Abstract
Objective
To report a validation of the Riester Big Ben Square Desk Aneroid Sphygmomanometer according to the international protocol developed by the Working Group on Blood Pressure Monitoring of the European Society of Hypertension 2002 (ESH-IP 2002) in the interest of transparency. This legacy publication is intended to assure users that that device satisfied the requirements in place at that time.
Methods
Performance of the device was assessed by participants’ age, sex, arm circumference and entry systolic/diastolic blood pressures (BP). Validation was performed in 33 participants. The sphygmomanometer was assessed according to the ESH-IP which defines zones of accuracy compared to the mercury standard as ≤ 5 mmHg, ≤ 10 mmHg, ≤ 15 mmHg or more.
Results
The mean (± SD) age was 50.5 ± 13.0 years, range 29-71 years, entry systolic BP (SBP) 142.6 ± 23.7 mmHg, entry diastolic BP (DBP) 89.0 ± 17.8 mmHg. The device passed all requirements listed and the validation protocol. The Riester Big Ben Square Desk aneroid sphygmomanometer slightly underestimated the observer-measured SBP, yet slightly over estimated DBP. The observer-device disagreement was -0.8 ± 6.4 mmHg SBP and +0.6 ± 4.0 mmHg DBP.
Conclusions
These data show that the Riester Big Ben Square Desk aneroid sphygmomanometer fulfilled the ESH-IP 2002 requirements for validation of BP monitors. It was on this basis that the British and Irish Hypertension Society recommended it for clinical use in the adult population.
Keywords: blood pressure, monitoring, validation, device
Introduction
The aim of the present study was to verify the accuracy of the Reister Big Ben Square Desk Aneroid Sphygmomanometer, (Rudolf Riester GmbH, Jungingen, Germany) for BP measurement in the upper arm, according to the European Society of Hypertension International Protocol (ESH-IP 2002) [1] for the validation of BP measuring in adults. The BIHS Working Party on Blood Pressure Measurement assessed the data presented and published an internal report in May 2009; at that time, the BIHS (then British Hypertension Society) did not have a policy of publishing their official validations. This report recommended the Riester Big Ben Square Desk for blood pressure assessment in the adult population. Based on these results, the recommendation was published on the BIHS website and the monitor has been in widespread use for many years since then.
In the interest of transparency, we believe it important to ensure that the data on which the original validation was made can be judged by the rest of the scientific community, especially in the absence of any alternative assessment. The validation study was carried out in 2009 prior to the 2010 update of the ESH protocol [2] and AAMI/ANSI/ISO 81060-2:2019 standard [3]. Many devices validated according to the ESH-IP 2002 protocol (the validation protocol at the time the work was undertaken) are still in use and, while recognising that new publications according to ESH-IP 2002 ceased to be recognised since 1st July 2011, this is a legacy presentation of an internal publication that predated that deadline.
Methods
Subjects
The ESH-IP 2002 for validation of blood pressure measuring devices in adults was used for this validation study. This monitor validation used the most contemporary protocol then available, long before subsequent protocol amendments had been developed. Korotkoff K5 was used for reference diastolic BP. The reference device details are as follows: sphygmomanometer: Accoson Dekamet Sphygmomanometer (A C COSSOR & SON (SURGICAL) LTD, Harlow, Essex, UK), stethoscope: Littmann Classic II S.E Teaching Stethoscope (3M United Kingdom PLC, Bracknell, Berkshire, UK), cuffs: Accoson Standard Adult cuff for arm circumferences 18.4 cm up to 28.75 cm; Accoson Alternative Adult Cuff for arm circumferences 28.75 cm up to 43.75 cm. (A C COSSOR & SON (SURGICAL) LTD, Harlow, Essex, UK).
Device
The Riester Big Ben Square Desk is an aneroid sphygmomanometer (Figure 1). It is a large device and suitable for desk use or wall mounting. The device can withstand pressure up to 600 mmHg, with a scale up to 300 mmHg. The cuffs are two-tubed with one tube connected to the latex bulb and air release valve and the other via an extendable tube to the sphygmomanometer. The device incorporates a large storage basket to the reverse. It has a large display with markings at 2 mm spacing, numbered at 20 mmHg increments (i.e. 20 mmHg, 40 mmHg, 60 mmHg). The display on the device is clear (white against a black background). Two bladder sizes were used: ‘Adult’ (24-32 cm) and ‘Adult Obese’ (32-42 cm) in our study. However, the new-born (5-7.5 cm) and small adult (17-26 cm) cuffs are also available. There are a full range of sizes in nylon and velcro and a limited number of cotton cuffs are available as well.
Figure 1. Reister Big Ben Square Desk Aneroid Sphygmomanometer, (Rudolf Riester GmbH, Jungingen, Germany).
The requirements of the ESH-IP 2002 for validation of blood pressure measuring devices in adults were followed precisely. The observers were blinded from each other's readings and the difference between each pair of their readings, both test and reference, was within ±4 mmHg, as required by the protocol.
Results
Results of the validation are presented in Table 1 and the number of recruitment SBPs in each of the ranges and recruitment DBPs in each of the ranges are presented in Table 2. For validation of this sphygmomanometer, 33 participants (16 males) that had the range of BP required by the ESH-IP rules were included (Table 1). The mean ± standard deviation (SD) age was 50.5 ± 13.0 years, range 29-71 years, entry systolic BP (SBP) was 142.6 ± 23.7 mmHg (range 98-178 mmHg), entry diastolic BP (DBP) was 89.0 ± 17.8 mmHg (range 61-120 mmHg) and arm circumference was 32.1 ± 3.9 cm (range 22-39 cm). In 18 patients (54.5 %), arm circumference was ≤ 32 cm and the standard cuff was used. In the remaining 15 patients (44.5 %), arm circumference was > 32 cm and the large cuff was used. Two subjects were aged 29 years old which is considered a minor violation (on this basis that no other protocol has an age limit of 30 years old). All participants provided informed consent to be included in the study.
Table 1. Device validation table for the Riester Big Ben Square Desk aneroid sphygmomanometer SBP, systolic blood pressure; DBP, diastolic blood pressure.
| Phase 1 (n=15) | within 5 mmHg | within 10 mmHg | within 15 mmHg | Recommendation | |||||||
| Required | One of | 25 | 35 | 40 | |||||||
| Achieved | SBP | 29 | 39 | 44 | Continue | ||||||
| DBP | 36 | 45 | 45 | Continue | |||||||
| Phase 2.1 (n=33) | within 5 mmHg | within 10 mmHg | within 15 mmHg | Recommendation | Mean difference (mmHg) | Standard deviation (mmHg) | |||||
| Required | Two of | 65 | 80 | 95 | |||||||
| All of | 60 | 75 | 90 | ||||||||
| Achieved | SBP | 61 | 89 | 97 | Pass | -0.8 | 6.4 | ||||
| DBP | 84 | 98 | 99 | Pass | 0.6 | 4.0 | |||||
| Phase 2.2 (n=33) | 2/3 within 5 mmHg | 0/3 within 5 mmHg | Recommendation | ||||||||
| Required | ≥ 22 | ≤ 3 | |||||||||
| Achieved | SBP | 22 | 2 | Pass | |||||||
| DBP | 32 | 0 | Pass |
Table 2.
The number of recruitment SBPs in each of the ranges Low (90-129 mmHg), Medium (130-160 mmHg) and High (161-180 mmHg) and recruitment DBPs in each of the ranges Low (40-79 mmHg), Medium (80-100 mmHg) and High (101-130 mmHg) are required. There are supposed to be 11 in each of these ranges.
| PHASE ONE | |||||
| Sys Group | Req’ | Achieved | Dias Group | Req’ | Achieved |
| Low | 5 | 5 | Low | 5 | 5 |
| Med | 5 | 5 | Med | 5 | 5 |
| High | 5 | 5 | High | 5 | 5 |
| TOTAL | 15 | 15 | |||
| PHASE TWO | |||||
| Sys Group | Req’ | Achieved | Dias Group | Req | Achieved |
| Low | 11 | 11 | Low | 11 | 11 |
| Med | 11 | 11 | Med | 11 | 11 |
| High | 11 | 11 | High | 11 | 11 |
| TOTAL | 33 | 33 | |||
In phase 1 (Table 1), the analysis was performed in a group of 15 participants. Each subject had BP measured three times, totalling 45 readings. For SBP, 29, 39 and 44 sphygmomanometer measurements fell in zones 0, 1 and 2, respectively. For DBP, 36, 45 and 45 sphygmomanometer measurements fell in zones 0, 1 and 2, respectively. The ESH-IP 2002 states that at least 25, 35 and 40 measurements must fall in zones 0, 1 and 2, respectively, and therefore phase 1 was passed.
In phase 2, 33 participants were included. For SBP, 61, 89 and 97 sphygmomanometer measurements fell in zones 0, 1 and 2 respectively. For DBP, 84, 98 and 99 sphygmomanometer measurements fell in zones 0, 1 and 2, respectively. Thus, phase 2 part 1 was successfully passed.
The sphygmomanometer also successfully passed phase 2 part 2 of the ESH-IP 2002. The protocol states that a minimum of 22 of the 33 subjects must have at least two of the three comparisons fall within 5 mmHg. Only a maximum of three subjects can have all three comparisons > 5 mmHg difference. For SBP, 22 participants had at least 2/3 readings within 5 mmHg and 2 had all 3 measurements above 5 mmHg. For DBP, these figures were 32 and 0, respectively. The mean SBP of the mercury (observer) measurements was 135.7 ± 21.7 mmHg compared to 134.9 ± 22.2 mmHg with the Riester device measurements. The mean DBP with the mercury measurement was 86.6 ± 16.4 mmHg compared to 87.2 ± 17.1 mmHg with the Riester device.
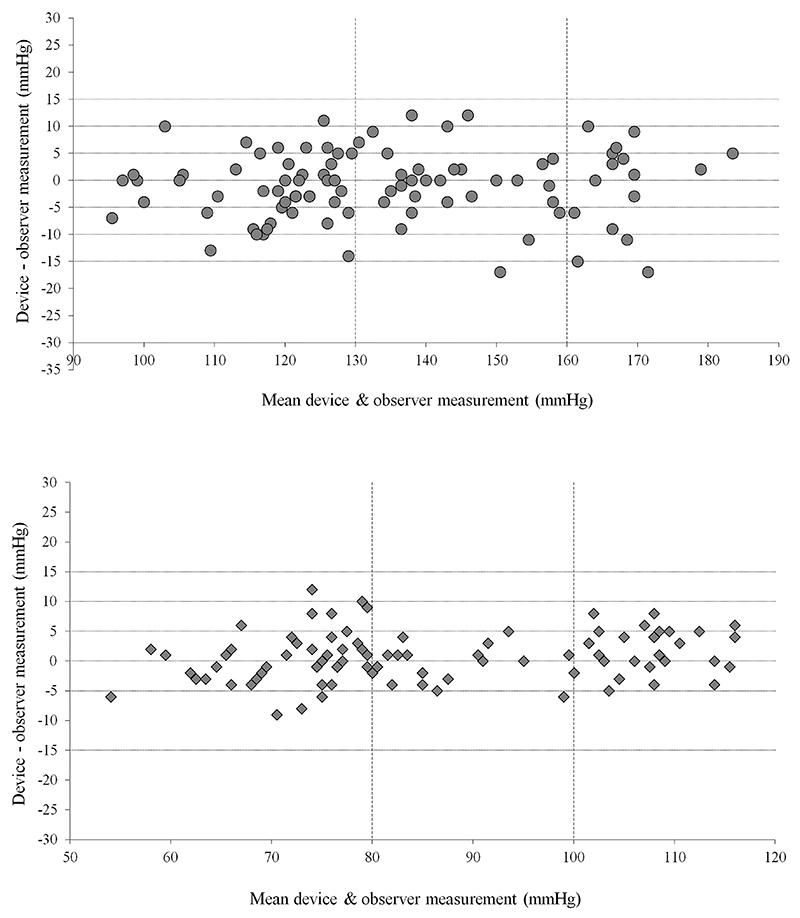
On average, the SBP was underestimated and DBP was overestimated when measurements were observed using the Riester Big Ben Square Desk aneroid sphygmomanometer compared to when measurements were observed using the standard mercury sphygmomanometer. The observer-device disagreement was -0.8 ± 6.4 mmHg for SBP and +0.6 ± 4.0 mmHg for DBP (Figure 2).
Figure 2.
Plot of the systolic (upper plot) and diastolic (lower plot) device – observer differences. The x-axis represents the mean of the device and observer measurements. Recruitment limits are represented by vertical lines. The y-axis represents the difference between the device and the observer measurements.
Conclusion
In 2009, when the validation was undertaken, the Riester Big Ben Square Desk aneroid sphygmomanometer passed the ESH-IP 2002 protocol which was recognised and widely used at that time. This legacy publication is intended to assure users that that device satisfied the requirements in place at that time and explains why the BIHS recommended, and continues to recommend, the Riester Big Ben Square Desk aneroid sphygmomanometer for clinical use in the adult population.
Acknowledgements
We would like to acknowledge support from the Blood Pressure Working Party of the British and Irish Hypertension Society (BIHS). We would also like to thank the nurses from Manchester Royal Infirmary who helped to deliver this study.
Sources of funding
None declared
Footnotes
Conflicts of interest: None declared
Contributor Information
British & Irish Hypertension Society:
Ryan John McNally, Janette Dunkerley, Maureen Holland, Ruth Eatough, Peter Lacy, Richard J McManus, Neil Chapman, Philip Chowienczyk, Philip Lewis, Christopher E Clark, Elizabeth Denver, Annette Neary, Sinead TJ McDonagh, and James P Sheppard
References
- 1.O’Brien E, Pickering T, Asmar R, Myers M, Parati G, Staessen J, et al. Working Group on Blood Pressure Monitoring of the European Society of Hypertension International Protocol for validation of blood pressure measuring devices in adults. Blood Press Monit. 2002;7:3–17. doi: 10.1097/00126097-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien E, Atkins N, Stergiou G, Karpettas N, Parati G, Asmar R, et al. European Society of Hypertension International Protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit. 2010;15:23–38. doi: 10.1097/MBP.0b013e3283360e98. [DOI] [PubMed] [Google Scholar]
- 3.ANSI/AAMI/ISO Noninvasive sphygmomanometers – Part 2: Clinical investigation of Intermittent Automated Measurement Type. American National Standards Institute. [Accessed August 2022];ANSI/AAMI/ISO 81060-2. 2019 http://webstore.ansi.org . [Google Scholar]