Introduction
NHS England issued guidance for anticoagulant services during the COVID-19 pandemic including advice on the safe switching of patients on warfarin to direct oral anticoagulants (DOACs), where appropriate(1). As DOACs require less frequent blood testing, this supported the reduction of pressures being experienced by the NHS and the lowering of the risk of virus transmission. We have previously shown that this guidance was followed by increased switching of anticoagulants to DOACs(2).
The use of DOACs in people with mechanical heart valves is not recommended and was explicitly advised against in the guidance issued during the switching programme(1). Despite this, the national patient safety team at NHS England received anecdotal reports of individuals with mechanical heart valves being inappropriately prescribed a DOAC as a result of the switching programme. A national audit would previously have been challenging on an issue such as this: however the establishment of the OpenSAFELY analytics platform for research and service improvement during COVID-19 raised the possibility of conducting a detailed analysis of adherence and breaches using the full raw pseudonymised linked electronic health records of almost the whole population.
We therefore set out to describe prescriptions of DOACs to people with a record of mechanical heart valves throughout the COVID-19 pandemic in England, using OpenSAFELY.
Material and Methods
Working on behalf of NHS England, we used the OpenSAFELY framework to conduct a retrospective cohort study of DOAC prescribing to people with a record of mechanical heart valves across the full pseudonymised patient primary care records for 57.9 million people (97%) registered at a general practice in England using either TPP or EMIS software.
Between January 2019 and December 2021, at the beginning of each month, we identified individuals aged 16 or older who had a SNOMED-CT code selected as being explicitly indicative of ever receiving a mechanical heart valve. We then ascertained the number of people who were also prescribed a DOAC in each month. People were classified as currently prescribed a DOAC if they had a DOAC prescription between October 1st 2021 and December 31st 2021. We describe the following characteristics of this population; age band, sex, ethnicity, quintile of Index of Multiple Deprivation (IMD), record of previous atrial fibrillation (AF),specific mechanical valve code and record of International Normalised Ratio (INR) self-monitoring as indicated by a prescription of INR monitoring reagents or SNOMED “Self monitoring of international normalised ratio” (309901000000107). All analytical code, results and clinical codelists used in this study are openly available for inspection and re-use at reports.opensafely.org.
Results
We identified 16,727 people in the study cohort coded as having a mechanical valve at any time before the start of December 2021. Of these, 1,149 (6.9%) were identified as having been prescribed a DOAC between January 2019 and December 2021 of which 664 were currently prescribed a DOAC as of December 31st 2021.
Among people coded to have a mechanical valve and currently prescribed a DOAC (Table 1), 62.7% were male and 37.3% were female. 66.9% were of white ethnicity, 4.7% were non-white and 28.5% did not have a recorded ethnicity. The most common codes were “mechanical prosthetic aortic valve replacement - 174929002” (n= 455, 68.5%) and “mechanical prosthetic mitral valve replacement - 431339008” (n = 159, 23.9%). 85.7% had a previously recorded AF diagnosis. In practices using EMIS software, 6.3% of people coded as having a mechanical valve were prescribed a DOAC, vs 2.9% in practices using TPP software. 2.7% of patients recorded as having a mechanical valve were identified as receiving a prescription of INR blood monitoring reagents and 0.9% had a recorded code for INR self monitoring between October 2021 and December 2021.
Table 1. Counts of people with mechanical valves currently prescribed a DOAC by demographic characteristics between October 2021 and December 2021.
| Characteristic | Count of individuals coded as having a mechanical valve prescribed a DOAC (column % within category) | Population coded as having a mechanical valve (column % within category) | |
|---|---|---|---|
| Total | 664 (100.0) | 13,982 (100.0) | |
| Sex | Male | 416 (62.7) | 8,990 (64.3) |
| Female | 248 (37.3) | 4,992 (35.7) | |
| Ethnicity | White | 444 (66.9) | 8,742 (62.5) |
| Non-white | 31 (4.7) | 1,277 (9.1) | |
| Unknown | 189 (28.5) | 3,963 (28.3) | |
| Age Band | 16-49 | 26 (3.9) | 2,144 (15.3) |
| 50-59 | 25 (3.8) | 2,471 (17.7) | |
| 60-69 | 69 (10.4) | 3,160 (22.6) | |
| 70-79 | 218 (32.8) | 3,686 (26.4) | |
| 80+ | 326 (49.1) | 2,521 (18.0) | |
| IMD | Most deprived or Missing | 85 (12.8) | 2,681 (19.2) |
| 2 | 105 (15.8) | 2,692 (19.3) | |
| 3 | 144 (21.7) | 2,983 (21.3) | |
| 4 | 154 (23.2) | 2,865 (20.5) | |
| Least deprived | 176 (26.5) | 2,761 (19.7) | |
| Mechanical valve code | Mechanical prosthetic aortic valve replacement (174929002) | 455 (68.5) | 9,797 (70.1) |
| Mechanical prosthetic mitral valve replacement (431339008) | 159 (23.9) | 3,372 (24.1) | |
| Other | 50 (7.5) | 813 (5.8) | |
| Record of previous atrial fibrillation | Yes | 569 (85.7) | 4,918 (35.2) |
| No | 95 (14.3) | 9,064 (64.8) | |
| EHR Software Vendor | TPP | 183 (27.6) | 6,381 (45.6) |
| EMIS | 481 (72.4) | 7,601 (54.4) | |
| Prescription indicative of Self-monitoring INR | Yes | - | 384 (2.7%) |
| No | - | 13,598 (97.3%) | |
| SNOMED code indicative of self-monitoring INR | Yes | - | 127 (0.9%) |
| No | - | 13,855 (99.1%) | |
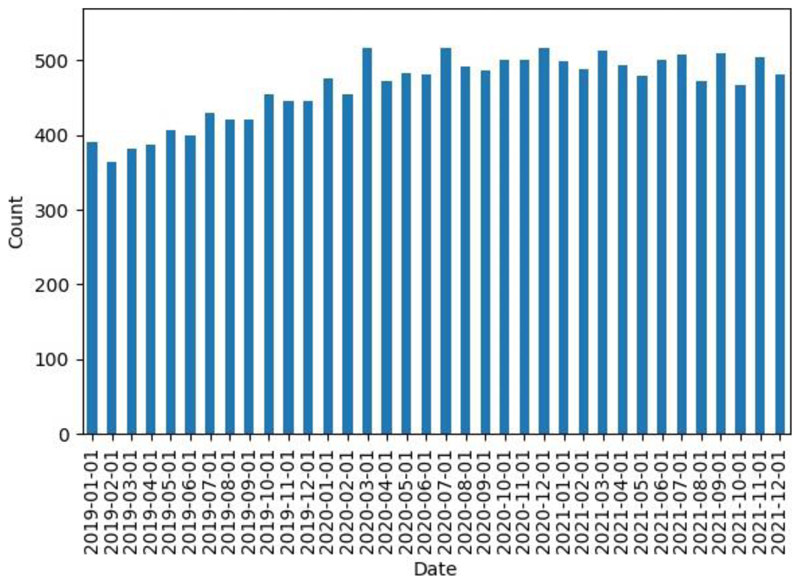
The monthly number of people with a record of a mechanical valve who are also prescribed a DOAC between January 2019 and December 2021 is shown in Figure 1. We observed a progressive increase in the monthly number of people with mechanical valves prescribed DOACs prior to national anticoagulation switching guidance in March 2020, with an increase from 390 (30.6 per 1000 patients coded as having a mechanical valve) in January 2019 to 454 (34.1 per 1000 patients coded as having a mechanical valve) in February 2020. In the six months following the guidance, we observed a mean number of 493 (95% C.I = 475, 511), compared to a mean of 449 (95% C.I = 432, 466) in the six months prior. The highest rate of 38.6 per 1000 patients coded as having a mechanical valve prescribed DOACs was observed in March 2020, the month the guidance was issued. The rate then plateaued, with a mean number of patients recorded as having a mechanical valve receiving a DOAC between January 2021 and July 2021, the month the National Patient Safety Alert was issued, of 495 (95% C.I = 477, 513). This remained similar in the last 5 months since issuing of a national safety alert in July 2021, with a mean count of 486 (95% C.I = 467, 505).
Figure 1. The monthly number of people with a record of a mechanical valve who are also prescribed a DOAC between January 2019 and December 2021.
Discussion
We observed a progressive increase of potentially inappropriate prescribing between January 2019 and March 2020, with a 9.8% increase in the mean number of patients with a record of a mechanical valve prescribed a DOAC in the six months after the anticoagulation switching guidance was issued compared with six months before. In July 2021, when a National Patient Safety alert was triggered(3), 663 patients with codes suggesting the presence of mechanical heart valves were currently prescribed a DOAC. As of December 2021, this number is 664.
Findings in context
In the UK there are approximately 10,000 valve replacement operations every year and in aortic valve replacement surgeries, a mechanical valve is used in approximately one in six(4,5). We identified 16,727 people with a code for ever receiving a mechanical heart valve suggesting that not all mechanical valve replacements are explicitly coded in GP records. A US study showed an overall 1% rate of postoperative DOAC prescribing following surgical aortic or mitral valve replacement with mechanical valves between 2014 and 2017, in 18,000 people with comparable sex and age distributions to our study(6). This is lower than the 6.9% rate observed in this study. We have previously described differences in the prescribing of certain medicines between GP clinical systems(7). The reasons for the difference between EHR systems in this study is unclear but could be explained by true differences between EHR systems, the geographic variation in deployment of the systems(8) and differences in the roll-out of the switching campaign at the height of a global health pandemic.
Strengths and Weakness
The key strength of this study is the scale and completeness of the underlying raw EHR coded data, covering 97% of the English population. A limitation is in the comprehensive identification of people with a mechanical valve, as coded records may not explicitly state whether a valve is mechanical (e.g. 50733009 Replacement of mitral valve with prosthesis) which may have led to under-ascertainment on the scale of the issue. We may also have counted a small number of people who were prescribed a DOAC before receiving a mechanical valve replacement, if it was fitted between October 2021 and December 2021. Finally, some people may be legitimately prescribed a DOAC whilst on a mechanical valve in rare clinical circumstances, with a justification recorded in the clinical record in free text. This data is not available but is likely to only account for a small number of all such patients.
Policy Implications and future research
Anticoagulants are considered high risk in terms of patient safety(9) and many national organisations have taken action to highlight specific clinical scenarios in breach of guidance through national safety alerts, cascaded via letters and e-mail. For more targeted communication, using the analytical code in this report TPP and EMIS have directly alerted clinicians, through their EHR software, to draw attention to patients prescribed a DOAC who may have a mechanical heart valve. In addition, following an earlier version of this analysis NHS England issued a National Patient Safety Alert highlighting that clinicians should review affected patients(3). Although this data is for the English NHS only, the findings may have implications for other health systems managing anticoagulation during the COVID-19 pandemic.
This study demonstrates that a service with access to raw electronic health records data in near real time, such as OpenSAFELY, can be used to rapidly evaluate the impact of clinical guidance, and support rapid dissemination of audit and feedback via EHR software providers by sharing detailed code to identify specific classes of patient. We have made OpenSAFELY available to national NHS organisations for real-time monitoring and feedback, to measure and rapidly mitigate the direct and indirect health impacts of COVID-19 on the NHS. A regularly updated report is openly available at reports.opensafely.org which can inform evaluation of further interventions on this safety issue.
Further areas of investigation include: evaluating the impact of the rapid alert issued by EMIS and TPP, evaluating outcomes for those with a mechanical valve who may have inappropriately received a DOAC, and ascertaining the number of people for whom there is ambiguity on the type of valve they have received.
Acknowledgements
We are very grateful for all the support received from the EMIS and TPP Technical Operations team throughout this work, and for generous assistance from the information governance and database teams at NHS England / NHSX, the national patient safety team at NHS England and NHS Improvement.
Funding
This work was supported by the Medical Research Council MR/V015737/1 and the Longitudinal Health and Wellbeing strand of the National Core Studies programme. EMIS and TPP provided technical expertise and infrastructure within their data environments pro bono in the context of a national emergency. The OpenSAFELY software platform is supported by a Wellcome Discretionary Award. BG’s work on clinical informatics is supported by the NIHR Oxford Biomedical Research Centre and the NIHR Applied Research Collaboration Oxford and Thames Valley. Funders had no role in the study design, collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. The views expressed are those of the authors and not necessarily those of the NIHR, NHS England, Public Health England or the Department of Health and Social Care.
Footnotes
Conflicts of Interest
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare the following: over the past five years BG has received research funding from the Laura and John Arnold Foundation, the NHS National Institute for Health Research (NIHR), the NIHR School of Primary Care Research, the NIHR Oxford Biomedical Research Centre, the Mohn-Westlake Foundation, NIHR Applied Research Collaboration Oxford and Thames Valley, the Wellcome Trust, the Good Thinking Foundation, Health Data Research UK (HDRUK), the Health Foundation, and the World Health Organisation; he also receives personal income from speaking and writing for lay audiences on the misuse of science. BMK is also employed by NHS England working on medicines policy and clinical lead for primary care medicines data. KB holds a Sir Henry Dale fellowship jointly funded by Wellcome and the Royal Society (107731/Z/15/Z). AYSW holds a fellowship from the British Heart Foundation. EJW holds grants from MRC. HF holds a UKRI fellowship. IJD has received unrestricted research grants and holds shares in GlaxoSmithKline (GSK).
Information governance and ethical approval
NHS England is the data controller; EMIS and TPP are the data processors; and the key researchers on OpenSAFELY are acting on behalf of NHS England. This implementation of OpenSAFELY is hosted within the EMIS and TPP environments which are accredited to the ISO 27001 information security standard and are NHS IG Toolkit compliant; patient data has been pseudonymised for analysis and linkage using industry standard cryptographic hashing techniques; all pseudonymised datasets transmitted for linkage onto OpenSAFELY are encrypted; access to the platform is via a virtual private network (VPN) connection, restricted to a small group of researchers; the researchers hold contracts with NHS England and only access the platform to initiate database queries and statistical models; all database activity is logged; only aggregate statistical outputs leave the platform environment following best practice for anonymisation of results such as statistical disclosure control for low cell counts. The OpenSAFELY research platform adheres to the obligations of the UK General Data Protection Regulation (GDPR) and the Data Protection Act 2018. In March 2020, the Secretary of State for Health and Social Care used powers under the UK Health Service (Control of Patient Information) Regulations 2002 (COPI) to require organisations to process confidential patient information for the purposes of protecting public health, providing healthcare services to the public and monitoring and managing the COVID-19 outbreak and incidents of exposure; this sets aside the requirement for patient consent. Taken together, these provide the legal bases to link patient datasets on the OpenSAFELY platform. GP practices, from which the primary care data are obtained, are required to share relevant health information to support the public health response to the pandemic, and have been informed of the OpenSAFELY analytics platform.
This study was approved by the Health Research Authority (REC reference 20/LO/0651) and by the LSHTM Ethics Board (reference 21863).
Guarantor
BG/LS are guarantors of the OpenSAFELY project.
Contributor Information
The OpenSAFELY Collaborative::
Louis Fisher, Victoria Speed, Helen J Curtis, Christopher T Rentsch, Angel YS Wong, Anna Schultze, Jon Massey, Peter Inglesby, Caroline E Morton, Marion Wood, Alex J Walker, Jessica Morley, Amir Mehrkar, Seb Bacon, George Hickman, Chris Bates, Richard Croker, David Evans, Tom Ward, Jonathan Cockburn, Simon Davy, Krishnan Bhaskaran, Becky Smith, Elizabeth Williamson, William Hulme, Amelia Green, Rosalind M Eggo, Harriet Forbes, John Tazare, John Parry, Frank Hester, Sam Harper, Jonathan Meadows, Shaun O’Hanlon, Alex Eavis, Richard Jarvis, Dima Avramov, Paul Griffiths, Aaron Fowles, Nasreen Parkes, Ian J Douglas, Stephen JW Evans, Liam Smeeth, Brian MacKenna, Laurie Tomlinson, and Ben Goldacre
References
- 1.Clinical guide for the management of anticoagulant services during the coronavirus pandemic. NHS England; [cited 2021 Sep 30]. [Internet]. Available from: https://www.nice.org.uk/Media/Default/About/COVID-19/Specialty-guides/specialty-guide-anticoagulant-services-and-coronavirus.pdf. [Google Scholar]
- 2.Curtis HJ, MacKenna B, Walker AJ, Croker R, Mehrkar A, Morton CE, et al. OpenSAFELY: impact of national guidance on switching from warfarin to direct oral anticoagulants (DOACs) in early phase of COVID-19 pandemic in England. openheart. 2021 Nov 16; doi: 10.1136/openhrt-2021-001784. [Internet]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inappropriate anticoagulation of patients with a mechanical heart valve. NHS England; [Internet]. Available from: https://www.england.nhs.uk/2021/07/inappropriate-anticoagulation-of-patients-with-a-mechanical-heart-valve/ [Google Scholar]
- 4.National Adult Cardiac Surgery Audit (NACSA) - 2020 Summary Report. NICOR; [cited 2021 Sep 30]. [Internet]. Available from: https://www.nicor.org.uk/wp-content/uploads/2020/12/National-Adult-Cardiac-Surgery-Audit-NACSA-FINAL.pdf. [Google Scholar]
- 5.Heart & Circulatory Disease Statistics 2020. BHF; [cited 2021 Sep 30]. [Internet]. Available from: https://www.bhf.org.uk/what-we-do/our-research/heart-statistics/heart-statistics-publications/cardiovascular-disease-statistics-2020. [Google Scholar]
- 6.Kalra A, Raza S, Jafry BH, King HE, Lahorra JA, Svensson LG, et al. Off-label Use of Direct Oral Anticoagulants in Patients Receiving Surgical Mechanical and Bioprosthetic Heart Valves. JAMA Netw Open. 2021 Mar 1;4(3):e211259. doi: 10.1001/jamanetworkopen.2021.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacKenna B, Bacon S, Walker AJ, Curtis HJ, Croker R, Goldacre B. Impact of Electronic Health Record Interface Design on Unsafe Prescribing of Ciclosporin, Tacrolimus, and Diltiazem: Cohort Study in English National Health Service Primary Care. J Med Internet Res. 2020 Oct 16;22(10):e17003. doi: 10.2196/17003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stocks SJ, Jill Stocks S, Kontopantelis E, Akbarov A, Rodgers S, Avery AJ, et al. Examining variations in prescribing safety in UK general practice: cross sectional study using the Clinical Practice Research Datalink. BMJ. 2015:h5501. doi: 10.1136/bmj.h5501. [Internet]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Actions that can make anticoagulant therapy safer: Alert and other information. National Patient Safety Agency; [cited 2021 Sep 30]. [Internet]. Available from: https://webarchive.nationalarchives.gov.uk/20171030131022/http://www.nrls.npsa.nhs.uk/resources/type/alerts/?entryid45=59814&p=3. [Google Scholar]