and interpreted the data. L.E.B., R.M.t.B., and A.H.M.v.d.H.-v.M. provided samples and clinical data on individuals with arthralgia and interpreted the data. H.S. and D.L.B. provided technology and immortalized B cell clones. T.W.J.H., R.E.M.T., and H.U.S. designed the experiments and interpreted the data. H.K., R.E.M.T., and H.U.S. drafted the manuscript. All authors revised the manuscript and approved the final submitted version.
Competing interests: D.L.B. is currently an employee of UCB Pharma. H.S. is shareholder of AIMM Therapeutics, has received consulting fees from GSK and UniQure, and holds a patent on the immortalization of B cells (patent title: Means and methods for influencing the stability of antibody producing cells; patent no. US10273454B2). The other authors declare that they have no competing interests.
Data and materials availability
All data associated with this study are present in the paper or the Supplementary Materials. The immortalized B cell lines were obtained using technology from AIMM Therapeutics and have been described previously (20); requests for these cell lines require written consent from the company AIMM.
References and Notes
- 1.Scherer HU, Huizinga TWJ, Krönke G, Schett G, Toes REM. The B cell response to citrullinated antigens in the development of rheumatoid arthritis. Nat Rev Rheumatol. 2018;14:157–169. doi: 10.1038/nrrheum.2018.10. [DOI] [PubMed] [Google Scholar]
- 2.Willemze A, Trouw LA, Toes RE, Huizinga TW. The influence of ACPA status and characteristics on the course of RA. Nat Rev Rheumatol. 2012;8:144–152. doi: 10.1038/nrrheum.2011.204. [DOI] [PubMed] [Google Scholar]
- 3.Burgers LE, van Steenbergen HW, Ten Brinck RM, Huizinga TW, van der Helm-van Mil AH. Differences in the symptomatic phase preceding ACPA-positive and ACPA-negative RA: A longitudinal study in arthralgia during progression to clinical arthritis. Ann Rheum Dis. 2017;76:1751–1754. doi: 10.1136/annrheumdis-2017-211325. [DOI] [PubMed] [Google Scholar]
- 4.van der Woude D, Rantapää-Dahlqvist S, Ioan-Facsinay A, Onnekink C, Schwarte CM, Verpoort KN, Drijfhout JW, Huizinga TW, Toes RE, Pruijn GJ. Epitope spreading of the anti-citrullinated protein antibody response occurs before disease onset and is associated with the disease course of early arthritis. Ann Rheum Dis. 2010;69:1554–1561. doi: 10.1136/ard.2009.124537. [DOI] [PubMed] [Google Scholar]
- 5.Suwannalai P, van de Stadt LA, Radner H, Steiner G, El-Gabalawy HS, Zijde CM, van Tol MJ, van Schaardenburg D, Huizinga TW, Toes RE, Trouw LA. Avidity maturation of anti-citrullinated protein antibodies in rheumatoid arthritis. Arthritis Rheum. 2012;64:1323–1328. doi: 10.1002/art.33489. [DOI] [PubMed] [Google Scholar]
- 6.van de Stadt LA, de Koning MH, van de Stadt RJ, Wolbink G, Dijkmans BA, Hamann D, van Schaardenburg D. Development of the anti-citrullinated protein antibody repertoire prior to the onset of rheumatoid arthritis. Arthritis Rheum. 2011;63:3226–3233. doi: 10.1002/art.30537. [DOI] [PubMed] [Google Scholar]
- 7.Hensvold AH, Magnusson PK, Joshua V, Hansson M, Israelsson L, Ferreira R, Jakobsson P-J, Holmdahl R, Hammarström L, Malmström V, Askling J, et al. Environmental and genetic factors in the development of anticitrullinated protein antibodies (ACPAs) and ACPA-positive rheumatoid arthritis: An epidemiological investigation in twins. Ann Rheum Dis. 2015;74:375–380. doi: 10.1136/annrheumdis-2013-203947. [DOI] [PubMed] [Google Scholar]
- 8.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 9.Chatzidionysiou K, Lie E, Nasonov E, Lukina G, Hetland ML, Tarp U, Gabay C, van Riel PL, Nordstrom DC, Gomez-Reino J, Pavelka K, et al. Highest clinical effectiveness of rituximab in autoantibody-positive patients with rheumatoid arthritis and in those for whom no more than one previous TNF antagonist has failed: Pooled data from 10 European registries. Ann Rheum Dis. 2011;70:1575–1580. doi: 10.1136/ard.2010.148759. [DOI] [PubMed] [Google Scholar]
- 10.Thurlings RM, Vos K, Wijbrandts CA, Zwinderman AH, Gerlag DM, Tak PP. Synovial tissue response to rituximab: Mechanism of action and identification of biomarkers of response. Ann Rheum Dis. 2008;67:917–925. doi: 10.1136/ard.2007.080960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerkman PF, Fabre E, van der Voort EI, Zaldumbide A, Rombouts Y, Rispens T, Wolbink G, Hoeben RC, Spits H, Baeten DL, Huizinga TW, et al. Identification and characterisation of citrullinated antigen-specific B cells in peripheral blood of patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1170–1176. doi: 10.1136/annrheumdis-2014-207182. [DOI] [PubMed] [Google Scholar]
- 12.Klein U, Rajewsky K, Küppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–1689. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerkman PF, Kempers AC, van der Voort EI, van Oosterhout M, Huizinga TW, Toes RE, Scherer HU. Synovial fluid mononuclear cells provide an environment for long-term survival of antibody-secreting cells and promote the spontaneous production of anti-citrullinated protein antibodies. Ann Rheum Dis. 2016;75:2201–2207. doi: 10.1136/annrheumdis-2015-208554. [DOI] [PubMed] [Google Scholar]
- 14.de Hair MJ, van de Sande MG, Ramwadhdoebe TH, Hansson M, Landewé R, van der Leij C, Maas M, Serre G, van Schaardenburg D, Klareskog L, Gerlag DM, et al. Features of the synovium of individuals at risk of developing rheumatoid arthritis: Implications for understanding preclinical rheumatoid arthritis. Arthritis Rheumatol. 2014;66:513–522. doi: 10.1002/art.38273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van de Sande MG, de Hair MJ, van der Leij C, Klarenbeek PL, Bos WH, Smith MD, Maas M, de Vries N, van Schaardenburg D, Dijkmans BA, Gerlag DM, et al. Different stages of rheumatoid arthritis: Features of the synovium in the preclinical phase. Ann Rheum Dis. 2011;70:772–777. doi: 10.1136/ard.2010.139527. [DOI] [PubMed] [Google Scholar]
- 16.Townsend SE, Goodnow CC, Cornall RJ. Single epitope multiple staining to detect ultralow frequency B cells. J Immunol Methods. 2001;249:137–146. doi: 10.1016/s0022-1759(00)00352-5. [DOI] [PubMed] [Google Scholar]
- 17.Ellebedy AH, Jackson KJ, Kissick HT, Nakaya HI, Davis CW, Roskin KM, McElroy K, Oshansky CM, Elbein R, Thomas S, Lyon GM, et al. Defining antigen-specific plasmablast and memory B cell subsets in human blood after viral infection or vaccination. Nat Immunol. 2016;17:1226–1234. doi: 10.1038/ni.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson SW, Kolhatkar NS, Rawlings DJ. B cells take the front seat: Dysregulated B cell signals orchestrate loss of tolerance and autoantibody production. Curr Opin Immunol. 2015;33:70–77. doi: 10.1016/j.coi.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dörner T, Jacobi AM, Lipsky PE. B cells in autoimmunity. Arthritis Res Ther. 2009;11:247. doi: 10.1186/ar2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwakkenbos MJ, Diehl SA, Yasuda E, Bakker AQ, van Geelen CM, Lukens MV, van Bleek GM, Widjojoatmodjo MN, Bogers WM, Mei H, Radbruch A, et al. Generation of stable monoclonal antibody–producing B cell receptor–positive human memory B cells by genetic programming. Nat Med. 2010;16:123–128. doi: 10.1038/nm.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Germar K, Fehres CM, Scherer HU, van Uden N, Pollastro S, Yeremenko N, Hansson M, Kerkman PF, van der Voort EIH, Reed E, Maassen H, et al. Generation and characterization of anti-citrullinated protein antibody-producing B cell clones from rheumatoid arthritis patients. Arthritis Rheumatol. 2019;71:340–350. doi: 10.1002/art.40739. [DOI] [PubMed] [Google Scholar]
- 22.Ioan-Facsinay A, el-Bannoudi H, Scherer HU, van der Woude D, Menard HA, Lora M, Trouw LA, Huizinga TW, Toes RE. Anti-cyclic citrullinated peptide antibodies are a collection of anti-citrullinated protein antibodies and contain overlapping and non-overlapping reactivities. Ann Rheum Dis. 2011;70:188–193. doi: 10.1136/ard.2010.131102. [DOI] [PubMed] [Google Scholar]
- 23.Russo RC, Garcia CC, Teixeira MM, Amaral FA. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert Rev Clin Immunol. 2014;10:593–619. doi: 10.1586/1744666X.2014.894886. [DOI] [PubMed] [Google Scholar]
- 24.Edwards SW, Hallett MB. Seeing the wood for the trees: The forgotten role of neutrophils in rheumatoid arthritis. Immunol Today. 1997;18:320–324. doi: 10.1016/s0167-5699(97)01087-6. [DOI] [PubMed] [Google Scholar]
- 25.Renner N, Krönke G, Rech J, Uder M, Janka R, Lauer L, Paul D, Herz B, Schlechtweg P, Hennig FF, Schett G, et al. Anti-citrullinated protein antibody positivity correlates with cartilage damage and proteoglycan levels in patients with rheumatoid arthritis in the hand joints. Arthritis Rheumatol. 2014;66:3283–3288. doi: 10.1002/art.38862. [DOI] [PubMed] [Google Scholar]
- 26.Sokolove J, Zhao X, Chandra PE, Robinson WH. Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll-like receptor 4 and Fcγ receptor. Arthritis Rheum. 2011;63:53–62. doi: 10.1002/art.30081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleyer A, Finzel S, Rech J, Manger B, Krieter M, Faustini F, Araujo E, Hueber AJ, Harre U, Engelke K, Schett G. Bone loss before the clinical onset of rheumatoid arthritis in subjects with anticitrullinated protein antibodies. Ann Rheum Dis. 2014;73:854–860. doi: 10.1136/annrheumdis-2012-202958. [DOI] [PubMed] [Google Scholar]
- 28.Trouw LA, Haisma EM, Levarht EW, van der Woude D, Ioan-Facsinay A, Daha MR, Huizinga TW, Toes RE. Anti–cyclic citrullinated peptide antibodies from rheumatoid arthritis patients activate complement via both the classical and alternative pathways. Arthritis Rheum. 2009;60:1923–1931. doi: 10.1002/art.24622. [DOI] [PubMed] [Google Scholar]
- 29.Laurent L, Clavel C, Lemaire O, Anquetil F, Cornillet M, Zabraniecki L, Nogueira L, Fournie B, Serre G, Sebbag M. Fcγ receptor profile of monocytes and macrophages from rheumatoid arthritis patients and their response to immune complexes formed with autoantibodies to citrullinated proteins. Ann Rheum Dis. 2011;70:1052–1059. doi: 10.1136/ard.2010.142091. [DOI] [PubMed] [Google Scholar]
- 30.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, Friday S, Li S, Patel RM, Subramanian V, Thompson P, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5:178ra140. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leyendeckers H, Odendahl M, Lohndorf A, Irsch J, Spangfort M, Miltenyi S, Hunzelmann N, Assenmacher M, Radbruch A, Schmitz J. Correlation analysis between frequencies of circulating antigen-specific IgG-bearing memory B cells and serum titers of antigen-specific IgG. Eur J Immunol. 1999;29:1406–1417. doi: 10.1002/(SICI)1521-4141(199904)29:04<1406::AID-IMMU1406>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 32.Good-Jacobson KL, Song E, Anderson S, Sharpe AH, Shlomchik MJ. CD80 expression on B cells regulates murine T follicular helper development, germinal center B cell survival, and plasma cell generation. J Immunol. 2012;188:4217–4225. doi: 10.4049/jimmunol.1102885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niu H, Cattoretti G, Dalla-Favera R. BCL6 controls the expression of the B7-1/CD80 costimulatory receptor in germinal center B cells. J Exp Med. 2003;198:211–221. doi: 10.1084/jem.20021395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vergroesen RD, Slot LM, Hafkenscheid L, Koning MT, van der Voort EIH, Grooff CA, Zervakis G, Veelken H, Huizinga TWJ, Rispens T, Scherer HU, et al. B-cell receptor sequencing of anti-citrullinated protein antibody (ACPA) IgG-expressing B cells indicates a selective advantage for the introduction of N-glycosylation sites during somatic hypermutation. Ann Rheum Dis. 2018;77:956–958. doi: 10.1136/annrheumdis-2017-212052. [DOI] [PubMed] [Google Scholar]
- 35.Sato S, Ono N, Steeber DA, Pisetsky DS, Tedder TF. CD19 regulates B lymphocyte signaling thresholds critical for the development of B-1 lineage cells and autoimmunity. J Immunol. 1996;157:4371–4378. [PubMed] [Google Scholar]
- 36.Inaoki M, Sato S, Weintraub BC, Goodnow CC, Tedder TF. CD19-regulated signaling thresholds control peripheral tolerance and autoantibody production in B lymphocytes. J Exp Med. 1997;186:1923–1931. doi: 10.1084/jem.186.11.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuasa T, Kubo S, Yoshino T, Ujike A, Matsumura K, Ono M, Ravetch JV, Takai T. Deletion of fcgamma receptor IIB renders H-2(b) mice susceptible to collagen-induced arthritis. J Exp Med. 1999;189:187–194. doi: 10.1084/jem.189.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X, Burch E, Cai L, So E, Hubbard F, Matteson EL, Strome SE. CD40 mediates downregulation of CD32B on specific memory B cell populations in rheumatoid arthritis. J Immunol. 2013;190:6015–6022. doi: 10.4049/jimmunol.1203366. [DOI] [PubMed] [Google Scholar]
- 39.O’Neill SK, Cao YX, Hamel KM, Doodes PD, Hutas G, Finnegan A. Expression of CD80/86 on B cells is essential for autoreactive T cell activation and the development of arthritis. J Immunol. 2007;179:5109–5116. doi: 10.4049/jimmunol.179.8.5109. [DOI] [PubMed] [Google Scholar]
- 40.Tak PP, Doorenspleet ME, de Hair MJH, Klarenbeek PL, van Beers-Tas MH, van Kampen HC, van Schaardenburg D, Gerlag DM, Baas F, de Vries N. Dominant B cell receptor clones in peripheral blood predict onset of arthritis in individuals at risk for rheumatoid arthritis. Ann Rheum Dis. 2017;76:1924–1930. doi: 10.1136/annrheumdis-2017-211351. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Schett G, Emery P, Tanaka Y, Burmester G, Pisetsky DS, Naredo E, Fautrel B, van Vollenhoven R. Tapering biologic and conventional DMARD therapy in rheumatoid arthritis: Current evidence and future directions. Ann Rheum Dis. 2016;75:1428–1437. doi: 10.1136/annrheumdis-2016-209201. [DOI] [PubMed] [Google Scholar]
- 42.van Steenbergen HW, Aletaha D, Beaart-van de Voorde LJ, Brouwer E, Codreanu C, Combe B, Fonseca JE, Hetland ML, Humby F, Kvien TK, Niedermann K, et al. EULAR definition of arthralgia suspicious for progression to rheumatoid arthritis. Ann Rheum Dis. 2017;76:491–496. doi: 10.1136/annrheumdis-2016-209846. [DOI] [PubMed] [Google Scholar]
- 43.van Steenbergen HW, Mangnus L, Reijnierse M, Huizinga TW, van der Helm-van Mil AH. Clinical factors, anticitrullinated peptide antibodies and MRI-detected subclinical inflammation in relation to progression from clinically suspect arthralgia to arthritis. Ann Rheum Dis. 2016;75:1824–1830. doi: 10.1136/annrheumdis-2015-208138. [DOI] [PubMed] [Google Scholar]
- 44.Kuhns DB, Priel DAL, Chu J, Zarember KA. Isolation and functional analysis of human neutrophils. Curr Protoc Immunol. 2015;111:7.23.1–7.23.16. doi: 10.1002/0471142735.im0723s111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Picelli S, Faridani OR, Björklund AK, Winberg G, Sagasser S, Sandberg R. Full-length RNA-seq from single cells using Smart-seq2. Nat Protoc. 2014;9:171–181. doi: 10.1038/nprot.2014.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this study are present in the paper or the Supplementary Materials. The immortalized B cell lines were obtained using technology from AIMM Therapeutics and have been described previously (20); requests for these cell lines require written consent from the company AIMM.