Abstract
Shigella-controlled human infection models (CHIMs) are an invaluable tool utilized by the vaccine community to combat one of the leading global causes of infectious diarrhea, which affects infants, children and adults regardless of socioeconomic status. The impact of shigellosis disproportionately affects children in low- and middle-income countries (LMICs) resulting in cognitive and physical stunting, perpetuating a cycle that must be halted. Shigella-CHIMs not only facilitate the early evaluation of enteric countermeasures and up-selection of the most promising products but also provide insight into mechanisms of infection and immunity that are not possible utilizing animal models or in vitro systems. The greater understanding of shigellosis obtained in CHIMs builds and empowers the development of new generation solutions to global health issues which are unattainable in the conventional laboratory and clinical settings. Therefore, refining, mining and expansion of safe and reproducible infection models hold the potential to create effective means to end diarrheal disease and associated co-morbidities associated with Shigella infection.
Abbreviations
- AMR
Antimicrobial resistance
- AR
Attack rate
- CCHMC
Cincinnati Children’s Hospital Medical Center
- CDC
US Center for Disease Control and prevention
- CFU
Colony forming units
- CHIM
Controlled human infection model
- CoP
Correlates of protection
- CRP
C-reactive protein
- EE
Environmental enteropathy
- EMA
European Medicines Agency
- ETEC
Enterotoxigenic Escherichia coli
- FDA
US Food and Drug Administration
- G4C
Group 4 capsule
- GMP
Good manufacturing practices
- HBD-1
Human β-defensin-1
- HICs
High-income countries
- IABS
International Alliance for Biological Standardisation
- Ipa
Invasion plasmid antigens
- LMICs
Low- and middle-income countries
- LPS
Lipopolysaccharide
- mAb
Monoclonal antibody
- MPO
Myeloperoxidase
- OAg
O-antigen
- SAGE
Strategic Advisory Group of Experts
- T3SS
Type-III secretion system
- T6SS
Type-VI secretion system
- TCV
Typhoid conjugate vaccine
- US
United States
- WHO
World Health Organization
1. Introduction
1.1. Shigella Disease Burden
Based on current estimates, 500,000 children under 5 years old die annually worldwide from diarrheal diseases, while millions more suffer multiple episodes during early childhood (GBD 2016 Diarrhoeal Disease Collaborators 2018). Our understanding of the global burden of Shigella-related disease has been greatly enhanced in recent years by the results of several large-scale epidemiological studies in South Asia, Africa and South America and by the application of new molecular-based diagnostics for case detection in both case-control and community-based prospective surveillance studies (GBD 2016 Diarrhoeal Disease Collaborators 2018; Livio et al. 2014; Liu et al. 2016; Tickell et al. 2017; WHO 2020). Shigella is the most common bacterial cause of moderate to severe diarrhea (bloody and non-bloody) in children under 5 years of age, with the current trends indicating the highest incidence occurring among children in their second year of life living in Africa and South Asia (Ahmed et al. 1997; Rogawski McQuade et al. 2020). The high burden of Shigella infections early in life has also been associated with the subsequent development of environmental enteropathy (EE) which can manifest as a “triple burden” of sequelae: (1) increased stunting-associated risk of mortality due to other infectious diseases, (2) poor cognitive development and subsequent reduction in educational outcomes and contribution to human capital and (3) increased risk of non-communicable diseases as adults (Guerrant et al. 2013, 2021; Lamberti et al. 2014; Rogawski et al. 2018; Anderson et al. 2019a, b). There is also the potential for post-infectious sequelae such as reactive arthritis and irritable bowel syndrome (Pogreba-Brown et al. 2020).
In addition to its impact in endemic settings, Shigella-attributable diarrhea among travelers and military populations can cause significant morbidity and incapacitation, requiring antibiotics, intravenous fluids and hospitalization (Riddle et al. 2006; Shah et al. 2009; Steffen 2017; Riddle 2018; Olson et al. 2019). Shigella is also considered an antimicrobial resistance (AMR) threat by the World Health Organization (WHO) and the US Center for Disease Control (CDC) (WHO 2017a; CDC 2019), leading the WHO Global Antimicrobial Resistance Surveillance System to identify Shigella as a priority pathogen for the development of new interventions. The WHO, in conjunction with the Wellcome Trust and Boston Consulting Group (Wellcome Trust and Boston Consulting Group 2021), has recommended acceleration of vaccine development for Shigella due to its high global burden of illness and the associated difficulty in effective treatment with commonly available antibiotics (CDC 2019).
The need to accelerate Shigella vaccine development has caused stakeholders, donors and the Shigella vaccine development community to consider how CHIMs could contribute to accelerated licensure of Shigella vaccines. CHIMs have been established for a broad range of enteric pathogens, including Shigella, and can provide insight into disease pathogenesis or the immune response to infection. Albeit in a small number of individuals, CHIMs can also be used to assess the early clinical efficacy of candidate vaccines and facilitate the identification of correlates of protection (CoP) by evaluating immune responses associated with protective efficacy (Holmgren et al. 2017; Giersing et al. 2019; MacLennan et al. 2019a; Clarkson et al. 2021a). For other enteric pathogens, CHIMs have been used to support licensure and recommendation by the WHO Strategic Advisory Group of Experts (SAGE) for vaccine uptake and expanded utilization (Giersing et al. 2019; MacLennan et al. 2019a).
The usefulness of Shigella-CHIMs is further solidified by the rapid expansion of Omics and systems biology technologies which can provide important information on host and bacterial factors that contribute to Shigella-associated disease and gut inflammation, as well as the cellular, molecular and biochemical basis of Shigellaspecific immunity. The challenges and potential benefits of the expanded use of standardized Shigella-CHIMs are further detailed below. This chapter also describes how host and bacterial factors can contribute to model consistency and how recent innovations align with existing animal models, as well as the newly developed in vitro human enteroid model. Finally, this chapter summarizes opportunities to expand challenge model capabilities to include new Shigella strains, clinical settings, microbiological and immunological outcomes and efforts to expand its application to LMICs and support policy recommendations for Shigella vaccine licensure, WHO prequalification and vaccine uptake in Shigella endemic areas.
1.2. Shigella Species and Global Distribution
The Shigella genus is currently classified into four species, each of which is human enteropathogens. These can be grouped based on serological reactivity with group-specific antisera into S. dysenteriae (serogroup A), S. flexneri (serogroup B), S. boydii (serogroup C) and S. sonnei (serogroup D). These bacteria can be further classified into serotypes, based on the chemical structures of the repeating saccharide units of the O-antigen (OAg), the surface exposed part of lipopolysaccharide (LPS) (Lindberg et al. 1991). However, the OAg of some Shigella species is structurally identical (Liu et al. 2008), serologically cross-react (Lefebvre et al. 1995) and shares gene clusters (Liu et al. 2008; Pupo et al. 2000; Yang et al. 2005) with some Escherichia coli, thus complicating the ability to distinguish these bacteria. Currently, there are at least 54 unique Shigella serotypes that are recognized (Muthuirulandi Sethuvel et al. 2017): 15 serotypes of S. dysenteriae, 19 serotypes and subserotypes of S. flexneri, 19 serotypes of S. boydii and a single serotype of S. sonnei.
Among the S. flexneri serogroup, there are molecular similarities between the structures of the LPS OAg repeating units. With the exception of S. flexneri 6, there is a common linear tetrasaccharide repeating unit composed of three a-L-rhamnosyl residues and one residue of N-acetyl-D-glucosamine [α-L-Rhap-(1 → 2)-α-L-Rhap-(1 → 3)-α-L-Rhap-(1 → 3)-β-D-GlucpNAc]; each of which is joined through a β-D-GlucpNAc-(1 → 2)-α-L-Rhap linkage (Carlin et al. 1984; Kenne et al. 1978). Antigenic diversity among the S. flexneri serogroup is due to additional α-D-glucopyranosyl and O-acetyl moieties at various positions on the tetrasaccharide subunit which are the basis for the designation of “type” (I, II, III, IV) and “group” (3,4; 6; 7,8) antigenic factors associated with S. flexneri serotypes (Perepelov et al. 2012; Knirel et al. 2015). Although S. flexneri 6 does not share the same linear tetrasaccharide repeating unit, the LPS does share a common O-acetylated disaccharide [α-L-Rhap-(1 → 2)-α-L-Rhap] with S. flexneri 2a.
Given that immunity to Shigella infection has been demonstrated to be serotype-specific, the generation of serotype-specific OAg-based immunity is the focus of many Shigella vaccines under development (Table 1). Nonetheless, other antigenic components may be important in Shigella pathogenesis. The virulence of Shigella is dependent upon the presence of a large virulence plasmid (>200 kbp) (Sansonetti et al. 1982), which encodes for the type-III secretion system (T3SS), and is necessary for the full pathogenicity of the bacterium (Schroeder and Hilbi 2008). Importantly, the T3SS syringe-like apparatus includes several invasion plasmid antigens (Ipa), which are shared across all invasive Shigella strains carrying the virulence plasmid. Other conserved virulence factors/antigens that are important in Shigella pathogenesis include VirG (IscA) (Shimanovich et al. 2017) and PSSP-1 (C-terminus of IcsP) (Kim et al. 2015). Both VirG and PSSP-1 proteins contribute to the cell-to-cell spread of Shigella among enterocytes in the large intestine and rectal mucosa.
Table 1. Shigella vaccines currently in clinical development*.
| Target | Serotype | Technology | Translational | Phase 1 | Phase 2a |
|---|---|---|---|---|---|
| Single Serotype | S. flexneri 2a | Bioconjugate | Flexyn2a a (LMTB) NCT02388009 | ||
| Synthetic Conjugate | Sf2a-TT15 (IP) NCT02797236 | Sf2a-TT15 (IP) NCT04602975 | |||
| Invasin Complex | InvaplexAR (WRAIR) NCT02445963 | ||||
| InvaplexAR-Detox (WRAIR) NCTO3869333 | |||||
| S. sonnei | GMMA | 1790GAHB a (GSK) NCT02017899 NCT02034500 NCT03089879 | 1790GAHB a (GSK) NCT02676895 | ||
| Live-Attenuated Oral | WRSs2 (WRAIR/NIAID) NCT01336699 | ||||
| Multiple Serotypes | S. sonnei and S. flexneri 2a, 3a, 6 | Bioconjugate | Shigella4V (LMTB) NCT04056117 | Shigella4V (LMTB) NCT04056117 | |
| S. sonnei and S. flexneri 1b, 2a, 3a | GMMA | altSonflex1-2−3 (GSK) NCT05073003 | |||
| Multi-Pathogen | S. flexneri 2a, ETEC | Live-Attenuated Oral | CVD1208S-122 (UMD) | ||
| NCT04634513 | |||||
| Live-Attenuated Oral | ShigETEC (Eveliqure) Harutyunyan et al. (2020) |
Single serotype candidate vaccine development terminated in favor of multi-serotype candidate vaccine
Trial status: Not yet recruiting/recruiting; Active/Ongoing; Completed
Product Developers
Eveliqure—Eveliqure Biotechnologies GmbH (Vienna, AT); GSK—GSK Vaccines Institute for Global Health (Siena, IT); IP—Institut Pasteur (Paris, FR); LMTB—LimmaTech Biotechnology (Zurich, CH); NIAID—National Institutes of Allergy and Infectious Diseases (Bethesda, MD, USA); UMD—University of Maryland (Baltimore, MD, USA); WRAIR—Walter Reed Army Institute of Research (Silver Spring, MD, USA)
The global distribution of Shigella species appears to follow a general trend that may reflect the predilection for certain species to have greater incidence according to the local availability of clean water, sanitation, hygiene and population nutritional status. Thus, S. flexneri represent the predominant species for the majority (>60%) of the burden of shigellosis in LMICs, whereas S. sonnei is the predominant species (>70%) afflicting high-income countries (HICs) (Livio et al. 2014; Gupta et al. 2004; Gu et al. 2012). Additionally, for countries that are economically transitioning from low- or middle-income to high-income status, these improvements have been associated with shifts away from S. flexneri toward S. sonnei predominance (Ram et al. 2008; Thompson et al. 2015; Qiu et al. 2015). An explanation for this observed epidemiological pattern is that individuals in LMICs have gained natural immunity to S. sonnei through frequent exposure to Plesiomonas shigelloides, which possesses a homologous LPS structure to S. sonnei and is often found in low-resource settings with poor infrastructure (Sack et al. 1994). Those living in higher income nations would not gain this natural immunity, as their improvements in infrastructure and sanitation measures have resulted in a low prevalence of P. shigelloides.
S. dysenteriae and S. boydii cause a minor proportion (≤ 5%) of overall shigellosis globally. In the past, large outbreaks of S. dysenteriae serotype A1 occurred generally once a decade in South Asia and central Africa particularly in refugee settings, where they were associated with a high case fatality rate and increasing antibiotic resistance. However, in recent years these outbreaks have inexplicably disappeared (Kerneis et al. 2009; Bardhan et al. 2010). The prevalent Shigella species, especially the S. flexneri serotypes, can be both temporally and geographically variable, as has been demonstrated over numerous attempts to characterize the epidemiology of shigellosis (Livio et al. 2014; Kotloff et al. 1999; Seidlein et al. 2006). Nonetheless, informed by epidemiological data on the prevalence of Shigella serogroups and serotypes, the largest proportion of the global disease burden has been attributed to the following serotypes: S. sonnei and S. flexneri 1b, 2a, 3a and 6 (Livio et al. 2014; Barry and Levine 2019). Multivalent vaccine approaches would be required to provide broad protection against these serogroups and serotypes, and others that may emerge (Seidlein et al. 2006).
1.3. S. sonnei Is a Unique Serogroup Among Shigella spp
Among the major Shigella serotypes associated with enteric illness, S. sonnei is unique for several reasons. S. sonnei encodes for a type-VI secretion system (T6SS) which is used to directly compete with and kill, not only other Shigella species but also other commensal enteric bacteria including E. coli (Anderson et al. 2017). Interestingly, the T6SS of S. sonnei has been cited as one of the reasons that S. sonnei has been able to rise to dominance in higher income countries. In the absence of natural immunity to P. shigelloides, S. sonnei has been able to out-compete other Shigella serotypes with the help of its T6SS (Sack et al. 1994). The T6SS can also play a role in altering the innate immune response and inflammatory environment during Shigella infection via the killing of intestinal microbiota and commensal microorganisms.
Other than S. flexneri 6, S. sonnei is also the only Shigella serotype known to express a group 4 capsule (G4C), characterized by structural similarity to the OAg (Caboni et al. 2015). The G4C is an important virulence factor, in that it creates a thick layer of polysaccharide on the surface of S. sonnei strains, reducing accessibility to the T3SS apparatus. The G4C also increases the resistance of S. sonnei to the bactericidal activity of antibodies (Caboni et al. 2015), which in conjunction with reduced cellular invasion creates a less inflammatory environment (Watson et al. 2019) and improves the ability of S. sonnei to persist in the human gut. Another unique attribute of S. sonnei is its adeptness in acquiring AMR genes compared to other Shigella serotypes. Thus, allowing S. sonnei to persist during infection and therapeutic treatment (Thompson et al. 2015).
1.4. Current Status of Shigella Vaccine Development
As shown in Table 1, the current portfolio of Shigella vaccine candidates target serotype-specific OAg alone (bioconjugates, synthetic conjugates and GMMA) or with conserved proteins (Invaplex) as well as multiple surface-expressed antigens (attenuated strains). These approaches target single serotypes, multiple serotypes and combinations of pathogens. At present, only single serotype vaccines have been evaluated in Shigella-CHIMs, revealing useful and unexpected data to further inform early clinical development. This expanded value of CHIMs has led to further efforts to harmonize the models and expand their application to LIMC settings. Similarly for ongoing and planned field evaluations of candidate vaccines in Kenya, sponsors and funders have harmonized the human trial protocols for clinical and immunologic readouts to streamline development among the most advanced OAg-based vaccines. In parallel, critical vaccine development efforts are ongoing to standardize the assays and tools as well as the optimal regulatory pathway and policy roadmap that will be needed for the roll-out and implementation of a safe and effective Shigella vaccine. The use of existing and new Shigella-CHIMs, both in HICs and their establishment in LMICs, will play a pivotal role.
2. Summary of Existing Shigella-CHIMs
The Shigella field currently utilizes two CHIMs developed for S. flexneri 2a and S. sonnei. To date, the majority of Shigella CHIMs have been conducted using the S. flexneri 2a model (Table 2). The limited experience with the S. sonnei CHIM has resulted in the use of outcome definitions, both for disease and immune responses, which were derived predominantly from S. flexneri 2a data. While many parameters of these two Shigella-CHIMs are similar, the evaluation of S. flexneri 2a and S. sonnei strains as challenge agents has shown differences in pathogenesis, translating into important distinctions in the spectrum of illness these strains induce (Clarkson et al. 2021b). Furthermore, evaluation of the immune responses induced by these two challenge agents has highlighted important differences in the magnitude of the innate inflammatory response, as well as the phenotype of the systemic and mucosal adaptive immune responses they trigger. The differences in immune response profiles may reflect different mechanisms of protection and immune CoP for shigellosis. By rigorously comparing and contrasting the key attributes of the S. flexneri 2a and S. sonnei CHIMs (outlined below), the field can be better informed of how new Shigella-CHIMs should be developed. It is likely that not all Shigella serotypes are created equal, and each new model will need to be treated as unique rather than as an extension of an existing model as was done for the S. sonnei (lyophilized) CHIM using S. flexneri 2a data. New Shigella-CHIMs should be established de novo and well-characterized during clinical setup to mitigate against important unappreciated differences among Shigella spp.
Table 2. Utilization of Shigella flexneri 2a and S. sonnei controlled human infection models.
| Evaluation | Challenge agent | Product under evaluation | Naïve attack rate (n/N) | Treated attack rate (n/ N) | Efficacy (%) a | References |
|---|---|---|---|---|---|---|
| Homologous re-challenge | S. flexneri 2a | Strain 2457T | 22/39 | 3/15 | 64 | DuPont et al. (1972b) |
| S. flexneri 2a | Strain 2457T | 11/12 | 3/11 | 71 | Kotloff et al. (1995a) | |
| S. sonnei | Strain 53G (frozen) | 8/12 | 0/6 | 100 | Herrington et al. (1990) | |
| Antibiotic | S. flexneri 2a | Rifaximin | 6/15 | 0/15 | 100 | Taylor et al. (2006) |
| S. flexneri 2a | Rifaximin | 13/15 | n/a | n/a | Taylor et al. (2008) | |
| Passive Ig | S. flexneri 2a | Bovine IgG | 5/11 | 0/10 | 100 | Tacket et al. (1992) |
| Homologous vaccine candidate | S. flexneri 2a b | Heat-killed whole cell | 19/30 | 18/25 | -14 | Shaughnessy and Olsson (1946) |
| S. flexneri 2a b | Irradiated whole cell | 19/30 | 23/28 | -30 | Shaughnessy and Olsson (1946) | |
| S. flexneri 2a | EcSF2a-1(E. coli K-12—S. flexneri 2a OAg hybrid live-oral) | 6/24, 52/88 | 1/15, 30/68 | 73, 25 | DuPont et al. (1972b) | |
| S. flexneri 2a | Streptomycin-dependent whole cell | 6/24, 52/88 | 3/31, 16/53 | 61, 49 | DuPont et al. (1972b) | |
| S. flexneri 2a | EcSF2a-2 (E. coli K-12 aroD mutant—S. flexneri 2a OAg hybrid live-oral) | 12/14 | 10/16 | 27 | Kotloff et al. (1995b) | |
| S. flexneri 2a | SC602 (live attenuated S. flexneri 2a icsA mutant) | 6/7 | 0/7 | 100 | Coster et al. (1999) | |
| S. flexneri 2a | Proteosome-Sflex2a LPS | 13/13 | 9/14 | 36 | Durbin et al. (2001) | |
| S. flexneri 2a | Invaplex50 NAT (Macromolecular complex containing IpaB, IpaC and S. flexneri 2a LPS) | 8/12 | 7/10 | -5 | NCT 00485134 | |
| S. flexneri 2a | Flexyn2a, bioconjugate (S. flexneri 2a OAg conjugated to P. aeruginosa exotoxin A) | 18/29 | 13/30 | 30 | Talaat et al. (2021) | |
| S. sonnei (Frozen) | WRSs1 (Live attenuated S. sonnei virG mutant) | 1/6 c | 0/13 | n/a c | Pitisuttithum et al. (2016) | |
| S. sonnei (Lyophilized) | 1790GAHB (S. sonnei GMMA-technology) | 15/32 | 12/28 | − 9.4 | Frenck et al. (2021) |
Efficacy outcomes presented as the per protocol definition for shigellosis
S. flexneri 2a strain was FW rather than the more commonly used 2457T
Study performed in Thai adults yielded lower than anticipated naïve AR, and the authors reported “no efficacy against dysentery and diarrhea”
2.1. Historical Context and Brief Overview of Established Models
The first experimental human Shigella challenge occurred in the 1940s using a S. flexneri strain that is no longer utilized (Shaughnessy and Olsson 1946). As late as the 1950s, despite multiple attempts, no effective vaccine against Shigella had been found, leading Colonel David Mel (Military Medical Academy, Yugoslavia) to conclude “protection against bacillary dysentery cannot be achieved by parenterally administered vaccines” (Mel et al. 1965a). Yet, his observations that “living cell” preparations administered by the oral route could achieve protection led to a series of field trials of live, attenuated, streptomycin-dependent Shigella vaccines (Mel et al. 1965b, c, 1968, 1971). These field trials in the 1960s were conducted in hyper-endemic regions in Yugoslavia and showed high efficacy in both adults (Mel et al. 1965c, 1968) and children (2–8 years of age) (Mel et al. 1971). An early live oral vaccine candidate requiring a 5-dose schedule (each dose spaced 3 days apart) elicited only short-lived immunity (Mel et al. 1974) and was found to be susceptible to genetic reversion (streptomycin-independence, but not invasion) (Levine et al. 1975). By the early 1970s, additional oral, live-attenuated Shigella vaccines were being evaluated (DuPont et al. 1972a, b; Levine et al. 1972, 1977). Using the established Shigella-CHIM (DuPont et al. 1969), positive efficacy was demonstrated in adults using a fully virulent Shigella flexneri 2a strain (DuPont et al. 1972b). These live-Shigella preparations used for oral vaccination could be considered early Shigella-CHIMs and could help to support the use of safer attenuated Shigella strains in experimental humans, if required.
The two Shigella strains currently available and characterized for use in CHIMs are 2457T, a S. flexneri 2a strain, and 53G, a S. sonnei strain (Text Box 1). Both of these challenge bacteria were wild-type antibiotic sensitive strains that have been well characterized and subsequently extensively evaluated in presumptively naïve subjects (DuPont et al. 1972b; Herrington et al. 1990; Kotloff et al. 1995a), as well as in studies of drugs (Taylor et al. 2006, 2008), passive immunoglobulins (Tacket et al. 1992) and novel vaccine candidates (Shaughnessy and Olsson 1946; DuPont et al. 1972b; Kotloff et al. 1995a; Coster et al. 1999; Pitisuttithum et al. 2016; Talaat et al. 2021) (Table 2). These bacteria, and their respective CHIMs, are a tremendous resource to the Shigella field, enabling detailed characterization of the clinical course of early disease that is not feasible in natural exposure, increasing the understanding of the host immune response to infection and the association between immune response and host susceptibility as well as aiding in the evaluation of multiple vaccines as promising candidates for further evaluation. Many of the operational aspects of the Shigella-CHIM are similar to those described for enterotoxigenic Escherichia coli (ETEC); the reader is referred to Fig. 1 of the ETEC-CHIM chapter for graphical representation of a CHIM study overview to supplement the information provided in this chapter on Shigella-CHIMs.
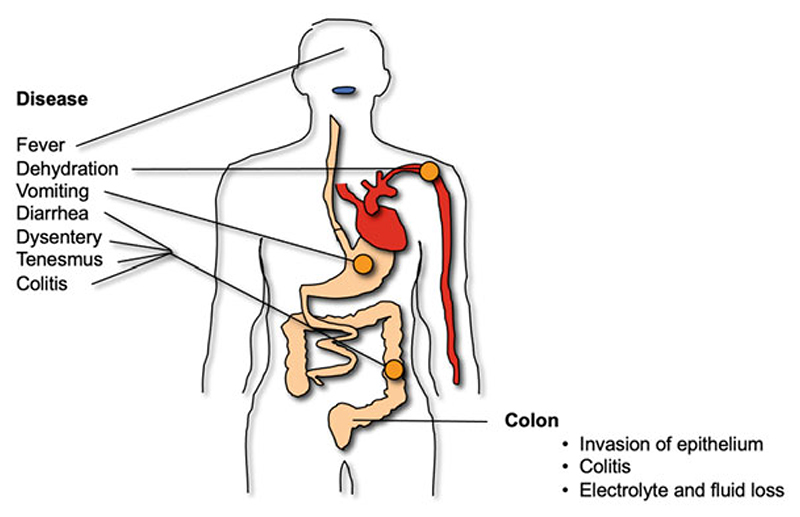
Fig. 1. Clinical symptoms of infection with Shigella spp.
Shigellosis is a self-limiting infection resulting in colonic epithelial lesions found in most patients, which is a consequence of innate and adaptive immune responses to infection. A hallmark of dysentery is small volume, bloody, mucoidal stools with a high number of neutrophils. Infection can also cause watery diarrhea, dehydration and contribute to malnutrition in endemic populations
2.2. Nature of Challenge Agents and Delivery Systems
One of the most successful efforts to standardize the Shigella (and other enteric) CHIMs came with the realization that the cholera challenge was refined by pre-administering sodium bicarbonate prior to challenge and also by providing the inoculum in a similar buffer (Cash et al. 1974). While head-to-head studies of various buffers are lacking, S. flexneri 2a 2457T administered in sodium bicarbonate demonstrated a consistent and high shigellosis attack rate (AR) (Kotloff et al. 1995a). Further efforts to refine and/or standardize the model have included the evaluation of lyophilized and frozen challenge strains directly diluted and used for inoculation.
In the most exhaustive head-to-head comparison of various delivery platforms, freshly grown S. sonnei 53G administered in either sodium bicarbonate or milk, as well as an inoculum prepared directly from a frozen suspension, were compared (Kirkpatrick et al. 2000). Briefly, an initial cohort received 2000 colony forming units (CFU) of the frozen S. sonnei 53G challenge strain, which caused a mild atypical gastrointestinal illness in most participants; however, one subject experienced a severe, sepsis-like illness which led to the abandonment of the frozen inoculum as a challenge strategy. All subsequent groups received approximately 400 CFU of fresh, plate-grown bacteria in either milk or bicarbonate buffer. The use of sodium bicarbonate increased the AR to 71% from the 40% seen with milk. More recently, a lyophilized lot of S. sonnei 53G was evaluated at Cincinnati Children’s Hospital Medical Center (CCHMC) using increasing doses ranging from approximately 500 CFU to 1500 CFU in bicarbonate buffer with an AR of 40% in the highest dose cohort (1760 CFU) (Frenck et al. 2020). Although there are differences in ARs across these two studies, it is important to consider that different primary outcome definitions were used in each study with a much stricter definition employed in the CHIM conducted at CCHMC. When a less severe disease definition was applied to the high dose cohort of the CCHMC study, the AR increased to 70% (Frenck et al. 2020) (see Sect. 2.5 for additional discussion on disease endpoint definitions).
The value of the lyophilized challenge strain is that it removes the inherent variability of using freshly grown and harvested cells from plate scrapes for inoculation. Prior to the use of the lyophilized strain, trained microbiologists initiated a multi-day process of inoculum preparation that consisted of isolating virulent (Form I) Shigella colonies following overnight incubation of a challenge stock (Talaat et al. 2019). Virulent colonies were expanded and freshly harvested for inoculation introducing potential variability in colony selection, reagents, growth, etc. Minimizing variability (along with harmonizing clinical endpoints, discussed below) enables direct comparison of study data across researchers, institutions and over time.
2.3. Volunteer Selection and Screening
The current strategy for subject selection is focused on identifying a healthy subset of the adult population that is susceptible to shigellosis (Talaat et al. 2019) while maintaining the ability to enroll sufficient numbers of subjects in a clinical trial to achieve the desired outcome and generate results that are externally valid to a broader population. Subject selection relies on self-reported prior Shigella exposure, travel to Shigella endemic regions and/or occupational or other known exposure that would potentially reduce susceptibility to challenge. Serologic screening based on anti-LPS serum IgG ELISA titers has also been utilized with the intent to complement other inclusion/exclusion criteria and to identify immunologically naïve or infection susceptible subjects.
To develop the serological screening assay, serum from individuals clinically diagnosed with Shigella infection was assayed by ELISA and western blot for antibody reactivity directed to IpaB, IpaC and IpaD proteins. Similarly, serum from individuals with no prior history of exposure to Shigella was also assessed by ELISA and western blot. Individuals with prior exposure history and serum reactive with the Ipa proteins were designated as “true positives”, whereas individuals with no prior exposure history and serum with undetectable reactivity were designated as “true negatives”. Serum from these positive and negative populations was then assayed for anti-S. flexneri 2a LPS serum IgG responses. A titer of 2500 differentiated subjects in the two populations (Kaminski, personal communication). The 2500 anti-S. flexneri 2a LPS serum IgG titer was subsequently used as an exclusion threshold for participation in S. flexneri 2a CHIMs. The same exclusions titer of anti-S. sonnei LPS serum IgG was later applied to the S. sonnei CHIM, in deference to the absence of data on its relationship to antibody levels or clinical outcome with this serotype.
In a refinement of the S. sonnei 53G (lyophilized) model, the 2500 serological screening threshold titer excluded a small subset (3%) of potential participants and led to comparable baseline S. sonnei IgG titers across study groups (Clarkson et al. 2020). In the 53G challenged subjects, there was no subsequent association between the baseline serum IgG titer and the primary shigellosis endpoint. This is unlike the negative correlation observed in vaccinated subjects but not placebo subjects of the S. flexneri 2a CHIM (Talaat et al. 2021). Further immune response evaluations in the S. sonnei 53G refinement study revealed that baseline S. sonnei anti-LPS serum IgA titers were heterogeneous across study groups and subjects with higher serum IgA titers were less likely to develop shigellosis. Similar trends were observed in baseline fecal and memory B cell antibodies, with higher IgA responses differentiating subjects progressing to shigellosis from those who did not (Clarkson et al. 2020).
Analysis of serum IgG screening titers from all potential participants in the S. sonnei 53G model refinement study revealed that 70% of screened subjects had IgG titers ≤ 625, at least fourfold lower than the exclusion threshold. However, using the same screening method in a subsequent vaccination-challenge study using S. sonnei 53G (Frenck et al. 2021), the 2500 titer screening threshold allowed enrollment of a large proportion of participants with baseline LPS-specific antibody levels close to or above the IgG threshold reported as protective for S. sonnei in field studies (Cohen et al. 1988). Across all cohorts in the vaccination-challenge trial (Frenck et al. 2021), subjects with higher anti-S. sonnei IgG levels at baseline were significantly less likely to develop shigellosis (p = 0.003). Serum IgA titers are yet to be determined from this vaccination-challenge study but may also reveal additional important differences in immune responses associated with disease progression across cohorts in this study.
Additional studies are needed to determine if the 2500 serum IgG screening threshold established for S. flexneri 2a is appropriate for the S. sonnei CHIM. One could offer that the threshold established as protective in field trials (Cohen et al. 1988) could serve as an appropriate alternative; however, differences between ELISA methods need to be considered as they can contribute to a misalignment of titer determinations. However, an immune response that protects in a field setting may be insufficient in the experimental CHIM, which is optimized to promote a specific disease outcome (e.g., pre-challenge fasting, gastric acid buffering and controlled inoculum dose). This, in conjunction with the association of baseline IgA responses and differences in disease progression during model setup, highlights the potential need for expansion and/or modification of the serologic prescreening processes, at least for S. sonnei 53G. It is possible that serum IgG levels may not be the only (or the most) appropriate marker of immunity or resistance to infection. Alternative immune parameters and thresholds should be evaluated and established for use as screening tools in these (and new) CHIMs.
Furthermore, during the S. sonnei 53G (lyophilized) CHIM development, the use of a high baseline IgG threshold, which could equate with prior exposure to S. sonnei or cross-reactive antigens, may have resulted in the selection of an inoculum dose that was higher than what would be required in a truly susceptible population; thus, leading to a more stringent CHIM that would overcome vaccine-induced immunity. Collectively, these observations point to a few of the challenges when establishing new Shigella-CHIMs. It will be essential to confirm these observations in future studies and identify immune exclusion criteria specific for each CHIM in order to accurately complement other exclusion criteria and assist in the recruitment of a naïve population.
2.4. Dose-Ranging and Attack Rates
Across CHIMs, there has been an unclear association between inoculation dose and disease (Porter et al. 2013); however, this may have been due to variability in strain preparation and administration (selection of virulent colonies, pre-challenge administration of a buffer, administering the challenge in skim milk or sodium bicarbonate), subject selection (immunology screening) and/or outcome definitions. Supporting this theory are several studies in which various inoculum doses have been administered as part of the same study. For example, a two to threefold increase in the proportion of subjects meeting various clinical endpoints has been reported when the S. flexneri 2a 2457T dose was increased from 140 to 1400 CFU (Kotloff et al. 1995a). A similar increase in disease rates has also been seen when evaluating a range of S. sonnei 53G doses from 500 to 1500 CFU (Frenck et al. 2020). However, both observations are based on a limited sample size and therefore may benefit from expanded evaluation.
The clinical course of disease in both Shigella-CHIMs is as expected in an otherwise healthy, immunocompetent adult with shigellosis and is characterized by gastrointestinal symptoms of diarrhea, dysentery, vomiting, nausea, anorexia and abdominal pain, and/or cramps. Additionally, systemic symptoms of fever, malaise, headache, myalgia and arthralgia are common (Fig. 1). While there are differences in the constellation of symptoms and their severity between the two evaluated strains, the proportion of exposed subjects experiencing each individual symptom is comparable (Table 3). Interestingly, fever and anorexia appear to be more prevalent in subjects challenged with S. flexneri 2a 2457T while dysentery appears to be more common in subjects challenged with S. sonnei 53G. It is important to note that while these potential differences are intriguing, no studies have been performed to date where the clinical profile of shigellosis induced by the two challenge strains has been studied simultaneously under the same clinical research protocol.
Table 3. Proportion of subjects developing symptoms or signs of disease in S. flexneri 2a (Porter et al. 2018) and S. sonnei CHIMs (Frenck et al. 2020).
| Symptom/sign | S. flexneri 2a, 2457T | S. sonnei, 53G a |
|---|---|---|
| Diarrhea | 75.9 | 70.0 |
| Fever | 50.0 | 23.9 |
| Nausea | 46.3 | 30.4 |
| Abdominal pain/cramps | 81.5 | 69.6 |
| Malaise | 57.4 | 43.4 |
| Headache | 66.7 | 69.6 |
| Myalgia | 42.6 | 47.8 |
| Arthralgia | 20.4 | 26.1 |
| Anorexia | 64.8 | 41.3 |
| Vomiting | 24.1 | 19.6 |
| Dysentery | 27.8 | 43.4 |
Excludes subjects receiving 500 CFU dose
2.5. Common Disease Endpoints Are Used Across Both Established Shigella-CHIMs
A critical aspect of CHIMs is the choice of endpoints and case definitions, as these have significant ramifications on the study results and consequently affect decision-making. Endpoints may represent a variety of disease/infection stages including carriage, shedding, infection (i.e., detection of the pathogen), clinically overt disease and its severity, including the extent of intestinal and systemic inflammation. While the choice of disease endpoints is specific to the infection of interest, endpoints are also required to be aligned with the target product profiles in order to assess intervention effects. Generally, endpoints that estimate vaccine efficacy against the most relevant aspect of the disease from a public health perspective should be deemed more relevant than others.
One of the complicating aspects of comparing the signs and symptoms of shigellosis over time and among institutions has been the inconsistency in primary endpoint definitions (Porter et al. 2019). For example, fever, diarrhea and dysentery have each been defined at least in six different ways in previously published Shigella-CHIMs and each has been included individually and collectively as components of a primary endpoint (Porter et al. 2019).
Recently, investigators representing multiple institutions utilizing Shigella-CHIMs gathered to evaluate previously utilized primary endpoint definitions and to evaluate a potentially harmonized primary endpoint that could be the standard for use in subsequent CHIMs (Text Box 2) (MacLennan et al. 2019b). One of the key elements in developing a consensus endpoint was to balance the AR with a clinically meaningful endpoint. For example, while a very high proportion of subjects develop some level of illness following the experimental Shigella challenge, some illnesses can be quite mild. While including overly mild illnesses as a primary endpoint may increase the AR, and it may also yield a model that does not represent the typical clinical picture of more severe disease, which in turn may decrease the ability to appropriately evaluate vaccine efficacy. In contrast, a model that has a too severe clinical endpoint may yield very low ARs requiring large sample sizes for sufficiently powered vaccine efficacy studies. Balancing these aspects, the investigators finalized a primary endpoint that comprised elements of diarrhea, fever, dysentery and other constitutional symptoms as a consensus shigellosis definition for future use in the currently established Shigella-CHIMs (MacLennan et al. 2019b).
2.6. Disease Endpoints Versus Severity Scores
Utilizing individual subject-level data from multiple S. flexneri 2a CHIMs, the co-occurrence and severity of the numerous signs and symptoms of shigellosis were evaluated (Porter et al. 2018). These analyses were used to develop a disease severity score to provide a more granular characterization of the disease-induced across the disease complex range. While the score was established using only data from S. flexneri 2a studies, it appeared to be equally applicable to disease following challenge with S. sonnei given the reported correlation of increasing disease severity score with increasing challenge dose of S. sonnei 53G (Frenck et al. 2020).
In addition to enabling a more robust characterization of the shigellosis disease complex in the Shigella-CHIM, the disease severity score offers an alternative endpoint for consideration in the assessment of candidate vaccines (Fedorov et al. 2009). Briefly, dichotomous or nominal endpoints are less statistically efficient than endpoints that are ordinal (or continuous). Thus, the use of the disease severity score in comparing disease between naïve and vaccinated subjects is likely to provide more statistical power to differentiate the disease across the two groups than what is afforded with a dichotomous endpoint such as shigellosis. The utility of the disease severity score has been demonstrated in evaluating the effect of a Shigella bioconjugate vaccine in reducing the severity of illness induced by S. flexneri 2a 2457T in a vaccination-challenge study (Talaat et al. 2021). The vaccine’s greatest effect appeared to be on reducing the severity of illness following the challenge. While the proportion of vaccine and placebo recipients meeting the a priori dichotomous shigellosis endpoint was not significantly different, vaccinated subjects had statistically significant lower disease severity compared to placebo subjects. Particularly interesting, the most striking difference in disease severity across treatment groups was among participants that met the a priori primary endpoint, highlighting the importance of improved granularity in characterizing shigellosis and disease outcomes in the CHIM.
The disease severity score may also provide a unique endpoint for the comparison of non-clinical responses, including immunologic outcomes (Talaat et al. 2021; Clarkson et al. 2020). Following challenge with S. sonnei 53G, a significant correlation between multiple post-challenge adaptive immune responses and the disease severity score was observed (Clarkson et al. 2020). Additionally, novel evaluations of fecal markers of intestinal inflammation post-challenge with S. sonnei 53G revealed a significant association with increasing intestinal inflammation and disease severity score, indicating that the severity of the disease may also be directly linked with the innate immune response. Similar associations of adaptive immune responses and disease severity score have also been reported in an S. flexneri 2a CHIM study where, following vaccination with a Shigella bioconjugate vaccine, pre-challenge anti-LPS serologic responses were significantly inversely correlated with disease severity score following challenge with S. flexneri 2a 2457T (Talaat et al. 2021). The Shigella disease severity score synthesizes multiple clinical signs and symptoms into a single endpoint, enabling a robust comparison across treatment groups; it also appears to correlate with non-clinical outcomes, potentially highlighting differences not readily apparent using traditional endpoints.
3. The Role of CHIMs in Shigella-Specific Immune Response Investigations
3.1. Shigella-Specific Immunity and Key Antigens
Homologous re-challenge studies in the same volunteers offer the opportunity to study Shigella serotype-specific immunity. Three separate challenge–re-challenge studies have demonstrated the ability of an initial experimental infection to induce a sufficiently robust immune response to protect against re-challenge with the same organism (DuPont et al. 1972b; Herrington et al. 1990; Kotloff et al. 1995a). In a seminal study of homologous protection, of the subjects previously challenged with S. flexneri 2a 2457T who developed shigellosis (n = 15), only 20% developed fever or diarrhea following re-challenge with 10,000 CFU of S. flexneri 2a 2457T (DuPont et al. 1972b). In contrast, naïve participants (n = 39) developed fever or diarrhea at a much higher proportion (56%). Interestingly, there was no difference in the proportion of subjects shedding the challenge strain in their stools across the two groups (67% and 69%). Following on from this work, the inoculation solution was changed from skim milk to sodium bicarbonate and a more consistent AR was achieved with additional gastric buffering. In the same study, among veterans previously challenged with 1000 CFU of 2457T (n = 5), when re-challenged with the same strain, subjects shed lower amounts of Shigella and only 40% experienced any illness while 20% had fever or dysentery and none had diarrhea (Kotloff et al. 1995a). In contrast, among naïve subjects (n = 12), 92% had some illness with 83% having either fever, diarrhea or dysentery.
There is a single published challenge–re-challenge study with S. sonnei 53G (400 CFU). Among the 12 naïve participants, 50% had fever, 58% diarrhea and 67% dysentery, while only 1 of 6 re-challenged participants developed dysentery (a single dysenteric stool) (Herrington et al. 1990). These challenge–re-challenge studies, in conjunction with epidemiological studies (DuPont et al. 1972b; Ferreccio et al. 1991; Formal et al. 1991), have been instrumental in demonstrating the role of serotype-specific immunity in protection from shigellosis. Although the exact mechanism of protection is unknown, the observed homotypic protection has solidified the OAg as a key protective antigen leading to the generation of serotype-specific OAg-based immune responses as the focus of most Shigella vaccines under development (Table 1).
Nonetheless, additional Shigella antigens, including highly conserved Ipa proteins as well as the essential virulence factors/antigens VirG (IscA) and PSSP-1, have also been associated with the development of protective immunity and may be the targets for vaccine development (Clarkson et al. 2021a, b; Shimanovich et al. 2017; Ndungo et al. 2018). Recently, a novel Shigella proteomic array was utilized to evaluate serum samples from subjects receiving the S. flexneri 2a 2457T challenge strain. Data indicated a strong association between antibody responses to IpaA, IpaB and IpaC and protection from shigellosis upon challenge (Ndungo et al. 2018). In a similar application of the proteome array using samples collected from the S. sonnei CHIM dose-finding/dose-verification study (Frenck et al. 2020; Clarkson et al. 2020), subjects that were resistant to infection had elevated antibodies directed to LPS and IpaB (Randall 2021) indicating that anti-Ipa antibodies may complement anti-LPS antibodies and further reduce the risk of developing shigellosis following challenge (Clarkson et al. 2021a; Shimanovich et al. 2017; Ndungo et al. 2018).
3.2. Potential for Cross-Protection Across Shigella Serotypes
Given the number of Shigella serotypes, investigating antigens that may provide cross-protection across multiple serotypes is of high interest. Some vaccine strategies have focused only on the highly conserved Ipa proteins in hopes of inducing pan protection from all Shigella species (Martinez-Becerra et al. 2012, 2013). Their potential value as vaccine antigens is further supported by animal studies that indicate induction of protective OAg-independent immunity (Martinez-Becerra et al. 2012, 2013). However, given the extensive evidence that protection from Shigella infection is serotype-specific, it is most likely that the OAg will be a required component of a protective vaccine. Nonetheless, given the homology of these protein antigens across Shigella serotypes, it is possible that robust immune responses to these highly conserved Ipa proteins may provide additional breadth of immunity and could potentially provide protection across heterologous serotypes (at least within a given serogroup) (Noriega et al. 1999).
The potential for cross-protection may be a more realistic goal within certain serogroups of Shigella species. For example, there are structural similarities between the LPS OAg repeating units of S. flexneri serogroups with the potential for cross-reactivity between some of the serotypes and subserotypes. The overlapping presence of several type- and group-specific antigens on the respective OAg structures is the foundation for OAg-based vaccine strategies targeting cross-protection across multiple S. flexneri serotypes. For example, the OAg portion of LPS of S. flexneri 2a (representing group factor 3,4 and type factor II) and 3a (representing group factors 6 and 7,8 and type factor III) could theoretically provide protection against all known serotypes of S. flexneri, except serotype 6, which expresses a different linear tetrasaccharide repeating unit of LPS. This potential of cross-protection was demonstrated in guinea pigs immunized with live-attenuated S. flexneri 2a and 3a vaccine candidates, leading to the generation of cross-reactive serum and mucosal antibody responses against all S. flexneri serotypes, except for S. flexneri 6. When these immunized guinea pigs were challenged with virulent wild-type S. flexneri strains, vaccination provided significant cross-protection against serotypes 1b, 2b, 5b, and Y, but not serotypes 1a, 4b, or (as anticipated) 6 (Noriega et al. 1999).
In a field trial of S. flexneri 2a LPS-conjugate vaccine in 1–4-year-old Israeli children, * 52% efficacy against shigellosis caused by S. flexneri serotype 6 was observed (Passwell et al. 2010). Further investigation of the serological responses from field trials of the S. flexneri 2a conjugate vaccine demonstrated some cross-reactivity with S. flexneri 6, but serum responses from natural infection with S. flexneri 2a did not cross-react against serotype 6 (Farzam et al. 2017). The shared O-acetylated disaccharide of these two serotypes could be the explanation for this observed cross-reactivity.
The existing Shigella CHIMs have supported the development of single serotype Shigella vaccines by providing an early indication of homotypic clinical protection. The existing and future Shigella CHIMs will also provide valuable assessment of multi-serotype Shigella vaccine candidates, including an early indication of both homotypic and heterotypic protection resulting from immunologic cross reactivity.
3.3. Immune Correlates of Protection for Shigella Infection
When considering Shigella pathogenesis, several protective immune mechanisms could be proposed depending on the clinical endpoint of interest (Fig. 2). If sterilizing immunity and prevention of transcytosis across the mucosal epithelium is desired, antibodies actively secreted locally or passively transudated into the lumen may be required. Protection from mild to moderate disease could be achieved within the intestinal lamina propria via antibody-mediated neutralization, killing by opsonization or complement activation or, antibody-dependent cellular cytotoxicity. Cell-mediated immunity may be important in protecting from more severe disease by working to prevent or reduce the intercellular spread of Shigellae and subsequently reducing the frequency or severity of bloody, mucoidal stools and colonic ulceration.
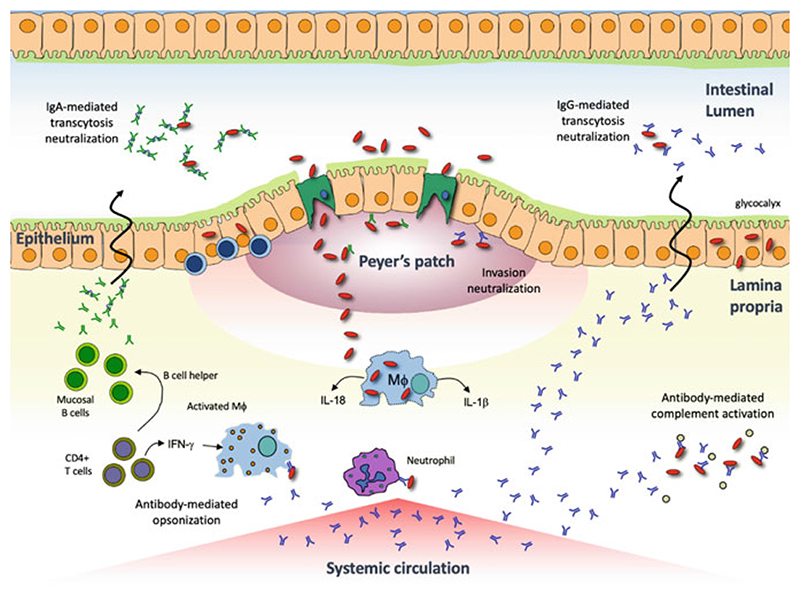
Fig. 2. Shigella pathogenesis and potential protective immune mechanisms.
Shigella is transmitted via the fecal-oral route and transits from the lumen into the lamina propria via M cells. Once in the basolateral space, Shigellae can induce pyroptosis in macrophages (MΦ) and utilize a type-III secretion system to translocate effector proteins (i.e., Ipa proteins) resulting in phagocytosis of the bacterium. Once intracellular, Shigellae multiply and spread to adjacent cells by reorganizing host cell actin polymerization. The intercellular bacterial spread and subsequent recruitment of neutrophils to the site of infection results in tissue destruction and the characteristic pathology of shigellosis. Although well-defined correlates of immunity have not been fully elucidated, protective immune responses may include neutralization of the bacteria in the gut lumen (prevention of M cell transit) or in the lamina propria (interference with host cell invasion), antibody-mediated complement activation (bacterial killing) and opsonization (marking bacteria for phagocytosis)
An immune CoP for Shigella infection has yet to be defined. LPS-specific serum IgG has been suggested as a CoP (Talaat et al. 2021; Passwell et al. 2001, 2010; Cohen et al. 1991, 2019; Robbins et al. 1992); however, it is yet to be defined if LPS-specific serum IgG is serving as a mechanistic correlate or as a surrogate measure for a yet-to-be-defined protective mechanism. An S. sonnei LPS-specific serum IgG threshold was previously established as protective in field trials (Cohen et al. 1988). However, in a recent vaccination-challenge study, comparable or higher anti-LPS IgG levels were achieved by nearly 70% of subjects yet were insufficient to prevent shigellosis following S. sonnei challenge (Frenck et al. 2021). Nonetheless, baseline and pre-challenge anti-LPS IgG levels were higher in subjects who did not develop shigellosis post-challenge, thus suggesting a role of these antibodies in clinical protection.
Additional CoP have also been suggested, including LPS-specific serum IgA and functional antibody responses (Clarkson et al. 2021a, 2020; Shimanovich et al. 2017), and several different protective mechanisms have been proposed as a result of data generated in Shigella-CHIMs. These infection models have demonstrated the importance of LPS-specific mucosal antibody responses, as measured either by mucosal IgA secreting B cells or by quantifying secretory IgA antibodies in mucosal secretions (Holmgren et al. 2017; Clarkson et al. 2021a, 2020; Coster et al. 1999; Schultsz et al. 1992; Cohen et al. 1996). Additionally, both LPS- and IpaB-specific memory B cell IgA responses have been associated with protective efficacy or a reduced risk of disease post-challenge in a CHIM (Clarkson et al. 2021a, 2020; Wahid et al. 2013). Although T cell immunity has been extensively investigated in the context of Shigella pathogenesis and immune evasion, there has been little evidence derived from CHIMs to suggest their utility as CoP for Shigella infection (Holmgren et al. 2017). Mathematical modeling or machine learning studies have described a combination of LPS-specific mucosal (IgA antibody secreting cells) and systemic (serum IgG) responses working synergistically together serving as co-correlates (Davis et al. 2013; Arevalillo et al. 2017).
Recently, anti-LPS IgG and serum bactericidal responses following vaccination with an S. flexneri 2a bioconjugate vaccine were associated with protection following challenge with S. flexneri 2a strain 2457T (Clarkson et al. 2021a). Using extensive methods to characterize the immune responses to vaccination and challenge in a controlled environment as afforded by the CHIM may offer an opportunity to differentiate a protective immune response that may otherwise be indiscernible in field studies. Similarly, placebo subjects challenged with either S. sonnei (Clarkson et al. 2020) or S. flexneri 2a (Clarkson et al. 2021a) can be compared and contrasted enabling dissection of potential differences across serotypes that may be essential for protective immunity.
3.4. Shigella-Specific Immune Profiles
Defining immune CoP can be complicated due to a variety of host and environmental factors (Katona and Katona-Apte 2008; Flores and Okhuysen 2009; Mondal et al. 2012; Zimmermann and Curtis 2019). Additionally, pathogen strain or serotype, route of infection and dose can influence the host’s immune response and pathogen virulence (Martins et al. 2013; Sela et al. 2018; Sperandio 2018). Furthermore, a protective immune response for Shigella infection may actually be composed of several highly correlated responses across a range of immunoassays measuring multiple different systemic, mucosal or cellular responses (Clarkson et al. 2021b). Consequently, attempts to define a single immune correlate or surrogate of protection may not adequately account for all of the described complexities and a broader definition of protective immunity describing an integrated and networked immune response profile should be considered (Clarkson et al. 2021b; Querec et al. 2009; Pulendran et al. 2010; Trautmann and Sekaly 2011; Li et al. 2013; Nakaya and Pulendran 2015; Haks et al. 2017; Hasin et al. 2017). Immune response profiles post-vaccination and/or challenge or across different vaccine constructs or exposure routes have been described for several pathogens including human immunodeficiency virus (HIV), polio, Salmonella Typhi and cholera (Querec et al. 2009; Ryan and Calderwood 2000; Plotkin 2001, 2010; Tomaras and Plotkin 2017; Alter and Barouch 2018; Dahora et al. 2019).
Recent data from two Shigella-CHIMs have described distinct protective immune profiles associated with different antigenic exposure routes: parenteral immunization with the S. flexneri 2a bioconjugate and oral challenge with either S. flexneri 2a 2457T or S. sonnei 53G. The protective immune profile associated with parenteral immunization was characterized by robust systemic IgG and IgA responses with milder functional antibody activity and mucosal IgG responses (Clarkson et al. 2021a, 2021b). As expected, a different immune profile was observed after oral challenge with S. flexneri 2a that induced a more “balanced” profile characterized by similar relative increases in both systemic and mucosal antibody responses with moderate memory immune responses and functional antibody activity. Surprisingly, an altogether different immune profile was observed after oral challenge with S. sonnei characterized by robust increases in mucosal IgG and IgA responses, clearly differentiating subjects without shigellosis from those that displayed clinical signs post-challenge (Clarkson et al. 2021b, 2020), while little to no differences in systemic responses were observed across these subjects. Data from these CHIMs suggest that: (1) different immune profiles can provide protection against Shigella infection and (2) different protective mechanisms may be associated with protection from different Shigella species. CHIMs provide a valuable tool to conduct in-depth immune response characterization and to investigate and describe early immune profiles associated with protection from shigellosis. Interestingly, the immune profile differences observed between S. flexneri 2a and S. sonnei are also consistent with the differences in pathogenesis between these two serotypes (see Sects. 1.2 and 1.3).
3.5. Vaccine Immunogenicity and Effectiveness in Target Age Group—Cautionary for CHIMs
A limited number of Shigella vaccines have been evaluated in children or infants living in LMICs. An orally delivered live-attenuated S. sonnei vaccine in Bangladeshi children aged 5–9 years lacked immunogenicity post-vaccination (Raqib et al. 2019). There was also no shedding of the vaccine strain among immunized children, indicating that the attenuated bacteria were unable to colonize the intestinal mucosa. Resistance to colonization is not uncommon with oral vaccines in endemic populations and has been reported with other enteric pathogens, including cholera and rotavirus (Qadri et al. 2005, 2007; Madhi et al. 2010). Although many factors likely contribute to a lack of vaccine colonization, consideration must be given to the high incidence of EE that exists in the target population. Environmental enteropathy significantly impacts proper gut immune function and intestinal permeability, contributing to a constant state of malnutrition combined with severe micronutrient deficiencies (Korpe and Petri 2012).
The impact of EE should also be specifically considered in the context of S. sonnei infection and S. sonnei live-attenuated vaccine strains given recent evidence that S. sonnei harbors a T6SS and uses it to kill commensal intestinal bacteria (Anderson et al. 2017). The importance of commensal bacteria has been well documented as they not only contribute to proper gut health but have also been shown to influence and modulate systemic immune responses to pathogenic bacteria (Molloy et al. 2012; Belkaid and Hand 2014). With S. sonnei infections increasing in prevalence in LMIC populations (Rogawski et al. 2018; Thompson et al. 2015; Obiero et al. 2017; Pavlinac et al. 2017) and, with the testing of live-attenuated S. sonnei vaccine strains, it is essential to consider how this serotype may exacerbate EE and further contribute to reduced intestinal barrier function and increased malnutrition in the target population. Furthermore, the killing of intestinal microbiota may increase the possible risk of systemic Shigella bacteremia, leading to serious health complications or death in an already susceptible population.
S. sonnei and S. flexneri 2a OAg-conjugate vaccines have also been studied in the target population and have been shown to be safe and immunogenic in Israeli children as young as 1-year-old (Passwell et al. 2010, 2003; Ashkenazi et al. 1999). The effectiveness of the S. sonnei and S. flexneri 2a OAg conjugates has also been evaluated with promising reports of * 70% efficacy in Israeli adults. The effectiveness of these candidate conjugate vaccines was also tested in 1–4-year-old Israeli children; however, the efficacy results were age-related with only children ≥ 3 years old protected from shigellosis (Passwell et al. 2010). This age-related efficacy in a Shigella endemic region, where the majority of infections occur prior to the age of three years, suggests that the observed efficacy in older populations may have partially resulted from the conjugate vaccine serving as a booster to previously exposed, and therefore mucosally primed, individuals.
Shigella-CHIMs are useful tools for preliminary readouts of vaccine efficacy; however, without defined CoP for Shigella infection, caution is required when extrapolating efficacy results from CHIMs to the target population. It is important to consider that CHIM participants are adults, typically living in high-income settings, and are distinctly different than the target population of young children living in LMICs. Infant health, nutritional status and immune status can vary greatly in endemic settings, all of which contribute to differences in immune and vaccine responses (Mondal et al. 2012; Zimmermann and Curtis 2019). Furthermore, the immune system does not fully mature until approximately 24 months of age and infants have been shown to have lower levels of circulating immunoglobulin and complement effectors (Simon et al. 2015), with a recent report indicating a gradual increase in both Shigella-specific serum IgG and IgA responses in infants over time (Chisenga et al. 2021). This is an important consideration in the context of the currently proposed CoP for Shigella infection, including serum IgG or functional antibody activity (Shimanovich et al. 2017; Cohen et al. 2019; Ndungo and Pasetti 2020). It is possible that protective mechanisms observed in one population may not extrapolate to all populations and that different protective immune mechanisms may be required depending on the population of interest.
4. Alternatives to Shigella-CHIMS
Animal models and cell culture systems using immortalized human cell lines or human tissue explants have historically been used to advance our understanding of the molecular and biochemical basis of the pathogenesis and immunology of enteric bacterial pathogens, like Shigella (Wenzel et al. 2017). However, it has long been recognized that these models have shortcomings that limit their translational applications, especially in drug and vaccine development. With the high burden of acute and long-term illness associated with Shigella, as well as the alarming rise in antibiotic resistance, the need to accelerate drug and vaccine development has become more urgent and, in addition to CHIMs, improved in vitro models are needed.
4.1. Commonly Used Animal Models
Several Shigella-specific animal models have been developed (Kim et al. 2013). For vaccine efficacy studies, two pre-clinical models have been utilized extensively. The mouse pulmonary lung model has been utilized by several groups to demonstrate preliminary immunogenicity and efficacy, largely as a stepping stone to testing in higher animal species (Mallett et al. 1993). The guinea pig keratocon-junctivitis or Sereny model has been utilized to evaluate immunogenicity and efficacy (Hartman et al. 1991). Both models rely on the protection of mucosal surfaces, albeit dissimilar to the intestinal tract and at bacterial doses that exceed those utilized in CHIMs. Although some evaluations have determined that mucosal antibody (ocular IgA) correlates with protection from disease (Kaminski et al. 2014), transferability to the human model has not been established.
A guinea pig rectocolitis model has been described (Shim et al. 2007) and with some slight technical modifications has been utilized successfully in vaccine efficacy studies; however, its use is not widespread. The advantage of the guinea pig rectocolitis model is that infection closely replicates human disease with bloody mucoidal stools, tenesmus and intestinal inflammation. More recently, a mouse diarrhea model has been developed by investigators at the University of Virginia that utilizes mice maintained on a zinc-deficient diet (Medeiros et al. 2019). This promising diarrhea/dysentery model is potentially a significant advancement because it also mirrors the human clinical outcomes resulting from natural or experimental Shigella infection (Shim et al. 2007; Medeiros et al. 2019; Barry et al. 2019). Both the guinea pig rectocolitis and mouse zinc-deficient models may also enable investigators to evaluate vaccine impact on other negative health outcomes from Shigella infection, e.g., gut inflammation and growth impairment (Medeiros et al. 2019, 2020). The new zinc-deficient mouse model has been used successfully to evaluate both active (vaccine) and passive (monoclonal antibody, mAb) interventions for the prevention of shigellosis (Medeiros et al. 2020; Teh et al. 2021).
In addition to small animal models, two non-human primate models have been utilized to evaluate potential Shigella countermeasures. The rhesus macaque model has proven extremely useful in the identification of Shigella antigens recognized by the immune system after oral infection (Oaks et al. 1986, 1996; Turbyfill et al. 1995) and establishing that prior infection with one Shigella serotype offers resistance to infection with the homologous serotype but not heterologous serotypes (Formal et al. 1991). Rhesus macaques have also been used in early efficacy studies of promising Shigella vaccine candidates (Formal et al. 1965, 1984). More recently, an Aotus nancymaae model was established and utilized to evaluate Shigella vaccine candidates (Gregory et al. 2014). The Aotus model has the added advantage of being capable of potentially evaluating combination enteric vaccines as the model has also been established for both enterotoxigenic Escherichia coli (ETEC) (Jones et al. 2006a; Rollenhagen et al. 2019) and Campylobacter spp. (Jones et al. 2006b).
However, the benefits of both non-human primate models must be considered against several inherent constraints, which include the requirement for a high inoculum dose to achieve reproducible attack rates and the development of gastric lesions, which are neither associated with human shigellosis nor evident in CHIMs. Additionally, it is unclear if protection seen in these models directly correlates to human efficacy (in CHIM studies or field efficacy trials). Costs and ethical considerations also must be factored into the decision matrix, which can reduce the attractiveness and value of these non-human primate models for vaccine efficacy assessments.
4.2. Human Enteroid Model
Over the last 10 years, the human enteroid model has rapidly evolved to become a more human-relevant research tool, with the potential to be transformational in drug and vaccine development (Ranganathan et al. 2020; Foulke-Abel et al. 2020). The full research potential of this ex vivo model is yet to be realized but early application to Shigella indicates that it is suitable for studies of pathogenesis and early events such as colonization, invasion and activation of innate immunity, as well as triggers of gut inflammation (Ranganathan et al. 2019; Koestler et al. 2019). The model has recently shown that exposure of S. flexneri 2a to glucose and bile salts upregulates the expression of multiple adhesins suggesting that Shigellae, as highly human-adapted pathogens, have evolved to regulate virulence gene expression for efficient colonization and infection of the human host (Chanin et al. 2019). This highlights the importance of this new enteroid model for studies of bacterial enteric pathogens and, in the case of Shigella, the identification of new adherence factors as novel targets for future vaccine development efforts and the exploration of their role in facilitating infection in future CHIMs with S. flexneri and S. sonnei strains. The encouraging early enteroid model results of Shigella pathogenesis complement observations made in the CHIMs (Bourgeois, personal communication) and also suggest that further modification of the enteroid model, using cells derived from Shigella immune individuals, CHIM-infected individuals or individuals immunized with leading vaccine candidates, should be pursued to better understand bacterial and host cell interactions.
5. Next Steps in Shigella-CHIMs: Expanding the Footprint
5.1. Extending Established Models to Endemic Populations and Regions
The conduct of CHIMs, including those for Shigella, has expanded into individuals living in endemic areas (Bodhidatta et al. 2012). This has largely been the result of increased funding for these studies in endemic populations and regions. Recent international meetings sponsored by the Wellcome Trust, International Alliance for Biological Standardisation (IABS) (Pollard et al. 2020), WHO and the Gates Foundation have all recognized that CHIMs performed in endemic populations would differ from those in non-endemic settings. Potential distinctions may be due to differences in pathogen exposure and acquisition of naturally acquired immunity, host genetic background, gut microbiome and co-morbidities. As a result, the findings from CHIMs undertaken in non-endemic populations may not be generalizable (WHO 2016; Gordon et al. 2017; Elliott et al. 2018; Baay et al. 2019; Kunda-Ng’andu et al. 2021). Establishing CHIMs in endemic settings requires close collaboration between laboratories and clinical sites that have well-established CHIMs in order to benefit from previous experience, allowing for model transfer, as well as proactive dialogues with regulatory and ethical agencies of endemic countries (Kunda-Ng’ andu et al. 2021).
Key attributes of endemic CHIMs would be the ability to evaluate disease susceptibility or severity known to be impacted by population diversity or genetics (e.g., prevalence of sickle cell anemia in African populations, or lower neutrophil counts in persons of African origin and development of benign neutropenia following potent immune stimulation, etc.). Particularly for Shigella-CHIM outcomes, pre-existing immunity, co-infections, exposure to cross-reacting pathogens, co-morbidities or infectious disease history, diet and enteric microbiome may alter responses by skewing the subject’s immune response pattern. Thus, CHIMs in endemic settings can confirm critical safety and end-point assessments of the model and as well aid in establishing early insight into the efficacy and possibly CoP in the target population, albeit perhaps not in the target age group.
Limitations for CHIMs are similar when carried out in unexposed populations and endemic settings (differences between natural and experimental infection, inoculum sizes, challenge strains, lack of accurate patient medical history, etc.). CHIMs have been conducted in LMICs since the 1990s involving cholera, rotavirus, malaria, Shigella, pneumococcus and dengue, thus allowing local capacity building and local investigators to play a pivotal role in the development of novel disease interventions and control measures that are relevant to their settings.
Transition of a CHIM to an area in which the pathogen is endemic requires additional development to re-assess parameters of the model including safety, clinical endpoints, infective doses and intervention efficacy in a potentially pre-exposed population. Typically, individuals repeatedly exposed to a pathogen over time will acquire immunologic responses that can impact disease susceptibility and/or resistance. Natural immunity is reflected by modified disease characteristics, morbidity and mortality compared to a naïve population, with profound impact on observed drug treatment or vaccine efficacy (Zimmermann and Curtis 2019). Level of prior exposure, and hence baseline immunity (Cohen et al. 1991, 1989, 1992; Obiero et al. 2017; Raqib et al. 2002), may influence the challenge dose needed to replicate a similar AR or illness compared to those observed in naïve population CHIMs. However, this may change the safety profile of the challenge. Alternatively, CHIMs in endemic populations provide an excellent opportunity for the identification of immunological signatures that would improve our understanding of host–pathogen and/or host–vaccine interactions and the spectrum of inflammatory and antigen-specific humoral and cellular responses associated with infection, immunity and protection in the context of naturally acquired immunity and historic exposure (Table 4). Findings in adult populations from endemic settings also need to be compared to findings in naïve adult populations, as for many prophylactic products protecting the individual, naïve or exposed, is a common goal.
Table 4. Conduct of Shigella-CHIMs in different population settings.
| Pros |
|---|
5.2. Learnings from Conduct of Shigella-CHIMs in Endemic Settings
To date, there have only been two Shigella-CHIM studies in an endemic country, both in Thailand. The studies were conducted among healthy Thai adults at Mahidol University first as an infectivity dose-finding study (Bodhidatta et al. 2012) and then as a vaccine efficacy study (Pitisuttithum et al. 2016). In an attempt to recruit an infection-susceptible population from within the Shigella endemic setting, a more stringent screening threshold was applied to the Thai population compared to studies conducted in North America. In the dose-finding study (Bodhidatta et al. 2012), 3 cohorts of 12 subjects each were challenged with S. sonnei 53G strain (frozen). Volunteers with a baseline anti-S. sonnei LPS IgG > 1:800 (20% of those screened) were excluded to minimize the impact of pre-existing antibody titers from interfering with the infectivity. The 1:800 threshold was chosen (Dilara Islam, personal communication), based on previous field trial data collected in Israel (Cohen et al. 1989); however, as outlined earlier, different screening thresholds may be required in different CHIM settings, even across different Shigella endemic areas. Target inocula were 100, 400 and 1600 CFU, which were achieved for each of the cohorts. While the majority of volunteers in each group shed the challenge strain, 41–75% of the volunteers had at least one episode of dysentery, and only 1 volunteer in the highest dose cohort met the pre-specified criteria for shigellosis (diarrhea and/or dysentery with fever, ≥ 1 severe intestinal symptom and shedding). A single volunteer in each of the mid- and high-challenge doses met the definition of diarrhea. At the highest challenge dose, 75% of the Thai volunteers met the primary disease endpoint (diarrhea, dysentery and/or fever).
To achieve the desired AR, the target 1600 CFU dose was higher in Thai volunteers than previously used in immunologically naïve volunteers. In HICs, the S. sonnei 53G (frozen) challenge strain delivered at 400–500 CFU was able to induce shigellosis in 5/12, 6/11 or 5/9 controls (approximately 50%) in three independent studies (Herrington et al. 1990; Munoz et al. 1995; Black et al. 1987). The need for a greater inoculum in the Thai study, as compared to that used in North America, may be due to the underlying immunity in the Thai population despite screening for S. sonnei-specific anti-LPS antibodies. Given the small cohort size, the investigators were unable to determine if baseline antibody responses correlated with infection or clinical disease.
In a follow-up vaccine efficacy challenge study in Thailand evaluating the live-attenuated S. sonnei WRSS1 vaccine, the investigators were unable to reproduce the 75% AR observed in the dose-finding study at the highest challenge dose (Bodhidatta et al. 2012) as only a 20% AR was achieved (Clarkson et al. 2020). All volunteers in this study were screened for anti-S. sonnei LPS antibodies (1:800 threshold) and 24% were excluded from participation due to high baseline antibody titers; this was comparable with the previous study. Interestingly, a key finding of the second study was that anti-LPS IgA titers were identified as associated with protection. This finding has since been corroborated in S. sonnei 53G (lyophilized) challenge infectivity study in naïve populations in the US (Clarkson et al. 2020). However, it is important to note that the challenge inoculum using traditional methods of preparation by plate scrapes was identified as not being reproducible and could have accounted for the low AR and findings of low vaccine efficacy in Thai adults. Additionally, differences in endpoint definitions and other factors unique to the endemic Thai population that were not investigated may account for the lack of reproducibility of the model.
The use of lyophilized inoculum preparations, particularly when transferring CHIMs to disease-endemic regions, would help mitigate differences in reproducibility. The data from the S. sonnei 53G CHIM has shown that each new lyophilized inoculum stock needs to be clinically characterized for the desired AR in different settings; S. sonnei 53G dose was 1500 CFU when lyophilized or 400–500 CFU when frozen in North America, whereas a rather different frozen inoculum dose, 1600 CFU, was used in Thailand. Future CHIMs, regardless of geographical location, should implement lyophilized challenge strains as this standardization will be key to unraveling other factors important in either susceptibility or resistance to infection and allow better comparison between studies conducted in naïve versus exposed populations.
Shigella-CHIMs are now being translated to Kenya, where the regulatory framework already exists due to the ongoing malaria-CHIMs (Kapulu et al. 2018). Field trials of the leading Shigella vaccine candidates are ongoing in Kenya, making Kenya a logical place for the establishment of a Shigella-CHIM to allow direct comparison of experimental and field infection as well as generating evidence of cross-protection. Initially, the established S. sonnei 53G (lyophilized) model will be implemented (Wellcome and grants awarded 2005), but extending other Shigella CHIMs to this area is warranted. Several stakeholder consultations have been held to consider the ethical and regulatory frameworks for setup and conduct of CHIMs in the LMIC setting (Gordon et al. 2017; Elliott et al. 2018; Kunda-Ng’andu et al. 2021; Kapumba et al. 2020). While each region, country and setting are unique, common themes have emerged, highlighting the need to work closely with ethicists and regulators at the national and local levels. When considering a CHIM in an endemic country, the embedding of social science and empirical ethics substudies to highlight ethical dilemmas, perceptions and experiences for participation are required (Njue et al. 2018; Jao et al. 2020).
5.3. Expanding Shigella-CHIMs Beyond the Currently Established Serotypes
Typically, CHIMs use a single available challenge strain for each Shigella serotype, which should adequately represent the strain(s) circulating in the setting where the infection occurs. This however may pose some obvious complications as often many serotypes of a disease-causing strain exist in the field (e.g., influenza, pneumococcus, Shigella). Nevertheless, the use of a single-strain CHIM may still be meaningful in de-risking a multivalent vaccine candidate. In any case, caution should be exercised when extending results from one strain to other strains in order to avoid over-estimation of protection (Sack et al. 1994; Kerneis et al. 2009). Choice of the challenge strain may be influenced by epidemiology, need for homologous or heterologous protection studies and (presumed) mechanism of vaccine-conferred protection (antibody or cell-mediated; mucosal or systemic).
It is generally accepted that demonstration of safety, immunogenicity and efficacy will be required in the target population for a novel Shigella vaccine. The existing S. sonnei and S. flexneri 2a CHIMs can predict homotypic protection and may also be useful in the evaluation of multivalent Shigella vaccine candidates. However, the current epidemiology of other S. flexneri serotypes, targeted by multivalent vaccine candidates, may not support feasible field efficacy studies, in which case new S. flexneri serotype challenge strains may be useful for early evaluations as well as heterotypic protection resulting from immunologic cross reactivity. Thus, there is an urgent need to develop additional challenge strains of Shigella including but not limited to S. flexneri 3a and S. flexneri 6 which are currently described as globally important and relevant, especially in LMIC settings but are of lower prevalence compared with S. sonnei and S. flexneri 2a (Livio et al. 2014). To this end, a GMP lyophilized lot of S. flexneri 3a J17B is being manufactured by Walter Reed Army Institute of Research and, after identifying a dose that safely and reproducibly induces an AR of * 60–70%, it will be made available to the global community (Kaminski, personal communication).
Earlier efforts to develop a Shiga toxin mutant of S. dysenteriae for future vaccine studies should also be re-considered (Samandari et al. 2000) because this serotype is sporadically associated with increased morbidity and mortality during epidemic outbreaks. Similarly, the use of existing attenuated Shigella vaccine strains, in a broader sense, might enable CHIMs to be conducted in an outpatient setting due to their reduced fitness, enhanced safety and lower transmissibility. Collectively, these additional challenge strains may enable evaluation of vaccine efficacy against different Shigella species and serotypes for which current epidemiology limits the feasibility of classical Phase 3 efficacy studies. Additional Shigella CHIMs would also further aid the interrogation of heterologous protection and definition of cross-strain correlates of protective immunity.
The relationship between the safety profile of the CHIM and virulence of the challenge agent requires careful balance. The use of attenuated strains or live-attenuated vaccine pathogens in CHIMs can be useful in achieving this balance, especially when used in populations with increased disease susceptibility. This approach can reduce risks associated with severe disease complications and has been employed for viremia (e.g., dengue). Challenge agents may be genetically modified (naturally or via laboratory manipulation) to partially attenuate their activity as long as the changes do not dramatically impact the infectious process or the desired CHIM endpoint, thus restricting the adequate extrapolation of results into clinical settings. For example, the need to retain a viable pathogen is paramount if the intervention is believed to impact organism replication, dissemination within the host (cell to cell spread) and reduction or elimination of diseases. However, if the intervention is believed to act via transmission blocking or generation of sterile immunity, an attenuated challenge agent may be sufficient.
Likely, this narrow balance between strain attenuation and potential CHIM outcomes must be determined experimentally. Appropriate attenuated vaccine strains have been used as challenge agents to assess the efficacy of subunit vaccines (e.g., rotavirus) (Chilengi et al. 2020), and although a promising approach, there is no guarantee that protection against an attenuated strain will necessarily lead to protection against the wild-type strain. An additional complexity is that a naturally attenuated challenge strain may be considered similar to a genetically modified organism from a regulatory perspective and require higher levels of biological containment. An additional environmental risk assessment may be required to decide on contained use or level of physical isolation for use.
In the interest of model standardization, it has been suggested to generate well-characterized challenge strain banks. While bringing benefits of reproducibility and thus comparability between cohorts and study centers, it also requires that the strains have undergone extensive ex vivo manipulation and long-term storage (sometimes for decades) which may raise concerns around their representativeness of strains circulating in the field.
5.4. Feasibility of Phase 3 Trials and Role of CHIMs in Shigella Vaccine Licensure and Full Value of Vaccine Assessments
Like any in vivo model system, CHIMs represent a sophisticated opportunity to assess host–pathogen interactions. However, there are limitations including small sample sizes, selected from within a very specific population. This means results may lack external validity. The small sample size of CHIMs limits the statistical power to detect only the most overt differences. Additionally, while the lack of efficacy in a CHIM may discourage further evaluation of the candidate vaccine, demonstration of efficacy in a CHIM likely does not preclude the need for a field-based efficacy trial to confirm the CHIM results and allow licensure and policy recommendations.
There are only a limited number of viral or bacterial CHIMs that have been confirmed against real-life or field efficacy studies (Memoli et al. 2015; Darton et al. 2016). Typically, CHIMs use a single available challenge strain, which should adequately represent the strain(s) circulating in the setting where the infection occurs. This however poses some obvious challenges for Shigella as several serotypes cause disease in the field, whereas only two CHIMs have been established for S. flexneri 2a and S. sonnei. The reliability and validity of potential immunological CoP between CHIM serotypes are unknown between the two Shigella strains used in experimental infections (Giersing et al. 2019). Thus for interventions with proposed broad coverage against multiple serotypes, at least some efficacy will need to be (1) generated in field trials, in the target population, (2) extrapolated across strains based on field data, or (3) demonstrated in large randomized control Phase 3 trials. Well-developed and well-characterized CHIMs are a precious tool to assist in identifying the most promising candidates, defining immunological readouts relevant for clinical protection (Clarkson et al. 2021a) and providing insight for additional field studies to validate CHIM data and investment in candidate development (WHO 2020; MacLennan et al. 2019a).
There is no general acceptance or explicit guidance documents from regulators on the use of CHIM data in the licensure pathway for a vaccine (WHO 2016, 2017b; Ramanathan et al. 2019). Initially, CHIMs are unlikely to replace large Phase 3 trials, particularly for pediatric LMIC indications. If CHIM data are used to accelerate licensure, there is the implicit expectation that effectiveness studies will be completed post-licensure. CHIM data have supported US Food and Drug Administration (FDA) licensure of a cholera vaccine (Vaxchora ®) for the traveler’s market (Mosley et al. 2017). In this case, field trials were very difficult to perform due to the low incidence rate of seasonal disease in the target populations, and the inability to predict the timing and location of outbreaks. CHIMs demonstrated > 90% protective efficacy 10 days after vaccination and nearly 80% after 3 months. This, along with demonstrated safety in a larger cohort and confirmed immune responses, was sufficient for licensure in healthy adults at risk of contracting the disease during travel to highly endemic areas. Alternatively, Salmonella Typhi CHIM data were considered as a factor in the WHO policy recommendation of typhoid conjugate vaccines (TCV) (WHO 2017b; Burki 2018). In this instance, there was no good animal model available and the CHIM provided valuable information on the immune response and a serologic bridge to an effective licensed vaccine.
A key aspect of the full value of vaccines assessment is to de-risk and incentivize investment in vaccine development and testing. By reducing the reliance on large phase 3 efficacy studies, CHIMs can reduce both development timelines and cost. Positive efficacy results of a single Shigella serotype candidate vaccine in a homologous CHIM may support intervention selection, generate early efficacy signals and contribute to higher success rate of late-stage clinical development. Successful evaluation of a single Shigella serotype candidate vaccine in a homologous CHIM may also help to benchmark expected immune responses in a multivalent candidate vaccine.
5.5. Expanding CHIMs to Other Age Groups
In selected cases, CHIMs may also include vulnerable populations (e.g., immunocompromised, elderly, young children or pregnant women) to assess, in well-controlled situations, CoP and in some cases efficacy that may differ in these populations (e.g., elderly) compared to healthy young adults typically enrolled in CHIMs. Previous arguments have been made to support the extension of Shigella-CHIMs to children in endemic areas, as they are likely to encounter Shigella normally (Giersing et al. 2019). However, the potential risks of childhood experimental challenges currently outweigh any potential benefits, and as a result, Shigella-CHIM development in young children is not recommended. Early efficacy indication of treatment in elderly adults with reduced ability to mount appropriate immune responses might be warranted when balancing the risks associated with a CHIM and the risks associated with large, lengthy and costly field trials. Presently, National Regulatory Agencies in the US (FDA) and in Europe (European Medicine Agency, EMA) do not support the conduct of CHIMs in pediatric populations, pregnant women or immunocompromised subjects (FDA 2011; EMA 2018).
5.6. Expanding CHIMs for Use Beyond Vaccines
Besides their use in vaccine development, CHIMs can be very useful in the evaluation of other prevention or treatment modalities and can contribute to a better understanding of naturally acquired immunity when conducted in exposed populations. For the former, Shigella-CHIMs have demonstrated that rifaximin can be used prophylactically to prevent illness after challenge (Taylor et al. 2006; Tacket et al. 1992). As the Shigella-CHIMs are translated to exposed populations, they may be useful tools in unraveling and identifying key pathogen-specific antigen targets and host immune signatures that will inform second and third-generation Shigella vaccines. In addition, CHIMs can assist in the evaluation of other therapeutic products, including but not limited to prophylactics, antibiotics and phages-targeting Shigella species (Bernasconi et al. 2018; Shahin et al. 2020). In the era of innovative technological advancements, CHIMs will be used for the discovery of therapeutics such as pathogen-specific mAbs in addition to their subsequent testing for efficacy. The potential for new therapeutic solutions, such as mAbs, should have a high impact on combating the growing threat of AMR strains.
6. Outlook
The acquisition of natural immunity following repeated infections with Shigella suggests that a vaccine is feasible. However, the large number of Shigella serotypes complicates the development of a broadly protective vaccine. Thus, a vaccine must contain either multiple components, if based on the serogroup-specific OAg or several highly conserved protein antigens with varied virulence targets. Currently, several diverse technologies are being applied (alone or in combination) to Shigella vaccine development (Table 1), including novel glycoconjugation techniques (bioconjugation or synthetic conjugation), subunit protein approaches, GMMA technology (outer membrane exosomes) and live-attenuated strains. These approaches may well have differing abilities to potentiate immune responses in the pediatric age group as a result of their modes of delivery, antigen presentation and different mechanisms of action. Shigella-CHIMs will help to clarify the mechanism by which immunity is generated and provide insight into which platform may elicit the most appropriate immune responses.
The recent Shigella-CHIM efforts to better grow and deliver strains, assess the impact of pre-existing immunity on outcomes and develop more standardized clinical endpoints are initial steps needed to make Shigella-CHIMs a better tool, supporting product development and licensure. However, there is more to be done to expand the application of CHIMs, the strain serotypes that can be studied as well as the potential for antigen discovery and identification of CoP. Each of these contributes to maximizing the potential and value of multivalent Shigella vaccine candidates in development and possible combination vaccines that include multiple Shigella components. To achieve this, aspects of the CHIMs’ development and application need to be further explored to enable the platform to reach its full potential aiding both basic and applied research supporting the ongoing international Shigella prevention and control efforts.
6.1. Role of CHIMs in Vaccine Development
The high global Shigella burden and difficulty in effectively treating shigellosis has heightened interest in accelerating the development of new vaccines. The growing interest in accelerating innovative vaccine development has led to a number of international convening’s that have critically evaluated the fundamental processes by which vaccines are traditionally developed (Giersing et al. 2019). The major driver for this assessment is that historically there has been a low probability of success for candidate vaccines entering advanced clinical development. As a result, better tools are needed to de-risk projects by more rapidly identifying the better vaccine candidates. Current timelines for vaccine development are lengthy and often vaccines that reach Phase 3 field trials, if feasible, fail to provide the desired level of protective efficacy. The broader application of an experimental medicine platform that expands the use of experimental human models of infection and disease, used at the appropriate stages of development, could accelerate candidate vaccines and better ensure that only candidates with the highest probability of success advance to costly Phase 3 testing. There is now growing consensus that expanded use of CHIMs for enteric pathogens could be a potential paradigm shift and significantly shorten timelines of vaccines to licensure and WHO pre-qualification. For the most common bacterial cases of diarrhea, dysentery and enteric fever among young children and infants in LMICs and international travelers, CHIMs have for the most part been established and utilized in the US and to a lesser extent in Europe. The utility of CHIMs in accelerating vaccine development has previously been demonstrated through its central role in the recent FDA licensure of the Vaxchora oral cholera vaccine (Mosley et al. 2017) and WHO’s pre-qualification of Typbar-TCV, a TCV, for typhoid fever (Jin et al. 2017).
International conferences on CHIMs sponsored by IABS (Pollard et al. 2020), WHO and the Wellcome Trust in 2017 and more recently by the United Kingdom’s Academy for Medical Sciences in 2018 (AMS 2005) have all reaffirmed the pivotal importance of CHIMs in vaccine development, being particularly useful in early “proof of concept” efficacy studies (Gordon et al. 2017). These international workshops identified a number of issues that were pertinent to further maximize the impact of CHIMs on the development of new preventive and treatment interventions for shigellosis. Paramount among these was the recommendation to expand and build capacity to perform CHIMs in LIMCs with an important rationale being that research conducted within areas of unmet medical needs will result in outcomes more relevant to the at-risk population while boosting the quality of research conducted in these areas. These conferences also discussed the benefits and barriers to performing CHIMs in LMICs and identified the growing interest among LMIC investigators to explore the use of CHIMs in their respective countries to help in the development of new interventions that are more appropriate for use in their communities. These international meetings were a very important first step in expanding CHIMs application and the recent decision by the Wellcome Trust to support Shigella-CHIMs development in Kenya (Wellcome and grants awarded 2021) under the oversight of the US FDA and local regulatory authority is a clear indication that public health stakeholders appreciate the value of CHIM expansion to LMICs. To support the vaccine development paradigm shift, the models must be conducted using high research, regulatory and ethical standards.
6.2. Role of Challenge Strains
As the application of Shigella CHIMs expand, international stakeholders, such as WHO and the Wellcome Trust (Giersing et al. 2019; Wellcome Human infection studies for vaccine development 2021), have stressed the need for available Shigella challenge strains to support broad coverage vaccine development and other preventive interventions. To date, the 2457T strain of S. flexneri 2a and the 53G strain of S. sonnei have been the most commonly used challenge strains and only the S. sonnei 53G strain is available in a lyophilized form. Given that most Shigella vaccines under development are envisioned to be multivalent formations, providing coverage for at least three S. flexneri serotypes as well as S. sonnei, it will be useful that GMP challenge strains, preferably lyophilized, be prepared for at least S. flexneri 3a and S. flexneri 6 and their respective challenge doses and ARs be established in dose-ranging studies. Shigella flexneri 1b, 3a and 6 are also epidemiological important strains associated with diarrhea and dysentery among infants and children in LMICs but their overall incidence would make Phase 3 efficacy studies against these serotypes very difficult and costly to carry out. Thus, it is important to work in parallel to develop challenge strains for epidemiologically relevant S. flexneri serotypes to support the range of protection afforded by these multivalent vaccines. As new challenge strains are developed, it will also be important to evaluate strain delivery across serotypes to ensure optimal buffering and protection against gastric acid during the dosing process. Similarly, insights from recent human enteroid models and transcription studies on the role of factors impacting the expression of adhesins and other Shigella virulence factors (i.e., component of bile salts, sodium glycocholate hydrate) might serve to increase ARs and improve the reproducibility/reliability of the model, if they were incorporated into the growth media and/or delivery buffer of the Shigella challenge strains (Chanin et al. 2019; Morris et al. 2013).
6.3. Role in Understanding Markers of Inflammation and Innate Immunity
The significance of Shigella as an enteric pathogen with global public health impact has been enhanced in recent years by a growing awareness that infection and illness resulting from this bacterium contributes to the causal pathways for gut enteropathy, malnutrition, stunting and poor cognitive development (Guerrant et al. 2021; Kosek and Investigators 2017; Donowitz et al. 2018). This has increased interest in Shigella vaccines that may also reduce the level of intestinal colonization and both gut and systemic inflammation, given that both asymptomatic colonization and higher levels of inflammatory biomarkers, like C-reactive protein (CRP) and myeloperoxidase (MPO), have been associated with an increased risk of stunting and poor cognitive development among infants and young children in LMICs. With the increasing interest in Shigella vaccine impact on these secondary endpoints, CHIMs may enable an early signal of an effect. In addition to MPO and CRP, other markers of inflammation or gut health that should be considered for assessment in CHIMs include calprotectin, stool alpha anti-trypsin, plasma intestinal fatty acid-binding protein, plasma soluble CD14, soluble CD163, serum amyloid A protein and LPS-binding protein (Guerrant et al. 2021; Munoz et al. 1995; Kosek and Investigators 2017). All these markers could be easily measured at baseline, pre- and post-challenge over the course of the experimental infection period by more conventional methods and potentially by new multiplex technologies like multi-micronutrient and environmental enteric dysfunction assessment tool (MEEDAT) platform (Arndt et al. 2020). MEEDAT is a prototype assay to quantitate multiple markers of environmental enteric dysfunction, systemic inflammation, growth hormone resistance and micronutrient use in low-resource settings.
Although not generally considered as part of the assessment of post-challenge inflammation or activation of other innate immunity mediators, studies of Shigella pathogenesis have shown that in order for the bacteria to reach the large intestine, its site of infection, the bacteria must first pass through the stomach and small intestine. Highly effective acid resistance systems allow the bacteria to safely travel through the acidic environment within the stomach (Schroeder and Hilbi 2008; Gorden and Small 1993; Kararli 1995; Islam et al. 2001). Once the small intestine is reached, Shigella make use of host bile salts to induce the up-regulation of bacterial survival genes but also down-regulate the expression of host antimicrobial peptides, such as LL-37 and HBD-1, which are released at mucosal surfaces acting as barrier effectors to infection (Islam et al. 2001). Together, these virulence factors allow the safe passage of Shigella species through the stomach and small intestine, and they ultimately contribute to the low infectious dose observed with Shigella infections in nature, as well as documented in CHIMs, and to intestinal colonization and subsequent invasive enteric illness. In recent studies of a live-attenuated Shigella vaccine in adults and young children, it was observed that host defensins and antimicrobial peptides (e.g., LL-37 and HBD-1) did not increase post-vaccination in the youngest age group (Sarkar et al. 2021). However, they were elevated in adults, suggesting that this innate response might be accentuated in primed individuals and contribute to protection. Consequently, they should be further evaluated in CHIMs, and especially those conducted in exposed populations, where they could be easily measured along with other markers of inflammation and innate immune activation.
6.4. Role in Understanding Signatures of Immunity
The application of advanced Omics and systems biology technologies to better characterize host and bacterial interactions, in CHIMs of ETEC (Clarkson et al. 2021b; Ndungo et al. 2018; Crofts et al. 2018a, b; Chakraborty et al. 2019; Jin et al. 2021), Campylobacter (Crofts et al. 2018a, b; Chakraborty et al. 2019; Jin et al. 2021) and Salmonella Typhi (Clarkson et al. 2021b; Ndungo et al. 2018; Crofts et al. 2018a, b; Chakraborty et al. 2019; Jin et al. 2021), have given investigators greater insights into important antigens as well as innate and adaptive immune responses associated with protective immunity (Clarkson et al. 2021b; Ndungo et al. 2018; Crofts et al. 2018a, b; Chakraborty et al. 2019; Jin et al. 2021). These new analytical tools have not yet been applied to Shigella-CHIMs in a systematic way. With the planned expansion of Shigella-CHIMs, now would be an opportune time to integrate these technologies in the analysis plans for future trials as they would provide a unique opportunity to compare host and bacterial responses in individuals from developed countries versus those from Africa and potentially other LMIC sites. Early application of proteomic array analyses in S. flexneri 2a and S. sonnei CHIMs have already identified an immune signature or profile that may be predictive of protection, but this observation needs confirmation especially with other serotypes. Proteomic array studies may be particularly important in identifying threshold baseline levels of anti-OAg and/or anti-Ipa antibodies that are associated with protection in CHIMs. These observations may also help identify more relevant pre-existing antibody screening criteria that could better determine subject eligibility for enrolment in a CHIM and by so doing help to further ensure more reliable ARs for shigellosis.
The application of a systems serology approach to the analysis of samples from typhoid- and malaria-CHIMs has helped identify immune signatures associated with protection (Jin et al. 2021; Minassian et al. 2021). Recently, a system serology approach to analyze immune response profiles induced by experimental infection with S. flexneri 2a or S. sonnei strains has been described (Clarkson et al. 2021b), and this novel analysis has identified differences in immune response patterns induced by the two different species. These Shigella immune response profiles need to be further evaluated in future CHIMs because of their potential role in guiding vaccine development. Shigella heterotypic studies are currently planned that will examine cross-protection between S. flexneri 2a and S. sonnei induced by the initial infection with either serotype followed by challenge with the heterologous serotype (NCT04992520). The application of systems serology may be instrumental in defining the contribution of OAg epitopes and conserved protein antigens, like PSSP-1 (Kim et al. 2015) to cross-protective immunity.
6.5. Role in Evaluation of Combination Vaccines
Finally, WHO’s recently published preferred product characteristics for a Shigella vaccine notes that the full public health value proposition can be improved by inducing protection with fewer doses (WHO 2020) and combining delivery with other enteric vaccines, such as TCV. The various CHIMs are well suited to aid this development effort and may be critical to ensuring a greater impact for Shigella vaccines. Single-dose or novel prime-boost options (Keren et al. 1988; Hartman et al. 1994), as well as the value of adjuvants in improving vaccine-induced protection, can also be evaluated in CHIMs. With CHIMs now well-established for Shigella (Talaat et al. 2019; MacLennan et al. 2019b), ETEC (Crofts et al. 2018a), Salmonella Typhi and Paratyphi A (Dobinson et al. 2017; Waddington et al. 2014; Gibani et al. 2020), Campylobacter (Crofts et al. 2018b) and cholera (Shirley 2011), novel combination vaccines can be evaluated for efficacy early in clinical development so that the optimal pathogen and antigenic combinations can be advanced to Phase 3 trials and licensure.
6.6. Recap
Our appreciation of the high burden of Shigella-associated disease and the growing AMR threat among infants and children in LMIC has increased. Similarly, the role of Shigella infection as a potential trigger for both acute and longer-term functional bowel disorder in travelers has gained more widespread acceptance. There has also been growing consensus that the development of more effective treatments and preventative interventions for shigellosis needs to be accelerated. As a result, the number of promising Shigella vaccine candidates in the current pipeline is unprecedented. It is generally agreed among international public health stake-holders and donors that the Shigella-CHIMs will play a central role in fast-tracking the development of better prevention and control measures.
Text Box 1. Key features in the conduct of Shigella-CHIMs.
The standardized methods for performing Shigella CHIMs are comprehensively presented in Talaat et al. (Talaat et al. 2019). Shigella CHIMs are exclusively conducted as inpatient studies due to the potential severity of illness and the need for careful monitoring, the clinical endpoints to be measured and transmissibility of Shigella (infection control).
Bacterial challenge strains
Shigella-CHIMs have been established based upon historical and contemporary studies. Both S. flexneri 2a 2457T and S. sonnei 53G were isolated from patients with clinical disease in the 1950s (DuPont et al. 1969, 1989) and manufactured to GMP standards as frozen or lyophilized challenge agents, respectively, for administration to healthy volunteers. Both GMP-prepared cell banks are highly antibiotic sensitive and remain susceptible to ciprofloxacin and trimethoprim-sulfamethoxazole, which are commonly used to treat shigellosis.
Challenge inoculum
Frozen or lyophilized challenge strains are used to prepare bacterial suspensions diluted to the defined target dose and delivered within 4 h. Results of proof-of-principle and dose-finding studies (Table 2) have led to the use of standard inoculum of 1500 colony forming units (CFU) for both S. flexneri 2a 2457T and S. sonnei 53G (lyophilized). For both CHIMs, this inoculum dose was selected for safety and to achieve the desired shigellosis attack rate (AR) of 60–70% in healthy volunteers aged 18–60 with no known prior history of shigellosis.
Challenge administration
To improve the AR prior to the challenge, volunteers fast for at least 90 min and drink 100–120 mL of bicarbonate buffer to neutralize gastric acid (Kotloff et al. 1995a). Subsequently, they drink a 30 mL solution containing the Shigella challenge inoculum.
Antibiotic therapy
Volunteers are drug treated once they meet the primary endpoint of shigellosis, have a fever ≥ 39 °C or at day 5 following administration of the challenge. All volunteers receive a 3–5 days treatment course and must have two consecutive Shigella-negative stool cultures prior to discharge.
Text Box 2. Clinical endpoints used in Shigella-CHIMs.
To enable comparison among sites conducting Shigella-CHIMs there have been recent efforts to harmonize primary and secondary clinical endpoints (Talaat et al. 2019; MacLennan et al. 2019b).
Primary endpoints
Severe diarrhea defined as ≥ 6 loose stools (grade 3 to 5) or > 800 g loose stools in a 24 h period
Moderate diarrhea defined as 4–5 loose stools or 400–800 g loose stools in a 24 h period with accompanying signs or symptoms
Dysentery defined as ≥ 2 loose hemoccult positive stools in a 24 h period with accompanying signs or symptoms
Accompanying signs or symptoms include: oral temperature ≥ 38 °C or ≥ 1 moderate constitutional/enteric symptom (nausea, abdominal pain/cramps, myalgia/arthralgia, malaise) or ≥ 2 episodes of vomiting in a 24 h period.
Secondary endpoints
Percentage of volunteers with diarrhea, dysentery or fever
Percentage of volunteers reporting moderate to severe nausea, vomiting, anorexia, gas or abdominal pain/cramps
Percentage of volunteers requiring early antibiotic therapy or intravenous fluids
Diarrhea characteristics including mean/median time to onset, mean/ median duration and maximum output total or in any 24 h period.
Shigella disease severity score (see Sect. 2.6).
Footnotes
Disclaimer and declaration of competing interest
KAC, CKP, RWK are employees of the U.S. Government and as such, the views expressed in this chapter are those of the authors and do not necessarily reflect the official policy or position of the Department of the Army, Department of the Navy, Department of Defense or the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U. S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
KRT has received research funding for Shigella-CHIMs from the Bill and Melinda Gates Foundation and the Wellcome Trust.
MCK reports research funding from Wellcome Trust for the conduct and development of Shigella-CHIM in exposed populations.
WHC reports research funding from the National Institute of Allergy and Infectious Diseases, the Bill and Melinda Gates Foundation, Institut Pasteur and Emergent Biosolutions for Shigella vaccine development.
RWF received funds from GSK to conduct a Shigella-CHIM trial of a candidate S. sonnei vaccine.
ALB reports research funding from the Bill and Melinda Gates Foundation, the Wellcome Trust and the British Foreign Commonwealth and Development Office (FCDO) for ETEC and Shigella vaccine development, as well as for further development of CHIMs models for Shigella.
LBM was an employee of the GSK group of companies when this chapter was written, owns GSK shares, holds patents related to Shigella topic vaccines and reports grants to GSK from Bill and Melinda Gates Foundation and Wellcome Trust for Shigella vaccine development.
Contributor Information
Kristen A. Clarkson, Department of Diarrheal Disease Research, Walter Reed Army Institute of Research, 503 Robert Grant Avenue, Silver Spring, MD 20910, USA
Chad K. Porter, Enteric Disease Department, Naval Medical Research Center, 503 Robert Grant Avenue, Silver Spring, MD 20910, USA
Kawsar R. Talaat, Center for Immunization Research, Johns Hopkins Bloomberg School of Public Health, 624 North Broadway Street Hampton House, Baltimore, MD 21205, USA
Melissa C. Kapulu, Center for Immunization Research, Johns Hopkins Bloomberg School of Public Health, 624 North Broadway Street Hampton House, Baltimore, MD 21205, USA
Wilbur H. Chen, Center for Vaccine Development and Global Health, University of Maryland School of Medicine, 685 West Baltimore Street, Baltimore, MD 21201, USA
Robert W. Frenck, Jr, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Avenue, Cincinnati, OH 45229, USA.
A. Louis Bourgeois, PATH Center for Vaccine Innovation and Access, 455 Massachusetts Avenue NW, Washington, DC 20001, USA
Robert W. Kaminski, Department of Diarrheal Disease Research, Walter Reed Army Institute of Research, 503 Robert Grant Avenue, Silver Spring, MD 20910, USA
Laura B. Martin, GSK Vaccines Institute for Global Health, Via Fiorentina 1, 53100 Siena, Italy
References
- Ahmed F, Clemens JD, Rao MR, Ansaruzzaman M, Haque E. Epidemiology of shigellosis among children exposed to cases of Shigella dysentery: a multivariate assessment. Am J Trop Med Hyg. 1997;56(3):258–264. doi: 10.4269/ajtmh.1997.56.258. [DOI] [PubMed] [Google Scholar]
- Alter G, Barouch D. Immune correlate-guided HIV vaccine design. Cell Host Microbe. 2018;24(1):25–33. doi: 10.1016/j.chom.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMS. Microbial challenge studies of human volunteers, a guidance document. Academy of Medical Sciences; 2005. [Google Scholar]
- Anderson MC, Vonaesch P, Saffarian A, Marteyn BS, Sansonetti PJ. Shigella sonnei encodes a functional T6SS used for interbacterial competition and Niche occupancy. Cell Host Microbe. 2017;21(6):769–776.:e763. doi: 10.1016/j.chom.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Anderson JDt, Bagamian KH, Muhib F, Amaya MP, Laytner LA, Wierzba T, Rheingans R. Burden of enterotoxigenic Escherichia coli and Shigella non-fatal diarrhoeal infections in 79 low-income and lower middle-income countries: a modelling analysis. Lancet Glob Health. 2019;7(3):e321–e330. doi: 10.1016/S2214-109X(18)30483-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JDt, Bagamian KH, Muhib F, Baral R, Laytner LA, Amaya M, Wierzba T, Rheingans R. Potential impact and cost-effectiveness of future ETEC and Shigella vaccines in 79 low- and lower middle-income countries. Vaccine X. 2019;2:100024. doi: 10.1016/j.jvacx.2019.100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevalillo JM, Sztein MB, Kotloff KL, Levine MM, Simon JK. Identification of immune correlates of protection in Shigella infection by application of machine learning. J Biomed Inform. 2017;74:1–9. doi: 10.1016/j.jbi.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt MB, Cantera JL, Mercer LD, Kalnoky M, White HN, Bizilj G, Boyle DS, de Hostos EL, Choy RKM. Validation of the micronutrient and environmental enteric dysfunction assessment tool and evaluation of biomarker risk factors for growth faltering and vaccine failure in young Malian children. PLoS Negl Trop Dis. 2020;14(9):e0008711. doi: 10.1371/journal.pntd.0008711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi S, Passwell JH, Harlev E, Miron D, Dagan R, Farzan N, Ramon R, Majadly F, Bryla DA, Karpas AB, Robbins JB, et al. Safety and immunogenicity of Shigella sonnei and Shigella flexneri 2a O-specific polysaccharide conjugates in children. J Infect Dis. 1999;179(6):1565–1568. doi: 10.1086/314759. [DOI] [PubMed] [Google Scholar]
- Baay MFD, Richie TL, Neels P Session chairs at the second Human Challenge Trials m. Human challenge trials in vaccine development, Rockville, MD, USA, 28-30 Sept 2017. Biologicals. 2019;61:85–94. doi: 10.1016/j.biologicals.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Bardhan P, Faruque AS, Naheed A, Sack DA. Decrease in shigellosis-related deaths without Shigella spp.-specific interventions, Asia. Emerg Infect Dis. 2010;16(11):1718–1723. doi: 10.3201/eid1611.090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry EM, Levine MM. A tale of two bacterial enteropathogens and one multivalent vaccine. Cell Microbiol. 2019;21(11):e13067. doi: 10.1111/cmi.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry E, Cassels F, Riddle M, Walker R, Wierzba T. Vaccines against Shigella and enterotoxigenic Escherichia coli a summary of the 2018 VASE Conference. Vaccine. 2019;37(34):4768–4774. doi: 10.1016/j.vaccine.2019.02.070. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernasconi OJ, Dona V, Tinguely R, Endimiani A. In vitro activity of 3 commercial bacteriophage cocktails against Salmonella and Shigella spp Isolates of Human Origin. Pathog Immun. 2018;3(1):72–81. doi: 10.20411/pai.v3i1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black RE, Levine MM, Clements ML, Losonsky G, Herrington D, Berman S, Formal SB. Prevention of shigellosis by a Salmonella typhi-Shigella sonnei bivalent vaccine. J Infect Dis. 1987;155(6):1260–1265. doi: 10.1093/infdis/155.6.1260. [DOI] [PubMed] [Google Scholar]
- Bodhidatta L, Pitisuttithum P, Chamnanchanant S, Chang KT, Islam D, Bussaratid V, Venkatesan MM, Hale TL, Mason CJ. Establishment of a Shigella sonnei human challenge model in Thailand. Vaccine. 2012;30(49):7040–7045. doi: 10.1016/j.vaccine.2012.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burki T. Typhoid conjugate vaccine gets WHO prequalification. Lancet Infect Dis. 2018;18(3):258. doi: 10.1016/S1473-3099(18)30088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caboni M, Pedron T, Rossi O, Goulding D, Pickard D, Citiulo F, MacLennan CA, Dougan G, Thomson NR, Saul A, Sansonetti PJ, et al. An O antigen capsule modulates bacterial pathogenesis in Shigella sonnei. PLoS Pathog. 2015;11(3):e1004749. doi: 10.1371/journal.ppat.1004749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin NI, Lindberg AA, Bock K, Bundle DR. The Shigella flexneri O-antigenic polysaccharide chain. Nature of the biological repeating unit. Eur J Biochem. 1984;139(1):189–194. doi: 10.1111/j.1432-1033.1984.tb07993.x. [DOI] [PubMed] [Google Scholar]
- Cash RA, Music SI, Libonati JP, Snyder MJ, Wenzel RP, Hornick RB. Response of man to infection with Vibrio cholerae. I. Clinical, serologic, and bacteriologic responses to a known inoculum. J Infect Dis. 1974;129(1):45–52. doi: 10.1093/infdis/129.1.45. [DOI] [PubMed] [Google Scholar]
- CDC. Antibiotic resistance threats in the United States. 2019. [DOI]
- Chakraborty S, Randall A, Vickers TJ, Molina D, Harro CD, DeNearing B, Brubaker J, Sack DA, Bourgeois AL, Felgner PL, Liang X, et al. Interrogation of a live-attenuated enterotoxigenic Escherichia coli vaccine highlights features unique to wild-type infection. NPJ Vaccines. 2019;4:37. doi: 10.1038/s41541-019-0131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanin RB, Nickerson KP, Llanos-Chea A, Sistrunk JR, Rasko DA, Kumar DKV, de la Parra J, Auclair JR, Ding J, Li K, Dogiparthi SK, et al. Shigella flexneri adherence factor expression in in vivo-like conditions. mSphere. 2019;4(6) doi: 10.1128/mSphere.00751-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilengi R, Simuyandi M, Chibuye M, Chirwa M, Sukwa N, Laban N, Chisenga C, Silwamba S, Grassly N, Bosomprah S. A pilot study on use of live attenuated rotavirus vaccine (Rotarix) as an infection challenge model. Vaccine. 2020;38(46):7357–7362. doi: 10.1016/j.vaccine.2020.09.023. [DOI] [PubMed] [Google Scholar]
- Chisenga CC, Bosomprah S, Simuyandi M, Mwila-Kazimbaya K, Chilyabanyama ON, Laban NM, Bialik A, Asato V, Meron-Sudai S, Frankel G, Cohen D, et al. Shigella-specific antibodies in the first year of life among Zambian infants: a longitudinal cohort study. PLoS ONE. 2021;16(5):e0252222. doi: 10.1371/journal.pone.0252222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson KA, Talaat KR, Alaimo C, Martin P, Bourgeois AL, Dreyer A, Porter CK, Chakraborty S, Brubaker J, Elwood D, Frolich R, et al. Immune response characterization in a human challenge study with a Shigella flexneri 2a bioconjugate vaccine. EBioMedicine. 2021a;66:103308. doi: 10.1016/j.ebiom.2021.103308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson KA, Frenck RW, Jr, Dickey M, Suvarnapunya AE, Chandrasekaran L, Weerts HP, Heaney CD, McNeal M, Detizio K, Parker S, Hoeper A, et al. Immune response characterization after controlled infection with lyophilized Shigella sonnei 53G. mSphere. 2020;5(5) doi: 10.1128/mSphere.00988-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson KA, P C, Talaat KR, Frenck RW, Alaimo C, Martin P, Bourgeois AL, Kaminski RW. Shigella-specific immune profiles induced after parenteral immunization or oral challenge with either S. flexneri 2a or S. sonnei. mSphere. 2021;6(4):e00122-00121. doi: 10.1128/mSphere.00122-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D, Green MS, Block C, Rouach T, Ofek I. Serum antibodies to lipopolysaccharide and natural immunity to shigellosis in an Israeli military population. J Infect Dis. 1988;157(5):1068–1071. doi: 10.1093/infdis/157.5.1068. [DOI] [PubMed] [Google Scholar]
- Cohen D, Block C, Green MS, Lowell G, Ofek I. Immunoglobulin M A, and G antibody response to lipopolysaccharide O antigen in symptomatic and asymptomatic Shigella infections. J Clin Microbiol. 1989;27(1):162–167. doi: 10.1128/jcm.27.1.162-167.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D, Green MS, Block C, Slepon R, Ofek I. Prospective study of the association between serum antibodies to lipopolysaccharide O antigen and the attack rate of shigellosis. J Clin Microbiol. 1991;29(2):386–389. doi: 10.1128/jcm.29.2.386-389.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D, Green MS, Block C, Slepon R, Lerman Y. Natural immunity to shigellosis in two groups with different previous risks of exposure to Shigella is only partly expressed by serum antibodies to lipopolysaccharide. J Infect Dis. 1992;165(4):785–787. doi: 10.1093/infdis/165.4.785. [DOI] [PubMed] [Google Scholar]
- Cohen D, Ashkenazi S, Green M, Lerman Y, Slepon R, Robin G, Orr N, Taylor DN, Sadoff JC, Chu C, Shiloach J, et al. Safety and immunogenicity of investigational Shigella conjugate vaccines in Israeli volunteers. Infect Immun. 1996;64(10):4074–4077. doi: 10.1128/iai.64.10.4074-4077.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D, Meron-Sudai S, Bialik A, Asato V, Goren S, Ariel-Cohen O, Reizis A, Hochberg A, Ashkenazi S. Serum IgG antibodies to Shigella lipopolysaccharide antigens—a correlate of protection against shigellosis. Hum Vaccin Immunother. 2019;15(6):1401–1408. doi: 10.1080/21645515.2019.1606971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coster TS, Hoge CW, VanDeVerg LL, Hartman AB, Oaks EV, Venkatesan MM, Cohen D, Robin G, Fontaine-Thompson A, Sansonetti PJ, Hale TL. Vaccination against shigellosis with attenuated Shigella flexneri 2a strain SC602. Infect Immun. 1999;67(7):3437–3443. doi: 10.1128/iai.67.7.3437-3443.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts AA, Poly FM, Ewing CP, Kuroiwa JM, Rimmer JE, Harro C, Sack D, Talaat KR, Porter CK, Gutierrez RL, DeNearing B, et al. Campylobacter jejuni transcriptional and genetic adaptation during human infection. Nat Microbiol. 2018b;3(4):494–502. doi: 10.1038/s41564-018-0133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts AA, Giovanetti SM, Rubin EJ, Poly FM, Gutierrez RL, Talaat KR, Porter CK, Riddle MS, DeNearing B, Brubaker J, Maciel M, Jr, et al. Enterotoxigenic E. coli virulence gene regulation in human infections. Proc Natl Acad Sci USA. 2018;115(38):E8968–E8976. doi: 10.1073/pnas.1808982115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahora LC, Jin C, Spreng RL, Feely F, Mathura R, Seaton KE, Zhang L, Hill J, Jones E, Alam SM, Dennison SM, et al. IgA and IgG1 specific to Vi polysaccharide of Salmonella Typhi correlate with protection status in a typhoid fever controlled human infection model. Front Immunol. 2019;10:2582. doi: 10.3389/fimmu.2019.02582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darton TC, Jones C, Blohmke CJ, Waddington CS, Zhou L, Peters A, Haworth K, Sie R, Green CA, Jeppesen CA, Moore M, et al. Using a human challenge model of infection to measure vaccine efficacy: a randomised, controlled trial comparing the typhoid vaccines M01ZH09 with placebo and Ty21a. PLoS Negl Trop Dis. 2016;10(8):e0004926. doi: 10.1371/journal.pntd.0004926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CL, Wahid R, Toapanta FR, Simon JK, Sztein MB, Levy D. Applying mathematical tools to accelerate vaccine development: modeling Shigella immune dynamics. PLoS ONE. 2013;8(4):e59465. doi: 10.1371/journal.pone.0059465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobinson HC, Gibani MM, Jones C, Thomaides-Brears HB, Voysey M, Darton TC, Waddington CS, Campbell D, Milligan I, Zhou L, Shrestha S, et al. Evaluation of the clinical and microbiological response to Salmonella paratyphi A infection in the first paratyphoid human challenge model. Clin Infect Dis. 2017;64(8):1066–1073. doi: 10.1093/cid/cix042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donowitz JR, Cook H, Alam M, Tofail F, Kabir M, Colgate ER, Carmolli MP, Kirkpatrick BD, Nelson CA, Ma JZ, Haque R, et al. Role of maternal health and infant inflammation in nutritional and neurodevelopmental outcomes of two-year-old Bangladeshi children. PLoS Negl Trop Dis. 2018;12(5):e0006363. doi: 10.1371/journal.pntd.0006363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPont HL, Hornick RB, Dawkins AT, Snyder MJ, Formal SB. The response of man to virulent Shigella flexneri 2a. J Infect Dis. 1969;119(3):296–299. doi: 10.1093/infdis/119.3.296. [DOI] [PubMed] [Google Scholar]
- DuPont HL, Levine MM, Hornick RB, Formal SB. Inoculum size in shigellosis and implications for expected mode of transmission. J Infect Dis. 1989;159(6):1126–1128. doi: 10.1093/infdis/159.6.1126. [DOI] [PubMed] [Google Scholar]
- DuPont HL, Hornick RB, Snyder MJ, Libonati JP, Formal SB, Gangarosa EJ. Immunity in shigellosis. I. Response of man to attenuated strains of Shigella. J Infect Dis. 1972;125(1):5–11. doi: 10.1093/infdis/125.1.5. [DOI] [PubMed] [Google Scholar]
- DuPont HL, Hornick RB, Snyder MJ, Libonati JP, Formal SB, Gangarosa EJ. Immunity in shigellosis. II. Protection induced by oral live vaccine or primary infection. J Infect Dis. 1972;125(1):12–16. doi: 10.1093/infdis/125.1.12. [DOI] [PubMed] [Google Scholar]
- Durbin ABA, McKenzie R, Moulton L, Mallett C, Harrington J, Linden J, Lowell G, Fries L. Intranasal immunization with proteosome-Shigella flexneri 2a LPS vaccine: factors associated with protection in a volunteer challenge model. Clin Infect Dis. 2001;33:1093. [Google Scholar]
- Elliott AM, Roestenberg M, Wajja A, Opio C, Angumya F, Adriko M, Egesa M, Gitome S, Mfutso-Bengo J, Bejon P, Kapulu M, et al. Ethical and scientific considerations on the establishment of a controlled human infection model for schistosomiasis in Uganda: report of a stakeholders’ meeting held in Entebbe, Uganda. AAS Open Res. 2018;1:2. doi: 10.12688/aasopenres.12841.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMA. Guideline on clinical evaluation of vaccines (draft) European Medicines Agency Committee on Human Medicinal Products (CHMP); 2018. Apr 26, edn 2018. [Google Scholar]
- Farzam N, Ramon-Saraf R, Banet-Levi Y, Lerner-Geva L, Ashkenazi S, Kubler-Kielb J, Vinogradov E, Robbins JB, Schneerson R. Vaccination with Shigella flexneri 2a conjugate induces type 2a and cross-reactive type 6 antibodies in humans but not in mice. Vaccine. 2017;35(37):4990–4996. doi: 10.1016/j.vaccine.2017.07.070. [DOI] [PubMed] [Google Scholar]
- FDA. Guidance for industry: general principles for the development of vaccines to protect against global infectious diseases (trans: Food and Drug Administration CfBEaR) US Department of Health and Human Services; 2011. [Google Scholar]
- Fedorov V, Mannino F, Zhang R. Consequences of dichotomization. Pharm Stat. 2009;8(1):50–61. doi: 10.1002/pst.331. [DOI] [PubMed] [Google Scholar]
- Ferreccio C, Prado V, Ojeda A, Cayyazo M, Abrego P, Guers L, Levine MM. Epidemiologic patterns of acute diarrhea and endemic Shigella infections in children in a poor periurban setting in Santiago, Chile. Am J Epidemiol. 1991;134(6):614–627. doi: 10.1093/oxfordjournals.aje.a116134. [DOI] [PubMed] [Google Scholar]
- Flores J, Okhuysen PC. Genetics of susceptibility to infection with enteric pathogens. Curr Opin Infect Dis. 2009;22(5):471–476. doi: 10.1097/QCO.0b013e3283304eb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formal SB, Labrec EH, Palmer A, Falkow S. Protection of monkeys against experimental shigellosis with attenuated vaccines. J Bacteriol. 1965;90(1):63–68. doi: 10.1128/jb.90.1.63-68.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formal SB, Hale TL, Kapfer C, Cogan JP, Snoy PJ, Chung R, Wingfield ME, Elisberg BL, Baron LS. Oral vaccination of monkeys with an invasive Escherichia coli K-12 hybrid expressing Shigella flexneri 2a somatic antigen. Infect Immun. 1984;46(2):465–469. doi: 10.1128/iai.46.2.465-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formal SB, Oaks EV, Olsen RE, Wingfield-Eggleston M, Snoy PJ, Cogan JP. Effect of prior infection with virulent Shigella flexneri 2a on the resistance of monkeys to subsequent infection with Shigella sonnei. J Infect Dis. 1991;164(3):533–537. doi: 10.1093/infdis/164.3.533. [DOI] [PubMed] [Google Scholar]
- Foulke-Abel J, Yu H, Sunuwar L, Lin R, Fleckenstein JM, Kaper JB, Donowitz M. Phosphodiesterase 5 (PDE5) restricts intracellular cGMP accumulation during enterotoxigenic Escherichia coli infection. Gut Microbes. 2020;12(1):1752125. doi: 10.1080/19490976.2020.1752125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenck R, Conti V, Ferruzzi P, Ndiaye A, Parker S, McNeal M, Dickey M, Granada J-P, Cilio G, De Ryck I, Necchi F, et al. Efficacy, safety, and immunogenicity of the Shigella sonnei 1790GAHB GMMA candidate vaccine: results from phase 2b randomized, placebo-controlled challenge study in adults. EClinicalMedicine. 2021;39:101076. doi: 10.1016/j.eclinm.2021.101076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenck RW, Jr, Dickey M, Suvarnapunya AE, Chandrasekaran L, Kaminski RW, Clarkson KA, McNeal M, Lynen A, Parker S, Hoeper A, Mani S, et al. Establishment of a controlled human infection model with a lyophilized strain of Shigella sonnei 53G. mSphere. 2020;5(5) doi: 10.1128/mSphere.00416-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2016 Diarrhoeal Disease Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the global burden of disease study 2016. Lancet Infect Dis. 2018;18(11):1211–1228. doi: 10.1016/S1473-3099(18)30362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibani MM, Jin C, Shrestha S, Moore M, Norman L, Voysey M, Jones E, Blackwell L, Thomaides-Brears H, Hill J, Blohmke CJ, et al. Homologous and heterologous re-challenge with Salmonella typhi and Salmonella paratyphi A in a randomised controlled human infection model. PLoS Negl Trop Dis. 2020;14(10):e0008783. doi: 10.1371/journal.pntd.0008783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giersing BK, Porter CK, Kotloff K, Neels P, Cravioto A, MacLennan CA. How can controlled human infection models accelerate clinical development and policy pathways for vaccines against Shigella? Vaccine. 2019;37(34):4778–4783. doi: 10.1016/j.vaccine.2019.03.036. [DOI] [PubMed] [Google Scholar]
- Gorden J, Small PL. Acid resistance in enteric bacteria. Infect Immun. 1993;61(1):364–367. doi: 10.1128/iai.61.1.364-367.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SB, Rylance J, Luck A, Jambo K, Ferreira DM, Manda-Taylor L, Bejon P, Ngwira B, Littler K, Seager Z, Gibani M, et al. A framework for Controlled Human Infection Model (CHIM) studies in Malawi: report of a wellcome trust workshop on CHIM in low income countries held in Blantyre, Malawi. Wellcome Open Res. 2017;2:70. doi: 10.12688/wellcomeopenres.12256.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory M, Kaminski RW, Lugo-Roman LA, Galvez Carrillo H, Tilley DH, Baldeviano C, Simons MP, Reynolds ND, Ranallo RT, Suvarnapunya AE, Venkatesan MM, et al. Development of an Aotus nancymaae model for Shigella vaccine immunogenicity and efficacy studies. Infect Immun. 2014;82(5):2027–2036. doi: 10.1128/IAI.01665-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu B, Cao Y, Pan S, Zhuang L, Yu R, Peng Z, Qian H, Wei Y, Zhao L, Liu G, Tong M. Comparison of the prevalence and changing resistance to nalidixic acid and ciprofloxacin of Shigella between Europe-America and Asia-Africa from 1998 to 2009. Int J Antimicrob Agents. 2012;40(1):9–17. doi: 10.1016/j.ijantimicag.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Guerrant RL, DeBoer MD, Moore SR, Scharf RJ, Lima AA. The impoverished gut-a triple burden of diarrhoea, stunting and chronic disease. Nat Rev Gastroenterol Hepatol. 2013;10(4):220–229. doi: 10.1038/nrgastro.2012.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant RL, Bolick DT, Swann JR. Modeling enteropathy or diarrhea with the top bacterial and protozoal pathogens: differential determinants of outcomes. ACS Infect Dis. 2021;7(5):1020–1031. doi: 10.1021/acsinfecdis.0c00831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Polyak CS, Bishop RD, Sobel J, Mintz ED. Laboratory-confirmed shigellosis in the United States, 1989-2002: epidemiologic trends and patterns. Clin Infect Dis. 2004;38(10):1372–1377. doi: 10.1086/386326. [DOI] [PubMed] [Google Scholar]
- Haks MC, Bottazzi B, Cecchinato V, De Gregorio C, Del Giudice G, Kaufmann SHE, Lanzavecchia A, Lewis DJM, Maertzdorf J, Mantovani A, Sallusto F, et al. Molecular signatures of immunity and immunogenicity in infection and vaccination. Front Immunol. 2017;8:1563. doi: 10.3389/fimmu.2017.01563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman AB, Powell CJ, Schultz CL, Oaks EV, Eckels KH. Small-animal model to measure efficacy and immunogenicity of Shigella vaccine strains. Infect Immun. 1991;59(11):4075–4083. doi: 10.1128/iai.59.11.4075-4083.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman AB, Van de Verg LL, Collins HH, Jr, Tang DB, Bendiuk NO, Taylor DN, Powell CJ. Local immune response and protection in the guinea pig keratoconjunctivitis model following immunization with Shigella vaccines. Infect Immun. 1994;62(2):412–420. doi: 10.1128/iai.62.2.412-420.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harutyunyan S, Neuhauser I, Mayer A, Aichinger M, Szijarto V, Nagy G, Nagy E, Girardi P, Malinoski FJ, Henics T. Characterization of ShigETEC, a novel live attenuated combined vaccine against Shigellae and ETEC. Vaccines (Basel) 2020;8(4) doi: 10.3390/vaccines8040689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin Y, Seldin M, Lusis A. Multi-omics approaches to disease. Genome Biol. 2017;18(1):83. doi: 10.1186/s13059-017-1215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington DA, Van de Verg L, Formal SB, Hale TL, Tall BD, Cryz SJ, Tramont EC, Levine MM. Studies in volunteers to evaluate candidate Shigella vaccines: further experience with a bivalent Salmonella typhi-Shigella sonnei vaccine and protection conferred by previous Shigella sonnei disease. Vaccine. 1990;8(4):353–357. doi: 10.1016/0264-410x(90)90094-3. [DOI] [PubMed] [Google Scholar]
- Holmgren J, Parashar UD, Plotkin S, Louis J, Ng SP, Desauziers E, Picot V, Saadatian-Elahi M. Correlates of protection for enteric vaccines. Vaccine. 2017;35(26):3355–3363. doi: 10.1016/j.vaccine.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam D, Bandholtz L, Nilsson J, Wigzell H, Christensson B, Agerberth B, Gudmundsson G. Downregulation of bactericidal peptides in enteric infections: a novel immune escape mechanism with bacterial DNA as a potential regulator. Nat Med. 2001;7(2):180–185. doi: 10.1038/84627. [DOI] [PubMed] [Google Scholar]
- Jao I, Marsh V, Che Chi P, Kapulu M, Hamaluba M, Molyneux S, Bejon P, Kamuya D. Deliberately infecting healthy volunteers with malaria parasites: perceptions and experiences of participants and other stakeholders in a Kenyan-based malaria infection study. Bioethics. 2020;34(8):819–832. doi: 10.1111/bioe.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Gibani MM, Moore M, Juel HB, Jones E, Meiring J, Harris V, Gardner J, Nebykova A, Kerridge SA, Hill J, et al. Efficacy and immunogenicity of a Vi-tetanus toxoid conjugate vaccine in the prevention of typhoid fever using a controlled human infection model of Salmonella typhi a randomised controlled, phase 2b trial. Lancet. 2017;390(10111):2472–2480. doi: 10.1016/S0140-6736(17)32149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Hill J, Gunn BM, Yu WH, Dahora LC, Jones E, Johnson M, Gibani MM, Spreng RL, Alam SM, Nebykova A, et al. Vi-specific serological correlates of protection for typhoid fever. J Exp Med. 2021;218(2) doi: 10.1084/jem.20201116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones FR, Hall ER, Tribble D, Savarino SJ, Cassels FJ, Porter C, Meza R, Nunez G, Espinoza N, Salazar M, Luckett R, et al. The new world primate, Aotus nancymae as a model for examining the immunogenicity of a prototype enterotoxigenic Escherichia coli subunit vaccine. Vaccine. 2006a;24(18):3786–3792. doi: 10.1016/j.vaccine.2005.07.029. [DOI] [PubMed] [Google Scholar]
- Jones FR, Baqar S, Gozalo A, Nunez G, Espinoza N, Reyes SM, Salazar M, Meza R, Porter CK, Walz SE. New world monkey Aotus nancymae as a model for Campylobacter jejuni infection and immunity. Infect Immun. 2006b;74(1):790–793. doi: 10.1128/IAI.74.1.790-793.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski RW, Wu M, Turbyfill KR, Clarkson K, Tai B, Bourgeois AL, Van De Verg LL, Walker RI, Oaks EV. Development and preclinical evaluation of a trivalent, formalin-inactivated Shigella whole-cell vaccine. Clin Vaccine Immunol. 2014;21(3):366–382. doi: 10.1128/CVI.00683-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapulu MC, Njuguna P, Hamaluba MM Team C-SS. Controlled human malaria infection in semi-immune Kenyan adults (CHMI-SIKA): a study protocol to investigate in vivo Plasmodium falciparum Malaria parasite growth in the context of pre-existing immunity. Wellcome Open Res. 2018;3:155. doi: 10.12688/wellcomeopenres.14909.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapumba BM, Jambo K, Rylance J, Gmeiner M, Sambakunsi R, Parker M, Gordon SB, Gooding K. Stakeholder views on the acceptability of human infection studies in Malawi. BMC Med Ethics. 2020;21(1):14. doi: 10.1186/s12910-020-0454-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos. 1995;16(5):351–380. doi: 10.1002/bdd.2510160502. [DOI] [PubMed] [Google Scholar]
- Katona P, Katona-Apte J. The interaction between nutrition and infection. Clin Infect Dis. 2008;46(10):1582–1588. doi: 10.1086/587658. [DOI] [PubMed] [Google Scholar]
- Kenne L, Lindberg B, Petersson K, Katzenellenbogen E, Romanowska E. Structural studies of Shigella flexneri O-antigens. Eur J Biochem. 1978;91(1):279–284. doi: 10.1111/j.1432-1033.1978.tb20963.x. [DOI] [PubMed] [Google Scholar]
- Keren DF, McDonald RA, Carey JL. Combined parenteral and oral immunization results in an enhanced mucosal immunoglobulin A response to Shigella flexneri. Infect Immun. 1988;56(4):910–915. doi: 10.1128/iai.56.4.910-915.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerneis S, Guerin PJ, von Seidlein L, Legros D, Grais RF. A look back at an ongoing problem: Shigella dysenteriae type 1 epidemics in refugee settings in Central Africa (1993–1995) PLoS ONE. 2009;4(2):e4494. doi: 10.1371/journal.pone.0004494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Yeo SG, Park JH, Ko HJ. Shigella vaccine development: prospective animal models and current status. Curr Pharm Biotechnol. 2013;14(10):903–912. doi: 10.2174/1389201014666131226123900. [DOI] [PubMed] [Google Scholar]
- Kim JO, Rho S, Kim SH, Kim H, Song HJ, Kim EJ, Kim RY, Kim EH, Sinha A, Dey A, Yang JS, et al. Shigella outer membrane protein PSSP-1 is broadly protective against Shigella infection. Clin Vaccine Immunol. 2015;22(4):381–388. doi: 10.1128/CVI.00661-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick BD, Sack DA, Bourgeois AL, Mallett CP, Shimko J, Gross LM, Linden J, Gomes G, Fries LF. A challenge model of Shigellosis; 49th annual meeting on American society of tropical medicine and hygiene; Houston, TX. October 2000; 2000. [Google Scholar]
- Knirel YA, Sun Q, Senchenkova SN, Perepelov AV, Shashkov AS, Xu J. O-antigen modifications providing antigenic diversity of Shigella flexneri and underlying genetic mechanisms. Biochem Mosc. 2015;80(7):901–914. doi: 10.1134/S0006297915070093. [DOI] [PubMed] [Google Scholar]
- Koestler BJ, Ward CM, Fisher CR, Rajan A, Maresso AW, Payne SM. Human intestinal enteroids as a model system of Shigella pathogenesis. Infect Immun. 2019;87(4) doi: 10.1128/IAI.00733-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpe PS, Petri WA., Jr Environmental enteropathy: critical implications of a poorly understood condition. Trends Mol Med. 2012;18(6):328–336. doi: 10.1016/j.molmed.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosek MN Investigators M-EN. Causal pathways from enteropathogens to environmental enteropathy: findings from the MAL-ED birth cohort study. EBioMedicine. 2017;18:109–117. doi: 10.1016/j.ebiom.2017.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotloff KL, Nataro JP, Losonsky GA, Wasserman SS, Hale TL, Taylor DN, Sadoff JC, Levine MM. A modified Shigella volunteer challenge model in which the inoculum is administered with bicarbonate buffer: clinical experience and implications for Shigella infectivity. Vaccine. 1995a;13(16):1488–1494. doi: 10.1016/0264-410x(95)00102-7. [DOI] [PubMed] [Google Scholar]
- Kotloff KL, Losonsky GA, Nataro JP, Wasserman SS, Hale TL, Taylor DN, Newland JW, Sadoff JC, Formal SB, Levine MM. Evaluation of the safety, immunogenicity, and efficacy in healthy adults of four doses of live oral hybrid Escherichia coli Shigella flexneri 2a vaccine strain EcSf2a-2. Vaccine. 1995b;13(5):495–502. doi: 10.1016/0264-410x(94)00011-b. [DOI] [PubMed] [Google Scholar]
- Kotloff KL, Winickoff JP, Ivanoff B, Clemens JD, Swerdlow DL, Sansonetti PJ, Adak GK, Levine MM. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ. 1999;77(8):651–666. [PMC free article] [PubMed] [Google Scholar]
- Kunda-Ng’andu EM, Simuyandi M, Kapulu M, Chirwa-Chobe M, Mwanyungwi-Chinganya H, Mwale S, Chilengi R, Sharma A. Engagement of ethics and regulatory authorities on human infection studies: proceedings of an engagement workshop in Zambia. Wellcome Open Res. 2021;6:31. doi: 10.12688/wellcomeopenres.16432.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberti LM, Bourgeois AL, Fischer Walker CL, Black RE, Sack D. Estimating diarrheal illness and deaths attributable to Shigellae and enterotoxigenic Escherichia coli among older children, adolescents, and adults in South Asia and Africa. PLoS Negl Trop Dis. 2014;8(2):e2705. doi: 10.1371/journal.pntd.0002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre J, Gosselin F, Ismail J, Lorange M, Lior H, Woodward D. Evaluation of commercial antisera for Shigella serogrouping. J Clin Microbiol. 1995;33(8):1997–2001. doi: 10.1128/jcm.33.8.1997-2001.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MM, Woodward WE, Formal SB, Gemski P, Jr, DuPont HL, Hornick RB, Snyder MJ. Studies with a new generation of oral attenuated shigella vaccine: Escherichia coli bearing surface antigens of Shigella flexneri. J Infect Dis. 1977;136(4):577–582. doi: 10.1093/infdis/136.4.577. [DOI] [PubMed] [Google Scholar]
- Levine MM, Dupont HL, Gangarosa EJ, Hornick RB, Snyder MJ, Libonati JP, Glaser K, Formal SB. Shigellosis in custodial institutions. II. Clinical, immunologic and bacteriologic response of institutionalized children to oral attenuated shigella vaccines. Am J Epidemiol. 1972;96(1):40–49. doi: 10.1093/oxfordjournals.aje.a121431. [DOI] [PubMed] [Google Scholar]
- Levine MM, Gangarosa EJ, Barrow WB, Morris GK, Wells JG, Weiss CF. Shigellosis in custodial institutions. IV. In vivo stability and transmissibility of oral attenuated streptomycin-dependent Shigella vaccines. J Infect Dis. 1975;131(6):704–707. [Google Scholar]
- Li S, Nakaya HI, Kazmin DA, Oh JZ, Pulendran B. Systems biological approaches to measure and understand vaccine immunity in humans. Semin Immunol. 2013;25(3):209–218. doi: 10.1016/j.smim.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg AA, Karnell A, Weintraub A. The lipopolysaccharide of Shigella bacteria as a virulence factor. Rev Infect Dis. 1991;13(Suppl 4):S279–284. doi: 10.1093/clinids/13.supplement_4.s279. [DOI] [PubMed] [Google Scholar]
- Liu B, Knirel YA, Feng L, Perepelov AV, Senchenkova SN, Wang Q, Reeves PR, Wang L. Structure and genetics of Shigella O antigens. FEMS Microbiol Rev. 2008;32(4):627–653. doi: 10.1111/j.1574-6976.2008.00114.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Platts-Mills JA, Juma J, Kabir F, Nkeze J, Okoi C, Operario DJ, Uddin J, Ahmed S, Alonso PL, Antonio M, et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet. 2016;388(10051):1291–1301. doi: 10.1016/S0140-6736(16)31529-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livio S, Strockbine NA, Panchalingam S, Tennant SM, Barry EM, Marohn ME, Antonio M, Hossain A, Mandomando I, Ochieng JB, Oundo JO, et al. Shigella isolates from the global enteric multicenter study inform vaccine development. Clin Infect Dis. 2014;59(7):933–941. doi: 10.1093/cid/ciu468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan CA, Aguilar AO, Steele AD. Consensus report on Shigella controlled human infection model: introduction and overview. Clin Infect Dis. 2019a;69(Suppl 8):S577–S579. doi: 10.1093/cid/ciz886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan CA, Riddle MS, Chen WH, Talaat KR, Jain V, Bourgeois AL, Frenck R, Kotloff K, Porter CK. Consensus report on Shigella controlled human infection model: clinical endpoints. Clin Infect Dis. 2019;69(Supplement_8):S591–S595. doi: 10.1093/cid/ciz891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, Ngwira B, Victor JC, Gillard PH, Cheuvart BB, Han HH, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362(4):289–298. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- Mallett CP, VanDeVerg L, Collins HH, Hale TL. Evaluation of Shigella vaccine safety and efficacy in an intranasally challenged mouse model. Vaccine. 1993;11(2):190–196. doi: 10.1016/0264-410x(93)90016-q. [DOI] [PubMed] [Google Scholar]
- Martinez-Becerra FJ, Kissmann JM, Diaz-McNair J, Choudhari SP, Quick AM, Mellado-Sanchez G, Clements JD, Pasetti MF, Picking WL. Broadly protective Shigella vaccine based on type III secretion apparatus proteins. Infect Immun. 2012;80(3):1222–1231. doi: 10.1128/IAI.06174-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Becerra FJ, Chen X, Dickenson NE, Choudhari SP, Harrison K, Clements JD, Picking WD, Van De Verg LL, Walker RI, Picking WL. Characterization of a novel fusion protein from IpaB and IpaD of Shigella spp. and its potential as a pan-Shigella vaccine. Infect Immun. 2013;81(12):4470–4477. doi: 10.1128/IAI.00859-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins NE, Faria VG, Teixeira L, Magalhaes S, Sucena E. Host adaptation is contingent upon the infection route taken by pathogens. PLoS Pathog. 2013;9(9):e1003601. doi: 10.1371/journal.ppat.1003601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros PHQS, Ledwaba SE, Bolick DT, Giallourou N, Yum LK, Costa DVS, Oria RB, Barry EM, Swann JR, Lima AAM, Agaisse H, et al. A murine model of diarrhea, growth impairment and metabolic disturbances with Shigella flexneri infection and the role of zinc deficiency. Gut Microbes. 2019;10(5):615–630. doi: 10.1080/19490976.2018.1564430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros PHQS, Bolick DT, Ledwaba SE, Kolling GL, Costa DVS, Oria RB, Lima AAM, Barry EM, Guerrant RL. A bivalent vaccine confers immunogenicity and protection against Shigella flexneri and enterotoxigenic Escherichia coli infections in mice. NPJ Vaccines. 2020;5(1):30. doi: 10.1038/s41541-020-0180-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mel DM, Arsic BL, Radovanovic ML, Litvinjenko SA. Live oral Shigella vaccine: vaccination schedule and the effect of booster dose. Acta Microbiol Acad Sci Hung. 1974;21(1-2):109–114. [PubMed] [Google Scholar]
- Mel DM, Terzin AL, Vuksic L. Studies on vaccination against bacillary dysentery. 1. Immunization of mice against experimental Shigella infection. Bull World Health Organ. 1965;32(5):633–636. [PMC free article] [PubMed] [Google Scholar]
- Mel DM, Papo RG, Terzin AL, Vuksic L. Studies on vaccination against bacillary dysentery. 2. Safety tests and reactogenicity studies on a live dysentery vaccine intended for use in field trials. Bull World Health Organ. 1965;32(5):637–645. [PMC free article] [PubMed] [Google Scholar]
- Mel DM, Terzin AL, Vuksic L. Studies on vaccination against bacillary dysentery. 3. Effective oral immunization against Shigella flexneri 2a in a field trial. Bull World Health Organ. 1965;32(5):647–655. [PMC free article] [PubMed] [Google Scholar]
- Mel DM, Arsic BL, Nikolic BD, Radovanic ML. Studies on vaccination against bacillary dysentery. 4. Oral immunization with live monotypic and combined vaccines. Bull World Health Organ. 1968;39(3):375–380. [PMC free article] [PubMed] [Google Scholar]
- Mel D, Gangarosa EJ, Radovanovic ML, Arsic BL, Litvinjenko S. Studies on vaccination against bacillary dysentery. 6. Protection of children by oral immunization with streptomycin-dependent Shigella strains. Bull World Health Organ. 1971;45(4):457–464. [PMC free article] [PubMed] [Google Scholar]
- Memoli MJ, Czajkowski L, Reed S, Athota R, Bristol T, Proudfoot K, Fargis S, Stein M, Dunfee RL, Shaw PA, Davey RT, et al. Validation of the wild-type influenza A human challenge model H1N1pdMIST: an A(H1N1)pdm09 dose-finding investigational new drug study. Clin Infect Dis. 2015;60(5):693–702. doi: 10.1093/cid/ciu924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian AMSS, Barret JR, Nielsen CM, Miura K, Diouf A, Loos C, Fallon JK, Michel AR, White MT, Edwards NJ, Poulton ID, et al. Reduced blood-stage malaria growth and immune correlates in humans following RH5 vaccination. Med. 2021 doi: 10.1016/j.medj.2021.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy MJ, Bouladoux N, Belkaid Y. Intestinal microbiota: shaping local and systemic immune responses. Semin Immunol. 2012;24(1):58–66. doi: 10.1016/j.smim.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal D, Minak J, Alam M, Liu Y, Dai J, Korpe P, Liu L, Haque R, Petri WA., Jr Contribution of enteric infection, altered intestinal barrier function, and maternal malnutrition to infant malnutrition in Bangladesh. Clin Infect Dis. 2012;54(2):185–192. doi: 10.1093/cid/cir807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CR, Grassel CL, Redman JC, Sahl JW, Barry EM, Rasko DA. Characterization of intracellular growth regulator icgR by utilizing transcriptomics to identify mediators of pathogenesis in Shigella flexneri. Infect Immun. 2013;81(9):3068–3076. doi: 10.1128/IAI.00537-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley JF, Smith LL, Brantley P, Locke D, Como M. Vaxchora: the first FDA-approved Cholera vaccination in the United States. P T. 2017;42(10):638–640. [PMC free article] [PubMed] [Google Scholar]
- Munoz C, Baqar S, van de Verg L, Thupari J, Goldblum S, Olson JG, Taylor DN, Heresi GP, Murphy JR. Characteristics of Shigella sonnei infection of volunteers: signs, symptoms, immune responses, changes in selected cytokines and acute-phase substances. Am J Trop Med Hyg. 1995;53(1):47–54. [PubMed] [Google Scholar]
- Muthuirulandi Sethuvel DP, Devanga Ragupathi NK, Anandan S, Veeraraghavan B. Update on: Shigella new serogroups/serotypes and their antimicrobial resistance. Lett Appl Microbiol. 2017;64(1):8–18. doi: 10.1111/lam.12690. [DOI] [PubMed] [Google Scholar]
- Nakaya HI, Pulendran B. Vaccinology in the era of high-throughput biology. Philos Trans R Soc Lond B Biol Sci. 2015;370(1671) doi: 10.1098/rstb.2014.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndungo E, Randall A, Hazen TH, Kania DA, Trappl-Kimmons K, Liang X, Barry EM, Kotloff KL, Chakraborty S, Mani S, Rasko DA, et al. A novel shigella proteome microarray discriminates targets of human antibody reactivity following oral vaccination and experimental challenge. mSphere. 2018;3(4) doi: 10.1128/mSphere.00260-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndungo E, Pasetti MF. Functional antibodies as immunological endpoints to evaluate protective immunity against Shigella. Hum Vaccin Immunother. 2020;16(1):197–205. doi: 10.1080/21645515.2019.1640427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njue M, Njuguna P, Kapulu MC, Sanga G, Bejon P, Marsh V, Molyneux S, Kamuya D. Ethical considerations in controlled human Malaria Infection studies in low resource settings: experiences and perceptions of study participants in a malaria challenge study in Kenya. Wellcome Open Res. 2018;3:39. doi: 10.12688/wellcomeopenres.14439.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriega FR, Liao FM, Maneval DR, Ren S, Formal SB, Levine MM. Strategy for cross-protection among Shigella flexneri serotypes. Infect Immun. 1999;67(2):782–788. doi: 10.1128/iai.67.2.782-788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaks EV, Hale TL, Formal SB. Serum immune response to Shigella protein antigens in rhesus monkeys and humans infected with Shigella spp. Infect Immun. 1986;53(1):57–63. doi: 10.1128/iai.53.1.57-63.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaks EV, Picking WD, Picking WL. Antibody response of monkeys to invasion plasmid antigen D after infection with Shigella spp. Clin Diagn Lab Immunol. 1996;3(2):242–245. doi: 10.1128/cdli.3.2.242-245.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obiero CW, Ndiaye AGW, Scire AS, Kaunyangi BM, Marchetti E, Gone AM, Schutte LD, Riccucci D, Auerbach J, Saul A, Martin LB, et al. A phase 2a randomized study to evaluate the safety and immunogenicity of the 1790GAHB generalized modules for membrane antigen vaccine against Shigella sonnei administered intramuscularly to adults from a Shigellosis-endemic country. Front Immunol. 2017;8:1884. doi: 10.3389/fimmu.2017.01884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson S, Hall A, Riddle MS, Porter CK. Travelers’ diarrhea: update on the incidence, etiology and risk in military and similar populations—1990-2005 versus 2005-2015, does a decade make a difference? Trop Dis Travel Med Vaccines. 2019;5:1. doi: 10.1186/s40794-018-0077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passwell JH, Harlev E, Ashkenazi S, Chu C, Miron D, Ramon R, Farzan N, Shiloach J, Bryla DA, Majadly F, Roberson R, et al. Safety and immunogenicity of improved Shigella O-specific polysaccharide-protein conjugate vaccines in adults in Israel. Infect Immun. 2001;69(3):1351–1357. doi: 10.1128/IAI.69.3.1351-1357.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passwell JH, Ashkenazi S, Harlev E, Miron D, Ramon R, Farzam N, Lerner-Geva L, Levi Y, Chu C, Shiloach J, Robbins JB, et al. Israel Shigella Study G. Safety and immunogenicity of Shigella sonnei-CRM9 and Shigella flexneri type 2a-rEPAsucc conjugate vaccines in one-to four-year-old children. Pediatr Infect Dis J. 2003;22(8):701–706. doi: 10.1097/01.inf.0000078156.03697.a5. [DOI] [PubMed] [Google Scholar]
- Passwell JH, Ashkenazi S, Banet-Levi Y, Ramon-Saraf R, Farzam N, Lerner-Geva L, Even-Nir H, Yerushalmi B, Chu C, Shiloach J, Robbins JB, et al. Israeli Shigella Study G Age-related efficacy of Shigella O-specific polysaccharide conjugates in 1–4-year-old Israeli children. Vaccine. 2010;28(10):2231–2235. doi: 10.1016/j.vaccine.2009.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlinac PB, Singa BO, John-Stewart GC, Richardson BA, Brander RL, McGrath CJ, Tickell KD, Amondi M, Rwigi D, Babigumira JB, Kariuki S, et al. Azithromycin to prevent post-discharge morbidity and mortality in Kenyan children: a protocol for a randomised, double-blind, placebo-controlled trial (the Toto Bora trial) BMJ Open. 2017;7(12):e019170. doi: 10.1136/bmjopen-2017-019170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perepelov AV, Shekht ME, Liu B, Shevelev SD, Ledov VA, Senchenkova SN, L’Vov VL, Shashkov AS, Feng L, Aparin PG, Wang L, et al. Shigella flexneri O-antigens revisited: final elucidation of the O-acetylation profiles and a survey of the O-antigen structure diversity. FEMS Immunol Med Microbiol. 2012;66(2):201–210. doi: 10.1111/j.1574-695X.2012.01000.x. [DOI] [PubMed] [Google Scholar]
- Pitisuttithum P, Islam D, Chamnanchanunt S, Ruamsap N, Khantapura P, Kaewkungwal J, Kittitrakul C, Luvira V, Dhitavat J, Venkatesan MM, Mason CJ, et al. Clinical trial of an oral live Shigella sonnei vaccine candidate, WRSS1, in Thai Adults. Clin Vaccine Immunol. 2016;23(7):564–575. doi: 10.1128/CVI.00665-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin SA. Immunologic correlates of protection induced by vaccination. Pediatr Infect Dis J. 2001;20(1):63–75. doi: 10.1097/00006454-200101000-00013. [DOI] [PubMed] [Google Scholar]
- Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17(7):1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogreba-Brown K, Austhof E, Armstrong A, Schaefer K, Villa Zapata L, McClelland DJ, Batz MB, Kuecken M, Riddle M, Porter CK, Bazaco MC. Chronic gastrointestinal and joint-related sequelae associated with common foodborne illnesses: a scoping review. Foodborne Pathog Dis. 2020;17(2):67–86. doi: 10.1089/fpd.2019.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard AJ, Sauerwein R, Baay M, Neels P, speakers HCT, session c Biologicals; Third human challenge trial conference, Oxford, United Kingdom, February 6-7, 2020, a meeting report; 2020. pp. 41–52. [DOI] [PubMed] [Google Scholar]
- Porter CK, Thura N, Ranallo RT, Riddle MS. The Shigella human challenge model. Epidemiol Infect. 2013;141(2):223–232. doi: 10.1017/S0950268812001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter CK, Lynen A, Riddle MS, Talaat K, Sack D, Gutierrez RL, McKenzie R, DeNearing B, Feijoo B, Kaminski RW, Taylor DN, et al. Clinical endpoints in the controlled human challenge model for Shigella: a call for standardization and the development of a disease severity score. PLoS ONE. 2018;13(3):e0194325. doi: 10.1371/journal.pone.0194325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter CK, Gutierrez RL, Kotloff KL. Clinical endpoints for efficacy studies. Vaccine. 2019;37(34):4814–4822. doi: 10.1016/j.vaccine.2019.03.051. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Li S, Nakaya HI. Systems vaccinology. Immunity. 2010;33(4):516–529. doi: 10.1016/j.immuni.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pupo GM, Lan R, Reeves PR. Multiple independent origins of Shigella clones of Escherichia coli and convergent evolution of many of their characteristics. Proc Natl Acad Sci USA. 2000;97(19):10567–10572. doi: 10.1073/pnas.180094797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadri F, Chowdhury MI, Faruque SM, Salam MA, Ahmed T, Begum YA, Saha A, Alam MS, Zaman K, Seidlein LV, Park E, et al. Peru-15 Study G Randomized, controlled study of the safety and immunogenicity of Peru-15, a live attenuated oral vaccine candidate for cholera, in adult volunteers in Bangladesh. J Infect Dis. 2005;192(4):573–579. doi: 10.1086/432074. [DOI] [PubMed] [Google Scholar]
- Qadri F, Chowdhury MI, Faruque SM, Salam MA, Ahmed T, Begum YA, Saha A, Al Tarique A, Seidlein LV, Park E, Killeen KP, et al. Peru-15, a live attenuated oral cholera vaccine, is safe and immunogenic in Bangladeshi toddlers and infants. Vaccine. 2007;25(2):231–238. doi: 10.1016/j.vaccine.2006.08.031. [DOI] [PubMed] [Google Scholar]
- Qiu S, Xu X, Yang C, Wang J, Liang B, Li P, Li H, Yi S, Liu H, Cui X, Wu Z, et al. Shift in serotype distribution of Shigella species in China, 2003-2013. Clin Microbiol Infect. 2015;21(3):252 e255-258. doi: 10.1016/j.cmi.2014.10.019. [DOI] [PubMed] [Google Scholar]
- Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, Pirani A, Gernert K, Deng J, Marzolf B, Kennedy K, et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10(1):116–125. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram PK, Crump JA, Gupta SK, Miller MA, Mintz ED. Part II Analysis of data gaps pertaining to Shigella infections in low and medium human development index countries, 1984-2005. Epidemiol Infect. 2008;136(5):577–603. doi: 10.1017/S0950268807009351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan R, Stibitz S, Pratt D, Roberts J. Use of controlled human infection models (CHIMs) to support vaccine development: US regulatory considerations. Vaccine. 2019;37(31):4256–4261. doi: 10.1016/j.vaccine.2019.06.009. [DOI] [PubMed] [Google Scholar]
- Randall A. Protein and LPS-based microarrays: a versatile immune-profiling platform for measuring antibody binding to vaccine-relevant Shigella and ETEC antigens; VASE virtual symposium, 28 and 30 Sept 2021; PATH|Washington, DC. 2021. 20001 [Google Scholar]
- Ranganathan S, Smith EM, Foulke-Abel JD, Barry EM. Research in a time of enteroids and organoids: how the human gut model has transformed the study of enteric bacterial pathogens. Gut Microbes. 2020;12(1):1795492. doi: 10.1080/19490976.2020.1795389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan S, Doucet M, Grassel CL, Delaine-Elias B, Zachos NC, Barry EM. Evaluating Shigella flexneri pathogenesis in the human enteroid model. Infect Immun. 2019;87(4) doi: 10.1128/IAI.00740-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raqib R, Qadri F, SarkEr P, Mia SM, Sansonnetti PJ, Albert MJ, Andersson J. Delayed and reduced adaptive humoral immune responses in children with shigellosis compared with in adults. Scand J Immunol. 2002;55(4):414–423. doi: 10.1046/j.1365-3083.2002.01079.x. [DOI] [PubMed] [Google Scholar]
- Raqib R, Sarker P, Zaman K, Alam NH, Wierzba TF, Maier N, Talukder K, Baqui AH, Suvarnapunya AE, Qadri F, Walker RI, et al. A phase I trial of WRSS1, a Shigella sonnei live oral vaccine in Bangladeshi adults and children. Hum Vaccin Immunother. 2019;15(6):1326–1337. doi: 10.1080/21645515.2019.1575165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle MS, Sanders JW, Putnam SD, Tribble DR. Incidence, etiology, and impact of diarrhea among long-term travelers (US military and similar populations): a systematic review. Am J Trop Med Hyg. 2006;74(5):891–900. [PubMed] [Google Scholar]
- Riddle MS. Is a Shigella vaccine needed for travellers and the military? J Travel Med. 2018;25(1) doi: 10.1093/jtm/tay049. [DOI] [PubMed] [Google Scholar]
- Robbins JB, Chu C, Schneerson R. Hypothesis for vaccine development: protective immunity to enteric diseases caused by nontyphoidal salmonellae and shigellae may be conferred by serum IgG antibodies to the O-specific polysaccharide of their lipopolysaccharides. Clin Infect Dis. 1992;15(2):346–361. doi: 10.1093/clinids/15.2.346. [DOI] [PubMed] [Google Scholar]
- Rogawski ET, Liu J, Platts-Mills JA, Kabir F, Lertsethtakarn P, Siguas M, Khan SS, Praharaj I, Murei A, Nshama R, Mujaga B, et al. Investigators M-EN Use of quantitative molecular diagnostic methods to investigate the effect of enteropathogen infections on linear growth in children in low-resource settings: longitudinal analysis of results from the MAL-ED cohort study. Lancet Glob Health. 2018;6(12):e1319–e1328. doi: 10.1016/S2214-109X(18)30351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski McQuade ET, Shaheen F, Kabir F, Rizvi A, Platts-Mills JA, Aziz F, Kalam A, Qureshi S, Elwood S, Liu J, Lima AAM, et al. Epidemiology of Shigella infections and diarrhea in the first two years of life using culture-independent diagnostics in 8 low-resource settings. PLoS Negl Trop Dis. 2020;14(8):e0008536. doi: 10.1371/journal.pntd.0008536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollenhagen JE, Jones F, Hall E, Maves R, Nunez G, Espinoza N, O’Dowd A, Prouty MG, Savarino SJ. Establishment, validation, and application of a new world primate model of enterotoxigenic Escherichia coli disease for vaccine development. Infect Immun. 2019;87(2) doi: 10.1128/IAI.00634-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan ET, Calderwood SB. Cholera vaccines. Clin Infect Dis. 2000;31(2):561–565. doi: 10.1086/313951. [DOI] [PubMed] [Google Scholar]
- Sack DA, Hoque AT, Huq A, Etheridge M. Is protection against shigellosis induced by natural infection with Plesiomonas shigelloides? Lancet. 1994;343(8910):1413–1415. doi: 10.1016/s0140-6736(94)92531-3. [DOI] [PubMed] [Google Scholar]
- Samandari T, Kotloff KL, Losonsky GA, Picking WD, Sansonetti PJ, Levine MM, Sztein MB. Production of IFN-gamma and IL-10 to Shigella invasins by mononuclear cells from volunteers orally inoculated with a Shiga toxin-deleted Shigella dysenteriae type 1 strain. J Immunol. 2000;164(4):2221–2232. doi: 10.4049/jimmunol.164.4.2221. [DOI] [PubMed] [Google Scholar]
- Sansonetti PJ, Kopecko DJ, Formal SB. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982;35(3):852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar P, Mily A, Ara A, Haque F, Maier N, Wierzba TF, Walker RI. Functional antibodies and innate immune responses to WRSS1, a live oral Shigella sonnei vaccine candidate in Bangladeshi adults and children. J Infect Dis. 2021;224(S7):S829–S839. doi: 10.1093/infdis/jiab395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder GN, Hilbi H. Molecular pathogenesis of Shigella spp.: controlling host cell signaling, invasion, and death by type III secretion. Clin Microbiol Rev. 2008;21(1):134–156. doi: 10.1128/CMR.00032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultsz C, Qadri F, Hossain SA, Ahmed F, Ciznar I. Shigella-specific IgA in saliva of children with bacillary dysentery. FEMS Microbiol Immunol. 1992;4(2):65–72. doi: 10.1111/j.1574-6968.1992.tb04972.x. [DOI] [PubMed] [Google Scholar]
- Sela U, Euler CW, Correa da Rosa J, Fischetti VA. Strains of bacterial species induce a greatly varied acute adaptive immune response: the contribution of the accessory genome. PLoS Pathog. 2018;14(1):e1006726. doi: 10.1371/journal.ppat.1006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah N, DuPont HL, Ramsey DJ. Global etiology of travelers’ diarrhea: systematic review from 1973 to the present. Am J Trop Med Hyg. 2009;80(4):609–614. [PubMed] [Google Scholar]
- Shahin K, Bouzari M, Komijani M, Wang R. A new phage cocktail against multidrug, ESBL-producer isolates of Shigella sonnei and Shigella flexneri with highly efficient bacteriolytic activity. Microb Drug Resist. 2020;26(7):831–841. doi: 10.1089/mdr.2019.0235. [DOI] [PubMed] [Google Scholar]
- Shaughnessy HJ, Olsson RC, et al. Experimental human bacillary dysentery; polyvalent dysentery vaccine in its prevention. J Am Med Assoc. 1946;132:362–368. doi: 10.1001/jama.1946.02870420002002. [DOI] [PubMed] [Google Scholar]
- Shim DH, Suzuki T, Chang SY, Park SM, Sansonetti PJ, Sasakawa C, Kweon MN. New animal model of shigellosis in the Guinea pig: its usefulness for protective efficacy studies. J Immunol. 2007;178(4):2476–2482. doi: 10.4049/jimmunol.178.4.2476. [DOI] [PubMed] [Google Scholar]
- Shimanovich AA, Buskirk AD, Heine SJ, Blackwelder WC, Wahid R, Kotloff KL, Pasetti MF. Functional and antigen-specific serum antibody levels as correlates of protection against shigellosis in a controlled human challenge study. Clin Vaccine Immunol. 2017;24(2) doi: 10.1128/CVI.00412-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley DA, McArthur MA. The utility of human challenge studies in vaccine development: lessons learned from cholera. Vaccine (auckland) 2011;1:3–13. doi: 10.2147/VDT.S23634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 2015;282(1821):20143085. doi: 10.1098/rspb.2014.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperandio V. Pathogens’ adaptation to the human host. Proc Natl Acad Sci U S A. 2018;115(38):9342–9343. doi: 10.1073/pnas.1813379115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen R. Epidemiology of travellers’ diarrhea. J Travel Med. 2017;24(suppl_1):S2–S5. doi: 10.1093/jtm/taw072. [DOI] [PubMed] [Google Scholar]
- Tacket CO, Binion SB, Bostwick E, Losonsky G, Roy MJ, Edelman R. Efficacy of bovine milk immunoglobulin concentrate in preventing illness after Shigella flexneri challenge. Am J Trop Med Hyg. 1992;47(3):276–283. doi: 10.4269/ajtmh.1992.47.276. [DOI] [PubMed] [Google Scholar]
- Talaat KR, Alaimo C, Martin P, Bourgeois AL, Dreyer AM, Kaminski RW, Porter CK, Chakraborty S, Clarkson KA, Brubaker J, Elwood D, et al. Human challenge study with a Shigella bioconjugate vaccine: analyses of clinical efficacy and correlate of protection. EBioMedicine. 2021;66:103310. doi: 10.1016/j.ebiom.2021.103310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talaat KR, Bourgeois AL, Frenck RW, Chen WH, MacLennan CA, Riddle MS, Suvarnapunya AE, Brubaker JL, Kotloff KL, Porter CK. Consensus report on Shigella controlled human infection model: conduct of studies. Clin Infect Dis. 2019;69(Supplement_8):S580–S590. doi: 10.1093/cid/ciz892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DN, McKenzie R, Durbin A, Carpenter C, Atzinger CB, Haake R, Bourgeois AL. Rifaximin, a nonabsorbed oral antibiotic, prevents shigellosis after experimental challenge. Clin Infect Dis. 2006;42(9):1283–1288. doi: 10.1086/503039. [DOI] [PubMed] [Google Scholar]
- Taylor DN, McKenzie R, Durbin A, Carpenter C, Haake R, Bourgeois AL. Systemic pharmacokinetics of rifaximin in volunteers with shigellosis. Antimicrob Agents Chemother. 2008;52(3):1179–1181. doi: 10.1128/AAC.01108-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh AY, Cavacini L, Hu Y, Kumru OS, Xiong J, Bolick DT, Joshi SB, Grunwald-Gruber C, Altmann F, Klempner M, Guerrant RL, et al. Investigation of a monoclonal antibody against enterotoxigenic Escherichia coli expressed as secretory IgA1 and IgA2 in plants. Gut Microbes. 2021;13(1):1–14. doi: 10.1080/19490976.2020.1859813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CN, Duy PT, Baker S. The rising dominance of Shigella sonnei: An intercontinental shift in the etiology of bacillary dysentery. PLoS Negl Trop Dis. 2015;9(6):e0003708. doi: 10.1371/journal.pntd.0003708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tickell KD, Brander RL, Atlas HE, Pernica JM, Walson JL, Pavlinac PB. Identification and management of Shigella infection in children with diarrhoea: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1235–e1248. doi: 10.1016/S2214-109X(17)30392-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaras GD, Plotkin SA. Complex immune correlates of protection in HIV-1 vaccine efficacy trials. Immunol Rev. 2017;275(1):245–261. doi: 10.1111/imr.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann L, Sekaly RP. Solving vaccine mysteries: a systems biology perspective. Nat Immunol. 2011;12(8):729–731. doi: 10.1038/ni.2078. [DOI] [PubMed] [Google Scholar]
- Turbyfill KR, Joseph SW, Oaks EV. Recognition of three epitopic regions on invasion plasmid antigen C by immune sera of rhesus monkeys infected with Shigella flexneri 2a. Infect Immun. 1995;63(10):3927–3935. doi: 10.1128/iai.63.10.3927-3935.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Seidlein L, Kim DR, Ali M, Lee H, Wang X, Thiem VD, Canh DG, Chaicumpa W, Agtini MD, Hossain A, Bhutta ZA, et al. A multicentre study of Shigella diarrhoea in six Asian countries: disease burden, clinical manifestations, and microbiology. PLoS Med. 2006;3(9):e353. doi: 10.1371/journal.pmed.0030353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington CS, Darton TC, Jones C, Haworth K, Peters A, John T, Thompson BA, Kerridge SA, Kingsley RA, Zhou L, Holt KE, et al. An outpatient, ambulant-design, controlled human infection model using escalating doses of Salmonella typhi challenge delivered in sodium bicarbonate solution. Clin Infect Dis. 2014;58(9):1230–1240. doi: 10.1093/cid/ciu078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahid R, Simon JK, Picking WL, Kotloff KL, Levine MM, Sztein MB. Shigella antigen-specific B memory cells are associated with decreased disease severity in subjects challenged with wild-type Shigella flexneri 2a. Clin Immunol. 2013;148(1):35–43. doi: 10.1016/j.clim.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JL, Sanchez-Garrido J, Goddard PJ, Torraca V, Mostowy S, Shenoy AR, Clements A. Shigella sonnei O-antigen inhibits internalization, vacuole escape, and inflammasome activation. mBio. 2019;10(6) doi: 10.1128/mBio.02654-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellcome grants awarded 1 Oct 2005-31 Mar 2021. [Accessed 02 June 2021]. https://wellcome.org/reports/grant-funding-data-2019-2020 .
- Wellcome Human infection studies for vaccine development. [Accessed 02 June 2021]. https://wellcome.org/grant-funding/schemes/human-infection-studies-vaccine-development .
- Wellcome Trust and Boston Consulting Group. Vaccines to tackle drug resistant infections An evaluation of R&D opportunities. 2021.
- Wenzel H, Kaminski RW, Clarkson KA, Maciel M, Jr, Smith MA, Zhang W, Oaks EV. Improving chances for successful clinical outcomes with better preclinical models. Vaccine. 2017;35(49 Pt A):6798–6802. doi: 10.1016/j.vaccine.2017.08.030. [DOI] [PubMed] [Google Scholar]
- WHO. Expert Committee on Biological Standardization, 17-21 Oct 2016. World Health Organization; Geneva: 2016. Human challenge trials for vaccine development: regulatory considerations. [Google Scholar]
- WHO. Prioritization of pathogens to guide discovery, research and development of new antibiotics for drug-resistant bacterial infections, including tuberculosis WHO/EMP/IAU/201712. World Health Organization; Geneva: 2017. [Google Scholar]
- WHO. WHO technical report series. Vol. 1004 Geneva: 2017. WHO expert committee on biological standardization, sixty-seventh report. [Google Scholar]
- WHO. WHO preferred product characteristics for vaccines against Shigella. World Health Organization; Geneva: 2020. draft. [Google Scholar]
- Yang F, Yang J, Zhang X, Chen L, Jiang Y, Yan Y, Tang X, Wang J, Xiong Z, Dong J, Xue Y, et al. Genome dynamics and diversity of Shigella species, the etiologic agents of bacillary dysentery. Nucleic Acids Res. 2005;33(19):6445–6458. doi: 10.1093/nar/gki954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019;32(2) doi: 10.1128/CMR.00084-18. [DOI] [PMC free article] [PubMed] [Google Scholar]