Abstract
Social rewards represent a strong driving force behind decisions and behaviors. Previous research suggests that the processing of a reward depends on the initial state of the individual. However, empirical research in humans on the influence of motivational states on reward processing is scant, especially for rewards of social nature. In the present study, we aimed at investigating how aversive and appetitive motivation affects the processing of social rewards, such as interpersonal touch. Participants (n = 102) were assigned to an appetitive (positive) or aversive (negative) motivational state condition (via modified versions of the Trier Social Stress Test) or to a control condition. After the state induction, their (a) self-reports of wanting and liking, (b) effort, and (c) hedonic facial reactions during anticipation and consumption of interpersonal touch, were measured. Participants in the aversive group showed higher subjective wanting of interpersonal touch, but no changes in subjective liking, compared to the control group. The aversive group also showed stronger positive hedonic facial reactions during reward anticipation, reflecting stronger anticipatory pleasure. No significant effects were found for the appetitive group. The results indicate that, after being exposed to an aversive experience, the motivation to obtain interpersonal touch, as well as the associated anticipatory pleasure, increase, without a corresponding change in liking during or after its consumption. The findings point to differential state-dependent effects on the processing of social rewards, possibly due to the action of different neurobiological systems regulating reward anticipation and consumption.
Keywords: motivational states, interpersonal touch, social reward, fEMG, wanting, liking
Introduction
We live in a social world and we are biologically driven to seek social contact with conspecifics (Baumeister and Leary, 1995). However, very different types of motivation can push us to look for proximity with others. Thinking of our daily life, the need for contact with our significant others can arise in situations characterized by negative affect and suffering (e.g., looking for being comforted by a friend after a hurtful break up), as well as positive emotions (e.g., celebrating our success and achievements with friends and family). In the first case, social contact helps us in alleviating negative emotions, while, in the latter, it represents a mean to get pleasure and joy, as well as to form and maintain social bonds.
Interpersonal touch, such as caressing and grooming, is one of the most archaic forms of social contact, common to most mammalian species and a fundamental component of attachment and affiliative behaviors (Dunbar, 2010). In humans, interpersonal touch can both evoke positive emotions and serve as a buffer for stress and negative affect, thus representing an attractive reward (Morrison et al., 2010; Ellingsen et al., 2016). Accordingly, interpersonal touch has been associated with strong pleasurable feelings (Morrison et al., 2010) and increased activity in brain circuits involved in reward processing (Perini et al., 2015; Sailer et al., 2016). In relation to stress and negative states, animal and human research has shown that interpersonal touch can be soothing, by acting on different physiological mechanisms (e.g., reducing heart rate and regulating the hypothalamic-pituitary-adrenocortical axis) and by producing a general effect of deep relaxation (Aureli et al., 1999; Dunbar, 2010; Morrison, 2016). In humans in particular, social support in the form of physical contact, such as caressing and holding hands, has been shown to decrease social pain (von Mohr et al., 2017; Morese et al., 2019), physical pain (Coan et al., 2006; Goldstein et al., 2018; López-Solà et al., 2019), and psychosocial stress (Ditzen et al., 2007), and to be generally more effective than verbal support or other kinds of aid in regulating negative emotions (Morese et al., 2019).
Therefore, interpersonal touch constitutes a powerful rewarding stimulus, which is, in appetitive contexts, positively reinforced by its evoked pleasure, and, in aversive contexts, negatively reinforced, by down-regulating negative emotions (Loseth et al., 2014). However, how these different contexts influence the processing of interpersonal touch remains largely unknown.
On a general level, reward processing involves at least two neurobiologically and psychologically distinct components: a predominantly dopamine-mediated ‘wanting’ or incentive salience (the motivation to mobilize effort to obtain the reward, associated to reward anticipation), and a predominantly opioid-mediated ‘liking’ (the hedonic response, evoked by reward consumption) (Berridge et al., 2009). Wanting and liking are strongly related, but under certain circumstances, wanting of a reward can be selectively enhanced, without a parallel change in the experienced liking. Accordingly, animal and human research has shown that acute stress is linked to increased reward motivation, mediated by increased dopamine levels in the brain reward circuitry, but unaffected/blunted hedonic responses to reward consumption (for a review see Bloomfield et al., 2019; Ironside et al., 2018). In humans, this preferential effect of acute stress on reward anticipation, rather than consumption, has been shown for food and monetary rewards, both at the behavioral and neural level (Kumar et al., 2014; Lewis et al., 2014; Pool et al., 2015; Boyle et al., 2020). Similarly, enhanced reward-related brain activity during reward anticipation (Wagner et al., 2012) and attentional bias to food cues (Hepworth et al., 2010) has been observed following negative mood induction. The relationship between positive mood and reward processing has been investigated far less, and the evidence remains equivocal. Positive mood induction has indeed been related to enhanced reward-related neural activity to anticipated monetary rewards (Young and Nusslock, 2016) and increased orientation towards reward-related words (Tamir and Robinson, 2007), but also to reduced selective orientation towards rewarding images (Wadlinger and Isaacowitz, 2006) and reduced activity in the reward circuitry during monetary reward anticipation (Green et al., 2019).
In summary, previous research has shown that aversive motivational states can selectively increase wanting (anticipatory motivational responses) of food and monetary rewards without affecting the liking component (consummatory hedonic responses). Similarly, being in an appetitive state can modulate reward anticipation, but its effect on reward consumption has not been tested yet. Notably, empirical research on the state-dependent effects on wanting and liking of social rewards is completely lacking.
To fill this gap in the literature, the present study aimed at investigating how aversive and appetitive motivational states affect the processing of social rewards. Motivational states were induced in 102 participants by means of variants of the Trier Social Stress Test paradigm (TSST; Kirschbaum et al., 1993), tailored to elicit positive, negative or neutral emotions. Subjective ratings of wanting and liking, real effort and facial hedonic reactions (via recording of the corrugator supercilii and zygomaticus major muscles) to anticipation and consumption of interpersonal touch, with different levels of pleasantness, were then assessed using a recently developed experimental paradigm (Korb et al., 2020a, 2020b).
Based on the animal and human literature, we hypothesized that participants in the aversive motivational state condition would seek social contact to decrease negative affect and restore homeostasis. We predicted wanting of interpersonal touch to increase, without a corresponding enhancement of liking, compared to the neutral state condition. In particular, we expected higher subjective ratings of wanting, and greater effort applied to obtain the announced reward, but no change in subjective ratings of liking after reward consumption. Previous studies have shown that both anticipation and consumption of rewarding stimuli, including interpersonal touch, are associated with relaxation of the corrugator (frowning) muscle and, to a lesser extent, with activation of the zygomaticus (smiling) muscle (Pawling et al., 2017; Mayo et al., 2018; Ree et al., 2019, 2020; Korb et al., 2020a, 2020b). Based on this and on the fact that hedonic facial reactions during reward anticipation reflect anticipatory pleasure in a period commonly associated with wanting (Korb et al., 2020a; Selby et al., 2020), we expected greater relaxation of the corrugator (frowning) muscle and/or greater activation of the zygomaticus (smiling) during reward anticipation (anticipatory liking) but not consumption (consummatory liking), compared to the neutral state condition. On the other hand, we hypothesized that participants in the appetitive state condition would seek social contact to gain pleasure. Given the lack of directional evidence, we did not predict a dissociation between wanting and liking for this group, but rather a general increase of both, compared to the neutral state condition. In particular, we expected increased subjective ratings of wanting and liking, greater effort, and changes in hedonic facial reactions (greater relaxation of corrugator and/or activation of zygomaticus) during both reward anticipation and consumption, compared to the neutral state condition. Based on previous evidence showing increased motivation towards highly palatable high-rewarding, as compared to unpalatable low-rewarding, food stimuli in stressed individuals (for a review see Adam and Epel, 2007; Sinha, 2018; Ulrich-Lai et al., 2015), we expected these effects to be more pronounced for the most preferred reward.
Experimental Procedures
Sample
The sample of the study consisted of 102 adult females, randomly assigned to one of the three experimental groups (34/group): appetitive, aversive, control. Each participant was accompanied by a close female friend, who was supposedly in charge of delivering the tactile stimulation (see Social Reward task section). In order to avoid possible sexual connotations of the interpersonal touch, only heterosexual female participants paired with a female friend were tested in this study.
Based on sample sizes of previous behavioral studies investigating the effect of stress on monetary and food rewards (Pool et al., 2015: 18 stress group and 18 control; Boyle et al., 2020: 34 stress group and 17 control), we aimed at a minimum sample size of 30 participants per group. The inclusion of only female participants was motivated by the fact that (1) same-gender touch is more frequent in women than men, and is generally perceived as more pleasant and less discomforting by women compared to men (Stier and Hall, 1984; Suvilehto et al., 2015) and (2) physiological responses to stress may differ a lot between men and women, therefore including only one gender is a common procedure to reduce variability. One participant in the aversive group was excluded from the analyses because she was fasting at the time of testing (hindering reliable analysis of saliva samples and possibly altering reward processing and effort capacity). Participants were recruited via flyers posted on social networks (e.g., Facebook groups) and via a database of potential participants of the University of Vienna. All participants reported to be right-handed and to be free of psychiatric and neurological disorders (see Supplementary Material for the full list of exclusion criteria).
The three experimental groups did not differ significantly (Table 1) in terms of age, relationship closeness with their friend, autistic traits, general anxiety and social anxiety, social touch appreciation, depression, and BMI (see Trait questionnaires for more details on the questionnaires used).
Table 1. Characteristics of the participants in the three experimental groups.
| N | 34 | 33 | 34 | |
| Age | 23.1 (3.8) | 21.7 (2.7) | 22.3 (3.0) | p = 0.18 |
| RCI | 12.5 (4.5) | 12.9 (4.8) | 12.9 (4.6) | p = 0.89 |
| AQ-k | 6.0 (3.9) | 5.8 (3.0) | 6.7 (3.2) | p = 0.52 |
| LSAS | 35.6 (16.9) | 39.9 (20.9) | 44.6 (21.3) | p = 0.19 |
| STQ | 26.3 (8.8) | 27.1 (9.3) | 28.0 (10.3) | p = 0.76 |
| BDI-II | 6.5 (7.6) | 6.7 (5.7) | 5.7 (4.7) | p = 0.82 |
| STAI | 40.6 (11.0) | 38.8 (10.9) | 39.2 (9.1) | p = 0.74 |
| BMI | 21.4 (2.2) | 21.2 (1.8) | 21.8 (2.6) | p = 0.57 |
RCI = Relationship Closeness Index; AQ-k = short version of the Autism Quotient; LSAS = Liebowitz Social Anxiety Scale; STQ = Social Touch Questionnaire; BDI-II = Beck Depression Inventory; STAI = State Trait Anxiety Inventory; BMI = Body Mass Index.
The study was approved by the Ethics Committee of the University of Vienna and was performed in line with the Declaration of Helsinki (World Medical Association, 2013). All participants signed an informed consent before taking part in the study. Participants and their friends received a monetary compensation of 25 € and 10 € respectively.
Procedure
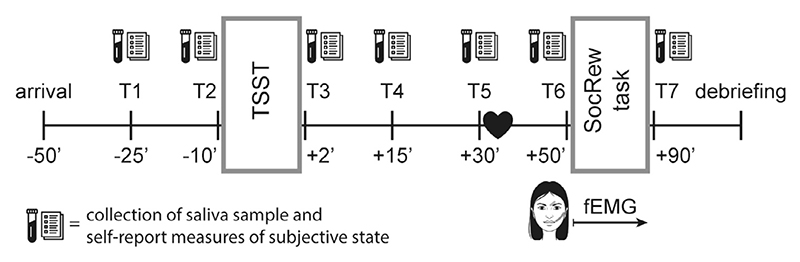
In order to control for cortisol diurnal fluctuations, all participants arrived alone at the laboratory either at 12:00 or at 15:30 (Labuschagne et al., 2019). After approximately 25 min from arrival, in which participants filled in the Relationship Closeness Inventory (Berscheid et al., 1989) and read magazines provided by the experimenter, the appetitive, aversive, or neutral motivational state was induced via modified versions of the TSST (Kirschbaum et al., 1993). After attaching of the facial electromyography (EMG) electrodes, participants performed the Social Reward task, which started 50 min after the state induction. Each participants’ friend was instructed to arrive 20 min before the start of the task, supposedly to be trained for the touch administration. Participants met their friends only immediately prior to the starting the Social Reward task.
In order to verify changes in the motivational state, physiological and self-report measures were collected at regular intervals, throughout the entire experimental session (Fig. 1). In particular, measures of subjective stress, anxiety (STAI; Spielberger et al., 1970), positive and negative affect (PANAS; Watson et al., 1988) were collected at baseline (T1), at the end the preparation phase of the TSST (T2), immediately after the TSST completion (T3), as well as immediately before and after the Social Reward Task (T6, T7). During the preparation phase of the TSST (T2), anticipatory cognitive appraisal (PASA; Gaab et al., 2005) was also assessed. Subjective stress was additionally measured during the interval between the motivational state induction and the Social Reward task (T4, T5). Saliva samples were collected via passive drool method at all seven time points (T1–T7), and analyzed in order to detect changes in salivary cortisol and alpha-amylase, which are respectively considered physiological indicators of hypothalamic–pituitary–adrenal and autonomic nervous system activity (see Supplementary Material for a detailed description of the used self-report and physiological measures, as well as for details regarding the determination of cortisol and alpha-amylase concentration).
Fig. 1.
Study procedure and timeline. The first rectangle represents the motivational state manipulation, i.e. speech and math task of aversive/appetitive/placebo TSST. The second rectangle represents the Social Reward task. The black heart represents the arrival of the participant´s friend. TSST, Trier Social Stress Test; fEMG, facial electromyography.
After completing the task, participants were debriefed about the employed deceptions and received monetary compensation for their time.
State induction
Two different motivational states were induced for the appetitive and aversive groups, and compared to the control group. Participants in both the appetitive and the aversive groups were instructed to deliver a five-minutes free speech in front of an evaluating panel consisting of two members (one female and one male). During the speech, participants had to apply for an open job position (their dream job) and they had to convince the panel of being the ideal candidates for that position. After the speech, participants were asked to perform a mental arithmetic task lasting five minutes.
Importantly, the appetitive and aversive conditions differed in the type of panel and the type of feedback the participants were confronted with, as well as in the level of difficulty of the math task. In doing so, we aimed at creating two situations that were similarly challenging and engaging for the participants, but leading to opposite affective states: positive in the case of the appetitive-TSST and negative in the case of the aversive-TSST (see Akinola and Mendes, 2008 for a similar procedure).
In particular, in the aversive-TSST participants delivered their speech in front of an evaluating panel, whose members were presented as psychologists who are experts in observation and analysis of verbal and non-verbal behavior. They were wearing white lab coats and were carefully trained to give an impression of authority and to act in a cold and reserved manner, showing negative non-verbal feedback (e.g., shaking of the head, frowning) in response to participants’ behavior. Moreover, participants were told that, in order to later analyze their performance and non-verbal behavior, the interview was going to be video recorded via a camera located behind the panel. If participants stopped to talk before the end of the five minutes period, the panel was instructed to wait for 20 s and then say “You still have time, please continue with your speech”. In case the participant was unable to continue after a few breaks, standardized questions were employed. Subsequently, participants were asked to count backwards from 2043 in steps of 17 as fast and as accurate as possible. For every mistake, the panel asked participants to start again from 2043.
In the appetitive-TSST, participants received the same instructions as in the aversive-TSST. However, the judging panel behaved in a very friendly and warm manner, by showing interest and asking follow-up questions. Moreover, positive non-verbal feedbacks, like smiling and nodding, were implemented. In this condition, the panel members were not wearing lab coats and the video-camera was not present in the room. In order to induce positive feelings of success and satisfaction in the participants, the math task was tailored to each individual’s abilities, and consisted of different subtasks with increasing difficulty: (1) Count backward from 250 to zero in steps of five; (2) Count backward from 250 to zero in steps of six; (3) Count backward from 543 to zero in steps of 13; (4) Count backward 2043 to zero in steps of 17. Every time the participants completed a subtask, they received a positive feedback from the panel and were instructed about the next subtask. When they committed a mistake, participants were informed about the correct answer by the panel and asked to continue in a friendly tone.
In the placebo-TSST (Het et al., 2009), which served as a control condition, participants were instructed to deliver a free speech aloud, alone in the room. During the speech, they had to describe their favourite book or movie. Following the speech, participants were asked to count forward from zero in steps of five.
After completion of the TSST, all participants were asked to rate their satisfaction of the speech and math performances using a VAS ranging from 0 (not at all) to 100 (very much).
Before their speech, participants in all groups underwent a preparation phase (10 min), during which they were asked to prepare for the speech by taking notes for three minutes, fill out some questionnaires and provide a saliva sample. To prolong the effects of the induced state, participants in the aversive and appetitive groups were informed that they were going to repeat the aversive/appetitive-TSST at the end of the testing session.
Social reward task
A modified version of a recently developed paradigm was used to assess explicit and implicit motivational and hedonic responses to social rewards (Korb et al., 2020a, 2020b). Social rewards consisted of gentle caresses that were delivered at three different speeds (6 cm/s, 21 cm/s and 27 cm/s) over a previously-marked nine–cm area on participants’ forearm (measurement started from the wrist towards the elbow). Velocity was varied in order to deliver touch in three different level of pleasantness (High, Low, Very Low). Speeds of touch were chosen based on (1) previous literature (Löken et al., 2009), indicating that, for the majority of individuals (but see Croy et al., 2020), pleasantness of touch is high between 1 and 10 cm/s, and decreases between 10 and 30 cm/s; and (2) extensive piloting, suggesting that such speeds give rise to three different level of pleasantness. Their suitability has been confirmed in two independent studies from our group (Korb et al., 2020a, 2020b). Participants were informed that their friend was going to apply the caresses.
In reality, in order to minimize variability in the delivery of the interpersonal touch, caresses were always performed by a female experimenter, moving her index and middle fingers back and forth in the marked area of the forearm. Participants and experimenter/friend were separated by a curtain, which prevented participants to see who was delivering the touch1. All experimenters were extensively trained in using the same pressure for all tactile stimuli and, during the task, they were guided by auditory rhythms, which matched the frequency of the stimulation, over headphones.
Task Preparation
As recently shown (Croy et al., 2020), individual differences exist in the liking of touch. In order to take this into account, prior to starting the Social Reward task, individual preferences for the different types of touch were assessed and used to individually calibrate the task. Participants experienced all stimuli three times and ranked them from the most to the least preferred. Stimuli were presented in a semi-random fashion and the ranking was used to categorize the three touches as “High” (most liked touch), “Low” and “Very Low” (least liked touch) rewards. Participants’ maximum voluntary contraction (MVC) was obtained by asking them to squeeze a hand-dynamometer (HD-BTA, Vernier Software & Technology, USA) at their maximum force for three consecutive times (three seconds each) using their right hand. The maximum value recorded represented the individual MVC (expressed in Newton), and was used to calibrate the hand-dynamometer for the Social Reward task. After completing the ranking and the MVC procedures, participants received instructions for the Social Reward task and completed two training trials.
Main task
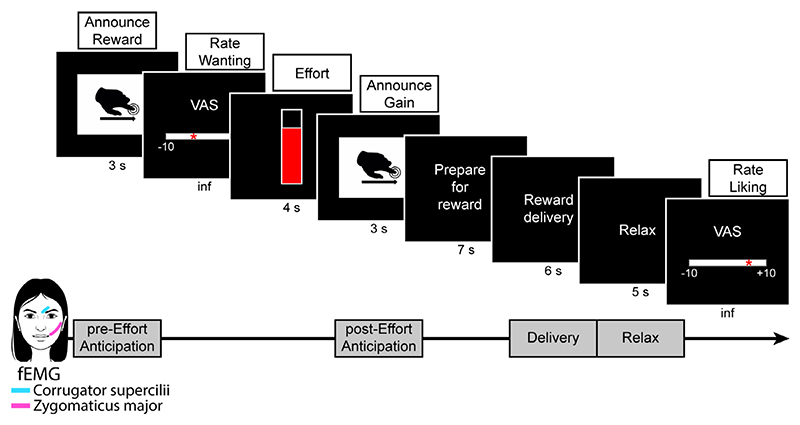
The Social Reward task consisted of two blocks of 16 trials separated by a five-minutes break (for a detailed description of the task see Korb et al., 2020a, 2020b). In each trial (Fig. 2), a cue announced the type of touch (High or Low reward) that could be gained (Pre-Effort Anticipation, 3 s). Participants rated their subjective wanting of the stimulus without time limit on a visual analogue scale (VAS) ranging from −10 (not at all) to +10 (very much). To obtain the announced reward, participants had to squeeze the hand-dynamometer (4 s). The applied force was expressed as percentage of the participants’ MVC, averaged with a 1-second sliding window, and translated into the probability of obtaining the announced reward (0–100%). An online visual-feedback was presented on the screen during pressing, indicating the level of effort exerted. In the Post-Effort Anticipation phase, a picture indicating the obtained reward (initially announced reward or, in case of insufficient effort, the Very Low reward) was shown (3 s), followed by the tactile stimulation (Delivery, 6 s) and a relaxation phase (Relax, 5 s). Immediately afterwards, participants rated their liking of the stimulus on a VAS ranging from −10 (not at all) to +10 (very much). The inter-trial interval varied randomly between three and four seconds. At the end of the Social Reward task, participants’ MVC was measured again. The task was run on a desktop computer with Windows XP using MATLAB 2014b and the Cogent 2000 and Cogent Graphics toolboxes, and presented on an LCD monitor with a resolution of 1280 × 1024 pixels.
Fig. 2.
Main elements in each trial of the Social Reward task. “Inf” (under ratings) indicates that the participants had no time limit to express their ratings. VAS, visual analogue scale; fEMG, facial electromyography.
Facial EMG
Facial EMG was recorded throughout the entire task, using Ag/AgCl electrodes attached bipolarly according to guidelines on the left corrugator supercilii and the left zygomaticus major muscles (Fridlund and Cacioppo, 1986). A ground electrode was attached to the forehead, next to the hairline. Before electrode application, participants’ face area was prepared using alcohol, water and an abrasive paste. Impedance was kept below 20 kΩ. EMG data were recorded using the TMS International Refa8 amplifier and the Portilab2 software (www.tmsi.com), with a sample rate of 1024 Hz.
Trait questionnaires2
Before coming to the laboratory, participants filled in an online survey consisting of socio-demographical and general health questions and questionnaires assessing autism spectrum disorders (Short version of the German Autism Spectrum Quotient, AQ-k; Freitag et al., 2007) social touch appreciation (Social touch questionnaire, STQ; Wilhelm et al., 2001), depression (German version of the Beck Depression Inventory II, BDI-II; Kühner et al., 2007), anxiety (STAI; Spielberger et al., 1970), and social anxiety (LSAS; Heimberg et al., 1999). On the day of testing, participants completed the Relationship Closeness Inventory (RCI; Berscheid et al., 1989), which was used to assess their relationship with the accompanying friend.
Statistical analyses
Data and analysis scripts are available online (https://osf.io/dw89j/). Statistical analyses were conducted in R (R Core Team, 2019) by means of linear mixed effects models (LMMs) using the lmer() function of the lme4 package. Group comparisons for age, trait questionnaires, BMI, MVC, satisfaction about the speech and math performances, and PASA were assessed with linear regressions (LM) using the lm() function, and group differences in stimulus rankings were investigated with Chi-squared tests using the chisq.test() function. For LMMs, Type-III F-tests were computed with the Satterth-waite degrees of freedom approximation, using the anova () function of the lmerTest package. For LMs, Type-III F-tests were computed using the Anova() function from the car package. Results from LMMs and LMs were controlled for the false discovery rate (FDR) associated with multiple testing using the Benjamini–Hochberg method (Benjamini and Hochberg, 1995). Significant interactions were further analyzed using the function emmeans() from the homonymous package. Planned comparisons were corrected using Tukey method. Figures were created in R using the packages ggplot2 and cowplot and modified using Illustrator (Adobe Inc., USA).
State induction: self-report and physiological measures of subjective state
Changes in self-reported stress (VAS), anxiety (STAI), positive and negative affect (PANAS), salivary cortisol and alpha-amylase were investigated using four LMMs including the fixed effects Group (appetitive, aversive, control) and Time (T1–T7)3. Scores of the PASA questionnaire were investigated using three LMs for the dependent variables (i) index of primary appraisal (threat and challenge subscales), (ii) index of secondary appraisal (self-concept of own abilities and control expectancy) and (iii) stress index (difference score between primary appraisal and secondary appraisal), including Group (appetitive, aversive, control) as between-subjects predictor. For salivary cortisol and alpha-amylase analyses, outliers were defined as subjects with a cortisol/alpha-amylase baseline (T1) higher than two SDs compared to the mean of the sample. Concerning cortisol data, seven outliers were excluded from statistical analyses (two aversive, one control, four appetitive), while for alpha-amylase data, five outliers were excluded (five appetitive). The majority of participants with outlier salivary cortisol or alpha-amylase baseline levels reported intense physical activity, or alcohol consumption in the previous 24 h, or food/coffee/tea consumption in the two hours before the test.
Ratings of wanting and liking, and effort
To assess the effect of different motivational states on social reward processing, three separate LMMs were implemented for each behavioral dependent variable (ratings of wanting and liking, and force exerted). The models included Group (appetitive, aversive, control) and Reward Level (High, Low, and Very Low in the case of liking) as fixed effects, and by-subject random intercepts and slopes for Reward Level. Given the long time interval between the manipulation and the Social Reward task, as well as the long duration of the task (40 min), the predictor Block was also included as fixed main effect and random slope, to take into account a possible decline of the manipulation effects along the first (block 1) and second (block 2) part of the task.
Facial EMG: Hedonic facial reactions during reward anticipation and consumption
EMG data were preprocessed in Matlab R2018a (www.themathworks.com), partly using the EEGLAB toolbox (Delorme and Makeig, 2004). The data were first filtered with a 20 to 400 Hz bandpass filter, then rectified and smoothed with a 40 Hz low-pass filter. In order to investigate facial reactions to reward anticipation and consumption, epochs were extracted for a Pre-Effort Anticipation period (3 s) at the beginning of each trial, a Post-Effort Anticipation period (3 s) following the effort phase and preceding reward delivery, a Delivery period (6 s) during which participants received the tactile stimulation, and a Relax period (5 s) immediately following reward delivery (Fig. 2). For each trial, values in these four epochs were expressed as percentage of the average amplitude during the fixation cross at the beginning of that trial. Due to technical failure, EMG was not recorded for one participant (aversive group). To reduce the effect of non-experimental movements, trials with activity of the corrugator (CS) and zygomaticus (ZM) muscles higher than three SDs compared to the subject’s mean were excluded from further analyses (average number of trials excluded per subject: CS = 2.49; ZM = 2.76). Despite this data cleaning procedure, skewness (S) and kurtosis (K) values were still high (CS: MS = 7.27, MK = 121.64; ZM: MS = 6.16, MK = 67.31) and EMG data were consequently transformed using the natural logarithm (log). The same LMMs described for the Social Reward task were used to analyze the EMG data. Since the reward level Very Low is not present in the Pre-Effort Anticipation phase, this phase was analyzed in a separate model. Post-Effort Anticipation, Delivery and Relax phases were analyzed in a single model including the additional fixed effect Phase (Post-Effort Anticipation, Delivery, Relax), as well as its interactions with Group and Reward Level. Random slopes were also added for Phase and its interaction with Reward Level. The two models were separately fitted for corrugator and zygomaticus muscles, leading to four models in total.
Results
We report here the significant (p < 0.05) or marginally significant (0.05 < p < 0.1) findings which were the focus of the study. For a complete description of all the significant effects, refer to the Supplementary Material.
State induction
Self-report measures of subjective state
All LMMs conducted on the self-report measures of the subjective state (stress, anxiety, negative and positive affect) revealed a significant Group by Time interaction effect (all F > 4.47, all p < 0.001; see Tables S1 and S2 of Supplementary Material). LMs conducted on PASA indexes (except for secondary appraisal) and ratings of participants’ satisfaction with their performance at the TSST revealed a significant main effect of Group (all F > 4.40, all p < 0.02; see Tables S1 and S3 of Supplementary Material).
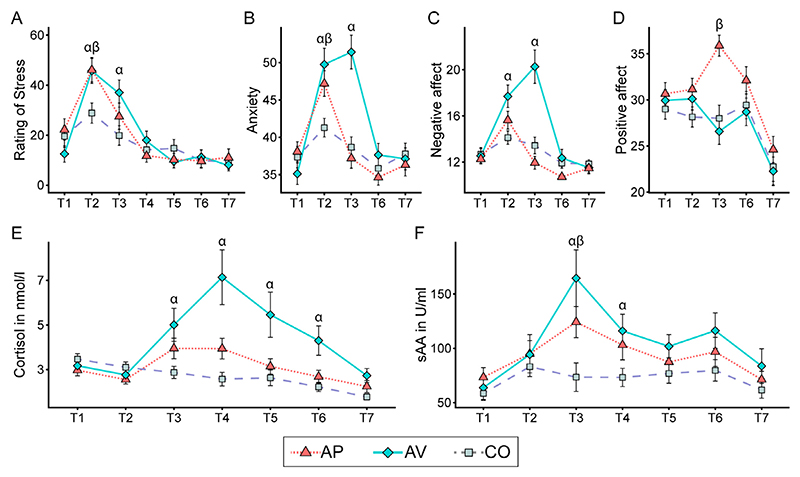
Self-reported stress and anxiety of both appetitive and aversive groups were significantly higher during the preparation phase of the TSST (T2) compared to the control group (all p < 0.05, Fig. 3A, B). Moreover, the aversive group presented also a significantly higher negative affect than the control group (p < 0.001, Fig. 3C). Regarding PASA scores (at T2), participants in both the aversive and appetitive groups appraised the situation as significantly more threatening and challenging than the control group (primary appraisal; all p < 0.001). Additionally, participants in the aversive group anticipated the TSST to be significantly more stressful than participants in the control group (stress index; p = 0.01). As expected, after the motivational state induction (T3), self-reported stress, anxiety and negative affect of the aversive group were significantly higher compared to the control group (all p < 0.001, Fig. 3A–C), while the appetitive group presented a significantly higher positive affect (p < 0.001, Fig. 3D). Finally, participants in the aversive group were less satisfied with their performance in both the speech (p = 0.05) and math (p < 0.001) tasks compared to participants in the control group, while participants in the appetitive group were significantly more satisfied with their performance at the speech task (p = 0.01) than participants in the control group.
Fig. 3.
(A) Stress ratings (VAS); (B) anxiety (STAI); (C) negative affect (subscale of PANAS); (D) positive affect (subscale of PANAS); (E) salivary cortisol in nanomole per liter (nmol/l); (F) salivary alpha-amylase (sAA) in units per milliliter (U/ml). Error bars represent standard error of the mean. AP = appetitive group; AV = aversive group; CO = control group. a denotes significant difference between aversive and control groups; α denotes significant difference between appetitive and control groups.
Physiological measures of subjective state
LMMs conducted on salivary cortisol and alpha-amylase revealed a significant Group by Time interaction effect (cortisol: F12,546 = 8.66, p < 0.001; alpha-amylase: F12,557.1 = 3.39, p < 0.001) (see Table S1 and S2 of Supplementary Material).
Participants in the aversive group had significantly higher salivary cortisol levels compared to the control group from the completion of the TSST until the starting of the Social Reward task (i.e., T3 to T6; all p < 0.01, Fig. 3E). No significant differences were found between the appetitive and control groups (all p > 0.1). Regarding salivary alpha-amylase, participants in the appetitive group had significantly higher alpha-amylase levels than participants in the control group immediately after the TSST (T3; p= 0.02, Fig. 3F). Participants in the aversive group had significantly higher alphaamylase levels following the TSST (T3: p < 0.001; T4: p = 0.05, Fig. 3F) compared to the control group.
Overall, the data suggest that both situations, aversive-TSST and appetitive-TSST, were similarly challenging and arousing for the participants (as indicated by comparable stress levels and anticipated threat and challenge during the preparation phase, as well as similarly elevated alpha-amylase concentration after the TSST), but markedly different in the resulting motivational state of the individuals: (i) an aversive/negative state for the aversive group, characterized by increased stress, anxiety, negative mood, elevated cortisol and (ii) an appetitive/positive state for the appetitive group, characterized by positive mood and a sense of satisfaction about the performance.
Social reward task
To exclude the possibility that differences between groups in the processing of interpersonal touch could be due to differences in the initial preferences (ranking) of the reward levels, three chi-squared tests were used. No group differences were found (all X2 < 4.67, all p > 0.32). In line with the previous literature (Löken et al., 2009), touch at six cm/s was ranked by most of the participants as High reward (62.4%), compared to 21 cm/s (24.7%) and 27 cm/s (12.9%) (for a complete description of the ranking of the touch stimuli see Table S4 of Supplementary Material).
Secondly, we assessed whether the number of High, Low and Very Low rewards obtained during the task were similar for the three groups. A LMM with number of trials as dependent variable, the fixed effects Group (appetitive, aversive, control) and Reward Level (High, Low, Very Low), and by-subject random intercept was fitted. The LMM revealed only a main effect of Reward Level (F2,290 = 20.58, p < 0.001), indicating that participants obtained more often High rewards (M = 12.19, SD = 2.82) as compared to Low (M = 11.15, SD = 2.89) and Very Low rewards (M = 9.02, SD = 4.67).
Finally, we tested for group differences in the average MVC assessed before and after the Social Reward task. No significant differences emerged (all F < 0.51, all p > 0.60), indicating that the induced motivational states did not affect participants’ grip force at rest.
Subjective ratings of wanting and liking (explicit wanting and liking)
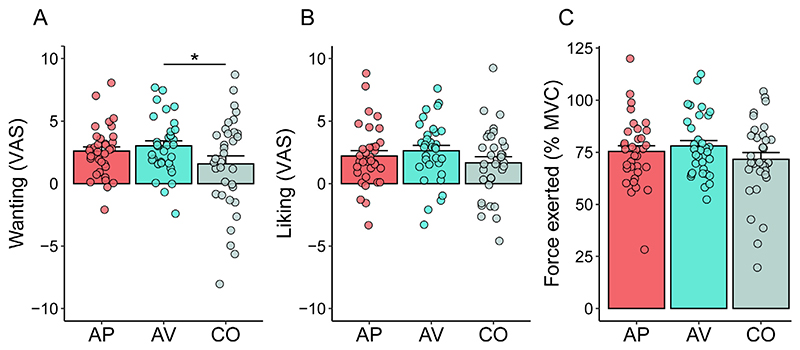
The analysis conducted on the ratings of wanting revealed significant main effects of Group (F2,98 = 3.50, p = 0.046), Reward Level (F1,98 = 69.51, p < 0.001) and Block (F1,100 = 8.80, p = 0.008). As expected, wanting was higher for High reward than Low reward and during the first block of the task as compared to the second one. Notably, post hoc tests conducted on the main effect of Group revealed that participants in the aversive group expressed higher ratings of wanting (M = 3.02, SD = 2.29; p = 0.03), compared to the control group (M = 1.57, SD = 3.75). No significant differences were observed when comparing the appetitive group (M = 2.59, SD = 2.05; p = 0.2) with the control group (Fig. 4A).
Fig. 4.
(A) Ratings of wanting and (B) ratings of liking expressed during the Social Reward task. (C) Force exerted in order to obtain the announced reward. AP = appetitive group; AV = aversive group; CO = control group. Error bars represent standard error of the mean. Points represent individual means. Asterisks indicate significant post hoc comparisons (*p < 0.05).
The LMM on the ratings of liking resulted in a significant main effect of Reward Level (F2,96.7 = 78.11, p < 0.001) and Block (F1,99.9 = 12.32, p = 0.001), but no significant main or interaction effect with the factor Group was found (all F < 1.58, p > 0.21). Post hoc comparisons showed that the ratings of wanting were higher for High reward than Low reward, which in turn was rated higher than Very Low reward (all p < 0.001). As for wanting, liking was generally higher in the first block of the task as compared to the second one (Fig. 4B).
Effort (implicit wanting)
The LMM on the effort exerted in order to obtain the tactile stimulation revealed a significant main effect of Reward Level (F1,98 = 40.80, p < 0.001) and Block (F1,100 = 46.46, p < 0.001). No significant main or interaction effect with the factor Group was found (all F < 1.24, all p > 0.30). Regarding the effect of Reward Level, as expected, participants exerted more effort in order to gain the High reward as compared to the Low reward. Moreover, force exerted was generally greater in the first block of the task than in the second one (Fig. 4C).
Facial EMG: Hedonic facial reactions during anticipation (implicit anticipatory liking) and consumption (implicit consummatory liking)
The LMMs conducted on corrugator and zygomaticus activity during the Pre-Effort Anticipation phase did not reveal any significant effects (all F < 4.57, all p > 0.14).
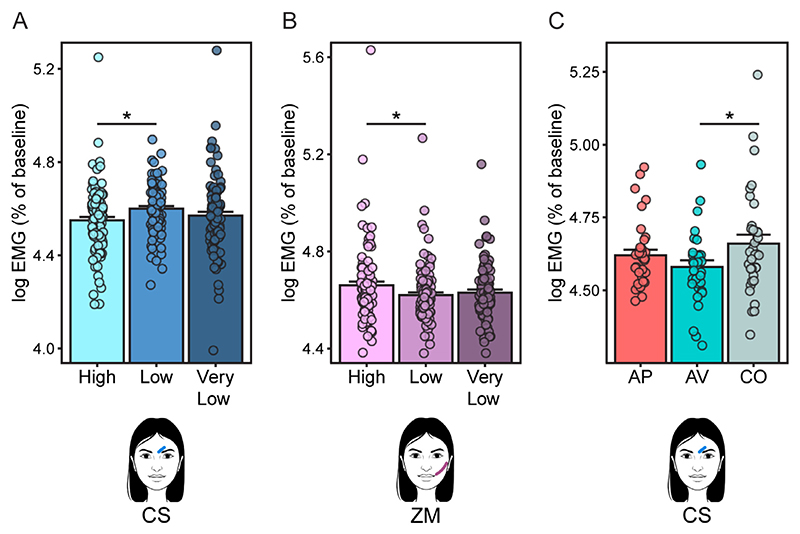
The LMM conducted on corrugator activity recorded during Post-Effort Anticipation, Delivery and Relax phases revealed a significant main effect of Reward Level (F2,97.5 = 4.22, p = 0.047), Phase (F2,150.2 = 11.74, p < 0.001) and Block (F1,98.6 = 6.20, p = 0.047), and a marginally significant Phase by Group interaction (F4,157.4 = 2.69, p < 0.066). Regarding the Group by Phase interaction, exploratory planned comparisons revealed that, within the Post-Effort Anticipation phase, activity of the corrugator muscle was significantly lower in the aversive group compared to the control group (p = 0.031) regardless of the type of reward, indicating higher reward anticipatory pleasure after stress and resembling the results found for the ratings of wanting (Fig. 5C). No other significant effects were observed (all p > 0.12). Post hoc comparisons of the main effects of Reward Level, Phase and Block showed that, as expected, corrugator activity was significantly greater for Low rewards compared to High rewards (p = 0.013) (Fig. 5A). Corrugator activity was also significantly greater during Post-Effort Anticipation as compared to Delivery and Relax (all p < 0.001), and in the second block of the task as compared to the first one.
Fig. 5.
Main effect of Reward Level for (A) the corrugator (CS) and (B) the zygomaticus (ZM) muscle, averaged activity over Post-Effort Anticipation, Delivery and Relax phases. (C) Group differences in corrugator (CS) muscle activity during the Post-Effort Anticipation phase. AP = appetitive group; AV = aversive group; CO = control group. Bars represent standard error of the mean. Points represent individual means. Asterisks indicate significant post hoc comparisons (*p < 0.05).
The LMM conducted on zygomaticus activity recorded during Post-Effort Anticipation, Delivery and Relax phases revealed a significant main effect of Phase (F2,120.6 = 13.43, p < 0.001), a marginally significant effect of Block (F1,98.9 = 4.80, p < 0.082) and a marginally significant effect of Reward Level (F2,99.2 = 3.72, p < 0.082). Exploratory planned comparisons revealed that, as expected, activity of the zygomaticus muscle was significantly greater for High rewards compared to Low rewards (p = 0.022) (Fig. 5B). Zygomaticus activity was also greater during the Post-Effort Anticipation phase compared to Delivery and Relax (all p < 0.001), and during the first block of the task compared to the second one.
Discussion
The present study investigated the effects of aversive and appetitive motivational states on the processing of interpersonal touch. By adapting the Trier Social Stress Test (Kirschbaum et al., 1993), we designed three different situations in order to induce aversive, appetitive or neutral motivational states in the participants. We then investigated the state-dependent effects on the anticipation and consumption of interpersonal touch of different levels of pleasantness, delivered to the participants’ fore-arm. In line with previous animal research on reward processing, effort and hedonic facial reactivity were assessed, in addition to self-reported ratings of wanting and liking.
As a first step, we checked whether the motivational state manipulation was effective in inducing positive or negative emotional states, compared to a neutral control state (Fig. 3). As expected, we found that participants reported higher levels of self-reported stress, anxiety and negative affect, as well as higher salivary cortisol, during the preparation and following the completion of the aversive-TSST, as compared to the control group. On the other hand, participants assigned to the appetitive-TSST showed higher stress and anxiety during preparation, but greater positive affect and higher satisfaction towards their performance after its completion, as compared to the control group. In addition, following the TSST, alpha-amylase levels were elevated in the appetitive and aversive groups compared to the control group, indicating similar levels of arousal in both motivational states. These findings indicate that the state induction was effective. We therefore subsequently explored its effect on the processing of social rewards.
Previous research on grooming in non-human primates and interpersonal touch in humans provided evidence that interpersonal physical contact can serve as a buffer against stress and can down-regulate negative emotions arising from aversive states (for a review see Morrison, 2016). We hypothesized that, during an aversive state, the main motivation that drives the individual to seek social contact is the reduction of distress and the return to homeostasis, rather than its hedonic properties. Consistent with this prediction, we found that experiencing an aversive situation led to greater self-reported wanting of interpersonal touch, without a corresponding increase of liking. This finding was corroborated by a greater relaxation of the corrugator muscle (reflecting a more positive facial response) in participants who experienced the aversive-TSST only during the anticipation of the interpersonal touch (anticipatory liking), but not during or after its delivery (consummatory liking).
The present findings extend prior research on the effect of stress and negative affect on reward processing to the domain of social rewards. Indeed, the results are consistent with previous research using both behavioral (Pool et al., 2015; Boyle et al., 2020) and neuroimaging techniques (Kumar et al., 2014; Lewis et al., 2014), demonstrating that stressful experiences, such as laboratory stress, can induce an amplification of wanting of food and monetary rewards, without a corresponding increase in the liking of those stimuli. It has to be noted though that, differently from the studies mentioned above, the enhanced wanting expressed through self-report ratings (explicit wanting) was not reflected in a greater effort (implicit wanting) mobilized to obtain the social reward. Importantly, a distinction between implicit and explicit wanting appears to exist (Berridge and Robinson, 2003; Anselme and Robinson, 2016; Pool et al., 2016). Implicit wanting (or incentive salience) is defined as a motivational reaction triggered by a reward-related cue present in the environment and does not necessarily require awareness. Explicit wanting (or cognitive incentives) is driven by goal-directed strategies of action (rather than by Pavlovian associations) and is influenced by subjective expectations of the hedonic experience that will be gained through reward consumption, as well as by episodic memories of past experiences with the same reward (Berridge and Robinson, 2003). Explicit and implicit wanting usually direct behaviors in a congruent manner, but under certain circumstances, their directions deviate (Anselme and Robinson, 2016). Several factors could have led to the discrepant results with the previous studies (e.g., type of stressor used, the time interval between the manipulation and the reward task, and the type of reward) and additional research is needed to understand the relationship between implicit/explicit wanting and aversive states in social reward processing.
Regarding the hedonic facial reactions measured via fEMG, in agreement with previous research using this method to assess anticipatory and consummatory liking (Pawling et al., 2017; Mayo et al., 2018; Ree et al., 2019, 2020; Korb et al., 2020a, 2020b), corrugator and zygomaticus activity recorded during reward anticipation and consumption was modulated by the level of pleasantness of the rewards. Importantly, the effect of the experimental manipulation on the behavioral results was in line with the fEMG findings. Indeed, we observed that during the announcement of the obtained reward, participants in the aversive group showed reduced frowning (increased hedonic reactions) as compared to the control group. This enhanced anticipatory pleasure was not accompanied by a similar increase during or immediately after the consumption of the social reward, as demonstrated by the absence of group differences in corrugator or zygomaticus activity during the delivery and relax phases. As previously mentioned, explicit wanting strongly relies on the anticipation of the pleasantness of the reward. The fact that both explicit wanting (measured with ratings) and anticipatory pleasure (measured with EMG during reward anticipation) are enhanced in the aversive group corroborate the hypothesis that in the current study the induction of an aversive state selectively modulated the goal-directed system of motivational control, rather than the incentive salience system.
Contrary to our on initial hypothesis of stronger effects of the experimental manipulation on the best possible (High) reward, we found a general increase of wanting across both High and Low rewards. One possibility is that stress affects anticipatory responses to touch rewards in a different way than it affects anticipation of food or other kinds of rewards. In addition, the type and duration of the employed stressor may also lead to different behaviors. Concerning this, the majority of the studies on food investigated the effects of chronic stress on eating behavior, while here participants were exposed to a short, single, acute stress manipulation.
Regarding the appetitive motivational state, we predicted that individuals’ main motivation to seek social contact is the gain of pleasure. We therefore expected that the induction of an appetitive motivational state would have been followed by an enhancement of liking, accompanied by an increase in wanting of the interpersonal touch. Contrary to our expectations, no significant effect was found on subjective ratings of wanting and liking, effort or hedonic facial reactions to anticipation and consumption of social touch. One intriguing possibility is that while interpersonal touch is one of the most effective social rewards to reduce aversive states, it might not be the most valuable social reward when we seek pleasure. Indeed, prior research in animals and humans suggests that specific types of physical contact are sought in different situations. For example, rodents in appetitive states engage in social play, while this social behavior is actually suppressed during periods of stress and instability (Loseth et al., 2014). In humans, Hertenstein and colleagues (Hertenstein et al., 2006, 2009) showed that different kinds of tactile interactions are used to communicate with high accuracy a wide range of emotions. For instance, in their studies, stroking was mainly associated with feelings of love, sympathy and sadness, while happiness was more often associated with more arousing types of physical contact, such as shaking, squeezing and lifting. Future studies should include different types of social rewards to investigate this hypothesis. Another possible explanation of the aforementioned findings could be related to the different duration of the effects of the manipulation. Aversive experiences with a self-threatening component, such as the employed aversive-TSST, can induce a strong and long-lasting physiological reaction. This was confirmed by the presence of elevated cortisol levels until 50 min after the stress induction in the aversive group. Our appetitive manipulation might have failed to induce a comparably long-lasting positive reaction. Therefore, the long interval between the manipulation and the Social Reward task might have blunted the effects of such manipulation on wanting and liking of interpersonal touch. Finally, it is also possible that the appetitive-TSST may not be ideal to induce an appetitive motivational state in the participants, although the self-report and physiological responses collected after the state induction (higher positive emotions, satisfaction and salivary alpha-amylase concentration compared to the control group), make this option unlikely. We nevertheless acknowledge that the preparation phase of the appetitive-TSST (T2) was perceived as stressful by the participants. Further studies are needed to investigate the effect of a purely positive situation on wanting and liking of interpersonal touch.
In general, and in line with studies using the same paradigm (Korb et al., 2020a, 2020b), implicit and explicit wanting and liking were higher for the High reward compared to the other reward levels. Moreover, wanting, liking, effort, and, in part, hedonic facial reactions were greater for the first half of the task compared to second half, presumably due to habituation and satiety effects (Triscoli et al., 2014).
Some limitations of the current study should be considered. First, as already mentioned, the time window between the state induction and the Social Reward task (ca. 50 min) is longer than that used in the majority of TSST studies and may have weakened the overall effects of the manipulation. This interval was used to test if the paradigm is suitable for pharmacological manipulations, which often require a certain amount of time to allow the administered active compound to reach the maximum serum concentration. However, in our study, subjective measures of the individuals’ state had returned to baseline levels at the moment of the administration of the Social Reward task, and only physiological indexes (cortisol) allowed to significantly differentiate the aversive group from the control group. Moreover, it is known that the physiological reaction to stress follows a precise temporal pattern, characterized by a fast response of the autonomic nervous system and a slower activation of the hypothalamic–pituitary–adrenal axis, which is responsible for cortisol release (Godoy et al., 2018).
Future studies should aim at replicating the results by assessing wanting and liking of interpersonal touch immediately after the induction of the motivational state. Second, the sample was composed exclusively of female participants, therefore the present findings need to be extended to the male population. Third, our participants could decide which touch frequency constituted the High, Low and Very Low reward in the experimental task. While this can help in reducing individual differences in subjective preferences, it also prevented us from investigating differences connected to the speed of the touch. Indeed, previous research showed that slow touch, as compared to fast touch, might be more effective in reducing distress caused by social exclusion (von Mohr et al., 2017). Last, even though we made an effort to increase the ecological validity of the study by using skin-to-skin touch and testing pairs of close friends, rather than strangers, the artificial lab situation and the fact that the touch was performed by the experimenter might have affected the results. Future studies should extend such findings to everyday life situations (e.g., via ecological momentary assessment; Shiffman et al., 2008).
To the best of our knowledge, this is the first study assessing the effect of aversive and appetitive motivation on the anticipation and consumption of interpersonal touch in humans. The present findings show that after an aversive experience, explicit wanting and anticipatory pleasure of interpersonal touch are enhanced, without a corresponding change in the liking expressed during and after consumption. No evidence was found concerning the influence of appetitive states on wanting and liking of interpersonal touch. The understanding of how different motivational states affect social contact seeking might be crucial for improving our knowledge of psychopathologies characterized by a dysregulation of social motivation and social pleasure. The present data provide a first step into this direction.
Supplementary Material
Acknowledgements
The project was funded by the University of Vienna with a Uni:docs scholarship awarded to C. Massaccesi, by the Austrian Science Fund (FWF; W1262-B29), by the Vienna Science and Technology Fund grants (WWTF; CS15-003 and VRG13-007) awarded to G. Silani, and C. Eisenegger. The study was supported by the research platform “The Stress of Life (SOLE) – Processes and Mechanisms underlying Everyday Life Stress” (PI: Nater, Co-PI: Silani). Funders had no role in study design, data collection and analyses, decision to publish or preparation of the manuscript. We thank Katharina Stiehl for commenting on an earlier version of the manuscript. We thank Ana Stjovic and all the students who contributed to data acquisition: Damian Bednarz, Johann Börner, Lukas Frieberger, Alexandra Ghamrawi, Franziska Hartmann, Laura Keil, Max Marschner, Ri Phan, Sara Potzöld, Manuela Rebel, Leopold Roth, Bojana Trajkovic, Markus Tünte. Finally, we thank Ina Dhrami (Biochemical Laboratory of the Faculty of Psychology, University of Vienna) for conducting salivary analysis.
Abbreviations
- AQ-k
short version of the Autism Quotient
- BDI-II
Beck Depression Inventory
- BMI
Body Mass Index
- fEMG
facial electromyography
- LM
linear model
- LMM
linear mixed model
- LSAS
Liebowitz Social Anxiety Scale
- MVC
maximum voluntary contraction
- PANAS
positive affect negative affect scale
- PASA
primary and secondary appraisal scale
- RCI
Relationship Closeness Index
- STAI
State Trait Anxiety Inventory
- STQ
Social Touch Questionnaire
- TSST
Trier Social Stress Test
- VAS
visual analogue scale.
Footnotes
Participants were informed that the curtain was used to let them focus on the tactile sensation without being distracted by the surrounding environment and that the experimenter was sitting next to the friend in order to check that the caresses were administered correctly.
Questionnaires scores, age and MVC were added as covariates to the analyses of wanting, liking and effort, to control for individual characteristics. Results did not change after adding those covariates.
T4 and T5 were included only in the case of self-reported stress, salivary cortisol and alpha-amylase.
Authors Contributions
CM and GS developed the hypothesis and research plan, and wrote the manuscript. CM collected and analyzed the data. SK contributed to the tasks scripts and the analysis plan. NS and UMN carried out the immunoassays for salivary cortisol and alpha-amylase and contributed to the design of the state manipulation. All authors contributed to and approved the final version of the manuscript for submission.
Declarations of Interest
None.
References
- Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91(4):449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Akinola M, Mendes WB. The dark side of creativity: biological vulnerability and negative emotions lead to greater artistic creativity. Pers Soc Psychol Bull. 2008;34(12):1677–1686. doi: 10.1177/0146167208323933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselme P, Robinson MJF. “Wanting”, “liking”, and their relation to consciousness. J Exp Psychol: Animal Learn Cognition. 2016;42(2):123–140. doi: 10.1037/xan0000090. [DOI] [PubMed] [Google Scholar]
- Aureli F, Preston SD, de Waal FBM. Heart rate responses to social interactions in free-moving rhesus macaques (Macaca mulatta): a pilot study. J Comp Psychol. 1999;113(1):59–65. doi: 10.1037/0735-7036.113.1.59. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Leary MR. The need to belong: desire for interpersonal attachments as a fundamental human motivation. Psychol Bull. 1995;117(3):497–529. doi: 10.1037/0033-2909.117.3.497. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc: Ser B (Methodol) 1995;57(1):289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26(9):507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: “liking”, “wanting”, and learning. Curr Opin Pharmacol. 2009;9(1):65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berscheid E, Snyder M, Omoto AM. The relationship closeness inventory: assessing the closeness of interpersonal relationships. J Pers Soc Psychol. 1989;57(5):792–807. doi: 10.1037/0022-3514.57.5.792. [DOI] [Google Scholar]
- Bloomfield MA, McCutcheon RA, Kempton M, Freeman TP, Howes O. The effects of psychosocial stress on dopaminergic function and the acute stress response. ELife. 2019;8:e46797. doi: 10.7554/eLife.46797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle CC, Stanton AL, Eisenberger NI, Seeman TE, Bower JE. Effects of stress-induced inflammation on reward processing in healthy young women. Brain Behav Immun. 2020;83:126–134. doi: 10.1016/j.bbi.2019.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA, Schaefer HS, Davidson RJ. Lending a hand: social regulation of the neural response to threat. Psychol Sci. 2006;17(12):1032–1039. doi: 10.1111/j.1467-9280.2006.01832.x. [DOI] [PubMed] [Google Scholar]
- Croy I, Bierling A, Sailer U, Ackerley R. Individual variability of pleasantness ratings to stroking touch over different velocities. Neuroscience. 2020;464:33–43. doi: 10.1016/j.neuroscience.2020.03.030. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Neumann ID, Bodenmann G, von Dawans B, Turner RA, Ehlert U, Heinrichs M. Effects of different kinds of couple interaction on cortisol and heart rate responses to stress in women. Psychoneuroendocrinology. 2007;32(5):565–574. doi: 10.1016/j.psyneuen.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM. The social role of touch in humans and primates: behavioural function and neurobiological mechanisms. Neurosci Biobehav Rev. 2010;34(2):260–268. doi: 10.1016/j.neubiorev.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Ellingsen DM, Leknes S, Løseth G, Wessberg J, Olausson H. The neurobiology shaping affective touch: expectation, motivation, and meaning in the multisensory context. Front Psychol. 2016;6 doi: 10.3389/fpsyg.2015.01986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag CM, Retz-Junginger P, Retz W, Seitz C, Palmason H, Meyer J, Rösler M, von Gontard A. Evaluation der deutschen Version des Autismus-Spektrum-Quotienten (AQ) - die Kurzversion AQ-k. Zeitschrift Für Klinische Psychologie Und Psychotherapie. 2007;36(4):280–289. doi: 10.1026/1616-3443.36.4.280. [DOI] [Google Scholar]
- Fridlund AJ, Cacioppo JT. Guidelines for human electromyographic research. Psychophysiology. 1986;23(5):567–589. doi: 10.1111/j.1469-8986.1986.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Gaab J, Rohleder N, Nater UM, Ehlert U. Psychological determinants of the cortisol stress response: the role of anticipatory cognitive appraisal. Psychoneuroendocrinology. 2005;30(6):599–610. doi: 10.1016/j.psyneuen.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Godoy LD, Rossignoli MT, Delfino-Pereira P, Garcia-Cairasco N, de Lima Umeoka EH. A Comprehensive overview on stress neurobiology: basic concepts and clinical implications. Front Behav Neurosci. 2018;12 doi: 10.3389/fnbeh.2018.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein P, Weissman-Fogel I, Dumas G, Shamay-Tsoory SG. Brain-to-brain coupling during handholding is associated with pain reduction. PNAS. 2018;115(11):E2528–E2537. doi: 10.1073/pnas.1703643115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green IW, Pizzagalli DA, Admon R, Kumar P. Anhedonia modulates the effects of positive mood induction on reward-related brain activation. NeuroImage. 2019;193:115–125. doi: 10.1016/j.neuroimage.2019.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimberg RG, Horner KJ, Juster HR, Safren SA, Brown EJ, Schneier FR, Liebowitz MR. Psychometric properties of the Liebowitz Social Anxiety Scale. Psychol Med. 1999;29(1):199–212. doi: 10.1017/S0033291798007879. [DOI] [PubMed] [Google Scholar]
- Hepworth R, Mogg K, Brignell C, Bradley BP. Negative mood increases selective attention to food cues and subjective appetite. Appetite. 2010;54(1):134–142. doi: 10.1016/j.appet.2009.09.019. [DOI] [PubMed] [Google Scholar]
- Hertenstein MJ, Holmes R, McCullough M, Keltner D. The communication of emotion via touch. Emotion. 2009;9(4):566–573. doi: 10.1037/a0016108. [DOI] [PubMed] [Google Scholar]
- Hertenstein MJ, Keltner D, App B, Bulleit BA, Jaskolka AR. Touch communicates distinct emotions. Emotion. 2006;6(3):528–533. doi: 10.1037/1528-3542.6.3.528. [DOI] [PubMed] [Google Scholar]
- Het S, Rohleder N, Schoofs D, Kirschbaum C, Wolf OT. Neuroendocrine and psychometric evaluation of a placebo version of the ‘Trier Social Stress Test’. Psychoneuroendocrinology. 2009;34(7):1075–1086. doi: 10.1016/j.psyneuen.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Ironside M, Kumar P, Kang M-S, Pizzagalli DA. Brain mechanisms mediating effects of stress on reward sensitivity. Curr Opin Behav Sci. 2018;22:106–113. doi: 10.1016/j.cobeha.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Korb S, Götzendorfer SJ, Massaccesi C, Sezen P, Graf I, Willeit M, Eisenegger C, Silani G. Dopaminergic and opioidergic regulation of implicit hedonic facial reactions during anticipation and consumption of social and nonsocial rewards. BioRxiv. 2020a:832196. doi: 10.1101/832196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korb S, Massaccesi C, Gartus A, Lundström JN, Rumiati R, Eisenegger C, Silani G. Facial responses of adult humans during the anticipation and consumption of touch and food rewards. Cognition. 2020b;194:104044. doi: 10.1016/j.cognition.2019.104044. [DOI] [PubMed] [Google Scholar]
- Kühner C, Bürger C, Keller F, Hautzinger M. Reliabilität und Validität des revidierten Beck-Depressions-inventars (BDI-II). Befunde aus deutschsprachigen Stichproben. [Reliability and validity of the Revised Beck Depression Inventory (BDI-II). Results from German samples.] Der Nervenarzt. 2007;78(6):651–656. doi: 10.1007/s00115-006-2098-7. [DOI] [PubMed] [Google Scholar]
- Kumar P, Berghorst LH, Nickerson LD, Dutra SJ, Goer FK, Greve DN, Pizzagalli DA. Differential effects of acute stress on anticipatory and consummatory phases of reward processing. Neuroscience. 2014;266(Supplement C):1–12. doi: 10.1016/j.neuroscience.2014.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuschagne I, Grace C, Rendell P, Terrett G, Heinrichs M. An introductory guide to conducting the Trier Social Stress Test. Neurosci Biobehav Rev. 2019;107:686–695. doi: 10.1016/j.neubiorev.2019.09.032. [DOI] [PubMed] [Google Scholar]
- Lewis AH, Porcelli AJ, Delgado MR. The effects of acute stress exposure on striatal activity during Pavlovian conditioning with monetary gains and losses. Front Behav Neurosci. 2014;8 doi: 10.3389/fnbeh.2014.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löken LS, Wessberg J, Morrison I, McGlone F, Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nat Neurosci. 2009;12(5):547–548. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- López-Solà M, Geuter S, Koban L, Coan JA, Wager TD. Brain mechanisms of social touch-induced analgesia in females. Pain. 2019;160(9):2072–2085. doi: 10.1097/j.pain.0000000000001599. [DOI] [PubMed] [Google Scholar]
- Loseth GE, Ellingsen D-M, Leknes S. State-dependent μ-opioid modulation of social motivation. Front Behav Neurosci. 2014;8:430. doi: 10.3389/fnbeh.2014.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo LM, Lindé J, Olausson H, Heilig M, Morrison I. Putting a good face on touch: facial expression reflects the affective valence of caress-like touch across modalities. Biol Psychol. 2018;137:83–90. doi: 10.1016/j.biopsycho.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Morese R, Lamm C, Bosco FM, Valentini MC, Silani G. Social support modulates the neural correlates underlying social exclusion. Social Cognitive Affect Neurosci. 2019;14(6):633–643. doi: 10.1093/scan/nsz033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison I. Keep calm and cuddle on: social touch as a stress buffer. Adaptive Human Behav Physiol. 2016;2(4):344–362. doi: 10.1007/s40750-016-0052-x. [DOI] [Google Scholar]
- Morrison I, Löken LS, Olausson H. The skin as a social organ. Exp Brain Res. 2010;204(3):305–314. doi: 10.1007/s00221-009-2007-y. [DOI] [PubMed] [Google Scholar]
- Pawling R, Cannon PR, McGlone FP, Walker SC. C-tactile afferent stimulating touch carries a positive affective value. PLoS ONE. 2017;12(3):e0173457. doi: 10.1371/journal.pone.0173457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perini I, Olausson H, Morrison I. Seeking pleasant touch: neural correlates of behavioral preferences for skin stroking. Front Behav Neurosci. 2015;9 doi: 10.3389/fnbeh.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool E, Brosch T, Delplanque S, Sander D. Stress increases cue-triggered “wanting” for sweet reward in humans. J Exp Psychol Animal Learn Cognition. 2015;41(2):128–136. doi: 10.1037/xan0000052. [DOI] [PubMed] [Google Scholar]
- Pool E, Sennwald V, Delplanque S, Brosch T, Sander D. Measuring wanting and liking from animals to humans: a systematic review. Neurosci Biobehav Rev. 2016;63:124–142. doi: 10.1016/j.neubiorev.2016.01.006. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2019. [Google Scholar]
- Ree A, Bendas J, Pabel L, Croy I, Sailer U. Right between the eyes: corrugator muscle activity tracks the changing pleasantness of repeated slow stroking touch. Physiol Behav. 2020;222:112903. doi: 10.1016/j.physbeh.2020.112903. [DOI] [PubMed] [Google Scholar]
- Ree A, Mayo LM, Leknes S, Sailer U. Touch targeting C-tactile afferent fibers has a unique physiological pattern: a combined electrodermal and facial electromyography study. Biol Psychol. 2019;140:55–63. doi: 10.1016/j.biopsycho.2018.11.006. [DOI] [PubMed] [Google Scholar]
- Sailer U, Triscoli C, Häggblad G, Hamilton P, Olausson H, Croy I. Temporal dynamics of brain activation during 40 minutes of pleasant touch. NeuroImage. 2016;139:360–367. doi: 10.1016/j.neuroimage.2016.06.031. [DOI] [PubMed] [Google Scholar]
- Selby DL, Harrison AA, Fozard TE, Kolokotroni KZ. Dissociating wanting and anticipated liking from consummatory liking in smokers with different levels of nicotine dependence. Addict Behav. 2020;102:106185. doi: 10.1016/j.addbeh.2019.106185. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annual Rev Clin Psychol. 2008;4(1):1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Sinha R. Role of addiction and stress neurobiology on food intake and obesity. Biol Psychol. 2018;131:5–13. doi: 10.1016/j.biopsycho.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. 1970 http://ubir.buffalo.edu/xmlui/handle/10477/2895 . [Google Scholar]
- Stier DS, Hall JA. Gender differences in touch: an empirical and theoretical review. J Pers Soc Psychol. 1984;47(2):440–459. doi: 10.1037/0022-3514.47.2.440. [DOI] [Google Scholar]
- Suvilehto JT, Glerean E, Dunbar RIM, Hari R, Nummenmaa L. Topography of social touching depends on emotional bonds between humans. Proc Natl Acad Sci. 2015;112(45):13811–13816. doi: 10.1073/pnas.1519231112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir M, Robinson MD. The happy spotlight: positive mood and selective attention to rewarding information. Pers Soc Psychol Bull. 2007;33(8):1124–1136. doi: 10.1177/0146167207301030. [DOI] [PubMed] [Google Scholar]
- Triscoli C, Ackerley R, Sailer U. Touch satiety: differential effects of stroking velocity on liking and wanting touch over repetitions. PLoS ONE. 2014;9(11):e113425. doi: 10.1371/journal.pone.0113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Fulton S, Wilson M, Petrovich G, Rinaman L. Stress exposure, food intake, and emotional state. Stress (Amsterdam, Netherlands) 2015;18(4):381–399. doi: 10.3109/10253890.2015.1062981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Mohr M, Kirsch LP, Fotopoulou A. The soothing function of touch: affective touch reduces feelings of social exclusion. Sci Rep. 2017;7(1):13516. doi: 10.1038/s41598-017-13355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadlinger HA, Isaacowitz DM. Positive mood broadens visual attention to positive stimuli. Motivation Emotion. 2006;30(1):87–99. doi: 10.1007/s11031-006-9021-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DD, Boswell RG, Kelley WM, Heatherton TF. Inducing negative affect increases the reward value of appetizing foods in dieters. J Cognit Neurosci. 2012;24(7) doi: 10.1162/jocn_a_00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Kochar AS, Roth WT, Gross JJ. Social anxiety and response to touch: incongruence between self-evaluative and physiological reactions. Biol Psychol. 2001;58(3):181–202. doi: 10.1016/S0301-0511(01)00113-2. [DOI] [PubMed] [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- Young CB, Nusslock R. Positive mood enhances reward-related neural activity. Social Cognitive Affect Neurosci. 2016;11(6):934–944. doi: 10.1093/scan/nsw012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.