Abstract
The megatooth shark, †Otodus megalodon, which likely reached at least 15 m in total length, is an iconic extinct shark represented primarily by its gigantic teeth in the Neogene fossil record. As one of the largest marine carnivores to ever exist, understanding the biology, evolution, and extinction of †O. megalodon is important because it had a significant impact on the ecology and evolution of marine ecosystems that shaped the present-day oceans. Some attempts inferring the body form of †O. megalodon have been carried out, but they are all speculative due to the lack of any complete skeleton. Here we highlight the fact that the previous total body length estimated from vertebral diameters of the extant white shark (Carcharodon carcharias) for an †O. megalodon individual represented by an incomplete vertebral column is much shorter than the sum of anteroposterior lengths of those fossil vertebrae. This factual evidence indicates that †O. megalodon had an elongated body relative to the body of the modern white shark. Although its exact body form remains unknown, this proposition represents the most parsimonious empirical evidence, which is a significant step towards deciphering the body form of †O. megalodon.
Keywords: body form, fossil record, morphology, Neogene, vertebra
Introduction
The extinct megatooth shark, †Otodus megalodon (Lamniformes: †Otodontidae), is an iconic prehistoric shark that has captured the attention of both scientists and the public due to its large teeth. Yet, one major challenge palaeontologists have faced is exactly what †O. megalodon looked like because no complete skeleton of the fossil species is known to date. Traditionally, the extant white shark (Carcharodon carcharias) has been used as a model species to reconstruct the body form of †O. megalodon (e.g., Gottfried et al., 1996). The most recent attempts have been the 2D reconstruction work by Cooper et al. (2020), followed by Cooper et al.’s (2022) 3D model of the body of †O. megalodon. Cooper et al. (2020, 2022) used the extant white shark as a model representation of †O. megalodon because the fossil shark has been inferred to be regionally endothermic like the extant lamnid sharks that include the white shark (Ferrón, 2017). In particular, Cooper et al. (2022) used an extant juvenile white shark specimen to generate a 3D model of †O. megalodon first, and then conducted a ‘model adjustment’ using all the extant lamnids because of the uncertainty in the phylogenetic position of †O. megalodon within Lamniformes. Based on their body form reconstruction, they concluded that †O. megalodon was a fast-cruising shark much like the extant lamnids. However, using the extant white shark or other lamnids as a template to reconstruct the body form of †O. megalodon lacks empirical fossil support (Sternes et al., 2023). Furthermore, it is also tenuous on the phylogenetic basis because †O. megalodon, as an otodontid, lies outside of the Lamnidae and may not be closely related to the family at all (Sternes et al., 2023; Figure 1A; but see also Appendix 1).
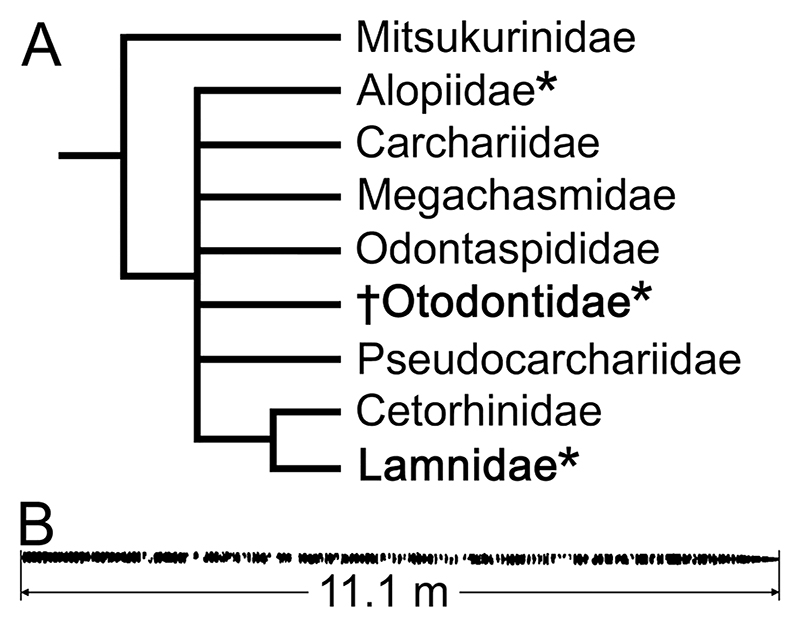
Figure 1.
Simplified family-level phylogenetic hypothesis of Lamniformes showing all extant clades and †Otodontidae (A: dagger [†] indicates extinct), and silhouette depiction of fossil vertebral column of †Otodus megalodon (B). A, Current understanding of lamniform phylogeny demonstrating that a large portion of the phylogenetic tree remains unresolved due to conflicting results based on various molecular and morphological studies (Sternes et al., 2023 and references therein); although the placement of †Otodontidae is tentative and other extinct families are not depicted in this tree, the main point of this illustration is to demonstrate that †Otodontidae lies outside of Lamnidae (both clades highlighted in bold letters) where clades containing one or more species with regional endothermy (indicated by an asterisk [*]) do not share an immediate common ancestry (Sternes et al., 2023). B, Reconstructed vertebral column and its total measured length by Cooper et al. (2022) based on an incomplete associated vertebral set from the Miocene of Belgium; this specific specimen (IRSNB P 9893) was previously estimated to have come from an individual that measured 9.2 m in total length, including the head and caudal fin (Gottfried et al., 1996) based on the modern white shark, not accounted for by Cooper et al. (2022).
One key question is: “Did †O. megalodon look like a large extant white shark?” It is true that the extant white shark has generally been used to estimate the body size of †O. megalodon (Shimada, 2019; Perez et al., 2021), but unlike preserved teeth that are at least tangibly comparable, the lack of any complete skeleton, or even a complete cranial skeleton or vertebral column, makes any skeletal or body reconstruction speculative. However, there are three critical pieces of information relevant to addressing the question that have become available since Cooper et al.’s (2022) study. First, on the basis of geochemical evidence, the endothermic physiology in †O. megalodon (specifically, likely regional endothermy) is empirically confirmed (Griffiths et al., 2023). Second, the newly described placoid scales of †O. megalodon, particularly the scales’ interkeel distances that vary independent of body sizes in sharks, indicate that the general cruising speed of †O. megalodon was likely slower than the cruising speeds of extant lamnids, including the white shark (Shimada et al., 2023). Third, and more significantly, two other lamniform species, the extant planktivorous basking shark (Cetorhinus maximus), which has traditionally been regarded as a sluggish shark, as well as the deep-water, benthopelagic smalltooth sand tiger (Odontaspis ferox) have both been reinterpreted to be endothermic (also likely regional endothermy: Dolton et al., 2023a, 2023b; despite at least O. ferox is suggested to be ectothermic based on isotopic analyses by Griffiths et al., 2023). Hence, while †O. megalodon was indeed ‘endothermic’ (Griffiths et al., 2023), the new palaeontological (Shimada et al., 2023) and neontological (at least Dolton et al., 2023a, at present) evidence do not corroborate the previous assumption and its rationale that †O. megalodon must have physically resembled the extant white shark or lamnids in general (Cooper et al., 2020, 2022). Therefore, the purpose of this paper is two-fold: 1) to re-evaluate the validity of the most recently proposed body form reconstruction of †O. megalodon; and 2) to provide a new hypothesis on the body form of †O. megalodon based on available evidence.
Materials and Methods
The main specimen used for the re-evaluation of the recently proposed body form of †O. megalodon and further discussion in this study is IRSNB P 9893, which is housed in the Royal Belgian Institute of Natural Sciences (IRSNB) in Brussels. This fossil specimen, formerly referred to as ‘IRSNB 3121’ (Gottfried et al., 1996), consists of 141 associated, but disarticulated, vertebral centra from an individual collected from the Miocene of Belgium (Shimada et al., 2021b; Cooper et al., 2022) (Figure 1B). Although it was not associated with any teeth, the specimen is broadly accepted to have come from †O. megalodon due to the large size and structure of the centra, which are consistent with non-cetorhinid lamniform vertebrae (Gottfried et al., 1996; Shimada et al., 2021b; Cooper et al., 2022). Based on the maximum width of the largest centrum in the specimen (‘vertebra #4’ measuring 155 mm in width), the †O. megalodon individual was estimated to be 9.2 m TL in life based on a linear regression function describing the quantitative relationship between the maximum vertebral width and TL measurements from 16 extant white sharks (Gottfried et al., 1996). Cooper et al. (2022, data S1) also took measurements of each vertebra of IRSNB P 9893 and presented the sum of anteroposterior lengths of all centra to be approximately 11.1 m (Figure 1B). Our study compared that measurement (11.1 m) with an estimated total length (9.2 m) for that specific †O. megalodon individual based on the extant white shark (Gottfried et al., 1996).
For comparisons, some preserved extant specimens housed in the following repository institutions were examined radiographically: Field Museum of Natural History (FMNH), Chicago, Illinois, USA; Natural History Museum of Los Angeles County (LACM), California, USA; and Florida Museum of Natural History, University of Florida (UF), Gainesville, USA. We used a Siemens Medical Systems’ SOMATOM Sensation 64-slice computed tomography (CT) scanner at the Children’s Memorial Hospital, Chicago, Illinois, USA, with the following settings: 120 kVp, effective mAs 200 with automatic exposure control activated, rotation time 0.33 sec, 0.75 pitch, 32 detectors using z-flying focal spot technique, 0.625-mm slice thickness and 0.4 mm overlapping slice reconstruction. Multiple CT images showing the skeletal elements of the specimens were generated using Siemens’ InS-pace software.
We acknowledge that different types of intra-specific variation may occur in sharks, including sexual dimorphism where, in many lamniform taxa, females tend to reach sexual maturity at larger body sizes or attain larger maximum body sizes (Compagno, 2002). However, for the purpose of re-evaluating the validity of Cooper et al.’s (2022) reconstructed vertebral column of †O. megalodon, we examined in detail the CT scans of a juvenile Carcharodon carcharias specimen (LACM 43805-1), which are available on the MorphoSource data-base: (https://www.morphosource.org/concern/media/000545335). Vertebral diameters were measured from this specimen by using the open-source web program postDICOM (Herten, The Netherlands; www.postdicom.com, last accessed July 25, 2023). Each measurement was taken three times to minimize possible measurement errors and to calculate a mean value that was subsequently used. A total of 163 vertebral centra were measured across the entire body of the specimen (see Appendix 2).
Results and Discussion
Re-evaluation of the Validity of the Recently Reconstructed Body Form of †O. megalodon
Cooper et al. (2022) proposed the most recent 3D model of †O. megalodon and used it to make various inferences on the ecology of the extinct shark. We re-evaluated their assumptions and propositions by considering available evidence and other recent discoveries. Our re-evaluation result is that there are at least four major concerns with their body reconstruction that are worthy of discussion.
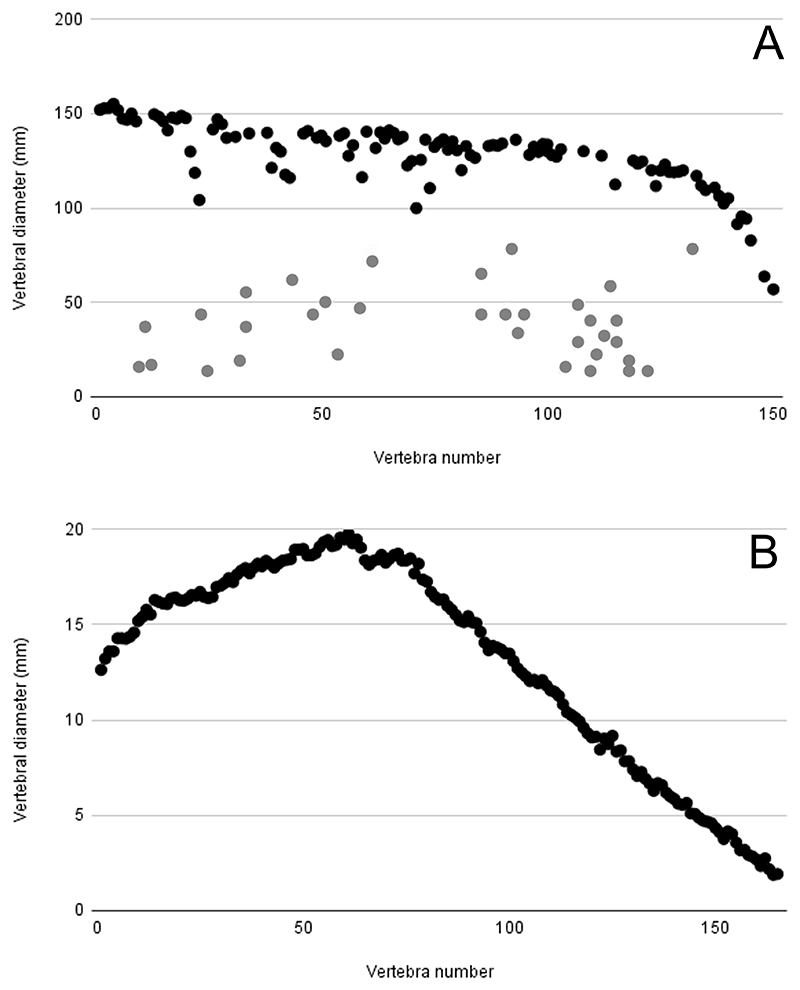
The first issue is the questionable accuracy of their reconstructed vertebral column of †O. megalodon. Cooper et al. (2022) used 141 associated vertebrae from an †O. megalodon individual (IRSNB P 9893) collected from a Miocene deposit in Belgium. Despite being the best-preserved vertebral column of †O. megalodon, there are several major concerns that must be taken into consideration about using this fossil specimen. As Cooper et al. (2022, p. 8) also pointed out, this set of vertebrae is most certainly incomplete. For instance, Cooper et al. (2022) followed the sequence of curatorially assigned vertebral numbers that do not represent the vertebral sequence in life and noted that “centra 30, 35 to 37, 45, 105, 131, 136, 141, 146, 147, 149 are missing from the column”. Although Cooper et al. (2022) accounted for those vertebrae with artificially and likely arbitrarily (Gottfried et al., 1996) assigned numbers that are interpreted to be missing, exactly how many more vertebrae were present in the vertebral column in life remains uncertain. In fact, vertebral counts are known to vary widely even among lamniform sharks (Springer and Garrick, 1964). It is therefore impossible to even decisively determine the total number of vertebrae, yet alone the total number of precaudal and caudal vertebrae, originally present in †O. megalodon. However, not only did Cooper et al. (2022) choose to assume that all preserved centra in the specimen represent precaudal vertebrae in their 3D model of †O. megalodon, they put the largest vertebrae near the neurocranium of their model (Figure 2). We point out that, in previous studies of both extinct (Conte et al., 2019) and extant (Natanson et al., 2018) lamniform sharks, the largest vertebrae are found in the girthiest portion of their trunk (mid-body), and this condition is also true for the extant white shark (vertebrae 54–64: Appendix 2; Figure 2). When plotting Cooper et al.’s (2022) reconstructed vertebral column, a gradual decline in vertebral diameter starting from the first vertebra is observed whereas the extant white shark shows a gradual increase in vertebral diameter and then a decline, which is the same pattern observed in other extant lamniform sharks (Natanson et al., 2018) (Figure 2). Furthermore, our reexamination of IRSNB P 9893 based on measurements provided by Cooper et al. (2022) suggests that not all centra in the specimen are precaudal vertebrae based on comparisons with a complete vertebral column in the extant white shark (Appendix 2). For example, in Cooper et al.’s (2022) computer model, the largest vertebra in IRSNB P 9893 (centrum 4) was 155 mm in diameter whereas the smallest vertebra (centrum 150) was 57 mm in diameter. When comparing the largest vertebra to the smallest in Cooper et al.’s (2022) model, this generates a ratio of 2.7. This same ratio (2.7) is present when comparing the largest vertebra found in the mid-body of the extant white shark to that of a vertebra found in its caudal fin, specifically, vertebrae #61 and #132 measuring 19.75 mm and 7.27 mm in diameter, respectively (Appendix 2). This fact strongly indicates that the reconstructed precaudal portion of the vertebral column of Cooper et al. (2022) indeed includes caudal vertebrae. Taking all the information into account, the model of the vertebral column created by Cooper et al. (2022) is most certainly incomplete and inaccurate.
Figure 2.
The distribution of vertebral diameters throughout each vertebral column, where vertebral number ‘1’ represents the anterior-most centrum in each specimen. A, Graph based on Cooper et al.’s (2022) Data S1 for the vertebral column of †Otodus megalodon from the Miocene of Belgium (IRSNB P 9893), where the vertebral column is most certainly incomplete and the vertebral numbers do not necessarily reflect the original anatomical sequence (grey plots represent significantly damaged vertebrae). B, Graph based on CT-scanned data of an extant white shark (Carcharodon carcharias) specimen (LACM 43805-1), where the vertebral column is complete and the vertebral numbers reflect the anatomical sequence.
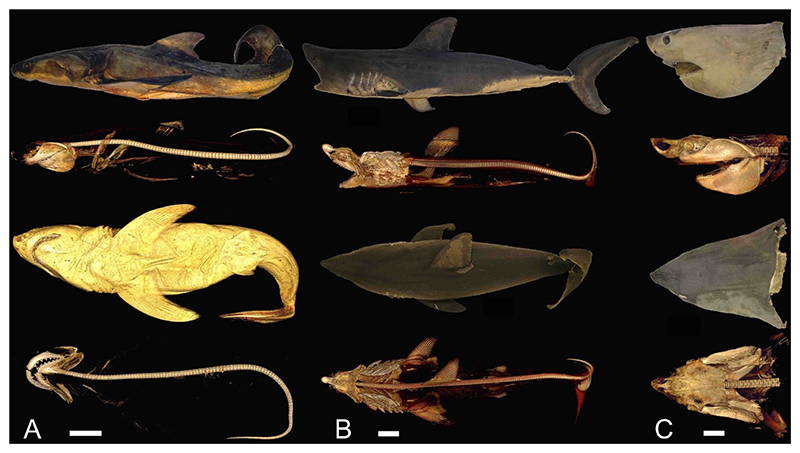
The second issue is the discrepancy in jaw size. The ratio of the anteroposterior upper jaw length to the largest vertebral diameter in two specimens of the extant white sharks we measured from CT images (Figure 3) is about 8.3. On the other hand, Cooper et al.’s (2022) 3D †O. megalodon skeletal model has a ratio of 10.6. This means that the jaw size in the 3D skeletal model is oversized relative to its vertebrae if the extant white shark is used. Such a discrepancy may indicate that there is a flaw in Cooper et al.’s (2022) skeletal reconstruction, the extant white shark may not necessarily be an appropriate body form analog for the extinct species (i.e., †O. megalodon could have had a different body form), or both. In addition, Cooper et al. (2022) noted that their reconstruction of the †O. megalodon head is slightly ‘undersized’ (p. 9), but we would argue that, while the overall length of the cranial region relative to its TL may be on par with that of the extant white shark (see above), at least their jaw reconstruction may actually be oversized relative to its body if the overall skeletal organization of the extant white shark (Figure 3), which Cooper et al. (2022) did not account for, is used as a model at face value.
Figure 3.
Photographic (*) and CT images (**) of preserved specimens of extant white shark (Carcharodon carcharias) and salmon shark (Lamna ditropis). A, Complete specimen of 126-cm-TL male C. carcharias caught off central California, USA (LACM 43805-1): from top to bottom, external body* and skeleton** in left lateral view and external body** and skeleton** in ventral view. B, Complete specimen of 151 cm TL male L. ditropis caught off central California (FMNH 117475): from top to bottom, external body* and skeleton** in left lateral view and external body* and skeleton** in dorsal view. C, Head specimen of estimated 271-cm-TL male C. carcharias caught off southern Florida, USA (FMNH 38335): from top to bottom, external head* and cranial skeleton** in left lateral view and external head* and cranial skeleton** in dorsal view. All scale bars equal 10 cm.
The third concern is the lack of ontogenetic consideration. The specific extant white shark specimen scanned for Cooper et al.’s (2022) †O. megalodon body reconstruction may not be ideal. Setting aside a slight upward bend of the head that is a rather unconventional posture compared to an otherwise fusiform body that typically characterizes the white shark and sharks in general (Sternes and Shimada, 2020; Paig-Tran et al., 2022; Sternes et al., 2023), the white shark specimen they used represents a 2.56-m-TL juvenile individual. Importantly, allometric changes in girth and the caudal fin morphology at various developmental stages are known for the white shark and other lamnids (Casey and Pratt, 1985; Lingham-Soliar, 2005; Tomita et al., 2018; Sternes et al., 2023). However, Cooper et al. (2022) did not address the possible effects of ontogenetic morphological differences in reconstructing the body form of †O. megalodon. Therefore, we question whether the use of a 2.6-m-TL juvenile white shark is appropriate for the extinct shark that likely reached at least 15 m TL (Shimada, 2019; Perez et al., 2021).
The fourth and perhaps the most critical issue is their method of body form reconstruction. Cooper et al. (2022) used a computer tomographic (CT) scan of an extant white shark cranial skeleton as a hypothetical substitute for that of †O. megalodon where they superimposed their artificially reconstructed dentition based on an incomplete associated tooth set of an †O. megalodon individual from the Pliocene of North Carolina, USA, estimated to be 17.3 m in total length (TL) (Perez et al., 2021) onto the digital image of the white shark jaws. Even though the exact size of the cranial skeleton relative to the vertebral column remains uncertain based on the present fossil record, Cooper et al. (2022) then attached their cranial reconstruction to their reconstructed vertebral column based on an incomplete associated set of vertebrae of another †O. megalodon individual from the Miocene of Belgium (Figure 1B). To reconstruct the body, they scaled the full-body scan of an extant white shark so that their reconstructed vertebral column “ended at the base of the caudal fin” (Cooper et al., 2022, p. 9). Effectively, their †O. megalodon skeletal reconstruction based on the two fossil specimens served practically no purpose in inferring the body shape of †O. megalodon because the entire head and body were based on the extant white shark. Therefore, by taking this methodological assessment along with the other three aforementioned concerns into account, the validity of their 3D model of †O. megalodon is highly questionable.
A New Interpretation of †O. megalodon Body Form
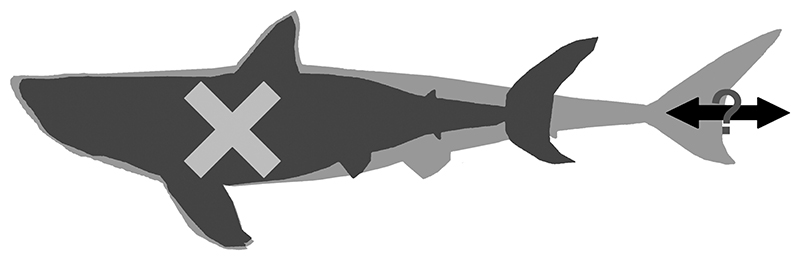
So, what did †O. megalodon actually look like? Despite their questionable reconstructions, we point out that Cooper et al.’s (2022) study is significant because it left an important clue about the body form of †O. megalodon. Their reconstructed vertebral column based on an associated vertebral set from the Miocene of Belgium was 11.1 m in length (Figure 1B) with the total length of their complete model measuring 15.9 m. The specimen is most certainly incomplete (Gottfried et al., 1996), missing an unknown number of vertebrae (see above). Yet, this specific †O. megalodon specimen was previously estimated to have come from an individual that measured 9.2 m TL (i.e., including the head and caudal fin) based on the quantitative relationship between the maximum vertebral width and TL measured from 16 extant white sharks that ranged 1.9–3.7 m TL (Gottfried et al., 1996; Shimada et al., 2021b). The vertebral centra of †O. megalodon are short, well mineralized and equipped with densely spaced radial lamellae (Leriche, 1926). This vertebral morphotype, which functionally adds architectural strength, is common within Lamniformes and characterizes both the extant white shark (Newbrey et al., 2015) and many other extinct apex predatory lamniform species (Shimada, 1997; Siverson, 1999; Amalfitano et al., 2022). Yet, the much longer vertebral column length measured by Cooper et al. (2022) (11.1 m) than the estimate based on the vertebral diameter sizes of the extant white shark (9.2 m TL) indicates that †O. megalodon had a more elongated body relative to the extant white shark (Figure 4).
Figure 4.
Previous and new schematic interpretations of †Otodus megalodon body form. A dark grey silhouette depicting the previously reconstructed †O. megalodon body form by Cooper et al. (2022) based on the extant white shark, superimposing a light grey outline showing the newly interpreted body form of †O. megalodon which is more elongated than the extant white shark. Note: it must be emphasized that this illustration should be strictly regarded as schematic as the exact extent of body elongation, the shape of the head, and the morphology and positions of the fins remain unknown based on the present fossil record.
Cooper et al. (2022) did also recognize that their reconstructed 3D model based on the Belgian fossil is “markedly longer than previously estimated for this specimen” (p. 4 of main text) and that their “initial [computer-generated] model [of †O. megalodon] appeared rather thin” (p. 16 of their Supplementary Methods). However, constrained by the underlying premise of their study using the extant white shark or Lamnidae as the modern analog for †O. megalodon, they did not consider the possibility that †O. megalodon could have had an elongated body form compared to the extant white shark. Instead, Cooper et al. (2022) attributed the discrepancy to 1) the distant phylogenetic relationship between †O. megalodon and the white shark, 2) the unknown total vertebral count and column structure in †O. megalodon, and 3) the uncertainty in whether the Miocene specimen from Belgium preserves the largest vertebral centrum from the individual. However, not only do these additional explanations make their proposition less parsimonious, their phylogenetic justification to explain the discrepancy is contradictory to their very premise of using the extant white shark as a model for †O. megalodon in the first place. Furthermore, whereas the likelihood of significantly larger vertebrae missing from the Belgian fossil specimen is rather low because diameter differences across the largest preserved centra are subtle and in a tight range (e.g., nearly 42% of the 141 preserved vertebrae measure 130–155 mm: Figure 2), the possibility that more vertebrae could be missing from the specimen would mean that their 11.1 m measurement must be regarded as the minimum possible length of the vertebral column. Alternatively, our proposition is based on evidence that is most parsimonious and empirical: i.e., 11.1 m [= minimum possible actual measured vertebral column length] > 9.2 m [total length of the same fossil individual estimated from the extant white shark].
Exactly how elongated †O. megalodon’s body was relative to the extant white shark is uncertain at the present time (Figure 4) because the extent of missing vertebrae in the associated vertebral set (Figure 1B) is unknown (Cooper et al., 2022; this study). However, besides the aforementioned new palaeontological (Shimada et al., 2023) and neontological (at least Dolton et al., 2023a, at present) evidence, our interpretation is further supported by additional anatomical evidence. In modern lamnids, centrum growth correlates with girth rather than body length (Natanson et al., 2018). White sharks have a thicker vertebral column than short-fin mako (Isurus oxyrinchus) and porbeagle (Lamna nasus) sharks at a comparable body length (Gottfried et al., 1996; Natanson et al., 2002; Doño et al., 2015) but with a similar mass (Kohler et al., 1995). More compression-resistant vertebrae may compensate for the structural issues associated with the thinner columns in shortfin makos and porbeagles (Ingle et al., 2018). The maximum diameter of the †O. megalodon vertebrae from Belgium along with the original vertebral column length of 11.1+ m indicates a vertebral column not only much thinner in relative terms than that of a white shark but also more gracile than those of smaller-bodied lamnids with known vertebral size data (Gottfried et al., 1996; Natanson et al., 2002; Doño et al., 2015). If anything, the data from living lamnids indicate a robust vertebral column in a hypothetical lamnid-like shark the size of an †O. megalodon. Therefore, the remarkably slender vertebral column of the Belgian †O. megalodon specimen raises concerns about the accuracy of girthy, lamnid-like reconstructions of this species suggested by Cooper et al. (2020, 2022). We also note that the body cross-sectional geometry in Cooper et al.’s (2022) 3D body reconstruction of †O. megalodon is rather rectangular and distorted, but it is generally elliptical in extant sharks (Tomita et al., 2021), suggesting that it is more parsimonious to consider †O. megalodon to also have had an elliptical body cross-section.
The exact body form of †O. megalodon (or any other otodontids: see Appendix 1) cannot be elucidated decisively based on the present fossil record (Sternes et al., 2023). Nevertheless, our new interpretation—that †O. megalodon had an elongated body relative to the extant white shark—has significant implications for the biology of the fossil shark, most notably because it would mean that its pleuroperitoneal cavity was likely elongated as well. †Otodus megalodon and its predecessors such as †O. chubutensis apparently occupied a trophic position similar to (McCormack et al., 2022), or possibly higher than (Kast et al., 2022), the extant white shark based on geochemical evidence, where its diet included marine mammals based on bite marks on fossil pinniped and cetacean bones (Aguilera et al., 2008; Collareta et al., 2017; Godfrey et al., 2018). The morphology of placoid scales suggests that the cruising speed of †O. megalodon was probably slower than that of the extant lamnids including the white shark, and its endothermic metabolism is thought to have been used largely to facilitate digesting large, ingested food items and enhancing nutrient absorption and processing (Shimada et al., 2023). Where digestion of food and absorption of nutrients are essential for every vertebrate (Tomita et al., 2023), endothermic fishes possess visceral countercurrent heat exchangers and retain an elevated metabolic rate from food processing (Dickson and Graham, 2004). Sharks have a spiral intestine with complex intestinal muscular activity (Tomita et al., 2023), that is thought to have evolved to increase the absorptive surface area and to reduce the unidirectional flow speed of digesta for prolonging absorptive time (Holmgren and Nilsson, 1999; Leigh et al., 2021). In fact, the spiral intestine is the warmest visceral organ in extant lamnids, along with their warm, large, lipid-rich liver associated with the suprahepatic rete (Carey et al., 1985; Bernal et al., 2001). The elongated body of †O. megalodon would imply that its liver as well as its alimentary canal, including the spiral intestine, within the body cavity may have also been long, which would have concomitantly provided more absorptive area and time with heat-induced nutrient processing efficiency. Furthermore, at least some endothermic fishes can exploit cool waters because of a warm viscera that further elevates the body core temperature (Dickson and Graham, 2004). It is conceivable that the worldwide occurrences of †O. megalodon fossils (Razak and Kocsis, 2018), including cool areas, may, at least in part, be attributed to this physiological condition.
Conclusions
Cooper et al.’s (2022) 3D reconstruction work is novel, but because the fundamental assumptions and accuracy of their 3D skeletal and body reconstructions are questionable in the first place, their entire conclusions about the lifestyle of †O. megalodon based on their 3D reconstruction must also be considered questionable. In fact, their conclusion that †O. megalodon was a fast or long-distance swimmer like the extant white shark is logically circular because their body reconstruction of the fossil shark was based on the fast-swimming, regionally endothermic lamnids including the white shark with known long-distance travel records (Weng et al., 2007; Jorgensen et al., 2010; Watanabe et al., 2015; Harding et al., 2021). The reality is that there is currently no scientific support for Cooper et al.’s (2022) or any of the previously published body forms of †O. megalodon (Gottfried et al., 1996; Cooper et al., 2020). Furthermore, our results indicate that the previously published †O. megalodon’s possible maximum body size estimates of 15–20 m TL (Shimada, 2019; Perez et al., 2021) as well as its ontogenetic growth model (Shimada et al., 2021b) based on the extant white shark are likely underestimated. We must acknowledge that, without direct fossil evidence such as a complete skeleton, extrapolation over 100 million years of otodontid or lamniform evolution and uniquely ‘off-the-scale’ gigantism of †O. megalodon among macrophagous lamniform sharks (Shimada et al., 2021a) make the direct comparison of body forms even within Lamniformes extremely challenging.
Supplementary Material
Acknowledgments
We thank A. Folie, S. Beaudart, C. Cousin, J. Lalanne, and U. Lefèvre (IRSNB) for supplying us with archival photographs and additional data of IRSNB P 9893. We also thank the following individuals who helped acquire, loaned, or conducted computer tomographic scanning and imaging of the extant shark specimens depicted in Figures: M.A. Rogers, M.W. Westneat, P. Willink, K. Swagel (FMNH), T. Clardy, W. Ludt, J.A. Seigel (LACM), S.R. Van Sommeran (Pelagic Shark Research Foundation, Capitola, California), L.M. Page, R.H. Robins (UF), C.K. Rigsby, A.C. Nicholas, K. Gray, B. Karl, and J. Hickey (Children’s Memorial Hospital, Chicago). In addition, we also thank all the anonymous reviewers, including those who reviewed the earlier versions of this manuscript, for their comments and suggestions that significantly improved the quality of this present paper. We are indebted to the editorial team of Palaeontologia Electronica for handling our manuscript with the utmost professionalism and helping make our paper further accessible to wider audiences. We acknowledge that not everyone (MS, AC) considers the megatooth species to belong to the genus †Otodus but to another otodontid genus †Carcharocles; however, this generic interpretation difference does not affect the content expressed in this paper.
Funding
National Science Foundation Sedimentary Geology and Paleobiology Award (1830581 to MLG and MAB; 1830858 to KS); University Research Council’s Competitive Research Grant, DePaul University, Chicago, Illinois (to KS); Austrian Science Fund (FWF: P33820 to JK).
Contributor Information
Phillip C. Sternes, Department of Evolution, Ecology, and Organismal Biology, University of California Riverside, Riverside, California, USA
Patrick L. Jambura, Email: patrick.jambura@gmail.com, Department of Palaeontology, University of Vienna, 1090 Vienna, Austria and Vienna Doctoral School of Ecology and Evolution (VDSEE), University of Vienna, 1030 Vienna, Austria
Julia Türtscher, Email: tuertscher.julia@gmail.com, Department of Palaeontology, University of Vienna, 1090 Vienna, Austria and Vienna Doctoral School of Ecology and Evolution (VDSEE), University of Vienna, 1030 Vienna, Austria.
Jürgen Kriwet, Email: juergen.kriwet@univie.ac.at, Department of Palaeontology, University of Vienna, 1090 Vienna, Austria and Vienna Doctoral School of Ecology and Evolution (VDSEE), University of Vienna, 1030 Vienna, Austria.
Mikael Siversson, Email: mikael.siversson@museum.wa.gov.au, Department of Earth and Planetary Sciences, Western Australian Museum, Welshpool, WA, Australia and School of Molecular and Life Sciences, Curtin University, Bentley, WA, Australia.
Iris Feichtinger, Email: Iris.Feichtinger@nhmwien.ac.at, Geological-Palaeontological Department, Natural History Museum, 1010 Vienna, Austria and University of Graz, NAWI Geocenter, Institute of Earth Sciences, Graz, Austria.
Gavin J.P. Naylor, Email: gnaylor@flmnh.ufl.edu, Florida Museum of Natural History, University of Florida, Gainesville, Florida, USA.
Adam P. Summers, Email: fishguy@uw.edu, Biology Department, University of Washington, Seattle, Washington, USA and Friday Harbor Laboratories, University of Washington, Friday Harbor, Washington, USA.
John G. Maisey, Email: maisey@amnh.org, Department of Vertebrate Paleontology, American Natural History Museum, New York, New York, USA.
Taketeru Tomita, Email: t-tomita@okichura.jp, Okinawa Churashima Research Center, Okinawa Churashima Foundation, Motobu-cho, Okinawa, Japan and Okinawa Churaumi Aquarium, Okinawa Churashima Foundation, Motobu-cho, Okinawa, Japan.
Joshua K. Moyer, Email: joshua.k.moyer@gmail.com, Department of Ecology and Evolutionary Biology, Yale University, New Haven, Connecticut, USA and Atlantic Shark Institute, Wakefield, Rhode Island, USA.
Timothy E. Higham, Email: thigham@ucr.edu, Department of Evolution, Ecology, and Organismal Biology, University of California Riverside, Riverside, California, USA.
João Paulo C.B. da Silva, Email: jpzoologia@dse.ufpb.br, Departamento de Sistemática e Ecologia, Centro de Ciências Exatas e da Natureza, Universidade Federal da Paraíba, Castelo Branco, João Pessoa, PB, 58051-900, Brazil.
Hugo Bornatowski, Email: anequim.bio@gmail.com, Center for Marine Studies, Universidade Federal do Paraná, Brazil.
Douglas J. Long, Email: dlong@calacademy.org, Department of Ichthyology, California Academy of Sciences, San Francisco, California, USA.
Victor J. Perez, Email: vjperez@smcm.edu, Environmental Studies Department, St. Mary’s College of Maryland, St. Mary’s City, Maryland, USA.
Alberto Collareta, Email: alberto.collareta@unipi.it, Dipartimento di Scienze della Terra, Università di Pisa, via S. Maria 53, 56126 Pisa, PI, Italy.
Charlie Underwood, Email: c.underwood@bbk.ac.uk, Department of Earth and Planetary Sciences, Birkbeck College, London, UK.
David J. Ward, Email: david@fossil.ws, Department of Earth Sciences, Natural History Museum, London, UK.
Romain Vullo, Email: romain.vullo@univ-rennes.fr, Univ Rennes, CNRS, Géosciences Rennes, UMR 6118, 35000 Rennes, France.
Gerardo González-Barba, Email: gerardo@uabcs.mx, Museo de Historia Natural-UABCS, Colonia El Mezquitito, CP 23080, La Paz, Baja California Sur, Mexico.
IV Harry M. Maisch, Email: hmaisch@fgcu.edu, Department of Marine and Earth Sciences, Florida Gulf Coast University, Fort Myers, Florida, USA.
Michael L. Griffiths, Email: GRIFFITHSM@wpunj.edu, Department of Environmental Science, William Paterson University of New Jersey, Wayne, New Jersey, USA.
Martin A. Becker, Email: BECKERM2@wpunj.edu, Department of Environmental Science, William Paterson University of New Jersey, Wayne, New Jersey, USA.
Jake J. Wood, Email: chondrichthyic@gmail.com, Department of Biological Sciences, DePaul University, Chicago, Illinois, USA.
Kenshu Shimada, Department of Biological Sciences, DePaul University, Chicago, Illinois, USA, Department of Environmental Science and Studies, DePaul University, Chicago, Illinois, USA, and Sternberg Museum of Natural History, Fort Hays State University, Hays, Kansas, USA.
References
- Aguilera OA, García L, Cozzuol MA. Giant-toothed white sharks and cetacean trophic interaction from the Pliocene Caribbean Paraguaná Formation. Paläontologische Zeitschrift. 2008;82:204–208. doi: 10.1007/BF02988410. [DOI] [Google Scholar]
- Amalfitano J, Dalla Vecchia FM, Carnevale G, Fornaciari E, Roghi G, Giusberti L. Morphology and paleobiology of the Late Cretaceous large-sized shark Cretodus crassidens (Dixon, 1850) (Neoselachii; Lamniformes) Journal of Paleontology. 2022;96:1155–1188. doi: 10.1017/jpa.2022.23. [DOI] [Google Scholar]
- Bernal D, Dickson KA, Shadwick RE, Graham JB. Review: analysis of the evolutionary convergence for high performance swimming in lamnid sharks and tunas. Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology. 2001;129:695–726. doi: 10.1016/s1095-6433(01)00333-6. [DOI] [PubMed] [Google Scholar]
- Carey FG, Casey JG, Pratt HL, Urquhart D, McCosker JE. Temperature, heat production and heat exchange in lamnid sharks. Memoirs of the Southern California Academy of Sciences. 1985;9:92–108. [Google Scholar]
- Casey JG, Pratt HL., Jr Distribution of the white shark, Carcharodon carcharias, in the western North Atlantic. Memoirs of the Southern California Academy of Sciences. 1985;9:2–14. [Google Scholar]
- Collareta A, Lambert O, Landini W, Di Celma C, Malinverno E, Varas-Malca R, Urbina M, Bianucci G. Did the giant extinct shark Carcharocles megalodon target small prey? Bite marks on marine mammal remains from the late Miocene of Peru. Palaeogeography, Palaeoclimatology, Palaeoecology. 2017;469:84–91. doi: 10.1016/j.palaeo.2017.01.001. [DOI] [Google Scholar]
- Compagno LJV. FAO species catalogue for fishery purposes. Food and Agriculture Organization of the United Nations; 2002. Sharks of the world: an annotated and illustrated catalogue of shark species known to date. Volume 2: bullhead, mackerel and carpet sharks (Heterodontiformes, Lamniformes and Orectolobiformes) pp. 1–269. [Google Scholar]
- Conte GL, Fanti F, Trevisani E, Gauschi P, Barbieri R, Bazzi M. Reassessment of a large lamniform shark from the Upper Cretaceous (Santonian) of Italy. Cretaceous Research. 2019;99:156–168. doi: 10.1016/j.cretres.2019.02.011. [DOI] [Google Scholar]
- Cooper JA, Pimiento C, Ferrón HG, Benton MJ. Body dimensions of the extinct megatooth shark Otodus megalodon: a 2D reconstruction. Scientific Reports. 2020;10:14596. doi: 10.1038/s41598-020-71387-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JA, Hutchinson JR, Bernvi DC, Cliff G, Wilson RP, Dicken ML, Menzel J, Wroe S, Pirlo J, Pimiento C. The extinct shark Otodus megalodon was a transoceanic superpredator: Inferences from 3D modeling. Science Advances. 2022;8:eabm9424. doi: 10.1126/sciadv.abm9424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson K, Graham JB. Evolution and consequences in endothermy in fishes. Physiological Biochemical Zoology. 2004;77:998–1018. doi: 10.1086/423743. [DOI] [PubMed] [Google Scholar]
- Dolton HR, Jackson AL, Deaville R, Hall J, Hall G, McManus G, Perkins MR, Rolfe RA, Snelling EP, Houghton JDR, Sims DW, et al. Regionally endothermic traits in the planktivorous basking sharks Cetorhinus maximus. Endangered Species Research. 2023a;51:227–232. doi: 10.3354/esr01257. [DOI] [Google Scholar]
- Dolton HR, Snelling EP, Deaville R, Jackson AL, Perkins MW, Bortoluzzi JR, Purves K, Curnick DJ, Pimiento C, Payne NL. Centralized red muscle in Odontaspis ferox and the prevalence of regional endothermy in sharks. Biology Letters. 2023b;19:20230331. doi: 10.1098/rsbl.2023.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doño F, Montealegre-Quijano S, Domingo A, Kinas PG. Bayesian age and growth analysis of the shortfin mako shark Isurus oxyrinchus in the western South Atlantic Ocean using a flexible method. Environmental Biology of Fishes. 2015;98:517–533. doi: 10.1007/s10641-014-0284-1. [DOI] [Google Scholar]
- Ferrón H. Regional endothermy as a trigger for gigantism in some extinct macropredatory sharks. PLoS ONE. 2017;12:e0185185. doi: 10.1371/journal.pone.0185185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey S, Ellwood M, Groff S, Verdin M. Carcharocles-bitten odontocete caudal vertebrae from the coastal eastern United States. Acta Palaeontologica Polonica. 2018;63:463–468. doi: 10.4202/app.00495.2018. [DOI] [Google Scholar]
- Gottfried MD, Compagno LJV, Bowman SC. In: Great White Sharks: The Biology of Carcharodon carcharias. Klimley AP, Ainley DG, editors. Academic Press; San Diego, CA: 1996. Size and skeletal anatomy of the giant megatooth shark Carcharodon megalodon; pp. 55–89. [Google Scholar]
- Griffths ML, Eagle RA, Kim SL, Flores RJ, Becker MA, Maisch HM, IV, Trayler RB, Chan RL, McCormack J, Akhtar AA, Tripati AK, et al. Endothermic physiology of extinct megatooth sharks. Proceedings of the National Academy of Sciences. 2023;120:e221815312. doi: 10.1073/pnas.2218153120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding L, Jackson A, Barnett A, Donohue I, Halsey L, Huveneers C, Meyer C, Papastamatiou Y, Semmens JM, Spencer E, Watanabe Y, et al. Endothermy makes fishes faster but does not expand their thermal niche. Functional Ecology. 2021;35:1951–1959. doi: 10.1111/1365-2435.13869. [DOI] [Google Scholar]
- Holmgren S, Nilsson S. In: Sharks, Skates, and Rays: The Biology of Elasmobranch Fishes. Hamlett WC, editor. Johns Hopkins University Press; Baltimore, U.S.A: 1999. Digestive system; pp. 144–173. [Google Scholar]
- Ingle DI, Natanson LJ, Porter ME. Mechanical behavior of shark vertebral centra at biologically relevant strains. Journal of Experimental Biology. 2018;221:188318. doi: 10.1242/jeb.188318. [DOI] [PubMed] [Google Scholar]
- Jorgensen SJ, Reeb CA, Chapple TK, Anderson S, Perle C, Van Sommeran SR, Fritz-Cope C, Brown AC, Klimley AP, Block BA. Philopatry and migration of Pacific white sharks. Proceedings of the Royal Society B. 2010;277:679–688. doi: 10.1098/rspb.2009.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast ER, Griffiths ML, Kim SL, Rao ZC, Shimada K, Becker MA, Maisch HM, Eagle RA, Clarke CA, Neumann AN, Karnes ME, et al. Cenozoic megatooth sharks occupied extremely high trophic positions. Science Advances. 2022;8:eabl6529. doi: 10.1126/sciadv.abl6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler NE, Casey JG, Turner PA. Length-weight relationships for 13 species of sharks from the western North Atlantic. Fishery Bulletin. 1995;93:412–418. [Google Scholar]
- Leigh SC, Summers AP, Hoffmann SL, German DP. Shark spiral intestines may operate as Tesla valves. Proceedings of the Royal Society B. 2021;288:20211359. doi: 10.1098/rspb.2021.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leriche M. Les poissons tertiaires de la Belgique IV Les poissons néogènes. Mémoires du Musée royal d’Histoire naturelle de Belgique. 1926;32:367–472. [Google Scholar]
- Lingham-Soliar T. Caudal fin allometry in the white shark Carcharodon carcharias: implications for locomotory performance and ecology. Naturwissenschaften. 2005;92:231–236. doi: 10.1007/s00114-005-0614-4. [DOI] [PubMed] [Google Scholar]
- McCormack J, Griffiths ML, Kim SL, Shimada K, Karnes M, Masich H, Pederzani S, Bourgon N, Jaouen K, Becker MA, Jöns N, et al. Trophic position Otodus megalodon and great white sharks through time revealed by zinc isotopes. Nature Communications. 2022;13:2980. doi: 10.1038/s41467-022-30528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natanson LJ, Mello JJ, Campana SE. Validated age and growth of the porbeagle shark (Lamna nasus) in the western North Atlantic Ocean. Fishery Bulletin. 2002;100:266–278. [Google Scholar]
- Natanson LJ, Skomal GB, Hoffmann SL, Porter ME, Goldman KJ, Serra D. Age and growth of sharks: do vertebral pairs record age? Marine and Freshwater Research. 2018;69:1440–1452. doi: 10.1071/MF17279. [DOI] [Google Scholar]
- Newbrey MG, Siversson M, Cook TD, Fotheringham AM, Sanchez RL. Vertebral morphology, dentition, age, growth and ecology of the large lamniform shark Cardabiodon ricki. Acta Palaeontologica Polonica. 2015;60:877–897. doi: 10.4202/app.2012.0047. [DOI] [Google Scholar]
- Paig-Tran EWM, Porter ME, Ferry LA, Whitenack LB. In: Biology of Sharks and Their Relatives. third edition. Carrier JC, Simpfendorfer CA, Heithaus MR, Yopak KE, editors. Boca Raton, U.S.A: CRC Press; 2022. How to build a shark: biomechanics and bioinspiration; pp. 59–103. [Google Scholar]
- Perez VJ, Leder RM, Badaut T. Body length estimation of Neogene macrophagous lamniform sharks (Carcharodon and Otodus) derived from associated fossil dentitions. Paleontologia Electronica. 2021;24:a09. doi: 10.26879/1140. [DOI] [Google Scholar]
- Razak H, Kocsis L. Late Miocene Otodus (Megaselachus) megalodon from Brunei Darussalam: body length estimation and habitat reconstruction. Neues Jahrbuch für Geologie und Paläontologie – Abhandlungen. 2018;288:299–306. doi: 10.1127/njgpa/2018/0743. [DOI] [Google Scholar]
- Shimada K. Skeletal anatomy of the Late Cretaceous lamniform shark, Cretoxyrhina mantelli from the Niobrara Chalk in Kansas. Journal of Vertebrate Paleontology. 1997;17:642–652. doi: 10.1080/02724634.1997.10011014. [DOI] [Google Scholar]
- Shimada K. The size of the megatooth shark, Otodus megalodon (Lamniformes: Otodontidae), revisited. Historical Biology. 2019;33:904–911. doi: 10.1080/08912963.2019.1666840. [DOI] [Google Scholar]
- Shimada K, Becker MA, Griffiths ML. Body, jaw, and dentition lengths of macrophagous lamniform sharks, and body size evolution in Lamniformes with special reference to ‘off-the-scale’ gigantism of the megatooth shark, Otodus megalodon. Historical Biology. 2021a;33:2543–2559. doi: 10.1080/08912963.2020.1812598. [DOI] [Google Scholar]
- Shimada K, Bonnan MF, Becker MA, Griffiths ML. Ontogenetic growth pattern of the extinct megatooth shark Otodus megalodon—implications for its reproductive biology, development, and life expectancy. Historical Biology. 2021b;33:3254–3259. doi: 10.1080/08912963.2020.1861608. [DOI] [Google Scholar]
- Shimada K, Yamaoka Y, Kurihara Y, Takakuwa Y, Maisch HM, IV, Becker MA, Eagle RA, Griffiths ML. Tessellated calcified cartilage and placoid scales of the Neogene megatooth shark, Otodus megalodon (Lamniformes: Otodontidae), offer new insights into its biology and the evolution of regional endothermy and gigantism in the otodontid clade. Historical Biology. 2023 doi: 10.1080/08912963.2023.2211597. [DOI] [Google Scholar]
- Siverson M. A new large lamniform shark from the uppermost Gearle Siltstone (Cenomanian, Late Cretaceous) of Western Australia. Transactions of the Royal Society of Edinburgh: Earth Sciences. 1999;90:49–66. [Google Scholar]
- Springer VG, Garrick JAF. A survey of vertebral numbers in sharks. Proceedings of the United States National Museum. 1964;116:73–96. doi: 10.5479/si.00963801.116-3496.73. [DOI] [Google Scholar]
- Sternes PC, Shimada K. Body forms in sharks (Chondrichthyes: Elasmobranchii), and their functional, ecological, and evolutionary implications. Zoology. 2020;140:125799. doi: 10.1016/j.zool.2020.125799. [DOI] [PubMed] [Google Scholar]
- Sternes PC, Wood JJ, Shimada K. Body forms of extant lamniform sharks (Elasmobranchii: Lamniformes), and comments on the morphology of the extinct megatooth shark, Otodus megalodon, and the evolution of lamniform thermophysiology. Historical Biology. 2023;35:139–151. doi: 10.1080/08912963.2021.2025228. [DOI] [Google Scholar]
- Tomita T, Toda M, Miyamoto K, Oka S, Ueda K, Sato K. Development of the lunate-shaped caudal fin in white shark embryos. Anatomical Record. 2018;301:1068–1073. doi: 10.1002/ar.23776. [DOI] [PubMed] [Google Scholar]
- Tomita T, Toda M, Murakumo K, Miyamoto K, Matsumoto R, Ueda K, Sato K. Volume of the whale shark and their mechanism of vertical feeding. Zoology. 2021;147:125932. doi: 10.1016/j.zool.2021.125932. [DOI] [PubMed] [Google Scholar]
- Tomita T, Murakumo K, Matsumoto R. Narrowing, twisting, and undulating: complicated movement in shark spiral intestine inferred using ultrasound. Zoology. 2023;157:126077. doi: 10.1016/j.zool.2023.126077. [DOI] [PubMed] [Google Scholar]
- Watanabe YY, Goldman KJ, Caselle JE, Chapman DD, Papastamatiou YP. Comparative analyses of animal-tracking data reveal ecological significance of endothermy in fishes. Proceedings of the National Academy of Sciences. 2015;112:6104–6109. doi: 10.1073/pnas.1500316112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng KC, Boustany AM, Pyle P, Anderson SD, Brown A, Block BA. Migration and habitat of white sharks (Carcharodon carcharias) in the eastern Pacific Ocean. Marine Biology. 2007;152:877–894. doi: 10.1007/s00227-007-0739-4. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.