Abstract
Diseases diagnosed in adulthood may have antecedents throughout (including prenatal) life. Gaining a better understanding of how exposures at different stages in the lifecourse influence health outcomes is key to elucidating the potential benefits of disease prevention strategies. Mendelian randomisation (MR) is increasingly used to estimate causal effects of exposures across the lifecourse on later life outcomes. This systematic literature review explores MR methods used to perform lifecourse investigations and reviews previous work that has utilised MR to elucidate the effects of factors acting at different stages of the lifecourse. We conducted searches in PubMed, Embase, Medline and MedRXiv databases. Thirteen methodological studies were identified. Four studies focused on the impact of time-varying exposures in the interpretation of “standard” MR techniques, five presented methods for repeat measures of the same exposure, and four described methodological approaches to handling multigenerational exposures. A further 127 studies presented the results of an applied research question. Over half of these estimated effects in a single generation and were largely confined to the exploration of questions regarding body composition. The remaining mostly estimated maternal effects. There is a growing body of research focused on the development and application of MR methods to address lifecourse research questions. The underlying assumptions require careful consideration and the interpretation of results rely on select conditions. Whilst we do not advocate for a particular strategy, we encourage practitioners to make informed decisions on how to approach a research question in this field with a solid understanding of the limitations present and how these may be affected by the research question, modelling approach, instrument selection, and data availability.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10654-023-01032-1.
Keywords: Lifecourse, Mendelian randomisation, Systematic literature review, Methodology
Introduction
Diseases diagnosed in adulthood often have antecedents throughout (including prenatal) life [1]. Gaining a better understanding of how exposures at different stages in the lifecourse influence health outcomes is key to elucidating the potential benefits of specific disease prevention strategies.
A lifecourse approach recognises the contribution of long-term biological, behavioural, and psychosocial processes that operate across an individual’s lifecourse, or across generations [2, 3]. Kuh et al. previously defined lifecourse epidemiology as the study of physical or social exposures during gestation, childhood, adolescence, earlier adulthood and later adult life on later health or disease risk [2]. In practice, operationalising this can be complex; by definition, exposures should precede outcomes, and so almost any study of an exposure in relation to an adult health outcome could arguably be considered a lifecourse study. Here, our focus is on methodological issues pertinent to the application of Mendelian randomisation (MR) to lifecourse studies; these issues are relevant where there is a large time gap between exposures and outcomes. Therefore, we consider the following types of study as falling within lifecourse epidemiology: (1) the effects of pre-gestation, gestation, early life, childhood, or adolescent exposures on adult outcomes; (2) the effects of adult exposures on adult outcomes when the adult exposure is related to a particular stage/phase of adulthood, such as menopause (e.g. the effects of age at menopause on cardiovascular disease), (3) the effects of repeated measures of a time-varying exposure on a later outcome.
Whilst a lifecourse approach provides a persuasive framework for conducting epidemiological research, mediation (and the effect of this on the interpretation of total effects), time-varying confounding (when confounders have values that change over time) and intermediate confounding (a confounder of the mediator-outcome relationship) are highly likely in studies with earlier life and time-varying exposures and later life health outcomes [4, 5]. Intergenerational and family level factors may also contribute to further distinctive sources of confounding in multigenerational studies. Approaches to interrogate causality by minimising confounding are therefore of importance to strengthen causal inference in a lifecourse setting [6, 7].
MR exploits the random assortment of genetic variants, independent of other traits, to enable analyses that largely mitigate against distortions resulting from confounding and reverse causality [8]. This is a key motivation behind using a MR approach, which estimates the causal effect of modifiable risk factors under three assumptions; the instrumental variables used must (1) be associated with the exposure of interest (‘relevance’), (2) not share common causes with the outcome (‘independence’ or ‘exchangeability’) and (3) not affect the outcome other than through the exposure (‘exclusion’). Several statistical methods have been proposed for MR with individual-level as well as summarised data. In a one-sample setting with individual-level data, a causal effect estimate is often obtained using the two-stage least-squares (2SLS) method [9]. It is more common for two-sample investigations to use summarised data. In addition, at the introduction of MR, it was recognised that the association of genetic variants with exposures could change with age, which needed to be considered in interpretation [10, 11].
The application of MR to lifecourse research questions has two key challenges. Firstly, we are interested in isolating the causal effects of age-specific exposures. MR studies typically use a single measurement of an exposure to estimate its effects on an outcome (henceforth termed “standard” MR) and genes are invariable across the lifecourse. As such, results obtained are often interpreted as the average lifetime effect of the genetically predicted exposure, or genetic liability for an exposure if that exposure is binary [12]. Whilst this approach is sufficient for some exposures, it requires extension to address lifecourse questions. This extension is possible in cases where inherited genetic variants have different effects at different time points in the lifecourse (within a population), allowing us to separate time-varying effects of certain exposures [13–15]. Secondly, some lifecourse research questions involve the exploration of parental exposures. The inclusion of multiple generations brings additional analytical and methodological challenges due to common confounding and genetic relatedness.
This systematic literature review has two core aims. Firstly, to identify MR methods that have been developed to evaluate or conduct lifecourse epidemiological investigations and secondly, to systematically review previous work that has utilised MR to elucidate the impacts of risk factors from different stages of the lifecourse on later life outcomes. These studies fulfil the criteria outlined in the STROBE-MR guidelines, and specifically to the criterion of whether effect estimates previously derived would generalise to other exposure periods [16, 17].
Methods
Search strategy and eligibility criteria
The protocol for this systematic literature review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) as CRD42022314287 and was conducted in line with the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [18]. We searched for lifecourse epidemiology studies, defined as: (1) the effects of pre-gestation, gestation, early life, childhood, or adolescent exposures on adult outcomes; (2) the effects of adult exposures on adult outcomes when the adult exposure is related to a particular stage/phase of adulthood, such as menopause (e.g. the effects of age at menopause on cardiovascular disease), (3) the effects of repeated measures of a time-varying exposure on a later outcome. (See Supplementary file 1) [19]. Studies were eligible from any geographical location, with individuals from any age group and which included a MR study design (i.e., a study using genetic variants to determine whether there is a causal relationship between a modifiable risk factor and an outcome). We include as an “MR study” any study that uses genetic variants related to an exposure of interest to understand the causal nature of the relationship between that exposure and an outcome of interest. This includes studies where the genetic variants are used as an instrumental variable, and those where the association between the genetic variants and the outcome under study is analysed outside of an instrumental variable framework. Searches included any papers published prior to 12 June 2023 in MEDLINE (PubMed), Embase (Ovid), Medline (Ovid) and MedRXiv. The search and full-text review were restricted to articles published in English. Outcome measures were any measure of health status or disease from a life stage after the exposure was measured. Study designs that do not use MR methods were not appraised. Treatment guidelines documents were excluded (Supplementary file 2).
Data extraction and analysis
Within the final list of papers, we separated methodological manuscripts that presented or tested an approach to lifecourse MR from applied papers that only presented the results of a specific lifecourse analysis. For methodological manuscripts that presented or tested an approach to lifecourse MR we recorded: author, baseline year of data collection, aim, methodological approach, challenges in methodological application, simulation scenarios, sample size, and assumptions. When an applied element was included in the manuscript, we also recorded: exposure, exposure age(s) in years, outcome and outcome age(s) in years. We extracted the following from applied studies that presented the results of a specific lifecourse analysis: author, baseline year of data collection, aim, exposure, exposure age(s) in years, outcome and outcome age(s) in years. Title and abstract and then full-text screening was conducted in duplicate by two investigators (G.M.P and P.P.) and extraction in duplicate by two investigators (G.M.P and C.P.). Discrepancies were resolved by consensus. A narrative synthesis was performed. The evaluation of study quality by conducting a bias assessment was not considered relevant here, since we were not collating evidence to answer one applied question [20, 21].
Results
Our search generated 407 records. Three additional records were identified through conversations with experts in the field. After screening titles and abstracts, 181 manuscripts were assessed for eligibility. Of these, 140 articles were deemed eligible for inclusion in this systematic review (Fig. 1). Thirteen studies presented or tested an approach to lifecourse MR [12–15, 22–30] and 127 presented the results of a specific lifecourse analysis without an emphasis on exploring or explaining a methodological approach [31–157]. If a study fit the criteria for the former section, it was not included in the latter.
Fig. 1.
PRISMA flow chart illustrating selection of studies. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Studies presenting or testing an approach to lifecourse MR
Of the 13 studies presenting and/or testing approaches to lifecourse MR, four focused on the impact of time-varying exposures on the interpretations of “standard” MR techniques [12, 23, 26, 27]. These additionally outline methods to assess and/or lessen potential bias. Five presented methods for analysing repeat measures of the same exposure. These comprised functional principal component (FPC) analysis through conditional expectation (PACE) followed by a two-stage functional residual inclusion (2SFRI) inverse variance weighted multivariable MR (IVW-MVMR), g-estimation of structural nested cumulative failure models (SNCFTMs) and g-estimation of structural mean models (SMM) [13, 15, 22, 25, 28]. Our definition of lifecourse studies, which includes the effects of repeated measures of the same time-varying exposure on a later outcome, connects lifecourse MR to g-estimation, which has been applied in several studies to adjust for time-varying confounding in traditional epidemiological settings [158, 159]. In addition, four studies described novel methods that have been developed for intergenerational studies investigating a parental or grandparental exposure whilst the outcome of interest is assessed in offspring. These have used structural equation models (SEM) or the statistically equivalent weighted linear model (WLM), as well as one-sample GRS analysis and gene-by-environment (G × E) MR [14, 24, 29, 30].
Implications of time-varying exposures for the interpretation of “standard” MR
There are potential limitations regarding the use of “standard” MR techniques to interpret relationships between an outcome and an exposure that change over the lifecourses. D’Urso et al. highlight issues when using MR to assess the validity of hypotheses relating to the Developmental Origins of Health and Disease (DOHaD), such as the Barker hypothesis, which proposes that the origins of chronic diseases of adult life lie in foetal responses to the intrauterine environment [26]. “Standard” MR methods do not take into account the relationship between maternal and offspring genotypes and, as a result, may produce inflated type 1 error rates. Standard errors may be too small in the presence of cryptic relatedness due to there being less genetic variation in the sample. A conditional analysis framework is recommended using an unweighted or weighted maternal allele score corrected for offspring genotypes [26].
Results from “standard” MR techniques are often interpreted as average lifetime effects of the exposure, i.e., the cumulative effect of the exposure level from conception and through the lifecourse. Labrecque et al. propose an alternative interpretation for exposures that vary over time. They suggest the effect should be interpreted using a counterfactual framework approach, shifting the entire exposure trajectory by one unit of time k (a timepoint of observation, where k = 0 at conception) [23]. Labrecque et al. argue that different effects would be estimated at different exposure time points if the relationship between the genetic variants and the exposure changes over time. Thus, a “standard” MR approach may produce biased results. They initially provided an empirical example to estimate the lifetime effect of body mass index (BMI) on systolic blood pressure using the rs9939609 variant. They then simulated a longitudinal relationship to estimate BMI as an exposure at age 30 and 50 years and concluded that when the genetic variable-exposure relationship was constant over time, estimates were unbiased with respect to the lifetime effect at both ages. In all other scenarios, however, they show the estimate differed, and this bias was sensitive to the strength of relationship between the genetic variant and exposure as well as the timing of measurement of both exposure window and outcome.
Previous studies have explored whether age modifies the relationship between the genetic variants and exposure [10], however, investigations are limited. Most studies that have addressed this have investigated body composition, BMI or other measures of body size. To assess how time-varying genetic effects may impact MR effect estimates, Labrecque et al. and others suggest looking at a statistical interaction between the genetic variant and age in relation to the exposure [13, 106, 107, 112, 115, 160]. Following this, Labracque et al. propose plotting the relationship between the genetic instrument and the exposure stratified by age in samples with sufficient variation in age. They additionally show that patterns in age-varying genetic relationships may be exposure specific [27]. This has been shown in applied studies [10, 13, 106, 107, 112, 115, 160].
Morris et al. clarify the causal estimates that are estimated by MR when applied to a single measure of a time-varying exposure with time-varying genetic effects [12]. They consider a situation where there is one genetic instrument, a time-varying continuous exposure assessed on two occasions, and a single measure of an outcome. They also note the genetic instrument cannot affect the exposure measured at different occasions in isolation. Instead, they argue that the instrument underlies all possible exposure measurements across the lifecourse through a genetic liability, so a change in genotype changes both measures of the exposure. Simulations demonstrate that the Wald Ratio MR estimator recovers the correct causal effect in all scenarios assessed, even where time-varying genetic associations were present. Morris et al. showed that MR estimates differ between measurements of time-varying exposures because MR is estimating the total effect of the exposure trajectory on the outcome rather than the effect of the exposure at a specific point in time. Further details of each of these approaches can be found in Supplementary file 3.
Methodological approaches to analysing repeat measures of the same exposure over the lifecourse in an MR framework
MR methods proposed to estimate the effects of repeat measures of the same exposure across the lifecourse have been developed in response to the concern that a single measurement of a time-varying exposure may not be adequate in capturing all time-varying information: a single measure of a time-varying exposure could underestimate the relationship between the exposure variable and the outcome variable due to the failure to capture long-term change [161]. Importantly, in this context, later stages of lifecourse exposures often depend on the earlier stages of the same exposure, whilst the reverse is not true.
Cao et al. developed two methods to combine functional data analysis (to describe the trajectory of the exposure) with MR, to test the causal effect of a time-varying exposure on a binary outcome [22]. They use functional principal component (FPC) analysis through conditional expectation (PACE) to model the exposure trajectories, and then test whether a summary measure of the trajectory is related to the outcome using the two-stage residual inclusion (2SRI) approach. Their methods examine the evidence against the null hypothesis of no causal effect, but do not estimate the causal effect. The first method (PACE + 2SRI) assumes that the time-varying exposure variable has a cumulative effect on the risk of disease, and that the genetic effects on the exposure do not vary over time. The cumulative value of the exposure between two timepoints can be obtained by integration. The first stage obtains the residuals from regressing this cumulative exposure on the instrument (and any non-time-varying covariates). The second stage then relates these residuals to the outcome via a logistic regression model. For the second method (PACE + 2SFRI), they allow a time-varying genetic effect on the exposure variable but assume that the effect of the exposure and the fitted residual on the outcome are constant over time. In this case, the first stage is a functional linear model for the time-varying exposure, and the second stage relates the outcome to the fitted residuals and to the detrended exposure (functional residual inclusion). The authors showed that this method outperformed “standard” MR analysis with a single measurement at one time point, with higher statistical power in simulation studies using the functional data analysis-based methods, even when the disease outcome was simulated to depend not on the cumulative exposure, but on the first three functional principal component scores from PACE.
Another method employed to assess repeat measures of the same exposure over the lifecourse is inverse variance weighted multivariable MR (IVW-MVMR) [13, 15]. IVW-MVMR can be used to estimate the independent direct effects of several highly correlated exposures on an outcome, conditional on all the other exposures included in the model. It is useful in the context of mediation analysis [162], to estimate the effects of several repeated measures of the same exposure, or to isolate the effects of related phenotypes. Sanderson et al. explore the use of IVW-MVMR to estimate the direct effect of a single exposure at different time points in an individual’s lifetime on an outcome (Fig. 2) [15]. For multiple measurements to be included in a IVW-MVMR the genetic variants must have different effects on each exposure included in the model and these effects must not be a linear function of the others. The interpretation of the estimate is the effect of having a liability associated with a unit higher level of exposure at one occasion while keeping the liability for exposure at a separate occasion constant. Richardson et al. applied this approach to evaluate whether body size in early life has an independent effect on risk of disease in later life, or whether the effect seen is a result of body size in childhood being mediated by body size in adulthood [13]. They use univariable MR to estimate total effects of early body size, and IVW-MVMR to estimate direct effects of early and adult body size. This approach suggests univariable analyses cannot identify critical or sensitive periods of exposure but can detect an effect of a difference in the cumulative lifetime exposure, which is a notion critiqued by Labrecque et al., highlighted earlier in this review [23, 27]. If measures of the exposure at different time periods are available, and genetic instruments capable of reliably separating time-varying effects exist, it is possible to identify whether the exposure effects are stable over time or whether sensitive/critical periods exist in the lifecourse using IVW-MVMR. In theory the more time periods we have should allow more granular inference into critical windows. However, whilst this method can narrow down or exclude periods, it cannot strictly identify important periods if the genetic effects on the periods included are correlated with genetic effects on excluded periods.
Fig. 2.
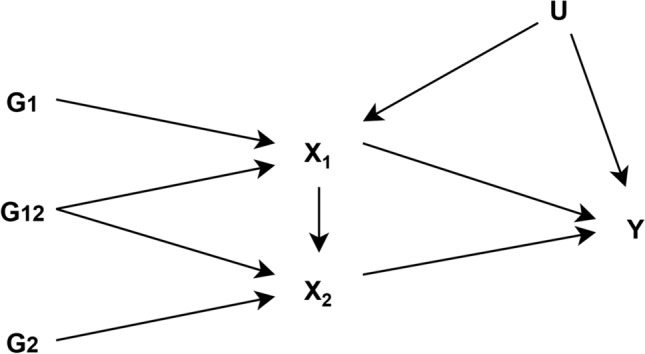
Latent exposure model with two periods of exposure (adapted from Sanderson et al. [163]). G1 is a set of genetic variants associated with the earlier exposure (X1) G2 is a set of genetic variants associated with the later exposure (X2), G12 is a set of genetic variants associated with both X1 and X2
Further attention has been bought to the importance of mitigating misspecification when running IVW-MVMR to estimate the effects of a single exposure during distinct time periods [164]. Tian and Burgess caution that this may otherwise result in the model’s poor performance with estimates suffering from unpredictable bias in both magnitude and direction [164, 165]. Correctly specifying when the outcome is a discrete function of the exposure at the precise time points at which the exposure was measured is therefore key. To run IVW-MVMR to answer lifecourse questions, Tian and Burgess argue that it is essential the exposure periods estimated represent distinct periods in the lifecourse where effects on the outcome are limited to a particular time period. This underlines ongoing methodological debates in this field. Sanderson et al. argue that any effect through a time period excluded from the model will form part of the effect estimated, asserting that, that effect can still be interpreted as the causal effect. Whilst being able to separate the genetic instruments for each period is important, running analyses on genetically predicted effects in small age-bands will almost certainly result in weak instruments and yield biased results.
The application of g-estimation of structural nested cumulative failure models (SNCFTMs) and g-estimation of structural mean models (SMM) was proposed by Shi et al. for the estimation of MR models with a time-varying exposure (Fig. 3) [25, 28]. The interpretation of results from estimation for these models depends on the availability of data for the time-varying exposure. SNCFTMs can be used to estimate the causal effect of a time-varying treatment on a failure time outcome under the assumption that all time-varying confounders have been measured and that failure is rare under all possible treatment values [166]. Shi et al. describe an adaptation of this use of SNCFTMs, incorporating IV-type assumptions [25]. Whilst confirmation of the validity of the method was achieved via simulations, analyses indicated that MR with time-varying treatments and failure time outcomes using SNCFTMs require large sample sizes (n = 10,000; n = 25,000 or n = 50,000). In addition, authors note that this method should only be used with rare outcomes. In the application of g-estimation of SMMs to MR analyses, Shi et al. consider three types of causal effects that can be targeted when the exposure is time-varying: the effect of exposure at a single time point on the outcome (point effect), the effect of exposure during a period on the outcome (period effect), and the effect of exposure throughout the lifetime on the outcome (lifetime effect) [28]. This approach highlighted two key challenges in estimating and interpreting period effects from MR analyses. The first is defining the period of interest. The second is the choice of time scale (e.g., time since conception or time since enrolment). In the context of additive causal effects for continuous outcomes, the authors note that g-estimation of SMMs and two-stage least squares (2SLS) MR yield similar estimates. SMMs can be naturally extended to many settings, including accommodating binary and failure-time outcomes and estimating effects on the multiplicative scale. SMMs are also semiparametric, and therefore avoid some of the parametric assumptions of 2SLS. Further details on these methodological approaches discussed along with their limitations are presented in Supplementary file 3.
Fig. 3.
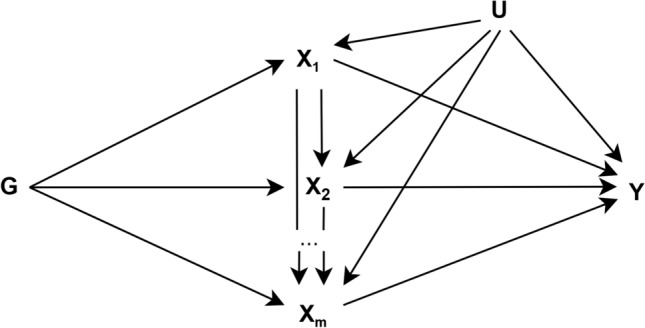
Causal diagram for instrumental variable analyses representing a scenario with a time-varying exposure (adapted from Shi et al. 2022 [28]). G indicates a set of genetic variants each associated with at least one of the exposures (Xm−p,…, Xm–1, Xm)
The methodological assumptions underlying the methods we present here vary greatly and require thorough consideration prior to running analyses. On top of this, very careful consideration is required for instrument selection when applying MR to lifecourse research questions. We therefore do not advocate for a particular strategy but encourage practitioners to think through their research question, instrumental variables, and data availability in-depth before pursuing a particular MR approach within a lifecourse setting. Table 1 comprises key considerations for analysts that are thinking about conducting a lifecourse investigation using MR techniques.
Table 1.
Key methodological considerations when implementing a Mendelian randomization approach to conduct lifecourse research
| Method | Phenotypic data required | SNP set required | Adjustments (if any) | Data level required | Generation (one or two) | MR effect | MR estimation | Estimate | Interpretation | Methodological assumptions | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure data | Outcome data | ||||||||||
| G-estimation of SMM | Measure of phenotype from specific timepoint | Measure of phenotype from a life stage after exposure data | SNPs only associated with exposure phenotype from specific timepoint and no other timepoints (association threshold may vary) | – | Individual | One | Period | Univariable MR | – | The effect of the phenotype of interest from a specific timepoint independent of pathways comprising considered phenotype measures from other timeframes in the lifecourse | Genetic variants strongly associated with the phenotype of interest from a specific timepoint do not have a pathway effect through another time period |
| G-estimation of SMM; IVW-MR; 2SLS-MR | Measure of phenotype from specific timepoint. | Measure of phenotype from a life stage after exposure data | Full set of SNPs associated with exposure phenotype from specific timepoint (association threshold may vary) | – | Individual; summary | One | Lifetime | Univariable MR | Total | The effect of the phenotype of interest from a specific timepoint including pathways comprising considered phenotype measures from different timeframes in the lifecourse | Genetic variants strongly associated with the phenotype of interest from a specific timepoint do not have a pathway effect through another time period |
| G-estimation of SMM; IVW-MVMR; 2SLS-MVMR | Measure of phenotype from specific timepoint. | Measure of phenotype from a life stage after exposure data. | Full set of SNPs associated with exposure phenotype from specific timepoint (association threshold may vary), plus full set of SNPs associated with alternative timepoint(s) | Control for measure of same phenotype as exposure from different timepoint(s) | Individual; summary | One | Period | Multivariable MR | Direct | The effect of the phenotype of interest from a specific timepoint including pathways comprising considered phenotype measures from different timeframes in the lifecourse, conditioning on phenotype measures from other time periods | There is variation in the genetic variant phenotype association across the time periods included in the estimation |
| G-estimation of SNCFTM | Repeat longitudinal measurements of phenotype between two time points. | Measure of phenotype from a life stage after exposure data. | Full set of SNPs associated with exposure phenotype (association threshold may vary) | – | Individual | One | Period or lifetime | – | Cumulative | The marginal cumulative risk under nondynamic treatment strategies across a period | Genetic variants strongly associated with the phenotype of interest from a specific timepoint do not have a pathway effect through another time period |
| PACE + 2SFRI | Repeat longitudinal measurements of phenotype between two time points | Measure of phenotype from a life stage after exposure data | Full set of SNPs associated with exposure phenotype (association threshold may vary) | – | Individual | One | Period or lifetime | – | Cumulative | The cumulative effect of the phenotype of interest across a period or the lifecourse | Genetic variants have time-varying effects on the exposure variable and the exposure variable has a cumulative effect on the disease risk |
| Genomic SEM or WLM (yielding a good approximation to the SEM) to conduct two-sample MR | Measure of phenotype in general population (i.e. does not specifically need to be in parents or offspring) | Measure of parental and offspring phenotype (these can come from separate samples) | Maternal (paternal) and offspring SNPs that are associated with maternal (paternal) exposure phenotype | Condition on parental or offspring genotype | Summary | Two | Parental | Univariable MR | Direct (offspring) + indirect (parental) | The causal effect of an intrauterine phenotype (separating direct offspring genotype effects from indirect maternal/paternal genotype effects) on an offspring outcome | Partitioning the outcome into maternal (paternal) and offspring specific genetic effects allows for the estimation of both parental and offspring causal effects on offspring outcomes |
| One-sample GRS analysis | Measure of parental/offspring phenotype | Measure of offspring phenotype | Maternal (paternal) and offspring SNPs that are associated with maternal (paternal) exposure phenotype | Adjust for offspring genotype (and other parent if relevant) | Individual | Two | Parental | Univariable MR | Direct (offspring) + indirect (parental) parental | The causal effect of an intrauterine phenotype (separating direct offspring genotype effects from indirect maternal/paternal genotype effects) on an offspring outcome | Partitioning the outcome into maternal (paternal) and offspring specific genetic effects allows for the estimation of both parental and offspring causal effects on offspring outcomes |
| G × E MR | Offspring measure on parental phenotype as proxy for parental phenotype | Measure of phenotype from a life stage after exposure data | Offspring SNPs associated with exposure phenotype as proxy for parental genetic data | – | Individual | Two | Parental | Univariable MR | – | The causal effect of an exposure in pregnancy when maternal genotype is unavailable | Offspring genotype is a suitable proxy for maternal genotype |
2SFRI = two-stage functional residual inclusion; 2SLS = Two-stage least-squares regression; G x E = gene-by-environment; GRS = genetic risk score; IVW = Inverse variance weighted; MR = Mendelian randomisation; MV = Multivariable; PACE = principal component analysis through conditional expectation; SEM = structural equation model; SMM = Structural mean model; SNCFTM = structural nested cumulative failure model; SNP = Single nucleotide polymorphisms; WLM = weighted linear model
Novel methodological approaches to handling parental exposures in relation to offspring outcomes
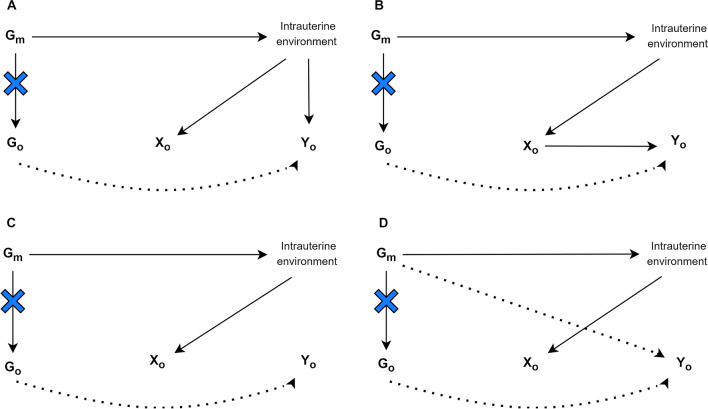
Novel methods have been developed for intergenerational studies investigating a parental or grandparental exposure whilst the outcome of interest is assessed in offspring. All of the studies we identified in this section relate maternal genotypes to offspring outcomes and establish the causal effect of a maternal exposure, e.g., smoking during pregnancy, on offspring health. Yang et al. used a proxy gene-by-environment (G × E) MR approach to explore maternal effects on offspring phenotypes where maternal genetic information was unavailable [30]. They validated this approach by replicating a known effect of maternal smoking heaviness on offspring birthweight using the rs16969968 variant in CHRNA5. They then applied it to explore effects of maternal smoking heaviness on offspring later life outcomes and on birthweight of participant’s children. Yang et al. demonstrated how G × E MR can be used to test transgenerational causal effects. Further studies included in this section emphasise the need to condition on offspring genotype to avoid including its effect on the outcome of interest. Earlier non-MR human genetic association studies have estimated maternal genetic effects on offspring phenotypes through conditional genetic association analysis of genotyped mother–offspring pairs [167]. This separation of genetic effects into maternal and offspring components is important as maternal and offspring genotypes are correlated. Consequently, any association between maternal genotype and offspring outcome may be mediated by offspring genotype (Fig. 4) [14, 29]. Thus, as described above, naïve two-sample MR approaches in unrelated sets of individuals without accounting for the correlation between maternal and offspring genotype effects may result in erroneous conclusions regarding causality.
Fig. 4.
Four credible ways in which maternal genetic variants can be related to an offspring exposure (XO) and offspring outcome (YO). Gm is a set of maternal genetic variants GO is a set of offspring genetic variants. Blue crosses indicate the act of conditioning on maternal or offspring genotype, blocking the association between maternal and offspring variables. Dotted paths show paths in which the maternal genotype can be related to offspring phenotype that are not to do with the intrauterine environment (adapted from Evans et al. [29] Moen et al. [95] and Warrington et al. [14])
Two MR approaches, described by Warrington et al. and Evans et al. use structural equation modelling (SEM) [166] to account for the correlation between maternal and offspring genotypes [14, 29]. Evans et al. developed a statistical model that can be used to estimate the effect of maternal genotypes on offspring outcomes, conditional on offspring genotype using both individual-level and summary data. The authors demonstrate this approach using the following example: birthweight of the individual, birthweight of the individual offspring, and the mother’s own genotype (SNP). The genotypes of the individual’s mother (their offspring’s grandmother) and the genotype of the individual’s offspring are considered latent unobserved variables. The causal path between the individual’s own genotype and both their mother and offspring’s latent genotype is set to 0.5, according to quantitative genetics theory. The estimated maternal and offspring effects on the observed phenotype, which refer to maternal and offspring genetic effects on birthweight, are also estimated. The resulting maternal and offspring genetic effects can subsequently be combined with SNP-exposure estimates for the maternal exposures that the investigator is interested in, in a two-sample MR framework.
Warrington et al. ran GWAS of own offspring genetic variants in relation to birthweight, and maternal genetic variants in relation to their offspring’s birthweight. They then partitioned the lead SNPs, representing independent association signals, into categories based on maternal and/or offspring genetic contributions to birth weight. To achieve this, they use the same SEM [166] as described in Evans et al. [29] to account for the correlation between offspring and maternal genotypes to provide unbiased estimates of maternal and offspring genetic effects on birthweight. This method gives an indication as to which genetic associations are driven by the maternal and which by the offspring genomes. To extend the estimates of adjusted maternal and foetal effects genome wide, the authors developed a weighted linear model (WLM) which yields a good approximation of the SEM but is less computationally intensive. They used WLM-adjusted estimates in downstream analyses to identify maternal and offspring specific mechanisms that regulate birthweight and to investigate genetic links between maternal traits and birthweight. The authors applied two-sample MR to estimate causal effects of intrauterine exposures on offspring birthweight. Authors selected SNPs associated with each exposure and regressed the WLM-adjusted maternal effects on birthweight for those SNPs against the effect estimates for the maternal exposure, weighting by the inverse of the variance of the maternal exposure effect estimates. Similarly, the authors used WLM-adjusted offspring effects to estimate the causal effect of the offspring’s genetic potential on their own birthweight and compare the results with the estimated maternal causal effects.
Moen et al. investigate whether a genetic risk score (GRS) of maternal SNPs associated with offspring birthweight is also associated with offspring cardiometabolic risk factors, after controlling for offspring GRS using a one-sample GRS analysis approach. They use a large dataset and perform primary analyses testing the relationship between maternal GRS and each of the offspring risk factors, whilst conditioning on the offspring GRS. They also explore father-offspring pairs to investigate whether there is evidence for a postnatal environmental effect (genetic nurture or dynastic effects) rather than an intrauterine environmental effect. In executing these analyses, the authors employ a LMM which accounts for the non-independence between siblings. They modelled the maternal (paternal) GRS, offspring GRS, age, sex and measurement occasion. The non-independence between siblings and relatedness between parents and offspring was modelled using a genetic relatedness matrix in the random effects part of the model [24]. Importantly, a one-sample GRS analysis can also be used in single generational setting. Further detail on applied results, assumptions and limitations for these methods are provided in Supplementary file 3. It may be helpful to consider some of the key aspects and requirements for running a multigenerational lifecourse MR analysis, presented in Table 1.
Applied MR studies presenting results of a lifecourse analysis
Of the 127 studies applying lifecourse MR methods, included in this review, 51% (65/127) estimated effects in just one generation, 42% (53/127) looked at intergenerational effects and 7% (9/127) estimating both. Of the one (and one and two) generational studies employed in this review, 51% (38/74) estimated the effect of exposures at birth, birth to/and childhood, birth to/and adolescence or birth to/and adulthood, 35% (26/74) at childhood, childhood to/and adolescence or childhood to/and adulthood, and 14% (10/74) at adolescence or adulthood. Within those focused on single generational effects, 42% (27/65) looked at birth weight, 38% (25/65) comprised other body composition measures, including adiposity traits, BMI, body size, obesity, waist-to-hip ratio, and body fat percent. Single generation studies additionally included estimating the genetically predicted effects of age at menarche, pubertal age (timing), first sexual intercourse, sleep duration, offspring fasting glucose and type 2 diabetes, genetic liability to juvenile idiopathic arthritis, disordered eating pattern, alcohol consumption and DNA methylation at the HLA locus. Amongst the studies that estimated intergenerational effects, 28% (15/53) examined body composition as exposure measures. These included maternal and paternal BMI as well as maternal adiposity, central obesity, and height. Other exposures examined in an intergenerational setting are included in Supplementary file 4. All of the two-generational studies estimated effects of maternal exposures, with two studies also examining paternal exposures [64, 73]. Outcomes addressed in the studies incorporated in this review are varied and can be found in Supplementary file 4.
Discussion
In this systematic literature review, we extracted and summarised findings from studies presenting and/or testing approaches to lifecourse MR as well as those presenting results of a specific lifecourse analysis. Among the former, we focused on papers addressing time-varying or lifecourse processes through interpretations of results from “standard” MR techniques. “Standard” MR techniques have focused on estimating lifetime effects of an exposure, i.e., the cumulative effect of the exposure level from conception and through the lifecourse. Labrecque et al. propose that MR estimates of the same exposure assessed at different ages vary in the presence of time-varying genotype-exposure associations, and this represents bias in estimates of a lifetime causal effect. In response, Morris et al. proposed that “standard” MR is not estimating the causal effect of an exposure as it manifests at a given time period, but the causal effect of the underlying exposure liability. Thus, a hypothetical change in genotype would affect all manifestations of the exposure.
In addition, we summarised papers employing a methodological approach for repeat measures of the same exposure over the lifecourse. The methods described here enhance capability for causal inference of lifecourse effects, however, there are clear limitations. One method comprised the FPC analysis through PACE, with the limitation that this method was developed for hypothesis testing, not for estimation of causal effects [22]. Another technique was IVW-MVMR, which can separate influences across the lifecourse under some but not all causal scenarios. Estimates used are based solely on body size and BMI data from the UK Biobank [168, 169]. These findings should be evaluated in more cohorts when sample sizes make this possible. This is particularly important as it has been shown that UK Biobank participants are highly selected, which can be problematic for instrumental variable analyses [168, 168]. In addition, a g-estimation of SNCFTMs was explored. If the rare failure assumption does not hold, however, estimates from this approach may be invalid. Informative MR analyses will additionally require sample sizes much larger than those presented. A g-estimation of SMM was also described. Due to wide variations in age at first visit and short duration of follow-up in the data used, authors were limited to using time since enrolment in the study as the time scale, which implies the added assumption that the period effect is homogeneous across age. The plausibility of this assumption is not only specific to the exposure–outcome relationship of interest, but also depends on the variability in age.
Papers comprising methodological approaches for intergenerational effects or pregnancy/birth exposures emphasised the importance of a statistical model that can estimate the effect of maternal genotypes on offspring outcomes, conditional on offspring genotype. On a related note, carrying out MR of own birthweight using only genetic variants of the individual is likely to result in inaccuracies. This is because foetal growth and subsequently birthweight may be influenced by both foetal and correlated maternal genotypes [72].
As a further test of model assumptions, negative controls may be employed when applying MR to lifecourse epidemiology. For example, in the investigation of repeat measures of the same exposure over the lifecourse, testing a negative control outcome by estimating the direct effect of an exposure in adulthood on an outcome at an earlier life stage will help to decipher whether results being generated are reliable [115].
Additional methodological studies have addressed the importance of gene–gene and gene-environment interactions in shaping the genetic architecture of certain phenotypes [170]. Whilst the current methods presented in our review are not suitable to address this research area, this provides an interesting area for future developments.
The aforementioned MR methods rely on genome-wide association studies (GWASs). Several GWASs are usually meta-analysed to increase power using a fixed-effect approach, which assumes a common true genetic effect across studies. Random-effects models are also employed, though have limited power in comparison. It has been observed that if the genetic effects change with age both fixed-effect or random-effects meta-analysis produce biased estimates of the combined genetic effect [171]. Since the MR methods presented in our review assume that genetic effects may vary with age, one option is to run GWASs on specific age categories and, if possible, apply meta-analysis in each age category. This is an approach most frequently taken in the studies presenting results of specific lifecourse analyses highlighted in this review. Alternatively, meta-regression may be used to relate between-study heterogeneity to age and estimate both main and age-varying genetic effects [171]. These data may then be applied within a MR framework.
Among the studies presenting results of specific lifecourse analyses, data availability limitations were apparent. Studies focusing on one generational research are largely confined to the exploration of questions regarding body composition, since these have the strongest instrumental variables. In addition, these data are often more commonly available on a large scale in most longitudinal cohorts. This emphasises the need for pooling data across studies to maximise power, highlighting the value of a Lifecourse MR consortium, which will enable the testing of key epidemiological hypotheses that have been advanced regarding critical period and cumulative effects on disease risk. For some phenotypes, however, lifecourse MR may not be able to usefully contribute. This could either be due to the lack of identified genetic variants allowing meaningful separation of measures at different life stages or because these do not exist. If the IV-exposure effects are relatively constant, “standard” MR may therefore be sufficient. Awareness of this may change over time as more data becomes available. The collection of these data is also likely to be useful to improve MR overall. For example, stratifying analyses by age could be of value for testing other MR assumptions. An instrument that has very little effect on the earlier life exposure whilst influencing a later-life exposure and associating with an early-life outcome may be indicative of violations of horizontal pleiotropy, correlated pleiotropy, as well as the gene-environment equivalence (‘consistency’) assumption. In addition, lifecourse data may be used for evidence of substantial in utero effects of variants on processes suggesting developmental trajectories.
Conclusions
There is a growing body of research focused on the development of lifecourse MR techniques and methods which are increasingly being applied to address lifecourse research questions. The possibility that genetic effects have different levels of importance in the development of an exposure at different time points should be more commonly considered for application when conducting MR investigations. The underlying assumptions for each of the methods presented in this review require careful consideration and interpretations following these analyses rely on specific condition’s which are dependent on the question being addressed, the model chosen, instruments selected and data available. We do not promote a particular strategy for conducting MR analyses in a lifecourse setting, however, we encourage practitioners to use this review to make informed decisions on how to approach a research question in this field with a solid understanding of the limitations present and how these may be affected by the aforementioned research conditions. Despite these challenges, the methodological developments and applied research being conducted using these approaches indicate the increase in opportunities becoming more available within this area.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Professor David Evans and Dr Gunn-Helen Moen at the Institute for Molecular Bioscience, The University of Queensland, Australia, for their intellectual input regarding the content of Section: Novel methodological approaches to handling parental exposures in relation to offspring outcomes.
Author contributions
GMP: conceptualisation, methodology, software, formal analysis, investigation, data curation, writing–original draft, writing–review and editing, visualisation. ES: writing–review and editing. PP: validation, writing–review and editing. AF: Writing–review and editing. TTM: writing–review and editing. CP: Validation. TMF: writing–review and editing. JH: writing–review and editing. TGR: writing–review and editing. RR: conceptualisation. JT: writing–review and editing. NW: Writing–review and editing. GDS: conceptualisation, writing–review and editing, supervision, funding acquisition. LDH: conceptualisation, writing–review and editing, supervision. KMT: conceptualisation, writing–review and editing, supervision.
Funding
This work was in part supported by the Integrative Epidemiology Unit which receives funding from the UK Medical Research Council and the University of Bristol (MC_UU_00032/01 and MC_UU_00032/02). GDS conducts research at the NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health. GMP is supported by the GW4 Biomed Doctoral Training Programme, awarded to the Universities of Bath, Bristol, Cardiff and Exeter from the Medical Research Council (MRC)/UKRI (MR/N0137941/1). TMF has received funding from the Medical Research Council (MR/T002239/1) EU-IMI SOPHIA and GSK. JT is supported by an Academy of Medical Sciences (AMS) Springboard award, which is supported by the AMS, the Wellcome Trust, GCRF, the Government Department of Business, Energy and Industrial strategy, the British Heart Foundation and Diabetes UK (SBF004\1079). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declarations
Competing interests
I have read the journal’s policy and the authors of this manuscript have the following competing interests: TGR is an employee of GlaxoSmithKline outside of this work. All other authors declare no competing interests.
Ethical approval
This study is a systematic review of available literature and did not involve direct access to participants of the primary research studies included. Research ethics approval was therefore not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Laura D. Howe and Kate M. Tilling contributed equally as last authors to this work.
References
- 1.Liu S, Jones RN, Glymour MM. Implications of lifecourse epidemiology for research on determinants of adult disease. Public Health Rev. 2010;32(2):489–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuh D, Ben-Shlomo Y, Lynch J, Hallqvist J, Power C. Life course epidemiology. J Epidemiol Community Health. 2003;57(10):778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lynch J, Davey Smith G. A life course approach to chronic disease epidemiology. Annu Rev Public Health. 2004;26(1):1–35. [DOI] [PubMed] [Google Scholar]
- 4.Davey Smith G. Assessing intrauterine influences on offspring health outcomes: can epidemiological studies yield robust findings? Basic Clin Pharmacol Toxicol. 2008;102(2):245–56. [DOI] [PubMed] [Google Scholar]
- 5.Santos S, Zugna D, Pizzi C, Richiardi L. Sources of confounding in life course epidemiology. J Dev Orig Health Dis. 2019;10(3):299–305. [DOI] [PubMed] [Google Scholar]
- 6.Davey Smith G, Leary S, Ness A, Lawlor DA. Challenges and novel approaches in the epidemiological study of early life influences on later disease. Adv Exp Med Biol. 2009;646:1–14. [DOI] [PubMed] [Google Scholar]
- 7.Lawlor D, Richmond R, Warrington N, McMahon G, Davey Smith G, Bowden J, et al. Using Mendelian randomization to determine causal effects of maternal pregnancy (intrauterine) exposures on offspring outcomes: sources of bias and methods for assessing them. Wellcome Open Res. 2017;2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kemp JP, Sayers A, Smith GD, Tobias JH, Evans DM. Using Mendelian randomization to investigate a possible causal relationship between adiposity and increased bone mineral density at different skeletal sites in children. Int J Epidemiol. 2016;45(5):1560–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgess S, Davey Smith G, Davies NM, Dudbridge F, Gill D, Glymour MM, et al. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res. 2019;4:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kivimäki M, Lawlor DA, Davey Smith G, Eklund C, Hurme M, Lehtimäki T, et al. Variants in the CRP gene as a measure of lifelong differences in average C-reactive protein levels: the cardiovascular risk in young finns study, 1980–2001. Am J Epidemiol. 2007;166(7):760–4. [DOI] [PubMed] [Google Scholar]
- 11.Davey Smith G, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32(1):1–22. [DOI] [PubMed] [Google Scholar]
- 12.Morris TT, Heron J, Sanderson ECM, Davey Smith G, Didelez V, Tilling K. Interpretation of Mendelian randomization using a single measure of an exposure that varies over time. Int J Epidemiol. 2022;139:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richardson TG, Sanderson E, Elsworth B, Tilling K, Davey Smith G. Use of genetic variation to separate the effects of early and later life adiposity on disease risk: mendelian randomisation study. BMJ. 2020;369:m1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warrington NM, Beaumont RN, Horikoshi M, Day FR, Helgeland Ø, Laurin C, et al. Maternal and fetal genetic effects on birth weight and their relevance to cardio-metabolic risk factors. Nat Genet. 2019;51(5):804–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanderson E, Richardson TG, Morris TT, Tilling K, Davey Smith G. Estimation of causal effects of a time-varying exposure at multiple time points through multivariable mendelian randomization. PLoS Genet. 2022;18(7):e1010290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skrivankova VW, Richmond RC, Woolf BAR, Davies NM, Swanson SA, VanderWeele TJ, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ. 2021;375:n2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: the STROBE-MR statement. JAMA. 2021;326(16):1614–21. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ben-Shlomo Y, Mishra G, Kuh D. Life Course Epidemiology. In: Ahrens W, Pigeot I, editors. Handbook of Epidemiology. New York: Springer; 2014. p. 1521–49. [Google Scholar]
- 20.Cheng TS, Day FR, Lakshman R, Ong KK. Association of puberty timing with type 2 diabetes: a systematic review and meta-analysis. PLoS Med. 2020;17(1):e1003017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spiga F, Gibson M, Dawson S, Davey Smith G, Munafò MR, Higgins JP. Tools for the assessment of quality and risk of bias in Mendelian randomization studies: a systematic review. medRxiv. 2021:2021.10.21.21265126. [DOI] [PMC free article] [PubMed]
- 22.Cao Y, Rajan SS, Wei P. Mendelian randomization analysis of a time-varying exposure for binary disease outcomes using functional data analysis methods. Genet Epidemiol. 2016;40(8):744–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labrecque JA, Swanson SA. Interpretation and potential biases of mendelian randomization estimates with time-varying exposures. Am J Epidemiol. 2019;188(1):231–8. [DOI] [PubMed] [Google Scholar]
- 24.Moen G-H, Brumpton B, Willer C, Åsvold BO, Birkeland KI, Wang G, et al. Mendelian randomization study of maternal influences on birthweight and future cardiometabolic risk in the HUNT cohort. Nat Commun. 2020;11(1):5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi J, Swanson SA, Kraft P, Rosner B, De Vivo I, Hernán MA. Instrumental variable estimation for a time-varying treatment and a time-to-event outcome via structural nested cumulative failure time models. BMC Med Res Methodol. 2021;21(1):258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Urso S, Wang G, Hwang L-D, Moen G-H, Warrington NM, Evans DM. A cautionary note on using Mendelian randomization to examine the barker hypothesis and developmental origins of health and disease (DOHaD). J Dev Orig Health Dis. 2021;12(5):688–93. [DOI] [PubMed] [Google Scholar]
- 27.Labrecque JA, Swanson SA. Age-varying genetic associations and implications for bias in Mendelian randomization. medRxiv. 2021:2021.04.28.21256235.
- 28.Shi J, Swanson SA, Kraft P, Rosner B, De Vivo I, Hernán MA. Mendelian randomization with repeated measures of a time-varying exposure: an application of structural mean models. Epidemiology. 2022;33(1):84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans DM, Moen G-H, Hwang L-D, Lawlor DA, Warrington NM. Elucidating the role of maternal environmental exposures on offspring health and disease using two-sample Mendelian randomization. Int J Epidemiol. 2019;48(3):861–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Q, Millard LAC, Davey Smith G. Proxy gene-by-environment Mendelian randomization study confirms a causal effect of maternal smoking on offspring birthweight, but little evidence of long-term influences on offspring health. Int J Epidemiol. 2020;49(4):1207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allard C, Desgagné V, Patenaude J, Lacroix M, Guillemette L, Battista MC, et al. Mendelian randomization supports causality between maternal hyperglycemia and epigenetic regulation of leptin gene in newborns. Epigenetics. 2015;10(4):342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alwan NA, Lawlor DA, McArdle HJ, Greenwood DC, Cade JE. Exploring the relationship between maternal iron status and offspring’s blood pressure and adiposity: a Mendelian randomization study. Clin Epidemiol. 2012;4:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arafat S, Minică CC. Fetal origins of mental disorders? An answer based on mendelian randomization. Twin Res Hum Genet. 2018;21(6):485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Au Yeung SL, Li AM, Schooling CM. A life course approach to elucidate the role of adiposity in asthma risk: evidence from a Mendelian randomisation study. J Epidemiol Community Health. 2021;75(3):277–81. [DOI] [PubMed] [Google Scholar]
- 35.Barry CS, Lawlor DA, Shapland CY, Sanderson E, Borges MC. Using mendelian randomisation to prioritise candidate maternal metabolic traits influencing offspring birthweight. Metabolites. 2022;12(6):537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bédard A, Lewis SJ, Burgess S, Henderson AJ, Shaheen SO. Maternal iron status during pregnancy and respiratory and atopic outcomes in the offspring: a Mendelian randomisation study. BMJ Open Respir Res. 2018;5(1):e000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belbasis L, Bellou V, Tzoulaki I, Evangelou E. Early-life factors and risk of multiple sclerosis: an MR-EWAS. Neuroepidemiology. 2020;54(6):433–45. [DOI] [PubMed] [Google Scholar]
- 38.Bell JA, Carslake D, Wade KH, Richmond RC, Langdon RJ, Vincent EE, et al. Influence of puberty timing on adiposity and cardiometabolic traits: a Mendelian randomisation study. PLoS Med. 2018;15(8):e1002641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bernard JY, Pan H, Aris IM, Moreno-Betancur M, Soh SE, Yap F, et al. Long-chain polyunsaturated fatty acids, gestation duration, and birth size: a mendelian randomization study using fatty acid desaturase variants. Am J Clin Nutr. 2018;108(1):92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bond TA, Richmond RC, Karhunen V, Cuellar-Partida G, Borges MC, Zuber V, et al. Exploring the causal effect of maternal pregnancy adiposity on offspring adiposity: mendelian randomisation using polygenic risk scores. BMC Med. 2022;20(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonilla C, Lawlor DA, Ben-Shlomo Y, Ness AR, Gunnell D, Ring SM, et al. Maternal and offspring fasting glucose and type 2 diabetes-associated genetic variants and cognitive function at age 8: a Mendelian randomization study in the avon longitudinal study of parents and children. BMC Med Genet. 2012;13:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonilla C, Lawlor DA, Taylor AE, Gunnell DJ, Ben-Shlomo Y, Ness AR, et al. Vitamin B-12 status during pregnancy and child’s IQ at age 8: a Mendelian randomization study in the Avon longitudinal study of parents and children. PLoS ONE. 2012;7(12):e51084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brand JS, Gaillard R, West J, McEachan RRC, Wright J, Voerman E, et al. Associations of maternal quitting, reducing, and continuing smoking during pregnancy with longitudinal fetal growth: findings from Mendelian randomization and parental negative control studies. PLoS Med. 2019;16(11):e1002972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brito Nunes C, Huang P, Wang G, Lundberg M, D’Urso S, Wootton RE, et al. Mendelian randomization study of maternal coffee consumption and its influence on birthweight, stillbirth, miscarriage, gestational age and pre-term birth. Int J Epidemiol. 2023;52(1):165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caramaschi D, Taylor AE, Richmond RC, Havdahl KA, Golding J, Relton CL, et al. Maternal smoking during pregnancy and autism: using causal inference methods in a birth cohort study. Transl Psychiatry. 2018;8(1):262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caramaschi D, Sharp GC, Nohr EA, Berryman K, Lewis SJ, Davey Smith G, et al. Exploring a causal role of DNA methylation in the relationship between maternal vitamin B12 during pregnancy and child’s IQ at age 8, cognitive performance and educational attainment: a two-step Mendelian randomization study. Hum Mol Genet. 2017;26(15):3001–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen C, Chen X, Wu D, Wang H, Wang C, Shen J, et al. Association of birth weight with cancer risk: a dose-response meta-analysis and Mendelian randomization study. J Cancer Res Clin Oncol. 2022;149(7):3925–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen J, Bacelis J, Sole-Navais P, Srivastava A, Juodakis J, Rouse A, et al. Dissecting maternal and fetal genetic effects underlying the associations between maternal phenotypes, birth outcomes, and adult phenotypes: a mendelian-randomization and haplotype-based genetic score analysis in 10,734 mother-infant pairs. PLoS Med. 2020;17(8):e1003305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen M, Wang Z, Xu H, Chen X, Teng P, Ma L. Genetic liability to age at first sex and birth in relation to cardiovascular diseases: a Mendelian randomization study. BMC Med Genomics. 2023;16(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen W, Yao D, Yan H, Wang M, Pan Y. Genetically predicted childhood obesity and adult atrial fibrillation: a mendelian randomization study. Nutr Metab Cardiovasc Dis. 2021;32(4):1019–26. [DOI] [PubMed] [Google Scholar]
- 51.Chen YC, Kuo HP, Hsia SM, Wu HT, Pan WH, Lee YL. Life course body mass index through childhood and young adulthood and risks of asthma and pulmonary function impairment. Pediatr Pulmonol. 2021;56(5):849–57. [DOI] [PubMed] [Google Scholar]
- 52.Clayton GL, Borges CM, Lawlor DA. From menarche to menopause: the impact of reproductive factors on the metabolic profile of over 65,000 women. medRxiv. 2023:2022.04.17.22273947.
- 53.Compton H, Smith ML, Bull C, Korologou-Linden R, Ben-Shlomo Y, Bell JA, et al. Effects of genetic liability to Alzheimer’s disease on circulating metabolites across the life course. medRxiv. 2022.
- 54.Decina CS, Hopkins R, Bowden J, Shields BM, Lawlor DA, Warrington NM, et al. Investigating a possible causal relationship between maternal serum urate concentrations and offspring birthweight: a Mendelian randomization study. Int J Epidemiol. 2023;52(1):178–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diemer EW, Labrecque J, Tiemeier H, Swanson SA. Application of the instrumental inequalities to a mendelian randomization study with multiple proposed instruments. Epidemiology. 2020;31(1):65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dong SS, Zhang K, Guo Y, Ding JM, Rong Y, Feng JC, et al. Phenome-wide investigation of the causal associations between childhood BMI and adult trait outcomes: a two-sample mendelian randomization study. Genome Med. 2021;13(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Esmeijer K, de Vries AP, Mook-Kanamori DO, de Fijter JW, Rosendaal FR, Rabelink TJ, et al. Low birth weight and kidney function in middle-aged men and women: the netherlands epidemiology of obesity study. Am J Kidney Dis. 2019;74(6):751–60. [DOI] [PubMed] [Google Scholar]
- 58.Fan HY, Huang YT, Hsieh RH, Chao JC, Tung YC, Lee YL, et al. Birthweight, time-varying adiposity growth and early menarche in girls: a Mendelian randomisation and mediation analysis. Obes Res Clin Pract. 2018;12(5):445–51. [DOI] [PubMed] [Google Scholar]
- 59.Gan Y, Lu D, Yan C, Zhang J, Zhao J. Maternal polycystic ovary syndrome and offspring birth weight: a Mendelian randomization study. J Clin Endocrinol Metab. 2021;107(4):1020–9. [DOI] [PubMed] [Google Scholar]
- 60.Geng TT, Huang T. Maternal central obesity and birth size: a Mendelian randomization analysis. Lipids Health Dis. 2018;17(1):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gill D, Sheehan NA, Wielscher M, Shrine N, Amaral AFS, Thompson JR, et al. Age at menarche and lung function: a Mendelian randomization study. Eur J Epidemiol. 2017;32(8):701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo X, Tang P, Hou C, Liu Y, Li R. Impaired pulmonary function mediates the impact of preterm birth on later-life stroke: a 2-step, multivariable Mendelian randomization study. Epidemiol Health. 2023. 10.4178/epih.e2023031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo X, Tang P, Zhang L, Cui J, Li R. Mendelian randomization approach shows no causal effects of gestational age on epilepsy in offspring. Epilepsy Res. 2023;191:107102. [DOI] [PubMed] [Google Scholar]
- 64.Havdahl A, Hughes A, Sanderson E, Ask H, Cheesman R, Reichborn-Kjennerud T, et al. Intergenerational effects of parental educational attainment on parenting and childhood educational outcomes: Evidence from MoBa using within-family Mendelian randomization. medRxiv. 2023.
- 65.Hawkes G, Beaumont RN, Tyrrell J, Power GM, Wood A, Laakso M, et al. Genetic evidence that high BMI in childhood has a protective effect on intermediate diabetes traits, including measures of insulin sensitivity and secretion, after accounting for BMI in adulthood. Diabetologia. 2023. [DOI] [PMC free article] [PubMed]
- 66.He R, Liu R, Wu H, Yu J, Jiang Z, Huang H. The causal evidence of birth weight and female-related traits and diseases: a two-sample mendelian randomization analysis. Front Genet. 2022;13:850892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.He R, Mo J, Zhu K, Luo Q, Liu X, Huang H, et al. The early life course-related traits with three psychiatric disorders: a two-sample Mendelian randomization study. Front Psych. 2023;14:1098664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hone L, Jacobs BM, Marshall C, Giovannoni G, Noyce A, Dobson R. Age-specific effects of childhood body mass index on multiple sclerosis risk. J Neurol. 2022;269(9):5052–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hou L, Li Y, Kang L, Li X, Li H, Xue F. The Long-term Mediation Role of Cytokines on the Causal Pathway from Maternal Gestational Age to Offspring Visual System: Lifecourse-Network Mendelian Randomization. medRxiv. 2022. [DOI] [PubMed]
- 70.Howe LJ, Sharp GC, Hemani G, Zuccolo L, Richmond S, Lewis SJ. Prenatal alcohol exposure and facial morphology in a UK cohort. Drug Alcohol Depend. 2019;197:42–7. [DOI] [PubMed] [Google Scholar]
- 71.Hu Z, Han L, Liu J, Fowke JH, Han JC, Kakhniashvili D, et al. Prenatal metabolomic profiles mediate the effect of maternal obesity on early childhood growth trajectories and obesity risk: the conditions affecting neurocognitive development and learning in early childhood (CANDLE) study. Am J Clin Nutr. 2022;116(5):1343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang T, Wang T, Zheng Y, Ellervik C, Li X, Gao M, et al. Association of birth weight with type 2 diabetes and glycemic traits: a mendelian randomization study. JAMA Netw Open. 2019;2(9):e1910915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hughes AM, Sanderson E, Morris T, Ayorech Z, Tesli M, Ask H, et al. Body mass index and childhood symptoms of depression, anxiety, and attention-deficit hyperactivity disorder: a within-family mendelian randomization study. Elife. 2022. 10.7554/eLife.74320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Humphriss R, Hall A, May M, Zuccolo L, Macleod J. Prenatal alcohol exposure and childhood balance ability: findings from a UK birth cohort study. BMJ Open. 2013;3(6):e002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hwang LD, Lawlor DA, Freathy RM, Evans DM, Warrington NM. Using a two-sample Mendelian randomization design to investigate a possible causal effect of maternal lipid concentrations on offspring birth weight. Int J Epidemiol. 2019;48(5):1457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jarvis D, Mitchell JS, Law PJ, Palin K, Tuupanen S, Gylfe A, et al. Mendelian randomisation analysis strongly implicates adiposity with risk of developing colorectal cancer. Br J Cancer. 2016;115(2):266–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jian Z, Yuan C, Ma L, Jin X. Genetic associations of birthweight, childhood, and adult bmi on testosterone levels: a mendelian randomization. J Clin Endocrinol Metab. 2022;107(7):1871–7. [DOI] [PubMed] [Google Scholar]
- 78.Jin S, Wang T, Wenying C, Wu Y, Huang S, Zeng P. Maternal and fetal origins of offspring blood pressure: statistical analysis using genetic correlation and genetic risk score-based Mendelian randomization. Int J Epidemiol. 2023. 10.1093/ije/dyad034. [DOI] [PubMed] [Google Scholar]
- 79.Kar SP, Andrulis IL, Brenner H, Burgess S, Chang-Claude J, Considine D, et al. The association between weight at birth and breast cancer risk revisited using Mendelian randomisation. Eur J Epidemiol. 2019;34(6):591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karhunen V, Bond TA, Zuber V, Hurtig T, Moilanen I, Järvelin MR, et al. The link between attention deficit hyperactivity disorder (ADHD) symptoms and obesity-related traits: genetic and prenatal explanations. Transl Psychiatry. 2021;11(1):455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kennedy OJ, Bafligil C, O’Mara TA, Wang X, Evans DG, Kar S, et al. Child and adult adiposity and subtype-specific endometrial cancer risk: a multivariable Mendelian randomisation study. Int J Obes (Lond). 2023;47(1):87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kirkbride JB, Susser E, Kundakovic M, Kresovich JK, Davey Smith G, Relton CL. Prenatal nutrition, epigenetics and schizophrenia risk: can we test causal effects? Epigenomics. 2012;4(3):303–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kjaergaard AD, Wu Y, Ming WK, Wang Z, Kjaergaard MN, Ellervik C. Homocysteine and female fertility, pregnancy loss and offspring birthweight: a two-sample Mendelian randomization study. Eur J Clin Nutr. 2022;76(1):40–7. [DOI] [PubMed] [Google Scholar]
- 84.Kong L, Ye C, Wang Y, Zheng J, Zhao Z, Li M, et al. Causal effect of lower birthweight on non-alcoholic fatty liver disease and mediating roles of insulin resistance and metabolites. Liver Int. 2023;43(4):829–39. [DOI] [PubMed] [Google Scholar]
- 85.Kupers LK, Monnereau C, Sharp GC, Yousefi P, Salas LA, Ghantous A, et al. Meta-analysis of epigenome-wide association studies in neonates reveals widespread differential DNA methylation associated with birthweight. Nature Communications. 2019;10(1) (no pagination). [DOI] [PMC free article] [PubMed]
- 86.Lawn RB, Sallis HM, Wootton RE, Taylor AE, Demange P, Fraser A, et al. The effects of age at menarche and first sexual intercourse on reproductive and behavioural outcomes: A Mendelian randomization study. PLoS ONE. 2020;15(6):e0234488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee HA, Park EA, Cho SJ, Kim HS, Kim YJ, Lee H, et al. Mendelian randomization analysis of the effect of maternal homocysteine during pregnancy, as represented by maternal MTHFR C677T genotype, on birth weight. J Epidemiol. 2013;23(5):371–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lewis SJ, Bonilla C, Brion MJ, Lawlor DA, Gunnell D, Ben-Shlomo Y, et al. Maternal iron levels early in pregnancy are not associated with offspring IQ score at age 8, findings from a Mendelian randomization study. Eur J Clin Nutr. 2014;68(4):496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li D, Zou Y. Causal effects of life course adiposity on chronic kidney disease: a Mendelian randomization study. Ann Palliat Med. 2021;10(10):10861–9. [DOI] [PubMed] [Google Scholar]
- 90.Li W, He Y, Zheng Q, Deng X. The causal effect of life course adiposity on periodontitis: a Mendelian randomization study. J Periodontol. 2023;94(2):256–62. [DOI] [PubMed] [Google Scholar]
- 91.Li X, Tian Y, Yang YX, Ma YH, Shen XN, Chen SD, et al. Life course adiposity and alzheimer’s disease: a mendelian randomization study. J Alzheimers Dis. 2021;82(2):503–12. [DOI] [PubMed] [Google Scholar]
- 92.Lin SL, Leung GM, Schooling CM. The effect of birth weight on academic performance: instrumental variable analysis. Am J Epidemiol. 2017;185(9):853–9. [DOI] [PubMed] [Google Scholar]
- 93.Ly A, Leppert B, Rai D, Jones H, Dardani C, Stergiakouli E. Genetic liability to rheumatoid arthritis on autism and autistic traits: polygenic risk score and Mendelian randomization analyses. Transl Psychiatry. 2022;12(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Madley-Dowd P, Dardani C, Wootton RE, Dack K, Palmer T, Thurston R, et al. Maternal vitamin D during pregnancy and offspring autism and autism-associated traits: a prospective cohort study. Mol Autism. 2022;13(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moen GH, Beaumont RN, Grarup N, Sommer C, Shields BM, Lawlor DA, et al. Investigating the causal effect of maternal vitamin B12 and folate levels on offspring birthweight. Int J Epidemiol. 2021;50(1):179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morales E, Vilahur N, Salas LA, Motta V, Fernandez MF, Murcia M, et al. Genome-wide DNA methylation study in human placenta identifies novel loci associated with maternal smoking during pregnancy. Int J Epidemiol. 2016;45(5):1644–55. [DOI] [PubMed] [Google Scholar]
- 97.O’Nunain K, Park C, Urquijo H, Leyden GM, Hughes AD, Davey Smith G, et al. A lifecourse mendelian randomization study highlights the long-term influence of childhood body size on later life heart structure. PLoS Biol. 2022;20(6):e3001656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Orri M, Pingault JB, Turecki G, Nuyt AM, Tremblay RE, Côté SM, et al. Contribution of birth weight to mental health, cognitive and socioeconomic outcomes: two-sample Mendelian randomisation. Br J Psychiatry. 2021;219(3):507–14. [DOI] [PubMed] [Google Scholar]
- 99.Pan M, Roe JM, Nudel R, Schork AJ, Iakunchykova O, Fjell AM, et al. Circulating S100B levels at birth and risk of six major neuropsychiatric or neurological disorders: a two-sample Mendelian randomization study. Transl Psychiatry. 2023;13(1):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Papadimitriou N, Bull CJ, Jenab M, Hughes DJ, Bell JA, Sanderson E, et al. Separating the effects of early and later life adiposity on colorectal cancer risk: a Mendelian randomization study. BMC Med. 2023;21(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pathak S, Richardson TG, Sanderson E, Åsvold BO, Bhatta L, Brumpton B.(2023) Investigating the causal effects of childhood and adulthood adiposity on later life mental health outcome: a Mendelian randomisation study. medRxiv. 2023:2023.05.09.23289512. [DOI] [PMC free article] [PubMed]
- 102.Pehkonen J, Viinikainen J, Kari JT, Böckerman P, Lehtimäki T, Raitakari O. Birth weight and adult income: an examination of mediation through adult height and body mass. Health Econ. 2021;30(10):2383–98. [DOI] [PubMed] [Google Scholar]
- 103.Pehkonen J, Viinikainen J, Kari JT, Böckerman P, Lehtimäki T, Viikari J, et al. Birth weight, adult weight, and cardiovascular biomarkers: evidence from the cardiovascular young finns study. Prev Med. 2022;154:106894. [DOI] [PubMed] [Google Scholar]
- 104.Pereira RD, Rietveld CA, van Kippersluis H (2020) The Interplay between Maternal Smoking and Genes in Offspring Birth Weight. medRxiv : the preprint server for health sciences. 2020.10.30.20222844.
- 105.Plotnikov D, Williams C, Guggenheim JA. Association between birth weight and refractive error in adulthood: a Mendelian randomisation study. Br J Ophthalmol. 2020;104(2):214–9. [DOI] [PubMed] [Google Scholar]
- 106.Power GM, Tobias JH, Frayling TM, Tyrrell J, Hartley A, Heron J, et al. Age-specific effects of weight-based body size on fracture risk in later life: a lifecourse Mendelian randomisation study. Eur J Epidemiol. 2023;38(7):795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Power GM, Tyrrell J, Frayling TM, Davey Smith G, Richardson TG. Mendelian randomization analyses suggest childhood body size indirectly influences end points from across the cardiovascular disease spectrum through adult body size. J Am Heart Assoc. 2021;10(17):e021503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Prince C, Howe LD, Sharp GC, Fraser A, Richmond RC (2023) Establishing the relationships between adiposity and reproductive factors: a multivariable Mendelian randomization analysis. medRxiv. [DOI] [PMC free article] [PubMed]
- 109.Probst-Hensch N, Jeong A, Stolz D, Pons M, Soccal PM, Bettschart R, et al. Causal effects of body mass index on airflow obstruction and forced mid-expiratory flow: a mendelian randomization study taking interactions and age-specific instruments into consideration toward a life course perspective. Front Public Health. 2021;9:584955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rathod R, Zhang H, Karmaus W, Ewart S, Mzayek F, Arshad SH, et al. Association of childhood BMI trajectory with post-adolescent and adult lung function is mediated by pre-adolescent DNA methylation. Respir Res. 2022;23(1):194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reed ZE, Micali N, Bulik CM, Davey Smith G, Wade KH. Assessing the causal role of adiposity on disordered eating in childhood, adolescence, and adulthood: a mendelian randomization analysis. Am J Clin Nutr. 2017;106(3):764–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Richardson TG, Crouch DJM, Power GM, Morales-Berstein F, Hazelwood E, Fang S, et al. Childhood body size directly increases type 1 diabetes risk based on a lifecourse mendelian randomization approach. Nat Commun. 2022;13(1):2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Richardson TG, Mykkanen J, Pahkala K, Ala-Korpela M, Bell JA, Taylor K, et al. Evaluating the direct effects of childhood adiposity on adult systemic metabolism: a multivariable mendelian randomization analysis. Int J Epidemiol. 2021;50(5):1580–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Richardson TG, Leyden GM, Davey Smith G. Time-varying and tissue-dependent effects of adiposity on leptin levels: a Mendelian randomization study. medRxiv. 2022. [DOI] [PMC free article] [PubMed]
- 115.Richardson TG, Power GM, Davey Smith G. Adiposity may confound the association between vitamin D and disease risk - a lifecourse Mendelian randomization study. Elife. 2022. 10.7554/eLife.79798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Richardson TG, Urquijo H, Holmes MV, Davey Smith G. Leveraging family history data to disentangle time-varying effects on disease risk using lifecourse mendelian randomization. European Journal of Epidemiology. 2023;32(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Richmond RC, Sharp GC, Ward ME, Fraser A, Lyttleton O, McArdle WL, et al. DNA methylation and BMI: Investigating identified methylation sites at HIF3A in a causal framework. Diabetes. 2016;65(5):1231–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Richmond RC, Timpson NJ, Felix JF, Palmer T, Gaillard R, McMahon G, et al. Using genetic variation to explore the causal effect of maternal pregnancy adiposity on future offspring adiposity: a mendelian randomisation study. PLoS Med. 2017;14(1):e1002221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ritchie SJ, Bates TC, Corley J, McNeill G, Davies G, Liewald DC, et al. Alcohol consumption and lifetime change in cognitive ability: a gene × environment interaction study. Age (Dordr). 2014;36(3):9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sallis HM, Wootton RE, Davey Smith G, Munafò MR. Proxy gene-by-environment Mendelian randomization study of the association between cigarette smoking during pregnancy and offspring mental health. Int J Epidemiol. 2023. 10.1093/ije/dyad022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shaheen SO, Rutterford C, Zuccolo L, Ring SM, Davey Smith G, Holloway JW, et al. Prenatal alcohol exposure and childhood atopic disease: a Mendelian randomization approach. J Allergy Clin Immunol. 2014;133(1):225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Su S, Fan J, Yang Y, Yang C, Jia X. Birth weight, cardiometabolic factors and coronary heart disease: a Mendelian randomization study. J Clin Endocrinol Metab. 2023. 10.1210/clinem/dgad308. [DOI] [PubMed] [Google Scholar]
- 123.Taylor AE, Howe LD, Heron JE, Ware JJ, Hickman M, Munafò MR. Maternal smoking during pregnancy and offspring smoking initiation: assessing the role of intrauterine exposure. Addiction. 2014;109(6):1013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Taylor K, McBride N, Zhao J, Oddie S, Azad R, Wright J, et al. The relationship of maternal gestational mass spectrometry-derived metabolites with offspring congenital heart disease: results from multivariable and mendelian randomization analyses. J Cardiovasc Dev Dis. 2022;9(8):237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Taylor K, Wootton RE, Yang Q, Oddie S, Wright J, Yang TC, et al. The effect of maternal BMI, smoking and alcohol on congenital heart diseases: a Mendelian randomisation study. BMC Med. 2023;21(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thompson WD, Beaumont RN, Kuang A, Warrington NM, Ji Y, Tyrrell J, et al. Higher maternal adiposity reduces offspring birthweight if associated with a metabolically favourable profile. Diabetologia. 2021;64(12):2790–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Thompson WD, Tyrrell J, Borges MC, Beaumont RN, Knight BA, Wood AR, et al. Association of maternal circulating 25(OH)D and calcium with birth weight: a mendelian randomisation analysis. PLoS Med. 2019;16(6):e1002828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tyrrell J, Richmond RC, Palmer TM, Feenstra B, Rangarajan J, Metrustry S, et al. Genetic evidence for causal relationships between maternal obesity-related traits and birth weight. JAMA. 2016;315(11):1129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang G, Bhatta L, Moen GH, Hwang LD, Kemp JP, Bond TA, et al. Investigating a potential causal relationship between maternal blood pressure during pregnancy and future offspring cardiometabolic health. Hypertension. 2022;79(1):170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang H, Jiang J, Jin T, Wang Y, Li M, Huang S, et al. Associations of circulation levels of cytokines with birthweight, preterm birth, spontaneous miscarriages, and stillbirth: a mendelian randomization analysis. Front Genet. 2023;14:1113804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang J, Kwok MK, Au Yeung SL, Zhao J, Li AM, Lam HS, et al. Age of puberty and sleep duration: observational and mendelian randomization study. Sci Rep. 2020;10(1):3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang J, Li AM, Lam H, Leung GM, Schooling CM. Sleep duration and adiposity in children and adults: observational and mendelian randomization studies. Obesity Silver Spring. 2019;27(6):1013–22. [DOI] [PubMed] [Google Scholar]
- 133.Wang T, Huang T, Li Y, Zheng Y, Manson JE, Hu FB, et al. Low birthweight and risk of type 2 diabetes: a Mendelian randomisation study. Diabetologia. 2016;59(9):1920–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang T, Tang Z, Yu X, Gao Y, Guan F, Li C, et al. Birth weight and stroke in adult life: genetic correlation and causal inference with genome-wide association data sets. Front Neurosci. 2020;14:479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Waterfield S, Richardson TG, Davey Smith G, O’Keeffe LM, Bell JA. Life course effects of genetic susceptibility to higher body size on body fat and lean mass: prospective cohort study. International Journal of Epidemiology. 2023 [DOI] [PMC free article] [PubMed]
- 136.Wei Y, Zhan Y, Löfvenborg JE, Tuomi T, Carlsson S. Birthweight, BMI in adulthood and latent autoimmune diabetes in adults: a Mendelian randomisation study. Diabetologia. 2022;65(9):1510–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wiklund P, Karhunen V, Richmond RC, Parmar P, Rodriguez A, De Silva M, et al. DNA methylation links prenatal smoking exposure to later life health outcomes in offspring. Clin Epigenetics. 2019;11(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Woolf B, Rajasundaram S, Gill D, Sallis HM, Munafò MR (2023) Assessing the causal effects of environmental tobacco smoke exposure: a meta-analytic mendelian randomisation study. medRxiv. 2023.03.30.23287949.
- 139.Xia JW, Zhang L, Li J, Yuan CD, Zhu XW, Qian Y, et al. Both indirect maternal and direct fetal genetic effects reflect the observational relationship between higher birth weight and lower adult bone mass. BMC Med. 2022;20(1):361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yajnik CS, Chandak GR, Joglekar C, Katre P, Bhat DS, Singh SN, et al. Maternal homocysteine in pregnancy and offspring birthweight: epidemiological associations and Mendelian randomization analysis. Int J Epidemiol. 2014;43(5):1487–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yan YS, Qu Z, Lv PP, Huang HF. Pediatric and adult obesity concerns in female health: a Mendelian randomization study. Endocrine. 2022;75(2):400–8. [DOI] [PubMed] [Google Scholar]
- 142.Yang Q, Borges MC, Sanderson E, Magnus MC, Kilpi F, Collings PJ, et al. Associations between insomnia and pregnancy and perinatal outcomes: evidence from mendelian randomization and multivariable regression analyses. PLoS Med. 2022;19(9):e1004090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yang Q, Magnus MC, Kilpi F, Santorelli G, Soares AG, West J, et al. Investigating causal relations between sleep duration and risks of adverse pregnancy and perinatal outcomes: linear and nonlinear Mendelian randomization analyses. BMC Med. 2022;20(1):295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ye J, Richardson TG, McArdle WL, Relton CL, Gillespie KM, Suderman M, et al. Identification of loci where DNA methylation potentially mediates genetic risk of type 1 diabetes. J Autoimmun. 2018;93:66–75. [DOI] [PubMed] [Google Scholar]
- 145.Yu XH, Wei YY, Zeng P, Lei SF. Birth weight is positively associated with adult osteoporosis risk: observational and Mendelian randomization studies. J Bone Miner Res. 2021;36(8):1469–80. [DOI] [PubMed] [Google Scholar]
- 146.Zanetti D, Tikkanen E, Gustafsson S, Priest JR, Burgess S, Ingelsson E. Birthweight, type 2 diabetes mellitus, and cardiovascular disease: addressing the barker hypothesis with mendelian randomization. Circ Genom Precis Med. 2018;11(6):e002054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zeng P, Yu X, Zhou X. Birth weight is not causally associated with adult asthma: results from instrumental variable analyses. Sci Rep. 2019;9(1):7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zeng P, Zhou X. Causal association between birth weight and adult diseases: evidence from a mendelian randomization analysis. Front Genet. 2019;10:618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang G, Bacelis J, Lengyel C, Teramo K, Hallman M, Helgeland Ø, et al. Assessing the causal relationship of maternal height on birth size and gestational age at birth: a mendelian randomization analysis. PLoS Med. 2015;12(8):e1001865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang L, Tang L, Huang T, Fan D. Life course adiposity and amyotrophic lateral sclerosis: a mendelian randomization study. Ann Neurol. 2020;87(3):434–41. [DOI] [PubMed] [Google Scholar]
- 151.Zhang M, Qiao J, Zhang S, Zeng P. Exploring the association between birthweight and breast cancer using summary statistics from a perspective of genetic correlation, mediation, and causality. J Transl Med. 2022;20(1):227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang X, Wu P, Chen Y, Zhang W, Xia K, Hu H, et al. Does maternal normal range thyroid function play a role in offspring birth weight? Evidence from a mendelian randomization analysis. Front Endocrinol (Lausanne). 2020;11:601956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhao J, Stewart ID, Baird D, Mason D, Wright J, Zheng J, et al. Causal effects of maternal circulating amino acids on offspring birthweight: a mendelian randomisation study. EBioMedicine. 2023;88:104441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zheng Y, Huang T, Wang T, Mei Z, Sun Z, Zhang T, et al. Mendelian randomization analysis does not support causal associations of birth weight with hypertension risk and blood pressure in adulthood. Eur J Epidemiol. 2020;35(7):685–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zheng BK, Sun XY, Xian J, Niu PP. Maternal testosterone and offspring birth weight a mendelian randomization study. J Clin Endocrinol Metab. 2022;107(9):2530–8. [DOI] [PubMed] [Google Scholar]
- 156.Zhou Y, Zha L, Pan S. The risk of atrial fibrillation increases with earlier onset of obesity: a mendelian randomization study. Int J Med Sci. 2022;19(9):1388–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zuccolo L, Lewis SJ, Smith GD, Sayal K, Draper ES, Fraser R, et al. Prenatal alcohol exposure and offspring cognition and school performance. A ‘Mendelian randomization’ natural experiment. Int J Epidemiol. 2013;42(5):1358–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tilling K, Sterne JA, Szklo M. Estimating the effect of cardiovascular risk factors on all-cause mortality and incidence of coronary heart disease using G-estimation: the atherosclerosis risk in communities study. Am J Epidemiol. 2002;155(8):710–8. [DOI] [PubMed] [Google Scholar]
- 159.Naimi AI, Cole SR, Hudgens MG, Richardson DB. Estimating the effect of cumulative occupational asbestos exposure on time to lung cancer mortality: using structural nested failure-time models to account for healthy-worker survivor bias. Epidemiology. 2014;25(2):246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jiang X, Holmes C, McVean G. The impact of age on genetic risk for common diseases. PLoS Genet. 2021;17(8):e1009723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Davis CE, Rifkind BM, Brenner H, Gordon DJ. A single cholesterol measurement underestimates the risk of coronary heart disease. An empirical example from the lipid research clinics mortality follow-up study. Jama. 1990;264(23):3044–6. [PubMed] [Google Scholar]
- 162.Carter AR, Sanderson E, Hammerton G, Richmond RC, Davey Smith G, Heron J, et al. Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Eur J Epidemiol. 2021;36(5):465–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sanderson E, Glymour MM, Holmes MV, Kang H, Morrison J, Munafò MR, et al. Mendelian randomization Nature Reviews Methods Primers. 2022;2(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tian H, Burgess S. Estimation of time-varying causal effects with multivariable Mendelian randomization: some cautionary notes. Int J Epidemiol. 2023;52(3):846–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vansteelandt S, Bekaert M, Claeskens G. On model selection and model misspecification in causal inference. Stat Methods Med Res. 2012;21(1):7–30. [DOI] [PubMed] [Google Scholar]
- 166.Picciotto S, Hernán MA, Page JH, Young JG, Robins JM. Structural nested cumulative failure time models to estimate the effects of interventions. J Am Stat Assoc. 2012;107(499):886–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Warrington NM, Freathy RM, Neale MC, Evans DM. Using structural equation modelling to jointly estimate maternal and fetal effects on birthweight in the UK Biobank. Int J Epidemiol. 2018;47(4):1229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, et al. Comparison of sociodemographic and health-related characteristics of UK biobank participants with those of the general population. Am J Epidemiol. 2017;186(9):1026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hughes RA, Davies NM, Davey Smith G, Tilling K. Selection bias when estimating average treatment effects using one-sample instrumental variable analysis. Epidemiology. 2019;30(3):350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Abadi A, Alyass A, Robiou du Pont S, Bolker B, Singh P, Mohan V, et al. Penetrance of polygenic obesity susceptibility loci across the body mass index distribution. Am J Hum Genet. 2017;101(6):925–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pagoni P, Higgins JPT, Lawlor DA, Stergiakouli E, Warrington NM, Morris TT, et al (2023) Meta-regression of Genome-Wide Association Studies to estimate age-varying genetic effects. medRxiv.2023.01.25.23284845. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.