Abstract
Long-term potentiation (LTP) of excitatory synapses is a leading model to explain the concept of information storage in the brain. Multiple mechanisms contribute to LTP, but central amongst them is an increased sensitivity of the postsynaptic membrane to neurotransmitter release. This sensitivity is predominantly determined by the abundance and localization of AMPA-type glutamate receptors (AMPARs). A combination of AMPAR structural data, super-resolution imaging of excitatory synapses, and an abundance of electrophysiological studies are providing an ever-clearer picture of how AMPARs are recruited and organized at synaptic junctions. Here, we review the latest insights into this process, and discuss how both cytoplasmic and extracellular receptor elements cooperate to tune the AMPAR response at the hippocampal CA1 synapse.
Keywords: AMPA receptor traffic, AMPA receptor structure, cryo-EM, long-term potentiation, synaptic plasticity, short-term plasticity
Introduction
Understanding how routes of neuronal communication are stored and later recalled has been a longstanding quest in neuroscience research. Synaptic plasticity, where connections between specific neurons are altered in response to ongoing activity, is thought to underlie much of this memory storage. Since the initial discovery of long-term potentiation (LTP) in the hippocampus,[1] the mechanisms controlling the strength of synaptic transmission have been intensely investigated. Two main mechanisms for this phenomenon have been proposed: a presynaptic change in L-glutamate release, and a change in the postsynaptic sensitivity to this neurotransmitter, which is mediated by the fast-acting ionotropic glutamate receptors (iGluRs).[2] The realization that the AMPAR response can selectively increase during LTP,[3–6] together with the demonstration that this increase can be triggered in the absence of a presynaptic terminal,[7] has shifted the study of AMPAR synaptic regulation into the limelight. Outlining the molecular dissection of AMPARs that has since followed in an attempt to locate these regulatory mechanisms will be the focus of this review (see also [8]).
AMPARs are a class of iGluRs: a family of glutamate-gated cation channels that also includes NMDA, kainate, and delta (GluD) receptors.[9] AMPARs and NMDARs are most widely expressed at excitatory synapses, where AMPARs mediate the majority of fast excitatory transmission, providing the initial postsynaptic depolarization that is essential for subsequent NMDAR activation; synapses lacking AMPAR are functionally silent.[4,5] At hippocampal Schaffer collateral-CA1 synapses, NMDAR activation allows the influx of Ca2+ ions, which in turn trigger downstream signaling processes, culminating in the expression of LTP through the recruitment and subsynaptic organization of AMPARs (Figure 1).
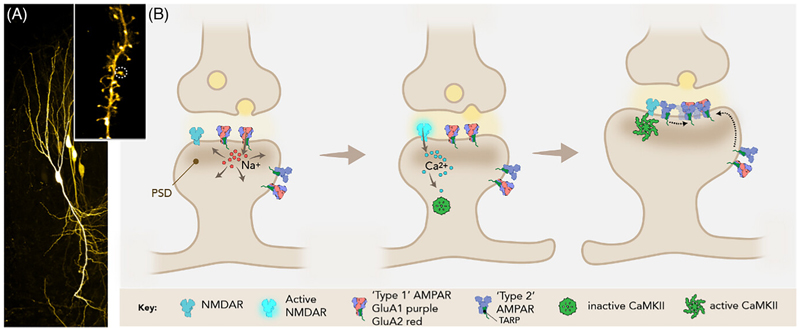
Figure 1.
(A) Confocal image of GFP-expressing hippocampal pyramidal neurons in an organotypic hippocampal slice. Inset shows a zoomed in region of dendrite, imaged by STED microscopy, with a postsynaptic spine circled in white. (B) Schematic of the postsynaptic molecular changes occurring during potentiation of an excitatory glutamatergic synapse. Glutamate release from presynaptic vesicles activates postsynaptic AMPARs, which enable the influx of Na+ ions to depolarize the postsynaptic cell (panel 1), thereby activating NMDARs and the influx of Ca2+ ions (panel 2). Subsequent postsynaptic Ca2+ signaling processes, such as the activation of CAMKII (panel 3), result in the recruitment of further AMPARs and their organization into transsynaptic nanocolumns, as well as the growth of the postsynaptic density and protrusion of the dendritic spine.
Ampars at Excitatory Synapses
Excitatory synapses are predominantly formed on dendritic spines, which are micron-sized membrane protrusions that make up the postsynaptic element opposing presynaptic axon terminals (Figure 1A). Spines are supported by a cytoskeletal framework enriched in filamentous (F)-actin, and harbor the postsynaptic density (PSD): a complex network of structural and signaling proteins that is aligned with presynaptic vesicle release sites.[10,11] AMPARs diffuse in the plane of the postsynapse [12] and connect to the MAGUK (membrane-associated guanylate kinase) family of PDZ-containing proteins, either directly via their C-terminal PDZ-binding motifs,[13,14] or indirectly via the C-tails of transmembrane AMPAR-associated auxiliary proteins (TARPs).[15,16] Interaction of the TARP cytosolic C-tail with PSD-93/95, the most abundant MAGUKs in the PSD, is a major mechanism of AMPAR synaptic recruitment.[17] This cytoplasmic anchor-age physically links receptors to the PSD, limiting their diffusion away from the critical sites for synaptic transmission.[18–21] Not only does the PSD capture and retain AMPARs in this manner, but is also able to organize them.[22] Superresolution imaging has demonstrated subsynaptic organization of MAGUKs, which can concentrate subsynaptic populations of AMPARs.[19,23,24] Optimizing the alignment of these clustered AMPARs with presynaptic neurotransmitter release sites may provide a mechanism for the control of synaptic strength.[24–26] A central player upstream of AMPAR recruitment is calcium calmodulin-dependent kinase II (CaMKII), which is activated by Ca2+ influx through NMDARs, upon which it translocates to the PSD (Figure 1B).[27] CAMKII is involved in the synaptic immobilization of AMPARs.[28] However, how CAMKII facilitates the recruitment of additional AMPARs during LTP is not established; this central issue remains a matter of ongoing debate.[29]
On the extracellular side, AMPARs face the synaptic cleft, an environment densely packed with various transsynaptic factors, including adhesion proteins, secreted synaptic organizers, and the extracellular matrix.[26] Whilst the cytoplasmic influence on AMPAR localization has been intensively studied for decades, only recently has the importance of the AMPAR extracellular domain in LTP been dissected.[30,31] Together, this dual setting of cleft and cytosol will determine the activity-dependent AMPAR subsynaptic organization.[26,32] Here, we review our current understanding of the contribution of cytosolic interactions of AMPAR-TARP complexes with the PSD on the one hand, and of receptor extracellular components in the synaptic cleft on the other.
Ampar Organisation
Like all iGluRs, AMPARs exist in the PSD as tetramers, assembled from four core subunits, GluA1-4, in various combinations.[33] Inclusion of the GluA2 subunit renders the tetramer impermeable to Ca2+ ions, and these “Type-1” AMPARs predominate in pyramidal neurons across the forebrain. GluA2-lacking, “Type-2” receptors on the other hand are Ca2+ permeable. They are abundantly expressed in interneurons and in glia, but are rare in pyramidal neurons, where the GluA1 homotetramer is thought to be the most prominent variety.[34] Various lines of evidence suggest that GluA1 homomers in CA1 pyramidal neurons can be induced transiently by LTP stimuli, with their Ca2+ signal contributing to the expression of LTP [35–37] (but see [38]). Type-2, Ca2+-permeable receptors would be subject to different regulatory trafficking mechanisms, due not only to sequence diversity in their C-tails and N terminal domains (NTDs), but also as a result of their distinct NTD structure [39] (see below).
AMPARs share the modular design of other iGluRs and are composed of four distinct domains: the extracellular NTD and ligand binding domain (LBD; binding the agonist L-glutamate), the transmembrane domain (TMD) forming the ion channel, and the unstructured intracellular C terminal domain (CTD) [9,40] (Figure 2A,B). The NTD and LBD form dimers of dimers, whilst the TMD is four-fold symmetric.[41] Contrary to the evolutionarily conserved LBD and TMD between the four AMPAR subunits, the NTD and CTD are the most sequence-diverse regions (Figure 2A), and therefore enable subunit-selective protein interactions and functions. The NTD encodes roughly 50 percent of an AMPAR subunit (~ 400 amino acids), whilst the CTD is only 50–80 amino acids in length.[13,42] Unique to AMPARs amongst iGluRs is the diversity of auxiliary subunits that they associate with. The type and expression level of auxiliary subunits varies by brain region, with consequences for gating and trafficking.[9,16,40,43] Both type and stoichiometry of auxiliary subunits on a given AMPAR will also significantly impact synaptic anchoring, as some auxiliaries interact with the PSD scaffold, while others do not.
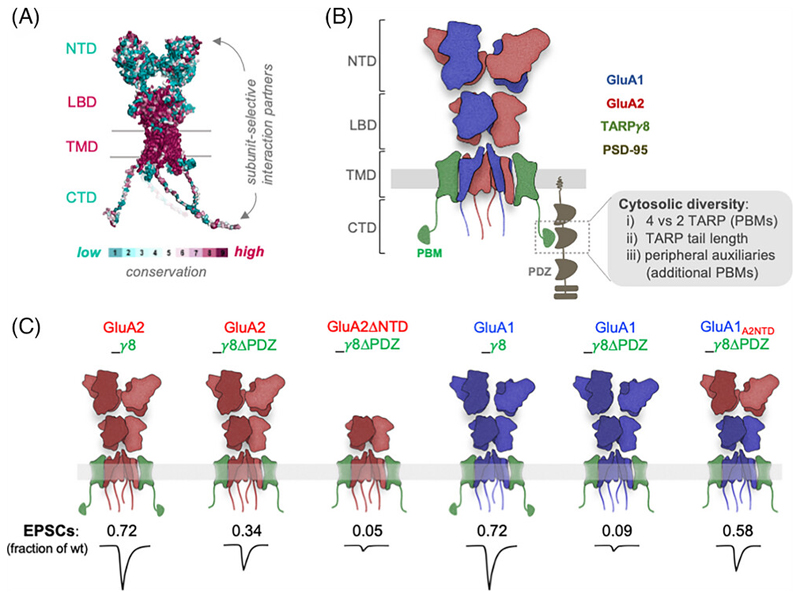
Figure 2.
(A) AMPAR structure colored by sequence conservation between the four subunits (NTD: N terminal domain, LBD: ligand-binding domain, TMD: transmembrane domain, CTD: C terminal domain). The LBD and TMD sequences are highly conserved (magenta), whilst the NTD and CTD show greater sequence diversity (cyan), enabling subunit-specific interactions. (B) Schematic of a heteromeric AMPAR on the postsynaptic membrane, held in the postsynaptic density by interactions between the associated auxiliary protein TARPγ8 and the three PDZ domains of PSD95, and the NTD that extends into the synaptic cleft. Cytosolic diversity of synaptic AMPAR complexes can arise by (i) the stoichiometry of associated TARPs (ii) TARP type, for example, different length C-tails of TARP γ2 and TARPγ8 (iii) PDZ anchoring by additional associated proteins, for example, CKAMPs. (C) Schematic depicting the subunit-specific effect of the NTD and TARPγ8 PDZ interactions on synaptic transmission. Excitatory postsynaptic current (EPSC) amplitude, normalized to a neighboring transfected neuron, is reduced when removing the TARPγ8 PDZ binding motif (γ8ΔPDZ). γ8 PDZ deletion prevents GluA1-mediated synaptic transmission, which can be partially rescued by the NTD of GluA2 (derived from Watson et al., 2021).
Role of Cytoplasmic Elements in Synaptic Recruitment
Initial studies of AMPAR synaptic trafficking and anchorage focused on cytoplasmic CTD interactions as the primary mechanism. This work was inspired by the observation that NMDARs are linked to PSD-95 through their extreme C-termini,[44] and what followed was extensive cloning of various multi-PDZ domain-containing proteins which interact with AMPARs.[13,14] Moreover, subunit differences in CTD sequence, together with the fact that GluA1 [45] but not GluA2 [46] is essential for various forms of LTP, focused further attention on the CTD. Hippocampal CA1 pyramidal neurons mainly express the GluA1 and GluA2 subunits, and to a lesser extent GluA3.[47] Interestingly, these three subunits are subject to different trafficking mechanisms, with GluA1 selectively accumulating at the cell surface to form an extra-synaptic “reserve” pool [47] that is critical to supply AMPARs during LTP.[48,49] Overexpression studies demonstrated distinct subunit-specific synaptic trafficking profiles, where GluA2 homomers (and GluA2/GluA3 heteromers) were shown to traffic into the synapse constitutively,[50] whilst the synaptic insertion of GluA1 required either LTP stimuli, constitutively active CAMKII,[50,51] or overexpressed PSD-95.[52,53] These differences were ascribed to the CTD, its cytoplasmic interactors and to subunit-selective CTD phosphorylation.[54] However, mutating the CTD protein interaction sites,[48,55] or even complete CTD removal, does not prevent synaptic targeting of GluA1 or the expression of LTP [48,56] (but see [57]). Therefore, subunit-specific trafficking as a principle has been established, but is not solely orchestrated by their CTDs.
An additional cytosolic role is played by AMPAR auxiliary subunits, primarily the six-membered TARP family,[17,58] which centrally regulate AMPAR trafficking and synaptic anchoring.[16,40,43] TARPs direct AMPARs both from the endoplasmic reticulum to the cell surface, and from the surface into the synapse, where they impact receptor stability.[17,21,59] A PDZ-binding motif (PBM) on the extreme C-tails of Type-1 TARPs (TTPV-COOH) connects AMPARs to PSD-93/95 [60–62] (Figure 2B). Deletion of the TARP-γ2 C-tail or of the TARP-γ8 PBM results in a more diffuse surface distribution of receptors,[55,61] highlighting the importance of TARP-PSD95 interactions in accumulating AMPARs at synaptic sites. Other than TARPs, CNIH (cornichon homolog) auxiliary subunits are strongly expressed in the hippocampus.[63,64] These proteins recognize the same four binding sites on the AMPAR TMD as the TARPs do,[65] but their C-terminus is extracellular and lacks a PBM.[65] Therefore, the type and stoichiometry of auxiliary subunits will determine the number of PSD-interacting anchors of a given AMPAR complex (Figure 2B). In hippocampal pyramidal neurons, the predominant AMPAR is the GluA1/2 heteromer,[47] which associates with two TARP and two CNIH subunits.[66,67] However, other minor receptor subtypes that are associated with different sets of auxiliaries likely exist in these neurons, and are expected to have very different functional,[68] trafficking, and anchoring properties.[43] Adding further complexity is the presence of peripheral auxiliary sub-units of the CKAMP/Shisha family.[69,70] These also harbour PBMs in their C-termini,[71] but their stoichiometry and mode of association with the AMPAR are currently elusive.
Given the prominent role of CaMKII in LTP, the regulatory effects of phosphorylation of the CTDs of both AMPARs and TARPs has been investigated as a possible downstream target for CaMKII.[27,29,54] In the case of TARPs, CTD phosphorylation has been suggested to cause their dissociation from negatively charged lipids of the inner membrane leaflet.[72] Release of the TARP tails from the membrane by charge repulsion would effectively increase their reach for the PDZ domains of PSD-95.[73] In addition, preventing the phosphorylation or dephosphorylation of TARP-γ2 blocked both LTP and LTD respectively.[74] LTP is also reduced (by 60%) in a phosphor-null mutant TARP-γ8 knockin mouse.[75] However, more recent experiments suggest that phosphorylation of the TARP tails reduces their binding to multiple sites on PSD-95, in addition to the canonical PDZ binding motif.[76] Moreover, in a study focusing on GluA1 homomers, the TARP-γ8 phospho-null mutant did not affect LTP,[77] suggesting that in some AMPAR/TARP complexes TARP phosphorylation has a non-essential role. Taken together, the mechanisms linking CaMKII activation to AMPAR synaptic insertion remain to be elucidated.
In addition to acting as a kinase, CaMKII has been shown to organize proteins by liquid-liquid phase separation, with Ca2+ triggering the segregation of PSD-95 and TARP-γ2 into a central condensate, thereby separating AMPAR and NMDAR nanoclusters.[78] The entire C-tail of TARP-γ2 has been shown to undergo phase separation with PSD-95 in vitro, with tail regions other than the PBM engaging PSD-95 and contributing to synaptic clustering.[76] An increase in Ca2+ concentration restricts the diffusion of AMPARs on the membrane,[79] likely a combined effect of the dual roles of CaMKII phosphorylation of TARP-γ2 trapping AMPARs at the synapse and causing phase separation.[28] Together, these mechanisms enrich the binding capacity of synaptic sites, thereby enhancing AMPAR recruitment and synaptic transmission.[80]
Subunit-Specific Requirement for Ntd Versus Tarp-Mediated Anchoring
Despite the nonselective interaction between TARPs and AMPAR subunits, there are nevertheless subunit-specific dependencies on TARP interactions. The TARP—PSD-95 anchor is of greater significance for GluA1 than GluA2, as seen using ‘tandem’ constructs, where the TARP is fused to the GluA C-tail (via the TARP N-terminus).[81] This setting allows control of both AMPAR subunit, and TARP-PDZ interactions. Deletion of the TARP-γ8 PBM in a GluA1-TARP-γ8 tandem (GluA1_γ8ΔPDZ) fails to rescue excitatory postsynaptic currents (EPSCs) when expressed in an AMPAR null genetic background [55,77] (Figure 2C). Interestingly, GluA2 is less sensitive to TARP PBM deletion, as the GluA2_γ8ΔPDZ receptor construct can partially rescue AMPAR synaptic currents. This effect is mediated by the GluA2 NTD, which also appears to have a strong affinity for synaptic sites, and when placed on GluA1, can facilitate synaptic anchoring in the absence of TARP interactions. Lastly, in a GluA1/GluA2 heteromeric receptor with both subunits lacking the TARP PBM (γ8ΔPDZ), the NTD of the GluA2 subunits is also capable of rescuing synaptic transmission.[55] These results add to our picture of subunit-specific recruitment: GluA2-lacking receptors are more dependent on TARPs than GluA2-containing receptors due to the dominating synaptic recruitment ability of the GluA2 NTD [55] (Figure 2C).
Role of the Ampar Ntd in Synaptic Recruitment
A role for the AMPAR NTD in synaptic anchoring was first suggested for the GluA4 subunit at interneuron synapses.[82–84] However only recently has the role of the NTD been fully appreciated at principal neuron synapses, with subunit specific effects on LTP.[30,31,55,56,85] It appears that historically used AMPAR N-terminal GFP tags,[50,86] occlude the contribution of GluA1 to synaptic transmission,[30,31] and therefore overestimated the role of CTD interactions in AMPAR targeting. The GFP tag may cause steric hindrance to the synaptic entry of GluA1, but also prevent contact with NTD interacting proteins critical for synaptic anchorage of the receptor. Of interest, an N-terminal GFP on GluA2 is less detrimental to synaptic transmission than on GluA1, with tagged GluA2 still entering the synapse readily.[30,31] The role of the NTD in AMPAR anchoring was further confirmed using NTD-deleted receptors, which have subunit-specific impairments in transmission. In particular, maintenance of LTP was prevented by GluA1 NTD deletion, but not GluA2.[30,31,55,85] The subunit-specific roles of NTD interactions, with GluA1 implicated in LTP and GluA2 for basal transmission levels, echo subunit trafficking rules ascribed to CTDs, yet how the NTD manifests these effects has not yet been resolved.[8] It should be noted that while the above experiments using receptor overexpression reported a clear difference between the GluA1 and GluA2 NTD in synaptic anchoring, GFP-tagged GluA1 knock-in mice do display normal receptor trafficking,[87,88] suggesting that the GFP appendage is not an absolute block to receptor localization under different experimental conditions.
The function of the NTD in the regulation of synaptic transmission may be multi-layered. This domain is not simply a static platform for interactions, but can be highly mobile.[42] Structural studies,[89–91] complemented by simulations [92,93] have demonstrated large motions of NTD dimers accompanying receptor gating, and an effect of domain removal on channel kinetics has been reported.[94] A recent atomic-force microscopy study even suggests NTD dimer splitting into monomers,[95] which is surprising given the low nanomolar interaction between NTD monomers,[33,96,97] but may permit clustering between adjacent receptors through NTD monomer interactions in trans.[95] Glutamate-induced receptor gating motions would have the potential to alter synaptic interactions, but this may be reduced in the context of a crowded synaptic cleft environment. Recent structural data has developed these ideas to demonstrate that the effect of NTD motions are also subunit-specific. Cryo-Electron Microscopy structures revealed that the NTD tier of GluA1 homomers is uniquely flexible compared to GluA2-containing receptors.[39] In GluA2, an interface between NTD dimers holds receptors in a compact “Y” shaped structure [41] (Figure 3A). This interface is absent in GluA1 (due to NTD sequence divergence), resulting in a wide spectrum of GluA1 NTD dimer configurations. Destabilizing the GluA2 NTD interface with a point mutation (F231A) renders the GluA2 NTDs equally flexible (Figure 3A).[39] Unlike unmodified GluA2 receptors,[30,31] the GluA2QF231A mutant fails to boost EPSCs in CA1 pyramidal neurons, thus closely mimicking GluA1 [39] (Figure 3A, bottom). How NTD dynamics affect AMPAR synaptic recruitment in LTP remains to be understood. The critical GluA2 NTD interface is important for more than just GluA2 homomers: the preferred building plan of heteromeric receptors (GluA1/2 and GluA2/3) places GluA2 subunits in position to maintain this interface in the majority of GluA2-containing receptors (Figure 2B).[98,99] Taken together, this supports the idea that the NTD may provide an “anchoring platform” that can adopt state and subunit-dependent conformations that affect receptor anchoring, and in turn impact various forms of synaptic plasticity (Figure 3).
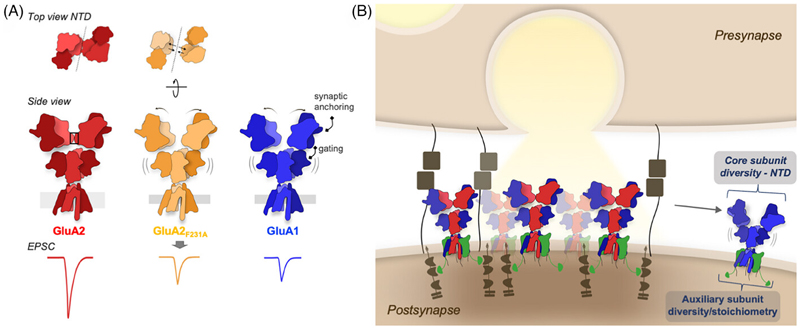
Figure 3.
(A) Proposed model of the role of the NTD in synaptic anchoring of AMPARs. The stability of the tetrameric interface in GluA2-containing receptors (highlighted in black; ‘side view’) may enable efficient synaptic anchoring, as receptors without this interface (GluA1 homomers) or with a disrupted interface (GluA2F231A) show reduced EPSCs following Schaffer collateral stimulation. The flexibility of the NTD with a disrupted interface has implications for both the gating and synaptic anchoring of the receptor. (B) Synaptic accumulation of AMPARs is maintained by TARP (green) PDZ interactions with PSD-95 (brown). Subsynaptic positioning into receptor clusters opposing vesicle release is influenced by both the core subunit, determining subunit-specific NTD interactions, and the associated auxiliary proteins providing PSD-95 anchoring. NTD interactions with synaptic cleft molecules may be disturbed in receptors with a broken NTD dimeric interface, making them less likely to be maintained in a stable synaptic position.
Based on these data, we propose that the constitutive recruitment of GluA2-containing AMPARs requires a compact, tetrameric sNTD platform, enabled by a GluA2-specific NTD interface (Figure 3A, top). Structural integrity of this platform determines interactions with anchoring proteins, and these interactions are altered or occluded by flexible NTD motions, occurring in GluA1, GluA2F231A, and in particular, desensitized receptor conformations.[39] This model explains both the strong ‘synapto-sticky’ phenotype of the GluA2 NTD, which can support synaptic anchoring alone, and the decreased synaptic anchoring of GluA1 and GluA2F231A.[31,39] The necessity of the GluA1 NTD for LTP suggests a role for specific interactors, which could act by stabilizing an otherwise mobile NTD tier. How NTDs contribute to subunit-selective delivery of heteromeric receptors is unclear. Both GluA1/GluA2 and GluA2/GluA3 heteromers are expected to exhibit a compact (tetrameric) NTD mediated by the GluA2-specific NTD interface (Figure 2B), leaving the GluA1 or GluA3 subunits to occupy the more exposed outer position within the tetramer. This has the potential to enable subunit-specific interactions and trafficking rules, which remain to be fully clarified.
Ntd Interactors in the Synaptic Cleft
The molecules directing AMPAR function through the NTD could extend from either pre or postsynapse, or be secreted factors. More generally, the mesh of proteoglycans and glycoproteins that makes up the extracellular matrix can constrain the diffusion of AMPARs but not NMDARs, affecting short term plasticity by limiting the exchange of AMPARs within the synapse.[100] Indeed, it is of interest that the synaptic occlusion of GluA1-GFP is not observed in dissociated neuronal culture lacking such an environment.[86] AMPARs uniquely form macromolecular complexes with numerous synaptic proteins,[42,69,101,102] amongst which the role of secreted neuronal pentraxins is the best studied. As pointed out above, these were the first NTD-interacting proteins described and have a capacity to cluster receptors within the synaptic cleft.[88] Pentraxins preferentially bind the NTD of GluA4 and act as a transsynaptic organizer in interneurons.[82,83] At excitatory synapses, the search for synaptic cleft interactions that could capture and retain AMPARs is still ongoing. A recent study has characterized neuroplastin-65, a single transmembrane postsynaptic cell adhesion molecule, showing it to interact specifically with the NTD of GluA1 and to be required for the maintenance of LTP,[85] but further work is required to understand how this anchor is employed at the synapse. Moreover, members of the Noelin/Olfactomedin family interact with both the AMPAR extracellular region and various extracellular proteins [103] to form a network of molecules potentially linking pre and postsynaptic neurons. Strong effects on both synaptic transmission and plasticity observed in Noelin knockout mice suggest a model whereby extracellular factors are required for the stable trapping of AMPARs at both the cell surface and synapse.[104] Transsynaptic receptor interactions are not just proposed for AMPARs; such interactions appear to be an organizing principle utilized across synapse types. For example, Cbln1, released from cerebellar granule cells, links presynaptic neurexins and the NTD of postsynaptic GluD2 to form a transsynaptic organizer complex.[105–107] Similarly, C1q-like secreted proteins bridge post-synaptic kainate receptors to presynaptic neurexin.[108] Given the dense protein network of the synaptic cleft, understanding receptor organization in their native context is becoming increasingly important to fully appreciate the mechanisms controlling synaptic transmission.
Transsynaptic Nanocolumns
Advances in super-resolution light microscopy has illuminated the subsynaptic organization of AMPARs. Both PALM and STORM imaging revealed the existence of AMPAR nanodomains of ~70–80 nm in diameter, containing 20–25 receptors per cluster.[19,23,24,109] Other iGluRs are also not homogeneously distributed at the synaptic membrane,[110] and are specifically localized for their signaling functions.[111] AMPARs have a relatively low affinity for glutamate, so clustering of receptors can efficiently increase synaptic currents without necessitating increased receptor production or trafficking.[112–114] Simulations suggest that the displacement of AMPAR clusters by at least 100 nm would result in a reduction in EPSC amplitude,[19] therefore a transsynaptic alignment of AMPARs will determine postsynaptic current amplitudes. Indeed presynaptic proteins, such as voltage-gated calcium channels and vesicle priming molecules required for transmitter release, for example, RIM and Munc-13, are similarly clustered.[26,115,116] Alignment of pre and postsynaptic nanoclusters into a transsynaptic “nanocolumn” [24,26,116] would enable efficient activation of postsynaptic receptors, and provide a mechanism for tuning the strength of transmission in addition to simply increasing the number of synaptic receptors. Yet, if and how these mechanisms occur and contribute across the timescales of synaptic plasticity remains to be answered. It should also be emphasized that AMPAR nanocolumns are not ubiquitously observed across excitatory synapses; they have not been seen in high-throughput synapses (that are tuned to coincidence detection),[117] and may be a hallmark for synapses optimized to integrate presynaptic signals.
Alignment of receptors with presynaptic release sites likely requires transsynaptic interactions. One recently identified interactor is LRRTM2, cleavage of which induces the dispersal of AMPARs from RIM1/2-labelled release sites.[118] LRRTMs plug into the PSD, so could align AMPARs to the presynapse indirectly, through TARP/PSD-95 linkage, or organize receptors through direct interactions.[69] The AMPAR NTD, projecting halfway into the synaptic cleft and reporting the conformational state of the receptor, would provide a prime anchor to link pre and post synapse. Whilst NTD deletion does not result in the diffuse surface distribution of receptors seen upon TARP-γ8 PBM deletion, some changes in the subsynaptic distribution of NTD-deleted receptors can be observed.[55] As PSD-95 appears to align with presynaptic vesicle release,[23,24] TARP interactions may be sufficient for the formation of AMPAR nanocolumns,[55] yet the role of each AMPAR interactor for anchoring, clustering, and alignment of the receptor requires careful further investigation.
Ampar Anchoring and Short-Term Plasticity
The interplay between AMPAR lateral diffusion by Brownian motion [20,22] and their anchoring within trans-synaptic nanocolumns is not only relevant for the expression of LTP,[48,49,119] but is also expected to impact short-term plasticity (STP).[79] The rate of AMPAR diffusion appears to be influenced by their conformation, with desensitized receptors showing an increased mobility.[79] This mobility had previously been linked to decreased TARP association,[120] however recent experiments demonstrate no effect of glutamate on TARP association in functional [121,122] and structural studies.[39,123–125] Desensitization-induced rearrangement of the NTD tier, followed by detachment from an anchoring protein is a possible alternative mechanism (Figure 3B), and may contribute to STP by altering paired-pulse facilitation.[39] These results suggest that an interplay between receptor desensitization kinetics and receptor diffusion is dictating STP, due to the need to stably position receptors opposing glutamate release. Subunit-specific NTD interactions likely contribute to these behaviors and thereby impact both STP and LTP.[126]
Conclusions and Outlook
The view that memory involves rapid and long-term changes in the strength of synaptic transmission is longstanding, persisting since Hebb’s 1949 postulation that memory formation involved “organization by structural modifications.” The current model of synaptic function is highly dynamic: receptor diffusion, phase separation and transsynaptic interactions cooperatively function to provide a modifiable channel of communication. With dynamic reconstruction occurring at both the presynaptic active zone and postsynaptic density, it is the alignment of receptors to glutamate release that can dramatically alter transmission strength.[26] As discussed, diverse AMPAR populations will have unique conformational landscapes, with the dynamics of receptor diffusion in the synaptic cleft being tuned by protein interactions. Linking the molecular architecture of the synapse to the long-term changes in in vivo synaptic strength has been a difficult yet essential aim for understanding synaptic information storage. With the diversity of AMPARs now well-established, understanding how this diversity is employed and regulated offers avenues to new view-points on information storage in the brain.
Realizing the central role of AMPARs in LTP, the last ~25 years have provided a wealth of insights into receptor regulation stemming from a multitude of experimental approaches. Sequence diversity in the NTD and CTD of the four core subunits, together with a wide variety of stably associating auxiliary subunits, enables fine control of receptor gating, trafficking and location through transient protein interactions, both in the cytosol and in the synaptic cleft. Yet how these anchor points co-operate under various modes of synaptic activity remains mostly enigmatic. Improved genetic and imaging tools, together with electrophysiology to interrogate the synapse on the one hand, and structural studies of the highly diverse (synapse-specific) AMPARs combined with simulations on the other, are expected to advance this central question in synaptic communication.
Acknowledgments
The authors thank Alexander Scrutton and James M. Krieger for comments on the manuscript. The authors also acknowledge Shraddha Nayak for help with Figure 1B design. This work was supported by grants from the Medical Research Council (MC_U105174197), the BBSRC (BB/N002113/1), and the Wellcome Trust (223194/Z/21/Z) to IHG.
Funding information
Medical Research Council, Grant/Award Number: MC_U105174197; Biotechnology and Biological Sciences Research Council, Grant/Award Number: BB/N002113/1; Wellcome Trust, Grant/Award Number: 223194/Z/21/Z
Footnotes
Conflict of interest statement
The authors declare no conflicts of interest.
Data Availability Statement
The data referenced in Figure 2 are openly available at https://doi.org/10.1038/s41467-021-25281-4.
References
- 1.Bliss TVP, Lømo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. The Journal of Physiology. 1973;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bliss TVP, Collingridge GL. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 3.Kauer JA, Malenka RC, Nicoll RA. A persistent postsynaptic modification mediates long-term potentiation in the hippocampus. Neuron. 1988;1(10):911–917. doi: 10.1016/0896-6273(88)90148-1. [DOI] [PubMed] [Google Scholar]
- 4.Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375(6530):400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- 5.Isaac JTR, Nicoll RA, Malenka RC. Evidence for silent synapses: Implications for the expression of LTP. Neuron. 1995;15(2):427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 6.Nicoll RA. A brief history of long-term potentiation. Neuron. 2017;93(2):281–290. doi: 10.1016/j.neuron.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 7.Matsuzaki M, Honkura N, Ellis-Davies GCR, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429(6993):761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Díaz-Alonso J, Nicoll RA. AMPA receptor trafficking and LTP: Carboxy-termini, amino-termini and TARPs. Neuropharmacology. 2021;197:108710. doi: 10.1016/j.neuropharm.2021.108710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen KB, Wollmuth LP, Bowie D, Furukawa H, Menniti FS, Sobolevsky AI, Swanson GT, Swanger SA, Greger IH, Nakagawa T, Mcbain CJ, et al. Structure, function, and pharmacology of glutamate receptor ion channels. Pharmacological Reviews. 2021;73(4):1469–1658. doi: 10.1124/pharmrev.120.000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennedy MB. The postsynaptic density at glutamatergic synapses. Trends in Neuroscience (Tins) 1997;20(6):264–268. doi: 10.1016/s0166-2236(96)01033-8. [DOI] [PubMed] [Google Scholar]
- 11.Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: A more quantitative view. Annual Review of Biochemistry. 2007;76:823–847. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- 12.Choquet D, Triller A. The dynamic synapse. Neuron. 2013;80(3):691–703. doi: 10.1016/j.neuron.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annual Review of Cell and Developmental Biology. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 14.Barry M. Receptor trafficking and the plasticity of excitatory synapses. Current Opinion in Neurobiology. 2002;12(3):279–286. doi: 10.1016/s0959-4388(02)00329-x. [DOI] [PubMed] [Google Scholar]
- 15.Zhu J, Shang Y, Zhang M. Mechanistic basis of MAGUK-organized complexes in synaptic development and signalling. Nature Reviews Neuroscience. 2016;17(4):209–223. doi: 10.1038/nrn.2016.18. [DOI] [PubMed] [Google Scholar]
- 16.Jackson AC, Nicoll RA. The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits. Neuron. 2011;70(2):178–199. doi: 10.1016/j.neuron.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408(6815):936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Levy JM, Hou A, Winters C, Azzam R, Sousa AA, Leapman RD, Nicoll RA, Reese TS. PSD-95 family MAGUKs are essential for anchoring AMPA and NMDA receptor complexes at the postsynaptic density. PNAS. 2015;112(50):E6983–E6992. doi: 10.1073/pnas.1517045112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nair D, Hosy E, Petersen JD, Constals A, Giannone G, Choquet D, Sibarita J-B. Super-resolution imaging reveals that AMPA receptors inside synapses are dynamically organized in nanodomains regulated by PSD95. Journal of Neuroscience. 2013;33(32):13204–13224. doi: 10.1523/JNEUROSCI.2381-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bessa-Neto D, Choquet D. Molecular mechanisms of AMPAR reversible stabilization at synapses. Molecular and Cellular Neuroscience. 2023;125:103856. doi: 10.1016/j.mcn.2023.103856. [DOI] [PubMed] [Google Scholar]
- 21.Bats C, Groc L, Choquet D. The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron. 2007;53(5):719–734. doi: 10.1016/j.neuron.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 22.Groc L, Choquet D. Linking glutamate receptor movements and synapse function. Science. 2020;368(6496):eaay4631. doi: 10.1126/science.aay4631. [DOI] [PubMed] [Google Scholar]
- 23.Macgillavry HD, Song Y, Raghavachari S, Blanpied TA. Nanoscale scaffolding domains within the postsynaptic density concentrate synaptic AMPA receptors. Neuron. 2013;78(4):615–622. doi: 10.1016/j.neuron.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang A-H, Chen H, Li TP, Metzbower SR, Macgillavry HD, Blanpied TA. A trans-synaptic nanocolumn aligns neurotransmitter release to receptors. Nature. 2016;536(7615):210–214. doi: 10.1038/nature19058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu KK, Hagan MF, Lisman JE. Gradation (approx. 10 size states) of synaptic strength by quantal addition of structural modules. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 2017;372(1715):20160328. doi: 10.1098/rstb.2016.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biederer T, Kaeser PS, Blanpied TA. Transcellular nanoalignment of synaptic function. Neuron. 2017;96(3):680–696. doi: 10.1016/j.neuron.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yasuda R, Hayashi Y, Hell JW. CaMKII: A central molecular organizer of synaptic plasticity, learning and memory. Nature Reviews Neuroscience. 2022;23(11):666–682. doi: 10.1038/s41583-022-00624-2. [DOI] [PubMed] [Google Scholar]
- 28.Opazo P, Labrecque S, Tigaret CM, Frouin A, Wiseman PW, De Koninck P, Choquet D. CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron. 2010;67(2):239–252. doi: 10.1016/j.neuron.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Nicoll RA, Schulman H. Synaptic memory and CaMKII. Physiological Reviews. 2023;103(4):2897–2945. doi: 10.1152/physrev.00034.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Díaz-Alonso J, Sun YJ, Granger AJ, Levy JM, Blankenship SM, Nicoll RA. Subunit-specific role for the amino-terminal domain of AMPA receptors in synaptic targeting. PNAS. 2017;114(27):7136–7141. doi: 10.1073/pnas.1707472114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watson JF, Ho H, Greger IH. Synaptic transmission and plasticity require AMPA receptor anchoring via its N-terminal domain. Elife. 2017;6:e23024. doi: 10.7554/eLife.23024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Droogers WJ, Macgillavry HD. Plasticity of postsynaptic nanostructure. Molecular and Cellular Neuroscience. 2023;124:103819. doi: 10.1016/j.mcn.2023.103819. [DOI] [PubMed] [Google Scholar]
- 33.Herguedas B, Krieger J, Greger IH. Receptor heteromeric assembly-how it works and why it matters: The case of ionotropic glutamate receptors. Progress in Molecular Biology and Translational Science. 2013;117:361–386. doi: 10.1016/B978-0-12-386931-9.00013-1. [DOI] [PubMed] [Google Scholar]
- 34.Wenthold RJ, Petralia RS, Blahos JI, Niedzielski A. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. Journal of Neuroscience. 1996;16(6):1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, Mcbain CJ, Collingridge GL, Isaac JTR. Transient incorporation of native GluR2-lacking AMPA receptors during shippocampal long-term potentiation. Nature Neuroscience. 2006;9(5):602–604. doi: 10.1038/nn1678. [DOI] [PubMed] [Google Scholar]
- 36.Park M. AMPA receptor trafficking for postsynaptic potentiation. Frontiers in Cell Neurosciences. 2018;12:361. doi: 10.3389/fncel.2018.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Purkey AM, Dell’acqua ML. Phosphorylation-dependent regulation of Ca2+-permeable AMPA receptors during hippocampal synaptic plasticity phosphorylation-dependent regulation of Ca. Frontiers in Synaptic Neurosciences. 2020;12:8. doi: 10.3389/fnsyn.2020.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adesnik H, Nicoll RA. Conservation of glutamate receptor 2-containing AMPA receptors during long-term potentiation. Journal of Neuroscience. 2007;27(17):4598–4602. doi: 10.1523/JNEUROSCI.0325-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang D, Ivica J, Krieger JM, Ho H, Yamashita K, Stockwell I, Baradaran R, Cais O, Greger IH. Structural mobility tunes signalling of the GluA1 AMPA glutamate receptor. Nature. 2023;621(7980):877–882. doi: 10.1038/s41586-023-06528-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greger IH, Watson JF, Cull-Candy SG. Structural and functional architecture of AMPA-type glutamate receptors and their auxiliary proteins. Neuron. 2017;94(4):713–730. doi: 10.1016/j.neuron.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 41.Sobolevsky AI, Rosconi MP, Gouaux E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. 2009;462(7274):745–756. doi: 10.1038/nature08624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.García-Nafría J, Herguedas B, Watson JF, Greger IH. The dynamic AMPA receptor extracellular region: A platform for synaptic protein interactions. The Journal of Physiology. 2016;594(19):5449–5458. doi: 10.1113/JP271844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamalova A, Nakagawa T. AMPA receptor structure and auxiliary subunits. The Journal of Physiology. 2021;599(2):453–469. doi: 10.1113/JP278701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kornau H-C, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the post-synaptic density protein PSD-95. Science. 1995;269(5231):1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- 45.Zamanillo D, Sprengel R, Hvalby Ø, Jensen V, Burnashev N, Rozov A, Kaiser KMM, KöSter HJ, Borchardt T, Worley P, LüBke J, et al. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science. 1999;284(5421):1805–1811. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]
- 46.Jia Z, Agopyan N, Miu P, Xiong Z, Henderson J, Gerlai R, Taverna FA, Velumian A, Macdonald J, Carlen P, Abramow-Newerly W, et al. Enhanced LTP in mice deficient in the AMPA receptor GluR2. Neuron. 1996;17(5):945–956. doi: 10.1016/s0896-6273(00)80225-1. [DOI] [PubMed] [Google Scholar]
- 47.Lu W, Shi Y, Jackson AC, Bjorgan K, During MJ, Sprengel R, Seeburg PH, Nicoll RA. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron. 2009;62(2):254–268. doi: 10.1016/j.neuron.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Granger AJ, Shi Y, Lu W, Cerpas M, Nicoll RA. LTP requires a reserve pool of glutamate receptors independent of subunit type. Nature. 2013;493(7433):495–500. doi: 10.1038/nature11775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Penn AC, Zhang CL, Georges F, Royer L, Breillat C, Hosy E, Petersen JD, Humeau Y, Choquet D. Hippocampal LTP and contextual learning require surface diffusion of AMPA receptors. Nature. 2017;549(7672):384–388. doi: 10.1038/nature23658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi S-H, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105(3):331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- 51.Hayashi Y, Shi S-H, Esteban JA, Piccini A, Poncer J-C, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: Requirement for GluR1 and PDZ domain interaction. Science. 2000;287(5461):2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- 52.Stein V, House DRC, Bredt DS, Nicoll RA. Postsynaptic density-95 mimics and occludes hippocampal long-term potentiation and enhances long-term depression. Journal of Neuroscience. 2003;23(13):5503–5506. doi: 10.1523/JNEUROSCI.23-13-05503.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ehrlich I, Malinow R. Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and 9 of 11 STOCKWELL ET AL. experience-driven synaptic plasticity. Journal of Neuroscience. 2004;24(4):916–927. doi: 10.1523/JNEUROSCI.4733-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diering GH, Huganir RL. The AMPA receptor code of synaptic plasticity. Neuron. 2018;100(2):314–329. doi: 10.1016/j.neuron.2018.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watson JF, Pinggera A, Ho H, Greger IH. AMPA receptor anchoring at CA1 synapses is determined by N-terminal domain and TARP γ8 interactions. Nature Communications. 2021;12(1):5083. doi: 10.1038/s41467-021-25281-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Díaz-Alonso J, Morishita W, Incontro S, Simms J, Holtzman J, Gill M, Mucke L, Malenka RC, Nicoll RA. Long-term potentiation is independent of the C-tail of the GluA1 AMPA receptor subunit. Elife. 2020;9:e58042. doi: 10.7554/eLife.58042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou Z, Liu A, Xia S, Leung C, Qi J, Meng Y, Xie W, Park P, Collingridge GL, Jia Z. The C-terminal tails of endogenous GluA1 and GluA2 differentially contribute to hippocampal synaptic plasticity and learning. Nature Neuroscience. 2018;21(1):50–62. doi: 10.1038/s41593-017-0030-z. [DOI] [PubMed] [Google Scholar]
- 58.Tomita S, Chen L, Kawasaki Y, Petralia RS, Wenthold RJ, Nicoll RA, Bredt DS. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. Journal of Cell Biology. 2003;161(4):805–816. doi: 10.1083/jcb.200212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choquet D. Linking nanoscale dynamics of AMPA receptor organization to plasticity of excitatory synapses and learning. Journal of Neuroscience. 2018;38(44):9318–9329. doi: 10.1523/JNEUROSCI.2119-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Opazo P, Sainlos M, Choquet D. Regulation of AMPA receptor surface diffusion by PSD-95 slots. Current Opinion in Neurobiology. 2012;22(3):453–460. doi: 10.1016/j.conb.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 61.Schnell E, Sizemore M, Karimzadegan S, Chen L, Bredt DS, Nicoll RA. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. PNAS. 2002;99(21):13902–13907. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sumioka A, Brown TE, Kato AS, Bredt DS, Kauer JA, Tomita S. PDZ binding of TARPγ-8 controls synaptic transmission but not synaptic plasticity. Nature Neuroscience. 2011;14(11):1410–1412. doi: 10.1038/nn.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schwenk J, Harmel N, Zolles G, Bildl W, Kulik A, Heimrich B, Chisaka O, Jonas P, Schulte U, Fakler B, KlöCker N. Functional proteomics identify cornichon proteins as auxiliary subunits of AMPA receptors. Science. 2009;323(5919):1313–1319. doi: 10.1126/science.1167852. [DOI] [PubMed] [Google Scholar]
- 64.Schwenk J, Baehrens D, Haupt A, Bildl W, Boudkkazi S, Roeper J, Fakler B, Schulte U. Regional diversity and developmental dynamics of the AMPA-receptor proteome in the mammalian brain. Neuron. 2014;84(1):41–54. doi: 10.1016/j.neuron.2014.08.044. [DOI] [PubMed] [Google Scholar]
- 65.Nakagawa T. Structures of the AMPA receptor in complex with its auxiliary subunit cornichon. Science. 2019;366(6470):1259–1263. doi: 10.1126/science.aay2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang D, Watson JF, Matthews PM, Cais O, Greger IH. Gating and modulation of a hetero-octameric AMPA glutamate receptor. Nature. 2021;594(7863):454–458. doi: 10.1038/s41586-021-03613-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu J, Rao P, Clark S, Mitra J, Ha T, Gouaux E. Hippocampal AMPA receptor assemblies and mechanism of allosteric inhibition. Nature. 2021;594(7863):448–453. doi: 10.1038/s41586-021-03540-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pampaloni NP, Plested AJR. Slow excitatory synaptic currents generated by AMPA receptors. The Journal of Physiology. 2022;600(2):217–232. doi: 10.1113/JP280877. [DOI] [PubMed] [Google Scholar]
- 69.Schwenk J, Harmel N, Brechet A, Zolles G, Berkefeld H, Müller CS, Bildl W, Baehrens D, Hüber B, Kulik A, Klöcker N, et al. High-resolution proteomics unravel architecture and molecular diversity of native AMPA receptor complexes. Neuron. 2012;74(4):621–633. doi: 10.1016/j.neuron.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 70.Von Engelhardt J. AMPA receptor auxiliary proteins of the CKAMP family. International Journalof Molecular Sciences. 2019;20(6):1460. doi: 10.3390/ijms20061460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Klaassen RV, Stroeder J, Coussen F, Hafner A-S, Petersen JD, Renancio C, Schmitz LJM, Normand E, Lodder JC, Rotaru DC, Rao-Ruiz P, et al. Shisa6 traps AMPA receptors at postsynaptic sites and prevents their desensitization during synaptic activity. Nature Communications. 2016;7:10682. doi: 10.1038/ncomms10682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sumioka A, Yan D, Tomita S. TARP phosphorylation regulates synaptic AMPA receptors through lipid bilayers. Neuron. 2010;66(5):755–767. doi: 10.1016/j.neuron.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hafner A-S, Penn AC, Grillo-Bosch D, Retailleau N, Poujol C, Philippat A, Coussen F, Sainlos M, Opazo P, Choquet D. Lengthening of the stargazin cytoplasmic tail increases synaptic transmission by promoting interaction to deeper domains of PSD-95. Neuron. 2015;86(2):475–489. doi: 10.1016/j.neuron.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 74.Tomita S, Stein V, Stocker TJ, Nicoll RA, Bredt DS. Bidirectional synaptic plasticity regulated by phosphorylation of stargazin-like TARPs. Neuron. 2005;45(2):269–277. doi: 10.1016/j.neuron.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 75.Park J, Chávez AE, Mineur YS, Morimoto-Tomita M, Lutzu S, Kim KS, Picciotto MR, Castillo PE, Tomita S. CaMKII phosphorylation of TARPγ-8 is a mediator of LTP and learning and memory. Neuron. 2016;92(1):75–83. doi: 10.1016/j.neuron.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zeng M, Díaz-Alonso J, Ye F, Chen X, Xu J, Ji Z, Nicoll RA, Zhang M. Phase separation-mediated TARP/MAGUK complex condensation and AMPA receptor synaptic transmission. Neuron. 2019;104(3):529–543.:e6. doi: 10.1016/j.neuron.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sheng N, Bemben MA, Díaz-Alonso J, Tao W, Shi YS, Nicoll RA. LTP requires postsynaptic PDZ-domain interactions with glutamate receptor/auxiliary protein complexes. PNAS. 2018;115(15):3948–3953. doi: 10.1073/pnas.1800719115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hosokawa T, Liu P-W, Cai Q, Ferreira JS, Levet F, Butler C, Sibarita J-B, Choquet D, Groc L, Hosy E, Zhang M, et al. CaMKII activation persistently segregates postsynaptic proteins via liquid phase separation. Nature Neuroscience. 2021;24(6):777–785. doi: 10.1038/s41593-021-00843-3. [DOI] [PubMed] [Google Scholar]
- 79.Heine M, Groc L, Frischknecht R, BéïQue J-C, Lounis B, Rumbaugh G, Huganir RL, Cognet L, Choquet D. Surface mobility of postsynaptic AMPARs tunes synaptic transmission. Science. 2008;320(5873):201–205. doi: 10.1126/science.1152089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hayashi Y. Molecular mechanism of hippocampal long-term potentiation—Towards multiscale understanding of learning and memory. Neuroscience Research. 2021;175:3–15. doi: 10.1016/j.neures.2021.08.001. [DOI] [PubMed] [Google Scholar]
- 81.Shi Y, Lu W, Milstein AD, Nicoll RA. The stoichiometry of AMPA receptors and TARPs varies by neuronal cell type. Neuron. 2009;62(5):633–640. doi: 10.1016/j.neuron.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sia G-M, Béïque J-C, Rumbaugh G, Cho R, Worley PF, Huganir RL. Interaction of the N-terminal domain of the AMPA receptor GluR4 subunit with the neuronal pentraxin NP1 mediates GluR4 synaptic recruitment. Neuron. 2007;55(1):87–102. doi: 10.1016/j.neuron.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 83.Pelkey KA, Barksdale E, Craig MT, Yuan X, Sukumaran M, Vargish GA, Mitchell R-M, Wyeth MS, Petralia RS, Chittajallu R, Karlsson RM, et al. Pentraxins coordinate excitatory synapse maturation and circuit integration of parvalbumin interneurons. Neuron. 2015;85(6):1257–1272. doi: 10.1016/j.neuron.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chang MC, Park JM, Pelkey KA, Grabenstatter HL, Xu D, Linden DJ, Sutula TP, Mcbain CJ, Worley PF. Narp regulates homeostatic scaling of excitatory synapses on parvalbumin-expressing interneurons. Nature Neuroscience. 2010;13(9):1090–1097. doi: 10.1038/nn.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jiang C-H, Wei M, Zhang C, Shi YS. The amino-terminal domain of GluA1 mediates LTP maintenance via interaction with neuroplastin-65. PNAS. 2021;118(9):e2019194118. doi: 10.1073/pnas.2019194118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shi S-H, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284(5421):1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- 87.Graves AR, Roth RH, Tan HL, Zhu Q, Bygrave AM, Lopez-Ortega E, Hong I, Spiegel AC, Johnson RC, Vogelstein JT, Tward DJ, et al. Visualizing synaptic plasticity in vivo by large-scale imaging of endogenous AMPA receptors. Elife. 2021;10 doi: 10.7554/eLife.66809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mack V, Burnashev N, Kaiser KMM, Rozov A, Jensen V, Hvalby Ø, Seeburg PH, Sakmann B, Sprengel R. Conditional restoration of hippocampal synaptic potentiation in Glur-A-deficient mice. Science. 2001;292(5526):2501–2504. doi: 10.1126/science.1059365. [DOI] [PubMed] [Google Scholar]
- 89.Nakagawa T, Cheng Y, Ramm E, Sheng M, Walz T. Structure and different conformational states of native AMPA receptor complexes. Nature. 2005;433(7025):545–549. doi: 10.1038/nature03328. [DOI] [PubMed] [Google Scholar]
- 90.Dürr KL, Chen L, Stein RA, De Zorzi R, Folea IM, Walz T, Mchaourab HS, Gouaux E. Structure and dynamics of AMPA receptor GluA2 in resting, pre-open, and desensitized states. Cell. 2014;158(4):778–792. doi: 10.1016/j.cell.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meyerson JR, Kumar J, Chittori S, Rao P, Pierson J, Bartesaghi A, Mayer ML, Subramaniam S. Structural mechanism of glutamate receptor activation and desensitization. Nature. 2014;514(7522):328–334. doi: 10.1038/nature13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Krieger J, Bahar I, Greger IH. Structure, dynamics, and allosteric potential of ionotropic glutamate receptor N-terminal domains. Biophysical Journal. 2015;109(6):1136–1148. doi: 10.1016/j.bpj.2015.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dutta A, Krieger J, Lee JY, Garcia-Nafria J, Greger IH, Bahar I. Cooperative dynamics of intact AMPA and NMDA glutamate receptors: similarities and subfamily-specific differences. Structure (London, England) 2015;23(9):1692–1704. doi: 10.1016/j.str.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Möykkynen T, Coleman SK, Semenov A, Keinänen K. The N-terminal domain modulates α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor desensitization. Journal of Biological Chemistry. 2014;289(19):13197–13205. doi: 10.1074/jbc.M113.526301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sumino A, Sumikama T, Zhao Y, Flechsig H, Umeda K, Kodera N, Hattori M, Shibata M. High-speed AFM reveals fluctuations and dimer splitting of the N-terminal domain of GluA2-y2. BioRxiv. 2023 doi: 10.1101/2023.12.19.572481. [DOI] [Google Scholar]
- 96.Rossmann M, Sukumaran M, Penn AC, Veprintsev DB, Babu MM, Greger IH. Subunit-selective N-terminal domain associations organize the formation of AMPA receptor heteromers. Embo Journal. 2011;30(5):959–971. doi: 10.1038/emboj.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhao H, Lomash S, Chittori S, Glasser C, Mayer ML, Schuck P. Preferential assembly of heteromeric kainate and AMPA receptor amino terminal domains. Elife. 2017;6:e32056. doi: 10.7554/eLife.32056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Herguedas B, Watson JF, Ho H, Cais O, García-Nafría J, Greger IH. Architecture of the heteromeric GluA1/2 AMPA receptor in complex with the auxiliary subunit TARP γ8. Science. 2019;364(6438):eaav9011. doi: 10.1126/science.aav9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao Y, Chen S, Swensen AC, Qian W-J, Gouaux E. Architecture and subunit arrangement of native AMPA receptors elucidated by cryo-EM. Science. 2019;364(6438):355–362. doi: 10.1126/science.aaw8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Frischknecht R, Heine M, Perrais D, Seidenbecher CI, Choquet D, Gundelfinger ED. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nature Neuroscience. 2009;12(7):897–904. doi: 10.1038/nn.2338. [DOI] [PubMed] [Google Scholar]
- 101.Yuzaki M. Two classes of secreted synaptic organizers in the central nervous system. Annual Review of Physiology. 2018;80:243–262. doi: 10.1146/annurev-physiol-021317-121322. [DOI] [PubMed] [Google Scholar]
- 102.Matthews PM, Pinggera A, Kampjut D, Greger IH. Biology of AMPA receptor interacting proteins—From biogenesis to synaptic plasticity. Neuropharmacology. 2021;197:108709. doi: 10.1016/j.neuropharm.2021.108709. [DOI] [PubMed] [Google Scholar]
- 103.Pandya NJ, Seeger C, Babai N, Gonzalez-Lozano MA, Mack V, Lodder JC, Gouwenberg Y, Mansvelder HD, Danielson UH, Li KW, Heine M, et al. Noelin1 affects lateral mobility of synaptic AMPA receptors. Cell Reports. 2018;24(5):1218–1230. doi: 10.1016/j.celrep.2018.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boudkkazi S, Schwenk J, Nakaya N, Brechet A, Kollewe A, Harada H, Bildl W, Kulik A, Dong L, Sultana A, Zolles G, et al. A Noelin-organized extracellular network of proteins required for constitutive and context-dependent anchoring of AMPA-receptors. Neuron. 2023;111(16):2544–2556.:e9. doi: 10.1016/j.neuron.2023.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Uemura T, Lee S-J, Yasumura M, Takeuchi T, Yoshida T, Ra M, Taguchi R, Sakimura K, Mishina M. Trans-synaptic interaction of GluRdelta2 and Neurexin through Cbln1 mediates synapse formation in the cerebellum. Cell. 2010;141(6):1068–1079. doi: 10.1016/j.cell.2010.04.035. [DOI] [PubMed] [Google Scholar]
- 106.Matsuda K, Miura E, Miyazaki T, Kakegawa W, Emi K, Narumi S, Fukazawa Y, Ito-Ishida A, Kondo T, Shigemoto R, Watanabe M, et al. Cbln1 is a ligand for an orphan glutamate receptor delta2, a bidirectional synapse organizer. Science. 2010;328(5976):363–368. doi: 10.1126/science.1185152. [DOI] [PubMed] [Google Scholar]
- 107.Matsuda K, Yuzaki M. Cbln family proteins promote synapse formation by regulating distinct neurexin signaling pathways in various brain regions. European Journal of Neuroscience. 2011;33(8):1447–1461. doi: 10.1111/j.1460-9568.2011.07638.x. [DOI] [PubMed] [Google Scholar]
- 108.Matsuda K, Budisantoso T, Mitakidis N, Sugaya Y, Miura E, Kakegawa W, Yamasaki M, Konno K, Uchigashima M, Abe M, Watanabe I, et al. Transsynaptic modulation of kainate receptor functions by C1q-like proteins. Neuron. 2016;90(4):752–767. doi: 10.1016/j.neuron.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 109.Hruska M, Cain RE, Dalva MB. Nanoscale rules governing the organization of glutamate receptors in spine synapses are subunit specific. Nature Communications. 2022;13(1):920. doi: 10.1038/s41467-022-28504-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Goncalves J, Bartol TM, Camus C, Levet F, Menegolla AP, Sejnowski TJ, Sibarita J-B, Vivaudou M, Choquet D, Hosy E. Nanoscale co-organization and coactivation of AMPAR, NMDAR, and mGluR at excitatory synapses. PNAS. 2020;117(25):14503–14511. doi: 10.1073/pnas.1922563117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kellermayer B, Ferreira JS, Dupuis J, Levet F, Grillo-Bosch D, Bard L, Linarès-Loyez J, Bouchet D, Choquet D, Rusakov DA, Bon P, et al. Differential nanoscale topography and functional role of GluN2-NMDA receptor subtypes at glutamatergic synapses. Neuron. 2018;100(1):106–119.:e7. doi: 10.1016/j.neuron.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 112.Savtchenko LP, Rusakov DA. Moderate AMPA receptor clustering on the nanoscale can efficiently potentiate synaptic current. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 2014;369(1633):20130167. doi: 10.1098/rstb.2013.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Foster KA, Kreitzer AC, Regehr WG. Interaction of postsynaptic receptor saturation with presynaptic mechanisms produces a reliable synapse. Neuron. 2002;36(6):1115–1126. doi: 10.1016/s0896-6273(02)01106-6. [DOI] [PubMed] [Google Scholar]
- 114.Raghavachari S, Lisman JE. Properties of quantal transmission at CA1 synapses. Journal of Neurophysiology. 2004;92(4):2456–2467. doi: 10.1152/jn.00258.2004. [DOI] [PubMed] [Google Scholar]
- 115.Südhof TC. The presynaptic active zone. Neuron. 2012;75(1):11–25. doi: 10.1016/j.neuron.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Heine M, Holcman D. Asymmetry between pre- and post-synaptic transient nanodomains shapes neuronal communication. Trends in Neuroscience (Tins) 2020;43(3):182–196. doi: 10.1016/j.tins.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 117.Tarusawa E, Matsui K, Budisantoso T, Molnár E, Watanabe M, Matsui M, Fukazawa Y, Shigemoto R. Input-specific intrasynaptic arrangements of ionotropic glutamate receptors and their impact on postsynaptic responses. Journal of Neuroscience. 2009;29(41):12896–12908. doi: 10.1523/JNEUROSCI.6160-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ramsey AM, Tang A-H, Legates TA, Gou X-Z, Carbone BE, Thompson SM, Biederer T, Blanpied TA. Subsynaptic positioning of AMPARs by LRRTM2 controls synaptic strength. Science Advances. 2021;7(34):eabf3126. doi: 10.1126/sciadv.abf3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Compans B, Choquet D, Hosy E. Review on the role of AMPA receptor nano-organization and dynamic in the properties of synaptic transmission. Neurophotonics. 2016;3(4):041811. doi: 10.1117/1.NPh.3.4.041811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Constals A, Penn AC, Compans B, Toulmé E, Phillipat A, Marais S, Retailleau N, Hafner A-S, Coussen F, Hosy E, Choquet D. Glutamate-induced AMPA receptor desensitization increases their mobility and modulates short-term plasticity through unbinding from Stargazin. Neuron. 2015;85(4):787–803. doi: 10.1016/j.neuron.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 121.Coombs ID, Maclean DM, Jayaraman V, Farrant M, Cull-Candy SG. Dual effects of TARP γ-2 on glutamate efficacy can account for AMPA receptor autoinactivation. Cell Reports. 2017;20(5):1123–1135. doi: 10.1016/j.celrep.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Baranovic J, Plested A., Jr Auxiliary subunits keep AMPA receptors compact during activation and desensitization. Elife. 2018;7 doi: 10.7554/eLife.40548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Herguedas B, Kohegyi BK, Dohrke J-N, Watson JF, Zhang D, Ho H, Shaikh SA, Lape R, Krieger JM, Greger IH. Mechanisms underlying TARP modulation of the GluA1/2-γ8 AMPA receptor. Nature Communications. 2022;13(1):734. doi: 10.1038/s41467-022-28404-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Twomey EC, Yelshanskaya MV, Grassucci RA, Frank J, Sobolevsky AI. Structural bases of desensitization in AMPA receptor-auxiliary subunit complexes. Neuron. 2017;94(3):569–580.:e5. doi: 10.1016/j.neuron.2017.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen S, Zhao Y, Wang Y, Shekhar M, Tajkhorshid E, Gouaux E. Activation and desensitization mechanism of AMPA receptor-TARP complex by cryo-EM. Cell. 2017;170(6):1234–1246.:e14. doi: 10.1016/j.cell.2017.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nowacka A, Getz AM, Zieger HL, Bessa-Neto D, Breillat C, Daburon S, Lemoigne C, Marais S, Ducros M, Penn AC, Sainlos M, et al. Synapse specific and plasticity-regulated AMPAR mobility tunes synaptic integration. BioRxiv. 2024 doi: 10.1101/2024.03.19.584837. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data referenced in Figure 2 are openly available at https://doi.org/10.1038/s41467-021-25281-4.