Abstract
Every organism interacts with a host of other organisms of the same and different species throughout its life. These biotic interactions have varying influences on the reproduction and dispersal of the organism, and hence also the population and species lineage to which the organism belongs. By extension, biotic interactions must contribute to the macroevolutionary patterns that we observe in the fossil record, but exactly how, when and why are research questions we have been asking before the start of the journal Paleobiology. In this contribution for Paleobiology’s 50th anniversary, we present a brief overview of how paleobiologists have studied biotic interactions and their macroevolutionary consequences, recognizing paleontology’s unique position to contribute data and insights to the topic of interspecies interactions. We then explore, in a semi free-form manner, what promising avenues might be open to those of us who use the fossil record to understand biotic interactions. In general, we emphasize the need for an increased effort in the understanding of ecological details, the integration of different types of information, and to strive for model-based approaches.
Keywords: biotic interactions, fossils, macroevolution, model-based, ecology, deep time
Introduction
There has been a historical tension in paleobiology with regards to the dominance of biotic versus abiotic forces in contributing to macroevolutionary patterns and processes (Vermeij 1994; Barnosky 2001; Jablonski 2008; Benton 2009). This academic dual, in retrospect, was a fruitful one as it steered the field towards a more nuanced view of how interactions among organisms, environmental change, phylogenetic constraints, and the interplay of all three, might lead to the macroevolutionary patterns that we infer (Jablonski 2008; Voje et al. 2015). There is no doubt that many biotic interactions, be they positive (mutualisms, symbioses) or negative (competition, predation, diseases), have consequences on the survival and reproduction of the organisms involved. This is despite the acceptance that it is not always straightforward to recognize biotic interactions and to understand their downstream effects, even among living organisms (Connell 1983, 1990). Specific biotic interactions may have minimal consequences when averaged over the lifespan of those individuals involved or may have huge impacts. Regardless of their impacts, biotic interactions are current and historical processes that contribute to the biological diversity we observe in the fossil record and in living, contemporary communities, when we study macroevolution. In this contribution for the celebration of Paleobiology’s 50th anniversary, we share our views on the way forward for further understanding how biotic interactions might influence macroevolutionary patterns and processes.
Paleobiology and its associated scientific community set the stage for our understanding of taxonomic diversity over geological time scales (Van Valen 1973; Raup 1978; Raup and Sepkoski 1982). Prominently, Sepkoski’s models demonstrated that some temporal diversity patterns are consistent with inter-clade competitive processes. While such patterns of what we could loosely term diversity-dependence offer a window into plausible long-term biotic controls on diversity (Sepkoski 1978, 1981), studies of biotic interactions in deep-time, many published in Paleobiology, have also been based on inferred direct interactions between individual fossil organisms. These interactions include competition (Stanley and Newman 1980; Lidgard et al. 1993), predation (Gahn and Baumiller 2005; Bellwood et al. 2014) including herbivory (Labandeira et al. 1994), parasitism (Baumiller 1990; De Baets et al. 2021) and mutualism, some of the most conspicuous cases being plant-pollinator interactions (Labandeira and Currano 2013) and coral-zooxanthellae symbiosis (Coates and Jackson 1987; Tornabene et al. 2017).
We begin by briefly discussing in three sections, how paleobiologists have used the fossil record i) to infer the effects of interspecific interactions on lineage diversification, ii) to understand changes in biogeographic distributions due to interspecific interactions and iii) to study trait evolution as a result of interspecific interactions, focusing on topics that were given less attention in recent reviews on a similar theme (Hembry and Weber 2020; Bush and Payne 2021; Fraser et al. 2021). We explicitly exclude intraspecific interactions, which can have important and detectable macroevolutionary consequences (Martins et al. 2018) and processes more amendable to study using contemporary species, such as introgression (Baack and Rieseberg 2007). We neither attempt to thoroughly review the literature nor critique specific methods that have been used in understanding biotic interactions and their consequences for macroevolution but point to approaches we feel are more promising after brief summaries. Because the specifics of the approaches we promote will depend on how the questions are formulated and which empirical systems are studied, we do not suggest concrete investigative paths or protocols, but hope to stimulate discussion and novel research. After each of the three sections, we suggest fruitful venues that could continue naturally in the vein of the research that has already been done and highlight some key remaining questions. We try to be explicit about the hierarchical level of organization on which data and/or processes are focused on both when discussing the literature and in suggesting new avenues of research (Fig. 1). All the levels illustrated in Fig. 1 are relevant to the study of biotic interactions. Being clear and explicit in the hierarchical level of analyses and inference can help in integrating findings from diverse approaches and aids us in examining our assumptions surrounding the evidence for past interactions between two organisms, and any detectable consequences for evolution. Then we turn to newer or rarer approaches, particularly those that “explicitly model species interactions and connect them to macroevolutionary patterns” (Harmon et al. 2019, p. 179). Ending with a list of general recommendations, we call for cross-disciplinary, integrative approaches in studying past biotic interactions. It is our hope to inspire another fun-filled half century of collaborative research harnessing the strength of the fossil record to answer questions on evolution and biodiversity.
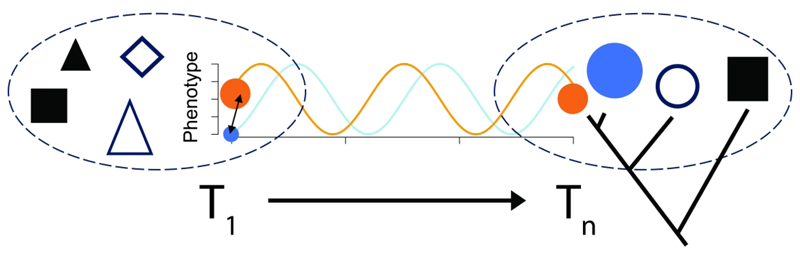
Figure. 1. A temporal, hierarchical view of the context of interspecific biotic interactions.
We illustrate two temporal, evolutionarily continuous communities (delimited by dashed ovals) observed in two separate time intervals T1 and Tn with some shared species represented by shapes and colors. The size of the shapes indicates phenotype (or abundance). The temporal instances between the snap shots of the communities at T1 and Tn are not preserved and hence not observable. Two of these species (orange and blue circles) are illustrated as directly interacting with each other, where their phenotypes (or abundance) cyclically change as a consequence of their interactions (indicated by an arrow between them in T1). In Tn we include a phylogenetic hypothesis that links the illustrated species, where the three circle-shaped species are more closely related, to indicate that phylogenetic/clade-level approaches have also been used in inferring biotic interactions and their consequences. While both the orange and blue species are extant in T1 and Tn, some other species have turned over, indicating that diffuse biotic interactions may be changing with respect to the two focal species. The levels of biological hierarchy directly illustrated in this figure include temporal populations (of the orange and blue species), the communities they have found themselves, and the clade they are part of (only drawn in Tn). Although individuals of the blue and orange species are not figured, it is implicit that the biotic interactions are between such individuals.
i). Species Interactions and Lineage Diversification
Most paleobiological work devoted to investigating the effect of species interactions on diversification dynamics has focused on interspecific competition (Van Valen 1973; Sepkoski 1978, 1996) and predation (Vermeij 1987; Dietl and Kelley 2002; Huntley and Kowalewski 2007; Stanley 2008). A common and superficially straightforward approach is to match a diversification scenario where interspecific interactions could have contributed substantially to temporal diversity patterns. For instance, interspecific competition is implied in equilibrium diversity, where the number of species reach a stable limit (Sepkoski 1978) or where the increase in diversity slows down due to diversity-dependent dynamics (Rabosky 2013; Moen and Morlon 2014; Foote et al. 2024). The underlying assumption here is that limited resources cause speciation rates to decrease and/or promote an increase in extinction rates as new species enter the system and ecological opportunities are exploited (Sepkoski 1978; Walker and Valentine 1984; Schluter 2000; Moen and Morlon 2014). Similarly, Van Valen’s Red Queen hypothesis is motivated by the pattern of age-independent extinction that he argued is consistent with interspecific competition and evolution (Van Valen 1973). Likewise, the so-called “double-wedge” diversity pattern that manifests as a temporally coincident rise and decline in taxonomic richness of two potentially competitor clades, has been used to argue for plausible role of interspecific competition on driving speciation and extinction dynamics (Krause 1986; Sepkoski 1996; Van Valkenburgh 1999). Comparisons of prey (and predator) temporal diversity changes and proxies of predation rates have also been presented to argue the case for negative biotic interactions being drivers of diversity (Huntley and Kowalewski 2007; Gorzelak et al. 2012; Klompmaker et al. 2017). In later studies, estimates of speciation and extinction rates, rather than taxonomic diversity, were used to infer diversity-dependent or competition-associated diversification dynamics both within (Alroy 1996, 2009) and between clades (Liow et al. 2015; Silvestro et al. 2015). Phylogenetic and functional diversity has also been used to investigate the signatures of interspecific competition and diversity saturation in local communities (Fraser and Lyons 2020). Several of the cited papers above used genus or higher taxonomic level data to study clade and/or community level dynamics but imply some form of individual-level interactions via resource competition (at the same trophic-level) or predation (across trophic-levels).
Consider Spatial Overlap and Interactions in Life
While the fossil record is irreplaceable for its direct temporal evidence of how diversity changes through time (Quental and Marshall 2010; Benton 2015; Benson et al. 2021), there is usually little direct evidence of how species interact ecologically, nor is there data explicitly collected in the majority of Phanerozoic-scale paleontological studies to robustly estimate spatial and resource-use overlap between purported interactors. Studies done at broad spatial scales i.e. globally or regionally (Gould and Calloway 1980; Alroy 1996, 2009; Liow et al. 2015; Silvestro et al. 2015) often do not explicitly verify the interactions implied. Some paleontological studies rely on a priori assumptions of relevant niche overlap, typically based on ecomorphological inferences and/or on the ecology of extant relatives (Van Valkenburgh 1995; Nascimento et al. 2024). Therefore, it is the initial choice of the species pool (e.g. species within a clade, or species of different clades), usually based on natural history knowledge (Van Valkenburgh 1999; Nascimento et al. 2024), that defines the ecological arena where interactions are thought to be relevant, and where we might expect to see changes in diversity or diversification rates. Because the fossil record is strongly unevenly distributed over space (Allison and Briggs 1993; McGowan and Smith 2008; Vilhena and Smith 2013; Close et al. 2020), and because some key ecological mechanisms (including biotic interactions) that control biodiversity change, operate at more local scales, spatial (Benson et al. 2021) and more direct ecological (Liow et al. 2016) information should be explicitly incorporated in these diversity and diversification analyses where possible (see section “Newer Avenues”). Some studies are more explicit about individual-level biotic interactions and their underlying mechanisms at local scales (e.g. Baumiller 1990; Robins and Klompmaker 2019), even when evaluating diversification dynamics over larger geographic areas (Lupia et al. 1999; Lidgard et al. 2021; Toivonen et al. 2022) and we encourage more of those. There should also be more studies that systematically explore the relationship between spatial overlap and potential interactions, including the extreme case of competitive exclusion (e.g. Klompmaker and Finnegan 2018).
Consider the Changing Intensity of Interactions Experienced by Species
A population or a species may be exposed to different important interactors through their evolutionary lifespans (Fig. 1). Capturing the changing composition (Fig. 2), and intensity of combined interspecific competition experienced through the evolutionary history of an individual lineage (e.g. a species) can give more information on ecological and evolutionary processes (Graciotti et al. 2023). Graciotti et. al (2023) characterized potentially shared ecological resources among temporally and spatially overlapping species (Fig. 2A) in the Canidae using similarity in tooth morphology and body size (Fig. 2B), spatial overlap (Fig. 2C) or the lack thereof due to competitive exclusion (Hardin 1960), with the aim of quantifying the average changing intensity of biotic interactions for species within a given clade (Fig. 2D). Although measuring ecological and spatial overlap per se is not new (Pineda-Munoz et al. 2021; Christison et al. 2022) such measurements can be used to explicitly investigate if substantial increase in temporal, spatial and/or ecological intersections among species are associated with changes in speciation and extinction regimes (Graciotti et. al 2023).
Figure. 2. The changing intensity of inter-specific interactions experienced by species within a given clade.
A, Estimates of species longevities allow one to estimate temporal coexistence among species within a given region. B, Morphospace characterization allows one to estimate the distance (similarity) of species within a region. C, The geographical coordinates of fossil specimens allow one to estimate the spatial overlap among different species. D, A time series (competition index) that takes into account temporal, spatial and morphological overlap is built to describe the average intensity of competition among species within a given clade. Here, smaller distances among species represent a more “crowded” scenario and hence higher intensity of competition.
Consider Trait-Based Diversification Models
It is increasingly common to apply trait-dependent diversification models, commonly known as SSE models (Maddison et al. 2007; FitzJohn 2010, 2012) to comparative data given the increasing availability of larger phylogenies. Here, phenotypic and behavioral traits can used as a proxies for specific biotic interactions, making it possible to investigate if biotic interactions are associated with different diversification regimes (Gómez and Verdú 2012; Foisy et al. 2019). For example, by characterizing plant species with respect to the presence or absence of traits that allow them to engage in mutualistic interaction with ants, it has been demonstrated plant lineages in mutualistic relationships with ants have higher diversification rates (Weber and Agrawal 2014). Along the same vein, how plant defenses affect diversification regimes has also been studied (Foisy et al. 2019). The effect of different diets on diversification regimes, where taxa with more omnivorous diets have lower diversification rates, possibly due to lower speciation rates rather than lower extinction rates (Price et al. 2012; Burin et al. 2016), can also be investigated in the fossil record, for instance in mammals (Jernvall and Fortelius 2004).
While many of the empirical examples cited above stem from extant lineages, it is highly feasible to apply similar trait-dependent diversification models (Porto et al. 2023) on the phylogenies of extinct taxa (Wright et al. 2022), or in the absence of phylogenies, by using traits as covariates in commonly used diversification rate models that are phylogeny-free (Liow and Nichols 2010; Silvestro et al. 2014; Warnock et al. 2020).
Open Questions in Species Interactions and Lineage Diversification
Many questions remain to be answered, for instance: do regional patterns and lower spatial scale processes simply scale-up to global levels, hence allowing us to detect global signals? In other words, what are the mechanisms that allow local ecological processes to cascade to macroevolutionary rates? Do interspecific biotic interactions have a major role in maintaining biological diversity? Are there circumstances under which ecological processes quantified as “strong” on short-time scales will be erased over macroevolutionary time scales? How do abiotic variables interact with ecological interactions to influence lineage diversification rates? Can we build trait-dependent models to infer the role of species interactions where the trait itself (and hence the description of how a species interacts with other species) changes through time? Are the macroevolutionary effects of species interactions predictable from their ecological effects on individual survival or population dynamics (sensu Jablonski 2008; Zeng and Wiens 2021)?
ii). Species Interactions and Biogeographical Dynamics
Interactions among species can influence the biogeographical distribution of whole clades by causing them to go extinct in some regions or by preventing them from entering others (Pires et al. 2015, 2017; Silvestro et al. 2015; Cantalapiedra et al. 2017; Stigall 2019). Dispersals into new regions range from ephemeral invasions of a single lineage to a restricted region to the synchronous invasions of multiple lineages at a global scale (Stigall 2019). Such dispersals might produce no noticeable changes in biodiversity structure at the local scale (Fraser and Lyons 2020) but at the other extreme may result in faunal homogenization and biotic crisis at a global scale (Stigall 2019). Even though the preservation of fossils is spatially heterogeneous (Benson et al. 2021), the fossil record is the only direct information on the past geographical distribution of species. Relying only on extant species for historical biogeographical inferences can be misleading (Lieberman 2002; Wisniewski et al. 2022). Previous paleontological studies have shown how different regions exchanged lineages over time (Marshall et al. 1982; Webb 1991; Webb and Opdyke 1995; Woodburne 2010), how continental isolation resulted in unique faunas (Simpson 1980), how immigration of new lineages changed the community structure (Patzkowsky and Holland 2007), and how the arrival of novel lineages into new regions might have affected the diversification dynamics of both the newcomers and the residents through ecological interactions (Silvestro et al. 2015; Pires et al. 2017; Stigall 2019).
In the terrestrial realm, for instance, it has been suggested that the Great American Biotic Exchange was strongly influenced by predation (Faurby and Svenning 2016), using arguments supported by phenotypic and behavioral traits. Here, successful mammal dispersal from South American into North America is associated with larger body size and arboreality, and presumably lower predation risks. In the marine realm, the Great Devonian Interchange is a well-documented event of biotic exchange (McGhee Jr 1996; Rode and Lieberman 2004; Stigall 2010, 2012, 2019) that could be further studied using model-based inference from a species interaction point of view (see below).
Interspecific interactions have been suggested as the underlying reason for clade replacement or the incumbency of resident species among different biogeographic regions, but largely based on indirect evidence including temporal patterns of waxing and waning in the taxonomic richness of different clades, or from fits to diversity-dependent models (Webb 1976; Marshall et al. 1982; Silvestro et al. 2015; Pires et al. 2017). However, some morphological and temporal analyses of fossil data have found no evidence for the role of species interactions in patterns suggesting clade-clade competition. For example, the “replacement” of Sparassodonta (a South American lineage of carnivorous marsupials) has been interpreted to result from the immigration of carnivores belonging to the placental Order Carnivora, suggesting that the negative interaction between these predators caused the extinction of Sparassodonta. Yet, the environmental changes due to the Andean uplift, changes in temperature and sea level, as well as changes in their prey diversity better explain the decline in Sparassodonta, than biotic interactions with their putative placental competitors as shown using model-based analysis including diversity-dependent models (Pino et al. 2022; Tarquini et al. 2022). Moreover, there is no evidence of substantial temporal overlap of ecomorphological similar species among Carnivora and Sparassodonta, suggesting that the later invasion by large predators such as felids and canids represent the occupation of ecological roles that have been left empty by the extinction of Sparassodonta (Prevosti et al. 2013; Pino et al. 2022; Tarquini et al. 2022). Yet, in all these studies, while patterns are observed at the community level (dashed ovals in Fig. 1) and/or at clade level, although the processes implied are at the level of individuals.
Modeling Immigration, Emigration and Regional Extinction
The Sparrasodonta/Carnivora case exemplifies the importance of considering ecological information and model-based approaches when studying biogeographic dynamics using fossil data (Silvestro et al. 2016). Dispersal, extinction and fossil sampling rates can be jointly estimated, while simultaneously examining the potential roles of phenotypic trait changes or external time series such as temperature via covariate modeling (Silvestro et al. 2016). One can allow diversity-dependent immigration and extinction rates and build models where the immigration of lineages to a new region is associated with higher extinction rates of resident lineages or with a given trait (Hauffe et al. 2022). An empirical application of such biogeographic models found higher immigration rates with increased body size for carnivore mammals that moved between North America and Eurasia (Hauffe et al. 2022). There are many ways to expand on the above-mentioned and other biogeographically explicit models (see Sukumaran and Knowles 2017 and references therein), for instance by considering the ecological similarity or functional overlap between invaders and residents, or by allowing for the explicit interaction between biotic (e.g. phenotypic traits that are demonstrably linked to ecological interactions) and abiotic environmental factors to dictate immigration and extinction dynamics. By building models that estimate comparable parameters, we might be able to start seeing commonalities among known invasions or biotic exchange e.g. Great American Biotic Exchange, Great Devonian Interchange mentioned above, and many others (Roy 1996; Patzkowsky and Holland 2007).
Integrate Trait and Biogeographical Evolution
Quintero and Landis (2020) presented a phylogenetic model where trait and geographical evolution affect each other, by expanding on a model where the traits on a set of lineages depend on the traits of other (interacting) lineages (Nuismer and Harmon 2015). This allows for a more coherent view of how species interactions might influence both traits and dispersal, and hence how such interactions mediate species and functional diversity in different regions. As Quintero and Landis (2020) admitted, their implementation does not include extinct species and their interactions. We could take advantage of being able to infer past spatial distributions and the changing occupation of morpho(eco)-space more directly using the fossil record when building such biogeographic models, with or without explicit topological information from phylogenies. Species distribution models, used with both fossil and contemporary data, also crucial to projecting future climate impacts including range shifts and extinction risk, should include biotic interactions for better inferences (Cosentino et al. 2023; Franklin 2023).
Include Biotic Interactions in Niche Modeling
The latitudinal diversity gradient (LDG) is a phenomenon well-documented in both contemporary species and in the fossil record (Fraser et al. 2014; Mannion et al. 2014; Marcot et al. 2016; Jablonski et al. 2017; Saupe 2023) with standing hypotheses that the LDG could be at least in part driven by the intensity of biotic interactions (Schemske et al. 2009). Yet, niche modeling, commonly pursued in paleobiology also when considering LDGs (Saupe et al. 2019), seldom explicitly consider species interactions. Because species interactions, rather than just dispersal limits or environmental constraints, can limit geographic range expansion over evolutionary time (Pigot and Tobias 2013), it will be fruitful to explicitly include species interactions when modeling niches to study the changing distribution of species, whether in an LDG context or beyond.
Open Questions in Species Interactions and Biogeographic Dynamics
What are the relative contributions of species interactions (versus abiotic niche or climate shifts) to successful dispersal and survival of residents in the face of invaders? Are “exchanges” and “invasions” predictable, given the potential invaders and residents and their traits, or given the spatial or environmental backdrop of such biogeographic dynamics? Are certain types of interactions (e.g. mutualisms, predation, or disease) more prevalent in restricting or permitting such dynamics? Are biogeographic processes dictated by biotic interactions different in the marine versus terrestrial realm? How do we incorporate information on interactions between individuals into biogeographic analyses?
iii). Species Interactions and Trait Evolution
Vermeij argued that interspecific enemies are the most important agents of evolution on geological time scales, where morphological traits pertaining to negative interactions are key inferential data (Vermeij 1987, 1994). Escalation, the evolution of functional traits to counter increasing pressure from predators and competitors, is thought to have promoted the Marine Mesozoic Revolution (Vermeij 1977), a restructuring of the functional ecology of especially the benthic realm, accompanied by the origination and decline of different groups. Evidence for (Aberhan et al. 2006; Sallan et al. 2011) and against escalation (Madin et al. 2006) as a driving force in diversity changes have been both presented, although traits relevant for the implied interactions are seldom the focus of such analyses. Lindberg and Pyenson (2007) argued that echolocation in early whales (implied by acoustically isolated fossil ear bones) could have been an adaptation for feeding on cephalopods, and used a combination of temporal, geographical and depth distribution data to test their idea (see also Kiel et al. 2022). A rare example with a trait focus is a recent study of body size evolution in lagomorphs (Tomiya and Miller 2021). Here, lagomorphs were found to be morphologically constrained by other distantly related herbivores that co-occurred and used similar resources.
Competition can occur across unrelated species (e.g. Tomiya and Miller 2021), or among individuals of the same species, or among closely related species (e.g. Meachen and Samuels 2012). Trait changes that occur as a consequence of interspecific interactions among closely related species were termed character displacement (and its converse, character release) in a seminal paper by Brown and Wilson (1956), a topic heavily researched among contemporary species. Such evolutionary trait changes attributable to competition and predation, while more easily demonstrated in contemporary species (Grant and Grant 2006) have also been demonstrated in scattered studies using the fossil record (Schindel and Gould 1977; Meachen and Samuels 2012). One particularly satisfying example fulfilling the criteria set up by Schluter and McPhail (1992) to identify character release is where a relaxation of competitive pressure from a close relative due to the latter’s extinction demonstrably led to morphological changes in the surviving Montastraea corals (Pandolfi et al. 2002). Ideas of character displacement have also been applied in clade-wide patterns of trait evolution that could arguably have results from clade-level competition (Benson et al. 2014).
Pursing more Fossil Studies of Character Displacement
There are few fossil studies as detailed and stringent like the one on Montastaea (Pandolfi et al. 2002) and without similar studies in different ecosystems and taxa, generalities in the relationship between trait evolution and biotic interactions may be difficult to uncover. It is perhaps challenging to fulfill criteria including co-existence, ruling out environmental drivers of phenotypic change, associating phenotypic change with resource shifts (Schulter and McPhail 1992), among others when studying species in the fossil record, which Pandolfi et al. (2002) achieved for their system. However, benthic systems in which sessile congeneric species (including corals, bryozoans, sponges, bivalves, all of which preserve in the fossil record) potentially co-occur could be good candidates for generating more case studies.
Focusing on Morphological Traits with Clear Implications for Biotic Interactions
Some commonly studied fossil traits like mammal teeth are gateways to understanding biotic interactions, because they reflect the types of plants and/or animal prey the organism is ingesting. For instance, Slater (2015) studied the ‘relative lower grinding area’ that exhibits distinctly different values in hyper- through hypocarnivorous canids in the context of ecological opportunity and interspecies competition. There is also a vast literature on vegetation/climate change and herbivore teeth evolution (MacFadden 2000; Mihlbachler et al. 2011; Strömberg et al. 2013; Toljagić et al. 2017). One natural expansion of these types of studies is to examine a phenotypic time series of an aspect of morphology that is demonstrably related to an “interactor” for single lineages with the main aim of estimating how much influence the interactor has on the morphology of the focal lineage. Dietary information can also be attained in the form of stable isotopes to answer different ecological questions (West et al. 2006; Clementz 2012), including the study of resource partitioning (MacFadden et al. 2004; Domingo et al. 2013; Hassler et al. 2018). Independent sources of phenotypic information such as stable isotopes and morphology can complement each other to better characterize the interspecific interactions of extinct species.
Open Questions in Species Interactions and Trait Evolution
Are traits better explained by constraints (developmental, genetic, geometric, phylogenetic), biotic interactions, the abiotic environment, their interactions, or some combination of these, and on what time scales? Do negative interactions (like predation) matter more than positive ones (like mutualism) for trait evolution, and on what time scales? How often are important biotic interaction traits not morphological ones (e.g. chemical defenses) or unpreserved (soft tissue) and do we have ways of inferring them using contemporary analogues or other signatures left in the fossil record or on inferred phylogenies? How can diverse sources of information (e.g. morphological and isotopic) be combined to better study biotic interactions in deep time?
Newer Avenues
In the previous sections, we described some natural directions, based on the more recent literature, in which to extend our research into realms of more nuanced understanding of the consequences of biotic interactions for diversification, biogeographic distributions and trait evolution. Below, we highlight and expand on these and more avenues that we feel are potentially fruitful.
1. Estimate Changing Biotic Interactions Perceived by Focal Lineages as They Age
Paleobiologists are in a particularly good position to study if and how the changing composition of species interactions might modulate rates of speciation and extinction rates. Using the fossil record we could more directly characterize how individual species interact with other species since its time of origin and extinction. This approach would represent a natural extension to the approach proposed by Graciotti et. al 2023. Instead of estimating an average "competition index" for a pool of species, we could estimate a "competition index" for each species individually, akin to what has been done for measuring niche overlap and partitioning for contemporary species (Lovari et al. 2015), but with one very important difference: temporal information. Here, the trait is viewed as dynamic over the evolutionary lifespan of each individual species. Under this approach the intensity of competition for each individual species can vary differently as new species are added to the system (Fig. 3), where the fossil record can help to estimate times of origination and extinction for individual species. While methodological development will be needed (such as building a trait-dependent process model e.g. using linear SDEs see Reitan and Liow 2019), this seems a plausible way forward (Kelley et al. 2024). This type of approach could also be adapted to evaluate the effect of predation on species extinction probability, by estimating the temporal and spatial overlap between each species and their putative predators.
Figure. 3. Illustration on how to consider the effect of competition on individual species.
A, Species longevities displaying the focal species (longevity shown in red) and the other species with which it coexists in space and time (longevities shown in blue). B, The resulting hypothetical index of competition, showing how the “crowding effect” (as measured by temporal, spatial and ecomorphological overlap) felt by the focal species (red) increases as it “ages” through time. The competition index could be estimated by measuring the temporal, spatial and niche overlap for each species individually in a similar manner as presented in Fig. 2. The main difference is that here the temporal, spatial and ecomorphological similarity is measured with respect to a single focal species.
2. Link Short-term Ecological Processes to the Long-Term Patterns
There is evidence that competition among species of planktonic foraminifera affected macroevolutionary patterns (Ezard et al. 2011), yet competition among individuals of different foraminifera species does not seem to drive shorter term population dynamics (Rillo et al. 2019). This mismatch between levels/scales suggests new questions and analyses that can help shed light on the underlying processes of biotic interactions, trait and lineage evolution. Trade-offs between individual survival and reproduction, both of which have consequences for population growth, dispersal, and the invasion of new areas, are processes that are modulated by resource competition, predation and disease, typifying the biotic interactions paleobiologists often study using fossils. Studies of inferred (fossil) population structure (Kurtén 1958; Van Valen 1963) shows us how to estimate population structure and infer reproduction and survival, the latter which can also be modeled directly, e.g. using predation scar data (Budd and Mann 2019). This can be combined with phenotypic time series and biotic interactions of the same species to understand how biotic interactions might influence longer term processes. One example of such a study uses cheilostome bryozoans to investigate any detectable signal of spatial competition on the evolution of trait size within a lineage (Di Martino and Liow 2021), given a signal of larger size being advantageous when encountering spatial competitors. In this example individual colonies with smaller autozooid present higher fecundity, but the long-term trend in autozooid size evolution does not suggest a consistent decrease through geological time, suggesting that biotic interactions (and perhaps climatic factors such as temperature), might influence long-term evolutionary outcomes (Di Martino & Liow 2021). In addition, studies of biotic interactions linked to macroevolutionary patterns often collate ecological characters at levels higher than the individual (Fig. 1). We suggest that it might be important to weigh the impact of any such biotic interactions by the size and density of the organisms involved (e.g. the abundance of parasites and competitors), rather than just the presence thereof. For example, in Fig. 3, many species spatially and temporally overlap with the focal (red) species, but commonly or densely occurring interacting species may impact the focal species more than rarer ones, and the abundance of each species are likely to change through their lineage duration (Foote et al. 2007; Liow et al. 2010). We also note that while some interactions are chronic (e.g. herbivory), others are rare but sometimes with extreme demographic consequences (e.g. disease that cause rapid mortality).
3. Use Phylogenetic Approaches Beyond Typical Trait-Based Diversification Models
Some species interactions are easier to observe than others. Parasites are pervasive and there is no known organism where parasites are absent, and their evolutionary consequences are well-studied in some groups (e.g. Wolbachia) (Werren et al. 2008). They are also postulated to drive adaptive radiations (Karvonen and Seehausen 2012). The fossil record of hosts, which are often more “visible” than their parasites, provide information on the timing of host diversification, which when combined with other types of information (e.g. the phylogeny of parasites) can help us estimate the evolutionary history of interactors that may not leave as much of a record in rocks (Warnock and Engelstädter 2021). Cophylogenetic models (Dismukes et al. 2022; Mulvey et al. 2022) could help estimate the timings and rates of evolutionary changes that can be potentially attributed to biotic interactions, even when one of the partners is “invisible”. Hence the combined use of fossil record and molecular phylogenies might help us better investigate types of interactions that are not easily studied when using either type of data alone.
4. Explore Theoretical Ecological Models Using Simulations
Ecological theory is rightly incorporated in studying the fossil record. For instance, as already mentioned, Sepkoski used growth models commonly used in population ecology to understand taxonomic diversification and inter-clade dynamics on the time scales of millions of years. Likewise, more recent studies use extended versions of island and historical biogeography models that to investigate the potential role of biotic interactions (by assuming a carrying capacity and a diversity-dependent dynamics) on determining lineage dispersal and extinction rates (Hauffe et al. 2020). Because paleoecological biotic interactions and communities are no different in their generating processes than contemporary ones, we should be seeing a greater use of established and/or promising ecological (and other biologically rooted) theories as conceptual frameworks to understand expectations and deviations (Hubbell 2005). One new example uses Hubbell’s neutral theory (Hubbell 2001), to explore models of population growth and species survival, the latter which can be studied best in the fossil record (Saulsbury et al. 2024). Although there is a body of work estimating past trophic networks within communities (Roopnarine et al. 2019; Shaw et al. 2021), inferring biotic interactions via proxies is not trivial (Morales-Castilla et al. 2015). A combination of modeling, simulations, whether at the level of populations and phenotypes (Yoder & Nuismer 2010) or individuals within networks (Maliet et al. 2020), may be very helpful in interpreting past communities and inferring the interactions among taxa. Even though there are difficult challenges of inference posed for contemporary (and past) communities (Morales-Castilla et al. 2015), some of these challenges are being gradually resolved (Pomeranz et al. 2019).
General Recommendations
1. Ditch the Dichotomy
Continued obsession in the dichotomous choice of trying to answer the question “is it climate or is it biotic interactions that drives diversity” is still commonplace in the literature. We advocate for including presumably important variables, both environmental and biotic, and for studying how environmental and biotic factors interact (Jablonski 2008) when the aim is to understand the contributions of each to evolution (Ezard et al. 2011; Condamine et al. 2019), rather than just using a single purported class of drivers as an alternative hypothesis to test against a strawman null.
2. Distinguish Correlation Versus Causation
Many studies still verbally interpret statistical associations (e.g. correlations, significant slopes) as causal relationships, but we argue that we should be mindful when talking about “drivers” when analyses are designed to demonstrate statistical associations rather than driven processes, and, when possible, use approaches that more directly establish causal connections (Hannisdal and Liow 2018). We should also explicitly consider unmeasured (aka hidden or latent) variables where reasonable (Reitan and Liow 2017; Boyko and Beaulieu 2021) as the measured/analyzed factor or variable may not be the (direct or even indirect) underlying force behind the pattern.
3. Focus on Ecological Mechanisms
Diversity-dependence and other approaches to study biotic interactions ultimately aim at understanding the macroevolutionary consequences of ecological processes happening at the level of the individual. Harnessing the limits of what is possible in the fossil record, we urge more effort on verifying purported ecological interactions using different lines of evidence beyond co-occurrence (Pandolfi et al. 2002; Tomiya and Miller 2021) with focus on (individual) specimen-level information, and/or devising time series that more closely depict the interaction (Graciotti et al. 2023). Can we eliminate other factors (e.g. environmental shifts, sampling artifacts) and attribute evolutionary change to biotic interactions? What mechanisms at the ecological level are responsible for the downstream evolutionary changes? Biotic interactions inferred from the fossil record and their downstream consequences can be “ground-truthed” using living relatives or analogous systems based on laboratory or natural experiments.
4. Emphasize Model-Based Inferences and Method Development
Model-based inferences in macroevolution (Harmon et al. 2019) and paleobiology are highly desirable as these can not only help us test hypotheses but also give estimates of evolutionary, ecological and observational parameters, to further understanding and help link empirical estimates in paleobiology to those in related fields. Much of paleontology is still descriptive, even though the descriptions are now more quantitative rather than verbal. A focus on method development, drawing strength from related, but more established fields, rather than ad hoc (re)-inventions can help paleobiology mature faster. For instance, Silvestro and colleagues build models that carefully consider speciation and extinction rates that are (more) equivalent to those estimated using birth-death processes with estimated genealogies (Silvestro et al. 2018, 2019). Similarly, statistical ecology is a well-established but rapidly developing field which has barely been harnessed in paleobiology (Nichols and Pollock 1983; Reitan et al. 2022; Smith et al. 2022); formal time-series analyses are crucial for our historical field but tidy tools developed in economics and finance need to be expanded to include un-evenly spaced, complex data like the paleontological and geological data we work with (Hannisdal 2011; Hannisdal and Liow 2018; Reitan and Liow 2019).
5. Contribute Ectively to Common, Integrative Goals
Sciences with greater maturity tend to have common goals and become more collaborative. Increasingly, macroevolutionary patterns and their potential drivers are inferred not just from the fossil record (Raup and Sepkoski 1982) or molecular phylogenies alone (Jetz et al. 2012) but using a combination of the two (Slater and Harmon 2013; Warnock and Engelstädter 2021; Wright et al. 2022). Diverse data, including morphology, spatial distribution, ecology, environment, biochemicals can all be used to model and/or constrain our inferences (Liow et al. 2023). It is time for paleobiology to become even more collaborative and coordinated. The Paleobiology Database serves us well for many purposes, but specimen-based interaction databases (Klompmaker et al. 2019; Petsios et al. 2021; Huntley et al. 2023) can help propel us into the next generation of analyses for biotic interactions. Currently, biotic interaction papers based on the fossil record are idiosyncratic, but if we have clearer ideas about what is important measure to estimate for drawing general inferences, e.g. interaction strength (Wootton and Emmerson 2005), we can all aim at comparable quantification.
Conclusion
Biotic interactions can seem to be more amenable to study among contemporary flora and fauna, yet without the fossil record, it is extremely challenging to estimate their contributions to macroevolution, especially extinction or to infer extinct interactors, traits and ancient environments. The deep past leaves marks of bottlenecks, constraints, and revolutions, which can influence the current day eco-evolutionary dynamics of species interactions. The fossil record is also a superior source of information for long-term population dynamics. By studying (paleo)ecology we can fruitfully consequences of biotic interactions on macroevolution and contribute to micro-macro debate.
Non-technical Summary.
Every animal and plant interact with many other individuals, including disease-causing organisms, prey items or pollinators, throughout their lives. These interactions necessarily contribute to the ecological and evolution processes that are associated with the diverse forms of life that we observe. It is comparatively easy to study such biotic interactions among living organisms, but more challenging to investigate such relationships when the organisms involved are dead or their species, extinct. We discuss how paleobiologists have studied biotic interactions in the last 50 years, then suggest new avenues of research we could continue to fruitfully explore.
Acknowledgments
This project has received funding from the European Research Council (ERC) under the Europoean Union’s Horizon 2020 research and innovation programme (grant agreement No. 724324 to L.H. Liow) and from FAPESP (grant#2021/06780-4 to T.B. Quental). We thank D. Fraser, an anonymous reviewer, G. Dietl, and K. De Baets for their insightful suggestions. L. H. Liow Thanks the BITE working group supported by the Paleosynthesis project at Friedrich-Alexander-Universität Erlangen-Nürnberg for continued discussion on biotic interactions.
Footnotes
Competing Interests. The authors declare no competing interests.
Literature Cited
- Aberhan M, Kiessling W, Fürsich FT. Testing the role of biological interactions in the evolution of mid-Mesozoic marine benthic ecosystems. Paleobiology. 2006;32:259–277. [Google Scholar]
- Allison PA, Briggs DEG. Paleolatitudinal sampling bias, Phanerozoic species diversity, and the end-Permian extinction. Geology. 1993;21:65–68. [Google Scholar]
- Alroy J. Constant extinction, constrained diversification, and uncoordinated stasis in North American mammals. Palaeogeography, Palaeoclimatology, Palaeoecology. 1996;127:285–311. [Google Scholar]
- Alroy J. In: Speciation and Patterns of Diversity. Roger JRB, Butlin K, Schulter D, editors. Cambridge University Press; 2009. Speciation and extinction in the fossil record of North American mammals. [Google Scholar]
- Baack EJ, Rieseberg LH. A genomic view of introgression and hybrid speciation. Genomes and Evolution. 2007;17:513–518. doi: 10.1016/j.gde.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnosky AD. Distinguishing the effects of the Red Queen and Court Jester on Miocene mammal evolution in the Northern Rocky Mountains. Journal of Vertebrate Paleontology. 2001;21:172–185. [Google Scholar]
- Baumiller T. Nonpredatory drilling of Mississippian crinoids by platyceratid gastropods. Paleontology. 1990;33:743–748. [Google Scholar]
- Bellwood DR, Hoey AS, Bellwood O, Goatley CHR. Evolution of long-toothed fishes and the changing nature of fish–benthos interactions on coral reefs. Nature Communications. 2014;5:3144. doi: 10.1038/ncomms4144. [DOI] [PubMed] [Google Scholar]
- Benson RBJ, Butler R, Close RA, Saupe E, Rabosky DL. Biodiversity across space and time in the fossil record. Current Biology. 2021;31:R1225–R1236. doi: 10.1016/j.cub.2021.07.071. [DOI] [PubMed] [Google Scholar]
- Benson RBJ, Frigot RA, Goswami A, Andres B, Butler RJ. Competition and constraint drove Cope’s rule in the evolution of giant flying reptiles. Nature Communications. 2014;5:3567. doi: 10.1038/ncomms4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton MJ. The Red Queen and the Court Jester: species diversity and the role of biotic and abiotic factors through time. Science. 2009;323:728–732. doi: 10.1126/science.1157719. [DOI] [PubMed] [Google Scholar]
- Benton MJ. Exploring macroevolution using modern and fossil data. Proceedings of the Royal Society B: Biological Sciences. 2015;282:20150569. doi: 10.1098/rspb.2015.0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyko JD, Beaulieu JM. Generalized hidden Markov models for phylogenetic comparative datasets. Methods in Ecology and Evolution. 2021;12:468–478. [Google Scholar]
- Brown WL, Wilson EO. Character displacement. Systematic Zoology. 1956;5:49–64. [Google Scholar]
- Budd GE, Mann RP. Modeling durophagous predation and mortality rates from the fossil record of gastropods. Paleobiology. 2019;45:246–264. [Google Scholar]
- Burin G, Kissling WD, Guimarães Ç, Şekercioğlu ÇH, Quental TB. Omnivory in birds is a macroevolutionary sink. Nature Communications. 2016;7:11250. doi: 10.1038/ncomms11250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush AM, Payne JL. Biotic and abiotic controls on the Phanerozoic history of marine animal biodiversity. Annual Review of Ecology, Evolution, and Systematics. 2021;52:269–289. [Google Scholar]
- Cantalapiedra JL, Prado JL, Fernández Hernández M, Alberdi MT. Decoupled ecomorphological evolution and diversification in Neogene-Quaternary horses. Science (New York, NY) 2017;355:627–630. doi: 10.1126/science.aag1772. [DOI] [PubMed] [Google Scholar]
- Christison BE, Gaidies F, Pineda-Munoz S, Evans AR, Gilbert MA, Fraser D. Dietary niches of creodonts and carnivorans of the late Eocene Cypress Hills Formation. Journal of Mammalogy. 2022;103:2–17. doi: 10.1093/jmammal/gyab123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz MT. New insight from old bones: stable isotope analysis of fossil mammals. Journal of Mammalogy. 2012;93:368–380. [Google Scholar]
- Close RA, Benson RBJ, Alroy J, Carrano MT, Cleary TJ, Dunne EM, Mannion PD, et al. The apparent exponential radiation of Phanerozoic land vertebrates is an artefact of spatial sampling biases. Proceedings of the Royal Society B: Biological Sciences. 2020;287:20200372. doi: 10.1098/rspb.2020.0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates AG, Jackson JBC. Clonal growth, algal symbiosis, and reef formation by corals. Paleobiology. 1987;13:363–378. [Google Scholar]
- Condamine FL, Romieu J, Guinot G. Climate cooling and clade competition likely drove the decline of lamniform sharks. Proceedings of the National Academy of Sciences. 2019;116:20584–20590. doi: 10.1073/pnas.1902693116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell JH. On the rrevalence and relative importance of interspecific competition: evidence from field experiments. American Naturalist. 1983;122:661–696. [Google Scholar]
- Connell JH. Perspectives on Plant Competition. Academic Press; 1990. Apparent versus “real” competition in plants; pp. 9–23. [Google Scholar]
- Cosentino F, Seamark ECJ, Van Cakenberghe V, Maiorano L. Not only climate: The importance of biotic interactions in shaping species distributions at macro scales. Ecology and Evolution. 2023;13:e9855. doi: 10.1002/ece3.9855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Baets K, Huntley JW, Scarponi D, Klompmaker AA, Skawina A. Phanerozoic parasitism and marine metazoan diversity: dilution versus amplification. Philosophical transactions of the Royal Society of London Series B, Biological Sciences. 2021;376:20200366. doi: 10.1098/rstb.2020.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino E, Liow LH. Trait-fitness associations do not predict within-species phenotypic evolution over 2 million years. Proceedings of the Royal Society B-Biological Sciences. 2021;288:20202047. doi: 10.1098/rspb.2020.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietl GP, Kelley PH. The fossil record of predator-prey arms races: coevolution and escalation hypotheses. The Paleontological Society Papers. 2002;8:353–374. [Google Scholar]
- Dismukes W, Braga MP, Hembry DH, Heath TA, Landis MJ. Cophylogenetic methods to untangle the evolutionary history of ecological interactions. Annual Review of Ecology, Evolution, and Systematics. 2022;53:275–298. [Google Scholar]
- Domingo MS, Domingo L, Badgley C, Sanisidro O, Morales J. Resource partitioning among top predators in a Miocene food web. Proceedings of the Royal Society B: Biological Sciences. 2013;280:20122138. doi: 10.1098/rspb.2012.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezard THG, Aze T, Pearson PN, Purvis A. Interplay between changing climate and species’s ecology drives macroevolutionary dynamics. Science. 2011;332:349–351. doi: 10.1126/science.1203060. [DOI] [PubMed] [Google Scholar]
- Faurby S, Svenning J-C. The asymmetry in the Great American Biotic Interchange in mammals is consistent with differential susceptibility to mammalian predation. Global Ecology and Biogeography. 2016;25:1443–1453. [Google Scholar]
- FitzJohn RG. Quantitative traits and diversification. Syst Biol. 2010;59:619–633. doi: 10.1093/sysbio/syq053. [DOI] [PubMed] [Google Scholar]
- FitzJohn RG. Diversitree: comparative phylogenetic analyses of diversification in R. Methods in Ecology and Evolution. 2012;3:1084–1092. [Google Scholar]
- Foisy MR, Albert LP, Hughes DWW, Weber MG. Do latex and resin canals spur plant diversification? Re-examining a classic example of escape and radiate coevolution. Journal of Ecology. 2019;107:1606–1619. [Google Scholar]
- Foote M, Crampton JS, Beu AG, Marshall BA, Cooper RA, Maxwell PA, Matcham I. Rise and fall of species occupancy in Cenozoic fossil molluscs. Science. 2007;318:1131–1134. doi: 10.1126/science.1146303. [DOI] [PubMed] [Google Scholar]
- Foote M, Edie SM, Jablonski D. Ecological structure of diversity-dependent diversification in Phanerozoic marine bivalves. Biology Letters. 2024;20:20230475. doi: 10.1098/rsbl.2023.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortelius M, Solounias N. Functional characterization of ungulate molars using the abrasion-attrition wear gradient: a new method for reconstructing paleodiets. 2000 [Google Scholar]
- Franklin J. Species distribution modelling supports the study of past, present and future biogeographies. Journal of Biogeography. 2023;50:1533–1545. [Google Scholar]
- Fraser D, Hassall C, Gorelick R, Rybczynski N. Mean annual precipitation explains spatiotemporal patterns of Cenozoic mammal beta diversity and latitudinal diversity gradients in North America. PloS One. 2014;9:e106499. doi: 10.1371/journal.pone.0106499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser D, Lyons SK. Mammal community structure through the Paleocene-Eocene Thermal Maximum. The American Naturalist. 2020;196:271–290. doi: 10.1086/709819. [DOI] [PubMed] [Google Scholar]
- Fraser D, Soul LC, Tóth AB, Balk MA, Eronen JT, Pineda-Munoz S, Shupinski AB, et al. Investigating biotic interactions in deep time. Trends in Ecology & Evolution. 2021;36:61–75. doi: 10.1016/j.tree.2020.09.001. [DOI] [PubMed] [Google Scholar]
- Gahn FJ, Baumiller TK. Arm regeneration in Mississippian crinoids: evidence of intense predation pressure in the Paleozoic? Paleobiology. 2005;31:151–164. [Google Scholar]
- Gómez JM, Verdú M. Mutualism with plants drives primate diversification. Systematic Biology. 2012;61:567–577. doi: 10.1093/sysbio/syr127. [DOI] [PubMed] [Google Scholar]
- Gorzelak P, Salamon MA, Baumiller TK. Predator-induced macroevolutionary trends in Mesozoic crinoids. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:7004–7007. doi: 10.1073/pnas.1201573109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould SJ, Calloway CB. Clams and brachiopods - ships that pass in the night. Paleobiology. 1980;6:383–396. [Google Scholar]
- Graciotti RP, Porto L, Quental T. Ecological and spatial overlap indicate interspecific competition during North American Canid radiation. bioRxiv. 2023:2023.12.14.571772 [Google Scholar]
- Grant PR, Grant BR. Evolution of character displacement in Darwin’s finches. Science. 2006;313:224–226. doi: 10.1126/science.1128374. [DOI] [PubMed] [Google Scholar]
- Hannisdal B. Non-parametric inference of causal interactions from geological records. American Journal of Science. 2011;311:315–334. [Google Scholar]
- Hannisdal B, Liow LH. Causality from palaeontological time series. Palaeontology. 2018;61:495–509. [Google Scholar]
- Hardin G. The competitive exclusion principle. Science. 1960;131:1292–1297. doi: 10.1126/science.131.3409.1292. [DOI] [PubMed] [Google Scholar]
- Harmon LJ, Andreazzi CS, Débarre F, Drury J, Goldberg EE, Martins AB, Melián CJ, et al. Detecting the macroevolutionary signal of species interactions. Journal of Evolutionary Biology. 2019;32:769–782. doi: 10.1111/jeb.13477. [DOI] [PubMed] [Google Scholar]
- Hassler A, Martin JE, Amiot R, Tacail T, Godet FA, Allain R, Balter V. Calcium isotopes offer clues on resource partitioning among Cretaceous predatory dinosaurs. Proceedings Biological Sciences. 2018;285 doi: 10.1098/rspb.2018.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauffe T, Delicado D, Etienne RS, Valente L. Lake expansion elevates equilibrium diversity via increasing colonization. Journal of Biogeography. 2020;47:1849–1860. [Google Scholar]
- Hauffe T, Pires MM, Quental TB, Wilke T, Silvestro D. A quantitative framework to infer the effect of traits, diversity and environment on dispersal and extinction rates from fossils. Methods in Ecology and Evolution. 2022;13:1201–1213. [Google Scholar]
- Hembry DH, Weber MG. Ecological interactions and macroevolution: a new field with old roots. Annual Review of Ecology, Evolution, and Systematics. 2020;51:215–243. [Google Scholar]
- Hopkins SSB, Price SA, Chiono AJ. Influence of phylogeny on the estimation of diet from dental morphology in the Carnivora. Paleobiology. 2022;48:324–339. [Google Scholar]
- Hubbell SP. The Unified Neutral Theory of Biodiversity and Biogeography. Princeton University Press; 2001. [DOI] [PubMed] [Google Scholar]
- Hubbell SP. The neutral theory of biodiversity and biogeography and Stephen Jay Gould. Paleobiology. 2005;31:122–132. [Google Scholar]
- Huntley JW, Kowalewski M. Strong coupling of predation intensity and diversity in the Phanerozoic fossil record. Proceedings of the National Academy of Sciences. 2007;104:15006. doi: 10.1073/pnas.0704960104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley JW, Skawina A, Dowding EM, Dentzien-Dias P, De Baets K, Kocsis ÁT, Labandeira CC, et al. Biotic Interactions in Deep Time (BITE): developing a specimen-level database to address fundamental questions in ecology and evolution; Presented at the Geological Society of America; 2023. [Google Scholar]
- Jablonski D. Biotic interactions and macroevolution: extensions and mismatches across scales and levels. Evolution. 2008;62:715–739. doi: 10.1111/j.1558-5646.2008.00317.x. [DOI] [PubMed] [Google Scholar]
- Jablonski D, Huang S, Roy K, Valentine JW. Shaping the latitudinal diversity gradient: new perspectives from a synthesis of paleobiology and biogeography. American Naturalist. 2017;189:1–12. doi: 10.1086/689739. [DOI] [PubMed] [Google Scholar]
- Jernvall J, Fortelius M. Maintenance of trophic structure in fossil mammal communities: Site occupancy and taxon resilience. American Naturalist. 2004;164:614–624. doi: 10.1086/424967. [DOI] [PubMed] [Google Scholar]
- Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO. The global diversity of birds in space and time. Nature. 2012;491:444–448. doi: 10.1038/nature11631. [DOI] [PubMed] [Google Scholar]
- Karvonen A, Seehausen O. The role of parasitism in adaptive radiations—when might parasites promote and when might they constrain ecological speciation? International Journal of Ecology. 2012;2012:280169 [Google Scholar]
- Kelley PH, Dietl GP, Handley JC. Coevolutionary alternation as an ecological cause for stasis. Paleobiology. 2024 (ms in review) [Google Scholar]
- Kiel S, Goedert JL, Tsai C-H. Seals, whales and the Cenozoic decline of nautiloid cephalopods. Journal of Biogeography. 2022;49:1903–1910. [Google Scholar]
- Klompmaker AA, Finnegan S. Extreme rarity of competitive exclusion in modern and fossil marine benthic ecosystems. Geology. 2018;46:723–726. [Google Scholar]
- Klompmaker AA, Kelley PH, Chattopadhyay D, Clements JC, Huntley JW, Kowalewski M. Predation in the marine fossil record: Studies, data, recognition, environmental factors, and behavior. Earth-Science Reviews. 2019;194:472–520. [Google Scholar]
- Klompmaker AA, Kowalewski M, Huntley JW, Finnegan S. Increase in predator-prey size ratios throughout the Phanerozoic history of marine ecosystems. Science. 2017;356:1178–1180. doi: 10.1126/science.aam7468. [DOI] [PubMed] [Google Scholar]
- Krause DW. Competitive exclusion and taxonomic displacement in the fossil record; the case of rodents and multituberculates in North America. Rocky Mountain Geology. 1986;24:95–117. [Google Scholar]
- Kurtén B. The life and death a Pleistocene cave bear, a study in paleoecology. Acta Zoologica Fennica. 1958;95:1–59. [Google Scholar]
- Labandeira CC, Currano ED. The fossil record of plant-insect dynamics. Annual Review of Earth and Planetary Sciences. 2013;41:287–311. [Google Scholar]
- Labandeira CC, Dilcher DL, Davis DR, Wagner DL. 97-Million years of angiosperm-insect association – Paleobiological insights into the meaning of coevolution. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:12278–12282. doi: 10.1073/pnas.91.25.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidgard S, Di Martino E, Zagorsek K, Liow LH. When fossil clades ‘compete’: local dominance, global diversification dynamics and causation. Proceedings of the Royal Society B-Biological Sciences. 2021;288:20211632. doi: 10.1098/rspb.2021.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidgard S, McKinney FK, Taylor PD. Competition, clade replacement, and a history of cyclostome and cheilostome bryozoan diversity. Paleobiology. 1993;19:352–371. [Google Scholar]
- Lieberman BS. Phylogenetic biogeography with and without the fossil record: gauging the effects of extinction and paleontological incompleteness. Palaeogeography, Palaeoclimatology, Palaeoecology. 2002;178:39–52. [Google Scholar]
- Lindberg DR, Pyenson ND. Things that go bump in the night: evolutionary interactions between cephalopods and cetaceans in the tertiary. Lethaia. 2007;40:335–343. [Google Scholar]
- Liow LH, Di Martino E, Voje KL, Rust S, Taylor PD. Interspecific interactions through 2 million years: are competitive outcomes predictable? Proceedings of the Royal Society B-Biological Sciences. 2016;283:20160981. doi: 10.1098/rspb.2016.0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liow LH, Nichols JD. Hunt G, Alroy J, editors. Estimating rates and probabilities of origination and extinction using taxonomic occurrence data: Capture-recapture approaches. Short Courses in Paleontology: Quantitative Paleobiology Paleontological Society. 2010:81–94. [Google Scholar]
- Liow LH, Reitan T, Harnik PG. Ecological interactions on macroevolutionary time scales: clams and brachiopods are more than ships that pass in the night. Ecology Letters. 2015;18:1030–1039. doi: 10.1111/ele.12485. [DOI] [PubMed] [Google Scholar]
- Liow LH, Skaug HJ, Ergon T, Schweder T. Global occurrence trajectories of microfossils: environmental volatility and the rises and falls of individual species. Paleobiology. 2010;36:224–252. [Google Scholar]
- Liow LH, Uyeda J, Hunt G. Cross-disciplinary information for understanding macroevolution. Trends in Ecology & Evolution. 2023;38:250–260. doi: 10.1016/j.tree.2022.10.013. [DOI] [PubMed] [Google Scholar]
- Lovari S, Pokheral CP, Jnawali SR, Fusani L, Ferretti F. Coexistence of the tiger and the common leopard in a prey-rich area: the role of prey partitioning. Journal of Zoology. 2015;295:122–131. [Google Scholar]
- Lupia R, Lidgard S, Crane PR. Comparing palynological abundance and diversity: implications for biotic replacement during the Cretaceous angiosperm radiation. Paleobiology. 1999;25:305–340. [Google Scholar]
- MacFadden BJ. Cenozoic mammalian herbivores from the Americas: Reconstructing ancient diets and terrestrial communities. Annual Review of Ecology and Systematics. 2000;31:33–59. [Google Scholar]
- MacFadden BJ, Higgins P, Clementz MT, Jones DS. Diets, habitat preferences, and niche differentiation of Cenozoic sirenians from Florida: evidence from stable isotopes. Paleobiology. 2004;30:297–324. [Google Scholar]
- Maddison WP, Midford PE, Otto SP. Estimating a binary character’s effect on speciation and extinction. Syst Biol. 2007;56:701–710. doi: 10.1080/10635150701607033. [DOI] [PubMed] [Google Scholar]
- Madin JS, Alroy J, Aberhan M, Fursich FT, Kiessling W, Kosnik MA, Wagner PJ. Statistical independence of escalatory ecological trends in Phanerozoic marine invertebrates. Science. 2006;312:897–900. doi: 10.1126/science.1123591. [DOI] [PubMed] [Google Scholar]
- Maliet O, Loeuille N, Morlon H. An individual-based model for the eco-evolutionary emergence of bipartite interaction networks. Ecology Letters. 2020;23:1623–1634. doi: 10.1111/ele.13592. [DOI] [PubMed] [Google Scholar]
- Mannion PD, Upchurch P, Benson RBJ, Goswami A. The latitudinal biodiversity gradient through deep time. Trends in Ecology & Evolution. 2014;29:42–50. doi: 10.1016/j.tree.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Marcot JD, Fox DL, Niebuhr SR. Late Cenozoic onset of the latitudinal diversity gradient of North American mammals. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:7189–7194. doi: 10.1073/pnas.1524750113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall LG, Webb SD, Sepkoski JJ, Raup DM. Mammalian evolution and the Great American Interchange. Science. 1982;215:1351–1357. doi: 10.1126/science.215.4538.1351. [DOI] [PubMed] [Google Scholar]
- Martins MJF, Puckett TM, Lockwood R, Swaddle JP, Hunt G. High male sexual investment as a driver of extinction in fossil ostracods. Nature. 2018;556:366–369. doi: 10.1038/s41586-018-0020-7. [DOI] [PubMed] [Google Scholar]
- McGhee GR., Jr . The late Devonian mass extinction: the Frasnian/Famennian crisis. Columbia University Press; 1996. [Google Scholar]
- McGowan AJ, Smith AB. Are global Phanerozoic marine diversity curves truly global? A study of the relationship between regional rock records and global Phanerozoic marine diversity. Paleobiology. 2008;34:80–103. [Google Scholar]
- Meachen JA, Samuels JX. Evolution in coyotes (Canis latrans) in response to the megafaunal extinctions. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:4191–4196. doi: 10.1073/pnas.1113788109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihlbachler MC, Rivals F, Solounias N, Semprebon GM. Dietary change and evolution of horses in North America. Science. 2011;331:1178–1181. doi: 10.1126/science.1196166. [DOI] [PubMed] [Google Scholar]
- Moen D, Morlon H. Why does diversification slow down? Trends in Ecology & Evolution. 2014;29:190–197. doi: 10.1016/j.tree.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Morales-Castilla I, Matias MG, Gravel D, Araújo MB. Inferring biotic interactions from proxies. Trends in Ecology & Evolution. 2015;30:347–356. doi: 10.1016/j.tree.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Mulvey LPA, Warnock RCM, De Baets K. Where traditional extinction estimates fall flat: using novel cophylogenetic methods to estimate extinction risk in platyhelminths. Proceedings Biological Sciences. 2022;289:20220432. doi: 10.1098/rspb.2022.0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento JCS, Blanco F, Domingo MS, Cantalapiedra JL, Pires MM. The reorganization of predator–prey networks over 20 million years explains extinction patterns of mammalian carnivores. Ecology Letters. 2024;27:e14448. doi: 10.1111/ele.14448. [DOI] [PubMed] [Google Scholar]
- Nichols JD, Pollock KH. Estimating taxonomic diversity, extinction rates, and speciation rates from fossil data using capture-recapture models. Paleobiology. 1983;9:150–163. [Google Scholar]
- Nuismer SL, Harmon LJ. Predicting rates of interspecific interaction from phylogenetic trees. Ecology Letters. 2015;18:17–27. doi: 10.1111/ele.12384. [DOI] [PubMed] [Google Scholar]
- Pandolfi JM, Lovelock CE, Budd AF. Character release following extinction in a Caribbean reef coral species complex. Evolution. 2002;56:479–501. doi: 10.1111/j.0014-3820.2002.tb01360.x. [DOI] [PubMed] [Google Scholar]
- Patzkowsky ME, Holland SM. Diversity partitioning of a Late Ordovician marine biotic invasion: controls on diversity in regional ecosystems. Paleobiology. 2007;33:295–309. [Google Scholar]
- Petsios E, Portell RW, Farrar L, Tennakoon S, Grun TB, Kowalewski M, Tyler CL. An asynchronous Mesozoic marine revolution: the Cenozoic intensification of predation on echinoids. Proceedings of the Royal Society B: Biological Sciences. 2021;288:20210400. doi: 10.1098/rspb.2021.0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigot AL, Tobias JA. Species interactions constrain geographic range expansion over evolutionary time. Ecology Letters. 2013;16:330–338. doi: 10.1111/ele.12043. [DOI] [PubMed] [Google Scholar]
- Pineda-Munoz S, Jukar AM, Tóth AB, Fraser D, Du A, Barr WA, Amatangelo KL, et al. Body mass-related changes in mammal community assembly patterns during the late Quaternary of North America. Ecography. 2021;44:56–66. [Google Scholar]
- Pineda-Munoz S, Lazagabaster IA, Alroy J, Evans AR. Inferring diet from dental morphology in terrestrial mammals. Methods in Ecology and Evolution. 2017;8:481–491. [Google Scholar]
- Pino K, Vallejos-Garrido P, Espinoza-Aravena N, Cooper RB, Silvestro D, Hernández CE, Rodríguez-Serrano E. Regional landscape change triggered by Andean uplift: The extinction of Sparassodonta (Mammalia, Metatheria) in South America. Global and Planetary Change. 2022;210:103758 [Google Scholar]
- Pires MM, Silvestro D, Quental TB. Continental faunal exchange and the asymmetrical radiation of carnivores. Proceedings of the Royal Society of London B: Biological Sciences. 2015;282 doi: 10.1098/rspb.2015.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires MM, Silvestro D, Quental TB. Interactions within and between clades shaped the diversification of terrestrial carnivores. Evolution; International Journal of Organic Evolution. 2017;71:1855–1864. doi: 10.1111/evo.13269. [DOI] [PubMed] [Google Scholar]
- Pomeranz JPF, Thompson RM, Poisot T, Harding JS. Inferring predator–prey interactions in food webs. Methods in Ecology and Evolution. 2019;10:356–367. [Google Scholar]
- Porto LMV, Maestri R, Janzen T, Etienne RS. From fossils to living Canids: two contrasting perspectives on biogeographic diversification. bioRxiv. 2023 [Google Scholar]
- Prevosti FJ, Forasiepi A, Zimicz N. The evolution of the Cenozoic terrestrial mammalian predator guild in South America: competition or replacement? Journal of Mammalian Evolution. 2013;20:3–21. [Google Scholar]
- Price SA, Hopkins SSB, Smith KK, Roth VL. Tempo of trophic evolution and its impact on mammalian diversification Samantha A. Price. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:7008–7012. doi: 10.1073/pnas.1117133109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quental T, Marshall CR. Diversity dynamics: molecular phylogenies need the fossil record. Trends in Ecology & Evolution. 2010;25:434–441. doi: 10.1016/j.tree.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Quintero I, Landis MJ. Interdependent phenotypic and biogeographic evolution driven by biotic interactions. Systematic Biology. 2020;69:739–755. doi: 10.1093/sysbio/syz082. [DOI] [PubMed] [Google Scholar]
- Rabosky DL. Diversity-dependence, ecological speciation, and the role of competition in macroevolution. Annual Review of Ecology, Evolution, and Systematics. 2013;44:481–502. [Google Scholar]
- Raup DM. Cohort analysis of generic survivorship. Paleobiology. 1978;4:1–15. [Google Scholar]
- Raup DM, Sepkoski JJ. Mass extinctions in the marine fossil record. Science. 1982;215:1501–1503. doi: 10.1126/science.215.4539.1501. [DOI] [PubMed] [Google Scholar]
- Reitan T, Ergon TH, Liow LH. Relative species abundance and population densities of the past: developing multispecies occupancy models for fossil data. Paleobiology. 2022;49:23–38. [Google Scholar]
- Reitan T, Liow LH. An unknown Phanerozoic driver of brachiopod extinction rates unveiled by multivariate linear stochastic differential equations. Paleobiology. 2017;43:1–13. [Google Scholar]
- Reitan T, Liow LH. layeranalyzer: Inferring correlative and causal connections from time series data in R. Methods in Ecology and Evolution. 2019;10:2183–2188. [Google Scholar]
- Robins CM, Klompmaker AA. Extreme diversity and parasitism of Late Jurassic squat lobsters (Decapoda: Galatheoidea) and the oldest records of porcellanids and galatheids. Zoological Journal of the Linnean Society. 2019;187:1131–1154. [Google Scholar]
- Rode AL, Lieberman BS. Using GIS to unlock the interactions between biogeography, environment, and evolution in Middle and Late Devonian brachiopods and bivalves. Palaeogeography, Palaeoclimatology, Palaeoecology. 2004;211:345–359. [Google Scholar]
- Roopnarine PD, Angielczyk KD, Weik A, Dineen A. Ecological persistence, incumbency and reorganization in the Karoo Basin during the Permian-Triassic transition. Sedimentology as a Key to Understanding Earth and Life Processes. 2019;189:244–263. [Google Scholar]
- Roy K. The roles of mass extinction and biotic interaction in large-scale replacements: a reexamination using the fossil record of stromboidean gastropods. Paleobiology. 1996;22:436–452. [Google Scholar]
- Sallan LC, Kammer TW, Ausich WI, Cook LA. Persistent predator–prey dynamics revealed by mass extinction. Proceedings of the National Academy of Sciences. 2011;108:8335–8338. doi: 10.1073/pnas.1100631108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saulsbury JG, Parins-Fukuchi CT, Wilson CJ, Reitan T, Liow LH. Age-dependent extinction and the neutral theory of biodiversity. Proceedings of the National Academy of Sciences. 2024;121:e2307629121. doi: 10.1073/pnas.2307629121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saupe EE. Explanations for latitudinal diversity gradients must invoke rate variation. Proceedings of the National Academy of Sciences of the United States of America. 2023;120:e2306220120. doi: 10.1073/pnas.2306220120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saupe EE, Myers CE, Townsend Peterson A, Soberón J, Singarayer J, Valdes P, Qiao H. Spatio-temporal climate change contributes to latitudinal diversity gradients. Nature Ecology & Evolution. 2019;3:1419–1429. doi: 10.1038/s41559-019-0962-7. [DOI] [PubMed] [Google Scholar]
- Schemske DW, Mittelbach GG, Cornell HV, Sobel JM, Roy K. Is there a latitudinal gradient in the importance of biotic interactions? Annual Review of Ecology, Evolution, and Systematics. 2009;40:245–269. [Google Scholar]
- Schindel DE, Gould SJ. Biological interaction between fossil species: character displacement in Bermudian land snails. Paleobiology. 1977;3:259–269. [Google Scholar]
- Schluter D. The Ecology of Adaptive Radiation. Oxford University Press; Oxford, UK: 2000. [Google Scholar]
- Schluter D, McPhail JD. Ecological character displacement and speciation in sticklebacks. The American Naturalist. 1992;140:85–108. doi: 10.1086/285404. [DOI] [PubMed] [Google Scholar]
- Sepkoski JJ. A kinetic model of Phanerozoic taxonomic diversity I. Analysis of marine orders. Paleobiology. 1978;4:223–251. [Google Scholar]
- Sepkoski JJ. A factor analytic description of the Phanerozoic marine fossil record. Paleobiology. 1981;7:36–53. [Google Scholar]
- Sepkoski JJ. In: Evolutionary Paleobiology. Jablonski D, Erwin DH, Lipps JH, editors. The University of Chicago Press; Chicago & London: 1996. Competition in macroevolution: the double wedge revisited; pp. 211–255. [Google Scholar]
- Shaw JO, Coco E, Wootton K, Daems D, Gillreath-Brown A, Swain A, Dunne JA. Disentangling ecological and taphonomic signals in ancient food webs. Paleobiology. 2021;47:385–401. [Google Scholar]
- Silvestro D, Antonelli A, Salamin N, Quental TB. The role of clade competition in the diversification of North American canids. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:8684–8689. doi: 10.1073/pnas.1502803112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestro D, Salamin N, Antonelli A, Meyer X. Improved estimation of macroevolutionary rates from fossil data using a Bayesian framework. Paleobiology. 2019;45:546–570. [Google Scholar]
- Silvestro D, Salamin N, Schnitzler J. PyRate: A new program to estimate speciation and extinction rates from incomplete fossil data. Methods in Ecology and Evolution. 2014;5:1126–1131. [Google Scholar]
- Silvestro D, Warnock RCM, Gavryushkina A, Stadler T. Closing the gap between palaeontological and neontological speciation and extinction rate estimates. Nature Communications. 2018;9:5237. doi: 10.1038/s41467-018-07622-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestro D, Zizka A, Bacon CD, Cascales-Minana B, Salamin N, Alexander A. Fossil biogeography: a new model to infer dispersal, extinction and sampling from palaeontological data. Philosophical Transactions of the Royal Society B-Biological Sciences. 2016;371:201502225. doi: 10.1098/rstb.2015.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GG. The Curious History of South American Mammals. Yale University Press; New Haven, London: 1980. Splendid Isolation. [Google Scholar]
- Slater GJ. Iterative adaptive radiations of fossil canids show no evidence for diversity-dependent trait evolution. Proceedings of the National Academy of Sciences. 2015;112:4897–4902. doi: 10.1073/pnas.1403666111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater GJ, Harmon LJ. Unifying fossils and phylogenies for comparative analyses of diversification and trait evolution. Methods in Ecology and Evolution. 2013;4:699–702. [Google Scholar]
- Smith JA, Handley JC, Dietl GP. Accounting for uncertainty from zero inflation and overdispersion in paleoecological studies of predation using a hierarchical Bayesian framework. Paleobiology. 2022;48:65–82. [Google Scholar]
- Stanley SM. Predation defeats competition on the seafloor. Paleobiology. 2008;34:1–21. [Google Scholar]
- Stanley SM, Newman WA. Competitive exclusion in evolutionary time: the case of the acorn barnacles. Paleobiology. 1980;6:173–183. [Google Scholar]
- Stigall AL. Invasive species and biodiversity crises: testing the link in the Late Devonian. PLoS ONE. 2010;5:e15584. doi: 10.1371/journal.pone.0015584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stigall AL. Speciation collapse and invasive species dynamics during the Late Devonian “Mass Extinction”. GSA Today. 2012;22:4–9. [Google Scholar]
- Stigall AL. The invasion hierarchy: ecological and evolutionary consequences of invasions in the fossil record. Annual Review of Ecology, Evolution, and Systematics. 2019;50:355–380. [Google Scholar]
- Strömberg CAE, Dunn RE, Madden RH, Kohn MJ, Carlini AA. Decoupling the spread of grasslands from the evolution of grazer-type herbivores in South America. Nature Communications. 2013;4:1478. doi: 10.1038/ncomms2508. [DOI] [PubMed] [Google Scholar]
- Sukumaran J, Knowles LL. Multispecies coalescent delimits structure, not species. Proceedings of the National Academy of Sciences. 2017 doi: 10.1073/pnas.1607921114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarquini SD, Ladevèze S, Prevosti FJ. The multicausal twilight of South American native mammalian predators (Metatheria, Sparassodonta) Scientific Reports. 2022;12:1224. doi: 10.1038/s41598-022-05266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toivonen J, Fortelius M, Žliobaitė I. Do species factories exist? Detecting exceptional patterns of evolution in the mammalian fossil record. Proceedings Biological Sciences. 2022;289:20212294. doi: 10.1098/rspb.2021.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toljagić O, Voje KL, Matschiner M, Liow LH, Hansen TF. Millions of years dehind: slow adaptation of ruminants to grasslands. Syst Biol. 2017;67:145–157. doi: 10.1093/sysbio/syx059. [DOI] [PubMed] [Google Scholar]
- Tomiya S, Miller LK. Why aren’t rabbits and hares larger? Evolution. 2021;75:847–860. doi: 10.1111/evo.14187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornabene C, Martindale RC, Wang XT, Schaller MF. Detecting photosymbiosis in fossil scleractinian corals. Scientific Reports. 2017;7:9465. doi: 10.1038/s41598-017-09008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Valen L. Selection in natural populations: Merychippus primus, a fossil horse. Nature. 1963;197:1181–1183. [Google Scholar]
- Van Valen LM. A new evolutionary law. Evolutionary Theory. 1973;1:1–30. [Google Scholar]
- Van Valkenburgh B. Tracking ecology over geological time - evolution with guilds of vertebrates. Trends in Ecology & Evolution. 1995;10:71–76. doi: 10.1016/S0169-5347(00)88980-6. [DOI] [PubMed] [Google Scholar]
- Van Valkenburgh B. Major patterns in the history of carnivorous mammals. Annual Review of Earth and Planetary Sciences. 1999;27:463–493. [Google Scholar]
- Vermeij GJ. The Mesozoic Marine Revolution: evidence from snails, predators and grazers. Paleobiology. 1977;3:245–258. [Google Scholar]
- Vermeij GJ. Evolution and Escalation: An Ecological History of Life. Princeton University Press; Princeton, N.J: 1987. [Google Scholar]
- Vermeij GJ. The evolutionary interaction among species: selection, escalation and coevolution. Annual Review of Ecology and Systematics. 1994;25:219–236. [Google Scholar]
- Vilhena DA, Smith AB. Spatial bias in the marine fossil record. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0074470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voje KL, Holen ØH, Liow LH, Stenseth NChr. The role of biotic forces in driving macroevolution: beyond the Red Queen. Proceedings of the Royal Society of London B: Biological Sciences. 2015;282 doi: 10.1098/rspb.2015.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker TD, Valentine JW. Equilibrium models of evolutionary species diversity and the number of empty niches. American Naturalist. 1984;124:887–889. [Google Scholar]
- Warnock RCM, Heath TA, Stadler T. Assessing the impact of incomplete species sampling on estimates of speciation and extinction rates. Paleobiology. 2020;46:137–157. [Google Scholar]
- Warnock Rachel CM, Engelstädter J. The Evolution and Fossil Record of Parasitism. Coevolution and Paleoparasitological Techniques; 2021. The molecular clock as a tool for understanding host-parasite evolution; pp. 417–450. [Google Scholar]
- Webb SD. Mammalian faunal dynamics of the Great American Interchange. Paleobiology. 1976;2:220–234. [Google Scholar]
- Webb SD. Ecogeography and the Great American Interchange. Paleobiology. 1991;17:266–280. [Google Scholar]
- Webb SD, Opdyke ND. Effects of past global change on life. National Academy Press; Washington, D. C: 1995. Global climatic influence on Cenozoic land mammal faunas; pp. 184–208. [Google Scholar]
- Weber MG, Agrawal AA. Defense mutualisms enhance plant diversification. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:16442–16447. doi: 10.1073/pnas.1413253111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nature Reviews Microbiology. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- West JB, Bowen GJ, Cerling TE, Ehleringer JR. Stable isotopes as one of nature’s ecological recorders. Trends in Ecology & Evolution. 2006;21:408–414. doi: 10.1016/j.tree.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Wisniewski AL, Lloyd GT, Slater GJ. Extant species fail to estimate ancestral geographical ranges at older nodes in primate phylogeny. Proceedings of the Royal Society B: Biological Sciences. 2022;289:20212535. doi: 10.1098/rspb.2021.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodburne MO. The Great American Biotic Interchange: dispersals, tectonics, climate, sea Level and holding pens. Journal of Mammalian Evolution. 2010;17:245–264. doi: 10.1007/s10914-010-9144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootton JT, Emmerson M. Measurement of interaction strength in nature. Annual Review of Ecology Evolution and Systematics. 2005;36:419–444. [Google Scholar]
- Wright AM, Bapst DW, Barido-Sottani J, Warnock RCM. Integrating fossil observations into phylogenetics using the fossilized birth–death model. Annual Review of Ecology, Evolution, and Systematics. 2022;53:251–273. [Google Scholar]
- Zeng Y, Wiens JJ. Species interactions have predictable impacts on diversification. Ecology Letters. 2021;24:239–248. doi: 10.1111/ele.13635. [DOI] [PubMed] [Google Scholar]