Summary
Staphylococcus aureus bacteraemia (SAB) continues to affect ~25,000 patients in the UK per year with a high crude mortality of 30% at 90 days. Prompt source control improves outcomes in sepsis and SAB and is included in sepsis guidelines. A recent clinical trial of adjunctive antibiotic treatment in SAB found that the majority of recurrences of SAB were associated with a failure of source management. In this condition, the ability to control the source of infection may be limited by the ability to detect a focus of infection. Echocardiogram is now a routinely used tool to detect such unknown foci in the form of unexpected infectious vegetations. We review the literature to explore the utility of advanced imaging techniques, such as [18F]fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) and magnetic resonance imaging (including whole-body MRI), to detect foci which may otherwise be missed. As unknown foci are associated with increased mortality, we propose that increasing the detection of foci could enable improved source control and result in improved outcomes in SAB.
Keywords: Staphylococcus aureus, bacteraemia, Diagnostic imaging, Magnetic resonance imaging, Positron emission tomography, Computed tomography, Focus of infection, Standard of care
Introduction
Staphylococcus aureus bacteraemia (SAB) has a high crude 90-day mortality of 30%, leading to 2−10 deaths per 100,000 population per year [1]. This incidence rate is higher than recorded mortality rates from tuberculosis and mortality rates are similar to those seen in Gram-negative bacteraemia [2–4]. Detecting infectious foci improves clinical outcomes, presumably by enabling more effective source control. However, in 15−20% of cases of SAB no infectious focus can be found [1]. This may relate to the sensitivity level of current tests. The absence of an identifiable focus is associated with 50% greater mortality from SAB [1–3]. The conclusion of the largest recent trial of antibiotics in SAB was that recurrences were ‘more commonly caused by failure to recognize or remove the primary infection focus than a failure of antibiotic treatment’ [3]. This highlights the importance of having more sensitive tests for detecting the focus of infection in SAB and, where possible, subsequently removing it. Early cessation of antibiotics relies on the reliable exclusion of metastatic infectious foci. We discuss the role of imaging and how advanced imaging may be harnessed to improve diagnosis in this life-threatening condition.
Positron emission tomography/computed tomography (usually [18F]fluorodeoxyglucose (FDG), denoted PET/CT subsequently) has traditionally been used primarily in oncology but is increasingly used to assess abnormal glucose metabolism in infection. This is possible due to the increase in glycolysis occurring in activated white blood cells in response to infection (or inflammation). PET/CT has become more widely available in the UK in regional hospitals and tertiary centres over the last five years. During that time the use of PET/CT in England has more than doubled and the median time from request to procedure is now seven days, including the two working days required to report the scan [5]. This enables its use within a realistic time-frame for the diagnosis of infectious foci in SAB.
Magnetic resonance imaging (MRI) has the benefit of avoiding radiation exposure. However, it is time-consuming and may be uncomfortable for the acutely unwell patient who is required to lie flat for a prolonged period to obtain imaging, particularly if they have back pain. Focused MRI selects one area to investigate but the location of infection may not be obvious in SAB. Whole-body MRI (WB-MRI) has been enabled by recent advances in MRI technology and has an increasing role to play in diagnostics [6]. This technology allows a broad investigation of the whole body in a single visit to the radiology department, as opposed to as many as 12 visits reported in studies to date in SAB patients [7]. A recent case report has highlighted the potential utility of WB-MRI in SAB when the scan is optimized to detect septic foci [8]. The protocol for investigation of SAB takes ~1 h, which is comparable to focused MRI, though on occasion a further focused MRI may be required for an area of interest. The use of coils in WB-MRI may be uncomfortable for the patient but in our clinic >90% of patients with myeloma were able to tolerate the entire procedure and in patients with lung cancer this pathway to diagnosis was preferred to standard imaging staging pathways [9]. There is limited experience of the use of WB-MRI in SAB but WB-MRI has been found to improve diagnosis in chronic recurrent multifocal osteomyelitis and be more sensitive than PET/CT in the assessment of myeloma [10,11]. Figure 1 shows a volunteer receiving WB-MRI and wearing coils.
Figure 1. A volunteer wearing coils as would be worn for a whole-body magnetic resonance imaging and demonstrating the procedure.
Foci of infection
There is a wide range of potential locations for SAB foci including:
-
–
infectious endocarditis (IE, heart valve);
-
–
osteoarticular (bone and joint);
-
–
lung (pneumonia, lung abscess or pleural infection);
-
–
skin and soft-tissue infection (SSTI);
-
–
intravenous catheter (IVC);
-
–
other (for example central nervous system (CNS) infection, implant infection, intravascular infection, genitourinary tract infection, wound infection, hepatobiliary infection or splenic abscesses);
-
–
not determined (‘unknown’).
Although contamination should always be considered in the differential of a blood culture containing S. aureus it is estimated that this is rare, representing ~1.5% of cases [4].
In some studies, foci of infection are divided into endovascular and non-endovascular. They may then be further divided into removable (e.g. intravenous catheter) and non-removable (e.g. endovascular graft infection) due to the prognostic implications of infected device removal. Where more than one focus occurs, the literature may fail to detail the full range of foci present when reporting the detail of cases as a hierarchy of foci is utilized [1].
Almost one-fifth of cases are concluded to be due to ‘unknown’ foci [12]. These occult foci may represent micro-foci, which cannot be detected, removed or treated. Alternatively, these cases may represent missed diagnoses of deep or difficult-to-diagnose foci.
Infective endocarditis
The recommendation to perform echocardiography routinely in SAB differs from recommendations in the investigation of other bacteraemia and demonstrates how SAB requires particular investigation of its source [13–16]. More than two decades ago Fowler et al. prospectively screened 103 patients with SAB using echocardiography and found a rate of 25% of endocarditis, compared to just 7% of cases that would have been detected due to clinical suspicion alone [17]. They recommended the increased use of invasive transoesophageal echocardiography (TOE) in SAB and routine use of transthoracic echocardiography (TTE) and this change in practice was adopted widely. International guidelines now recommend echocardiography be performed routinely in all cases of SAB bacteraemia [13–16]. American guidelines suggest that TOE is preferred in some circumstances, though further work is needed to clarify which cases these would be [15,16].
TOE is more sensitive for IE than TTE alone (90–100% vs 70–80% respectively) [13]. Despite the improved sensitivity of TOE, as it is an invasive test it is performed less often. A multi-centre study in Denmark prospectively screened 244 patients with SAB with TTE/TOE [18]. The majority of patients in this study had TOE (62%) and the remaining patients had TTE alone. The authors found an overall rate of IE, defined as definite IE according to the modified Duke criteria, of 22%. They explored the pre-existing risk factors and clinical signs present in those cases of IE. They found that patients with an unknown source of SAB were significantly more likely to have IE (20 (38%) vs 31 (16%), P = 0.001). In patients whom they considered had a low-risk of infection (based on pre-test clinical probability) they found a low rate of IE (5%). They suggested such calculation of risk could be used to stratify cases for TTE/TOE in SAB. This suggestion of pre-TOE risk assessment was supported by a subsequent review of the literature which suggested that, although TOE is preferred when feasible, there may be identifiable low-risk patients in whom TOE is not required [19]. This was based on a rate of major complications from TOE of 1 in 5000 but, given the high mortality of SAB, it remains possible that the benefits of TOE outweigh the risks, particularly when the source is not known pre-imaging. Repeated echocardiograms may be required to demonstrate vegetations, so in the presence of risk factors or clinical features, or where the source of SAB remains unknown, a single echocardiogram should not be used alone as an exclusion of IE.
There is an increasing interest in the role of cross-sectional imaging in the detection of IE in SAB. In mice S. aureus-induced IE can be detected using MRI technology [20]. A review of the benefit of cardiac MRI found an increasing role in the diagnosis of valvular lesions, though vegetations can be easily missed due to the fine imaging slices used in cine [21]. In a small single-centre study in Turkey cardiac MRI was requested on 16 patients with presumed IE to obtain additional information, particularly in six patients who had been unable to complete a TOE [22]. In this study the diagnosis of IE was made by the authors based on clinical, laboratory and echocardiographic findings. The modified Duke criteria and microbiological results were not reported. In these 16 patients 11 had lesions visible on cardiac MRI and 12 had lesions visible using echocardiogram, with 15 out of 16 patients having lesions visible using one of these modalities.
Cross-sectional imaging has benefits beyond simply determining the source of infection in IE; specifically, it can assist with the detection of septic emboli. Such emboli may be missed clinically and their detection may alter clinical management. Prospective screening with brain MRI of those with left-sided IE can reveal unexpected brain lesions [23]. In addition, when PET/CT is used, ~10% of patients have an unexpected additional (non-bacteraemic) finding on imaging, and these findings may be clinically relevant [7]. In a meta-analysis of 13 studies (including 537 patients) of the utility of PET/CT imaging in IE this was found to be a useful adjunctive tool when TTE/TOE was performed [24]. The sensitivity and specificity of PET/CT combined with echocardiography in this study for IE, as defined by the authors using modified Duke criteria or alternative methods, were found to be 77% and 78% respectively, and this was found to be higher in cases of prosthetic valves and more recent studies. In native valve endocarditis protocols, detection of cardiac involvement on PET/CT ideally requires prolonged fasting to minimize normal myocardial uptake. In one study of 303 episodes of endocarditis the sensitivity of PET/CT in prosthetic valve endocarditis (PVE) was 93% with a specificity of 90%, whereas for native valve endocarditis the corresponding values were 22% and 100%, demonstrating the utility of PET/CT in the exclusion of PVE in diagnostic work-up [25].
Osteoarticular infection
There is a lack of clear national or international guidelines on the management of SAB with an osteoarticular source, though it falls within other guidelines such as endocarditis and meticillin-resistant Staphylococcus aureus (MRSA) [13,14]. Where diagnostic algorithms are present within national guidelines in Australia and in Scotland, these recommend a symptoms/signs-based approach to osteoarticular imaging [26,27]. Australian SAB guidelines advise that MRI should be considered in the case of new or worsening back, joint, or bone pain [26]. Alternative tests include bone scan (bone/joint or muscular pain) or ultrasound-guided aspiration of swollen tender joints [26]. US guidelines for MRSA advise the use of MRI with gadolinium for detection of early osteomyelitis [14]. In a large Danish cohort study of 10,891 patients with SAB, osteomyelitis was found to be more common (11%) than IE (4%), though in this study rates of IE were low [28]. In a pooled analysis of five prospective studies in SAB, similarly rates of osteoarticular infection (13%) were found to be higher than rates of IE (8%) [12]. These high rates highlight the importance of accurate detection of osteoarticular foci. The investigation of osteoarticular sites of infection has traditionally been symptom-based, but the high incidence of back pain, for example, may mean that such foci are easily missed. It is recognized that on detecting a lesion of bacterial spondylodiscitis at one level of the spine, a whole-spine MRI should be considered to avoid missing other asymptomatic lesions [29].
The current preferred imaging technology for osteoarticular infection is usually MRI, as demonstrated by its widespread use in detecting spinal infection, epidural abscesses and osteomyelitis. Early studies in rabbits involved the introduction of S. aureus into the medullary space and subsequent comparative imaging using CT or MRI [30]. MRI was found to detect osteoarticular changes earlier than CT in this situation.
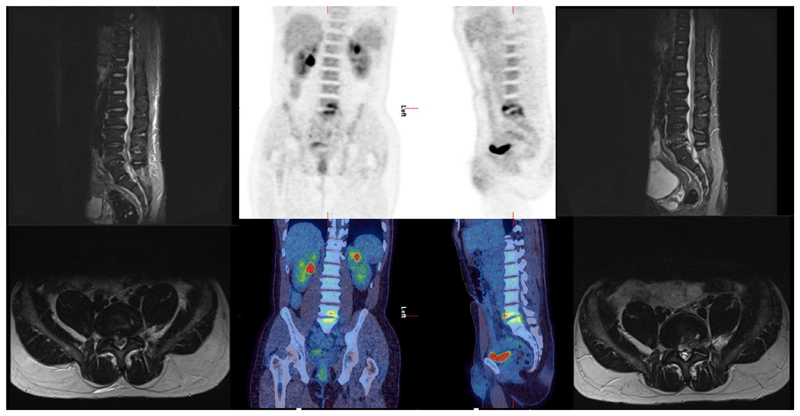
It is unclear whether PET/CT or MRI is the optimal imaging modality when seeking to detect osteoarticular infection in SAB. Head-to-head trials of PET/CT versus MRI or PET/CT or MRI versus no bone imaging in SAB are rare. A prospective study of PET/CT compared to MRI in suspected vertebral osteomyelitis included 11 cases of SAB [31]. In this study 32 patients with suspected vertebral osteomyelitis had both PET/CT and MRI performed in the same clinical episode. The authors found both imaging modalities to be 100% sensitive for vertebral osteomyelitis and the specificity of PET/CT to be 83% and MRI 92%. MRI detected more epidural/spinal abscesses and PET/CT detected metastatic infection in 16 patients, half of those scanned. An earlier prospective study compared MRI to PET/CT in 26 patients with suspected haematogenous infective spondylodiscitis [32]. Eighteen out of 26 patients were confirmed to have infective spondylodiscitis including eight patients with S. aureus infection. In those with S. aureus infection half (four out of eight) were found to have a further unexpected focus of infection on PET/CT (three cases of IE and one of shoulder septic arthritis). In this study the authors reported sensitivity of PET/CT as 83% and MRI 94% for vertebral osteomyelitis. They reported specificity of 88% and 38% for PET/CT and MRI, respectively, as they found they were unable to exclude infection in five out of eight (62%) patients with MRI. Both studies highlight the potential additional utility of PET/CT, as both modalities were highly sensitive for discitis but PET/CT provided additional relevant information in half of the cases. In our clinical practice we have seen cases in which early MRI can seemingly miss an infective vertebral focus which is later evident on PET/CT (Figure 2). In a prospective study of 20 patients with persistent foot ulcers and diabetes, MRI was found to detect confirmed osteomyelitis in six out of seven cases and PET did so in two out of seven cases [33]. These numbers are too small to draw conclusions and, although the patients had evidence of S. aureus infection in six cases, none had SAB.
Figure 2.
A case of a patient who underwent magnetic resonance imaging (MRI) on day 2 following Staphylococcus aureus bacteraemia (SAB) which did not reveal a focus (left), then a positron emission tomography (PET)/computed tomography (CT) (middle) on day 10 which revealed a spinal focus (L4/5) with a repeat MRI on day 17 (right) to confirm this diagnosis. Although this may represent progression in imaging over time, it highlights the potential utility of PET/CT when the focus is unclear. A bone single-photon emission CT was also performed at day 6 and activity at L4/5 was reported on this as degenerative.
An alternative to CT and MRI for detection of bone pathology in SAB is bone scintigraphy. Australian guidelines suggest this as a potential imaging investigation when determining the infective focus in SAB [26]. Bone scintigraphy has historically been found to be abnormal at an earlier time-point than conventional radiography or CT [34]. However, in UK practice it is rarely used. In a recent UK randomized control trial (RCT) of SAB just 2% of patients had a bone scan, in contrast to 3% who had a PET/CT and 36% who had a CT scan [35]. Of the 494 patients for whom data was available, only one patient had a white blood cell scan [35]. For diabetic foot osteomyelitis, for example, which may be associated with SAB, bone scintigraphy has a sensitivity of 80–90% but a specificity of <50% [36].
Lung infection (pneumonia, lung abscess, and pleural infection)
A chest radiograph (CXR) is a routine investigation in sepsis and therefore likely to have been performed prior to the identification of SAB in patients with this condition. It is included as part of the diagnostic pathway in the Australian algorithm for investigation of SAB [26]. The CXR may be abnormal due to the source of infection (e.g. pneumonia, lung abscess, necrotising pneumonia, empyema) or the dissemination of infection (septic emboli) or from parenchymal changes related to the deterioration of the patient (e.g. fluid overload or acute respiratory distress syndrome). An abnormal CXR appearance, for example of a lung abscess in an intravenous drug user, may highlight the possibility of SAB prior to the diagnosis being made on blood culture. A CT chest is not indicated as a routine investigation in SAB but may be performed where investigation of abscesses or pleural infection is required. Ultrasound scan may additionally enable image-guided drainage of pleural collections.
Skin and soft-tissue infection
Clinical examination rather than imaging is the key assessment in the case of SSTI in SAB. However, techniques such as PET/CT and WB-MRI may detect skin lesions when these are missed clinically. In the case of a wound or recent surgery a high-index of clinical suspicion needs to be present regarding this site for the infection and focused imaging may be required of a deep surgical site or wound.
Intravenous catheter infection
IVC infection is a common source of infection in SAB, responsible for up to 28% of cases [12]. The device may be central or peripheral. The diagnosis is usually made through the collection of blood cultures from an infected device, prompting early device removal. In all cases of SAB a review of intravenous catheters is required. There may be thrombus at the site of the line and there may be a need to differentiate between infected and non-infected thrombus in such cases. IVC infection is often described as uncomplicated if the IVC (source) is rapidly removed. Reviews describe the potential for shorter antibiotic courses in catheter-associated bacteraemia and SAB but the possibility of dissemination remains so imaging such as echocardiogram may still be required despite early device removal [19].
Central nervous system infection
SAB may rarely occur due to CNS infection (e.g. meningitis associated with SAB or invasion from an infected surgical site in the CNS). MRI is the imaging modality of choice in such infections and may detect silent cerebral involvement in S. aureus infective endocarditis. Although a CT brain may initially detect an area of concern, this would then be accompanied by MRI including diffusion-weighted and post-gadolinium contrast sequences to further detail the CNS involvement. Knowledge of CNS involvement may guide drug choice, e.g. to include antibiotics that penetrate the blood–brain barrier.
Implant or vascular infection
An area in which PET/CT has been particularly helpful is that of prosthetic vascular graft infection [37]. For example, a prospective study of 33 patients with suspected aortic graft infection in Japan assessed by PET/CT versus CT alone found PET/CT to have higher sensitivity but lower specificity for infection in the graft [38]. The complexity in this setting is to differentiate uptake secondary to chronic low-grade inflammation from that of infection when interpreting the imaging results. In addition, PET/CT is increasingly used in the diagnostic assessment of native vascular infections and may demonstrate this focus when it had not previously been suspected clinically in SAB.
Not determined (‘unknown’)
The ‘unknown’ (or occult) focus of infection group is of great concern in SAB due to the association with increased mortality [1–3]. In a retrospective observational study in the Netherlands, PET/CT within 14 days of the first positive blood culture with Gram-positive bacteria in patients with suspected metastatic infectious foci was associated with significantly lower three-month mortality (19% vs 32% without PET/CT; P = 0.01; N = 435) [39]. In addition, PET/CT was the first test performed to detect a focus of SAB infection in 30% of these cases. A further retrospective observational study, which focused on SAB (also in the Netherlands), demonstrated that those patients with high-risk SAB who underwent PET/CT had one-third of the mortality of those who did not [40]. In this study, 105 out of 184 patients (57%) with SAB underwent PET/CT. The imaging result informed clinical practice, leading to modifications of treatment in 75% of high-risk SAB cases in this study, demonstrating how such imaging can alter patient management. The authors therefore conducted a subsequent study to demonstrate that early use of PET/CT in the SAB pathway can enable safe early cessation of antibiotics (N = 76) [41].
In Belgium, a single-centre, retrospective observational study of 102 patients with ‘high-risk’ SAB (as defined using their risk profile and from a total of 196 patients with SAB) found that 48 patients had been investigated using PET/CT [42]. The one-year mortality of those who had PET/CT was 8 out of 48 (17%) compared to 24 out of 52 (44%) in those who did not have a PET/CT (P = 0.002). A single-centre retrospective study in Denmark reviewed data from 157 patients with bacteraemia of unknown origin with catalase-negative Gram-positive cocci (excluding pneumococci and enterococci) or S. aureus bacteraemia from 2009 to 2013 [7]. They found that PET/CT was the first imaging modality to identify sites of infection in 41% of bacteraemia cases, that it led to change of antimicrobial therapy in 15%, and that it established a new diagnosis unrelated to bacteraemia in 10%. In this study, IE was frequently diagnosed using PET/CT. However, a major challenge with observational studies in this field is reverse causality – it is difficult to exclude the possibility that scans were not done partly because patients were too unwell, or had already moved to a palliative pathway.
Imaging costs
In the UK, NHS Reference Costs of PET/CT were £909 in 2017 [43]. The alternative imaging modalities remain cheaper. A transthoracic echocardiogram had a reference cost in the same year of £71 (simple; £253 for complex), localized MRI £208, CT £140 and white cell scan £205. A whole-body MRI currently costs approximately £500 for comparison. The imaging cost per episode of SAB in a recent UK SAB trial was £395.60 for those 494 patients (65% of the total 757 analysed) who had imaging [35]. To understand these costs in context, the average cost of a single episode of SAB was £12,197 in this trial. If PET/CT can be used to reduce mortality and morbidity it is likely to be cost-effective despite its apparently high cost. The cost also needs to account for the prevention of other futile imaging or investigation and their impact [7].
Radiation exposure
The overall effective dose from PET/CT, using a low-dose CT protocol, is ~8 mSv from the [18F]FDG and 6 mSv from the CT. This equates to approximately five years’ background radiation that we all experience. The use of MRI would prevent such exposure to radiation so has obvious benefits.
Discussion
Failure to identify a focus of infection is associated with high mortality in SAB [1–3]. Cross-sectional imaging has been associated with improved outcomes in retrospective studies in SAB but few studies have directly compared imaging modalities or compared optimized imaging to standard of care [39,40,42]. In order to influence clinical outcomes it is likely that additional imaging is required within seven to 14 days. However, some modalities may be falsely negative, particularly early in disease evolution.
Globally there are no previously published trials of enhanced imaging in SAB but there are two current trials, both focusing on PET/CT. PETCT4SAB (NCT02476487) is a single-site single-arm interventional trial, which recently closed having recruited 143 participants [44]. TEPSTAR (NCT03419221) is an RCT randomizing 290 adults with SAB to PET/CT vs standard of care (expected recruitment completion July 2020) [45].
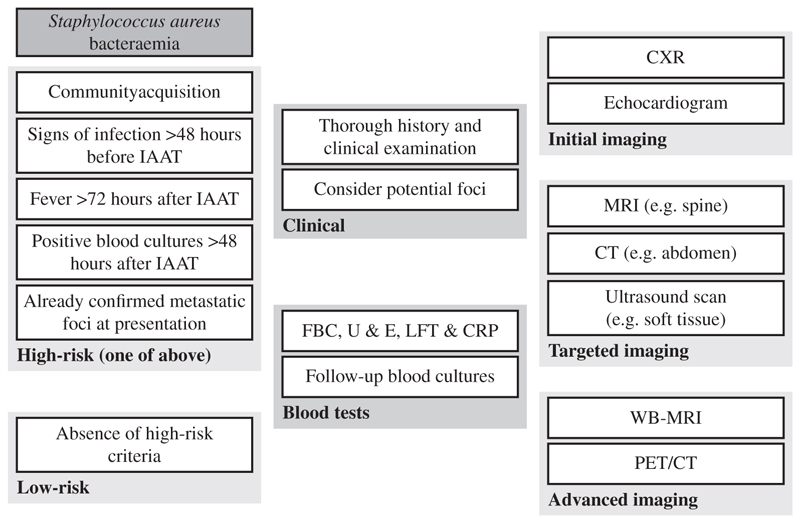
It is likely that imaging is under-utilized in current diagnostic pathways and that unsuspected infectious foci are thus likely to be underdiagnosed [39]. We have found that the impact of imaging on SAB is an area requiring further investigation and analysis (Figure 3). Previous studies in this field are small and findings inconclusive. Reported clinician decision-making appears to be improved by imaging results but it is to be expected that opinions will change when increased information is available. What is not clear is whether increased or optimized imaging can subsequently impact on patient outcomes. In order to answer such a question the ideal method would be an RCT incorporating either WB-MRI or PET/CT as routine to increase diagnostic yield and then explore clinical outcomes.
Figure 3.
An overview of investigations in Staphylococcus aureus bacteremia incorporating the role of whole-body imaging. High-risk criteria as used in reference [41]; IAAT, initiation of appropriate antibiotic therapy; FBC, full blood count; U & E, urea and creatinine and electrolytes (renal profile); LFT, liver function tests; CRP, C-reactive protein; CXR, chest radiograph PET/CT, positron emission tomography/computed tomography; MRI, magnetic resonanceimaging; WB-MRI, whole-body MRI.
This approach to the optimal diagnosis and detection of infectious foci, combined with the reassurance of imaging demonstrating absence of foci, has the potential to enable tailored antibiotic selection and potentially earlier cessation of unnecessary antibiotics. In an era of personalized medicine and improved availability of imaging at lower prices, we anticipate increased future utilization and seek evidence to support such an approach.
Funding sources
The authors acknowledge financial support from the Medical Research Council Clinical Trials Unit at University College London and the King’s College London/UCL Comprehensive Cancer Imaging Centres funded by Cancer Research UK and Engineering and Physical Sciences Research Council in association with the Medical Research Council and the Department of Health (C1519/A16463), the Wellcome Trust EPSRC Centre for Medical Engineering at King’s College London (WT203148/Z/16/Z), and UK Research & Innovation London Medical Imaging and Artificial Intelligence Centre.
Footnotes
Conflict of interest statement
None declared.
References
- [1].Nambiar K, Seifert H, Rieg S, Kern WV, Scarborough M, Gordon NC, et al. Survival following Staphylococcus aureus bloodstream infection: a prospective multinational cohort study assessing the impact of place of care. J Infect. 2018;77:516–25. doi: 10.1016/j.jinf.2018.08.015. [DOI] [PubMed] [Google Scholar]
- [2].Van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB. Predictors of mortality in Staphylococcus aureus bacteremia. Clin Microbiol Rev. 2012;25:362–86. doi: 10.1128/CMR.05022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Thwaites GE, Scarborough M, Szubert A, Nsutebu E, Tilley R, Greig J, et al. Adjunctive rifampicin for Staphylococcus aureus bacteraemia (ARREST): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391:668–78. doi: 10.1016/S0140-6736(17)32456-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Thwaites GE, Edgeworth JD, Gkrania-Klotsas E, Kirby A, Tilley R, Török ME, et al. Clinical management of Staphylococcus aureus bacteraemia. Lancet Infect Dis. 2011;11:208–22. doi: 10.1016/S1473-3099(10)70285-1. [DOI] [PubMed] [Google Scholar]
- [5].NHS England diagnostic imaging dataset annual statistical release 2017/18. [last accessed 17 December 2019]. Available at: https://www.england.nhs.uk/statistics/wp-content/uploads/sites/2/2018/11/Annual-Statistical-Release-2017-18-PDF-1.6MB-1.pdf.
- [6].Pasoglou V, Michoux N, Larbi A, Van Nieuwenhove S, Lecouvet F. Whole body MRI and oncology: recent major advances. Br J Radiol. 2018;91:20170664. doi: 10.1259/bjr.20170664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Brøndserud MB, Pedersen C, Rosenvinge FS, Høilund-Carlsen PF, Hess S. Clinical value of FDG-PET/CT in bacteremia of unknown origin with catalase-negative Gram-positive cocci or Staphylococcus aureus . Eur J Nucl Med Mol Imaging. 2019;46:1351–8. doi: 10.1007/s00259-019-04289-5. [DOI] [PubMed] [Google Scholar]
- [8].Khattak SG, Dady I, Mukherjee D. Unusual presentation of late-onset disseminated staphylococcal sepsis in a preterm infant. BMJ Case Rep. 2019;12(3):e226325. doi: 10.1136/bcr-2018-226325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Miles A, Taylor SA, Evans REC, Halligan S, Beare S, Bridgewater J, et al. Patient preferences for whole-body MRI or conventional staging pathways in lung and colorectal cancer: a discrete choice experiment. Eur Radiol. 2019;29:3889–900. doi: 10.1007/s00330-019-06153-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Andronikou S, Mendes da Costa T, Hussien M, Ramanan AV. Radiological diagnosis of chronic recurrent multifocal osteomyelitis using whole-body MRI-based lesion distribution patterns. Clin Radiol. 2019;74:737. doi: 10.1016/j.crad.2019.02.021. [DOI] [PubMed] [Google Scholar]
- [11].Gariani J, Westerland O, Natas S, Verma H, Cook G, Goh V. Comparison of whole body magnetic resonance imaging (WBMRI) to whole body computed tomography (WBCT) or 18F-fluorodeoxyglucose positron emission tomography/CT (18F-FDG PET/CT) in patients with myeloma: systematic review of diagnostic performance. Crit Rev Oncol Hematol. 2018;124:66–72. doi: 10.1016/j.critrevonc.2018.02.012. [DOI] [PubMed] [Google Scholar]
- [12].Kaasch AJ, Barlow G, Edgeworth JD, Fowler VG, Jr, Hellmich M, Hopkins S, et al. Staphylococcus aureus bloodstream infection: a pooled analysis of five prospective, observational studies. J Infect. 2014;68:242–51. doi: 10.1016/j.jinf.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gould FK, Denning DW, Elliott TS, Foweraker J, Perry JD, Prendergast BD, et al. Guidelines for the diagnosis and antibiotic treatment of endocarditis in adults: a report of the Working Party of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother. 2012;67:269–89. doi: 10.1093/jac/dkr450. [DOI] [PubMed] [Google Scholar]
- [14].Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18–55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- [15].Baddour LM, Wilson WR, Bayer AS, Fowler VG, Jr, Tleyjeh IM, Rybak MJ, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132:1435–86. doi: 10.1161/CIR.0000000000000296. [DOI] [PubMed] [Google Scholar]
- [16].Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC) Eur Heart J. 2015;36:3075–128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- [17].Fowler VG, Jr, Li J, Corey GR, Boley J, Marr KA, Gopal AK, et al. Role of echocardiography in evaluation of patients with Staphylococcus aureus bacteremia: experience in 103 patients. J Am Coll Cardiol. 1997;30:1072–8. doi: 10.1016/s0735-1097(97)00250-7. [DOI] [PubMed] [Google Scholar]
- [18].Rasmussen RV, Høst U, Arpi M, Hassager C, Johansen HK, Korup E, et al. Prevalence of infective endocarditis in patients with Staphylococcus aureus bacteraemia: the value of screening with echocardiography. Eur J Echocardiogr. 2011;12:414–20. doi: 10.1093/ejechocard/jer023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Holland TL, Arnold C, Fowler VG., Jr Clinical management of Staphylococcus aureus bacteremia: a review. JAMA. 2014;312:1330–41. doi: 10.1001/jama.2014.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ring J, Hoerr V, Tuchscherr L, Kuhlmann MT, Löffler B, Faber C, et al. MRI visualization of Staphyloccocus aureus-induced infective endocarditis in mice. PLoS One. 2014;9:e107179. doi: 10.1371/journal.pone.0107179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mathew RC, Löffler AI, Salerno M. Role of cardiac magnetic resonance imaging in valvular heart disease: diagnosis, assessment, and management. Curr Cardiol Rep. 2018;20:119. doi: 10.1007/s11886-018-1057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dursun M, Yılmaz S, Yılmaz E, Yılmaz R, Onur İ, Oflaz H, et al. The utility of cardiac MRI in diagnosis of infective endocarditis: preliminary results. Diagn Interv Radiol. 2015;21:28–33. doi: 10.5152/dir.2014.14239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Iung B, Tubiana S, Klein I, Messika-Zeitoun D, Brochet E, Lepage L, et al. Determinants of cerebral lesions in endocarditis on systematic cerebral magnetic resonance imaging: a prospective study. Stroke. 2013;44:3056–62. doi: 10.1161/STROKEAHA.113.001470. [DOI] [PubMed] [Google Scholar]
- [24].Mahmood M, Kendi AT, Ajmal S, Farid S, O’Horo JC, Chareonthaitawee P, et al. Meta-analysis of 18F-FDG PET/CT in the diagnosis of infective endocarditis. J Nucl Cardiol. 2019;26:922–35. doi: 10.1007/s12350-017-1092-8. [DOI] [PubMed] [Google Scholar]
- [25].de Camargo RA, Bitencourt MS, Meneghetti JC, Soares J, Gonçalves LFT, Buchpiguel CA, et al. The role of 18F-FDG-PET/CT in the diagnosis of left-sided endocarditis: native vs. prosthetic valves endocarditis. Clin Infect Dis. 2019 Apr 5;:pii: ciz267. doi: 10.1093/cid/ciz267. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [26].Staphylococcus aureus bacteraemia (SAB) management clinical guideline version No.: 1.2 2019. Government of South Australia Policy No CG167; [last accessed December 2019]. Available at: https://www.sahealth.sa.gov.au/wps/wcm/connect/450f0b80469722d7b4bdf6b0ec6dccc9/Guideline_SABManagement_v1.2_18.07.2019.pdf?MOD=AJPERES&CACHEID=ROOTWORKSPACE-450f0b80469722d7b4bdf6b0ec6dccc9-mN5AptG. [Google Scholar]
- [27].Healthcare Improvement Scotland. Guidance on management of proven or suspected Staphylococcus aureus bacteraemia in adults. 2019. [last accessed December 2019]. Available at: https://www.sapg.scot/media/4706/sab-algorithm.pdf.
- [28].Wiese L, Mejer N, Schønheyder HC, Westh H, Jensen AG, Larsen AR, et al. A nationwide study of comorbidity and risk of reinfection after Staphylococcus aureus bacteraemia. J Infect. 2013;67:199–205. doi: 10.1016/j.jinf.2013.04.018. [DOI] [PubMed] [Google Scholar]
- [29].Abdelrahman AE, Siam H, Allouch H, Boehm H. Multi-level non-contiguous spinal infections. Series of 77 cases in a single institution. 8th Annual Meeting of the German Spine Society, Frankfurt, Germany. Eur Spine J. 2013;22:2582–669. [Google Scholar]
- [30].Spaeth HJ, Chandnani VP, Beltran J, Lucas JG, Ortiz I, King MA, et al. Magnetic resonance imaging detection of early experimental periostitis. Comparison of magnetic resonance imaging, computed tomography, and plain radiography with histopathologic correlation. Invest Radiol. 1991;26:304–8. doi: 10.1097/00004424-199104000-00003. [DOI] [PubMed] [Google Scholar]
- [31].Kouijzer IJE, Scheper H, de Rooy JWJ, Bloem JL, Janssen MJR, van den Hoven L, et al. The diagnostic value of 18F-FDG-PET/CT and MRI in suspected vertebral osteomyelitis – a prospective study. Eur J Nucl Med Mol Imaging. 2018;45:798–805. doi: 10.1007/s00259-017-3912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fuster D, Tomás X, Mayoral M, Soriano A, Manchón F, Cardenal C, et al. Prospective comparison of whole-body (18)F-FDG PET/CT and MRI of the spine in the diagnosis of haematogenous spondylodiscitis. Eur J Nucl Med Mol Imaging. 2015;42:264–71. doi: 10.1007/s00259-014-2898-0. [DOI] [PubMed] [Google Scholar]
- [33].Schwegler B, Stumpe KD, Weishaupt D, Strobel K, Spinas GA, von Schulthess GK, et al. Unsuspected osteomyelitis is frequent in persistent diabetic foot ulcer and better diagnosed by MRI than by 18F-FDG PET or 99mTc-MOAB. J Intern Med. 2008;263:99–106. doi: 10.1111/j.1365-2796.2007.01877.x. [DOI] [PubMed] [Google Scholar]
- [34].Jensen AG, Espersen F, Skinhøj P, Frimodt-Møller N. Bacteremic Staphylococcus aureus spondylitis. Arch Intern Med. 1998;158:509–17. doi: 10.1001/archinte.158.5.509. [DOI] [PubMed] [Google Scholar]
- [35].Thwaites GE, Scarborough M, Szubert A, Saramago Goncalves P, Soares M, Bostock J, et al. Adjunctive rifampicin to reduce early mortality from Staphylococcus aureus bacteraemia: the ARREST RCT. Health Technol Assess. 2018;22:1–148. doi: 10.3310/hta22590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Malhotra R, Chan CS, Nather A. Osteomyelitis in the diabetic foot. Diabet Foot Ankle. 2014;30:5. doi: 10.3402/dfa.v5.24445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lyons OT, Baguneid M, Barwick TD, Bell RE, Foster N, Homer-Vanniasinkam S, et al. Diagnosis of aortic graft infection: a case definition by the Management of Aortic Graft Infection Collaboration (MAGIC) Eur J Vasc Endovasc Surg. 2016;52:758–63. doi: 10.1016/j.ejvs.2016.09.007. [DOI] [PubMed] [Google Scholar]
- [38].Fukuchi K, Ishida Y, Higashi M, Tsunekawa T, Ogino H, Minatoya K, et al. Detection of aortic graft infection by fluorodeoxyglucose positron emission tomography: comparison with computed tomographic findings. J Vasc Surg. 2005;42:919–25. doi: 10.1016/j.jvs.2005.07.038. [DOI] [PubMed] [Google Scholar]
- [39].Vos FJ, Bleeker-Rovers CP, Sturm PD, Krabbe PF, van Dijk AP, Cuijpers ML, et al. 18F-FDG PET/CT for detection of metastatic infection in Gram-positive bacteremia. J Nucl Med. 2010;51:1234–40. doi: 10.2967/jnumed.109.072371. [DOI] [PubMed] [Google Scholar]
- [40].Berrevoets MAH, Kouijzer IJE, Aarntzen EHJG, Janssen MJR, De Geus-Oei LF, Wertheim HFL, et al. 18F-FDG PET/CT optimizes treatment in Staphylococcus aureus bacteremia and is associated with reduced mortality. J Nucl Med. 2017;58:1504–10. doi: 10.2967/jnumed.117.191981. [DOI] [PubMed] [Google Scholar]
- [41].Berrevoets MAH, Kouijzer IJE, Slieker K, Aarntzen EHJG, Kullberg BJ, Oever JT, et al. 18F-FDG PET/CT-guided treatment duration in patients with high-risk Staphylococcus aureus bacteremia: a proof of principle. J Nucl Med. 2019;60:998–1002. doi: 10.2967/jnumed.118.221929. [DOI] [PubMed] [Google Scholar]
- [42].Yildiz H, Reychler G, Rodriguez-Villalobos H, Orioli L, D’Abadie P, Vandeleene B, et al. Mortality in patients with high risk Staphylococcus aureus bacteremia undergoing or not PET-CT: a single center experience. J Infect Chemother. 2019;25:880–5. doi: 10.1016/j.jiac.2019.04.016. [DOI] [PubMed] [Google Scholar]
- [43].NHS reference costs 2017 to 2018 national schedule of reference costs. [last accessed July 2019]. Available at: https://www.gov.uk/government/collections/nhs-reference-costs.
- [44].Rambam Health Care Campus. The benefit of FDG PET CT in the treatment algorithm of Staphylococcus aureus bacteremia (PETCT4SAB) trials identifier NCT02476487. [last accessed December 2019]. Available at: https://clinicaltrials.gov/ct2/show/NCT02476487.
- [45].University Hospital, Montpellier. Impact of 18 FDG PET/CT on the management of patients with Staphylococcus aureus bloodstream infection (TEPSTAR) [last accessed December 2019]. Available at: https://clinicaltrials.gov/ct2/show/NCT03419221.