Worldwide, more than 850 million people are estimated to be living with kidney disease— chronic kidney disease (CKD), acute kidney injury/disease, or kidney failure treated with kidney replacement therapy.1 This burden is increasing. Between 1990 and 2017, the prevalence of CKD increased by 29% and CKD moved from being the 17th to the 12th most common cause of death.2 The burden is also distributed unevenly. The age-standardized disability-adjusted life years lost from CKD is 15 times higher in American Samoa, El Salvador, Federated States of Micronesia, Marshall Islands, and Mauritius than in the best performing high-income countries.2 Robust longitudinal data are needed to increase the awareness of the burden of CKD and kidney failure and to advocate for resources to prevent and treat kidney diseases, yet low- and middle-income countries are much less likely to have surveillance systems, such as renal registries, in place.3 The International Society of Nephrology (ISN) registry initiative Sharing Expertise to Support the setup of Renal Registries (SharE-RR) was established in 2017 to address this.4
The main focus of the ISN SharE-RR Advisory Group since the completion of its pilot phase in 20194 has been the development of a toolkit to assist countries and regions in the development and growth of renal registries. The potential impact of this ambition has been greatly enhanced by its incorporation as a deliverable into the ISN’s 2021-2023 Collaboration Plan with the World Health Organization. Looking forward, the implementation of the toolkit has been incorporated into the ISN/World Health Organization 2024-2026 Collaboration Plan. The toolkit is being launched at the World Congress of Nephrology 2024 and can be found online at www.theisn.org/inaction/research/share-rr.
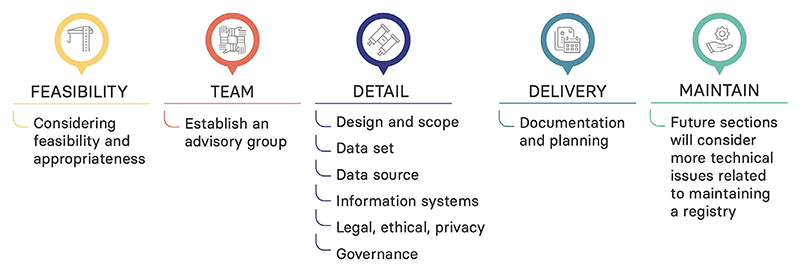
Although there is substantial registry expertise among the global kidney community,4,5 the toolkit is based on 2 existing, highly systematic resources on developing registries in general: 1 produced by the Agency for Healthcare Research and Quality (AHRQ) in the United States6 and 1 produced by a Joint Action funded by the European Commission, the Patient Registries iNiTiative (PARENT).7 These 2 comprehensive guidelines act as an extensive resource underpinning the ISN SharE-RR Toolkit, thereby providing lessons from the wider registry community. They allow the toolkit to be written as a brief, accessible, overarching resource that takes the aspiring registry developer on a journey from considering the feasibility and appropriateness [of setting up a registry] all the way to maintaining a registry (Figure 1). There is signposting to the relevant sections in the AHRQ and PARENT guidelines and the opportunity to link to other resources as they become available. The advisory group developed a structure for the toolkit and, assisted by representatives from the ISN Young Nephrologist Committee and the ISN Emerging Leaders Program, distilled the key lessons from the guidelines and drafted the chapters. As appropriate, wording was adapted for renal registries on the basis of the experience of the advisory group and nephrologists with limited registry experience working in low- and middle-income countries. Both AHRQ and PARENT gave permission for the inclusion of their guidelines in this toolkit.
Figure 1. Steps for a successful registry.
The toolkit begins with 2 often-overlooked (or at least underappreciated) preparatory steps that highlight “prerequisites” for a national registry, such as institutional and financial support, stakeholder involvement, and quality procedures and feedback to establish and maintain trust.
-
(1)
Feasibility and appropriateness of setting up a registry. The aspiring registry developer is asked to consider 3 key questions: What is the health service or public health goal that needs to be achieved? Is a registry the best way to accomplish this? Is setting up a registry in a particular country or region feasible? The first 2 questions remind them that setting up a registry is not always the right approach and encourage them to consider more agile and efficient approaches, such as surveys and cohort studies. The third touches on practical considerations, such as health care system, political situation, and funding. AHRQ and PARENT are quite strict and stress the need to have a plan for funding before you get started. The experience of the advisory group, however, is that several successful renal registries would never have started if they had taken this approach. Therefore, the guidance in the toolkit instead encourages the consideration of costs and funding options and the involvement of stakeholders early in the process and recognizes the role of pilot feasibility work. Potential sources of funding include industry, charities, insurance providers, public-private partnerships, and ministries of health, with the last being particularly effective and sustainable if linked to treatment center accreditation or reimbursement.
-
(2)
Establishing your team. While registries are often driven by clinical experts, a wide range of expertise is required to plan and implement a sustainable registry. In broad terms this will include experts on the subject matter (clinicians and patient representatives), registry science (epidemiologists, biostatisticians, and health economists), database management (data scientists and technicians/programmers), legal and data privacy, and project management. All potential stakeholders should be included early in the work to define the purpose and scope, making it more likely that they will support the registry strategically and/or financially.
Having established the feasibility and appropriateness of a registry and identified the team, the toolkit moves on to address a number of key considerations: design and scope; data set; data source; information systems; legal, ethical, and privacy; and governance. Although many of these can be worked on in parallel, the first—designing the registry and setting its scope—needs to be completed before work begins on others. The following questions are posed: Is the purpose of the registry to describe the incidence and/or prevalence of a disease, treatment rates, the natural history of a disease, or the effectiveness or safety or quality of a treatment? Linked to this will be the target population, often defined by an exposure such as diagnosis (e.g., a rare disease), treatment (e.g., hemodialysis), or program (e.g., a CKD screening program).
One strength of population-based registries is that they can provide representative data. To achieve this, attention must be paid to the individual patients, the sites included, and their coverage of the general population. Mandatory reporting of all patients at all sites is the most obvious way to achieve a representative sample. If this is not possible, or if the goal is to gather more detailed data on a subset of patients, sites can be randomly or purposively sampled and patient eligibility criteria kept broad to ensure representativeness. In decisions around data collection, potential consequences need to be considered. The importance of a minimal data set is often acknowledged, but difficult to enact. Strict discipline is required, mapping every potential variable back to the primary purpose of the registry and understanding the burden of data collection on sites and patients. Where possible, standard data terminologies and code lists should be used.
Data for a registry can come from multiple sources, depending on the local health information system infrastructure, with linkage to other routine health records increasingly an option. Patients might also enter their own data, such as patient-reported outcomes. The optimal combination will be driven by the primary purpose of the registry, as well as by local considerations such as data quality, interoperability (i.e., for sharing and comparing data, nationally or internationally), and the legal framework (e.g., consent or waiver) for data linkage, processing, and storing data for audit and research. These governance aspects will require robust systems and oversight, with authentic patient and public engagement going a long way toward ensuring transparency about how data are processed and stored securely.
Alongside all these decisions will be a technical one about the information system that will be required to house the registry data. Broadly, 3 options exist: to buy a solution “off-the-shelf,” to build one “in-house,” or to build one through an external partner. Each of these has pros and cons, which are briefly summarized in the toolkit and explored more fully in the AHRQ and PARENT guidelines.
Once all the details have been worked out, it is good practice to document everything in a protocol and standard operating procedures. In addition to being needed when applying for various permissions, these will make the registry robust to staffing changes over time. Recognizing the complexity of the task at hand, it is advisable to include someone in the team with project management experience to keep things on track. The final section of the toolkit addresses maintaining (and evolving) a renal registry.
In addition to the written guidance with signposting to the relevant sections in the AHRQ and PARENT guidelines, the ISN SharE-RR Toolkit contains a searchable world map of renal registries. Talks will be prepared by content experts and recorded as a resource in the ISN Academy, linked with the toolkit. The framework of the toolkit is now starting to be piloted in a small number of countries setting up a renal registry, with lessons being learned about what advice is core to success and what is optional; all will be situation dependent. ISN SharE-RR will continue its precongress workshops and exchange sessions, sharing knowledge and connecting experts. Ultimately, the success of ISN SharE-RR will be judged on the generation of new robust data on kidney health that can underpin advocacy, service redesign, research, and public health intervention that will improve the health of populations worldwide.
Acknowledgments
We are grateful to the Agency for Healthcare Research and Quality in the United States and the Patient Registries iNiTiative in the European Union for their permission to link to their guideline documents. This work was supported by in-kind administrative support from the International Society of Nephrology.
Footnotes
Disclosure
MP was funded by the National Institute of Health Research (NIHR). KJJ received funding from the European Renal Association (ERA) and the European Society for Paediatric Nephrology (ESPN) for running the ERA Registry and the ESPN/ERA Registry. JBW received funding from the National Institute of Diabetes and Digestive and Kidney Disease (USA) for serving as the United States Renal Data System Coordinating Center. SK is a consultant for George Clinical. JH received funding from the Japan Agency for Medical Research and Development, a Grant-in-Aid for Intractable Renal Diseases Research, Research on Rare and Intractable Diseases, and Health and Labour Sciences Research grants from the Ministry of Health, Labour and Welfare of Japan. VK was funded by India Alliance (Department of Biotechnology, Government of India and Wellcome Trust, UK) through a Clinical and Public Health Intermediate Fellowship grant (grant no. IA/CPHI/18/1/503954). AH, SA, and SD received personal fees from the International Society of Nephrology. All the other authors declared no competing interests.
References
- 1.Jager KJ, Kovesdy C, Langham R, et al. A single number for advocacy and communication—worldwide more than 850 million individuals have kidney diseases. Kidney Int. 2019;96:1048–1050. doi: 10.1016/j.kint.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng MSY, Charu V, Johnson DW, et al. National and international kidney failure registries: characteristics, commonalities, and contrasts. Kidney Int. 2022;101:23–35. doi: 10.1016/j.kint.2021.09.024. [DOI] [PubMed] [Google Scholar]
- 4.Hole BD, Evans KM, Pyart R, et al. International collaborative efforts to establish kidney health surveillance systems. Kidney Int. 2020;98:812–816. doi: 10.1016/j.kint.2020.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.See EJ, Bello AK, Levin A, et al. Availability, coverage, and scope of health information systems for kidney care across world countries and regions. Nephrol Dial Transplant. 2021;37:159–167. doi: 10.1093/ndt/gfaa343. [DOI] [PubMed] [Google Scholar]
- 6.Gliklich RE, Leavy MB, Dreyer NA. Registries for evaluating patient outcomes: a user’s guide. Agency for Healthcare Research and Quality; 2020. [Accessed June 6, 2021]. https://effectivehealthcare.ahrq.gov/products/registries-guide-4th-edition/users-guide . [PubMed] [Google Scholar]
- 7.Zaletel M, Kralj M, editors. Methodological guidelines and recommendations for efficient and rational governance of patient registries. National Institute of Public Health; 2015. [Accessed June 25, 2020]. http://www.nijz.si/ [Google Scholar]