Hypertrophic cardiomyopathy (HCM) is diagnosed in 1/500 individuals in the population. Up to 70% of individuals with HCM have resting or provocable obstructive HCM (oHCM), in which cardiac outflow is compromized. Treatment options are symptomatic rather than disease-modifying. Mavacamten is a first-in-class, orally administered, cardiac-specific, small-molecule allosteric modulator of β-cardiac myosin. Mavacamten reversibly inhibits the binding of β-cardiac myosin to actin to reduce hypercontractility in an exposure-dependent manner. Mavacamten treatment can reduce outflow tract obstruction and improve exercise capacity, symptoms, and health status1. Heart failure due to reversible systolic dysfunction is a recognized adverse consequence of treatment in some (5% of treated individuals with HCM in phase 3 clinical studies).
DNA variants can alter the therapeutic and adverse effects of drugs at recommended dosages. Variants in genes encoding cytochrome P450 enzymes, expressed in the liver and small intestine, are associated with variable drug metabolism. Individuals can be categorized into five metabolizer phenotypes: poor, intermediate, extensive (sometimes described as normal), rapid, and ultra-rapid. CYP2C19 is the dominant metabolizer of mavacamten, responsible for 74% of mavacamten metabolism. CYP3A4/5 and CYP2C9 also contribute significantly, with CYP3A4 being the dominant metabolizer in individuals with reduced CYP2C19 activity2.
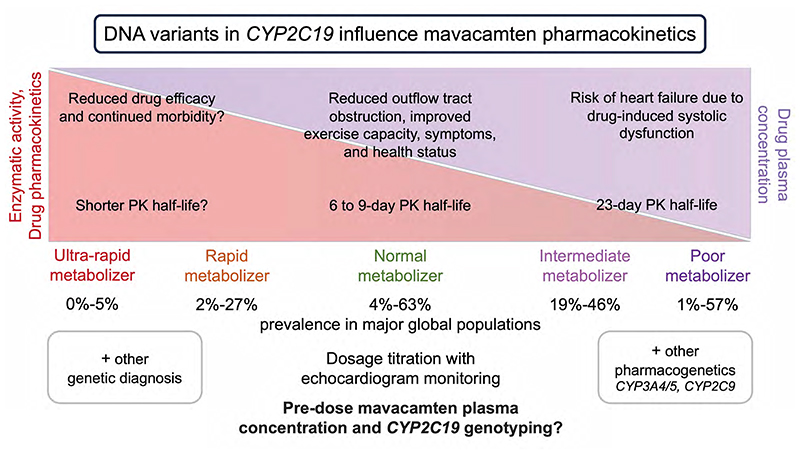
Poor metabolizers, due to diplotypes of two CYP2C19 alleles, may be at increased risk of systolic dysfunction from mavacamten treatment at the recommended dose. The prevalence of poor metabolizers varies between major global populations (Figure 1). While less common in European ancestry (2%), they comprise 57% of Oceanic populations, 13% of East Asian populations, and 8% of Central or South Asian populations.
Figure 1. The impact of CYP2C19 pharmacogenetics on mavacamten pharmacokinetics.
The figure depicts the variation in CYP2C19 enzymatic activity, the impact on mavacamten pharmacokinetics, and the alteration in the plasma concentration of mavacamten due to genetic variants. The prevalence of metabolizer status of global populations for CYP2C19 is adapted from the Clinical Pharmacogenetics Implementation Consortium. PK, pharmacokinetics.
The U.S. Food and Drug Administration (FDA; 2022) labelling for mavacamten includes a pharmacogenetic report: “mavacamten AUCinf [area under the plasma concentration-time curve from time 0 to infinity] increased by 241% and Cmax [the maximum plasma concentration following administration] increased by 47% in CYP2C19 poor metabolizers compared to normal metabolizers following a single dose of 15 mg mavacamten. Mean half-life is prolonged in CYP2C19 poor metabolizers compared to normal metabolizers (23 days vs. 6 to 9 days, respectively)”. It takes approximately four weeks to eliminate mavacamten from the body after treatment discontinuation.
Mavacamten is approved for adults with symptomatic New York Heart Association Class II-III oHCM by the FDA, Health Canada (Santé Canada), the Australian Therapeutic Goods Administration (TGA), the European Medicines Agency (EMA), the UK National Institute for Health and Care Excellence (NICE), and the UK Medicines and Healthcare products Regulatory Agency (MHRA). NICE recommends treatment only as an add-on to individually optimized standard care (beta-blockers, non-dihydropyridine calcium-channel blockers or disopyramide, unless contraindicated) and discusses potential feasibility issues of echocardiogram monitoring due to long waiting times and a lack of trained echocardiographers. Dosage titration is based on echocardiogram monitoring of ejection fraction (LVEF) and outflow tract obstruction gradients (LVOT) which reduce in a concentration-dependent manner.
The EMA and MHRA recommend genotyping for CYP2C19 to determine the appropriate dose. If treatment is initiated without metabolizer status determination, dosage should follow as described for poor metabolizers (starting at 2.5 mg once daily and a maximum dose of 5 mg once daily). The recommended starting dose for all other metabolizers is 5 mg once daily and a maximum dose of 15 mg once daily. Dose modifications are provided for concomitant medicinal products including CYP2C19 and CYP3A4 inhibitors and inducers. The EMA and TGA suggest a simulated 5 mg dose in a poor metabolizer is similar to the maximum dose in a normal metabolizer.
It remains unclear how the EMA genotyping recommendation will be implemented across diverse European healthcare systems. Genetic testing for variants in sarcomere-encoding genes is used for individuals with HCM to establish the molecular aetiology: pharmacogenetic analysis could be incorporated for individuals who have not already undergone testing and allow for the EMA recommended dosage stratification.
There is limited data on the safety of mavacamten in poor metabolizers. A trial of Chinese participants treated 7 poor metabolizers and 24 intermediate metabolizers and assessed genotype and pre-dose plasma mavacamten levels3. The authors support the titration scheme from 2.5 mg without the need for CYP2C19 genotyping as no participant experienced an ejection fraction of less than 50%. However, adverse events were not reported stratified by metabolizer status and an immediate metabolizer had dose interruption due to >1000 ng/mL mavacamten plasma concentration. The study allowed for a reduced dose of 1 mg from 6 weeks and poor metabolizers underwent additional monitoring until 20 weeks post-treatment.
Further studies of CYP2C19 genotyping and pre-dose mavacamten plasma concentration measurement with risk/benefit assessments are needed. The Phase 2 trial4 of 21 individuals with oHCM suggested that >1000 ng/mL mavacamten plasma concentration was associated with systolic dysfunction. Transient systolic dysfunction occurred in ~10% of phase 3 trial1 individuals despite dosing dictated by plasma mavacamten concentration and echocardiography5.
Providing the appropriate mavacamten dosage to each patient with oHCM from treatment initiation should allow for improved quality of life at the lowest risk of adverse events, cost, and burden to healthcare systems. Prescribers must be aware of the potential for metabolic variability across and within different ancestries and clinical vigilance with close monitoring will be required to avoid adverse events. Successful treatment requires improving symptoms of oHCM in rapid metabolizers and minimising the risk of drug-induced systolic dysfunction in poor metabolizers. Treatment without genotyping risks reduced ejection fraction in poor metabolizers and/or increased time to therapeutic dose in normal metabolizers. With limited medications for the management of oHCM, or where there is limited access to septal reduction therapy, effective titration of cardiac myosin inhibitors is vital to the success of treatment. While future clinical trials with improved metabolizer and ancestral representation will aid our understanding in this area, pre-dose mavacamten plasma concentration measurement and CYP2C19 genotyping may allow for less frequent clinical monitoring and improved costs.
Acknowledgements and funding sources
K.A.M. is supported by the British Heart Foundation [FS/IPBSRF/22/27059, RE/18/4/34215] and the NIHR Imperial College Biomedical Research Centre. N.B. is supported by the Imperial College London Undergraduate Research Opportunities Programme. J.S.W. is supported by the Sir Jules Thorn Charitable Trust [21JTA], Medical Research Council (UK), British Heart Foundation [RE/18/4/34215], and the NIHR Imperial College Biomedical Research Centre. The views expressed in this work are those of the authors and not necessarily those of the funders. For open access, the authors have applied a CC BY public copyright license to any Author Accepted Manuscript version arising from this submission.
Footnotes
CrediT statement
Conceptualization and guarantor: K.A.M.; Formal analysis: K.A.M., N.B.; Writing – original draft: K.A.M.; Writing – review & editing: K.A.M, N.B., J.S.W.
Conflict of interest
J.S.W. has consulted for MyoKardia, Inc., Pfizer, Foresite Labs, and Health Lumen, and receives research support from Bristol Myers-Squibb. None of these activities are directly related to the work presented here. All other authors declare no competing interests.
References
- 1.Olivotto I, Oreziak A, Barriales-Villa R, Abraham TP, Masri A, Garcia-Pavia P, Saberi S, Lakdawala NK, Wheeler MT, Owens A, Kubanek M, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet. 2020;396:759–769. doi: 10.1016/S0140-6736(20)31792-X. [DOI] [PubMed] [Google Scholar]
- 2.Grillo MP, Erve JCL, Dick R, Driscoll JP, Haste N, Markova S, Brun P, Carlson TJ, Evanchik M. In vitro and in vivo pharmacokinetic characterization of mavacamten, a first-in-class small molecule allosteric modulator of beta cardiac myosin. Xenobiotica. 2019;49:718–733. doi: 10.1080/00498254.2018.1495856. [DOI] [PubMed] [Google Scholar]
- 3.Tian Z, Li L, Li X, Wang J, Zhang Q, Li Z, Peng D, Yang P, Ma W, Wang F, Jin W, et al. Effect of Mavacamten on Chinese Patients With Symptomatic Obstructive Hypertrophic Cardiomyopathy: The EXPLORER-CN Randomized Clinical Trial. JAMA Cardiol. 2023;8:957–965. doi: 10.1001/jamacardio.2023.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heitner SB, Jacoby D, Lester SJ, Owens A, Wang A, Zhang D, Lambing J, Lee J, Semigran M, Sehnert AJ. Mavacamten treatment for obstructive hypertrophic cardiomyopathy a clinical trial. Ann Intern Med. 2019;170:741–748. doi: 10.7326/M18-3016. [DOI] [PubMed] [Google Scholar]
- 5.Maron MS, Ommen SR. Exploring New and Old Therapies for Obstructive Hypertrophic Cardiomyopathy: Mavacamten in Perspective. Circulation. 2021;143:1181–1183. doi: 10.1161/CIRCULATIONAHA.120.051330. [DOI] [PubMed] [Google Scholar]