Abstract
Age-associated B cells (ABCs) accumulate during infection, aging, and autoimmunity, contributing to lupus pathogenesis. Here, we screen for transcription factors driving ABC formation and find ZEB2 is required for human and mouse ABC differentiation in-vitro. ABCs are reduced in ZEB2 haploinsufficient individuals and in mice lacking Zeb2 in B cells. In mice with TLR7-driven lupus, ZEB2 is essential for ABC formation and autoimmune pathology. ZEB2 binds to +20kb Mef2b’s intronic enhancer, repressing MEF2B-mediated germinal center B cell differentiation and promoting ABC formation. ZEB2 also targets genes important for ABC specification and function including Itgax. Zeb2-driven ABC differentiation requires JAK–STAT signaling, and treatment with JAK1/3 inhibitor reduces ABC accumulation in autoimmune mice and patients. Thus, ZEB2 emerges as a driver of B cell autoimmunity.
Age-associated B cells (ABCs) are a distinct effector B cell subset found at increased numbers in aged female mice, infection models, and systemic autoimmune diseases (1). ABCs are identified as CD11c+CD11b+CD21−CD23−T-bet+ in mice (2, 3) and CD11c+CD21−CD27−CXCR5− FCRL5+IgD−T-bet+ in humans (4, 5). In autoimmune settings, these B cells are enriched for autoantibody specificities and are thought to be antigen-experienced. Moreover, there is evidence that ABCs can persist in tissues and rapidly differentiate into antibody-secreting cells (ASCs) upon antigen re-encounter or innate stimulation (1).
The transcription factors (TFs) T-bet, IRF5, and IRF8 are highly expressed in ABCs and have been put forward as functional regulators of ABC differentiation (6–9). However, except for IgG2a/c isotype switching, T-bet is dispensable for ABC accumulation and maintenance of ABC features (9–11), and IRF5 and IRF8 are broadly expressed in other B cell subsets and have been reported to be involved in cell activation, proliferation, differentiation, and function (12–14). To determine the TF(s) essential for ABC formation, we screened all TFs expressed by these cells and identified ZEB2 as the key regulator required for ABC specification and differentiation in mice and humans.
Screen for TFs directing ABC differentiation
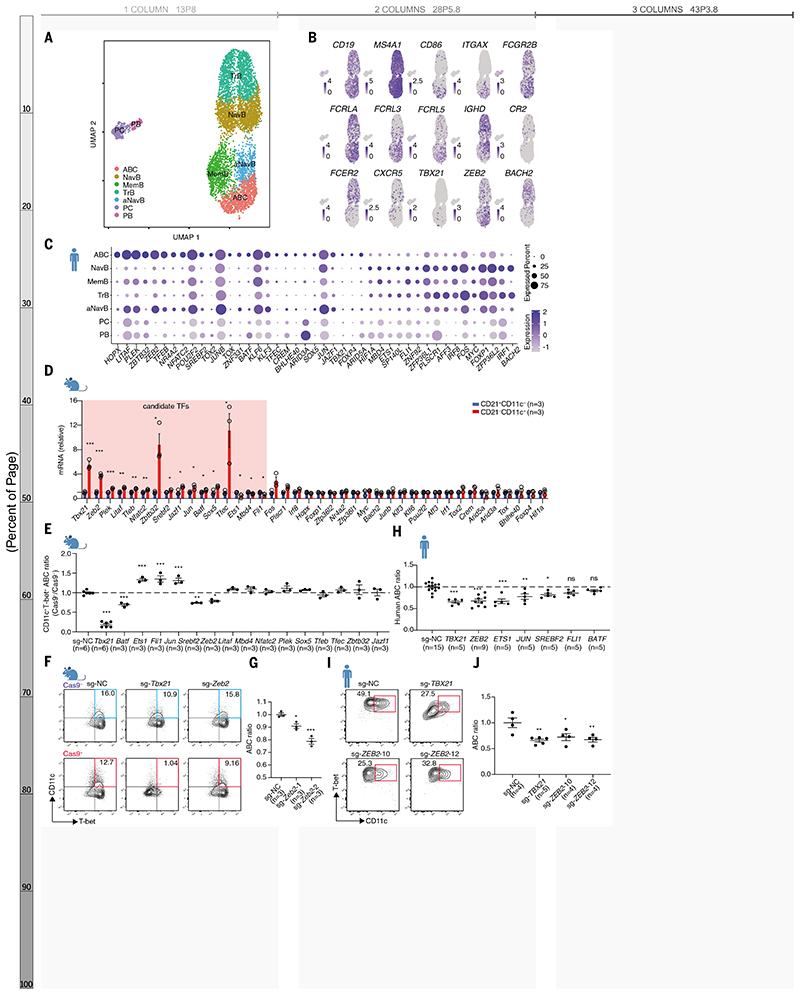
To gain insights into the nature of ABCs, we sorted peripheral B cells from a patient with new-onset SLE (table S1) and performed droplet-based scRNA-seq. Seven distinct clusters were revealed by unsupervised clustering with a two-dimensional uniform manifold approximation and projection (UMAP) (Fig. 1A and fig. S1A and B). These clusters were assigned to known peripheral B cell subsets including transitional B cells, naïve B cells, activated naïve B cells, ABCs, memory B cells, plasmablasts, and plasma cells by comparing differentially expressed genes with established landmark genes (5, 15) (fig. S1A and C). ABCs preferentially expressed genes encoding key surface markers CD19, CD86, FCRLA, FCRL3/5, FCGR2B, MS4A1 and ITGAX, and they lacked CD27, CR2, CXCR5, FCER2, and IGHD (Fig. 1B and fig. S1C). We found 43 differentially expressed TFs: 27 upregulated and 16 downregulated (Fig. 1C). We sorted murine CD19+CD11c+CD21− B cells from the bm12 induced lupus mouse model and validated 40 murine homologues of the differentially expressed TFs identified in the human scRNA-seq data (Fig. 1D, and fig. S2A-C). Among these, 13 upregulated (Tbx21, Zeb2, Plek, Litaf, Tfeb, Nfatc2, Zbtb32, Srebf2, Jazf1, Jun, Sox5, Tfec, and Batf) and three downregulated TFs (Ets1, Mbd4, and Fli1) were identically regulated in human and mouse ABCs and were therefore considered potential transcriptional regulators of ABC differentiation.
Fig. 1. CRISPR/Cas9 based screen of transcription factors for ABC differentiation.
(A) Single-cell RNAseq of CD19+ peripheral B cells isolated from a patient with new-onset SLE. Seven clusters were defined as transitional B cells (TrB), naïve B cells (NavB), activated naïve B cells (aNavB), age-associated B cells (ABC), memory B cells (MemB), plasmablasts (PB), and plasma cells (PC). (B) UMAP plots of select genes expression distinguishing ABCs. (C) Dot plots of 43 differentially expressed transcription factors (TFs) in ABCs. (D) Relative expression of mouse homologue genes (encoding equivalent TFs in (C)) in CD19+CD11c+CD21− and CD19+CD11c−CD21+ B cells sorted from bm12-induced lupus mice. (E) Mouse ABC ratio in groups targeting indicated genes. The ratio defined by comparing Cas9+CD11c+T-bet+ to Cas9−CD11c+T-bet+ in the coculture was normalized with sg-NC. (F) Flow cytometry plots of ABCs (CD11c+T-bet+) derived from B cells transuded with sgRNA targeting Tbx21 or Zeb2. (G) ABC ratio in groups with two distinct sgRNAs targeting Zeb2. (H) Human ABC ration in groups targeting indicated genes. The human ABC ratio was defined by normalizing the frequency of CD27−IgD−CD11c+T-bet+ B cell with sg-NC group. (I and J) Representative plots (I) and ABC ratio (J) from B cells electroporated with Cas9-gRNA (RNP) complex targeting TBX21 and ZEB2. n represents distinct samples (biological repeats). Data are representative of 3-4 independent experiments. Data are mean ± SEM values. *P<0.05, **P<0.01, ***P<0.001, ns, not significant, unpaired Student’s t test (D) and ordinary one-way ANOVA with Dunnett’s multiple comparisons testing (E, H, G, and J).
To identify which of these 16 TFs were driving ABC differentiation, B cells from Cas9 transgenic mice and CD45.1 congenic mice were retrovirally infected with sgRNA plasmids targeting each TF and co-expressing blue fluorescent protein (BFP), and cultured with the ABC differentiation cocktail (7, 16) (fig. S3A). The ratio between live BFP+ CD45.1− and BFP+ CD45.1+ ABCs was determined and genome-editing was validated with a sequence specific for Itgax (fig. S3B and C). We identified seven TFs that could significantly (P<0.05) alter ABC formation (Fig. 1E and F, and fig. S3D). Except for Zeb2, ablation of the other six TFs predominantly influenced cell viability (fig. S3E and F). Two different sgRNAs targeting Zeb2 in separate Cas9+ B cell cultures led to reduced ABC formation (Fig. 1G), excluding an off-target editing effect. To determine whether any of the other six TFs was required for human ABC lineage formation, we transduced Cas9-guide RNA ribonucleoprotein (RNP) complexes by electroporation and cultured edited cells with the ABC differentiation cocktail (4–6). (fig. S4A and B). Ablation of TBX21, ZEB2, and SREBF2 dampened ABC differentiation in both human and mouse B cells but TBX21 and SREBF2 deficiency also led to altered B cell viability (Fig. 1H, and fig. S4C-E). Gene editing of ETS1 and JUN had opposite effects and BATF and FLI1 did not change human ABCs (P<0.05) (Fig. 1H and fig. S4C). Two different guide RNAs targeting ZEB2 impaired ABC induction (Fig. 1I and J) emerging thus as the most promising putative ABC transcriptional regulator.
ZEB2 happloinsufficiency impairs human ABC formation
ZEB2, a member of the zinc-finger E homeobox-binding protein family, is pivotal in early fetal development and cancer progression by driving the epithelial-to-mesenchymal transition (17). Loss-of-function heterozygous gene variants lead to Mowat–Wilson syndrome (MWS), a rare genetic disorder in which ZEB2 happloinsufficency causes intellectual disability, distinctive facial features, seizures, and a predisposition to Hirschsprung disease (18, 19). Though mouse studies have shown ZEB2's role in immune cell differentiation and function (20), insights into the immunological consequences of reduced ZEB2 function in MWS patients are limited.
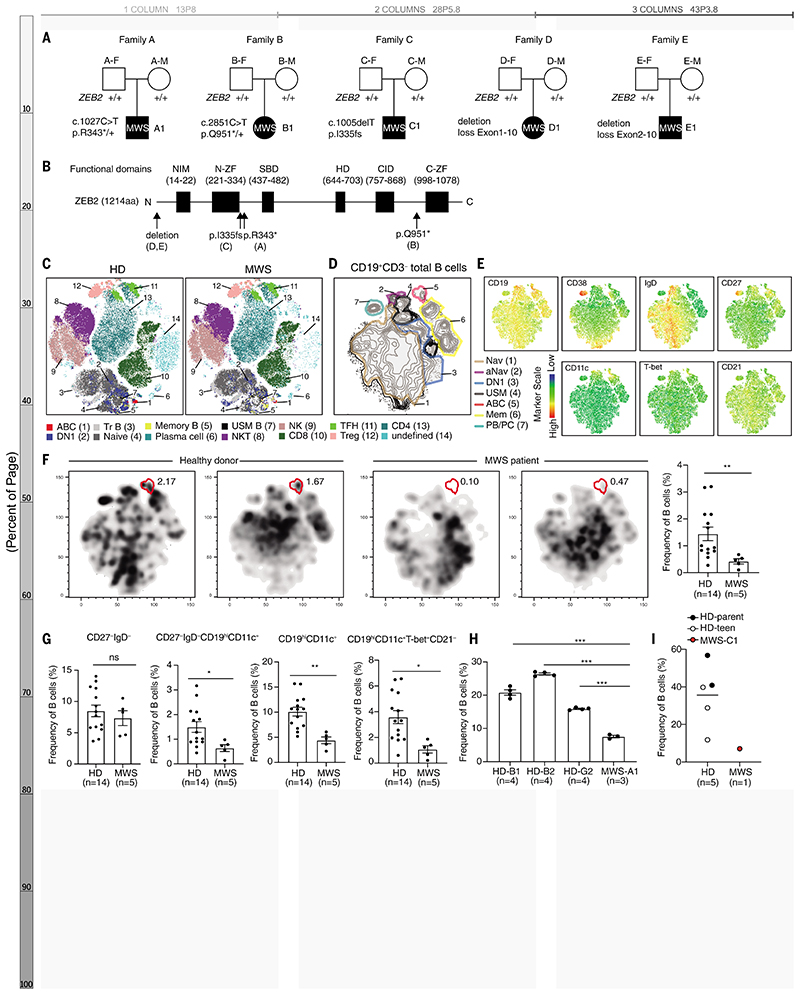
We examined peripheral blood mononuclear cells from five unrelated MWS patients (tables S2 and S3) and identified 5 de novo heterozygous germline ZEB2 variants through whole-exome sequencing (Fig. 2A and B). ZEB2 deficiency dramatically decreased ABC frequency (Fig. 2C and fig. S5A). Detailed B cell profiling revealed seven prominent B cell clusters (Fig. 2, D and E). In MWS patients, ABC frequency was notably reduced by 71% accompanied by a significant decline in activated naïve B cells and ABC progenitors (5) (Fig. 2F and fig. S5B). Although switched memory B cells were decreased by 46%, DN1 B cells—an alternative trajectory for effector B cells—were increased (fig. S5B) (5). These changes were confirmed using manual gating with specific markers (Fig. 2G and fig. S5, C and D). We further studied ZEB2's regulatory effects on ABC formation by isolating B cells from MWS patients and inducing ABC differentiation in vitro. Corroborating our in vivo findings, in vitro ABC formation was also impaired (Fig. 2, H and I, and fig. S5, E and F). Thus, ZEB2 loss-of-function variants confirm that ZEB2 is required for human ABC formation.
Fig. 2. ZEB2 is required for human ABC formation.
(A) Family pedigrees showing de novo heterozygous mutations of ZEB2 in Mowat–Wilson syndrome (MWS) patients. (B) Schematic of the general, linear structure of the functional domain composition of ZEB2 protein. The black arrows show the location of ZEB2 mutation described in (A). (C) t-SNE plots of lymphocytes clusters in PBMC of a healthy donor (HD) and a MWS patient by flow cytometry. (D) t-SNE plots of peripheral B cell clusters for MWS patients and healthy donors. Seven B cell clusters were identified based on lineage marker expression as naïve B cells (Nav), activated naïve B cells (aNav), CXCR5+ double-negative B cells (DN1), unswitched memory B cells (USM), age-associated B cells (ABC), memory B cells (Mem), plasmablasts (PB), and plasma cells (PC). (E) t-SNE plots of peripheral B cells displaying CD11c, CD19, CD21, CD27, CD38, IgD, and T-bet expression. (F) Representative t-SNE plots and frequency of ABCs (red frame) in peripheral B cells from MWS patient and HDs. (G) Frequency of DN, DN2, CD11c+B, and ABCs in PBMCs from MWS patient and HDs analyzed by manual gating. (H and I) Representative plots and frequency of in vitro–induced ABCs derived from B cells of MWS-A1 (H), MWS-C1 (I), and healthy donors. n represents distinct samples (biological repeats except (H)). Data are mean ± SEM values. *P<0.05, **P<0.01, ***P<0.001, ns, not significant, unpaired Student’s t test (G and H) with Welch’s correction (F).
ZEB2 determines ABCs pathogenicity in lupus
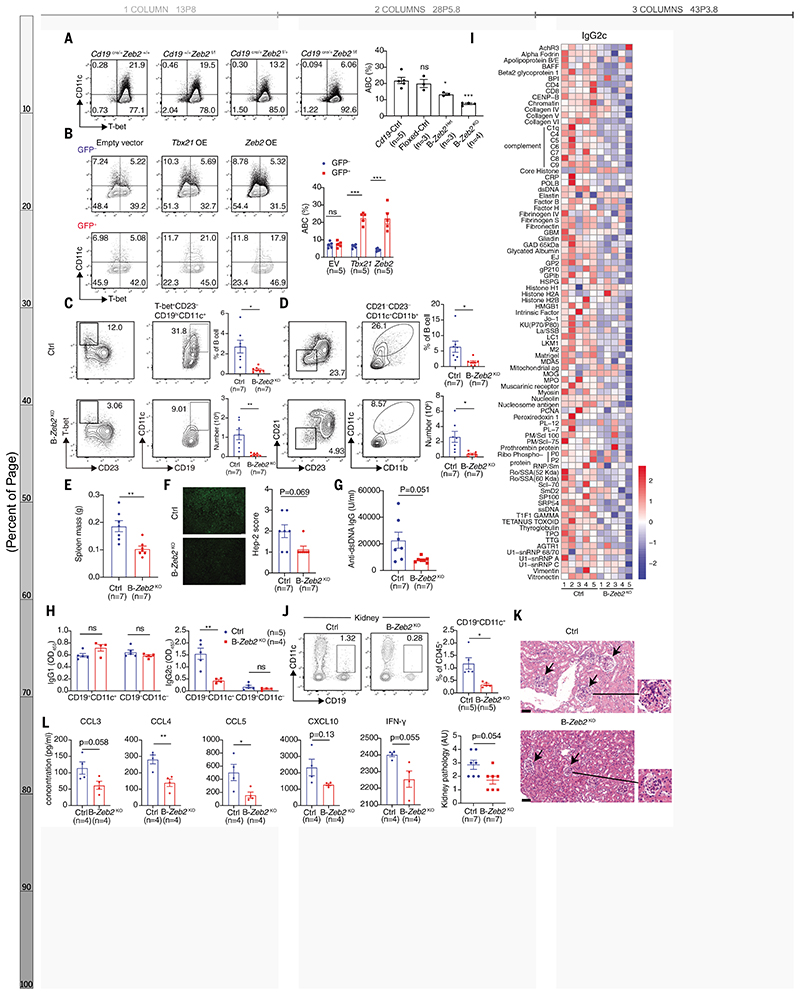
To further explore ZEB2’s role in ABC formation, we generated mice selectively lacking Zeb2 in B cells by crossing Zeb2 floxed mice with Cd19-cre mice (fig. S6, A to C). Zeb2 deficiency in B cells reduced ABC differentiation by over 50% (Fig. 3A). Even mice hemizygous for Zeb2 in B cells (B-Zeb2Het) displayed reduced ABC formation, providing evidence of Zeb2 haploinsufficiency in mice. Moreover, overexpression of Zeb2 in splenic B cells promoted ABC formation, indicating that Zeb2 is sufficient for ABC differentiation in vitro (Fig. 3B).
Fig. 3. Zeb2 deficiency impairs ABC formation and alleviates lupus pathogenesis.
(A) Representative plots and frequency of in vitro induced ABCs (CD19+CD11c+T-bet+) derived from splenic B cells of Zeb2+/+Cd19Cre/+ (CD19-Ctrl), Zeb2f/fCd19+/+ (Floxed-Ctrl), Zeb2f/+Cd19cre/+(B-Zeb2Het), Zeb2f/fCd19cre/+(B-Zeb2KO) mice. (B) Representative plots and frequency of ABCs (CD19+CD11c+T-bet+) in GFP+ (infected) and GFP− (uninfected) B cells transduced with empty plasmid, Tbx21 or Zeb2 cDNA sequence. (C and D) Representative plots, frequency and absolute number of splenic ABCs identified by CD19+CD23−CD11c+T-bet+ (C) and CD19+CD21−CD23−CD11c+CD11b+ (D) in IMQ-induced B-Zeb2KO and Cd19Cre/+ (Ctrl) mice. (E to G) Spleen weight (E), ANA (F), and anti-dsDNA (G) in serum from mice described in (C and D). (H) The IgG1 and IgG2c antibody titers in the culture supernatants from CD19+CD21−CD11c+ and CD19+CD21+CD11c− B cells sorted from mice described in (C and D). (I) Autoantigen microarray showing the relative IgG2c-isotype autoantibody levels in the serum of mice described in (C and D). (J) Representative plots and frequency of renal ABCs (CD19+CD11c+) from mice described in (C and D). (K) H&E staining (right) and pathology assessment (left) of kidneys from mice described in (C and D). (L) The concentration of cytokine and chemokine in the culture supernatants described in (H). n represents distinct samples (biological repeats). Data are representative of 2-3 independent experiments. Data are mean ± SEM values. *P<0.05, **P<0.01, ***P<0.001, ns, not significant, unpaired Student’s t test (B, E, H, L for CCL3, CCL4 and CCL5) with Welch’s correction (C, D, G, J, and L for CXCL10 and IFN-γ), Mann–Whitney U test (F and K) and ordinary one-way ANOVA with Dunnett’s multiple comparisons testing (A).
ABCs are a unique effector B cell subset that arises during immune responses to nuclear acid–related antigens (1), developing separately from the germinal center (GC) pathway (21, 22). To investigate how ZEB2 impacts pathogenic ABCs, we investigated the consequences of Zeb2 deficiency in B cells using two lupus mouse models (lupus induced by the TLR7 agonist imiquimod (IMQ) and bm12 cell transfer) as well as an acute LCMV infection model. B cell–intrinsic Zeb2 deficiency significantly impaired ABC formation in all three models (Fig. 3, C and D, and figs. S6, D to F, and S7, A to C). Furthermore, GC B cells increased in B cell–intrinsic Zeb2-deficient mice in the acute bm12-induced and LCMV infection models (fig. S7 D and E) suggesting a competitive relationship between GC B cells and ABCs. Zeb2 neither directly instructed GC B cell differentiation nor promoted antibody responses to an ABC-irrelevant protein antigens however (fig. S7F and G). Detailed profiling of ABCs in IMQ-induced lupus confirmed that ABCs were phenotypically distinct from CD38−GL-7+ GC B cells (fig. S8A). A significant proportion of CD19hiCD11c+IgD− ABCs exhibited CD38+GL-7− memory markers, while also displaying a unique hyperactivated state (CD95+CD80+) in comparison to other memory B cells (Fig. S8A). Zeb2 deficiency selectively impacted the distribution and hyperactivation of CD11c+ memory-like B cells, without affecting the frequency of the CD11c− memory B cell subset (fig. S8B). A subpopulation of ABCs acquired a phenotype (CD38+GL-7+) consistent with precursors of GC (pre-GC) B cells (fig. S8C), suggesting that like conventional memory B cells, ABCs could contribute to secondary GCs, seeding a chronic GC response. Notably, ABCs reside at the pre-plasma cell stage and can quickly differentiate into plasma cells (1). Such chronic GC responses and terminally differentiated plasma cells were reduced in IMQ-induced B-Zeb2KO mice, likely due to reduced replenishment from ABCs (fig. S8 D to E). Thus, rather than broadly promoting all effector B cell responses, ZEB2 selectively drives ABCs and their progeny. Moreover, although these cells develop extrafollicularly, their progeny may participate in GC responses in the context of chronic inflammation.
TLR7-driven lupus is GC-independent and mostly ABC-dominant (21). We therefore examined whether ZEB2 regulated ABC-mediated autoimmunity in lupus induced by IMQ, a TLR7 agonist. Zeb2 deficiency in B cells significantly ameliorated splenomegaly (Fig. 3E) and decreased serum antinuclear (ANA) and dsDNA autoantibodies (Fig. 3, F and G). ABCs are particularly pathogenic due to secretion of antibodies of the IgG2a/c isotype (1). Compared to non-ABCs, ABCs secreted the highest levels of IgG2c isotype antibodies upon restimulation. By contrast, residual ABC-like cells isolated from B-Zeb2KO mice were unable to produce comparable amounts of IgG2c antibodies (Fig. 3H). Similarly, B-Zeb2KO mice treated with IMQ produced much lower IgG2c autoantibodies (Fig. 3I). In lupus nephritis (LN), ABCs correlate with tissue damage (21, 23) and are known to produce proinflammatory cytokines and chemokines like CCL5, CXCL10, IFNγ, and IL-6 (8). In IMQ-treated B-Zeb2KO mice, kidney-infiltrating ABCs were substantially decreased (Fig. 3J and fig. S8G) as was tissue damage (Fig. 3K). The residual CD11c+CD21− B cells from B-Zeb2KO mice also produced reduced quantities of CCL5 and CXCL10 ex vivo (Fig. 3L). Thus, ZEB2 is essential for ABC-mediated autoimmunity and the proinflammatory properties of ABCs.
ZEB2 controls the lineage specification and cellular identity of ABCs
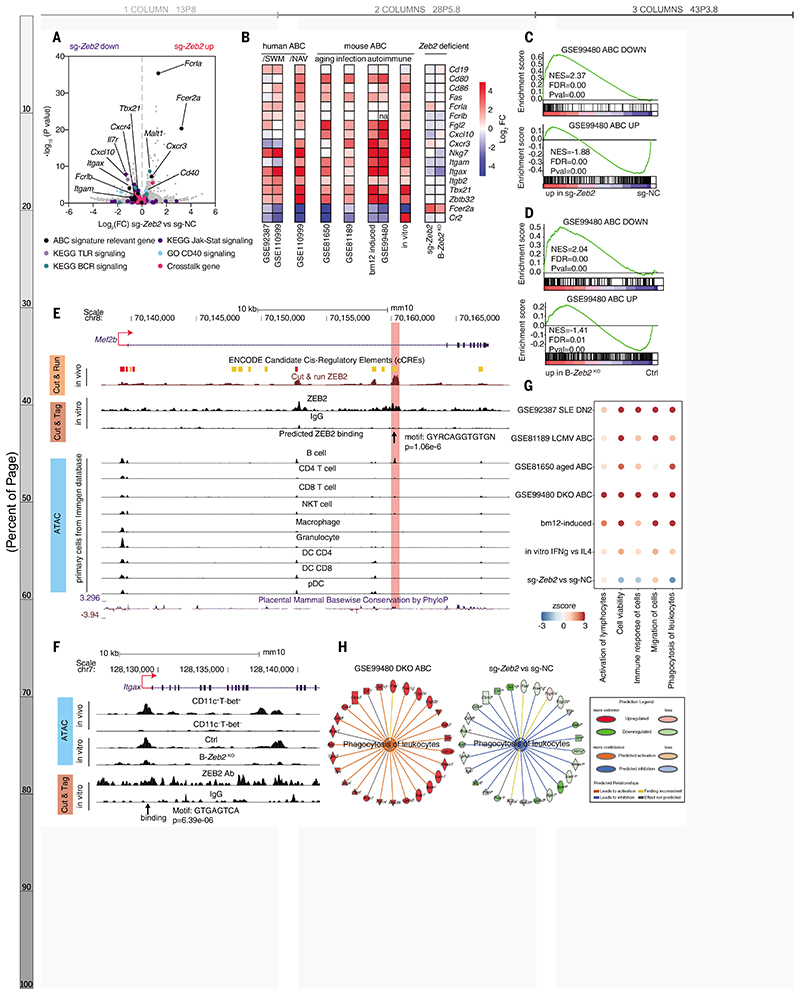
To investigate the consequences of ZEB2 regulation of gene transcription, RNA sequencing was performed on sorted Zeb2 deficient B cells after in vitro ABC induction. ABC signature genes including Itgax, Itgam, Itgb2, Nkg7, Tbx21, Zbtb32 and Fcer2a/Fcer2 (encoding CD23a/CD23) (4, 5, 8, 24–26) were reversly expressed after Zeb2 deficiency (Fig. 4, A and B). Gene set enrichment analysis (GSEA) revealed that Zeb2-deficient B cells lacked expression of the “ABC upregulated” gene set while it was enriched in the “ABC downregulated” gene set from the public dataset GSE99480 (8) (Fig. 4C and D).
Fig. 4. Zeb2 regulates specification and cellular identity of ABCs.
(A) Volcano graph showing the transcriptional profiles in Zeb2 edited B cells. ABC-signature genes, genes associated with BCR, TLR, JAK–STAT, and CD40 signaling, and crosstalk genes were labeled with colored dots. (B) Heatmap showing expression of representative ABC signature genes in public RNA-seq datasets: GSE92387, GSE110999, GSE81650, GSE81189, GSE99480 and RNA-seq of ABCs sorted from bm12-induced lupus mice (bm12-induced), ABC-polarized B cells (in vitro), ABC-polarized B cells derived from Zeb2 edited B cells (sg-Zeb2) and Zeb2-knockout B cells (B-Zeb2KO). (C and D) GSEA showing the enrichment of the ABC-down geneset and ABC-up geneset from GSE99480 in ABC-polarized B cells derived from Zeb2 deficiency B cells. Zeb2 deficiency was mediated by sg-Zeb2 editing (C) or knockout (D). (E) CUT & RUN, CUT & Tag, and ATAC-seq tracks display ZEB2 binding around the Mef2b locus, visualized by the UCSC genome browser. The chromatin accessibility in mouse primary immune cell subsets was from public Immgene database. (F) ATAC and CUT& Tag tracks display ZEB2 binding around the Itgax locus in ex vivo sorted ABCs and ABC-polarized B cells. (G) Dot plot showing the activation Z-score of predicated biological function in RNA-seq datasets mainly described as in (B). (H) Network diagram representing phagocytosis pathway in sg-Zeb2 versus sg-NC ABCs by IPA. The color of each node indicates change in the expression: red (upregulated) and green (downregulated). The edges indicate the predicted relationship between nodes and biological function: orange representing activation, blue representing inhibition, and gray representing effect not predicted.
To elucidate ZEB2's direct targets in ABCs, we performed high-throughput sequencing of the regulome by ATAC-seq, CUT & Tag, and CUT & RUN, leading to the identification of 4338 genes annotated by 6733 accessible sites with ZEB2 binding. Among the genes differentially expressed in Zeb2-deficient cells, we found 33 candidate direct targets: 22 repressed and 11 activated by ZEB2 (fig. S9A). Notably, Mef2b, an essential TF for GC development (27), was repressed by ZEB2. This direct regulation was mapped to a conserved region ~20 kb downstream of Mef2b's exon 1 TSS, enriched with enhancer-associated features in both human and mouse (Fig. 4E and fig. S9, B to D). We validated ZEB2 suppression of Mef2b expression in Zeb2-deficient and Zeb2-overxpressing B cells and confirmed opposing expression patterns of Zeb2 and Mef2b in ABCs and GC B cells from public datasets (22) (fig. S9, E to H). MEF2B can directly regulate S1pr2 (27) and Zeb2-deficient B cells upregulated S1pr2 expression (fig. S9E). Thus, ZEB2 appears to foster ABC differentiation by directly repressing Mef2b to constrain GC B cells, in alignment with our observations in the bm12 and LCMV models (fig. S7, D and E).
Additionally, ZEB2-specific peaks from CUT & Tag were matched to motifs of GATA3, FOSL2, and ZEB2 (fig. S10, A to C), consistent with existing ZEB2 Chip-seq data (fig. S10D). We identified a ZEB2-specific peak residing in the promoter of Itgax, containing a ZEB2-binding sequence (Fig. 4F). Zeb2 deficiency altered the chromatin accessibility of the ABC-specific opening in the Itgax promoter, further confirming that ZEB2 controls transcription of ABC signature gene. CD11c (Itgax), an important alpha-subunit member of beta2 integrins, can pair with the beta-subunit CD18 to form heterodimeric cell surface receptors important for immune cell adhesion and recruitment to tissues (28), which is a unique property of ABCs (4, 21, 23). In kidney biopsies from patients with LN (table S4), CD11c+ B cells were found in affected tissues, constituting approximately 50% of total B cells with IgD−CD27−CD11c+ ABCs comprising about 20% (fig. S11, A and B). We validated the enhanced migratory capacity of ABCs in vitro, which was modulated by CD11c blockade and Zeb2 deficiency (fig. S11, C to G). Thus, Zeb2 plays a crucial role in orchestrating ABC specification by directly suppressing other effector B cell subsets and inducing the ABC signature.
ZEB2 drives distinct functional properties of ABCs
To better define the function of ABCs, we applied ingenuity pathway analysis (IPA) to a public dataset (GSE99480) (8). Among the top 35 significantly increased predicted functions (table S5), ABCs shared five features: “enhanced viability”, “migration”, “activation”, “immune response”, and “phagocytosis/engulfment” (fig. S12A). These were validated accross several transcriptomes (Fig. 4G). Selected transcripts linked to these biological functions formed a network (fig. S12A). Pathway analysis further supported our finding that ABC-enriched pathways were linked to these five functional features (fig. S12B). ABCs have also been characterized by a hyperactivation state, long-term survival, and unique migration/distribution pattern in published studies (4, 5, 29). ABCs also exhibited a unique phagocytic capacity, identified by enriched phagosome formation and Fc-receptor pathways (fig. S12B). The ability of ABCs to both perform typical B cell functions and co-opt myeloid markers like CD11c, as well as cytotoxic molecules like NKG7, granzyme A, and perforin have been previously described (3, 4). In line with ZEB2’s critical role in ABC function, Zeb2 editing in B cells dampened their viability, immune response, and phagocytosis/engulfment (Fig. 4, G and H).
To experimentally test the phagocytic capacity of ABCs, we incubated splenic B cells with apoptotic thymocytes labeled with pHrodo and monitored apoptotic cell internalization (fig. S12C). CD19hiCD11c+ B cells exhibited markedly enhanced uptake evidenced by both an increased pHrodo+ fraction and signal intensity (fig. S12D). In vitro–generated ABCs were also able to engulf apoptotic cells, which was dampened by Zeb2 deficiency (fig. S12E and F). Thus ABCs exhibit unique biological functions that are regulated by ZEB2.
The ZEB2–JAK–STAT axis governs ABC differentiation
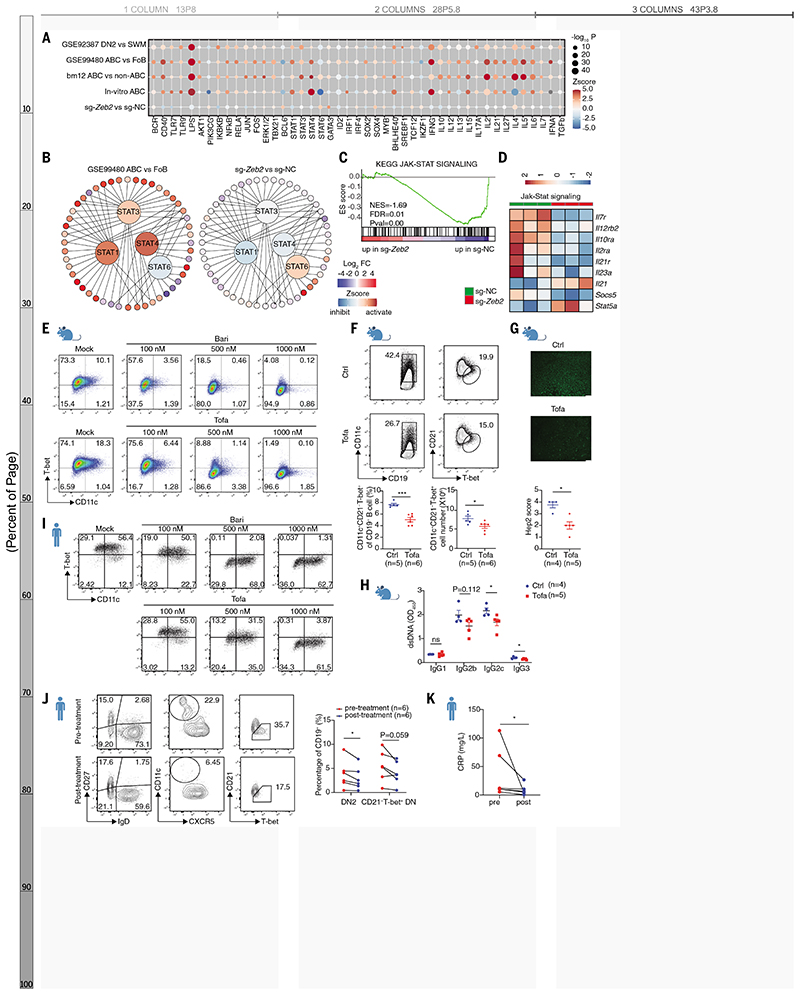
To elucidate the signaling pathways by which ZEB2 influences ABC formation, we performed upstream regulator analysis (URA) in IPA on both public and our own datasets. As anticipated, BCR, CD40, TLR and key downstream pathways like NF-κB and AKT were predicted to be activated in ABCs (Fig. 5A). Regulatory effects of cytokines IFNγ, IL-10, and IL-21, along with their JAK–STAT signals were also detected (Fig. 5A and B). Specifically STAT1, STAT3, and STAT4 were activated, whereas STAT6 was inhibited (Fig. 5, A and B) consistent with previous findings (1). Zeb2 deficiency altered STAT signals (Fig. 5, A and B), reflecting opposite expression pattern of STATs’ target genes in Zeb2 edited B cells (Fig. 5B). GSEA and KEGG analysis produced similar findings, supporting an important role of JAK–STAT signaling in ZEB2’s function (Fig. 5, C and D, and fig. S13A). We also confirmed that gene expression altered by Zeb2 deficiency largely overlapped with the transcriptional program affected by inhibition of JAK–STAT signaling (Fig. S13, B and C).
Fig. 5. Zeb2-JAK–STAT axis controls ABC differentiation.
(A) Activation Z-score heatmap for IPA-predicted upstream regulators in indicated datasets mainly described in Fig. 4B. P-values were log-normalized and presented by the size of the plot. The color of the plot indicates the activated (orange) or inhibited (blue) regulation of the predicted regulators. (B) Upstream regulator analysis showing STAT1, STAT3, and STAT4 activated and STAT6 inhibited in ABCs (GSE99480), whereas the opposite effects were observed in sg-Zeb2 vs sg-NC dataset. The color of the surrounding circles indicates the change in the expression: red (upregulated) and blue (downregulated). The color of the center circles indicates predicted regulators: orange (activated) and blue (inhibited). (C) GSEA showing impaired JAK–STAT pathway in Zeb2 edited B cells. (D) Heatmap showing expression of selected JAK–STAT signaling genes in sg-NC+ and sg-Zeb2+ B cells. (E) Flow cytometry plots of in vitro induced mouse ABCs with different concentrations of baricitinib (Bari) or tofacitinib (Tofa). (F to H) Frequency and absolute number of ABCs (F), ANA titer (G), and anti-dsDNA Ig titers (H) in serum from bm12-induced lupus mice treated with tofacitinib. (I) Flow cytometry plots of in vitro induced human ABCs with different concentrations of baricitinib or tofacitinib. (J) Flow cytometry plots of human ABCs in PBMCs from patients with rheumatoid arthritis before and after JAK–STAT inhibitor tofacitinib treatment for 4 weeks. (K) The change of CRP level in RA patients described in (J). n represents distinct samples (biological repeats). Data are representative of 2-3 independent experiments (E to I). Data are mean ± SEM values. *P<0.05, **P<0.01, ***P<0.001, ns, not significant, unpaired Student’s t test (F and H), Mann–Whitney U test (G), paired Student’s t test (J and K).
JAK–STAT inhibitors, such as baricitinib and tofacitinib, have proven to be effective in dampening intracellular cytokine signaling (30, 31). We therefore tested their effects on mouse ABC formation and found they impaired in vitro ABC differentiation in a dose-dependent manner (Fig. 5E and fig. S13, D to G). Tofacitinib administration reduced ABC accumulation and splenomegaly, lowered autoantibody titers, and decreased ABC-relevant cytokines in a manner likely to be B cell–intrinsic (Fig. 5, F to H, and fig. S14, A to E). Human ABC differentiation was also inhibited by these drugs (Fig 5I and fig. S14F). Furthermore, tofacitinib treatment decreased circulating ABCs in RA patients (Fig. 5J and fig. S14G) and ameliorated systemic inflammation (Fig. 5K). Thus, targeting the JAK–STAT pathway can block ABC differentiation in both mice and humans, making it a promising strategy for the treatment of ABC-mediated autoimmunity.
Discussion
We have identified ZEB2 as an essential TF that drives human and mouse ABC differentiation, antinuclear antibody formation, proinflammatory cytokine and chemokine production, and ABC migration to inflamed tissues. ZEB2 drives the ABC gene signature including Itgax, and suppresses Mef2b, which causes activated B cells to deviate from GCs and differentiate extrafollicularly. Differentiation of ABCs in a GC-independent manner has raised questions about the role of GCs in autoimmunity (21, 22). GC reactions comprise several tolerance checkpoints that are lacking in ABC development. Although ZEB2 appears to be essential for ABC formation, the upstream physiological signals and cells that turn on Zeb2 expression in vivo remain unclear. Zeb2 is likely to act in concert with other transcription or epigenetic factors including IRF5, T-bet, and metabolic regulators, to shape a regulatory complex in ABCs, mirroring ZEB2’s regulatory programs in NK cells (32) and CD8 T cells (33).
Our study highlights the innate ability of ABCs to phagocytose apoptotic cells, a function that may underpin TLR7 activation and self-antigen presentation to T cells, as well as explaining their hyperactivated status. The requirement of the JAK-STAT pathway to exert Zeb2-mediated ABC development and pathogenicity offers promising therapeutic prospects through Jak inhibitors. These insights extend to conditions where ABCs are expanded and may exert pathogenic roles such as aging.
Supplementary Material
One-Sentence Summary.
ZEB2 is essential for age-associated B cell differentiation and function.
Acknowledgments
We thank Z. Liu, Y. Ma, Q. Hu, Y. Hu, Y. Chen and S. Zhou for providing experimental help; J. Li, X. Xu for provding the LCMV Amstrong virus; X. Song for suggestion; J. Qin and J. Huang for sample collection and Y. Yu for reagents support.
Funding
National Natural Science Foundation of China (31630021, 31930037, 82071843, and 81901637); National Human Genetic Resources Sharing Service Platform (2005DKA21300); Shanghai Municipal Key Medical Center Construction Project (2017ZZ01024-002); Shenzhen Science and Technology Project (JCYJ20180504170414637 and JCYJ20180302145033769); Shenzhen Futian Public Welfare Scientific Research Project (FTWS2018005); and Sanming Project of Medicine in Shenzhen (SZSM201602087).
Footnotes
Author contributions: Conceptualization: N.S., D.D., and C.G.V. Methodology: D.D. and S.G. Investigation: D.D., S.G., X.H., H.D., Y.J., X.Z., C.Y., S.H., J.Z., G.H., B.Q., H.Z., Y.Q., Y.H., J.M., Z.Yin, Z.Ye, J.Q., Q.J., L.W., Q.G., S.C., C.H., L.C.K., and M.T.W. Visualization: D.D., S.G., and Y.J. Funding acquisition: N.S., D.D., Z.Ye, and S.C. Supervision: N.S. and C.G.V. Writing—original draft: D.D. and G.S.S. Writing—review and editing: N.S. and C.G.V.
Competing interests: The authors declare no competing interests.
Data and materials availability
All data are available in the main text or the supplementary materials. The scRNA-seq, RNA-seq, ATAC-seq, CUT&Tag, and CUT&RUN sequencing data are deposited in GEO under accession numbers GSE242615, GSE242607, and GSE242611. Plasmids are available upon establishment of an MTA with Shanghai Jiaotong University. All data are available in the main text or the supplementary materials.
References and Notes
- 1.Cancro MP. Age-Associated B Cells. Annu Rev Immunol. 2020;38:315–340. doi: 10.1146/annurev-immunol-092419-031130. [DOI] [PubMed] [Google Scholar]
- 2.Hao Y, O'Neill P, Naradikian MS, Scholz JL, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118:1294–1304. doi: 10.1182/blood-2011-01-330530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubtsov AV, et al. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c(+) B-cell population is important for the development of autoimmunity. Blood. 2011;118:1305–1315. doi: 10.1182/blood-2011-01-331462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang S, et al. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11c(hi)T-bet(+) B cells in SLE. Nat Commun. 2018;9:1758. doi: 10.1038/s41467-018-03750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jenks SA, et al. Distinct Effector B Cells Induced by Unregulated Toll-like Receptor 7 Contribute to Pathogenic Responses in Systemic Lupus Erythematosus. Immunity. 2018;49:725–739.:e726. doi: 10.1016/j.immuni.2018.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zumaquero E, et al. IFNgamma induces epigenetic programming of human T-bet(hi) B cells and promotes TLR7/8 and IL-21 induced differentiation. Elife. 2019;8 doi: 10.7554/eLife.41641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stone SL, et al. T-bet Transcription Factor Promotes Antibody-Secreting Cell Differentiation by Limiting the Inflammatory Effects of IFN-gamma on B Cells. Immunity. 2019;50:1172–1187.:e1177. doi: 10.1016/j.immuni.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manni M, et al. Regulation of age-associated B cells by IRF5 in systemic autoimmunity. Nat Immunol. 2018;19:407–419. doi: 10.1038/s41590-018-0056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricker E, et al. Altered function and differentiation of age-associated B cells contribute to the female bias in lupus mice. Nature Communications. 2021;12:4813. doi: 10.1038/s41467-021-25102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du SW, Arkatkar T, Jacobs HM, Rawlings DJ, Jackson SW. Generation of functional murine CD11c(+) age-associated B cells in the absence of B cell T-bet expression. Eur J Immunol. 2019;49:170–178. doi: 10.1002/eji.201847641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levack RC, Newell KL, Popescu M, Cabrera-Martinez B, Winslow GM. CD11c(+) T-bet(+) B Cells Require IL-21 and IFN-gamma from Type 1 T Follicular Helper Cells and Intrinsic Bcl-6 Expression but Develop Normally in the Absence of T-bet. J Immunol. 2020;205:1050–1058. doi: 10.4049/jimmunol.2000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lien C, et al. Critical role of IRF-5 in regulation of B-cell differentiation. Proc Natl Acad Sci U S A. 2010;107:4664–4668. doi: 10.1073/pnas.0911193107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu H, et al. Regulation of bifurcating B cell trajectories by mutual antagonism between transcription factors IRF4 and IRF8. Nature Immunology. 2015;16:1274–1281. doi: 10.1038/ni.3287. [DOI] [PubMed] [Google Scholar]
- 14.De S, et al. B Cell-Intrinsic Role for IRF5 in TLR9/BCR-Induced Human B Cell Activation, Proliferation, and Plasmablast Differentiation. Front Immunol. 2017;8:1938. doi: 10.3389/fimmu.2017.01938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glass DR, et al. An Integrated Multi-omic Single-Cell Atlas of Human B Cell Identity. Immunity. 2020;53:217–232.:e215. doi: 10.1016/j.immuni.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naradikian MS, et al. Cutting Edge: IL-4, IL-21, and IFN-gamma Interact To Govern T-bet and CD11c Expression in TLR-Activated B Cells. J Immunol. 2016;197:1023–1028. doi: 10.4049/jimmunol.1600522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vandewalle C, et al. SIP1/ZEB2 induces EMT by repressing genes of different epithelial cell-cell junctions. Nucleic Acids Res. 2005;33:6566–6578. doi: 10.1093/nar/gki965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mowat DR, et al. Hirschsprung disease, microcephaly, mental retardation, and characteristic facial features: delineation of a new syndrome and identification of a locus at chromosome 2q22-q23. J Med Genet. 1998;35:617–623. doi: 10.1136/jmg.35.8.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wakamatsu N, et al. Mutations in SIP1, encoding Smad interacting protein-1, cause a form of Hirschsprung disease. Nat Genet. 2001;27:369–370. doi: 10.1038/86860. [DOI] [PubMed] [Google Scholar]
- 20.Scott CL, Omilusik KD. ZEBs: Novel Players in Immune Cell Development and Function. Trends Immunol. 2019;40:431–446. doi: 10.1016/j.it.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Brown GJ, et al. TLR7 gain-of-function genetic variation causes human lupus. Nature. 2022;605:349–356. doi: 10.1038/s41586-022-04642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song W, et al. Development of Tbet- and CD11c-expressing B cells in a viral infection requires T follicular helper cells outside of germinal centers. Immunity. 2022;55:290–307.:e295. doi: 10.1016/j.immuni.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu C, et al. Lupus-associated atypical memory B cells are mTORC1-hyperactivated and functionally dysregulated. Ann Rheum Dis. 2019;78:1090–1100. doi: 10.1136/annrheumdis-2019-215039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell Knode LM, et al. Age-Associated B Cells Express a Diverse Repertoire of VH and Vkappa Genes with Somatic Hypermutation. J Immunol. 2017;198:1921–1927. doi: 10.4049/jimmunol.1601106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnett BE, et al. Cutting Edge: B Cell-Intrinsic T-bet Expression Is Required To Control Chronic Viral Infection. J Immunol. 2016;197:1017–1022. doi: 10.4049/jimmunol.1500368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han X, et al. Amelioration of Autoimmunity in a Lupus Mouse Model by Modulation of T-Bet-Promoted Energy Metabolism in Pathogenic Age/Autoimmune-Associated B Cells. Arthritis Rheumatol. 2023;75:1203–1215. doi: 10.1002/art.42433. [DOI] [PubMed] [Google Scholar]
- 27.Brescia P, et al. MEF2B Instructs Germinal Center Development and Acts as an Oncogene in B Cell Lymphomagenesis. Cancer Cell. 2018;34:453–465.:e459. doi: 10.1016/j.ccell.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Wang H. Integrin signalling and function in immune cells. Immunology. 2012;135:268–275. doi: 10.1111/j.1365-2567.2011.03549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson JL, et al. The Transcription Factor T-bet Resolves Memory B Cell Subsets with Distinct Tissue Distributions and Antibody Specificities in Mice and Humans. Immunity. 2020;52:842–855.:e846. doi: 10.1016/j.immuni.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A. Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis. 2013;72 Suppl 2:ii111–115. doi: 10.1136/annrheumdis-2012-202576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Traves PG, et al. JAK selectivity and the implications for clinical inhibition of pharmacodynamic cytokine signalling by filgotinib, upadacitinib, tofacitinib and baricitinib. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2020-219012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Helden MJ, et al. Terminal NK cell maturation is controlled by concerted actions of T-bet and Zeb2 and is essential for melanoma rejection. J Exp Med. 2015;212:2015–2025. doi: 10.1084/jem.20150809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dominguez CX, et al. The transcription factors ZEB2 and T-bet cooperate to program cytotoxic T cell terminal differentiation in response to LCMV viral infection. J Exp Med. 2015;212:2041–2056. doi: 10.1084/jem.20150186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klarquist J, Janssen EM. The bm12 Inducible Model of Systemic Lupus Erythematosus (SLE) in C57BL/6 Mice. J Vis Exp. 2015:e53319. doi: 10.3791/53319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yokogawa M, et al. Epicutaneous application of toll-like receptor 7 agonists leads to systemic autoimmunity in wild-type mice: a new model of systemic Lupus erythematosus. Arthritis Rheumatol. 2014;66:694–706. doi: 10.1002/art.38298. [DOI] [PubMed] [Google Scholar]
- 36.Cossarizza A, et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition) Eur J Immunol. 2019;49:1457–1973. doi: 10.1002/eji.201970107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heinen AP, et al. Improved method to retain cytosolic reporter protein fluorescence while staining for nuclear proteins. Cytometry A. 2014;85:621–627. doi: 10.1002/cyto.a.22451. [DOI] [PubMed] [Google Scholar]
- 38.Montague TG, Cruz JM, Gagnon JA, Church GM, Valen E. CHOPCHOP: a CRISPR/Cas9 and TALEN web tool for genome editing. Nucleic Acids Res. 2014;42:W401–407. doi: 10.1093/nar/gku410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conant D, et al. Inference of CRISPR Edits from Sanger Trace Data. Crispr j. 2022;5:123–130. doi: 10.1089/crispr.2021.0113. [DOI] [PubMed] [Google Scholar]
- 40.Zheng GXY, et al. Massively parallel digital transcriptional profiling of single cells. Nature Communications. 2017;8:14049. doi: 10.1038/ncomms14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stuart T, et al. Comprehensive Integration of Single-Cell Data. Cell. 2019;177:1888–1902.:e1821. doi: 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henikoff S, Henikoff JG, Kaya-Okur HS, Ahmad K. Efficient chromatin accessibility mapping in situ by nucleosome-tethered tagmentation. Elife. 2020;9 doi: 10.7554/eLife.63274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tarasov A, Vilella AJ, Cuppen E, Nijman IJ, Prins P. Sambamba: fast processing of NGS alignment formats. Bioinformatics. 2015;31:2032–2034. doi: 10.1093/bioinformatics/btv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramírez F, et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 2016;44:W160–165. doi: 10.1093/nar/gkw257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grant CE, Bailey TL, Noble WS. FIMO: scanning for occurrences of a given motif. Bioinformatics. 2011;27:1017–1018. doi: 10.1093/bioinformatics/btr064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the main text or the supplementary materials. The scRNA-seq, RNA-seq, ATAC-seq, CUT&Tag, and CUT&RUN sequencing data are deposited in GEO under accession numbers GSE242615, GSE242607, and GSE242611. Plasmids are available upon establishment of an MTA with Shanghai Jiaotong University. All data are available in the main text or the supplementary materials.