Summary
Tuberculous meningitis (TBM) causes death or disability in approximately 50% of those affected and kills approximately 78200 adults every year. Antimicrobial treatment is based on regimens used for pulmonary tuberculosis which overlooks important differences between lung and brain drug distributions. TBM has a profound inflammatory component, yet only adjunctive corticosteroids have shown clear benefit. There is an active pipeline of new antitubercular drugs, and the advent of biological agents targeted at specific inflammatory pathways promise a new era of improved TBM treatment and outcomes. Yet, to date, TBM trials have been relatively small, underpowered, heterogeneous, poorly generalizable, and have had little impact on policy and practice. Progress is slow, and a new approach is required. Here, a global consortium of TBM researchers articulate a coordinated, definitive way ahead via globally conducted clinical trials of novel drugs and regimens to advance treatment and improve outcomes from this life-threatening infection.
Introduction
Tuberculous meningitis (TBM) is the most severe form of tuberculosis, causing death or disability in 50% of those affected1. An estimated 78,200 adult deaths result from TBM annually, with 35% of these in people living with HIV/AIDS (PWH). Despite such poor patient outcomes, little progress has been made over the last 40 years to identify evidence-based therapies and strategies that reduce death and disability from TBM; the exception being trials demonstrating that adjunctive corticosteroids reduce death in HIV-negative adults and children with TBM2,3.
Randomised controlled trials (RCT) in TBM are urgently required. This urgency comes at a time of opportunity for improving patient outcomes: the pipeline of new antitubercular drugs is more active than it has been for 50 years, and the advent of biological agents targeted at specific inflammatory molecules or pathways (e.g. TNF), both promise a new era of improved TBM therapy. Yet, to date, TBM trials have been relatively small, underpowered, heterogeneous, poorly generalisable, and have had little impact on policy. Progress is slow, and a new approach is due.
Recognising TBM to be a neglected form of tuberculosis, the Tuberculous Meningitis International Research Consortium was established in 2009. The consortium has contributed several reviews on aspects of epidemiology, pathogenesis, diagnosis, and management of TBM4–18. Membership includes over 100 researchers and TBM trial centres in India, Indonesia, Madagascar, South Africa, Uganda, and Vietnam. At consortium meetings in Oxford (2022) and in Cape Town (2023), a new approach to TBM trials and therapeutic development was conceived. A working group was formed, seeking to reach consensus agreement on the design, execution, and funding of future TBM clinical trials. This personal view summarises current knowledge on the therapeutic landscape for TBM, articulates a consensus view on research priorities, and sets out recommendations to accelerate improvements in TBM outcomes through globally conducted clinical trials.
Therapeutic goals
Effectively control Mycobacterium tuberculosis
TBM became a treatable disease in the 1940s with the discovery of the first antitubercular drugs, streptomycin and para-aminosalicylic acid. The bacterial killing induced by these two drugs reduced mortality from 100% to around 70%19. However, whilst bacterial killing is necessary for survival from TBM, the correlation between increased bacterial killing and better clinical outcomes has been elusive. This may be due to the difficulties of measuring killing with relatively few bacteria in cerebrospinal fluid (CSF), and the confounding influence of inflammation upon outcome. Clinical studies of TBM caused by isoniazid-resistant bacteria showed that resistance was associated with reduced times to CSF sterility, which in turn was associated with worse outcomes, especially in PWH20. However, clinical trials of ‘optimised’ antitubercular regimens that result in greater brain drug exposures have had mixed results. A trial of higher dose rifampicin (13 mg/kg given intravenously) confirmed higher drug exposure and documented increased survival21, but a much larger trial did not show any benefit of adding higher dose rifampicin (15 mg/kg orally) and levofloxacin to standard therapy unless disease was caused by isoniazid-resistant bacteria22. Nevertheless, despite equivocal findings of previous studies, improved brain penetration and thus faster bacterial killing may lead to better outcomes. This motivates testing new and potentially more active antitubercular drugs for TBM, and mandates associated pharmacometric studies that will enable better understanding of the complex relationship between drug exposure, bacterial killing, and clinical outcome23.
Control host inflammation
Excessive host inflammation contributes to death and disability from TBM. Whilst limited evidence exists to guide adjunctive therapies, a landmark trial in Vietnam demonstrated that 6-8 weeks adjunctive dexamethasone reduced 9-month mortality in a predominantly HIV-negative group of adults and adolescents with TBM, though with no impact on disability3. However, current doses of corticosteroids may be insufficient to prevent and reduce host inflammation in all TBM patients, particularly in PWH24 and in the context of high dose rifampicin which increases corticosteroid clearance25. The recently published ACT-HIV trial (dexamethasone for TBM in PWH) from Vietnam and Indonesia did not conclusively establish a benefit of dexamethasone on survival in PWH26. In paediatric TBM, corticosteroids have also shown clinical benefit27. However, more targeted approaches to reducing tissue damaging host responses are emerging as understanding of TBM immunopathology deepens.
Prevent and manage secondary neurological complications
Neurological complications majorly contribute to poor outcome from TBM. Most manifestations, including raised intracranial pressure, hydrocephalus, cerebral infarction, paradoxical reactions, and seizures, are a direct consequence of intracranial inflammation, emphasising the need to prioritise evaluation of more effective anti-inflammatory therapies. However, evidence-based interventions for adjunctive neurocritical care in TBM are also needed28. Trials defining the management of these important complications have never been conducted. This is partly because of resource constraints in high burden settings, for example limited access to expertise for ventricular drainage, and because of substantial challenges in trial design and implementation.
Antitubercular chemotherapy
General considerations
Current antitubercular chemotherapy for TBM remains based on that used in pulmonary tuberculosis and does not account for distinct disease characteristics in TBM that require specific therapeutic considerations. Use of the present regimen for TBM is not based on bespoke trials, rather on progress made in the derivation of ‘short course’ regimens for pulmonary tuberculosis. In contrast with pulmonary tuberculosis, where overall mortality is relatively low and the treatment goal is long-term relapse-free cure, TBM therapy must reduce early mortality and longer-term neurological disability. A primary consideration for TBM treatment is to select effective antitubercular drugs that rapidly achieve therapeutic concentrations at the site of disease. Drug penetration into brain tissue is key: CSF drug concentrations are often used but are an indirect and suboptimal measure of brain tissue penetration. There is limited pharmacometric evidence to support the composition, doses, or duration of the current standard of care for TBM. Other characteristics of an ideal TBM regimen include activity against drug-resistant M. tuberculosis strains, particularly isoniazid-resistant M. tuberculosis, which is common but infrequently detected in TBM; a low propensity for serious adverse reactions that may lead to premature discontinuation; and comprising drugs that can be dosed in neurocritical illness and that are accessible in high burden settings.
Selection of new antitubercular drugs and regimens
Ongoing and recently completed unpublished trials are shown in Table 1. All actively recruiting phase 3 trials of antitubercular therapy are investigating high-dose rifampicin (35 mg/kg), often in combination with linezolid; several are powered to demonstrate reductions in mortality. Higher dose isoniazid is also being evaluated. Doses and composition of these experimental regimens were selected based on pharmacokinetic (PK)/pharmacodynamic (PD) data from patients with pulmonary tuberculosis (using sputum culture conversion as the efficacy measure) and small trials in TBM showing inconsistent effects on clinical outcome. Nevertheless, these ongoing studies will provide high-quality evidence to inform treatment guidelines. Future trials should avoid repeating evaluation of these regimens (which may become standard of care and serve as a control in future trials, if successful) and take different approaches to regimen selection.
Table 1. Current and planned clinical trials in tuberculous meningitis.
| # | Trial | N | Enrolment Status | Estimated Completion | Registration | Population | Intervention |
|---|---|---|---|---|---|---|---|
| 1 | ACT TBM | 237 | 100% | Complete | CTRI/2019/08/020488 | Adults | ASA or CLOPI or standard care |
| 2 | SIMPLE | 36 | 100% | Complete | NCT03537495 | Adult | High dose RIF + LZD |
| 3 | ALTER | 40 | 100% | Complete, unpublished | NCT04021121 | Adults | High or standard dose RIF + LZD |
| 4 | LAST ACT | 720 | 100% | Mar 2024 | NCT03100786 | HIV- Adults | Corticosteroids stratified by LTA4H genotype |
| 5 | HARVEST | 500 | 53% | Nov 2024 | ISRCTN15668391 | Adults | High dose RIF |
| 6 | INTENSE TBM | 768 | 43% | Aug 2025 | NCT04145258 | Adults, >15yo | High dose RIF+LZD +/-ASA |
| 7 | TIMPANI | 130 | 0% | Dec 2025 | NCT05590455 | HIV+ Adults | Adalimumab (TNF inhibitor) |
| 8 | SURE | 400 | 80% | Dec 2025 | ISRCTN40829906 | Children + adolescents | High dose RIF and INH + LFX w/ or w/o ASA |
| 9 | IMAGINE TBM | 330 | 5% | Feb 2027 | NCT05383742 | Adults, >15yo | High dose RIF and INH + LZD x 6 mos. |
| 10 | INSHORT | 372 | 0% | Sept 2027 | NCT05917340 | Adults | High dose RIF + moxifloxacin + ASA x 6 mos. |
Short form titles or descriptions: ACT TBM = A Randomised Trial to Assess the efficacy or add on therapy with Aspirin or Clopidogrel to the standard medical therapy alone in patients with Tuberculous meningitis. SIMPLE = Pharmacokinetic Study of Linezolid for TB Meningitis. ALTER = Adjunctive Linezolid for the Treatment of Tuberculous Meningitis. LAST ACT = Leukotriene A4 Hydrolase Stratified Trial of Adjunctive Corticosteroids for HIV-uninfected Adults with Tuberculous Meningitis. TIMPANI = TNF Inhibitors to Reduce Mortality in HIV-1 Infected Patients with Tuberculous meningitis. HARVEST = High-dose oral rifampicin to improve survival from adult tuberculous meningitis. INTENSE-TBM = Intensified Tuberculosis Treatment to Reduce the Mortality of Patients with Tuberculous Meningitis. SURE = Short intensive treatment for children with tuberculous meningitis. IMAGINE-TBM = Improved Management with Antimicrobial Agents Isoniazid Rifampicin Linezolid for TBM. HDH Trial = Optimizing Antituberculosis Therapy in Adults with Tuberculous Meningitis. ASA=aspirin. CLOPI=clopidogrel. HIV=human immunodeficiency virus. INH=isoniazid. LTA4H=leukotriene A4 hydrolase. LFX=levofloxacin. LZD=linezolid. NAT-2. N-acetyltransferease-2. RIF=rifampicin. TNF=tumour necrosis factor.
Given the inability to perform frequent direct brain sampling in patients with TBM and the limitations of using CSF as a proxy for site of disease drug exposure, pre-clinical disease models are a promising strategy to optimise and select new drugs to enter TBM trials29. A translational TBM model should recapitulate key elements of human disease, including similar time course and clinical manifestations, typical histopathology, and compatible bacterial load and distribution. Importantly, TBM models should employ dosing schedules that are used in patients30. These features enable better predictive ability and may serve as potential efficacy markers. Animal models enable estimation of drug penetration within any CNS compartment at human-equivalent doses (determined by approximating plasma drug exposures from patients), which can be extrapolated to inform regimen design for trials. The underlying hypothesis is that rapid attainment of maximal effect exposures for a potent antitubercular agent at the site of disease in a relevant TBM model may translate into clinical efficacy. A major limitation of TBM models (and clinical trials) is lack of a predictive PD marker for treatment response. Application of efficacy measures from pulmonary tuberculosis, such as decline in bacterial load, do not necessarily predict clinical treatment response in TBM. This may lead to incorrect conclusions around clinical effectiveness of new drugs, with a risk of down-selecting potentially good candidates or promoting drugs that may not perform well in patients. Other limitations of preclinical models in TBM include altered oral bioavailability of combination regimens due to intolerance of high drug volumes during administration and differences in drug metabolism across species limiting evaluation of PK variability and drug-drug interactions.
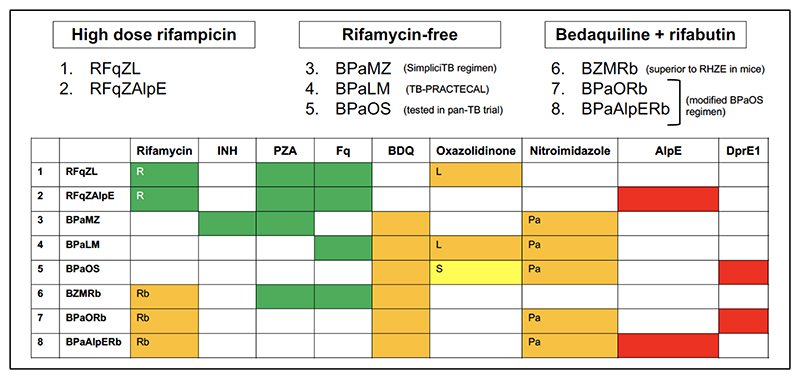
Pre-clinical TBM models provide invaluable information about site-of-disease drug exposure but, because of their inherent limitations, TBM regimen selection needs to be informed by additional parameters aligned with the target regimen profile. These include antitubercular activity; observed clinical efficacy in TBM; safety and tolerability; potential for PK drug-drug interactions; and access in high burden settings. We propose a ranking system to select individual drugs for inclusion in experimental regimens for TBM (Table 2), using the approach adopted by the NIH ACTIV platform COVID-19 trials therapeutic agent selection committee (https://www.nih.gov/research-training/medical-research-initiatives/activ). New regimens of interest are constructed from individual agent rankings (Table 3), plus other considerations including in vitro synergy, combining different mechanisms of action, efficacy in pulmonary tuberculosis, and activity against drug resistant tuberculosis. Using this approach, three regimen categories emerge: (1) rifampicin-based; (2) bedaquiline-based; and (3) rifabutin-bedaquiline based (Figure 1).
Table 2. A scoring system to select drugs for inclusion in experimental regimens.
| Anti-TB activity | Clinical efficacy data in TBM | Site of disease exposure | Safety/tolerability | Drug-drug interactions | Access | |
|---|---|---|---|---|---|---|
| 3 | Potent bactericidal sterilising activity in vivo | Potentially therapeutic concentrations in brain parenchyma from animal models and/or non-invasive human studies at human-equivalent doses | ||||
| 2 | Moderate in vivo activity, mainly related to EBA | Benefit in RCT | Detectable concentrations in brain parenchyma but possibly below therapeutic thresholds | Well- tolerated at optimised doses with low toxicity potential | Affordable and available in target countries, oral administration | |
| 1 | Weak in vivo activity at tolerable doses | Benefit in non- randomised studies, sub- groups in RCT, or case reports/series | Detectable in CSF only or not yet studied in brain parenchyma | Generally well-tolerated but may have treatment- limiting AE | No clinically relevant DDI | Affordable and available in target countries, IV administration |
| 0 | In vitro data only | No data from trials or case reports | Insufficient / no data to judge | Poorly tolerated but acceptable safety profile | Clinically important DDI | Expensive and/or not registered in target countries |
| No go | No in vivo activity (EBA) attolerable doses | RCT data shows no effect | Undetectable in brain parenchyma at human- equivalent doses | Poorly tolerated and/or frequent treatment- limiting AE | Precludes use in TBM regimens | Not currently manufactured |
Individual drugs are ranked by adding points from each parameter; the higher number of points the higher the priority for inclusion in experimental TBM regimens. Extra weighting is applied to anti-TB activity and site of disease exposure by creating categories for higher point allocation. Lower weighting is applied to clinical efficacy in TBM because new agents are less likely to have been evaluated in clinical trials. Similarly, safety/tolerability is weighted less because drug efficacy is prioritised for this condition with high early mortality. Drug-drug interactions and access are assigned fewer available points because of limited categories. AE=adverse events. DDI=drug-drug interactions. EBA=early bactericidal activity. IV=intravenous. RCT=randomised controlled trial. TBM=tuberculous meningitis.
Table 3. Characteristics of individual drugs for use in TBM.
| Anti-TB activity |
Clinical efficacy in TBM |
Site of disease exposure |
Safety/ tolerability |
Drug-drug interactions |
Access | |
|---|---|---|---|---|---|---|
| Rifampicin | 3 | 2 | 3 | 2 | 0 | 2 |
| Isoniazid | 3 | 2 | 3 | 2 | 1 | 2 |
| Linezolid 74 | 2 | 2 | 3 | 1 | 1 | 2 |
| Fluoroquinolones 50 | 3 | 2 | 3 | 2 | 1 | 2 |
| Pyrazinamide | 2 | 2 | 3 | 2 | 1 | 2 |
| Pretomanid/delamanid 75 | 3 | 1 | 3 | 2 | 1 | 2 |
| Bedaquiline | 3 | 1 | 2 | 2 | 0 | 2 |
| Alpibectir/ethionamide | 2 | 0 | 3 | 2 | 1 | |
| Rifabutin | 3 | 0 | 2 | 2 | 0 | 2 |
| Clofazimine | 1 | 0 | 1 | 2 | 1 | 2 |
| Ethionamide | 1 | 2 | 2 | 1 | 2 | |
| Cycloserine | 1 | 0 | 2 | 1 | 2 | |
| Ethambutol | 1 | 2 | 1 | 2 | ||
| DprE1 inhibitors | 3 | 0 | 0 | 2 | 1 |
Scores derived from the scoring system in Table 2.
Figure 1. Potential regimens based on individual drug rankings.
B = bedaquiline, H = isoniazid, L = linezolid, M = moxifloxacin, O = DprE1 inhibitor, Pa = pretomanid, Rb = rifabutin, S = sutezolid, AlpE = alpibectir/ethionamide, Z = pyrazinamide, R = rifampicin (high dose), Fq = fluoroquinolone, E = ethambutol. Coloured cells relate to individual drug scores on the ranking system, indicated in Table 3: green ≥ 12; orange 9 – 11; yellow < 9; red not currently manufactured.
Towards rifampicin-free regimens for TBM
It remains possible that the ongoing trials, particularly if data are pooled, will establish the role of high-dose rifampicin in TBM. Future trials should therefore only plan evaluation of rifampicin-based regimens under a scenario where results of current studies are equivocal or indicate a need for further evaluation of high-dose rifampicin, possibly in combination with other agents. The development of an effective rifampicin-free regimen is a priority given the global threat of rifampicin-resistant tuberculosis. Drug-resistant TBM is associated with extremely high mortality and is under-recognised because of limited diagnostic sensitivity31. An individualised approach to treatment of TBM is therefore not possible and treatment regimens that cover drug-resistant disease are needed. An expanding evidence base supports this approach and creates conditions of potential equipoise to exclude rifampicin from treatment regimens in TBM. First, rifampicin concentrations at the site of disease in TBM animal models are variable and spatially heterogeneous32. In patients, rifampicin penetration into CSF is relatively poor compared to other antitubercular drugs33–35. Second, although several PK endpoint trials have suggested improved outcomes, rifampicin use has not conclusively led to survival benefit in randomised trials and pooled analyses, even at higher doses22,34,36. The perceived essentiality of rifampicin therapy for TBM is further undermined by case reports of treatment success with rifamycin-free regimens in patients with recognised rifampicin-resistant TBM. Third, rifamycin-free, bedaquiline-containing regimens perform better (cure and bactericidal) in mouse models of pulmonary tuberculosis and are highly successful in trials and clinical practice for patients with pulmonary tuberculosis, achieving comparable cure rates to standard rifampicin-based therapy for drug-susceptible tuberculosis37–39 In this context, investigating the effectiveness of bedaquiline (BDQ)-based regimens for TBM is a priority.
Considerations for bedaquiline-based regimens
A highly lipophilic drug with extensive tissue distribution, bedaquiline has potential to concentrate within brain tissue. However, there are analytical challenges to quantifying bedaquiline in CNS compartments (binding to collection tubes, requirement for highly sensitive assays) and physiological barriers to CNS entry (extensive protein binding). Pre-clinical and clinical data on the CNS distribution of bedaquiline are limited; early investigations have shown measurable concentrations of total (bound and unbound fractions) drug in CSF from patients with pulmonary tuberculosis and relatively higher brain exposures in rodents40–42. The bedaquiline brain concentration associated with efficacy is unknown, but even limited exposure of this potent drug at the site of disease may provide benefit in TBM which is paucibacillary, particularly in combination with other effective agents. More detailed study of bedaquiline PK in representative animal disease models is a research priority.
Synergies with existing and new antitubercular agents that achieve high site of disease exposures may enhance potential efficacy of bedaquiline in TBM. Combining bedaquiline with pretomanid, linezolid, and moxifloxacin (BPaLM) has been highly successful for pulmonary tuberculosis and represents a promising regimen for TBM because of favourable PK characteristics of individual drugs. Similarly, the combination of BPaM plus pyrazinamide (BPaMZ), which was highly effective in the SimpliciTB trial for pulmonary tuberculosis, would likely achieve therapeutic concentrations in the CNS. However, hepatic toxicity from the combination of pyrazinamide plus pretomanid reduces enthusiasm for evaluation in TBM43. While fluoroquinolones have been extensively investigated and there is accumulating experience with linezolid in TBM, human pharmacometric data are limited for pretomanid (and delamanid, a different nitroimidazole that can be used in children) and there are no clinical studies investigating use in TBM (Table 3).
Another strategy is to combine bedaquiline with rifabutin, a rifamycin drug that, unlike rifampicin, does not result in clinically important increases in bedaquiline clearance44,45. Rifabutin has similar efficacy to rifampicin in (non-TBM) pre-clinical models and in observational patient cohorts46. Although there are no data on CNS penetration of rifabutin from TBM patients, several lines of evidence support potential efficacy in this condition. Rifabutin achieves high concentrations in CSF of healthy non-human primates47 (estimated free rifabutin CSF/plasma ratio 2.4–3.4), it is measurable in CSF of PWH48 and was effective in a rabbit model of pneumococcal meningitis49. Addition of rifabutin to a core regimen of bedaquiline plus pyrazinamide and moxifloxacin (BZM), both of which have excellent CNS penetration35,50, resulted in additive efficacy and had similar bactericidal and superior sterilising activity to standard therapy in a pulmonary tuberculosis mouse model experiment51. This regimen should be prioritised for clinical evaluation pending additional data confirming rifabutin exposure at site of disease in animal TBM models and CSF from tuberculosis patients.
Future drug options
New compounds emerging from the pulmonary tuberculosis development pipeline may have a role in future TBM regimens. Sutezolid, a new generation oxazolidinone with a lower propensity for mitochondrial toxicity, is being evaluated as a replacement for linezolid in bedaquiline-based regimens for pulmonary tuberculosis. DprE1 inhibitors, offering a novel mechanism of action and potent antitubercular activity, are also in clinical development for pulmonary tuberculosis, including in combination with bedaquiline, pyrazinamide and moxifloxacin. Pharmacokinetic data need to be generated for sutezolid and DprE1 inhibitors before entering clinical trials for TBM. Another prospect is alpibectir (BVL-GSK098), a novel compound that increases bioactivation of ethionamide, requiring lower ethionamide doses to obtain rapid bactericidal activity when the two agents are combined (AlpE). AlpE is in active development for TBM based on a suite of favourable characteristics including good CNS penetration, activity against isoniazid resistant M. tuberculosis, improved tolerance, and limited drug-drug interactions, including with rifampicin. Ganfeborole (formerly GSK 3036656) targets M. tuberculosis leucyl-tRNA synthetase, inhibiting protein synthesis with rapid mycobacterial killing and sterilising ability52. Ganfeborole is not expected to be affected by drug-drug interactions with rifampicin, raising possibilities for combination with rifamycins and other novel antitubercular agents for TBM if CNS penetration is confirmed.
Host-directed therapy (HDT)
General considerations
Interventions with anti-inflammatory effects are required to reduce immunopathology and consequent mortality and disability. There is rationale to investigate targeted therapies, directed at inflammatory molecules or pathways central to TBM pathophysiology that may complement or replace corticosteroids. The heterogeneity of inflammatory response between individuals suggest certain subgroups may derive more benefit from HDT than others. Examples include TBM in PWH, which is associated dysregulated inflammation, high mortality, and unclear benefit from corticosteroids; and patients developing paradoxical worsening during treatment who often require intensification of corticosteroid therapy. Identification of specific clinical phenotypes (for example, severity and nature of inflammation at baseline estimated by clinical, radiological or CSF markers) or genotypes (for example, variations in leukotriene A4 hydrolase (LTA4H) associated with distinct inflammatory phenotypes) that predict individual risk and treatment response is a priority. Mechanistic investigations nested in interventional trials can provide important insights. The LAST-ACT trial, which provides corticosteroids based on LTA4H genotype, is an example of targeted anti-inflammatory approach developed from translational studies in TBM53. However, until such time as other treatment-defining subgroups emerge, the priority is to evaluate an intervention that offers potential benefit to all patients, with possibility to identify subgroups for targeted intervention at a later stage.
TNF-α antagonists
Tumour necrosis factor (TNF) is central to the immunopathology of TBM54. Retrospective case series data are promising for anti-TNF directed therapies of thalidomide and infliximab55–59. Initial enthusiasm for thalidomide was diminished after a trial among children with TBM in South Africa found an association with increased adverse effects and death when dosed at 24 mg/kg/day60. A more recent retrospective cohort study showed that much lower doses of 3-5 mg/kg/day thalidomide demonstrated satisfactory clinical and radiological response in 37/38 children with CNS tuberculosis-related complications56. However, there are ongoing barriers to thalidomide use for TBM including limited accessibility high cost, concerns about teratogenicity, and dose-related toxicity (e.g. neuropathy).
Specific TNF antagonists, particularly infliximab, are attractive options for definitive evaluation in TBM trials. A large case series provides strong preliminary support for safety and efficacy in TBM58, and there is accumulating clinical experience with use for paradoxical reactions in CNS TB. Infliximab is widely used for other inflammatory conditions with established safety in adults and children61–63 and no signal of major infection complications, including inflammatory bowel disease where there is high risk of bacterial translocation. Increased risk of tuberculosis is a lesser concern as all individuals will receive antitubercular chemotherapy. Cost is not expected to be a major limitation as generic preparations are now available, although consideration must be given to availability of, and access to, infliximab or biosimilars after the trial. The optimal dose and number of infusions of infliximab for TBM remains uncertain and pharmacometric data would help to optimise use. An additional event-driven randomisation to a second dose of infliximab (or another host directed therapy) for a subgroup with neuro-deterioration with inflammatory complications may be considered. This decision could be based on the performance of infliximab when provided to all participants at study entry (if successful it may substantially reduce delayed complications and reduce the need for a second randomisation).
Other considerations for host directed therapy
Detailed investigations of TBM immunopathology from phase 2 trials, observational studies, and animal models can generate alternatives to corticosteroids (Table 4). Small trials suggest that adjunctive aspirin may provide safe and beneficial anti-inflammatory effects in children and adults with TBM64–67, supporting ongoing phase 3 (Table 1).
Table 4. Host directed therapies for TBM.
| Activity in TBM | Clinical use | CNS exposure | Safety | |
|---|---|---|---|---|
| Corticosteroids | Large RCTs: Adults: 25% lower mortality 3, smaller effect for MRC grade 2/3 and with longer follow up 71; no effect on disability; uncertain effect in HIV 26. Scarce data among African adults and Asian paediatric TBM | Guideline- recommended for all patients with TBM, including IRIS and paradoxical reactions | Good |
Excellent in
TBM RCT 76 |
| Aspirin | Small RCT: Possibly fewer new-onset strokes at high doses among adults with TBM 65 | Not in routine clinical use, evaluated in adults and children with new TBM diagnosis | Good | No signal of severe bleeding events 77 |
| Thalidomide | Individual case reports of resolution from mass lesions and blindness related to optochiasmatic arachnoiditis (children) | Steroid-refractory TBM or paradoxical reactions | Good | Dose related toxicity, paediatric RCT stopped prematurely for safety 60 |
| TNF blockers (infliximab) | Case series 57,59,78 and matched retrospective cohort 58 showing clinical benefit in TBM | Steroid-refractory TBM or paradoxical reactions | Good | No serious safety signals, Risk of secondary infection |
|
Anti-IL1
(anakinra) |
Case reports in TBM 70,79 | Steroid-refractory TBM or paradoxical reactions | Good | Good safety profile, associated with mild neutropenia |
| mTOR inhibitors | RCT: Less post-TB lung disease | No experience in TBM | Unknown | Well tolerated in an RCT for PTB |
| JAKi |
Cases reports for HLH /
HLH-TB 80 |
No experience in TBM | Good safety profile, associated with VZV/HSV |
Immunopathological studies have implicated multiple cytokines in TBM pathogenesis68,69. Use of the IL-1 receptor antagonist anakinra has been described in both PWH and TBM and HIV-negative TBM70. However, costs and accessibility currently preclude use in low-resource settings, and a need for daily intravenous or subcutaneous administration present challenges. Other immunomodulatory agents may offer potential benefit, such as JAK-inhibitors (e.g. baricitinib) which have broader anti-inflammatory activity and good safety profile, however, their evaluation in any form of tuberculosis hitherto has been very limited.
Evaluating therapies in a global trial
Key study populations
TBM affects all age groups but is especially common amongst young children and in PWH. Therefore, therapeutic trials, particularly phase 3 trials, should include these patient groups. It is also essential that all disease severities are included in future trials. Some previous trials of corticosteroids excluded those with mild disease (MRC grade 1), believing inflammation, and therefore likely benefit, was less in these patients27. However, the 2004 Vietnam trial showed that whilst dexamethasone benefited all three MRC severity grades, and sustained benefit beyond 2 years was only seen in those with grade 1 disease71. Conversely, there is risk of systematic exclusion of patients with more severe disease without capacity to provide informed consent themselves, and ethics committees should support mechanisms for obtaining surrogate consent to provide the sickest patients an opportunity for trial participation.
The limited sensitivity of current diagnostic tests for TBM means ascertaining the true population with TBM is difficult. In 2010, the TBM consortium published a uniform case definition for TBM that is now widely used to categorise TBM research participants into definite, probable, possible, and not TBM72. This case classification is applied retrospectively after all diagnostic information has returned thus is not practical for eligibility at enrolment. Enrolling cases of suspected TBM, based on clinician intention to treat for TBM, is the most pragmatic approach for phase 3 trials and reflects real-world clinical practice. However, this may result in enrolment of cases eventually re-classified with a different diagnosis. Cases of possible TBM who are treated for TBM are heterogenous across different settings, with approaches to commencing antitubercular chemotherapy particularly influenced by the HIV and tuberculosis prevalence in that population. Therefore, increased sample sizes might be required recognising that a small proportion might not have TBM and therefore may respond differently to new interventions.
Sites and countries
TBM is a disease of poverty. It is commonest in settings least able to deliver the clinical care and research required to reduce its frequently fatal consequences. TBM research must therefore promote and expand research capacity in less well-resourced or developed centres, building a sustainable global infrastructure and community capable of performing high-quality clinical research. There is a core of centres, developed over the last 20 years, that now have established track records of performing TBM trials, in India, Indonesia, Madagascar, South Africa, Uganda, and Vietnam. To date, they have tended to conduct their trials independently. Coordination and collaboration between these centres could enable annual trial recruitment rates of around 1000 participants/year, which would have a dramatic effect on the speed and power of future TBM trials.
Trial design
For some drugs and regimens, further evidence is required from phase 2 evaluation of safety and PK before they can enter practice-defining phase 3 trials. Phase 2 trials can also be exploited for mechanistic investigation that may identify targets for immune modulation and translational evaluation. However, in the absence of predictive treatment response biomarkers, phase 2 trials are unable to provide actionable information on efficacy because they are underpowered for disability and mortality, the only available efficacy measures in TBM. The current approach, where independent centres conduct small and sequential phase 2 then 3 trials of single interventions leads to decades-long delays before new drugs or regimens benefit patients. In this context, phase 3 evaluation of promising interventions may be justifiable without phase 2 trials with demonstrable site of disease exposure from preclinical models and safety data from PTB.
A global multi-arm, potentially multi-stage, factorially randomised (antitubercular chemotherapy and anti-inflammatory drugs), controlled trial could address many of the current obstacles to improving outcomes from TBM. A platform trial offers the ability to study different interventions in parallel and to introduce new interventions over time, including at site level based on accessibility and other local considerations. Having a master protocol agnostic to interventions studied with the ability to add a next intervention could also maximise overall trial impact, if one intervention ends early (for superiority or harm). This design has much appeal but introduces funding challenges that need further exploration with relevant agencies.
Statistical considerations
A large trial is needed to demonstrate superiority of new antitubercular and anti-inflammatory regimens over standard of care. Assuming typical mortality, a trial of 900 patients per arm would be required to detect a 20% reduction in mortality with 90% power. Making no presumption of interaction between antitubercular and anti-inflammatory drugs, an efficient design is factorial randomization across two domains:
Domain 1: Antitubercular therapy. The trial would ideally investigate at least three regimens, with one of them being a standard-of-care regimen (following WHO recommendations). For practical reasons (rifampicin stains secretions red) this randomisation would likely be open label, but outcome assessors would be blinded.
Domain 2: HDT. The priority intervention is infliximab versus standard of care (corticosteroids). Infliximab would be combined with corticosteroids in the experimental groups. This randomisation could be blinded with a placebo saline infusion. As the efficacy of HDT regimens may differ between people with and without HIV, a basket trial design could be adopted with partial pooling across the two groups.
Outcomes
The TBM consortium have published two consensus statements (2017 and 2019) concerning standardised methods for enhancing the quality and comparability of TBM studies, with recommendations for primary and secondary outcomes for phase 2 and 3 TBM clinical trials (Table 5)12,15. Improving disability-free survival is the primary objective of successful TBM treatment. Thus, a composite endpoint of death and severe disability at 1 year from randomisation is recommended for phase 3 TBM trials. The Modified Rankin Score (MRS) is widely used to assess functional disability after stroke and has been used in many recent TBM trials73. Choosing an appropriate MRS cut-off to define disability is complicated by differing cultural perceptions and consequences of disability, often related to the resources available for the long-term care of disabled individuals. Patient and community stakeholder engagement is needed to identify culturally appropriate outcomes that are desired by persons afflicted with TBM. Health economic endpoints (DALY, QALY) should be acquired to understand the impacts of new interventions on individuals and society and provide essential information for policy makers.
Table 5. Outcomes according to phase.
| Phase | Primary endpoint(s) | Secondary endpoint(s) |
|---|---|---|
| II | Adverse events of special interest (AESI) Serious adverse events (SAE) Pharmacokinetic analyses |
Drug-drug interactions Treatment interruption (tolerability) Mortality and disability Biomarkers of treatment response (pathogen and host inflammation) |
| III | Mortalitya at 12 mo. Disability by MRS at 12 mo. |
Change in GCS or MRC grade Change in neuroimaging Occurrence of new events Occurrence of paradoxical deterioration or TBM-IRIS Cognitive status at 12 mo. SAE with grade and relatedness Duration of hospitalisation AESI Treatment interruption (with reason and duration) Health Economic (improvement in DALY and QALY) |
Important secondary outcomes include the occurrence of the common intracerebral (e.g. stroke) and extracerebral (e.g. hyponatraemia) disease complications. Longer-term cognitive impairment is well recognised, especially in children, but poorly studied, partly because of the complexity of assessment methods15. Cognitive assessments may therefore only be possible in selected centres, but they represent an essential substudy.
Some pragmatism is justified to support delivery of a global phase 3 trial, particularly if the safety profile of the intervention is well-known. Safety reporting should focus on serious treatment-related adverse events rather than those related to the severity and complications of the disease.
Governance and sponsorship
Models of sponsorship and governance should reflect the importance of keeping the centre of gravity of TBM research within low- and middle-income countries (LMIC), thus enabling local decision-making and building capacity, expertise and leadership for future research. Engagement with industry will be essential, given the need to test new drugs. Trials will therefore need to meet the regulatory standards of conduct, necessary to allow global approval of new drugs for TBM treatment. This will undoubtedly be challenging but forms an essential part of building a global infrastructure to conduct TBM trials. Given the need to test multiple drugs, potentially from different companies, an umbrella sponsorship model will be required, ideally from an LMIC-based academic institution. The role for contract research organisations is anticipated to be small, as they are perceived to substantially increase cost and complexity, to excessively emphasize regulatory compliance above operational efficiency, and fail to build local trial infrastructure and expertise for future trials.
Conclusions and future directions
Only adequately powered, definitive trials with clinical endpoints that are relevant to patients and their carers can address the unacceptable outcomes in TBM. This goal requires a recognition from funders that it is not reasonable to neglect the most serious form of tuberculosis on the grounds that it does not contribute to transmission and thus global elimination targets. To do so marginalises already vulnerable populations in which this disease is common and misses opportunity to improve overall treatment of tuberculosis, with enormous potential benefits to individuals and communities. EndTB targets – 90% reduction in deaths by 2030 - can only be achieved if the most severe forms of TB are tackled. Efficient and pragmatic clinical trials, presented in this personal view, would evaluate several readily implementable interventions for communities most affected by TBM in a cost-effective manner. This represents an opportunity for funders to make investments with direct and lasting benefit for many people in LMIC. Risk could be mitigated through seamless phase 2/3 evaluation of novel therapies and with innovative funding models that involve multiple stakeholders, including drug manufacturers. The Tuberculous Meningitis International Research Consortium will continue working towards improving treatment for people with TBM through high quality clinical trials.
Funding
This work was funded by an Academy of Medical Sciences Global Challenges Research Fund Networking Grant awarded to Dr Sean Wasserman (University of Cape Town, South Africa) and Dr Joseph Donovan (London School of Hygiene and Tropical Medicine, London, United Kingdom) (GCRFNGR8\1090). Additional support was provided by Wellcome (203135). RJW and JRB is funded by the Francis Crick Institute which receives support from Wellcome (CC2112), UKRI-Medical Research Council (CC2112) and Cancer Research UK (CC2112). RJW also receives support from NIH (R01AI145436), Meningitis Now, EDCTP (RIA2017T-2019 109237) and in part from the NIHR Biomedical Research Centre of Imperial College NHS Trust. DRB receives support from the National Institute of Allergy and Infectious Diseases (R01AI162786, R01AI145437). SW is supported by NIH (K43TW011421, U01AI170426, and UM1AI068636) and the Bill & Melinda Gates Foundation. RvC (and GT, AR, DI and NTTT) receive funding for TBM research from NIH (R01AI145781 and R01AI165721). SM received funding from the National Research Foundation of South Africa [Grant Number 132051]. JAW is a Sir Henry Dale Fellow funded by the Wellcome Trust (223253/Z/21/Z). FVC is funded by Wellcome (300088/Z/23/Z). AGD receives funding from Meningitis Now UK. SM received funding from the National Research Foundation of South Africa [Grant Number 132051]. UKR is supported by the Wellcome Trust (224176/Z/21/Z). RSS received funding from the National Research Foundation of South Africa [Grant Number 150174].
Footnotes
Author contributions
RJW and GET conceived the idea of a large-scale trial and sought engagement from suitably qualified investigator groups potentially capable of contribution to such a study. Online and an in-person meetings were convened to define key themes and responsibility to develop those themes was devolved to interest groups who in turn report back to plenary calls. JD, SW, EK, GET, JAW and RJW wrote the personal view with editorial input and consensus from all other authors.
Conflict of interest statement
FVC has received grant funding from Johnson & Johnson and ViiV. GET has acted as a consultant for Bioversys. The following grants to institutions are declared: Wellcome (RJW, AF, KHS, FVC), Cancer Research UK (RJW); Medical Research Council UK (RJW); National Institutes of Health (RJW, FC, KED, DRB, RvC); Meningitis Now (RJW); National Research Foundation of South Africa (RS, RJW, SM, AF); South African Medical Research Council (AF); UK Academy of Medical Sciences (JD); European and Developing Countries Clinical Trials Partnership (RJW, MR). The other authors declared no conflicts of interest.
References
- 1.Dodd PJ, Osman M, Cresswell FV, et al. The global burden of tuberculous meningitis in adults: A modelling study. PLOS Glob Public Health. 2021;1(12):e0000069. doi: 10.1371/journal.pgph.0000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schoeman JF, Elshof JW, Laubscher JA, Janse van Rensburg A, Donald PR. The effect of adjuvant steroid treatment on serial cerebrospinal fluid changes in tuberculous meningitis. Ann Trop Paediatr. 2001;21(4):299–305. doi: 10.1080/07430170120093481. [DOI] [PubMed] [Google Scholar]
- 3.Thwaites GE, Nguyen DB, Nguyen HD, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351(17):1741–51. doi: 10.1056/NEJMoa040573. [DOI] [PubMed] [Google Scholar]
- 4.Bahr NC, Marais S, Caws M, et al. GeneXpert MTB/Rif to Diagnose Tuberculous Meningitis: Perhaps the First Test but not the Last. Clin Infect Dis. 2016;62(9):1133–5. doi: 10.1093/cid/ciw083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyles TH, Lynen L, Seddon JA Tuberculous Meningitis International Research C. Decision-making in the diagnosis of tuberculous meningitis. Wellcome Open Res. 2020;5:11. doi: 10.12688/wellcomeopenres.15611.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cresswell FV, Davis AG, Sharma K, et al. Recent Developments in Tuberculous Meningitis Pathogenesis and Diagnostics. Wellcome Open Res. 2019;4:164. doi: 10.12688/wellcomeopenres.15506.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis AG, Donovan J, Bremer M, et al. Host Directed Therapies for Tuberculous Meningitis. Wellcome Open Res. 2020;5:292. doi: 10.12688/wellcomeopenres.16474.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis AG, Nightingale S, Springer PE, et al. Neurocognitive and functional impairment in adult and paediatric tuberculous meningitis. Wellcome Open Res. 2019;4:178. doi: 10.12688/wellcomeopenres.15516.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donovan J, Cresswell FV, Thuong NTT, et al. Xpert MTB/RIF Ultra for the Diagnosis of Tuberculous Meningitis: A Small Step Forward. Clin Infect Dis. 2020;71(8):2002–5. doi: 10.1093/cid/ciaa473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donovan J, Rohlwink UK, Tucker EW, et al. Checklists to guide the supportive and critical care of tuberculous meningitis. Wellcome Open Res. 2019;4:163. doi: 10.12688/wellcomeopenres.15512.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imran D, Hill PC, McKnight J, van Crevel R Tuberculous Meningitis International Research C. Establishing the cascade of care for patients with tuberculous meningitis. Wellcome Open Res. 2019;4:177. doi: 10.12688/wellcomeopenres.15515.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marais BJ, Heemskerk AD, Marais SS, et al. Standardized Methods for Enhanced Quality and Comparability of Tuberculous Meningitis Studies. Clin Infect Dis. 2017;64(4):501–9. doi: 10.1093/cid/ciw757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marais S, Van Toorn R, Chow FC, et al. Management of intracranial tuberculous mass lesions: how long should we treat for? Wellcome Open Res. 2019;4:158. doi: 10.12688/wellcomeopenres.15501.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Misra UK, Kalita J Tuberculous Meningitis International Research C. Mechanism, spectrum, consequences and management of hyponatremia in tuberculous meningitis. Wellcome Open Res. 2019;4:189. doi: 10.12688/wellcomeopenres.15502.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohlwink UK, Chow FC, Wasserman S, et al. Standardized approaches for clinical sampling and endpoint ascertainment in tuberculous meningitis studies. Wellcome Open Res. 2019;4:204. doi: 10.12688/wellcomeopenres.15497.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seddon JA, Tugume L, Solomons R, Prasad K, Bahr NC Tuberculous Meningitis International Research C. The current global situation for tuberculous meningitis: epidemiology, diagnostics, treatment and outcomes. Wellcome Open Res. 2019;4:167. doi: 10.12688/wellcomeopenres.15535.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seddon JA, Wilkinson R, van Crevel R, Figaji A, Thwaites GE Tuberculous Meningitis International Research C. Knowledge gaps and research priorities in tuberculous meningitis. Wellcome Open Res. 2019;4:188. doi: 10.12688/wellcomeopenres.15573.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkinson RJ, Rohlwink U, Misra UK, et al. Tuberculous meningitis. Nat Rev Neurol. 2017;13(10):581–98. doi: 10.1038/nrneurol.2017.120. [DOI] [PubMed] [Google Scholar]
- 19.Loffler W, Moeschlin S. [Combined streptomycin and paraaminosalicylic acid (PAS) therapy of tuberculous meningitis, compared with streptomycin therapy alone] Schweiz Med Wochenschr. 1950;80(15):365–72. [PubMed] [Google Scholar]
- 20.Thwaites GE, Lan NT, Dung NH, et al. Effect of antituberculosis drug resistance on response to treatment and outcome in adults with tuberculous meningitis. J Infect Dis. 2005;192(1):79–88. doi: 10.1086/430616. [DOI] [PubMed] [Google Scholar]
- 21.Ruslami R, Ganiem AR, Dian S, et al. Intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis: an open-label, randomised controlled phase 2 trial. Lancet Infect Dis. 2013;13(1):27–35. doi: 10.1016/S1473-3099(12)70264-5. [DOI] [PubMed] [Google Scholar]
- 22.Heemskerk AD, Bang ND, Mai NT, et al. Intensified Antituberculosis Therapy in Adults with Tuberculous Meningitis. N Engl J Med. 2016;374(2):124–34. doi: 10.1056/NEJMoa1507062. [DOI] [PubMed] [Google Scholar]
- 23.Heemskerk AD, Nguyen MTH, Dang HTM, et al. Clinical Outcomes of Patients With Drug-Resistant Tuberculous Meningitis Treated With an Intensified Antituberculosis Regimen. Clin Infect Dis. 2017;65(1):20–8. doi: 10.1093/cid/cix230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thuong NTT, Heemskerk D, Tram TTB, et al. Leukotriene A4 Hydrolase Genotype and HIV Infection Influence Intracerebral Inflammation and Survival From Tuberculous Meningitis. J Infect Dis. 2017;215(7):1020–8. doi: 10.1093/infdis/jix050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McAllister WA, Thompson PJ, Al-Habet SM, Rogers HJ. Rifampicin reduces effectiveness and bioavailability of prednisolone. Br Med J (Clin Res Ed) 1983;286(6369):923–5. doi: 10.1136/bmj.286.6369.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donovan J, Bang ND, Imran D, et al. Adjunctive Dexamethasone for Tuberculous Meningitis in HIV-Positive Adults. N Engl J Med. 2023;389(15):1357–67. doi: 10.1056/NEJMoa2216218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoeman JF, Van Zyl LE, Laubscher JA, Donald PR. Effect of corticosteroids on intracranial pressure, computed tomographic findings, and clinical outcome in young children with tuberculous meningitis. Pediatrics. 1997;99(2):226–31. doi: 10.1542/peds.99.2.226. [DOI] [PubMed] [Google Scholar]
- 28.Donovan J, Figaji A, Imran D, Phu NH, Rohlwink U, Thwaites GE. The neurocritical care of tuberculous meningitis. Lancet Neurol. 2019;18(8):771–83. doi: 10.1016/S1474-4422(19)30154-1. [DOI] [PubMed] [Google Scholar]
- 29.Litjens CHC, Aarnoutse RE, Te Brake LHM. Preclinical models to optimize treatment of tuberculous meningitis - A systematic review. Tuberculosis (Edinb) 2020;122:101924. doi: 10.1016/j.tube.2020.101924. [DOI] [PubMed] [Google Scholar]
- 30.Lanni F, Antilus Sainte R, Hansen M, Jr, et al. A preclinical model of TB meningitis to determine drug penetration and activity at the sites of disease. Antimicrob Agents Chemother. 2023;67(12):e0067123. doi: 10.1128/aac.00671-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huynh J, Donovan J, Phu NH, Nghia HDT, Thuong NTT, Thwaites GE. Tuberculous meningitis: progress and remaining questions. Lancet Neurol. 2022;21(5):450–64. doi: 10.1016/S1474-4422(21)00435-X. [DOI] [PubMed] [Google Scholar]
- 32.Ruiz-Bedoya CA, Mota F, Tucker EW, et al. High-dose rifampin improves bactericidal activity without increased intracerebral inflammation in animal models of tuberculous meningitis. J Clin Invest. 2022;132(6) doi: 10.1172/JCI155851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cresswell FV, Meya DB, Kagimu E, et al. High-Dose Oral and Intravenous Rifampicin for the Treatment of Tuberculous Meningitis in Predominantly Human Immunodeficiency Virus (HIV)-Positive Ugandan Adults: A Phase II Open-Label Randomized Controlled Trial. Clin Infect Dis. 2021;73(5):876–84. doi: 10.1093/cid/ciab162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding J, Thuy Thuong Thuong N, Pham TV, et al. Pharmacokinetics and Pharmacodynamics of Intensive Antituberculosis Treatment of Tuberculous Meningitis. Clin Pharmacol Ther. 2020;107(4):1023–33. doi: 10.1002/cpt.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pouplin T, Bang ND, Toi PV, et al. Naive-pooled pharmacokinetic analysis of pyrazinamide, isoniazid and rifampicin in plasma and cerebrospinal fluid of Vietnamese children with tuberculous meningitis. BMC Infect Dis. 2016;16:144. doi: 10.1186/s12879-016-1470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Svensson EM, Dian S, Te Brake L, et al. Model-Based Meta-analysis of Rifampicin Exposure and Mortality in Indonesian Tuberculous Meningitis Trials. Clin Infect Dis. 2020;71(8):1817–23. doi: 10.1093/cid/ciz1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conradie F, Bagdasaryan TR, Borisov S, et al. Bedaquiline-Pretomanid-Linezolid Regimens for Drug-Resistant Tuberculosis. N Engl J Med. 2022;387(9):810–23. doi: 10.1056/NEJMoa2119430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conradie F, Diacon AH, Ngubane N, et al. Treatment of Highly Drug-Resistant Pulmonary Tuberculosis. N Engl J Med. 2020;382(10):893–902. doi: 10.1056/NEJMoa1901814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nyang’wa BT, Berry C, Kazounis E, et al. A 24-Week, All-Oral Regimen for Rifampin-Resistant Tuberculosis. N Engl J Med. 2022;387(25):2331–43. doi: 10.1056/NEJMoa2117166. [DOI] [PubMed] [Google Scholar]
- 40.Upton CM, Steele CI, Maartens G, Diacon AH, Wiesner L, Dooley KE. Pharmacokinetics of bedaquiline in cerebrospinal fluid (CSF) in patients with pulmonary tuberculosis (TB) J Antimicrob Chemother. 2022;77(6):1720–4. doi: 10.1093/jac/dkac067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ordonez AA, Carroll LS, Abhishek S, et al. Radiosynthesis and PET Bioimaging of (76)Br-Bedaquiline in a Murine Model of Tuberculosis. ACS Infect Dis. 2019;5(12):1996–2002. doi: 10.1021/acsinfecdis.9b00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pamreddy A, Baijnath S, Naicker T, et al. Bedaquiline has potential for targeting tuberculosis reservoirs in the central nervous system. RSC Adv. 2018;8(22):11902–7. doi: 10.1039/c8ra00984h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cevik M, Thompson LC, Upton C, et al. Bedaquiline-pretomanid-moxifloxacin-pyrazinamide for drug-sensitive and drug-resistant pulmonary tuberculosis treatment: a phase 2c, open-label, multicentre, partially randomised controlled trial. Lancet Infect Dis. 2024 doi: 10.1016/S1473-3099(24)00223-8. [DOI] [PubMed] [Google Scholar]
- 44.Healan AM, Griffiss JM, Proskin HM, et al. Impact of Rifabutin or Rifampin on Bedaquiline Safety, Tolerability, and Pharmacokinetics Assessed in a Randomized Clinical Trial with Healthy Adult Volunteers. Antimicrob Agents Chemother. 2018;62(1) doi: 10.1128/AAC.00855-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Healan AM, Salata RA, Griffiss JM, et al. Effects of Rifamycin Coadministration on Bedaquiline Desmethylation in Healthy Adult Volunteers. Clin Pharmacol Drug Dev. 2019;8(4):436–42. doi: 10.1002/cpdd.639. [DOI] [PubMed] [Google Scholar]
- 46.Ji B, Truffot-Pernot C, Lacroix C, et al. Effectiveness of rifampin, rifabutin, and rifapentine for preventive therapy of tuberculosis in mice. Am Rev Respir Dis. 1993;148(6 Pt 1):1541–6. doi: 10.1164/ajrccm/148.6_Pt_1.1541. [DOI] [PubMed] [Google Scholar]
- 47.Strolin Benedetti M, Pianezzola E, Brughera M, Fraier D, Castelli MG. Concentrations of rifabutin in plasma and cerebrospinal fluid in cynomolgus monkeys. J Antimicrob Chemother. 1994;34(4):600–3. doi: 10.1093/jac/34.4.600. [DOI] [PubMed] [Google Scholar]
- 48.Siegal FP, Eilbott D, Burger H, et al. Dose-limiting toxicity of rifabutin in AIDS-related complex: syndrome of arthralgia/arthritis. AIDS. 1990;4(5):433–41. doi: 10.1097/00002030-199005000-00009. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt H, Zysk G, Reinert RR, et al. Rifabutin for experimental pneumococcal meningitis. Chemotherapy. 1997;43(4):264–71. doi: 10.1159/000239577. [DOI] [PubMed] [Google Scholar]
- 50.Thwaites GE, Bhavnani SM, Chau TT, et al. Randomized pharmacokinetic and pharmacodynamic comparison of fluoroquinolones for tuberculous meningitis. Antimicrob Agents Chemother. 2011;55(7):3244–53. doi: 10.1128/AAC.00064-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tasneen R, Garcia A, Converse PJ, et al. Novel Regimens of Bedaquiline-Pyrazinamide Combined with Moxifloxacin, Rifabutin, Delamanid and/or OPC-167832 in Murine Tuberculosis Models. Antimicrob Agents Chemother. 2022;66(4):e0239821. doi: 10.1128/aac.02398-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diacon AH, Barry CE, 3rd, Carlton A, et al. A first-in-class leucyl-tRNA synthetase inhibitor, ganfeborole, for rifampicin-susceptible tuberculosis: a phase 2a open-label, randomized trial. Nat Med. 2024;30(3):896–904. doi: 10.1038/s41591-024-02829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Donovan J, Phu NH, Thao LTP, et al. Adjunctive dexamethasone for the treatment of HIV-uninfected adults with tuberculous meningitis stratified by Leukotriene A4 hydrolase genotype (LAST ACT): Study protocol for a randomised double blind placebo controlled non-inferiority trial. Wellcome Open Res. 2018;3:32. doi: 10.12688/wellcomeopenres.14007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barnacle JR, Davis AG, Wilkinson RJ. Recent advances in understanding the human host immune response in tuberculous meningitis. Front Immunol. 2023;14:1326651. doi: 10.3389/fimmu.2023.1326651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marais BJ, Cheong E, Fernando S, et al. Use of Infliximab to Treat Paradoxical Tuberculous Meningitis Reactions. Open Forum Infect Dis. 2021;8(1):ofaa604. doi: 10.1093/ofid/ofaa604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Toorn R, Zaharie SD, Seddon JA, et al. The use of thalidomide to treat children with tuberculosis meningitis: A review. Tuberculosis (Edinb) 2021;130:102125. doi: 10.1016/j.tube.2021.102125. [DOI] [PubMed] [Google Scholar]
- 57.Armange L, Lacroix A, Petitgas P, et al. The use of TNF-alpha antagonists in tuberculosis to control severe paradoxical reaction or immune reconstitution inflammatory syndrome: a case series and literature review. Eur J Clin Microbiol Infect Dis. 2023;42(4):413–22. doi: 10.1007/s10096-023-04564-2. [DOI] [PubMed] [Google Scholar]
- 58.Manesh A, Gautam P, Kumar DS, et al. Effectiveness of Adjunctive High-Dose Infliximab Therapy to Improve Disability-Free Survival Among Patients With Severe Central Nervous System Tuberculosis: A Matched Retrospective Cohort Study. Clin Infect Dis. 2023;77(10):1460–7. doi: 10.1093/cid/ciad401. [DOI] [PubMed] [Google Scholar]
- 59.Santin M, Escrich C, Majos C, Llaberia M, Grijota MD, Grau I. Tumor necrosis factor antagonists for paradoxical inflammatory reactions in the central nervous system tuberculosis: Case report and review. Medicine (Baltimore) 2020;99(43):e22626. doi: 10.1097/MD.0000000000022626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schoeman JF, Springer P, van Rensburg AJ, et al. Adjunctive thalidomide therapy for childhood tuberculous meningitis: results of a randomized study. J Child Neurol. 2004;19(4):250–7. doi: 10.1177/088307380401900402. [DOI] [PubMed] [Google Scholar]
- 61.Kelsen JR, Grossman AB, Pauly-Hubbard H, Gupta K, Baldassano RN, Mamula P. Infliximab therapy in pediatric patients 7 years of age and younger. J Pediatr Gastroenterol Nutr. 2014;59(6):758–62. doi: 10.1097/MPG.0000000000000533. [DOI] [PubMed] [Google Scholar]
- 62.Mori M, Imagawa T, Hara R, et al. Efficacy and limitation of infliximab treatment for children with Kawasaki disease intractable to intravenous immunoglobulin therapy: report of an open-label case series. J Rheumatol. 2012;39(4):864–7. doi: 10.3899/jrheum.110877. [DOI] [PubMed] [Google Scholar]
- 63.Burns JC, Roberts SC, Tremoulet AH, et al. Infliximab versus second intravenous immunoglobulin for treatment of resistant Kawasaki disease in the USA (KIDCARE): a randomised, multicentre comparative effectiveness trial. Lancet Child Adolesc Health. 2021;5(12):852–61. doi: 10.1016/S2352-4642(21)00270-4. [DOI] [PubMed] [Google Scholar]
- 64.Davis AG, Wilkinson RJ. Aspirin in tuberculous meningitis. EClinicalMedicine. 2021;35:100871. doi: 10.1016/j.eclinm.2021.100871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mai NTH, Dobbs N, Phu NH, et al. A randomised double blind placebo controlled phase 2 trial of adjunctive aspirin for tuberculous meningitis in HIV-uninfected adults. Elife. 2018;7 doi: 10.7554/eLife.33478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Misra UK, Kalita J, Sagar B, Bhoi SK. Does adjunctive corticosteroid and aspirin therapy improve the outcome of tuberculous meningitis? Neurol India. 2018;66(6):1672–7. doi: 10.4103/0028-3886.246278. [DOI] [PubMed] [Google Scholar]
- 67.Schoeman JF, van Rensburg Janse, Laubscher JA, Springer P. The role of aspirin in childhood tuberculous meningitis. J Child Neurol. 2011;26(8):956–62. doi: 10.1177/0883073811398132. [DOI] [PubMed] [Google Scholar]
- 68.Misra UK, Kalita J, Srivastava R, Nair PP, Mishra MK, Basu A. A study of cytokines in tuberculous meningitis: clinical and MRI correlation. Neurosci Lett. 2010;483(1):6–10. doi: 10.1016/j.neulet.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 69.Simmons CP, Thwaites GE, Quyen NT, et al. The clinical benefit of adjunctive dexamethasone in tuberculous meningitis is not associated with measurable attenuation of peripheral or local immune responses. J Immunol. 2005;175(1):579–90. doi: 10.4049/jimmunol.175.1.579. [DOI] [PubMed] [Google Scholar]
- 70.van Arkel C, Boeree M, Magis-Escurra C, et al. Interleukin-1 receptor antagonist anakinra as treatment for paradoxical responses in HIV-negative tuberculosis patients: A case series. Med. 2022;3(9):603–11.:e2. doi: 10.1016/j.medj.2022.07.001. [DOI] [PubMed] [Google Scholar]
- 71.Torok ME, Nguyen DB, Tran TH, et al. Dexamethasone and long-term outcome of tuberculous meningitis in Vietnamese adults and adolescents. PLoS One. 2011;6(12):e27821. doi: 10.1371/journal.pone.0027821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marais S, Thwaites G, Schoeman JF, et al. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis. 2010;10(11):803–12. doi: 10.1016/S1473-3099(10)70138-9. [DOI] [PubMed] [Google Scholar]
- 73.Davis AG, Wasserman S, Stek C, et al. A Phase 2A Trial of the Safety and Tolerability of Increased Dose Rifampicin and Adjunctive Linezolid, With or Without Aspirin, for Human Immunodeficiency Virus-Associated Tuberculous Meningitis: The LASER-TBM Trial. Clin Infect Dis. 2023;76(8):1412–22. doi: 10.1093/cid/ciac932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abdelgawad N, Wasserman S, Abdelwahab MT, et al. Linezolid population pharmacokinetic model in plasma and cerebrospinal fluid among patients with tuberculosis meningitis. J Infect Dis. 2023 doi: 10.1093/infdis/jiad413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mota F, Ruiz-Bedoya CA, Tucker EW, et al. Dynamic (18)F-Pretomanid PET imaging in animal models of TB meningitis and human studies. Nat Commun. 2022;13(1):7974. doi: 10.1038/s41467-022-35730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prasad K, Singh MB, Ryan H. Corticosteroids for managing tuberculous meningitis. Cochrane Database Syst Rev. 2016;4(4):CD002244. doi: 10.1002/14651858.CD002244.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rohilla R, Shafiq N, Malhotra S. Efficacy and safety of aspirin as an adjunctive therapy in tubercular meningitis: A systematic review and meta-analysis. EClinicalMedicine. 2021;34:100819. doi: 10.1016/j.eclinm.2021.100819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abo YN, Curtis N, Osowicki J, et al. Infliximab for Paradoxical Reactions in Pediatric Central Nervous System Tuberculosis. J Pediatric Infect Dis Soc. 2021;10(12):1087–91. doi: 10.1093/jpids/piab094. [DOI] [PubMed] [Google Scholar]
- 79.Keeley AJ, Parkash V, Tunbridge A, et al. Anakinra in the treatment of protracted paradoxical inflammatory reactions in HIV-associated tuberculosis in the United Kingdom: a report of two cases. Int J STD AIDS. 2020;31(8):808–12. doi: 10.1177/0956462420915394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hansen S, Alduaij W, Biggs CM, et al. Ruxolitinib as adjunctive therapy for secondary hemophagocytic lymphohistiocytosis: A case series. Eur J Haematol. 2021;106(5):654–61. doi: 10.1111/ejh.13593. [DOI] [PubMed] [Google Scholar]